 Open Access
Open Access
ARTICLE
Exogenous Application of Hesperidin Ameliorates Chromium Toxicity in Wheat Irrigated with Tannery Wastewater
1 Department of Environmental Sciences, Government College University Faisalabad, Faisalabad, 38000, Pakistan
2 Department of Biological Sciences and Technology, China Medical University, Taichung, 40402, Taiwan
3 Department of Botany, Government College University Faisalabad, Faisalabad, 38000, Pakistan
4 Department of Plant Biotechnology, College of Life Sciences, Korea University, Seoul, 02841, Republic of Korea
5 State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, 210023, China
6 Botany and Microbiology Department, College of Science, King Saud University, Riyadh, 11451, Saudi Arabia
* Corresponding Author: Shafaqat Ali. Email:
Phyton-International Journal of Experimental Botany 2025, 94(3), 929-951. https://doi.org/10.32604/phyton.2025.059288
Received 03 October 2024; Accepted 26 January 2025; Issue published 31 March 2025
Abstract
Chromium (Cr), a persistent soil pollutant, has detrimental effects on plants and living things, and its contamination in soil increased as a result of human-induced activities. Pakistan suffers from a lack of fresh water supplies; hence most people use metal-containing water and wastewater to irrigate their crops. Exposure to Cr toxicity, the plant reduces their morphological and physiological growth which ultimately decreases crop productivity. The current study was designed to investigate the foliar application of hesperidin (HSP) at varying effluent rates (25, 50, 75, and 100 mg L−1) on wheat growth under tannery wastewater irrigated soil. Cr toxicity caused a change in the concentration of chlorophyll molecules, indicating early signs of stress. Modifications in the ultrastructure of chloroplasts, the elevated activity of chlorophyllase, and the generation of reactive oxygen species were causing the reduction in chlorophyll. Cr stress disrupted total soluble protein concentrations and the activity of antioxidation-related enzymes and NRA, suggesting the onset of oxidative stress. On the other hand, the application of HSP reduced oxidative damage by improving protein concentration (37%), chlorophyll concentration (37%), and antioxidant enzyme activity such as CAT (65%), SOD (46%), and POD (68%). Furthermore, HSP raised the concentrations of non-enzymatic antioxidant molecules, which may indicate better redox homeostasis and stress tolerance. These molecules include GSH, GSSG, ascorbic acid, flavonoids, phenolics, and anthocyanins. HSP therapy lessened the impact of Cr stress on lipid peroxidation markers. HSP enhanced these measures during the investigation. Cr stress raised the concentrations of total free amino acids and nitrogen oxide and decreased the radical scavenging activity in wheat. Cr stress raised the concentration of all soluble sugars, primarily reducing and non-reducing sugars, whereas the application of HSP strengthened these osmo protectants even more results of the present investigation indicate that exogenous HSP is a feasible and eco-friendly approach to improving plant resistance against Cr toxicity by efficiently reducing the physiological strain and metabolic stress caused by Cr in wheat plants.Keywords
Long-term use of irrigation water causes heavy metals to build up in agricultural soils, disrupting the food chain [1,2]. Industrial and urban regions are directly causing an alarming increase in environmental pollution worldwide [3]. Unintentional changes in the physical, chemical, and biological properties of the water, air, and soil result from this, and eventually, living organisms will be negatively affected by these changes [4]. It is essential to implement strategies that both halt contamination from spreading and remove existing contamination to feed a world population that is growing [5].
The production of leather tanning has rapidly increased throughout the world as well as in Pakistan. Tan tanning production frequently uses salts containing Cr [6]. When human activities such as electroplating and mining fail to manage tanning industry waste properly, Cr accumulation in soils occurs significantly [7,8]. Among the heavy metals, Cr is considered as dangerous to plants, animals, and also humans [4]. The deposition of Cr in soil poses a serious threat to agricultural production due to its detrimental effects on crop growth and development and food security [9–11].
Industrial operations, mining, and electroplating waste are major contributors to Cr deposition in soil due to improper management [12,13]. Heavy metals, including Cr, are frequently known to be extremely toxic to all living things when compared to other elements [14]. One of the main causes for concern is the adverse impact of Cr buildup in agricultural soils on crop development and food safety [12,15]. Several plant species developed reduced growth, chlorosis of leaves and roots, and decreased grain output when exposed to Cr poisoning [9]. Dangerous Cr concentrations can cause delays in the maturity of plants, disturb photosynthesis, mitigate uptake of essential growth nutrients, and produce lower-quality crops [16] Humans, through their manufacturing activities, have been steadily increasing the amount of Cr in the environment [17]. Plants are vulnerable to chromium poisoning, which can cause uncontrolled oxidation cascades that damage their cells [18]. Excessive absorption of Cr leads to soil degradation; moreover, the combustion of coal, oil, and waste from metallurgical, chemical, and tannery industries are major causes the excess Cr deposition in soil [19].
The morphology of roots and growth of plants are affected by overexposure to Cr because it stimulates the generation of reactive oxygen species (ROS), particularly H2O2, which damages cell membranes, tissue injuries as well as lipid-based peroxidation [20,21]. Additionally, higher production of ROS damages proteins, RNA, and lipids. It also increases membrane absorbency and promotes the breakdown of chlorophyll [22]. Crop plants have their complex oxidative protection mechanism to protect plants from Cr-induced oxidative damage, which includes antioxidants of enzymes (APX, CAT, SOD, and POD) [23] and non-enzymatic antioxidants (anthocyanins, α-tocopherols, ascorbic acid, phenolics, reduced glutathione (GSH), carotenoids, and flavonoids) [24]. The increased antioxidant levels and activities of crop plants closely correlate with their ability to withstand stress. Glutathione is an enduring non-enzymatic antioxidant present in every cell organelle [25]. GR and GSH are important components of the ascorbate-glutathione (AsA-GSH) cycle in agricultural plants [26]. In addition to protecting the proteins from denaturation, GR can protect the integrity of the cellular plasma membrane, whereas glutathione functions as an H2O2 scavenger to reduce metal toxicity [27]. In agricultural plants injured by Cr, the GSH plays a role in producing phytochelatins that bind the metal and transport it into the vacuole, changes in the GSH/GSSG ratio are important for controlling the redox-signaling pathway and cellular homeostasis [28,29]. However, in extreme stress conditions, crop plants’ antioxidant capacities might not be sufficient to protect plants from the detrimental impact of metal stressors, which mitigate plant development and yield [30,31]. Crop plants have been protected from damage and minimized Cr uptake through various techniques. Using exogenous application of antioxidants to reduce metal-induced injuries in agricultural plants under harsh ecological conditions is one of the novel approaches [32]. Dietary polyphenols demonstrate advantageous antioxidant and metal-chelating qualities [33]. Hesperidin (HSP) is a major flavonoid found in citrus fruits. It is an effective approach that decreases inflammation, regulates blood pressure, and prevents cancer [34,35]. In animal systems, certain poisons decrease free species and raise antioxidant levels, shielding cells from harm according to research [36]. Nevertheless, there is currently limited investigation regarding the exogenous HSP treatments in crop plants that affect radical scavenging or antioxidant capacity. Hesperidin coordination with redox homeostasis under Cr stress in wheat is unclear. Understanding wheat and optimizing mitigating strategies to boost crop output is crucial.
Heavy metals can susceptible wheat (Triticum aestivum L.), a staple crop that is widely grown worldwide, to environmental stress [28]. Contamination of heavy metals with Cr has a major impact on wheat growth which ultimately reduces yield [37]. The general public faces a serious concern due to wheat’s high accumulation of Cr [38]. As a result, the reduction in Cr concentration that wheat takes up from the soil is essential for ensuring food security [39]. Therefore, it is important to prioritize several approaches in addition to food crops to reduce the injurious impacts of metal contamination [40]. Despite numerous results regarding the physiological functions and production pathways of these compounds in many plant species [41]. Hypothesizing that exogenous application of HSP would enhance wheat plants’ tolerance to chromium stress and alter their growth and antioxidant activity. Specifically, the present study aims to evaluate the effects of HSP on the growth, uptake of Cr, photosynthetic pigments, and antioxidant actions of wheat crops grown under tannery wastewater irrigated soil. This work aids in the analysis of the parameters of Cr stress on various physiological, morphological, and biochemical aspects of wheat species and identifies, via histochemical research, alterations in ROS formation caused by HSP. Overall, research examining the mechanism by which hesperidin mediates chromium stress tolerance in wheat is infrequently explored. Therefore, due to the significant potential of hesperidin in safe crop production, we proposed that hesperidin might effectively alleviate chromium toxicity. This study specifically examined the influence of foliar hesperidin on plant physiological parameters, biochemical features, and Cr uptake while chromium stress. This research underscores global problems related to food security and food safety difficulties, offering insights into sustainable and eco-friendly approaches to improve growing wheat in chromium-contaminated soil.
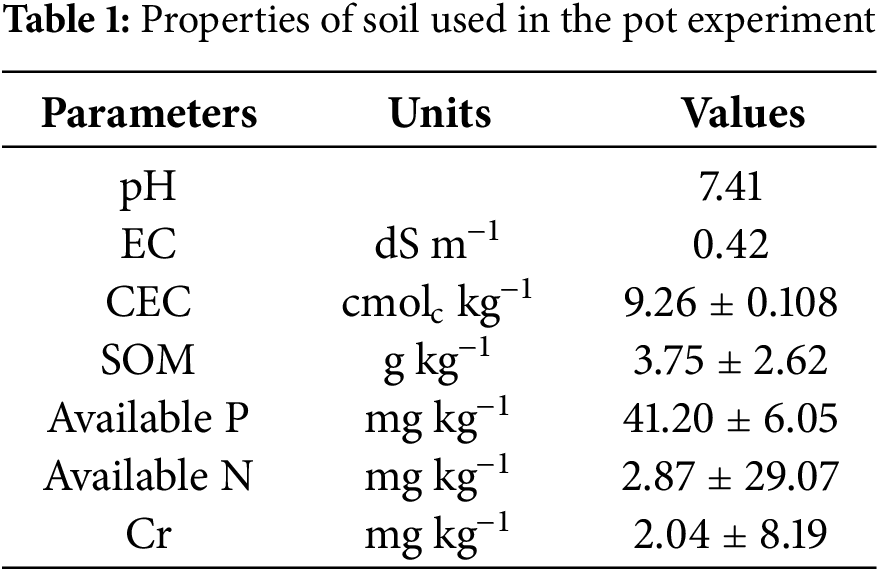
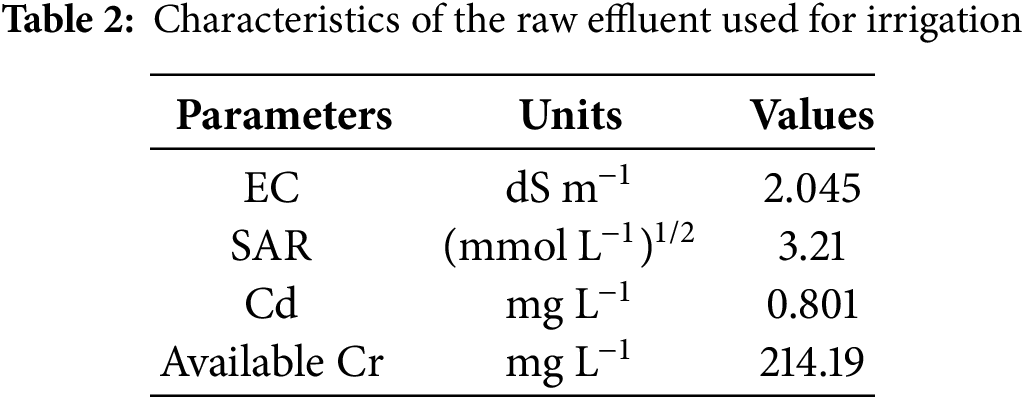
Wheat seeds were acquired from Wheat Research Institute, Ayyub Agricultural Research Institute (AARI) located in Faisalabad, Pakistan. The experiment soil was collected from the research farm of the University of Agriculture Faisalabad at 0–20 cm depth. Additionally, several traits were examined for it after sieved through a 2 mm mesh sieve (Table 1). Experts gathered and examined tannery effluents according to the established procedure (Table 2).
In a completely randomized design (CRD), the soil was gathered and filled into pots for arrangement (7 kg soil pot−1). After 15 min of disinfection with 2% H2O2, the wheat seeds were thoroughly cleaned with d3H2O. In three iterations, every treatment plan was completed. The pot trial was conducted in a botanic plot with sowing and harvesting temperatures of 20°C–27°C and 65%–80% relative humidity, respectively. After 15 days, we thinned the wheat plants and crushed and carefully incorporated the removed plants into comparable pots. A 500-mL combination containing 0.6 g (1−1) P (as (NH4)2HPO4), 2.20 g (1−1) N (as (NH2)2CO), and 2.15 g (1−1) K (as K2SO4) was used to enrich the soil pot. After 16 days of germination, we applied half of the fertilizer combination. After an additional 16 days, we applied the remaining half. Synthetic Hoagland solution (Sigma) containing the following macro elements: 0.2 M Ca (NO3)2, 0.01 M FeSO4, 0.09 M MgSO4, 0.3 M KNO3, 0.4 M KH2PO4; and microelements: 1.4 mM ZnSO4, 10 mM H2MoO4, 0.5 mM H3BO3, 0.4 mM CuSO4 as needed [42]. This was applied to all of the sand pots. Irrigators supplied wheat plants with fresh water or tannery wastewater (TWW) (0%, 50%, and 100%) one week after thinning. The foliar application of Hesperidin (0, 25, 50, 75, and 100 mg L−1) was started one week after the irrigation with tannery effluents. At various stages of plant development, we gathered the desired information. Wild plants were manually removed from their pots and switched out regularly.
2.2 Estimation of Chlorophyll and Other Pigments
Following 90 days of HSP administration, the concentration of carotenoid and β-carotene, as well as chlorophylls (a, b, ab−1, and total chlorophylls). The uppermost fully expanded young leaves were assessed. We utilized the altered disappearance coefficients and estimations to deliberate on the concentrations of β-carotene, carotenoids, and chlorophylls [43]. Using a SPAD meter (SPAD-502), the second highest and most fully developed leaf reached the required chlorophyll amount based on the soil plant analysis development (SPAD) rate. Using this method, the concentration of β-cyanin and β-xanthin was determined [44].
2.3 Estimation of Proteins, Enzymatic Antioxidant Activity
Freshly harvested leaf flesh weighing 0.5 g was used to prepare enzyme extracts. Tris-HCl solution with a pH of: 7.5, 25 mM, and 1% TritonX-100 was utilized for homogenization. Using a pestle and mortar for assistance, the testers centrifuged for 20 min at 13,000 rpm. Using the technique described in [45], we quantified and used the Total Soluble Proteins (TSP), amounting to (0.5 g, from a fresh leaf sample in enzyme action experiment). Using a spectrophotometer set up according to their indicated wavelengths, the activities of superoxide dismutase (SOD) [46], ascorbate peroxidase (APX) [47], peroxidase (POD) [48] and catalase (CAT) [49] were evaluated from the fresh leaf section. Using the method of Hossain et al. (2006) at 340 nm, the application of glutathione S-Transferase (GST) was seen spectrophotometrically. Using the following protocol, the Nitrate Reductase Activity (NRA) of the fresh leaf sample was assessed [50].
2.4 Estimation of Non-Enzymatic Antioxidant Activity
The glutathione concentration ratio was calculated by applying some modifications to the method [51]. We measured the quantities of oxidized glutathione (GSSG) and reduced glutathione (GSH) using this approach [52]. Using a spectrophotometer, the response blend optical density was measured at 412 nm. We ascertained the ascorbic acid concentration of wheat plants after extracting them in TCA using the specified protocol [53]. We determined the flavonoid concentration of the foliage sample by measuring its absorbance spectrophotometrically at 510 nm in aqueous ethanol (10 mL) we used the method described in [54] for this determination. The Wolfe et al. [55] method was applied to determine the phenolic concentration of a fresh leaves sample (0.5 g) to extract the supernatant containing anthocyanins. We standardized leaf tissue in a methanol solution with 1% HCl and centrifuged it at 657 and 530 nm; we determined the absorbance value of the supernatant [56].
2.5 Estimation of Hydrogen Peroxide Concentration, Superoxide Radicals, Methylglyoxal Concentration, Lipid Peroxidation Rate, and Hydrogen Sulfide (H2S)
New leaf samples (0.30 g) were measured for O2•− and H2O2 using the methods described in [57] and [58], respectively. Suggested determining the methylglyoxal (MG) level of the new leaf sample using the specified technique [59]. To control the MDA level, a fresh foliage sample (0.30 g) was taken and measured at 532 nm OD using a spectrophotometer, as described in [60]. Using the method of [61], the amount of H2S in the leaf tissue tester (0.3 g) was measured, and a spectrophotometer was used to determine the absorbance value at 412 nm.
2.6 Nitric Oxide, Total Free Amino Acids, and Radical Scavenging Activity
By applying the method of Zhou et al. [62] at 540 nm absorbance of the reaction mix using a spectrophotometer, the amount of NO in foliage was measured. The TFAA level was determined by the protocol described in [63]. Using a spectrophotometer, measured the DPPH activity of (0.3 g of fresh plant tissue) at 517 nm, following the methodology outlined in [64].
2.7 Total Soluble Sugars and Reducing Sugars
The new leaf material (0.3 g) preserved in 70% ethanol was used to calculate the total soluble sugars (TSS). We measured TSS [65] using this extract, and we followed this process to track reducing sugars (RS) [66]. Using a UV-VIS spectrophotometer (Hitachi U-2910), the absorbance of TSS and RS was measured at 625 nm.
2.8 Estimation of Actions of Lipoxygenase, Polyphenol Oxidase, and Phenyl Ammonia-Lyase
Ref. [67] used their approach to measure the PPO action, while Ref. [68] used their technique to document the PAL activity. We used a spectrophotometer to measure the activity of LOX at 234 nm in the presence of linoleic acid [69].
2.9 Determination of Chromium in Plants
After harvesting plants and soil samples were analyzed for Cr. Samples of plants were oven-dried and ground to make fine powder for further analysis. A mixture of 70% HNO3, 70% H2SO4, and 65% HClO4 (5:1:1) were used for digestion of samples at 80°C. For analysis of Cr, carried out the filtration and dilution of digested transparent samples, and then run the samples on an atomic absorption spectrophotometer.
Three replicates were used to determine the mean as well as the standard deviation (±SD). IBM SPSS 20 was used to assess the experimental results through a one-way analysis of variance (ANOVA) at a level of significance of 5% (p ≤ 0.05). The Tukey (HSD) test for multiple comparisons was used to determine particular pairwise differences.
3.1 Effect of Foliar Application Hesperidin on Chlorophyll and Other Pigments of Wheat Plants under Chromium Stress
While 100% Cr stress increased the Chlorophyll ab−1 concentration in wheat cultivar and 50% Cr stress reduced the Chlorophyll ab−1 concentration in wheat cultivar, tannery wastewater (chromium stress) considerably decreased the Chl. a, b, and total Chl. concentration, as well as SPAD chlorophyll values. At the tannery, the greatest decrease or rise in 100% was seen after the application of its wastewater. In both stressed and non-stressed wheat cultivars, the application of exogenous hesperidin (HSP) with different treatments facilitated to improve the level of leaf photosynthetic pigments (Fig. 1). In this case, compared to control plants, 25, 50, 75, and 100 mg L−1 HSP supplementation significantly increased chlorophyll a (18%, 41%, 45% and 37%), chlorophyll b (7%, 23%, 25% and 12%), total chlorophyll concentration (14%, 34%, 37% and 27%), and chlorophyll ab−1 (10%, 14%, 16%, and 22%) under 50% Cr stress. Following 100% Cr stress, these same plants showed the greatest increases in chlorophyll a (14%, 28%, 54% and 20%), chlorophyll b (26%, 45%, 50% and 34%), and total chlorophyll concentration (18%, 34%, 53% and 25%) respectively. Control wheat plants were linked with these results. When wheat plants are stressed with chromium (Cr), their levels of carotenoids, β-cyanin, and β-xanthin increase in contrast to normal conditions while their β-carotene concentration decreased (Fig. 1).
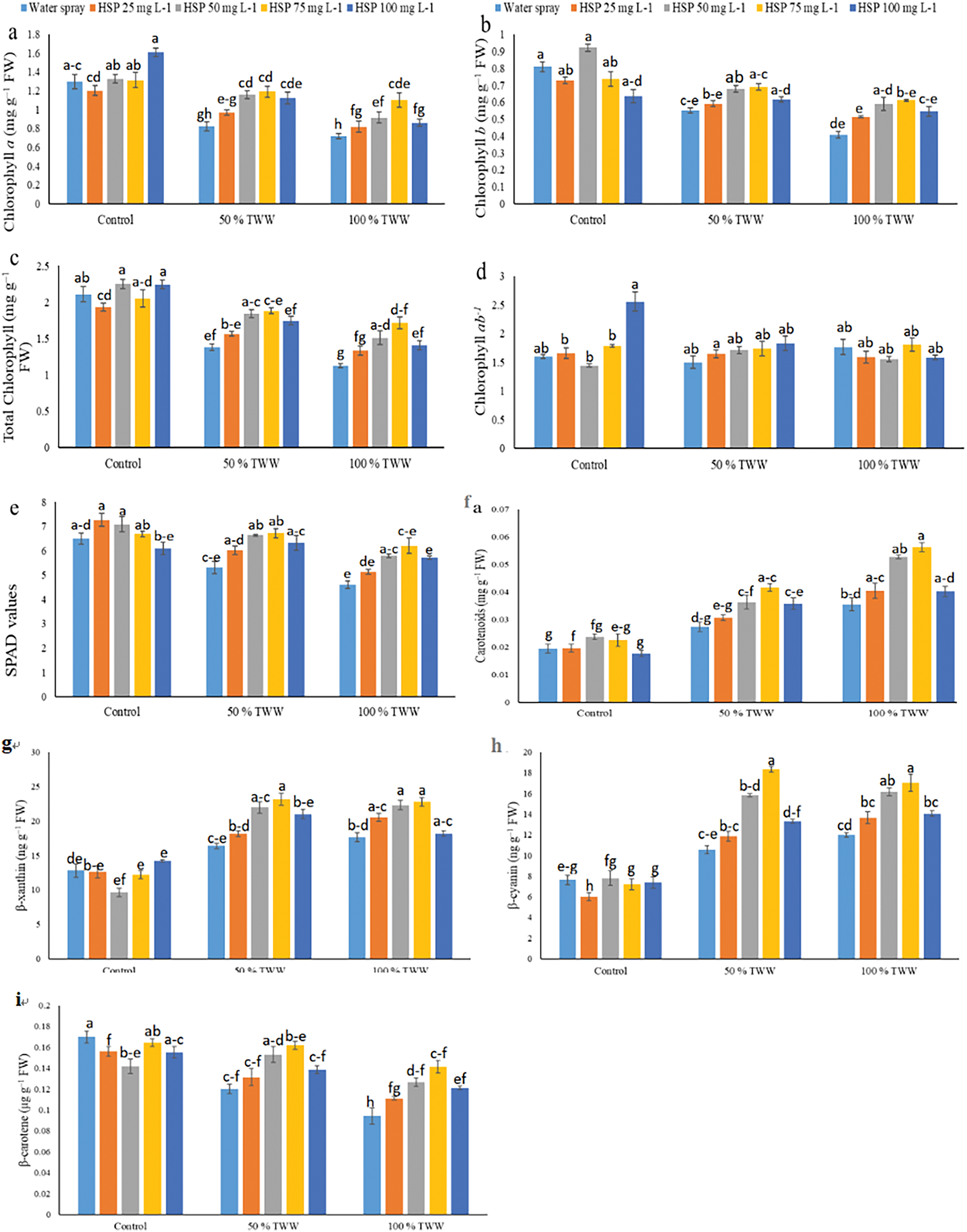
Figure 1: Impact of exogenous hesperidin (HSP) on (a) chlorophyll a, (b) chlorophyll b (c) total chlorophyll (d) chlorophyll ab−1 (e) SPAD values, (f) carotenoids, (g) β-xanthin, (h) β-cyanin and (i) and β-carotene in wheat plants under chromium (Cr) stress. Bars symbolize mean ± standard error values of three repeats (n = 3). Means were compared using a one-way analysis of variance followed by Tukey’s post-hoc test (p ≤ 0.05). In each panel, bars with different letters demonstrated significant differences among treatments
Nonetheless, the application of HSP considerably mitigates the detrimental effects of Cr on several pigments in stressed plants. In this instance, compared to control plants, 25, 50, 75, and 100 mg L−1 HSP supplementation increased the levels of carotenoids (12%, 32%, 52% and 30%), β-carotene (9%, 27%, 35% and 15%), β-xanthin (11%, 34%, 41% and 28%), and β-cyanin (12%, 50%, 74% and 26%), exposing 50% Cr stress (Fig. 1). Furthermore, when 100% Cr stress was applied, carotenoids (14%, 49%, 59% and 13%), β-carotene (18%, 34%, 50% and 28%), β-xanthin (17%, 26%, 29% and 03%), and β-cyanin (14%, 35%, 42% and 17%) increased further in response to 25, 50, 75, and 100 mg L−1 exogenous HSP supplementation, in comparison to control plants (Fig. 1). When wheat plants were exposed to 50 or 100 nM stress, they showed the greatest increase in leaf photosynthetic and other pigment levels following the application of exogenous HSPs at a dose of 75 mg L−1, but exogenous HSP application with 25 mg L−1 produced only slight changes in these parameters (Fig. 1).
3.2 Effect of Foliar Application Hesperidin on Non-Enzymatic Antioxidant Activity of Wheat Plants under Chromium Stress
In Cr-stressed wheat plants, tannery wastewater increased the levels of oxidized glutathione (GSSG) in the current study ascorbic acid (AsA), reduced glutathione (GSH), flavonoids, phenolics, and anthocyanins, and lowered total glutathione (GSH: GSSG ratio) In both stressed and non-stressed wheat cultivars, we refined the GSH: GSSG ratio, GSH levels, GSSG levels, AsA levels, flavonoid production, and phenolic concentration through the use of exogenous hesperidin (HSP) with different treatments and anthocyanins (Fig. 2). In this instance, the following GSH levels were significantly increased by 25, 50, 75, and 100 mg L−1 HSP supplementation: GSSG ratio (14%, 87%, 180%, and 49%), GSH (11%, 52%, 84% and 37%), ASA (11%, 25%, 34% and 12%), flavonoids (04%, 19%, 25% and 06%), phenolics (16%, 47%, 75% and 09%), and anthocyanins (41%, 63%, 97% and 79%) levels, and under 50% Cr stress, respectively. Additionally, when 100% Cr stress was applied, the 25, 50, 75, and 100 mg L−1 HSP supplementation caused a reduction in GSSG (−07%, −23%, −31% and −12%) and an increase in GSH: GSSG ratio (18%, 74%, 114% and 32%), GSH (10%, 34%, 48% and 16%), ASA (12%, 18%, 28% and 12%), flavonoids (14%, 27%, 38% and 23%), phenolics (18%, 37%, 49% and 29%), and anthocyanins (14%, 77%, 97%, and 56%) levels, respectively. When exogenous HSP was applied at a dosage of 75 mg L−1, a greater increase in the non-enzymatic antioxidant activities of wheat plants occurred, when Cr was applied at a dosage of 25 mg/L, they were stressed which only slightly altered the non-enzymatic antioxidant activities of the plants under 50% and 100% Cr stress.
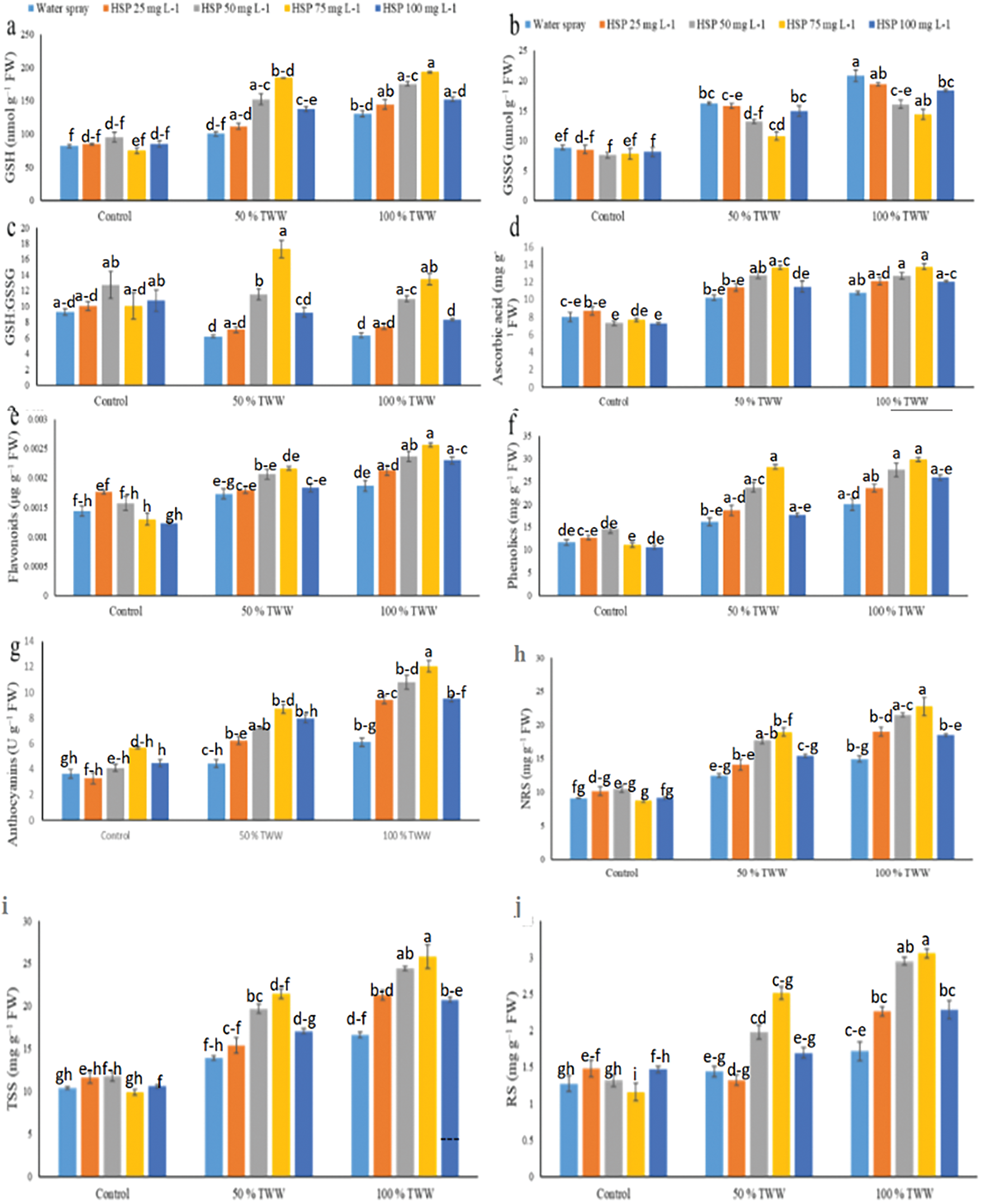
Figure 2: Impact of exogenous hesperidin (HSP) on (a) reduced glutathione (GSH), (b) oxidized glutathione (GSSG) (c) total glutathione (GSH: GSSG) (d) ascorbic acid, (e) flavonoids, (f) phenolics, (g) anthocyanins, (h) non-reducing sugars (NRS), (i) total soluble sugars (TSS) and (j) reducing sugars (RS) in wheat plants under chromium (Cr) stress. Bars symbolize mean ± standard error values of three repeats (n = 3). Means were compared using a one-way analysis of variance followed by Tukey’s post-hoc test (p ≤ 0.05). In each panel, bars with different letters demonstrated significant differences among treatments
The experiments evaluated the effect of chromium on the increased levels of reducing sugars (RS), non-reducing sugars (NRS), and total soluble sugars (TSS). Exogenous Hesperidin (HSP) application effectively further increased TSS, RS and NRS concentration under Cr stress in Wheat (Fig. 2). In the particular context, 25 and 50 mg L−1, 75 and 100 mg L−1 HSP supplementation greatly raised TSS (11%, 42%, 54% and 23%), RS (08%, 37%, 75% and 18%), and NRS (13%, 42%, 52% and 24%) concentration respectively under 50% Cr stress, as compared to wheat plants under control (Fig. 2). Moreover, 25, 50, 75, and 100 mg L−1 HSP application also caused an increase in TSS (28%, 47%, 55% and 25%), RS (32%, 72%, 78% and 33%), and NRS (27%, 44%, 52% and 24%) concentration respectively when 100% Cr stress was applied, as compared to control wheat plants (Fig. 2). Exogenous HSP application @75 mg L−1 caused a maximum upsurge in TSP, TSS, RS, and NRS levels of Cr stressed wheat plants than other treatments under 50% and 100% Cr-stress while exogenous HSP application @25 mg L−1 caused a minute change in TSP, TSS, RS, and NRS levels.
3.3 Effect of Foliar Application Hesperidin on Proteins, Enzymatic Antioxidant Activity of Wheat Plants under Chromium Stress
Total soluble protein (TSP) concentrations increased at 50% Cr stress while decreasing at 100% Cr stress in wheat plants (Fig. 3). The effects of antioxidant enzymes (APX, POD, CAT, GST, and SOD) were amplified under stress conditions, and the NRA’s activity in wheat plants decreased (Fig. 3). Application of exogenous hesperidin (HSP) improved the protein concentration and antioxidant enzyme activities of wheat plants under Cr stress. HSP 75 mg L−1 enhanced the maximum amount of protein and antioxidant enzymes under Cr stress in wheat plants. In this situation, 25, 50, 75 and 100 mg L−1 HSP supplementation caused a further increase in TSP (04%, 34%, 37% and 11%), SOD (22%, 37%, 46% and 09%), POD (28%, 53%, 68% and 13%), CAT (08%, 41%, 65% and 24%), APX (16%, 39%, 68% and 12%), GST (11%, 53%, 57% and 31%) and NRA (09%, 35%, 46% and 21%) 50% Cr stresses the plants respectively, compared to control plants (Fig. 4). Moreover, 25, 50, 75 and 100 mg L−1 HSP supplementation also caused a further increase in TSP (02%, 26%, 34% and 15%), SOD (29%, 41%, 63% and 15%), POD (25%, 46%, 91% and 40%), CAT (11%, 56%, 72% and 25%), APX (27%, 43%, 80% and 29%), When we apply 100% Cr stress to GST (06%, 49%, 73% and 28%), and NRA (19%, 44%, 88% and 30%), respectively, their behaviors differ from that of the control plant (Fig. 4). Applying exogenous HSPs at a concentration of 75 mg L−1 caused maximum increases in TSP and antioxidant enzyme activities in wheat plants subjected to Cr stress, surpassing the effects of other treatments at 50% and 100% Cr stress while exogenous TSP application with 25 mg L−1 caused a minute change activities.
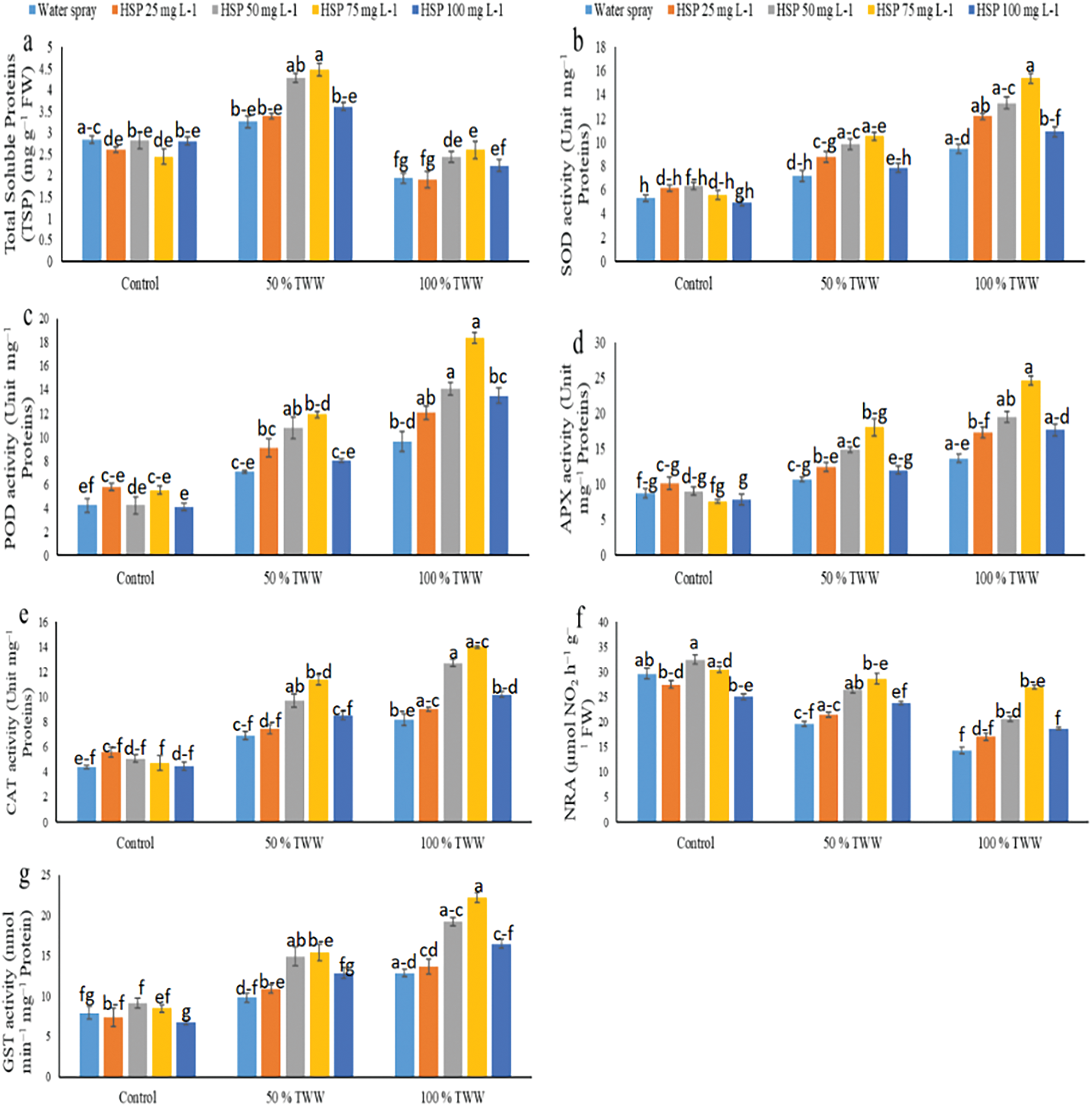
Figure 3: Impact of exogenous hesperidin (HSP) on (a) total soluble proteins (TSP), (b) superoxide dismutase (SOD) activity, (c) peroxidase (POD) activity, (d) ascorbate peroxidase APX activity, (e) catalase (CAT) activity, (f) Nitrate reductase (NRA) activity, and (g) glutathione S-Transferase (GST) in wheat plants exposed to chromium (Cr) stress. The three replicates (n = 3), mean ± standard error values are represented by Bars. Means were compared using a one-way analysis of variance followed by Tukey’s post-hoc test (p ≤ 0.05). In each panel, bars with different letters demonstrated significant differences among treatments
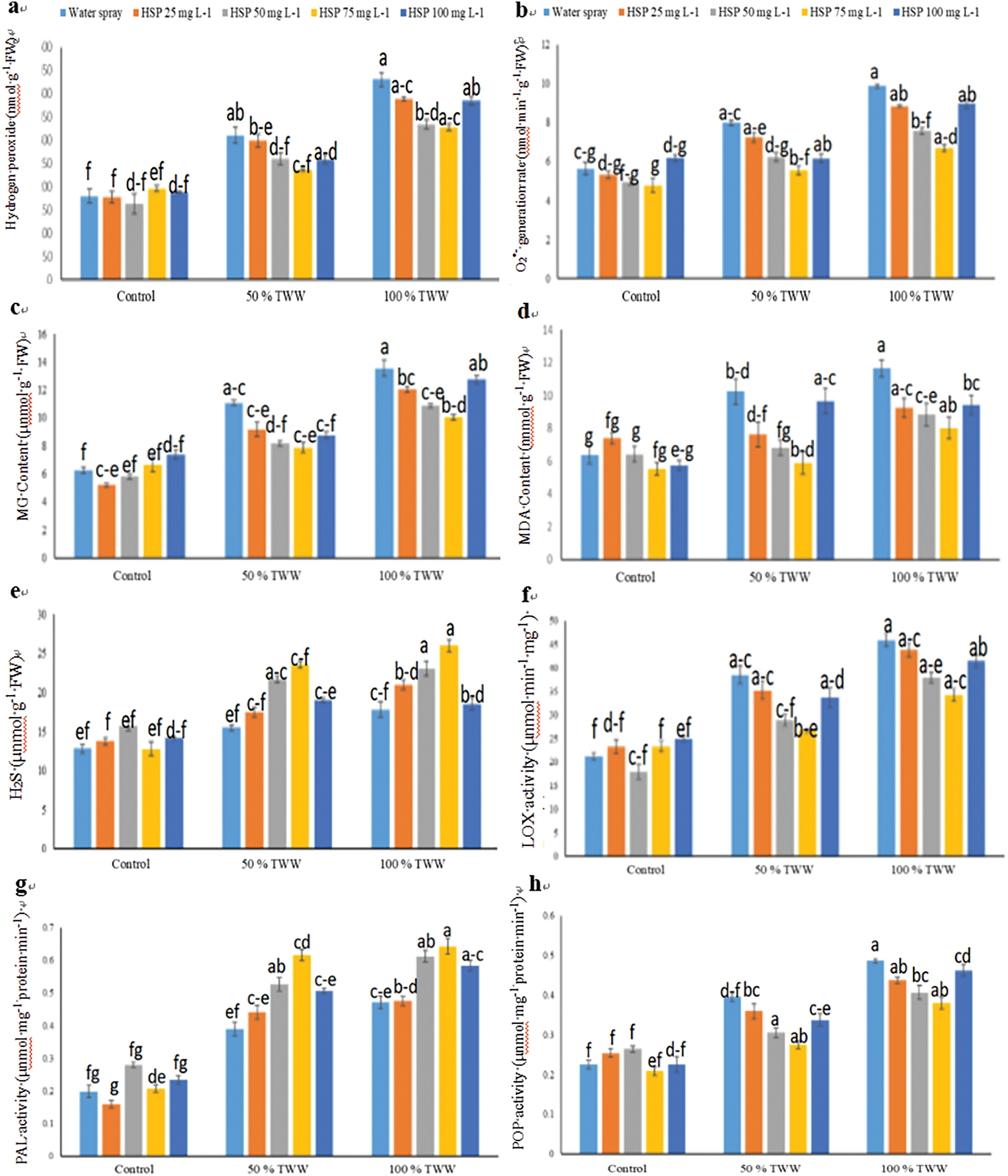
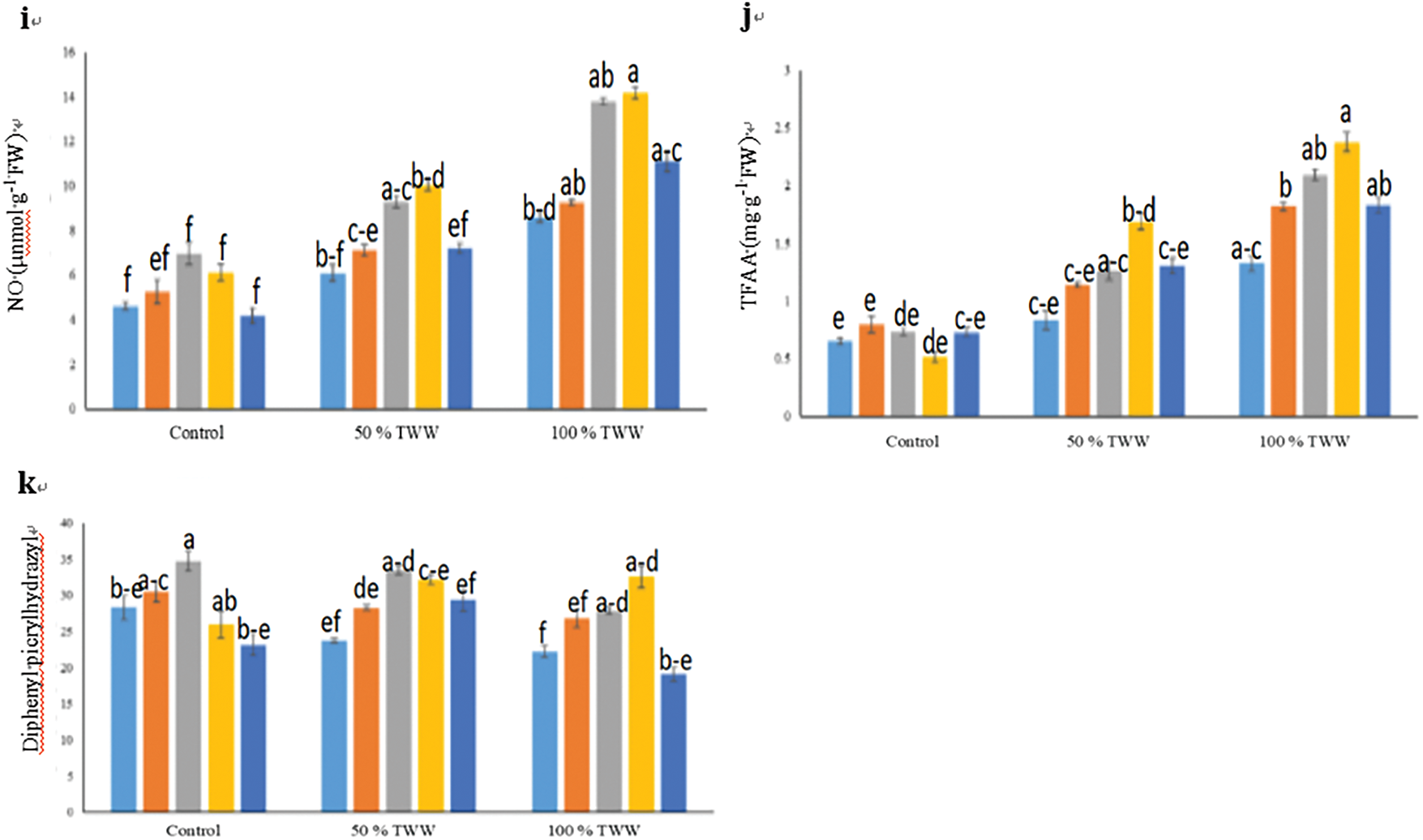
Figure 4: Impact of exogenous hesperidin (HSP) on (a) Hydrogen peroxide (H2O2), (b) O2•− generation rate, (c) MG concentration, (d) Malondialdehyde (MDA), (e) Hydrogen sulfide (H2S), (f) Lipoxygenase (LOX) activity, (g) Phenylalanine ammonia-lyase (PAL) activity, (h) PPO activity, (i) Nitric oxide (NO), (j) Total free amino acids (TFFA) and (k) Diphenyl picrylhydrazyl (DPPH) in wheat plants under chromium (Cr) stress. Mean ± standard error values of three repeats (n = 3) are represented by bars. Means were compared using a one-way analysis of variance followed by Tukey’s post-hoc test (p ≤ 0.05). In each panel, bars with different letters demonstrated significant differences among treatments
3.4 Effect of Foliar Application Hesperidin on Hydrogen Peroxide, Superoxide Radicals, Methylglyoxal, Lipid Peroxidation Rate, Hydrogen Sulfide, Phenyl Ammonia Lyase, Polyphenol Oxidase and Lipoxygenase Activity of Wheat Plants under Chromium Stress
In the present investigation, augmented levels of (H2O2), O2•− generation rate, MG concentration, Malondialdehyde (MDA), and Hydrogen sulfide (H2S) were found in the wheat plants under chromium (Cr) stress (Fig. 4). The application of hesperidin (HSP) helps wheat plants defend against oxidative stress by increasing their H2O2 and O2•− generation rates. MG concentration, Malondialdehyde (MDA), and Hydrogen sulfide (H2S) in wheat plants (Fig. 5). In this situation, 25, 50, 75, and 100 mg L−1 HSP supplementation caused a decrease in H2O2 (−04%, −16%, −24%, and −17%), O2•− generation rate (−09%, −22%, −30%, and −23%), MG concentration (−17%, −26%, −29%, and −21%), MDA (−26%, −33%, −43%, and −06%), and further increase in H2S level (13%, 40%, 52%, and 23%) respectively under 50% Cr stress, compared to control plants (Fig. 4). Moreover, 25, 50, 75, and 100 mg L−1 HSP supplementation also caused a decrease in H2O2 (−10%, −23%, −24%, and −11%), O2•− generation rate (−10%, −23%, −32% and −09%), MG concentration (−11%, −20%, −26%, and −06%), when 100% Cr stress was applied, MDA (−21%, −24%, −31%, and −19%), as well as the H2S levels (18%, 29%, 46%, and 04%), significantly increased compared to the control plant (Fig. 4). Exogenous HSP application @75 mg L−1 caused a maximum reduction in activities of biomarkers of oxidative stress under Cr stressed wheat plants than other treatments under 50% and 100% Cr stress while exogenous HSP application @25 mg L−1 caused a minute change in activities of biomarkers of oxidative stress.
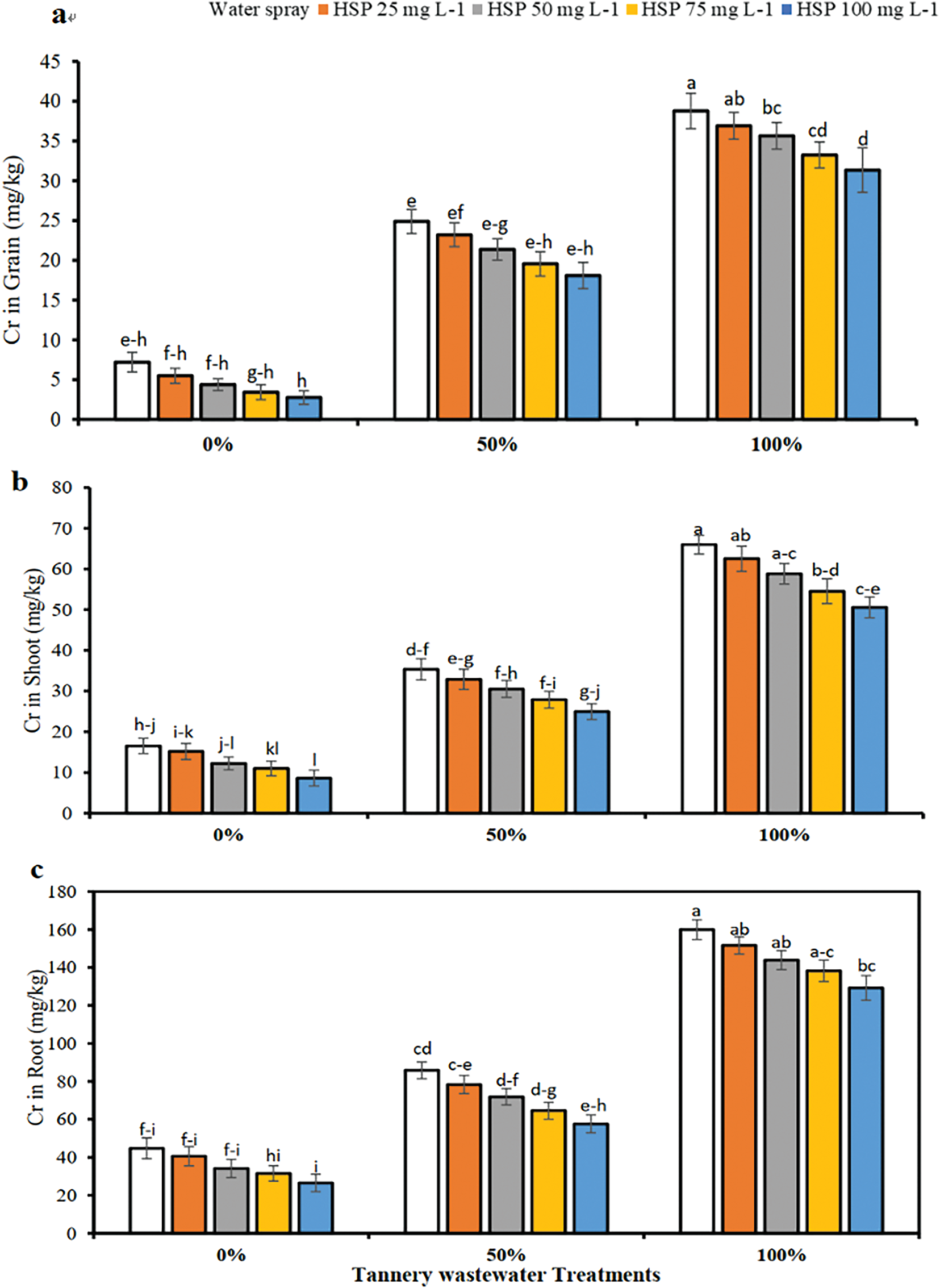
Figure 5: Impact of exogenous hesperidin (HSP) on (a) Cr in grain, (b) Cr in shoot and (c) Cr in roots in wheat plants under chromium (Cr) stress. Mean ± standard error values of three repeats (n = 3) are represented by bars. Means were compared using a one-way analysis of variance followed by Tukey’s post-hoc test (p ≤ 0.05). In each panel, bars with different letters demonstrated significant difference among treatments
In our experiment, it was noticed that Cr toxicity increased nitrogen oxide (NO) and the concentration of total free amino acids (TFAA) in wheat plants decreases under chromium stress, while DPPH Radical Scavenging activity becomes less prominent. NO, TFAA and DPPH activity increased when exogenous hesperidine (HSP) was applied to the wheat genotype (Fig. 4). In this context, 25, 50, 75, and 100 mg L−1 HSP supplementation greatly raised NO concentration (17%, 55%, 65%, and 18%), TFAA concentration (36%, 51%, 102%, and 56%), and DPPH activity (19%, 41%, 35%, and 23%) respectively under 50% Cr stress, as compared with plants under control (Fig. 4). We administered 25 mg L−1 less than 0.1 mg L−1 HSP supplementation, and 0.75 mg L−1 and less than 0.1 mg L−1 HSP supplementation caused an increase in NO concentration by (08%, 60%, 65%, and 29%) units, and TFAA concentration by (37%, 58%, 79%, and 38%) units. and DPPH activity (21%, 25%, 47%, and −14%) respectively when 100% Cr stress was applied, as compared to wheat plants under control (Fig. 4). Applying exogenous HSPs at a concentration of 75 mg L−1 caused maximum increases in NO, TFAA concentration, and DPPH activity in Cr-stressed wheat plants than other treatments under 50% and 100% Cr stress while exogenous HSP application @25 mg L−1 caused a minute change in NO, TFAA concentration and DPPH activity.
The wheat genotype involved in activities of PAL, PPO, and LOX depicts its effects, due to the application of Hesperidin (HSP) (Fig. 4). The use of exogenous HSP in the wheat genotype boosts the activity of PAL, while it decreases the activities of PPO and LOX. In this situation, 25, 50, 75, and 100 mg L−1 HSP supplementation caused an increase in PAL activity (13%, 35%, 58%, and 30%), and decrease in PPO activity (−09%, −23%, −31%, and −15%), and LOX activity (−08%, −25%, −31%, and −13%) respectively under 50% Cr stress, compared to control plants (Fig. 4). Moreover, 25, 50, 75, and 100 mg L−1 HSP supplementation also caused a further increase in PAL activity (01%, 30%, 36%, and 24%), and decline in PPO activity (−10%, −16%, −22%, and −05%), and LOX activity (−05%, −17%, −25%, and −10%) respectively when 100% Cr stress was applied, relative to plants under control (Fig. 4). Under Cr-stressed wheat plants, the application of exogenous HSPs at 75 mg L−1 caused a greater increase in PAL activity and a more significant decrease in PPO and LOX activities than other treatments at 50% and 100% Cr stress while exogenous HSP application with 25 mg L−1 caused a little change in activities of LOX, PAL, and PPO.
3.5 Effect of Foliar Application Hesperidin on Metal Concentration in Wheat Plants
The Cr concentration in roots, shoots, and grains reduced to 19.19%, 23.33%, and 19.15%, respectively (Fig. 5). 100% Cr stress caused an increase in Cr concentration to 272.12%, 298.87% in roots, 439.24% in shoots, and NUM3 in grains, compared to the control.
Heavy metal toxicity has catastrophic effects on aquatic along with terrestrial life [70], and it has considerably disturbed the normal environment [71]. Globally, cultivated territories have become an ecological issue due to the recent prevalence of crop adulteration [72]. In the current investigation, we evaluated the potential part of exogenous hesperidin (HSP) on the biochemical and physiological factors of wheat plants under Cr stress. We found that tannery wastewater (Cr stress) reduced the concentrations of total Chl. concentration, i.e., Chl. a, b, and SPAD chlorophyll values in wheat cultivars while 100% Cr stress increased the Chlorophyll ab values and 50% Cr stress decreased the Chlorophyll a and b values in wheat cultivars which demonstrates that chlorophyll concentration might provide initial signs of toxic effects of Cr in wheat crop. In scientific investigations, excess chromium causes a decline in photosynthesis pigments in wheat and other crop foliage [73–75]. While, our study witnessed the enhanced level of β-xanthin, β-cyanin concentration, and reduction in carotenoids such as β-carotene concentration in wheat plants under Cr stress. Photosynthetic pigments and chloroplast ultrastructure, as well as gas exchange factors, have been attributed to the upsurge in Chromium’s influence, resulting in modifications of photosynthetic pigments because of reduction in carotenoids as well as in Chl, a, b concentration [76–78]. Reduction in chlorophyll concentration might also be because of the ROS production in plants under abiotic stress [76,79]. Plants experience damage as their carotenoid concentration increases and these pigments become involved in the defense mechanism against photo-oxidative damage to chlorophyll [80]. Application of exogenous hesperidin (HSP) improved the carotenoid absorptions and leaf chlorophyll concentration of wheat plants under Cr toxicity. Specifically, the application of HSP enhanced photosynthetic pigments in Celosia argentea plants under abiotic stresses [81].
Non-enzymatic antioxidants including reduced oxidized glutathione (GSSG), glutathione (GSH), the GSH ratio, flavonoids, phenolics, anthocyanins, and ascorbic acid, are crucial redox-protecting intermediaries in cells. The cell maintains a balanced redox state and neutralizes excess ROS, thereby normalizing oxidative stress. Furthermore, these antioxidants play important roles in various physiological activities such as phytohormone homeostasis, cell division, and pollen development and differentiation [82,83]. The outcomes of our research designated raised levels of reduced oxidized glutathione (GSSG), flavonoids, ascorbic acid, phenolics, anthocyanins, and glutathione (GSH) along with a reduced GSH ratio in chromium-treated wheat crop. These outcomes are reliable with interpretations in Oryza sativa L. [84], Brassica napus L. [85], and Zea mays L. [86] in chromium stress. We also evaluated that the total soluble protein (TSP) level increased under 50% chromium stress but reduced under 100% chromium stress in wheat plants, supporting the outcomes of [77]. The hostile effects of chromium on photosynthetic pigments or the increased chromium uptake may cause a decrease in protein concentration. Heavy metal stress is recognized to generate ROS that can lead to the alteration or degradation of proteins [87]. In Cr stress conditions, the actions of antioxidant enzymes (SOD, APX, CAT, GST, and POD) amplified, whereas the action of NRA declined in wheat plants.
These results oppose the outcomes of [12] who found that chromium reduced the activity of several antioxidant enzymes in wheat plants Gill et al. [77] found that CAT action amplified while APX activity lessened in B. napus under Cr stress. A previous study by Khan et al. [88] concluded that leaf APX activity amplified in wheat under cadmium stress. Cr stress in wheat plants leads to an increased production of antioxidant actions, effectively enhancing their ability to scavenge reactive oxygen species, and consequently reducing plant growth inhibition caused by excess ROS. The experimental reduction in total soluble proteins and the modification in antioxidant enzyme activity in wheat exposed to Cr stress designate the presence of oxidative stress. Wheat plants in chromium stress experienced a decrease in oxidative stress as a result of the use of exogenous hesperidin (HSP), which enhanced protein concentration and antioxidant enzyme actions. Previous investigation also revealed that the exogenous use of HSP boosted the antioxidant system in Celosia argentea plants in metal stresses [81].
In the current research, improved levels of (H2O2), O2•− generation rate, Malondialdehyde (MDA), MG concentration, and Hydrogen sulfide (H2S) were found under chromium (Cr) stress in wheat plants. In parallel with the current study, we observed an increase in oxidative injury ourselves, i.e., the concentration of lipid peroxidation, production of superoxide (O2•−) and hydrogen peroxide (H2O2) generation, and level of methylglyoxal (MG) in Brassica juncea L [89]. Chromium stress causes plants to face adversity. Similarly, over-production of MDA and H2O2 was observed in wheat plants exposed to chromium toxicity. Cr toxicity is attributed to stimulating the ROS injuries including superoxide anion (O2•−), hydrogen peroxide (H2O2), Malondialdehyde (MDA), and Hydrogen sulfide (H2S) as a curative approach modified by the plants to change the cellular redox position of the plant, that consequences in oxidative damages [90–92]. In our study, the application of exogenous HSP improved the stress tolerance to protect the wheat plants from oxidative stress due to reduced levels of O2•− production, MDA, MG concentration, and H2S in wheat plants.
Nitrogen oxide (NO) acts as an important signaling molecule, modifying numerous physiological processes such as organogenesis, germination, and plant development [93]. In our experimentation, we examined that Cr toxicity reduced NO absorption inside wheat plants, consequently disturbing overall growth. While application of exogenous HSP with the wheat genotype, both TFAA and NO levels showed improvements under stress conditions. The application of exogenous HSP encouraged the DPPH activity in wheat plants. Moreover, the results of our present study align with the previous study [94]. In a Cr-contaminated environment, rice plants developed and I tested the effect of DPPH radical exposing action, which deteriorated inside them. Likewise, Kundu et al. [95] noticed a substantial decrease in the radical of DPPH remediating action in Plantago under Cr stress.
Our research also presented that Cr toxicity reduced reducing sugars (RS), non-reducing sugars (NRS), and total soluble sugars (TSS), in wheat plants. The application of exogenous HSP proficiently improved the concentration of TSS, RS, and NRS under the Cr stress. Numerous primary and secondary metabolic mechanisms play an energetic role in cell osmotic alterations. These discoveries support the outcomes described by [96]. The improvement of osmoprotectants, such as TSS, RS, and NRS, may be accredited to improved meristematic action, leading to enhanced cell division and development, which eventually increases osmoprotectant action [97] (Fig. 6). Phenylalanine ammonia-lyase (PAL) generates precursors of various secondary metabolites, including phytoalexins, phenolic and lignin composites. It is the primary enzyme involved in the phenylpropanoid passageway, playing a major impact during growth, its adaptation, development, and alleviation responses to different pathogen and environmental stresses through constructing secondary metabolites that control plant growth reactions [98,99]. Polyphenol oxidase (PPO) decreases oxidative injuries in wheat plants during the grain-filling stage [100], while lipoxygenase (LOX) is a sign of oxidative stress, catalyzes the process of oxygenation in unsaturated fatty acids that results in the formation of lipid hydroperoxides [91]. In our research, the enzymatic actions of LOX, PAL, and PPO, considerably improved in the leaves of wheat genotypes under Cr stress. While the action of PAL further improved with the application of HSP, while the actions of PPO and LOX declined. A decline in PPO and LOX actions in response to both abiotic and biotic environmental stresses has also been evaluated by previous studies [97,99,101,102]. Moreover, in our research, Cr with 100% enhanced accumulation of Cr concentration in the root as well as aerial part of the plant. On the other hand, application of HSP with 100 mg L−1 under both cases significantly reduced the accumulation of Cr in root, shoot, and grain as well. Previous studies by Kumar Singh et al. [103] and Nawaz et al. [104] also depicted that Cr toxicity reduced stimulated oxidative stress and promoted translocation of Cr metal from root to grains in rice and wheat crops. Furthermore, the results of previous studies by Hussain et al. [104] showed that the application of HSP significantly enhanced plant growth, photosynthetic pigments, and nutrient acquisition as well as reduced the Cr accumulation in Bassia Scoparia and Celosia argentea plants.
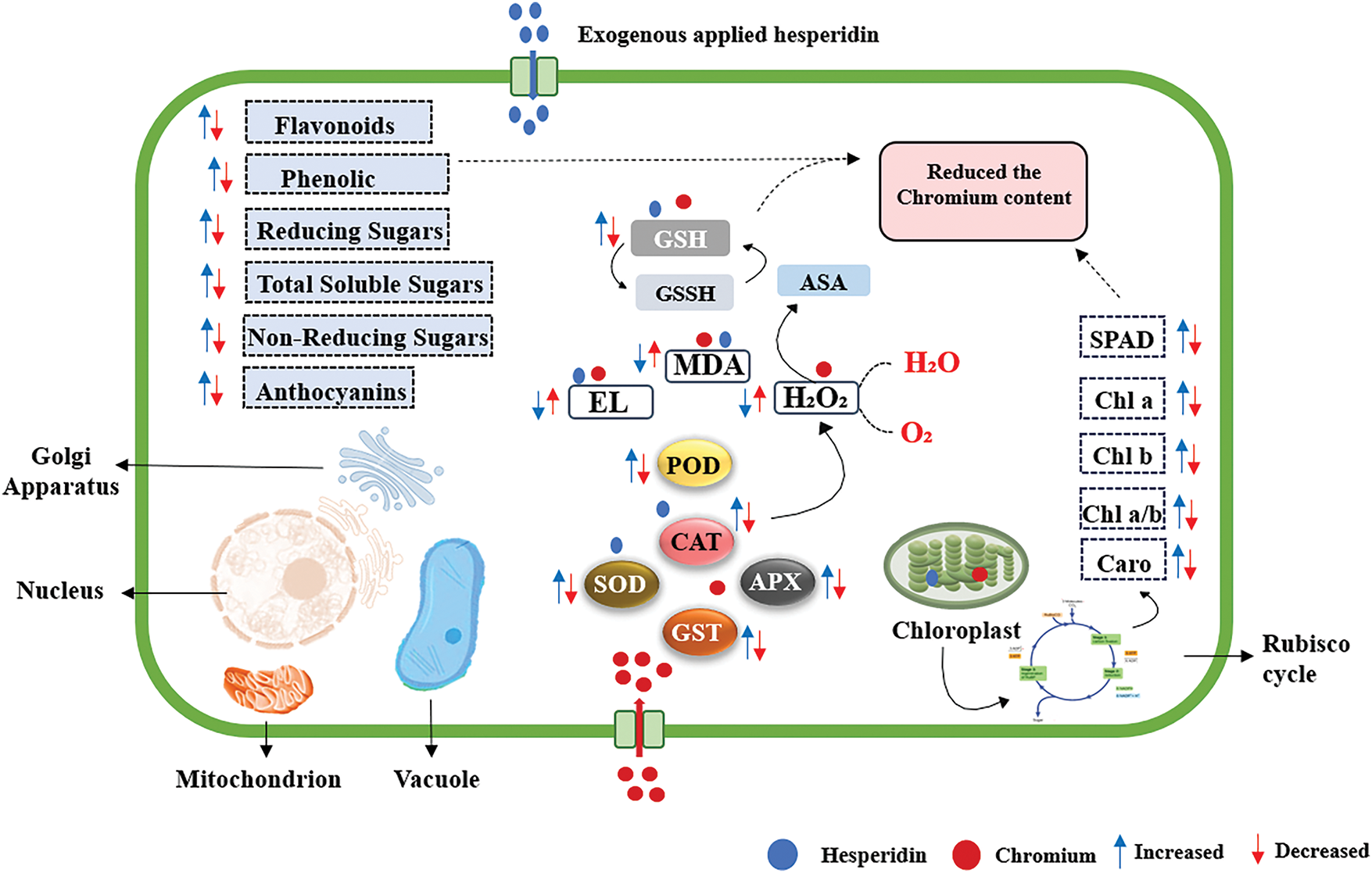
Figure 6: Proposed mechanism for Chromium toxicity alleviation by foliar application Hesperidin
This study examined the impact of foliar HSP spray on mitigating Cr toxicity in wheat cultivated in soil irrigated with tannery wastewater. Chromium stress produces oxidative damage in wheat by promoting the generation of reactive oxygen species (ROS), resulting in membrane damage, lipid peroxidation, and cellular damage. Conversely, the application of exogenous HSP effectively mitigates Cr-induced oxidative stress in wheat plants. The implementation of HSP markedly diminished Cr stress by attenuating the activity of enzymatic antioxidants and non-enzymatic antioxidants, hence reducing ROS accumulation and lipid peroxidation, while enhancing cellular viability. This enables wheat genotypes to thrive under chromium stress. This study offers valuable insight into the stress-reducing properties of HSP by histochemical, biochemical, and essential antioxidant enzyme analysis. The results provide a comprehensive understanding of how HSP enhances stress alleviation, aiding researchers in formulating strategies to enhance plant production and growth in chromium-contaminated soils. Improving these techniques for diverse crop species and varying levels of heavy metal load could advance agricultural efficiency in these settings. Furthermore, identifying the primary regulators involved in the stress amelioration process may facilitate the development of genetically modified crops optimized for high yield under chromium stress.
Acknowledgement: The authors would like to extend their sincere appreciation to the financial support for the Government College University, Faisalabad. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2025R182), King Saud University, Riyadh, Saudi Arabia.
Funding Statement: The authors would like to extend their sincere appreciation to the Higher Education Commission, Pakistan for their financial support. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2025R182), King Saud University, Riyadh, Saudi Arabia.
Author Contributions: Gohar Ayoub: Performed the design of the experiment, formal analysis, investigation, carried out the experiment, originally drafted the manuscript. Muhammad Arslan Ashraf: Methodology, writing—review and editing. Shoaib Ahmad: Formal analysis, writing—review and editing. Mohamed A. El-Sheikh: Validation, writing-review and editing. Shafaqat Ali: Supervision, conceptualization, resources, funding acquisition. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data analyzed during this study were included.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Rashid A, Schutte BJ, Ulery A, Deyholos MK, Sanogo S, Lehnhoff EA, et al. Heavy metal contamination in agricultural soil: environmental pollutants affecting crop health. Agronomy. 2023;13(6):1521. doi:10.3390/agronomy13061521. [Google Scholar] [CrossRef]
2. Soleimani H, Mansouri B, Kiani A, Omer AK, Tazik M, Ebrahimzadeh G, et al. Ecological risk assessment and heavy metals accumulation in agriculture soils irrigated with treated wastewater effluent, river water, and well water combined with chemical fertilizers. Heliyon. 2023;9(3):e14580. doi:10.1016/j.heliyon.2023.e14580. [Google Scholar] [PubMed] [CrossRef]
3. Awewomom J, Dzeble F, Takyi YD, Ashie WB, Ettey ENYO, Afua PE, et al. Addressing global environmental pollution using environmental control techniques: a focus on environmental policy and preventive environmental management. Discov Environ. 2024;2(1):8. doi:10.1007/s44274-024-00033-5. [Google Scholar] [CrossRef]
4. Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9):e04691. doi:10.1016/j.heliyon.2020.e04691. [Google Scholar] [PubMed] [CrossRef]
5. Pakdel M, Olsen A, Bar EMS. A review of food contaminants and their pathways within food processing facilities using open food processing equipment. J Food Prot. 2023;86(12):100184. doi:10.1016/j.jfp.2023.100184. [Google Scholar] [PubMed] [CrossRef]
6. Ali HQ, Yasir MU, Farooq A, Khan M, Salman M, Waqar M. Tanneries impact on groundwater quality: a case study of Kasur city in Pakistan. Environ Monit Assess. 2022;194(11):823. doi:10.1007/s10661-022-10502-0. [Google Scholar] [PubMed] [CrossRef]
7. Araujo ASF, Pereira APA, Mendes LW. Applications of Cr-rich composted tannery sludge in the soil decrease microbial biomass and select specific bacterial groups. Environ Sci Pollut Res. 2022;29(50):75113–8. doi:10.1007/s11356-022-22933-w. [Google Scholar] [PubMed] [CrossRef]
8. Ullah S, Liu Q, Wang S, Jan AU, Sharif HMA, Ditta A, et al. Sources, impacts, factors affecting Cr uptake in plants, and mechanisms behind phytoremediation of Cr-contaminated soils. Sci Total Environ. 2023;899(2):165726. doi:10.1016/j.scitotenv.2023.165726. [Google Scholar] [PubMed] [CrossRef]
9. Zulfiqar U, Haider FU, Ahmad M, Hussain S, Maqsood MF, Ishfaq M, et al. Chromium toxicity, speciation, and remediation strategies in soil-plant interface: a critical review. Front Plant Sci. 2023;13:1081624. doi:10.3389/fpls.2022.1081624. [Google Scholar] [PubMed] [CrossRef]
10. Vaiopoulou E, Gikas P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: a review. Water Res. 2012;46(3):549–70. doi:10.1016/j.watres.2011.11.024. [Google Scholar] [PubMed] [CrossRef]
11. Wang J. Reuse of heavy metal from industrial effluent water. In: IOP Conference Series: Earth and Environmental Science; 2018 Nov 9–11; Tokyo, Japan. doi:10.1088/1755-1315/199/4/042002. [Google Scholar] [CrossRef]
12. Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, et al. Alleviation of chromium toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed chromium uptake and oxidative stress in wheat (Triticum aestivum L.). Environ Sci Pollut Res. 2015;22:10669–78. doi:10.1007/s11356-015-4193-4. [Google Scholar] [PubMed] [CrossRef]
13. Saha TR, Khan MAR, Kundu R, Naime J, Karim KMR, Ara MH. Heavy metal contaminations of soil in waste dumping and non-dumping sites in Khulna: human health risk assessment. Results Chem. 2022;4:100434. doi:10.1016/j.rechem.2022.100434. [Google Scholar] [CrossRef]
14. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Mol Clin Environ Toxicol. 2012;1:133–64. doi:10.1007/978-3-7643-8340-4_6. [Google Scholar] [PubMed] [CrossRef]
15. Angon PB, Islam MS, Kc S, Das A, Anjum N, Poudel A, et al. Sources, effects and present perspectives of heavy metals contamination: soil, plants and human food chain. Heliyon. 2024;10(7):e28357. doi:10.1016/j.heliyon.2024.e28357. [Google Scholar] [PubMed] [CrossRef]
16. Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS, et al. Chromium bioaccumulation and its impacts on plants: an overview. Plants. 2020;9(1):100. doi:10.3390/plants9010100. [Google Scholar] [PubMed] [CrossRef]
17. Mitra S, Chakraborty AJ, Tareq AM, Emran TB, Nainu F, Khusro A, et al. Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci. 2022;34(3):101865. doi:10.1016/j.jksus.2022.101865. [Google Scholar] [CrossRef]
18. Ali S, Mir RA, Tyagi A, Manzar N, Kashyap AS, Mushtaq M, et al. Chromium toxicity in plants: signaling, mitigation, and future perspectives. Plants. 2023;12(7):1502. doi:10.3390/plants12071502. [Google Scholar] [PubMed] [CrossRef]
19. Kapoor RT, Mfarrej MFB, Alam P, Rinklebe J, Ahmad P. Accumulation of chromium in plants and its repercussion in animals and humans. Environ Pollut. 2022;301(1):119044. doi:10.1016/j.envpol.2022.119044. [Google Scholar] [PubMed] [CrossRef]
20. Guo W, Xing Y, Luo X, Li F, Ren M, Liang Y. Reactive oxygen species: a crosslink between plant and human eukaryotic cell systems. Int J Mol Sci. 2023;24(17):13052. doi:10.3390/ijms241713052. [Google Scholar] [PubMed] [CrossRef]
21. Mansoor S, Ali A, Kour N, Bornhorst J, AlHarbi K, Rinklebe J, et al. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants. 2023;12(16):3003. doi:10.3390/plants12163003. [Google Scholar] [PubMed] [CrossRef]
22. Ahmad P, Tripathi DK, Deshmukh R, Singh VP, Corpas FJ. Revisiting the role of ROS and RNS in plants under changing environment. Environ Exp Bot. 2019;161:1–3. [Google Scholar]
23. Yengkokpam P, Mazumder PB. Antioxidant enzymatic activities and profiling of gene expression associated with organophosphate stress tolerance in Solanum melongena L. cv. Longai. 3 Biotech. 2021;11(12):510. doi:10.1007/s13205-021-03061-7. [Google Scholar] [PubMed] [CrossRef]
24. Zandi P, Schnug E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology. 2022;11(2):155. doi:10.3390/biology11020155. [Google Scholar] [PubMed] [CrossRef]
25. Mishra N, Jiang C, Chen L, Paul A, Chatterjee A, Shen G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front Plant Sci. 2023;14:1110622. doi:10.3389/fpls.2023.1110622. [Google Scholar] [PubMed] [CrossRef]
26. Hasanuzzaman M, Bhuyan MHMB, Anee TI, Parvin K, Nahar K, Mahmud JA, et al. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8(9):384. doi:10.3390/antiox8090384. [Google Scholar] [PubMed] [CrossRef]
27. Chaudhary P, Janmeda P, Docea AO, Yeskaliyeva B, Razis AFA, Modu B, et al. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023;11:1158198. doi:10.3389/fchem.2023.1158198. [Google Scholar] [PubMed] [CrossRef]
28. Ahmad S, Mfarrej MFB, El-Esawi MA, Waseem M, Alatawi A, Nafees M, et al. Chromium-resistant Staphylococcus aureus alleviates chromium toxicity by developing synergistic relationships with zinc oxide nanoparticles in wheat. Ecotoxicol Environ Saf. 2022;230(4):113142. doi:10.1016/j.ecoenv.2021.113142. [Google Scholar] [PubMed] [CrossRef]
29. Vitelli V, Giamborino A, Bertolini A, Saba A, Andreucci A. Cadmium stress signaling pathways in plants: molecular responses and mechanisms. Current Issues Mol Biol. 2022;46(6):6052–68. doi:10.3390/cimb46060361. [Google Scholar] [PubMed] [CrossRef]
30. Adrees M, Ali S, Iqbal M, Bharwana SA, Siddiqi Z, Farid M, et al. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and Cr uptake in sand and soil media. Ecotoxicol Environ Saf. 2015;122:1–8. doi:10.1016/j.ecoenv.2015.07.003. [Google Scholar] [PubMed] [CrossRef]
31. Farooq MA, Ali S, Hameed A, Bharwana SA, Rizwan M, Ishaque W, et al. Cadmium stress in cotton seedlings: physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S Afr J Bot. 2016;104:61–8. doi:10.1016/j.sajb.2015.11.006. [Google Scholar] [CrossRef]
32. Ball K, Sadhukhan S. Chromium toxicity in plants: an overview of plant signaling. Met Met Plant Signal. 2024;29:143–69. doi:10.1007/978-3-031-59024-5. [Google Scholar] [CrossRef]
33. Kanner J. Food polyphenols as preventive medicine. Antioxidants. 2023;12(12):2103. doi:10.3390/antiox12122103. [Google Scholar] [PubMed] [CrossRef]
34. Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact. 2017;277(2):146–58. doi:10.1016/j.cbi.2017.09.015. [Google Scholar] [PubMed] [CrossRef]
35. Mahmoud AM, Bautista RJH, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxidative Med Cell Longev. 2019;2019(1):5484138. doi:10.1155/2019/5484138. [Google Scholar] [PubMed] [CrossRef]
36. Arikan B, Ozfidan-Konakci C, Yildiztugay E, Zengin G, Alp FN, Elbasan F. Exogenous hesperidin and chlorogenic acid alleviate oxidative damage induced by arsenic toxicity in Zea mays through regulating the water status, antioxidant capacity, redox balance and fatty acid composition. Environ Pollut. 2022;292:118389. doi:10.1016/j.envpol.2021.118389. [Google Scholar] [PubMed] [CrossRef]
37. Seleiman MF, Ali S, Refay Y, Rizwan M, Alhammad BA, El-Hendawy SE. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere. 2020;250(6):126239. doi:10.1016/j.chemosphere.2020.126239. [Google Scholar] [PubMed] [CrossRef]
38. Nawaz H, Anwar-ul-Haq M, Akhtar J, Arfan M. Cadmium, chromium, nickel and nitrate accumulation in wheat (Triticum aestivum L.) using wastewater irrigation and health risks assessment. Ecotoxicol Environ Saf. 2021;208(3):111685. doi:10.1016/j.ecoenv.2020.111685. [Google Scholar] [PubMed] [CrossRef]
39. Ali W, Zhang H, Mao K, Shafeeque M, Aslam MW, Yang X, et al. Chromium contamination in paddy soil-rice systems and associated human health risks in Pakistan. Sci Total Environ. 2022;826(5):153910. doi:10.1016/j.scitotenv.2022.153910. [Google Scholar] [PubMed] [CrossRef]
40. Narayanan M, Ma Y. Mitigation of heavy metal stress in the soil through optimized interaction between plants and microbes. J Environ Manag. 2023;345:118732. doi:10.1016/j.jenvman.2023.118732. [Google Scholar] [PubMed] [CrossRef]
41. Zuo S, Zuo Y, Gu W, Wei S, Li J. Exogenous proline optimizes osmotic adjustment substances and active oxygen metabolism of maize embryo under low-temperature stress and metabolomic analysis. Processes. 2022;10(7):1388. doi:10.3390/pr10071388. [Google Scholar] [CrossRef]
42. Kanwal R, Pandey M, Bhaskaran N, Maclennan GT, Fu P, Ponsky LE, et al. Protection against oxidative DNA damage and stress in human prostate by glutathione S-transferase P1. Mol Carcinog. 2014;53(1):8–18. doi:10.1002/mc.21939. [Google Scholar] [PubMed] [CrossRef]
43. Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–82. doi:10.1016/0076-6879(87)48036-1. [Google Scholar] [CrossRef]
44. Sarker U, Oba S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 2018;252:72–83. doi:10.1016/j.foodchem.2018.01.097. [Google Scholar] [PubMed] [CrossRef]
45. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi:10.1016/0003-2697(76)90527-3. [Google Scholar] [PubMed] [CrossRef]
46. Giannopolitis CN, Ries SK. Superoxide dismutases: i. occurrence in higher plants. Plant Physiol. 1977;59(2):309–14. doi:10.1104/pp.59.2.309. [Google Scholar] [PubMed] [CrossRef]
47. Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–80. doi:10.1093/oxfordjournals.pcp.a076232. [Google Scholar] [CrossRef]
48. Polle A, Otter T, Seifert F. Apoplastic peroxidases and lignification in needles of norway spruce (Picea Abies L.). Plant Physiol. 1994;106(1):53–60. doi:10.1104/pp.106.1.53. [Google Scholar] [PubMed] [CrossRef]
49. Chance B, Maehly AC. Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–75. doi:10.1002/9780470110171.ch14. [Google Scholar] [PubMed] [CrossRef]
50. Joyia FA, Ashraf MY, Shafiq F, Anwar S, Nisa Z, Khaliq B, et al. Phytotoxic effects of varying concentrations of leather tannery effluents on cotton and brinjal. Agric Water Manag. 2021;246(8):106707. doi:10.1016/j.agwat.2020.106707. [Google Scholar] [CrossRef]
51. Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–12. doi:10.1016/0003-2697(80)90139-6. [Google Scholar] [PubMed] [CrossRef]
52. Hasanuzzaman M, Hossain MA, Fujita M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep. 2011;5(4):353–65. doi:10.1007/s11816-011-0189-9. [Google Scholar] [CrossRef]
53. Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in vigna seedlings. Physiol Plant. 1983;58(2):166–70. doi:10.1111/j.1399-3054.1983.tb04162.x. [Google Scholar] [CrossRef]
54. Marinova D, Ribarova F, Atanassova M. Total phenolics and flavonoids in bulgarian fruits and vegetables. J Univ Chem Technol Metall. 2005;40:255–60. [Google Scholar]
55. Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51(3):609–14. doi:10.1021/jf020782a. [Google Scholar] [PubMed] [CrossRef]
56. Mita S, Murano N, Akaike M, Nakamura K. Mutants of arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 1997;11(4):841–51. doi:10.1046/j.1365-313X.1997.11040841.x. [Google Scholar] [PubMed] [CrossRef]
57. Yang H, Wu F, Cheng J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011;127(3):1237–42. doi:10.1016/j.foodchem.2011.02.011. [Google Scholar] [PubMed] [CrossRef]
58. Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000;151(1):59–66. doi:10.1016/S0168-9452(99)00197-1. [Google Scholar] [CrossRef]
59. Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf. 2016;126:245–55. doi:10.1016/j.ecoenv.2015.12.026. [Google Scholar] [PubMed] [CrossRef]
60. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. Arch Biochem Biophys. 1968;125(1):189–98. doi:10.1016/0003-9861(68)90654-1. [Google Scholar] [PubMed] [CrossRef]
61. Nashef AS, Osuga DT, Feeney RE. Determination of hydrogen sulfide with 5,5′-dithiobis-(2-nitrobenzoic acidn-ethylmaleimide, and parachloromercuribenzoate. Anal Biochem. 1977;79:394–405. doi:10.1016/0003-2697(77)90413-4. [Google Scholar] [PubMed] [CrossRef]
62. Zhou B, Guo Z, Xing J, Huang B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in stylosanthes guianensis. J Exp Bot. 2005;56(422):3223–8. doi:10.1093/jxb/eri319. [Google Scholar] [PubMed] [CrossRef]
63. Hamilton PB, Van Slyke DD. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J Biol Chem. 1943;150(1):231–50. doi:10.1016/S0021-9258(18)51268-0. [Google Scholar] [CrossRef]
64. Sarker U, Oba S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected amaranthus leafy vegetables. J Sci Food Agric. 2019;99(5):2275–84. doi:10.1002/jsfa.9423. [Google Scholar] [PubMed] [CrossRef]
65. Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57(3):508–14. doi:10.1042/bj0570508. [Google Scholar] [PubMed] [CrossRef]
66. Nelson NA. Photometric adaptation of the somogyi method for the determination of glucose. J Biol Chem. 1944;153(2):375–80. doi:10.1016/S0021-9258(18)71980-7. [Google Scholar] [CrossRef]
67. Edwards R, Kessmann H. Isoflavonoid phytoalexins and their biosynthetic enzymes. In: Gurr SJ, Mcpherson MJ, Bowles DJ, editors. Molecular plant pathology. Oxford, UK: Oxford University; 1992. p. 45–62. [Google Scholar]
68. Kar M, Mishra D. Peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976;57(2):315–9. doi:10.1104/pp.57.2.315. [Google Scholar] [PubMed] [CrossRef]
69. Doderer A, Kokkelink I, Van Der Veen S, Valk BE, Schram A, Douma AC. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. BBA—Protein Struct Mol Enzymol. 1992;1120(1):97–104. doi:10.1016/0167-4838(92)90429-H. [Google Scholar] [PubMed] [CrossRef]
70. Pushkar B, Sevak P, Parab S, Nilkanth N. Chromium pollution and its bioremediation mechanisms in bacteria: a review. J Environ Manag. 2021;287:112279. doi:10.1016/j.jenvman.2021.112279. [Google Scholar] [PubMed] [CrossRef]
71. Zulfiqar U, Jiang W, Xiukang W, Hussain S, Ahmad M, Maqsood MF, et al. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils. A comprehensive review. Front Plant Sci. 2022;13:773815. doi:10.3389/fpls.2022.773815. [Google Scholar] [PubMed] [CrossRef]
72. Xu S, Yu C, Wang Q, Liao J, Liu C, Huang L, et al. Chromium contamination and health risk assessment of soil and agricultural products in a rural area in Southern China. Toxics. 2022;11(1):27. doi:10.3390/toxics11010027. [Google Scholar] [PubMed] [CrossRef]
73. Ali S, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, et al. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf. 2013;89:66–72. doi:10.1016/j.ecoenv.2012.11.015. [Google Scholar] [PubMed] [CrossRef]
74. Dey SK, Jena PP, Kundu S. Antioxidative efficiency of Triticum aestivum L. Expo Chromium Stress J Environ Biol. 2009;30:539–44. [Google Scholar]
75. Diwan H, Ahmad A, Iqbal M. Characterization of chromium toxicity in food crops and their role in phytoremediation. J Bioremediation Biodegrad. 2012;3:159. doi:10.4172/2155-6199.1000159. [Google Scholar] [CrossRef]
76. Ali S, Zeng F, Cai S, Qiu B, Zhang G. The interaction of salinity and chromium in the influence of barley growth and oxidative stress. Plant Soil Environ. 2011;57(4):153–9. [Google Scholar]
77. Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, et al. Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere. 2015;120:154–64. doi:10.1016/j.chemosphere.2014.06.029. [Google Scholar] [PubMed] [CrossRef]
78. Rodriguez E, Santos C, Azevedo R, Moutinho-Pereira J, Correia C, Dias MC. Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem. 2012;53:94–100. doi:10.1016/j.plaphy.2012.01.013. [Google Scholar] [PubMed] [CrossRef]
79. Ehsan S, Ali S, Noureen S, Mahmood K, Farid M, Ishaque W, et al. Citric acid assisted phytoremediation of cadmium by Brassica napus L. Ecotoxicol Environ Saf. 2014;106:164–72. doi:10.1016/j.ecoenv.2014.03.007. [Google Scholar] [PubMed] [CrossRef]
80. Pérez-Gálvez A, Viera I, Roca M. Carotenoids and chlorophylls as antioxidants. Antioxidants. 2020;9:505. doi:10.3390/antiox9060505. [Google Scholar] [PubMed] [CrossRef]
81. Hussain M, Hafeez A, AL-Huqail AA, Alsudays IM, Alghanem SMS, Ashraf MA, et al. Effect of hesperidin on growth, photosynthesis, antioxidant systems and uptake of cadmium, copper, chromium and zinc by celosia argentea Plants. Plant Physiol Biochem. 2024;207:108433. doi:10.1016/j.plaphy.2024.108433. [Google Scholar] [PubMed] [CrossRef]
82. Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–84. doi:10.1111/j.1365-3040.2011.02400.x. [Google Scholar] [PubMed] [CrossRef]
83. Potter AJ, Trappetti C, Paton JC. Streptococcus pneumoniae uses glutathione to defend against oxidative stress and metal ion toxicity. J Bacteriol. 2012;194(22):6248–54. doi:10.1128/jb.01393-12. [Google Scholar] [PubMed] [CrossRef]
84. Chen Q, Zhang X, Liu Y, Wei J, Shen W, Shen Z, et al. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 2017;81:253–64. doi:10.1007/s10725-016-0202-y. [Google Scholar] [CrossRef]
85. Ulhassan Z, Gill RA, Huang H, Ali S, Mwamba TM, Ali B, et al. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol Biochem. 2019;145:142–52. doi:10.1016/j.plaphy.2019.10.035. [Google Scholar] [PubMed] [CrossRef]
86. Adhikari A, Adhikari S, Ghosh S, Azahar I, Shaw AK, Roy D, et al. Imbalance of redox homeostasis and antioxidant defense status in maize under chromium (VI) Stress. Environ Exp Bot. 2020;169:103873. doi:10.1016/j.envexpbot.2019.103873. [Google Scholar] [CrossRef]
87. Habiba U, Ali S, Farid M, Shakoor MB, Rizwan M, Ibrahim M, et al. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Env Sci Pollut Res. 2015;22:1534–44. doi:10.1007/s11356-014-3431-5. [Google Scholar] [PubMed] [CrossRef]
88. Khan NA, Singh SS, Nazar R. Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. J Agron Crop Sci. 2007;193:435–44. doi:10.1111/j.1439-037X.2007.00272.x. [Google Scholar] [CrossRef]
89. Akbari B, Najafi F, Bahmaei M, Mahmoodi NM, Sherman JH. Modeling and optimization of malondialdehyde (MDA) Absorbance behavior through response surface methodology (RSM) and artificial intelligence network (AINan endeavor to estimate lipid peroxidation by determination of MDA. J Chemom. 2023;37:e3468. doi:10.1002/cem.3468. [Google Scholar] [CrossRef]
90. Mahmud JA, Hasanuzzaman M, Nahar K, Rahman A, Hossain MDS, Fujita M. γ-Aminobutyric Acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology. 2017;26:675–90. doi:10.1007/s10646-017-1800-9. [Google Scholar] [PubMed] [CrossRef]
91. Zhang H, Hu LY, Li P, Hu KD, Jiang CX, Luo JP. Hydrogen sulfide alleviated chromium toxicity in wheat. Biol Plant. 2010;54:743–7. doi:10.1007/s10535-010-0133-9. [Google Scholar] [CrossRef]
92. Ma Q, Cao X, Ma J, Tan X, Xie Y, Xiao H, et al. Hexavalent chromium stress enhances the uptake of nitrate but reduces the uptake of ammonium and glycine in pak choi (Brassica chinensis L.). Ecotoxicol Environ Saf. 2017;139:384–93. doi:10.1016/j.ecoenv.2017.02.009. [Google Scholar] [PubMed] [CrossRef]
93. Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S. Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under Drought Stress. Protoplasma. 2018;255:163–74. doi:10.1007/s00709-017-1140-x. [Google Scholar] [PubMed] [CrossRef]
94. Tripathi DK, Singh VP, Kumar D, Chauhan DK. Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol Plant. 2012;34:279–89. doi:10.1007/s11738-011-0826. [Google Scholar] [CrossRef]
95. Kundu D, Dey S, Raychaudhuri SS. Chromium (VI)—induced stress response in the plant plantago ovata forsk in vitro. Genes Env. 2018;40:21. doi:10.1186/s41021-018-0109-0. [Google Scholar] [PubMed] [CrossRef]
96. Noreen S, Faiz S, Akhter MS, Shah KH. Influence of foliar application of osmoprotectants to ameliorate salt stress in sunflower (Helianthus annuus L.). Sarhad J Agric. 2019;35:1316–25. doi:10.17582/journal.sja/2019/35.4.1316.1325. [Google Scholar] [CrossRef]
97. Hussain S, Irfan M, Sattar A, Hussain S, Ullah S, Abbas T, et al. Alleviation of cadmium stress in wheat through the combined application of boron and biochar via regulating morpho-physiological and antioxidant defense mechanisms. Agronomy. 2022;12:434. doi:10.3390/agronomy12020434. [Google Scholar] [CrossRef]
98. Rasool F, Uzair M, Naeem MK, Rehman N, Afroz A, Shah H, et al. Phenylalanine Ammonia-Lyase (PAL) genes family in wheat (Triticum aestivum L: genome-wide characterization and expression profiling. Agronomy. 2021;11:2511. doi:10.3390/agronomy11122511. [Google Scholar] [CrossRef]
99. Li G, Wang H, Cheng X, Su X, Zhao Y, Jiang T, et al. Comparative genomic analysis of the PAL genes in five rosaceae species and functional identification of Chinese white pear. PeerJ. 2019;7:e8064. doi:10.7717/peerj.8064. [Google Scholar] [PubMed] [CrossRef]
100. Ibrahim HA, Abdellatif YMR. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Ann Agric Sci. 2016;61:267–74. doi:10.1016/j.aoas.2016.05.002. [Google Scholar] [CrossRef]
101. Beshamgan ES, Sharifi M, Zarinkamar F. Crosstalk among polyamines, phytohormones, hydrogen peroxide, and phenylethanoid glycosides responses in scrophularia striata to Cd stress. Plant Physiol Biochem. 2019;143:129–41. doi:10.1016/j.plaphy.2019.08.028. [Google Scholar] [PubMed] [CrossRef]
102. Jańczak-Pieniążek M, Cichoński J, Michalik P, Chrzanowski G. Effect of heavy metal stress on phenolic compounds accumulation in winter wheat plants. Molecules. 2022;28:241. doi:10.3390/molecules28010241. [Google Scholar] [PubMed] [CrossRef]
103. Singh KS, Suhel M, Husain T, Prasad SM, Singh VP. Hydrogen sulfide manages hexavalent chromium toxicity in wheat and rice seedlings: the role of sulfur assimilation and ascorbate-glutathione cycle. Environ Pollut. 2022;307(1–3):119509. doi:10.1016/j.envpol.2022.119509. [Google Scholar] [PubMed] [CrossRef]
104. Hussain M, Hafeez A, Rizwan M, Rasheed R, Seleiman MF, Ashraf MA, et al. Pervasive influence of heavy metals on metabolic pathways is potentially relieved by hesperidin to enhance the phytoremediation efficiency of Bassia scoparia. Environ Sci Pollut Res. 2024;31(23):34526–49. doi:10.1007/s11356-024-33530-4. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools