 Open Access
Open Access
ARTICLE
Prognostic Value of Circulating Tumor Cells and Cancer Associated Macrophage-Like Cells in Metastatic Non-Small Cell Lung Cancer Patients: A Retrospective Exploratory Analysis
1 Department of Molecular Medicine, Sapienza University of Rome, Rome, 00161, Italy
2 Medical Oncology, Sant’Andrea University Hospital, Sapienza University of Rome, Via di Grottarossa 1035–1039, Rome, 00189, Italy
3 Medical Oncology, Department of Radiological, Oncological and Pathological Science, Policlinico Umberto I, Sapienza University of Rome, Viale Regina Elena 324, Rome, 00161, Italy
4 Department of Life Science, Health and Health Professions, Link Campus University, Via del Casale di San Pio V, Rome, 00165, Italy
5 Department of Experimental Medicine, Sapienza University, Rome, 00161, Italy
* Corresponding Author: Marco Siringo. Email:
# These authors contributed equally to this work
§ These authors share senior authorship
(This article belongs to the Special Issue: Liquid Biopsy: A Powerful Tool for Exploring Tumor Biology)
Oncology Research 2026, 34(2), 11 https://doi.org/10.32604/or.2025.069832
Received 01 July 2025; Accepted 26 November 2025; Issue published 19 January 2026
Abstract
Objectives: Although immune checkpoint inhibitors (ICIs) and targeted therapies have reshaped treatment non-small cell lung cancer (NSCLC) paradigms, prognosis remains poor for many patients due to delayed diagnosis and resistance mechanisms. Liquid biopsy offers a minimally invasive approach to monitoring tumor evolution. Among circulating biomarkers, circulating tumor cells (CTCs) and cancer-associated macrophage-like cells (CAM-Ls) may provide complementary prognostic insights. The study aimed to evaluate the prognostic role of CTC and CAM-Ls dynamic in metastatic NSCLC patients. Methods: We retrospectively analyzed 77 patients with metastatic NSCLC who underwent CTC and CAM-L evaluation via the CellSearch® system at baseline (T0) and after three months of first-line treatment (T1) including chemotherapy, targeted therapy, or ICIs. Survival outcomes were analyzed using Kaplan-Meier and Cox regression analyses. Results: Conversion to CTC-negative status at T1 was associated with improved outcomes, with median overall survival (OS) and progression-free survival (PFS) of 33 and 18 months, respectively, vs. 10 and 6 months in persistently positive patients (both p < 0.001). CTC negativity at T1 remained an independent prognostic factor for OS (HR: 6.68) and PFS (HR: 5.91, both p < 0.0001). CAM-L positivity at T1 also correlated with longer OS (30 vs. 12 months) and PFS (13 vs. 6 months, both p < 0.0001), particularly among ICI-treated patients. Combined CTC and CAM-L assessment further refined risk stratification. Conclusions: Dynamic monitoring of CTCs and CAM-Ls provides actionable prognostic information in metastatic NSCLC. CTC-negative status predicted longer OS and PFS, while CAM-L positivity at T1 was associated with improved outcomes, particularly in ICI-treated patients. Combined assessment of both biomarkers may directly inform therapeutic decision-making, through early detection of outcomes.Keywords
Non-Small Cell Lung Cancer (NSCLC) represents approximately 85% of all lung cancer cases and remains the leading cause of cancer-related mortality worldwide [1]. Advances in molecular biology and tumor genomics have identified actionable alterations, leading to targeted therapies that improved prognosis and quality of life in selected patients [2]. In parallel, immune checkpoint inhibitors (ICIs), particularly those targeting the PD-1/PD-L1 axis, have transformed treatment of oncogene-negative NSCLC and are now standard first-line options, either alone or in combination with chemotherapy, according to PD-L1 expression levels [3–5].
Despite significant advancements in the therapeutic landscape the prognosis for many NSCLC patients remains poor, largely due to late-stage diagnosis and the development of resistance mechanisms [6,7]. Consequently, there is a critical need for robust biomarkers that can guide therapeutic decision-making, enable real-time monitoring of treatment response, and predict disease progression.
In recent years, liquid biopsy has emerged as a minimally invasive approach capable of capturing the molecular and cellular heterogeneity of tumors through the analysis of circulating biomarkers, including circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), extracellular vesicles (EVs) and microRNAs (miRNAs) [8,9].
Among these, CTCs have attracted particular attention due to their involvement in the metastatic cascade and their potential to offer a real-time snapshot of tumor evolution. Despite their promise, the clinical utility of CTCs in NSCLC is still limited by challenges in detection sensitivity, inter-platform variability, and the absence of standardized methodologies [10].
The CellSearch® system, the first FDA-approved platform for the enumeration of CTCs in certain metastatic malignancies, including breast, prostate, and colorectal cancers, has also demonstrated prognostic significance in NSCLC [11,12]. However, its broader clinical utility in NSCLC is limited due to inherently low CTC counts in many patients, variability in detection thresholds, and a lack of consensus on standardized cut-offs for clinical decision-making. These limitations contribute to inconsistent sensitivity and challenge the integration of CellSearch® into routine oncological workflows for NSCLC [13].
Notably, this system also enables the identification of additional blood-based biomarkers, including Cancer-Associated Macrophage-Like Cells (CAM-Ls), a recently described population of circulating myeloid cells characterized by their phagocytosis of tumor-derived material [14–16]. CAM-Ls have been identified across a range of malignancies and are notably absent in benign conditions, indicating potential diagnostic specificity. Furthermore, their presence, as well as morphological characteristics such as size and quantity, has been correlated with poor prognosis in several solid tumors. While preliminary data indicate that CAM-Ls may serve as indicators of treatment response, their clinical utility in NSCLC remains underexplored [17,18].
The aim of the present study to assess the potential for clinical utility of serial CTC and CAM-L enumeration as a tool for early treatment monitoring for populations defined by different first-line treatment type (ICIs vs. non-ICIs).
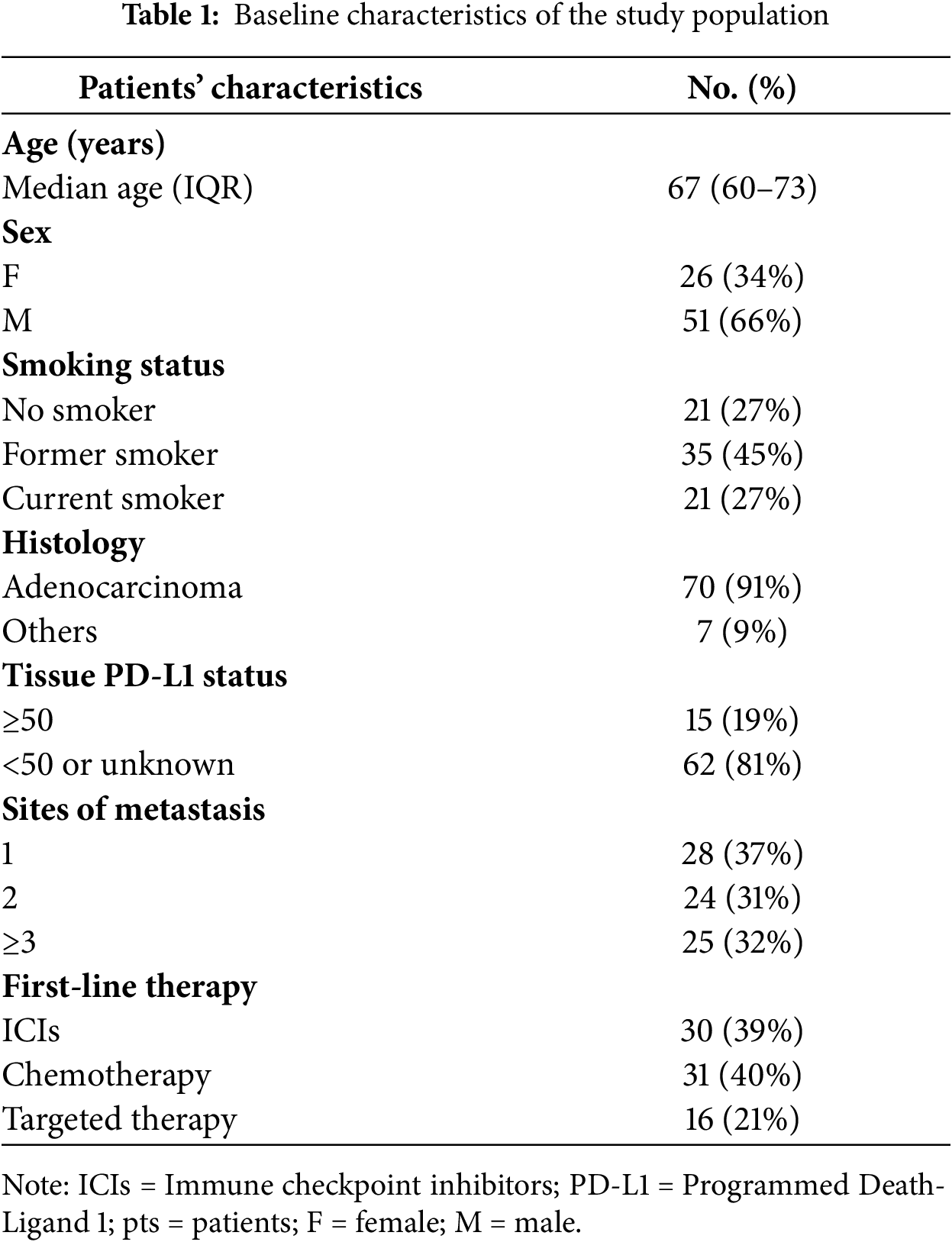
One hundred twenty patients were consecutively enrolled at Policlinico Umberto I of Rome, Italy. All patients had a diagnosis of metastatic NSCLC. Tissue PD-L1 status, valuated by immunohistochemistry, and molecular profile, assessed through next generation sequencing (NGS), were determined according to Italian guidelines. The mandatory inclusion criteria were the availability of CTC and CAM-L counts both at baseline (T0) and after three months of first-line treatment (T1), diagnosis of metastatic NSCLC. We excluded patients who received previous systemic treatment, had concomitant tumors, or had incomplete clinical data. Detailed information can be found in Table 1.
Additional data collected included patient demographics, tumor histology, date of metastatic disease onset, number of metastatic sites, and treatment history.
Treatments received in the metastatic setting included chemotherapy, immunotherapy with ICIs and targeted therapy (EGFR and ALK inhibitors). Population was subclassified in two groups according to therapeutic treatment: ICIs and non-ICIs. ICIs population included patients without actionable alterations and PD-L1 status ≥50%, according to international guidelines.
Informed consent was obtained from all patients. This study was approved by the Institutional Review Board of Policlinico Umberto I of Rome (protocol n. 668/09, 09 July 2009; amended protocol 179/16, 01 March 2016) and is valis throughout the study period. The study was conducted in accordance with the Declaration of Helsinki.
2.2 CTC and CAM Categorizations
Patients were first categorized as CTC-negative or CTC-positive at baseline and at first follow-up (T1), using CTC ≥1 as cut-off for positivity, consistent with prior publications studies [18,19]. The same classification was performed on CAM-L, considering the cut-off of CAM-L ≥1 as positivity.
The first follow-up point (T1) was defined as 3 months ± 15 days after initiation of first-line treatment, allowing a 2-week window to accommodate scheduling variations and ensure comparability of results across patients.
All CTC and CAM-L analyses were performed in a single certified laboratory by operators blinded to patients’ clinical outcomes. To ensure consistency and reproducibility, quality control procedures included internal calibration, standardized processing protocols, repeated measurements for a subset of samples, standardized positive and negative control samples in each batch and verification of inter-operator consistency through repeated measurements on selected samples.
2.3 CTC and CAM-L Analysis Procedure
Peripheral blood (7.5 mL) was obtained from each patient at baseline and after three months of first-line treatment. Samples were drawn into CellSave tubes (Menarini Silicon Biosystems, Castel Maggiore, Italy), which contain EDTA and a cell-stabilizing fixative. Tubes were kept at room temperature and processed within 72 h in accordance with standardized laboratory procedures. No sample exceeded the 72-h processing window. These uniform collection and handling conditions ensured consistent preservation of cellular integrity and minimized technical variability.
Circulating tumor cells (CTCs) were quantified using the CellSearch® platform and the CellSearch® Epithelial Cell Kit (Menarini Silicon Biosystems). This system enriches epithelial cells through an anti-EpCAM–coated ferrofluid, followed by immunofluorescent staining for cytokeratins (CK), 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclei, and CD45 to exclude leukocytes. Cells were classified as CTCs if they exhibited round or oval morphology, contained a clearly identifiable nucleus, were CK-positive, and lacked CD45 expression.
Cancer-associated macrophage-like cells (CAM-Ls) were defined as CK+ and CD45+ cells measuring at least 30 μm, with one or more nuclei, an oblong or irregular contour, and a diffuse CK staining pattern (17, 20). CAM-L assessment was performed independently by two experienced operators blinded to clinical information. Any uncertain cases were jointly reviewed until consensus was reached.
Based on CTC assessments at both time points, patients were categorized into the four groups: neg/neg, neg/pos, pos/neg and pos/pos.
Furthermore, we performed a binary analysis by grouping patients based on their CTC at T0 and T1. Patients were classified into two main groups: those who were negative at T1 (including patients who were negative at both time points and those who converted from positive at T0 to negative at T1), and those who were positive at T1 (including patients who converted from negative to positive and those who remained positive at both time points). This dichotomization was adopted due to the limited sample size and to improve statistical power for analyses based on T1 CTC status. Among patients who were CTC-positive at T0, patients becoming CTC negative at T1 (CTC pos/neg) were termed CTC responders, while patients that remained positive at T1 (CTC pos/pos) were termed CTC non-responders. This classification was validated against clinical outcomes, including progression-free survival (PFS) and overall survival (OS), and analyses were stratified by treatment type.
CAM-L cells were assessed at baseline (T0) and at the first follow-up (T1). At T1, patients were categorized as CAM-L positive if at least one CAM-L cell was detected, and CAM-L negative otherwise. Dynamic changes in CAM-L (e.g., conversion from positive to negative) were not analyzed, as they were not considered relevant for this study. The cut-off for positivity was set at ≥1 cell. All CTC and CAM-L measurements were performed on the same blood specimen to ensure consistency and comparability.
Continuous variables are reported as medians and ranges, whereas categorical variables are reported as counts and percentages. Continuous variables were analyzed as continuous, and their normality was assessed using the Shapiro–Wilk test. The primary objective of the study was to evaluate the prognostic significance of CTC dynamic changes (baseline to T1) in patients with metastatic NSCLC receiving first-line therapy. Secondary objectives included: evaluating the association of CAM-L positivity at T1 with clinical outcomes, exploring correlations between CTC and CAM-L status with different first-line treatments.
Survival analyses were performed in the overall population (patients with both CTC and CAM-L assessments at T0 and T1) and the defined subgroups according to treatment received (chemotherapy, ICIs and targeted therapy). Overall survival (OS) was defined as time from first-line treatment start to death from any cause; if a patient was not known to have died, survival time was censored at the last date the patient was known to be alive. Progression-free survival (PFS) was defined as the time from first-line treatment start to progression disease or death. Survival parameters were estimated based on the Kaplan-Meier method and summarized with medians and 95% confidence intervals (CI). In addition, univariate and multivariate Cox regressions were performed to obtain hazard ratio (HR) and 95% CI for death and progression disease. Multivariate models included clinically relevant covariates such as age, number of metastatic sites, treatment type, and tissue PD-L1 expression. The correlations between categorical variables were analyzed via chi-square tests and binary logistic regression. Missing data were minimal cases with missing values for a specific variable were excluded from analyses. Statistical significance was defined at p ≤ 0.05. Given the exploratory nature of this study, no formal correction for multiple testing was applied. All the statistical analyses were performed via IBM SPSS Statistics version 23 (IBM, Armonk, NY, USA).
3.1 Patient Cohort, Tumor Subtypes and Baseline Characteristics
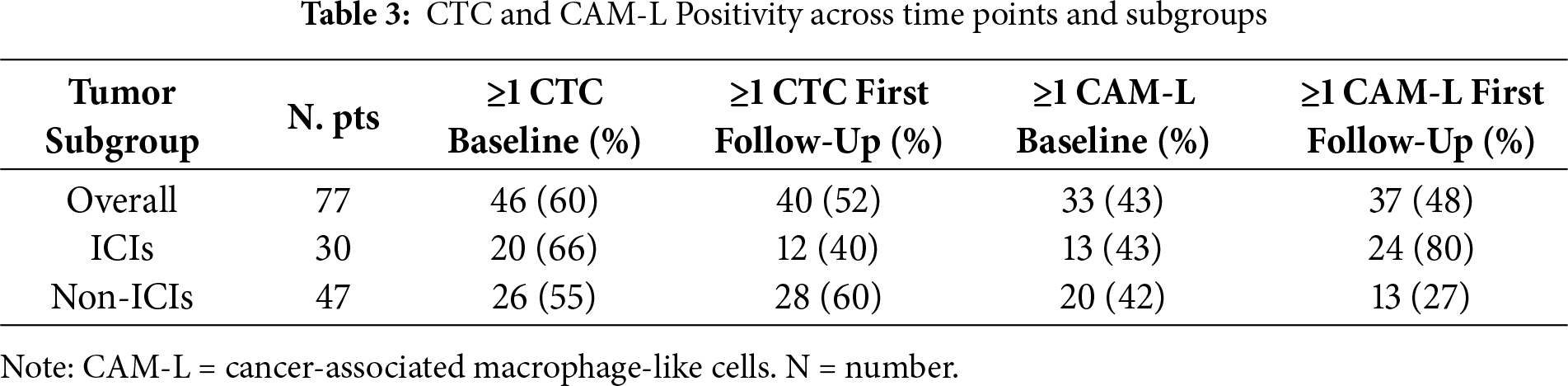
Individual patient data from 120 metastatic NSCLC patients treated between January 2019 and December 2024 were provided. A total of 43 patients were excluded because they did not meet the inclusion criteria. We selected 77 patients who received CellSearch CTC and CAM-L assessment at baseline (before starting first-line treatment), and at least one follow-up time after 3 months of first-line treatment.
The overall population was divided into three categories according to first-line treatment received. Thirty-one patients received immune checkpoint inhibitors (ICIs) alone or with chemotherapy according to PD-L1 status (15 patients receive ICIs alone because of PD-L1 expression ≥50% and the remaining received chemo + ICIs combination because of PD-L1 expression <50% or unknown.
Seventeen patients received targeted therapies because of oncogenic driver mutations detected on tissue samples through NGS: 11 were treated with EGFR inhibitors, 6 with ALK inhibitors. Chemotherapy treatment was platinum doublet in the remaining 30 patients, according to tumor histology (Table 1).
Clinical data of the overall population and distribution according to treatment received are resumed in Tables 2, A1 and A2.
3.2 CTC and CAM-L at Baseline and First Follow-Up
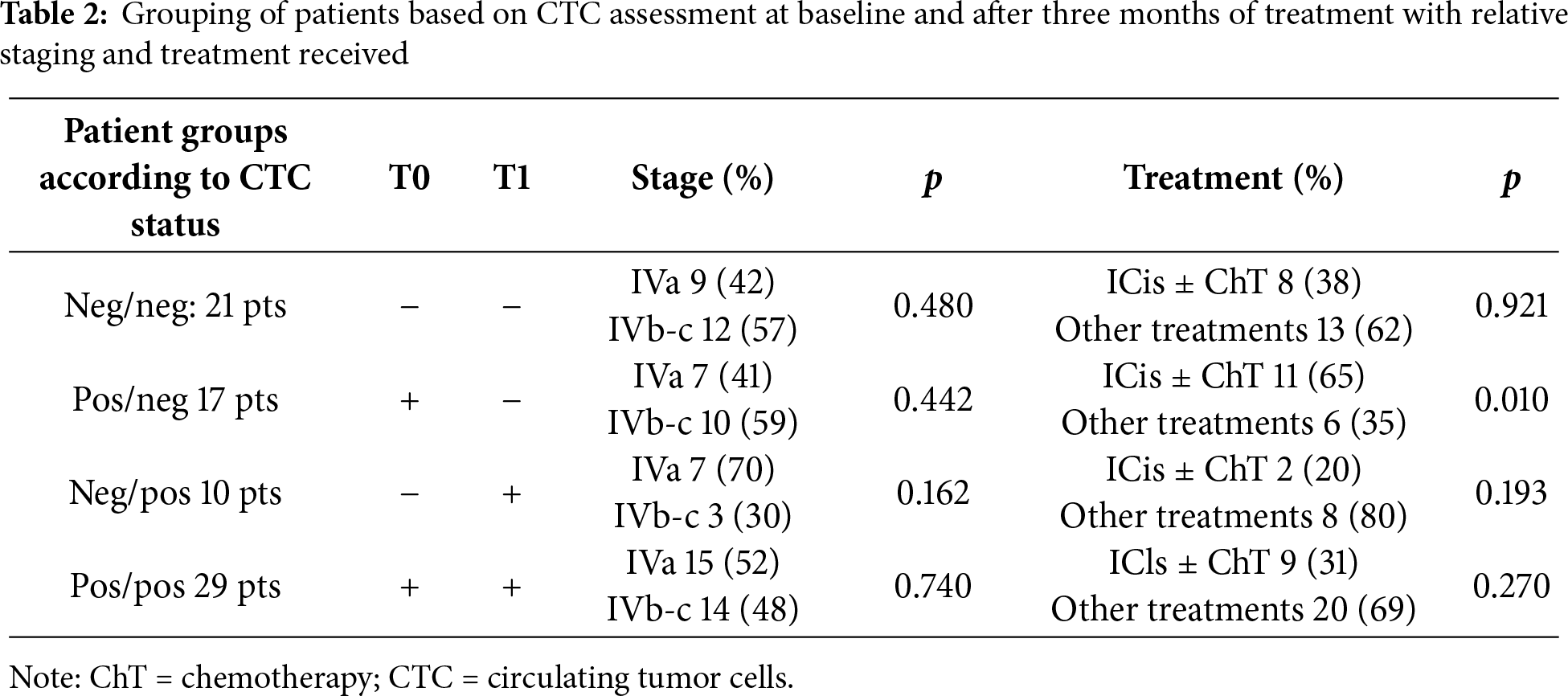
In total, 46 patients had ≥1 CTCs at baseline and 40 at first follow-up, respectively. Overall, 21 patients had <1 CTC both at baseline and at first follow-up (neg/neg). In contrast, 10 patients had <1 CTC at baseline but ≥1 CTC at first follow-up (neg/pos); 17 patients converted from ≥1 CTC at baseline to <1 CTC at first follow-up (pos/neg) and 29 patients had ≥1 at both points (pos/pos). Meanwhile, 33 patients had ≥1 CAM-L at baseline and 37 patients at T1.
A total of 38 patients remained negative (<1 CAM-L) at both points, whereas 8 patients converted from negative at baseline to positive at T1. Additionally, 4 patients changed from positive at baseline to negative at T1, and 29 patients were positive at both time points (Table 3).
3.3 Survival Outcomes According to Serial CTC Level Status
As noted above, we identified four separate patient groups according to the status of CTC at T0 and T1: neg/neg, neg/pos, pos/neg, pos/pos. Using the cut-off of ≥1 CTC, OS among these four CTC change groups differed significantly (Fig. 1). Median OS for neg/neg, pos/neg, neg/pos, and pos/pos group was 26 months (95% CI 5.74–46.25), 33 months (95% CI 21.51–44.58), 10 months (95% CI 7.52–12.47) and 10 months (95% CI 9.14–10.85), respectively (p < 0.0001). Hazard ratios (reference group neg/neg) were 5.11 (95% CI 2.11–12.36) for the neg/pos group, 5.20 (95% CI 2.60–10.37) for the pos/pos group and 1.23 (95% CI 0.58–2.62) for the pos/neg group (Fig. 1a). Similarly, median PFS for neg/neg, pos/neg, neg/pos, and pos/pos group was 13 months (95% CI 8.51–17.48), 18 months (95% CI 10.48–25.51), 5 months (95% CI 2.93–7.06) and 6 months (95% CI 5.24–6.75), respectively (p < 0.0001). Hazard ratios (reference group neg/neg) were 2.81 (95% CI 1.25–6.31) for the neg/pos group, 5.06 (95% CI 2.62–9.81) for the pos/pos group and 0.63 (95% CI 0.31–1.30) for the pos/neg group (Fig. 1b).

Figure 1: (a) Overall Survival (OS) and (b) Progression Free Survival (PFS) analysis of overall population according to circulating tumor cell (CTC) status at T0 and T1
Among patients who were CTC-positive at T0, CTC non-responders (pos/pos) had a significantly higher risk of disease progression compared to CTC responders (pos/neg), with a hazard ratio of 15.47 (95% CI: 3.63–65.95; p < 0.0001) (Fig. 2a). Similarly, CTC non-responders showed an increased risk of death relative to responders, with a hazard ratio of 8.94 (95% CI: 2.35–33.92; p = 0.001) (Fig. 2b).
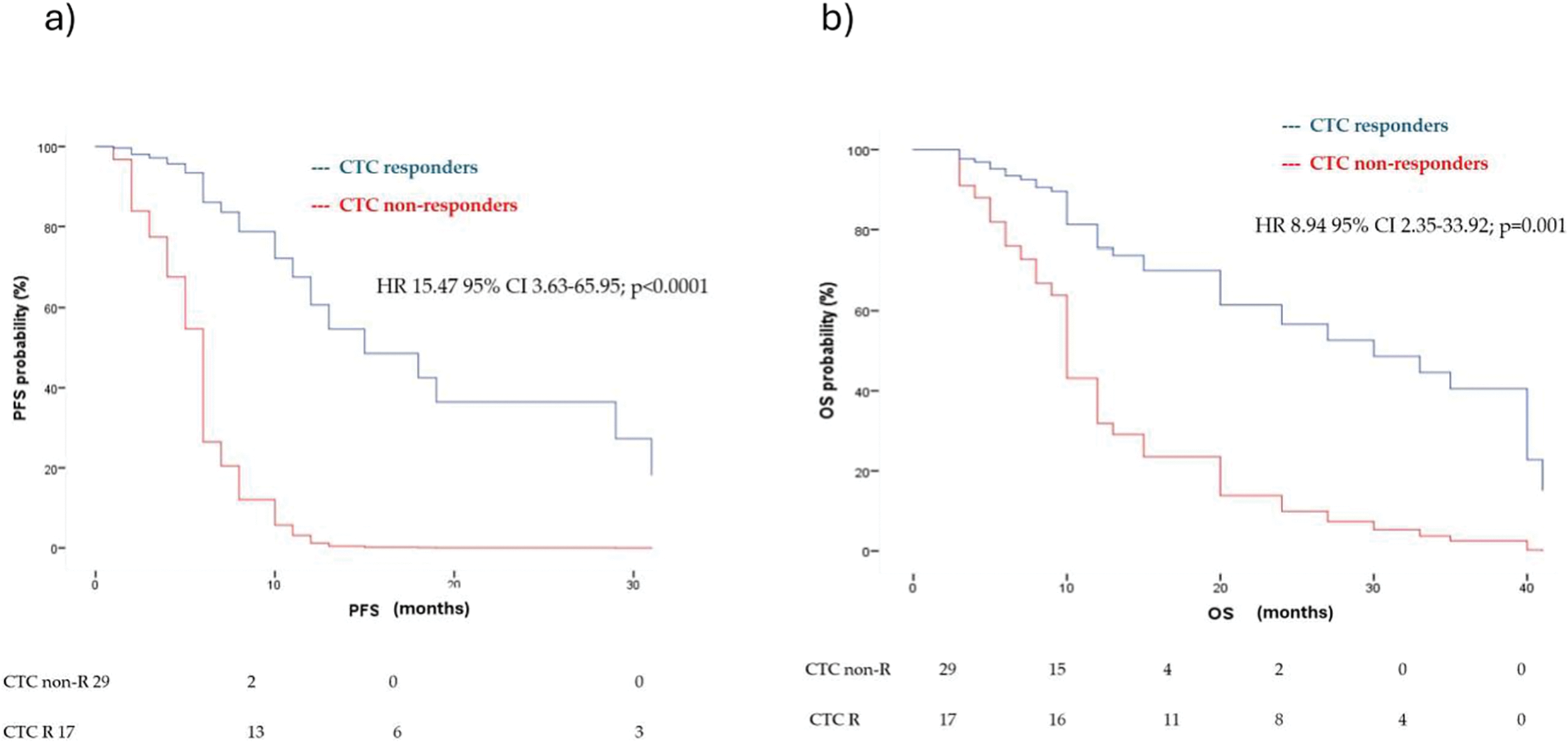
Figure 2: Multivariate analysis for (a) PFS and (b) OS in CTC responders (CTC pos/neg) and CTC non-responders (pos/pos) according to first-line treatment (immunotherapy vs. chemotherapy vs. target therapy), age, sites of metastasis and tissue Programmed Death-Ligand 1 (PD-L1) status. HR = hazard ratio, CI = confidence interval
Furthermore, T1 CTC-negative patients (neg/neg, pos/neg) demonstrated significantly longer OS, with a median OS of 30 months (95% CI: 19.48–40.52), compared to T1 CTC-positive patients (pos/pos, neg/pos), who had a median OS of 10 months (95% CI: 9.14–10.86) (p < 0.0001) (Fig. 3a).
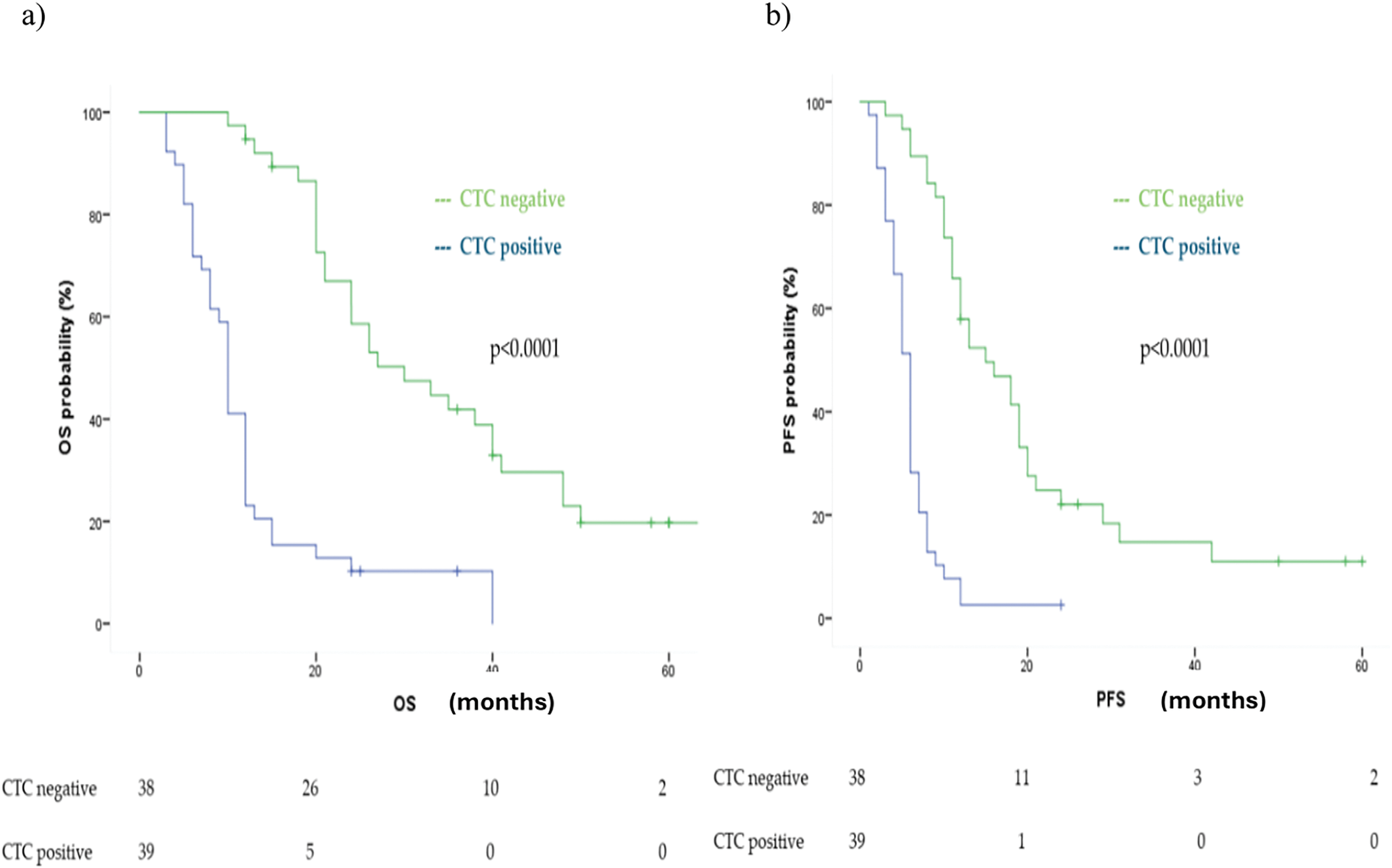
Figure 3: Kaplan Meier analysis of (a) OS and (b) PFS comparing CTC positive vs. CTC negative at first follow up (T1)
Similarly, PFS was significantly longer in the T1 CTC-negative group, with a median PFS of 15 months (95% CI: 9.19–20.80), compared to 6 months (95% CI: 5.26–6.73) in the CTC-positive group (p < 0.0001) (Fig. 3b).
Both findings were confirmed in multivariate analysis adjusted for age, number of metastatic sites, treatment type, and tissue PD-L1 expression, showing a HR for OS of 6.68 (95% CI: 2.72–16.44, p < 0.0001) and for PFS of 5.91 (95% CI: 2.58–13.54, p < 0.0001).
3.4 Survival Outcomes According to CAM-L Trends
CAM-L cells were assessed at baseline (T0) and at the first follow-up (T1). A sample was considered positive if at least one CAM-L cell was detected. Based on the T1 CAM-L status, patients were stratified into two groups.
Unlike CTCs, T1 CAM-L positivity was significantly associated with improved median OS: 30 months (95% CI: 19.48–40.51) in CAM-L-positive patients, compared to 12 months (95% CI: 10.75–13.24) in CAM-L-negative patients (p < 0.0001). The corresponding HR was 0.28 (95% CI: 0.16–0.48, p < 0.0001). This association remained statistically significant in multivariate analysis adjusted for age, number of metastatic sites, treatment type, and PD-L1 expression (adjusted HR: 0.28, 95% CI: 0.12–0.62, p = 0.002) (Fig. 4a).
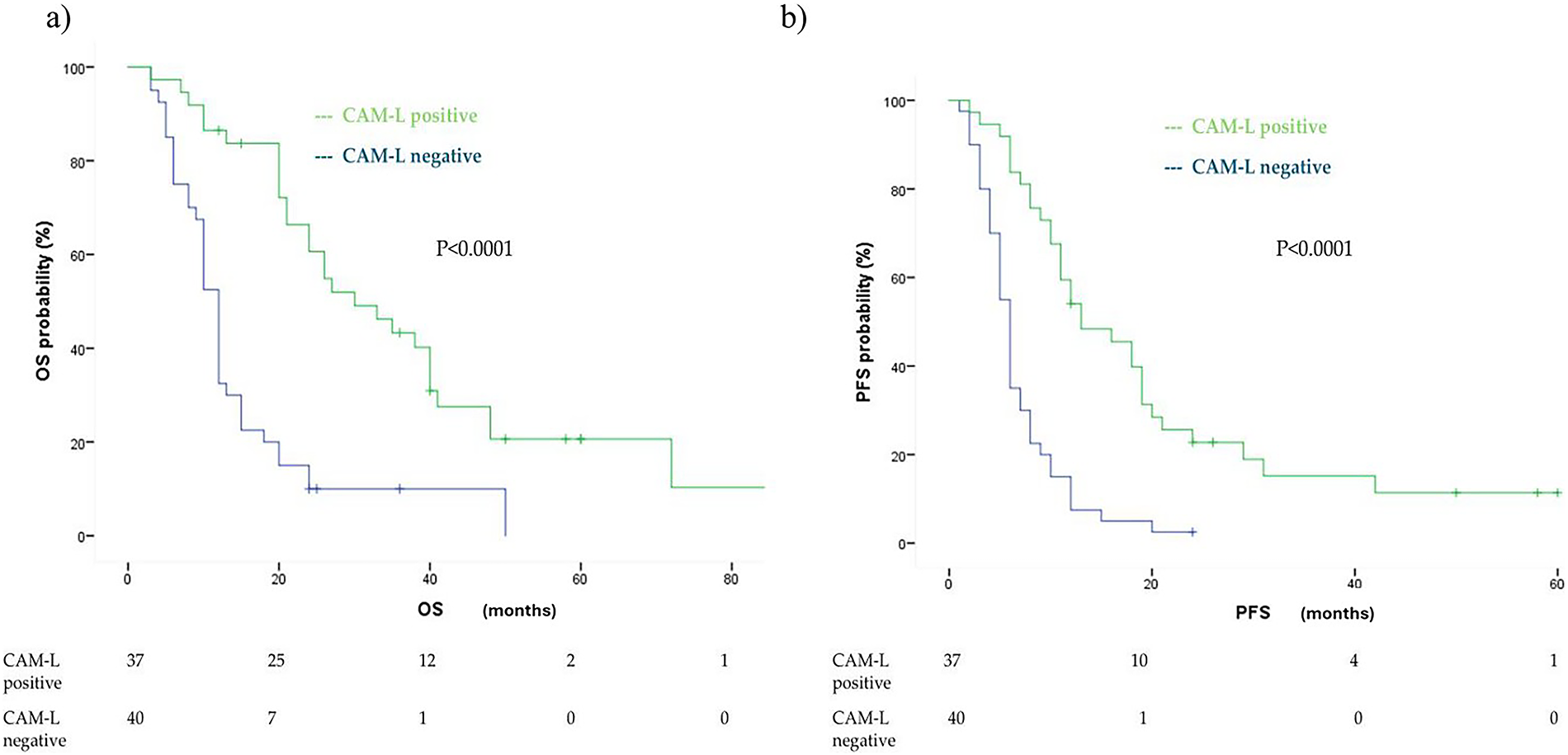
Figure 4: Kaplan Meier analysis of OS (a) and PFS (b) comparingcancer-associated macrophage-like cell (CAM-L) positive vs. CAM-L negative at first follow up (T1)
However, this association appeared to be influenced by the CTC status at T1, which showed a non-significant trend (HR: 0.59, 95% CI: 0.28–1.23, p = 0.16).
A similar pattern was observed for first-line PFS. Patients who were T1 CAM-L positive showed a longer median PFS of 13 months (95% CI: 7.22–18.77) compared to 6 months in T1 CAM-L-negative patients (95% CI: 5.26–6.74), with a highly significant difference (p < 0.0001) (Fig. 4b). The corresponding HR was 0.30 (95% CI: 0.18–0.50) in univariate analysis. This association was maintained after adjusting for age, number of metastatic sites, treatment type, and PD-L1 expression (adjusted HR: 0.23, 95% CI: 0.11–0.48, p < 0.0001), but was attenuated when accounting for T1 CTC status (HR: 0.72, 95% CI: 0.34–1.50, p = 0.38).
3.5 Survival Outcomes According to CTC Trends by First-Line Treatment Subgroups
As in the overall cohort, OS and PFS were compared between T1 CTC-negative and T1 CTC-positive patients within both ICIs and non-ICIs treatment subgroups. In the ICIs-treated subgroup, patients who were CTC-negative at T1 (including both neg/neg and pos/neg patterns) exhibited a significantly longer median OS of 38 months (95% CI: 28.65–47.34) compared to CTC-positive patients at T1 (neg/pos and pos/pos), who had a median OS of 10 months (95% CI: 7.91–12.08) (p < 0.0001). The corresponding univariate HR was 6.29 (95% CI: 2.37–16.72, p < 0.0001), which remained significant in multivariate analysis adjusted for age, number of metastatic sites, and PD-L1 status (HR: 8.17, 95% CI: 2.41–27.64, p = 0.001). Similarly, median PFS was significantly longer in T1 CTC-negative patients (18 months; 95% CI: 5.92–30.08) compared to T1 CTC-positive patients (5 months; 95% CI: 2.57–7.43) (p < 0.0001). The univariate HR for PFS was 4.44 (95% CI: 1.80–10.94, p = 0.001), and the multivariate HR was 5.25 (95% CI: 1.70–16.25, p = 0.004) (Fig. A1).
Similar findings were observed in the non-ICIs subgroup. T1 CTC-negative patients showed a significantly longer median OS of 21 months (95% CI: 15.31–26.68) compared to 10 months (95% CI: 7.95–12.05) in T1 CTC-positive patients (p < 0.0001). The univariate HR was 3.84 (95% CI: 1.89–7.80, p < 0.0001), and this was confirmed in multivariate analysis (HR: 3.84, 95% CI: 1.83–8.03, p < 0.0001). PFS analysis in the non-ICIs group confirmed these trends. T1 CTC-negative patients had a median PFS of 12 months (95% CI: 8.80–15.20), while T1 CTC-positive patients had a median PFS of 6 months (95% CI: 5.18–6.82), with a statistically significant difference (p < 0.0001). The univariate HR was 6.04 (95% CI: 2.73–13.38, p < 0.0001), and the multivariate HR was 7.63 (95% CI: 3.20–18.21, p < 0.0001). In summary, the HRs for death associated with CTC positivity were similar and statistically significant in both non-ICI (HR 3.84) and ICI (HR 4.17) groups, even after multivariate adjustment for age, number of metastases, and PD-L1 status.
3.6 Survival Outcomes According to CAM-L Trends by First-Line Treatment Subgroups
CAM-L trends were analyzed within both the ICIs-treated and non-ICIs-treated patient populations.
Patients receiving ICIs were significantly more likely to exhibit T1 CAM-L positivity, with an odds ratio (OR) of 3.53 (95% CI: 1.34–9.25, p = 0.009) (Fig. A2).
As expected, in the ICIs subgroup, T1 CAM-L positivity (neg/pos and pos/pos) was associated with markedly improved OS. Median OS in CAM-L-positive patients was 38 months (95% CI: 32.29–43.71), compared to 10 months (95% CI: 8.33–11.67) in CAM-L-negative patients (neg/neg and pos/neg), (p < 0.0001). The univariate HR was 0.08 (95% CI: 0.02–0.27, p < 0.0001), and this association remained significant in multivariate analysis adjusted for age, number of metastatic sites, and PD-L1 expression (adjusted HR: 0.07, 95% CI: 0.01–0.28, p < 0.0001).
A similar benefit was observed in terms of PFS. CAM-L-positive patients had a median PFS of 18 months (95% CI: 1.36–34.63), whereas CAM-L-negative patients had a median PFS of 5 months (95% CI: 3.32–6.67), (p < 0.0001). The univariate HR was 0.11 (95% CI: 0.03–0.34, p < 0.0001), and the multivariate HR was 0.10 (95% CI: 0.02–0.41, p < 0.0001).
In contrast, within the non-ICIs subgroup, no statistically significant difference in OS was observed between T1 CAM-L-positive patients (median OS: 21 months; 95% CI: 15.49–26.50) and CAM-L-negative patients (median OS: 12 months; 95% CI: 10.44–13.55), with an adjusted HR for age, number of metastasis, PD-L1 status of 0.54 (95% CI: 0.28–1.06; p = 0.073). However, CAM-L positivity was significantly associated with longer progression-free survival (median PFS: 11 vs. 6 months), with an adjusted HR for age and number of metastases of 0.41 (95% CI: 0.20–0.86; p = 0.019). (Fig. 5).
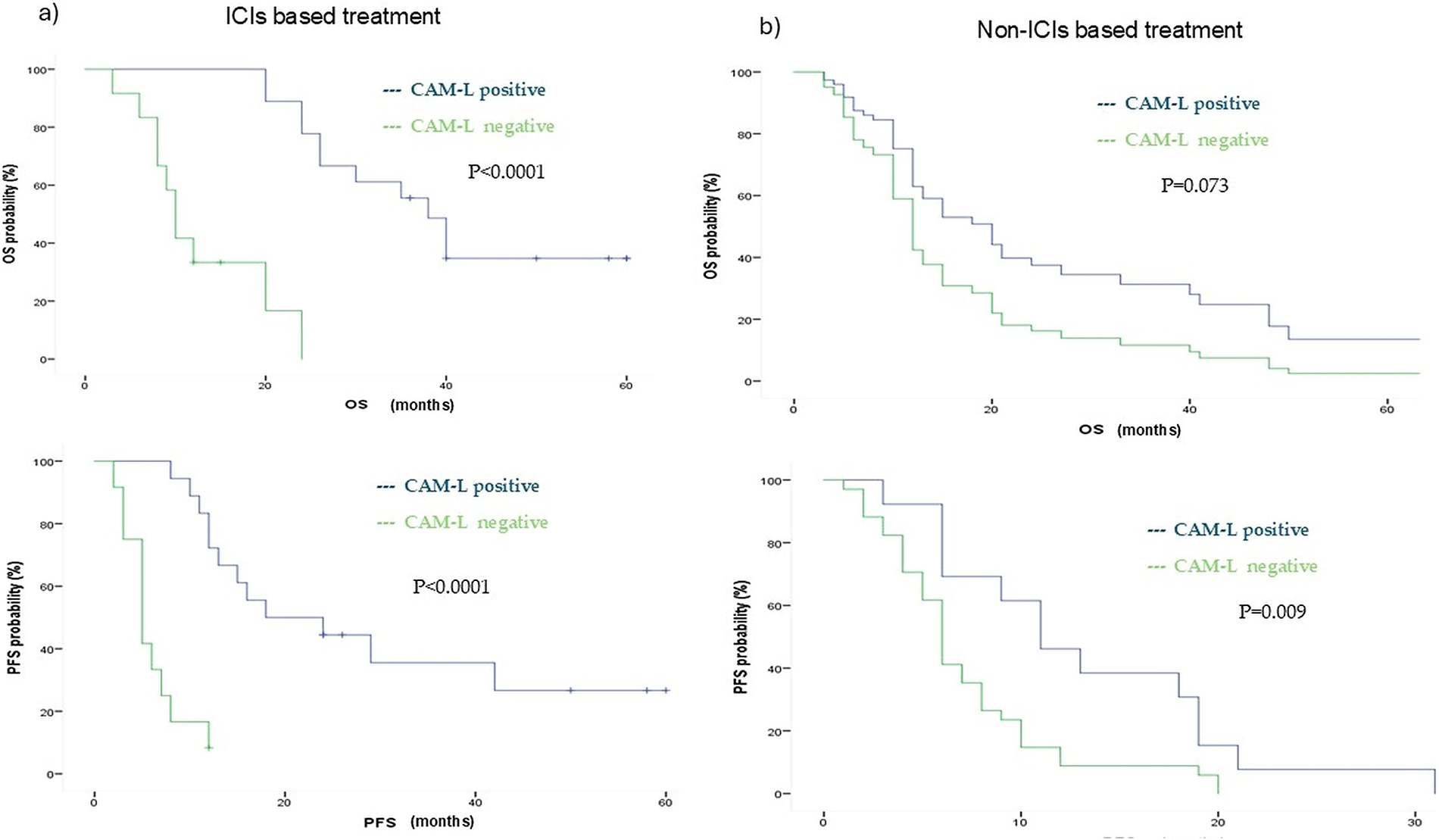
Figure 5: Kaplan Meyer showing ICIs (a) and non-ICIs (b) population OS and PFS outcomes according to CAM-L positivity. ICIs = Immune checkpoint inhibitors
This study provides valuable insights into the prognostic significance of CTCs and CAM-L in patients with metastatic NSCLC undergoing different first-line treatment regimens, including chemotherapy, ICIs, and targeted therapies. Our findings suggest that both CTC dynamics and CAM-L positivity provide robust prognostic information, which could help guide personalized treatment strategies, particularly in the context of immunotherapy.
In the context of NSCLC, establishing a reliable baseline cut-off value for CTCs is crucial for their clinical utility in prognosis and treatment monitoring. Although CTCs are rare in peripheral blood, their enumeration can provide meaningful insights into tumor dynamics, metastatic potential, and treatment efficacy. However, a standardized cut-off for CTC positivity in NSCLC has not been universally established, leading to variability in results across different studies and detection platforms [11,12,18]. Therefore, in our study we considered CTC ≥1 as cut-off for CTC positivity to maximize the number of patients that were CTC-positive according to relevant previous studies [19–22].
The concept of monitoring CTC dynamics over time has gained substantial interest in the context of cancer treatment, with several studies indicating that CTC count changes during therapy reflect treatment efficacy and tumor response [23,24]. Our study extends these findings by demonstrating that early reductions in CTC levels, especially within the first few months of treatment, are significantly associated with better OS and PFS in metastatic NSCLC. Specifically, patients who became CTC-negative at T1 exhibited significantly improved survival outcomes, which is consistent with previous studies highlighting the prognostic utility of CTCs as markers for treatment response and disease progression [25–28]. This emphasizes the importance of dynamic monitoring of CTCs during treatment, as a single point assessment may fail to capture the evolving tumor biology and the patient’s response to therapy.
Consistent with previous studies, CTC positivity correlated with worse OS across treatment subgroups. It is possible that CTCs play a dual role in the context of immunotherapy. On the one hand, higher CTC number could be associated with a higher burden of disease and poorer prognosis. On the other, CTCs may act as sources of tumor-associated antigens that can be recognized by the immune system, potentially enhancing antitumor responses in the presence of ICIs. In this context, the immunological effect of CTC biology warrants further mechanistic and clinical exploration [29].
While CTCs are well-established as prognostic markers, our study also underscores the potential role of CAM-L in assessing treatment outcomes. CAM-L positivity at T1 was strongly associated with improved OS and PFS, particularly in patients treated with ICIs. This result is consistent with previous studies suggesting that CAM-L, as a component of the tumor microenvironment, may provide additional prognostic value [18,30]. Our findings expand on show that CAM-L positivity, when combined with CTC data, may offer more robust predictive information, especially for patients receiving immunotherapy. The presence of CAM-L cells could indicate a more favorable immune response or better treatment efficacy, particularly with ICIs. However, this association was attenuated when adjusting for CTC status at T1, which suggests that the prognostic power of CAM-L may be influenced by the concurrent assessment of CTCs.
Importantly, the association between CAM-L positivity and improved OS was more pronounced in patients receiving ICIs than in those treated with chemotherapy or targeted therapies. This may reflect the role of tumor-associated macrophages in facilitating immune activation and enhancing the effects of checkpoint blockade. ICIs are known to modulate macrophage polarization toward an antitumor (M1-like) phenotype, which could explain the survival advantage observed in CAM-L–positive patients undergoing immunotherapy. This finding suggests that CAM-Ls may not only serve as prognostic markers but also reflect a favorable immune microenvironment that synergizes with ICIs [31].
The differential impact of CTC and CAM-L in the context of various treatments highlights the potential for personalized monitoring strategies.
While the findings of this study are promising, several limitations must be considered. First, the observational design and relatively small sample size limit the generalizability of the results. Additionally, although CTC and CAM-L assessments were conducted at baseline and after three months of treatment, a longer follow-up period would provide more robust data on the long-term prognostic value of these biomarkers.
Another limitation is the lack of a standardized cut-off value for CAM-L, along with the inability to distinguish between M1 and M2 macrophage polarization within CAM-Ls. The polarization of these macrophages may be influenced by the tumor microenvironment and treatment modality, especially ICIs. Notably, ICIs can promote M1 polarization, which is associated with an active immune response. To address this, incorporating a CD38 fluorescent-labeled antibody into the fourth channel of the CellSearch® system could enable more accurate differentiation of M1 and M2 macrophages. Alternatively, utilizing PD-L1 fluorescent-labeled antibodies in the fourth channel could offer valuable insights into the immune dynamics of CTCs and CAM-Ls, enhancing our understanding of their role in immunotherapy responses [32].
A further methodological limitation is that we applied binary thresholds for CTC and CAM-L positivity to align with prior CTC cut-off and to facilitate clinical interpretability. However, this approach may reduce statistical power compared to continuous modeling of biomarker counts. It is important to note that a subset of patients in this study underwent liquid biopsy analysis prior to the introduction of immunotherapy and chemotherapy combinations as first-line treatments for PD-L1 <50% tumors, which could have influenced the observed results.
Larger studies are warranted to validate the prognostic utility of CTCs and CAM-L and the possible added value of the CAM-L/CTC ratio, including those undergoing various treatment regimens, and to evaluate their potential to predict long-term clinical outcomes.
In the context of improving precision medicine in NSCLC, where ctDNA analysis can complement, and in some cases replace tissue biopsies for the detection of predictive biomarkers, the integrated assessment of CTCs and other blood-based analyses may provide valuable prognostic information and assist clinicians in monitoring disease progression [8,33]. In line with these objectives, our group is currently conducting a prospective, multicenter trial investigating the prognostic relevance of PD-L1–positive CTCs in patients with metastatic NSCLC receiving first-line immunotherapy, with or without chemotherapy through CellSearch.
In conclusion, this study highlights the prognostic significance of serial CTC and CAM-L assessments in metastatic NSCLC, with CTC dynamics providing important insights into treatment response and survival outcomes. Our results suggest that these biomarkers, may help stratify patients based on their likelihood of benefiting from different first-line treatments, including immunotherapy. Early biomarker monitoring may enable prediction of outcomes and support treatment escalation or de-escalation strategies. These findings highlight the potential of integrating CTC and CAM-L testing into clinical practice to advance personalized treatment in metastatic NSCLC. Given their potential to guide personalized treatment, the integration of CTC and CAM-L testing into clinical practice warrants further investigation.
Acknowledgement: The authors sincerely thank all the patients who generously donated their blood, thereby contributing to scientific research, the advancement of knowledge, and the improvement of patient care.
Funding Statement: This research was funded by Sapienza University PNRR-RT_SPOKE_1—ROME TECHNOPOLE—Spoke 1—B83C22002820006—ECS00000024 and FO R.O. onlus.
Author Contributions: Marco Siringo: Writing—review & editing, Writing—original draft, Validation, Methodology. Michela De Meo: Writing—review & editing, Methodology, Validation. Alain Jonathan Gelibter: Validation, Methodology, Formal analysis. Chiara Nicolazzo: Validation, Supervision, Writing—review & editing. Paola Gazzaniga: Writing—review & editing, Funding acquisition, Conceptualization. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author, [Marco Siringo], upon reasonable request.
Ethics Approval: The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Policlinico Umberto I of Rome (protocol n. 668/09, 09 July 2009; amended protocol 179/16, 01 March 2016).
Informed Consent: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Appendix
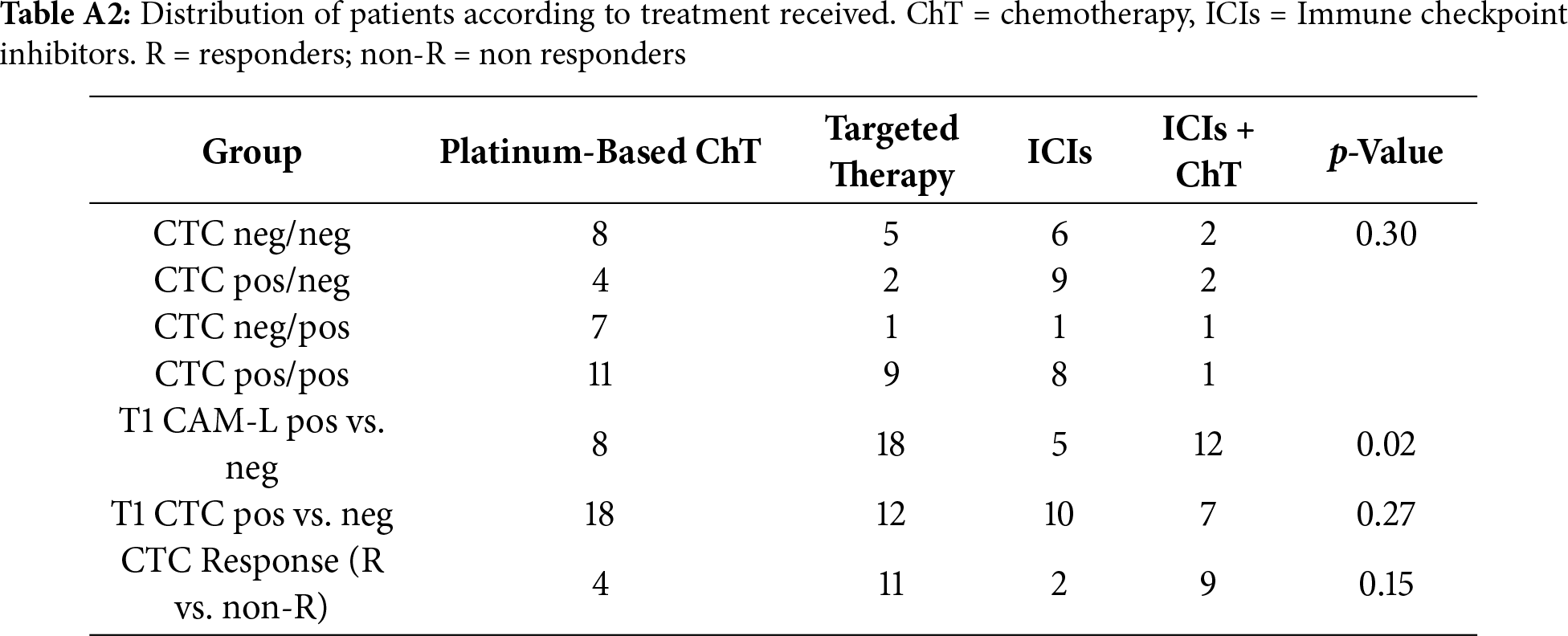
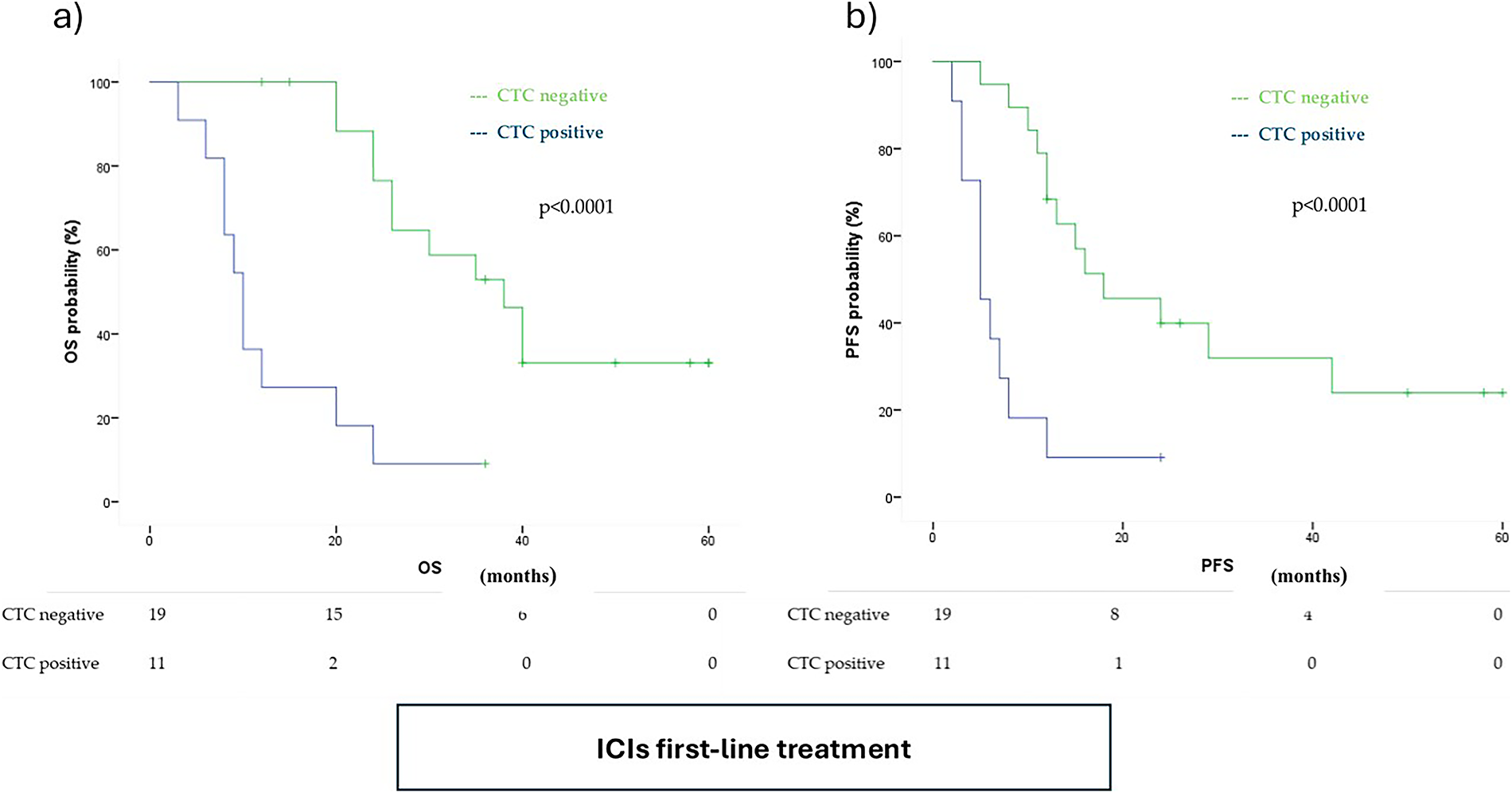
Figure A1: Kaplan Meyer of (a) Overall Survival (OS) and (b) Progression Free Survival (PFS) according to T1 CTC positivity in immune check point inhibitors (ICIs) population
Figure A2: Distribution of cancer-associated macrophage-like cells (CAM-L) positivity in ICIs and non-ICIs populations
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654. [Google Scholar] [PubMed] [CrossRef]
2. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–57. doi:10.1016/j.annonc.2022.12.009. [Google Scholar] [PubMed] [CrossRef]
3. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab vs. chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. doi:10.1056/nejmoa1606774. [Google Scholar] [PubMed] [CrossRef]
4. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. doi:10.1056/nejmoa1801005. [Google Scholar] [PubMed] [CrossRef]
5. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. N Engl J Med. 2018;379(21):2040–51. doi:10.1056/nejmoa1810865. [Google Scholar] [PubMed] [CrossRef]
6. Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):358–76. doi:10.1016/j.annonc.2022.12.013. [Google Scholar] [PubMed] [CrossRef]
7. Siringo M, Baena J, Bote de Cabo H, Torres-Jiménez J, Zurera M, Zugazagoitia J, et al. Future perspectives in the second line therapeutic setting for non-oncogene addicted non-small-cell lung cancer. Cancers. 2023;15(23):5505. doi:10.3390/cancers15235505. [Google Scholar] [PubMed] [CrossRef]
8. Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the international association for the study of lung cancer. J Thorac Oncol. 2021;16(10):1647–62. doi:10.1016/j.jtho.2021.06.017. [Google Scholar] [PubMed] [CrossRef]
9. Bertoli E, De Carlo E, Basile D, Zara D, Stanzione B, Schiappacassi M, et al. Liquid biopsy in NSCLC: an investigation with multiple clinical implications. Int J Mol Sci. 2023;24(13):10803. doi:10.3390/ijms241310803. [Google Scholar] [PubMed] [CrossRef]
10. Reduzzi C, Nicolo’ E, Singhal S, Venetis K, Ortega-Franco A, de Miguel-Perez D, et al. Unveiling the impact of circulating tumor cells: two decades of discovery and clinical advancements in solid tumors. Crit Rev Oncol Hematol. 2024;203:104483. doi:10.1016/j.critrevonc.2024.104483. [Google Scholar] [PubMed] [CrossRef]
11. Alix-Panabières C, Pantel K. Liquid biopsy: from discovery to clinical application. Cancer Discov. 2021;11(4):858–73. doi:10.1158/2159-8290.cd-20-1311. [Google Scholar] [PubMed] [CrossRef]
12. Lindsay CR, Blackhall FH, Carmel A, Fernandez-Gutierrez F, Gazzaniga P, Groen HJM, et al. EPAC-lung: pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur J Cancer. 2019;117:60–8. doi:10.1016/j.ejca.2019.04.019. [Google Scholar] [PubMed] [CrossRef]
13. Liu C, Cai Y, Mou S. Liquid biopsy in lung cancer: the role of circulating tumor cells in diagnosis, treatment, and prognosis. Biomed Pharmacother. 2024;181:117726. doi:10.1016/j.biopha.2024.117726. [Google Scholar] [PubMed] [CrossRef]
14. Sutton TL, Patel RK, Anderson AN, Bowden SG, Whalen R, Giske NR, et al. Circulating cells with macrophage-like characteristics in cancer: the importance of circulating neoplastic-immune hybrid cells in cancer. Cancers. 2022;14(16):3871. doi:10.3390/cancers14163871. [Google Scholar] [PubMed] [CrossRef]
15. Nitschke C, Markmann B, Konczalla L, Kropidlowski J, Pereira-Veiga T, Scognamiglio P, et al. Circulating cancer associated macrophage-like cells as a potential new prognostic marker in pancreatic ductal adenocarcinoma. Biomedicines. 2022;10(11):2955. doi:10.3390/biomedicines10112955. [Google Scholar] [PubMed] [CrossRef]
16. Adams DL, Martin SS, Alpaugh RK, Charpentier M, Tsai S, Bergan RC, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci U S A. 2014;111(9):3514–9. doi:10.1073/pnas.1320198111. [Google Scholar] [PubMed] [CrossRef]
17. Pore AA, Dhanasekara CS, Navaid HB, Vanapalli SA, Rahman RL. Comprehensive profiling of cancer-associated cells in the blood of breast cancer patients undergoing neoadjuvant chemotherapy to predict pathological complete response. Bioengineering. 2023;10(4):485. doi:10.3390/bioengineering10040485. [Google Scholar] [PubMed] [CrossRef]
18. Magri V, De Renzi G, Marino L, De Meo M, Siringo M, Gelibter A, et al. Circulating cancer-associated macrophage-like cells as a blood-based biomarker of response to immune checkpoint inhibitors. Int J Mol Sci. 2024;25(7):3752. doi:10.3390/ijms25073752. [Google Scholar] [PubMed] [CrossRef]
19. Tamminga M, de Wit S, Schuuring E, Timens W, Terstappen LWMM, Hiltermann TJN, et al. Circulating tumor cells in lung cancer are prognostic and predictive for worse tumor response in both targeted- and chemotherapy. Transl Lung Cancer Res. 2019;8(6):854–61. doi:10.21037/tlcr.2019.11.06. [Google Scholar] [PubMed] [CrossRef]
20. de Miguel-Pérez D, Bayarri-Lara CI, Ortega FG, Russo A, Moyano Rodriguez MJ, Alvarez-Cubero MJ, et al. Post-surgery circulating tumor cells and AXL overexpression as new poor prognostic biomarkers in resected lung adenocarcinoma. Cancers. 2019;11(11):1750. doi:10.3390/cancers11111750. [Google Scholar] [PubMed] [CrossRef]
21. Peng H, Tan X, Wang Y, Dai L, Liang G, Guo J, et al. Clinical significance of red cell distribution width and circulating tumor cells with an epithelial-mesenchymal transition phenotype in lung adenocarcinoma. Cancer Manage Res. 2020;12:5105–17. doi:10.2147/CMAR.S251271. [Google Scholar] [PubMed] [CrossRef]
22. Jin F, Zhu L, Shao J, Yakoub M, Schmitt L, Reißfelder C, et al. Circulating tumour cells in patients with lung cancer universally indicate poor prognosis. Eur Respir Rev. 2022;31(166):220151. doi:10.1183/16000617.0151-2022. [Google Scholar] [PubMed] [CrossRef]
23. Yang B, Qin A, Zhang K, Ren H, Liu S, Liu X, et al. Circulating tumor cells predict prognosis following tyrosine kinase inhibitor treatment in EGFR-mutant non-small cell lung cancer patients. Oncol Res. 2017;25(9):1601–6. doi:10.3727/096504017X14928634401178. [Google Scholar] [PubMed] [CrossRef]
24. Janni W, Friedl TWP, Yab TC, Bidard FC, Cristofanilli M, Hayes DF, et al. Clinical validity of repeated circulating tumor cell enumeration as an early treatment monitoring tool for metastatic breast cancer in the PREDICT global pooled analysis. Clin Cancer Res. 2025;31(11):2196–209. doi:10.1158/1078-0432.ccr-24-3108. [Google Scholar] [PubMed] [CrossRef]
25. Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, Greystoke A, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non–small-cell lung cancer. J Clin Oncol. 2011;29(12):1556–63. doi:10.1200/jco.2010.28.7045. [Google Scholar] [PubMed] [CrossRef]
26. Jiang SS, Deng B, Feng YG, Qian K, Tan QY, Wang RW. Circulating tumor cells prior to initial treatment is an important prognostic factor of survival in non-small cell lung cancer: a meta-analysis and system review. BMC Pulm Med. 2019;19(1):262. doi:10.1186/s12890-019-1029-x. [Google Scholar] [PubMed] [CrossRef]
27. Purcell E, Niu Z, Owen S, Grzesik M, Radomski A, Kaehr A, et al. Circulating tumor cells reveal early predictors of disease progression in patients with stage III NSCLC undergoing chemoradiation and immunotherapy. Cell Rep. 2024;43(2):113687. doi:10.1016/j.celrep.2024.113687. [Google Scholar] [PubMed] [CrossRef]
28. Gallo M, De Luca A, Maiello MR, D’Alessio A, Esposito C, Chicchinelli N, et al. Clinical utility of circulating tumor cells in patients with non-small-cell lung cancer. Transl Lung Cancer Res. 2017;6(4):486–98. doi:10.21037/tlcr.2017.05.07. [Google Scholar] [PubMed] [CrossRef]
29. Dotse E, Lim KH, Wang M, Wijanarko KJ, Chow KT. An immunological perspective of circulating tumor cells as diagnostic biomarkers and therapeutic targets. Life. 2022;12(2):323. doi:10.3390/life12020323. [Google Scholar] [PubMed] [CrossRef]
30. Pirrello A, Killingsworth M, Spring K, Rasko JEJ, Yeo D. Cancer-associated macrophage-like cells as a prognostic biomarker in solid tumors. J Liq Biopsy. 2024;6:100275. doi:10.1016/j.jlb.2024.100275. [Google Scholar] [PubMed] [CrossRef]
31. Xiong H, Mittman S, Rodriguez R, Moskalenko M, Pacheco-Sanchez P, Yang Y, et al. Anti–PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79(7):1493–506. doi:10.1158/0008-5472.can-18-3208. [Google Scholar] [PubMed] [CrossRef]
32. Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. Sci Rep. 2016;6:31726. doi:10.1038/srep31726. [Google Scholar] [PubMed] [CrossRef]
33. Bote-de Cabo H, Siringo M, Conde E, Hernández S, López-Ríos F, Castelo-Loureiro A, et al. Clinical utility of combined tissue and plasma next-generation sequencing in patients with advanced, treatment-naïve NSCLC. JTO Clin Res Rep. 2024;6(3):100778. doi:10.1016/j.jtocrr.2024.100778. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools