 Open Access
Open Access
ARTICLE
Humic Acid Mediates Drought Tolerance in Wheat through the Modulation of Morphophysiological Traits, Leading to Improve the Grain Yield in Wheat
1 Department of Crop Physiology and Ecology, Hajee Mohammad Danesh Science and Technology University, Dinajpur, 5200, Bangladesh
2 Department of Agronomy, Hajee Mohammad Danesh Science and Technology University, Dinajpur, 5200, Bangladesh
3 Department of Chemistry, Hajee Mohammad Danesh Science and Technology University, Dinajpur, 5200, Bangladesh
4 Department of Biology, College of Science, Taif University, P.O. Box 11099, Taif, 21944, Saudi Arabia
5 Soil Science Division, Bangladesh Wheat and Maize Research Institute, Dinajpur, 5200, Bangladesh
* Corresponding Authors: Akbar Hossain. Email: ; Subrota Kumer Pramanik. Email:
Phyton-International Journal of Experimental Botany 2025, 94(3), 763-779. https://doi.org/10.32604/phyton.2025.062717
Received 25 December 2024; Accepted 26 February 2025; Issue published 31 March 2025
Abstract
The increasing frequency and intensity of drought caused by climate change necessitate the implementation of effective ways to increase the ability of wheat to withstand drought, with humic acid being a promising approach. Therefore, a pot experiment was conducted to determine the efficacy of exogenous humic acid on wheat under water deficit stress via a completely randomized design (CRD) with three replications. The impacts of four growing conditions, i.e., well water (65% field capacity), water deficit stress (35% field capacity), soil application of humic acid (44 mg kg−1 soil) under water deficit stress and foliar feeding of humic acid (200 ppm) under water deficit stress, were investigated on two wheat varieties (BWMRI Gom 1 and BWMRI Gom 3). The results demonstrated that water deficit stress substantially decreased the studied morphological and physiological traits, yield components and yield, in both genotypes, with the exception of the proline content of flag leaves. Compared with soil application, foliar feeding of humic acid promoted the ability of wheat to overcome stress conditions better. In the present study, humic acid as a soil application increased the grain yield by 9.13% and 13.86% and the biological yield by 9.94% and 5.19%, whereas foliar treatment increased the grain output by 24.76% and 25.19% and the biological yield by 19.23% and 6.50% in BWMRI Gom 1 and BWMRI Gom 3, respectively, under water deficit stress. Therefore, exogenous foliar humic acid treatment was more effective than soil application in alleviating the effects of drought stress on wheat.Keywords
Wheat is a major cereal, accounting for approximately 29% (806.50 million tons) of total cereal production from 2022–2023, as reported by the FAO [1]. Additionally, after rice, it is Bangladesh’s 2nd most important cereal crop, with an area of 0.32 million ha under production and a yearly yield of 1.17 million metric tons, but the average yield (3.69 t ha−1) is lower than that of other countries [2]. According to the IPCC (Intergovernmental Panel on Climate Change) awareness report, the potential yield of wheat in Bangladesh is projected to decline by 32% by the year 2050 [3]. On the other hand, the worldwide demand for wheat will be increased by as much as 60% by 2050 [4] because of the faster growth of the economy, expanding urbanization, and consequent changes in lifestyle [5]. National wheat production can only fulfil approximately 20% of its overall consumption, creating a demand–supply gap that imports can only fill [6].
The rapid expansion of the world population has led to a problematic situation regarding food security. This is caused primarily by global climate change and increased unpredictability in weather precedents, resulting in drought-prone regions worldwide, including the crop-growing areas in northwestern Bangladesh, which severely hinder crop productivity [7]. Bangladesh is confronted with a significant risk of moisture stress, as almost 3.50 million hectares of land are experiencing drought, accounting for approximately 47% of the country’s total area [8].
Drought has been identified as a polygenic stress that primarily occurs due to a reduction in rainfall and the subsequent period of drought [9], which limits crop productivity and quality as well as the overall potential of land globally [10]. The detrimental impacts of drought on crops are well documented. Drought inhibits nutrient uptake, shortens phenology during regular growth and development periods, and modifies physio-biochemical processes. As a result, it decreases the production of dry matter, grain output, and quality of wheat [11]. The main constraint of water shortages during wheat grain filling can shorten its duration, lowering grain size and quantity and ultimately leading to a decrease in grain output [12]. The effects of drought stress on wheat may be minimized in two ways: first, by managing optimal agronomic practices efficiently, and second, by developing drought-resistant wheat cultivars [13]. Researchers have developed strategies to mitigate drought stress through the use of film mulch [14], drought-tolerant varieties [15], nanoparticles [16], superabsorbent hydrogels and biochar [17], humic acid [18], and plant growth-promoting rhizobacteria [19]. In fact, in China, humic acid has been widely added to common urea and phosphate fertilizers to increase their effectiveness.
Humic acid is a vital element of soil organic materials that strengthens the physicochemical and biological aspects of soil while enhancing grain production and crop quality [20]. It is an active ingredient of organic fertilizers and may provide a replacement for conventional soil fertilization and quick reservoirs of nitrogen, especially in semiarid areas [21]. Humic acid applied to leaves improves photosynthesis activity, gas exchange capacity, chlorophyll pigments, protein and starch ratios, and physiological processes and increases tolerance to abiotic stress [22]. Several researchers have reported that the application of humic acid under drought stress conditions can reduce drought-induced adverse impacts on wheat [23], maize, sorghum, mungbean [24] and rapeseed [25]. Therefore, the present study aimed to reduce the adverse effects of drought stress on wheat through the administration of humic acid and to observe the effectiveness of various application methods.
2.1 Location of the Observations
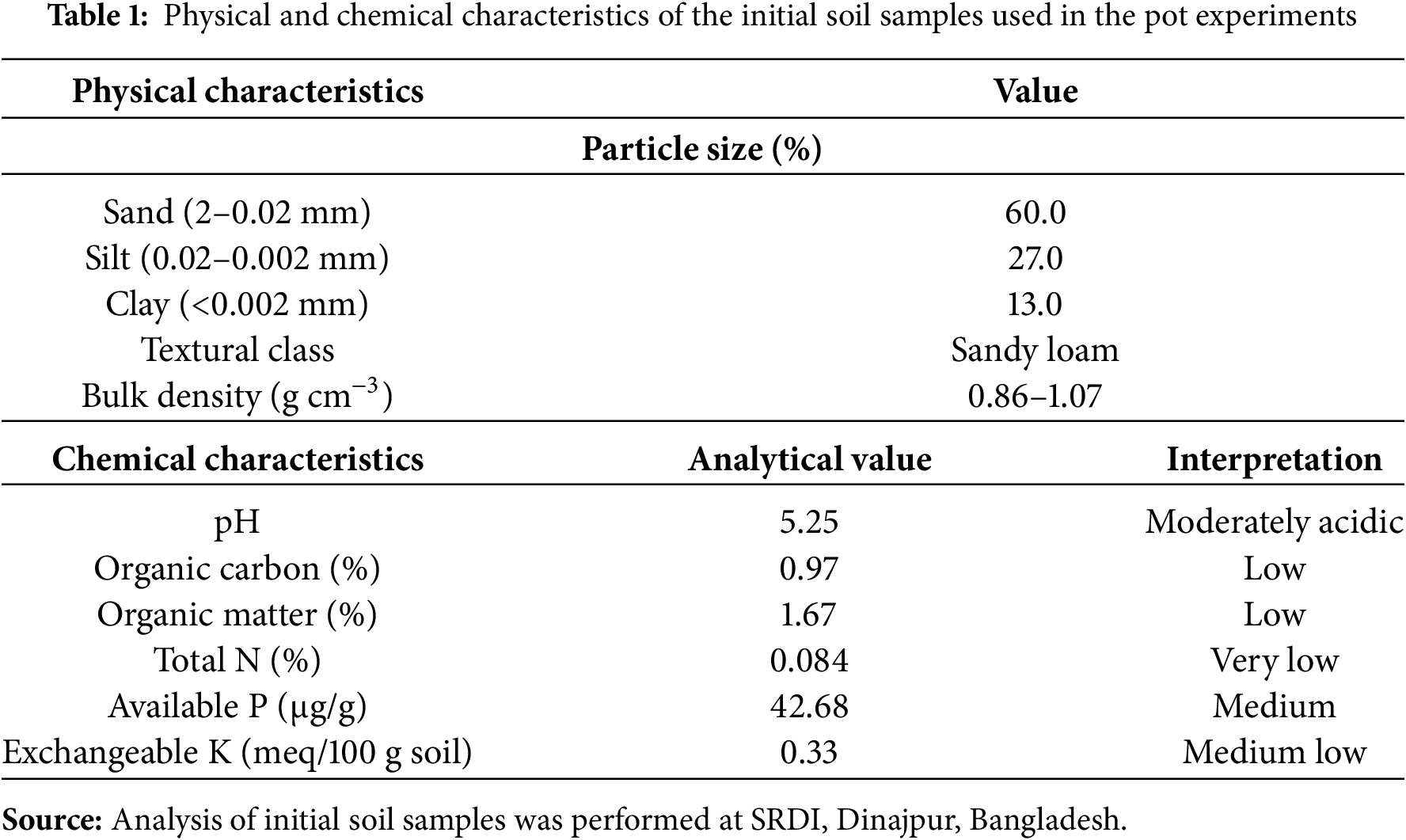
A pot experiment was administered at the Department of Crop Physiology and Ecology (CPE) research farm at Hajee Mohammad Danesh Science and Technology University (HSTU), Dinajpur, from November 2022 to March 2023. This area is situated under AEZ-1 and has medium–high terrain with an elevation of 37.58 m above sea level. The weather conditions during the crop growth stage are presented in Fig. 1. The physical and chemical characteristics of the initial soil samples used in the pot experiments are available in Table 1.
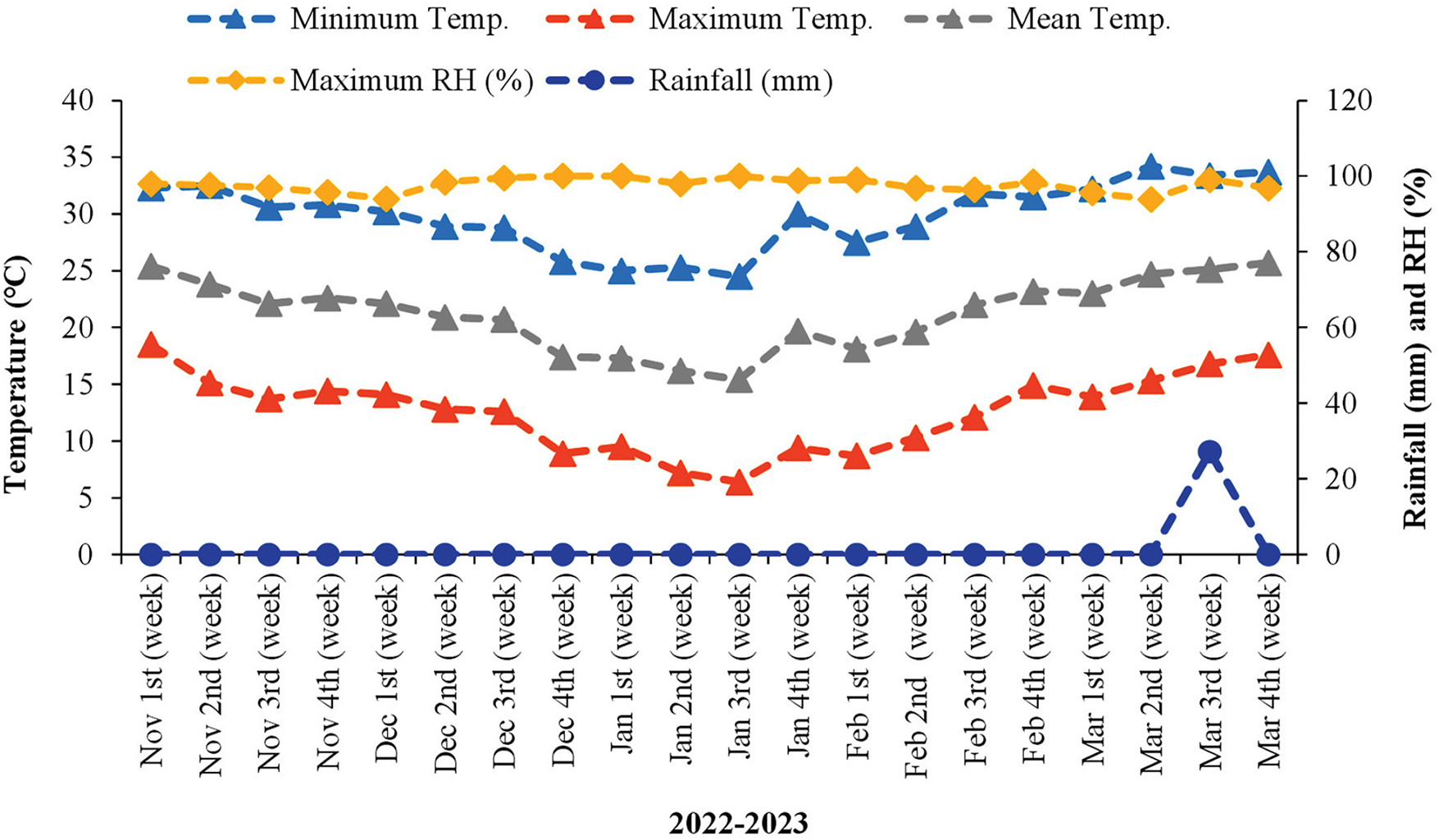
Figure 1: Weekly average weather data during the wheat growing season from 2022–23. Source: Bangladesh Wheat and Maize Research Institute, Dinajpur
The sources of both wheat varieties ‘BWMRI Gom 1 and BWMRI Gom 3’ are from ‘Bangladesh Wheat and Maize Research Institute, Dinajpur, 5200, Bangladesh’.
Available link (BWMRI Gom 1): https://bwmri.portal.gov.bd/site/page/3c2b3935-f97b-4182-9034-45a9d2718d14/- (accessed on 24 December 2024).
Available link (BWMRI Gom 3): https://bwmri.portal.gov.bd/site/page/606e5786-c25f-4bb1-b01a-2d7172985069/- (accessed on 25 December 2024).
2.3 Treatments and Experimental Design
This experiment was performed via a completely randomized design (CRD) comprising two factors with three replications. Factor A included four growing conditions: (i) good water conditions (65% of the field capacity of the pot’s soil), (ii) water deficit stress conditions (35% of the field capacity of the pot’s soil), (iii) soil application of humic acid (44 mg kg−1 soil) under water deficit stress, and (iv) foliar feeding of humic acid (200 ppm) under water deficit stress. Factor B: two wheat varieties: (i) BWMRI Gom 1 and (ii) BWMRI Gom 3.
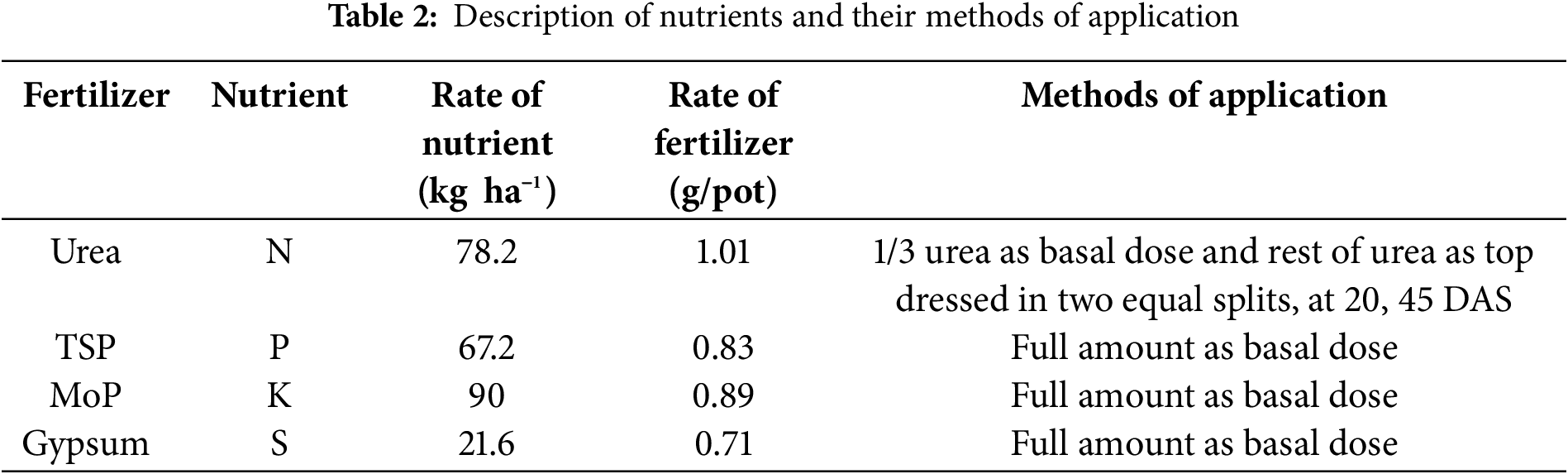
Twenty-four plastic pots (26 cm in diameter and 30 cm in height) were collected, and a small pore was made at the bottom of each pot for the drainage system. The dirt was collected from a depth of 15 cm in the CPE research field, which was free from contamination, pulverized well and dried in the sun. The pots were filled with equal amounts (11 kg pot−1) of a combination of soil and compost (weight ratio = 3:1). Each pot was fertilized with urea, triple superphosphate (TSP), Muriate of Potash (MoP) or gypsum as described in Table 2.
A modest amount of irrigation was applied after sowing to ensure equal germination. All the experimental pots were subjected to field-controlled soil moisture until 30 days after sowing (DAS) for better plant growth. After 30 DAS, irrigation water was regulated by weight to apply well water (65% of field capacity) and stressful water (35% of field capacity) at one-day intervals. Water loss was measured via daily pot weight measurements. The study avoided precipitation by covering it with plastic. During the growing season, necessary actions are taken to guard the crop from several insect pests and diseases.
2.5 Preparation and Application of Humic Acid
As a soil application, humic acid was applied to the potted soil (44 mg kg−1 soil) at 30 days after sowing when water deficit stress was applied. A solution of 200 ppm humic acid was prepared for foliar application during tillering (27 DAS), booting (55 DAS), and anthesis (72 DAS) through a hand sprayer.
The number of leaves plant−1 and the length and breadth of the flag leaf blade were manually measured at 71 DAS from three plants in each treatment replication, and the average values were calculated.
Relative Leaf Water Content (RLWC)
The relative water content of the flag leaf was measured at 10 days after anthesis, following the procedure described by Kocheva et al. [26]. The RLWC was computed via the following formula:
SPAD Values
The SPAD values of the flag leaves were measured via a portable SPAD meter at 15 days after anthesis.
Leaf Temperature
A portable infrared thermometer was used to evaluate this characteristic at 16 days after anthesis in the presence of strong sunlight and minimal breeze at midday.
Total Chlorophyll
The quantification of the photosynthetic pigment content (total chlorophyll) of flag leaves was conducted at 10 days after anthesis via the method outlined by Witham et al. [27] and measured on the basis of fresh weight. The total chlorophyll content was calculated via the following formula:
Total chlorophyll (mg g−1 FW) = [20.2 (D645) + 8.02 (D663)] × [V/(1000 × W)]
where V = volume of 80% aqueous acetone (ml); W = weight of fresh leaf (g); D645 = absorbance at a wavelength of 645 nm; and D663 = absorbance at a wavelength of 663 nm.
Proline Content
The proline content of the flag leaf was assessed at 15 days after anthesis via the Bates et al. [28] method. The proline content was estimated via a standard curve, and the fresh weight was measured on the basis of the following formula:
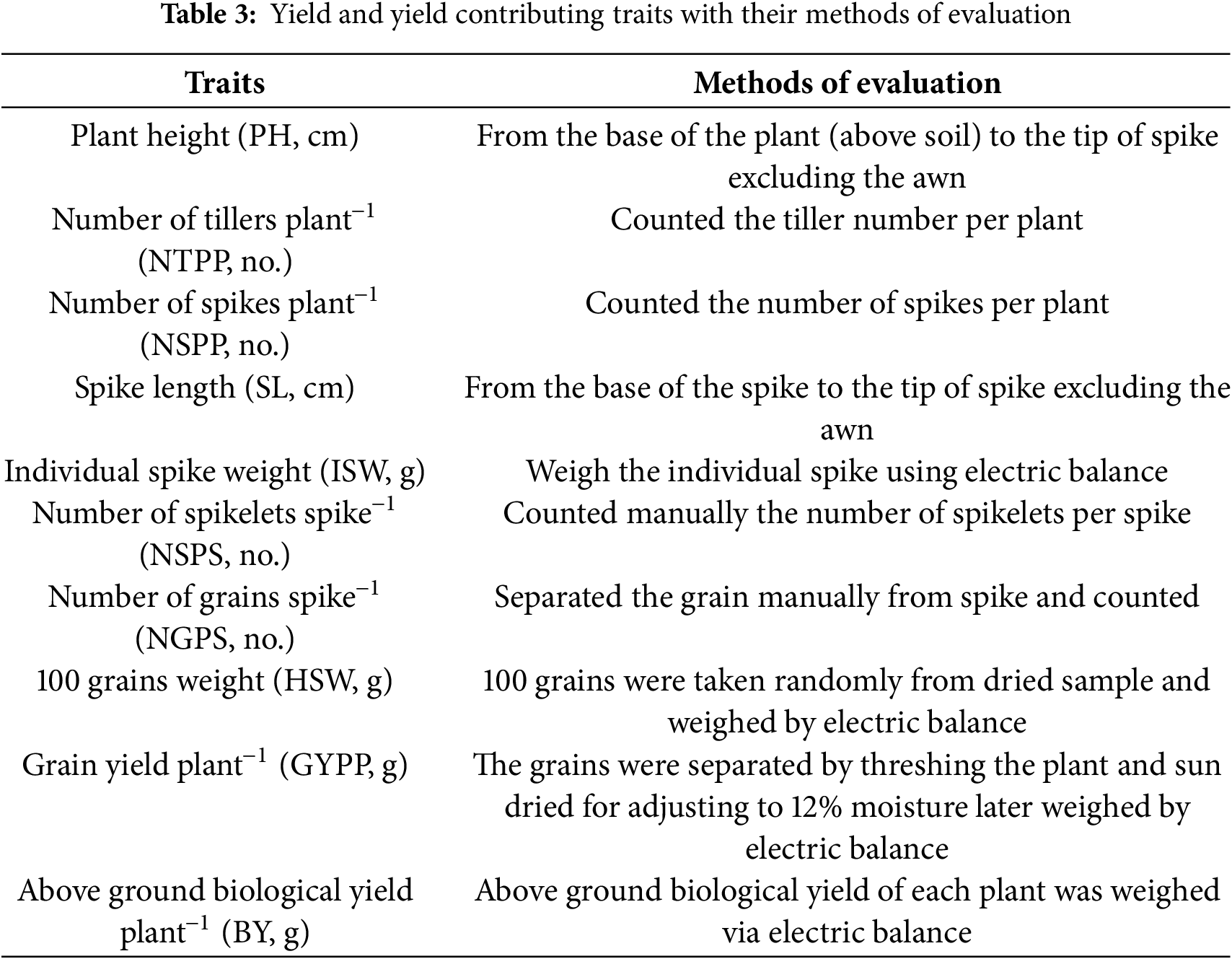
Three plant samples were chosen randomly from each replication of every treatment to record the features mentioned in Table 3. The average value was then computed.
The software package “STAR” [29] was used to establish the ANOVA table. The average means of the parameters were then compared via Tukey’s test.
Under well-watered conditions (WW), BWMRI Gom 3 had a greater number of leaves than that of BWMRI Gom 1. When subjected to water deficit stress (WDS), both varieties presented reductions in leaf count and flag leaf dimensions (Table 4). The length of the flag leaf for both varieties was markedly decreased under WDS, with the BWMRI Gom 1 decreasing from 27.78 to 21.78 cm (−21.59%) and the BWMRI Gom 3 decreasing from 27.71 to 19.83 cm (−28.43%) (Table 4). The soil application of humic acid was less effective than the foliar methods in mitigating the adverse effects of water deficit. For example, the soil application resulted in a flag leaf length of 21.09 cm for BWMRI Gom 1, showing a reduction of only 24.08%. The foliar application led to a length of 25.56 cm (+17.36%). In terms of leaf breadth, both varieties also experienced reductions under WDS, with the BWMRI Gom 1 decreasing from 1.80 to 1.56 cm (−13.34%) and the BWMRI Gom 3 decreasing from 1.68 to 1.44 cm (−14.29%). The use of humic acid improved leaf breadth, with the BWMRI Gom 1 reaching at 1.62 cm (+3.85%) with soil application and 1.72 cm (+10.26%) with foliar application. Similarly, the BWMRI Gom 3 improved with the soil and foliar application of humic acid (Table 4).
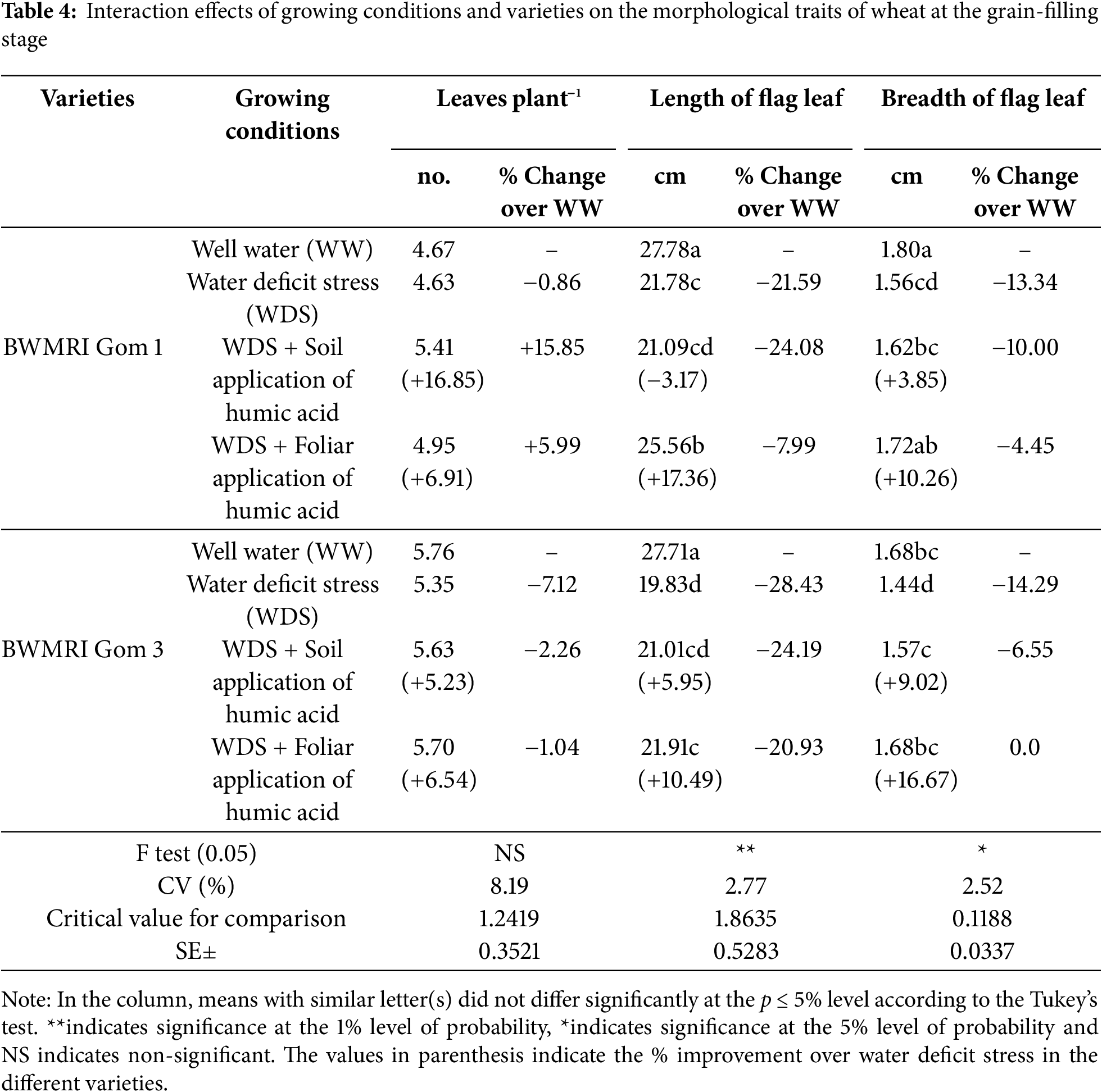
The relative leaf water content (RLWC) was significantly decreased under WDS for both varieties. The BWMRI Gom 1 decreased from 78.62% to 67.00% (−14.78%), whereas the BWMRI Gom 3 decreased from 73.43% to 60.52% (−17.58%) (Table 5). The application of humic acid, both through soil and foliar methods, improved the RLWC in stressed plants. Notably, foliar application resulted in a significant increase to 75.89% (+13.27%) for BWMRI Gom 1, demonstrating its effectiveness in enhancing water retention and mitigating stress effects. The total chlorophyll content was also adversely affected by WDS, decreasing for both varieties. The BWMRI Gom 1 decreased from 2.29 mg g−1 FW to 1.95 mg g−1 FW (−14.84%), whereas the BWMRI Gom 3 decreased more severely, from 1.74 mg g−1 FW to 1.23 mg g−1 FW (−29.31%). Both types of humic acid application showed the potential to increase chlorophyll levels. In BWMRI Gom 1, foliar application increased the total chlorophyll content to 2.28 mg g−1 FW (+16.92%), whereas soil application resulted in a smaller increase to 2.01 mg g−1 FW (+3.07%). For BWMRI Gom 3, the soil application improved the chlorophyll content to 1.60 mg g−1 FW (+30.08%), indicating that humic acid can support chlorophyll synthesis even under stress. Proline, an osmoprotectant, was increased significantly under WDS, with BWMRI Gom 1 increasing to 2.41 μmol g−1 FW (+21.71%) and BWMRI Gom 3 increasing to 2.37 μmol g−1 FW (+65.73%). Following humic acid application, proline levels varied slightly (Table 5). In BWMRI Gom 1, the soil application resulted in a decrease to 2.23 μmole g−1 FW (−7.47%), whereas the foliar application caused a decrease to 2.00 μmole g−1 FW (–17.01%). The SPAD values, which are indicative of leaf greenness and chlorophyll content, decreased under WDS. The BWMRI Gom 1 decreased from 54.40 to 53.78 (–1.13%), whereas the BWMRI Gom 3 decreased from 56.67 to 52.47 (–7.41%).
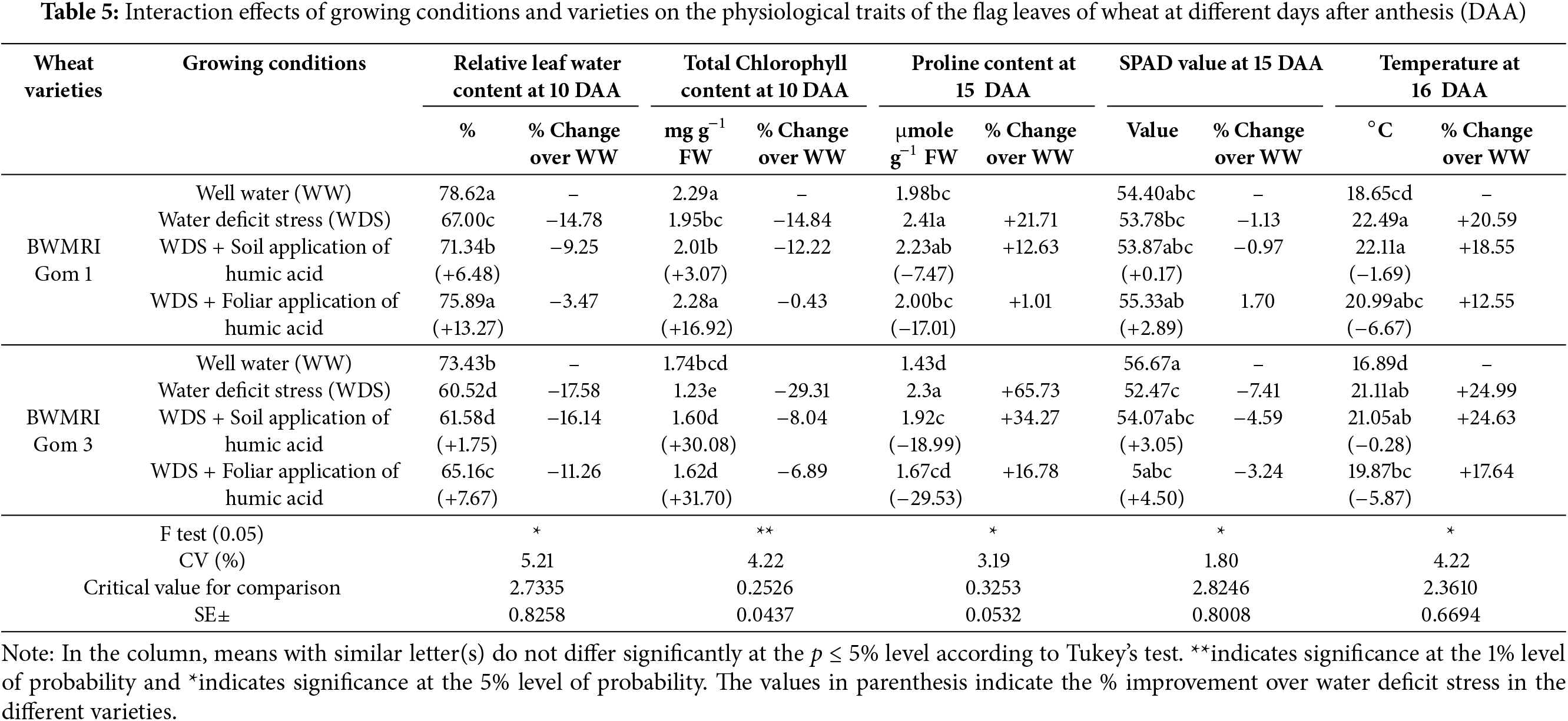
The use of humic acid also positively influenced the SPAD values (Table 5). The foliar application of BWMRI Gom 1 resulted in a slight increase to 55.33 (+2.89%), indicating improved chlorophyll retention. For BWMRI Gom 3, the soil application improved the SPAD value to 54.07 (+3.05%). The leaf temperature increased under WDS, with the BWMRI Gom 1 increasing to 22.49°C (+20.59%) and the BWMRI Gom 3 increasing to 21.11°C (+24.99%). The application of humic acid helped to regulate the leaf temperature. For BWMRI Gom 1, the soil application maintained a relatively stable temperature at 22.11°C (−1.69%), whereas the foliar application reduced it to 20.99°C (−6.67%). For BWMRI Gom 3, the temperature was slightly lower at 21.05°C (−0.28%) following soil application (Table 5).
3.3 Yield and Yield Contributing Traits
Under optimal watering conditions, the height of the wheat plants reached a maximum, with the BWMRI Gom 1 reaching 81.86 cm and the BWMRI Gom 3 reaching 73.13 cm (Table 6). However, water deficit stress resulted in a reduction in plant height for both varieties. Specifically, plant height decreased to 77.90 cm for BWMRI Gom 1 (−4.83%) and to 68.87 cm for BWMRI Gom 3 (−5.82%). The foliar application of humic acid was the most effective treatment, particularly for BWMRI Gom 1, which reached a height of 81.19 cm (+4.22%) (Table 6).
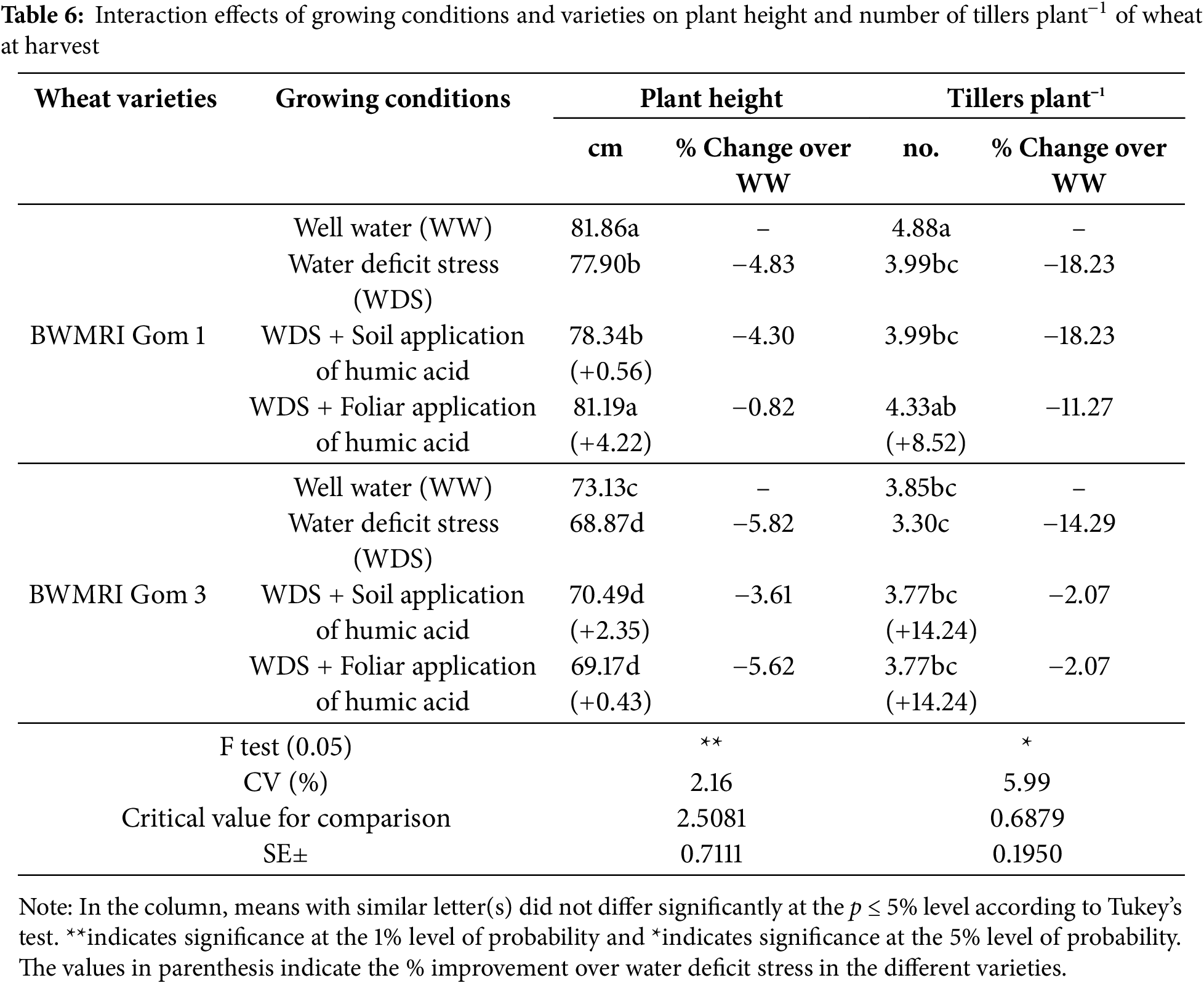
Under optimal conditions, the number of tillers per plant was maximized, with BWMRI Gom 1 producing 4.88 tillers and BWMRI Gom 3 producing 3.85 tillers. Under WDS, a meaningful decrease in tiller number was observed for both varieties. The BWMRI Gom 1 decreased to 3.99 tillers (−18.23%), whereas the BWMRI Gom 3 decreased to 3.30 tillers (−14.29%). The foliar application of humic acid led to an increase in tiller number for BWMRI Gom 1, which reached 4.33 tillers (+8.52%) (Table 6).
Under well-watered conditions, BWMRI Gom 1 had the highest number of spikes plant−1 (4.40), whereas WDS significantly reduced the count by 16.59% (Table 7). The application of humic acid under WDS improved the spike number, with foliar application yielding the highest recovery (+25.61%). Spike length was longest in BWMRI Gom 1 under well-watered conditions (13.65 cm) and decreased most significantly under WDS (−24.83%). Compared with WDS alone, the soil and foliar applications of humic acid improved the spike length by 2.24% and 9.25%, respectively (Table 7).
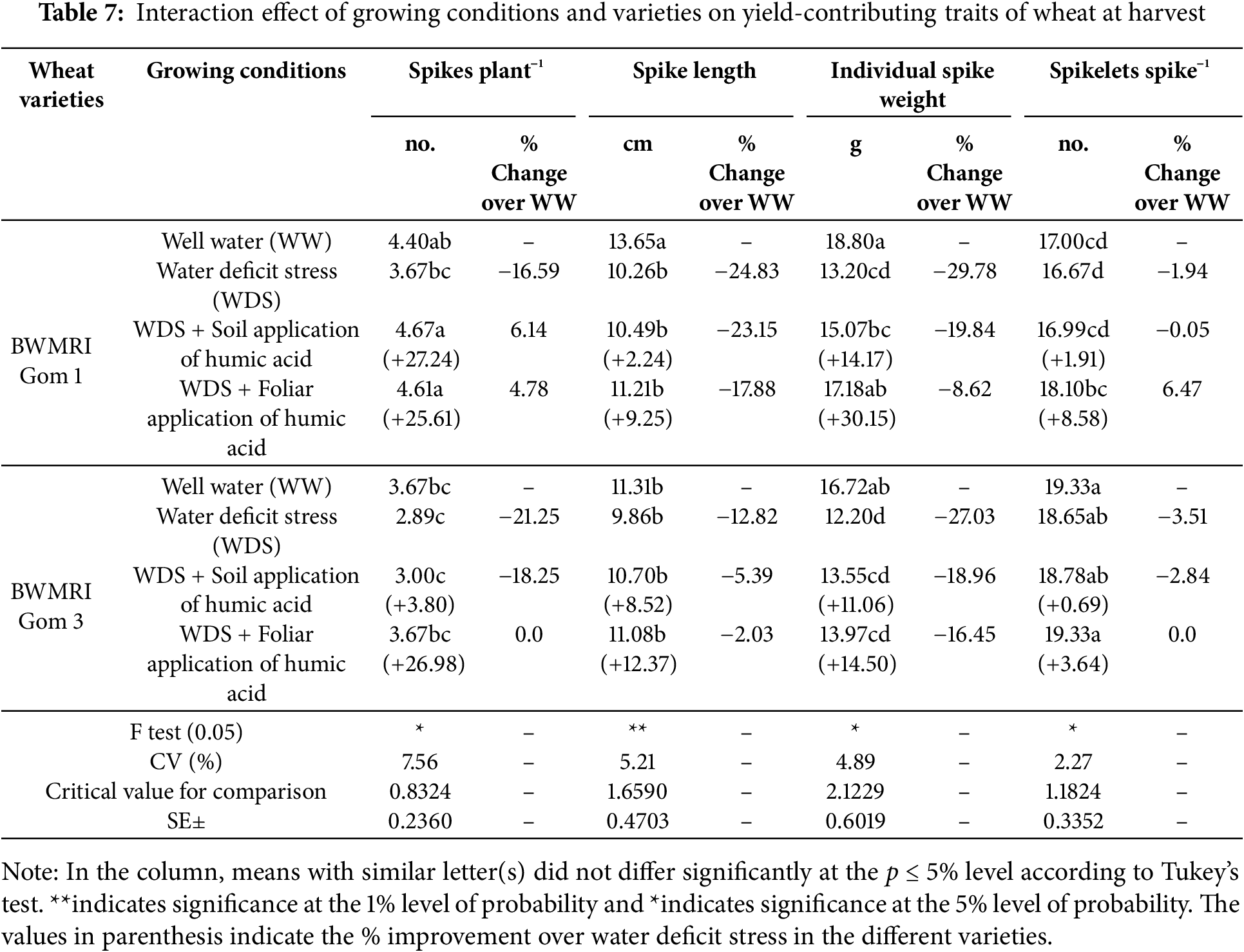
Under WW, BWMRI Gom 1 presented the heaviest spike weight (18.80 g), whereas WDS decreased it by 29.78%. Foliar application of humic acid resulted in the most significant improvement in individual spike weight (+30.15%) under WDS. The BWMRI Gom 3 produced the greatest number of spikelets spike−1 under WW (19.33). In BWMRI Gom 1, spikelet count was minimally affected under WDS conditions, while with foliar application of humic acid showing a slight increase (+6.47%) relative to WDS (Table 7).
The BWMRI Gom 3 produced a greater number of spike−1 grains under watered conditions than did the BWMRI Gom 1. Water deficit stress reduced the number of grains in both varieties, with decreases of 8.38% for BWMRI Gom 1 and 4.85% for BWMRI Gom 3 (Table 8). The application of humic acid mitigated some of this loss, especially in BWMRI Gom 3, where foliar application increased the grain number by 3.24% over WDS alone. The highest 100-grain weight was observed in the BWMRI Gom 1 under well-watered conditions (5.09 g). WDS reduced the 100-grain weight for both varieties, with decreases of 8.05% for BWMRI Gom 1 and 14.60% for BWMRI Gom 3 (Table 8).
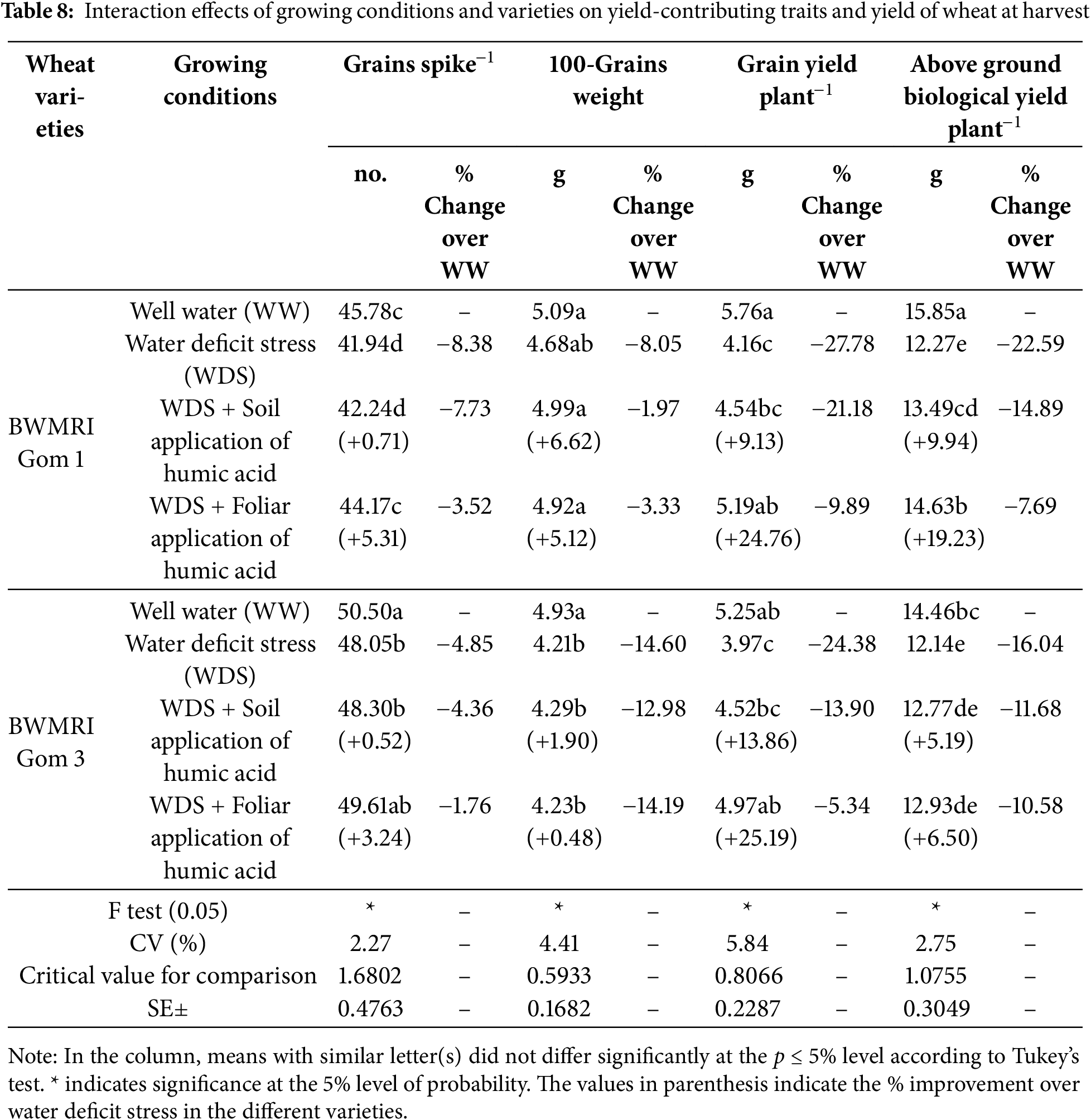
The soil application of humic acid slightly improved the grain weight in BWMRI Gom 3 (+1.90%) under WDS, although foliar applications resulted in limited improvement. The grain yield was highest in BWMRI Gom 1 under well-watered conditions (5.76 g), which was decreased significantly under WDS by 27.78%. Foliar application of humic acid markedly improved yield under WDS for BWMRI Gom 1 (+24.76%) and BWMRI Gom 3 (+25.19%), suggesting that foliar application may effectively support yield resilience under water stress. The biological yield was highest in BWMRI Gom 1 (15.85 g) under well-watered conditions and was significantly lower in WDS (22.59% decrease). Compared with WDS alone, foliar application of humic acid increased the biological yield under WDS for BWMRI Gom 1 by 19.23%, indicating that humic acid application may increase aboveground biomass retention during drought stress (Table 8).
The grain yield of agricultural crops depends on photosynthesis, and leaves are vital determinants for improved photosynthetic capacity [30]. According to the results of the present study, the use of humic acid led to an increase in the number of leaves compared with that of stressed plants. Research conducted by de Freitas Moura et al. [31] also corroborates our findings. Additionally, Arslan et al. [32] reported that humic acid suppressed the activity of the GRF gene, which is known to be sensitive to drought in wheat, promoting wheat development in environments prone to drought. The flag leaf is the primary supplier of photosynthates and acts as a nearby food source for the grain during the reproduction stage [33]. We observed that water deficit stress substantially hampers the morphological traits of the flag leaves of wheat plants. These results align with the findings reported by Ahmad et al. [34]. However, humic acid accelerates nutrient uptake, photosynthesis, translocation, and photosynthate utilization, resulting in rapid cell division, which might be the reason behind the increase in leaf morphology [35].
The data in Table 3 also confirmed that, compared with soil application, exogenous application of humic acid in adverse situations improved flag leaf shape. Sindabad et al. [36] reported that water deficiency stress considerably reduces the relative leaf water content in wheat. In our investigation, the use of humic acid considerably improved the RLWC of the flag leaves of wheat compared with that under water scarcity stress. Research conducted by Abou Tahoun et al. [37] indicated that humic acid molecules increase the osmotic adjustability and water retention of plants and maintain their photosynthetic and antioxidant metabolism when they are subjected to drought stress. The findings of Wang et al. [38] align with our findings, indicating that drought reduces photosynthetic activity because of both stomatal and nonstomatal restrictions. However, humic acid enhances photosynthesis, respiration, hormone synthesis, and protein synthesis in plants [39]. Humic acid promotes chlorophyll production by stimulating ascorbate peroxidase activity, which removes ROS in the thylakoids and minimizes chlorophyll breakdown in drought-stressed plants [39]. Tohidi [40] reported that humic acid increased the wheat chlorophyll content under water deficit stress, which justifies our present findings. Proline acts as an enzyme stabilizing agent that helps control and decrease water loss from cells during periods of water scarcity. It also helps to maintain the osmotic equilibrium and contributes to the structure of subcellular components [41]. A study by Nowsherwan et al. [42] demonstrated that higher crop plants significantly increase proline levels in response to abiotic stresses. Similarly, Liang et al. [41] reported countable increases in proline concentrations in wheat leaves under conditions of water scarcity, which is in accordance with our present results. The decline in greenness (SPAD value) under water deficit stress may be explained by photooxidation and degradation of chlorophyll in the leaves [43]. Water deficit stress impairs the greenness and photosynthetic machinery of green plants.
In our study (data in Table 4), humic acid remarkably increased the SPAD value of wheat leaves under stress conditions. As stated by Nikbakht et al. [44], humic acid improves the nutrient uptake rate (N and K) in plant shoots, thereby increasing the number of chloroplasts per cell and increasing the synthesis of chlorophyll as well as the greenness of green plants such as wheat [45]. Leaf temperature serves as a valuable measure of drought tolerance because of the significant role of stomata in regulating water loss. Plants respond to drought by experiencing a reduction in water content, which decreases leaf water potential. Thus, there is a growing inclination towards higher leaf temperatures and a decline in leaf area, leading to a decrease in both crop productivity and resilience. Humic acid, when applied to plants, particularly through foliar application, enhances stomatal conductance, leading to stomatal transpiration. This process results in the cooling of plant leaves and a decrease in leaf temperature. Water scarcity can lead to stunted plant growth through dehydration of the protoplasm, decreased turgidity, and impaired cell growth and division [46]. Our results agree with the studies of Jannat et al. [47], who demonstrated that water deficit stress lowered the plant height and tillering capacity of wheat. The decrease in the number of tillers in plant−1 might be due to the roots receiving less water and nutrient availability under drought conditions, which disrupts plant tissue and reduces meristematic activity and cell proliferation. Humic acid enhances the transportation and accessibility of nutrients [48], which encourages the growth and development of plants during both vegetative and reproductive stages. Haghighi et al. reported that organic compounds such as humic acid decrease watering intervals, improve WUE, and minimize the effects of drought stress on crop plants, ultimately improving vegetative development. El-Sayed et al. [49] reported that humic acid administration during water stress increased the tiller density of bread wheat cultivars. According to Forgane [50], spikes plant−1 are a significant factor that has a favourable relationship with grain yield. In this study, water deficit stress significantly reduced this component. These findings are supported by those of Jannat et al. [47], who confirmed that water shortage diminished the number of spikes and spike length compared with nonstress conditions. In our investigation, humic acid produced a greater increase in the spikes of wheat than that of under stressful conditions. Nasiroleslami et al. [51] reported a positive relationship between humic acid application and the number of spikes. Humic acid may improve wheat spike length by adjusting the cellular osmotic pressure and increasing enzymatic and antioxidant activities [52], which increase plant reproduction. The spike weight is a crucial component that is strongly correlated with the overall grain yield. Reduced photosynthesis and a lower supply of photosynthates due to water shortage are the primary drivers that hinder the growth of vegetative and reproductive development [15]. Nowsherwan et al. [42] reported that water scarcity stress decreased spike length, spikelets, and grains per spike, lowering grain yield. Water shortage stress reduces the number of grains, affecting dry matter production, pollen viability and ovule function. Our findings align with those of Mehrinfar et al. [53], who reported that foliar humic acid spray altered bread wheat cultivar grain yield and quality under water stress. Rana et al. [54] reported that water deficiency stress reduces grain weight, which is consistent with our findings. Humic acid increases plant photosynthetic pigments and biochemical activity. Arjumend et al. [55] reported that varying humic acid rates and NPK mineral fertilizers affected wheat thousand grain weight and yield, that is supporting our findings on 100-grain weight. In this study, both varieties presented decreased grain yield (plant−1) under water deficit stress. This might be because stress may reduce photosynthetic activity and assimilate supply, reducing grain number and size [15]. Similarly, Helmy Hegab et al. [56] demonstrated that humic acid increased wheat grain yield and components. Our study revealed that above-ground biological yield decreased due to water stress at the vegetative stage, resulting in the production of fewer tillers and less biomass than under well-watered conditions. Other authors, such as Zhang et al. [57], reported that drought lowered the biomass and yield of wheat by 25.00% and 27.50%, respectively that is congruent to our present findings on above ground biological yield.
The current investigation revealed that both wheat varieties, BWMRI Gom 1 and BWMRI Gom 3, presented significant reductions in key growth and yield parameters under water deficit stress compared with well-watered conditions. All the parameters (except proline) were declined considerably under WDS, indicating the detrimental impact of water stress on wheat productivity. However, the application of humic acid, particularly via foliar spraying, has shown promising effectiveness in mitigating these negative effects. Foliar application of humic acid consistently improved plant resilience under WDS, with increases in spike number, spike weight, grain number, grain yield, and biological yield in both varieties. Finally, BWMRI Gom 3 appears to be more resistant to drought conditions than BWMRI Gom 1, and foliar application of humic acid was found to be comparatively more effective than soil application in mitigating the adverse effects of water deficit stress on wheat.
Acknowledgement: The authors extend their appreciation to the chairman of the Department of Crop Physiology and Ecology, Hajee Mohammad Danesh Science and Technology University, Dinajpur 5200 Bangladesh, for supporting all facilities in this study. The authors also extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number TU-DSPP-2024-07.
Funding Statement: The current work was funded by Department of Crop Physiology and Ecology, Hajee Mohammad Danesh Science and Technology University, Dinajpur 5200 Bangladesh and Taif University, Saudi Arabia, Project No. TU-DSPP-2024-07.
Author Contributions: Conceptualization, Subrota Kumer Pramanik, Dristy Roy; Methodology, Dristy Roy, Md. Zakarya Ibne Sayed, Durjay Mondal; Validation, Subrota Kumer Pramanik, Md. Maniruzzaman Bahadur, Md. Rabiul Islam; Formal analysis, Dristy Roy, Md. Zakarya Ibne Sayed, Ahmed Gaber, Md. Parvez Kabir, Akbar Hossain, Durjay Mondal; Investigation, Durjay Mondal, Md. Zakarya Ibne Sayed, Banosree Saha Bandhan, Akbar Hossain; Data curation, Dristy Roy, Md. Zakarya Ibne Sayed, Ahmed Gaber, Md. Parvez Kabir, Akbar Hossain, Durjay Mondal; Writing—original draft preparation, Dristy Roy, Md. Zakarya Ibne Sayed, Durjay Mondal; Writing—review and editing, Md. Zakarya Ibne Sayed, Ahmed Gaber, Md. Parvez Kabir, Akbar Hossain, Durjay Mondal, Subrota Kumer Pramanik; supervision, Subrota Kumer Pramanik, Md. Maniruzzaman Bahadur, Md. Rabiul Islam, Akbar Hossain; Funding acquisition, Ahmed Gaber, Md. Parvez Kabir, Akbar Hossain. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data recorded in the current study are available in all tables and figures of the manuscript.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflict of interests to report regarding the present study.
References
1. FAO (Food and Agriculture Organization of the United Nations). Food outlook—biannual report on global food markets. In: Food outlook. Rome, Italy: FAO; 2024. [Google Scholar]
2. FAO (Food and Agriculture Organization of the United Nations). FAOSTAT analytical brief 79. In: Agricultural production statistics 2000–2022. Rome, Italy: FAO; 2023. [Google Scholar]
3. Hossain A, da Silva JAT. Wheat production in Bangladesh: its future in the light of global warming. AoB Plants. 2013;5:pls042. doi:10.1093/aobpla/pls042. [Google Scholar] [PubMed] [CrossRef]
4. Jahan MAHS, Hossain A, Teixeira Da Silva JA, El Sabagh A, Rashid MH, Barutçular C. Effect of naphthaleneacetic acid on root and plant growth and yield of ten irrigated wheat genotypes. Pak J Bot. 2019;51(2):451–9. doi:10.30848/PJB2019-2(11). [Google Scholar] [CrossRef]
5. Mottaleb KA, Rahut DB, Kruseman G, Erenstein O. Changing food consumption of households in developing countries: a Bangladesh case. J Int Food Agribus Mark. 2018;30(2):156–74. doi:10.1080/08974438.2017.1402727. [Google Scholar] [CrossRef]
6. Timsina J, Wolf J, Guilpart N, van Bussel LJ, Grassini P, van Wart J, et al. Can Bangladesh produce enough cereals to meet future demand? Agric Syst. 2018;163:36–44. doi:10.1016/j.agsy.2016.11.003. [Google Scholar] [PubMed] [CrossRef]
7. Alam E, Hridoy AE, Tusher SMSH, Islam ARMT, Islam MK. Correction: climate change in Bangladesh: temperature and rainfall climatology of Bangladesh for 1949–2013 and its implication on rice yield. PLoS One. 2023;18(12):e0295718. doi:10.1371/journal.pone.0295718. [Google Scholar] [PubMed] [CrossRef]
8. Alam K. Farmers’ adaptation to water scarcity in drought-prone environments: a case study of Rajshahi District, Bangladesh. Agric Water Manag. 2015;148(1):196–206. doi:10.1016/j.agwat.2014.10.011. [Google Scholar] [CrossRef]
9. Gogoi A, Tripathi B, 42% India’s land area under drought, worsening farm distress in election year. Mumbai, India: India Spend; 2019[cited 2025 Jan 1]. Available from: https://www.indiaspend.com/42-indias-land-area-underdrought-worsening-farm-distress-in-election-year/. [Google Scholar]
10. Waraich EA, Ahmad R, Ashraf MY. Role of mineral nutrition in alleviation of drought stress in plants. Aust J Crop Sci. 2010;5:764–77. [Google Scholar]
11. EL Sabagh A, Hossain A, Barutçular C, Islam MS, Ahmad Z, Wasaya A, et al. Adverse effect of drought on quality of major cereal crops: implications and their possible mitigation strategies. In: Hasanuzzaman M, editor. Agronomic crops. Singapore: Springer; 2020. p. 635–58. doi:10.1007/978-981-15-0025-1_31. [Google Scholar] [CrossRef]
12. Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, et al. Crop production under drought and heat stress: plant responses and management options. Front Plant Sci. 2017;8:1147. doi:10.3389/fpls.2017.01147. [Google Scholar] [PubMed] [CrossRef]
13. Farooq M, Hussain M, Siddique KHM. Drought stress in wheat during flowering and grain-filling periods. Crit Rev Plant Sci. 2014;33(4):331–49. doi:10.1080/07352689.2014.875291. [Google Scholar] [CrossRef]
14. Dong W, Zhang L, Duan Y, Sun L, Zhao P, van der Werf W, et al. Ridge and furrow systems with film cover increase maize yields and mitigate climate risks of cold and drought stress in continental climates. Field Crops Res. 2017;207:71–8. doi:10.1016/j.fcr.2017.03.003. [Google Scholar] [CrossRef]
15. Ahmed S, Kouser S, Asgher M, Gandhi SG. Plant aquaporins: a frontward to make crop plants drought resistant. Physiol Plant. 2021;172(2):1089–105. doi:10.1111/ppl.13416. [Google Scholar] [PubMed] [CrossRef]
16. Heikal YM, El-Esawi MA, El-Ballat EM, Abdel-Aziz HMM. Applications of nanoparticles for mitigating salinity and drought stress in plants: an overview on the physiological, biochemical and molecular genetic aspects. N Z J Crop Hortic Sci. 2023;51(3):297–327. doi:10.1080/01140671.2021.2016870. [Google Scholar] [CrossRef]
17. Islam S, Hasan MM, Sayed MZI, Sikder S, Chowdhury AK. Amelioration of the detrimental effects of water deficit stress on lentil (lens culinaris medik) through the utilization of poultry litter-based compost. Turkish JAF SciTech. 2024;12(6):1080–7. doi:10.24925/turjaf.v12i6.1080-1087.6739. [Google Scholar] [CrossRef]
18. Abu-Ria ME, Elghareeb EM, Shukry WM, Abo-Hamed SA, Ibraheem F. Mitigation of drought stress in maize and Sorghum by humic acid: differential growth and physiological responses. BMC Plant Biol. 2024;24(1):514. doi:10.1186/s12870-024-05184-4. [Google Scholar] [PubMed] [CrossRef]
19. Abdelaal K, AlKahtani M, Attia K, Hafez Y, Király L, Künstler A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology. 2021;10(6):520. doi:10.3390/biology10060520. [Google Scholar] [PubMed] [CrossRef]
20. Mutlu A, Tas T. Foliar application of humic acid at heading improves physiological and agronomic characteristics of durum wheat (Triticum durum L.). J King Saud Univ Sci. 2022;34(8):102320. doi:10.1016/j.jksus.2022.102320. [Google Scholar] [CrossRef]
21. Karakurt Y, Unlu H, Unlu H, Padem H. The influence of foliar and soil fertilization of humic acid on yield and quality of pepper. Acta Agric Scand Sect B Soil Plant Sci. 2009;59(3):233–7. doi:10.1080/09064710802022952. [Google Scholar] [CrossRef]
22. Khedr RA, Sorour SGR, Aboukhadrah SH, El Shafey NM, Abd Elsalam HE, El-Sharnouby ME, et al. Alleviation of salinity stress effects on agro-physiological traits of wheat by auxin, Glycine betaine, and soil additives. Saudi J Biol Sci. 2022;29(1):534–40. doi:10.1016/j.sjbs.2021.09.027. [Google Scholar] [PubMed] [CrossRef]
23. El-Hashash EF, Abou El-Enin MM, Abd El-Mageed TA, Attia MAE, El-Saadony MT, El-Tarabily KA, et al. Bread wheat productivity in response to humic acid supply and supplementary irrigation mode in three northwestern coastal sites of Egypt. Agronomy. 2022;12(7):1499. doi:10.3390/agronomy12071499. [Google Scholar] [CrossRef]
24. Alsamadany H. Physiological, biochemical and molecular evaluation of mungbean genotypes for agronomical yield under drought and salinity stresses in the presence of humic acid. Saudi J Biol Sci. 2022;29(9):103385. doi:10.1016/j.sjbs.2022.103385. [Google Scholar] [PubMed] [CrossRef]
25. Lotfi R, Gharavi-Kouchebagh P, Khoshvaghti H. Biochemical and physiological responses of Brassica napus plants to humic acid under water stress. Russ J Plant Physiol. 2015;62(4):480–6. doi:10.1134/s1021443715040123. [Google Scholar] [CrossRef]
26. Kocheva K, Nenova V, Karceva T, Petrov P, Georgiev G, Börner A, et al. Changes in water status, membrane stability and antioxidant capacity of wheat seedlings carrying different rht-B1 dwarfing alleles under drought stress. J Agron Crop Sci. 2014;200(2):83–91. doi:10.1111/JAC.12047. [Google Scholar] [CrossRef]
27. Witham FH, Blaydes DF, Devlin RM, Exercise in plant physiology. 2nd ed. Boston, MA, USA: Prindle Weber and Schmidt Publishers; 1986. p. 128–31. [Google Scholar]
28. Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–7. doi:10.1007/bf00018060. [Google Scholar] [CrossRef]
29. IRRI (International Rice Research Institute). STAR, version 2.0.1. In: Biometrics and breeding informatics, PBGB division. LosBaños, Laguna: International Rice Research Institute; 2014. [Google Scholar]
30. Makino A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011;155(1):125–9. doi:10.1104/pp.110.165076. [Google Scholar] [PubMed] [CrossRef]
31. de Freitas Moura LM, da Costa AC, Müller C, de Oliveira Silva-Filho R, Almeida GM, da Silva AA, et al. Morpho-physiological traits and oil quality in drought-tolerant Raphanus sativus L. used for biofuel production. Plants. 2024;13(12):1583. doi:10.3390/plants13121583. [Google Scholar] [PubMed] [CrossRef]
32. Arslan E, Agar G, Aydin M. Humic acid as a biostimulant in improving drought tolerance in wheat: the expression patterns of drought-related genes. Plant Mol Biol Rep. 2021;39(3):508–19. doi:10.1007/s11105-020-01266-3. [Google Scholar] [CrossRef]
33. Racz I, Hirişcău D, Berindean I, Kadar R, Muntean E, Tritean N, et al. The influence of flag leaf removal and its characteristics on main yield components and yield quality indices on wheat. Agronomy. 2022;12(10):2545. doi:10.3390/agronomy12102545. [Google Scholar] [CrossRef]
34. Ahmad A, Aslam Z, Javed T, Hussain S, Raza A, Shabbir R, et al. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy. 2022;12(2):287. doi:10.3390/agronomy12020287. [Google Scholar] [CrossRef]
35. Lamlom SF, Irshad A, Mosa WFA. The biological and biochemical composition of wheat (Triticum aestivum) as affected by the bio and organic fertilizers. BMC Plant Biol. 2023;23(1):111. doi:10.1186/s12870-023-04120-2. [Google Scholar] [PubMed] [CrossRef]
36. Sindabad MAS, Pramanik SK, Rahman MA, Bahadur MM, Sikder S. Mitigation of water deficit stress in wheat by foliar feeding of potassium. J Sci Technol. 2023;21(1):1–11. doi:10.59125/jst.21101. [Google Scholar] [CrossRef]
37. Abou Tahoun AMM, Abou El-Enin MM, Mancy AG, Sheta MH, Shaaban A. Integrative soil application of humic acid and foliar plant growth stimulants improves soil properties and wheat yield and quality in nutrient-poor sandy soil of a semiarid region. J Soil Sci Plant Nutr. 2022;22(3):2857–71. doi:10.1007/s42729-022-00851-7. [Google Scholar] [PubMed] [CrossRef]
38. Wang Z, Li G, Sun H, Ma L, Guo Y, Zhao Z, et al. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol Open. 2018;7(11):bio035279. doi:10.1242/bio.035279. [Google Scholar] [PubMed] [CrossRef]
39. Olk DC, Dinnes DL, Rene Scoresby J, Callaway CR, Darlington JW. Humic products in agriculture: potential benefits and research challenges a review. J Soils Sediment. 2018;18(8):2881–91. doi:10.1007/s11368-018-1916-4. [Google Scholar] [CrossRef]
40. Tohidi HR. The study of humic acid foliar application on physiological and biochemical changes in wheat under irrigation withholding at different growth stages. Int J Nat Sci. 2016;5(1):1–7. doi:10.3329/ijns.v5i1.28604. [Google Scholar] [CrossRef]
41. Liang X, Zhang L, Natarajan SK, Becker DF. Proline mechanisms of stress survival. Antioxid Redox Signal. 2013;19(9):998–1011. doi:10.1089/ars.2012.5074. [Google Scholar] [PubMed] [CrossRef]
42. Nowsherwan I, Shabbir G, Malik SI, Ilyas M, Iqbal MS, Musa M. Effect of drought stress on different physiological traits in bread wheat. SAARC J Agric. 2018;16(1):1–6. doi:10.3329/sja.v16i1.37418. [Google Scholar] [CrossRef]
43. Keyvan S. The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J Anim Plant Sci. 2010;8:1051–60. [Google Scholar]
44. Nikbakht A, Kafi M, Babalar M, Xia YP, Luo A, Etemadi NA. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of Gerbera. J Plant Nutr. 2008;31(12):2155–67. doi:10.1080/01904160802462819. [Google Scholar] [CrossRef]
45. Ju F, Pang J, Huo Y, Zhu J, Yu K, Sun L, et al. Potassium application alleviates the negative effects of salt stress on cotton (Gossypium hirsutum L.) yield by improving the ionic homeostasis, photosynthetic capacity and carbohydrate metabolism of the leaf subtending the cotton boll. Field Crops Res. 2021;272:108288. doi:10.1016/j.fcr.2021.108288. [Google Scholar] [CrossRef]
46. Buckley TN. How do stomata respond to water status? New Phytol. 2019;224(1):21–36. doi:10.1111/nph.15899. [Google Scholar] [PubMed] [CrossRef]
47. Jannat S, Abu Hasan M, Bari CAKMM, Hafiz MHR, Sinthy TA, Pramanik SK, et al. Physiological indices and yield traits of wheat genotypes under drought stress condition. Int J Agric Sci Vet Med. 2023;11(1):21–30. doi:10.25303/1101ijasvm021030. [Google Scholar] [CrossRef]
48. Liu M, Wang C, Wang F, Xie Y. Maize (Zea mays) growth and nutrient uptake following integrated improvement of vermicompost and humic acid fertilizer on coastal saline soil. Appl Soil Ecol. 2019;142(62):147–54. doi:10.1016/j.apsoil.2019.04.024. [Google Scholar] [CrossRef]
49. El-Sayed ME, Soliman GM, Ahmed AF. Impact of silicon and humic acid application under water stress condition on some bread wheat cultivars and some soil properties. Assiut J Agric Sci. 2018;49(4):138–57. doi:10.21608/ajas.2019.28885. [Google Scholar] [CrossRef]
50. Forgone AG. Physiological indicators of drought tolerance of wheat. Szeged, Hungary, Szeged: Department of plant Biology, Faculty of Science and Informatics, University of Szeged; 2009. Biology PhD Program. [cited 2025 Jan 1]. http://doktori.bibl.u-szeged.hu/id/eprint/1003/3/Thesis_Guoth.pdf. [Google Scholar]
51. Nasiroleslami E, Mozafari H, Sadeghi-Shoae M, Habibi D, Sani B. Changes in yield, protein, minerals, and fatty acid profile of wheat (Triticum aestivum L.) under fertilizer management involving application of nitrogen, humic acid, and seaweed extract. J Soil Sci Plant Nutr. 2021;21(3):2642–51. doi:10.1007/s42729-021-00552-7. [Google Scholar] [CrossRef]
52. Alsudays IM, Alshammary FH, Alabdallah NM, Alatawi A, Alotaibi MM, Alwutayd KM, et al. Applications of humic and fulvic acid under saline soil conditions to improve growth and yield in barley. BMC Plant Biol. 2024;24(1):191. doi:10.1186/s12870-024-04863-6. [Google Scholar] [PubMed] [CrossRef]
53. Mehrinfar AR, Rezaei MM, Mirmahmoody T, Yazdan SS, Yazdan SS. The effect of foliar application of nutrients and humic acid on grain yield and quality of bread wheat cultivars under drought stress. Environ Stress Crop Sci. 2023;16(1):1–18. doi:10.22077/escs.2022.4234.2000. [Google Scholar] [CrossRef]
54. Rana MS, Ma H, Bahadur MM, Islam MR. Physiological evaluation of wheat genotypes for tolerance to water deficit stress. Bangladesh Agron J. 2018;20(2):37–52. doi:10.3329/baj.v20i2.37086. [Google Scholar] [CrossRef]
55. Arjumend T, Abbasi MK, Rafique E. Effects of lignite-derived humic acid on some selected soil properties, growth and nutrient uptake of wheat (Triticum aestivum L.) grown under greenhouse conditions. Pak J Bot. 2015;47:2231–8. [Google Scholar]
56. Helmy Hegab R, Abd El-atty Fawy H, Ahmed Mohamed Habib A. Evaluates effect of amino acids, humic acid and antioxidants as foliar application on the biochemical content and productivity of wheat under north Sinai soils conditions. Am J Agric For. 2020;8(4):167. doi:10.11648/j.ajaf.20200804.19. [Google Scholar] [CrossRef]
57. Zhang J, Zhang S, Cheng M, Jiang H, Zhang X, Peng C, et al. Effect of drought on agronomic traits of rice and wheat: a meta-analysis. Int J Environ Res Public Health. 2018;15(5):839. doi:10.3390/ijerph15050839. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools