 Open Access
Open Access
ARTICLE
GC-MS Profiling, In Vitro and In Silico Antibacterial and Antioxidant Potential of Origanum elongatum Essential Oil: Novel Source against Phytopathogenic Bacteria
1 Laboratory of Applied Organic Chemistry, Faculty of Sciences and Techniques, Sidi Mohamed Ben Abdellah University, Imouzzer Road, Fez, 30000, Morocco
2 Plant Protection Research Unit, National Institute of Agronomic Research, Regional Center of Agronomic Research of Meknes INRA-CRRA, Meknes, 50000, Morocco
3 LIMAS Laboratory, Faculty of Sciences Dhar El Mahraz, Sidi Mohamed Ben Abdellah University, Fez, 30000, Morocco
4 Faculty of Medicine and Pharmacy, Ibn Zohr University, Guelmim, 81000, Morocco
5 Laboratoire d’Amélioration des Productions Agricoles, Biotechnologie et Environnement (LAPABE), Faculté des Sciences, Université Mohammed Premier, Oujda, 60000, Morocco
6 Laboratory of Microbial Biotechnology and Bioactive Molecules, Faculty of Sciences and Technologies Faculty, Sidi Mohamed Ben Abdellah University, Imouzzer Road, Fez, P.O. Box 2202, Morocco
7 Laboratory of Biotechnology and Bio-Resources Valorization, Faculty of Sciences, Moulay Ismail University, Meknes, 50000, Morocco
8 Department of Biology, College of Sciences, Princess Nourah Bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
* Corresponding Authors: Naoufal El Hachlafi. Email: ; Amine Elbouzidi. Email:
(This article belongs to the Special Issue: Biological Activities of Essential Oils)
Phyton-International Journal of Experimental Botany 2025, 94(2), 481-501. https://doi.org/10.32604/phyton.2025.059841
Received 18 October 2024; Accepted 23 January 2025; Issue published 06 March 2025
Abstract
This study highlights the regulatory potential antibacterial and antiradical of Origanum elongatum essential oil (EO), an endemic medicinal plant of Morocco used for its various properties. The chemical composition of the EO was characterized using gas chromatography-mass spectrometry (GC-MS). The antibacterial activity against different agricultural phytopathogens was determined by disc diffusion and microatmosphere methods, as well as by the determination of minimum inhibitory concentrations (MIC) and minimum bactericidal concentration (MBC), while the antioxidant activity was evaluated by DPPH and FRAP assays. To complement the experimental analyses, a molecular docking approach was used to predict and elucidate the mechanisms of action of the identified bioactive compounds, both for their antioxidant and antibacterial properties. The GC-MS analysis revealed a chemical composition dominated by the major compounds: p-cymene-2-ol (25.31%), thymol (23.88%), and γ-terpinene (19.26%). Furthermore, antibacterial analyses performed using different methodological approaches (disc diffusion, microatmosphere, MIC, and MBC) showed significant inhibitory activity against all phytopathogens tested. Moreover, O. elongatum EO exhibited interesting antioxidant ability with an IC50 value of 168.25 ± 1.14 µg/mL for DPPH assay and EC50 value of 164.22 ± 1.04 µg/mL for FRAP assay. Furthermore, in silico molecular docking demonstrated further insights into the interactions between the oil’s active components and bacterial targets, supporting its mode of action. This in-depth characterization highlights the potential of O. elongatum EO as a natural alternative for the biocontrol of plant pathogens. It opens new perspectives for developing natural solutions to protect crops against plant diseases.Keywords
Bacterial resistance represents a major challenge to human health and food production, highlighting the urgent need for the ongoing discovery of new natural antimicrobial compounds [1]. While chemical products are often effective and affordable, their residual toxicity and potential carcinogenic and teratogenic effects raise substantial concerns [2]. Consequently, there is growing attention in evaluating the antibacterial and antioxidant properties of natural substances for potential use as functional foods and therapeutic agents [3]. Essential oils (EOs) from aromatic and medicinal plants have demonstrated notable biological activity [4,5]. These oils, composed of a wide range of bioactive substances responsible for potent antimicrobial, anti-inflammatory, and antioxidant activities [6]. By targeting various microbial processes, EOs effectively prevent the emergence of resistant strains, thereby maintaining their efficacy as natural antimicrobial agents [7,8].
Within the Lamiaceae family, the genus Origanum includes species with strongly aromatic leaves and bioactive compounds [9]. Origanum elongatum, endemic to Morocco, thrives in temperate biomes at altitudes above 1000 m and is particularly rich in proteins, fibers, carbohydrates, and EOs [10]. The chemical composition of O. elongatum, which varies with genetic and environmental factors, includes bioactive substances such as thymol, carvacrol and p-cymene [11]. These constituents contribute to the plant’s biological effects, including hepatoprotective, antioxidant, antiparasitic, antiviral, and antibacterial properties [12].
The practical applications of EOs are becoming increasingly of interest, particularly in the agricultural, food, and pharmaceutical sectors [13–15]. However, a number of studies have evidenced the potential utility of plant EOs in the development of biocontrol and food preservation products, due to their established antimicrobial and antioxidant properties [16,17].
Despite the effectiveness of O. elongatum in inhibiting various microorganisms [18,19], no studies have explored its effects against phytopathogens such as Allorhizobium vitis, Agrobacterium tumefaciens, and Erwinia amylovora. The defense against plant bacterial pathogens in agriculture predominantly relies on preventive treatments with copper-based chemicals [20]. While advantageous due to their high toxicity towards pathogens, low mammalian toxicity, cost-effectiveness, and prolonged stability, excessive use of copper compounds has led to soil accumulation and the selection of copper-resistant strains [21]. Therefore, finding alternatives or optimizing the use of copper is critical, and EOs present a promising, environmentally sustainable solution for plant and human health.
Allorhizobium vitis, the causative agent of Crown Gall in grapevines, is facilitated by the pathogen’s presence in the soil and entry through wounds on the host plant [22]. Biological control methods, including the use of essential oils from Origanum and Eucalyptus species, have shown promise in managing this disease [23]. Similarly, Agrobacterium tumefaciens, another significant phytopathogen responsible for Crown Gall, induces tumor formation by transferring its T-DNA into host plants, with research focusing on various strains and mutants to inhibit tumor growth [24]. Erwinia amylovora, the agent behind fire blight in apple and pear trees, spreads through rain, insects, and pruning tools, with control measures primarily involving copper-based sprays and resistant cultivars [25].
The current exploration aims to assess the antibacterial and antioxidant effects of EO obtained from O. elongatum via in vitro and in silico experiments. This is achieved through the utilization of both experimental and computational docking techniques. To our knowledge, this work is the first to investigate the antibacterial activity and molecular docking patterns of O. elongatum EO against phytopathogenic bacteria, namely Allorhizobium vitis, Agrobacterium tumefaciens, and Erwinia amylovora, offering valuable insights into its potential applications as biocontrol agents.
The identification of chemical compounds and the determination of their quality in our OEEO sample was performed employing GC-MS analysis. The method that was adopted to analyze our sample was described by [26]. The analysis was done using a TRACE GC ULTRA coupled to the mass spectrometer (Polaris Q), with an electron initiation energy of 70 eV. A non-polar VB5 capillary was used. It measured 30 m long, 0.25 mm in diameter, and 0.25 um thick. The temperature parameters were carefully set: The injector at 250°C and the detector at 300°C; the oven was then programmed to increase from 40°C to 180°C at a speed of 4°C/min, then from 180°C to 300°C at a speed of 20°C/min. An injection of 0.5 µL of OEEO was performed without fractionation. The OE molecules were characterized by making comparisons of their kovats indices with those of n-alkanes series (C8 to C24) reported in the literature. The results obtained were compared with known references in order to determine the specific constituents of OEEO and evaluate their relative concentration. As a final step, the oil fractions were then carried out by comparing the MS fragmentation patterns of spectra with those reported by the NIST library data.
Three phytopathogens were used to determine the antibacterial effect of OEEO (Erwinia amylovora, Agrobacterium tumefaciens C58, Allorhizobium vitis S4). All the phytopathogens employed in this work were from the Laboratory of Phytopathology, Regional Centre of Agronomic Research of Meknes INRA-CRRA, Meknes. To make the bacterial solution for inoculation, two or three colonies were taken from a fresh culture on LPGA and then suspended in a sterile 0.9% NaCl solution. The final bacterial density was around 106 CFU/mL.
A preliminary antibacterial screening was done adopting the disc diffusion method, with certain modifications, according to a previously outlined methodology [27]. One plate of LB agar medium was inoculated with a solution of a bacterial culture. Next, 8 µL of OEEO was used to soak 6 mm sterile filter discs, which were then placed on each plate. Streptomycin (15 µg/disc) was served as a standard. The inhibition zone (IZ) was determined and expressed in millimeters after 24 h of incubation at 37°C. The mean ± SD of three different measurements is presented for each data set.
The micro-atmospheric technique described by Reference [28] with minor modifications was also adapted to indicate the antibacterial capacity of OEEO by gas contact. In short, after plating bacterial strains (108 CFU) on LB agar medium, Whatman discs (6 mm) soaked with 8 µL of OEEO were placed in each Petri dish lid. Inhibitory zone growth was measured by incubation for 24 h at 37°C.
MIC of OEEO was examined using a technique that was reported in the literature, with some minor modifications [6]. A 96-well polypropylene microplate was used. In brief, 100 µL of double serial dilutions of OEEO (5% DMSO) and positive control (streptomycin) were added to each well in each row of the microplate. Then 10 µL of the bacterial suspension was calibrated against the 0.5 McFarland standard and 50 µL of double-strength LB broth medium was added to the microplates. The incubation period was 24 h at 37°C. A medium containing no bacterial suspension and 5% DMSO was used as a negative control of the experiment. A volume of 20 μL of resazurin was added to measure the development of the bacteria.
The MBC tests were performed after MIC determination. 50 µL of each MIC tube was plated on LB agar plates specially prepared for this purpose. The plates were then incubated at 25°C to 35°C for 24 h. MIC at which no bacterial growth was observed was called the MBC. The MBC/MIC ratio was used to assess whether the effect was bacteriostatic or bactericidal [29].
2.3.1 DPPH Free Radical-Scavenging Assay
In the presence of a DPPH radical the antioxidant substances present in the EO can reduce the radical, resulting in a decrease in absorbance measured spectrophotometrically. In this study, the ability of OEEO to trap the DPPH radical was determined by the standard method described by [30] with a few modifications. Ascorbic acid and BHT were employed as standards.
2.3.2 Reducing Ferric Power Determination TEST FRAP
The test was carried out to investigate the ability of OEEO to convert Fe3+ into Fe2+. The method used is a modified version described by Reference [31]. The BHT was used as a standard for the assay. The test was carried out in three independent experiments and the IC50 values were expressed as the mean ± SD.
2.4 Physicochemical and ADMET Pharmacokinetic Predictions
In the current study, two major compounds namely Thymol and p-cymene-2-ol extracted from Origanum elongatum essential oil with areas of 23.88% and 25.31%, respectively, were tested using in silico investigations, including their physicochemical and pharmacokinetic features of absorption, distribution, excretion, and toxicity (ADMET) [32], more than drug-likeness ability based on Lipinski rules of five, predictive model of Egan’s boiled-egg, and oral bioavailability radars which were carried out with the assistance of Pkcsm and Swiss ADME servers [33]. In addition, a sum of ten molecular docking simulations was equally performed between both candidate ligands and all five targeted receptors including NADPH oxidase (2CDU.pdb) and 5-LOX Lipoxygenase (1N8Q.pdb) enzymes [34] to examine their antioxidant and anti-inflammatory effects. Subsequently, they were tested for their antimicrobial potential through three antibacterial proteins against Erwinia amylovora (4D74.pdb), Agrobacterium tumefaciens (5J9R.pdb), and Allorhizobium vitis (4WT7.pdb) pathogenic strains. The molecular docking processes were performed using Autodock software in which all five targeted proteins were prepared adding the Gasteiger charges and removing all water molecules and other suspended ligands [35,36]. At the same time, the produced intermolecular interactions were visualized in 2 and 3D using Discovery Studio software [37,38].
The data analyses were done using Minitab Statistical Software 21. The statistical significance of the observed differences was assessed by employing a one-way analysis of variance (ANOVA) adopting Tukey’s test.
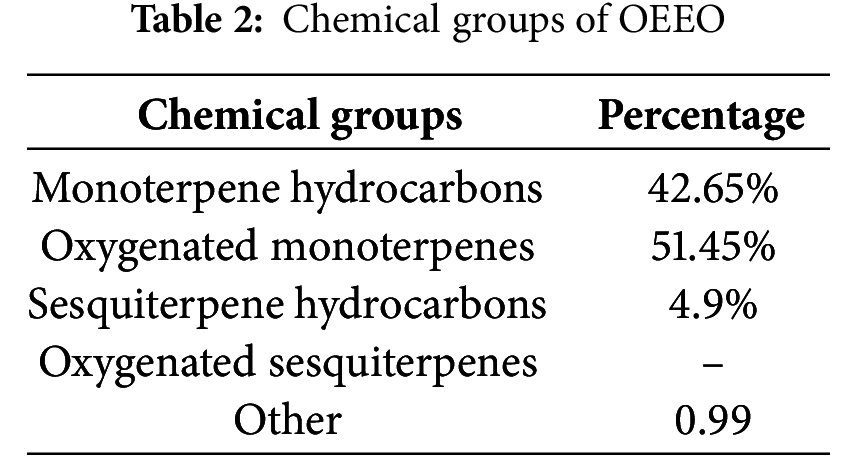
According to GC-MS analysis, the OEEO has a distinctive chemical profile (Table 1). Our OEEO is particularly rich in oxygenated monoterpenes, representing 51.45% of its total composition, and monoterpene hydrocarbons 42.65% (Table 2). Among the many compounds identified, p-cymene-2-ol stands out as the major component with a percentage of 25.31%. It was closely followed by thymol (23.88%). The third most abundant component is γ-terpinene which accounts for 19.26% of the total sample.
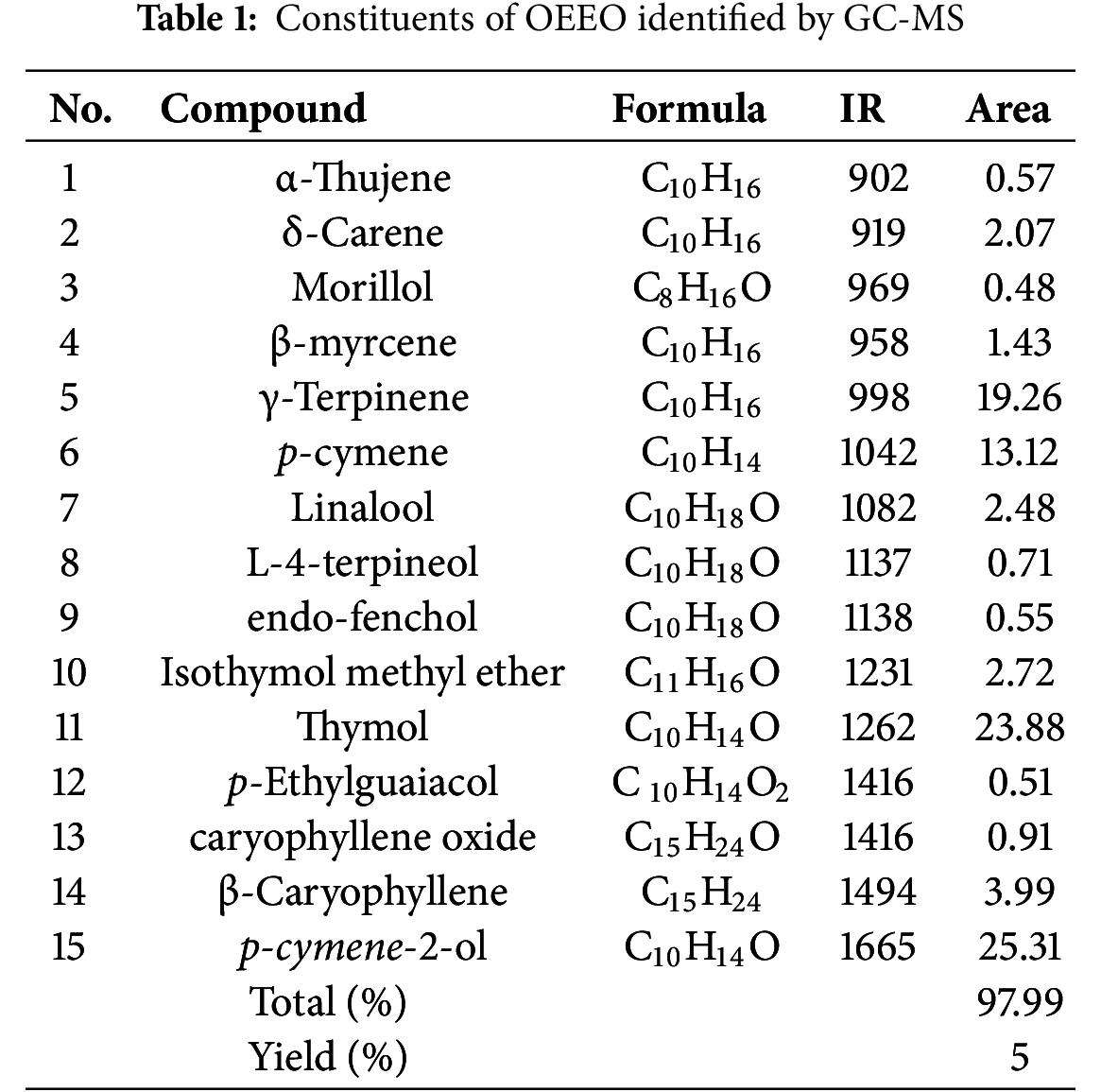
A comparison of our results with other research on the OEEO reveals some interesting similarities and differences. For example, analysis by GC/MS showed that OEEO contains 27 compounds covering 99.08% of its constituents, with carvacrol 57.32% as the major compound, subsequently, p-cymene (14.70%) and γ-terpinene (10.0%) [39]. In another study, the OEEO from Moroccan Rif has reported 11 major constituents, with carvacrol (60.4%), p-cymene (14.0%) and γ-terpinene (9.4%) [11]. A study conducted in the same region identified 28 compounds in the OEEO, the most prevalent of which were carvacrol, thymol, and p-cymene [40]. Other studies have also found that the main compounds in the OEEO are carvacrol (63.0%), γ-terpinene (16.0%), and p-cymene (9.5%) [18]. An earlier study identified oxygenated compounds as the dominant component (65.14%), followed by hydrocarbon molecules (28.0%), with thymol representing the primary constituent at 63.4% [19]. Other work also noted that the major OEEO constituent is carvacrol (67.3%) [41]. Moussaoui, in 2013 had identified carvacrol (40.1%), thymol (14.2%), p-cymene (16.2%) and γ-terpinene (13.48%) as the major constituents of OEEO [42]. Similarly, GC-MS of OEEO seeds revealed a high chemical composition dominated by carvacrol (79.2%), with the presence of p-cymene (5.2%), γ-terpinene (3.7%), and linalool (2.4%) [43,44].
These comparisons show the diversity of the chemical profiles of OEEO while confirming the presence of some key compounds such as carvacrol, γ-terpinene, p-cymene, and thymol. However, contrary to what is generally expected in the literature, our OEEO does not contain carvacrol. Instead, it is rich in p-cymene, known as a precursor of the main monoterpenes such as carvacrol.
There are several possible explanations for this paradox. It is possible that the biosynthetic conditions or enzymes necessary to successfully convert p-cymene to carvacrol were not present or active at the time when our OEEO was formed [45]. In addition, ecological factors, genetic diversity, environmental variables, geographic location, and harvest season can all have an impact on chemical composition [40]. Indeed, the harvest site’s latitude and altitude, temperature, solar radiation, and phylogenetic stage, all of which can modify the composition of EO [46]. However, different cultural practices can have an impact on how plants are grown and consequently, the quality and the quantity of the oil. Nevertheless, the plant’s harvest season can have a big impact on the composition of its oils. In actuality, the oil content and composition can be significantly influenced by the age of the plant during harvest, it’s possible that younger plants have distinct oil profiles from older ones [47]. Furthermore, differences in lighting and temperature throughout the year might impact oil synthesis, resulting in different compositions at different times of harvest. Seasons of harvest can also cause variations in the physiology and metabolism of plants, which can impact the properties of oil [48].
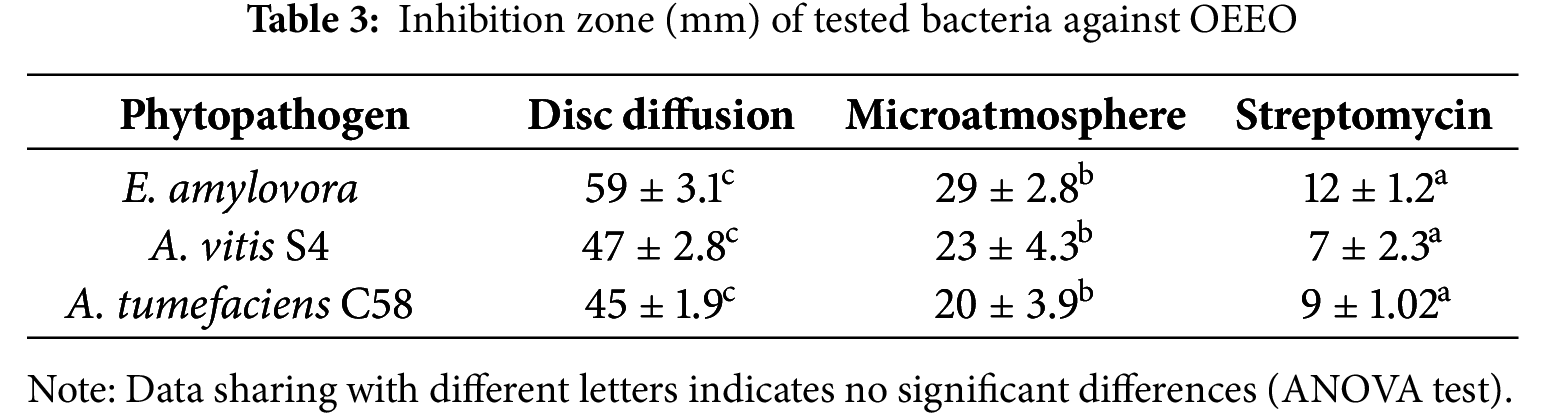
The results indicate significant variability in the sensitivity of the different bacteria to OEEO. E. amylovora was the most sensitive with a mean zone inhibition of 59 ± 3.1 mm. It was followed by A. vitis S4 with an inhibition zone (IZ) of 47 ± 2.8 mm. Finally, an IZ of 45 ± 1.9 mm was observed for A. tumefaciens C58.
The micro-atmosphere test was used to assess the effect of OEEO on bacterial growth in a closed atmosphere. The findings revealed that OEEO demonstrated notable antibacterial efficacy against all bacterial strains examined. E. amylovora showed the most sensitivity, with an average IZ of 29 mm. A. vitis S4 was the next most sensitive with an IZ of 23 mm. while A. tumefaciens C58 had an IZ of 20 mm (Table 3).
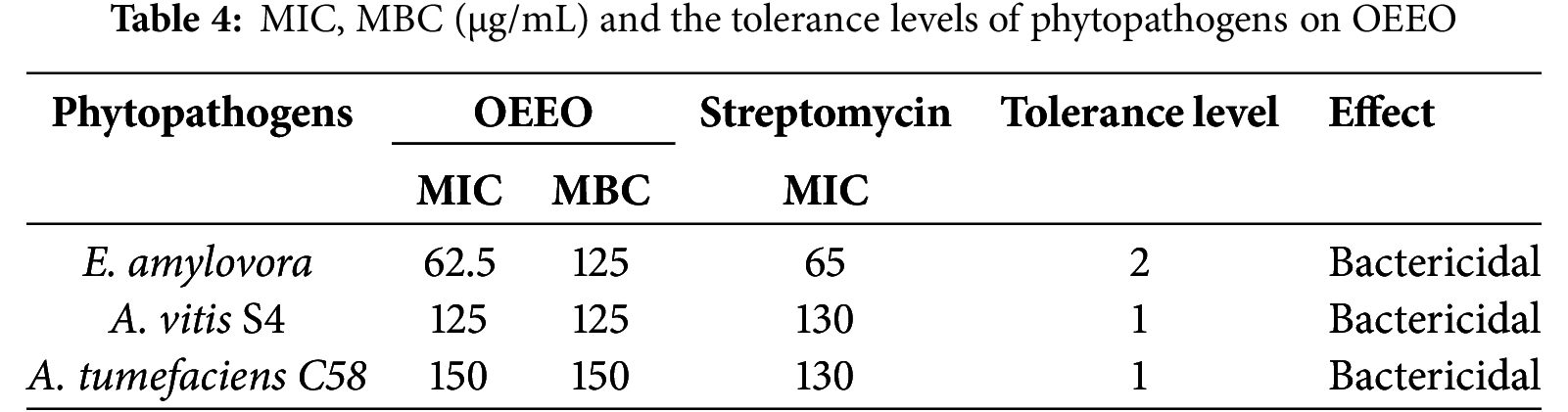
The objective of the MIC test was to ascertain the lowest concentration of OEEO that could be demonstrated to visibly inhibit the visible development of plant pathogen bacteria: E. amylovora, A. tumefaciens C58, A. vitis S4. The MIC of E. amylovora, was 62.5 µg/mL, that of A. vitis S4 was 125 µg/mL and for A. tumefaciens C58 the MIC was 150 µg/L. In particular, the MBC results showed that the minimum lethal concentration of OEEO for E. amylovora was 125 µg/mL. In contrast, the concentration required to kill A. vitis S4 was 125 µg/mL and for A. tumefaciens C58 it was 150 µg/mL. Consequently, OEEO has bactericidal activity against all phytopathogens tested, depending on her level of tolerance. MBC/MIC ratio was 2 against E. amylovora and 1 against A. vitis S4, and A. tumefaciens C58 (Table 4). The results of the positive controls demonstrated that the standard antibiotic exhibited MICs ranging between 65 to 130 µg/mL, contingent on the bacterial strains subjected to analysis. In comparison, the OEEO demonstrated remarkable antibacterial ability, particularly against E. amylovora, which is approximately 10 times more efficacious than the positive control. With regard to A. vitis S4 and A. tumefaciens C58, the MICs obtained were comparable to those of standard. Furthermore, the bactericidal effect observed for all the strains tested lends additional support to the hypothesis that this EO may serve as a valuable natural alternative to conventional antibacterials in the treatment of phytopathogenic bacterial infections.
The analysis of the antibacterial effect of OEEO on plant pathogens provides valuable insights into the potential for agricultural crop protection. The findings showed that our EO had very potent antibacterial capabilities against all bacteria tested, which could potentially be used in agriculture. The ability of OEEO to control the growth of E. amylovora, A. vitis S4, and A. tumefaciens C58 could lead to a major revolution in plant disease management methods. Based on a literature review of existing research, there is a wide collection of studies demonstrating the efficacy of OEEO against various pathogens [18,19,39]. However, no published work has been done on the effect of OEEO on the antibacterial ability of the three phytopathogenic bacteria investigated.
The results of our work confirm those already reported in the literature on the efficacy of EO in the biocontrol of the three phytopathogens tested. Significant antibacterial activity of Origanum vulgare EO has been reported in a study, with a MIC range between 7.8 and 625 µg/mL and a zone of inhibition of 15 ± 0.5 mm against E. amylovora. Furthermore, the EO of Origanum compactum showed an IZ of 40 mm, suggesting an even stronger antibacterial activity against E. amylovora [49]. However, the EOs from Foeniculum vulgare and Pimpinella anisum also showed significant antibacterial activity with a MIC of 7.8 µg/mL and zones of inhibition of 36.33 and 26.31 mm respectively vs. E. amylovora [50]. Nevertheless, research conducted by [51] highlighted the potential of Rosa damascene EO against E. amylovora, providing a 45 mm zone of inhibition. The essential oil of Satureja L. on E. amylovora, also had a high antibacterial activity, MBC/MIC ranging from 0.09 and 0.18 µg/mL [52]. Furthermore, Cinnamomum cassia EO also reported an inhibition zone of 37.7 mm and an MBC of 1.04 µg/mL [53]. On the other hand, A. vitis has also shown sensitivity to the Eos. In a study by Habbadi and colleagues on Origanum compactum and Thymus. Vulgaris EOs vs. A. vitis S4 showed significant in vitro activity, with MICs of 156 and 312 µg/mL, respectively [54]. In parallel, a study of EOs from different plants reported percentage inhibitions ranging from 7.5% to 25.88% and MICs between 0.15 and 20 mg/mL [55]. In addition, the efficacy of four Eucalyptus EOs against A.vitis S4 has demonstrated inhibition percentages ranging from 13.67% to 20.50% and MICs from 20 to 40 mg/mL [56]. Moreover, A. tumefaciens has also exhibited notable sensitivity to essential oils effects as highlighted by many studies. However, Cuminum cyminum EO showed inhibitory activity with a MIC ranging from 170.2 to 7280 µg/mL. Similarly, Thymus vulgaris EO showed a MIC ranging from 75 to 1100 µg/mL, with an IZ measuring 11 mm [57]. Mentha piperita L EO was particularly effective against A. tumefaciens, with an IZ of 36 mm, and lowest MIC (0.02 mg/mL), and MBC of 0.39 mg/mL [58]. A study of five Eos against A. tumefaciens showed that Thuja occidentalis exhibited significant inhibition with an MIC of 400 mg/mL, closely followed by Pelargonium graveolens with an MIC of 540 mg/mL. In contrast, Citrus sinensis, Myrtus communis, and Schinus terebinthifolius Eos failed to inhibit A. tumefaciens development even at high concentrations (MIC > 1000 mg/mL) [59]. In addition, a study on Eos from Cinnamon, Thyme, and Oregano, provided the highest inhibition zones against A. tumefaciens with diameters of 44, 32.6, and 32.3 mm, respectively [49].
In the present work, the antioxidant’s ability of OEEO to reduce ferric ions, and to scavenge DPPH free radical were determined. Our results indicate that OEEO has a significant anti-free DPPH radical potential, with an IC50 of 168.25 ± 1.14 μg/mL, clearly demonstrating its strong efficacy. In the FRAP assay, OEEO also exhibited remarkable antioxidant capacity with an EC50 value of 164.22 ± 1.04 μg/mL (Table 5). highlighting its ability to effectively reduce ferric ions. When compared to standards such as BHT and ascorbic acid, which have IC50 values of 63.21 ± 0.03 μg/mL and 41.32 ± 0.08 μg/mL, respectively, in the DPPH assay, OEEO shows effective antioxidant potency. Furthermore, in the FRAP assay, BHT had an EC50 of 89.44 ± 0.13 μg/mL, a value closely matched by OEEO, underscoring its potential as a potent natural antioxidant.
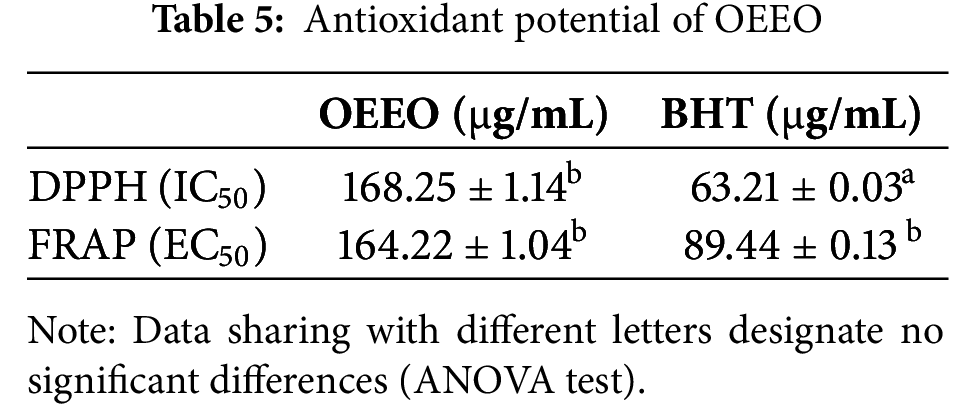
Our results on the antioxidant activity of OEEO are in arrangement with those proved in the literature. In the study by Tagnaout and colleagues, OEEO was shown to have significant antiradical potential with an IC50 value of 2.855 ± 0.018 µg/mL in the DPPH assay and an EC50 value of 0.124 ± 0.013 µg/mL in the FRAP assay [39]. Another study revealed a significant antiradical activity with an EC50 value of 1.20 g extract/g DPPH [11]. Many other researches have demonstrated the reducing power of EOs from the Origanum genus. A study on Origanum majorana showed very interesting results with an IC50 of 503.08 ± 0.06 µg/mL for the DPPH assay and an EC50 of 511.43 ± 0.61 µg/mL for FRAP [60]. Furthermore, a study on the antioxidant activity of Eos from Origanum vulgare at several phenological phases showed an IC50 of the DPPH radical ranging from 59 to 89 mg/L [61]. Another study found that the EO of Origanum vulgare obtained from the leaves, roots, and stems, exhibited significant antioxidant activity for each plant part. The IC50 values measured were 332 µg/mL for leaves, 357 µg/mL for roots, and 501 µg/mL for stems [62]. Similarly, in a previous study on Origanum compactum EO from Boulemane and Taounate regions was found to have strong antioxidant potential. In the case of Boulemane, the IC50 was 0.27 ± 0.01 mg/mL (DPPH) and 0.19 ± 0.03 mg/mL (FRAP), while in Taounate it was 37 µg/mL (DPPH) and 25 µg/mL (FRAP) [63].
Natural antioxidant compounds with and without phenolic characteristics contribute to oxidative inhibition through different mechanisms. Phenolic compounds, are known for their strong radical-scavenging potency, interact directly with free radicals, while non-phenolic and non-oxygenated compounds, such as terpenes and hydrocarbons, may exert their antioxidant effects by interrupting lipid peroxidation or stabilizing free radicals through hydrophobic interactions [64]. This study’s comprehensive chemical profiling underscores the significant role of various constituents, beyond phenolic compounds, in the overall antioxidant activity of OEEO. Indeed, the powerful antioxidant potency of our OEEO is related to the high concentration of non-oxygenated substances, such as p-cymene, thymol, and terpenes, which have been clearly described in the published literature [45].
A significant correlation has been found between antioxidant properties and phenolic compound content, enabling them to act as reducing agents, as hydrogen donors, as singlet oxygen scavengers, as lipid peroxidation inhibitors, and as metal chelators [65]. Furthermore, thymol has been shown to have the highest DPPH radical scavenging activity [66]. However, p-cymene is characterized by its powerful antioxidant properties, playing an essential role in neutralizing free radicals and protecting cells from the harmful effects of oxidation [45]. Terpenes also have a strong antioxidant capacity. In particular, they prevent lipid peroxidation [67]. Overall, treatment strategies based on free radical scavenging antioxidants have demonstrated the potential to protect cells from delay, control, or ameliorate many complex diseases. Antioxidants may also be important in avoiding or reducing cellular injury and subsequent cellular alteration, including DNA mutations, lipid peroxidation in the cell membrane, and mitochondrion malfunction [68,69].
3.4 Physicochemical and ADMET Pharmacokinetic Predictions
As a result of in silico investigations applied to Thymol and p-cymene-2-ol as the main compounds extracted from OEEO, we noticed that both molecules labeled C11 and C15 were predicted with good physicochemical profiles meeting all five Lipinski rules, justified by molecular weight less than 500 g/mol threshold, molar refractivity index included in [40, 130] range, lipophilicity in solvent inferior to five, acceptors and donors of Hydrogen bond not exceed ten and five, respectively, as resulted in Table 6. Moreover, both chemical compounds were equally predicted with a desired ADME profile, explained by excellent human intestinal absorption (HIA of 93%), good levels of permeability to the central nervous system (CNS) and blood-brain barrier (BBB), any inhibitory effect on human cytochromes of 2C9, 2C19, 1A2, 2D6, and 3A4, except for Thymol (C11) which was predicted with positive impact to inhibit 1A2 cytochrome. In addition, according to the AMES toxicity test, both compounds were indicated as not toxic agents as shown in Table 7. Furthermore, the candidate’s ligands were also predicted as CNS inhibitors as they are part of the yellow Egan’s egg as displayed in Fig. 1, so they can cross the BB barrier with the highest possible probability. Then, the predictive model of bioavailability radars confirms that both ligands were predicted with good oral bioavailability as their associated radars are part of ideal zones as colored in pink (Fig. 2) [70]. Both molecules were therefore evaluated with desirable physicochemical and pharmacokinetic profiles, providing significant similarities to drug candidates [71].
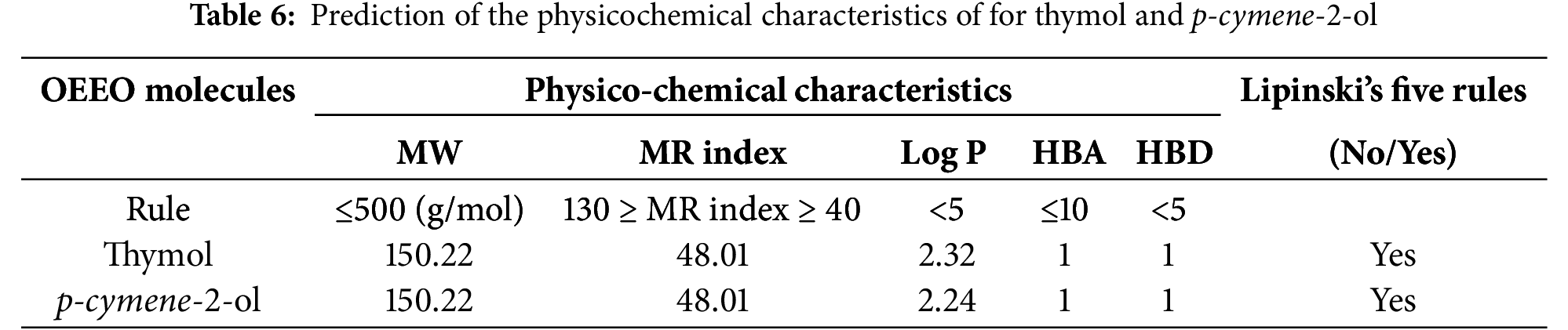
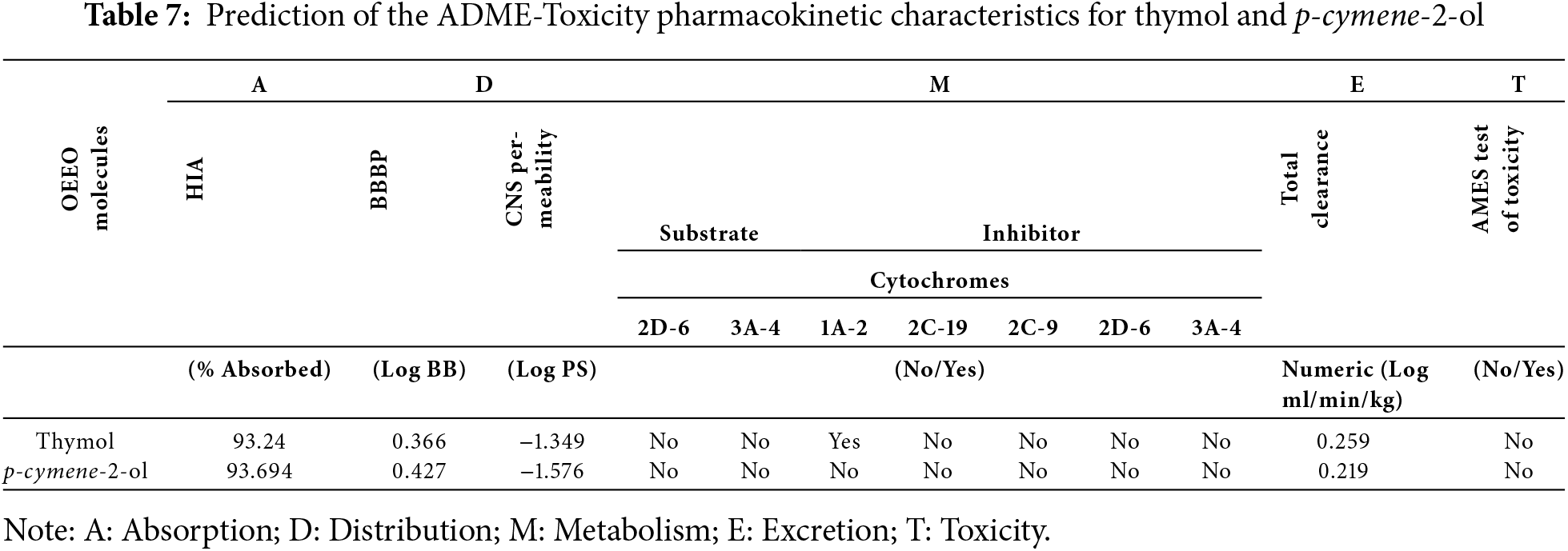
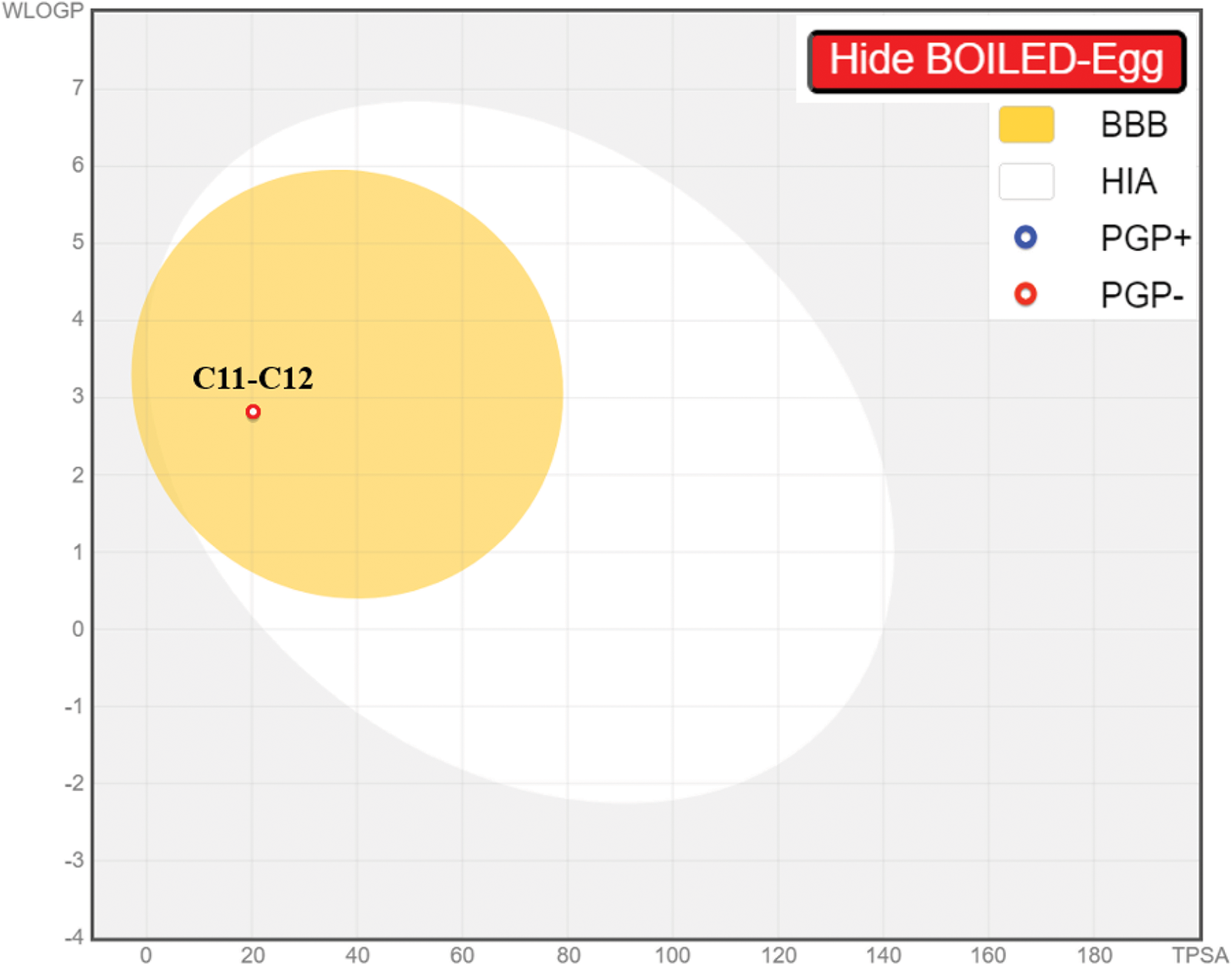
Figure 1: Egan’s boild egg model for thymol and p-cymene-2-ol
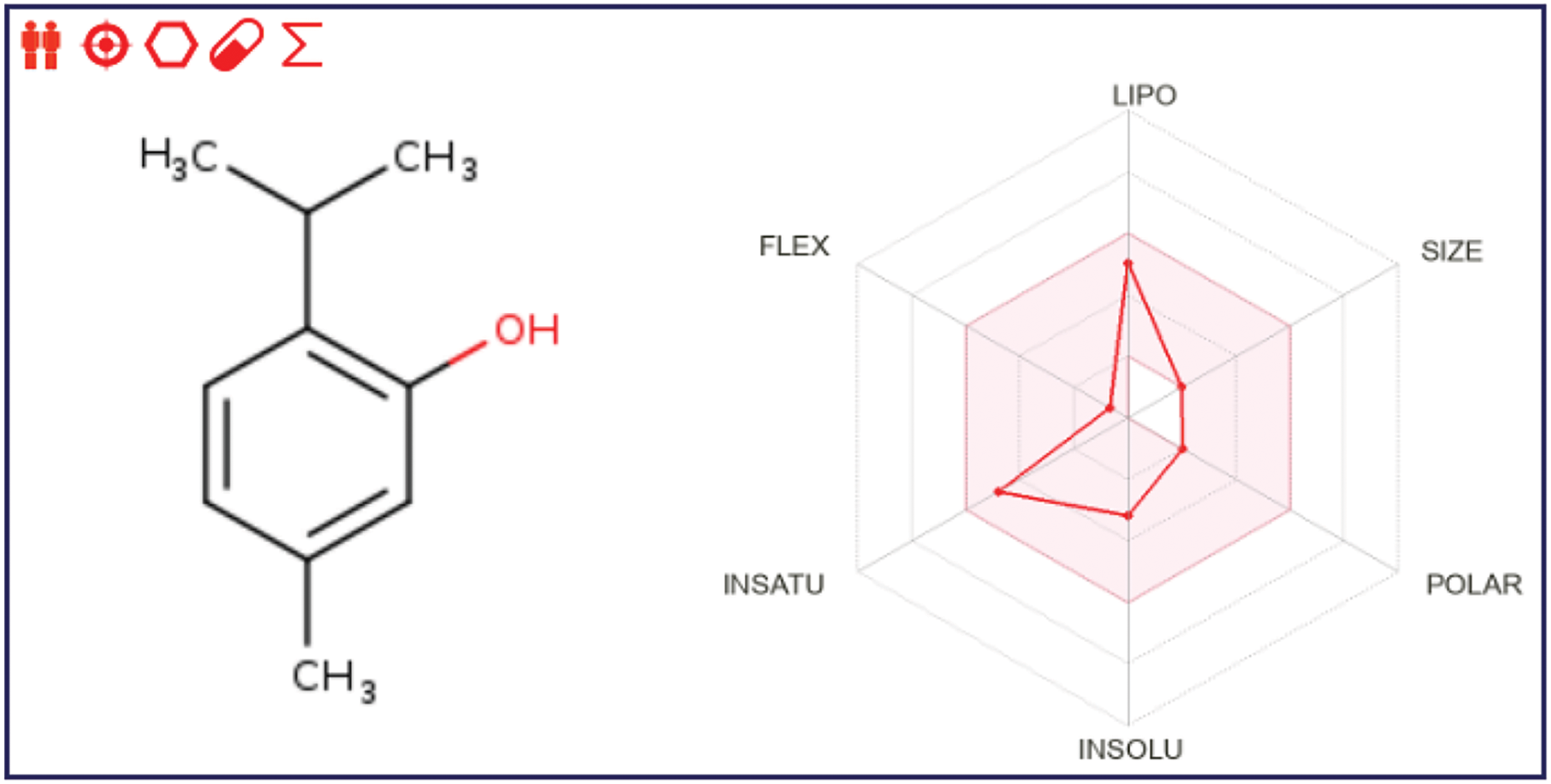
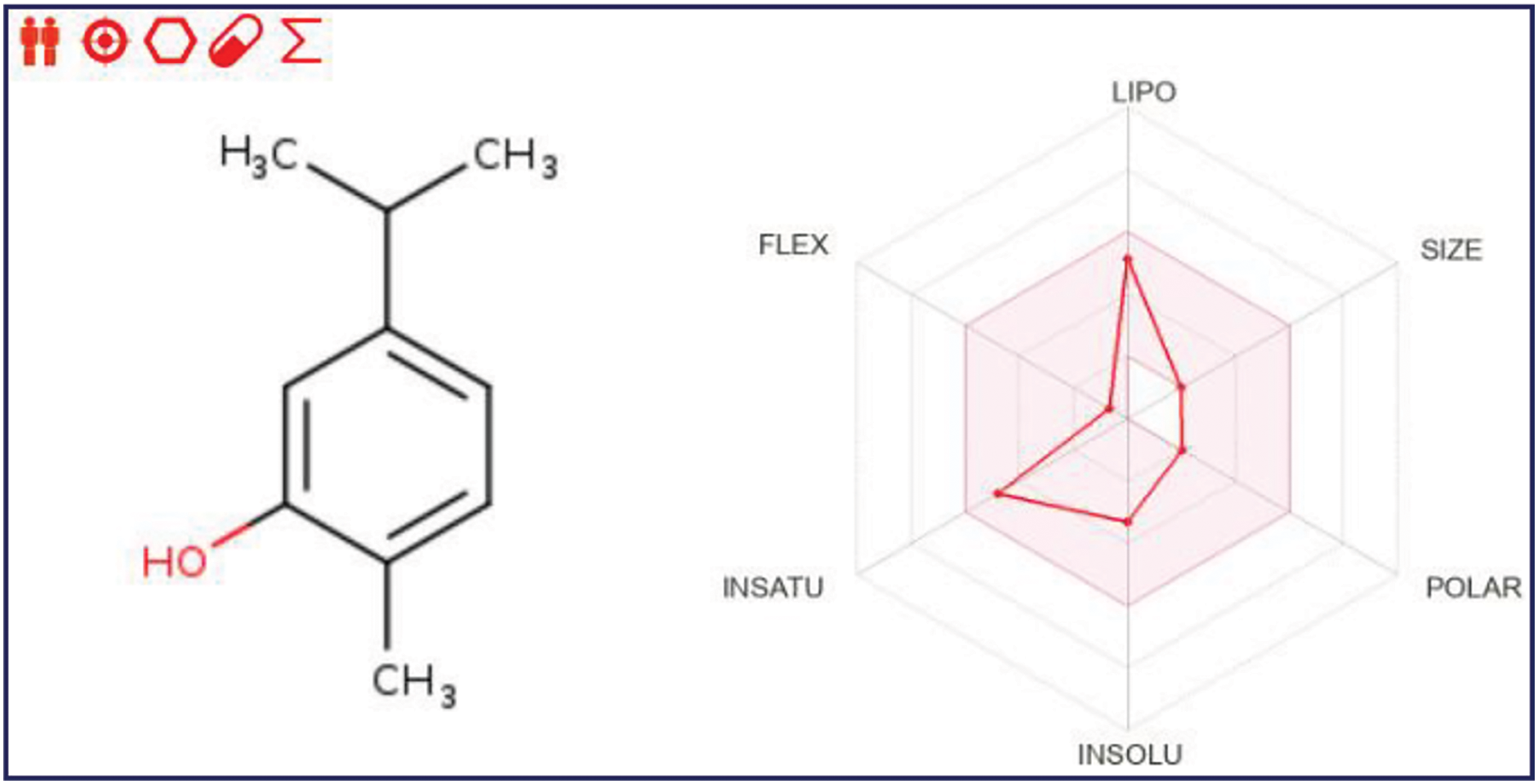
Figure 2: Bioavailability radars for thymol and p-cymene-2-ol, respectively
3.5 Molecular Docking Simulations
The docking simulation revealed that Thymol and p-cymene-2-ol ligands were docked for the first time to NADPH oxidase protein encoded in the PDB by 2CDU with the lowest binding energies (BE) of −6.24 and −6.26 kcal/mol respectively, producing several intermolecular interactions, including one Hydrogen bond fixed to Asp282 active site, more than two active sits detected through two Alkyl and Pi-Alkyl bonds fixed with Ala10 and Ala300 AA residues as resulted in Fig. 3. The same substances were secondly docked to 5-LOX Lipoxygenase enzyme encoded by 1N8Q.pdb with BE of −5.86 and −6.13 kcal/mol respectively, in which various intermolecular interactions such as Sigma bond detected towards His523 and Pi-sulfur bond fixed to Lys545 amino acid residues could justify the anti-inflammatory activities of both docked ligands as presented in Fig. 4. In the next stage, the candidate’s ligands were equally complexed to the antibacterial protein of Erwinia amylovora pathogenic strain (4D74.pdb) with BE of −6.27 and −6.31 kcal/mol, respectively, demonstrating two common interactions of Pi-Sulfur bond type detected with Cys9 AA residue more than one common interaction of Pi-Anion type established with Asp115 amino acid residue, more than Alky, Pi-Alkyl, and Pi-Donor Hydrogen bonds detected with Arg115, Cys14, Tyr117, and Glu11 AA residues, as presented in Fig. 5. Moreover, Thymol and p-cymene-2-ol compounds were also complexed to the antibacterial protein of Agrobacterium tumefaciens pathogenic strain encoded by 5J9R.pdb with BE of −5.61 and −5.37 kcal/mol, respectively, in which three Hydrogen bonds were created towards Il191, Gly193, and Asp85 AA residues in A chain as displayed in Fig. 6. Finally, the same major compounds were complexed to the antibacterial protein from Allorhizobium vitis pathogenic strain (4WT7.pdb) with BE of −5.03 and −5.08 kcal/mol, respectively, in which the Thymol compound interacted with the targeted receptor forming one Hydrogen bond with Gln290 active site in A chain, while p-cymene-2-ol compound complexed to the same protein creating two Hydrogen bonds detected towards Arg297 and Glu294 AA residues as resulted in Fig. 7.
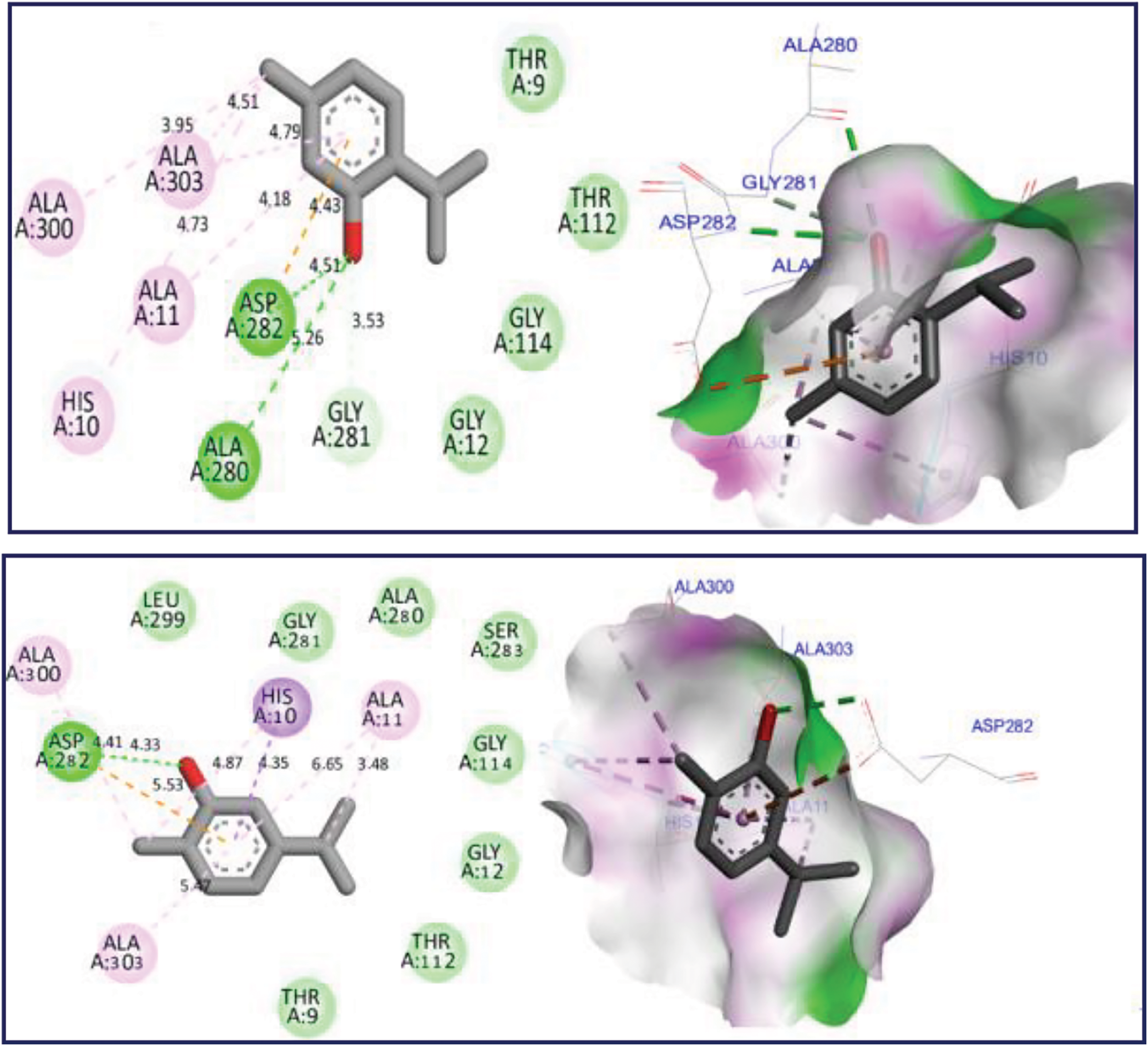
Figure 3: 2D and 3D views of molecular docking interactions against NADPH oxidase protein (2CDU.pdb) for thymol and p-cymene-2-ol, respectively
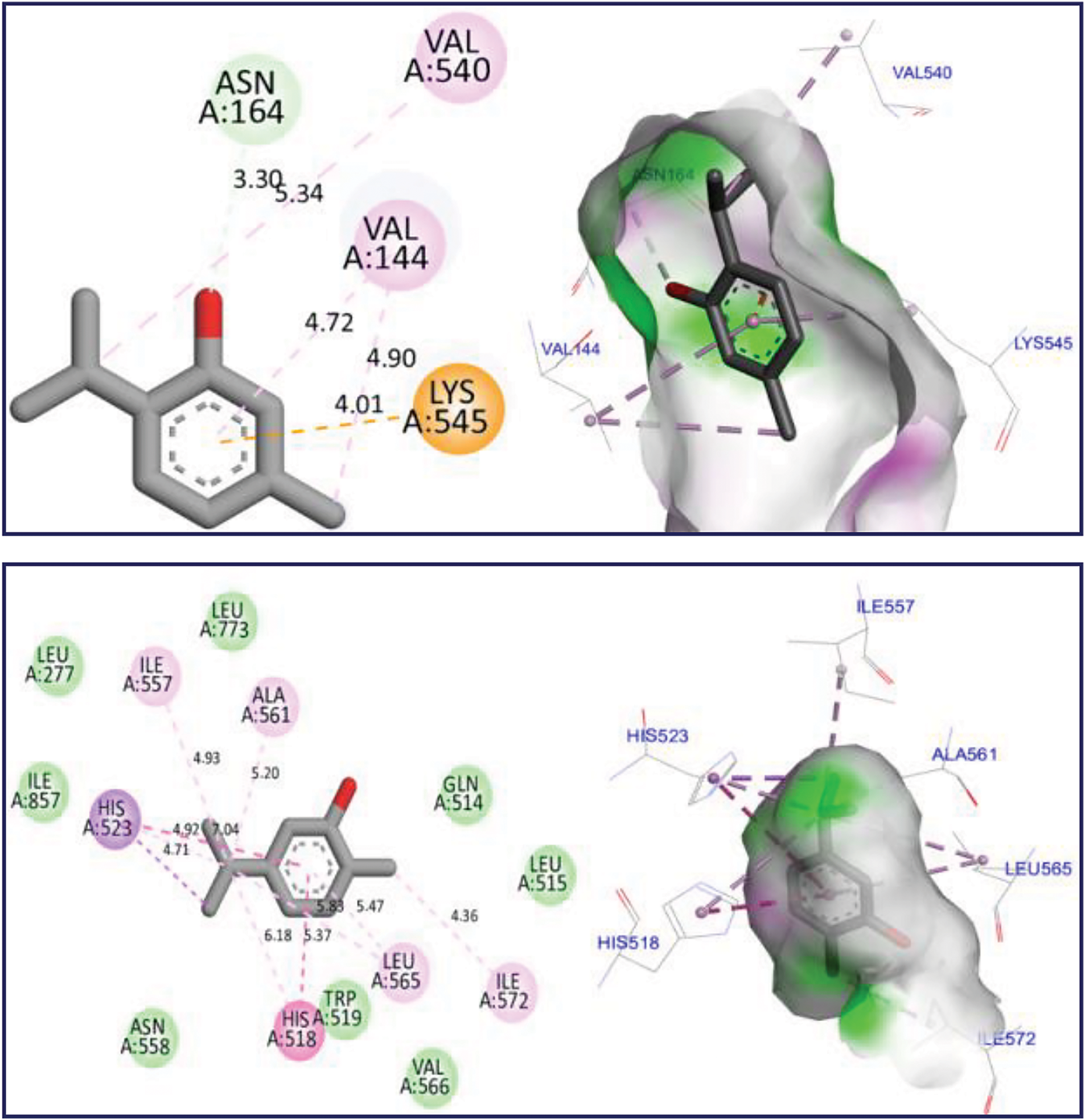
Figure 4: 2D and 3D views of molecular docking interactions against 5-LOX Lipoxygenase enzyme (1N8Q.pdb) for thymol and p-cymene-2-ol, respectively
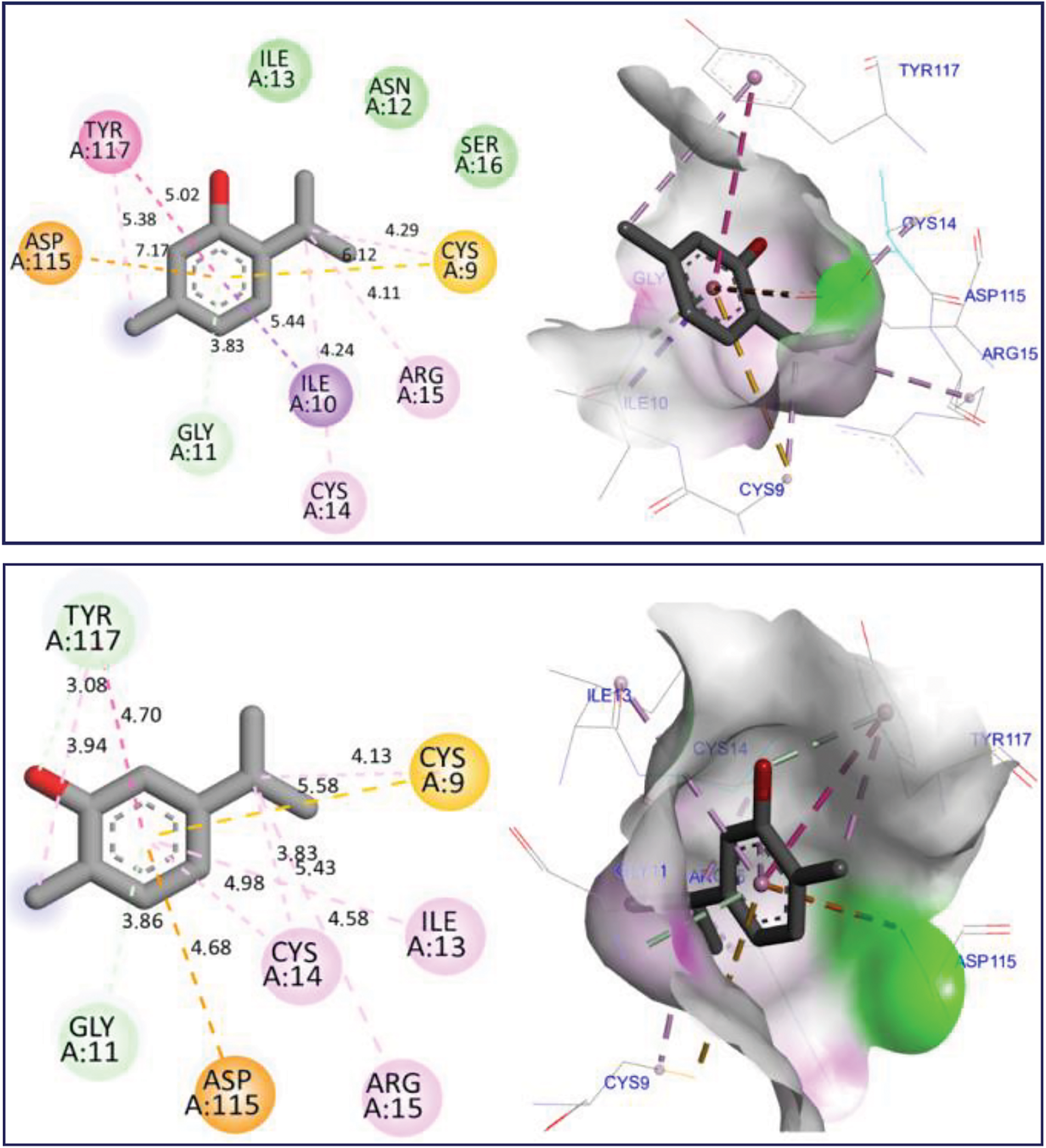
Figure 5: 2D and 3D views of molecular docking interactions against Erwinia amylovora pathogenic strain (4D74.pdb) for major compounds thymol and p-cymene-2-ol, respectively
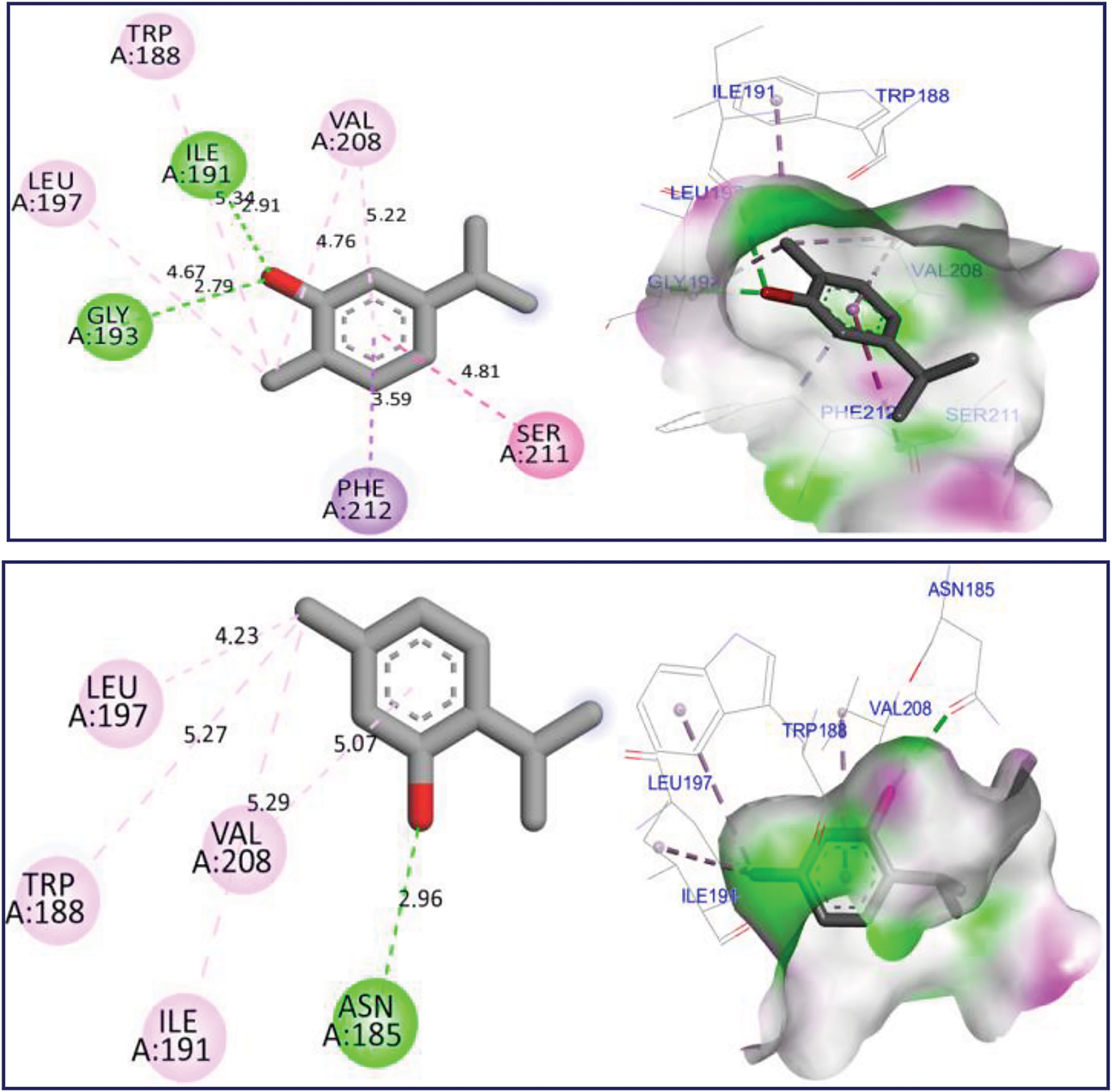
Figure 6: 2D and 3D views of molecular docking interactions against Agrobacterium tumefaciens (5J9R.pdb) pathogenic strain for thymol and p-cymene-2-ol, respectively
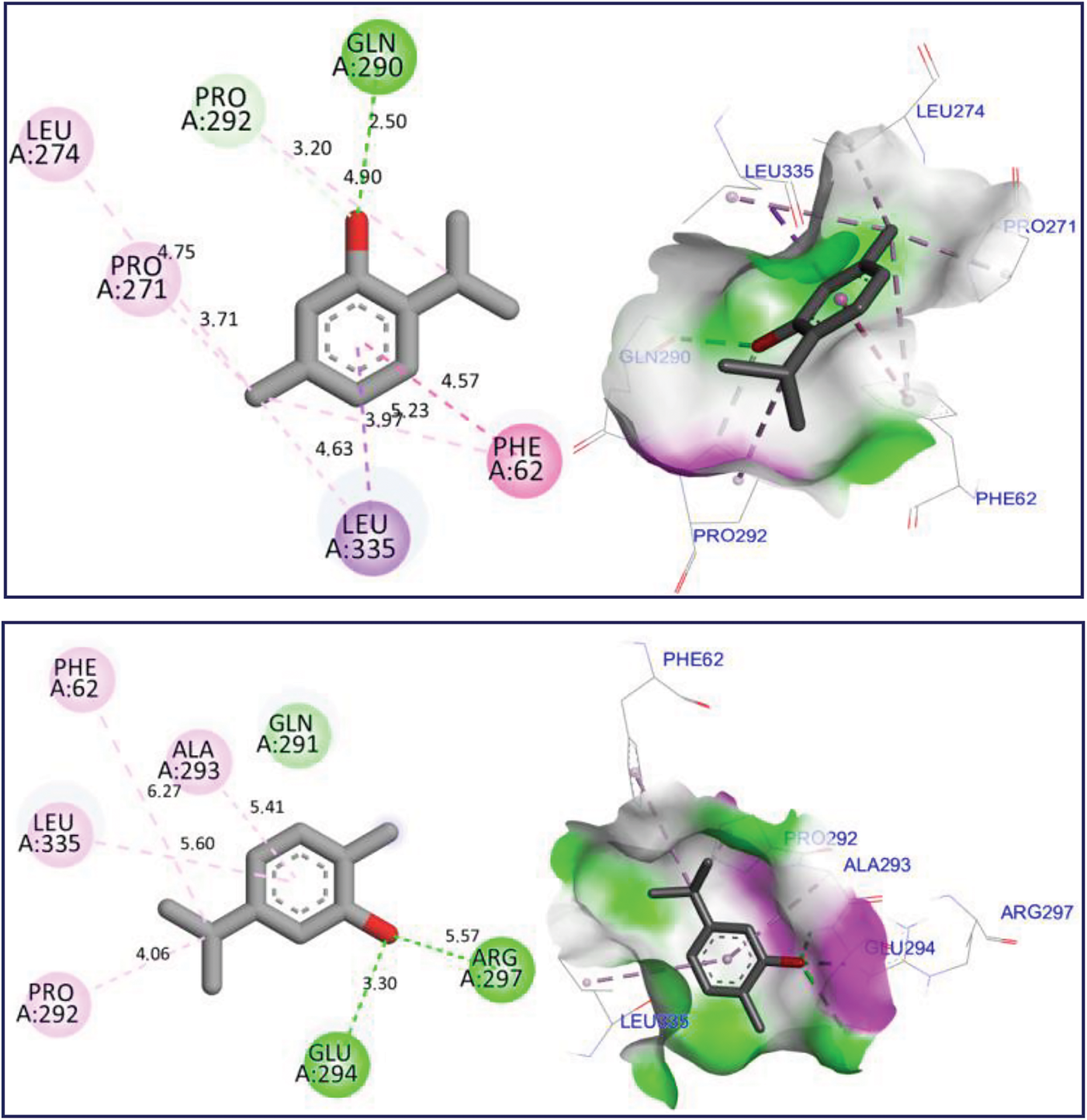
Figure 7: 2D and 3D views of molecular docking interactions against Allorhizobium vitis pathogenic strain (4WT7.pdb) for thymol and p-cymene-2-ol, respectively
O. elongatum EO has shown to be a novel and potent natural source of active substances with significant antibacterial and antioxidant properties. Through GC-MS analysis, a diverse array of chemical constituents, including phenolic compounds and monoterpenes, were identified in O. elongatum EO, with several known for their remarkable antibacterial and antioxidant properties. In vitro assays demonstrated strong antibacterial efficacy against various phytopathogenic bacteria such as Erwinia amylovora, Agrobacterium tumefaciens, and Allorhizobium vitis, as well as robust antioxidant properties. These dual activities suggest a possible correlation, as the antioxidant capacity may contribute to the oil’s antibacterial efficacy by mitigating oxidative stress in bacterial cells.
Furthermore, in silico molecular docking provided further insights into the interactions between the oil’s active components and bacterial targets, supporting its mode of action.
The combination of experimental and computational results suggests that O. elongatum EO could be a valuable natural agent in the fight against phytopathogens, offering a sustainable and eco-friendly solution for agricultural applications. Our results pave the way for the practical implementation of O. elongatum EO as an eco-friendly biocontrol agent in agricultural systems. Its potent antibacterial activity against phytopathogenic bacteria positions it as a viable alternative to synthetic antimicrobial drugs, which are often accompanied by environmental degradation and the emergence of resistant pathogens. By integrating this EO into crop management practices, farmers can enhance disease control while minimizing harmful chemical residues in the soil and water. Moreover, its antioxidant potential contributes to plant health and stress resilience, further supporting sustainable agriculture. This nature-based solution not only endorses ecological balance but also strengthens agricultural productivity and resilience, addressing key challenges in modern farming. Further studies, including field trials and toxicity assessments, are recommended to fully explore its practical applicability.
Acknowledgement: Princess Nourah Bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R158) Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study concept and design: Amine Batbat, Khaoula Habbadi and Hassane Greche; data acquisition: Amine Batbat, Mohamed El Fadili, Naoufal El Hachlafi, Samiah Hamad Al-Mijalli and Amine Elbouzidi; methodology: Amine Batbat, Mohamed Addi, Chaymae Hmimen, Hiba Yahyaoui, Samir Jeddi, Abdellatif Benbouazza and Samiah Hamad Al-Mijalli; software: Mohamed El Fadili, Samir Jeddi, Kawtar Fikri-Benbrahim and Mohamed Addi; analysis and interpretation of data: Naoufal El Hachlafi, Amine Batbat, Mohamed El Fadili and Samiah Hamad Al-Mijalli; drafting of the manuscript: Naoufal El Hachlafi and Amine Batbat; critical revision of the manuscript: Naoufal El Hachlafi, Kawtar Fikri-Benbrahim and Amine Elbouzidi, supervision: Khaoula Habbadi and Hassane Greche. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No datasets were generated or analyzed during this study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Salam MA, Al-Amin MY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare. 2023;11(13):1946. doi:10.3390/healthcare11131946. [Google Scholar] [PubMed] [CrossRef]
2. Yadav D, Rangabhashiyam S, Verma P, Singh P, Devi P, Kumar P, et al. Environmental and health impacts of contaminants of emerging concerns: recent treatment challenges and approaches. Chemosphere. 2021;272:129492. doi:10.1016/j.chemosphere.2020.129492. [Google Scholar] [PubMed] [CrossRef]
3. Verrillo M, Salzano M, Savy D, Di Meo V, Valentini M, Cozzolino V, et al. Antibacterial and antioxidant properties of humic substances from composted agricultural biomasses. Chem Biol Technol Agric. 2022;9:28. doi:10.1186/s40538-022-00291-6. [Google Scholar] [CrossRef]
4. Hüsnü Can Başer K, Buchbauer G. Handbook of essential oils: science, technology, and applications; 2015 [cited 2024 Jul 5]. Available from: https://www.cabidigitallibrary.org/doi/full/10.5555/20163380803. [Google Scholar]
5. Tajkarimi MM, Ibrahim SA, Cliver DO. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–218. doi:10.1016/j.foodcont.2010.02.003. [Google Scholar] [CrossRef]
6. Mutlu-Ingok A, Devecioglu D, Dikmetas DN, Karbancioglu-Guler F, Capanoglu E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: an updated review. Molecules. 2020;25:4711. doi:10.3390/molecules25204711. [Google Scholar] [PubMed] [CrossRef]
7. Bueno J, Demirci F, Baser KHC. Essential oils against microbial resistance mechanisms, challenges and applications in drug discovery. Essent Oils Nanotechnol Treat Microb Dis. 2017:143–58. doi:10.1201/9781315209241-6. [Google Scholar] [CrossRef]
8. Trifan A, Luca SV, Greige-Gerges H, Miron A, Gille E, Aprotosoaie AC. Recent advances in tackling microbial multidrug resistance with essential oils: combinatorial and nano-based strategies. Crit Rev Microbiol. 2020;46:338–57. [Google Scholar] [PubMed]
9. Lombrea A, Antal D, Ardelean F, Avram S, Pavel IZ, Vlaia L, et al. A recent insight regarding the phytochemistry and bioactivity of Origanum vulgare L. essential oil. Int J Mol Sci. 2020;21:9653. doi:10.3390/ijms21249653. [Google Scholar] [PubMed] [CrossRef]
10. Batbat A, Habbadi K, El Hachlafi N, El Allaoui N, Yahyaoui H, Ferioun M, et al. Unveiling germination conditions for Origanum elongatum: strategic insights into temperature, salinity, and pH for agricultural propagation and conservation management. Ecol Front. 2024:S2950509724001163. doi:10.1016/j.ecofro.2024.10.015. [Google Scholar] [CrossRef]
11. Oualili H, Nmila R, Chibi F, Lasky M, Mricha A, Rchid H. Chemical composition and antioxidant activity of Origanum elongatum essential oil. Phcog Res. 2019;11:283. doi:10.4103/pr.pr_157_18. [Google Scholar] [CrossRef]
12. Miere F, Vicas SI, Timar AV, Ganea M, Zdrinca M, Cavalu S, et al. Preparation and characterization of two different liposomal formulations with bioactive natural extract for multiple applications. Processes. 2021;9:432. doi:10.3390/pr9030432. [Google Scholar] [CrossRef]
13. Hrytsyk Y, Koshovyi O, Lepiku M, Jakštas V, Žvikas V, Matus T, et al. Phytochemical and pharmacological research in galenic remedies of Solidago canadensis L. Herb Phyton. 2024;93:2303–15. doi:10.32604/phyton.2024.055117. [Google Scholar] [CrossRef]
14. Król B, Kołodziej B, Kędzia B, Hołderna-Kędzia E, Sugier D, Luchowska K. Date of harvesting affects yields and quality of Origanum vulgare ssp. hirtum (Link) Ietswaart. J Sci Food Agric. 2019;99:5432–43. doi:10.1002/jsfa.9805. [Google Scholar] [PubMed] [CrossRef]
15. Assadpour E, Can Karaça A, Fasamanesh M, Mahdavi SA, Shariat-Alavi M, Feng J, et al. Application of essential oils as natural biopesticides; recent advances. Crit Rev Food Sci Nutr. 2024;64:6477–97. doi:10.1080/10408398.2023.2170317. [Google Scholar] [PubMed] [CrossRef]
16. Hrytsyk Y, Koshovyi O, Hrytsyk R, Ain R. Extracts of the Canadian goldenrod (Solidago canadensis L.)–promising agents with antimicrobial, anti-inflammatory and hepatoprotective activity. ScienceRise: Pharm Sci. 2024:78–87. doi:10.15587/2519-4852.2024.310837. [Google Scholar] [CrossRef]
17. Koshovyi O, Vlasova I, Jakštas V, Vilkickytė G, Žvikas V, Hrytsyk R, et al. American cranberry (Oxycoccus macrocarpus (Ait.) Pursh) leaves extract and its amino-acids preparation: the phytochemical and pharmacological study. Plants. 2023;12:2010. doi:10.3390/plants12102010. [Google Scholar] [PubMed] [CrossRef]
18. Boukhira S, Bousta F, Moularat S, Abdellaoui A, Ouaritini ZB, Bousta D. Evaluation of the preservative properties of origanum elongatum essential oil in a topically applied formulation under a challenge test. Phytothérapie. 2018;18:92–8. doi:10.3166/phyto-2018-0067. [Google Scholar] [CrossRef]
19. El Harsal A, Ibn Mansour A, Skali Senhaji N, Ouardy Khay E, Bouhdid S, Amajoud N, et al. Influence of extraction time on the yield, chemical composition, and antibacterial activity of the essential oil from Origanum elongatum (E. & M.) harvested at northern Morocco. J Essent Oil Bearing Plants. 2018;21:1460–74. doi:10.1080/0972060x.2019.1572545. [Google Scholar] [CrossRef]
20. Lamichhane JR, Osdaghi E, Behlau F, Köhl J, Jones JB, Aubertot J-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron Sustain Dev. 2018;38:28. doi:10.1007/s13593-018-0503-9. [Google Scholar] [CrossRef]
21. La Torre A, Iovino V, Caradonia F. Copper in plant protection: current situation and prospects. Phytopathol Mediterr. 2018;57:201–36. doi:10.14601/Phytopathol_Mediterr-23407. [Google Scholar] [CrossRef]
22. Faist H, Keller A, Hentschel U, Deeken R. Grapevine (Vitis vinifera) crown galls host distinct microbiota. Appl Environ Microb. 2016;82(18):5542–52. doi:10.1128/aem.01131-16. [Google Scholar] [CrossRef]
23. Habbadi K, Salma El Iraqui EL, Benbouazza A, Benali T. Rendement, caractérisation phytochimique et activité anti-Allorhizobium vitis des huiles essentielles de quatre espèces marocaines d’Eucalyptus. Afr Med Agric J-Al Awamia. 2022;137:87–101. [Google Scholar]
24. Ben Abdallah D, Frikha-Gargouri O, Tounsi S. Bacillus amyloliquefaciens strain 32a as a source of lipopeptides for biocontrol of Agrobacterium tumefaciens strains. J Appl Microbiol. 2015;119:196–207. doi:10.1111/jam.12797. [Google Scholar] [PubMed] [CrossRef]
25. Ryu DK, Adhikari M, Choi DH, Jun KJ, Kim DH, Kim CR, et al. Copper-based compounds against Erwinia amylovora: response parameter analysis and suppression of fire blight in apple. Plant Pathol J. 2023;39:52. doi:10.5423/PPJ.OA.07.2022.0100. [Google Scholar] [PubMed] [CrossRef]
26. El-Nashar HA, Eldehna WM, Al-Rashood ST, Alharbi A, Eskandrani RO, Aly SH. GC/MS analysis of essential oil and enzyme inhibitory activities of Syzygium cumini (Pamposia) grown in Egypt: chemical characterization and molecular docking studies. Molecules. 2021;26:6984. doi:10.3390/molecules26226984. [Google Scholar] [PubMed] [CrossRef]
27. Kačániová M, Galovičová L, Valková V, Tvrdá E, Terentjeva M, Žiarovská J, et al. Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation. Open Chem. 2021;19:214–27. doi:10.1515/chem-2021-0191. [Google Scholar] [CrossRef]
28. Lavin P, De Saravia SG, Guiamet P. Scopulariopsis sp. and Fusarium sp. in the documentary heritage: evaluation of their biodeterioration ability and antifungal effect of two essential oils. Microb Ecol. 2016;71:628–33. doi:10.1007/s00248-015-0688-2. [Google Scholar] [PubMed] [CrossRef]
29. Sipahi N, Kekeç AI, Halaç B. In vitro effect of some essential oils against multiple antibiotic-resistant bacteria from cats and dogs. Pak Vet J. 2022;42:561–5. [Google Scholar]
30. El Hachlafi N, Elbouzidi A, Batbat A, Taibi M, Jeddi M, Addi M, et al. Chemical composition and assessment of the anti-inflammatory, antioxidant, cytotoxic and skin enzyme inhibitory activities of Citrus sinensis (L.) osbeck essential oil and its major compound limonene. Pharmaceuticals. 2024;17:1652. doi:10.3390/ph17121652. [Google Scholar] [PubMed] [CrossRef]
31. Johari A, Khan MH. Evaluation of antioxidant activity of essential oils of some Indian medicinal plants by DPPH, FRAP and ABTS assay. J Pharm Negat Results. 2022;13:2602–7. [Google Scholar]
32. Anza M, Endale M, Cardona L, Cortes D, Eswaramoorthy R, Zueco J, et al. Antimicrobial activity, in silico molecular docking, ADMET and DFT analysis of secondary metabolites from roots of three ethiopian medicinal plants. AABC. 2021;14:117–32. doi:10.2147/AABC.S323657. [Google Scholar] [PubMed] [CrossRef]
33. Azzam KA. SwissADME and pkCSM webservers predictors: an integrated online platform for accurate and comprehensive predictions for in silico ADME/T properties of artemisinin and its derivatives. Complex Use Miner Resour. 2023;325:14–21. doi:10.31643/2023/6445.13. [Google Scholar] [CrossRef]
34. Kour J, Singh K. Virtual screening using the ligand ZINC database for novel lipoxygenase-3 inhibitors. Bioinformation. 2013;9:583. doi:10.6026/97320630009583. [Google Scholar] [PubMed] [CrossRef]
35. Satpute UM, Rohane SH. Efficiency of AUTODOCK: insilico study of pharmaceutical drug molecules; 2021 [cited 2025 Jan 8]. Available from: https://www.indianjournals.com/ijor.aspx?target=ijor:ajrc&volume=14&issue=1&article=016. [Google Scholar]
36. Xue Q, Liu X, Russell P, Li J, Pan W, Fu J, et al. Evaluation of the binding performance of flavonoids to estrogen receptor alpha by Autodock, Autodock Vina and Surflex-Dock. Ecotoxicol Environ Saf. 2022;233:113323. doi:10.1016/j.ecoenv.2022.113323. [Google Scholar] [PubMed] [CrossRef]
37. Sharma S, Sharma A, Gupta U. Molecular docking studies on the anti-fungal activity of Allium sativum (Garlic) against Mucormycosis (black fungus) by BIOVIA discovery studio visualizer 21.1.0.0. Ann Antivir Antiretrovir. 2021;5:028–32. [Google Scholar]
38. Alesawy MS, Elkaeed EB, Alsfouk AA, Metwaly AM, Eissa IH. In silico screening of semi-synthesized compounds as potential inhibitors for SARS-CoV-2 papain-like protease: pharmacophoric features, molecular docking, ADMET, toxicity and DFT studies. Molecules. 2021;26:6593. doi:10.3390/molecules26216593. [Google Scholar] [PubMed] [CrossRef]
39. Tagnaout I, Zerkani H, Bencheikh N, Amalich S, Bouhrim M, Mothana RA, et al. Chemical composition, antioxidants, antibacterial, and insecticidal activities of Origanum elongatum (Bonnet) emberger & maire aerial part essential oil from Morocco. Antibiotics. 2023;12:174. doi:10.3390/antibiotics12010174. [Google Scholar] [PubMed] [CrossRef]
40. Bakha M, El Mtili N, Machon N, Aboukhalid K, Amchra FZ, Khiraoui A, et al. Intraspecific chemical variability of the essential oils of Moroccan endemic Origanum elongatum L. (Lamiaceae) from its whole natural habitats. Arab J Chem. 2020;13:3070–81. doi:10.1016/j.arabjc.2018.08.015. [Google Scholar] [CrossRef]
41. Ramzi H, Ismaili MR, Aberchane M, Zaanoun S. Chemical characterization and acaricidal activity of Thymus satureioides C. & B. and Origanum elongatum E. & M. (Lamiaceae) essential oils against Varroa destructor Anderson & Trueman (Acari: Varroidae). Ind Crops Prod. 2017;108:201–7. doi:10.1016/j.indcrop.2017.06.031. [Google Scholar] [CrossRef]
42. Moussaoui N. Antibacterial and antiviral activities of essential oils of northern Moroccan plants. BBJ. 2013;3:318–31. doi:10.9734/BBJ/2013/3596. [Google Scholar] [CrossRef]
43. Figueredo G. Etude chimique et statistique de la composition d’huiles essentielles d’origans (Lamiaceae) cultivés issus de graines d’origine méditerranéenne [Internet] [Ph.D. thesis]. France: Université Blaise Pascal-Clermont-Ferrand II; 2007 [cited 2024 Jul 2]. Available from: https://theses.hal.science/tel-00717749/. [Google Scholar]
44. Figuérédo G, Cabassu P, Chalchat J-C, Pasquier B. Studies of mediterranean oregano populations. VI: chemical composition of essential oils of Origanum elongatum Emberger et Maire from Morocco. J Essent Oil Res. 2006;18:278–80. [Google Scholar]
45. Balahbib A, El Omari N, Hachlafi NEI, Lakhdar F, El Menyiy N, Salhi N, et al. Health beneficial and pharmacological properties of p-cymene. Food Chem Toxicol. 2021;153:112259. doi:10.1016/j.fct.2021.112259. [Google Scholar] [PubMed] [CrossRef]
46. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46:446–75. doi:10.1016/j.fct.2007.09.106. [Google Scholar] [PubMed] [CrossRef]
47. Calo JR, Crandall PG, O’Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems—a review. Food Control. 2015;54:111–9. doi:10.1016/j.foodcont.2014.12.040. [Google Scholar] [CrossRef]
48. Hazrati S, Mousavi Z, Nicola S. Harvest time optimization for medicinal and aromatic plant secondary metabolites. Plant Physiol Biochem. 2024;108735. doi:10.1016/j.plaphy.2024.108735. [Google Scholar] [PubMed] [CrossRef]
49. Hassan EA, El-Ouazna PB, Naima PEG. Evaluation de l’activité antibactérienne de certaines huiles essentielles sur Allorhizobium vitis, Agrobacterium tumefaciens et Erwinia amylovora “in vitro”; 2021. [Google Scholar]
50. Akhlaghi M, Tarighi S, Taheri P. Effects of plant essential oils on growth and virulence factors of Erwinia amylovora. J Plant Pathol. 2020;102:409–19. [Google Scholar]
51. Basım E, Basım H. Note: evaluation of antibacterial activity of essential oil of Rosa damascena on Erwinia amylovora. Phytoparasitica. 2004;32:409–12. doi:10.1007/BF02979853. [Google Scholar] [CrossRef]
52. Mihajilov-Krstev T, Radnović D, Kitić D. Antimicrobial activity of Satureja L. essential oils against phytopathogenic bacteria Erwinia amylovora. Biologica Nyssana. 2010;1:95–8. doi:10.2478/V10133-009-0018-2. [Google Scholar] [CrossRef]
53. Doukkali E, Radouane N, Tahiri A, Tazi B, Guenoun F, Lahlali R. Chemical composition and antibacterial activity of essential oils of Cinnamomum cassia and Syzygium aromaticum plants and their nanoparticles against Erwinia amylovora. Arch Phytopathol Plant Prot. 2022;55:217–34. doi:10.1080/03235408.2021.2015865. [Google Scholar] [CrossRef]
54. Habbadi K, Meyer T, Vial L, Gaillard V, Benkirane R, Benbouazza A, et al. Essential oils of Origanum compactum and Thymus vulgaris exert a protective effect against the phytopathogen Allorhizobium vitis. Environ Sci Pollut Res. 2018;25:29943–52. doi:10.1007/s11356-017-1008-9. [Google Scholar] [PubMed] [CrossRef]
55. Habbadi K, Sabri M, Benbouazza A, Vial L, Lavire C, Kerzaon I, et al. La composition chimique et l’activité antibactérienne des huiles essentielles de quatre plantes aromatiques et médicinales marocaines contre Allorhizobium vitis. Afr Mediterr Agric J-Al Awamia. 2021;131:117–35. [Google Scholar]
56. Khaoula H, El Iraqui EL, Abdellatif B, Taoufiq B, El Hassan A. Yield, phytochemical characterization and anti-Allorhizobium vitis activity of essential oils from four eucalyptus species growing in Morocco; 2022 [cited 2024 Jun 30]. Available from: https://www.researchgate.net/profile/Khaoula-Habbadi/publication/366881083_Yield_phytochemical_characterization_and_anti-_Allorhizobium_vitis_activity_of_essential_oils_from_four_Eucalyptus_species_growing_in_Morocco/links/63b6c48a097c7832ca9192fb/Yield-phytochemical-characterization-and-anti-Allorhizobium-vitis-activity-of-essential-oils-from-four-Eucalyptus-species-growing-in-Morocco.pdf. [Google Scholar]
57. Abd-El-Aziz R, Abd El-Rahman A, Hendi D. Control of Agrobacterium tumefaciens with essential oils compared to antagonistic agrobacterium radiobacter strain K84. Egypt J Phytopathol. 2021;49:80–92. [Google Scholar]
58. Hsouna AB, Touj N, Hammami I, Dridi K, Al-Ayed AS, Hamdi N. Chemical composition and in vivo efficacy of the essential oil of Mentha piperita L. in the suppression of crown gall disease on tomato plants. J Oleo Sci. 2019;68:419–26. doi:10.5650/jos.ess18261. [Google Scholar] [PubMed] [CrossRef]
59. Badawy MEI, Abdelgaleil SAM. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind Crops Prod. 2014;52:776–82. doi:10.1016/j.indcrop.2013.12.003. [Google Scholar] [CrossRef]
60. Paudel PN, Satyal P, Satyal R, Setzer WN, Gyawali R. Chemical composition, enantiomeric distribution, antimicrobial and antioxidant activities of Origanum majorana L. Essent Oil Nepal Mol. 2022;27:6136. doi:10.3390/molecules27186136. [Google Scholar] [PubMed] [CrossRef]
61. Mechergui K, Coelho JA, Serra MC, Lamine SB, Boukhchina S, Khouja ML. Essential oils of Origanum vulgare L. subsp. glandulosum (Desf.) Ietswaart from Tunisia: chemical composition and antioxidant activity. J Sci Food Agric. 2010;90:1745–9. doi:10.1002/jsfa.4011. [Google Scholar] [PubMed] [CrossRef]
62. Han F, Ma G, Yang M, Yan L, Xiong W, Shu J, et al. Chemical composition and antioxidant activities of essential oils from different parts of the oregano. J Zhejiang Univ Sci B. 2017;18:79–84. doi:10.1631/jzus.B1600377. [Google Scholar] [PubMed] [CrossRef]
63. Al-Mijalli SH, Mrabti NN, Ouassou H, Sheikh RA, Assaggaf H, Bakrim S, et al. Chemical composition and antioxidant, antimicrobial, and anti-inflammatory properties of origanum compactum benth essential oils from two regions: in vitro and in vivo evidence and in silico molecular investigations. Molecules. 2022;27:7329. doi:10.3390/molecules27217329. [Google Scholar] [PubMed] [CrossRef]
64. López PL, Enemark GKG, Grosso NR, Olmedo RH. Antioxidant effectiveness between mechanisms of “Chain breaking antioxidant” and “Termination enhancing antioxidant” in a lipid model with essential oils. Food Biosci. 2024;57:103498. doi:10.1016/j.fbio.2023.103498. [Google Scholar] [CrossRef]
65. Yanishlieva NV, Marinova EM, Gordon MH, Raneva VG. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999;64:59–66. doi:10.1016/S0308-8146(98)00086-7. [Google Scholar] [CrossRef]
66. Sarikurkcu C, Zengin G, Oskay M, Uysal S, Ceylan R, Aktumsek A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind Crops Prod. 2015;70:178–84. doi:10.1016/j.indcrop.2015.03.030. [Google Scholar] [CrossRef]
67. Foti MC, Ingold KU. Mechanism of inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J Agric Food Chem. 2003;51:2758–65. doi:10.1021/jf020993f. [Google Scholar] [PubMed] [CrossRef]
68. Li G, Ye C, Zhu Y, Zhang T, Gu J, Pan J, et al. Oxidative injury in ischemic stroke: a focus on NADPH oxidase 4. Oxid Med Cell Longev. [Google Scholar]
69. Sindhu RK, Kaur P, Kaur P, Singh H, Batiha GE-S, Verma I. Exploring multifunctional antioxidants as potential agents for management of neurological disorders. Environ Sci Pollut Res. 2022;29:24458–77. [Google Scholar]
70. Karakoti H, Mahawer SK, Tewari M, Kumar R, Prakash O, de Oliveira MS, et al. Phytochemical profile, in vitro bioactivity evaluation, in silico molecular docking and ADMET study of essential oils of three Vitex species grown in Tarai Region of Uttarakhand. Antioxidants. 2022;11:1911. doi:10.3390/antiox11101911. [Google Scholar] [PubMed] [CrossRef]
71. Alminderej F, Bakari S, Almundarij TI, Snoussi M, Aouadi K, Kadri A. Antioxidant activities of a new chemotype of Piper cubeba L. fruit essential oil (Methyleugenol/Eugenolin silico molecular docking and ADMET studies. Plants. 2020;9:1534. doi:10.3390/plants9111534. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools