 Open Access
Open Access
ARTICLE
Thymus serpyllum L. Essential Oil: Phytochemistry and in Vitro and in Silico Screening of Its Antimicrobial, Antioxidant and Anti-Inflammatory Properties
1 Department of Biology, College of Sciences, Princess Nourah bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
2 Department of Pharmcology, Laboratory of Epidemiology and Research in Health Sciences, Faculty of Medicine, Pharmacy and Dentistry, Sidi Mohamed Ben Abdellah University, Fez, 30000, Morocco
3 Laboratoire d’Amélioration des Productions Agricoles, Biotechnologie et Environnement (LAPABE), Faculté des Sciences, Moroccodes Sciences, Université Mohammed Premier, Oujda, 60000, Morocco
4 Department of Pharmaceutical Sciences, College of Pharmacy, Gulf Medical University, Ajman, P.O. Box 4184, United Arab Emirates
5 Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Abbassia, Cairo, 11566, Egypt
6 Laboratory of Applied Organic Chemistry, Faculty of Sciences and Techniques, Sidi Mohamed Ben Abdellah University, Imouzzer Road, Fez, 30000, Morocco
7 Department of Biology, College of Science, Qassim University, Qassim, 51452, Saudi Arabia
8 High Institute of Nursing Professions and Health Techniques Fez, Fez, 30050, Morocco
9 Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, Buraydah, 51452, Saudi Arabia
10 Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Buraydah, 51452, Saudi Arabia
11 Department of Pharmacology and Toxicology, College of Pharmacy, Qassim University, Buraydah, 52571, Saudi Arabia
12 Faculty of Medicine and Pharmacy, Ibn Zohr University, Guelmim, 81000, Morocco
* Corresponding Author: Amine Elbouzidi. Email:
(This article belongs to the Special Issue: Biological Activities of Essential Oils)
Phyton-International Journal of Experimental Botany 2025, 94(1), 209-227. https://doi.org/10.32604/phyton.2025.060438
Received 01 November 2024; Accepted 26 December 2024; Issue published 24 January 2025
Abstract
Thymus serpyllum L., often known as wild thyme, has been used since ancient times due to its multifaceted culinary and medicinal attributes. It is usually utilized in folk medicine to manage different health issues. This work aimed to investigate the chemical composition and biological characteristics of T. serpyllum essential oil (EO), including its antimicrobial, antioxidant, and anti-inflammatory capabilities. Moreover, we have prompted an in-silico simulation to reveal the underlying mode of action of these properties. The chemical characterization of T. serpyllum (EO) by Gas Chromatography-Mass Spectrometry (GC-MS) indicated sabinene (17.33%), terpinen-4-ol (11.73%), phellandral (13.18%), and thymol (10.54%) as main components. The antimicrobial screening utilized the disc-diffusion technique, MIC, and MBC assays. The disc-diffusion test’s results revealed significant anti-Candida activity and notable antibacterial efficacy. The MIC and MBC tests showed that T. serpyllum EO effectively stops bacterial growth, including Gram-positive and Gram-negative strains and Candida strains. The tolerance level ratio demonstrated that this EO exhibits bactericidal and fungicidal effects on all tested bacteria and Candida strains. Also, T. serpyllum EO presented effective inhibitory activity against the 5-lipoxygenase (5-LOX) enzyme (IC50 = 744.19 ± 0.1 µg/mL) (p < 0.05). It also effectively affected FRAP, β-carotene, DPPH, and ABTS radicals. In light of these findings, T. serpyllum holds promise for diverse applications across pharmaceuticals, nutraceuticals, and the food industry. However, further research and collaboration between traditional knowledge and modern medicine are crucial to fully realizing its potential benefits in these fields.Keywords
Since ancient times, medicinal plants have been used to cure various illnesses, and still, they remain a vital source of pharmaceutical chemicals [1]. Medicinal plants are a rich source of chemical compounds that are responsible for their anticancer, antioxidant, and antidiabetic as well as antibacterial, antiviral, and antifungal activities [2–5]. Interest in natural antimicrobial agents has increased due to the speculation about drugs’ safety and selectivity [6]. Antimicrobial resistance is currently a worldwide problem, and the World Health Organization (WHO) encourages the discovery of novel antibiotic resources [7,8]. Many scientists believe that the post-antibiotic era has entered due to pathogenic microbes’ heightened aggressiveness and the antibiotics’ diminishing efficacy. This underscores the necessity to develop new drugs to counteract these microbes [9]. In 2017, the WHO identified the most significant antibiotic-resistant bacteria globally, underscoring the urgent requirement for new treatment options. Pathogens such as multi-drug resistant Acinetobacter spp., Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa; along with MRSA and vancomycin-resistant Enterococcus faecium, have presented formidable challenges, making their eradication extremely difficult [10,11].
EOs obtained from plants have been acknowledged for their varied biological activities, which include antioxidant, anticancer, and antimicrobial properties [12,13]. EOs are widely reported for their bioactive chemical compounds of therapeutic potential, making them play a crucial role in drug discovery. In fact, the investigation into the antimicrobial attributes of these natural compounds has been a focus of extensive research in recent times and their investigation will provide a source of active agents against infections [14].
Thymus serpyllum L. (Lamiaceae), also known as wild thyme, is an aromatic plant native to Mediterranean Europe and Africa, and typically, it vegetates at higher altitudes [5]. T. serpyllum is rich in essential oils, flavonoids, and phenolic acids [15]. T. serpyllum EOs are reported for their characteristic chemical composition, rich in bioactive compounds with important therapeutic potential [16]. These properties play a critical role in the development of treatments aimed at reducing inflammation, managing bacterial infections, and targeting cancerous cells [17,18].
In the present exploration, we designed to determine the volatile compounds and biological effects, including antibacterial, anticandidal, antioxidant, and anti-inflammatory activities of Moroccan T. serpyllum EO. Moreover, we have prompted an in-silico simulation to reveal the underlying molecular mechanisms of these activities. Notably, to our knowledge, no previous study has reported the in vitro and in-silico anti-inflammatory attributes of T. serpyllum EO.
Lipoxygenase (5-LOX), p-iodonitrotetrazoliumchloride, 2,2-diphenyl-1-picrylhydrazyl (DPPH), methanol, ethanol, butylhydroxytoluene (BHT), acid 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonique (ABTS), linoleic acid, potassium ferricyanide K3Fe(CN)6, Butylated hydroxyanisole (BHA), trichloroacetic acid (TCA), ferric chloride (FeCl3), and quercetin were obtained from Sigma-Aldrich. Luria-Bertani (LB), Potato dextrose agar (PDA), Yeast Extract-Peptone-Dextrose (YPD) agar, dimethyl sulfoxide (DMSO), clotrimazole, chloramphenicol, fluconazole and clotrimazole were acquired from labKem, Spain and Biokar Diagnostics, France.
Thymus serpyllum L. fresh leaves were harvested in March 2023 from the province of Taounate, Morocco (34° 39′ 03″ N, 4° 16′ 40″ W).
The plant taxonomical classification was done at the Scientific Institute, Mohammed V University in Rabat, where a voucher specimen was deposited under the reference number RAB 11502.
200 g of the fresh leave were dried at 25°C for 6 days and then hydrodistilled for 3 h using a Clevenger-type glass apparatus. EOs separately isolated and dehydrated with anhydrous sodium sulphate (Na2SO4), before its storage. This experiment was conducted four times.
GC chromatograms and mass spectra were used to analyze the obtained oil samples using Shimadzu GC/MS-QP 2010 (Kyoto, Japan) coupled to a mass spectrometer (SSQ 7000 quadrupole: Thermo-Finnigan, Bremen, Germany). The capillary column used was Rtx-5MS, 30 m in length, 0.25 mm internal diameter, and 0.25 µm film thickness (Restek, Bellefonte, PA, USA). The run started initially at 45°C for 2 min, then increased gradually to 300°C (heating rate of 10–20°C/min). The temperature of the injector was kept at 250°C, whereas the detector’s temperature was held at 280°C. Automatic sample injection was applied (1 µL, split ratio of 1:15), and the carrier gas used was Helium with a flow rate of 1.41 mL/min. The following conditions were applied for the mass spectrometry: the ion source temperature was set at 200°C, an ionization voltage of 70 eV, and the scan range was performed from 35 to 500 amu.
The mass spectra of the individual GC peaks were identified through a computerized search using commercial libraries (WILEY, NIST). The identification was further confirmed by comparing the results with published data and calculating the retention indices (RI) relative to a series of n-alkanes (C6–C22) [19,20].
This study employed seven microbial strains, encompassing two Gram-positive bacterial species, specifically Staphylococcus aureus ATCC 29213 and Bacillus subtilis ATCC 6633. Three Gram-negative bacterial species were included: Klebsiella aerogenes ATCC 13048, Escherichia coli ATCC 27853, and Salmonella enterica serotype Typhi. Furthermore, two clinical isolates of fungal strains, namely Candida albicans and Candida tropicalis, were incorporated.
The preliminary screening of the antimicrobial effects of T. serpyllum EO was conducted using the disc diffusion method, incorporating minor modifications as per a previously outlined methodology [21]. LB agar medium was inoculated with the bacterial culture suspension, and YPD agar was inoculated with Candida strains using a swab. Subsequently, 6 mm sterile filter paper discs, saturated with 10 μL of TSEO, were positioned on respective plates. Positive control for bacteria included chloramphenicol (15 μg/disc), while clotrimazole (20 μg/disc) was a reference drug for yeasts. The inoculated plates were incubated at appropriate conditions; following the incubation period, the inhibitory diameters were measured in millimeters. The results were then expressed as the mean ± SD based on three independent replicates.
The Minimum Inhibitory Concentration (MIC) of T. serpyllum EO against the examined microorganisms that exhibited noticeable results was determined using the micro-broth dilution technique, employing 96-well microplates with slight modifications as previously mentioned [21]. The MIC values were determined as the lowest concentrations that did not exhibit detectable microbial growth in the examined wells.
Following the completion of the MIC test, assessments for minimum bactericidal activity (MBC) and minimum fungicidal activity (MFC) were carried out, as previously reported [22]. Additionally, the MBC/MIC was analyzed to differentiate between bacteriostatic and bactericidal effects, and the MFC/MIC was calculated to classify the activity as either fungistatic or fungicidal.
The antioxidant potential of T. serpyllum EO has been investigated using four known complementary methods, including DPPH, ABTS, reductive power and β-carotene/linoleic acid tests as indicated in the literature [23,24]. The experiments were performed in three independent replicates (n = 3), and IC50 was calculated from inhibition curves and presented as mean ± SD. BHT and BHA were used as standard antioxidants.
2.11 In Vitro Anti-Inflammatory Assay
The anti-inflammatory effects of T. serpyllum EO were assessed in vitro using the 5-Lipoxygenase (5-LOX) inhibition method, following linoleic acid oxidation at 234 nm, as outlined by [25]. In summary, a mixture of 20 µL T. serpyllum EO and 20 µL 5-LOX from glycine max (100 U/mL) was combined with 200 µL phosphate buffer (0.1 M, pH 9), then incubated at 25°C for 6 min. Subsequently, 20 µL linoleic acid (4.18 mM) was added, and the mixture was monitored for 3 min at 234 nm. Results were presented as IC50 ± SD from three replicates, with quercetin serving as the standard compound.
2.12 Molecular Docking Protocol
The molecular docking simulation was utilized to anticipate the likely binding configurations and strengths of T. serpyllum components with precise target biomolecules [26]. We employed a computational docking technique to forecast the identified compounds’ potential antibacterial and anti-inflammatory properties. We chose specific proteins based on previous studies: dihydrofolate reductase (DHFR) enzyme (PDB ID: 4M6J) for antibacterial effects [27], 5-Lipoxygenase (PDB ID: 1N8Q) for anti-inflammatory activity [28], and NADPH oxidase (PDB ID: 2CDU) for the antioxidant activity [27].
The target proteins were prepared and labeled as macromolecules within PyRx [29]. The 3D ligand conformers in SDF format were optimized in PyRx and converted to pdbqt format using Open Babel in AutoDock Vina, selecting the best-fit structure [30].
Statistical analysis was performed in triplicate (n = 3), with results expressed as mean ± SD. Data were analyzed using GraphPad Prism 9 and XLSTAT 2016, applying one-way ANOVA followed by Tukey’s test, with significance set at p < 0.05.
The EO yield (v/w) of T. serpyllum extracted from the fresh leaves using a hydrodistillation Clevenger-type method was 2.17%. GC/MS analysis determined the plant EO’s chemical composition. Eighteen components representing 99.99% of T. serpyllum leaves EO were identified (Table 1 and Fig. 1).
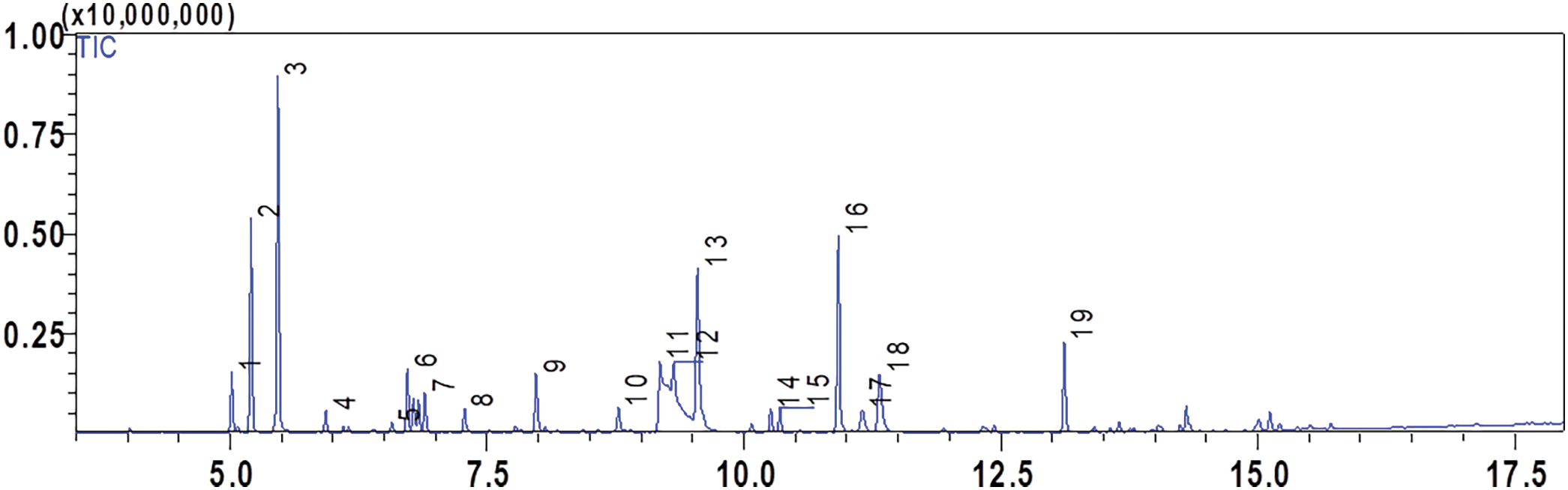
Figure 1: Total ion chromatogram (TIC) of GC-MS analysis of T. serpyllum EO
Sabinene (17.33%), terpinen-4-ol (11.73%), phellandral (13.18%), thymol (10.54%), and α-pinene (9.64%) were the principal detected molecules from T. serpyllum EO. Oxygenated monoterpenes represent the main class of compounds in the EO of T. serpyllum, accounting for 59.82%. Although oxygenated monoterpenes have been reported previously as the key components class in T. serpyllum oil, the major compounds identified in the present study from the isolated oil are different. Thymol was reported previously as the major component of T. serpyllum EO with a percent composition of (30%) [31], (38.5%) [32] and (18.8%) [33]. Meanwhile, thymol is identified in our study in a lesser amount (10.54%). Moreover, carvacrol was reported in one study as the major component (49.4%) of the EO isolated from wild thyme, and it was reported in another study among the major components of T. serpyllum EO with a percentage of (17.4%) [34]. In a study comparing the oil composition of T. serpyllum in both pre and flowering stages, carvacrol was identified with a percent composition of 1.34% and 0.4% respectively [35]. In our study, carvacrol was not detected among the oil components of T. serpyllum.
On the other hand, sabinene is identified in the present study as the major component (17.33%); however, it was reported earlier in a trace amount (0.6%) in the EO of the wild thyme. Furthermore, our results show terpinen-4-ol (11.73%) as one of the major components of the oil, while, it was identified in another study in a small amount with a percentage of 1.5% [31]. Moreover, phellandral (13.18%) is among the major component of the isolated oil and reported in our study for the first time from the T. serpyllum oil.
Additionally, although α-pinene was reported as a minor component in the previous studies with a composition of 2.4% [31], 0.6% [36], and 1.2% [33], it is identified in our study among the main components with a percent abundancy of 9.64%.
These findings show differences in EO’s chemical composition, which may be attributed to many factors. Generally, plants chemical composition is reported to differ due to the age of the plant organs, time of collection, geographical factors, and climate variation [37–39]. Moreover, the EO composition may vary due to the volatile oil extraction method, which significantly impacts the oils’ profiles [40].
In Fig. 2, the results of the disc diffusion test revealed that, among the tested bacterial and fungal strains, yeast exhibited the highest susceptibility. Candida albicans recorded 26.10 ± 1.16 mm, while Candida tropicalis recorded 23.67 ± 2.08 mm, respectively. The inhibition zones (in mm) were higher than those of the reference drug (clotrimazole), and this difference was statistically significant using the ANOVA test at p < 0.05. On the other hand, bacterial strains also exhibited remarkable and significant susceptibility to T. serpyllum EO compared to the reference drug (Chloramphenicol). Bacillus subtilis showed the highest susceptibility to T. serpyllum EO with a mean value of 20.16 ± 1.34 mm, followed by K. aerogenes (18.04 ± 0.93 mm), S. aureus (17.69 ± 1.45 mm), S. enterica (12.19 ± 1.23 mm), and E. coli (11.07 ± 2.25 mm), respectively. These results indicate that this EO has broad-spectrum antibacterial Effect. Our findings regarding the high anticandidal properties of T. serpyllum EO are of significant interest, considering the substantial challenges in treating infections triggered by yeasts of the Candida genus.
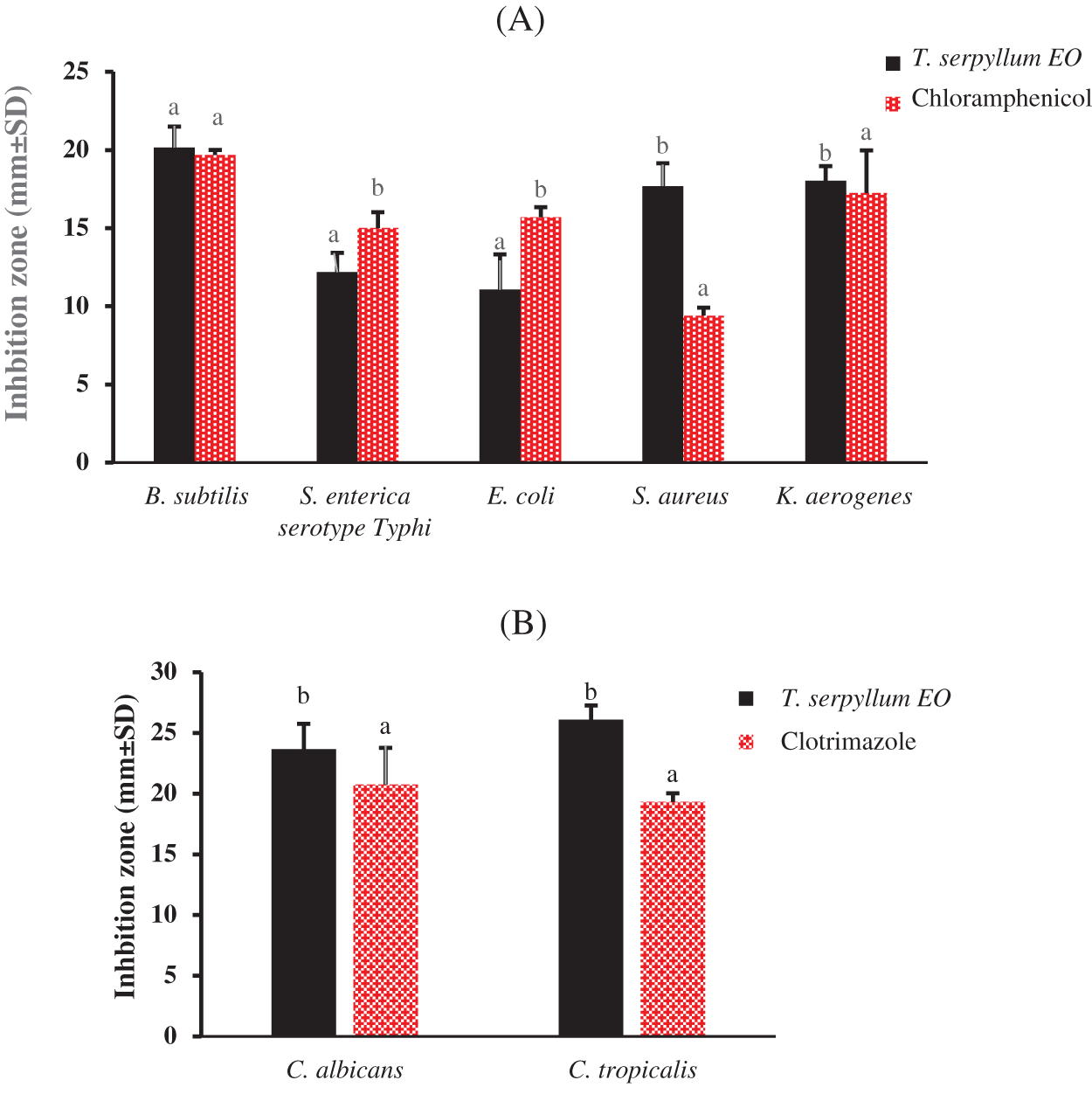
Figure 2: Antimicrobial activity of T. serpyllum EO using disc diffusion method; Data with different letters indicate no significant differences, as determined by Tukey’s multiple range test (p < 0.05). (A) inhibition zones of T. serpyllum EO and chloramphenicol against bacteria. (B) inhibition zones of T. serpyllum EO, and clotrimazole against the two Candida species
Noteworthy clinical species include C. albicans, C. tropicalis, C. auris, C. krusei, C. parapsilosis, and C. glabrata [41]. Furthermore, our results align with prior research. In a study on Moroccan T. serpyllum, strong anticandidal activity was observed against four Candida species, with inhibition zones ranging from 17.33 ± 1.15 to 12.0 ± 1.0 mm [42]. This variability was attributed to seasonal influences affecting the chemical composition and antimicrobial activities of plants [43]. Regarding antibacterial activity, our findings are consistent with existing literature. Previous reports highlighted that among three Thymus species (T. algeriensis, T. serpyllum, and T. vulgaris), T. serpyllum exhibited the highest antibacterial activity (3). Another study noted that the ethanol extract of T. serpyllum demonstrated broad-spectrum activity against S. aureus, B. subtilis, E. coli, and P. aeruginosa [44]. Historically, aromatic plants of the Thymus genus have been recognized for their antibacterial properties [45].
The antibacterial potential of T. serpyllum EO was also investigated through MIC, MBC/MFC, and by determining the tolerance levels of the tested bacteria. As shown in Table 2, the lowest MIC values were recorded by S. aureus (0.125%), followed by C. tropicalis and B. subtilis (0.25%), K. aerogenes and E. coli (0.5%), and C. albicans and S. enterica (1.0%). The MIC results of the EO were significantly higher than those of the reference drugs for bacteria and fungi. The observed variability of T. serpyllum EO in MIC values across different microorganisms suggests a nuanced response to T. serpyllum EO. Factors influencing this variability may include variations in cell wall structure, membrane permeability, or specific resistance mechanisms inherent to each microorganism [46].
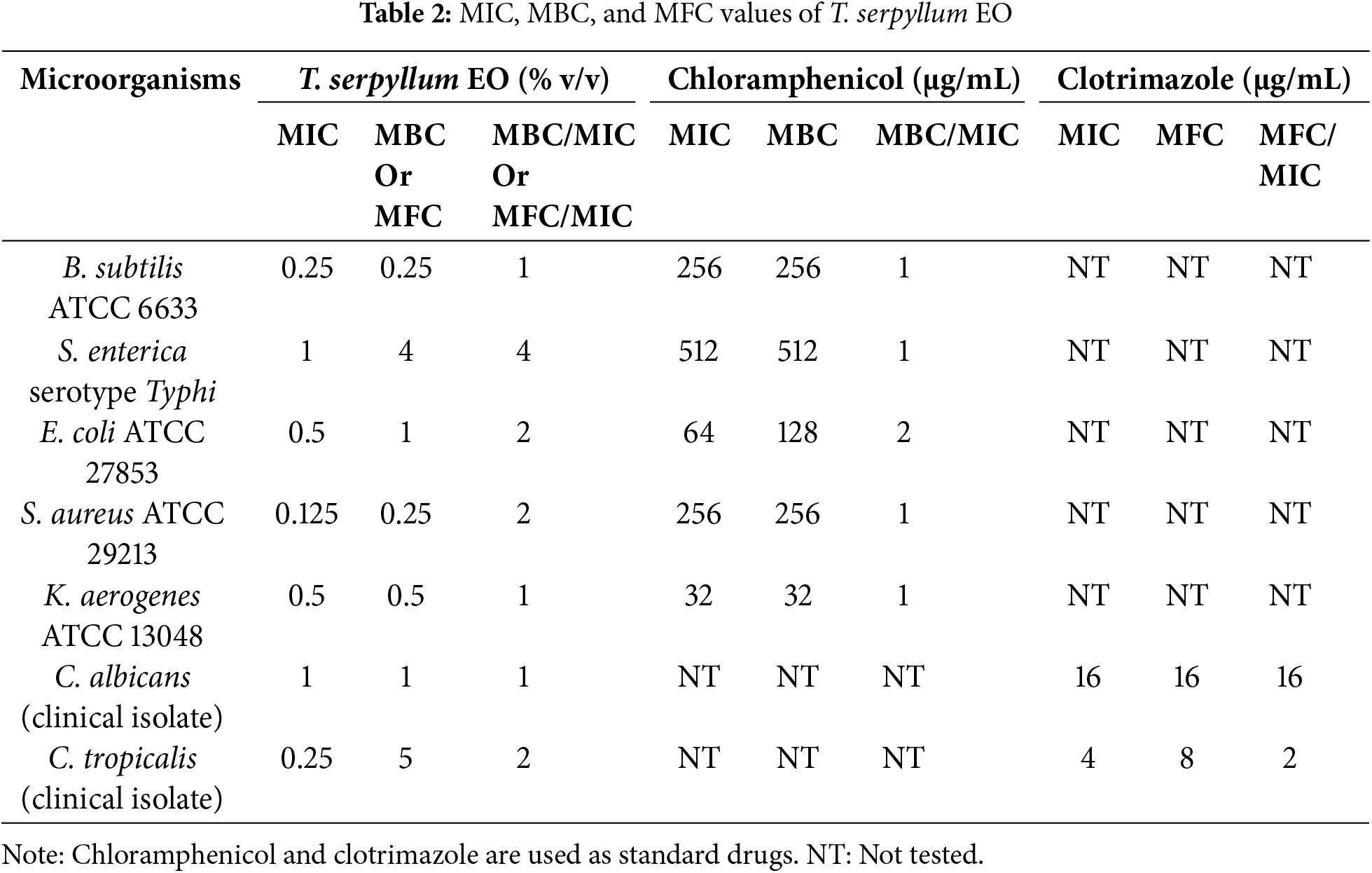
Additionally, the diverse nature of microbial species may result in distinct interactions with the EO, influencing the observed MIC values. Understanding these variations is crucial for elucidating the broad-spectrum potential of T. serpyllum EO. Furthermore, this study’s range of MIC values holds significant clinical implications. The efficacy of T. serpyllum EO, particularly against prominent pathogens like S. aureus and Candida species, suggests its potential application as an alternative or adjunctive therapeutic agent. Remarkably, the outcomes of the MBC and MFC tests revealed that T. serpyllum EO could eliminate all tested bacteria, encompassing both Gram-positive and Gram-negative strains, at concentrations ranging from 4.0% to 0.25%. In the case of Candida spp., the effective concentration spanned from 5.0% to 1.0%. Consequently, considering the tolerance level (MBC/MIC or MBC/MFC), T. serpyllum EO exhibited a bactericidal effect against the entire spectrum of tested bacteria, with MBC/MIC ratios ranging from 4.0 to 1.0. Additionally, it demonstrated a fungicidal effect specifically against C. albicans and C. tropicalis, indicated by MFC/MIC ratios of 1.0% and 2.0%, respectively. As a general rule, antimicrobial agents are classified as either bactericidal or fungicidal when the MBC/MIC and MFC/MIC ratios are 4.0 or less. In such instances, achieving concentrations of the tested agent sufficient for eliminating 99.9% of the treated organisms is considered feasible. Conversely, if these ratios exceed 4.0, administering doses of the tested agent capable of eradicating 99.9% of microorganisms may become impractical, resulting in the categorization of the agent as bacteriostatic.
Previous investigations are consistent with our study; it was reported that T. serpyllum EO was listed as potent antifungal against clinical Candida isolates with MIC values ranging from 0.49 to 3.9 μg/mL [47]. Furthermore, T. serpyllum EO exhibits antimicrobial activity against a broad spectrum of oral microbes, encompassing Streptococcus spp., Lactobacillus acidophilus, Enterococcus faecalis, P. aeruginosa, S. aureus, and Candida spp., with MIC values ranging from 1.0 to 5.0 μg/mL and MBC/MFC spanning from 2.0 to 10.0 μg/mL [45]. In additional studies, its efficacy was assessed against gram-negative bacterial isolates, such as P. aeruginosa, Salmonella, and others, revealing MIC values within the range of 0.2 to 12.5 μL/mL [45]. In conclusion, this oil has the potential to function as a natural antimicrobial for addressing a wide range of infections triggered by bacterial or fungal pathogens. It is advisable to conduct thorough research on multidrug-resistant pathogens, along with other pertinent biological studies.
The antioxidant activity of T. serpyllum EO has garnered significant interest due to its potential health benefits. Several studies have explored its antioxidant properties using different in vitro assays, shedding light on its potential applications in food preservation, pharmaceuticals, and cosmetics. Various investigations have proved the powerful antioxidant profile of T. serpyllum [48]. The obtained IC50 values agree with other study [49], which indicated T. serpyllum EO possesses strong antioxidant ability compared to other oils from Thymus species.
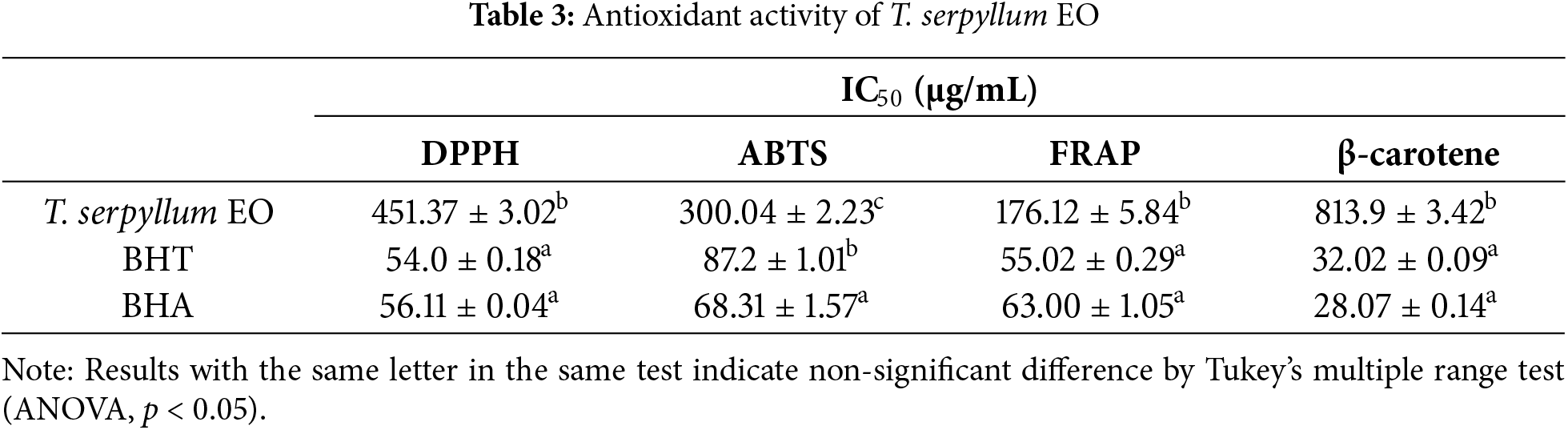
According to Fig. 3 findings, T. serpyllum EO demonstrates significant antioxidant properties compared to the standard antioxidants BHT and BHA, used as reference points (p < 0.05). T. serpyllum EO shows strong scavenging action on DPPH and ABTS radicals, with IC50 values of 451.37 ± 3.02 and 300.04 ± 2.23 µg/mL, respectively (Table 3). The results from the FRAP technique suggest that T. serpyllum EO possess valuable capacity for reducing ferric ions, with an IC50 value of 176.12 ± 5.84 µg/mL. Nevertheless, this reducing power is still less active as of the synthetic antioxidants BHT (IC50 = 55.02 ± 0.29 µg/mL) and BHA (IC50 = 63.00 ± 1.05 µg/mL). Furthermore, in the β-carotene-bleaching test, T. serpyllum EO significantly inhibits lipid peroxidation with an IC50 of 813.9 ± 3.42 µg/mL (p < 0.05).
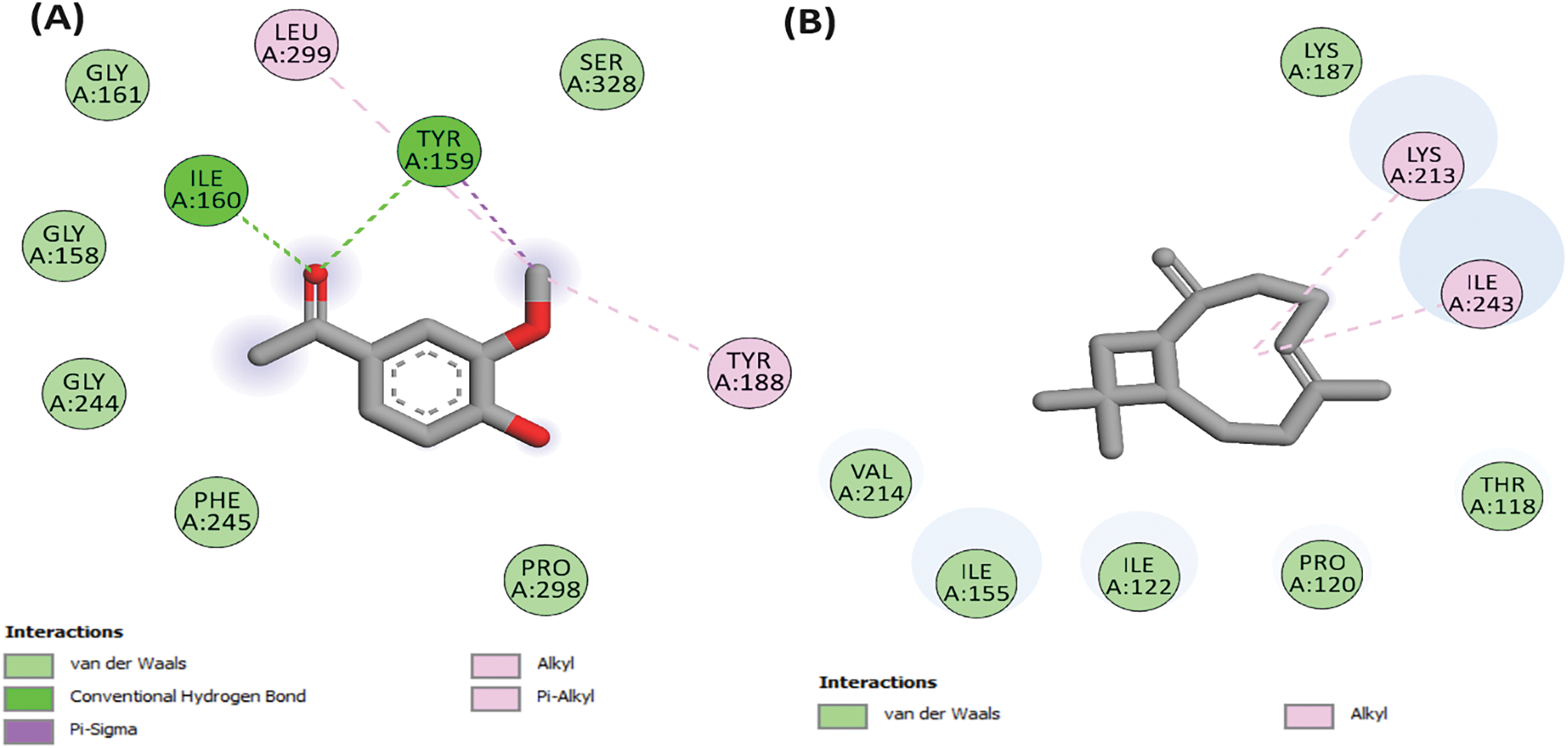
Figure 3: 2D Molecular docking interactions of apocynin (A), and caryophyllene (B), with NADPH oxidase (PDB: 2CDU)
Our results confirm those reported in the literature, including the work of [49] who indicated that T. serpyllum EO exhibited significant antioxidant activity, attributed to its high content of phenolic compounds such as thymol and carvacrol. The study by Pruteanu et al. [50] recently showed that T. serpyllum EO collected from Western Romania possesses significant antioxidant potential, with high levels of phenolic compounds contributing to its free radical scavenging ability. Interestingly, Mrkonjić and his collaborators [51] the volatile content and antioxidant properties of T. serpyllum EO obtained by different extraction methods, including hydrodistillation, soxhlet and supercritical fluid extraction (SFE). The results revealed that the supercritical fluid extraction method yielded EO with remarkable antioxidant activity, suggesting the potential of T. serpyllum as a natural antioxidant agent.
The antioxidant activity of T. serpyllum EO underscores its potential therapeutic applications in combating oxidative stress-related disorders, such as diabetes, inflammation and cancer preserving the quality of food and cosmetic products. However, it is crucial to consider factors such as extraction method, geographical origin, and storage conditions, which can influence the antioxidant potency of the EO.
3.4 Anti-Inflammatory Activity
Inflammation is a multifaceted biological reaction triggered within the body as a protective response to harmful stimuli, including pathogens, toxins, or tissue damage. While inflammation plays a vital role in the immune system’s defense mechanism, persistent inflammation can foster the onset of diverse ailments, encompassing cardiovascular disease, cancer, and autoimmune disorders [52,53]. One significant aspect of inflammation is the implication of lipoxygenases (LOX), enzymes that play a crucial role in producing lipid mediators called leukotrienes. These leukotrienes are involved in various inflammatory processes, including immune cell recruitment, vascular permeability, and tissue damage [54,55].
LOX enzymes catalyze the oxidation of polyunsaturated fatty acids, such as arachidonic acid, leading to the formation of leukotrienes. These lipid mediators act as signaling molecules, triggering inflammatory responses by binding to specific receptors on immune cells and other target cells. Understanding the role of LOX in inflammation offers valuable insights into the mechanisms underlying inflammatory diseases and may lead to the elaboration of innovative therapeutic approaches targeting these enzymes [56]. Inhibitors of LOX activity is being investigated as potential anti-inflammatory agents, aiming to modulate inflammatory responses and reduce tissue damage associated with chronic inflammation.
Natural compounds sourced from plants, especially EOs have garnered attention for their anti-inflammatory attributes. These compounds typically influence pivotal inflammatory pathways and mediators in the body.
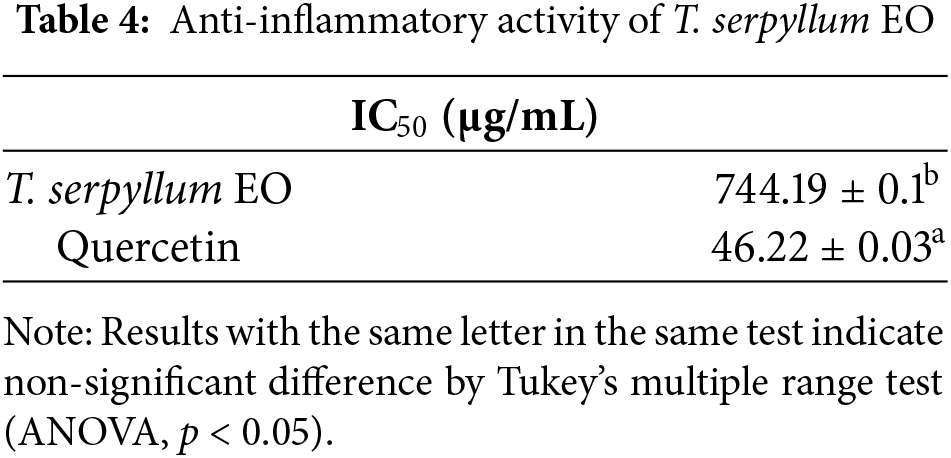
In this investigation, T. serpyllum EO has shown remarkable inhibition on the 5-LOX enzyme, with an IC50 value of 744.19 ± 0.1 μg/mL. This effect was less effective than the standard drug quercetin, which had an IC50 value of 46.22 ± 0.03 μg/mL (Table 4).
Although T. serpyllum EO is mainly renowned for its antimicrobial and antioxidant properties, emerging evidence suggests its potential anti-inflammatory effects. Indeed, T. serpyllum EO contains high levels of the compounds sabinene, thymol, and carvacrol, which have been shown to possess effective anti-inflammatory activity. These compounds have been shown to inhibit inflammatory pathways such as NF-kappaB and MAPK signaling, which play key roles in regulating the expression of pro-inflammatory genes [57–60].
Additionally, T. serpyllum EO has exhibited antimicrobial and anti-biofilm properties, which can help reduce inflammation by inhibiting pathogenic microorganisms. The oil has shown potent activity against bacteria like P. aeruginosa, Salmonella, and biofilm-forming B. subtilis [33,61].
Conversely, T. serpyllum extract has been found to protect against inflammatory states. Studies have found that T. serpyllum aqueous extracts can inhibit inflammatory responses and modulate gut dysbiosis (imbalance of gut microbiome) [62]. The antioxidant activity of the T. serpyllum extract reduced inflammation markers and improved the gut microbiome composition in experimental models of colitis [62].
To understand how the T. serpyllum EO affects pharmacological activities, we employed computational methods to conduct molecular docking of its bioactive components with corresponding molecular receptors.
The strength of binding is indicated by the numerical value of the binding affinity (kcal/mol), with higher values indicating weaker binding. Our docking predictions revealed a high level of accuracy, demonstrating an expected binding affinity with a mean square deviation of zero [63].
In this investigation, we utilized a methodology to evaluate the binding affinities of 19 compounds found in the essential oil. These compounds were assessed against three proteins associated with the two investigated biological activities: dihydrofolate reductase (DHFR) enzyme (PDB ID: 4M6J) for antibacterial effects [27], 5-Lipoxygenase (PDB ID: 1N8Q) for anti-inflammatory activity [28] and NADPH oxidase (PDB ID: 2CDU) for the antioxidant activity [64]. The findings of the molecular docking analyses are visually represented systematically, offering a graphical approach that significantly aids in identifying chemical compounds capable of inhibiting specific biological targets. This graphical depiction facilitates an intuitive visual examination of interactions between the tested molecules and the active sites of the target proteins.
3.6 Antioxidant Activity: Interactions with NADPH Oxidase (PDB: 2CDU)
NADPH oxidase, a multi-subunit enzyme complex primarily found in phagocytes, plays a pivotal role in generating reactive oxygen species (ROS) as part of the immune response [65]. While ROS serve important functions in cellular signaling and host defense, excessive production due to dysregulation of NADPH oxidase can lead to oxidative stress [66]. This oxidative stress contributes to various pathological conditions, including inflammation, neurodegenerative diseases, and cardiovascular disorders [52]. Consequently, inhibition of NADPH oxidase is a promising therapeutic strategy to mitigate oxidative damage and alleviate associated diseases [67]. By targeting this enzyme, researchers aim to regulate ROS levels effectively, thereby preventing the detrimental effects of oxidative stress on cellular components and overall tissue homeostasis [67]. The protein structure, identified by its PDB UD 2CDU, was acquired from the RCSB Protein Data Bank and represents the Crystal Structure of NADPH Oxidase from Lactobacillus sanfranciscensis [68].
In this investigation, eight molecules were found to have comparable binding affinity values in comparison with apocynin, a natural compound derived from the roots of certain plants, notably the Picrorhiza kurroa and Apocynum cannabinum species, well-known for its ability to inhibit the activity of NADPH oxidase, an enzyme complex involved in the production of reactive oxygen species (ROS) within cells [69]. These molecules are caryophyllene, linalyl anthranilate, 2-caren-10-al, p-menth-1-en-4-ol, (+)-4-carene, p-cumic aldehyde, β-cymene, phellandral, with docking scores of −7.2, −6.6, −6.1, −5.9, −5.8, −5.8, −5.7, and −5.7 kcal/mol, respectively. Caryophyllene, a naturally occurring sesquiterpene abundant in various plants and renowned for its characteristic spicy, woody aroma and taste, exhibited a remarkable docking score of −7.2 kcal/mol. Apocynin was used as a reference; its docking score was found to be −5.7 kcal/mol), suggesting that caryophyllene from our study as a more potent inhibitor of caryophyllene (Fig. 4). Moreover, the molecular interaction analysis revealed intriguing insights into the mode of action of caryophyllene. While apocynin demonstrated binding interactions via two hydrogen bonds with specific amino acid residues within the protein’s binding pocket of the NADPH oxidase enzyme, namely TYR A: 159 and ILE A: 160 (Fig. 3), caryophyllene, intriguingly, did not engage in any hydrogen bond formation with the amino acid residues from the active site.
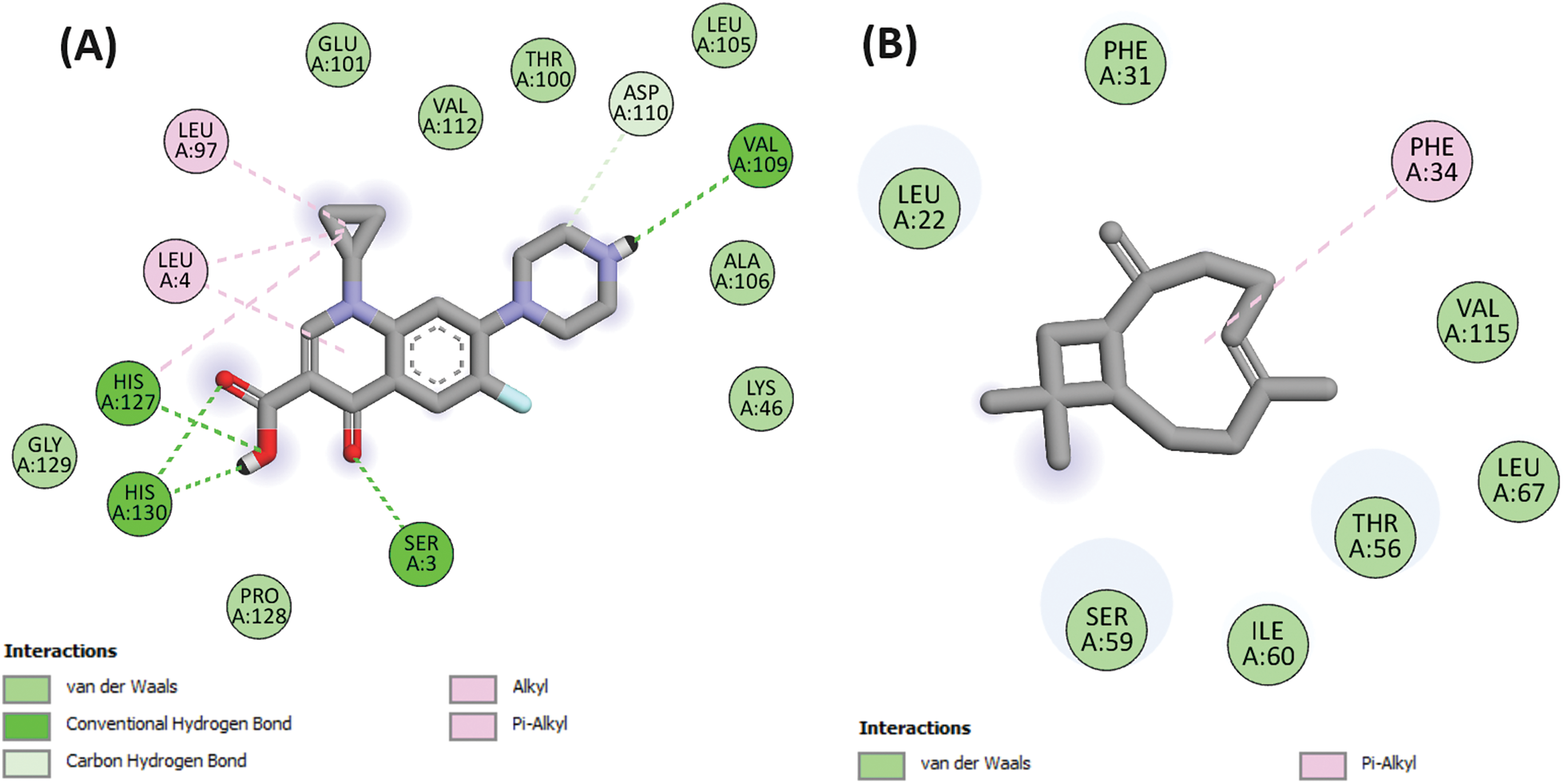
Figure 4: 2D Molecular docking interactions of ciprofloxacin (A), and caryophyllene (B), with dihydrofolate reductase (PDB: 4M6J)
3.7 Antibacterial Activity: Interactions with Dihydrofolate Reductase (PDB: 4M6J)
Dihydrofolate reductase (DHFR) is a crucial enzyme involved in synthesizing tetrahydrofolate, which is essential for producing purines, pyrimidines, and certain amino acids [70]. Inhibiting DHFR has been a primary objective in developing antibacterial, antifungal, anti-tuberculosis, and anticancer drugs, as disrupting this pathway can hinder cell replication and growth [71,72].
The protein’s structure, identified by its PDB ID 4M6J, was obtained from the RCSB Protein Data Bank and represents the crystallographic depiction of DHFR from Staphylococcus aureus [73]. This structural understanding presents a promising avenue for potential antibacterial therapeutic development, particularly against S. aureus, a virulent pathogenic bacterium implicated in various infections, including skin and soft tissue disorders and bone infections [73]. Targeting DHFR is strategically significant as inhibiting this enzyme disrupts tetrahydrofolate biosynthesis, depleting crucial folate coenzymes necessary for nucleic acid (DNA and RNA) and specific amino acid synthesis [74]. Consequently, DHFR represents an attractive target for advancing antibacterial drugs, effectively impeding bacterial growth and proliferation [75].
Caryophyllene exhibited a remarkable docking score of −8 kcal/mol in this investigation. This score, comparable to that of ciprofloxacin, a widely-used broad-spectrum antibiotic belonging to the fluoroquinolones class, suggests the potential of caryophyllene as a potent antibacterial agent within the essential oil composition (Fig. 4).
Moreover, the molecular interaction analysis revealed intriguing insights into the mode of action of caryophyllene. While ciprofloxacin demonstrated binding interactions via three hydrogen bonds with specific amino acid residues within the protein’s binding pocket, namely SER A:3, VAL A:109, and HIS A:130 (Fig. 4), caryophyllene, intriguingly, did not engage in any hydrogen bond formation with the amino acid residues from the active site. This observation suggests a potentially unique mechanism of action for caryophyllene compared to traditional antibiotics like ciprofloxacin. Instead of directly forming hydrogen bonds with the target protein, caryophyllene may exert its antibacterial effects through alternative mechanisms such as membrane disruption, interference with bacterial cell signaling pathways, or modulation of host immune responses. Further elucidation of these mechanisms could unveil novel strategies for combating bacterial infections and potentially reduce the risk of antibiotic resistance development.
3.8 Anti-Inflammatory Activity: Interactions with 5-LOX (PDB: 1N8Q)
Lipoxygenases (LOXs) are widely dispersed across both the plant and animal kingdoms, representing a diverse array of enzymes [76]. Their primary function revolves around catalyzing reactions on polyunsaturated fatty acids (PUFA) with cis double bonds, with the 20-carbon arachidonic acid (AA) being a prominent substrate in animals [76]. LOX enzymes are named after the specific carbon they oxygenate, exemplified by 9-LOX and 13-LOX in plants, and 5-LOX, 12-LOX, and 15-LOX in animals [77]. Nonetheless, the reactions catalyzed by LOXs may also yield undesirable outcomes [55]. For instance, excessive activation of 5-LOX in humans leads to heightened leukotrienes (LTs) levels, thereby instigating inflammation and associated conditions such as bronchoconstriction.
In this study, 14 compounds had potent theoretical inhibitory potential against lipoxygenase (Fig. 5), with binding affinity values ranging from −5.9 to −7.5 kcal/mol (see Fig. 6). Linalyl anthranilate, a commonly utilized chemical compound in the fragrance industry, which is classified as an ester and is derived from linalool and anthranilic acid, was found to have the lowest binding affinity value of −7.5 kcal/mol among the compounds identified in TSEO, in comparison with protocatechuic acid (−5.9 kcal/mol), a recognized inhibitor of lipoxygenases.
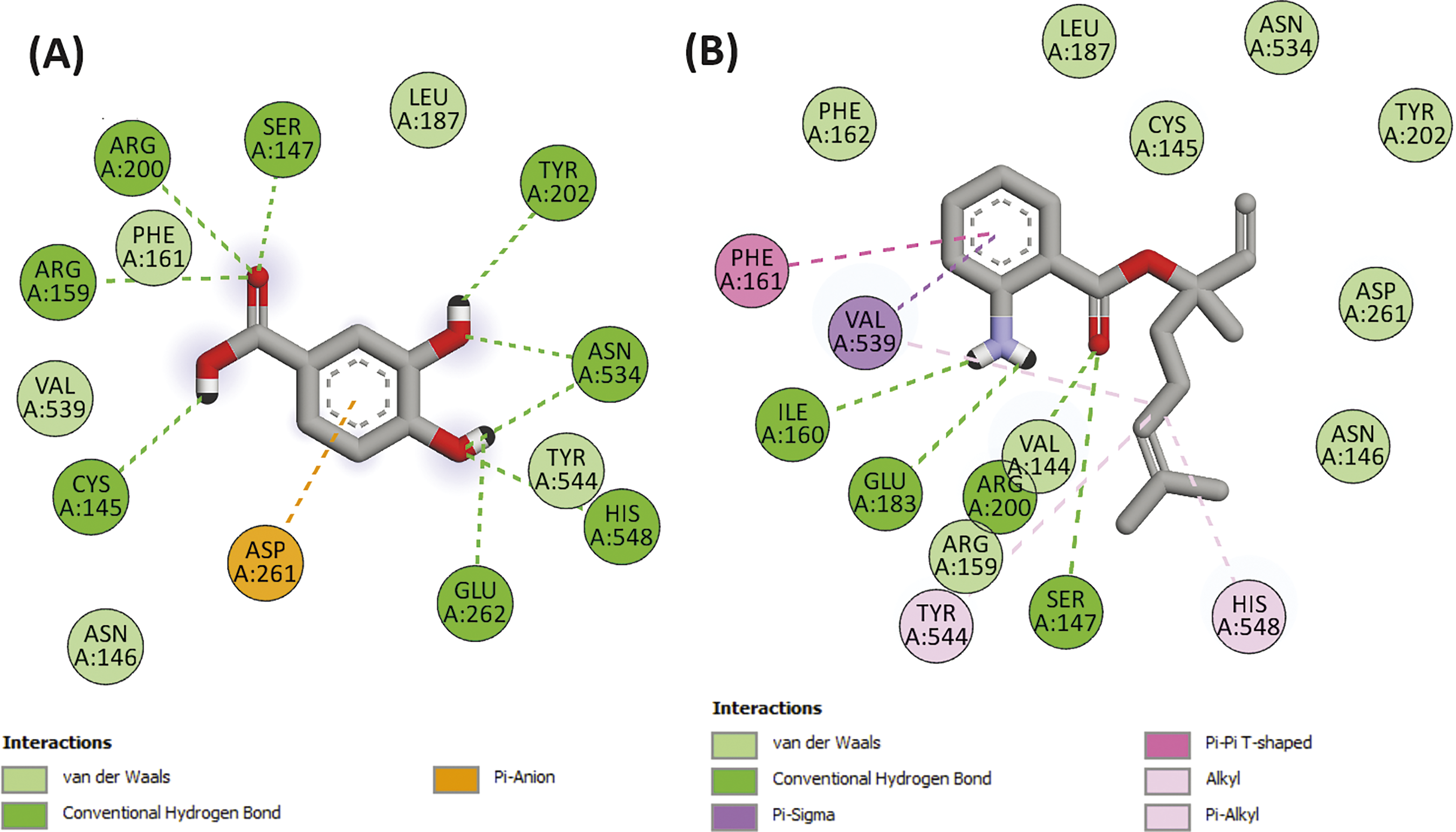
Figure 5: 2D Molecular docking interactions of protocatechuic acid ((A), native ligand of 5-LOX), and linalyl anthranilate (B), with 5-lipoxygenase (PDB: 1N8Q)
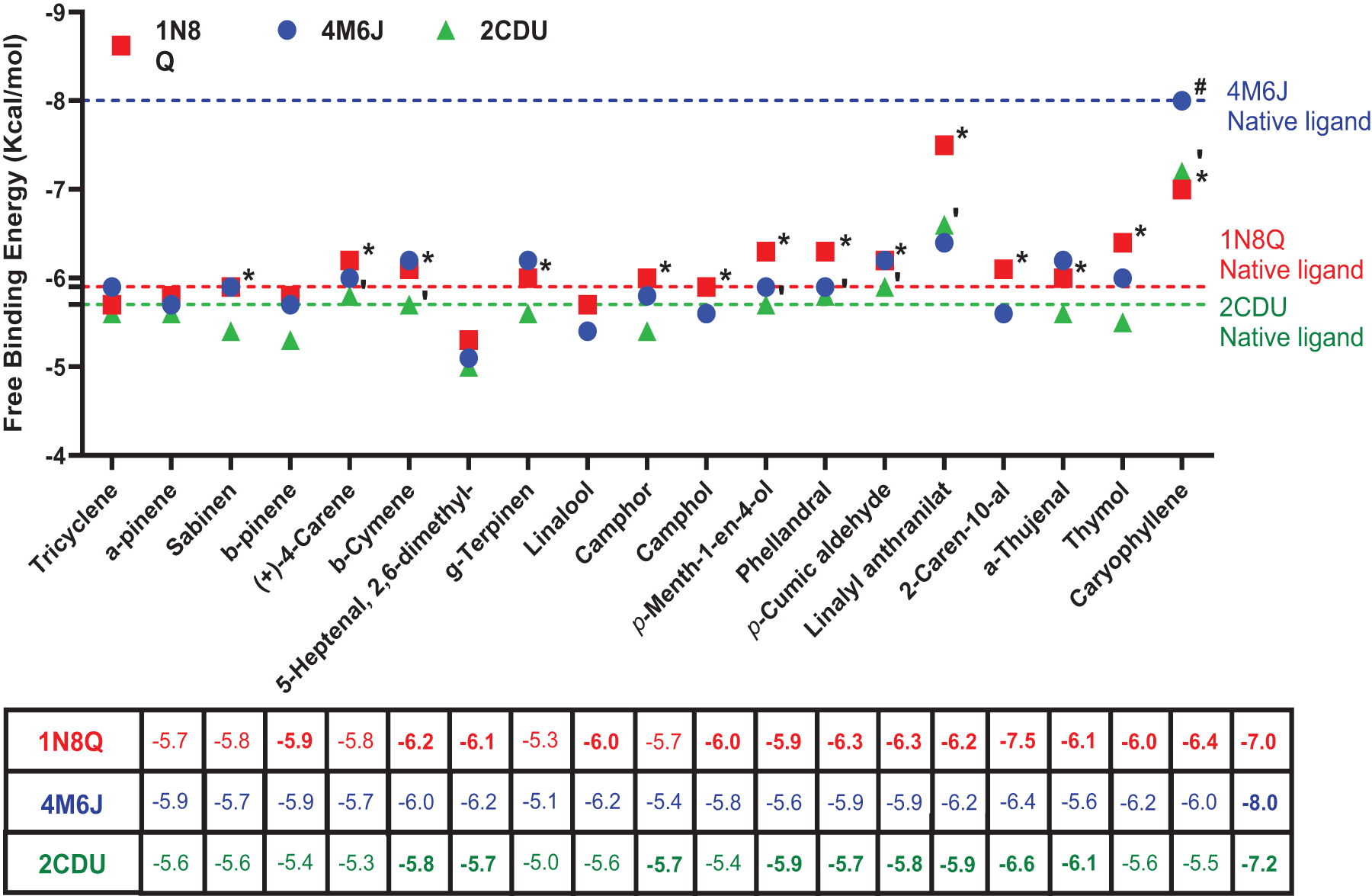
Figure 6: Free binding energy values (kcal/mol) of the 19 compounds identified in TSEO. Dihydrofolate reductase (PDB ID: 4M6J) inhibitor: Ciprofloxacin; Lipoxygenase (PDB ID: 1N8Q) native ligand: protocatechuic acid; NADPH oxidase (PDB ID: 2CDU): apocynin. * indicates the potent ligands against lipoxygenase; # indicates the potent ligand against dihydrofolate reductase; indicates potent ligands against NADPH oxidase
The interaction of linalyl anthranilate with the protein involved the formation of four hydrogen bonds with specific amino acid residues from the binding site, namely SER A:147, ILE A:160, GLU A:183, and ARG A:200. It is noteworthy that when compared to the native ligand, we observed that the native ligand formed seven hydrogen bonds, as depicted in Fig. 3. These findings highlight the potential of linalyl anthranilate as a potent inhibitor of the studied protein. Moreover, the diverse nature of their binding interactions underscores their promise as a candidate for further exploration in developing novel therapeutic agents.
Based on the analysis of the chemical composition and biological activity of T. serpyllum EO, it can be concluded that the oil possesses a diverse array of chemical constituents and exhibits significant biological effects. The oil’s composition includes various compounds known for their therapeutic properties, suggesting its potential for medicinal use. Furthermore, the biological activities demonstrated by T. serpyllum EO, such as antimicrobial and antioxidant effects, indicate its potential applications in various fields, including pharmaceuticals, cosmetics, and food preservation. However, more research is warranted to fully elucidate its mechanisms of action and explore its potential therapeutic benefits in clinical settings. Moreover, although preliminary evidence suggests that T. serpyllum EO may possess promising anti-inflammatory effects, further preclinical and clinical investigation is needed to fully understand its anti-inflammatory properties and potential therapeutic applications in humans.
Acknowledgement: Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R158), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study concept and design: Hanae Naceiri Mrabti and Naoufal El Hachlafi; data acquisition: Samiah Hamad Al-Mijalli, Hanae Naceiri Mrabti and Amine Elbouzidi; methodology: Samiah Hamad Al-Mijalli, Naglaa S. Ashmawy, Emad M. Abdallah, Mohammed Aladhadh and Naoufal El Hachlafi; software: Fahad M. Alshabrmi and Sulaiman Mohammed Alnasser; analysis and interpretation of data: Hanae Naceiri Mrabti, Wafa Laaboudi, Amine Elbouzidi, Emad M. Abdallah and Samiah Hamad Al-Mijalli; drafting of the manuscript: Naoufal El Hachlafi, Amine Batbat; critical revision of the manuscript: Hanae Naceiri Mrabti; supervision: Hanae Naceiri Mrabti, Mohamed Addi and Naoufal El Hachlafi. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Pandey M, Debnath M, Gupta S, Chikara SK. Phytomedicine: an ancient approach turning into future potential source of therapeutics. J Pharmacogn Phytother. 2011;3:27–37. [Google Scholar]
2. Benkhaira N, Zouine N, Fadil M, Koraichi SI, Hachlafi NE, Jeddi M, et al. Application of mixture design for the optimum antibacterial action of chemically-analyzed essential oils and investigation of the antiadhesion ability of their optimal mixtures on 3D printing material. Bioprinting. 2023;34:e00299. doi:10.1016/j.bprint.2023.e00299. [Google Scholar] [CrossRef]
3. Batbat A, Habbadi K, El Hachlafi N, El Allaoui N, Yahyaoui H, Ferioun M, et al. Unveiling germination conditions for Origanum elongatum: strategic insights into temperature, salinity, and pH for agricultural propagation and conservation management. Ecol Front. 2024. doi:10.1016/j.ecofro.2024.10.015. [Google Scholar] [CrossRef]
4. Bouyahya A, Bakrim S, Chamkhi I, Taha D, El Omari N, El Mneyiy N, et al. Bioactive substances of cyanobacteria and microalgae: sources, metabolism, and anticancer mechanism insights. Biomedicine Pharmacotherapy. 2024;170:115989. doi:10.1016/j.biopha.2023.115989. [Google Scholar] [PubMed] [CrossRef]
5. Cheminal A, Kokkoris IP, Strid A, Dimopoulos P. Medicinal and aromatic Lamiaceae plants in Greece: linking diversity and distribution patterns with ecosystem services. Forests. 2020;11:661. doi:10.12735/AS.V4I1P09. [Google Scholar] [CrossRef]
6. Boren K, Crown A, Carlson R. Multidrug and pan-antibiotic resistance—the role of antimicrobial and synergistic essential oils: a review. Nat Prod Commun. 2020;15:1934578X20962595. doi:10.1177/1934578x20962595. [Google Scholar] [CrossRef]
7. Castro-Sánchez E, Moore LS, Husson F, Holmes AH. What are the factors driving antimicrobial resistance? Perspectives from a public event in London. England BMC Infect Dis. 2016;16:1–5. doi:10.1186/s12879-016-1810-x. [Google Scholar] [PubMed] [CrossRef]
8. Ouedrhiri W, Mechchate H, Moja S, Baudino S, Saleh A, Al Kamaly OM, et al. Optimized antibacterial effects in a designed mixture of essential oils of myrtus communis, artemisia herba-alba and thymus serpyllum for wide range of applications. Foods. 2022;11:132. doi:10.3390/foods11010132. [Google Scholar] [PubMed] [CrossRef]
9. Jeddi M, El Hachlafi N, El Fadili M, Benkhaira N, Al-Mijalli SH, Kandsi F, et al. Antimicrobial, antioxidant, α-amylase and α-glucosidase inhibitory activities of a chemically characterized essential oil from Lavandula angustifolia Mill.: in vitro and in silico investigations. Biochem Syst Ecol. 2023;111:104731. doi:10.1016/j.bse.2023.104731. [Google Scholar] [CrossRef]
10. Abebe GM. The role of bacterial biofilm in antibiotic resistance and food contamination. Int J Microbiol. 2020;2020(1):1705814. doi:10.1155/2020/1705814. [Google Scholar] [PubMed] [CrossRef]
11. Savoldi A, Carrara E, Gladstone BP, Azzini AM, Göpel S, Tacconelli E. Gross national income and antibiotic resistance in invasive isolates: analysis of the top-ranked antibiotic-resistant bacteria on the 2017 WHO priority list. J Antimicrob Chemother. 2019;74:3619–25. doi:10.1093/jac/dkz381. [Google Scholar] [PubMed] [CrossRef]
12. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol. 2008;46:446–75. doi:10.1016/j.fct.2007.09.106. [Google Scholar] [PubMed] [CrossRef]
13. Calo JR, Crandall PG, O’Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems—a review. Food control. 2015;54:111–9. doi:10.1016/j.foodcont.2014.12.040. [Google Scholar] [CrossRef]
14. Seow YX, Yeo CR, Chung HL, Yuk H-G. Plant essential oils as active antimicrobial agents. Crit Rev Food Sci Nutr. 2014;54:625–44. doi:10.1080/10408398.2011.599504. [Google Scholar] [PubMed] [CrossRef]
15. Tohidi B, Rahimmalek M, Arzani A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017;220:153–61. doi:10.1016/j.foodchem.2016.09.203. [Google Scholar] [PubMed] [CrossRef]
16. Jarić S, Mitrović M, Pavlović P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid Based Complement Alternat Med. 2015;1:101978. doi:10.1155/2015/101978. [Google Scholar] [PubMed] [CrossRef]
17. Ayoub Z, Mehta A. Medicinal plants as potential source of antioxidant agents: a review. Asian J Pharm Clin Res. 2018;11:50–6. doi:10.22159/ajpcr.2018.v11i6.24725. [Google Scholar] [CrossRef]
18. Kandsi F, Elbouzidi A, Lafdil FZ, Meskali N, Azghar A, Addi M, et al. Antibacterial and antioxidant activity of Dysphania ambrosioides (L.) Mosyakin and clemants essential oils: experimental and computational approaches. Antibiotics. 2022;11:482. doi:10.3390/antibiotics11040482. [Google Scholar] [PubMed] [CrossRef]
19. Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. TX, USA: Texensis Publishing; 2017. [Google Scholar]
20. Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. USA: Allured Publishing Corporation; 2001. p. 175–469. [Google Scholar]
21. Benouaklil F, Hamaidi-Chergui F, Hamaidi MS, Saidi F. Chemical composition and antimicrobial properties of Algerian Cedrus atlantica M. essential oils. Revue Agrobiologia. 2017;7:355–62. doi:10.3390/pharmaceutics14081608. [Google Scholar] [PubMed] [CrossRef]
22. Nouioura G, El Hachlafi N, Abuelizz HA, Elidrissi AE, Ferioun M, Soulo N, et al. Petroselinum crispum L., essential oil as promising source of bioactive compounds, antioxidant, antimicrobial activities: in vitro and in silico predictions. Heliyon. 2024;10(8):e29520. doi:10.1016/j.heliyon.2024.e29520. [Google Scholar] [PubMed] [CrossRef]
23. Chebbac K, Moussaoui AE, Bourhia M, Salamatullah AM, Alzahrani A, Guemmouh R. Chemical analysis and antioxidant and antimicrobial activity of essential oils from Artemisia negrei L. against drug-resistant microbes. Evid-Based Complement Altern Med. 2021;2021:1–9. doi:10.1155/2021/5902851. [Google Scholar] [PubMed] [CrossRef]
24. Mssillou I, Agour A, El Ghouizi A, Hamamouch N, Lyoussi B, Derwich E. Chemical composition, antioxidant activity, and antifungal effects of essential oil from Laurus nobilis L. flowers growing in Morocco. J Food Qual. 2020;2020:1–8. doi:10.1155/2020/8819311. [Google Scholar] [CrossRef]
25. El Hachlafi N, Mrabti HN, Al-Mijalli SH, Jeddi M, Abdallah EM, Benkhaira N, et al. Antioxidant, Volatile compounds; antimicrobial, anti-inflammatory, and dermatoprotective properties of Cedrus atlantica (Endl.) manetti ex carriere essential oil: in vitro and in silico investigations. Molecules. 2023;28:5913. doi:10.3390/molecules28155913. [Google Scholar] [PubMed] [CrossRef]
26. Elbouzidi A, Taibi M, Laaraj S, Loukili EH, Haddou M, El Hachlafi N, et al. Chemical profiling of volatile compounds of the essential oil of grey-leaved rockrose (Cistus albidus L.) and its antioxidant, anti-inflammatory, antibacterial, antifungal, and anticancer activity in vitro and in silico. Front Chem. 2024;12:1334028. doi:10.3389/fchem.2024.1334028. [Google Scholar] [CrossRef]
27. Khatun MCS, Muhit MA, Hossain MJ, Al-Mansur MA, Rahman SMA. Isolation of phytochemical constituents from Stevia rebaudiana (Bert.) and evaluation of their anticancer, antimicrobial and antioxidant properties via in vitro and in silico approaches. Heliyon. 2021;7(12):e08475. doi:10.1016/j.heliyon.2021.e08475. [Google Scholar] [PubMed] [CrossRef]
28. Tomy MJ, Sharanya CS, Mahapatra DK, Suresh KI, Sabu A, Haridas M. In vitro assessment of selected benzoic acid derivatives as anti-inflammatory compounds. J Scientific Ind Res. 2018;77(6):330–6. [Google Scholar]
29. Pawar RP, Rohane SH. Role of autodock vina in PyRx molecular docking. Asian J Res Chem. 2021;14(2):132–4. doi:10.5958/0974-4150.2021.00024.9. [Google Scholar] [CrossRef]
30. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:1–14. doi:10.1186/1758-2946-3-33. [Google Scholar] [PubMed] [CrossRef]
31. Raal A, Paaver U, Arak E, Orav A. Content and composition of the essential oil of Thymus serpyllum L. growing wild in Estonia. Medicina. 2004;40:795–800. doi:10.4236/fns.2018.95034. [Google Scholar] [CrossRef]
32. Nikolić M, Glamočlija J, Ferreira IC, Calhelha RC, Fernandes Â., Marković T, et al. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind Crops Prod. 2014;52:183–90. doi:10.1016/j.indcrop.2013.10.006. [Google Scholar] [CrossRef]
33. Galovičová L, Borotová P, Valková V, Vukovic NL, Vukic M, Terentjeva M, et al. Thymus serpyllum essential oil and its biological activity as a modern food preserver. Plants. 2021;10:1416. doi:10.3390/molecules29245826. [Google Scholar] [CrossRef]
34. Aitbekov R, Zhamanbayeva G, Aralbaeva A, Zhunussova G, Zhumina A, Zhusupova A, et al. Pharmacological composition of Thymus serpyllum and its components. ES Food Agrofor. 2024;17:1244. doi:10.30919/esfaf1244. [Google Scholar] [CrossRef]
35. Rasooli I, Mirmostafa SA. Antibacterial properties of Thymus pubescens and Thymus serpyllum essential oils. Fitoterapia. 2002;73:244–50. doi:10.1016/S0367-326X(02)00064-3. [Google Scholar] [PubMed] [CrossRef]
36. Kulisic T, Radonic A, Milos M. Antioxidant properties of thyme (Thymus vulgaris L.) and wild thyme (Thymus serpyllum L.) essential oils. Italian J Food Sci. 2005;17:315. [Google Scholar]
37. Pokajewicz K, Białoń M, Svydenko L, Fedin R, Hudz N. Chemical composition of the essential oil of the new cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules. 2021;26:5681. doi:10.3390/molecules26185681. [Google Scholar] [PubMed] [CrossRef]
38. Moumni M, Romanazzi G, Najar B, Pistelli L, Ben Amara H, Mezrioui K, et al. Antifungal activity and chemical composition of seven essential oils to control the main seedborne fungi of cucurbits. Antibiotics. 2021;10:104. doi:10.3390/antibiotics10020104. [Google Scholar] [PubMed] [CrossRef]
39. Youssef FS, Eid SY, Alshammari E, Ashour ML, Wink M, El-Readi MZ. Chrysanthemum indicum and Chrysanthemum morifolium: chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities. Foods. 2020;9:1460. doi:10.3390/foods9101460. [Google Scholar] [PubMed] [CrossRef]
40. Benkhaira N, El Hachlafi N, Jeddi M, Abdnim R, Bnouham M, Koraichi SI, et al. Unveiling the phytochemical profile, in vitro bioactivities evaluation, in silico molecular docking and ADMET study of essential oil from Clinopodium nepeta grown in Middle Atlas of Morocco. Biocatal Agri Biotechnol. 2023;54:102923. doi:10.1016/j.bcab.2023.102923. [Google Scholar] [CrossRef]
41. Malinovská Z, Čonková E, Váczi P. Biofilm formation in medically important Candida species. J Fungi. 2023;9:955. doi:10.3390/jof9100955. [Google Scholar] [PubMed] [CrossRef]
42. Jamali CA, El Bouzidi L, Bekkouche K, Lahcen H, Markouk M, Wohlmuth H, et al. Chemical composition and antioxidant and anticandidal activities of essential oils from different wild Moroccan Thymus species. Chem Biodivers. 2012;9:1188–97. doi:10.1002/cbdv.201200041. [Google Scholar] [PubMed] [CrossRef]
43. Assaggaf H, Jeddi M, Mrabti HN, Ez-Zoubi A, Qasem A, Attar A, et al. Design of three-component essential oil extract mixture from Cymbopogon flexuosus, Carum carvi, and Acorus calamus with enhanced antioxidant activity. Sci Rep. 2024;14:9195. doi:10.1038/s41598-024-59708-x. [Google Scholar] [PubMed] [CrossRef]
44. Gahlot K, Kumar S, Sahu R. Evaluation of antibacterial activity of aerial parts of Thymus serpyllum Linn. J Pharm Res. 2011;4:641–2. doi:10.1155/2015/265425. [Google Scholar] [PubMed] [CrossRef]
45. Nabavi SM, Marchese A, Izadi M, Curti V, Daglia M, Nabavi SF. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015;173:339–47. doi:10.1016/j.foodchem.2014.10.042. [Google Scholar] [PubMed] [CrossRef]
46. Van de Vel E, Sampers I, Raes K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit Rev Food Sci Nutr. 2019;59:357–78. doi:10.1080/10408398.2017.1371112. [Google Scholar] [PubMed] [CrossRef]
47. Shapoval OG, Sheremetyeva AS, Dumova NA, Mukhamadiev NQ, Rabbimova GT, Nazirbekov MH. Antimicrobial activity of Thymus serpyllum L. and Thymus marschallianus Willd. Essential oils against candida albicans. Pharm Chem J. 2023:1–5. doi:10.4315/0362-028x-62.9.1017. [Google Scholar] [PubMed] [CrossRef]
48. Stanisavljević D, Stojičević S, Karabegović I, Đorđević S, Veličković D, Lazić M. Antioxidant activity of the essential oils of five species of the family Lamiaceae. Planta Med. 2011;77:34. doi:10.1055/s-0031-1282365. [Google Scholar] [CrossRef]
49. Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–53. doi:10.1016/j.ijfoodmicro.2004.03.022. [Google Scholar] [PubMed] [CrossRef]
50. Pruteanu A, Popescu C, Vladut V, Gageanu G. Biochemical analysis of some vegetal extracts obtained from indigenous spontaneous species of (Thymus serpyllum L.). Romanian Biotechnol Letters. 2018;23:14013–24. doi:10.25083/rbl/26.3/2594.2605. [Google Scholar] [CrossRef]
51. Mrkonjić Ž., Kaplan M, Milošević S, Božović D, Sknepnek A, Miletić D, et al. Green extraction approach for isolation of bioactive compounds in wild thyme (Thymus serpyllum L.) Herbal dust—chemical profile, antioxidant and antimicrobial activity and comparison with conventional techniques. Plants. 2024;13:897. doi:10.3390/plants13060897. [Google Scholar] [PubMed] [CrossRef]
52. Chatterjee S. Oxidative stress, inflammation, and disease. In: Oxidative stress and biomaterials. Elsevier; 2016. p. 35–58. [Google Scholar]
53. Ouadja B, Katawa G, Toudji GA, Layland L, Gbekley EH, Ritter M, et al. Anti-inflammatory, antibacterial and antioxidant activities of Chenopodium ambrosioides L.(Chenopodiaceae) extracts. J Appl Biosci. 2021;162:16764–94. doi:10.35759/JABs.162.7. [Google Scholar] [CrossRef]
54. Drikvandi P, Bahramikia S, Alirezaei M. Modulation of the antioxidant defense system in liver, kidney, and pancreas tissues of alloxan-induced diabetic rats by camphor. J Food Biochem. 2020;44:e13527. doi:10.1111/jfbc.13527. [Google Scholar] [PubMed] [CrossRef]
55. Manju SL, Ethiraj KR, Elias G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: a structure-based approach. Eur J Pharm Sci. 2018;121:356–81. doi:10.1111/j.1365-2036.2006.02903.x. [Google Scholar] [CrossRef]
56. Biltekin SN, Karadaǧ AE, Demirci B, Demirci F. ACE2 and LOX enzyme inhibitions of different lavender essential oils and major components linalool and camphor. ACS omega. 2022;7:36561–6. doi:10.1021/acsomega.2c04518. [Google Scholar] [PubMed] [CrossRef]
57. Costa MF, Durço AO, Rabelo TK, de Barreto RSS, Guimarães AG. Effects of Carvacrol, Thymol and essential oils containing such monoterpenes on wound healing: a systematic review. J Pharm Pharmacol. 2019;71:141–55. doi:10.1111/jphp.13054. [Google Scholar] [PubMed] [CrossRef]
58. Fachini-Queiroz FC, Kummer R, Estevao-Silva CF, de Carvalho MDB, Cunha JM, Grespan R, et al. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid Based Complement Alternat Med. 2012;2012:1–10. doi:10.1155/2012/657026. [Google Scholar] [PubMed] [CrossRef]
59. Gholijani N, Gharagozloo M, Farjadian S, Amirghofran Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J Immunotoxicol. 2016;13:157–64. doi:10.3109/1547691X.2015.1029145. [Google Scholar] [PubMed] [CrossRef]
60. Valente J, Zuzarte M, Gonçalves MJ, Lopes MC, Cavaleiro C, Salgueiro L, et al. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem Toxicol. 2013;62:349–54. doi:10.1016/j.fct.2013.08.083. [Google Scholar] [PubMed] [CrossRef]
61. Roselló J, Llorens-Molina JA, Larran S, Sempere-Ferre F, Santamarina MP. Biofilm containing the Thymus serpyllum essential oil for rice and cherry tomato conservation. Front Plant Sci. 2024;15:1362569. doi:10.3389/fpls.2024.1362569. [Google Scholar] [PubMed] [CrossRef]
62. Ruiz-Malagón AJ, Rodríguez-Sojo MJ, Hidalgo-García L, Molina-Tijeras JA, García F, Pischel I, et al. The antioxidant activity of Thymus serpyllum extract protects against the inflammatory state and modulates gut dysbiosis in diet-induced obesity in mice. Antioxidants. 2022;11:1073. doi:10.3390/antiox11061073. [Google Scholar] [PubMed] [CrossRef]
63. Jain AS, Sushma P, Dharmashekar C, Beelagi MS, Prasad SK, Shivamallu C, et al. In silico evaluation of flavonoids as effective antiviral agents on the spike glycoprotein of SARS-CoV-2. Saudi J Biol Sci. 2021;28:1040–51. doi:10.1016/j.sjbs.2020.11.049. [Google Scholar] [PubMed] [CrossRef]
64. Taibi M, Elbouzidi A, Ou-Yahia D, Dalli M, Bellaouchi R, Tikent A, et al. Assessment of the Antioxidant and antimicrobial potential of ptychotis verticillata duby essential oil from eastern morocco: an in vitro and in silico analysis. Antibiotics. 2023;12. doi:10.3390/antibiotics12040655. [Google Scholar] [PubMed] [CrossRef]
65. Quinn MT. NADPH oxidases: structure and function. Molecular basis of oxidative stress: chemistry, mechanisms, and disease pathogenesis. 2013:137–78. [Google Scholar]
66. Tarafdar A, Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci. 2018;19:3824. doi:10.3390/ijms19123824. [Google Scholar] [PubMed] [CrossRef]
67. Teixeira G, Szyndralewiez C, Molango S, Carnesecchi S, Heitz F, Wiesel P, et al. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br J Pharmacol. 2017;174:1647–69. doi:10.1111/bph.13532. [Google Scholar] [PubMed] [CrossRef]
68. Lountos GT, Jiang R, Wellborn WB, Thaler TL, Bommarius AS, Orville AM. The crystal structure of NAD(P)H oxidase from Lactobacillus sanfranciscensis: insights into the conversion of O2 into two water molecules by the flavoenzyme. Biochemistry. 2006;45:9648–59. doi:10.1021/bi060692p. [Google Scholar] [PubMed] [CrossRef]
69. Petrônio MS, Zeraik ML, Da Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18:2821–39. doi:10.3390/molecules18032821. [Google Scholar] [PubMed] [CrossRef]
70. Andrews BA, Dyer RB. Comparison of the role of protein dynamics in catalysis by dihydrofolate reductase from E. coli and H. sapiens. J Phys Chem B. 2022;126:7126–34. doi:10.1021/acs.jpcb.2c05112. [Google Scholar] [CrossRef]
71. Bhagat K, Kumar N, Kaur Gulati H, Sharma A, Kaur A, Singh JV, et al. Dihydrofolate reductase inhibitors: patent landscape and phases of clinical development (2001–2021). Expert Opin Ther Pat. 2022;32:1079–95. [Google Scholar] [PubMed]
72. Chawla P, Teli G, Gill RK, Narang RK. An insight into synthetic strategies and recent developments of dihydrofolate reductase inhibitors. ChemistrySelect. 2021;6:12101–45. [Google Scholar]
73. Bhabha G, Ekiert DC, Jennewein M, Zmasek CM, Tuttle LM, Kroon G, et al. Divergent evolution of protein conformational dynamics in dihydrofolate reductase. Nature Struct Mol Biol. 2013;20:1243–9. [Google Scholar]
74. Wróbel A, Arciszewska K, Maliszewski D, Drozdowska D. Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors. J Antibiot. 2020;73:5–27. [Google Scholar]
75. Bayazeed A, Alenazi NA, Alsaedi AMR, Ibrahim MH, Al-Qurashi NT, Farghaly TA. Formazan analogous: synthesis, antimicrobial activity, dihydrofolate reductase inhibitors and docking study. J Mol Struct. 2022;1258:132653. [Google Scholar]
76. Baysal T, Demirdöven A. Lipoxygenase in fruits and vegetables: a review. Enzyme Microb Technol. 2007;40:491–6. [Google Scholar]
77. Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–82. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools