 Open Access
Open Access
ARTICLE
Impact of Seed Halopriming on Germination, Morphological Traits, and Cry1Ac Gene Expression in Bt Cotton (Gossypium hirsutum)
1 College of Agriculture, Guizhou University, Guiyang, 550025, China
2 Faculty of Medicine and Health Technology, Tampere University, Tampere, 33720, Finland
3 Department of Life Sciences, University of Trieste, Via Licio Giorgieri 5, Trieste, 34127, Italy
4 National Institute for Genomics and Advanced Biotechnology (NIGAB), National Agricultural Research Centre (NARC), Islamabad, 45500, Pakistan
* Corresponding Authors: Ruhong Xu. Email: ; Luhua Li. Email:
# These authors equally contributed to this work
(This article belongs to the Special Issue: Recent Research Trends in Genetics, Genomics, and Physiology of Crop Plants)
Phyton-International Journal of Experimental Botany 2025, 94(1), 229-241. https://doi.org/10.32604/phyton.2025.059315
Received 04 October 2024; Accepted 12 December 2024; Issue published 24 January 2025
Abstract
Seed priming is an effective seed pretreatment technology that enhances germination and overall crop performance by optimizing seed hydration and metabolic processes before planting. Seed quality is a critical determinant of cotton (Gossypium hirsutum) crop performance, influencing germination, plant vigor, and yield. This study evaluates the effects of seed priming with potassium salts (1% and 2% KCl and K2SO4) on germination, morphological traits, and Cry1Ac gene expression in three Bt cotton cultivars (IUB-2013, NIAB-878B, FH-142) as Cry1Ac enhance the pest resistance in Bt cotton and reduce the plant’s dependence on chemical insecticides. Seeds were primed for six hours, air-dried, and sown in the field. Germination rates, plant height, number of bolls per plant, boll weight, seed cotton yield, and ginning outturn (GOT) were assessed at crop maturity. Cry1Ac gene expression was quantified to explore the influence of priming treatments on transgene activity. Results demonstrated that 1% K2SO4 priming significantly enhanced germination and yield-related traits, with Cry1Ac expression peaking in the IUB-2013 cultivar under 1% K2SO4 treatment. These findings suggest that potassium-based halopriming improves cotton seedling establishment and Bt gene expression. This study addresses the critical gaps in understanding the effects of seed halopriming on morphological traits, germination, and expression of the Cry1Ac gene in Bt cotton while providing a novel eco-friendly and cost-effective halopriming approach, offering the potential to improve cotton production.Keywords
Cotton (Gossypium hirsutum) is the most widely cultivated natural fiber crop and ranks third among global oilseed crops. With an estimated annual production of 25 million tons, cotton is critical to agricultural economies and plays a pivotal role in the textile industry [1–3]. Projections suggest that global cotton production will increase by 1.5% annually, reaching approximately 30 million tons by 2029 [4]. Cotton significantly contributes to the economic development of many nations, especially in countries like Pakistan, where it accounts for 50% of industrial employment and over 60% of agricultural exports [5]. It is primarily cultivated in tropical and subtropical regions across the globe. The leading cotton-producing countries include China, India, the United States, Brazil, Pakistan, Australia, Turkey, and Uzbekistan [6]. Meeting the rising industrial demand and improving the economic conditions of cotton farmers require expanding cotton cultivation into new areas and enhancing crop productivity [7]. Growth in cotton production is expected to stem from higher global yields and the expansion of cultivation areas. However, achieving sustainable yield growth remains challenging due to climate change, poor seed quality, genetic limitations, and pest pressures, including whitefly infestations, cotton leaf curl virus (CLCuV), and pink bollworm attacks [5,8]. Addressing these challenges necessitates the development of resilient genetic varieties and the adoption of improved agronomic practices to secure the future of cotton production.
Biotic and abiotic stressors substantially threaten cotton productivity [9], leading to the development of transgenic cotton varieties like Bt cotton, which express Cry1Ac genes from Bacillus thuringiensis [10]. The Cry1Ac gene encodes an endotoxin toxic to lepidopteran pests such as cotton bollworms [11]. In Bt cotton, constitutive plant promoters regulate Cry1Ac gene expression, ensuring endotoxin production throughout the plant life cycle. However, variations in gene expression can occur due to environmental factors, genetic backgrounds, and plant developmental stages [12]. Agronomic practices, including nutrient management and seed priming, have been reported to influence Cry1Ac gene expression and pest resistance [13]. These findings underscore the need for targeted strategies to maximize the benefits of Bt cotton in diverse agricultural systems.
Bt cotton offers several advantages, such as reduced pesticide use, enhanced pest resistance, and improved yield potential [14]. It has demonstrated high efficacy against bollworms, with insecticidal efficiency higher in leaf tissues, particularly in younger leaves [15]. However, poor access to high-quality seeds remains a significant challenge, negatively affecting germination, crop establishment, and yield potential [16]. Enhancing seed quality and availability is therefore essential to optimize Bt cotton performance and ensure sustainable production.
Seed priming, a low-cost pre-sowing technique, has shown potential in improving seed germination and seedling vigor under stress conditions. By enabling controlled hydration, seed priming activates pre-germinative metabolic processes without initiating germination, enhancing metabolic activity and nutrient uptake [17]. This technique is effective in improving tolerance to biotic and abiotic stresses [18], promoting uniform germination [19], and increasing water-use efficiency [20]. Halopriming, which involves treating seeds with salt solutions, has been found to enhance chilling tolerance, improve germination indices, and boost seedling vigor [21]. Potassium-based priming, such as with KNO3, has demonstrated significant improvements in germination rates, growth, and hormonal responses in cotton [22,23].
Furthermore, seed priming has been linked to gene expression changes influencing germination and stress responses. For example, priming can modulate the balance of plant hormones like gibberellins (GA) and abscisic acid (ABA) [24] and enhance the expression of stress-related genes [25]. Studies in other crops have shown that priming alters the expression of over 1300 genes, affecting key metabolic events such as DNA replication [26] and protein synthesis [27]. However, the specific impact of priming on transgene expression, particularly Cry1Ac, in Bt cotton remains underexplored. The present study aims to evaluate the effects of potassium-based halopriming on germination, morphological traits, and Cry1Ac gene expression in three Bt cotton cultivars (IUB-2013, NIAB-878B, FH-142). By investigating how stress mitigation techniques like halopriming enhance seedling establishment and Cry1Ac gene expression, this research seeks to develop sustainable strategies for improving Bt cotton performance in stress-prone environments. These findings will contribute to optimizing Bt cotton cultivation across diverse agroclimatic conditions, promoting resilient and sustainable cotton production.
In this study, seeds from three cotton (Gossypium hirsutum) varieties, FH-142, NIAB-878B, and IUB-2013 were obtained from the Crop Sciences Institute (CSI), National Agriculture Research Council (NARC), Islamabad, Pakistan, during the 2022–2023 growing season.
2.2 Cotton Seed Priming Treatments
Seeds from each cotton variety were subjected to halopriming treatments. The seeds were immersed for six hours in priming solutions of 1% and 2% concentrations of potassium chloride (KCl) and potassium sulfate (K2SO4). The temperature of the priming solution was maintained at room temperature (approximately 25 ± 1°C) to avoid thermal stress and the pH of the priming solution was maintained near neutral (approximately 6.5–7) to avoid Ph-induced stress. After the priming treatment, seeds were air-dried on filter paper to remove excess moisture. Once completely dried, the seeds were prepared for sowing under field conditions.
Field plots were prepared and the primed seeds were manually sown in field tunnels using the dibbing method for precise seed placement. The experiment followed a randomized complete block design (RCBD), with three replicates per treatment. Uniform agronomic practices, including fertilizer application and plant protection measures, were applied to all treatments. Seeds treated with the priming solutions and control seeds treated with normal water were sown for comparative analysis.
2.4 Data Collection of Morphological and Yield-Related Traits
Germination data (%) for all treatments were recorded on the 15–20 days after sowing. At maturity, morphological parameters were assessed for both primed and control plants of the cotton cultivars. From control and each seed primed plant, randomly selected the five full-length cotton plants to measure the plant height (cm), internode distance (cm), number of bolls per plant, boll weight (g), seeds per boll, ginning outturn (%), and yield (kg/acre).
2.5 Expression Analysis of Bt Gene
Quantification of Cry1Ac protein expression in Bt cotton plants was performed using a sandwich ELISA kit. A 0.05 g leaf sample was homogenized in 0.5 mL of sample extraction buffer and mixed for 5 min. The extract was centrifuged at 4000 rpm for 3 min, and 100 μL of the supernatant was added to ELISA wells. Samples were incubated in the dark for 45 min, after which the wells were washed 4–5 times with washing buffer. After washing, 100 μL of enzyme solution was added to each well and incubated in the dark for 30 min. The washing step was repeated, and 100 μL of chromogenic substrate was added. Samples were incubated for 15 min at room temperature in the dark. To quantify Bt protein expression, 100 μL of stop buffer was added to each well to terminate the enzymatic reaction. Absorbance (O.D.) was then measured at 450 nm using a microplate reader, following the protocol provided by Envirologix Inc., USA.
Data were recorded in triplicate for each treatment and control group, and mean values were subjected to analysis of variance (ANOVA) using Statistix 8.1 software. The experiment followed a randomized complete block design (RCBD). ANOVA was performed on all morphological parameters and Cry1Ac gene expression data to assess the effects of individual treatments and cotton varieties. A two-way factorial ANOVA was conducted to analyze treatment-by-variety interactions.
3.1 Statistical Analysis of Morphological Traits
Significant differences among treatments were observed when compared to the control group (p < 0.01). The impact of halopriming on the morphological traits was robust, as demonstrated by the two-factor ANOVA (Table 1).
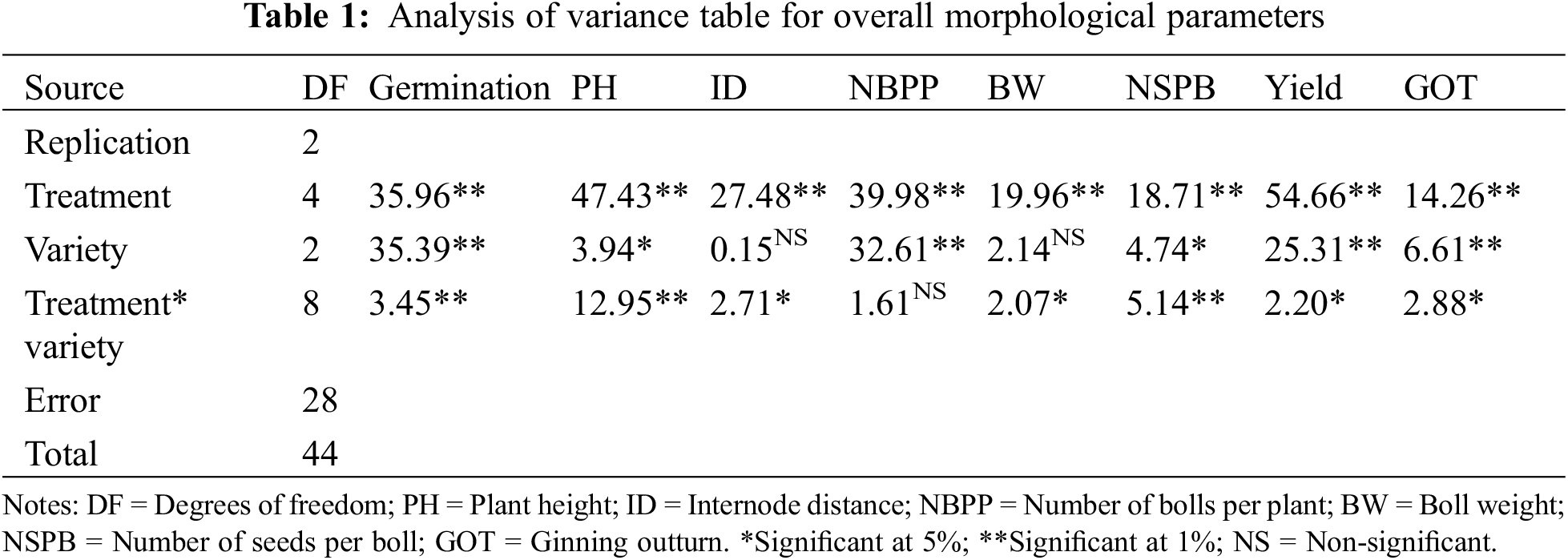
3.2 Effect of Halopriming on Germination
Germination rates were significantly influenced by halopriming treatments. The cultivar FH-142 exhibited the highest germination percentage at 82.6% under the 1% K2SO4 treatment, followed by NIAB-878 at 80%. Conversely, the cultivar IUB-2013 had the lowest germination percent (60%) with the same treatment (Fig. 1). The results indicated significant differences in germination percentages both between treatments and among cultivars (p < 0.01).
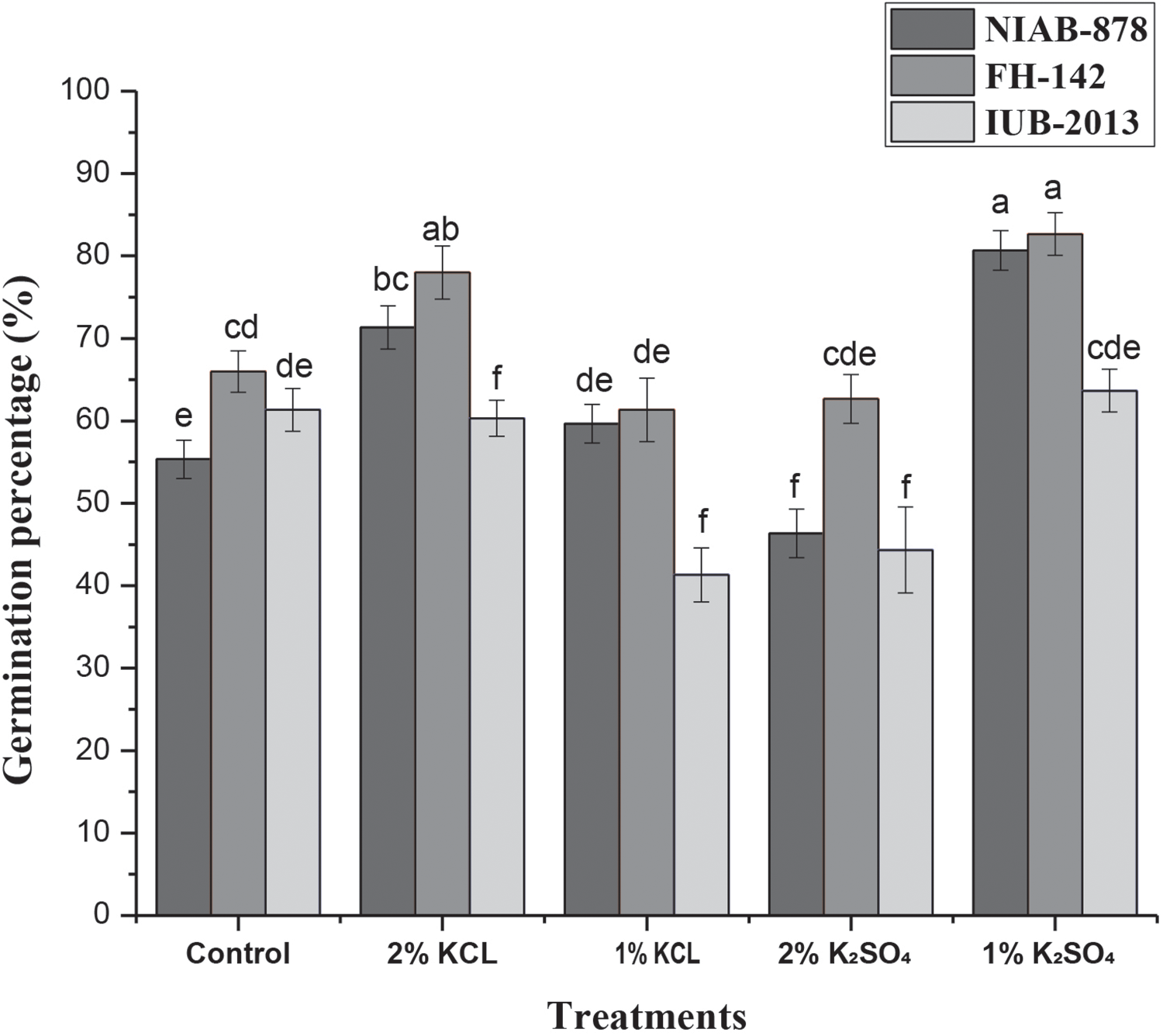
Figure 1: Effect of seed priming on cotton germination and selected morphological parameters in three cotton cultivars (NIAB-878, FH-142, and IUB-2013). The mean values for each parameter are shown with error bars representing the standard error of the mean (SEM). Bars marked with the same letter indicate no significant difference between treatments according to Tukey’s test at the 5% significance level (p < 0.05)
3.3 Effect of Halo-Priming on Morphological Data and Number of Bolls per Plant
The mean plant height for all treatments and varieties was assessed in comparison to the control. Results indicated that the variety NIAB-878 exhibited the highest plant height across all treatments. Specifically, 2% KCl and 1% K2SO4 treatments resulted in the greatest plant heights of 167 and 166 cm, respectively, in the NIAB-878 cultivar. In contrast, the other two varieties, FH-142 and IUB-2013, displayed shorter plant heights compared to NIAB-878. Overall, all cultivars performed best under the 1% K2SO4 treatment, followed closely by 2% KCl, compared to the other treatments (Fig. 2A). The number of bolls is directly correlated with yield. In this study, after calculating the mean number of bolls per plant for all treatments and the control, results indicated that the 1% K2SO4 treatment produced the highest number of bolls per plant across all varieties. Specifically, NIAB-878 had the greatest number of bolls, reaching 78 at 1% K2SO4. However, under all other priming conditions, FH-142 exhibited the highest number of bolls. The effect of cultivar on boll number was significant at the 1% level (p < 0.01). A significant interaction was observed between treatment and variety in terms of boll number per plant (Fig. 2B).
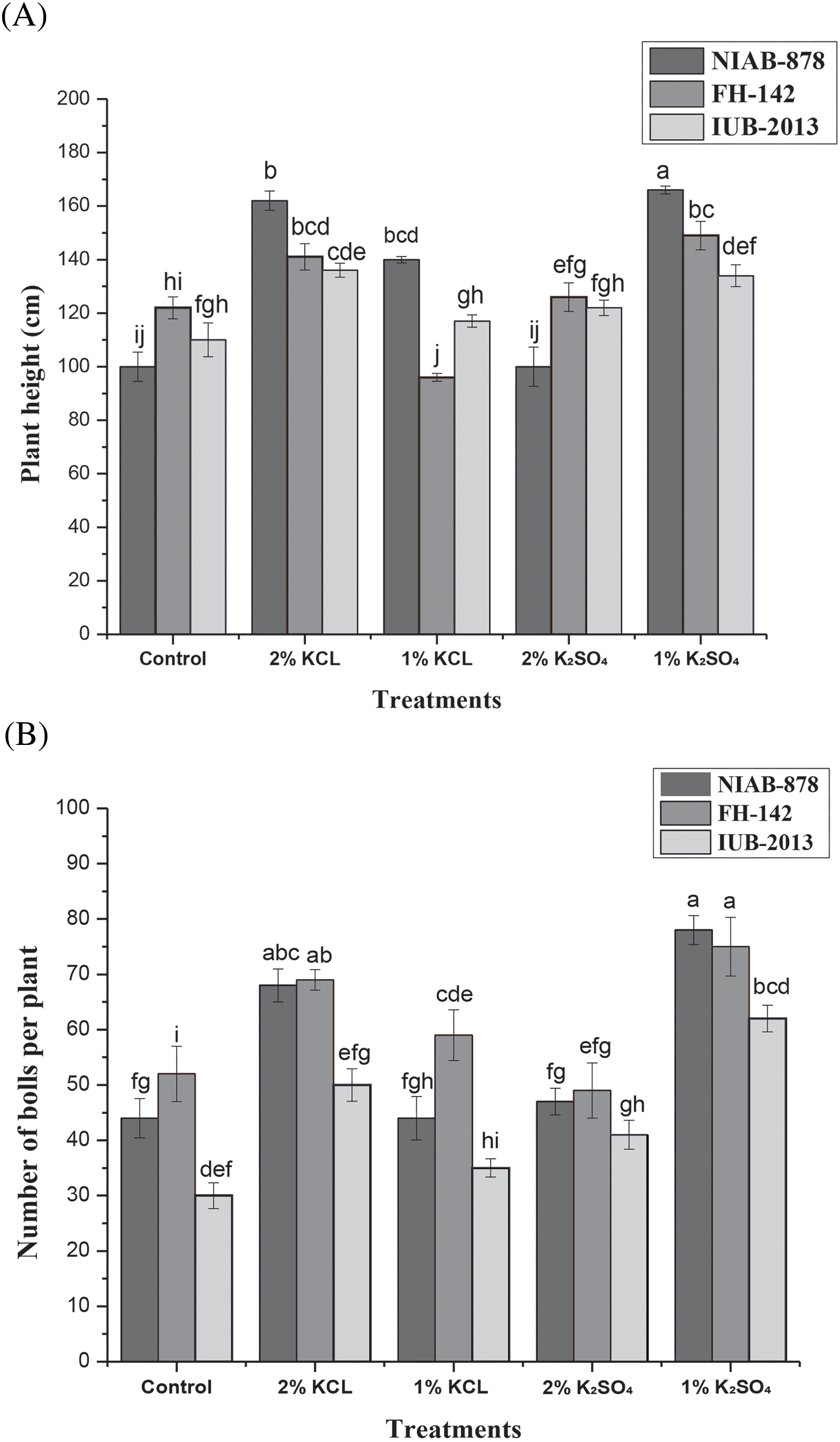
Figure 2: Effect of seed priming on plant height (A) and number of bolls per plant (B) in three cotton cultivars (NIAB-878, FH-142, and IUB-2013). Panel A shows the mean plant height for each cultivar under different priming treatments, with NIAB-878 exhibiting the maximum height, particularly under 2% KCl and 1% K2SO4 treatments. Panel B displays the number of bolls per plant, with 1% K2SO4 resulting in the highest boll count across all cultivars. NIAB-878 recorded the greatest number of bolls at 1% K2SO4, while FH-142 outperformed under other priming conditions. Error bars represent the standard error of the mean (SEM), and bars with the same letter indicate no significant difference (p < 0.05)
3.4 Number of Seeds per Boll and Boll Weight
The number of seeds per boll was highest in NIAB-878 under the 1% K2SO4 treatment, with a mean of 38 seeds per boll, while the lowest number (25 seeds) was observed at 2% K2SO4 compared to the control and KCl treatments. Similarly, variety FH-142 showed the highest number of seeds per boll at 1% K2SO4 and the lowest at 1% KCl. The cultivar IUB-2013 consistently produced fewer seeds per boll. Statistical analysis revealed significant differences between varieties at the 5% probability level (p < 0.05). Additionally, there was a highly significant treatment × variety interaction effect (p < 0.01) on the number of seeds per boll, as illustrated in Fig. 3A. The boll weight for variety NIAB-878 was highest with 2% KCl and 1% K2SO4 treatments, recording weights of 5.2 and 4.8 g, respectively, compared to the control. For variety NIAB-878, the 1% K2SO4 treatment resulted in the highest boll weight of 5.2 g relative to the FH-142 and IUB-2013 with 5 g boll weight. The lowest boll weight was at 2% K2SO4 in variety NIAB-878 with 3.7 g. Varieties IUB-2013 and NIAB-878 exhibited similar boll weights across all primed treatments. Results indicated that the individual effects of varieties on boll weight were not significant; however, the interaction between variety and treatment showed highly significant differences at the 5% probability level (p < 0.05), as depicted in Fig. 3B.
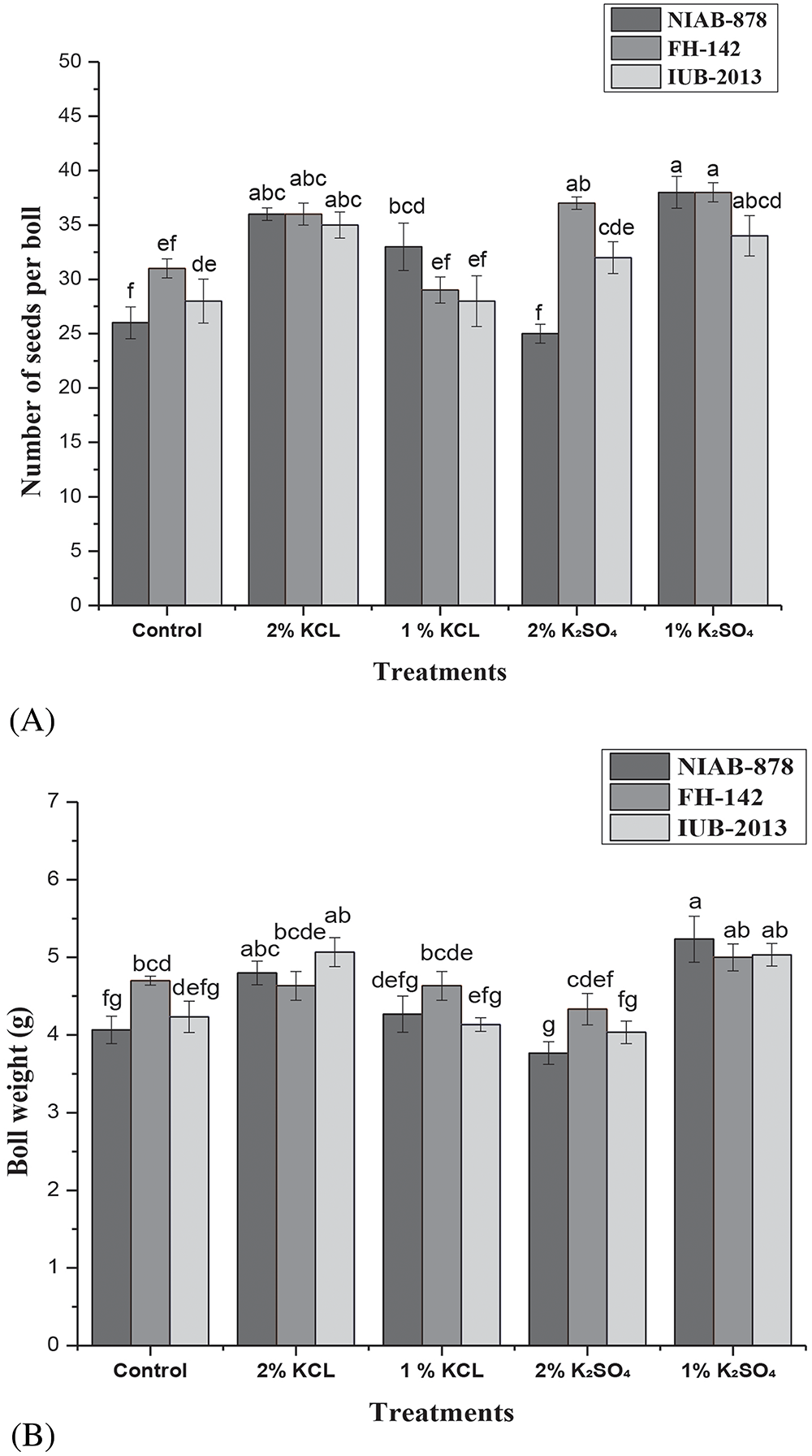
Figure 3: Effect of seed priming on the number of seeds per boll (A) and boll weight (B) in three cotton cultivars (NIAB-878, FH-142, and IUB-2013). Panel A illustrates the mean number of seeds per boll for each cultivar under different priming treatments, with NIAB-878 demonstrating the highest seed count at 1% K2SO4. Panel B shows boll weight, where NIAB-878 exhibited maximum weight with 2% KCl and 1% K2SO4 treatments. Error bars represent the standard error of the mean (SEM). Bars labeled with the same letter indicate no significant difference between treatments (p < 0.05)
In this study, cotton cultivars NIAB-878, FH-142, and IUB-2013 exhibited the lowest internode distances of 3, 3, and 2.7 cm, respectively, under the 1% K2SO4 treatment. Both the 1% K2SO4 and 2% KCl treatments resulted in significantly lower mean internode distances compared to the other treatments. Conversely, the 2% K2SO4 treatments produced the highest internode distances of 4.6 cm for variety IUB-2013 in comparison to all other varieties and treatments. The lowest internode distance of 2.7 g was seen for 1% K2SO4 treatment in IUB-2013 variety. While most internode distance measurements were non-significant, the interaction between variety and treatment revealed significant differences at the 5% LSD level, as depicted in Fig. 4.
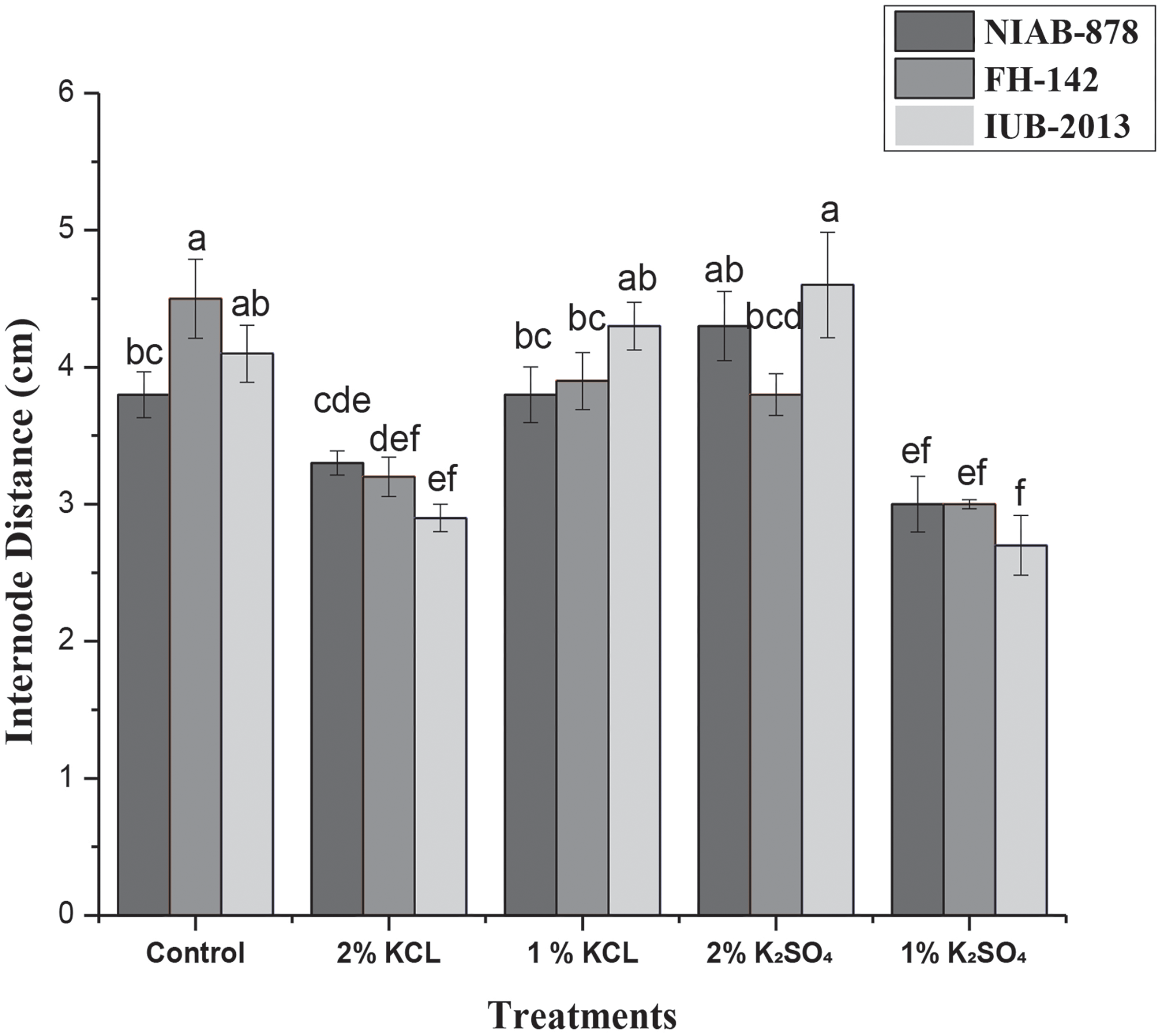
Figure 4: Effect of seed priming on internode distance in three cotton cultivars (NIAB-878, FH-142, and IUB-2013). The graph displays the mean internode distance for each cultivar under different priming treatments, with the 1% K2SO4 treatment resulting in the shortest distances. Error bars indicate the standard error of the mean (SEM). Bars labeled with the same letter indicate no significant difference between treatments (p < 0.05)
3.6 Seed Cotton Yield (SCY) and Ginning Outturn (OUT)
Halopriming exhibited promising effects on seed cotton yield. The results indicated that the 1% K2SO4 treatment yielded the highest results in the NIAB-878 cultivar, with a yield of 920 kg/acre, surpassing all other treatments and cultivars. This was followed by FH-142, which achieved yields of 880 kg/acre under 1% K2SO4 and 810 kg/acre under 2% KCl. In contrast, IUB-2013 recorded the lowest yield under the 1% KCl treatment. Statistical analysis revealed significant differences in the interaction between priming and variety, with yield differences being significant at the 1% level (p < 0.01). Additionally, seed cotton yield exhibited significant differences at the 5% LSD level for the variety × treatment interaction, as shown in Table 2.
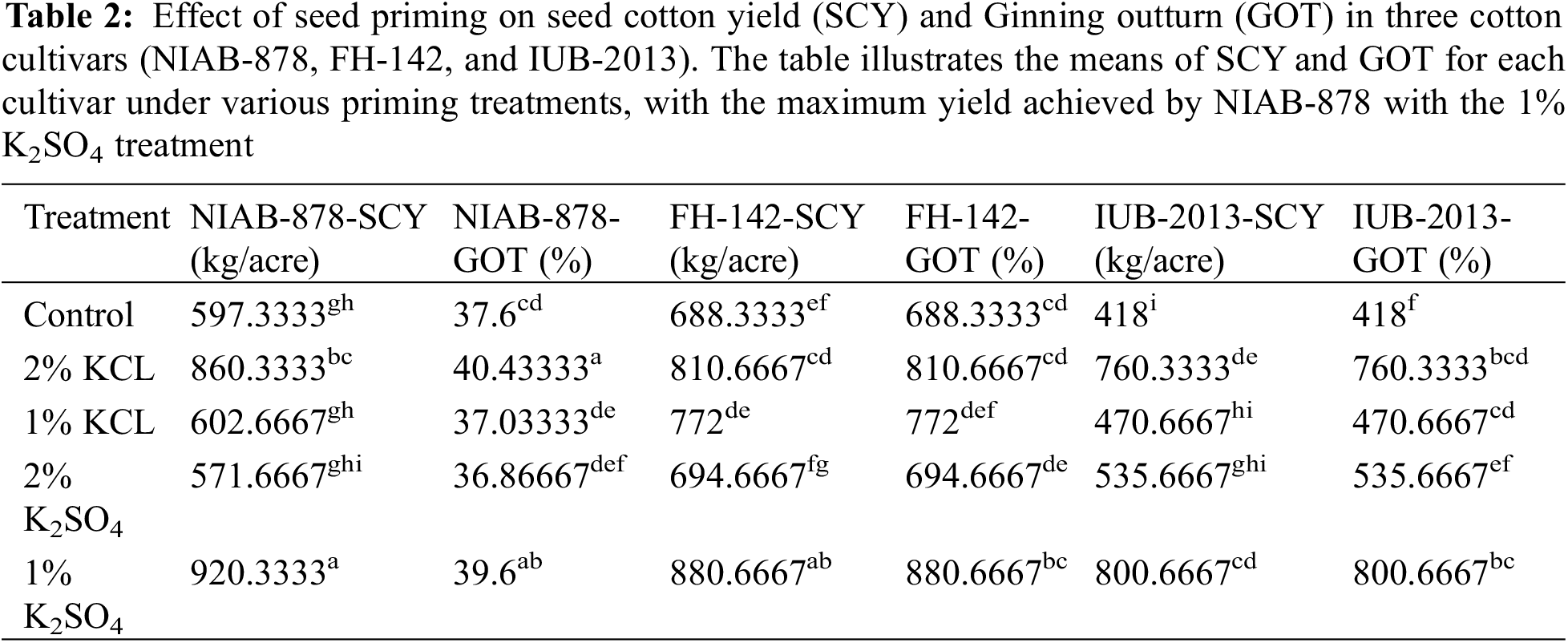
The results of the mean ginning outturn (GOT) percentage indicated that the cultivar NIAB-878 achieved the highest GOT values, recording 40.4% and 39.6% with 2% KCl and 1% K2SO4 treatments, respectively, compared to the other varieties, treatments, and control. Conversely, the treatments with 1% KCl and 2% K2SO4 resulted in lower GOT percentages. The cultivar FH-142 showed no significant variation in GOT across all treatments. The minimum GOT was observed in IUB-2013 under the 2% K2SO4 treatment. Statistical analysis revealed significant differences in GOT among the varieties at the 1% level (p < 0.01). Additionally, significant differences in GOT were found at the 5% level for the interaction between variety and treatment, as illustrated in Table 2.
3.7 Effect of Halopriming on Bt Gene Expression
Analysis of variance (ANOVA) was conducted to assess the effects of various treatments and cultivars on Bt protein expression, including the interaction between treatment and variety, using Statistix 8.1 software. Significant differences were observed among all treatments, with a high level of significance at the 1% probability level (p < 0.01) compared to the control. The results indicate a strong correlation between halopriming treatment and Bt protein levels as determined through two-factorial ANOVA. Additionally, the effect of cultivar on Bt toxin levels was also highly significant. However, no significant differences were found in Bt toxin levels concerning the treatment × variety interaction (Fig. 5).
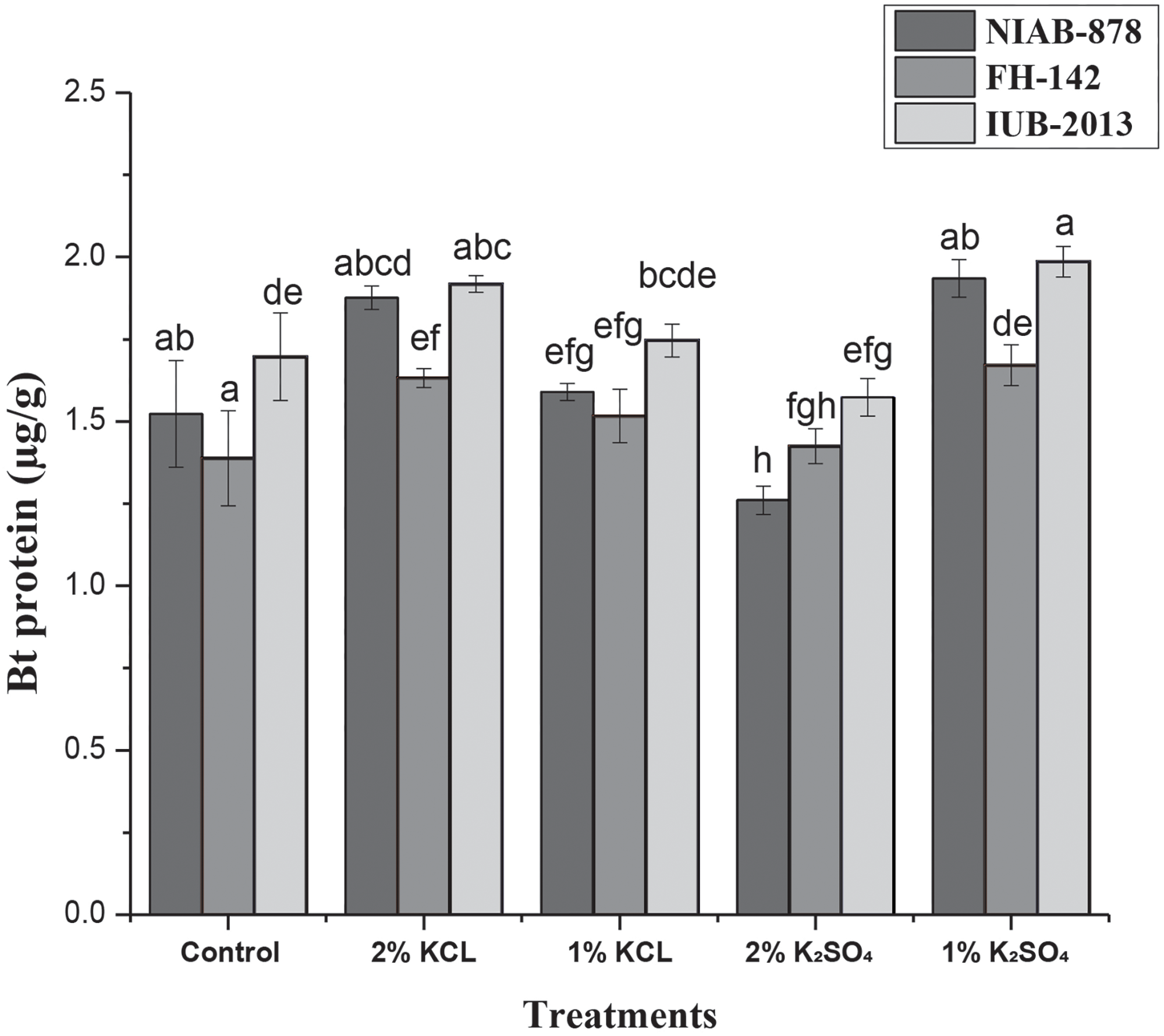
Figure 5: Effect of seed priming on Bt gene expression in three cotton cultivars (NIAB-878, FH-142, and IUB-2013). The graph illustrates the mean levels of Bt protein (Cry1Ac) for each cultivar under various priming treatments, highlighting the maximum expression observed with the 1% K2SO4 and 2% KCl treatments. Error bars represent the standard error of the mean (SEM). Bars labeled with the same letter indicate no significant difference between treatments (p < 0.05)
The cultivar IUB-2013 exhibited the highest level of Bt toxin at 1.9 μg/g with the 1% K2SO4 treatment. IUB-2013 and NIAB-878 showed comparable levels of Bt toxin under the 2% KCl treatment. Results of the Bt gene (Cry1Ac) expression indicate that the 1% K2SO4 and 2% KCl treatments resulted in the highest concentrations of Bt protein compared to the control. Conversely, the 1% KCl and 2% K2SO4 treatments exhibited lower concentrations of Cry1Ac protein compared to the control. Overall, the data on Bt gene expression suggest that the 1% K2SO4 and 2% KCl treatments yield the highest Bt protein contents, as illustrated in Fig. 5.
The global cotton industry faces numerous challenges, with poor seed germination and low seedling vigor being significant constraints on production. Seed priming has emerged as an effective strategy to enhance seedling growth and ultimately increase cotton yield. Previous studies have demonstrated that halopriming, which involves the treatment of seeds with various salts, can significantly improve the rate of seedling emergence and overall seed vigor under stress conditions [16,28]. Potassium (K), an essential macronutrient, plays a pivotal role in osmotic regulation and is integral to the development of cotton plants. The response of crops to priming is highly dependent on the concentration, composition of the priming medium, and exposure duration [29].
In our study, we explored the effects of halopriming on various cotton cultivars and found that treatments with 1% KCl and 2% K2SO4 significantly improved germination rates, consistent with findings by Singh et al. [30] and Khalequzzaman et al. [28], where lower concentrations of potassium salts also enhanced germination in other crops such as rice. Moreover, seed priming accelerated germination and improved plant growth, as demonstrated in other studies [31,32]. Our results showed that the 1% K2SO4 treatment led to the shortest internode distance, suggesting that potassium salts not only enhance germination but also influence other growth parameters such as plant height, leaf area, and internode distance, which aligns with previous reports on cotton and other crops [19,33,34]. Further, potassium salts significantly impacted yield-related parameters in cotton. Treatments with 1% K2SO4 and 2% KCl led to a higher number of bolls, seeds per boll, boll weight, and ginning outturn (GOT) percentage, ultimately improving overall yields. Among the cotton cultivars tested, NIAB-878 showed the highest yield, confirming the positive role of potassium salts in enhancing cotton productivity [33]. The beneficial effects of potassium sulfate may also be attributed to the presence of sulfate ions (SO42−), which have been shown to improve plant growth and yield [35].
In addition to growth and yield improvements, our study highlights the potential for seed priming to influence gene expression. Specifically, seed priming with potassium salts enhanced the expression of the Cry1Ac gene in Bt cotton, which is critical for pest resistance. The introduction of the Bt gene has been shown to lead to reduced pesticide usage and increased yields [3,36]. Our findings suggest that halopriming not only improves cotton physiology but may also enhance the expression of key traits, such as the Cry1Ac protein, contributing to better pest resistance and improved yield outcomes. This is consistent with research showing that seed priming can influence gene expression in various crops, improving plant health through enhanced RNA stimulation, protein synthesis, expression at the transcriptional and translational level by various internal and external factors and hormone regulation [37–39]. Moreover, seed priming has been linked to enhanced DNA repair and the stimulation of mRNA and protein synthesis machinery, further supporting its role in improving plant growth and stress resistance [40,41]. Finally, our study underscores the potential of halopriming as a strategy to enhance cotton germination, growth, and yield. The positive effects of potassium salts on cotton physiological traits, combined with the improved expression of pest resistance genes like Cry1Ac, offer promising avenues for enhancing cotton productivity while managing pest threats in an environmentally sustainable manner.
The application of halopriming, specifically using 1% K2SO4 and 2% KCl, on cotton seeds from three cultivars, NIAB-878B, FH-142, and IUB-2013 demonstrated significant positive effects on various morphological traits and Bt gene expression. Among the treatments, 1% K2SO4 and 2% KCl emerged as particularly effective in enhancing yield-related parameters and boosting Bt protein levels. To further advance cotton cultivation, future studies should explore additional priming methods and their respective treatments to assess their impact on yield-related attributes. Additionally, it is essential to evaluate other high-yielding Bt cotton varieties in conjunction with priming treatments. This study represents the first investigation into the effects of halopriming on Bt gene expression in cotton. Therefore, further molecular-based research is warranted to provide a more comprehensive understanding of Bt gene expression at the transcriptomic, metabolomic, and proteomic levels.
Acknowledgement: Thanks to Crop Sciences Institute (CSI), National Agriculture Research Council (NARC), Islamabad, Pakistan, for providing Seeds.
Funding Statement: This project was supported by National Natural Science Foundation of China (32160456; 32360474; 32360486), grants from the Provincial Basic Research Program (Natural Science) ([2020] 1Z018), Provincial Key Technology R&D Program ([2021] YiBan272).
Author Contributions: Study conception and design: Luhua Li and Muhammad Arif; data collection: Wenqi Shi, Binyameen Bin Shafqat and Muhammad Arif; analysis and interpretation of results: Wenqi Shi, Binyameen Bin Shafqat and Muhammad Arif; writing original draft preparation: Wenqi Shi, Binyameen Bin Shafqat and Muhammad Arif; review and editing: Ayesha Fazal Nawaz, Muhammad Amir Zia, Xu Ling, Dingli Hong, Ruhong Xu and Luhua Li; funding: Luhua Li and Ruhong Xu. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All the data that support the findings of this study are available in the manuscript.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Paparella S, Araújo SS, Rossi G, Wijayasinghe M, Carbonera D, Balestrazzi A, et al. OECD, Food and agriculture organization of the United Nations. In: OECD-FAO agricultural outlook. Paris: Rome/OECD Publishing; 2020. p. 2020–9. [Google Scholar]
2. Tokel D, Genc BN, Ozyigit II. Economic impacts of Bt (Bacillus thuringiensis) cotton. J Nat Fibers. 2022;19(12):4622–39. doi:10.1080/15440478.2020.1870613. [Google Scholar] [CrossRef]
3. Peshin R, Hansra BS, Singh K, Nanda R, Sharma R, Yangsdon S, et al. Long-term impact of Bt cotton: empirical evidence from North India. J Clean Prod. 2021;312:127575. doi:10.1016/j.jclepro.2021.127575. [Google Scholar] [CrossRef]
4. OECD/FAO, OECD-FAO agricultural outlook 2020–2029. Paris/FAO, Rome: OECD Publishing; 2020. doi:10.1787/1112c23b-en. [Google Scholar] [CrossRef]
5. Abbas G. Cotton in Pakistan: status, challenges and prospects. Pakistan J Agric Res. 2020;33(4):450–60. [Google Scholar]
6. Hasan MMU, Ma F, Prodhan ZH, Li F, Shen H, Chen Y, et al. Molecular and physio-biochemical characterization of cotton species for assessing drought stress tolerance. Int J Mol Sci. 2018;19(9):2636. doi:10.3390/ijms19092636. [Google Scholar] [PubMed] [CrossRef]
7. Bezerra BG, da Silva BB, Bezerra JR, Sofiatti V, dos Santos CA. Evapotranspiration and crop coefficient for sprinkler-irrigated cotton crop in Apodi Plateau semiarid lands of Brazil. Agric Water Manag. 2012;107:86–93. doi:10.1016/j.agwat.2012.01.013. [Google Scholar] [CrossRef]
8. Razzaq M, Zafar Y, Mahmood T, Mansoor S, Qureshi JA. Cotton leaf curl disease and its management strategies. Virol J. 2021;12(1):1–10. [Google Scholar]
9. Gomez S, Oosterhuis DM, Holaday AS. Photosynthesis, leaf respiration, and carbohydrate production of cotton under high night temperatures. J Cotton Sci. 2006;10(3):154–60. [Google Scholar]
10. Kouser S, Qaim M. Impact of Bt cotton on pesticide poisoning in smallholder agriculture: a panel data analysis. Ecol Econ. 2011;70(11):2105–13. doi:10.1016/j.ecolecon.2011.06.008. [Google Scholar] [CrossRef]
11. Hanif M, Saeed S, Ali M, Ishtiaq M, Khan Z. Evaluation of Cry1Ac and Cry2A endotoxins in transgenic cotton cultivars with respect to plant growth periods and stages; 2023. Available from: https://www.tandfonline.com/doi/full/10.1080/21645698.2020.1799644. [Accessed 2024]. [Google Scholar]
12. Khan MI, Khan AA, Cheema HMN, Khan RSA. Spatio-temporal and intra-plant expression variability of insecticidal gene (Cry1Ac) in upland cotton; 2018. doi:10.17957/IJAB/15.0546. [Google Scholar] [CrossRef]
13. Khan S, Ali S, Shah S, Zia M, Shoukat S, Hussain Z, et al. Impact of nitrogen and phosphorus fertilizers on Cry1Ac protein contents in transgenic cotton. Braz J Biol. 2021;83:e246436. [Google Scholar] [PubMed]
14. Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, et al. Food security: the challenge of feeding 9 billion people. Science. 2010;327(5967):812–8. doi:10.1126/science.1185383. [Google Scholar] [PubMed] [CrossRef]
15. Shen J, Lin H, Jiang X, Wang L, Liu X. Distribution and dynamic expression of Cry1Ac in different plant parts of Bt cotton. Plant Breed. 2010;129(4):386–92. [Google Scholar]
16. Afzal I, Shabir G, Basra SMA, Rehman H, Farrukh M. Seed priming for improving seed vigour, germination and crop stand in cotton. Int J Agric Biol. 2019;21(1):85–92. [Google Scholar]
17. Rhaman H, Rauf A, Ahmad M, Anwar M. Role of seed priming in improving drought tolerance in cotton (Gossypium hirsutum L.). Pakistan J Bot. 2020;52(1):15–21. [Google Scholar]
18. Jisha KC, Vijayakumari K, Puthur JT. Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant. 2013;35:1381–96. doi:10.1007/s11738-012-1186-5. [Google Scholar] [CrossRef]
19. Rhaman MS, Rauf F, Tania SS, Bayazid N, Tahjib-ul-Arif M, Robin AHK, et al. Proline and glycine betaine: a dynamic duo for enhancing salt stress resilience in maize by regulating growth, stomatal size, and oxidative stress responses. Plant Stress. 2024;14:100563. doi:10.1016/j.stress.2024.100563. [Google Scholar] [CrossRef]
20. Borges AA, Jiménez-Arias D, Expósito-Rodríguez M, Sandalio LM, Pérez JA. Priming crops against biotic and abiotic stresses: MSB as a tool for durable resistance. Front Plant Sci. 2014;5:642. [Google Scholar] [PubMed]
21. Farooq M, Aziz T, Basra SMA, Cheema MA, Wahid A. Evaluating the role of seed priming in improving drought tolerance in maize (Zea mays L.). Int J Agric Biol. 2008;10(3):238–44. [Google Scholar]
22. Çokkizgin A, Bölek Y. The effects of potassium nitrate priming on germination and seedling growth of cotton (Gossypium hirsutum L.). J Plant Nutr. 2015;38(11):1747–56. [Google Scholar]
23. Mustafa G, Mahmood T, Rasool A, Arshad M, Shahzad A. Influence of priming on seed germination and seedling development in wheat under drought conditions. J Crop Improv. 2017;31(1):99–108. [Google Scholar]
24. El-Araby MM, Moustafa YA, Abd El-Wahed MS. Gibberellic acid and abscisic acid levels in cotton seeds in response to priming with potassium salts. Egypt J Agron. 2006;28(1):77–87. [Google Scholar]
25. Falourd X, Natali L, Sautron E, Ducournau S. Transcriptomic changes associated with rapid germination induced by priming in tomato (Solanum lycopersicum L.) seeds. BMC Genomics. 2014;15:168. [Google Scholar]
26. Lanteri S, Saracco F, Acquadro A, Portis E. Analysis of physiological and biochemical changes induced by seed priming in peppers. Seed Sci Technol. 1994;22(1):181–9. [Google Scholar]
27. Bailly C, Benamar A, Corbineau F, Côme D. Antioxidant systems in sunflower (Helianthus annuus L.) seeds as affected by priming. Seed Sci Res. 2000;10(1):35–42. doi:10.1017/S0960258500000040. [Google Scholar] [CrossRef]
28. Khalequzzaman UH, Himanshu SK, Islam NET, Tisarum R, Cha-um S, Datta A. Seed priming improves germination, yield, and water productivity of cotton under drought stress. J Soil Sci Plant Nutr. 2023;23(2):2418–32. doi:10.1007/s42729-023-01196-5. [Google Scholar] [CrossRef]
29. Singh K, Gupta N, Dhingra M. Effect of temperature regimes, seed priming and priming duration on germination and seedling growth on American cotton. J Environ Biol. 2018;39(1):83–91. doi:10.22438/jeb/. [Google Scholar] [CrossRef]
30. Singh H, Jassal RK, Kang JS, Sandhu SS, Kang H, Grewal K. Seed priming techniques in field crops–A review. Agric Rev. 2015;36(4):251–64. [Google Scholar]
31. Kanjevac M, Bojović B, Todorović M, Stanković M. Effect of seed halopriming on improving salt tolerance in Raphanus sativus L.; 2021. Available from: https://www.pmf.kg.ac.rs/KJS/en/images/volumes/vpl43/kjs43kanjevac87.pdf. [Accessed 2024]. [Google Scholar]
32. Minhas WA, Mehboob N, Shahzad M, Khan MA, Hussain M. The impact of seed priming techniques and drying methods on germination and early seedling growth of Mashbean. J Field Crops. 2017;1:1–7. [Google Scholar]
33. Chakma SP, Chileshe SM, Thomas R, Krishna P. Cotton seed priming with brassinosteroid promotes germination and seedling growth. Agronomy. 2021;11(3):566. doi:10.3390/agronomy11030566. [Google Scholar] [CrossRef]
34. Khalequzzaman UH, Himanshu SK, García-Caparrós P, Tisarum R, Praseartkul P, Datta A. Growth, yield, and fiber quality of cotton plants under drought stress are positively affected by seed priming with potassium nitrate. J Plant Nutr. 2024;47(19):3646–64. doi:10.1080/01904167.2024.2380784. [Google Scholar] [CrossRef]
35. Shafiq F, Batool H, Raza SH, Hameed M. Effect of potassium nitrate seed priming on allometry of drought-stressed cotton (Gossypium hirsutum L.). J Crop Sci Biotechnol. 2015;18:195–204. doi:10.1007/s12892-015-0035-7. [Google Scholar] [CrossRef]
36. Ali S, Hameed S, Masood SHAHID, Ali GM, Zafar Y. Status of Bt cotton cultivation in major growing areas of Pakistan. Pakistan J Bot. 2010;42(3):1583–94. [Google Scholar]
37. Cheema HMN, Khan AA, Khan MI, Aslam U, Rana IA, Khan IA. Assessment of Bt cotton genotypes for the Cry1Ac transgene and its expression. J Agric Sci. 2016;154(1):109–17. doi:10.1017/S0021859615000325. [Google Scholar] [CrossRef]
38. Biswas S, Seal P, Majumder B, Biswas AK. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: an overview of the progress and achievements. Plant Stress. 2023;9:100186. doi:10.1016/j.stress.2023.100186. [Google Scholar] [CrossRef]
39. Hussain S, Hussain S, Khaliq A, Ali S, Khan I. Physiological, biochemical, and molecular aspects of seed priming. In: Priming and pretreatment of seeds and seedlings: implication in plant stress tolerance and enhancing productivity in crop plants. Singapore: Springer; 2019. p. 43–62. [Google Scholar]
40. Dawood MG. Stimulating plant tolerance against abiotic stress through seed priming. Adv Seed Priming. 2018;147–83. doi:10.1007/978-981-13-0032-5_10. [Google Scholar] [CrossRef]
41. Garcia D, Arif S, Zhao Y, Zhao S, Ming LC, Huang D. Seed priming technology as a key strategy to increase crop plant production under adverse environmental conditions. J Agris Hortic Res. 2021. doi:10.20944/preprints202109.0364.v1. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools