 Open Access
Open Access
ARTICLE
Cerium Oxide Nanoparticles Alleviate Enhanced UV-B Radiation-Induced Stress in Wheat Seedling Roots by Regulating Reactive Oxygen Species
1 College of Chemistry and Materials Science, Shanxi Normal University, Taiyuan, 030000, China
2 College of Life Sciences, Shanxi Normal University, Taiyuan, 030000, China
3 Shanxi Provincial Key Laboratory of Plant Macromolecular Adversity, Taiyuan, 030000, China
* Corresponding Author: Rong Han. Email:
Phyton-International Journal of Experimental Botany 2025, 94(2), 455-479. https://doi.org/10.32604/phyton.2025.061462
Received 25 November 2024; Accepted 23 January 2025; Issue published 06 March 2025
Abstract
Enhanced UV-B radiation represents a major environmental factor impacting global cereal production. Researchers have explored various approaches to reduce the detrimental impact of UV-B radiation on crops. Recently, engineered nanoparticles, particularly cerium oxide nanoparticles (CeO2-NPs), have attracted widespread interest for their ability to boost plant tolerance to a range of abiotic stresses. This study investigates how CeO2-NPs application affects the morphology, physiology, biochemistry, and transcriptomics profiles of wheat seedling roots subjected to enhanced UV-B stress. The findings demonstrate that CeO2-NPs notably promoted root length, fresh and dry weights, and root activity (p < 0.05) under enhanced UV-B stress. CeO2-NP treatment reduced the content of hydrogen peroxide (H2O2) and malondialdehyde (MDA) in wheat, alleviating oxidative damage in seedling roots and partially restoring the root phenotype. Under non-UV-B stress conditions, CeO2-NP treatment triggered the difference of 237 transcripts in plants relative to the control group. Under enhanced UV-B stress, CeO2-NP treatment exhibited differentially expressed genes (DEGs) linked to the antioxidant defense mechanism responsible for reactive oxygen species (ROS) scavenging, compared to the non-nanoparticle control. This suggests that ROS scavenging may be a key mechanism by which CeO2-NPs enhance wheat resistance to enhanced UV-B radiation. This study elucidates a potential molecular mechanism through which CeO₂ nanoparticles may enhance wheat tolerance to UV-B stress.Keywords
Supplementary Material
Supplementary Material FileIn recent decades, human activities, such as increased use of refrigerators and automobile emissions, have led to a rise in bromine- and chlorine-containing compounds in the environment, including chlorofluorocarbons (CFCs). This has exacerbated ozone depletion, resulting in increased solar radiation dominated by UV-B [1,2]. UV-B radiation can be absorbed by essential molecules (lipids, proteins, hormones, and nucleic acids) in terrestrial plants, causing such as lipid peroxidation at the molecular level. This affects plant physiology and morphology (e.g., reduced plant height and inhibited root growth), ultimately leading to decreased cereal yield [3–5]. UV-B radiation induces physiological alterations in plants primarily through the production of ROS and the subsequent oxidative stress. These highly cytotoxic ROS, including superoxide anions (O2•−), hydroxyl radical (•OH), H2O2, and singlet oxygen (1O2), can damage cellular components such as membrane lipids, nucleic acids, and proteins [6]. To counteract the radical-induced damage caused by UV-B stress, plants have evolved both enzymatic and non-enzymatic antioxidant mechanisms. The enzymatic defense system consists of enzymes like superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), and glutathione reductase (GR), while the latter comprises flavonoids, ascorbic acid (AsA), glutathione (GSH), and carotenoids. For improved plant resistance to UV-B stress, maintaining equilibrium in ROS levels and regulating antioxidant activity is vital [7].
Engineered nanoparticles have demonstrated significant potential in recent years for enhancing crop yields to address future global food demands, owing to their capacity to promote plant growth and augment stress resistance. Consequently, their application in agricultural research has increased [8]. Among these, CeO2-NPs are particularly noteworthy. When applied at appropriate dosages, they promote plant growth through various mechanisms, primarily by enhancing photosynthetic pigments, improving photosynthesis, balancing mineral nutrition, promoting root elongation, and increasing biomass [9]. Plant responses to CeO2-NPs are influenced by their physicochemical characteristics, level, type, and the growth conditions of the crops [10–13]. Notably, CeO2-NPs are capable of replicating the functions of several antioxidant enzymes, including SOD, CAT, POD, and oxidase, due to the Ce3+ and Ce4+ surface dangling bonds, thus functioning as effective scavengers of ROS [14]. In nanomedicine, abnormal ROS accumulation leads to oxidative stress and inflammation, which are pathological foundations of numerous chronic diseases, including cancer and neurodegenerative diseases. CeO₂-NPs, with their broad-spectrum catalytic antioxidant activity, can target ROS clearance and possess self-regeneration ability, making them ideal candidates for purposes in nanobiology and tissue regeneration [15]. Furthermore, their excellent biocompatibility has promoted the integration of ceria NPs in nanomedicine. For instance, Yang et al. enhanced pharmacological bioavailability by manipulating the surface property of ceria nanocages, proposing a nanomedicine strategy for treating chemical eye injuries [16]. In the field of agriculture, previous research has demonstrated that CeO2-NPs can catalyze the scavenging of ˙OH in plants and notably increase K+ content, thereby boosting the photosynthetic efficiency of Arabidopsis in the presence of salt stress [17]. Moreover, CeO2-NPs can boost the inherent antioxidant defense ability of plants and improve their resistance to exposure to various abiotic stressors [14,18]. For instance, Mohammadi et al. demonstrated that CeO2-NPs significantly improved the agronomic traits and photosynthetic pigment components of Moldavian balm in the presence of salt stress by boosting the action of antioxidant enzymes [such as APX, guaiacol peroxidase (GP), SOD] and proline content, as reducing electrolyte leakage (EL), MDA, and H2O2 levels [19]. Similarly, Gohari et al. reported that CeO2-NP treatment enhanced antioxidant levels and stabilized the ratios of the elements in the presence of salt stress, helping grapevines (Vitis vinifera L) mitigate salt stress-induced damage [20]. CeO2-NPs also reduce oxidative stress and promote plant growth in sorghum under drought stress and in Vigna radiata under mercury stress [21,22].
Wheat has emerged as a crucial raw material for various food products, including bread, and holds a strategic position among global crops due to its capacity to provide carbohydrates, proteins, and other essential nutrients, as well as its extensive cultivation area and high yield [23,24]. C3 plants, such as wheat, display heightened susceptibility to UV-B in contrast to C4 plants, with the early seedling stage being particularly vulnerable to environmental stress [25]. While previous research has primarily concentrated on the impact of UV-B on the plants’ aboveground portions, limited knowledge exists regarding the impact it has on root structure and development from a molecular perspective [26]. Plant roots serve a pivotal function in growth and progression by absorbing water and nutrients, delivering them to aboveground parts, and releasing specific hormones and organic compounds, hence acting as a critical link between plants and their growing environment. Consequently, this study selected wheat seedling roots as the primary research subject.
The aforementioned findings underscore the critical roles of ROS production and scavenging in the consequences of UV-B and CeO2-NPs on plant progression. We hypothesized that CeO2-NPs might enhance plants’ ability to scavenge ROS by modulating the antioxidant defence system, thereby improving seedling tolerance to enhanced UV-B and promoting plant progression under adverse conditions. To explore the implications of CeO2-NP application on root development in wheat seedlings under UV-B, we employed molecular biology, physiological, and biochemical analytical methods. Specifically, the research content was as follows: (1) seedling phenotype and physiological indicators, (2) absorption of CeO2-NPs by seedlings, (3) root vitality, (4) H2O2 and MDA contents, and (5) transcriptome profile changes in the antioxidant defence system associated with ROS scavenging. This study elucidated the relationship between CeO2-NP-induced changes in wheat seedling genetic control mechanisms governing developmental processes and antioxidant defence pathways.
2.1 Characterization of Nanomaterials
The CeO2-NPs nanomaterial was acquired from Beijing Dekedao Gold Technology Co., Ltd. (Beijing, China). Scanning electron microscope (SEM, FEI G2F20, USA) and transmission electron microscopy (TEM, FEI Talos F200X, USA) were implemented to investigate the morphology of nanomaterials. The SEM image was provided by Beijing Dekedao Gold Technology Co., Ltd. X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, USA) was applied to detect the exterior elements.
2.2 Preparation of Nanomaterials Suspension
Suspensions of CeO2-NPs were made in ultrapure water within varying concentrations (0, 20, 60, 100, 120, 140, and 160 mg/L) and subjected to sonication in a 25°C water bath for 1 h.
2.3 Plant Cultivation and Treatment
“Jinmai-79” wheat (Triticum aestivum L.) seeds, obtained from the Shanxi Academy of Agricultural Sciences’ Wheat Research Institute, were surface-sterilized with 1.5% sodium hypochlorite for 10 min and rinsed with sterile water. After 24 h of dark incubation at 25°C on moist gauze to induce germination (before coleoptile emergence), at least 30 germinated seeds were transferred to gauze-lined Petri dishes. The seedlings were cultivated in an intelligent light incubator (TOP Cloud-agri Technology Co., Ltd., Zhejiang, China) at 25°C (16 h)/18°C (8 h) for 5 d. The Petri dishes containing wheat seeds were categorized into four groups. Untreated seedlings served as controls (CK). Plants cultured in Petri dishes containing 120 mg/L CeO2-NPs solution were designated as the CeO2-NP treatment group (CeO2-NPs). Wheat subjected to UV-B for 5 d by suspending a UV lamp (FLB30T8E/5C, Nanjing Huaqiang Electronics Co., Ltd., China) at an appropriate distance above the plants, with the dose adjusted to 10.08 KJ/m2, comprised the UV-B treatment group. Wheat treated with CeO2-NPs combined with enhanced UV-B was classified as the CeO2-NPs+UV-B group.
2.4 Measurement of Wheat Root Length and Biomass
A minimum of 30 plants were randomly chosen from each experimental group. Surface moisture was removed from these seedlings using filter paper before phenotypic measurements. A steel ruler was employed to measure plant height and root length. The fresh, dry weights of each plant were measured by a BT25S electronic analytical scale (Sartorius Scientific Instruments Co., Ltd., Beijing, China).
2.5 Determination of Ce Content in Wheat
The wheat seedling tissues were thoroughly washed with distilled water and desiccated at 70°C for 48 h. Following desiccation, the samples underwent digestion with HNO3 and H2O2 (4:1) at 120°C for 1 h. The Ce concentration in the resulting digest was analyzed using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (NexION 300D, Perkin Elmer, Waltham, MA, USA).
The H2O2 concentration was measured following Yu’s method [27]. Root samples were ground with cold acetone (1.5 mL), and then centrifuged. 1 mL of supernatant was mixed with 0.1 mL of 5% (w/v) TiSO4 and 0.1 mL of cold ammonia. After a 10 min incubation, the mixture was centrifuged (3000 g, 10 min). The resulting pellet was then redissolved in 4 mL of 2 M H2SO4, and absorbance was measured at 415 nm using a spectrophotometer (Shanghai Yidian Analysis Instrument Co., Ltd., Shanghai, China). H2O2 content was determined using a standard curve generated with known H2O2 concentrations.
2.7 Antioxidant Enzyme-Mimicking Activities of CeO2-NPs and Determination of Wheat MDA Content, Root Vitality, and Antioxidant Activity
Wheat taproots from distinct treatment groups were separately chopped and homogenized. A 0.1 g sample of each mixture was utilized for the assessment of physiological and biochemical indices. The SOD-mimicking and CAT-mimicking activities of CeO2-NPs, along with the MDA concentration, root vitality, enzymatic activities of antioxidants (SOD, POD, APX, CAT, and GR), and non-enzymatic antioxidant (flavonoid, AsA, and GSH) contents of wheat were quantified by enzyme-linked immunosorbent assay (ELISA) kits (Jiangsu Jingmei Biotechnology Co., Ltd., Yancheng, China).
2.8 Transcriptome Sequencing Analysis
RNA was extracted from fresh wheat seedlings roots from each treatment group using the TRIzol method, with three biological replicates per group utilized for the RNA-Seq experiment. Libraries were constructed following the NEB standard library method employing the NEBNext Ultra RNA Library Prep Kit for Illumina. The cDNA library underwent sequencing on the Illumina NovaSeq 6000 platform (Illumina, USA). Raw data went through filtering to eliminate adapter-containing reads, reads with N, and low-quality reads, resulting in clean data. HISAT2 aligned the clean reads to the wheat reference genome (https://ftp.ncbi.nlm.nih.gov/genomes/all/GCF/018/294/505/GCF_018294505.1_IWGSC_CS_RefSeq_v2.1/GCF_018294505.1_IWGSC_CS_RefSeq_v2.1_genomic.fna.gz, accessed on 20 December 2024). Gene expression levels in each library were normalized to fragments per kilobase of the exon model per million mapped reads (FPKM). Differential expression analysis employed DESeq2 software (version 1.20.0), identifying differentially expressed genes (DEGs) with criteria of |log2FoldChange| > 1 and p < 0.05.
To confirm the transcriptome analysis results’ reliability, RNA was extracted from the roots of plants, and cDNA was synthesized via reverse transcription using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, China). Gene expression changes were analyzed with the QuantStudio 3 Real-Time PCR System (Applied Biosystems, Wakefield, RI, USA), and relative expression levels were determined employing the comparative 2−ΔΔCt method [28], with ACTIN serving as the internal reference gene. Table S1 contains all primer sequences.
Triplicate data were subjected to one-way ANOVA based on Newman–Keuls multiple comparison tests (p ≤ 0.05). Data processing and figure creation were carried out with GraphPad Prism 5.
3.1 Characterization of CeO2-NPs
As depicted in the figure, CeO2-NPs exhibited predominantly a uniformly dispersed sphere morphology with an approximate particle size of 20 nm (Fig. 1a,b). The XPS analysis revealed that the Ce over CeO2-NPs existed in both Ce3+ and Ce4+ forms (Fig. 1c). Binding energies at 882.06, 885.37, 897.91, 900.07, 907.05, and 916.1 eV were attributed to Ce4+, while those at 888.53 and 903.19 eV corresponded to Ce3+. Subsequent calculations indicated that the area ratio (i.e., molar ratio) of Ce3+ to Ce4+ was approximately 0.19.
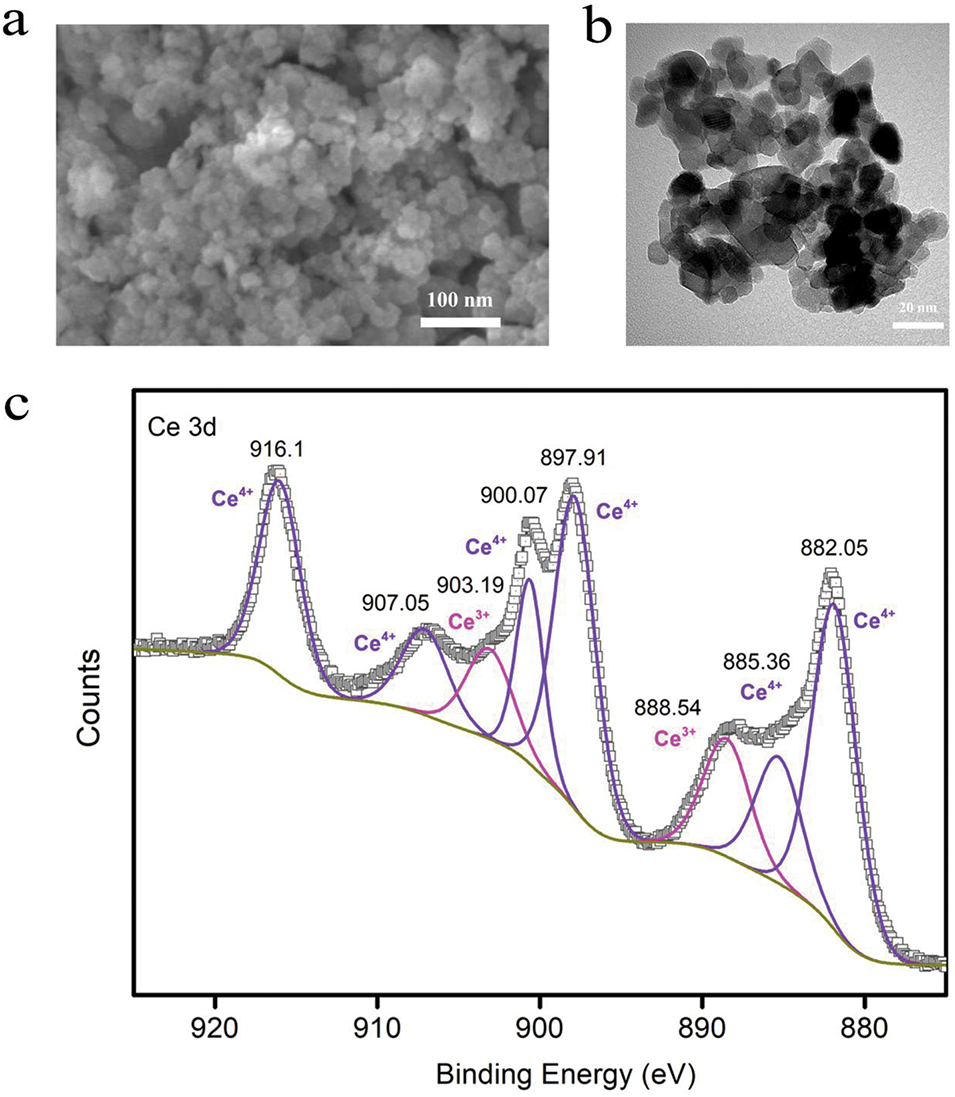
Figure 1: Characterization of CeO2-NPs: (a) SEM image of CeO2-NPs (100 nm), (b) TEM image of CeO2-NPs (20 nm), and (c) XPS narrow spectrum of CeO2-NPs
3.2 Effects of UV-B and CeO2-NP Treatments on Wheat Growth
Initially, various CeO2-NPs concentrations (0, 20, 60, 100, 120, 140, and 160 mg/L) were applied to wheat for 5 days to evaluate the impact on seedling growth. The wheat seedling phenotype diagram illustrates that different concentrations of CeO2-NPs enhanced plant growth to varying degrees (Fig. S1a) in comparison to the control group. Statistical analysis of root length and plant height revealed that root length in the C1, C2, C3, C4, C5 and C6 treatment groups increased by 11.1%, 17.4%, 29.9%, 48%, 33.5%, and 10.04%, respectively, relative to the control group (p < 0.05). However, plant height remained relatively unchanged (Fig. S1b,c). Based on these results, 120 mg/L CeO2-NPs was chosen for continued research thanks to its most significant promotion of root length.
The impact of CeO2-NPs on UV-stressed wheat was assessed by measuring root length, fresh and dry biomass, and root viability in each group of seedlings. Compared to the CK group, the phenotype diagram revealed significant inhibition of wheat seedling root growth and impeded root development under UV-B (Fig. 2a), UV-B exposure significantly reduced root length, fresh and dry biomass, and root vitality (p < 0.05). In contrast, CeO2-NP treatment alone promoted root elongation, increased root weights, and enhanced root vitality. Under elevated UV-B treatment, adding CeO2-NPs effectively mitigated the adverse effects of enhanced UV-B on seedlings, with increases in root length (76.3%), root fresh weight (52.1%), dry weight (38.5%), and root vitality (42.5%) compared to the UV-B treatment (Fig. 2b–f). Notably, a CeO2 treatment group (CeO2) and enhanced UV-B combined with a CeO2 treatment group (CeO2+UV-B) were employed to determine whether the UV resistance effect of CeO2-NPs was linked to the properties of the nanomaterial itself or the CeO2 substance. The concentration of the solution, UV-B dose, and plant growth conditions were consistent with those of the CeO2-NPs group and CeO2-NPs+UV-B groups, with only the added substances differing. Neither the CeO2 treatment alone nor the simultaneous augmentation UV-B and CeO2 processing significantly impacted wheat seedling root length, biomass, or vitality. This suggests that the nanomaterial, rather than CeO2, facilitated the enhancement of UV stress resistance in wheat. This can be attributed to nanomaterials possessing a large specific surface area and high reactivity, with significantly superior physicochemical and biological properties compared to conventional materials [29].
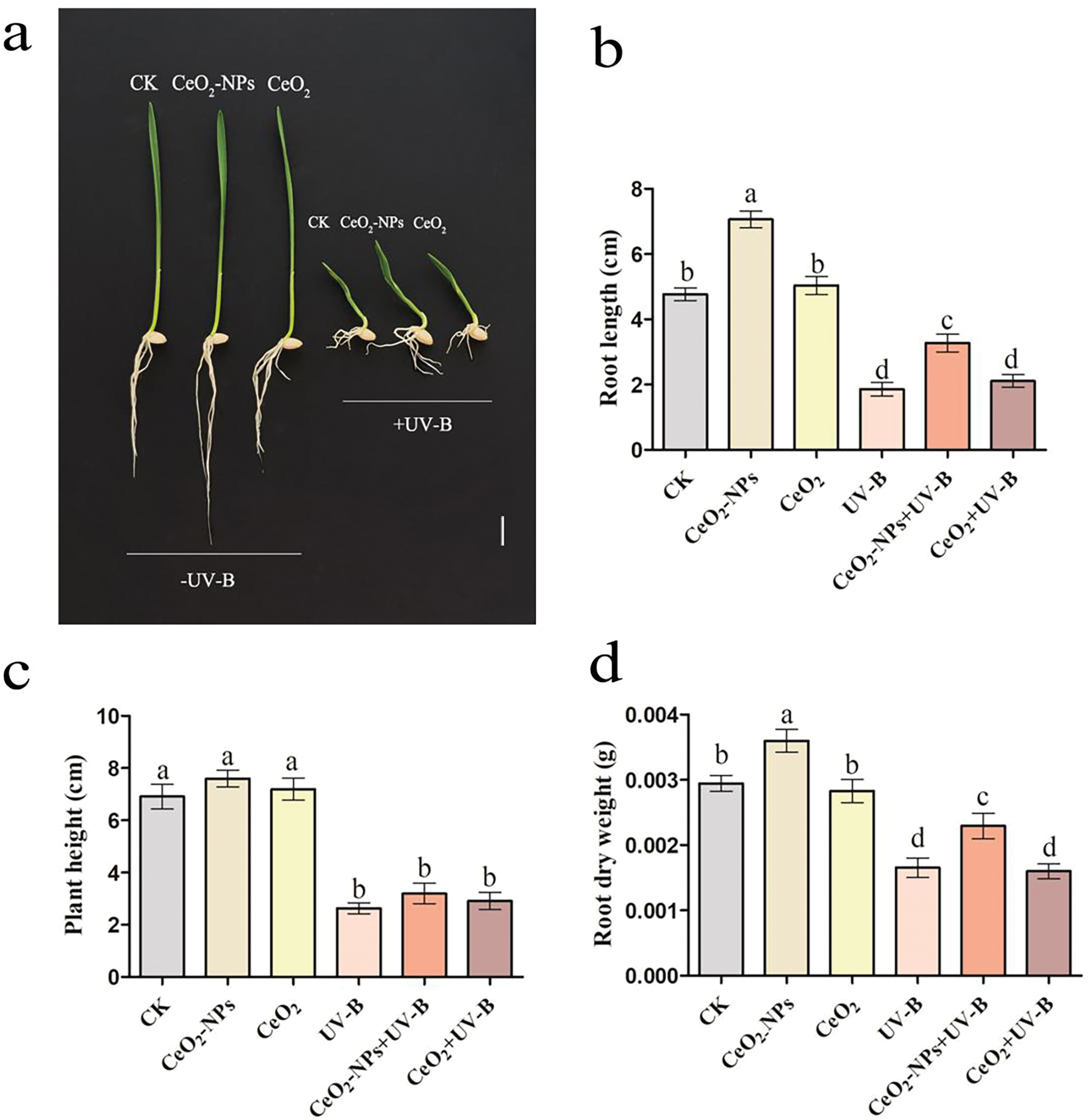
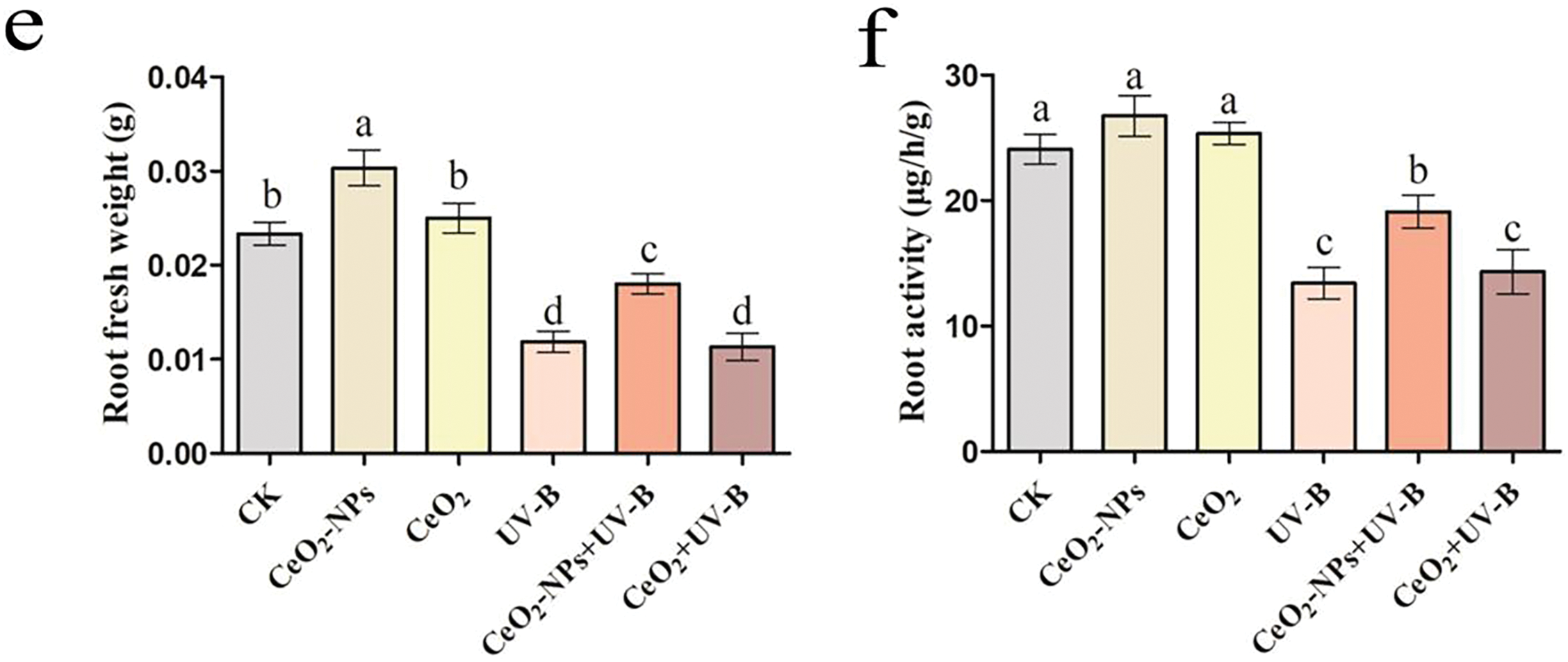
Figure 2: Influence of UV-B and CeO2-NP application to wheat development: (a) phenotype, (b) root length, (c) root fresh mass, (d) root dry mass, (e) root viability, and (f) root activity. Statistically significant differences are indicated by different letters (p < 0.05). Scale bar: 1 cm
3.3 Uptake of CeO2-NPs by Wheats
The Ce content in wheat roots, stems, and leaves was quantified to assess the absorption and transfer of CeO2-NPs by wheat. The analysis revealed that the Ce content in different parts of wheats intervened with CeO2-NPs was markedly higher than other treatment groups (p < 0.05) (Fig. 3).
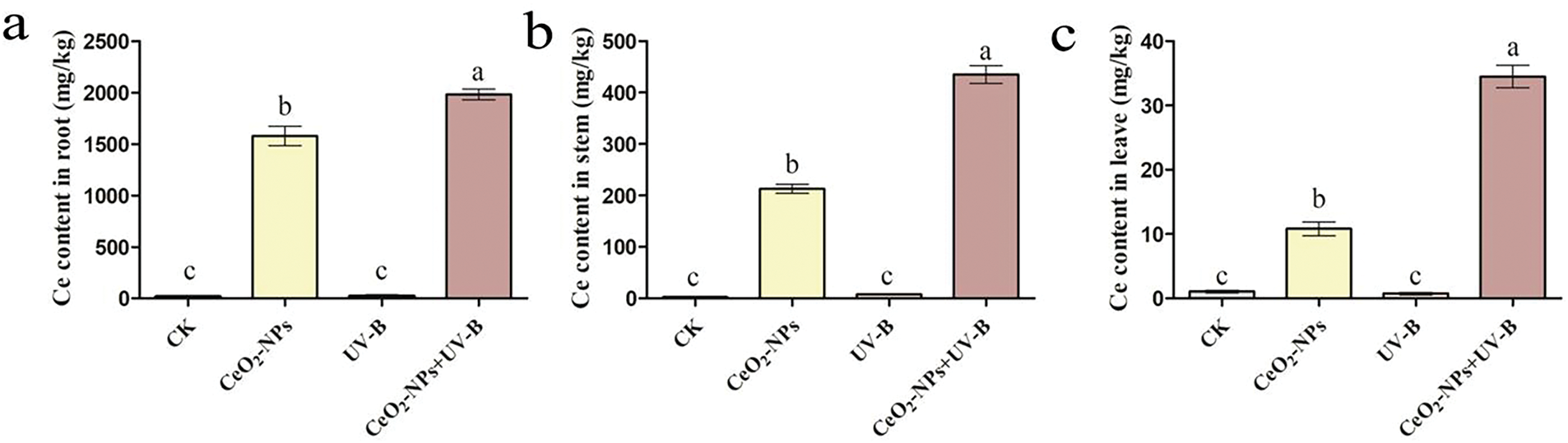
Figure 3: The effects of various therapies on cerium (Ce) content in wheat, specifically in (a) roots, (b) stems, and (c) leaves. Statistically distinct letters signify significant differences (p < 0.05)
3.4 Antioxidant Enzyme-Mimicking Activities of CeO2-NPs
O2•− is among the most destructive ROS generated in abiotically stressed plants. SOD is essential for catalyzing the conversion of O2•− into H2O2 and oxygen (O2). CAT is a key ROS-scavenging enzyme that decomposes H2O2 into H2O and O2 [30]. CeO2-NPs at 120 mg/L showed excellent performance in scavenging ROS (O2•−, H2O2), with SOD-mimicking activity and CAT-mimicking activity measured at 18.62 and 35.58 U/g, respectively.
3.5 Cellular Damage Indicators (H2O2 and MDA Contents) in Wheat
H2O2, as a representative of ROS, can oxidize proteins and react with O2•− to produce highly reactive •OH, which has strong reactivity toward all biomolecules through the Haber-Weiss cycle [6]. MDA, as a membrane lipid oxidation product induced by ROS, can be used for reporting ROS [7]. Under non-UV irradiation conditions, compared with the CK group, CeO2-NPs treatment reduced H2O2 content (Fig. 4a). MDA content did not differ significantly among the CK and CeO2-NPs groups (Fig. 4b). Enhanced UV-B led to marked cellular damage, accompanied by increased H2O2 and MDA accumulation (p < 0.05). Under UV-B stress, CeO2-NP treatment effectively reduced the content of H2O2 and MDA.
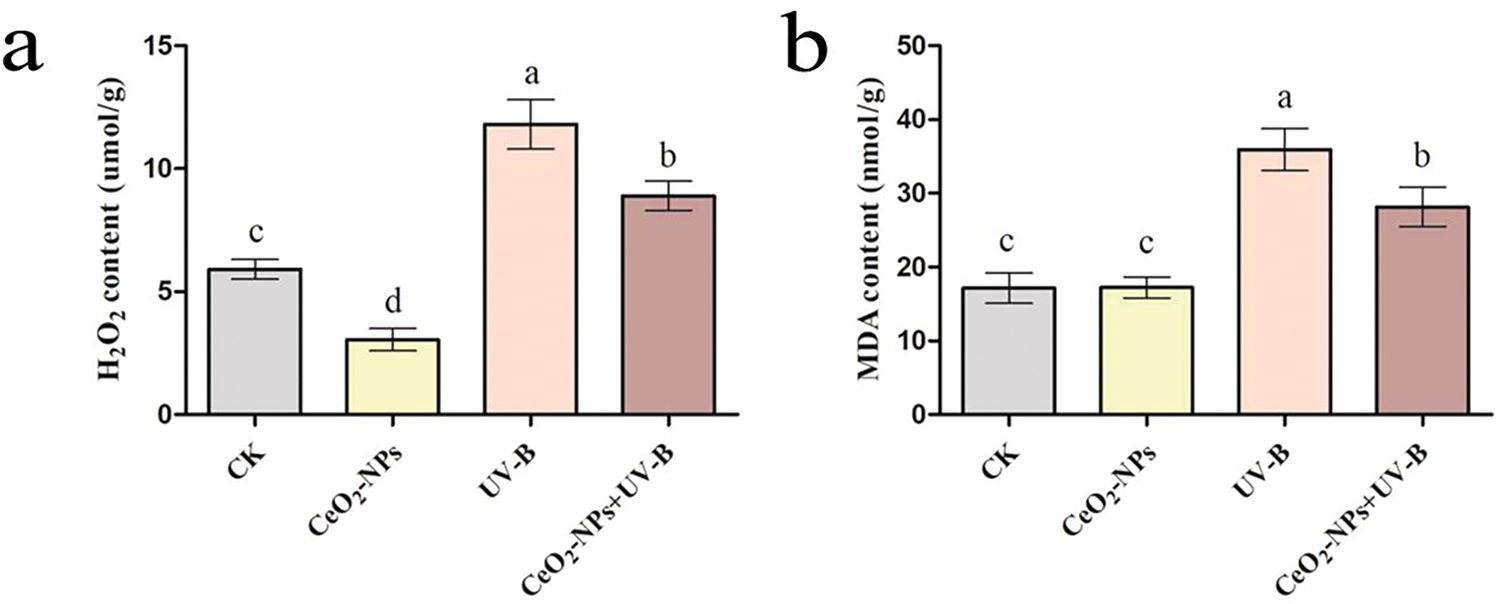
Figure 4: Cellular damage indicators in wheat: (a) H2O2 content, and (b) MDA content. Different letters denote marked differences (p < 0.05)
3.6 Effects of Enhanced UV-B and CeO2-NP Treatments on the Transcriptomics of Wheats
To elucidate the molecular basis of differential responses in wheat seedling roots to enhanced UV-B and CeO2-NP treatments, as well as the potential alterations in mRNA levels induced by CeO2-NP treatment following UV-B exposure, RNA-seq analysis was performed to examine the gene expression profiles of each treatment group.
3.6.1 Global Transcriptomic Responses of Wheats to Enhanced UV-B and CeO2-NP Treatments
Wheat in the UV-B and UV-B+CeO2-NPs groups exhibited distinct responses to UV stress, with the UV-B+CeO2-NPs group demonstrating enhanced UV adaptation compared to the UV-B group. The observed phenotypic differences in UV resistance between these groups were likely attributable to specific DEGs. Volcano plots illustrate the differential gene expression patterns between the UV-B exposure and CeO2-NP treatment groups in comparison to the CK group. In the CeO2-NP treatment, 237 genes were differentially expressed in wheat seedling roots between the CeO2-NPs and CK groups (T1 vs. CK), comprising 197 upregulated and 40 downregulated genes (Fig. 5a). Following enhanced UV-B treatment, 26,703 DEGs (15,178 upregulated and 11,525 downregulated) were identified compared to the CK group (T2 vs. CK) (Fig. 5b). Under enhanced UV-B conditions, CeO2-NPs addition resulted in a decrease of DEGs vs. the UV-B group, with 5301 genes upregulated and 2706 genes downregulated (T3 vs. CK) (Fig. 5c).
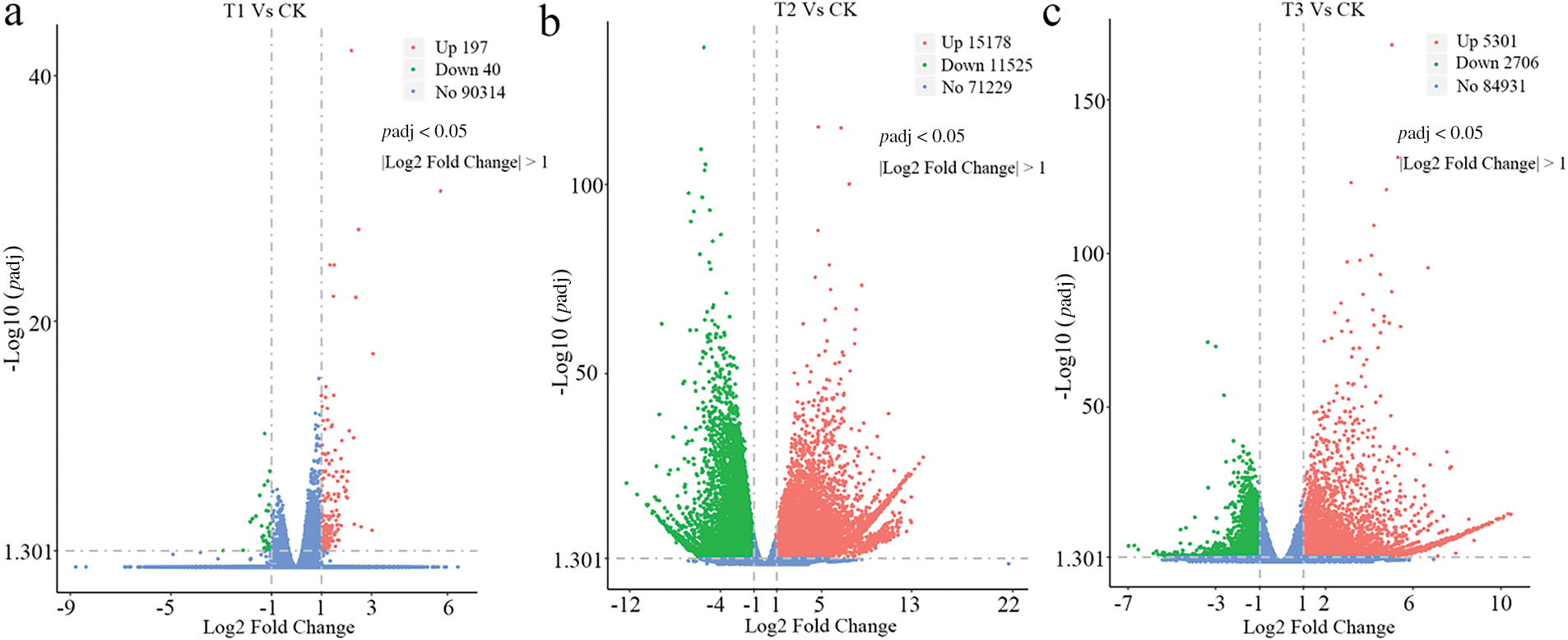
Figure 5: Volcano plots of DEGs between various treatments and the control group: (a) CeO2-NP treatment, (b) enhanced UV-B treatment, and (c) UV-B+CeO2-NP treatment
A Venn diagram was created to pinpoint the unique and shared DEGs in wheat under the enhanced UV-B and CeO2-NP treatments (Fig. 6). The comparison of CeO2-NPs vs. CK (T1 vs. CK) revealed 237 shared DEGs and 15 unique DEGs. UV-B vs. CK (T2 vs. CK) exhibited 26,703 shared DEGs and 3283 unique DEGs. The combination of UV-B+CeO2-NPs vs. CK demonstrated 8007 shared DEGs and 699 unique DEGs.
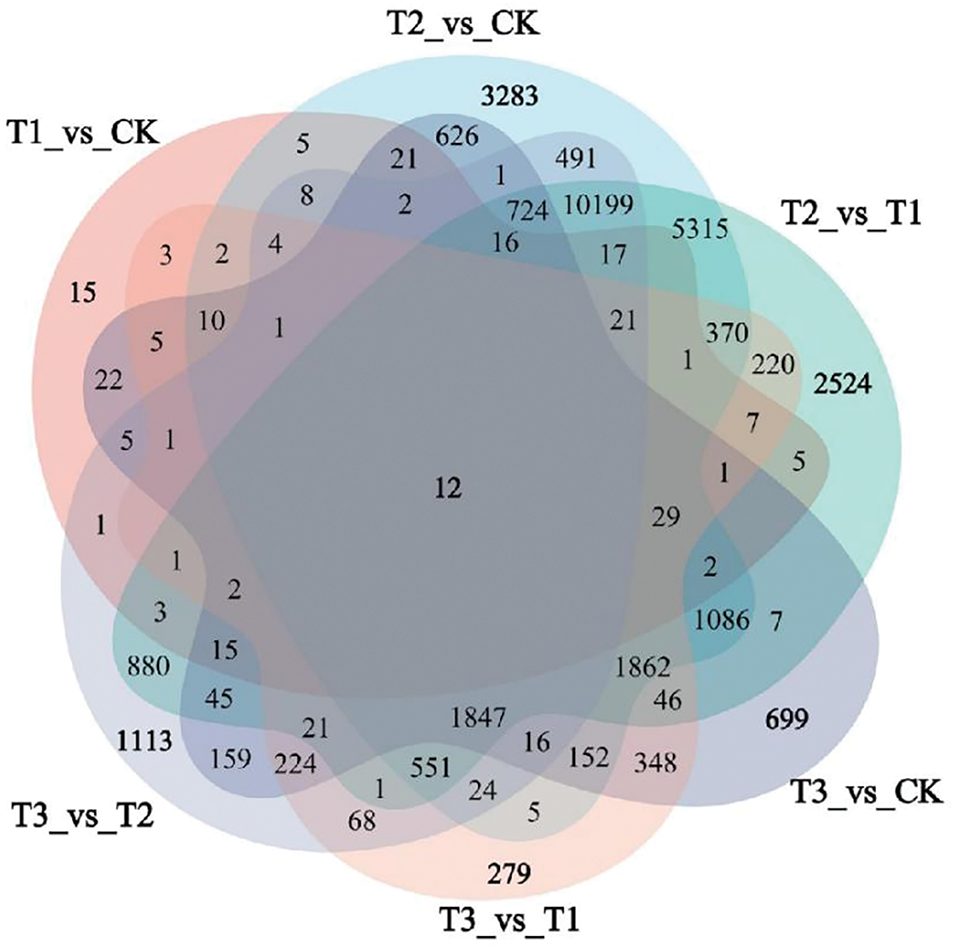
Figure 6: Venn diagram of DEGs for all treatments
As demonstrated by the volcano plots and Venn diagrams, CeO2-NP treatment in isolation exerted minimal influence on the wheat transcriptome. Under UV stress, the number of DEGs in plants within the UV-B group increased dramatically. However, CeO2-NPs regulated wheat at the molecular degree under UV-B, consequently reducing the number of DEGs.
3.6.2 Kyoto Encyclopaedia of Genes and Genomes (KEGG) Analysis
We performed cluster analysis on genes with transcripts per kilobase of exon model per million mapped reads (TPM) > 0 using the Mfuzz package to reveal the primary functions and activated routes of DEGs with similar expression patterns under various treatments. The DEGs were categorized into four groups based on their expression trends, and the results are presented as a clustering heatmap (Fig. S2). ClusterProfiler was employed to perform KEGG enrichment analysis on each cluster of DEGs, using a p-value < 0.05 as the selection criterion, with results depicted in Fig. 7. As previously noted, UV-B triggers the formation of ROS within plants, consequently elevating their oxidant pressure and inflicting injury, while CeO2-NPs enhance plant resistance to UV-B stress by scavenging ROS. Considering the interconnection between plant antioxidant defense modulation and ROS elimination, this investigation centered on clarifying the influences of CeO2-NPs on the antioxidant protection mechanism within wheat roots under UV-B stress. KEGG enrichment analysis of the different gene clusters revealed that the antioxidant defences related to ROS scavenging were primarily concentrated in the following metabolic pathways: antioxidant enzyme (SOD, POD, and CAT) synthesis in peroxisomes, flavonoid biosynthesis, and ascorbate biosynthesis and metabolism. The DEGs encoding key enzymes in the aforementioned metabolic pathways were analyzed, and the reliability of the transcriptomic results was validated using RT-qPCR. Additionally, the activity and content of relevant metabolites were measured.
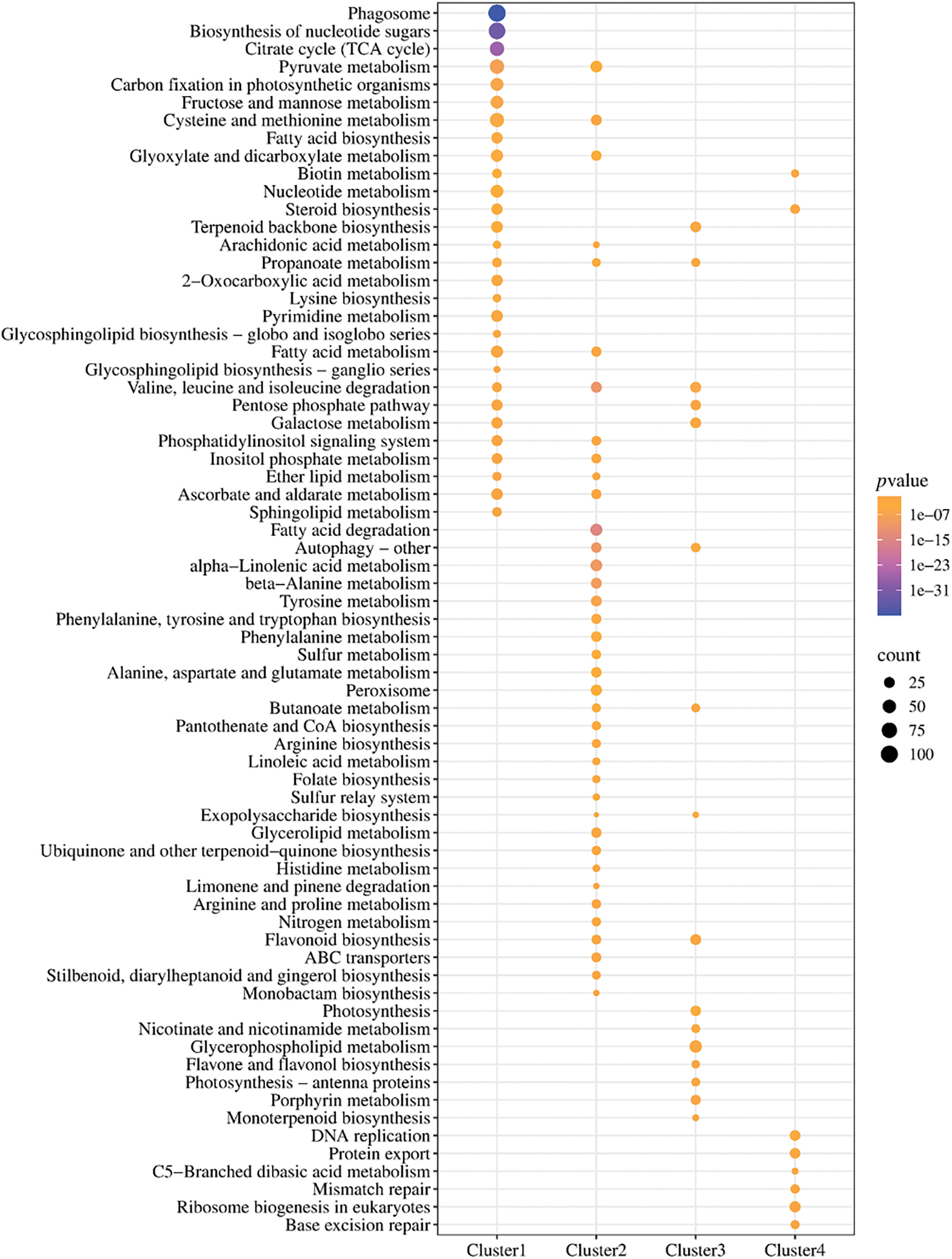
Figure 7: KEGG enrichment analysis of the four clusters of DEGs
3.6.3 Response of Antioxidant Enzymes Genes and Enzyme Activity in Peroxisomes
Fig. 8a illustrates the DEGs encoding oxidative stress-reducing enzymes SOD, CAT, POD, cationic peroxidase SPC4, and other peroxidases associated with ROS scavenging in peroxisomes. Relative to the CK group, CeO2-NPs induced upregulation of genes encoding CAT and POD, with minimal effects on other genes. Enhanced UV-B treatment significantly downregulated genes encoding SOD, cationic peroxidase SPC4, and other peroxidases compared to the CK group, while most genes were only slightly downregulated in the UV-B+CeO2-NPs group. Genes encoding CAT and POD were upregulated under UV-B stress, and the addition of CeO2-NPs prompted the upregulation of POD-encoding genes compared to the UV-B group, with minimal impact on CAT-encoding genes. The principal roles of these genetic sequences are cataloged within Table S2. Among them, POD is a class of oxidoreductases that utilize H2O2 functioning as an electron recipient to catalyze the phenolics, with dual roles in detoxifying H2O2 and phenolics, and serves as a marker enzyme for peroxisomes. CAT is predominantly found in peroxisomes [7]. RT-qPCR validation of the genes encoding SOD and POD showed results consistent with the RNA-seq datas (Fig. 8b,c). SOD, CAT, and POD activities in each group were quantified. The results indicated that compared to the control group, CeO2-NPs treatment alone had a minimal effect on SOD activity, whereas CAT and POD activities increased. UV-B treatment decreased SOD activity but increased CAT and POD catalytic action. The addition of CeO2-NPs under enhanced UV-B irradiation partially restored SOD activity and CAT and POD catalytic action was elevated compared to the UV-B group (Fig. 8d–f).
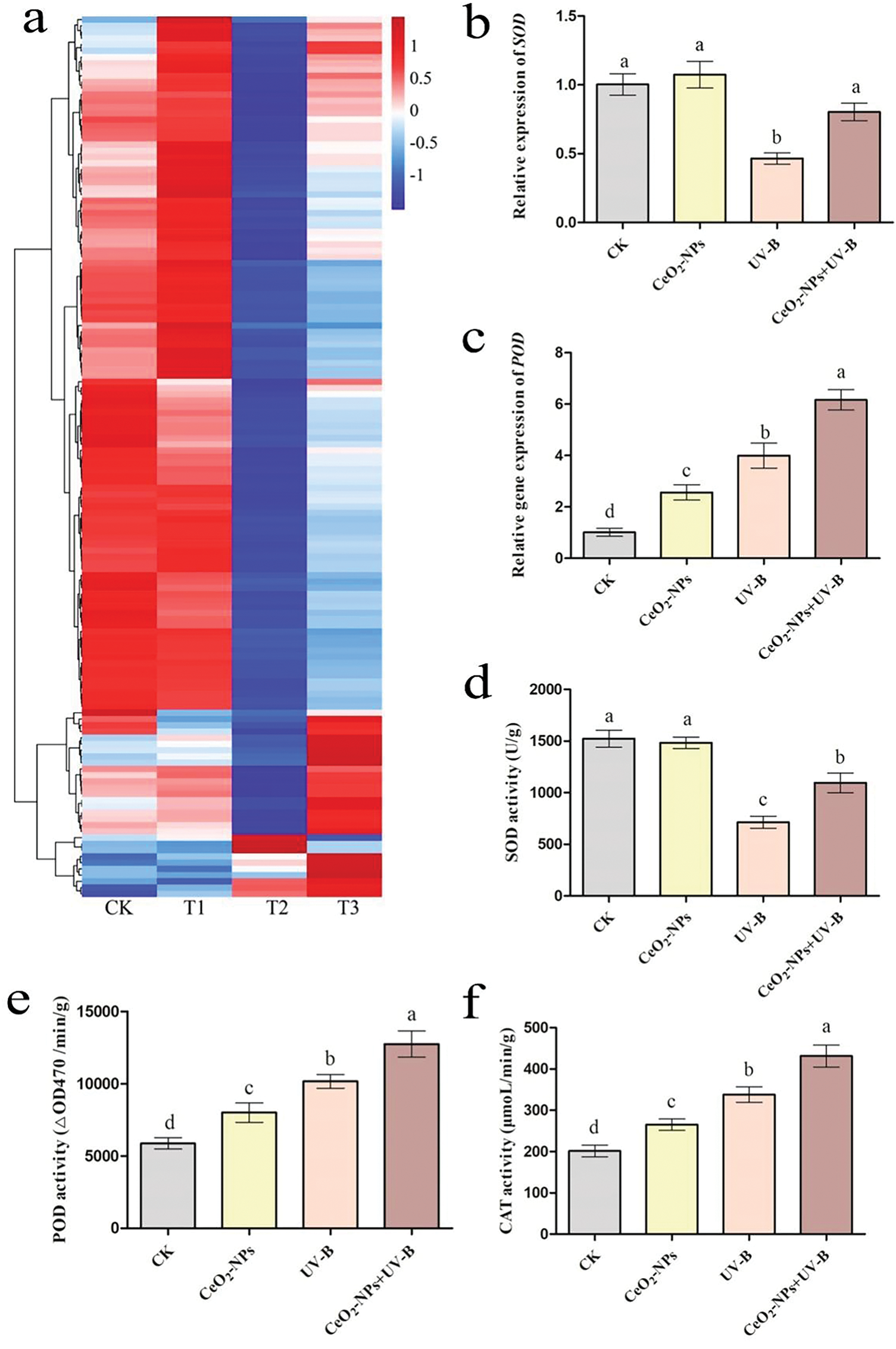
Figure 8: Antioxidant enzymes (SOD, POD, and CAT) in wheat seedling peroxisomes: (a) A heatmap depicting DEGs associated with antioxidant enzymes. The gene names are listed in Table S2. CK, T1, T2, and T3 denote the CK, CeO2-NPs, UV-B, and UV-B+CeO2-NPs groups, respectively. Relative expression levels of (b) SOD and (c) POD. Enzymatic activities of (d) SOD, (e) POD, and (f) CAT. Variations in lettering denote statistically significant differences (p < 0.05)
3.6.4 Response of Flavonoid Biosynthesis-Related Genes and Metabolites
The general phenylpropanoid pathway, a well-established route in plant secondary metabolism, serves as the origin for all flavonoid compounds in plants, with phenylalanine as the precursor [31]. Chalcone synthase (CHS) is a crucial rate-limiting enzyme in the biosynthesis of flavonoids. catalyzes the production of chalcone, marking the initiation of specific flavonoid compound synthesis. In tomatoes (Solanum lycopersicum), suppression of CHS expression resulted in decreased total flavonoid levels [32]. Chalcone isomerase (CHI), the second crucial rate-limiting enzyme, exhibits a positive correlation with flavonoid content in Arabidopsis thaliana [33]. As illustrated in the figures, CeO2-NP treatment alone had minimal impact on flavonoid biosynthesis genes and content compared to the CK group (Fig. 9a,d). Conversely, enhanced UV-B treatment upregulated multiple DEGs in this pathway, encoding key enzymes for flavonoid biosynthesis [ammonia-lyase (PAL), trans-cinnamate 4-monooxygenase (C4H), 4-coumarate-CoA ligase (4CL), CHS, FLS, and CHI.] (Fig. 9a). The RT-qPCR results corroborated the transcriptome analysis findings (Fig. 9b,c), ultimately leading to flavonoid accumulation in wheat (Fig. 9d). However, the addition of CeO2-NPs under UV-B stress caused a reduction of flavonoid biosynthesis-related genes compared to the UV-B group, consequently reducing flavonoid content (Fig. 9).
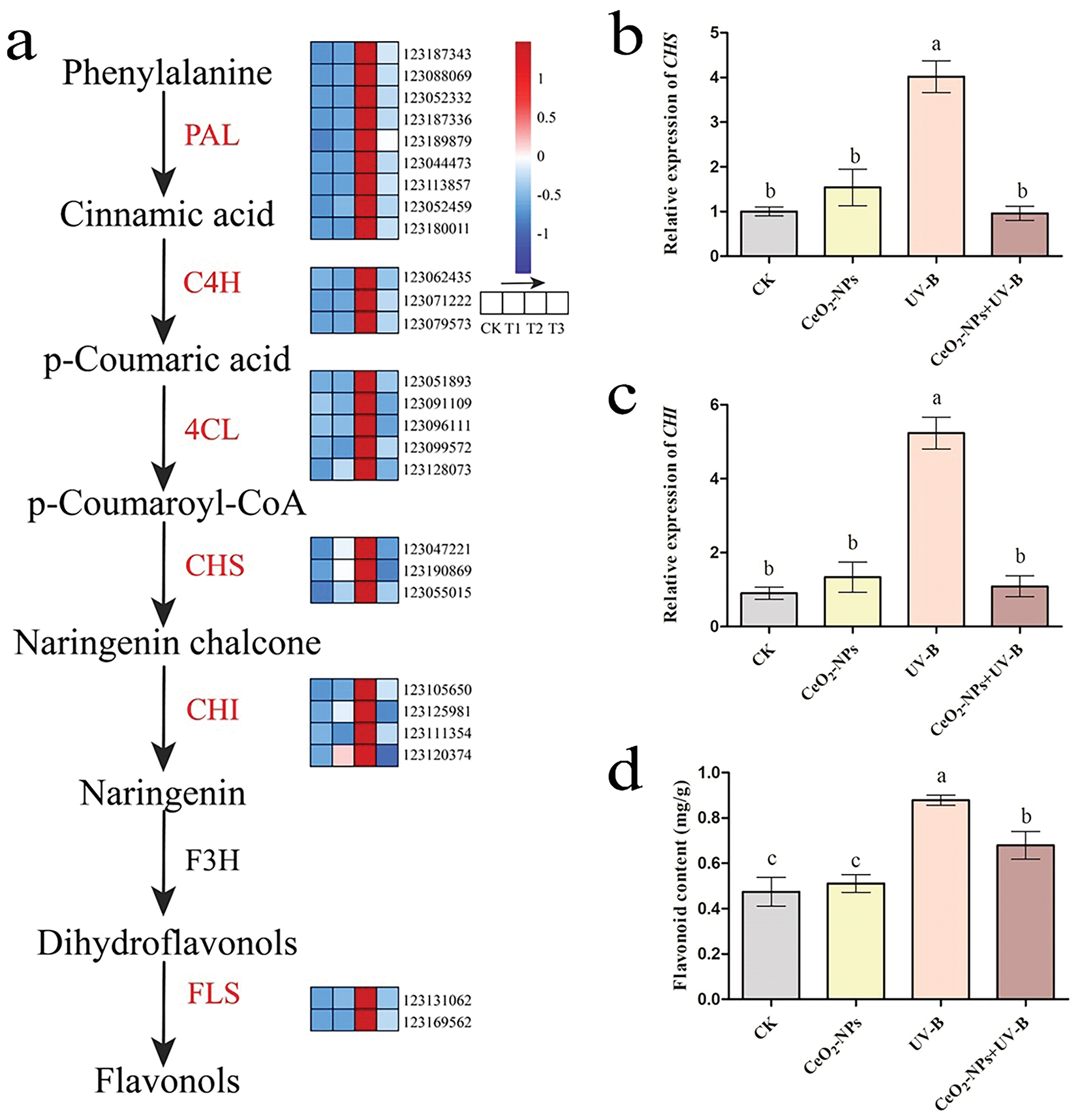
Figure 9: Flavonoid biosynthesis-related genes and metabolites. (a) A heat map of DEGs associated with flavonoid biosynthesis. CK, T1, T2, and T3 represent the CK group, CeO2-NPs group, UV-B, and UV-B+CeO2-NPs group, respectively; Relative expression levels of (b) CHS and (c) CHI; (d) flavonoid concentration. Distinct letters represent statistically significant variations (p < 0.05)
3.6.5 Response of Ascorbic Acid Biosynthesis and Metabolism-Related Genes and Metabolites
The Smirnoff-Wheeler pathway involves intermediates like GDP-D-mannose, GDP-L-galactose, L-galactose, and L-galactose-1,4-lactone, which is the primary pathway for the biosynthesis of AsA in plants [34]. Under non-UV-stress, AsA biosynthesis-related gene expression levels in the CeO2-NPs treated plants were comparable to the CK group. UV-B treatment upregulated DEGs encoding GDP-L-galactose phosphatase (GGP) and L-galactose-1-phosphate phosphatase (GPP) while downregulating DEGs encoding GDP-mannose-3,5-epimerase (GME) relative to the control group. Furthermore, CeO2-NPs treatment mitigated the UV-B-induced suppression of the GME-encoding gene. In comparison to the CK group, the combination of CeO2-NPs and UV-B treatment upregulated DEGs encoding GGP (Fig. 10a).
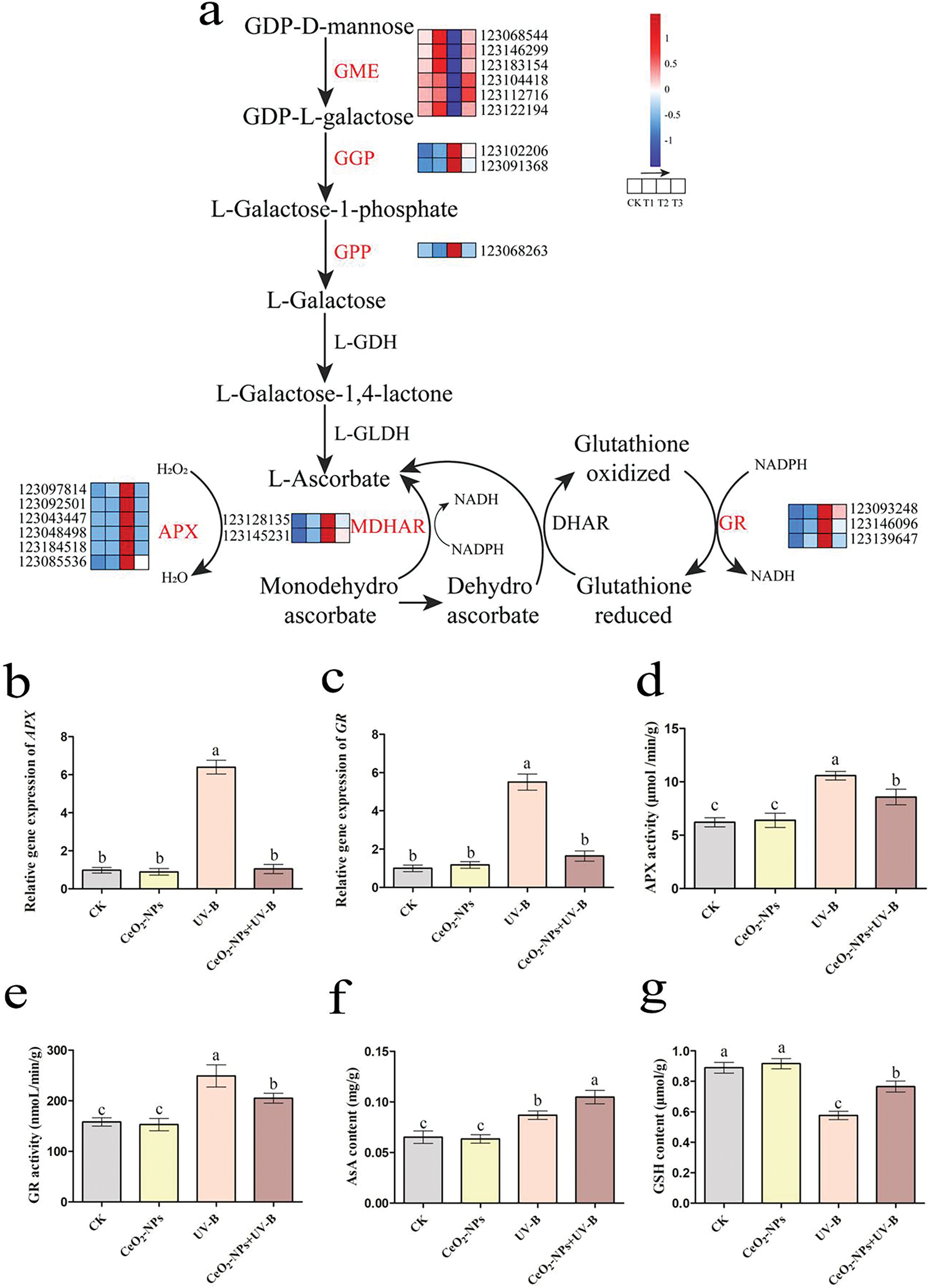
Figure 10: Effects of different treatments on genes and metabolites associated with ascorbic acid synthesis and regulation inplants. (a) A heatmap of ascorbic acid synthesis and regulation-related DEGs. CK, T1, T2, and T3 represent the CK group, CeO2-NPs group, UV-B, and UV-B+CeO2-NPs group, respectively. Relative expression of (b) APX and (c) GR, (d) APX activity, (e) GR activity, (f) AsA concentration, and (g) GSH levels. Dissimilar letters signify statistical significance (p < 0.05)
In plant cells, APX utilizes AsA to reduce H2O2 to water, generating monodehydroascorbate (MDHA). MDHA can be catalysed by monodehydroascorbic acid reductase (MDHAR) to AsA, or via rapid disproportionation to produce dehydroascorbic acid (DHA). DHA can then be converted into AsA by dehydroascorbate reductase (DHAR) utilizing GSH as a reducing substrate. GSH is subsequently regenerated through the reduction of glutathione disulfide (GSSG) by GR. This series of metabolic processes is denoted as the AsA-GSH cycle, which functions as a key mechanism for ROS scavenging in plant chloroplasts and cytoplasm, having a vital role in plant reactions to environmental stress [35,36]. In comparison to the CK group, the CeO2-NPs group exhibited no significant effect on the DEGs encoding enzymes participating in the above cycle, with minimal alterations in APX and GR activities. In contrast, UV-B exposure triggered the expression of APX, MDHAR, and GR DEGs, leading to elevated APX and GR activities. The addition of CeO2-NPs under UV-B stress downregulated these DEGs compared to the UV-B group, resulting in reduced APX and GR activities (Fig. 10a,d,e). The RT-qPCR validation results for APX and GR aligned with the transcriptome data (Fig. 10b,c).
Considering that both the de novo synthesis of AsA and the AsA-GSH cycle collectively influence AsA content in plants [37], this study further examined AsA and GSH contents in wheat under various treatments. In comparison to the control group, CeO2-NP treatment exhibited minimal impact on AsA and GSH levels. However, UV-B exposure induced AsA expression while downregulating GSH content. Notably, the addition of CeO2-NPs under UV-B resulted in elevated AsA and GSH content in plants when compared to the UV-B group alone (Fig. 10f,g).
In summary, our results indicate that increased transcriptional activities of certain enzymes in the AsA biosynthesis pathway following UV-B exposure contribute to AsA accumulation. However, increased APX activity simultaneously consumes AsA. UV-B-induced GR activity enhances GSH production, which supplements AsA content, ultimately resulting in higher AsA levels relative to the CK group, while GSH contents decrease. The introduction of CeO2-NPs under UV stress upregulates the expression of genes associated with AsA biosynthesis, thereby promoting AsA synthesis. Additionally, it downregulates APX activity, reducing AsA consumption and allowing plants to accumulate more AsA. Conversely, in the UV-B+CeO2-NPs group, the AsA content is sufficient, negating the need for excess GSH conversion. Furthermore, increased GR activity is observed. These two factors raise GSH concentration compared to the UV-B group.
Consistent with our research findings, enhanced UV-B radiation inhibits root development, adversely affecting plant growth performance and biomass production, as extensively documented in the literature [38–40]. The impact of CeO2-NPs treatment on roots through soil or nutrient solution, concerning growth-related parameters (root length, plant height, and biomass yield), is closely associated with nanoparticle concentration and varies among plant species [9]. For instance, CeO2-NPs at concentrations of 500 to 4000 mg/L notably enhanced root elongation and accumulation of biomass in soybeans [41]. Likewise, 1000 mg/L CeO2-NPs stimulated root growth in alfalfa, while inhibiting it in tomatoes [42]. Levels of 10 mg/L and 100 mg/kg CeO2-NPs boosted the total fruit weight of tomatoes and the biomass of lettuce (Lactuca sativa L.), respectively [43,44]. Our research results confirm that CeO2-NPs promote root length and biomass accumulation in wheat within a specific concentration range, with an optimal promotional effect at an intermediate concentration (120 mg/L). Corroborating our findings, Abbas et al. showed that lower concentrations of CeO2-NPs enhanced wheat stem growth and boosted biomass, while higher concentrations reduced root length and biomass production [45]. Morales et al. found that cilantro grown in soil amended with 0–500 mg/kg CeO2-NPs, only 125 mg/kg CeO2-NPs promoted root and shoot growth [46]. The growth-promoting effect of lower concentrations of CeO2-NPs may be attributed to their antioxidant activity and enhancement of macronutrient absorption in plant tissues. Conversely, high concentrations of CeO2-NPs lead to delayed root elongation, possibly due to alterations in cell division and an increase in cortical cell columns [45,47].
Earlier research has demonstrated that NPs application can notably mitigate UV-B stress in plants by modifying the levels of genes associated with stress signaling and responses, activating the plant’s antioxidant defense mechanisms, and improving photosynthetic traits [48]. For instance, Tripathi et al. showed that under increased UV-B exposure, pre-added SiNp enhanced photosynthesis by triggering the NO-mediated antioxidant defense system in wheat. In contrast to previous studies highlighting nanoparticles’ potential to mitigate morphological changes in plant aboveground parts under UV stress, this study’s findings indicate that under elevated UV-B exposure, CeO2-NPs had minimal impact on leaf damage [49,50]. However, they significantly reduced root damage, as evidenced by increased wheat root length, biomass, and root vitality. The discrepancy in experimental results may be attributed to variations in nanoparticle application methods, nanoparticle types, plant types, growth phases, UV-B intensity, and exposure duration. Roots are crucial in plant responses to abiotic stress [51]. Root activity, to some extent, reflects the quality of root development and metabolic status, serving as an indicator of root damage [52]. CeO2-NPs enhanced root activity in plants under UV-B stress, aligning with An et al.’s findings in cotton (Gossypium hirsutum L.), where CeO2-NPs seed priming increased root activity and biomass under salt stress, enhancing salt tolerance by reducing ROS accumulation [53]. Furthermore, CeO2-NPs have shown the capacity to enhance plant growth and boost biomass under various stress conditions [54].
CeO2-NP is one of the few engineered nanoparticles that can be transported from plant roots to stems [42]. A previous study by Ma et al. utilized a split-root system to demonstrate that CeO2-NPs were capable of being transported from the roots to the aerial parts of cucumber through the xylem [55]. According to research on tomatoes conducted by Wang et al., CeO2-NPs were absorbed by the roots and then transported to the fruits via the phloem [43]. Schwabe et al. reported the movement of CeO2-NPs within pumpkin stems [56]. The concentration of Ce in various wheat tissues followed the pattern: roots > stems > leaves, suggesting that CeO2-NPs were taken up by wheat seedling roots and translocated to the stems and distant leaves. This study further confirms the absorption and accumulation of CeO2-NPs in plants.
Various environmental stresses can induce excessive ROS accumulation in plants, exceeding the scavenging capacity of their antioxidant systems and resulting in oxidative stress [57]. ROS accumulation can lead to lipid peroxidation and free radical generation, causing lipid degeneration and the formation of aldehydes such as MDA, which are often considered indicators of toxic ROS presence and oxidative stress [58–60]. Research has confirmed that UV-B can trigger the buildup of plants’ H2O2 and MDA [61,62]. The rise in H2O2 (ROS) levels in wheats under UV-B stress corresponded with the increase in MDA, indicating that the seedlings were in a severe oxidative stress state. Nanoceria have been demonstrated in numerous studies to simulate antioxidant enzyme activities, thereby neutralizing ROS. For instance, negatively charged nanoceria efficiently scavenged ROS, aiding Arabidopsis in reducing oxidative damage under intense light, elevated temperatures, and darkness [63]. This indicates that CeO2-NPs possess SOD and CAT activity, as well as the ability to scavenge hydroxyl radicals. Mukherjee et al. also confirmed that CeO2-NPs effectively reduced H2O2 and MDA induced by HgCl2, alleviating oxidative stress and reducing mercury toxicity in Vigna radiata [22]. The catalytic activity of CeO2-NPs is influenced by the surface Ce3+/Ce4+ ratio of Ce atoms: a higher Ce3+/Ce4+ ratio corresponds to higher simulated SOD activity, while a higher Ce4+/Ce3+ ratio correlates with higher CAT simulation activity [64,65]. Our research corroborates these findings. In the present study, CeO2-NPs reduced MDA levels under UV-B stress were associated with their ability to rapidly scavenge excessive ROS (reduced H2O2 content) accumulation in plants. It is hypothesized that the enhancement of wheat seedling antioxidant stress capacity induced by CeO2-NPs is not only related to their enzyme-like catalytic performance but also to the activation of plant antioxidants by CeO2-NPs.
Previous studies have reported varied responses of SOD, POD, and CAT to UV-B exposure in different plants, with inconsistent results. For instance, enhanced UV-B increased SOD activity in indoor experiments on peas, wheat, soybeans, poplars, as well as field experiments on spruce (Picea asperata) and beans, but decreased SOD activity in sunflowers [66–70]. Our study suggests that the reduction in SOD activity under enhanced UV-B irradiation is linked to the suppression of SOD gene levels by excessive UV-B doses. Consistent with our findings, UV-B exposure triggered a t increase in POD transcript levels in cucumbers, with increased gene expression and enzyme activity [71]. Furthermore, UV-B enhanced POD activity in mung beans, wheat, peanuts, and Hibiscus rosa-sinensis [7]. UV-B radiation-induced increases in CAT activity have been observed in spruce (Picea asperata), tomatoes (Lycopersicum esculentum), and common wheat (Triticum aestivum) [6], whereas Agrawal and Rathore observed decreased CAT activity in mung beans [72]. This decrease in CAT activity may result from enhanced lipid peroxidation under UV-B stress, which damages peroxisomes [73]. CeO2-NPs have been widely reported to boost plant antioxidant activity under various abiotic stressors. Seed priming with CeO2-NPs enhanced salt tolerance in cotton by modulating the levels of ROS enzyme gene family members (upregulating POD and glutathione S-transferase, and downregulating peroxidase) [53]. CeO2-NPs exhibited strong antioxidant properties in sorghum under drought stress, enhancing the activities of SOD, CAT, and POD, effectively scavenging O2·− and H2O2, and increasing yield [21]. Zhang et al. demonstrated that nano-anatase can activate protective enzymes in spinach, scavenging ROS under UV-B radiation and improving antioxidant capacity [74]. Based on a comprehensive analysis of transcriptomics and enzyme activities measurement results, compared with the CK group, CeO2-NP treatment alone increased the transcription levels of genes encoding CAT and POD, and boosted enzyme activities. Enhanced UV-B decreased SOD transcript accumulation, gene expression, and enzyme activities in wheat roots. Although the elevation in CAT and POD activities could partially enhance antioxidant capacity, it was insufficient to eliminate the excessive accumulation of ROS caused by high-intensity UV-B, leading to severe damage in wheat. CeO2-NP treatment upregulated antioxidant enzyme gene families (SOD and POD) gene levels under UV-B stress and enhanced SOD, POD, and CAT activities, contributing to ROS scavenging and alleviating oxidative damage in seedlings under UV stress. Compared with the UV-B group, the minimal impact of CeO2-NPs on CAT-encoding genes under UV-B stress, coupled with increased CAT activity, is hypothesized to be related to the inherent CAT-mimicking activity of CeO2-NPs.
Flavonoids, a class of polyphenolic secondary metabolites, which are essential for plant growth, including auxin transport, male fertility, and seed formation. Their ROS-scavenging capabilities contribute significantly to plant stress defense against drought, cold, and salinity [75]. As non-enzymatic antioxidants, flavonoids mitigate UV-B-induced oxidative stress by absorbing UV-B and scavenging ROS generated from UV-B exposure [6]. Gao et al. confirmed that UV-B triggers an adaptive response in alfalfa seedlings by inducing flavonoid production [76]. Similarly, UV-B treatment increased flavonoid biosynthesis-related genes levels (CHS, CHI, and FLS) in rice seedlings [77]. Our research also indicated that enhanced UV-B induced flavonoid biosynthesis pathway genes levels in wheats and promoted flavonoid compound accumulation, aiding in ROS scavenging. Contrary to a previous study demonstrating that nano-titanium dioxide acts as an inducer of flavonoid and total phenol biosynthesis in Salvia officinalis, in this study, treatment with CeO2-NPs alone had no significant effect on flavonoid biosynthesis genes or content compared to the CK group [78]. Under UV-B stress, CeO2-NPs downregulated wheats flavonoid biosynthesis-related genes levels and reduced the content of flavonoid, contradicting results from a study in Arabidopsis showing that TiO2 NPs could promote flavonoid transcription under UV-B stress [79]. These discrepancies may be ascribed to differences in crop types and nanomaterials. Furthermore, these results suggest that CeO2-NPs may mitigate oxidative stress in wheat seedings under UV-B stress through alternative antioxidant pathways, thereby conferring resistance to changes in flavonoids induced by enhanced UV-B.
AsA can react with O2•− and •OH in plants to produce water, functioning as an effective scavenger of oxygen-free radicals. It is the primary and most important non-enzymatic antioxidant involved in ROS detoxification in plants. •OH is the most damaging ROS in plants, and no enzymatic scavenger has been identified to mitigate its effects [63,80]. The AsA response to UV-B exposure differs across plant species and experimental conditions. Liu et al. demonstrated that UV-B can upregulate the transcription levels of APX, GR and DHAR in cucumbers, thereby promoting AsA accumulation [81]. An UV-B-induced rise in AsA levels has been reported in numerous plants such as wheat, cucumber, cowpea, and soybean, which helps mitigate ROS damage [7,82,83]. A UV-B-triggered increase in AsA content has been documented in wheat and mung beans under field conditions [72]. GSH reacts with H2O2, 1O2, and •OH, and works synergistically with AsA to scavenge ROS [84]. Additionally, GSH has various important functions, including signal transduction [85]. Research has demonstrated that UV-B can elevate GSH levels in wheat and mung beans, but has no effect on Vaccinium myrtillus L [72,86]. The AsA-GSH cycle is crucial for maintaining the reduced form of AsA and for scavenging ROS in plants exposed to UV-B [87]. The accumulation of AsA and GSH in lettuce under UV-B irradiation was primarily attributed to changes in the the above cycle [88]. Furthermore, APX, primarily scavenges H2O2 produced by SOD, whereas high GR activity promotes the production of GSH, which helps replenish reduced AsA [7]. UV-B increased APX activity in various plants, such as cucumber, cotton, and sunflower [89–91], while increased GR activity has been observed in barley [92]. Our results indicated that enhanced APX and GR activity and increased AsA content in wheats under UV-B stress could help alleviate ROS harm induced by elevated UV-B. However, these changes were insufficient to counteract oxidative stress triggered by intense UV-B, which ultimately inhibited wheat root growth. Previously, CeO2-NPs have been reported to improve APX activity under salt stress, enhancing plant growth performance in a salt-stress environments [19]. Our study confirmed that CeO2-NPs, by regulating encoding key enzymes-related genes levels in the AsA-associated pathway under UV-B stress, further influenced APX and GR activities and promoted the accumulation of AsA and GSH, thereby enhancing seedling resistance to UV stress. Similar to our findings, Zhao et al. reported that nano-silicon mitigated Brassica napus L. damage under cadmium stress by elevating AsA and GSH levels [93].
This research underscores the vital role of CeO₂-NPs in mitigating UV stress in wheat roots through controlling ROS levels and enhancement of stress tolerance. Elevated UV-B disrupted the balance between ROS generation and elimination in wheat roots, leading to severe oxidative damage (evidenced by increased H2O2 and MDA levels), which consequently reduced root biomass and vitality, significantly impeding wheat root growth. CeO2-NPs exhibited dual functionality: they demonstrated intrinsic SOD and CAT enzyme-mimetic activities to scavenge ROS and modulated the levels of the antioxidant defense system-related genes in wheat, thereby activating both enzymatic (increased SOD, CAT, and POD activities) and non-enzymatic (increased AsA and GSH contents) antioxidants. This led to decreased oxidative damage in wheat, enhanced growth performance (greater root vitality and biomass), and alleviation of the harmful impacts of intensified UV-B. The application of CeO2-NPs presents a sustainable, practical, and scalable approach to enhancing wheat root resistance to UV stress. However, this experiment focused primarily on the effect of CeO2-NPs on early root development in hydroponic wheat. The efficacy of CeO2-NPs in mitigating damage to wheat and other crops grown in various soil types (such as acidic, alkaline, or sandy soils) under UV stress remains unclear. Additionally, it is uncertain whether the stress resistance conferred by CeO2-NPs is influenced by the plant growth stage. Therefore, enhancing the stability and reproducibility of CeO2-NPs is crucial to ensure their catalytic activity in diverse agricultural environments. Furthermore, the potential sustained impacts of CeO2-NPs on human health through the food chain warrant in-depth attention and research. Future studies should focus on optimizing synthesis methods to minimize the potential toxicity of CeO2-NPs to living systems, ensuring their safe application in agriculture.
Acknowledgement: We would like to thank Tech Science Press (www.techscience.com/ndetail/languageservice, accessed on 24 December 2024) for English language editing.
Funding Statement: This research was supported by Graduate Innovation Project of Shanxi Normal University (Grant No. 2021Y443).
Author Contributions: Cheng Sun and Rong Han designed the research. Cheng Sun designed research, performed experiments and analyzed date. Chen Zhao, Qianwen Mao and Guohua Wang performed experiments. Rong Han and Cheng Sun wrote the article. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Transcriptome data generated or analyzed during this study are available in the NCBI repository (Project Number: PRJNA1124367, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1124367, accessed on 24 December 2024).
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/phyton.2025.061462.
Abbreviations
| CeO2-NPs | Cerium oxide nanoparticles |
| H2O2 | Hydrogen peroxide |
| MDA | Malondialdehyde |
| DEGs | Differentially expressed genes |
| CFCs | Chlorofluorocarbons |
| O2•− | Superoxide anions |
| •OH | Hydroxyl radical |
| 1O2 | Singlet oxygen |
| SOD | Dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| APX | Ascorbate peroxidase |
| GR | Glutathione reductase |
| AsA | Ascorbic acid |
| GSH | Glutathione |
| GP | Guaiacol peroxidase |
| EL | Electrolyte leakage |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| O2 | Oxygen |
| ELISA | Enzyme-linked immunosorbent assay |
| FPKM | Fragments per kilobase of the exon model per million mapped reads |
| ANOVA | Analysis of variance |
| TPM | Transcripts per kilobase of exon model per million mapped reads |
| KEGG | Kyoto Encyclopaedia of Genes and Genomes |
| SPC4 | Cationic peroxidase |
| CHS | Chalcone synthase |
| CHI | Chalcone isomerase |
| C4H | 4-monooxygenase |
| 4CL | 4-coumarate–CoA ligase |
| FLS | Flavonol synthase |
| GGP | GDP-L-galactose phosphatase |
| GPP | L-galactose-1-phosphate phosphatase |
| GME | GDP-mannose-3,5-epimerase |
| MDHA | Monodehydroascorbate |
| MDHAR | Monodehydroascorbic acid reductase |
| DHA | Dehydroascorbic acid |
| DHAR | Dehydroascorbate reductase |
| GSSG | Glutathione disulfide |
References
1. Hossain F. Fate and transport of stack emissions in the environment and potential reduction of pollutants in the context of global warming. J Environ Eng. 2023;149(1):04022080. doi:10.1061/(ASCE)EE.1943-7870.0002078. [Google Scholar] [CrossRef]
2. Zhang Y, Zhan L, Xu YZ. Simultaneous harmless ionization of CFC and resource utilization of waste solar panel through one-pot hydrothermal treatment. J Hazard Mater. 2023;441(6):129918. doi:10.1016/j.jhazmat.2022.129918. [Google Scholar] [CrossRef]
3. Robson TM, Aphalo PJ, Banaś AK, Barnes PW, Brelsford CC, Jenkins GI, et al. A perspective on ecologically relevant plant-UV research and its practical application. Photochem Photobiolo Sci. 2019;18(5):970–88. doi:10.1039/c8pp00526e. [Google Scholar] [PubMed] [CrossRef]
4. Xie C, Wang P, Gu Z, Yang R. Spermidine alleviates oxidative damage and enhances phenolic compounds accumulation in barley seedlings under UV-B stress. J Sci Food Agricult. 2023;103(2):648–56. doi:10.1002/jsfa.12176. [Google Scholar] [PubMed] [CrossRef]
5. Liaqat W, Altaf MT, Barutçular C, Nawaz H, Ullah I, Basit A, et al. Ultraviolet-B radiation in relation to agriculture in the context of climate change: a review. Cereal Res Commun. 2024;52(1):1–24. doi:10.1007/s42976-023-00375-5. [Google Scholar] [PubMed] [CrossRef]
6. Pathak J, Rajneesh, Ahmed H, Singh DK, Singh PR, Kumar D, et al. Oxidative stress and antioxidant defense in plants exposed to ultraviolet radiation. In: Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. Hoboken: Wiley-Blackwell; 2019. p. 371–420. [Google Scholar]
7. Kataria S. Oxidative stress and antioxidative defence system in plants in response to UV-B stress. In: UV-B radiation: from environmental stressor to regulator of plant growth. Hoboken: John Wiley & Sons, Inc.; 2017. p. 99–121. [Google Scholar]
8. Zhao L, Lu L, Wang A, Zhang H, Huang M, Wu H, et al. Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J Agricul Food Chem. 2020;68(7):1935–47. doi:10.1021/acs.jafc.9b06615. [Google Scholar] [PubMed] [CrossRef]
9. Pietrzak M, Skiba E, Wolf WM. Root-applied cerium oxide nanoparticles and their specific effects on plants: a review. Int J Mol Sci. 2024;25(7):4018. doi:10.3390/ijms25074018. [Google Scholar] [PubMed] [CrossRef]
10. Rico CM, Hong J, Morales MI, Zhao L, Barrios AC, Zhang JY, et al. Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol. 2013;47(11):5635–42. doi:10.1021/es401032m. [Google Scholar] [PubMed] [CrossRef]
11. Rico CM, Lee SC, Rubenecia R, Mukherjee A, Hong J, Peralta-Videa JR, et al. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (Triticum aestivum L.). J Agricul Food Chem. 2014;62(40):9669–75. doi:10.1021/jf503526r. [Google Scholar] [PubMed] [CrossRef]
12. Cao Z, Rossi L, Stowers C, Zhang W, Lombardini L, Ma X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ Sci Pollut Res. 2018;25(1):930–9. doi:10.1007/s11356-017-0501-5. [Google Scholar] [PubMed] [CrossRef]
13. Jahani S, Saadatmand S, Mahmoodzadeh H, Khavari-Nejad RA. Effect of foliar application of cerium oxide nanoparticles on growth, photosynthetic pigments, electrolyte leakage, compatible osmolytes and antioxidant enzymes activities of Calendula officinalis L. Biologia. 2019;74:1063–75. doi:10.2478/s11756-019-00239-6. [Google Scholar] [CrossRef]
14. Lord MS, Berret JF, Singh S, Vinu A, Karakoti AS. Redox active cerium oxide nanoparticles: current status and burning issues. Small. 2021;17(51):2102342. doi:10.1002/smll.202102342. [Google Scholar] [PubMed] [CrossRef]
15. Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine. 2013;8(9):1483–508. doi:10.2217/nnm.13.133. [Google Scholar] [PubMed] [CrossRef]
16. Yang CJ, Nguyen DD, Lai JY. Poly(L-histidine)-mediated on-demand therapeutic delivery of roughened ceria nanocages for treatment of chemical eye injury. Adv Sci. 2023;10(26):2302174. doi:10.1002/advs.202302174. [Google Scholar] [PubMed] [CrossRef]
17. Wu H, Shabala L, Shabala S, Giraldo JP. Hydroxyl radical scavenging by cerium oxide nanoparticles improves Arabidopsis salinity tolerance by enhancing leaf mesophyll potassium retention. Environ Sci: Nano. 2018;5(7):1567–83. doi:10.1039/C8EN00323H. [Google Scholar] [CrossRef]
18. Liu Y, Xiao Z, Chen F, Yue L, Zou H, Lyu J, et al. Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: trends, meta-analysis, and prospect. Sci Total Environ. 2021;780:146578. doi:10.1016/j.scitotenv.2021.146578. [Google Scholar] [PubMed] [CrossRef]
19. Mohammadi MHZ, Panahirad S, Navai A, Bahrami MK, Kulak M, Gohari G. Cerium oxide nanoparticles (CeO2-NPs) improve growth parameters and antioxidant defense system in Moldavian Balm (Dracocephalum moldavica L.) under salinity stress. Plant Stress. 2021;1:100006. doi:10.1016/j.stress.2021.100006. [Google Scholar] [CrossRef]
20. Gohari G, Zareei E, Rostami H, Panahirad S, Kulak M, Farhadi H, et al. Protective effects of cerium oxide nanoparticles in grapevine (Vitis vinifera L.) cv. Flame Seedless under salt stress conditions. Ecotoxicol Environ Saf. 2021;220:112402. doi:10.1016/j.ecoenv.2021.112402. [Google Scholar] [PubMed] [CrossRef]
21. Djanaguiraman M, Nair R, Giraldo JP, Prasad PVV. Cerium oxide nanoparticles decrease drought-induced oxidative damage in sorghum leading to higher photosynthesis and grain yield. ACS Omega. 2018;3(10):14406–16. doi:10.1021/acsomega.8b01894. [Google Scholar] [PubMed] [CrossRef]
22. Mukherjee S, Krishnamoorthy SB, Subrayan R, Goswami A, Mitra S. A brief study on the role of cerium oxide nanoparticles in growth and alleviation of mercury-induced stress in Vigna radiata and soil bacteria Bacillus coagulans. Environ Sci Pollut Res. 2023;30(29):73952–63. doi:10.1007/s11356-023-27496-y. [Google Scholar] [PubMed] [CrossRef]
23. Erenstein O, Jaleta M, Mottaleb KA, Sonder K, Donovan J, Braun H-J. Global trends in wheat production, consumption and trade. In: Wheat improvement: food security in a changing climate. Cham: Springer International Publishing; 2022. p. 47–66. [Google Scholar]
24. Kheiralipour K, Brandão M, Holka M, Choryński A. A review of environmental impacts of wheat production in different agrotechnical systems. Resources. 2024;13(7):93. doi:10.3390/resources13070093. [Google Scholar] [CrossRef]
25. Gao W, Zheng Y, Slusser JR, Heisler GM. Impact of enhanced ultraviolet-B irradiance on cotton growth, development, yield, and qualities under field conditions. Agric For Meteorol. 2003;120(1–4):241–8. doi:10.1016/j.agrformet.2003.08.019. [Google Scholar] [CrossRef]
26. Kul R, Ekinci M, Turan M, Ors S, Yildirim E. How abiotic stress conditions affects plant roots. In: Plant roots. London: IntechOpen; 2020. [Google Scholar]
27. Yu Y, Zhou W, Zhou K, Liu W, Liang X, Chen Y, et al. Polyamines modulate aluminum-induced oxidative stress differently by inducing or reducing H2O2 production in wheat. Chemosphere. 2018;212:645–53. doi:10.1016/j.chemosphere.2018.08.133. [Google Scholar] [PubMed] [CrossRef]
28. Livak KJ, Schmittgen TD. Analysis of relative gene expr data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–8. doi:10.1006/meth.2001.1262. [Google Scholar] [PubMed] [CrossRef]
29. Kaneko K, Inoke K, Freitag B, Hungria AB, Midgley PA, Hansen TW, et al. Structural and morphological characterization of cerium oxide nanocrystals prepared by hydrothermal synthesis. Nano Letters. 2007;7(2):421–5. doi:10.1021/nl062677b. [Google Scholar] [PubMed] [CrossRef]
30. Huang H, Ullah F, Zhou D-X, Yi M, Zhao Y. Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci. 2019;10:800. doi:10.3389/fpls.2019.00800. [Google Scholar] [PubMed] [CrossRef]
31. Liu W, Feng Y, Yu S, Fan Z, Li X, Li J, et al. The flavonoid biosynthesis network in plants. Int J Mol Sci. 2021;22(23):12824. doi:10.3390/ijms222312824. [Google Scholar] [PubMed] [CrossRef]
32. Schijlen EG, de Vos CR, Martens S, Jonker HH, Rosin FM, Molthoff JW, et al. RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol. 2007;144(3):1520–30. doi:10.1104/pp.107.100305. [Google Scholar] [PubMed] [CrossRef]
33. Jiang W, Yin Q, Wu R, Zheng G, Liu J, Dixon RA, et al. Role of a chalcone isomerase-like protein in flavonoid biosynthesis in Arabidopsis thaliana. J Experimen Bot. 2015;66(22):7165–79. doi:10.1093/jxb/erv413. [Google Scholar] [PubMed] [CrossRef]
34. Suekawa M, Kondo T, Fujikawa Y, Esaka M. Regulation of ascorbic acid biosynthesis in plants. In: Ascorbic acid in plant growth, development and stress tolerance. Berlin: Springer; 2017. p. 157–76. [Google Scholar]
35. Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Rev Plant Biol. 1998;49(1):249–79. doi:10.1146/annurev.arplant.49.1.249. [Google Scholar] [PubMed] [CrossRef]
36. Gallie DR. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J Experiment Bot. 2013;64(2):433–43. doi:10.1093/jxb/ers330. [Google Scholar] [PubMed] [CrossRef]
37. Wang J, Zhang Z, Huang R. Regulation of ascorbic acid synthesis in plants. Plant Signal Behav. 2013;8(6):e24536. doi:10.4161/psb.24536. [Google Scholar] [PubMed] [CrossRef]
38. Chen H, Gong Y, Han R. Cadmium telluride quantum dots (CdTe-QDs) and enhanced ultraviolet-B (UV-B) radiation trigger antioxidant enzyme metabolism and programmed cell death in WS. PLoS One. 2014;9(10):e110400. doi:10.1371/journal.pone.0110400. [Google Scholar] [PubMed] [CrossRef]
39. Zhang R, Huang G, Wang L, Zhou Q, Huang X. Effects of elevated ultraviolet-B radiation on root growth and chemical signaling molecules in plants. Ecotoxicol Environ Saf. 2019;171:683–90. doi:10.1016/j.ecoenv.2019.01.035. [Google Scholar] [PubMed] [CrossRef]
40. Nassour R, Ayash A. Effects of ultraviolet-B radiation in plant physiology. Agriculture (Pol’nohospodárstvo). 2021;67(1):1–15. doi:10.2478/agri-2021-0001. [Google Scholar] [CrossRef]
41. Lopez-Moreno ML, de la Rosa G, Hernandez-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR, et al. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants. Environ Sci Technol. 2010;44(19):7315–20. doi:10.1021/es903891g. [Google Scholar] [PubMed] [CrossRef]
42. López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agricul Food Chem. 2010;58(6):3689–93. doi:10.1021/jf904472e. [Google Scholar] [PubMed] [CrossRef]
43. Wang Q, Ma X, Zhang W, Pei H, Chen Y. The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics. 2012;4(10):1105–12. doi:10.1039/c2mt20149f. [Google Scholar] [PubMed] [CrossRef]
44. Gui X, Zhang Z, Liu S, Ma Y, Zhang P, He X, et al. Fate and phytotoxicity of CeO2 nanoparticles on lettuce cultured in the potting soil environment. PLoS One. 2015;10(8):e0134261. doi:10.1371/journal.pone.0134261. [Google Scholar] [PubMed] [CrossRef]
45. Abbas Q, Liu G, Yousaf B, Ali MU, Ullah H, Munir MAM, et al. Biochar-assisted transformation of engineered-cerium oxide nanoparticles: effect on wheat growth, photosynthetic traits and cerium accumulation. Ecotoxicol Environ Saf. 2020;187:109845. doi:10.1016/j.ecoenv.2019.109845. [Google Scholar] [PubMed] [CrossRef]
46. Morales MI, Rico CM, Hernandez-Viezcas JA, Nunez JE, Barrios AC, Tafoya A, et al. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J Agricul Food Chem. 2013;61(26):6224–30. doi:10.1021/jf401628v. [Google Scholar] [PubMed] [CrossRef]
47. Cui D, Zhang P, Ma Y, He X, Li Y, Zhang J, et al. Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium. Environ Sci: Nano. 2014;1(5):459–65. doi:10.1039/C4EN00025K. [Google Scholar] [CrossRef]
48. Soni S, Jha AB, Dubey RS, Sharma P. Application of nanoparticles for enhanced UV-B stress tolerance in plants. Plant Nano Biology. 2022;2:100014. doi:10.1016/j.plana.2022.100014. [Google Scholar] [CrossRef]
49. Azadi M, Moghaddam SS, Rahimi A, Pourakbar L, Popović-Djordjević J. Biosynthesized silver nanoparticles ameliorate yield, leaf photosynthetic pigments, and essential oil composition of garden thyme (Thymus vulgaris L.) exposed to UV-B stress. J Environ Chem Eng. 2021;9(5):105919. doi:10.1016/j.jece.2021.105919. [Google Scholar] [CrossRef]
50. Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, Chauhan DK. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem. 2017;110:70–81. doi:10.1016/j.plaphy.2016.06.026. [Google Scholar] [PubMed] [CrossRef]
51. Ceccoli G, Ramos JC, Ortega LI, Acosta JM, Perreta MG. Salinity induced anatomical and morphological changes in Chloris gayana Kunth roots. Biocell. 2011;35(1):9. [Google Scholar] [PubMed]
52. Liu JJ, Wei Z, Li JH. Effects of copper on leaf membrane structure and root activity of maize seedling. Botanical Studies. 2014;55:1–6. [Google Scholar]
53. An J, Hu P, Li F, Wu H, Shen Y, White JC, et al. Emerging investigator series: molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ Sci: Nano. 2020;7(8):2214–28. doi:10.1039/D0EN00387E. [Google Scholar] [CrossRef]
54. Preetha JSY, Sriram D, Premasudha P, Pudake RN, Arun M. Cerium oxide as a nanozyme for plant abiotic stress tolerance: an overview of the mechanisms. Plant Nano Biology. 2023;6:100049. doi:10.1016/j.plana.2023.100049. [Google Scholar] [CrossRef]
55. Ma Y, He X, Zhang P, Zhang Z, Ding Y, Zhang J, et al. Xylem and phloem based transport of CeO2 nanoparticles in hydroponic cucumber plants. Environ Sci Technol. 2017;51(9):5215–21. doi:10.1021/acs.est.6b05998. [Google Scholar] [PubMed] [CrossRef]
56. Schwabe F, Schulin R, Limbach LK, Stark W, Bürge D, Nowack B. Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere. 2013;91(4):512–20. doi:10.1016/j.chemosphere.2012.12.025. [Google Scholar] [PubMed] [CrossRef]
57. Hasanuzzaman M, Bhuyan MB, Parvin K, Bhuiyan TF, Anee TI, Nahar K, et al. Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. Int J Mol Sci. 2020;21(22):8695. doi:10.3390/ijms21228695. [Google Scholar] [PubMed] [CrossRef]
58. Demidchik V. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Experim Bot. 2015;109:212–28. doi:10.1016/j.envexpbot.2014.06.021. [Google Scholar] [CrossRef]
59. Banerjee A, Roychoudhury A. Abiotic stress, generation of reactive oxygen species, and their consequences: an overview. In: Reactive oxygen species in plants: boon or bane-revisiting the role of ROS. Hoboken: Wiley; 2017. p. 23–50. [Google Scholar]
60. Ahmad P, Ahanger MA, Alam P, Alyemeni MN, Wijaya L, Ali S, et al. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J Plant Growth Regulat. 2019;38:70–82. doi:10.1007/s00344-018-9810-2. [Google Scholar] [CrossRef]
61. Wang M, Leng C, Zhu Y, Wang P, Gu Z, Yang R. UV-B treatment enhances phenolic acids accumulation and antioxidant capacity of barley seedlings. LWT. 2022;153:112445. doi:10.1016/j.lwt.2021.112445. [Google Scholar] [CrossRef]
62. Ma M, Wang P, Yang R, Zhou T, Gu Z. UV-B mediates isoflavone accumulation and oxidative-antioxidant system responses in germinating soybean. Food Chem. 2019;275(1):628–36. doi:10.1016/j.foodchem.2018.09.158. [Google Scholar] [PubMed] [CrossRef]
63. Wu H, Tito N, Giraldo JP. Anionic cerium oxide nanoparticles protect plant photosynthesis from abiotic stress by scavenging reactive oxygen species. ACS Nano. 2017;11(11):11283–97. doi:10.1021/acsnano.7b05723. [Google Scholar] [PubMed] [CrossRef]
64. Singh S. Cerium oxide based nanozymes: redox phenomenon at biointerfaces. Biointerphases. 2016;11(4):04B202. doi:10.1116/1.4966535. [Google Scholar] [PubMed] [CrossRef]
65. Damle MA, Jakhade AP, Chikate RC. Modulating pro-and antioxidant activities of nanoengineered cerium dioxide nanoparticles against Escherichia coli. ACS Omega. 2019;4(2):3761–71. doi:10.1021/acsomega.8b03109. [Google Scholar] [CrossRef]
66. Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell Environ. 2001;24(12):1337–44. doi:10.1046/j.1365-3040.2001.00778.x. [Google Scholar] [CrossRef]
67. Costa H, Gallego SM, Tomaro ML. Effect of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci. 2002;162(6):939–45. doi:10.1016/S0168-9452(02)00051-1. [Google Scholar] [CrossRef]
68. Prasad SM, Dwivedi R, Zeeshan M. Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica. 2005;43(2):177–85. doi:10.1007/s11099-005-0031-0. [Google Scholar] [CrossRef]
69. Ren J, Yao Y, Yang Y, Korpelainen H, Junttila O, Li C. Growth and physiological responses to supplemental UV-B radiation of two contrasting poplar species. Tree Physiol. 2006;26(5):665. doi:10.1093/treephys/26.5.665. [Google Scholar] [PubMed] [CrossRef]
70. Yao X, Liu Q. Changes in photosynthesis and antioxidant defenses of Picea asperata seedlings to enhanced ultraviolet-B and to nitrogen supply. Physiol Planta. 2010;129(2):364–74. doi:10.1111/j.1399-3054.2006.00815.x. [Google Scholar] [CrossRef]
71. Cantarello C, Volpe V, Azzolin C, Bertea C. Modulation of enzyme activities and expression of genes related to primary and secondary metabolism in response to UV-B stress in cucumber (Cucumis sativus L.). J Plant Interact. 2005;1(3):151–61. doi:10.1080/17429140600831581. [Google Scholar] [CrossRef]
72. Agrawal S, Rathore D. Changes in oxidative stress defense system in wheat (Triticum aestivum L.) and mung bean (Vigna radiata L.) cultivars grown with and without mineral nutrients and irradiated by supplemental ultraviolet-B. Environ Experim Bot. 2007;59(1):21–33. doi:10.1016/j.envexpbot.2005.09.009. [Google Scholar] [CrossRef]
73. Ravindran KC, Indrajith A, Pratheesh PV, Sanjiviraja K, Balakrishnan V. Effect of ultraviolet-B radiation on biochemical and antioxidant defence system in Indigofera tinctoria L. seedlings. Int J Eng Sci Technol. 2010;2(5). doi:10.4314/ijest.v2i5.60154. [Google Scholar] [CrossRef]
74. Zheng L, Su M, Wu X, Liu C, Qu C, Chen L, et al. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biolo Trace Elem Researc. 2008;121:69–79. doi:10.1007/s12011-007-8028-0. [Google Scholar] [PubMed] [CrossRef]
75. Ferreyra MLF, Serra P, Casati P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiologia Plantarum. 2021;173(3):736–49. doi:10.1111/ppl.13543. [Google Scholar] [PubMed] [CrossRef]
76. Gao L, Liu Y, Wang X, Li Y, Han R. Lower levels of UV-B light trigger the adaptive responses by inducing plant antioxidant metabolism and flavonoid biosynthesis in Medicago sativa seedlings. Funct Plant Biol. 2019;46(10):896–906. doi:10.1071/FP19007. [Google Scholar] [PubMed] [CrossRef]
77. Kim G-E, Kim M-S, Sung J. UVB Irradiation-induced transcriptional changes in lignin-and flavonoid biosynthesis and indole/tryptophan-auxin-responsive genes in rice seedlings. Plants. 2022;11(12):1618. doi:10.3390/plants11121618. [Google Scholar] [PubMed] [CrossRef]
78. Ghorbanpour M. Major essential oil constituents, total phenolics and flavonoids content and antioxidant activity of Salvia officinalis plant in response to nano-titanium dioxide. Indian J Plant Physiol. 2015;20:249–56. doi:10.1007/s40502-015-0170-7. [Google Scholar] [CrossRef]
79. Wang J, Li M, Feng J, Yan X, Chen H, Han R. Effects of TiO2-NPs pretreatment on UV-B stress tolerance in Arabidopsis thaliana. Chemosphere. 2021;281:130809. doi:10.1016/j.chemosphere.2021.130809. [Google Scholar] [PubMed] [CrossRef]
80. Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Critical Rev Plant Sci. 2000;19(4):267–90. doi:10.1080/10409230008984166. [Google Scholar] [PubMed] [CrossRef]
81. Liu P, Li Q, Gao Y, Wang H, Chai L, Yu H, et al. A new perspective on the effect of UV-B on L-ascorbic acid metabolism in cucumber seedlings. J Agricul Food Chem. 2019;67(16):4444–52. doi:10.1021/acs.jafc.9b00327. [Google Scholar] [PubMed] [CrossRef]
82. Xu MJ, Dong JF, Zhu MY. Effects of germination conditions on ascorbic acid level and yield of soybean sprouts. J Sci Food Agricul. 2005;85(6):943–7. doi:10.1002/jsfa.2050. [Google Scholar] [CrossRef]
83. Dwivedi R, Singh VP, Kumar J, Prasad SM. Differential physiological and biochemical responses of two Vigna species under enhanced UV-B radiation. J Radiat Res Appl Sci. 2015;8(2):173–81. doi:10.1016/j.jrras.2014.12.002. [Google Scholar] [CrossRef]
84. Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, et al. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003;133(2):443–7. doi:10.1104/pp.103.028399. [Google Scholar] [PubMed] [CrossRef]
85. Gomez L, Noctor G, Knight M, Foyer C. Regulation of calcium signalling and gene expression by glutathione. J Experim Bot. 2004;55(404):1851–9. doi:10.1093/jxb/erh202. [Google Scholar] [PubMed] [CrossRef]
86. Taulavuori E, Bäckman M, Taulavuori K, Gwynn-Jones D, Johanson U, Laine K, et al. Long-term exposure to enhanced ultraviolet-B radiation in the sub-arctic does not cause oxidative stress in Vaccinium myrtillus. The New Phytol. 1998;140(4):691–7. doi:10.1046/j.1469-8137.1998.00302.x. [Google Scholar] [PubMed] [CrossRef]
87. Chen Z, Young TE, Ling J, Chang S-C, Gallie DR. Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Nat Acad Sci. 2003;100(6):3525–30. doi:10.1073/pnas.0635176100. [Google Scholar] [PubMed] [CrossRef]
88. Liu S, Yu L, Liu L, Yang A, Huang X, Zhu A, et al. Effects of ultraviolet-B radiation on the regulation of ascorbic acid accumulation and metabolism in lettuce. Horticulturae. 2023;9(2):200. doi:10.3390/horticulturae9020200. [Google Scholar] [CrossRef]
89. Kataria S, Jain K, Guruprasad KN. UV-B induced changes in antioxidant enzymes and their isoforms in cucumber (Cucumis sativus L.) cotyledons. Indian J Biochem Biophy. 2007;44(1):31–7. [Google Scholar] [PubMed]
90. Dehariya P, Kataria S, Pandey G, Guruprasad K. Assessment of impact of solar UV components on growth and antioxidant enzyme activity in cotton plant. Physiol Molec Biol Plants. 2011;17:223–9. doi:10.1007/s12298-011-0071-9. [Google Scholar] [PubMed] [CrossRef]
91. Hagh AG, Khara J, Darvishzadeh R. Effect of UV-B radiation on activity of antioxidant enzymes in four sunflower cultivars. Int J Agricul Res Rev. 2012;2:528–34. [Google Scholar]
92. Cakirlar H, Cicek N, Ekmekci Y. Is the induction of H_2O_2-detoxifying antioxidant enzyme activities sufficient to protect barley cultivars from oxidative stress by UV-B irradiation alone or pretreatment with high temperature and NaCl? Turkish J Biol. 2011;35(1):59–68. doi:10.3906/biy-0904-21. [Google Scholar] [CrossRef]
93. Zhao S, Kamran M, Rizwan M, Ali S, Yan L, Alwahibi MS, et al. Regulation of proline metabolism, AsA-GSH cycle, cadmium uptake and subcellular distribution in Brassica napus L. under the effect of nano-silicon. Environ Pollut. 2023;335:122321. doi:10.1016/j.envpol.2023.122321. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools