 Open Access
Open Access
ARTICLE
Substrate, Hormone, Winnowing, and Stratification Influence the Seed Germination of Ilex asprella (Hook. et Arn.) Champ. ex Benth
1 Guangxi Key Laboratory of Medicinal Resources Protection and Genetic Improvement, Guangxi Botanical Garden of Medicinal Plants, Nanning, 530000, China
2 Research and Development Center, China Resources Sanjiu Medical & Pharmaceutical Co., Ltd., Shenzhen, 518000, China
* Corresponding Authors: Yanxia Zhu. Email: ; Danfeng Tang. Email:
Phyton-International Journal of Experimental Botany 2023, 92(7), 2105-2116. https://doi.org/10.32604/phyton.2023.029205
Received 07 February 2023; Accepted 14 March 2023; Issue published 29 May 2023
Abstract
Ilex asprella (Hook. et Arn.) Champ. ex Benth is one of the most important traditional Chinese medicines in southern China. The seeds of Ilex asprella usually have extremely low germination due to their dormancy characteristics, which severely impacts the efficiency of seedling raising and increases labor costs. In this study, to improve the seed germination of I. asprella, the effects of germination substrate, hormone, winnowing, and stratification treatments on the seed germination of I. asprella were investigated. The results of the germination matrix showed that the highest germination percentage of 45.2% was achieved under the 20°C/10°C day/night temperature and vermiculite germination medium conditions. The results of hormone treatments revealed that 100–400 mg/L of gibberellin (GA) and 50–100 mg/L of salicylic acid (SA) were found to be effective in releasing the dormancy of I. asprella seeds. Moreover, winnowing could effectively eliminate unsaturated seeds and impurities, thus improving the seed germination of I. asprella. Furthermore, warm temperature (15°C) stratification could expand the temperature range of I. asprella’s seed germination, which was beneficial for seed germination of I. asprella and for seed nursery at room temperature in production practice. The present study obtained a method to break dormancy and increase seed germination in I. asprella, thereby forming a groundwork for improving the efficiency of large-scale planting of I. asprella.Keywords
Ilex asprella (Hook. et Arn.) Champ. ex Benth., commonly referred to as “Gangmei” in Chinese, belongs to the genus of holly in the Holly family and is native to Southern China [1,2]. The roots and leaves of Ilex asprella are primarily used as traditional medicine [3]. Its main active ingredients include polysaccharides, triterpenoid saponins, chlorogenic acids, phenols, and flavonoids [1,3,4], with a series of pharmacological and biological properties such as anti-inflammatory [5], antiviral [6,7], immunomodulatory [8], antitumor [9], antibacterial [10], antifungal [11], and hypolipidemic [12] activity. As a local medicinal herb in Lingnan regions, I. asprella is one of the main raw materials of many Chinese patent medicines and herbal teas [1,13].
Wild I. asprella is mainly distributed in the low mountainous areas of Guangdong, Guangxi, Fujian, and Jiangxi, with a slow growth rate naturally. In recent years, due to the destruction of the ecological environment and the large increase in demand for Chinese patent medicines, the supply of wild I. asprella has become scarce, leading to overexploitation of the resources and a huge disparity between supply and demand [2]. Therefore, it is imperative to carry out artificial cultivation of I. asprella in order to ensure sufficient market supply. Seed propagation is a common approach to artificially cultivate I. asprella. However, studies have shown that I. asprella’s seeds have a characteristic for dormancy, slow germination, and low germination rate [14–16].
Seed dormancy occurs when viable seeds fail to germinate under suitable conditions. It can be induced by several factors, such as immature seed embryos, immature seeds, seed coat impermeability, and the presence of germination-inhibiting substances [15,17]. To overcome seed dormancy, stratification and hormonal treatments are widely used. Stratification can be more effective in breaking seed dormancy by softening the seed coat and increasing permeability, as well as by enabling the seeds to undergo a series of physiological changes and maturation processes, and reducing or eliminating germination inhibitory substances [15]. Depending on the stratification temperatures, the stratification treatment can be categorized into low-temperature stratification (0°C–5°C), warm temperature stratification (15°C–25°C or 20°C–30°C), and variable temperature stratification [18]. Significant results have been achieved by using this stratification treatment on I. argentina, I. ficifolia, and I. triflora [19–21].
Compared with the stratification treatment, the application of exogenous plant hormones and growth regulators has emerged as a common method to promote seed germination. The commonly used exogenous hormones include gibberellins (GAs) and abscisic acid (ABA). As is well known, GAs are the primary hormones that facilitate the breakdown of seed dormancy by promoting the synthesis of auxin and cytokinin [17]. This also increases the activity of enzymes in the seed and promotes metabolic processes, resulting in the termination of seed dormancy and enhanced seed germination [22,23]. ABA is considered to play a major role in guiding seed maturation and sustaining seed dormancy under adverse environmental conditions until it is antagonized by GA and some environmental signals, thus allowing seed germination when the environmental conditions are favorable [24]. Additionally, in recent years, exogenous hormones such as salicylic acid (SA), melatonin (MT), and mepiquat chloride (MC/DPC) have received a lot of attention due to their role in breaking seed dormancy and promoting seed germination. SA, being a small phenolic molecule, has strong physiological effects under adverse plant conditions. It can improve seed germination under environmental stress, promote seedling growth and regulate various physiological and biochemical indices of stress resistance in plants [25–27]. Melatonin, a small indole-like molecule compound commonly found in plants, has been shown to improve seed germination and induce root production and growth. This is likely due to its involvement in the scavenging of reactive oxygen species and its vital role in physiological processes such as mature senescence, stress resistance, and immune regulation [28,29]. MC/DPC is a plant growth regulator with growth retarding functions that can increase seed vigor by promoting reductive and respiratory rates, thus increasing seed germination [30,31].
Winnowing grading is a method of removing impurities by using the difference in suspension speed between good seeds and unqualified seeds, and there is a significant difference between good seeds and unqualified seeds in terms of thousand-grain weight, so the purity and quality of seeds can be improved by winnowing [32]. The differences in nutrient content and physical structure of different substrates lead to differences in seed germination and growth, which are mainly reflected in the physiological indicators of seed germination rate, germination potential and seedling height [33]. Many studies have demonstrated that different substrates affect seed germination and that seed germination varies significantly under different substrate treatments [34,35].
During the harvesting, there are differences in the morphology of fruit and seeds of different batches of I. asprella, leading to fluctuating seed germination percentages that have a direct impact on the planting plan and planting scale of I. asprella. In addition, the seeds of I. asprella have a prolonged dormancy period, which results in a long period of seed germination and seedling growth. These two issues, namely the low and unstable seed germination and the extended seedling-raising period are the key constraints limiting the production and planting of I. asprella. Here, the effects of germination medium, hormone, winnowing, and stratification treatments were studied to improve the seed germination of I. asprella and shorten the seedling raising time. This study aimed to develop an effective method for the germination of I. asprella seeds and provide a scientific reference for improving the efficiency of large-scale planting of I. asprella.
On June 26th, 2022, the mature seeds of 4-year-old I. asprella plants were collected from the Maodaoyuan I. asprella planting base in Hezhou, Guangxi, China. The average weight of 100 seeds was 0.513 g, with a water content of 7.73%.
2.2 Germination Medium Screening Test
A germination box measuring 12 cm × 6 cm × 6 cm was filled with moist fine sand (particle size 0.1–0.2 mm, 200 g river sand + 40 g purified water), moist perlite (particle size 3–5 mm, 15 g perlite + 60 g purified water), moist vermiculite (particle size 1–2 mm, 30 g vermiculite + 80g purified water), and moist filter paper (2 layers of filter paper +10 ml purified water) to represent the germination bed. Subsequently, 200 seeds were evenly placed throughout the germination box. Then the germination box was placed in a light incubator for germination culture with a photoperiod of 12 h light/day, and a constant temperature of 10°C, 15°C, 20°C, 25°C, and 30°C, as well as a day/night variable temperature of 20°C/10°C, 25°C/15°C, and 30°C/20°C, respectively. Each treatment was repeated three times. The germination percentage was calculated on the 100th day after sowing.
To further observe the germination progress of I. asprella seeds, the germination percentage was recorded over time under the optimum germination medium (vermiculite) and temperature (20°C/10°C day/night).
2.4 Hormonal Treatment Experiment
For the hormonal treatment experiment, 3 g of dried seeds were soaked in gibberellin (50, 100, 200, 400 mg/L), melatonin (1, 5, 10, 20, 40 mg/L), abscisic acid (1, 5, 10, 20, 40 mg/L), salicylic acid (1, 10, 20, 50, 100 mg/L), and mepiquat chloride (1, 5, 10, 20, 40 mg/L) solutions for 10 h, with the distilled water immersion as the control, then rinsed 3~5 times with clean water. Subsequently, the seeds were evenly placed on a moist vermiculite germination bed and placed in a light incubator for germination (photoperiod of 12/12 h light/dark, the temperature of 20°C/10°C day/night). Each treatment was repeated four times and each repetition contained 100 seeds. After 90 days, the germination percentage was calculated.
3 g of dried seeds were placed in a fanning mill (model CFY-4, Zhejiang Topyunnong Technology Co., Ltd., China) with a wind flow of 2.5 m3/min for 120 s. The seeds (wind-selected and control) were placed on the wet vermiculite germination bed and then were laid in the light incubator for germination (the photoperiod of 12 h light/day, the germination temperatures of 20°C/10°C day/night, 10°C, and 15°C). After 90 days, we calculated the germination percentage. Each treatment was replicated four times and each repeat contained 100 seeds.
The seeds that had been selected by the wind were mixed with the wet perlite (the weight ratio of perlite: water is 1:2) in a volume ratio of 1:1 and incubated for 2 months at a constant temperature of 5°C, 15°C, and 25°C for stratification treatment. Then, these seeds were placed on the wet vermiculite germination bed and germinated in the light incubator (the photoperiod was 12 h light/d, and the temperature was set to be a series of the constant temperatures of 10°C, 15°C, 20°C, 25°C, and 30°C and a series of day/night variable temperatures of 20°C/10°C, 25°C/15°C and 30°C/20°C, respectively). After 80 days, we calculated the germination percentage. The dry seeds without stratification were set as the control. Each treatment was repeated three times and each repeat contained 100 seeds.
2.7 Germination Standard and Calculation
When the radicle emerged through the seed coat and its length exceeded 2 mm, the seed was deemed to have germinated. The germination ended when the number of germinated seeds did not increase for 7 consecutive days.
Germination percentage = Number of germinated seeds/number of tested seeds × 100%.
SPSS 23.0 software was used for statistical analysis and the data means were analyzed using the Duncan test for statistical significance (p < 0.05). Each test was analyzed independently and the test for homogeneity of variance was performed before ANOVA. WPS software was employed for graphing.
3.1 Germination Medium Screening Test
Table 1 showed that 20°C/10°C had the highest germination percentage among the different germination temperatures tested, followed by 10°C and 15°C, conversely, the seeds of I. asprella barely germinated at the other temperatures. At 20°C/10°C, the germination percentage of vermiculite treatment was significantly higher than that of the fine sand, perlite, and filter paper treatments and increased by 13.4%, 19.9%, and 411.6%, respectively. Under a constant temperature of 10°C, the germination percentage of I. asprella seeds varied as follows: vermiculite (35%) > fine sand (30.8%) > perlite (16.3%) > filter paper (4.3%), while at a constant temperature of 15°C, the order of the germination rate of I. asprella seeds was fine sand (17.7%) > vermiculite (4.5%) > perlite (3.2%) > filter paper (2.7%). In a word, the highest germination percentage (45.2%) was obtained under the treatment of 20°C/10°C day/night temperature and vermiculite germination medium conditions, which was more conducive to the seed germination of I. asprella. This could be because the vermiculite germination medium was more effective in providing insulation and moisture for the seeds of I. asprella, thus making the germination of the seeds more favorable.
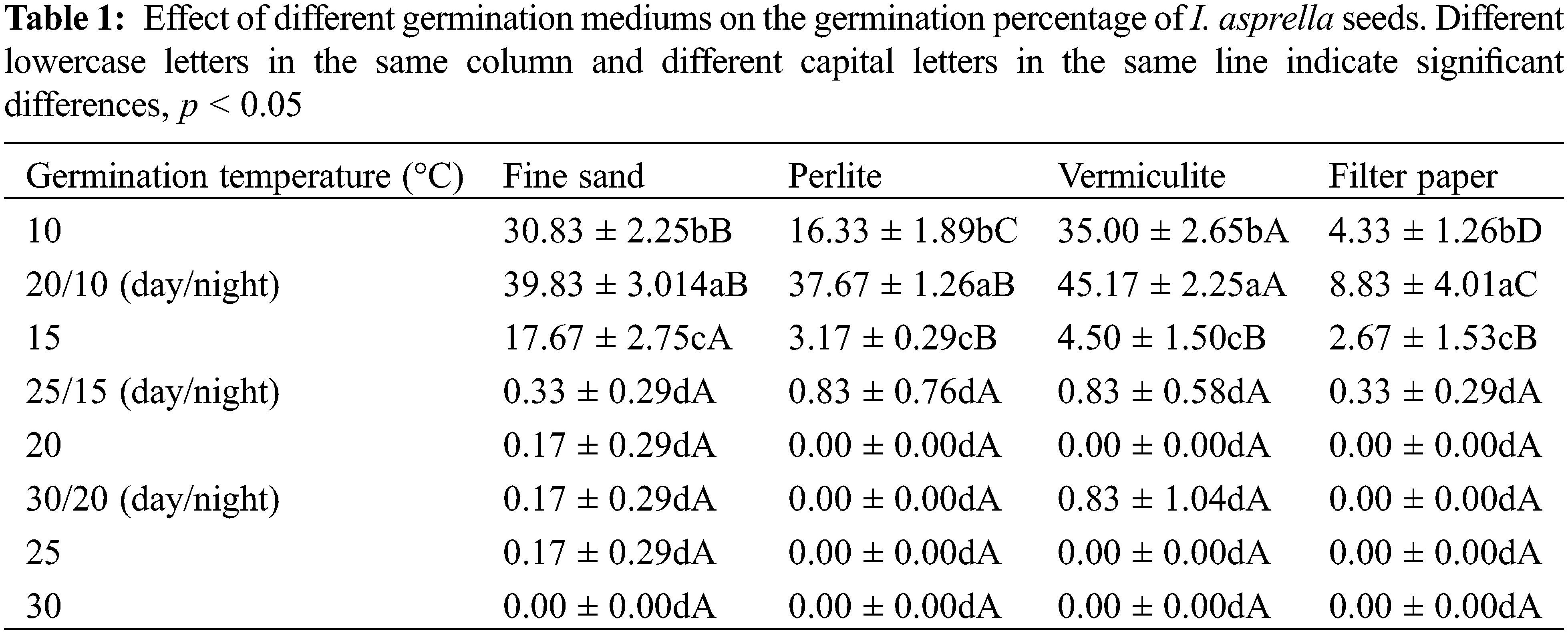
3.2 Germination Progress of I. asprella Seeds
As could be observed from Fig. 1, the germination of I. asprella seeds commenced on the 50th day, with subsequent germination percentages calculated every 10 days from the 60th to the 100th day being 3.8%, 20.2%, 32.3%, 45.2%, and 47.7%, respectively. Particularly, during the time intervals of 60th–70th, 70th–80th, and 80th–90th, the germination percentage of I. asprella seeds had a growth rate greater than 10%, with respective figures of 16.4%, 12.1%, and 14.9%. However, from the 90th day to the 100th day, the germination percentage increased by a rate of 5.5%. This indicated that the seed germination percentage of I. asprella tended to be flat.
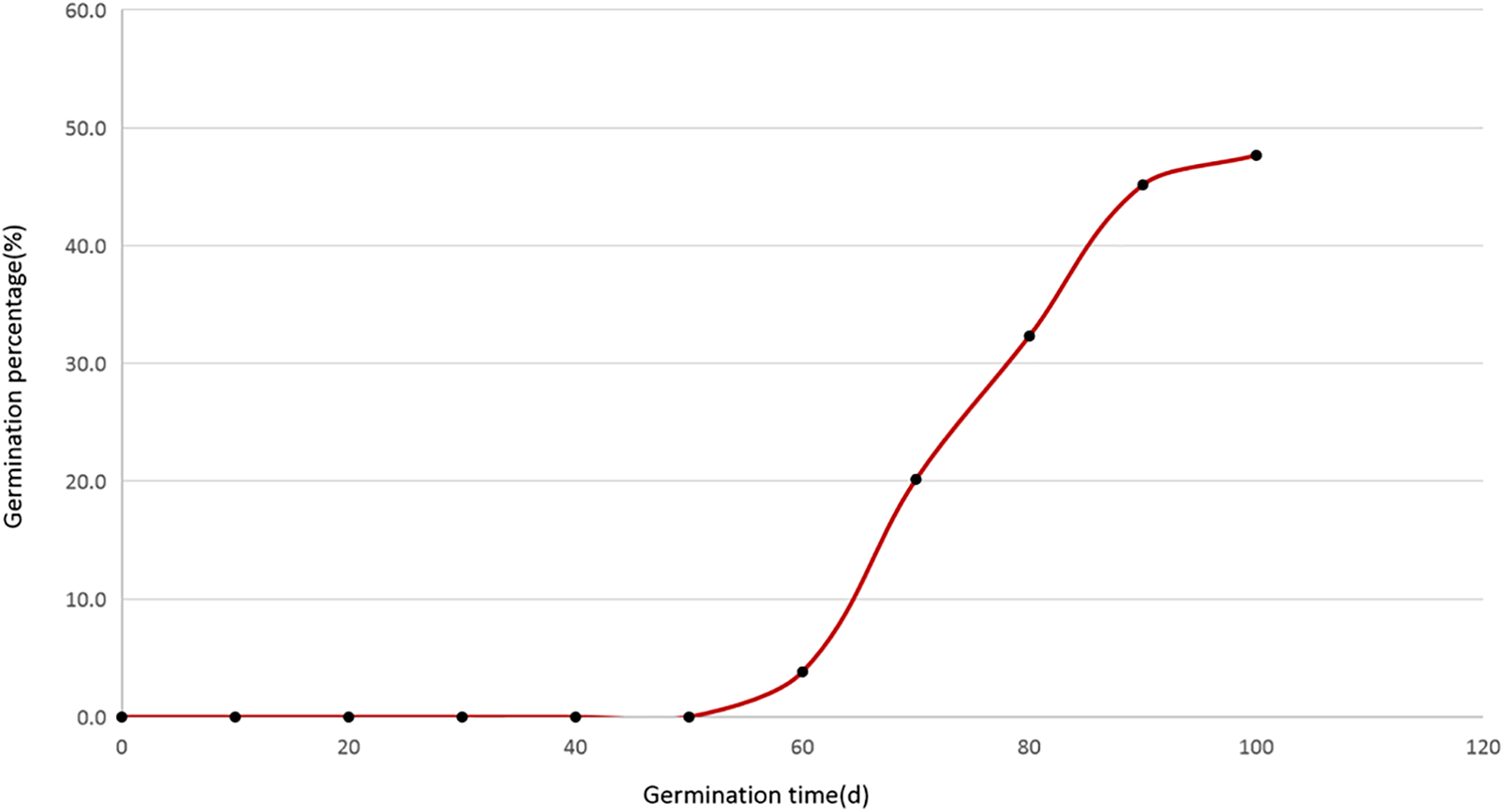
Figure 1: Germination progress of I. asprella seeds under 20°C/10°C day/night and vermiculite condition
3.3 Hormonal Treatment Experiment
Different hormones had different effects on the germination percentage of I. asprella seeds. The germination percentage of I. asprella seeds was significantly affected by the concentration of GA (p < 0.05; Fig. 2A). With the increase in GA concentration, the germination percentage of I. asprella seeds increased first and then became flat. The germination percentage of the seeds treated with different concentrations of GA decreased in the order of 400 mg/L GA (60.13%) > 100 mg/L GA (58.25%) > 200 mg/L GA (56.88%) > 50 mg/L GA (52.5%) > CK (42.88%).
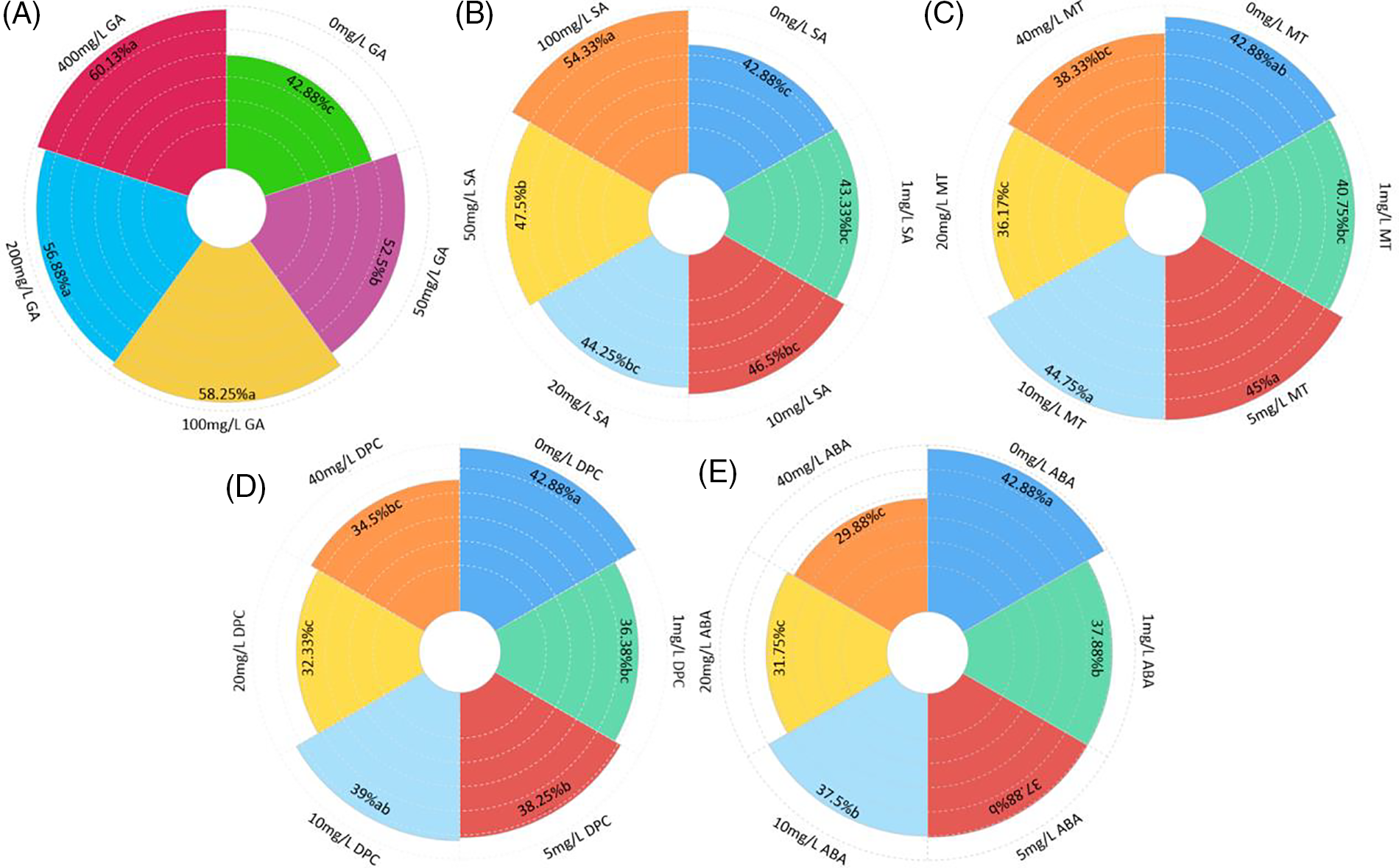
Figure 2: Effects of hormone soaking on the seed germination of I. asprella. (A) The effects of different GA concentrations on seed germination. (B) The effects of different SA concentrations on seed germination. (C) The effects of different MT concentrations on seed germination. (D) The effects of different DPC concentrations on seed germination. (E) The effects of different ABA concentrations on seed germination
No statistically significant difference in seed germination was observed at 1, 10, and 20 mg/L SA compared to the control (p > 0.05). However, a significant increase in seed germination percentage was observed at 50 mg/L SA (47.50%) when compared to the control (42.88%). The treatment with 100 mg/L SA had the most positive effect on germination, resulting in a germination percentage of 54.33%, which was 14.38%, 22.78%, 16.84%, 25.39%, and 26.70% higher than that of the treatments with 50, 20, 10, 1 mg/L SA and the control, respectively (Fig. 2B). It was indicated that the high concentration of SA had a promoting effect on the seed germination of I. asprella. On the contrary, the MT, ABA, and DPC treatments dramatically reduced the seed germination percentage of I. asprella (p < 0.05), compared with the control (Figs. 2C–2E). Conversely, with the increase in the concentration of MT, ABA, and DPC, the germination percentage of I. asprella seeds tended to decrease, indicating that MT, ABA, and DPC had an inhibitory effect on the germination of I. asprella seeds.
There was a significant difference (p < 0.05) in the 100-grain weight of the winnowing seeds of I. asprella compared with the control (Fig. 3A). The winnowing seeds had a 100-grain weight of 0.62 g, which increased by 21.57% in comparison with the control. The winnowing treatment also significantly affected the seed germination percentage of I. asprella seeds (p < 0.05) (Fig. 3B). At 20°C/10°C, 15°C, and 10°C, the germination percentage of the winnowing seeds were 68.3%, 51.3%, and 41.3%, respectively. The highest germination percentage was 68.3% at 20°C/10°C, representing an increase of 23% higher than that of the seeds without winnowing.
Figure 3: Effects of winnowing on the seed germination of I. asprella. (A) The comparison of 100-grain weight between the winnowing seeds and the control. (B) The comparison of germination percentage between the winnowing seeds and the control under different germination temperatures. CK = No stratification
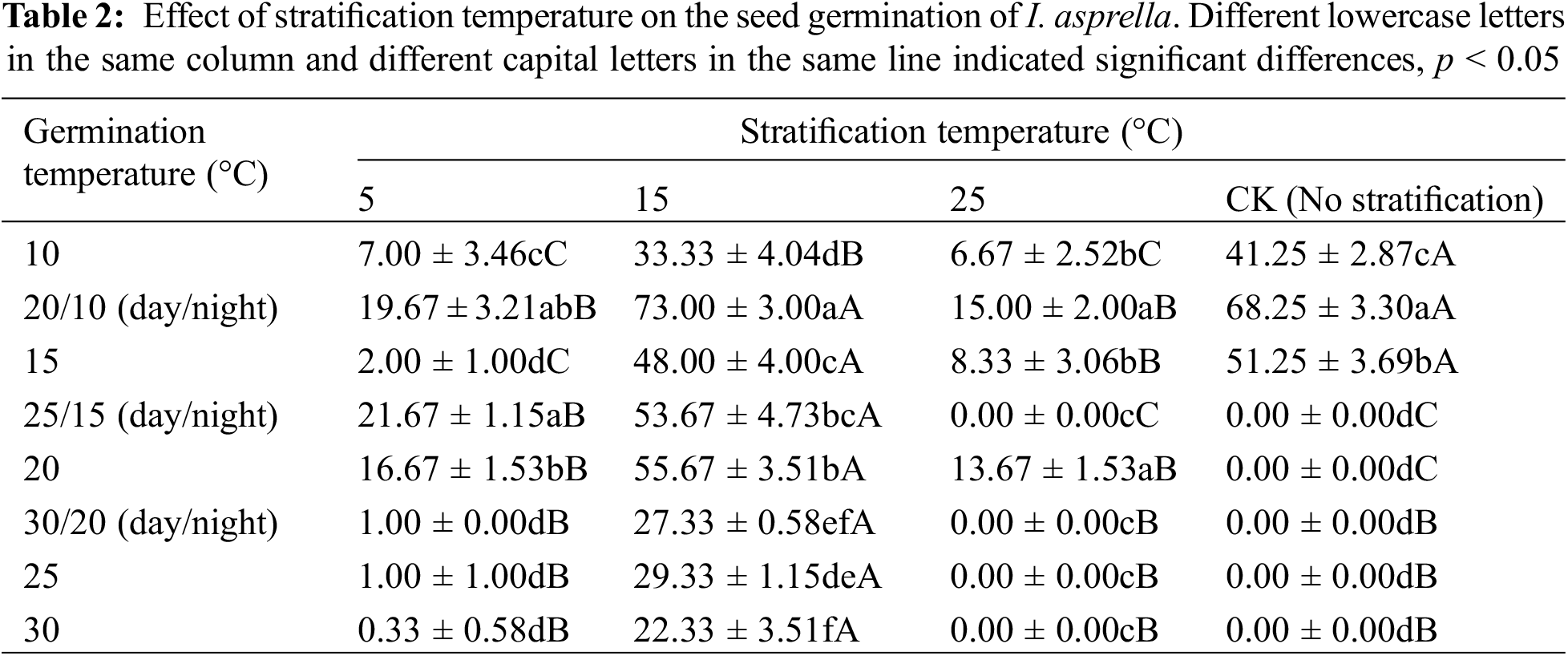
To observe the effects of stratification temperature on the seed germination of I. asprella, the changing trend of the germination of the wind-selected seeds in perlite was monitored after stratification at 5°C, 15°C, and 25°C for 2 months. As was evident in Table 2, the stratification treatment not only improved the seed germination percentage but also expanded the temperature range in which I. asprella seeds could germinate. The seeds without stratification treatment hardly germinated under higher temperature conditions (25°C/15°C, 20°C, 30°C/20°C, 25°C, and 30°C). The highest germination percentage without stratification was 68.3% under the 20°C/10°C condition. It was indicated that low temperature was favorable for the seed germination of I. asprella. In particular, after the 15°C stratification treatment, the seeds germinated at different germination temperatures, and the germination percentage was higher than 20%. After the stratification treatment at 15°C, the highest germination percentage was 73% under the germination condition of 20°C/10°C, which was 4.7% higher than that of the control (p > 0.05). It was inferred that the 15°C stratification treatment could significantly increase the seed germination and expand the temperature range of seed germination.
Seed formation is closely related to the plant’s genetic features and environmental conditions, and it is generally believed that larger seeds are considered to facilitate the reproduction and development of the species [36]. The winnowing treatment could remove unsaturated seeds and impurities and improves the clarity and purity of the seeds. Previous studies have shown that the thousand-grain weight of I. asprella seeds is positively correlated with germination percentage [37]. In the present study, after winnowing treatment, the 100-grain weight of the seeds was heavier and the seed germination percentage observed was significantly higher than that of the control seeds (Fig. 3), indicating that the results were consistent with the above findings [37].
Substrate, a light material, is an effective substitute for soil, as it provides plants with the necessary nutrients and growth environment. It is abundant in key nutrients such as nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), and other essential nutrients to support plant growth and development, thus reducing the need for fertilizers. Common substrates include vermiculite, perlite, fine sand, etc. [38]. In this study, at the optimum germination temperature (20°C/10°C), vermiculite had the best treatment effects (Table 1). It might be because vermiculite harbored the characteristics of improving the soil aggregate structure and storing water and moisture, resulting in better moisturizing and fertilizing, which was more conducive to the seed germination of I. asprella [39].
The internationally acceptable hierarchical system of classification for seed dormancy includes five classes of dormancy: physiological dormancy (PD), morphological dormancy (MD), morphophysiological dormancy (MPD), physical dormancy (PY), and combinational dormancy (PY+PD) [17]. Based on cold and/ or warm stratification requirements for germination, temperature requirements for embryo growth, and response to GA3, MPD can be classified into nine types: non-deep simple, intermediate simple, deep simple, deep simple epicotyl, non-deep simple epicotyl, deep simple double, non-deep complex, intermediate complex, and deep complex MPD [40]. Among them, the feature of non-deep simple is that the seed embryo grows and develops under warm stratification (>15°C). Additionally, GA can shorten the stratification time and reduce the depth of dormancy [40]. In this study, the effect of warm temperature stratification at 15°C was significantly better than that at 5°C and 25°C (Table 2), and the concentration of GA at 100–400 mg/L could significantly improve the seed germination of I. asprella (Fig. 2). Therefore, it was indicated that the seeds of I. asprella might belong to the non-deep simple MPD type. Moreover, only the high concentration of SA significantly increased the germination of I. asprella seeds (Fig. 2). This indicated that the most suitable concentration of SA for the germination of I. asprella seeds needed to be further studied. In addition, with the increase of the concentrations of MT, ABA, and DPC, the seed germination of I. asprella tended to decrease, suggesting that MT, ABA, and DPC had an inhibitory effect on the germination of I. asprella seeds.
In the Lingnan region, the ideal climatic conditions for I. asprella growth are an annual average temperature of 20°C–28°C, with an average of 24°C–31°C in summer and 13°C–25°C during the spring and winter. Even if the seeds are collected and sown immediately in July of that year, they will not germinate until the next spring [16]. In addition to the dormancy characteristics of the seeds, the reasons might also be related to the high temperature in summer, which was not suitable for the germination of the seeds. In this study, the seeds without stratification (CK) were capable of germinating under constant temperatures of 10°C and 15°C, as well as altering temperature of 20°C/10°C. This also confirmed that the germination of the seeds of I. asprella prefered the seasons and temperatures of autumn, winter, and spring in South China.
Studies have shown that seed germination in many species requires temperature fluctuations [41–43]. In the present study, regardless of whether the seeds of I. asprella were treated by stratification or not, the germination of I. asprella seeds under 20/10 variable temperature conditions was significantly higher than that under other constant temperature conditions. I. asprella is a deciduous shrub, which often grows in roadside bushes or broad-leaved forests in the valley at an altitude of 400–1000 m. The suitable temperature for growth is 16°C–25°C, and it especially prefers semi-shady and semi-sunny environments [2]. Therefore, it was inferred that the lack of fluctuation of ambient temperature might also be one of the important reasons limiting the germination of I. asprella seeds.
Stratification treatment is a useful physical method to release plant seed dormancy, which can break seed dormancy and improve seed germination. In this study, the seeds from low-temperature stratification (5°C) and high-temperature stratification (25°C) had a low germination percentage (<22%) at all germination temperatures, while the seeds from warm-temperature stratification (15°C) at all germination temperatures harbored higher germination (Table 2). Although the germination percentage of the seeds from warm temperature stratification was not significantly different from that of the control at the germination temperatures of 20°C/10°C, the temperature range of seed germination of I. asprella was greatly expanded after warm temperature stratification. It was indicated that the low germination temperature and warm temperature stratification conditions were favorable for seed germination of I. asprella and for seed nursery at room temperature in production practice.
In this study, vermiculite was found to be the most suitable germination medium, and the optimum germination temperature was 20°C/10°C day/night for the seeds of I. asprella. 100–400 mg/L gibberellin (GA) and 50–100 mg/L salicylic acid (SA) were effective methods to break the dormancy of I. asprella seeds. In addition, winnowing was found to be an effective method for removing unsaturated seeds and impurities, thus improving seed germination. A warm temperature (15°C) stratification was also shown to expand the temperature range of seed germination of I. asprella. Overall, the present study provided the conditions where a high germination percentage of I. asprella seeds could be obtained.
Funding Statement: This study was supported by the Fund Projects of the Central Government in Guidance of Local Science and Technology Development (GuiKeZY22096020), Natural Science Foundation of Guangxi (2019GXNSFBA245073), National Natural Science Foundation of China (82260750, 82260749), and Cooperative Project of Guangxi Botanical Garden of Medicinal Plants with China Resources Sanjiu Medical & Pharmaceutical Co., Ltd. (202112-1).
Author Contributions: Conceptualization: D.F.T. and Y. X. Z.; methodology: Y.H., Q.M., Y.L. and J.H.; formal analysis: F.W.; writing-review and editing: D.F.T. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this article.
Ethics Approval: In this study, the collection of plant materials complies with relevant institutional, national, and international guidelines and legislation.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Chen, W. S., Zhou, L. L., Qiao, Y., Qi, J. Z., Fu, G. C. et al. (2019). Quality evaluation of Ilex asprella based on simultaneous determination of five bioactive components, chlorogenic acid, luteoloside, quercitrin, quercetin, and kaempferol, using UPLC-Q-TOF MS study. Journal of AOAC International, 102(5), 1414–1422. [Google Scholar] [PubMed]
2. Zeng, S. Y., Liu, H. N., Zhang, D. S., Zhu, X. J., Fan, X. Q. et al. (2020). Experiment on seed breeding Ilex asprella. Hunan Forestry Science & Technology, 47(6), 20–23. [Google Scholar]
3. Editorial Committee of Flora of China, Chinese Academy of Sciences (1993). Flora of China. China: Science Press. [Google Scholar]
4. Xing, J. Y., Du, B. Z., Feng, X., Zhang, H. X. G., Han, Z. Z. et al. (2018). Phenolic constituents from stems of Ilex asprella. China Journal of Chinese Materia Medica, 43(21), 4267–4273. [Google Scholar] [PubMed]
5. Yang, X. Y., Gao, X. L., Du, B. Z., Zhao, F., Feng, X. et al. (2018). Ilex asprella aqueous extracts exert in vivo anti-inflammatory effects by regulating the NF-κB, JAK2/STAT3, and MAPK signaling pathways. Journal of Ethnopharmacol, 225, 234–243. [Google Scholar]
6. Zhang, W., Chen, S. T., He, Q. Y., Huang, L. Q., Li, X. et al. (2019). Asprellcosides B of Ilex asprella ihibits influenza a virus infection by blocking the hemagglutinin-mediated membrane fusion. Frontiers in Microbiology, 9, 3325. [Google Scholar] [PubMed]
7. Peng, M. H., Dai, W. P., Liu, S. J., Yu, L. W., Wu, Y. N. et al. (2016). Bioactive glycosides from the roots of Ilex asprella. Pharmaceutical Biology, 54(10), 2127–2134. [Google Scholar] [PubMed]
8. Meng, F. C., Li, Q., Qi, Y. N., He, C. W., Wang, C. M. et al. (2018). Characterization and immunoregulatory activity of two polysaccharides from the root of Ilex asprella. Carbohydrate Polymers, 197, 9–16. [Google Scholar] [PubMed]
9. Lei, Y., Shi, S. P., Song, Y. L., Bi, D., Tu, P. F. (2014). Triterpene saponins from the roots of Ilex asprella. Chemistry & Biodiversity, 11(5), 767–775. [Google Scholar]
10. He, S. Z., Zhang, Y. P., Yu, L. Y. (2008). Research on the antimicrobial function of CTM Ilicis asprellae. Modern Hospitals, 8(5), 12. [Google Scholar]
11. Xiao, C. H. (2014). Studies on antioxidant, antimicrobial and efficacy components of holly root (Master Thesis). South China University of Technology, China. [Google Scholar]
12. Hu, X. Y., Shu, X. C., Guo, Y., Ma, Y. (2012). Effect of an Ilex asprella root decoction on the related genes of lipid metabolism from chronic stress and hyperlipidemic fatty liver in rats. Chinese Medical Journal, 125(19), 3539–3542. [Google Scholar] [PubMed]
13. Du, B. Z., Yang, X. Y., Feng, X., Yin, X., Zhang, H. X. G. et al. (2017). A phytochemical and pharmacological advance on Ilex asprella. China Journal of Chinese Materia Medica, 42(1), 20–28. [Google Scholar] [PubMed]
14. Zeng, X. D., Li, Y. K., Zhuo, Y. N., Wang, M. L., He, R. et al. (2018). Preliminary study on germination inhibitors from Ilex asprella fruits. Northern Horticulture, 7, 133–138. [Google Scholar]
15. Wang, N. (2006). Research on the dormancy mechanism of holly seeds (Master Thesis). Henan Agricultural University, China. [Google Scholar]
16. Mei, Y., Zhou, Z. X., Wang, J. H. (2020). Advances in research of Ilex asprella. Chinese Journal of Tropical Agriculture, 40(2), 31–38. [Google Scholar]
17. Baskin, J. M., Baskin, C. C. (2004). A classification system for seed dormancy. Seed Science Research, 14, 1–16. [Google Scholar]
18. Wei, C. Y. (2007). Studies on the mechanisms and breaking methods of Ilex purpurea seed dormancy (Master Thesis). Nanjing Forestry University, China. [Google Scholar]
19. Galíndez, G., Ceccato, D., Bubillo, R., Lindow-López, L., Malagrina, G. et al. (2018). Three levels of simple morphophysiological dormancy in seeds of Ilex (Aquifoliaceae) species from Argentina. Seed Science Research, 28(2), 131–139. [Google Scholar]
20. Pan, W. W., Hong, X., Lian, F. L. (2017). Effects of different treatments on seed germination of Ilex ficoidea. Seed, 36(3), 99–101. [Google Scholar]
21. Deng, Y. D., Pan, W. B., Chen, H. Z. (2013). Effects of different treatment methods on seeding emergence of Ilex triflora. Journal of Zhangzhou Institute of Technology, 15(2), 20–23+33. [Google Scholar]
22. Herrera, C. M., Cerdá, X., García, M. B., Guitián, J., Medrano, M. et al. (2010). Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. Journal of Evolutionary Biology, 15(1), 108–121. [Google Scholar]
23. Castro-Camba, R., Sánchez, C., Vidal, N., Vielba, J. M. (2022). Plant development and crop yield: The role of gibberellins. Plants, 11(19), 2650. [Google Scholar] [PubMed]
24. Yan, A., Chen, Z. (2016). The pivotal role of abscisic acid signaling during transition from seed maturation to germination. Plant Cell Reports, 6(5), 689–703. [Google Scholar]
25. Sun, M. D., Liu, C., Feng, G. J., Liu, D. J., Yan, Z. S. et al. (2022). Effect of salicylic acid on seed germination and cold resistance of kidney bean under low temperature stress. Journal of Engineering of Heilongjiang University, 13(2), 91–96. [Google Scholar]
26. Zhang, Y. K., Zhang, S. S. (2022). Effects of seed soaking with salicylic acid on seed germination and early seedling growth of Mastixia euonymoides under drought stress. Hunan Forestry Science & Technology, 49(3), 24–29. [Google Scholar]
27. Szalai, G., Horgosi, S., Soós, V., Majláth, I., Balázs, E. et al. (2010). Salicylic acid treatment of pea seeds induces its de novo synthesis. Journal of Plant Physiology, 168(3), 213–219. [Google Scholar] [PubMed]
28. Li, Y. L., Gao, Y. Q. H., Xin, L. M., Li, Q., Li, N. (2022). Effect of melatonin soaking on alfalfa seed under NaCl stress. Molecular Plant Breeding. https://kns.cnki.net/kcms/detail/46.1068.S.20220524.1440.007.html [Google Scholar]
29. Arnao, M. B., Hernández-Ruiz, J. (2018). Melatonin and its relationship to plant hormones. Annals of Botany, 121(2), 195–207. [Google Scholar] [PubMed]
30. Wang, N., Tian, X. L., Duan, L. S., Yan, G. T., Huang, Q. et al. (2014). Metabolism of reactive oxygen species involved in increasing root vigour of cotton seedlings by soaking seeds with Mepiquat chloride. Acta Agronomica Sinica, 40(7), 1220–1226. [Google Scholar]
31. Wang, N., Wang, X. R., Shi, J. B., Liu, X. H., Xu, Q. H. et al. (2019). Mepiquat chloride-priming induced salt tolerance during seed germination of cotton (Gossypium hirsutum L.) through regulating water transport and K/Na homeostasis. Environmental and Experimental Botany, 159, 168–178. [Google Scholar]
32. Ji, Y. H., Wu, P., Wang, C., Zhong, Q. W., Wu, Z. H. et al. (2020). Effects of winnowing and priming on the seed germination and seedling growth of pepper. China Cucurbits and Vegetables, 33(1), 29–32. [Google Scholar]
33. Zhou, Y. D., Zhou, Y. B., Yan, S. L., Luo, C., Qi, J. S. et al. (2013). Effects of different substrates on seed germination and seedling growth of Firmiana pulcherrima Hsue. Journal of Tropical Biology, 4(4), 322–326. [Google Scholar]
34. Huang, G. H., Liang, K. N., Zhou, Z. Z., Ma, H. M., Lin, M. P. (2009). The effect of different media on seeds budding and seedlings growth of Tectona Grandis. Seed, 28(10), 86–87+90. [Google Scholar]
35. Dong, Q., He, Z., Xu, Y. P., Zhang, X. M., Li, C. Y. (2012). Effect of different basal fertilizers on Cyphomandra betacea plug seeding breeding. Seed, 31(5), 50–53. [Google Scholar]
36. Gómez, J. M. (2004). Bigger is not always better: Conflicting selective pressures on seed size in Quercus ilex. Evolution, 58(1), 71–80. [Google Scholar]
37. Lan, J. X., Wang, J., Liu, H. H., Chen, S. Q., Guo, X. K. (2019). Effects of plant, fruit and seed morphology on seed germination rate of Ilex asprella. Journal of Chinese Medicinal Materials, 42(12), 2755–2758. [Google Scholar]
38. Zhang, Y. T. (2011). Screening of cheap substrate for industrialization of fruit vegetable seedling production (Master Thesis). Inner Mongolia Agricultural University, China. [Google Scholar]
39. Liu, Y. C. (2022). The formula screening of pepper seedlings and cultivation substrates in solar green house with vermiculite as the main ingredient (Master Thesis). Tarim University, China. [Google Scholar]
40. Yao, L. J., Zhang, K. L., Xiong, Z. M., Tao, J. (2019). Research advances in seed morphophysiological dormancy. Chinese Journal of Ecology, 38(1), 247–255. [Google Scholar]
41. Yang, L. E., Peng, D. L., Li, Z. M., Huang, L., Yang, J. et al. (2020). Cold stratification, temperature, light, GA3, and KNO3 effects on seed germination of Primula beesiana from Yunnan. China Plant Diversity, 42(3), 168–173. [Google Scholar] [PubMed]
42. Liu, K., Baskin, J. M., Baskin, C. C., Bu, H. Y., Du, G. Z. et al. (2013). Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the Eastern Tibet Plateau. PLoS One, 8(7), e69364. [Google Scholar] [PubMed]
43. Thompson, K., Grime, J. P. (1983). A comparative study of germination responses to diurnally-fluctuating temperatures. Journal of Applied Ecology, 20(1), 141–156. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools