 Open Access
Open Access
REVIEW
Reviving Contaminated Soils: Microbe-Aided Phytoremediation for Sustainable Metal Pollution Cleanup
1 School of Agriculture, Forestry and Food Engineering, Yibin University, Yibin, 644000, China
2 Department of Botany, Government College University, Faisalabad, 38000, Pakistan
3 Inner Mongolia Saikexing Institute of Breeding and Reproductive Biotechnology in Domestic Animals Co., Ltd., Hohhot, 011500, China
4 Department of Agronomy, PMAS Arid Agriculture University, Rawalpindi, 46300, Pakistan
* Corresponding Authors: Sara Zafar. Email: ; Zuhair Hasnain. Email:
(This article belongs to the Special Issue: Soil Microbe-Plant Interactions: Unveiling Mechanisms of Biostimulants in Stress Tolerance)
Phyton-International Journal of Experimental Botany 2025, 94(3), 603-621. https://doi.org/10.32604/phyton.2025.062560
Received 20 December 2024; Accepted 10 February 2025; Issue published 31 March 2025
Abstract
Soil metal pollution is a global issue due to its toxic nature affecting ecosystems and human health. This has become a concern since metals are non-biodegradable and toxic. Most of the reclamation methods currently used for soils rely on the use of physical and chemical means, which tend to be very expensive and result in secondary environmental damage. However, microbe-aided phytoremediation is gaining attention as it is an eco-friendly, affordable, and technically advanced method to restore the ecosystem. It is essential to understand the complex interaction between plants and microbes. The primary function of plant growth-promoting bacteria (PGPB) is to stimulate plant development, aid in metal elimination, and reduce their bioavailability in the soil. These microbes regulate phytohormones, stimulate processes such as phytoextraction and phyto-stabilization, and improve the uptake of essential nutrients, such as nitrogen and phosphorus. PGPBs secrete a range of enzymes and chemicals, fix nitrogen, solubilize minerals, increase the bioavailability of nutrients under diverse biological environments with high salinities, excessive metal-contaminated soil, and organic pollutants, increase the soil fertility and help in the reclamation of agriculture and regenerate the native flora. The integration of CRISPR-Cas9 gene-editing technology with microbial-aided phytoremediation and the use of genetically modified microbes with nanomaterials further enhance the efficacy of the approaches in polluted environments for sustainable restoration of the soil.Graphic Abstract
Keywords
The accelerated pace of urbanization and industrial growth creates significant environmental concerns, particularly about metal contamination [1]. The elevated concentration of harmful metals in soils raises critical issues for both environmental safety as well as human well-being [2]. Highly beneficial and strongly recommended is the use of chemical and physical remediation methods to address soil metal pollution. The synthetic approaches have the highest frequency of changing the qualities of the soil, have high labor intensiveness, and destroy the soil microorganisms, thereby enhancing the impurities to the ecosystem [3]. Plants-based remediation is an eco-friendly approach to minimize the lethal effects of contaminants in the ecosystem. The strategy entails the application of plants together with the soil microbiota [4]. In contrast to conventional, phytotechnique approaches are recognized as being reasonable and appropriate for extensive use in the field [5,6]. Although a lot of improvements have been observed over the last three decades, the approaches are yet under development [7]. The use of bio-agents, sequestering materials, and inoculation of microorganisms, are some of the practices that are considered essential for the effective bio-remediation of soils [8]. To restore ecosystem variability and functionality, microorganisms play a vital role, making it essential to fully comprehend the association of soil microbiome [9,10]. Metal-tolerant plants can thrive in metal-contaminated soil by enhancing their uptake [11].
This article brings out the significance of the remediation of metal-contaminated lands and the crucial function of microbes. Abiotic stresses such as extreme weather or climate change increase soil deterioration rates; the result of which has led to losses of natural ecosystems over the past several centuries [12]. Overuse of fertilizer poses dangerous consequences on soil sustainability. Although a major portion of the global food demand is maintained by land, the solution henceforth must be curative in an attempt to control land degradation. Mankind’s priorities have destroyed the environment and ecosystems, lessening the output of farming and balanced living. The main causes of soil degradation include overgrazing, intensive farming, forestry, wood production for fuel, and modernization. Cultivating energy crops in these areas can aid in land restoration and significantly lower greenhouse gas emissions [13]. Much of the Earth’s surface is impacted by various abiotic stresses, including contamination with toxic heavy metals (HMs), organic solvents, increased acidity, high salinity, and water scarcity.
Much of the Earth’s surface is impacted by numerous abiotic stresses, involving contamination by toxic heavy metals (HMs), organic solvents, high acidity, elevated salinity, and water scarcity [14]. Salinity stress or HM toxicity, pH stress, water scarcity, and other negative factors highly hinder the growth of plants. The interruption of plant physiological activities under stress leads to the loss of leaves, wilting, and less water transpired from leaves. Under such conditions, a drop in turgor pressure limits cell expansion under stress. The loss of turgor pressure is one of the more sensitive biological processes that limit cells from growing under stressful conditions.
Hydrodynamic changes stimulate the enzymatic synthesis of compounds and help to counteract the effects brought about by stress through balancing cellular water [15]. Plants use avoidance and tolerance as strategies to tolerate different stresses, and some species have been reported for their potential to remediate polluted soils [16]. PGPB increases the remediation potential of plants by producing hormones and metabolites, solubilization of minerals, fixation of nitrogen, and protection of plants from infections. PGPB also assists plants in dealing with both biotic and abiotic stresses [17]. Phytoremediation techniques effectively remove pollutants from the environment with less secondary waste, in an economical and eco-friendly way [18]. Such techniques include phyto-stabilization, rhizo-degradation, phyto-desalination, phytovolatilization, phyto-filtration, phytoaccumulation, phyto-transformation, phytoextraction and phytodegradation [19]. While the decomposition of plants and rhizo-degradation deal with biological contaminants, techniques like phytoextraction, phyto-filtration, and phyto-stabilization are mostly applied for soils contaminated with heavy metals. This involves plant species with high efficiency in extracting or immobilizing metals, which possess a strong tolerance to metal contamination, are applied in phytoremediation [20]. The integration of CRISPR-Cas9 gene-editing technology with microbial-aided phytoremediation further enhances the efficacy of the approaches in polluted environments. Genetically modified microbes along with nanomaterials, further accelerate the effectiveness in detoxifying contaminated soils. Moreover, the article brings out the synergistic role of PGPB in phytoremediation and how it could alleviate abiotic stresses and enhance metal uptake, which would be more sustainable and advanced in the restoration of soil.
2 Role of PGPB in Alleviating Abiotic Stresses
Abiotic and biotic stresses decrease the agricultural output. Productivity declines by 50% under abiotic stress and by 30% under biotic stress [21]. It is generally known that PGPBs can reduce the harmful effects of extreme stress on vegetation. In the natural habitat, plants collaborate with a variety of microorganisms, from several kingdoms and domains, comprising of viruses, bacteria, fungi, and archaea. Microorganisms (PGPB) yield valuable compounds, like phytohormones, which shield plants from extreme conditions, whereas the rhizosphere provides the ecological habitats and nutrients for the emergence of microbiota [22]. By using PGPB products, contaminated and unusable land can be turned into fertile ground, which is beneficial for plant growth [23,24].
2.1 PGPB Mitigates Salinity Stress
Translocation of sodium to vesicles lowers the concentration of salts in the cells and is the primary mechanism by which plants tolerate salt [25]. According to research, PGPB phytoremediation is linked to higher expression of the Salt Overly Sensitive 1 (SOS1) and the other genes related to the SOS trail. To increase salt resistance, phytohormones like ethylene, salicylic acid, and abscisic acid are synthesized more often in response to salinity stress. These hormones are liable for stimulating the signaling pathway of several genes [26]. It is reported that inoculation of soil with Bacillus aryabhattai H19-1 and Bacillus mesonae H20-5, PGPB strains may boost the working of antioxidant enzymes, the breakdown of abscisic acid, and the increase of proline under saline stress [27,28]. According to research done on uninoculated plants and plants infected with mutant Pseudomonas species, the manufacture of ACC deaminase (lowers the level of ethylene) by PGPB is the technique that allows plants to survive salinity. It is reported by Girolkar et al. [29], that Streptomyces, Arthrobacter, and Bacillus sp. increased the root growth in wheat, maize, and rice crops. In another study, B. pumilus FAB10 (a salt-resistant phosphate solubilizing bacteria) enhanced the yield of wheat under 25 dSm−1 NaCl stress. Phosphate solubilizing strains enhanced the growth of shoots in pepper and rice under 20 and 15 dSm−1 NaCl [30]. The synthesis of exopolysaccharides (EPS) and the development of biofilms are crucial defensive mechanisms under salt stress. The fresh weight of the chickpea increased by 153% and 177%, respectively, after being inoculated with strain Planococcus rifietoensis RT4 and Halomonas variabilis HT1 of bacteria at a 100 mM NaCl concentration [31].
2.2 Plant Growth Increased by PGPB on High-Salinity Marginal Land
By producing ACC deaminase and biofilms that cover the outside of the roots, which reduce ethylene precursors, PGPB aids plants in alleviating salinity stress in marginal lands [32]. It also increases the effectiveness of water consumption by controlling transpiration, regulating stomatal conductance, and lowering the concentrations of ROS in inoculated species [33]. Inoculating Pseudomonas species that produce ACC deaminase in barley and oats for phytoremediation of saline soils, the plants’ roots grew by 200% and 50%, respectively, and their shoot biomass increased by 100%–1500% [34]. Also, Novosphingobium sp. HR1a and Pseudomonas putida KT2440 enhanced Citrus macrophylla development. Under salt stress, the strain KT2440 avoided stem chloride and proline accumulation, and the strain HR1a enhanced IAA accumulation in leaves (Fig. 1) [35]. Similarly, increased plant development and decreased salinity were observed in spinach (Spinacia oleracea L.) after inoculation with chitinolytic (Sanguibacter spp., Pseudomonas spp., Bacillus spp.) and halotolerant (Pseudomonas spp., Thalassobacillus spp.) bacterial strains with high antifungal activity. On marginal fields with organic compound contamination, PGPB increased plant growth. The efficacy of phytoextraction is typically lesser than that of phytodegradation or phyto-stimulation mainly because many organic contaminants have strong repellent characteristics [36]. Bacteria increase their ability to biodegrade by using the metabolites that plants release as carbon sources. By doing so, the contaminant’s stress is reduced and plant growth is promoted. High hydrocarbon-resistant bacteria which break down organic pollutants aerobically, are among the PGPB strains beneficial in phyto-stimulation technique. Environmental organic pollutants that can degrade petroleum hydrocarbons are the most common type of pollution in any country [37]. PGPB is commonly obtained from the autochthonic microbiota, for soil bioaugmentation and exogenous pools of microorganisms. Although there is a diversity among these strains, the rhizosphere bacteria are thought to be the best at degrading hydrocarbons [38]. These bacterial strains produce biosurfactants that increase the bioavailability of hydrocarbons, essential for biodegradation. The Bassia scoparia in conjunction with rhizosphere microorganisms exhibited the high efficacy of phyto-stimulation in soil contaminated with crude oil [39]. Cajanus cajan, rhizospheric bacteria, and Zea mays L. were used to phyto-remediate petroleum oily sludge, Italian ryegrass was used to break down petroleum hydrocarbons, similar effects of PGPB were seen supported by alkane-degrading bacterial strains [40], and the degradation of diesel contaminants by Zea mays. The cleanup of organic compounds in polluted areas may also use phyto-stimulation as an additional technique. Using plant-beneficial bacteria, phyto-stimulation helped switch grass to eliminate polychlorinated biphenyls (PCBs). In a study, Burkholderia xenovorans LB400 was used as an adjunct to the key treatment, i.e., phytoextraction [41]. It is feasible to use a range of biotechnological methods to improve phytoremediation, such as changing the genes for HM transporters and the mechanisms that allow them to be absorbed, as well as boosting the production of HM ligands. The HM transporter gene was overexpressed in Arabidopsis thaliana to increase sensitivity and accumulate Pb and Cd (YCF1). Likewise, Nicotiana tabacum NtCBP4 protein overexpression in transgenic plants led to increased Pb accumulation and hypersensitivity. For HM detoxification, ligands that bind to HM, glutathione, phyto-chelatins, and cystine-rich peptides like metallothioneins are used. Significant Cu accumulation in roots was seen in peas as a result of the metallothionein (PsMTA) being overexpressed. Similar, results were seen in transgenic Brassica juncea that had the gene for E. coli GSH synthetase overexpressed [42].
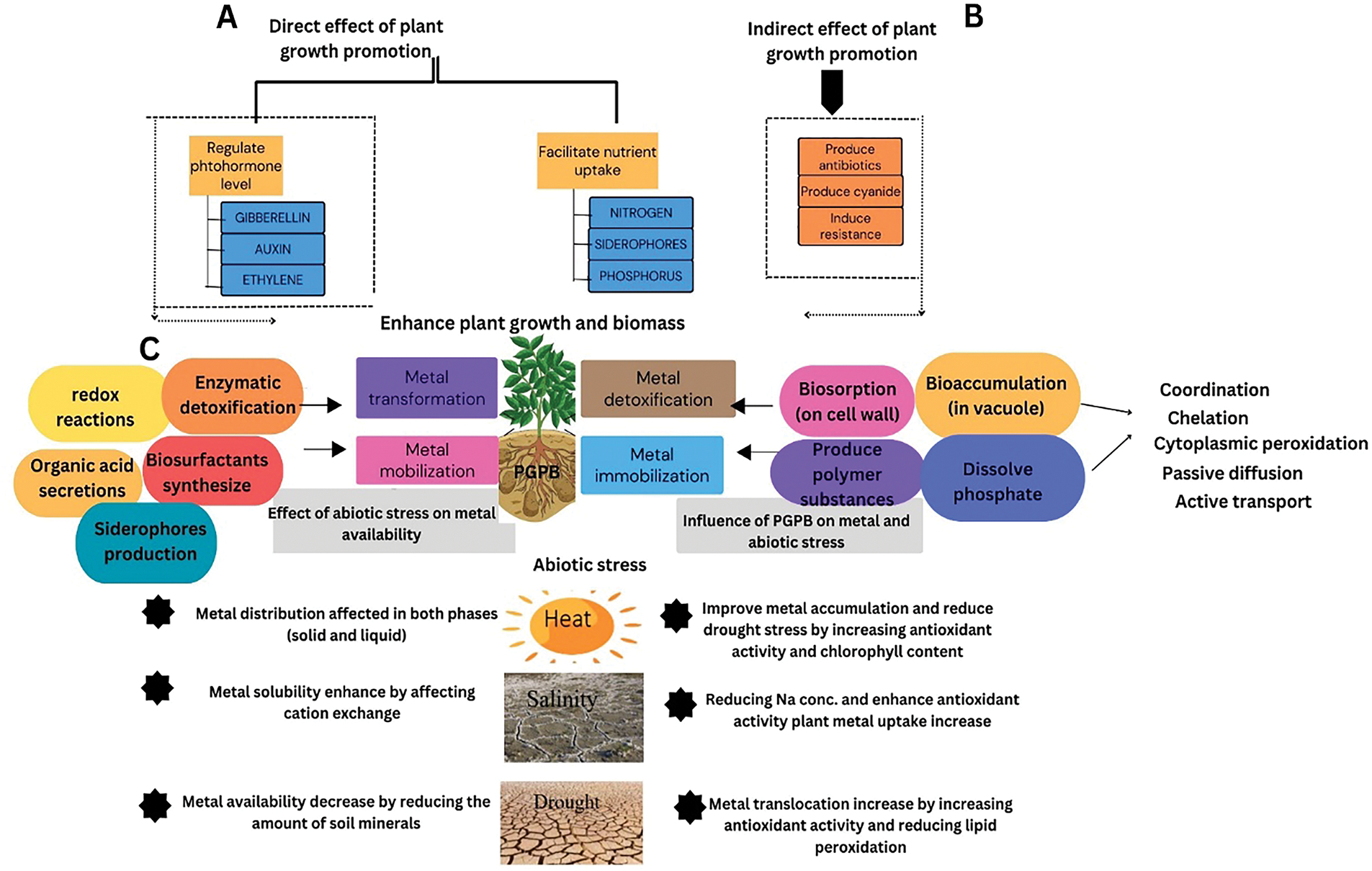
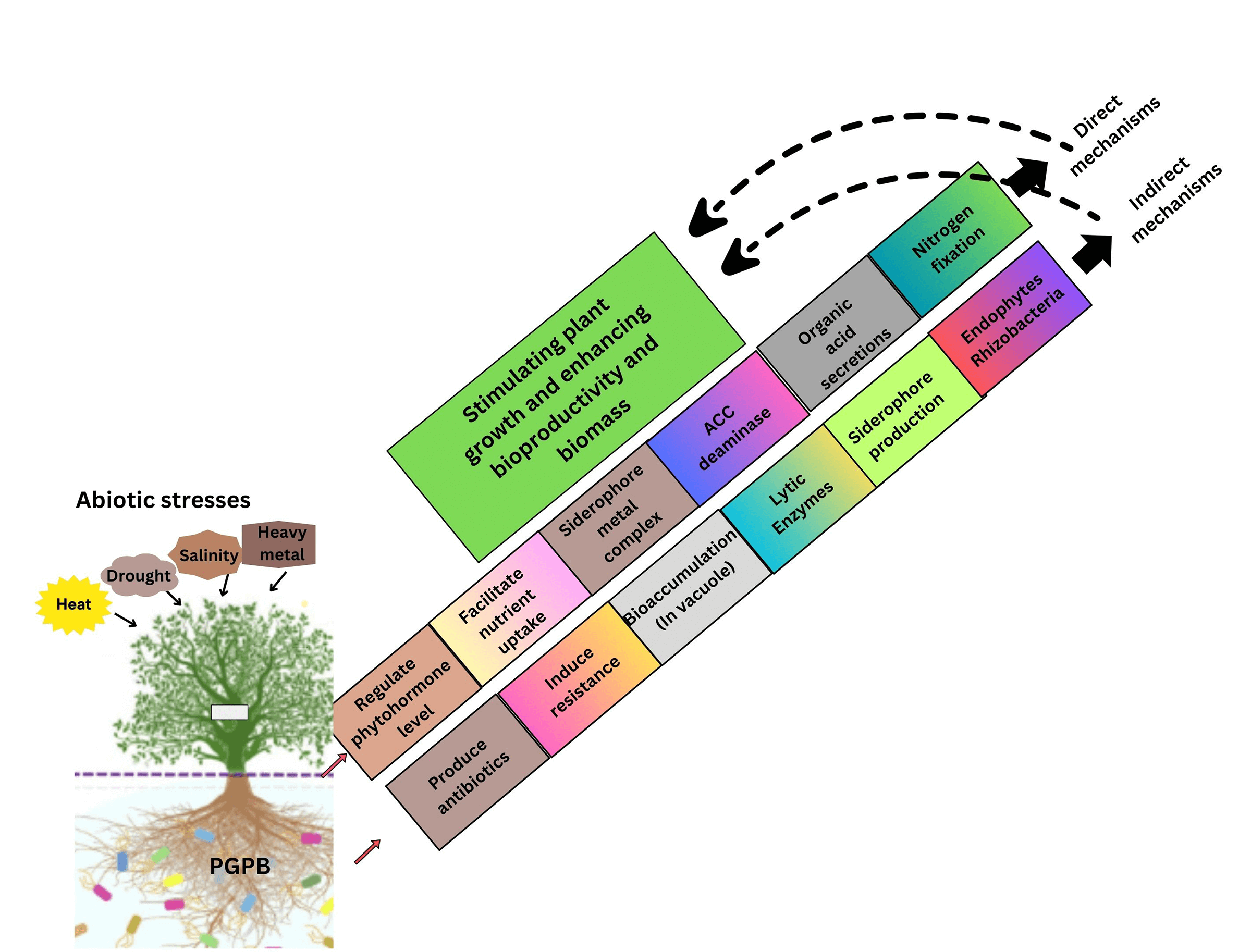
Figure 1: Methods to stimulate plant growth promotion under abiotic stress by plant growth promoting bacteria (PGPB)
2.3 Role of PGPB in Drought-Tolerance of Plants
As a result of climate change, water shortage would impact more areas of the world as climate change is predicted to increase by 1.6°C in semi-arid areas of South Africa and 0.2°C–0.5°C per decade for Asia [43]. Reactive oxygen species (ROS) are accumulated in plants as a result of a signaling cascade activated by drought, and in the absence of detoxifying systems, these ROS can harm proteins, cell membranes, and DNA [44]. In a study, Azosprillum brasilense was used to inoculate Urochloa ruziziensis leaves, which improved the level of CAT and POD in the plant tissues and enhanced the plant’s resistance to water shortage [45]. Exopolysaccharides secreted by PGPB are involved in the manufacture of ACC deaminase, which assists the plant in coping with drought stress. The Methylobacterium oryzae (LMG23582(T)) strain stimulated the growth of shoots (32%) and roots (51%), enhanced photosynthetic activity (4.85 µmolCO2/m2/s), and improved harvest index (4-fold) under drought stress in lentil plants, The stress mitigation effect was due to microbial cytokinins delivered to plants [46].
2.4 Role of PGPB in Inducing HM-Tolerance in Plants
According to research by Liu et al. [47], HM (Heavy metal) pollution affects about 20 Mha of land worldwide. HMs are naturally occurring in the Earth’s shell and are used excessively in industry. Exposure to HMs can have an impact on key enzymes and cellular organelles in plants. PGPB can improve plant biomass while reducing the adverse properties of HM exposure on plants (Fig. 2) [48] either by aiding phyto-extraction or by changing it into a form that isn’t bioavailable [49]. The PGPB may develop tolerance to HMs by developing metal-protein complexes or by using methylation, demethylation, and biotransformation techniques [49], thus reducing the lethal effects of Pb, Ni, Cu As, Cd, Zn by preventing their accumulation in the aerial plants of Alnus firma [50]. Maize plants inoculated by Proteus mirabilis strain T2Cr and CrP450 improved Cr tolerance and decreased Cr toxicity [51]. The metal-resistant PGPR Pseudomonassp and Bacillus enhanced the ability of sunflowers to hyperaccumulate metals and caused a 1.7 to 2.5-fold rise in zinc and cadmium in the shoots [52]. The yellow stripe-like (YSL), copper (COPT/Ctr), P1B-type metallic ATPase (HMA), cation efflux, Zn- and Fe-controlled ZIP transporter protein, ZIF1 carrier or Zn-induced facilitator1 families are examples of Zn-related transporters that regulate Zn absorption, interpreting, intracellular transport, and efflux. The ZNT family of micronutrient transporters includes zinc and iron-tolerant transporters like proteins to increase plants’ resistance to heavy metals. Similarly, by enhancing nutrient uptake and controlling the absorption of Zn at the gene level, Arbuscular mycorrhiza fungi (AMF) improved E. grandis resistance to elevated stress of Zn. AMF resulted in the upregulation of ZNT:4, COPT/Ctr:2, YSL:3, CE:1 genes and the downregulation of ZNT:9, COPT/Ctr:2, YSL:3, ZIFL:4, CE:1 genes under increased Zn soil environment [53].
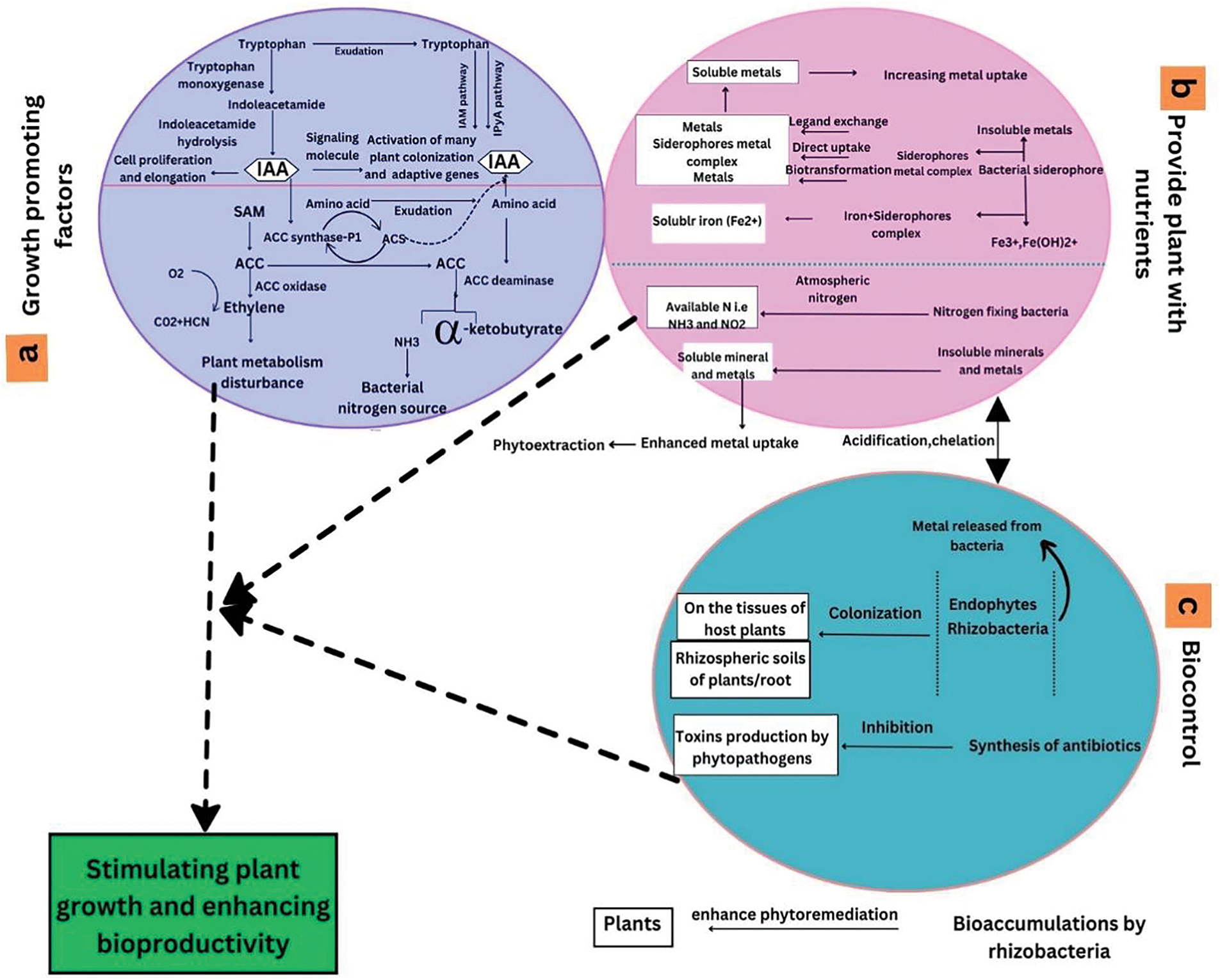
Figure 2: Plant growth-promoting bacteria stimulate phytoremediation of metal-polluted soil by accelerating plant growth-enhancing parameters
2.5 PGPB’s in Metal Phytoremediation
The effectiveness of phytoremediation is affected by extremely hazardous pollutants or other stressed factors. Using microorganisms either exogenously or endogenously can sustain plant growth in these stressful situations and aid in the mobility of pollutants in soil, phytoremediation can be made successful (Fig. 3) [54]. Mello et al. [55] isolated eleven phosphate-solubilizing bacteria from the quinoa rhizosphere, all of which produced plant growth-promoting substances and showed tolerance to various heavy metals and salinity. Of these, Bacillus atrophaeus S8 and Enterobacter asburiae QB1 promoted enhanced seed germination in quinoa and increased seedling growth, with great potential to be used as inoculants for enhanced quinoa growth in salty or heavy metal-contaminated soils.
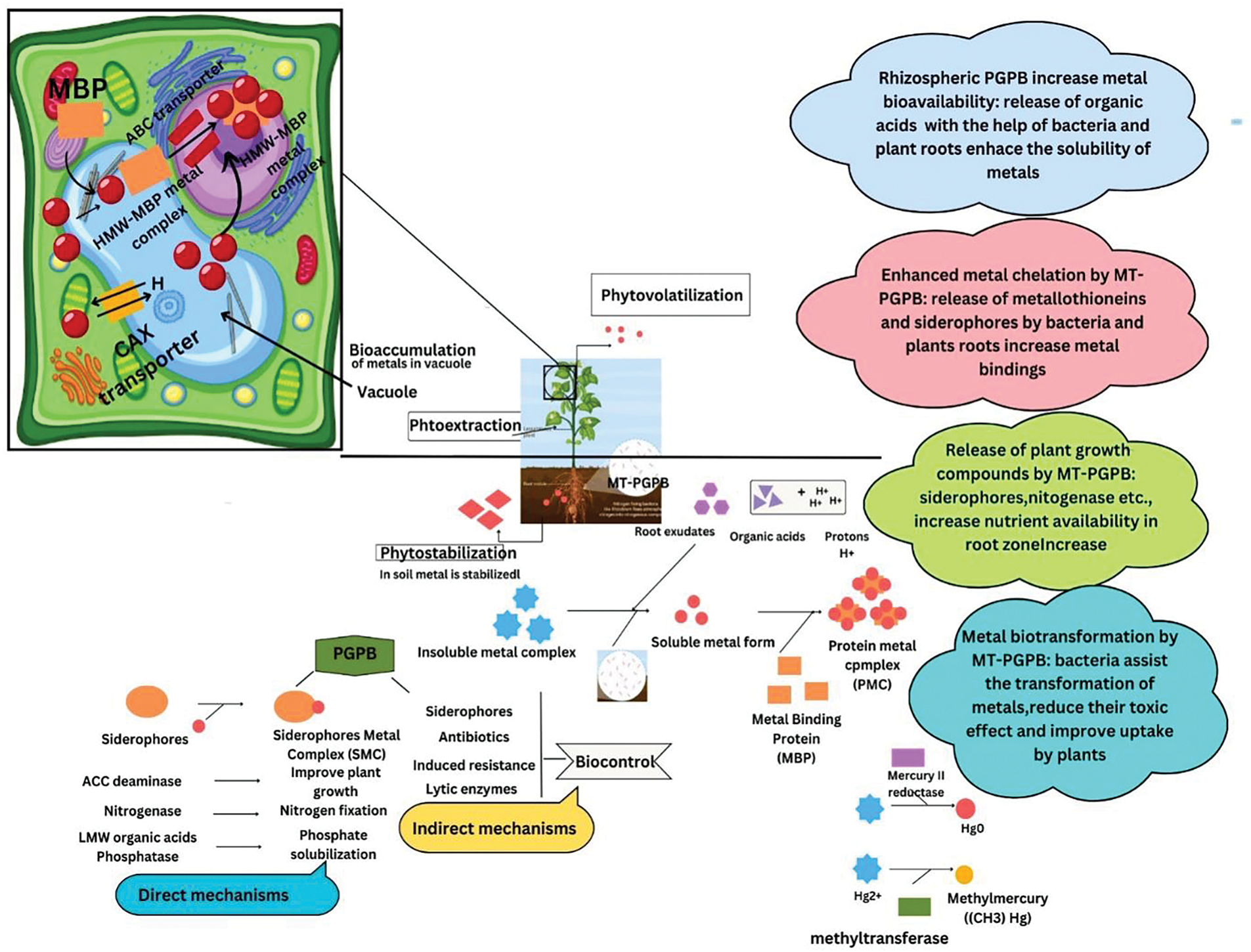
Figure 3: Phytoremediation of metal contaminated soil by plant growth promoting bacteria (PGPB)
In soils that have been affected by HMs, microbes could promote plant development due to the mobility of HMs or the biological transformation into less dangerous chemical complexes of elements. In a study, endophytic Cd-resistant P. fluorescens Sasm05 increased Sedum alfredi’s growth and capacity to accumulate Cd by upregulating the gene expression maintaining Cd absorption and transportation by plants, increasing the production and raising IAA levels. According to Ke et al. [56], PGPR inoculated ryegrass showed improved growth and reduced Cu- and Cd uptake. The lead uptake increased (1mM Pb) in Lathyrus sativus plants inoculated with I5 strains, with enhanced chlorophyll retention, carotenoid levels, and the upsurge of antioxidant activity suggesting an improved tolerance to metal uptake [57]. Klebsiella pneumoniae HR1 biosorb Cd(II) enhanced Vigna mungo plants’ tolerance to Cd(II) stress, through systemic tolerance mechanisms [58]. In a study, Zn-tolerant bacterium Serratia sp. accumulated very high concentrations of Zn and produced large quantities of EPS (extracellular polymeric substances) helping in the sorption of Zn through complexation. Further, secretion of growth hormones, inhibitory compounds, and solubilization of essential nutrients proved it a good bioenhancer for maize crop cultivation [59].
In M. lupulina, the chimeric strain of Sinorhizobium meliloti enhanced development as well as copper tolerance, with an improved antioxidant defense system [60]. It is reported by Akhtar et al. [61] that Bacillus sp. CIK-516 inoculation facilitated radish growth with bacterial-assisted phytoextraction of Ni in contaminated soils. Streptomyces pactum (Act12) improved growth metal uptake in roots and shoots of wheat plants, making it a promising eco-friendly solution for phytoremediation in contaminated soils (Table 1) [62]. Streptomyces rapamycinicus and S. cyaneus promoted sunn hemp growth and Cd accumulation in polluted soils [63]. The Providencia sp. bacteria strain 7MM11, produced high levels of Indole-3-acetic acid (IAA), enhancing growth in tomato plants. Brassica oxyrrhina accumulated Cu and Zn under HMs stress and drought stress due to IAA synthesis of metal-resistant P. libanensis TR1 and P. reactans Ph3R3 [64]. In a study inoculating B. tymphaea–N. tymphaea with isolated Variovorax strains significantly enhanced shoot biomass with enhanced uptake of nickel (Ni) in the plants. Inoculation of endophytic plant growth-promoting bacteria improved the growth and stress tolerance of Noccaea caerulescens and Rumex acetosa in metal-contaminated soils [65] (Fig. 4).
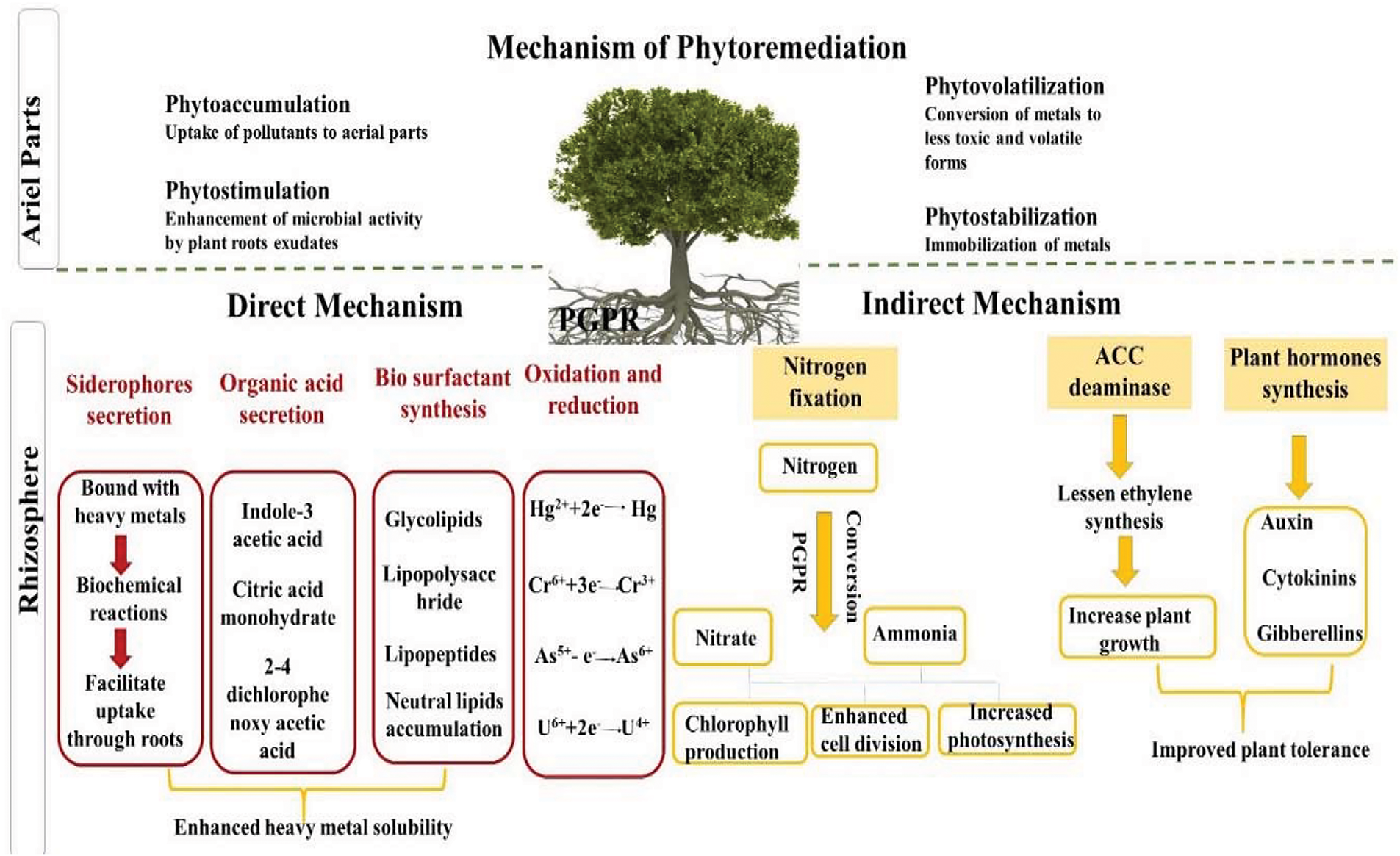
Figure 4: Mechanisms involved in bacterial-assisted phytoremediation
In the case of Pb, the significance of microbes in plant extraction is further reinforced [66], Cd [67], Mn [68] and Cr [69], all provided evidence supporting the involvement of microorganisms in phytoextraction. R. communis and H. annuus showed significant growth promotion as a result of psychrobactersp. SRS8 soil biological enhancement with nickel-resistant bacteria and their use increased the effectiveness of Ni phytoextraction [70]. With reduced Cd and Mn heavy metal uptake, making it a potential inoculum for phytoremediation of contaminated soils [71].
By enhancing plant tissues’ resistance to Ni by PGPB activity, the microbes further boosted Ni bioaccumulation in them [93]. The bacterial strain Sinorhizobium saheli YH1, isolated from V-Ti magnetite tailings, produced IAA, solubilized phosphate, and enhanced plant growth Inoculation of Bacillus sp. QX8 and QX13 enhanced Solanum nigrum development and increased resistance to Pb and Cd stress [94]. The use of bacterial consortium (Streptomyces pactum Act12, Bacillus subtilis, and Bacillus licheniformis) in mono- and co-applications enhanced the phytoextraction of toxic metals (Cd, Cu, Pb, Zn) using Brassica juncea and improved soil enzyme activities, plant growth and antioxidant levels, metal bioavailability and uptake, making it a promising strategy for improving phytoremediation and reducing health risks from contaminated soils. It is reported by Ma et al. [95], Cd-immobilizing PGPB, TZ5, loaded onto biochar for preparation of the biochemical composite material used in bioremediation of Cd-polluted soil. The biochar treatment significantly decreased extractable Cd in soils, increased ryegrass dry weight by 77.78%, decreased Cd concentration in the plant by 48.49%, and increased microbial activity and abundance of the Bacillus genus in the rhizosphere, thereby providing a feasible approach for Cd remediation.
The microbes have the possibility of lowering Cr(IV) and reducing As(III) toxicity of soil contaminated with Cr and As [96]. The role of Providencia sp. (TCR05) and Proteus mirabilis (TCR20) in enhancing Zea mays growth and phytoremediation efficiency under drought and chromium (Cr) stress revealed that the bacteria improved plant growth, photosynthesis, and stress tolerance, reduced lipid peroxidation and proline levels, and immobilized Cr in roots, making them promising bio-inoculants for improving plant growth and phytostabilization in Cr-contaminated and drought-prone soils.
In a study PGPR consortium and M. luteus reduced arsenic concentration in berries and leaves, while P. fluorescens enhanced arsenic tolerance in grapevine [97]. The capacity of plants to transport copper from roots to aerial plant parts has an impact on the HM phytoremediation approach. Plant species with translocation factor values >1 are regarded as strong candidates for phytoextraction, while species with translocation factor <1 are regarded as suitable phyto-stabilizers [98]. When the concentration of HM in the soil is less and its possible release into other ecosystems does not pose a significant problem, phyto-stabilization is typically used. In a study regarding Ricinus communis and Brassica juncea infected with Pseudomonas sp. (A3R3) and Psychrobacter sp. (SRS8) the phytoremediation technique is employed to phytostabilize Zn. In another study, Sedum plumbizincicola rhizoaccumulation of Cd and Zn was enhanced by the E6S strain like Achromobacter piechaudii [99]. Decreased HM transport and/or accessibility in the ground as well as plants, are the main causes of the microbial increasing phyto-stabilization. Some of these processes include adsorption, bioaccumulation, biosorption, biotransformation, HM complexation and (bio) precipitation, and soil alkalinization. Anionic-charged EPS plays an important part in absorption and cationic-charged metals adsorbed by extracellular membrane functional groups. Metals are then transported to microbial cells via passive or active processes known as biosorption and bioaccumulation, where they are subjected to intracellular precipitation, sequestration, and accumulation. Biotransformation of HMs by microbes can also reduce their bioavailability and mobility. The final step in the process of phytoremediation of fields with HM contamination is called phytovolatilization. HMs that can be bio-transformed into less hazardous volatile molecules, e.g., As, Se, and Hg-contaminated fields, and the effectiveness may also be enhanced by the activity of PGPB. The beneficial bacteria for plants are Agrobacterium sp. and Stenotrophomonas maltophilia were helpful in the efficient phytovolatilization of As in a study using the phytoremediation method on Arundo donax L [100]. For plants to decrease As(V) within their cells to As(III) or subsequently reduce the As into its methylated form or volatilization to reduce toxic As in grains. The exact mechanism underlying this process is dependent on the As-speciation in the soil. Increased soil absorption efficiency is necessary to support microorganisms since it leads to greater As phytovolatilization. In the case of Se contamination, a microbial increase of phytovolatilization was also confirmed. Phytovolatilization depends on the conversion of lethal Se (as selenate) into harmless dimethyl selenide gas. Bacteria boosted Se’s high rate of combustion and deposition in plant cells by 35% and 70%, respectively. By switching organomercurials from poisonous and combustible Hg(I) to Hg(II) or from Hg(II) to Hg(0), it is possible to increase the effectiveness of Hg phytovolatilization by microbes as observed by Serratia marcescens BacI56 and Pseudomonas sp. BacI38 inoculation into Zea mays [101].
3 Biotechnological Advancements in Phytoremediation
Recent advancements in genetic engineering and the development of genetically modified microbes (GEMs) have significantly improved bioremediation processes. Genetic modification, especially by CRISPRCas9, has made it easier to manipulate the microbial gene for better heavy metal remediation. Altered metabolic pathways in microbes due to activation of specific genes allow them to focus on specifically targeting environmental pollution. Research studies on genomics, metagenomics, and other ‘omics’ technologies have shed more light on the interaction of microbes with pollutants by identification of different genes and pathways important for remediation [102].
The CRISPR-Cas9 gene-editing tool is used to introduce or modify genes within microbes, increasing their ability to degrade metals, such as Cd, Cu, Hg, Ni, and Fe. It has proven effective in engineering microbes to resist metal toxicity and increase the rate of bioremediation. Other gene-editing tools, for instance, TALENs, and ZFNs have also been utilized to design microbes to degrade HMs. TALENs, which stands for Transcription Activator-Like Effector Nucleases, work by using a DNA-binding module that targets specific sequences in the host genome, creating double-stranded breaks with “sticky ends.” This process helps ensure genetic modifications are stable. Another similar tool is zinc finger nucleases (ZFNs), which use a 30-amino acid DNA-binding domain to create DSBs at specific locations in the genome. Hybrid nucleases, such as TALENs and ZFNs, have emerged through improvement studies over the years that scientists have encountered in genetic engineering. These innovations eventually set the stage for CRISPR-Cas technology, a stronger system capable of making multiple gene edits simultaneously with high accuracy. The CRISPR-Cas system operates by using guide RNA, which consists of two types of RNA: crisper-derived RNA (crRNA) and trans-acting antisense RNA (trcRNA). This creates a defense mechanism where the gRNA (guide RNA) guides the Cas9 enzyme to specific DNA sequences, where it creates DSBs by recognizing the matching sequence.
CRISPR has allowed multiple genes in microbes to be targeted at a single time thereby efficiently performing remediation processes using microbes. Genome sequencing, metabolomics, and computational biology have been applied to the identification of potentially useful microbes that could be developed for bioremediation while enhancing their tolerance to pollutants. CRISPR-Cas9 has been successfully used in E. coli and Pseudomonas for targeted remediation of HMs. In parallel, nanomaterials are also being studied for their potential in the enhancement of microbial bioremediation. Nanoparticles, because of their high surface area, reactivity, and ability to interact with heavy metals, reduce and degrade pollutants. Nanomaterials like metal oxide nanoparticles, carbon-based nanomaterials, and nanocomposites enhance electron transfer, which supports microbial activity in breaking down toxic metals. Moreover, Nano biosensors are being developed to monitor the progress of the remediation process. Nanomaterials can also serve as substitutes for conventional biosorbents, with various functional groups improving their efficiency in capturing heavy metals. When combined with bacteria, these nanoparticles enhance the bioremediation process, making it more effective than using microbes alone. The interaction between nanoparticles and microbes depends on several factors, including the properties of the nanoparticles, such as size, shape, and surface coating. The CRISPR-Cas9 gene-editing technology and microbial-aided nanomaterials present a highly effective method to enhance microbial-assisted phytoremediation of contaminated soils, potentially resulting in faster detoxification of heavy metals [103].
Plants used in bioremediation may effectively utilize contaminated biomass to create a variety of value-added goods like pigments, chemicals, etc. Burning plant debris can recover metals that can be utilized as starting points for production processes. There are many untapped potential topics for research in the future. The first step is determining how different pollutants interact with one another and how hazardous they are to soil microbial communities when those populations include specific plant species like Miscanthus sp. It’s also important to look into genetically modified microbes that support phytoremediation, mineral dynamics, carbon from plants, and biodiversity. Due to the extreme sensitivity to a certain metal, several traditional plants could not be used in contaminated soils. However, addressing how climate change affects the dynamics of different chemicals in crops and the discharge of microbial metabolites is urgently needed.
Combining microbes with approaches, such as plants, nanoparticles, or soil additives, further enhances remediation outcomes. Furthermore, pairing microbes with organic or carbon-based materials should also be explored. The behavior of microbes in heavy metal-contaminated environments is essential to understand, and further research is needed to understand the physiochemical, biological, and molecular characteristics that allow microbes to thrive in these conditions. Key research gaps that need to be filled include the understanding of microbial diversity, mechanisms of resistance to metals, and the nature of microbe-soil interaction. The study needs to explore, potential mixed microbial communities, scale-up from laboratory-scale to field application, and the integration with other techniques as well. Addressing the gaps involving safety aspects of the environment, and reduced costs of the technology in targeting heavy metals by phytoremediation will enhance the efficiency and sustainability for large-scale environmental cleanup.
Both plants and bacteria aid in the phytoremediation of harmful chemicals. Additionally, bacteria can shield plants from environmental stresses such as HM toxicity, water stress, salt stress, etc., and stimulate growth in plants through a number of PGP processes like hormone and siderophores production, mineral solubilization, nitrogen fixation, and various other mechanisms. By employing microorganisms that are crucial to the phytoremediation of soils, phytoremediation continues to be seen as a relatively easy approach for lowering or bio-transforming contaminants and indirectly increasing plant development. The range of currently employed phytoremediation techniques can be increased by studying bacteria that have changed to be tolerant of high chemicals and their environmental associations, in particular. By employing PGPB and metal-solubilizing bacteria to inoculate the soil, hyperaccumulators’ health, biomass, yield, and ability to accumulate metal can all be enhanced. The efficiency of biological microbe-assisted phytoremediation conditions like drought and salinity due to climate change requires more investigation. Additionally, more study is required to comprehend the interactions between microorganisms and plants in an ecosystem that has been contaminated by metal.
Acknowledgement: The authors are very grateful to the kind administration of Yibin University, Yibin, China, and Government College University, Faisalabad, Pakistan for providing us with such a prestigious and well-equipped platform for research and development. The authors are also grateful to the kind administration of the Department of Science and Technology of Sichuan Province, China.
Funding Statement: This work was supported by the Yibin Science and Technology Plan (2022NY011).
Author Contributions: Conceptualization, Chengyi Zou and Sara Zafar; methodology, Chengyi Zou, Zuhair Hasnain, and Sara Zafar; formal analysis, Chengyi Zou, Manzar Abbas, Umbreen Bibi, and Sara Zafar; investigation, Chengyi Zou, Sara Zafar, Manzar Abbas, Umbreen Bibi, and Zuhair Hasnain; data curation, Chengyi Zou, Sara Zafar, Umbreen Bibi, Zuhair Hasnain, and Manzar Abbas; writing—original draft preparation, Chengyi Zou and Sara Zafar; writing—review and editing, Manzar Abbas and Sara Zafar; visualization, Sara Zafar; supervision, Zuhair Hasnain. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The study included in the article can be inquired further from the corresponding authors.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| PGPB | Plant growth promoting bacteria |
| CRISPR-Cas9 | Clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 |
| HM | Heavy metal |
| SOS1 | Salt Overly Sensitive 1 |
| EPS | Exopolysaccharides |
| Pb | Lead |
| Cd | Cadmium |
| Cu | Copper |
| ROS | Reactive oxygen species |
| Ni | Nickel |
| As | Arsenic |
| Zn | Zinc |
| Cr | Chromium |
| YSL | Yellow stripe-like |
| AMF | Arbuscular mycorrhiza fungi |
| GEMs | Genetically modified microbes |
| ZFN | Zinc finger nucleases |
| TALENS | Transcription Activator-Like Effector Nucleases |
| crRNA | Crisper-derived RNA |
| gRNA | Guide RNA |
| DSBs | Double-stranded breaks |
References
1. Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals—concepts and applications. Chemosphere. 2013;91(7):869–81. doi:10.1016/j.chemosphere.2013.01.075. [Google Scholar] [PubMed] [CrossRef]
2. Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9):1–11. doi:10.1016/j.heliyon.2020.e04691. [Google Scholar] [PubMed] [CrossRef]
3. Raffa CM, Chiampo F, Shanthakumar S. Remediation of metal/metalloid-polluted soils: a short review. Appl Sci. 2021;11(9):4134. doi:10.3390/app11094134. [Google Scholar] [CrossRef]
4. Greipsson S. Phytoremediation. Nat Educat Know. 2011;2(10):2. [Google Scholar]
5. Bernardino CA, Mahler CF, Alvarenga P, Castro PM, da Silva EF, Novo LA. Recent advances in phytoremediation of soil contaminated by industrial waste: a road map to a safer environment. Bioremed Indus Waste Environ Safety: Indus Waste Manag. 2020;1:207–21. doi:10.1007/978-981-13-1891-7. [Google Scholar] [CrossRef]
6. Beans C. Phytoremediation advances in the lab but lags in the field. Proc Nat Acad Sci. 2017;114(29):7475–7. doi:10.1073/pnas.1707883114. [Google Scholar] [PubMed] [CrossRef]
7. Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN. Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol Environ Saf. 2019;174(13):714–27. doi:10.1016/j.ecoenv.2019.02.068. [Google Scholar] [PubMed] [CrossRef]
8. Nkrumah PN, Baker AJ, Chaney RL, Erskine PD, Echevarria G, Morel JL, et al. Current status and challenges in developing nickel phytomining: an agronomic perspective. Plant Soil Till Res. 2016;406(1–2):55–69. doi:10.1007/s11104-016-2859-4. [Google Scholar] [CrossRef]
9. Harris J. Soil microbial communities and restoration ecology: facilitators or followers? Science. 2009;325(5940):573–4. doi:10.1126/science.1172975. [Google Scholar] [PubMed] [CrossRef]
10. Barra Caracciolo A, Terenzi V. Rhizosphere microbial communities and heavy metals. Microorganisms. 2021;9(7):1462. doi:10.3390/microorganisms9071462. [Google Scholar] [PubMed] [CrossRef]
11. Thakare M, Sarma H, Datar S, Roy A, Pawar P, Gupta K, et al. Understanding the holistic approach to plant-microbe remediation technologies for removing heavy metals and radionuclides from soil. Curr Res Biotechnol. 2021;3(6):84–98. doi:10.1016/j.crbiot.2021.02.004. [Google Scholar] [CrossRef]
12. Ahemad M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab J Chem. 2019;12(7):1365–77. doi:10.1016/j.arabjc.2014.11.020. [Google Scholar] [CrossRef]
13. Schröder R, Glandorf S, Kiehl K. Temporal revegetation of a demolition site—a contribution to urban restoration? J Urban Ecol. 2018;4(1):10. doi:10.1093/jue/juy010. [Google Scholar] [CrossRef]
14. Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, et al. Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere. 2017;171:710–21. doi:10.1016/j.chemosphere.2016.12.116. [Google Scholar] [PubMed] [CrossRef]
15. Yadav S, Modi P, Dave A, Vijapura A, Patel D, Patel M. Effect of abiotic stress on crops. In: Sustainable crop production. Rijeka: IntechOpen; 2020. doi:10.5772/intechopen.88434. [Google Scholar] [CrossRef]
16. Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z. Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci. 2020;11:359. doi:10.3389/fpls.2020.00359. [Google Scholar] [PubMed] [CrossRef]
17. Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1473. doi:10.3389/fpls.2018.01473. [Google Scholar] [PubMed] [CrossRef]
18. Shah V, Daverey A. Phytoremediation: a multidisciplinary approach to clean up heavy metal contaminated soil. Environ Technol Innov. 2020;18(5):100774. doi:10.1016/j.eti.2020.100774. [Google Scholar] [CrossRef]
19. Mohan I, Goria K, Dhar S, Kothari R, Bhau BS, Pathania D. Phytoremediation of heavy metals from the biosphere perspective and solutions. Pollut Water Manag: Res, Strat Scar. 2021:95–127. doi:10.1002/9781119693635. [Google Scholar] [CrossRef]
20. Visconti D, Ventorino V, Fagnano M, Woo SL, Pepe O, Adamo P, et al. Compost and microbial biostimulant applications improve plant growth and soil biological fertility of a grass-based phytostabilization system. Environ Geochem Hlth. 2023;45(3):787–807. doi:10.1007/s10653-022-01235-7. [Google Scholar] [PubMed] [CrossRef]
21. Kumar A, Verma JP. Does plant—microbe interaction confer stress tolerance in plants: a review? Microbiol Res. 2018;207(2):41–52. doi:10.1016/j.micres.2017.11.004. [Google Scholar] [PubMed] [CrossRef]
22. Das PP, Singh KR, Nagpure G, Mansoori A, Singh RP, Ghazi IA, et al. Plant-soil-microbes: a tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ Res. 2022;214(6):113821. doi:10.1016/j.envres.2022.113821. [Google Scholar] [PubMed] [CrossRef]
23. Ramakrishna W, Rathore P, Kumari R, Yadav R. Brown gold of marginal soil: plant growth promoting bacteria to overcome plant abiotic stress for agriculture, biofuels and carbon sequestration. Sci Total Environ. 2020;711:135062. doi:10.1016/j.scitotenv.2019.135062. [Google Scholar] [PubMed] [CrossRef]
24. Anand U, Pal T, Yadav N, Singh VK, Tripathi V, Choudhary KK, et al. Current scenario and future prospects of endophytic microbes: promising candidates for abiotic and biotic stress management for aricultural and environmental sustainability. Microb Ecol. 2023;86(3):1455–86. doi:10.1007/s00248-023-02190-1. [Google Scholar] [PubMed] [CrossRef]
25. Zafar S, Hasnain Z, Aslam N, Mumtaz S, Jaafar HZ, Wahab PE, et al. Impact of Zn nanoparticles synthesized via green and chemical approach on okra (Abelmoschus esculentus L.) growth under salt stress. Sustainability. 2021;13(7):3694. doi:10.3390/su13073694. [Google Scholar] [CrossRef]
26. Ku YS, Sintaha M, Cheung MY, Lam HM. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci. 2018;19(10):3206. doi:10.3390/ijms19103206. [Google Scholar] [PubMed] [CrossRef]
27. Yoo SJ, Weon HY, Song J, Sang MK. Induced tolerance to salinity stress by halotolerant bacteria Bacillus aryabhattai H19-1 and B. mesonae H20-5 in tomato plants. J Microbiol Biotechnol. 2019;829(7):1124–36. doi:10.4014/jmb.1904.04026. [Google Scholar] [PubMed] [CrossRef]
28. Gupta A, Mishra R, Rai S, Bano A, Pathak N, Fujita M, et al. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int J Mol Sci. 2022;23(7):3741. doi:10.3390/ijms23073741. [Google Scholar] [PubMed] [CrossRef]
29. Girolkar S, Thawale P, Juwarkar A. Bacteria-assisted phytoremediation of heavy metals and organic pollutants: challenges and future prospects. Bioremed Environ Sustain. 2021;117(1):247–67. doi:10.1016/B978-0-12-820318-7.00012-5. [Google Scholar] [CrossRef]
30. Msimbira LA, Smith DL. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front Sustain Food Syst. 2020;4:106. doi:10.3389/fsufs.2020.00106. [Google Scholar] [CrossRef]
31. Kumawat KC, Nagpal S, Sharma P. Potential of plant growth-promoting rhizobacteria-plant interactions in mitigating salt stress for sustainable agriculture: a review. Pedosphere. 2022;32(2):223–45. doi:10.1016/S1002-0160(21)60070-X. [Google Scholar] [CrossRef]
32. Ilangumaran G, Smith DL. Plant growth promoting rhizobacteria in amelioration of salinity stress: a systems biology perspective. Front Plant Sci. 2017;8:1768. doi:10.3389/fpls.2017.01768. [Google Scholar] [PubMed] [CrossRef]
33. Bitla UM, Shinde AL, Sorty AM, Meena KK, Singh NP. Microbial mitigation of abiotic stress responses in legumes. In: Microbial mitigation of stress response of food legumes. 1st ed. Boca Raton: CRC Press (Taylor & Francis Group); 2020. [Google Scholar]
34. Chang P, Gerhardt KE, Huang XD, Yu XM, Glick BR, Gerwing PD, et al. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: implications for phytoremediation of saline soils. Int J Phytoremediation. 2014;16(1):1133–47. doi:10.1080/15226514.2013.821447. [Google Scholar] [PubMed] [CrossRef]
35. Vives-Peris V, De Ollas C, Gómez-Cadenas A, Pérez-Clemente RM. Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep. 2020;39(1):3–17. doi:10.1007/s00299-019-02447-5. [Google Scholar] [PubMed] [CrossRef]
36. Schwitzguébel JP. Phytoremediation of soils contaminated by organic compounds: hype, hope and facts. J Soils Sediments. 2017;17(5):1492–502. doi:10.1007/s11368-015-1253-9. [Google Scholar] [CrossRef]
37. Ławniczak Ł, Woźniak-Karczewska M, Loibner AP, Heipieper HJ, Chrzanowski Ł. Microbial degradation of hydrocarbons—basic principles for bioremediation: a review. Molecules. 2020;25(4):856. doi:10.3390/molecules25040856. [Google Scholar] [PubMed] [CrossRef]
38. Ruley JA, Tumuhairwe JB, Amoding A, Westengen OT, Vinje H. Rhizobacteria communities of phytoremediation plant species in petroleum hydrocarbon contaminated soil of the Sudd ecosystem, South Sudan. Int J Microbiol. 2020;2020(2):639118–13. doi:10.1155/2020/6639118. [Google Scholar] [PubMed] [CrossRef]
39. Moubasher HA, Hegazy AK, Mohamed NH, Moustafa YM, Kabiel HF, Hamad AA. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int Biodeter Biodegr. 2015;98:113–20. doi:10.1016/j.ibiod.2014.11.019. [Google Scholar] [CrossRef]
40. Hussain F, Hussain I, Khan AH, Muhammad YS, Iqbal M, Soja G, et al. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ Expe Bot. 2018;153:80–8. doi:10.1016/j.envexpbot.2018.05.012. [Google Scholar] [CrossRef]
41. Liang Y, Meggo R, Hu D, Schnoor JL, Mattes TE. Enhanced polychlorinated biphenyl removal in a switchgrass rhizosphere by bioaugmentation with Burkholderia xenovorans LB400. Ecol Eng. 2014;71:215–22. doi:10.1016/j.ecoleng.2014.07.046. [Google Scholar] [PubMed] [CrossRef]
42. Rai GK, Bhat BA, Mushtaq M, Tariq L, Rai PK, Basu U, et al. Insights into decontamination of soils by phytoremediation: a detailed account on heavy metal toxicity and mitigation strategies. Physiol Plant. 2021;173(1):287–304. doi:10.1111/ppl.13433. [Google Scholar] [PubMed] [CrossRef]
43. Berger J, Palta J, Vadez V. An integrated framework for crop adaptation to dry environments: responses to transient and terminal drought. Plant Sci. 2016;253:58–67. doi:10.1016/j.plantsci.2016.09.007. [Google Scholar] [PubMed] [CrossRef]
44. Zafar S, Ashraf A, Ijaz MU, Muzammil S, Siddique MH, Afzal S, et al. Eco-friendly synthesis of antibacterial zinc nanoparticles using Sesamum indicum L. extract. J King Saud Univ-Sci. 2020;32(1):1116–22. doi:10.1016/j.jksus.2019.10.017. [Google Scholar] [CrossRef]
45. Bulegon LG, Guimarães VF, Laureth JCU. Azospirillum brasilense affects the antioxidant activity and leaf pigment content of Urochloa ruziziensis under water stress. Pesqui Agropecu Trop. 2016;46:343–9. doi:10.1590/1983-40632016v4641489. [Google Scholar] [CrossRef]
46. Jorge GL, Kisiala A, Morrison E, Aoki M, Nogueira AP. Emery RN.Endosymbiotic Methylobacterium oryzae mitigates the impact of limited water availability in lentil (Lens culinaris Medik.) by increasing plant cytokinin levels. Environ Experi Bot. 2019;162:525–40. doi:10.1016/j.envexpbot.2019.03.028. [Google Scholar] [CrossRef]
47. Liu L, Li W, Song W, Guo M. Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ. 2018;633:206–19. doi:10.1016/j.scitotenv.2018.03.161. [Google Scholar] [PubMed] [CrossRef]
48. Wang Q, Zhou Q, Huang L, Fu Y, Hou D, Feng Y, et al. Cadmium phytoextraction through Brassica juncea L. under different consortia of plant growth-promoting bacteria from different ecological niches. Ecotoxicol Environ Saf. 2022;237:113541. doi:10.1016/j.ecoenv.2022.113541. [Google Scholar] [PubMed] [CrossRef]
49. Tak HI, Ahmad F, Babalola OO. Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev Environ Cont Toxicol. 2012;223:33–52. doi:10.1007/978-1-4614-5577-6_2. [Google Scholar] [PubMed] [CrossRef]
50. Janeeshma E, Puthur JT. Potential role of microbial endophytes in xenobiotic stress management. Sustain Environ Clean-up. 2021;62:165–85. doi:10.1016/B978-0-12-823828-8.00008-6. [Google Scholar] [CrossRef]
51. Egamberdieva D, Wirth SJ, Alqarawi AA, Abd_Allah EF, Hashem A. Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol. 2017;8:2104. doi:10.3389/fmicb.2017.02104. [Google Scholar] [PubMed] [CrossRef]
52. Poria V, Dębiec-Andrzejewska K, Fiodor A, Lyzohub M, Ajijah N, Singh S, et al. Plant Growth-Promoting Bacteria (PGPB) integrated phytotechnology: a sustainable approach for remediation of marginal lands. Front Plant Sci. 2022;13:999866. doi:10.3389/fpls.2022.999866. [Google Scholar] [PubMed] [CrossRef]
53. Wang X, Liang J, Liu Z, Kuang Y, Han L, Chen H, et al. Transcriptional regulation of metal metabolism-and nutrient absorption-related genes in Eucalyptus grandis by arbuscular mycorrhizal fungi at different zinc concentrations. BMC Plant Biol. 2022;22(1):1–20. doi:10.1186/s12870-022-03456-5. [Google Scholar] [PubMed] [CrossRef]
54. Thijs S, Langill T, Vangronsveld J. The bacterial and fungal microbiota of hyperaccumulator plants: small organisms, large influence. Vol. 83, In: Advances botanical research. Elsevier; 2017. p. 43–86. [Google Scholar]
55. Mello IS, Targanski S, Pietro-Souza W, Stachack FF, Terezo AJ, Soares MA. Endophytic bacteria stimulate mercury phytoremediation by modulating its bioaccumulation and volatilization. Ecotoxicol Environ Saf. 2020;202:110818. doi:10.1016/j.ecoenv.2020.110818. [Google Scholar] [PubMed] [CrossRef]
56. Ke T, Guo G, Liu J, Zhang C, Tao Y, Wang P, et al. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ Pollut. 2021;271:116314. doi:10.1016/j.envpol.2020.116314. [Google Scholar] [PubMed] [CrossRef]
57. Bhanse P, Kumar M, Singh L, Awasthi MK, Qureshi A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: opportunities, challenges, and prospects. Chemosphere. 2022;303:134954. doi:10.1016/j.chemosphere.2022.134954. [Google Scholar] [PubMed] [CrossRef]
58. Dutta S, Lanvin B, Wunsch-Vincent Seditors. The global innovation index 2018: energizing the world with innovation. Geneva: WIPO; 2018. [Google Scholar]
59. Rizvi A, Ahmed B, Khan MS, Rajput VD, Umar S, Minkina T, et al. Maize associated bacterial microbiome linked mitigation of heavy metal stress: a multidimensional detoxification approach. Environ Exp Bot. 2022;200:104911. doi:10.1016/j.envexpbot.2022.104911. [Google Scholar] [CrossRef]
60. Kong Z, Mohamad OA, Deng Z, Liu X, Glick BR, Wei G. Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ Sci Pollut Res. 2015;22:12479–89. doi:10.1007/s11356-015-4530-7. [Google Scholar] [PubMed] [CrossRef]
61. Akhtar MS, Hameed A, Aslam S, Ullah R, Kashif A. Phytoremediation of metal-contaminated soils and water in Pakistan: a review. Water Air Soil Pollut. 2023;234(1):11. doi:10.1007/s11270-022-06023-8. [Google Scholar] [CrossRef]
62. Ali I, Khan MJ, Shah A, Deeba F, Hussain H, Yazdan F, et al. Screening of various Brassica species for phytoremediation of heavy metals-contaminated soil of Lakki Marwat, Pakistan. Environ Sci Pollut Res. 2022;29(25):37765–76. doi:10.1007/s11356-021-18109-7. [Google Scholar] [PubMed] [CrossRef]
63. Thooppeng P, Junpradit C, Rongsayamanont W, Duangmal K, Prapagdee B. Cadmium-resistant Streptomyces stimulates phytoextraction potential of Crotalaria juncea L. in cadmium-polluted soil. Int J Phytoremediation. 2023;25(10):1318–27. doi:10.1080/15226514.2022.2152424. [Google Scholar] [PubMed] [CrossRef]
64. Abdelkrim S, Jebara SH, Saadani O, Jebara M. Potential of efficient and resistant plant growth-promoting rhizobacteria in lead uptake and plant defence stimulation in Lathyrus sativus under lead stress. Plant Biol. 2018;20(5):857–69. doi:10.1111/plb.12863. [Google Scholar] [PubMed] [CrossRef]
65. Deng S, Zhang X, Zhu Y, Zhuo R. Recent advances in phyto-combined remediation of heavy metal pollution in soil. Biotechnol Adv. 2024;7:108337. doi:10.1016/j.biotechadv.2024.108337. [Google Scholar] [PubMed] [CrossRef]
66. Durand A, Piutti S, Rue M, Morel JL, Echevarria G, Benizri E. Improving nickel phytoextraction by co-cropping hyperaccumulator plants inoculated by plant growth promoting rhizobacteria. Plant Soil. 2016;399(1):179–92. doi:10.1007/s11104-015-2691-2. [Google Scholar] [CrossRef]
67. Alves AR, Yin Q, Oliveira RS, Silva EF, Novo LA. Plant growth-promoting bacteria in phytoremediation of metal-polluted soils: current knowledge and future directions. Sci Total Environ. 2022;838:156435. doi:10.1016/j.scitotenv.2022.156435. [Google Scholar] [PubMed] [CrossRef]
68. Rushabh S, Kajal C, Prittesh P, Amaresan N, Krishnamurthy R. Isolation, characterization, and optimization of indole acetic acid-producing Providencia species (7MM11) and their effect on tomato (Lycopersicon esculentum) seedlings. Biocatal Agric Biotechno. 2020;28:101732. doi:10.1016/j.bcab.2020.101732. [Google Scholar] [CrossRef]
69. Akhtar MJ, Ullah S, Ahmad I, Rauf A, Nadeem SM, Khan MY, et al. Nickel phytoextraction through bacterial inoculation in Raphanus sativus. Chemosphere. 2018;190:234–42. doi:10.1016/j.chemosphere.2017.09.136. [Google Scholar] [PubMed] [CrossRef]
70. Ali A, Guo D, Li Y, Shaheen SM, Wahid F, Antoniadis V, et al. Streptomyces pactum addition to contaminated mining soils improved soil quality and enhanced metals phytoextraction by wheat in a green remediation trial. Chemosphere. 2021;273:129692. doi:10.1016/j.chemosphere.2021.129692. [Google Scholar] [PubMed] [CrossRef]
71. Oleńska E, Małek W, Wójcik M, Swiecicka I, Thijs S, Vangronsveld J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ. 2020;743(5):140682. doi:10.1016/j.scitotenv.2020.140682. [Google Scholar] [PubMed] [CrossRef]
72. Burges A, Epelde L, Blanco F, Becerril JM, Garbisu C. Ecosystem services and plant physiological status during endophyte-assisted phytoremediation of metal contaminated soil. Sci Total Environ. 2017;584:329–38. doi:10.1016/j.scitotenv.2016.12.146. [Google Scholar] [PubMed] [CrossRef]
73. Dutta P, Karmakar A, Majumdar S, Roy S. Klebsiella pneumoniae (HR1) assisted alleviation of Cd(II) toxicity in Vigna mungo: a case study of biosorption of heavy metal by an endophytic bacterium coupled with plant growth promotion. Euro-Mediterr J Environ Integr. 2018;3(1):1–10. doi:10.1007/s41207-018-0069-6. [Google Scholar] [CrossRef]
74. Rawat N, Singla‐Pareek SL, Pareek A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol Plant. 2021;171(4):653–76. doi:10.1111/ppl.13217. [Google Scholar] [PubMed] [CrossRef]
75. Aloo BN, Tripathi V, Makumba BA, Mbega ER. Plant growth promoting rhizobacterial biofertilizers for crop production. The past, present, and future. Front Plant Sci. 2022;13:1002448. doi:10.3389/fpls.2022.1002448. [Google Scholar] [PubMed] [CrossRef]
76. Jeyasundar PG, Ali A, Azeem M, Li Y, Guo D, Sikdar A, et al. Green remediation of toxic metals contaminated mining soil using bacterial consortium and Brassica juncea. Environ Pollut. 2021;277:116789. doi:10.1016/j.envpol.2021.116789. [Google Scholar] [CrossRef]
77. Ma H, Wei M, Wang Z, Hou S, Li X, Xu H. Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J Hazard Mat. 2020;388:122065. doi:10.1016/j.jhazmat.2020.122065. [Google Scholar] [PubMed] [CrossRef]
78. Mahdi I, Fahsi N, Hafidi M, Benjelloun S, Allaoui A, Biskri L. Rhizospheric phosphate solubilizing Bacillus atrophaeus GQJK17 S8 increases quinoa seedling, withstands heavy metals, and mitigates salt stress. Sustainability. 2021;13(6):3307. doi:10.3390/su13063307. [Google Scholar] [CrossRef]
79. Vishnupradeep R, Bruno LB, Taj Z, Karthik C, Challabathula D, Kumar A, et al. Plant growth promoting bacteria improve growth and phytostabilization potential of Zea mays under chromium and drought stress by altering photosynthetic and antioxidant responses. Environ Technol Innov. 2022;25:102154. doi:10.1016/j.eti.2021.102154. [Google Scholar] [CrossRef]
80. Seth K, Kumar A. Role of soil microflora in phytoremediation of heavy metal contaminated soils. In: Prasad R, editor. Phytoremediation for environmental sustainability. Singapore: Springer; 2022. doi:10.1007/978-981-16-5621-7_2. [Google Scholar] [CrossRef]
81. Burges A, Alkorta I, Epelde L, Garbisu C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int J Phytoremediat. 2018;20(4):384–97. doi:10.1080/15226514.2017.1365340. [Google Scholar] [PubMed] [CrossRef]
82. Lu M, Zhang ZZ, Wang JX, Zhang M, Xu YX, Wu XJ. Interaction of heavy metals and pyrene on their fates in soil and tall fescue (Festuca arundinacea). Environ Sci Technol. 2014;48(2):1158–65. doi:10.1021/es403337t. [Google Scholar] [PubMed] [CrossRef]
83. Zhu Y, Wang Y, Zheng H, Xiang X, Wang H, Xie M, et al. N fertilizers promote abscisic acid-catabolizing bacteria to enhance heavy metal phytoremediation from metalliferous soils. Sci Total Environ. 2023;894(1):164964. doi:10.1016/j.scitotenv.2023.164964. [Google Scholar] [PubMed] [CrossRef]
84. Aransiola SA, Ijah UJ, Peter AO, Bala JD. Microbial-aided phytoremediation of heavy metals contaminated soil. Eur J Biol Res. 2019;9(2):104–25. doi:10.5281/zenodo.3244176. [Google Scholar] [CrossRef]
85. Zhang M, Wang X, Yang L, Chu Y. Research on progress in combined remediation technologies of heavy metal polluted sediment. Int J Environ Res Public Health. 2019;16(24):5098. doi:10.3390/ijerph16245098. [Google Scholar] [PubMed] [CrossRef]
86. Ma Y. Biotechnological potential of plant-microbe interactions in environmental decontamination. Front Plant Sci. 2019;10:1519. doi:10.3389/fpls.2019.01519. [Google Scholar] [PubMed] [CrossRef]
87. Chen X, Liu X, Zhang X, Cao L, Hu X. Phytoremediation effect of Scirpus triqueter noculated plant-growth-promoting bacteria (PGPB) on different fractions of pyrene and Ni in co-contaminated soils. J Hazard Mater. 2017;325:319–26. doi:10.1016/j.jhazmat.2016.12.009. [Google Scholar] [PubMed] [CrossRef]
88. Kang X, Yu X, Zhang Y, Cui Y, Tu W, Wang Q, et al. Inoculation of Sinorhizobium saheli YH1 leads to reduced metal uptake for Leucaena leucocephala grown in mine tailings and metal-polluted soils. Front Microbiol. 2018;9:1853. doi:10.3389/fmicb.2018.01853. [Google Scholar] [PubMed] [CrossRef]
89. Raklami A, Meddich A, Oufdou K, Baslam M. Plant microorganisms-based bioremediation for heavy metal cleanup: recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int J Mol Sci. 2022;23(9):5031. doi:10.3390/ijms23095031. [Google Scholar] [PubMed] [CrossRef]
90. He X, Xu M, Wei Q, Tang M, Guan L, Lou L, et al. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol Environ Saf. 2020; 205(3–4):111333. doi:10.1016/j.ecoenv.2020.111333. [Google Scholar] [PubMed] [CrossRef]
91. Ma Y, Cheng L, Zhang D, Zhang F, Zhou S, Ma Y, et al. Stability of Pb, Cd, and Zn in soil by modified-zeolite: mechanisms and evaluation of effectiveness. Sci Total Environ. 2022;814(654):152746. doi:10.1016/j.scitotenv.2021.152746. [Google Scholar] [PubMed] [CrossRef]
92. Debiec-Andrzejewska K, Krucon T, Piatkowska K, Drewniak L. Enhancing the plants growth and arsenic uptake from soil using arsenite-oxidizing bacteria. Environ Pollut. 2020;264(331):114692. doi:10.1016/j.envpol.2020.114692. [Google Scholar] [PubMed] [CrossRef]
93. Manoj SR, Karthik C, Kadirvelu K, Arulselvi PI, Shanmugasundaram T, Bruno B, et al. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J Environ Manag. 2020;254(9–10):109779. doi:10.1016/j.jenvman.2019.109779. [Google Scholar] [PubMed] [CrossRef]
94. Pinter IF, Salomon MV, Gil R, Mastrantonio L, Bottini R, Piccoli P. Arsenic and trace elements in soil, water, grapevine and onion in Jáchal. Argentina Sci Total Environ. 2018;615(11):1485–98. doi:10.1016/j.scitotenv.2017.09.114. [Google Scholar] [PubMed] [CrossRef]
95. Ma Y, Rajkumar M, Zhang C, Freitas H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J Hazard Mater. 2016;320(16):36–44. doi:10.1016/j.jhazmat.2016.08.009. [Google Scholar] [PubMed] [CrossRef]
96. Munir N, Hanif M, Abideen Z, Sohail M, El-Keblawy A, Radicetti E, et al. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy. 2022;12(9):2069. doi:10.3390/agronomy12092069. [Google Scholar] [CrossRef]
97. Chen Y, Han YH, Cao Y, Zhu YG, Rathinasabapathi B, Ma LQ. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci. 2017;8(26):268. doi:10.3389/fpls.2017.00268. [Google Scholar] [PubMed] [CrossRef]
98. Khan N, Bano A. Role of PGPR in the phytoremediation of heavy metals and crop growth under municipal wastewater irrigation. Phytoremed: Manag Environl Contam. 2018;6:135–49. doi:10.1007/978-3-319-99651-6_5. [Google Scholar] [CrossRef]
99. Rajendran P, Muthukrishnan J, Gunasekaran P. Microbes in heavy metal remediation. Indian J Exp Biol. 2003;41:935–44. [Google Scholar] [PubMed]
100. Guarino F, Miranda A, Castiglione S, Cicatelli A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere. 2020;251:126310. doi:10.1016/j.chemosphere.2020.126310. [Google Scholar] [PubMed] [CrossRef]
101. Nayeri S, Dolatyari M, Mouladoost N, Nayeri S, Zarghami A, Mirtagioglu H, et al. Ag/ZnO core-shell NPs boost photosynthesis and growth rate in wheat seedlings under simulated full sun spectrum. Sci Rep. 2023;13(1):14385. doi:10.1038/s41598-023-41575-7. [Google Scholar] [PubMed] [CrossRef]
102. Tang M, Zhu KJ, Sun W, Yuan X, Wang Z, Zhang R, et al. Ultrasimple size encoded microfluidic chip for rapid simultaneous multiplex detection of DNA sequences. Biosens Bioelectron. 2024;253:116172. doi:10.1016/j.bios.2024.116172. [Google Scholar] [PubMed] [CrossRef]
103. Li J, Li X, Wang C, Liu JZ, Gao ZD, Li KM, et al. Pollution characteristics and probabilistic risk assessment of heavy metal(loid)s in agricultural soils across the Yellow River Basin. China Ecol Indic. 2024;167(2):112676. doi:10.1016/j.ecolind.2024.112676. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF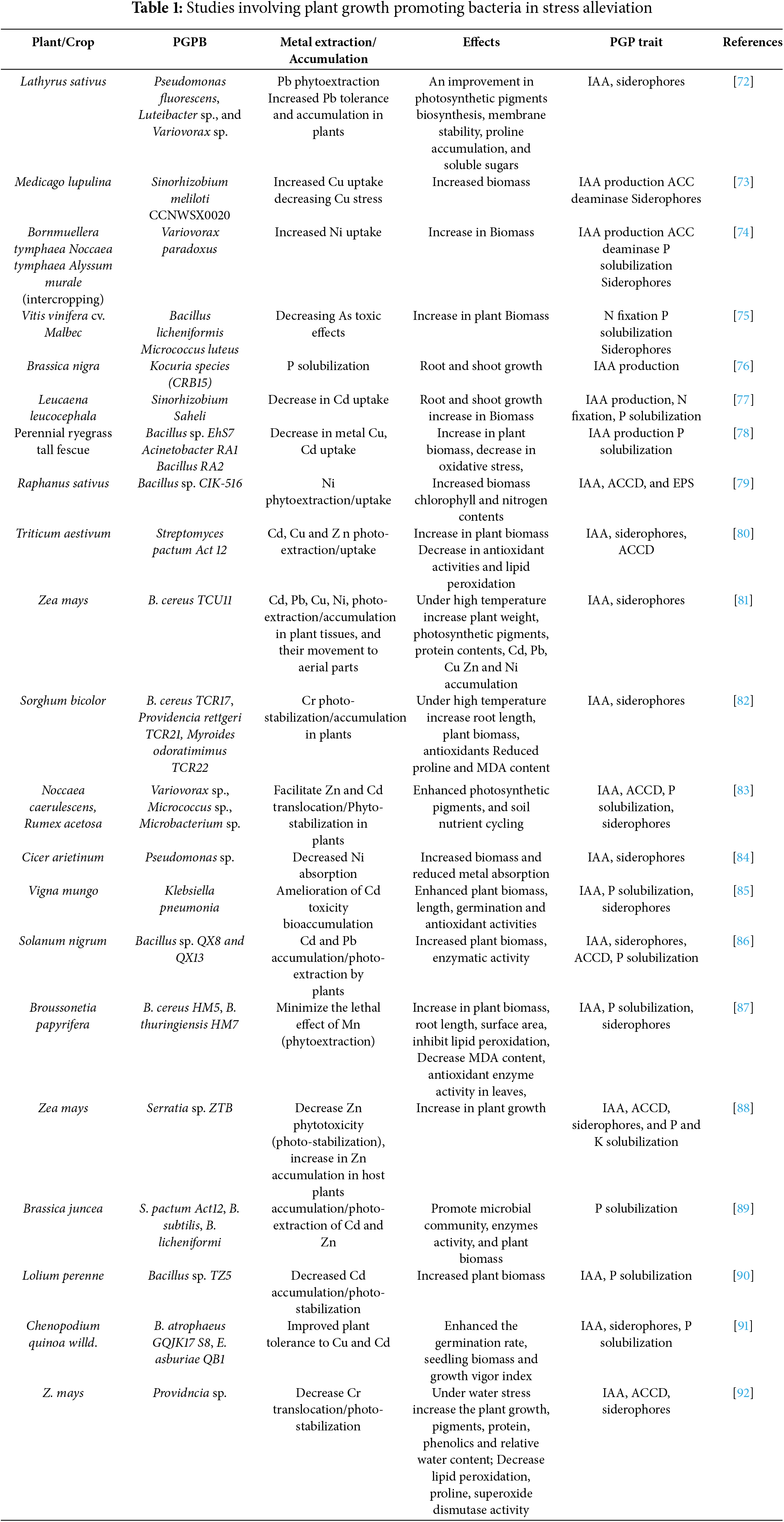
 Downloads
Downloads
 Citation Tools
Citation Tools