 Open Access
Open Access
REVIEW
From Cell Division to Stress Tolerance: The Versatile Roles of Cytokinins in Plants
1 Instituto de Ciências da Natureza, Universidade Federal de Alfenas, Alfenas, 37130-001, Brazil
2 Escuela de Ciencias Agrícolas y Veterinarias, Universidad Viña del Mar, Vina del Mar, 2572007, Chile
* Corresponding Author: Michele Carla Nadal. Email:
(This article belongs to the Special Issue: Emerging Insights into Phytohormonal Crosstalk in Plant Stress Tolerance)
Phyton-International Journal of Experimental Botany 2025, 94(3), 539-560. https://doi.org/10.32604/phyton.2025.061776
Received 03 December 2024; Accepted 06 February 2025; Issue published 31 March 2025
Abstract
Cytokinins are plant hormones that are essential for plant growth and development and are involved in a variety of processes. They are synthesized by the modification of adenine with an isoprenoid chain, resulting in cytokinins such as isopentenyladenine and zeatin. The levels of these hormones are regulated by conjugation, degradation and oxidation processes that modulate their activity. Cytokinins are perceived by cells through specific receptors that, when activated, trigger signaling cascades responsible for regulating the expression of genes critical for development. In addition, cytokinins interact with other hormones, such as auxins, to coordinate plant growth and architecture. They are transported by the xylem and phloem, allowing them to be distributed to different parts of the plant and to regulate processes such as cell division, morphogenesis and inhibition of leaf senescence, thereby prolonging the vegetative phase. Cytokinins also play a role in plant responses to biotic and abiotic stresses. They influence the expression of defense genes against pathogens and pests and adjust plant metabolism and growth in response to adverse conditions such as drought and salinity. Cytokinins interact in an integrated manner with other stress hormones, such as abscisic acid and ethylene, to coordinate plant responses to environmental challenges. In agriculture, the manipulation of cytokinins, whether by external application or genetic modification, shows great potential for increasing crop yields and improving plant resistance to stress. Advances in molecular biology and gene editing offer new opportunities to precisely modify these functions. This review elucidates recent research on cytokinins, covering their mechanisms of action, interactions with other hormones, and applications in agriculture.Keywords
The definition of plant hormones as substances that regulate growth at low concentrations began to be established in the late 19th century. These compounds, whether natural or synthetic, are characterized by their ability to act in small quantities, translocate between plant tissues and interact with specific protein receptors, playing roles in processes that enable development and environmental adaptation [1].
Phytohormones play central roles as signaling molecules, regulating plant growth and responses to adverse environmental conditions. Their action is controlled by complex systems of biosynthesis, transport, and signaling, operating in tightly regulated feedback cycles to ensure metabolic balance and physiological functionality [2].
Among phytohormones, cytokinins stand out for promoting cell division and coordinating various aspects of plant growth and development. These hormones regulate processes such as shoot branching, serrated leaf formation, root system development, increased seed yield, and, most notably, plant responses to environmental stresses. Additionally, cytokinins delay leaf senescence and prolong the functionality of photosynthetic tissues, maintaining efficient metabolic activity in plants over extended periods [2–5].
The occurrence and levels of cytokinins vary widely among plant species, specific tissues, developmental stages, and environmental conditions. Cytokinins integrate internal and external signals, enabling the fine-tuning of plant growth to environmental demands [1,6]. Recent studies demonstrate that cytokinins, in addition to their role in development, also interact with other phytohormones, such as auxins, to coordinate complex responses that ensure plant survival and productivity [7,8].
Based on this overview, the present article reviews recent advances in understanding the mechanisms of cytokinin regulation and transport, exploring their roles in cell division, senescence inhibition, and responses to biotic and abiotic stresses, with direct implications for applications in agriculture and biotechnology.
2 Regulatory Mechanisms of Cytokinins
2.1 Biosynthesis and Metabolism
The analysis of tissue-specific gene expression using a promoter-reporter system revealed that de novo cytokinin biosynthesis predominantly occurs in non-photosynthetic tissues, such as the phloem and pericycle [2,9].
Cytokinins are isoprenoids that can be biosynthesized via the Methylerythritol Phosphate (MEP) pathway (Fig. 1). This pathway is involved in plant growth, development, and responses to environmental stresses [10]. In the MEP pathway, the ratio of isopentenyl diphosphate to dimethylallyl diphosphate (DMAPP), synthesized from (E)-4-hydroxy-3-methyl-but-2-enyl diphosphate (HMBDP), is approximately 5:1, and subsequent isomerization equalizes the ratio between these two molecules [2,11,12].
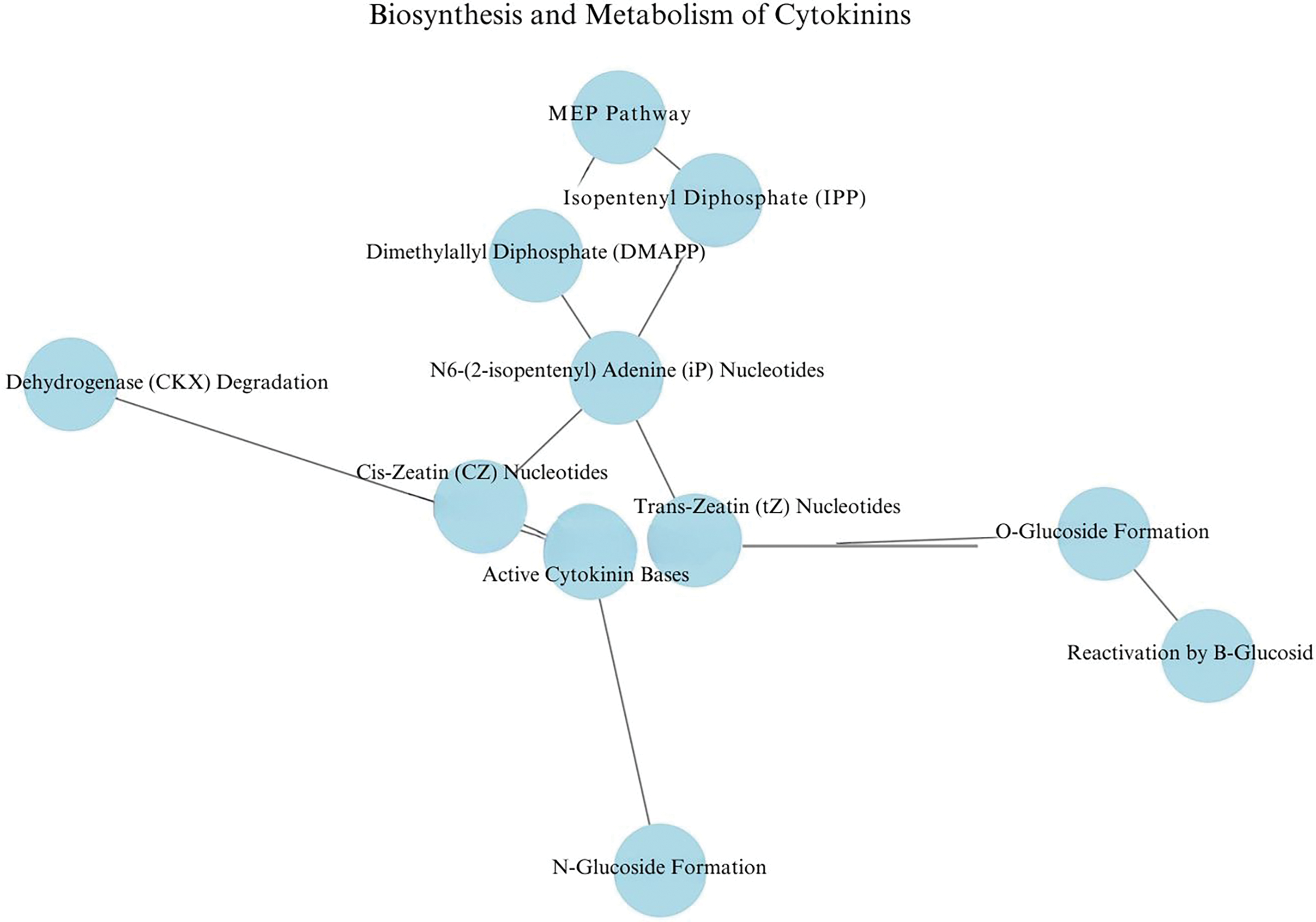
Figure 1: Biosynthesis and metabolism of cytokinins. MEP pathway: the initial metabolic pathway that generates the precursors isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). IPP and DMAPP: isoprenoid precursors used to synthesize cytokinin nucleotides. N6-(Δ2-isopentenyl) adenine (iP) nucleotides: initial products formed by the conjugation of the isoprenoid group to adenine phosphate. Trans-zeatin (tz) and cis-zeatin (cz) nucleotides: cytokinin nucleotides synthesized from iP. Active cytokinin bases: biologically active forms of cytokinins generated by enzymes such as LOG and GY3/CPN. Degradation by CKX: cytokinins are irreversibly degraded by the enzyme cytokinin dehydrogenase (CKX). O-glucoside formation: modification by the enzyme UGT85A1, enabling reactivation by β-glucosidase. N-glucoside formation: modification by the enzyme UGT76C2, altering cytokinin functionality
Cytokinins are adenine derivatives with a prenyl side chain at the N6 position of adenine, such as N6-(Δ2-isopentenyl) adenine (iP), trans-zeatin (tZ), cis-zeatin (cZ), and dihydrozeatin (DZ). They are first formed as nucleotide precursors and subsequently converted into active free bases, which can be recycled through purine-metabolizing enzymes. The initial reaction in cytokinin biosynthesis is catalyzed by adenosine phosphate-isopentenyltransferase (IPT), which conjugates the prenyl group to the N6 position of adenosine phosphate, using DMAPP as the donor [5,9,13–15].
Plant IPT preferentially uses ATP or ADP as acceptors of isoprenoids and DMAPP as the donor to form iP riboside 5’-triphosphate (iPRTP) and iP riboside 5’-diphosphate (iPRDP), respectively. The dephosphorylation of iPRTP and iPRDP by phosphatase, the phosphorylation of iPR by adenosine kinase (AK), and the conjugation of phosphoribosyl groups to iP by adenine phosphoribosyltransferase (APT) create the metabolic pool of iP riboside 5’-monophosphate (iPRMP) [2,16].
iP nucleotides are converted into the corresponding tZ nucleotides by the cytochrome P450 monooxygenase 735A (CYP735A). Cytokinin 5’-monophosphate nucleosides, such as iPRMP, tZ riboside 5’-monophosphate (tZRMP), DZRMP, and cZRMP, are activated into cytokinin nucleobases through a one-step reaction catalyzed by Lonely Guy (LOG) or a two-step reaction catalyzed by the 5’-ribonucleotide phosphohydrolase Grain Yield 3 (GY3) and cytokinin/purine riboside nucleosidase (CPN). Growth phenotype studies and stable isotope labeling experiments using higher-order T-DNA insertion mutants in Arabidopsis have demonstrated the central role of the LOG-mediated pathway in cytokinin activation for normal growth and development, including lateral root formation and root and shoot morphology [2,9,13,17].
iP, tZ, and their nucleosides can be catabolized by cytokinin oxidase (CKX) into adenine or adenosine. tZ can be converted into O-glucoside by the O-glucosyltransferase UGT85A1 and reactivated by β-glucosidase BGLU44. Cytokinin nucleobases can also be converted into N-glucoside by the N-glucosyltransferase UGT76C2. Both tZ and iP are synthesized by IPT and exhibit higher cytokinin activity than cZ in various plant species. The cZ form is believed to be produced through the modification of tRNA by tRNA-isopentenyltransferase (tRNA-IPT) following tRNA turnover [14,15].
In plants, cytokinins are degraded by cytokinin oxidase/dehydrogenase (CKO/CKX, EC 1.5.99.12), which catalyzes their irreversible oxidative degradation, thereby reducing their abundance. CKX proteins are encoded by a gene family, and individual enzymatic isoforms typically differ in their substrate specificity, spatial and temporal expression patterns, and subcellular localization [5,6].
Cytokinins are perceived by membrane-bound receptors located primarily in the endoplasmic reticulum. These receptors belong to the histidine kinase (HK) family and are central to the two-component signaling system, an adaptive mechanism originally observed in bacteria and later evolved in plants for hormone signal transduction [9]. In Arabidopsis thaliana, the principal cytokinin receptors include AHK2, AHK3, and AHK4 (CRE1). Each receptor possesses three essential domains: a ligand-binding domain for cytokinin recognition, a histidine kinase domain, and a receiver domain.
Upon binding of cytokinins to the extracellular ligand-binding domain, the receptor undergoes a conformational change. This conformational alteration activates the histidine kinase domain, initiating autophosphorylation at a conserved histidine residue. This initial step is crucial as it sets the stage for the subsequent phosphotransfer events within the signaling cascade [5,11,14,18,19].
The cytokinin signaling pathway utilizes a two-component system comprising a receptor kinase and response regulators (RRs) [4,9,13]. Upon cytokinin binding, the receptor’s histidine kinase domain autophosphorylates and subsequently transfers the phosphate group to a conserved aspartate residue in its receiver domain. This phosphorylated receiver domain then interacts with intermediary proteins called histidine phosphotransfer proteins (AHPs). These proteins play a dual role: they act as carriers of the phosphoryl group and as regulators ensuring specificity and fidelity of the signal transduction [20].
AHPs are soluble proteins that migrate from the cytosol to the nucleus, effectively translocating the phosphorylated signal. This step ensures that the hormonal signal reaches its nuclear effectors, the type-B response regulators (ARR-B). The ARR-B proteins, once phosphorylated, function as transcription factors, activating the expression of cytokinin-responsive genes. These genes are involved in diverse cellular processes, including cell division, differentiation, organogenesis, and responses to environmental stress [8,21,22].
In addition to activating type-B RRs, cytokinins induce the expression of type-A response regulators (ARR-A). The ARR-A proteins serve as negative feedback regulators, modulating the intensity and duration of cytokinin responses. By competing with ARR-Bs for phosphorylation or directly inhibiting ARR-B activity, ARR-As ensure that the signaling is finely tuned to prevent overactivation [4,23].
The genes activated by ARR-Bs play pivotal roles in regulating critical physiological processes. These include promoting cell cycle progression, initiating organ formation, and modulating developmental transitions. Additionally, cytokinin signaling influences responses to environmental stresses such as drought, salinity, and nutrient deficiency. Recent studies have highlighted the role of cytokinin signaling in root development, emphasizing its dual role in promoting lateral root growth and regulating root apical meristem activity [8].
Moreover, cytokinin signaling interacts with other hormonal pathways, including auxin and abscisic acid, forming complex networks that integrate environmental signals with endogenous developmental cues. This crosstalk ensures a coordinated response that optimizes growth and survival under varying environmental conditions [13,24].
Recent research has further elucidated the structural dynamics of cytokinin receptors and the molecular mechanisms underlying phosphorelay events. For instance, advanced imaging techniques and structural biology approaches have revealed how receptor conformational changes upon ligand binding facilitate precise signal relay. Additionally, genetic studies in Arabidopsis and crop species have identified novel components of the signaling pathway, expanding our understanding of its regulatory complexity [9].
In summary, cytokinin signaling represents a highly sophisticated network that integrates receptor activation, phosphorelay mechanisms, and transcriptional regulation. This pathway not only underscores the evolutionary adaptation of plants to utilize bacterial signaling strategies but also highlights its indispensable role in plant growth, development, and stress resilience.
Proper cytokinin transport is essential for these hormones to reach target tissues and efficiently perform their physiological functions. Although many aspects of cytokinin transport systems remain incompletely understood, recent advances have identified key genes and important mechanisms, such as the ATP-Binding Cassette Transporter G14 subfamily (ABCG14) and Purine Permease 14 (PUP14) (Fig. 2) [25].
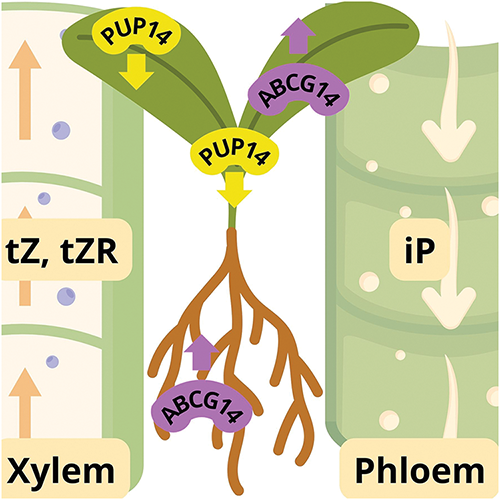
Figure 2: Diagram illustrating the movement of cytokinins facilitated by transporters over different distances
The ABCG14 gene plays a fundamental role in the transport of cytokinins from the root to the aerial organs of the plant via the xylem. Studies in Arabidopsis thaliana have shown that ABCG14 mutants exhibit cytokinin deficiency symptoms in leaves, such as reduced growth and abnormal bud development, confirming its essential function in long-distance translocation [18]. In rice, its homolog OsABCG18 performs a similar role, reinforcing functional conservation across species [26].
Although it is not yet fully clear which specific forms of cytokinins are transported by ABCG14, studies suggest that it may be responsible for the movement of cytokinin nucleotide precursors (such as isopentenyl-AMP) or bioactive forms, such as trans-zeatin [15]. The predominant localization of ABCG14 in xylem cells and its differential expression in transport tissues support its role in supplying cytokinins to organs that rely on these hormones for processes such as maintaining meristematic activity and promoting stem and leaf growth [25].
The PUP14 gene encodes a permease that functions as an importer of cytokinin nucleobases, modulating the apoplastic pool of these molecules. Unlike ABCG14, which regulates long-distance transport, PUP14 acts locally, controlling cytokinin availability in specific regions such as the apical meristem and the embryo. PUP14 expression is negatively correlated with cytokinin signaling, suggesting that it plays a regulatory role by limiting hormonal action in certain areas [27].
Loss of function in PUP14 results in enhanced cytokinin signaling, as evidenced by increased expression of cytokinin-regulated genes and augmented growth of lateral buds and other structures regulated by these hormones. This indicates that PUP14 functions as a fine-tuning mechanism, modulating the intensity and localization of hormonal responses [7].
In addition to ABCG14 and PUP14, other transporters contribute to cytokinin transport. ENT (Equilibrative Nucleoside Transporters) facilitate the bidirectional movement of nucleotide forms of cytokinins, while members of the SWEET (Sugars Will Eventually Be Exported Transporters) family have recently been implicated in cytokinin export in specific tissues. Additional ABC transporters, beyond ABCG14, may mediate the active transport of cytokinins across different cellular compartments [28].
The interaction between transporters and hormonal signaling pathways also plays a crucial role. For instance, cytokinins transported via the phloem can interact with auxins to regulate root development, while xylem transport is essential for controlling shoot growth, highlighting the coordination between hormone transport and plant development [29–31].
Transport mechanisms are closely linked to cytokinin metabolism. Cytokinin nucleotide precursors are often transported and converted into bioactive forms in target tissues through enzymes such as LOG (Lonely Guy), which catalyzes the conversion of cytokinin nucleotides into free bases. Similarly, efficient transport ensures that cytokinins are directed to areas of high metabolic demand, such as young growing tissues and zones of active cell division [2,9].
3.2 Spatial and Temporal Distribution
Cytokinins are primarily synthesized in the roots, but their production also occurs in other tissues such as leaves, seeds, and fruits. The primary biosynthetic pathway involves the conversion of adenines into isopentenyladenines through the action of the enzyme isopentenyltransferase (IPT). Depending on the physiological context, different IPT isoforms act in specific locations to produce cytokinins in either free or conjugated forms [32,33].
Cytokinin transport occurs via the xylem and phloem, enabling their redistribution to aerial organs and specific sites of action. In the xylem, cytokinins are transported from the roots to the shoot, promoting shoot growth and leaf expansion [34]. In the phloem, cytokinins can move bidirectionally, allowing for more precise regulation in response to metabolic and environmental signals [31].
Cytokinin concentrations vary significantly among different plant tissues and organs, creating gradients that influence development. For instance, higher cytokinin levels are often observed in apical and lateral meristems, where they promote cell division and maintain meristematic potential. In young leaves, these hormones support cell expansion, whereas in mature leaves, a decline in cytokinin levels is associated with the onset of senescence [35,36].
In the context of roots, cytokinins play an opposite role to auxins, inhibiting lateral root formation and promoting cell differentiation in the transition zone. Thus, the balance between cytokinins and auxins is crucial for the proper development of root architecture [37,38].
The temporal regulation of cytokinins is equally crucial. During germination, there is a rapid increase in cytokinin levels to promote initial cell division and seedling growth. As the plant matures, hormonal levels are adjusted according to the energy and resource demands for vegetative and reproductive growth [39,40].
Under stress conditions, such as drought or salinity, cytokinins play complex roles. They can be repressed to conserve energy resources or induced in specific tissues to promote recovery and adaptation. Cytokinin metabolism during stress is regulated by enzymes such as cytokinin oxidase/dehydrogenase (CKX), which control hormone degradation, thereby adjusting its availability [7].
Molecular and genetic studies have revealed that the differential expression of genes related to biosynthesis (IPT), transport (ATP-binding cassette transporters such as ABCG14), and degradation (CKX) is responsible for spatial and temporal variations in cytokinin distribution. Manipulation of these genes has been explored to optimize plant growth and productivity, particularly in agricultural crops [27].
For example, the overexpression of IPT genes under specific promoters enables localized cytokinin production, delaying leaf senescence and increasing biomass under adverse environments. Engineering plants with reduced CKX activity have demonstrated enhanced tolerance to water and salt stress, highlighting the biotechnological potential of cytokinins. Thus, the spatial and temporal distribution of cytokinins in plants is a highly dynamic and integrated process, essential for coordinated development and plant adaptation [7,13,25].
4 Hormonal Crosstalk and Cytokinins
Cytokinins are central regulators in the intricate network of hormonal interactions that govern plant growth, development, and environmental adaptation. Through crosstalk with auxins, abscisic acid (ABA), ethylene, gibberellins (GA), and jasmonates, cytokinins orchestrate complex physiological processes by integrating and modulating hormonal signals. These interactions ensure a dynamic balance between growth, resource allocation, and stress responses [41,42].
The interaction between cytokinins and auxins is fundamental for coordinating cell proliferation and differentiation, particularly in meristematic regions. While cytokinins promote cell division and maintain the pluripotency of meristematic cells through the activation of genes like WUSCHEL (WUS), auxins direct the differentiation of these cells into specialized structures. This balance is critical in shoot apical meristems for organogenesis and in root development, where cytokinins antagonize auxin signaling to inhibit lateral root formation while enhancing root apical meristem activity. These opposing actions create a finely tuned mechanism for adjusting root and shoot architecture in response to environmental conditions [43].
Cytokinins and ABA exhibit antagonistic interactions that are particularly evident under abiotic stress. In drought conditions, ABA promotes stomatal closure to conserve water, while cytokinins counterbalance this effect to maintain minimal gas exchange for photosynthesis. Additionally, cytokinins repress ABA-induced senescence by downregulating genes responsible for chlorophyll degradation and catabolic processes. This interplay ensures that plants can balance water use efficiency with the maintenance of photosynthetic activity, enhancing stress resilience [44].
Ethylene is a key promoter of leaf senescence, and cytokinins act as antagonists to delay this process. Cytokinins inhibit ethylene biosynthesis by reducing the expression of ACC synthase and oxidase genes, preserving the integrity of photosynthetic components and prolonging leaf functionality. However, in response to biotic stresses, cytokinins can synergistically interact with ethylene to activate defense-related pathways, demonstrating a context-dependent relationship that balances growth with immune responses [41].
Cytokinins and gibberellins interact to regulate growth processes such as stem elongation, flowering, and seed development. Cytokinins modulate GA biosynthesis and signaling, often acting synergistically to promote growth under favorable conditions. Conversely, under stress or resource limitations, cytokinins can limit GA activity to prioritize stress adaptation and resource conservation, showcasing their role in balancing vegetative and reproductive growth [45].
The interaction between cytokinins and jasmonates highlights their role in integrating growth and defense responses. Jasmonates are typically involved in stress-induced senescence and defense against herbivores and pathogens. Cytokinins modulate jasmonate signaling by repressing catabolic pathways and enhancing antioxidant defenses, ensuring that metabolic resources are efficiently allocated to maintain growth while responding to stress. This crosstalk is crucial in environments where plants must simultaneously manage growth and defense trade-offs [42].
5 Cytokinins in Cell Proliferation
Cytokinins play a central role in regulating the cell cycle, primarily influencing the critical transitions from the G1 to S phase and from the G2 to M phase, which are essential for cell division. This process is governed by the activation of genes encoding key proteins required for cell cycle progression, such as cyclins and cyclin-dependent kinases (CDKs). Cyclins, particularly type D cyclins (CycD), are crucial for entry into the S phase, promoting the formation of cyclin-CDK complexes. These complexes phosphorylate specific proteins, such as Retinoblastoma (Rb), which, once inactivated, release E2F-type transcription factors. These factors activate the expression of DNA synthesis-related genes, enabling the cell to progress to the S phase [46,47].
Additionally, cytokinins have the ability to suppress the activity of cell cycle inhibitors, such as kinase-related proteins (KRPs), which typically block CDK activity [46]. In this way, cytokinins ensure that meristematic cells can proliferate rapidly, which is essential for continuous growth and the expansion of plant tissues. Cytokinin regulation of cell division also involves control over entry into the mitotic (M) phase, ensuring proper segregation of genetic material [48–50].
The interaction between cytokinins and auxins plays a crucial role in balancing cell proliferation and differentiation. While cytokinins promote cell proliferation, auxins tend to encourage cell differentiation. This balance is particularly evident in apical meristems, where cytokinins maintain a population of undifferentiated cells that are continuously renewed, while auxins direct some of these cells toward differentiation to form new organs. This mechanism ensures sustained plant growth by preserving a reservoir of undifferentiated meristematic cells while enabling the formation of specialized structures [30,31,51].
In meristems, cytokinins play another vital role: maintaining the functionality and identity of these pluripotent cells. A clear example is the regulation of the WUSCHEL (WUS) gene, which is essential for the maintenance of the apical meristem. Cytokinins promote the expression of WUS, which, in turn, sustains the ability of meristematic cells to divide and contribute to stem and root growth. This mechanism illustrates how cytokinins not only promote cell division but also ensure the identity of the cells that make up the meristems, enabling them to continue proliferating indefinitely [52,53].
Despite their positive role in cell division, cytokinin activity must be carefully regulated to prevent uncontrolled growth. This regulation is achieved through intrinsic mechanisms, such as the action of negative regulators, including type-A ARR (Response Regulators type A) genes. These regulators are activated by cytokinin signaling and compete with type-B ARRs, which are responsible for activating the transcription of target genes. By inhibiting the action of type-B ARRs, type-A ARRs promote negative feedback, ensuring that cytokinin signaling is deactivated after a period of stimulation [54,55].
Moreover, cytokinin degradation is mediated by the enzyme cytokinin oxidase/dehydrogenase (CKX), which breaks down the active forms of the hormone. This degradation allows for precise spatial and temporal control of cytokinin action, ensuring that cell division occurs in a regulated manner and at specific locations. For instance, in tissues where growth has reached an advanced stage, cytokinin levels are reduced, halting cell proliferation and allowing cells to enter processes of differentiation or senescence [7,27].
These mechanisms demonstrate that cytokinins are more than mere promoters of cell division; they function as sophisticated regulators that balance plant growth and development, ensuring that each tissue and organ develops in a coordinated and efficient manner [51,56].
6 Cytokinins and Leaf Senescence
Cytokinins are widely recognized as key regulators of leaf senescence in plants, acting as inhibitors of this degenerative process. Senescence is a terminal phase of cellular development characterized by the breakdown of macromolecules, nutrient redistribution, and programmed cell death. The action of cytokinins in inhibiting senescence occurs through complex molecular mechanisms involving gene regulation, hormonal cross-signaling, and the maintenance of cellular homeostasis [57].
The inhibition of senescence by cytokinins begins with the activation of a network of regulatory genes. Upon being perceived by specific histidine kinase receptors (such as AHK2, AHK3, and CRE1/AHK4), cytokinins activate the two-component signaling cascade. Signals are transduced to the nucleus by mediator proteins, such as histidine phosphotransferases (AHPs), which then phosphorylate type-B response regulators (ARR-B) (Fig. 3) [5,14,19].
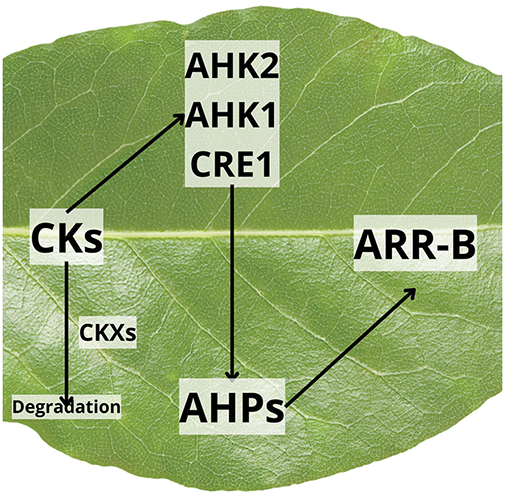
Figure 3: Action of cytokinins (CKs) through histidine kinase receptors (AHK1, AHK2, CRE1) to activate histidine phosphotransfer proteins (AHPs), which, in turn, modulate the activity of response regulators (ARR-B)
Activated ARR-B regulators modulate the expression of genes associated with senescence, such as those encoding proteins involved in chlorophyll degradation and proteases responsible for protein breakdown. Additionally, maintenance genes, including those related to the production of antioxidant defense proteins and enzymes involved in cellular metabolism, are induced, thereby delaying the metabolic collapse typical of senescence [41,58].
Cytokinins play a central role in maintaining chloroplast integrity, acting as crucial regulators in preventing leaf senescence, particularly in the context of the degradation of photosynthetic components. During senescence, chloroplasts are the first organelles to be degraded, leading to the loss of photosynthetic capacity. Cytokinins delay this degradation through specific mechanisms that ensure the preservation of the structure and functionality of the photosynthetic apparatus. Firstly, cytokinins sustain the expression of genes related to chlorophyll biosynthesis, pigments essential for light capture. This effect contributes to the stabilization of photosynthetic protein complexes, including photosystems I and II [59,60].
Additionally, cytokinins play a crucial role in activating antioxidant systems that protect chloroplasts from oxidative stress generated during senescence. Enzymes such as superoxide dismutase (SOD) and catalase (CAT) are induced by cytokinins, helping to neutralize reactive oxygen species (ROS) accumulated during cellular degradation. This antioxidant action is essential to prevent damage to thylakoid membranes and preserve the efficiency of photosynthetic processes. Complementarily, cytokinins inhibit the expression of proteases and other enzymes involved in the degradation of essential chloroplast proteins, such as Rubisco and the subunits of the light-harvesting complex (LHC), thereby delaying the disassembly of the photosynthetic apparatus [61,62].
Another crucial aspect of cytokinin action is its interaction with other phytohormones that regulate senescence, such as ethylene, abscisic acid (ABA), and jasmonates. Ethylene is known as one of the primary promoters of leaf senescence, inducing the degradation of chlorophylls and photosynthetic proteins. Cytokinins antagonize this action by repressing ethylene biosynthesis and inhibiting the expression of genes encoding key enzymes in the ACC (1-aminocyclopropane-1-carboxylate) production pathway, the immediate precursor of ethylene. Similarly, cytokinins negatively modulate ABA signaling, a hormone widely associated with stress responses and the onset of senescence. The presence of cytokinins reduces the expression of ABA-responsive genes and decreases its biosynthesis, thereby delaying the catabolic processes associated with cellular aging [31,63,64].
Regarding jasmonates, hormones often associated with defense and senescence, cytokinins also play a regulatory role by reducing the expression of genes involved in catabolic responses. This interaction between cytokinins and these hormones ensures finer control over the metabolic and physiological processes leading to senescence, thereby maintaining leaf functionality for extended periods [34,65].
Another significant mechanism in the cytokinin-mediated inhibition of senescence is their influence on nutrient redistribution. During senescence, proteins and nucleic acids are degraded, and the released nutrients, such as nitrogen and phosphorus, are redistributed to younger parts of the plant or reproductive organs. Cytokinins delay this process by inhibiting the transcription of genes encoding proteases, nucleases, and other enzymes involved in macromolecule degradation. This not only preserves the structural and functional components of leaf cells but also maintains the efficiency of cellular metabolism [59,66].
Furthermore, cytokinins promote the maintenance of the antioxidant system, which is crucial for mitigating oxidative stress caused by the accumulation of reactive oxygen species (ROS) during senescence. Reactive oxygen species, such as hydrogen peroxide (H2O2) and superoxide radical (O2−), are byproducts of cellular metabolism that, if uncontrolled, cause irreparable damage to cellular structures, including membranes, proteins, and nucleic acids. The activation of enzymes such as SOD, CAT, and glutathione reductase by cytokinins contributes to the detoxification of these species, protecting leaf cells and prolonging their functionality [67,68]. A detailed understanding of these interactions not only elucidates the role of cytokinins in plant development but also opens avenues for practical applications in agriculture, such as improving crops to enhance their productivity and resilience under adverse conditions [2–5].
7 Cytokinins and Stress Responses
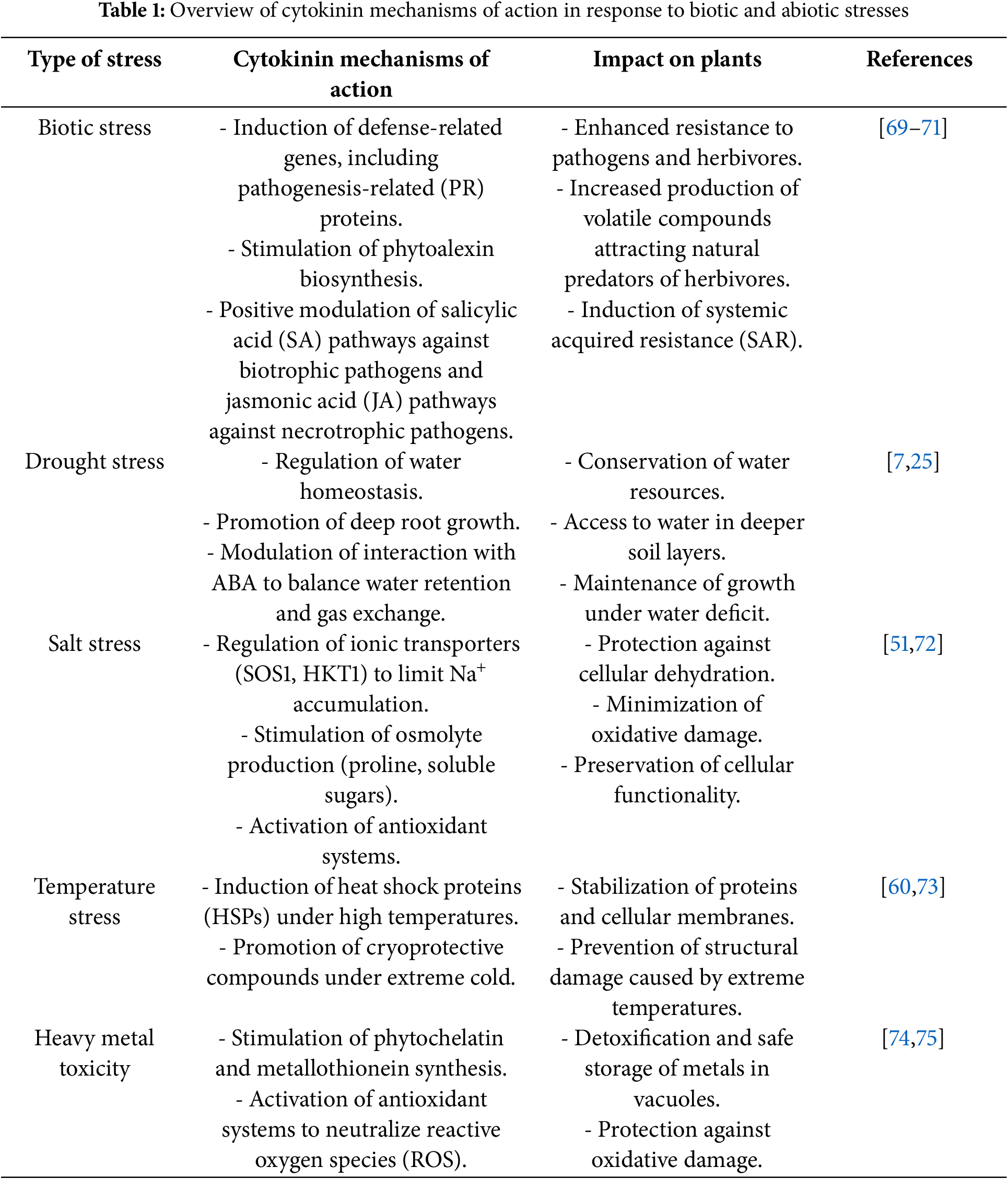
Cytokinins play a central role in regulating plant responses to various biotic and abiotic stresses, modulating physiological and metabolic processes that ensure adaptation and survival. These plant hormones interact with other hormonal pathways, activate defense-related genes, and adjust cellular mechanisms, promoting resilience under adverse conditions. Table 1 provides a synthesis of the main mechanisms of cytokinin action across different stress types, highlighting their physiological impacts and implications for plants.
7.1 Responses to Biotic Stress
Cytokinin signaling can induce both local and systemic immune responses. This is achieved by activating defense-related genes, including those encoding pathogenesis-related (PR) proteins, which directly inhibit pathogens. Additionally, cytokinins promote the expression of genes involved in the biosynthesis of antimicrobial compounds, such as phytoalexins, which restrict the growth and spread of invading agents [70].
Cytokinins play a central role in interacting with other phytohormones and regulating plant immune responses to biotic stresses, such as pathogen infections and herbivore attacks. These hormonal interactions shape defensive responses depending on the type of stressor and the plant’s physiological needs [69]. For instance, in cases of infection by necrotrophic pathogens, which feed on dead tissues, cytokinins modulate the jasmonic acid (JA) and ethylene (ET) pathways, which are responsible for activating effective defense mechanisms against these pathogens. Cytokinins amplify JA signaling, promoting the production of protease inhibitor proteins and toxic compounds that limit pathogen progression. In contrast, against biotrophic pathogens, which rely on living tissues to proliferate, cytokinins synergistically interact with salicylic acid (SA), a key hormone in systemic acquired resistance (SAR). This interaction enhances SA production and strengthens its signaling, leading to the activation of defense-related genes, such as those encoding pathogenesis-related (PR) proteins, which have antimicrobial functions [69,76,77].
In addition to regulating specific defense pathways against pathogens, cytokinins play an important role in the response to herbivores by interacting with JA to induce indirect defenses. They promote the production of volatile compounds that attract natural predators of herbivores and stimulate the synthesis of protease inhibitors that impair herbivore digestion. This coordination ability enables plants to develop robust defensive responses against different biotic agents [69].
Another essential mechanism promoted by cytokinins is their ability to induce systemic acquired resistance (SAR). Following the initial attack of a pathogen, cytokinins contribute to the transport of defense signals to uninfected parts of the plant, establishing a state of readiness in distant tissues. This is achieved through the production of signaling molecules, such as methyl salicylate, which is transported via the phloem to prepare other parts of the plant for potential future infections. In still-healthy tissues, cytokinins activate defense-related and antioxidant metabolism genes, fostering a form of ‘immune memory’ that strengthens the plant’s resistance to subsequent attacks [70,71].
In addition to modulating defense responses, cytokinins can directly affect the metabolism of pathogens and herbivores. In some interactions, cytokinins regulate the redistribution of nutrients in plant cells, reducing the availability of essential resources for invaders. For instance, during fungal and bacterial infections, cytokinins can limit the export of carbohydrates from cells, hindering the survival and proliferation of the pathogen [78]. Conversely, in beneficial interactions such as symbiosis with mycorrhizae or rhizobia, cytokinins promote the development of symbiotic structures like root nodules. They regulate the expression of genes associated with nodule formation and local hormone metabolism, facilitating nitrogen fixation and nutrient exchange between the plant and the symbiotic microorganism [79].
Another significant role of cytokinins in biotic stresses is their influence on the plant’s secondary metabolism. They induce the production of phenolic compounds, alkaloids, and flavonoids, which possess antimicrobial properties and play a critical role in resistance against pathogens and herbivores. These secondary metabolites not only exert toxic effects on invading agents but also reinforce cell wall structures, making it more difficult for pathogens to penetrate. Additionally, cytokinins stimulate the biosynthesis of phytoalexins, antimicrobial compounds produced in response to infections, which help contain pathogens at the initial sites of infection [71,80].
Experimental evidence reinforces the importance of cytokinins in these processes. Plants deficient in cytokinins or with impaired hormonal signaling exhibit increased susceptibility to pathogens, with reduced expression of defense genes and greater dissemination of infectious agents. In contrast, plants overexpressing cytokinin biosynthesis genes, such as the IPT gene, display enhanced resistance, with increased production of PR proteins, phytoalexins, and antioxidant compounds [16]. Additionally, studies on pathogens that produce cytokinins, such as Agrobacterium tumefaciens, show that these microorganisms use cytokinins to manipulate host plant metabolism, inducing gall formation to secure resources. This highlights the importance of balanced cytokinin signaling in protecting against pathogenic agents [70,79].
Cytokinins, therefore, play a central role in plant responses to biotic stresses by coordinating hormonal signaling, activating defense genes, and modulating secondary metabolism. These functions not only protect plants against pathogen and herbivore attacks but also ensure beneficial interactions with symbiotic organisms [80].
7.2 Responses to Abiotic Stresses
Cytokinins play a pivotal role in plant responses to abiotic stresses, regulating a range of physiological and biochemical mechanisms that enable adaptation and survival under adverse conditions such as drought, salinity, extreme temperatures, heavy metal toxicity, and nutrient deficiencies [51]. Their action is complex, involving the modulation of hormonal pathways, activation of antioxidant systems, and adjustment of cellular metabolism, underscoring their role as central components in integrating growth and environmental adaptation [81,82].
Under drought stress, cytokinins play a dual role by regulating water homeostasis and adjusting plant growth. In aerial tissues, such as leaves, cytokinin levels typically decrease, contributing to the suppression of vegetative growth and water conservation. Conversely, in roots, cytokinin synthesis may increase, promoting their transport to specific tissues and signaling the need for metabolic reorganization [7,25]. This adjustment is essential for maintaining vital processes. Cytokinins directly interact with abscisic acid (ABA), which plays a key role in the response to water deficit. While ABA induces stomatal closure to limit water loss, cytokinins can attenuate this response, ensuring a balance between water retention and maintaining minimal gas exchange for photosynthesis. Additionally, under drought conditions, cytokinins stimulate the growth of deeper roots, enabling the plant to access water resources in deeper soil layers [51,83,84].
In the context of salt stress, cytokinins play a crucial role in mitigating osmotic and ionic imbalances, which directly affect cellular functionality [51]. These hormones regulate ionic homeostasis by controlling the expression of transporters such as SOS1 and HKT1, which limit the toxic accumulation of sodium (Na+) in sensitive tissues and promote its compartmentalization in vacuoles. Additionally, cytokinins stimulate the production of osmolytes, such as proline and soluble sugars, which help maintain cellular osmotic pressure and protect cellular structures from dehydration [45,72]. The response to salt stress also involves the activation of antioxidant systems, such as superoxide dismutase (SOD) and catalase (CAT), which neutralize reactive oxygen species (ROS) generated by the stress, protecting proteins, membranes, and nucleic acids from oxidative damage [85,86].
In response to extreme temperatures, cytokinins modulate adaptive responses that protect cells from structural and metabolic damage. Under heat conditions, they induce the expression of heat shock proteins (HSPs), which stabilize and repair damaged proteins, as well as preserve the fluidity of cellular membranes, preventing ruptures caused by high temperatures. In extreme cold, cytokinins play a crucial role in the synthesis of cryoprotective compounds, such as sugars and amino acids, which prevent the formation of ice crystals within cells. They also regulate the production of unsaturated fatty acids, which enhance cellular membrane fluidity, ensuring functionality even at low temperatures [73,87].
In relation to heavy metal toxicity, such as cadmium (Cd) and lead (Pb), cytokinins play a significant protective role by promoting detoxification and compartmentalization of these elements. They stimulate the synthesis of phytochelatins and metallothioneins, chelating molecules that bind to metals, reducing their toxicity and directing them to vacuoles for safe storage [74]. Additionally, cytokinins activate antioxidant systems that neutralize ROS generated by heavy metal exposure, minimizing oxidative damage to membranes, proteins, and nucleic acids. At the level of the photosynthetic apparatus, cytokinins help preserve chloroplast functionality by regulating the synthesis and stability of chlorophylls and photosynthetic proteins [75].
Cytokinins also play an important role in responding to nutrient deficiencies, particularly nitrogen and phosphorus. Under low nitrogen availability, they promote the redistribution of nutrients from older leaves to younger ones by activating catabolic pathways that release resources and direct them to developing tissues. In the case of phosphorus deficiency, cytokinins increase the expression of phosphorus transporters and stimulate the exudation of organic acids by roots, enhancing the solubilization and availability of this element in the soil [88,89].
In addition to these specific roles, cytokinins integrate broader hormonal networks, interacting with other hormones to coordinate global stress responses. For instance, under drought or salinity conditions, they often antagonize ABA, helping to balance growth and adaptation. Interaction with auxins regulates root growth, while their interplay with jasmonic acid (JA) adjusts defensive responses to combined stresses [69].
Finally, cytokinins also influence epigenetic regulation under stress conditions, modulating gene expression through chromatin modifications, enabling a faster and more efficient response. These complex mechanisms highlight cytokinins as central components in plants’ adaptive capacity to adverse abiotic conditions [51,90].
8 Future Perspectives and Applications
The future perspectives and practical applications related to cytokinins are vast, given the advancements in understanding their biosynthesis, signaling, and regulatory functions. As key phytohormones in controlling growth, development, and stress responses, cytokinins hold the potential to revolutionize fields such as sustainable agriculture, biotechnology, and environmental management (Fig. 4).
Figure 4: Key applications of cytokinins in plants
8.1 Genetic Manipulation for Crop Improvement
Genetic engineering has proven to be a powerful tool for harnessing the potential of cytokinins in cultivated plants. Modulating cytokinin biosynthesis and degradation through the manipulation of key genes, such as IPT (isopentenyl transferase) and CKX (cytokinin oxidase/dehydrogenase), can optimize desirable traits. Transgenic plants overexpressing the IPT gene exhibit greater drought resistance, improved performance under salinity, and increased productivity due to cytokinins’ ability to delay senescence and enhance photosynthetic efficiency [82,91,92].
On the other hand, targeted inhibition of CKX, which is responsible for cytokinin degradation, can also increase endogenous hormone levels in specific tissues, improving resource allocation and the growth of economically important organs such as grains and fruits. This approach is particularly relevant in crops like rice, wheat, and maize, where enhancing grain filling efficiency can significantly boost productivity [91,93,94].
8.2 Applications in Sustainable Agriculture
The use of cytokinins in agricultural practices offers promising prospects for promoting sustainability. Exogenous applications of cytokinins can be employed to mitigate the effects of abiotic stress in crops exposed to drought, salinity, or extreme temperatures. This approach can be particularly valuable in regions affected by climate change, where growing conditions are becoming increasingly unpredictable [60,92,95,96].
Additionally, cytokinins have been explored as tools to improve nutrient use efficiency. In soils with low nitrogen or phosphorus availability, cytokinins can be used to stimulate root growth and enhance nutrient uptake. This application reduces the reliance on chemical fertilizers, minimizing the environmental impacts associated with their production and overuse [97,98].
Another promising field is the use of cytokinins to delay post-harvest senescence, particularly in fruits, vegetables, and cut flowers [92]. The application of cytokinins can extend the shelf life of these products, preserving their nutritional and visual quality, which has significant economic implications for the agricultural supply chain [99–101].
8.3 Control of Plant Growth and Development
Cytokinins hold potential for targeted growth management in ornamental and forest plants. In ornamental crops, their application can be used to regulate shoot development, promote lateral branching, and enhance the aesthetic quality of plants [100]. In forest species, cytokinins can be employed to regulate bud sprouting and increase biomass productivity [102,103].
Additionally, in intensive cropping systems, cytokinins can be combined with other growth regulators to balance vegetative and reproductive growth, promoting greater efficiency in the use of resources such as water and light [104,105].
8.4 Biotechnology and Production of Secondary Compounds
Cytokinins are also powerful tools in biotechnology, particularly inducing organogenesis and plant tissue culture. In micropropagation protocols, cytokinins are widely used to induce shoot proliferation in plant explants, playing an essential role in the cloning of genotypes of agricultural and forestry interest [106,107].
Another emerging field is the use of cytokinins to stimulate the production of secondary metabolites with high economic and pharmacological value. Many medicinal plants increase the production of bioactive compounds, such as alkaloids, flavonoids, and terpenes, in response to cytokinin application due to their ability to modulate specific metabolic pathways. This represents a significant advancement for the industrial production of therapeutic substances derived from plants [108,109].
8.5 Adaptation to Climate Change
Global climate change represents one of the greatest challenges to agriculture and food security. In this context, cytokinins play a central role in plant adaptation to adverse environmental conditions, such as drought, salinity, and extreme temperatures. Developing plant varieties with enhanced cytokinin synthesis or sensitivity can help mitigate the impacts of climate change, promoting crop resilience [51,110].
Studies indicate that cytokinins can also be used to increase water use efficiency in plants under water stress. By modulating root growth and stomatal opening, cytokinins enable plants to maintain a balance between growth and adaptation, even under water deficit conditions [111,112].
8.6 Environmental Management and Bioremediation
Cytokinins can be applied in environmental management projects, such as the bioremediation of soils contaminated with heavy metals. Their ability to stimulate the production of phytochelatins and metallothioneins in plants makes them essential for enhancing plant tolerance and facilitating the extraction of toxic metals from contaminated areas. Additionally, cytokinins can be used to promote the growth of pioneer plants in degraded environments, aiding in ecosystem restoration and soil stabilization [113,114].
Cytokinins stand out as key regulators in plant growth, development, and adaptation processes. Their roles in cell division, senescence regulation, and responses to biotic and abiotic stresses underscore their importance as versatile modulators in plant physiology.
Discoveries about the molecular and cellular mechanisms of cytokinins open possibilities for genetic and hormonal manipulation, enabling the development of more resilient and productive plants in adverse conditions. In agriculture, the use of cytokinins to improve efficiency of nutrient use, delay post-harvest senescence, and promote resistance to environmental stress offers sustainable alternatives to meet the growing global demand for food.
A detailed understanding of cytokinin interactions with epigenetic networks, their roles in non-model species, and the environmental impacts of their application present both challenges and opportunities for future research. Additionally, advancements in technologies such as CRISPR/Cas9 genetic editing and nanobiotechnology could enable even more precise and efficient applications of these molecules in enhancing agricultural performance.
Acknowledgement: Not applicable.
Funding Statement: This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [Funding Code 001], CAPES/BRASIL PDPG-POSDOC No. 2930/2022. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) [BPD-00571-22].
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Antonio Rodrigues da Cunha Neto, Breno Régis Santos, Michele Carla Nadal; methodology, Antonio Rodrigues da Cunha Neto, Alexandra dos Santos Ambrósio, Arlinda de Jesus Rodrigues Resende; formal analysis, Antonio Rodrigues da Cunha Neto, Alexandra dos Santos Ambrósio, Arlinda de Jesus Rodrigues Resende, Breno Régis Santos, Michele Carla Nadal; investigation, Antonio Rodrigues da Cunha Neto, Breno Régis Santos, Michele Carla Nadal; resources, Breno Régis Santos, Michele Carla Nadal; data curation, Alexandra dos Santos Ambrósio, Arlinda de Jesus Rodrigues Resende; writing—original draft preparation, Antonio Rodrigues da Cunha Neto, Alexandra dos Santos Ambrósio; writing—review and editing, Michele Carla Nadal; supervision, Breno Régis Santos, Michele Carla Nadal; project administration, Michele Carla Nadal; funding acquisition, Breno Régis Santos, Michele Carla Nadal. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka P. Evolution of cytokinin biosynthesis and degradation. J Exp Bot. 2011;62(8):2431–52. doi:10.1093/jxb/err004. [Google Scholar] [PubMed] [CrossRef]
2. Sakakibara H. Five unaddressed questions about cytokinin biosynthesis. J Exp Bot. 2024;115:erae348. doi:10.1093/jxb/erae348. [Google Scholar] [PubMed] [CrossRef]
3. Efroni I, Han S-K, Kim H J, Wu MF, Steiner E, Birnbaum K D, et al. Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev Cell. 2013;24(4):438–45. doi:10.1016/j.devcel.2013.01.019. [Google Scholar] [PubMed] [CrossRef]
4. Zubo YO, Schaller GE. Role of the cytokinin-activated type-B response regulators in hormone crosstalk. Plants. 2020;9(2):166. doi:10.3390/plants9020166. [Google Scholar] [PubMed] [CrossRef]
5. Li SM, Zheng HX, Zhang XS, Sui N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021;40(2):271–82. doi:10.1007/s00299-020-02612-1. [Google Scholar] [PubMed] [CrossRef]
6. Nisler J, Klimeš P, Končitíková R, Kadlecová A, Voller Jří, Chalaki M, et al. Cytokinin oxidase/dehydrogenase inhibitors: progress towards agricultural practice. J Exp Bot. 2024;75(16):erae239. doi:10.1093/jxb/erae239. [Google Scholar] [PubMed] [CrossRef]
7. Sakakibara H. Cytokinin biosynthesis and transport for systemic nitrogen signaling. Plant J. 2021;105(2):421–30. doi:10.1111/tpj.15011. [Google Scholar] [PubMed] [CrossRef]
8. Pokimica N, Ćosić T, Uzelac B, Ninković S, Raspor M. Dissecting the roles of the cytokinin signaling network: the case of De Novo shoot apical meristem formation. Biomolecules. 2024;14(3):381. doi:10.3390/biom14030381. [Google Scholar] [PubMed] [CrossRef]
9. Zhao J, Wang J, Liu J, Zhang P, Kudoyarova G, Liu CJ, et al. Spatially distributed cytokinins: metabolism, signaling, and transport. Plant Commun. 2024;5(7):100936. doi:10.1016/j.xplc.2024.100936. [Google Scholar] [PubMed] [CrossRef]
10. Movahedi A, Wei H, Pucker B, Ghaderi-Zefrehei M, Rasouli F, Kiani-Pouya A, et al. Isoprenoid biosynthesis regulation in poplars by methylerythritol phosphate and mevalonic acid pathways. Front Plant Sci. 2022;13:968780. doi:10.3389/fpls.2022.968780. [Google Scholar] [PubMed] [CrossRef]
11. He G, Yang P, Tang Y, Cao Y, Qi X, Xu L, et al. Mechanism of exogenous cytokinins inducing bulbil formation in Lilium lancifolium in vitro. Plant Cell Rep. 2020;39(7):861–72. doi:10.1007/s00299-020-02535-x. [Google Scholar] [PubMed] [CrossRef]
12. Oslovsky VE, Savelieva EM, Drenichev MS, Romanov GA, Mikhailov SN. Distinct peculiarities of in planta synthesis of isoprenoid and aromatic cytokinins. Biomolecules. 2020;10(1):86. doi:10.3390/biom10010086. [Google Scholar] [PubMed] [CrossRef]
13. Kieber JJ, Schaller GE. Cytokinin signaling in plant development. Development. 2018;145(4):dev149344. doi:10.1242/dev.149344. [Google Scholar] [PubMed] [CrossRef]
14. Horner W, Brunkard JO. Cytokinins stimulate plasmodesmatal transport in leaves. Front Plant Sci. 2021;12:674128. doi:10.3389/fpls.2021.674128. [Google Scholar] [PubMed] [CrossRef]
15. Shah S, Cai L, Li X, Fahad S, Wang D. Influence of cultivation practices on the metabolism of cytokinin and its correlation in rice production. Food Energy Secur. 2023;12(5):e488. doi:10.1002/fes3.488. [Google Scholar] [CrossRef]
16. Nguyen HN, Nguyen TQ, Kisiala AB, Emery RN. Beyond transport: cytokinin ribosides are translocated and active in regulating the development and environmental responses of plants. Planta. 2021;254(3):45. doi:10.1007/s00425-021-03693-2. [Google Scholar] [PubMed] [CrossRef]
17. Tsai YC, Weir NR, Hill K, Zhang W, Kim HJ, Shiu S-H, et al. Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiology. 2012;158(4):1666–84. doi:10.1104/pp.111.192765. [Google Scholar] [PubMed] [CrossRef]
18. Kubiasová K, Montesinos JC, Šamajová O, Nisler J, Mik V, Semerádová H, et al. Cytokinin fluoroprobe reveals multiple sites of cytokinin perception at plasma membrane and endoplasmic reticulum. Nat Commun. 2020;11(1):4285. doi:10.1038/s41467-020-17949-0. [Google Scholar] [PubMed] [CrossRef]
19. Hoang XLT, Prerostova S, Thu NBA, Thao NP, Vankova R, Tran LSP. Histidine kinases: diverse functions in plant development and responses to environmental conditions. Annu Rev Plant Biol. 2021;72(1):297–323. doi:10.1146/annurev-arplant-080720-093057. [Google Scholar] [PubMed] [CrossRef]
20. Powell AE, Heyl A. The origin and early evolution of cytokinin signaling. Front Plant Sci. 2023;14:1142748. doi:10.3389/fpls.2023.1142748. [Google Scholar] [PubMed] [CrossRef]
21. He L, Chen R, Chen Z, Lin J, Jin X, Ren C, et al. The molecular characteristics of soybean ARR-B transcription factors. BIOCELL. 2022;46(6):1575–92. doi:10.32604/biocell.2022.018762. [Google Scholar] [CrossRef]
22. Zhou CM, Li JX, Zhang TQ, Xu ZG, Ma ML, Zhang P, et al. The structure of B-ARR reveals the molecular basis of transcriptional activation by cytokinin. Proc Natl Acad Sci. 2024;121(3):e2319335121. doi:10.1073/pnas.2319335121. [Google Scholar] [PubMed] [CrossRef]
23. Lomin SN, Savelieva EM, Arkhipov DV, Pashkovskiy PP, Myakushina YA, Heyl A, et al. Cytokinin perception in ancient plants beyond angiospermae. Int J Mol Sci. 2021;22(23):13077. doi:10.3390/ijms222313077. [Google Scholar] [PubMed] [CrossRef]
24. Yao Y, Xiang D, Wu N, Wang Y, Chen Y, Yuan Y, et al. Control of rice ratooning ability by a nucleoredoxin that inhibits histidine kinase dimerization to attenuate cytokinin signaling in axillary buds. Mol Plant. 2023;16(12):1911–26. doi:10.1016/j.molp.2023.10.009. [Google Scholar] [PubMed] [CrossRef]
25. Pan Y, Zeng X, Wen S, Gao X, Liu X, Tian F, et al. Multiple ATP-binding cassette transporters genes are involved in thiamethoxam resistance in Aphis gossypii glover. Pestic Biochem Physiol. 2020;167:104558. doi:10.1016/j.pestbp.2020.104558. [Google Scholar] [PubMed] [CrossRef]
26. Kaur G, Mishra D. AtABCG14: a long-distance root-to-shoot carrier of cytokinin. Int J Plant Biol. 2022;13(3):352–5. doi:10.3390/ijpb13030029. [Google Scholar] [CrossRef]
27. Hu Y, Shani E. Cytokinin activity-transport and homeostasis at the whole plant, cell, and subcellular levels. New Phytol. 2023;239(5):1603–8. doi:10.1111/nph.19001. [Google Scholar] [PubMed] [CrossRef]
28. Zhao J, Deng X, Qian J, Liu T, Ju M, Li J, et al. Arabidopsis ABCG14 forms a homodimeric transporter for multiple cytokinins and mediates long-distance transport of isopentenyladenine-type cytokinins. Plant Commun. 2023;4(2):100468. doi:10.1016/j.xplc.2022.100468. [Google Scholar] [PubMed] [CrossRef]
29. Bishopp A, Lehesranta S, Vatén A, Help H, El-Showk S, Scheres B, et al. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Curr Biol. 2011;21(11):927–32. doi:10.1016/j.cub.2011.04.049. [Google Scholar] [PubMed] [CrossRef]
30. Petrasek J, Hoyerova K, Motyka V, Hejatko J, Dobrev P, Kaminek M, et al. Auxins and cytokinins in plant development 2018. Int J Mol Sci. 2019;20(4):909. doi:10.3390/ijms20040909. [Google Scholar] [PubMed] [CrossRef]
31. Hussain S, Nanda S, Zhang J, Rehmani MIA, Suleman M, Li G, et al. Auxin and cytokinin interplay during leaf morphogenesis and phyllotaxy. Plants. 2021;10(8):1732. doi:10.3390/plants10081732. [Google Scholar] [PubMed] [CrossRef]
32. Takei K, Sakakibara H, Sugiyama T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J Biol Chem. 2001;276(28):26405–10. doi:10.1074/jbc.M102130200. [Google Scholar] [PubMed] [CrossRef]
33. Dabravolski S. Multi-faceted nature of the tRNA isopentenyltransferase. Funct Plant Biol. 2020;47(6):475–85. doi:10.1071/FP19255. [Google Scholar] [PubMed] [CrossRef]
34. Zhao J, Ding B, Zhu E, Deng X, Zhang M, Zhang P, et al. Phloem unloading via the apoplastic pathway is essential for shoot distribution of root-synthesized cytokinins. Plant Physiol. 2021;186(4):2111–23. doi:10.1093/plphys/kiab188. [Google Scholar] [PubMed] [CrossRef]
35. Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Curr Biol. 2013;23(20):1979–89. doi:10.1016/j.cub.2013.08.008. [Google Scholar] [PubMed] [CrossRef]
36. Azizi P, Rafii MY, Maziah M, Abdullah SNA, Hanafi MM, Latif MA, et al. Understanding the shoot apical meristem regulation: a study of the phytohormones, auxin and cytokinin, in rice. Mech Dev. 2015;135:1–15. doi:10.1016/j.mod.2014.11.001. [Google Scholar] [PubMed] [CrossRef]
37. Rivas MÁ, Friero I, Alarcón MV, Salguero J. Auxin-cytokinin balance shapes maize root architecture by controlling primary root elongation and lateral root development. Front Plant Sci. 2022;13:836592. doi:10.3389/fpls.2022.836592. [Google Scholar] [PubMed] [CrossRef]
38. Jan M, Muhammad S, Jin W, Zhong W, Zhang S, Lin Y, et al. Modulating root system architecture: cross-talk between auxin and phytohormones. Front Plant Sci. 2024;15:1343928. doi:10.3389/fpls.2024.1343928. [Google Scholar] [PubMed] [CrossRef]
39. Wu W, Du K, Kang X, Wei H. The diverse roles of cytokinins in regulating leaf development. Hortic Res. 2021;8(1):118. doi:10.1038/s41438-021-00558-3. [Google Scholar] [PubMed] [CrossRef]
40. Zhang M, Zheng H, Jin L, Xing L, Zou J, Zhang L, et al. miR169o and ZmNF-YA13 act in concert to coordinate the expression of ZmYUC1 that determines seed size and weight in maize kernels. New Phytol. 2022;235(6):2270–84. doi:10.1111/nph.18317. [Google Scholar] [PubMed] [CrossRef]
41. Zdarska M, Dobisová T, Gelová Z, Pernisová M, Dabravolski S, Hejátko J. Illuminating light, cytokinin, and ethylene signalling crosstalk in plant development. J Exp Bot. 2015;66(16):4913–31. doi:10.1093/jxb/erv261. [Google Scholar] [PubMed] [CrossRef]
42. Samanta S, Roychoudhury A. Molecular crosstalk of jasmonate with major phytohormones and plant growth regulators during diverse stress responses. J Plant Growth Regul. 2024;44(1):1–27. doi:10.1007/s00344-024-11412-w. [Google Scholar] [CrossRef]
43. Sosnowski J, Truba M, Vasileva V. The impact of auxin and cytokinin on the growth and development of selected crops. Agriculture. 2023;13(3):724. doi:10.3390/agriculture13030724. [Google Scholar] [CrossRef]
44. Singh A, Roychoudhury A. Abscisic acid in plants under abiotic stress: crosstalk with major phytohormones. Plant Cell Rep. 2023;42(6):961–74. doi:10.1007/s00299-023-03013-w. [Google Scholar] [PubMed] [CrossRef]
45. Singh P, Choudhary KK, Chaudhary N, Gupta S, Sahu M, Tejaswini B, et al. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front Plant Sci. 2022;13:1006617. doi:10.3389/fpls.2022.1006617. [Google Scholar] [PubMed] [CrossRef]
46. Carneiro AK, Montessoro PDF, Fusaro AF, Araújo BG, Hemerly AS. Plant CDKs—driving the cell cycle through climate change. Plants. 2021;10(9):1804. doi:10.3390/plants10091804. [Google Scholar] [PubMed] [CrossRef]
47. Braat J, Havaux M. The SIAMESE family of cell-cycle inhibitors in the response of plants to environmental stresses. Front Plant Sci. 2024;15:1362460. doi:10.3389/fpls.2024.1362460. [Google Scholar] [PubMed] [CrossRef]
48. Tank JG, Pandya RV, Thaker VS. Phytohormones in regulation of the cell division and endoreduplication process in the plant cell cycle. RSC Adv. 2014;4(24):12605–12613. doi:10.1039/c3ra45367g. [Google Scholar] [CrossRef]
49. Shimotohno A, Aki SS, Takahashi N, Umeda M. Regulation of the plant cell cycle in response to hormones and the environment. Annu Rev Plant Biol. 2021;72(1):273–96. doi:10.1146/annurev-arplant-080720-103739. [Google Scholar] [PubMed] [CrossRef]
50. Soni N, Bacete L. The interplay between cell wall integrity and cell cycle progression in plants. Plant Mol Biol. 2023;113(6):367–82. doi:10.1007/s11103-023-01394-w. [Google Scholar] [PubMed] [CrossRef]
51. Bielach A, Hrtyan M, Tognetti VB. Plants under stress: involvement of auxin and cytokinin. Int J Mol Sci. 2017;18(7):1427. doi:10.3390/ijms18071427. [Google Scholar] [PubMed] [CrossRef]
52. Merelo P, González-Cuadra I, Ferrándiz C. A cellular analysis of meristem activity at the end of flowering points to cytokinin as a major regulator of proliferative arrest in Arabidopsis. Curr Biol. 2022;32(4):749–62. doi:10.1016/j.cub.2021.11.069. [Google Scholar] [PubMed] [CrossRef]
53. Lee JS. To overcome the limitations of fixed life patterns, plants can generate meristems throughout life. J Plant Physiol. 2023;291:154097. doi:10.1016/j.jplph.2023.154097. [Google Scholar] [PubMed] [CrossRef]
54. Kusnetsov VV, Bychkov IA, Kudryakova NV. Phytomelatonin as an element of the plant hormonal system. Russ J Plant Physiol. 2024;71(4):134. doi:10.1134/S1021443724606839. [Google Scholar] [CrossRef]
55. Qi X, Zhuang Z, Ji X, Bian J, Peng Y. The mechanism of exogenous salicylic acid and 6-benzylaminopurine regulating the elongation of maize mesocotyl. Int J Mol Sci. 2024;25(11):6150. doi:10.3390/ijms25116150. [Google Scholar] [PubMed] [CrossRef]
56. Zhumanova N, Akimbayeva N, Myrzakhmetova N, Dzhiembaev B, Ku A, Diyarova B, et al. A comprehensive review of new generation plant growth regulators. ES Food Agroforestry. 2024;17:1190. doi:10.30919/esfaf1190. [Google Scholar] [CrossRef]
57. Deveshwar P, Prusty A, Sharma S, Tyagi AK. Phytohormone-mediated molecular mechanisms involving multiple genes and QTL govern grain number in rice. Front Genet. 2020;11:586462. doi:10.3389/fgene.2020.586462. [Google Scholar] [PubMed] [CrossRef]
58. Sharma M, Laxmi A. Deciphering the physiological and molecular functions of phytohormones. In: Plant hormones in crop improvement. Academic Press; 2023. p. 15–40. doi:10.1016/B978-0-323-91886-2.00013-6. [Google Scholar] [CrossRef]
59. Guo Y, Ren G, Zhang K, Li Z, Miao Y, Guo H. Leaf senescence: progression, regulation, and application. Mol Hortic. 2021;1(1):1–25. doi:10.1186/s43897-021-00006-9. [Google Scholar] [PubMed] [CrossRef]
60. Mughal N, Shoaib N, Chen J, He Y, Fu M, Li X, et al. Adaptive roles of cytokinins in enhancing plant resilience and yield against environmental stressors. Chemosphere. 2024;364(2):143189. doi:10.1016/j.chemosphere.2024.143189. [Google Scholar] [PubMed] [CrossRef]
61. Lv X, Zhang Y, Zhang Y, Fan S, Kong L. Source-sink modifications affect leaf senescence and grain mass in wheat as revealed by proteomic analysis. BMC Plant Biol. 2020;20(1):1–17. doi:10.1186/s12870-020-02447-8. [Google Scholar] [PubMed] [CrossRef]
62. Rossi S, Huang B. Research advances in molecular mechanisms regulating heat tolerance in cool-season turfgrasses. Crop Sci. 2024;65(1):341. doi:10.1002/csc2.21339. [Google Scholar] [CrossRef]
63. Kaur H, Kohli SK, Khanna K, Bhardwaj R. Scrutinizing the impact of water deficit in plants: transcriptional regulation, signaling, photosynthetic efficacy, and management. Physiol Plant. 2021;172(2):935–62. doi:10.1111/ppl.13389. [Google Scholar] [PubMed] [CrossRef]
64. Shinozaki K, Yamaguchi-Shinozaki K. Functional genomics in plant abiotic stress responses and tolerance: from gene discovery to complex regulatory networks and their application in breeding. Proc Japan Acad, Ser B. 2022;98(8):470–92. doi:10.2183/pjab.98.024. [Google Scholar] [PubMed] [CrossRef]
65. Huang P, Li Z, Guo H. New advances in the regulation of leaf senescence by classical and peptide hormones. Front Plant Sci. 2022;13:923136. doi:10.3389/fpls.2022.923136. [Google Scholar] [PubMed] [CrossRef]
66. Baseer AQ, Niazi P, Monib AW, Hassand MH, Hejran AB, Sarwari A, et al. Lifecycle transitions in plant development: ripening, senescence, & cell death. J Pharma Insights Res. 2024;2(2):169–79, 2024. [Google Scholar]
67. Espinosa-Vellarino FL, Garrido I, Casimiro I, Silva AC, Espinosa F, Ortega A. Enzymes involved in antioxidant and detoxification processes present changes in the expression levels of their coding genes under the stress caused by the presence of antimony in tomato. Plants. 2024;13(5):609. doi:10.3390/plants13050609. [Google Scholar] [PubMed] [CrossRef]
68. Shahid M, Shafi Z, Ilyas T, Singh UB, Pichtel J. Crosstalk between phytohormones and pesticides: insights into unravelling the crucial roles of plant growth regulators in improving crop resilience to pesticide stress. Sci Hortic. 2024;338(1):113663. doi:10.1016/j.scienta.2024.113663. [Google Scholar] [CrossRef]
69. Akhtar SS, Mekureyaw MF, Pandey C, Roitsch T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front Plant Sci. 2020;10:1777. doi:10.3389/fpls.2019.01777. [Google Scholar] [PubMed] [CrossRef]
70. Gupta R, Anand G, Pizarro L, Laor D, Kovetz N, Sela N, et al. Cytokinin inhibits fungal development and virulence by targeting the cytoskeleton and cellular trafficking. Mbio. 2021;12(5):10–1128. doi:10.1128/mBio.03068-20. [Google Scholar] [PubMed] [CrossRef]
71. Meena M, Swapnil P, Divyanshu K, Kumar S, Harish, Tripathi YN, et al. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: current perspectives. J Basic Microbiol. 2020;60(10):828–61. doi:10.1002/jobm.202000370. [Google Scholar] [PubMed] [CrossRef]
72. Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, et al. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi:10.3390/biom9070285. [Google Scholar] [PubMed] [CrossRef]
73. Bhattacharya A. Plant growth hormones in plants under low-temperature stress: a review. Physiol Processes Plants Under Low Temp Stress. 2022;45(4):517–627. doi:10.1007/978-981-16-9037-2_6. [Google Scholar] [CrossRef]
74. Saini S, Kaur N, Pati PK. Phytohormones: key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ Saf. 2021;223(1):112578. doi:10.1016/j.ecoenv.2021.112578. [Google Scholar] [PubMed] [CrossRef]
75. Emamverdian A, Ding Y, Mokhberdoran F, Ahmad Z. Mechanisms of selected plant hormones under heavy metal stress. Pol J Environ Stud. 2021;30(1):497–507. doi:10.15244/pjoes/122809. [Google Scholar] [CrossRef]
76. Ma KW, Ma W. Phytohormone pathways as targets of pathogens to facilitate infection. Plant Mol Biol. 2016;91(6):713–25. doi:10.1007/s11103-016-0452-0. [Google Scholar] [PubMed] [CrossRef]
77. Checker VG, Kushwaha HR, Kumari P, Yadav S. Role of phytohormones in plant defense: signaling and cross talk. Molecular Aspects Plant-Pathogen Interaction. 2018;40(1):159–84. doi:10.1007/978-981-10-7371-7_7. [Google Scholar] [CrossRef]
78. McIntyre KE, Bush DR, Argueso CT. Cytokinin regulation of source-sink relationships in plant-pathogen interactions. Front Plant Sci. 2021;12:677585. doi:10.3389/fpls.2021.677585. [Google Scholar] [PubMed] [CrossRef]
79. Hai NN, Chuong NN, Tu NHC, Kisiala A, Hoang XLT, Thao NP. Role and regulation of cytokinins in plant response to drought stress. Plants. 2020;9(4):422. doi:10.3390/plants9040422. [Google Scholar] [PubMed] [CrossRef]
80. Riseh RS, Vazvani MG. Unveiling methods to stimulate plant resistance against pathogens. Front Biosci (Landmark Ed). 2024;29(5):188. doi:10.31083/j.fbl2905188. [Google Scholar] [PubMed] [CrossRef]
81. EL Sabagh A, Islam MS, Hossain A, Iqbal MA, Mubeen M, Waleed M, et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front Agron. 2022;4:765068. doi:10.3389/fagro.2022.765068. [Google Scholar] [CrossRef]
82. Mandal S, Ghorai M, Anand U, Samanta D, Kant N, Mishra T, et al. RETRACTED: cytokinin and abiotic stress tolerance-what has been accomplished and the way forward? Front Genet. 2022;13:943025. doi:10.3389/fgene.2022.943025. [Google Scholar] [PubMed] [CrossRef]
83. Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, et al. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell. 2010;22(12):3905–20. doi:10.1105/tpc.109.072694. [Google Scholar] [PubMed] [CrossRef]
84. Wang XL, Qin RR, Sun RH, Wang JJ, Hou XG, Qi L, et al. No post-drought compensatory growth of corns with root cutting based on cytokinin induced by roots. Agric Water Manag. 2018;205:9–20. doi:10.1016/j.agwat.2018.04.035. [Google Scholar] [CrossRef]
85. Singh M, Kumar J, Singh S, Singh VP, Prasad SM. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Bio/Technol. 2015;14(3):407–26. doi:10.1007/s11157-015-9372-8. [Google Scholar] [CrossRef]
86. Iqbal S, Wang X, Mubeen I, Kamran M, Kanwal I, Díaz GA, et al. Phytohormones trigger drought tolerance in crop plants: outlook and future perspectives. Front Plant Sci. 2022;12:799318. doi:10.3389/fpls.2021.799318. [Google Scholar] [PubMed] [CrossRef]
87. Zheng XW, Cao XY, Jiang WH, Xu GZ, Liang QZ, Yang ZY. Cryoprotectant-mediated cold stress mitigation in litchi flower development: transcriptomic and metabolomic perspectives. Metabolites. 2024;14(4):223. doi:10.3390/metabo14040223. [Google Scholar] [PubMed] [CrossRef]
88. Kudoyarova GR, Vysotskaya LB, Arkhipova TN, Kuzmina LY, Galimsyanova NF, Sidorova LV, et al. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol Plant?. 2017;39(11):1–8. doi:10.1007/s11738-017-2556-9. [Google Scholar] [CrossRef]
89. Jindo K, Canellas LP, Albacete A, Figueiredo dos Santos L, Frinhani Rocha RL, Carvalho Baia D, et al. Interaction between humic substances and plant hormones for phosphorous acquisition. Agronomy. 2020;10(5):640. doi:10.3390/agronomy10050640. [Google Scholar] [CrossRef]
90. Yamamuro C, Zhu JK, Yang Z. Epigenetic modifications and plant hormone action. Mol Plant. 2016;9(1):57–70. doi:10.1016/j.molp.2015.10.008. [Google Scholar] [PubMed] [CrossRef]
91. Chen L, Zhao J, Song J, Jameson PE. Cytokinin dehydrogenase: a genetic target for yield improvement in wheat. Plant Biotechnol J. 2020;18(3):614–30. doi:10.1111/pbi.13305. [Google Scholar] [PubMed] [CrossRef]
92. Aremu AO, Fawole OA, Makunga NP, Masondo NA, Moyo M, Buthelezi NM, et al. Applications of cytokinins in horticultural fruit crops: trends and future prospects. Biomolecules. 2020;10(9):1222. doi:10.3390/biom10091222. [Google Scholar] [PubMed] [CrossRef]
93. Mao C, He J, Liu L, Deng Q, Yao X, Liu C, et al. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol J. 2020;18(2):429–42. doi:10.1111/pbi.13209. [Google Scholar] [PubMed] [CrossRef]
94. Ramireddy E, Nelissen H, Leuendorf JE, Van Lijsebettens M, Inzé D, Schmülling T. Root engineering in maize by increasing cytokinin degradation causes enhanced root growth and leaf mineral enrichment. Plant Mol Biol. 2021;106(6):555–67. doi:10.1007/s11103-021-01173-5. [Google Scholar] [PubMed] [CrossRef]
95. Ali J, Mukarram M, Ojo J, Dawam N, Riyazuddin R, Ghramh HA, et al. Harnessing phytohormones: advancing plant growth and defence strategies for sustainable agriculture. Physiol Plant. 2024;176(3):e14307. doi:10.1111/ppl.14307. [Google Scholar] [PubMed] [CrossRef]
96. He S, Li L, Lv M, Wang R, Wang L, Yu S, et al. PGPR: key to enhancing crop productivity and achieving sustainable agriculture. Curr Microbiol. 2024;81(11):1–17. doi:10.1007/s00284-024-03893-5. [Google Scholar] [PubMed] [CrossRef]
97. Kapoore RV, Wood EE, Llewellyn CA. Algae biostimulants: a critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol Adv. 2021;49:107754. doi:10.1016/j.biotechadv.2021.107754. [Google Scholar] [PubMed] [CrossRef]
98. Lastochkina O, Aliniaeifard S, SeifiKalhor M, Bosacchi M, Maslennikova D, Lubyanova A. Novel approaches for sustainable horticultural crop production: advances and prospects. Horticulturae. 2022;8(10):910. doi:10.3390/horticulturae8100910. [Google Scholar] [CrossRef]
99. Rani P, Singh N. Senescence and postharvest studies of cut flowers: a critical review. Pertanika J Trop Agric Sci. 2014;37(2):159–201. [Google Scholar]
100. Xiang W, Wang HW, Sun DW. Phytohormones in postharvest storage of fruit and vegetables: mechanisms and applications. Crit Rev Food Sci Nutr. 2021;61(18):2969–83. doi:10.1080/10408398.2020.1864280. [Google Scholar] [PubMed] [CrossRef]
101. Janowska B, Andrzejak R. The role of cytokinins and gibberellins on post-harvest longevity of florists’ greens. Agriculture. 2022;12(9):1375. doi:10.3390/agriculture12091375. [Google Scholar] [CrossRef]
102. Buttò V, Deslauriers A, Rossi S, Rozenberg P, Shishov V, Morin H. The role of plant hormones in tree-ring formation. Trees. 2020;34(2):315–35. doi:10.1007/s00468-019-01940-4. [Google Scholar] [CrossRef]
103. Chen Z, Chen Y, Shi L, Wang L, Li W. Interaction of phytohormones and external environmental factors in the regulation of the bud dormancy in woody plants. Int J Mol Sci. 2023;24(24):17200. doi:10.3390/ijms242417200. [Google Scholar] [PubMed] [CrossRef]
104. Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proc Natl Acad Sci. 2001;98(18):10487–92. doi:10.1073/pnas.171304098. [Google Scholar] [PubMed] [CrossRef]
105. Zaman M, Kurepin LV, Catto W, Pharis RP. Enhancing crop yield with the use of N-based fertilizers co-applied with plant hormones or growth regulators. J Sci Food Agric. 2015;95(9):1777–85. doi:10.1002/jsfa.6938. [Google Scholar] [PubMed] [CrossRef]
106. Oliveira LS, Brondani GE, Molinari LV, Dias RZ, Teixeira GL, Gonçalves AN, et al. Optimal cytokinin/auxin balance for indirect shoot organogenesis of Eucalyptus cloeziana and production of ex vitro rooted micro-cuttings. J For Res. 2022;33(5):1573–84. doi:10.1007/s11676-022-01454-9. [Google Scholar] [CrossRef]
107. Correia M, Lopes T, Puga AP, Pinto G, Canhoto J, Correia S. Morpho-physiological evaluation of Solanum betaceum Cav. In Vitro cloned plants: a comparison of different micropropagation methods. Plants. 2023;12(9):1884. doi:10.3390/plants12091884. [Google Scholar] [PubMed] [CrossRef]
108. Chandran H, Meena M, Barupal T, Sharma K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep. 2020;26(5):e00450. doi:10.1016/j.btre.2020.e00450. [Google Scholar] [PubMed] [CrossRef]
109. Yeshi K, Crayn D, Ritmejerytė E, Wangchuk P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules. 2022;27(1):313. doi:10.3390/molecules27010313. [Google Scholar] [PubMed] [CrossRef]
110. Gray SB, Brady SM. Plant developmental responses to climate change. Dev Biol. 2016;419(1):64–77. doi:10.1016/j.ydbio.2016.07.023. [Google Scholar] [PubMed] [CrossRef]
111. Liu Y, Zhang M, Meng Z, Wang B, Chen M. Research progress on the roles of cytokinin in plant response to stress. Int J Mol Sci. 2020;21(18):6574. doi:10.3390/ijms21186574. [Google Scholar] [PubMed] [CrossRef]
112. Nazli F, Mustafa A, Ahmad M, Hussain A, Jamil M, Wang X, et al. A review on practical application and potentials of phytohormone-producing plant growth-promoting rhizobacteria for inducing heavy metal tolerance in crops. Sustainability. 2020;12(21):9056. doi:10.3390/su12219056. [Google Scholar] [CrossRef]
113. Raza MAS, Zaheer MS, Saleem MF, Khan IH, Ahmad S, Iqbal R. Drought ameliorating effect of exogenous applied cytokinin in wheat. Pakistan J Agric Sci. 2020;57–3. doi:10.21162/PAKJAS/20.8183. [Google Scholar] [CrossRef]
114. Yu S, Zehra A, Sahito ZA, Wang W, Chen S, Feng Y, et al. Cytokinin-mediated shoot proliferation and its correlation with phytoremediation effects in Cd-hyperaccumulator ecotype of Sedum alfredii. Sci Total Environ. 2024;912(2):168993. doi:10.1016/j.scitotenv.2023.168993. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools