 Open Access
Open Access
REVIEW
Enhancing Plant Resilience to Abiotic Stress: The Power of Biostimulants
1 Centre for Research in Biotechnology for Agriculture, Universiti Malaya, Kuala Lumpur, 50603, Malaysia
2 Faculty of Science, Universiti Malaya, Kuala Lumpur, 50603, Malaysia
3 School of Biological Sciences, Universiti Sains Malaysia, George Town, 11800, Malaysia
4 School of Biology, Faculty of Applied Sciences, UiTM Shah Alam, Shah Alam, 40450, Malaysia
5 Department of Biotechnology, Faculty of Applied Sciences, UCSI University, Cheras, 56000, Malaysia
6 Department of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, 43400, Malaysia
* Corresponding Authors: Su-Ee Lau. Email: ; Boon Chin Tan. Email:
(This article belongs to the Special Issue: Recent Bio-stimulants and Their Physiological Effects in Alleviating or Overcoming Various Environmental Stresses)
Phyton-International Journal of Experimental Botany 2025, 94(1), 1-31. https://doi.org/10.32604/phyton.2025.059930
Received 10 October 2024; Accepted 12 December 2024; Issue published 24 January 2025
Abstract
Abiotic stresses such as drought, heat, salinity, and heavy metal contamination severely affect global agricultural productivity. Between 2005 and 2015, droughts caused losses of approximately USD 29 billion in developing countries, and from 2008 to 2018, droughts accounted for over 34% of crop and livestock yield losses, totaling about USD 37 billion. To support the growing human population, agricultural output must increase substantially, necessitating a 60%–100% rise in crop productivity to meet the escalating demand. To address environmental challenges, organic, inorganic, and microbial biostimulants are increasingly employed to enhance plant resilience through various morphological, physiological, and biochemical modifications. Plant biostimulants enhance plant resilience under abiotic stress through mechanisms such as abscisic acid signaling modulation, which regulates stomatal closure to reduce water loss during drought and heat stress. Additionally, they aid in scavenging reactive oxygen species and stabilizing ion channels, mitigating oxidative damage, and maintaining ionic balance under stress conditions such as salinity. This review summarizes recent advancements in applying these biostimulants, focusing on their roles in triggering morphological, physiological, biochemical, and molecular changes that collectively enhance plant resilience under stress conditions. It also includes a bibliometric analysis of all articles published on biostimulants from 2019 to 2024 and explores future research directions. Emphasis was placed on optimizing biostimulant formulations and understanding their synergistic effects to maximize their efficacy under various stress conditions. By integrating biostimulants into agricultural practices, we can adopt a sustainable strategy to safeguard crop productivity in the face of climate change and environmental stressors.Keywords
Abiotic stresses like drought, heat stress, and salinity pose major challenges to global agriculture, contributing significantly to crop yield losses and global soil degradation. These stresses are particularly damaging as climate change accelerates, intensifying extreme weather patterns and altering precipitation and temperature regimes worldwide. Drought is one of the most devastating abiotic stressors responsible for billions of dollars in agricultural losses. Between 2005 and 2015, it caused losses of approximately USD 29 billion in developing countries [1]. Similarly, from 2008 to 2018, drought was the leading cause of crop and livestock yield losses, accounting for over 34% of the total losses, or about USD 37 billion [2]. For crops like soybeans, extreme heat conditions exceeding 30°C can reduce soybean yield by up to 6% under rainfed conditions in the USA [3]. Similarly, rising temperatures and shifting precipitation patterns in Malaysia are expected to reduce rice yield by 12% during the primary season and 31.3% during the off-season by 2030 [4]. Salinity stress, typically caused by high concentrations of Na+ and Cl- in soil, interferes with essential metabolic processes, such as seed germination and photosynthesis, potentially causing severe damage to plant tissues or the death of plants [5]. Additionally, heavy metals, such as iron (Fe), manganese (Mn), copper (Cu), nickel (Ni), cobalt (Co), cadmium (Cd), zinc (Zn), mercury (Hg), lead (Pb), and arsenic (As), can accumulate in soil due to industrial waste and sewage disposal. Although some of these metals are essential in small amounts for plant development, their excessive accumulation disrupts plant metabolism and impairs growth [6]. Together, these abiotic stresses impose significant challenges to crop production and necessitate adaptive strategies to secure food supplies in the face of environmental change.
One effective strategy to enhance food production under such challenging conditions is cultivating crops with enhanced resilience to environmental stress. Traditionally, improving crop resilience has relied on breeding programs. However, these programs often encounter difficulties due to the complex genetic basis of abiotic stress tolerance, which involves multiple genes with intricate interactions. This complexity makes identifying and incorporating these traits into new cultivars time-consuming and resource-intensive. Moreover, breeding programs require several generations to produce stable, high-yielding varieties, making them too slow to keep pace with the accelerating impact of climate-induced stresses. Given these constraints, plant biostimulants (PBs) have emerged as a promising alternative, attracting considerable interest from researchers for their ability to enhance plant growth, overall fitness, and resilience to abiotic stresses. PBs are formulated with diverse microorganisms or naturally occurring bioactive compounds and work by promoting nutrient uptake and supporting plant growth and development rather than directly supplying nutrients or targeting pests and pathogens.
Biostimulants, including both microbial and non-microbial formulations, enhance nutrient use efficiency, improve crop tolerance to stress, support soil health, and reduce the need for chemical fertilizers and pesticides [7]. For example, a case study on maize showed that Kappaphycus alvarezii seaweed extract allowed for a 50% reduction in fertilizer input without compromising yield, thereby decreasing reliance on chemical fertilizers [8]. Additionally, biostimulants have been shown to reduce the carbon footprint, saving approximately 2.06 kg CO2 equivalent per ton of cane produced [9]. PBs growing market presence has made them a focal point in agricultural discussions and has been the subject of extensive reviews [7]. The availability and use of these products among growers have significantly increased, with the global market for PBs valued at approximately USD 2.6 billion in 2019 and projected to exceed USD 4 billion by 2025 [10]. This market growth reflects their practical potential, as demonstrated by Jiménez-Arias et al. [11], who conducted lab-to-field research using glycine betaine to cultivate maize under water-deprivation conditions. With an added cost of just 4.3 € per hectare, this treatment increased profits by 154.4 to 386.7 € per hectare under drought conditions. Such results highlight the effectiveness of biostimulants in mitigating drought-related losses and bridging the gap between research and practical solutions [11]. This review provides an overview of PBs and highlights recent advancements in applying PBs in agriculture, particularly their role in enhancing plant resilience under stress conditions.
2 Plant Biostimulants: Definition
The definition of PBs has been a subject of rigorous debate over the past decade, with various authors defining them based on product composition, source material, and mode of action. However, the complexity of PB efficacy, which arises from interactions between multiple compounds rather than a single active ingredient, complicates the understanding of their most active constituents and mechanisms. Recognizing this complexity, the European Commission identified the necessity to update existing fertilizer regulations to encompass a broader range of organic products, including PBs [12].
PBs, by definition, are claims-based according to the function of the product, encompass substances or microorganisms applied to plants to facilitate nutrient uptake, enhance environmental stress tolerance, and boost crop quality traits while ensuring good yields without being nutrients, soil improvers or pesticides [13]. Initially defined by Zhang et al. [14] as “materials that, in minute quantities, promote plant growth”, PBs are distinguished from nutrients, soil improvers, and pesticides due to their minimal application quantities. Before the adoption of Regulation (EU) No 2019/1009 (European Parliament and European Council Regulation (EU) 2019/1009), PBs were defined by what they are not, differentiating them from fertilizers and pesticides.
The European Union Fertilizing Products Regulation (FPR) redefined PBs as “fertilizing products stimulating plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: nutrient use efficiency, tolerance to abiotic stress, quality traits, availability of confined nutrients in soil or rhizosphere” (European Parliament and European Council Regulation (EU) 2019/1009). This updated definition clarifies the categorization of PB products. Although PBs have been excluded from the Plant Protection Products Regulation, they remain within the purview of Regulation (EC) 1907/2006, also known as the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) (European Parliament and European Council Commission Implementing Regulation (EU) No 354/2014). These new regulations are geared toward enhancing internal market operations for fertilizer products within the European Union, fostering investment, and promoting the development of effective and safe fertilizers [15].
PBs differ from direct nutrient supplements by improving nutrient use efficiency without directly supplying nutrients. PBs enhance root growth and modulate nutrient transporter activity, allowing plants to absorb and utilize available nutrients more effectively even in low-nutrient environments. The application of PBs leads to higher nutrient use efficiency than direct nutrient supplements, which provide nutrients but do not actively enhance plant nutrient uptake or stress tolerance. While nutrient supplements address specific deficiencies, biostimulants promote holistic plant metabolism, adaptability, and efficient nutrient use, reducing the risk of excess nutrient accumulation in the soil [16].
3 Non-Microbial Plant Biostimulants in Alleviating Abiotic Stresses
Non-microbial PBs, encompassing bioactive substances like seaweed extracts, humic substances, protein hydrolysates, chitosan, biopolymers, and inorganic compounds, play a significant role in the global biostimulant market. Seaweed extracts, the most dominant among these, accounted for 33.3% of the market in 2022, valued at approximately EUR 1.45 billion [17]. Their rich nutritional profile and historical use in enhancing nutrient efficiency, mitigating environmental stresses, and promoting root growth and microbial activity make them a favored choice. The biostimulant market is expected to grow to around EUR 2.66 billion, driven by the increasing demand for safe and sustainable agricultural products [18]. Organic PBs, such as seaweed extract, humic acids (HAs) derived from soil organic materials, chitosan, protein hydrolysate, and other plant-derived organic compounds, have attracted increasing attention from scientists. These substances have been shown to significantly enhance plant tolerance to abiotic stresses, thereby improving crop production and supporting plant physiological development. They help plants cope with major abiotic stressors, including drought, heat, and salinity [7,19]. The following examples illustrate the successful application of organic PBs in crop improvement.
Seaweed, or macroalgae, are crucial to marine and coastal ecosystems, significantly enhancing their biodiversity and contributing to the broader biosphere. Rich in bioactive compounds, seaweeds contribute significantly to plant stress tolerance. These compounds, including alginate, laminarins, phlorotannins, betaines, and glycerol, play crucial roles in osmotic adjustment, ROS scavenging, and hormonal regulation. Seaweeds are classified into three main groups based on their color: (i) Phaeophyta (brown), (ii) Rhodophyta (red), and (iii) Chlorophyta (green) [20]. Each group has been commercially exploited for various agricultural applications, with each producing a distinct set of bioactive compounds that enhance plant stress resilience.
Brown algae, such as Ascophyllum nodosum, are well-known for their high content of laminarins and phlorotannins [21]. Laminarins, a type of β-glucan, act as osmoprotectants, stabilizing cellular structures and helping plants manage osmotic stress. Phlorotannins, a group of polyphenolic compounds, serve as potent antioxidants, scavenging reactive oxygen species (ROS) and thereby protecting plants from oxidative damage under stress conditions. These compounds also act as elicitors, priming plants for enhanced stress responses by activating plant defense pathways.
Red algae (e.g., Kappaphycus alvarezii) are rich in sulfated polysaccharides, which contribute to stress resilience by enhancing the plant’s ability to manage oxidative stress and improve cellular water retention. These compounds, along with betaines such as γ-aminobutyric acid (GABA) (found in both brown and red algae), help regulate water balance and protect against osmotic stress.
Green algae, like Ulva rigida, contain high levels of glycerol and betaines, including glycine betaine (GB), which act as osmolytes, stabilizing proteins and cell membranes during stress [22]. GB is critical in enhancing plant tolerance to abiotic stresses like drought and salinity by maintaining cellular hydration and protecting against oxidative damage.
Seaweed is often used in foliar applications. For example, Trivedi et al. [8] applied an extract of the red algae Kappaphycus alvarezii to drought-stressed maize. Their study revealed significant improvements in plant growth, with enhancements of 4% in plant height, 16% in leaf length, 19% in green corn cob yield, and 17% in cob girth [8]. Similarly, the foliar application of brown algae (Sargassum angustifolium) extracts increased drought tolerance in canola, leading to greater shoot height and dry weight [23]. Additionally, the use of green algae (Ulva rigida) improved salinity tolerance in salinity-stressed wheat, resulting in increased plant growth compared with non-primed control plants [22]. These studies also reported a significant increase in photosynthetic activity. However, some studies suggest these treatments may induce stomatal closure under certain circumstances [24].
To further enhance plant tolerance and recovery from abiotic stresses, many PBs incorporate osmoprotectants such as proline, amides, GABA, and GB. These compounds, particularly betaines like GB, GABA, and proline, are abundant in marine algae. For instance, GB accounts for approximately 2% of the dry weight of green algae (Chaetomorpha capillaris), whereas other algae contain less than 1%. GABA, a primary betaine found in brown algae (Ascophyllum nodosum), is vital in stress resilience. Betaines function as osmolytes and ROS scavengers, protecting cellular structures by stabilizing molecules and membranes, thus aiding in the plant’s ability to tolerate environmental stresses [25].
At the biochemical level, priming Arabidopsis seeds with Super Fifty (SF) PBs extracted from brown algae, Ascophyllum nodosum, alleviated drought stress by reducing ROS accumulation and ion leakage [24]. Similarly, the drought-stressed tomato plants foliar-sprayed with Ascophyllum nodosum (brown algae) extract exhibited increased antioxidant enzyme activity and higher osmolyte levels, including proline and GB [26]. These treated plants also showed significantly greater plant height and total yield under reduced irrigation than control plants [26]. The beneficial effects of brown algae and GB in alleviating drought stress were further demonstrated in a field trial on grapevines [27]. Foliar application of Ascophyllum nodosum extract and GB improved the physiological and biochemical performance of grapevine cv. ‘Touriga Franca’ under summer stress in the Douro Demarcated Region, particularly within the ‘Douro Superior’ sub-region. These biostimulants, especially GB, showed significant potential in mitigating the adverse effects of summer stress, leading to increased levels of bioactive compounds, including total phenolics, flavonoids, and ortho-diphenols [27]. Under salinity conditions, wheat treated with Ulva rigida extract (a green algae) exhibited increased antioxidant enzyme activities, including superoxide dismutase (SOD), isocitrate dehydrogenase (ICDH), glutathione peroxidase (GPx), and glutathione reductase (GR) activities [22]. In addition to the increased activity of antioxidant enzymes, proline content was also elevated. This adaptive reaction is attributed to the upregulation of delta-1-pyrroline-5-carboxylate synthase, an enzyme involved in proline biosynthesis, and the downregulation of proline dehydrogenase, which catalyzes proline degradation [23]. Similarly, an extract from the green algae, Ulva lactuca, and its GB-rich fractions were found to significantly alleviate salinity stress in tomato plants [28]. At the highest GB concentration, the residual fraction acted as an osmoprotectant and ROS scavenger, reducing hydrogen peroxide (H2O2) levels and increasing tomato fresh weight under salinity stress conditions. These findings further support the crucial role of GB from seaweed extracts in mitigating salinity stress [28].
Brown algae extract priming alleviated drought-related damage in Arabidopsis thaliana through multiple genetically regulated mechanisms, underscoring its effectiveness as a drought-mitigation tool. Notably, RESPONSIVE TO DESICCATION 26 (RD26), a drought-induced gene associated with abscisic acid (ABA) signaling and salinity response, was upregulated, whereas the cell cycle marker gene HISTONE H4 (HIS4) was repressed. The brown algae extract priming also promotes stomatal closure during drought, minimizing water loss through the upregulation of RCAR3 and RBOHD, which are key genes in the ABA-dependent signaling pathway for stomatal regulation [24]. Additionally, applying red algae (Kappaphycus alvarezii) to drought-stressed maize led to the upregulation of genes associated with photosynthesis, signal transduction, transmembrane transport, nitrogen assimilation, and carbohydrate metabolism. Simultaneously, genes involved in the catabolism of macromolecules, including starch, chitin, and protein degradation, are downregulated [8]. Overall, the seaweed extracts modulate the internal balance of plant hormones to maintain hormonal homeostasis, regulate the expression of key transporters to enhance nutrient uptake and assimilation, stimulate and protect photosynthesis, and reduce plant stress-induced responses [29].
In addition to seaweed extracts, certain medicinal plant extracts have shown effectiveness in enhancing plant tolerance to abiotic stress. For example, moringa seed extract has been shown to mitigate heat stress in cancer bushes (Lessertia frutescens L.), a medicinal and ornamental plant, by promoting growth, increasing carotenoid content, reducing electrolyte leakage, and preventing wilting. Plants treated with moringa seed extract also exhibit more branching and higher levels of ABA, jasmonic acid (JA), and indole-3-acetic acid (IAA), while showing reduced levels of superoxide and H2O2 levels [30]. Similarly, seven medicinal plant extracts, Adathoda vasica, Cordia dichotoma, Asparagus racemosus, Saraca asoca, Kalanchoe pinnata, Andrographis paniculata, and Morus alba, were evaluated for their effectiveness in alleviating drought stress in maize. Among these, Adathoda vasica leaf extract was the most effective. When combined with Pseudomonas putida, it significantly improved growth and reduced oxidative stress in drought-stressed maize by increasing chlorophyll, sugar, and phenolic levels, lowering proline, and upregulating defense gene expression (ZmAPX, ZmSOD, ZmCAT, ZmNAC, ZmWRKY, and ZmMYB) [31]. Overall, medicinal plant extracts, whether applied alone or synergistically with other biostimulants, offer a sustainable and eco-friendly approach to enhancing plant tolerance and productivity under abiotic stress.
Humic and fulvic acids, derived from plant and animal wastes through biological or chemical processes, are vital to plant physiology. These substances enhance nutrient uptake and distribution, improving crop growth and increasing stress tolerance. Typically applied via soil drenching or foliar spraying depending on the crop’s needs, humic substances offer several benefits: they improve soil structure, increase phosphorus availability, neutralize soil pH, promote lateral root growth, and stimulate nitrate assimilation in crops. Despite their well-documented advantages, the complex nature of humic substances and the varying responses they trigger in different plants make it challenging to elucidate their mechanisms of action [7,32].
HA-primed maize and sorghum seeds exhibited enhanced vegetative growth and physiological responses under varying levels of drought stress (100%, 80%, and 60% field capacity) [33]. In maize, biomass accumulation was reduced by 37.0% and 58.7% under moderate and severe drought conditions, respectively, while sorghum experienced 21.2% and 32.3% reduction under the same conditions compared to HA-treated seeds. HA priming significantly improved the photosynthesis rates, with increases of 29.2% in maize and 15.0% in sorghum under severe drought conditions [33]. This improvement was further reflected in higher levels of total chlorophyll, stomatal conductance, relative water content, and increased concentrations of sugars, proline, and soluble proteins in both maize and sorghum plants [33]. Another study showed similar findings, demonstrating that intermediate doses of HA (8 and 12 mL/L) enhanced drought tolerance in cowpeas [34]. This was achieved by reducing water potential and root nodule formation while increasing the fresh and dry weight of the shoots and roots, as well as total soluble protein content [34]. Like seaweed extracts, HA priming also exhibited enhanced enzymatic antioxidants such as catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD), and polyphenol oxidase (PPO) enzymes, as well as non-enzymatic antioxidants (phenolic compounds) in maize and sorghum plants [33]. These findings suggest HA is a promising PB for enhancing crop drought tolerance. A 2023–2024 field trial on mango production also demonstrated the synergistic effects of combining wood vinegar, seaweed extract, and HA, resulting in significant improvements in chlorophyll content, leaf mineral levels, and fruit quality, with the 2 L/ha application of each showing the greatest impact by enhancing leaf nutrient uptake, antioxidant response, and sugar accumulation [35].
The role of humic substances in modulating ion transporters at the root-soil interface under salinity stress has been elucidated by Khaleda et al. [36] using Arabidopsis. Humic substances enhance salt stress tolerance by regulating ion transporters, specifically the sodium influx transporter HIGH-AFFINITY K+ TRANSPORTER 1 (HKT1). The application of HA restored root growth and prevented the degradation of HKT1 protein in salt-stressed HKT1-overexpressing plants while also promoting sodium reabsorption in the roots [36]. Furthermore, HA can enhance plant nutrient uptake under stress by modulating critical processes at the root-soil interface. It may stimulate proton pump activity, exhibit auxin-like effects to promote lateral root emergence and improve nutrient availability, making essential nutrients more accessible to plants during abiotic stress conditions [37].
Overall, HAs are valuable additions to crop management strategies for enhancing plant resilience to drought, salinity, and nutrient stress. Their ability to improve soil health, nutrient uptake, and physiological responses makes them versatile tools for sustainable agriculture. As we explore further, the combination of humic substances with other biostimulants, such as seaweed extracts, may yield synergistic effects that amplify stress tolerance and improve crop quality and yield.
Protein hydrolysates, derived from plant materials and animal by-products, consist of a mixture of free amino acids, oligo- and polypeptides. These substances are also commonly applied as foliar sprays or, occasionally, as substrate drenches or seed treatments. Protein hydrolysates are known for their biostimulatory effects, including the activation of key enzymes involved in nitrogen and carbon metabolism, as well as the enhancement of antioxidant enzyme activity and secondary metabolite production.
Applying protein hydrolysates, including brassinosteroid (BR), amino acids (AA), nitrophenolates (NP), and botanical extracts (BE), to heat-stressed rice genotypes ‘F67’ and ‘F60’ enhanced the heat tolerance by improving leaf gas exchange parameters. These improvements included an increase in net photosynthesis rate (PN) (~14.5 µmol CO2 m−2 s−1), stomatal conductance (gs) (~0.46 mmol m−2 s−1), transpiration rate (E) (~43.9 H2O day−1 plant−1), leaf saturation (LS), PN/Ci ratio, and intrinsic water use efficiency (WUEi) [38]. BR, AA, and NP protein hydrolysates enhance plant stress tolerance through various physiological mechanisms: BR promotes carbon metabolite accumulation and chlorophyll biosynthesis, AA supports nitrogen metabolism and glutamate receptor synthesis for stress response [39], and NP improves photosynthetic capacity by reducing stomatal resistance and enhancing chlorophyll stability [40]. Similarly, in Chinese cabbage, applying glutamic acid (Glu) and poly-γ-glutamic acid (γ-PGA) PBs improved heat stress tolerance by enhancing carotenoid biosynthesis, photosynthesis, and ROS signaling [41]. Together, protein hydrolysates act as effective biostimulants that support photosystem stability and enhance photosynthesis through multiple mechanisms, including stabilizing photosystem proteins and promoting chlorophyll biosynthesis or protecting it from degradation.
In addition, treatments with Glu and γ-PGA resulted in a notable increase in fresh weight (47.58% for Glu and 37.32% for γ-PGA) and dry weight (51.52% for Glu and 39.39% for γ-PGA) in Chinese cabbage compared to control plants [41]. Other morphological parameters, such as plant height, hypocotyl diameter, leaf length, and leaf width, also showed notable improvements following priming with Glu and γ-PGA [41]. In contrast, malondialdehyde (MDA) levels, electrolyte leakage, and superoxide radical content were significantly reduced, indicating a decrease in oxidative stress. These effects were accompanied by elevated activities of antioxidant enzymes, which help detoxify the ROS produced during heat stress, thereby reducing oxidative damage [41,42]. Transcriptomic analysis has revealed elevated expression levels of genes crucial for DNA repair, mitigating direct DNA damage caused by heat stress [43]. Notably, the RPA1 gene, which encodes a 70 kDa DNA-binding replication protein, was differentially expressed. RPA1 plays a vital role in DNA replication, repair, and recombination [44]. Similarly, in salt-stressed lettuce, priming with Graminaceae-derived PBs, including protein hydrolysates and their lighter molecular fraction F3 (<1 kDa), led to a significant reduction in proline, MDA levels, and osmolyte content, with a 35% decrease compared to control plants [45]. In protein hydrolysates-primed plants, genes involved in cell wall organization and biogenesis, carbohydrate catabolic processes, and polysaccharide metabolism, including putative linoleate 9S-lipoxygenase, were upregulated. Additionally, protein hydrolysates influence the structural components of chromatin and protein dimerization activity under salt stress. In contrast, F3 PBs affected a smaller set of genes, primarily affecting hormone-mediated signaling pathways, cellular responses to endogenous stimuli, and hormonal responses, notably through the upregulation of ethylene-responsive transcription factors. This study provides valuable insight into the molecular bioactivity of PBs, laying the groundwork for their application in agricultural practices to enhance plant stress resilience [45].
Hamedeh et al. [46] examined the use of EnNuVi® ALPAN®, a plant-derived biostimulant enriched with essential nutrients (Cu, Mg, Mn, and Zn) to mitigate drought stress in tomatoes, analyzing its effects at the phenomics and transcriptomics levels. From a photosynthetic perspective, ALPAN® treatment effectively maintained stable levels of photosynthetic pigments (chlorophyll a/b and carotenoids) across all time points (days 1, 7, and 8). In contrast, the untreated plants experienced significant fluctuations in pigment levels during water stress and recovery. ALPAN®-treated plants also exhibited lower electrolyte leakage and MDA and proline concentrations than the untreated controls, indicating reduced cellular damage. Transcriptomic analysis revealed that ALPAN® treatment upregulated the drought-responsive gene SlTAS14, which is associated with ABA and water-deficit responses, making it a key drought tolerance marker. Other genes such as SlPDH1, SlFN1, SlTRX, and SlPAL1 were also expressed at higher levels in ALPAN®-treated plants, particularly on day 1 of water stress, supporting rapid adaptation and resilience in young seedlings under desiccation conditions [46].
Collectively, organic PBs have demonstrated significant potential for enhancing plant growth and development under abiotic stress. They improve nutrient uptake efficiency, increase photosynthetic pigments, and promote higher photosynthetic rates across various growth stages. Additionally, PBs promote the synthesis of non-enzymatic antioxidants like phenolic compounds, flavonoids, and ascorbic acid, leading to higher proline accumulation and reduced oxidative damage, as indicated by lower MDA levels. However, most studies have been conducted in controlled environments, highlighting the need for more field studies to understand PBs’ effects in real-world conditions.
4 Inorganic Non-Microbial Plant Biostimulants
Several inorganic elements have been shown to benefit specific plant taxa. These include trace elements from the 16 essential nutrients required for plant growth and non-essential elements, often referred to as beneficial elements. Cobalt, sodium, silicon (Si), selenium (Se), and vanadium are some of the essential elements that can enhance physiological and biochemical activities, promote growth, and improve plants’ adaptation to abiotic stress [47]. While an adequate supply of these trace elements can improve plant growth and development, excessive amounts may lead to toxicity and impair plant development [47,48]. Given the potential of inorganic PBs to enhance plant tolerance to abiotic stressors, research in this area is ongoing. Scientists are actively investigating how these inorganic PBs strengthen plants and optimize growth in an ever-changing environment.
The application of Se has shown promising results in mitigating the adverse effects of environmental stresses. For instance, applying Se at a rate of 60 g/ha to onions grown in semi-arid regions, where heat and drought stresses are prevalent, has been shown to increase total bulb yield and the Se content of the onions. This not only enhances the nutritional value for consumers, as Se is an essential element for human health, but also enhances crop resilience [49]. Similarly, the foliar application of Se at 20 mg/L significantly improved several growth parameters in drought-stressed rice, including plant height, number of tillers, filled grains/m2, total grains/m2, grain yield (kg/ha), and straw yield by 5.1%, 12.6%, 21.8%, 4.3%, 11.0%, and 10.1%, respectively, compared to the control [50]. Physiologically, Se application increased chlorophyll stability, membrane stability, proline content, and relative water content in drought-stressed rice.
Si, another well-known beneficial element for plants, plays a crucial role in enhancing drought stress tolerance. Once taken up by plants, Si accumulates primarily beneath the cuticles and within the cell walls as silica deposits, forming a physical barrier that strengthens the cell wall structure and helps plant cells mitigate the harmful effects of stress [51]. Si reinforcement reduces transpiration water loss by creating denser cell walls, limiting stomatal opening, and minimizing cuticular transpiration. This water retention is crucial under drought stress, as it maintains cellular turgor and prevents wilting [52]. Seed priming with Si has been shown to induce significant morpho-physiological and biochemical changes in drought-stressed maize plants, leading to notable improvements in growth parameters, such as shoot length, root length, and both fresh and dry weights of shoots and roots [53]. Additionally, higher stomatal conductance was observed in Si-primed drought-stressed tomato plants [54]. At the biochemical level, Si priming significantly reduces the accumulation of ROS, as evidenced by lower levels of H2O2, MDA, and proline [53]. It also decreases the concentration of phenolic compounds, glycine betaine, and total soluble sugars in maize plants. This reduction in ROS is coupled with an enhanced activity of antioxidant enzymes [53].
Advances such as nano-encapsulation have introduced unique physicochemical properties such as high reactivity, precise surface structures, and large surface-area-to-volume ratios, which differentiate them from traditional molecular formulations [55]. In biostimulant applications, nano-encapsulation enhances bioavailability, stability, and uptake efficiency by enabling the controlled and targeted release of active compounds into plant tissues. This approach not only boosts nutrient-use efficiency but also reduces the need for frequent applications, thereby promoting more sustainable crop practices [56]. Additionally, the transition from conventional to green synthesis in nanoparticle development offers an eco-friendly alternative that relies on natural agents, such as plant extracts, microbes, or biomolecules. This approach minimizes the use of toxic chemicals and reduces energy consumption. Green-synthesized nanoparticles further support sustainable agriculture by improving nutrient delivery, enabling controlled release, and enhancing pest resistance, all while reducing environmental impact [57]. For instance, silicon nanoparticles (Si-NPs) have been shown to enhance plant growth and photosynthesis while reducing Cd and lead (Pb) concentrations in wheat [58] and maize [59], respectively. Si-NPs treatment has also been shown to alleviate oxidative stress in these plants, evidenced by lower levels of H2O2, electrolyte leakage, and MDA content [58,59]. The potential of Si-NPs in mitigating heavy metal stress is increasingly recognized, as demonstrated by Hussain et al. [60], who explored the synergistic effects of combining different nanoparticles (SiNPs, ZnONPs, and FeNPs) in wheat grown in Cd-contaminated fields. Their study demonstrated that the combination of these three NPs significantly reduced Cd uptake in wheat straw and grains by 84% and 99%, respectively, while increasing Zn and Fe levels in the grains. In line with other research, this study also found improvements in chlorophyll and carotenoid contents, increased antioxidant enzyme activities, and reduced electrolyte leakage compared to the control [60]. Similarly, Khan et al. [61] showed that applying Si-NPs to wheat under combined heavy metal and drought stress conditions improved growth, enhanced photosynthesis, and lowered Cd levels in plant tissues, especially in grains. Si-NPs also reduce oxidative stress by decreasing H2O2, electrolyte leakage, and MDA, while boosting antioxidant enzyme activity. These findings underscore the potential of nano-encapsulation biostimulants as powerful tools for enhancing plant resilience to multiple abiotic stresses, supporting productivity under challenging conditions [61].
Conversely, the application of calcium-silicon nanoparticles (Ca-SiNPs) has been shown to mitigate salinity stress in Lilium plants, leading to enhanced flower quality, as evidenced by increased flower size and fresh biomass [62]. Moreover, Ca-SiNP treatment boosted chlorophyll content, ascorbic acid levels, and antioxidant capacity, as measured by DPPH (1,1-diphenyl-2-picrylhydrazyl) assays in the leaves, despite an observed increase in MDA levels under salinity stress [62]. These findings highlight the potential of combining different NPs to mitigate heavy metal stress and improve plant resilience.
In addition to Se and Si, silver (Ag) also plays a vital role in salinity stress, a common stressor that can negatively impair plants’ growth and crop productivity. Barley seed primed with polyvinylpyrrolidone-coated silver nanoparticles (PVP-AgNPs) exhibited a 100% germination rate [63]. Similarly, applying AgNPs to lily plants improved flowering, plant height, petal width, fresh bulb weight, bulb diameter, number of scales in a bulb, and enhanced the levels of essential nutrients in the plant tissue (N, K, Ca, Cu, Mn, and Zn), compared to control under salt stress conditions [64]. Other nanoparticles, such as carbon nanotubes and graphene, have also demonstrated significant benefits. Research indicates that carbon nanoparticles improved tomato plant growth under salt stress [65]. The treated tomato plants showed an increase in chlorophyll, ascorbic acid, glutathione, protein, and phenolic compounds while also boosting the activity of antioxidant enzymes, such as CAT, APX, and GPx. These treatments also improved tomato fruit quality, characterized by higher total soluble solids, phenolic compounds, flavonoids, ascorbic acid, and lycopene [65].
Interestingly, the combination of various inorganic substances, such as HA, Si, and biochar, has been shown to significantly reduce ABA levels while increasing salicylic acid (SA) concentration and enhancing antioxidant activity, particularly polyphenol production, in rice [66]. The synergistic interaction between HAs, biochar, and Si highlights the complex relationship between phytohormones, particularly ABA and SA, and antioxidant responses in rice plants under heavy metal, drought, and salinity stress. In rice, ABA activation is facilitated by a Ca2+/calmodulin-dependent kinase, which triggers the activation of antioxidant enzymes such as SOD and CAT. These enzymes manage oxidative stress by converting harmful O2 to H2O2, which is further detoxified, thereby reducing cellular damage [67,68]. SA production in plants is often induced under stress conditions and works synergistically with ABA. Under stress, the production of phenolic compounds not only contributes to SA biosynthesis but also facilitates the scavenging of ROS. These compounds are critical in metal ion chelation, activate phytohormones, and improve nutrient uptake [69]. Together, these mechanisms highlight the potential of HA, biochar, and Si to bolster plant resilience against environmental stress.
An example of a commercial product that enhances plant resilience is Quantis™, a PB derived from sugar cane by-products fermented with yeast and enriched with potassium and calcium [70]. In potato plants, the application of the PB Quantis™ significantly improved heat tolerance compared to untreated plants. Quantis™ enhanced Photosystem II photochemical efficiency and reduced thermal dissipation in heat-stressed potatoes, leading to a 4% increase in tuber weight and a 40% increase in tuber size compared with the control. Furthermore, the Quantis™ application upregulated the gene StFKF1, which promotes tuberization, resulting in larger tubers. It also increased the expression of heat-defense genes, such as PEN1, PR4, and MEE59, contributing to leaf photoprotection and thermal protection in roots. Additionally, Quantis™ treatment was linked to increased cytokinin signaling in roots and reduced levels of endogenous ABA and cytokinin in leaves, essential factors in mitigating stress responses [70].
The success of inorganic PBs in alleviating abiotic stresses presents a promising strategy for enhancing plant resilience under challenging environmental conditions. By improving plant growth, photosynthesis, and stress tolerance, these biostimulants can contribute significantly to sustainable agricultural practices. Continued research and application of inorganic PBs may be key to addressing the challenges of feeding a growing global population in the face of climate change and environmental degradation.
5 Role of Microbial Plant Biostimulants in Alleviating Abiotic Stresses
Plant growth-promoting rhizobacteria (PGPR), such as Salinispora arenicola [71], Burkholderia sp. BK01 [72], and B. velezensis HY23 [73], have been reported to alleviate salt stress in various plants, including tomatoes [71], Arabidopsis [71], and soybeans [73]. These PGPR enhance germination and shoot and root growth under saline conditions. They also increase chlorophyll content and activities of SOD, POD and CAT in Arabidopsis under salinity stress while reducing MDA levels [72]. Notably, Burkholderia sp. BK01 produces polysaccharides that could serve as biostimulants to further enhance plant growth and salt tolerance. Both Burkholderia sp. BK01 [72] and B. velezensis HY23 [73] produce exopolysaccharides that improve salt tolerance in soybeans and Arabidopsis by modulating the antioxidant defense system and regulating the expression of ion transporters. Moreover, Pseudomonas thivervalensis SC5, which produces 1-aminocyclopropane-1-carboxylate deaminase, increases osmolyte and polyamine production, which are essential for improving osmotic stress tolerance in cucumber plants [74].
Acinetobacter calcoaceticus AC06 and Bacillus amyloliquefaciens BA01 [75] have been identified as effective osmolyte-producing microbial biostimulants under drought stress. These microbes significantly increase proline, SA, trehalose, and glycine betaine levels, thereby enhancing drought tolerance in groundnut plants. Their presence leads to improved plant biomass, higher concentrations of photosynthetic pigments, increased relative water content, and elevated levels of proline and soluble sugars compared to control plants. Additionally, A. calcoaceticus AC06 and B. amyloliquefaciens BA01 reduce the electrolyte leakage and MDA content in drought-stressed groundnut while enhancing antioxidant enzyme activities, such as CAT, APX, and SOD [75].
Other studies have highlighted the benefits of different PGPR strains in stress tolerance. For example, B. butanolivorans KJ40 and B. siamensis H30-3, when used to prime soybean seeds, promote osmotic stress tolerance during germination and mitigate cellular damage from secondary oxidative stress by increasing enzyme activities [76]. Similarly, Lozo et al. [77] explored the effects of drought-tolerant Bacillus strains (B. safensis SS-2.7 and B. thuringiensis SS-29.2) on four sweet pepper genotypes. While genotype 274 exhibited the best establishment under normal conditions, it was the most drought-sensitive. In contrast, Matica, though slower to establish under normal conditions, along with CalW, demonstrated superior drought tolerance. The authors found that antioxidant enzyme activity varied by genotype and treatment, with CalW showing the best bacterial response, while genotype 133 declined more rapidly under drought, even with treatment. These results highlight the potential of Bacillus strains in breeding programs to select biostimulant-compatible, drought-resilient pepper genotypes and other crops [77].
Several studies suggest that the beneficial effects of microbial PBs may result from the unique metabolites they produce, such as IAA, siderophores, and other compounds. For example, a comparison of 15 PGPR isolates for alleviating drought stress in oilseed crops revealed that A. calcoaceticus AC06 exhibited the highest IAA production (128.82 μg ml−1) and siderophore production (84.87%), along with significant osmotic resistance to −0.73 MPa [75]. In osmotically stressed wheat, A. brasilense produced approximately twice the phytohormone levels (IAA, GA, and ABA) than B. subtilis. IAA promotes cell division, elongation, and differentiation, whereas GA and ABA serve as signaling molecules in plants that enhance plant stress tolerance under adverse conditions [78,79]. Additionally, siderophore production facilitates iron uptake by chelating ferric ions, which is particularly beneficial in nutrient-poor soils and improving drought tolerance [80]. Altogether, these findings suggest that Azospirillum sp. confer greater drought stress tolerance than Bacillus sp. The natural symbiotic relationship between arbuscular mycorrhizal fungi (AMF) and many plant species positively affects plant water relations, especially under water deficit conditions. AMF improves soil-plant hydraulic conductance and enhances the water status of tomato plants in drying soil by increasing the effective root radius [81]. This expansion reduces water flow at the root-soil boundary and eases the decrease in water potential in the surrounding soil, thereby promoting drought tolerance in tomato plants [81]. Similarly, Serendipita indica, a culturable endophytic fungus that can colonize various host roots, including citrus plants, has been shown to enhance plant drought tolerance. It achieves this by reducing growth inhibition, increasing the photosynthetic rate, chlorophyll index, and water use efficiency, and boosting antioxidant enzyme activities in citrus plants [82]. Moreover, the endosymbiosis of Piriformospora indica with Andrographis paniculata not only promotes the production of andrographolide, but also enhances antioxidant enzyme activities and proline content, thereby improving drought tolerance in A. paniculata [83].
Building on the success of single-strain microbial biostimulants, researchers are now exploring the use of multistrain biostimulants to enhance their effectiveness in promoting abiotic stress tolerance. For example, a combination of multistrain microbial biostimulants, including Claroideoglomus claroideum BEG96, Funneliformis caledonium BEG97 and F. geosporum BEG199, along with a mix of Glomus spp. and rhizospheric bacteria has been shown to improve drought tolerance in tomato plants [84]. These plants showed higher IAA production, chlorophylls, and polyphenols under drought stress [84]. Similarly, combining Glomus spp., Bacillus spp., Streptomyces spp., Pseudomonas spp., and Trichoderma spp. significantly increased the total phenolic compound content in drought-stressed green beans compared to control plants [85]. Furthermore, a study on the synergistic effects of microbial and non-microbial biostimulants demonstrated that a combination of AMF and protein hydrolysates could alleviate heavy metal stress caused by iodine fortification in eggplants [86]. This combination enhances nutrient uptake through root system expansion, increases proline content, and boosts ROS-scavenging enzyme activity, thereby reducing oxidative damage caused by iodine [86]. Among non-microbial biostimulants, HAs are particularly effective in improving soil fertility [87]. They can act as carriers for introducing beneficial microorganisms into the soil as they are resistant to microbial degradation [88]. A synergistic effect was observed with the combined application of HA and endophytic bacteria in tomato plants [89]. These bacterial strains demonstrated active root colonization in tomato seedlings, supported by their biofilm-forming capability, which helps them survive in challenging environments and improves their persistence [90]. These mechanistic insights provide valuable guidance for developing innovative formulation strategies, leading to the development of more effective and resilient biostimulant products.
In summary, the use of PGPR and AMF as biostimulants has demonstrated significant potential in enhancing plant tolerance to abiotic stresses, such as drought and salinity. These beneficial microbes improve plant growth, stress resilience, and overall productivity through the production of various metabolites and the modulation of plant defense mechanisms. When combined with non-microbial biostimulants, the benefits are further amplified, offering new avenues for developing more robust agricultural practices to address environmental challenges. Notably, some bioactive compounds and natural plant hormones, such as melatonin, fall within the broader category of PBs. Although it is not traditionally grouped with common biostimulant types like HA, amino acids, seaweed extracts, or microbial inoculants, melatonin functions similarly by enhancing abiotic stress tolerance in horticultural crops, strengthening resilience to cold, drought, heat, salinity, and heavy metal stress. As a potent antioxidant, melatonin regulates stress-responsive genes, promotes water-use and photosynthetic efficiency, and interacts synergistically with other plant hormones to support growth under adverse environmental conditions [91]. Examples of the non-microbial and microbial PBs that have been reported to alleviate abiotic stresses are listed in Table 1.
More than 260 scientific papers on PBs and abiotic stresses have been published in the last five years (2019–2024) from a single database (www.scopus.com) (accessed on 12 December 2024). A list of relevant publications from 2019–2024 with the co-occurrence of terms in titles and abstracts from journal articles and conference proceedings from the database were compiled. The strings of keywords used when searching were: “Plant” AND “Biostimulant” AND “Abiotic stress” AND “Microbial” OR “Microbes” OR “Plant growth-promoting bacteria” OR “Plant growth-promoting rhizobacteria” OR “Mycorrhizal fungi” OR “Humic” OR “Seaweed” OR “Organic” OR “protein hydrolysate” OR “inorganic”. Bibliographic maps and networks were generated using VOSviewer, version 1.6.18. The eligibility criteria were set as follows: the terms repeated at least five times were selected, singular and plural forms were standardized to singular forms to avoid redundancies, and full names and abbreviations were standardized to full names.
Based on Fig. 1, the central role of “biostimulant” is expected, given its widespread application and interest in improving plant growth and stress tolerance. However, the network also highlights specific biostimulant types and their benefits, indicating targeted research efforts. The keyword “drought” having a high link strength (98) with “biostimulant” suggests a significant research focus on developing drought-tolerance crops through biostimulant application (Table 2). This aligns with global agricultural challenges, where water scarcity necessitates innovative solutions for crop sustainability. Additionally, “protein hydrolysate” with a notable link strength (84), indicates substantial research into how these substances can enhance nutrient uptake and soil fertility, reflecting a trend towards sustainable agriculture practices that reduce dependency on synthetic fertilizers.
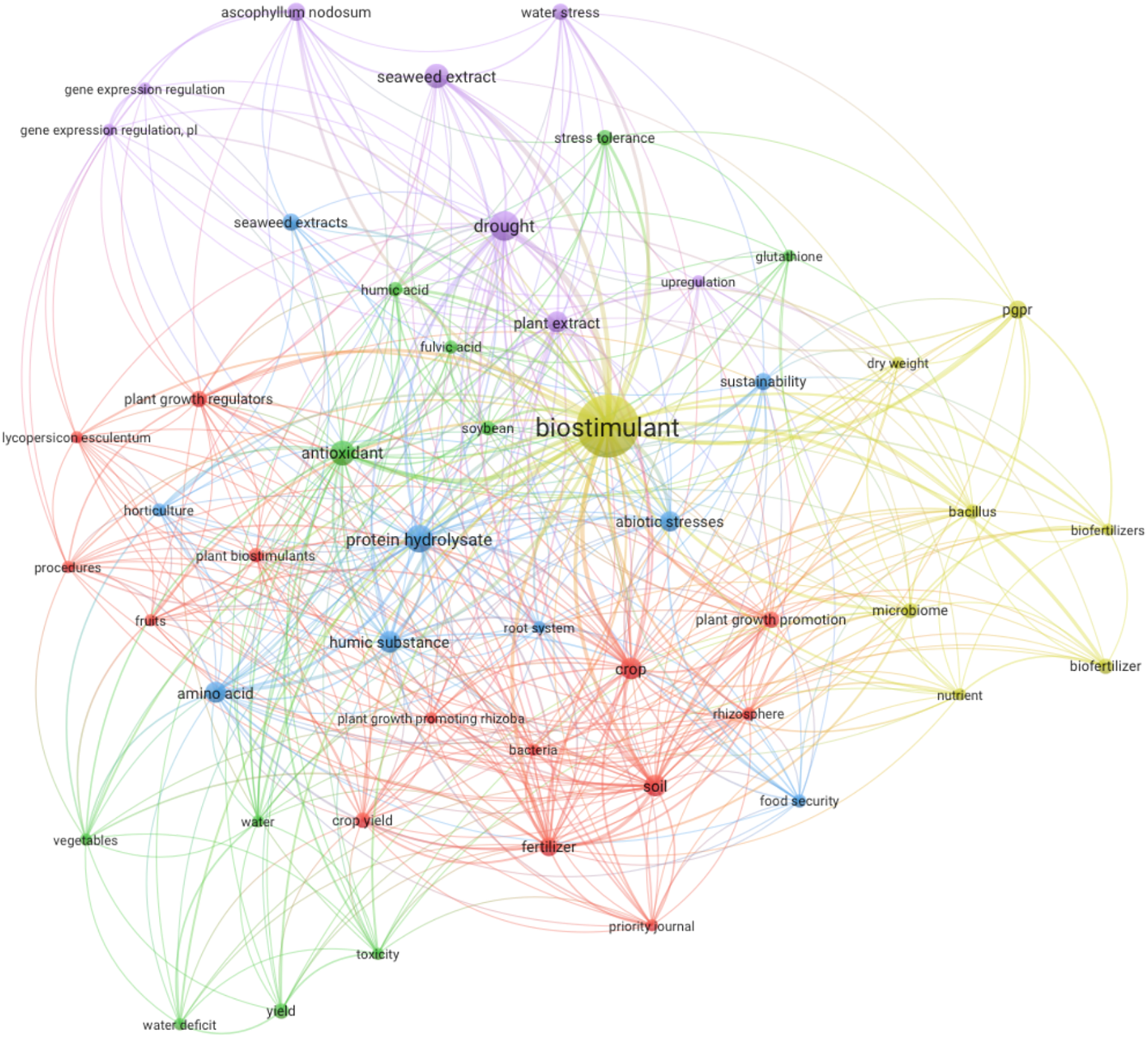
Figure 1: Network map in biostimulant research. The node (circle) size represents the occurrence frequency of the keyword in the dataset, with larger nodes indicating higher frequency. Line thickness represents the link strength between keywords, with thicker edges indicating stronger connections. Node and line colors represent clusters of related keywords, with different colors indicating clusters or thematic groups within the research network. The map contains six distinct colors: yellow for core biostimulant research and general plant growth promotion, purple for drought resistance and stress tolerance, blue for protein hydrolysates and related soil health topics, green for antioxidants and nutrient management, red for soil health, microbiome interactions, and crop yield, and light blue for specific biostimulants like amino acids and humic substances

The presence of “antioxidant” and “humic substance” as key terms with strong link strengths (72 and 55, respectively) highlights the dual focus on improving plant resilience and soil health. Antioxidants are crucial in mitigating oxidative stress in plants, while humic substances are known for their soil conditioning properties, enhancing nutrient availability and microbial activity. This suggests an integrated approach in biostimulant research, targeting plant and soil health for overall ecosystem benefits. Moreover, the inclusion of “microbiome” and “rhizosphere” with considerable link strengths (35 each) indicates an emerging interest in the role of microbial communities in plant health. This reflects a broader shift towards understanding and leveraging plant-microbe interactions to boost plant growth and stress resistance. The keyword “plant growth promoting rhizobacteria (PGPR)” with a link strength of 25, further supports this trend, showcasing the potential of beneficial microbes as biostimulants.
Overall, the network map reveals a comprehensive and multifaceted research landscape. It indicates a synergistic approach where biostimulants are applied directly to plants, soil, and microbial environments to optimize plant growth and resilience. This holistic strategy underscores the complexity and interconnectedness of modern agricultural research, aiming for sustainable and resilient crop production systems. Limitations of this approach include the reliance on Scopus alone, which may omit relevant articles indexed in other databases like Web of Science or PubMed. Additionally, our strategy might have excluded some niche studies due to the specificity of the keywords used, potentially overlooking articles that discuss PBs indirectly or under different terminologies. Despite these limitations, the selected approach offers a comprehensive view of recent PB research.
Organic, inorganic, and microbial biostimulants each offer unique advantages in enhancing crop resilience under drought, heat, salinity, and heavy metal stresses, but they also present notable challenges. Organic biostimulants are limited by variability in composition, potential contamination, and slower response times, whereas inorganic biostimulants can lead to environmental pollution, nutrient imbalances, and short-lived efficacy. Microbial biostimulants, although promising, face issues related to specificity, survivability under stress, and biosafety concerns. In addition, formulating effective biostimulants using nano-enabled methods across diverse soil types and plant species is challenging. Key issues include enabling nanoparticles to penetrate cell membranes and walls and understanding the growth-enhancing effects of specific nanomaterials, such as carbon nanotubes. Despite the potential of PBs, conducting field trials with PBs presents several challenges, including environmental variability, complex plant-microbe-soil interactions, and difficulties in optimizing application methods and dosages. Most PBs showed no phytotoxic effects on treated plants. However, applying more than 10 µM of Se led to phytotoxicity in cucumber plants [69], and protein hydrolysate at dilutions of 10−3 or higher inhibited maize germination [58]. The lack of standardized protocols for assessing PB efficacy and the need for long-term studies add to its complexity. Additionally, regulatory issues, economic constraints, and the challenge of ensuring reproducibility across diverse conditions must be considered. Addressing these challenges requires careful experimental design, standardized methods, and a deep understanding of environmental and biological factors influencing PB effectiveness.
In conclusion, the use of various PBs, including seaweed extracts, HAs, organic and inorganic PBs, PGPR, and mycorrhizal fungi, offers significant promise in enhancing plant resilience to abiotic stresses, such as drought, salinity, and nutrient limitations. These biostimulants function through various mechanisms and support osmoregulation, antioxidant activity, hormonal balance, and nutrient uptake efficiency, ultimately improving growth, yield, and stress tolerance in crops such as maize, wheat, and tomatoes (Fig. 2). Organic PBs, such as seaweed extracts and HAs, have proven effective in promoting photosynthesis, enhancing antioxidant defenses, and reducing oxidative damage, whereas inorganic PBs contribute to stress resilience and plant productivity. Furthermore, the integration of microbial biostimulants, such as PGPR and mycorrhizal fungi, enhances plant defence systems, further improving stress tolerance. Synergy between microbial and non-microbial biostimulants offers an exciting pathway for the development of sustainable agricultural strategies. However, further field studies are necessary to validate these findings under real-world conditions, making the continued exploration of biostimulants crucial to addressing the challenges posed by climate change and ensuring food security for the future.
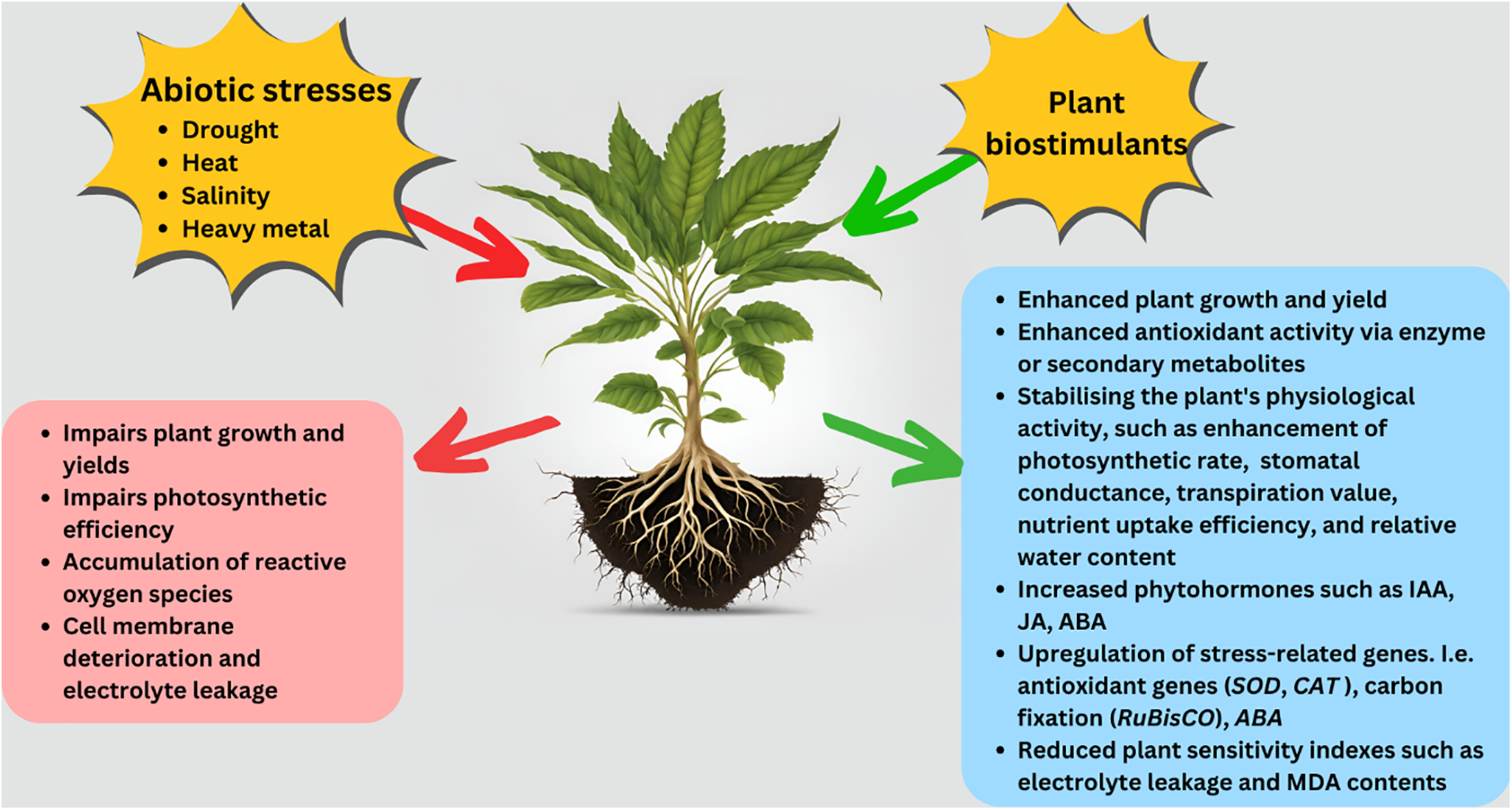
Figure 2: Overview of the role of plant biostimulants in modulating plant responses to abiotic stress. Abbreviations: ABA: Abscisic acid; CAT: Catalase; IAA: Indole-3-acetic acid; JA: Jasmonic acid; MDA: Malondialdehyde; SOD: Superoxide dismutase
Acknowledgement: Not applicable.
Funding Statement: This research was funded by the University Consortium (UC) Student Grant for Research Activities (GBG22-0919) and Hadiah Latihan Persekutuan (HLP).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Su-Ee Lau, Boon Chin Tan; draft manuscript preparation: Su-Ee Lau, Lucas Wei Tze Lim, Mohd Fadhli Hamdan, Colin Chan, Noor Baity Saidi, Janna Ong-Abdullah and Boon Chin Tan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Lau S-E, Pua T-L, Saidi NB, Ong-Abdullah J, Lamasudin DU, Tan BC. Combined proteomics and physiological analyses reveal drought and recovery response mechanisms in banana leaves. J Plant Growth Regul. 2023;42(12):7624–48. doi:10.1007/s00344-023-11039-3. [Google Scholar] [CrossRef]
2. FAO. The impact of disasters and crises on agriculture and food security. Rome: Food and Agriculture Organization of the United Nations (FAO); 2021. doi:10.4060/cb3673en. [Google Scholar] [CrossRef]
3. Schauberger B, Archontoulis S, Arneth A, Balkovic J, Ciais P, Deryng D, et al. Consistent negative response of US crops to high temperatures in observations and crop models. Nat Commun. 2017;8(1):13931. doi:10.1038/ncomms13931. [Google Scholar] [PubMed] [CrossRef]
4. Vaghefi N, Shamsudin MN, Radam A, Rahim KA. Impact of climate change on food security in Malaysia: economic and policy adjustments for rice industry. J Integr Environ Sci. 2016;13(1):19–35. doi:10.1080/1943815X.2015.1112292. [Google Scholar] [CrossRef]
5. Balasubramaniam T, Shen G, Esmaeili N, Zhang H. Plants’ response mechanisms to salinity stress. Plants. 2023;12(12):2253. doi:10.3390/plants12122253. [Google Scholar] [PubMed] [CrossRef]
6. Ghori N-H, Ghori T, Hayat M, Imadi S, Gul A, Altay V, et al. Heavy metal stress and responses in plants. Int J Environ Sci Technol. 2019;16:1807–28. doi:10.1007/s13762-019-02215-8. [Google Scholar] [CrossRef]
7. Lau S-E, Teo WFA, Teoh EY, Tan BC. Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discov Food. 2022;2(1):9. doi:10.1007/s44187-022-00009-5. [Google Scholar] [CrossRef]
8. Trivedi K, Gopalakrishnan VAK, Kumar R, Ghosh A. Transcriptional analysis of maize leaf tissue treated with seaweed extract under drought stress. Front Sustain Food Syst. 2021;5:774978. doi:10.3389/fsufs.2021.774978. [Google Scholar] [CrossRef]
9. Singh I, Anand KV, Solomon S, Shukla SK, Rai R, Zodape ST, et al. Can we not mitigate climate change using seaweed based biostimulant: a case study with sugarcane cultivation in India. J Cleaner Prod. 2018;204:992–1003. doi:10.1016/j.jclepro.2018.09.070. [Google Scholar] [CrossRef]
10. Sible CN, Seebauer JR, Below FE. Plant biostimulants: a categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy. 2021;11(7):1297. doi:10.3390/agronomy11071297. [Google Scholar] [CrossRef]
11. Jiménez-Arias D, Hernándiz AE, Morales-Sierra S, García-García AL, García-Machado FJ, Luis JC, et al. Applying biostimulants to combat water deficit in crop plants: research and debate. Agronomy. 2022;12(3):571. doi:10.3390/agronomy12030571. [Google Scholar] [CrossRef]
12. European Union. A legal framework for plant biostimulants and agronomic fertiliser additives in the EU: final report. Available from: Http://Op.Europa.Eu. [Accessed 2024]. [Google Scholar]
13. Ricci M, Tilbury L, Daridon B, Sukalac K. General principles to justify plant biostimulant claims. Front Plant Sci. 2019;10:494. doi:10.3389/fpls.2019.00494. [Google Scholar] [PubMed] [CrossRef]
14. Zhang X, Schmidt R. The impact of growth regulators on the α-tocopherol status in water-stressed Poa pratensis L. Int Turfgrass Soc Res J. 1997;8:1364–2137. [Google Scholar]
15. Caradonia F, Battaglia V, Righi L, Pascali G, La Torre A. Plant biostimulant regulatory framework: prospects in Europe and current situation at international level. J Plant Growth Regul. 2019;38:438–48. doi:10.1007/s00344-018-9853-4. [Google Scholar] [CrossRef]
16. Rouphael Y, Colla G. Biostimulants in agriculture. Front Plant Sci. 2020;11:40. doi:10.3389/fpls.2020.00040. [Google Scholar] [PubMed] [CrossRef]
17. El Boukhari MEM, Barakate M, Bouhia Y, Lyamlouli K. Trends in seaweed extract based biostimulants: manufacturing process and beneficial effect on soil-plant systems. Plants. 2020;9(3):359. doi:10.3390/plants9030359. [Google Scholar] [PubMed] [CrossRef]
18. Critchley AT, Critchley JSC, Norrie J, Gupta S, Van Staden J. Perspectives on the global biostimulant market: applications, volumes, and values, 2016 data and projections to 2022. In: Gupta S, Van Staden J, editors. Biostimulants for crops from seed germination to plant development. New York, NY, USA: Academic Press; 2021. p. 289–96. [Google Scholar]
19. Oyebamiji YO, Adigun BA, Shamsudin NAA, Ikmal AM, Salisu MA, Malike FA, et al. Recent advancements in mitigating abiotic stresses in crops. Horticulturae. 2024;10(2):156. doi:10.3390/horticulturae10020156. [Google Scholar] [CrossRef]
20. Ali O, Ramsubhag A, Jayaraman J. Biostimulant properties of seaweed extracts in plants: implications towards sustainable crop production. Plants. 2021;10(3):531. doi:10.3390/plants10030531. [Google Scholar] [PubMed] [CrossRef]
21. Silva A, Cassani L, Grosso C, Garcia-Oliveira P, Morais SL, Echave J, et al. Recent advances in biological properties of brown algae-derived compounds for nutraceutical applications. Crit Rev Food Sci Nutr. 2024;64(5):1283–311. doi:10.1080/10408398.2022.2115004. [Google Scholar] [PubMed] [CrossRef]
22. Latique S, Mrid RB, Kabach I, Kchikich A, Sammama H, Yasri A, et al. Foliar application of Ulva rigida water extracts improves salinity tolerance in wheat (Triticum durum L.). Agronomy. 2021;11(2):265. doi:10.3390/agronomy11020265. [Google Scholar] [CrossRef]
23. Shahriari AG, Mohkami A, Niazi A, Parizipour MHG, Habibi-Pirkoohi M. Application of brown algae (Sargassum angustifolium) extract for improvement of drought tolerance in canola (Brassica napus L.). Iran J Biotechnol. 2021;19(1):e2775. [Google Scholar]
24. Rasul F, Gupta S, Olas JJ, Gechev T, Sujeeth N, Mueller-Roeber B. Priming with a seaweed extract strongly improves drought tolerance in Arabidopsis. Int J Mol Sci. 2021;22(3):1469. doi:10.3390/ijms22031469. [Google Scholar] [PubMed] [CrossRef]
25. Carillo P, Ciarmiello LF, Woodrow P, Corrado G, Chiaiese P, Rouphael Y. Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology. 2020;9(9):253. doi:10.3390/biology9090253. [Google Scholar] [PubMed] [CrossRef]
26. Ali O, Farrell AD, Ramsubhag A, Jayaraman J. Beneficial effects of an Ascophyllum nodosum extract on tomato (Solanum lycopersicum L.) during water stress. J Appl Phycol. 2024;36(1):385–97. doi:10.1007/s10811-023-03156-z. [Google Scholar] [CrossRef]
27. Monteiro E, Baltazar M, Pereira S, Correia S, Ferreira H, Alves F, et al. Ascophyllum nodosum extract and glycine betaine preharvest application in grapevine: enhancement of berry quality, phytochemical content and antioxidant properties. Antioxid. 2023;12(10):1835. doi:10.3390/antiox12101835. [Google Scholar] [PubMed] [CrossRef]
28. El Boukhari MEM, Barakate M, Choumani N, Bouhia Y, Lyamlouli K. Ulva lactuca extract and fractions as seed priming agents mitigate salinity stress in tomato seedlings. Plants. 2021;10(6):1104. doi:10.3390/plants10061104. [Google Scholar] [PubMed] [CrossRef]
29. De Saeger J, Van Praet S, Vereecke D, Park J, Jacques S, Han T, et al. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J Appl Phycol. 2020;32(1):573–97. doi:10.1007/s10811-019-01903-9. [Google Scholar] [CrossRef]
30. Buthelezi NMD, Mugivhisa LL, Gololo SS. Foliar application of moringa seed extract alone or in combination with salicylic acid enhanced growth, bioactive, and phytohormone compositions of cancer bush plants under heat stress. HortScience. 2024;59(5):587–94. doi:10.21273/HORTSCI17691-24. [Google Scholar] [PubMed] [CrossRef]
31. Mishra A, Kar S, Bisht N, Mishra SK, Chauhan PS. Synergistic effect of Adathoda vasica plant-derived biostimulant and PGPR on Zea mays L. for drought stress management. Microbiol Res. 2024;290:127968. [Google Scholar] [PubMed]
32. Ampong K, Thilakaranthna MS, Gorim LY. Understanding the role of humic acids on crop performance and soil health. Front Agron. 2022;4:848621. doi:10.3389/fagro.2022.848621. [Google Scholar] [CrossRef]
33. Abu-Ria ME, Elghareeb EM, Shukry WM, Abo-Hamed SA, Ibraheem F. Mitigation of drought stress in maize and sorghum by humic acid: differential growth and physiological responses. BMC Plant Biol. 2024;24(1):514. doi:10.1186/s12870-024-05184-4. [Google Scholar] [PubMed] [CrossRef]
34. de Jesus ALN, de Andrada LVP, dos Santos Silva LF, Santos NA, Cruz FB, Cumbana NCA, et al. Humic and fulvic acid influence the morphophysiological and biochemical properties of cowpea (Vigna unguiculata) under water deficit. Crop Pasture Sci. 2023;75(1):CP23250. [Google Scholar]
35. Abdel-Sattar M, Mostafa LY, Rihan HZ. Enhancing mango productivity with wood vinegar, humic acid, and seaweed extract applications as an environmentally friendly strategy. Sustainability. 2024;16(20):8986. doi:10.3390/su16208986. [Google Scholar] [CrossRef]
36. Khaleda L, Park HJ, Yun D-J, Jeon J-R, Kim MG, Cha J-Y, et al. Humic acid confers high-affinity K+ transporter 1-mediated salinity stress tolerance in Arabidopsis. Mol Cells. 2017;40(12):966–75. [Google Scholar] [PubMed]
37. Canellas LP, da Silva RM, Busato JG, Olivares FL. Humic substances and plant abiotic stress adaptation. Chem Biol Technol Agric. 2024;11(1):66. doi:10.1186/s40538-024-00575-z. [Google Scholar] [CrossRef]
38. Quintero-Calderon EH, Sanchez-Reinoso AD, Chavez-Arias CC, Garces-Varon G, Restrepo-Diaz H. Rice seedlings showed a higher heat tolerance through the foliar application of biostimulants. Not Bot Horti Agrobo. 2021;49(1):12120. doi:10.15835/nbha49112120. [Google Scholar] [CrossRef]
39. Price MB, Jelesko J, Okumoto S. Glutamate receptor homologs in plants: functions and evolutionary origins. Front Plant Sci. 2012;3:235. [Google Scholar] [PubMed]
40. Kazda J, Herda G, Spitzer T, Řičařová V, Przybysz A, Gawrońska H. Effect of nitrophenolates on pod damage caused by the brassica pod midge on the photosynthetic apparatus and yield of winter oilseed rape. J Pest Sci. 2015;88:235–47. doi:10.1007/s10340-014-0603-5. [Google Scholar] [CrossRef]
41. Quan J, Zheng W, Tan J, Li Z, Wu M, Hong S-B, et al. Glutamic acid and poly-γ-glutamic acid enhanced the heat resistance of Chinese cabbage (Brassica rapa L. ssp. pekinensis) by improving carotenoid biosynthesis, photosynthesis, and ROS signaling. Int J Mol Sci. 2022;23(19):11671. doi:10.3390/ijms231911671. [Google Scholar] [PubMed] [CrossRef]
42. Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M. Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants. 2021;10(2):277. doi:10.3390/antiox10020277. [Google Scholar] [PubMed] [CrossRef]
43. Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:273. [Google Scholar] [PubMed]
44. Aklilu BB, Culligan KM. Molecular evolution and functional diversification of replication protein A1 in plants. Front Plant Sci. 2016;7:33. [Google Scholar] [PubMed]
45. Monterisi S, Zhang L, Garcia-Perez P, Alzate Zuluaga MY, Ciriello M, El-Nakhel C, et al. Integrated multi-omic approach reveals the effect of a Graminaceae-derived biostimulant and its lighter fraction on salt-stressed lettuce plants. Sci Rep. 2024;14(1):10710. doi:10.1038/s41598-024-61576-4. [Google Scholar] [PubMed] [CrossRef]
46. Hamedeh H, Antoni S, Cocciaglia L, Ciccolini V. Molecular and physiological effects of magnesium-polyphenolic compound as biostimulant in drought stress mitigation in tomato. Plants. 2022;11(5):586. doi:10.3390/plants11050586. [Google Scholar] [PubMed] [CrossRef]
47. Sarraf M, Janeeshma E, Arif N, QudratUllahFarooqi M, Kumar V, Ansari NA, et al. Understanding the role of beneficial elements in developing plant stress resilience: signalling and crosstalk with phytohormones and microbes. Plant Stress. 2023;10:100224. doi:10.1016/j.stress.2023.100224. [Google Scholar] [CrossRef]
48. Chen L, Liu J-R, Hu W-F, Gao J, Yang J-Y. Vanadium in soil-plant system: source, fate, toxicity, and bioremediation. J Hazard Mater. 2021;405:124200. doi:10.1016/j.jhazmat.2020.124200. [Google Scholar] [PubMed] [CrossRef]
49. Paiva LGD, Grangeiro LC, Nascimento CWD, Costa RM, Pereira NA, Lima RBD, et al. Selenium as an inorganic biostimulant in onion grown in a semi-arid climate. Rev Bras Eng Agric Ambiental. 2024;28(4):e279061. doi:10.1590/1807-1929/agriambi.v28n4e279061. [Google Scholar] [CrossRef]
50. Patnaik GP, Monisha V, Thavaprakaash N, Djanaguiraman M, Sachin S, Vikram K, et al. Selenium application improves drought tolerance during reproductive phase of rice. Sustainability. 2023;15(3):2730. doi:10.3390/su15032730. [Google Scholar] [CrossRef]
51. Exley C. A possible mechanism of biological silicification in plants. Front Plant Sci. 2015;6(112):853. doi:10.3389/fpls.2015.00853. [Google Scholar] [PubMed] [CrossRef]
52. Ali AM, Bijay-Singh. Silicon: a crucial element for enhancing plant resilience in challenging environments. J. Plant Nutr. 2024;13(16):1–36. doi:10.1080/01904167.2024.2406479. [Google Scholar] [CrossRef]
53. Parveen A, Liu W, Hussain S, Asghar J, Perveen S, Xiong Y. Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants. 2019;8(10):431. doi:10.3390/plants8100431. [Google Scholar] [PubMed] [CrossRef]
54. Cao B, Ma Q, Xu K. Silicon restrains drought-induced ROS accumulation by promoting energy dissipation in leaves of tomato. Protoplasma. 2020;257:537–47. doi:10.1007/s00709-019-01449-0. [Google Scholar] [PubMed] [CrossRef]
55. Rodrigues SM, Demokritou P, Dokoozlian N, Hendren CO, Karn B, Mauter MS, et al. Nanotechnology for sustainable food production: promising opportunities and scientific challenges. Environ Sci: Nano. 2017;4(4):767–81. [Google Scholar]
56. Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS. Nanoparticulate material delivery to plants. Plant Sci. 2010;179(3):154–63. doi:10.1016/j.plantsci.2010.04.012. [Google Scholar] [CrossRef]
57. Arsenov D, Beljin J, Jović D, Maletić S, Borišev M, Borišev I. Nanomaterials as endorsed environmental remediation tools for the next generation: eco-safety and sustainability. J Geochem Explor. 2023;253:107283. doi:10.1016/j.gexplo.2023.107283. [Google Scholar] [CrossRef]
58. Ali S, Rizwan M, Hussain A, ur Rehman MZ, Ali B, Yousaf B, et al. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol Biochem. 2019;140:1–8. doi:10.1016/j.plaphy.2019.04.041. [Google Scholar] [PubMed] [CrossRef]
59. Maryam H, Abbasi GH, Waseem M, Ahmed T, Rizwan M. Preparation and characterization of green silicon nanoparticles and their effects on growth and lead (Pb) accumulation in maize (Zea mays L.). Environ Pollut. 2024;346:123691. doi:10.1016/j.envpol.2024.123691. [Google Scholar] [PubMed] [CrossRef]
60. Hussain A, Rizwan M, Ali S, ur Rehman MZ, Qayyum MF, Nawaz R, et al. Combined use of different nanoparticles effectively decreased cadmium (Cd) concentration in grains of wheat grown in a field contaminated with Cd. Ecotoxicol Environ Saf. 2021;215:112139. doi:10.1016/j.ecoenv.2021.112139. [Google Scholar] [PubMed] [CrossRef]
61. Khan ZS, Rizwan M, Hafeez M, Ali S, Adrees M, Qayyum MF, et al. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ Sci Pollut Res. 2020;27:4958–68. doi:10.1007/s11356-019-06673-y. [Google Scholar] [PubMed] [CrossRef]
62. Gómez-Santos M, González-García Y, Pérez-Álvarez M, Cadenas-Pliego G, Juárez-Maldonado A. Impact of Calcium-Silicon nanoparticles on flower quality and biochemical characteristics of Lilium under salt stress. Plant Stress. 2023;10:100270. doi:10.1016/j.stress.2023.100270. [Google Scholar] [CrossRef]
63. Cembrowska-Lech D, Rybak K. Nanopriming of barley seeds—a shotgun approach to improve germination under salt stress conditions by regulating of reactive oxygen species. Plants. 2023;12(2):405. doi:10.3390/plants12020405. [Google Scholar] [PubMed] [CrossRef]
64. Byczyńska A, Zawadzińska A, Salachna P. Colloidal silver nanoparticles enhance bulb yield and alleviate the adverse effect of saline stress on lily plants. J Ecol Eng. 2023;24(6):338–47. doi:10.12911/22998993/163173. [Google Scholar] [CrossRef]
65. González-García Y, López-Vargas ER, Pérez-Álvarez M, Cadenas-Pliego G, Benavides-Mendoza A, Valdés-Reyna J, et al. Seed priming with carbon nanomaterials improves the bioactive compounds of tomato plants under saline stress. Plants. 2022;11(15):11–5. doi:10.3390/plants11151984. [Google Scholar] [PubMed] [CrossRef]
66. Adhikari A, Aneefi AG, Sisuvanh H, Singkham S, Pius MV, Akter F, et al. Dynamics of humic acid, silicon, and biochar under heavy metal, drought, and salinity with special reference to phytohormones, antioxidants, and melatonin synthesis in rice. Int J Mol Sci. 2023;24(24):17369. doi:10.3390/ijms242417369. [Google Scholar] [PubMed] [CrossRef]
67. Jiang M, Zhang J. Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defence in leaves of maize seedlings. Plant Cell Environ. 2003;26(6):929–39. doi:10.1046/j.1365-3040.2003.01025.x. [Google Scholar] [PubMed] [CrossRef]
68. You J, Chan Z. ROS regulation during abiotic stress responses in crop plants. Front Plant Sci. 2015;6:1092. doi:10.3389/fpls.2015.01092. [Google Scholar] [PubMed] [CrossRef]
69. Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24(13):2452. doi:10.3390/molecules24132452. [Google Scholar] [PubMed] [CrossRef]
70. Jayaweera DP, Dambire C, Angelopoulou D, Munné-Bosch S, Swarup R, Ray RV. Physiological, molecular, and genetic mechanism of action of the biostimulant QuantisTM for increased thermotolerance of potato (Solanum tuberosum L.). Chem Biol Technol Agric. 2024;11(1):9. doi:10.1186/s40538-023-00531-3. [Google Scholar] [CrossRef]
71. Becerril-Espinosa A, Hernández-Herrera RM, Meza-Canales ID, Perez-Ramirez R, Rodríguez-Zaragoza FA, Méndez-Morán L, et al. Habitat-adapted heterologous symbiont Salinispora arenicola promotes growth and alleviates salt stress in tomato crop plants. Front Plant Sci. 2022;13:920881. doi:10.3389/fpls.2022.920881. [Google Scholar] [PubMed] [CrossRef]
72. Chen E, Yang C, Tao W, Li S. Polysaccharides produced by plant growth-promoting rhizobacteria strain Burkholderia sp. BK01 enhance salt stress tolerance to Arabidopsis thaliana. Polymers. 2024;16(1):145. doi:10.3390/polym16010145. [Google Scholar] [PubMed] [CrossRef]
73. Zou P, Ma S, Yuan Y, Ma J, Yang X, Hu X, et al. A glucomannan produced by Bacillus velezensis HY23 and its growth promoting effect on soybeans under salt stress. Int J Biol Macromol. 2024;275:133474. doi:10.1016/j.ijbiomac.2024.133474. [Google Scholar] [PubMed] [CrossRef]
74. Nascimento FX, Urón P, Glick BR, Giachini A, Rossi MJ. Genomic analysis of the 1-aminocyclopropane-1-carboxylate deaminase-producing Pseudomonas thivervalensis sc5 reveals its multifaceted roles in soil and in beneficial interactions with plants. Front Microbiol. 2021;12:752288. doi:10.3389/fmicb.2021.752288. [Google Scholar] [PubMed] [CrossRef]
75. Eswaran SUD, Sundaram L, Perveen K, Bukhari NA, Sayyed R. Osmolyte-producing microbial biostimulants regulate the growth of Arachis hypogaea L. under drought stress. BMC Microbiol. 2024;24(1):165. doi:10.1186/s12866-024-03320-6. [Google Scholar] [PubMed] [CrossRef]
76. Kim ST, Sang MK. Enhancement of osmotic stress tolerance in soybean seed germination by bacterial bioactive extracts. PLoS One. 2023;18(10):e0292855. doi:10.1371/journal.pone.0292855. [Google Scholar] [PubMed] [CrossRef]
77. Lozo J, Danojević D, Jovanović Ž, Nenadović Ž, Fira D, Stanković S, et al. Genotype-dependent antioxidative response of four sweet pepper cultivars to water deficiency as affected by drought-tolerant Bacillus safensis SS-2.7 and Bacillus thuringiensis SS-29.2 strains. Horticulturae. 2022;8(3):236. doi:10.3390/horticulturae8030236. [Google Scholar] [CrossRef]
78. Daniel A. Screening of rice apoplast associated endophytic bacterial isolates for moisture stress tolerance and plant growth promoting traits. Madras Agric J. 2019;106:1. [Google Scholar]
79. Ghosh D, Gupta A, Mohapatra S. A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J Microbiol Biotechnol. 2019;35:1–15. [Google Scholar]
80. Ilyas N, Mumtaz K, Akhtar N, Yasmin H, Sayyed R, Khan W, et al. Exopolysaccharides producing bacteria for the amelioration of drought stress in wheat. Sustainability. 2020;12(21):8876. doi:10.3390/su12218876. [Google Scholar] [CrossRef]
81. Abdalla M, Ahmed MA. Arbuscular mycorrhiza symbiosis enhances water status and soil-plant hydraulic conductance under drought. Front Plant Sci. 2021;12:722954. doi:10.3389/fpls.2021.722954. [Google Scholar] [PubMed] [CrossRef]
82. Wang Y, Cao J-L, Hashem A, Abd_Allah EF, Wu Q-S. Serendipita indica mitigates drought-triggered oxidative burst in trifoliate orange by stimulating antioxidant defense systems. Front Plant Sci. 2023;14:1247342. doi:10.3389/fpls.2023.1247342. [Google Scholar] [PubMed] [CrossRef]
83. Nair DS, Manjula S. Induction of root endosymbiosis as a highly sustainable and efficient strategy for overproduction of the medicinally important diterpenoid lactone-andrographolide in Andrographis paniculata (Burm. F.) Wall. ex Nees. Ind Crops Prod. 2020;156:112835. doi:10.1016/j.indcrop.2020.112835. [Google Scholar] [CrossRef]
84. Mannino G, Nerva L, Gritli T, Novero M, Fiorilli V, Bacem M, et al. Effects of different microbial inocula on tomato tolerance to water deficit. Agronomy. 2020;10(2):170. doi:10.3390/agronomy10020170. [Google Scholar] [CrossRef]
85. Petropoulos SA, Taofiq O, Fernandes Â, Tzortzakis N, Ciric A, Sokovic M, et al. Bioactive properties of greenhouse-cultivated green beans (Phaseolus vulgaris L.) under biostimulants and water-stress effect. J Sci Food Agric. 2019;99(13):6049–59. doi:10.1002/jsfa.v99.13. [Google Scholar] [CrossRef]
86. Consentino BB, Vultaggio L, Allevato E, Sabatino L, Ntatsi G, Ciriello M, et al. Plant protein hydrolysate and arbuscular mycorrhizal fungi synergistically orchestrate eggplant tolerance to iodine supply: a two-year study. Sci Hortic. 2024;336:113437. doi:10.1016/j.scienta.2024.113437. [Google Scholar] [CrossRef]
87. Olivares FL, Busato JG, de Paula AM, da Silva Lima L, Aguiar NO, Canellas LP. Plant growth promoting bacteria and humic substances: crop promotion and mechanisms of action. Chem Biol Technol Agric. 2017;4:1–13. [Google Scholar]
88. Canellas LP, Olivares FL. Physiological responses to humic substances as plant growth promoter. Chem Biol Technol. 2014;1:1–11. [Google Scholar]
89. Galambos N, Compant S, Moretto M, Sicher C, Puopolo G, Wäckers F, et al. Humic acid enhances the growth of tomato promoted by endophytic bacterial strains through the activation of hormone-, growth-, and transcription-related processes. Front Plant Sci. 2020;11:582267. doi:10.3389/fpls.2020.582267. [Google Scholar] [PubMed] [CrossRef]
90. Seneviratne G, Weerasekara M, Seneviratne K, Zavahir J, Kecskés M, Kennedy I. Importance of biofilm formation in plant growth promoting rhizobacterial action. In: Maheshwari D, editor. Plant growth and health promoting bacteria. Microbiology monographs. Berlin, Heidelberg: Springer; 2010. vol. 18, p. 81–95. doi:10.1007/978-3-642-13612-2. [Google Scholar] [CrossRef]
91. Ahmad J, Hayat F, Khan U, Ahmed N, Li J, Ercisli S, et al. Melatonin: a promising approach to enhance abiotic stress tolerance in horticultural plants. S Afr J Bot. 2024;164:66–76. doi:10.1016/j.sajb.2023.10.045. [Google Scholar] [CrossRef]
92. Campobenedetto C, Grange E, Mannino G, Van Arkel J, Beekwilder J, Karlova R, et al. A biostimulant seed treatment improved heat stress tolerance during cucumber seed germination by acting on the antioxidant system and glyoxylate cycle. Front Plant Sci. 2020;11:836. doi:10.3389/fpls.2020.00836. [Google Scholar] [PubMed] [CrossRef]
93. Carmody N, Goñi O, Łangowski Ł, O’Connell S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front Plant Sci. 2020;11:807. doi:10.3389/fpls.2020.00807. [Google Scholar] [PubMed] [CrossRef]
94. Rossi S, Huang B. Carotene-enhanced heat tolerance in creeping bentgrass in association with regulation of enzymatic antioxidant metabolism. J Am Soc Hortic Sci. 2022;147(3):145–51. doi:10.21273/JASHS05201-22. [Google Scholar] [CrossRef]
95. Vaseva II, Simova-Stoilova L, Kostadinova A, Yuperlieva-Mateeva B, Karakicheva T, Vassileva V. Heat-stress-mitigating effects of a protein-hydrolysate-based biostimulant are linked to changes in protease, DHN, and HSP gene expression in maize. Agronomy. 2022;12(5):1127. doi:10.3390/agronomy12051127. [Google Scholar] [CrossRef]
96. Demehin O, Attjioui M, Goñi O, O’Connell S. Chitosan from mushroom improves drought stress tolerance in tomatoes. Plants. 2024;13(7):1038. doi:10.3390/plants13071038. [Google Scholar] [PubMed] [CrossRef]
97. Naboulsi I, Ben Mrid R, Ennoury A, Zouaoui Z, Nhiri M, Ben Bakrim W, et al. Crataegus oxyacantha extract as a biostimulant to enhance tolerance to salinity in tomato plants. Plants. 2022;11(10):1283. doi:10.3390/plants11101283. [Google Scholar] [PubMed] [CrossRef]
98. Ben Saad R, Ben Romdhane W, Wiszniewska A, Baazaoui N, Taieb Bouteraa M, Chouaibi Y, et al. Rosmarinus officinalis L. essential oil enhances salt stress tolerance of durum wheat seedlings through ROS detoxification and stimulation of antioxidant defense. Protoplasma. 2024;261:1–14. [Google Scholar]
99. Sun C, Chen J, Wang L, Li J, Shi Z, Yang L, et al. Thymol deploys multiple antioxidative systems to suppress ROS accumulation in Chinese cabbage seedlings under saline stress. Agronomy. 2024;14(5):1059. doi:10.3390/agronomy14051059. [Google Scholar] [CrossRef]
100. Campobenedetto C, Mannino G, Beekwilder J, Contartese V, Karlova R, Bertea CM. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci Rep. 2021;11(1):354. doi:10.1038/s41598-020-79770-5. [Google Scholar] [PubMed] [CrossRef]
101. Del Buono D, Regni L, Del Pino AM, Bartucca ML, Palmerini CA, Proietti P. Effects of megafol on the olive cultivar ‘Arbequina’ grown under severe saline stress in terms of physiological traits, oxidative stress, antioxidant defenses, and cytosolic Ca2+. Front Plant Sci. 2021;11:603576. doi:10.3389/fpls.2020.603576. [Google Scholar] [PubMed] [CrossRef]
102. Jóźwiak W, Politycka B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants. 2019;8(7):217. doi:10.3390/plants8070217. [Google Scholar] [PubMed] [CrossRef]
103. Silva S, Dias MC, Silva AM. Potential of MgO and MgCO3 nanoparticles in modulating lettuce physiology to drought. Acta Physiol Plant. 2023;45(2):31. doi:10.1007/s11738-022-03507-2. [Google Scholar] [CrossRef]
104. Avestan S, Ghasemnezhad M, Esfahani M, Byrt CS. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy. 2019;9(5):246. doi:10.3390/agronomy9050246. [Google Scholar] [CrossRef]
105. Morales-Espinoza MC, Cadenas-Pliego G, Pérez-Alvarez M, Hernández-Fuentes AD, Cabrera de la Fuente M, Benavides-Mendoza A, et al. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules. 2019;24(17):3030. doi:10.3390/molecules24173030. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF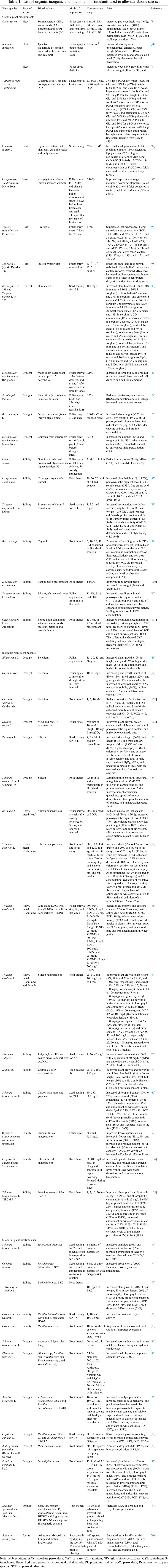
 Downloads
Downloads
 Citation Tools
Citation Tools