 Open Access
Open Access
ARTICLE
Effects of Drought Stress on the Physiological Characteristics of Flue-Cured Tobacco during the Vigorous Growing Period
1 Upland Flue-Cured Tobacco Quality and Ecology Key Laboratory, Guizhou Academy of Tobacco Science, Guiyang, 550081, China
2 College of Juncao Science and Ecology, Fujian Agriculture and Forestry University, Fuzhou, 350002, China
3 Guizhou Provincial Tobacco Company of China National Tobacco Corporation, Guiyang, 550018, China
4 School of Geography and Resources, Guizhou Education University, Guiyang, 550018, China
* Corresponding Author: Zhaowei Li. Email:
(This article belongs to the Special Issue: Abiotic Stress in Agricultural Crops)
Phyton-International Journal of Experimental Botany 2025, 94(4), 1287-1298. https://doi.org/10.32604/phyton.2025.062385
Received 17 December 2024; Accepted 21 March 2025; Issue published 30 April 2025
Abstract
To systematically examine the tolerance of flue-cured tobacco K326 to soil drought stress, this study set up different water content trials for K326 at 60%, 40%, and 20% levels during the vigorous growing period by greenhouse pot planting. Pigment content, chlorophyll fluorescence parameters, antioxidant enzyme activity, and SPS and GS activities associated with carbon and nitrogen metabolism were investigated in the K326 tobacco leaves. The results showed that chlorophyll a and chlorophyll b contents decreased, non-photochemical quenching coefficient Y(NPQ) decreased in the later stage of vigorous growth, and energy dissipation quantum yield Y(NO) levels increased under the drought stresses, suggesting a decrease in the efficiency of conversion by light to electricity in the PSII reaction center. SOD and CAT activities were elevated during the early stage of drought stresses but sharply declined during the later stage of drought stresses. POD enzyme activity was less affected by moderate drought stress, but it was inhibited by severe drought stress. Additionally, moderate drought stress increased the SPS activity and reduced GS activity in K326 tobacco leaves in the later growth stage, indicating that moderate drought stress can promote the transduction from nitrogen metabolism to carbon metabolism in tobacco leaves in the later growth stage, which will help to improve the internal chemical quality of tobacco leaves.Keywords
Tobacco originates in tropical regions and has high water requirements during the growth, development, and quality formation stages [1]. The production and quality of tobacco leaves can be altered by an insufficient or excessive water supply, which can also affect tobacco plants’ growth status and physiological metabolism [2]. As one of China’s most significant economic crops, tobacco requires much water during its growing season. Water scarcity during seed germination and seedling stage can seriously alter seed vitality, germination rate, and emergence uniformity. After transplanting, seedlings are vulnerable to drought stress, which can cause growth and development abnormalities once suffer an insufficient water supply [3]. Tobacco fields with less than 50% field water holding capacity will significantly impact the growth status of tobacco leaves during the vigorous growth stage, resulting in decreased tobacco output and quality [4,5].
The primary pigment for photosynthesis in plants is chlorophyll. Tobacco leaves’ chlorophyll is extremely vulnerable to water stress. Water deficit significantly reduces the chlorophyll in tobacco leaves, affecting the leaves absorbing solar energy. Because of this, the photosynthetic capacity of tobacco leaves is reduced, which hinders tobacco leaf growth and inhibits the normal development of tobacco plants [6]. The antioxidant enzyme system plays a major role in controlling the plant stress response. Moderate water stress can significantly affect the antioxidant enzyme activity in tobacco leaf cells, whereas extreme drought stress can significantly reduce the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), which can affect the development of tobacco leaves [7]. Furthermore, carbohydrates and nitrogen-containing compounds are important intermediates that affect tobacco leaf quality, acting as regulators in tobacco plant growth and development [8,9]. Tobacco leaves transport and convert photosynthetic products in the form of sucrose via controlling carbon metabolism pathways [10]. Among them, sucrose phosphate synthase (SPS), an enzyme that catalyzes the reaction between fructose-6-phosphate and UDPG to produce sucrose, is one of the most important enzymes in the sucrose synthesis and a key participant in the carbon metabolism of tobacco leaves [11]. Nitrogen metabolism consists of the reduction and assimilation of inorganic nitrogen, as well as the synthesis, transformation, and breakdown of organic nitrogen-containing compounds. The amount of protein and other nitrogen-containing compounds in tobacco leaves is influenced by the intensity of nitrogen metabolism, which controls the growth status, yield, and quality of tobacco leaves [9,12]. Glutamine synthase (GS), a crucial enzyme that catalyzes glutamine synthesis, is a useful physiological biomarker of plant nitrogen assimilation. It is essential for nitrogen utilization and protein synthesis in tobacco leaves [13].
In most regions of China, Nicotiana tabacum K326 (K326, flue-cured tobacco) can complete its life cycle due to its strong temperature and light tolerance. However, K326 is not drought-tolerant and requires much water during its growth and development, particularly in northern regions with limited water supplies [14,15]. Long-term drought activates the gene regulatory networks of hormones and photosynthesis in K326 [15]. Tobacco leaves can grow and develop normally when the soil moisture level of 60%. When there is a severe water deficit in the soil, tobacco leaves shrink, leaf opening is prevented, tissue cell expansion slows, and plant elongation delays or even stagnates. For tobacco plants, the vigorous growing period is the most active and vigorous stage for dry matter accumulation. During this period, tobacco plants require the greatest water. Lack of water supply can easily result in the premature blooming of tobacco plants, poor leaf opening, and smooth leaf formation. The flue-curing characteristics of tobacco leaves decrease, severely limiting the output and quality of cigarette products [1,5]. Therefore, this study combined the arid climate conditions that tobacco in the Guizhou region is prone to encountering in the vigorous period, set different degrees of water stress, and investigated the photosynthetic characteristics, antioxidant enzymes, and key enzyme activities of carbon and nitrogen metabolism of tobacco K326 in the vigorous period. The effects of different water stresses on the physiological characteristics of tobacco K326 were elucidated, providing valuable insights for optimizing water-saving tobacco cultivation.
The experiment was conducted in the greenhouse at the Longgang Base of the Guizhou Academy of Tobacco Science using soil cultured in pots. The experimental pot was 40 cm in height, 40 cm in mouth diameter, and 35 cm in bottom diameter, with a row spacing of 110 cm by 55 cm. The test material was Nicotiana tabacum K326; it has a high yield and is very highly resistant to disease. The original tobacco is orange-yellow and has a high oil content. It is the most widely grown variety in China and is known for its strong aroma and burning ability. Using a floating method for tobacco seedling cultivation, the seedlings were transplanted on 13 May. Each pot contained a single tobacco plant, and each pot received two kilograms of water every three days. When the tobacco plants reached the vigorous growth stage (about 25 days after transplantation), the water supply was restricted and the soil moisture content was maintained at 60% (control), 40% (moderate drought stress), and 20% (severe drought stress), by watered normally to maintain water content. The water content was real-time monitored with FieldScout TDR 300 Soil Moisture Meter (Spectrum, Inc., Aurora, IL, USA). Fresh tobacco leaves were collected at 10, 25, 40, and 55 days after water stress treatment to measure the photosynthetic pigment content and chlorophyll fluorescence parameters of tobacco leaves; leaf samples were taken at 0, 10, 20, 30, 40, and 50 days after water stress treatment to measure the activities of antioxidant enzymes, sucrose phosphate synthase (SPS), and glutamine synthetase (GS).
2.2 Determination of Photosynthetic Pigments
Photosynthetic pigment content in tobacco leaves was measured by a spectrophotometer [16]. Weigh 0.1 g of freshly cut and mixed tobacco leaf samples, then transfer them to a glass test tube with a stoppered cover. Next, quickly add 20 mL of 95% ethanol over the leaf sample, and then place them in a dark environment until the samples become white. The extraction solution’s absorbance values were measured at 665, 649, and 470 nm, respectively. The photosynthetic pigment content was calculated using the formula below:
Chlorophyll a concentration (μg/mL) = 13.95 A665 − 6.88 A649
Chlorophyll b concentration (μg/mL) = 24.96 A649 − 7.32 A665
Carotenoid concentration (μg/mL) = (1000 A470 − 2.05 Ca − 114 Cb)/245
Chlorophyll component content (μg/mg) = (chlorophyll concentration × extraction solution volume)/sample fresh weight
A665, A649, and A470 represents the absorbance value of the extraction solution at 665, 649, and 470 nm, respectively.
2.3 Determination of Chlorophyll Fluorescence Parameters
After 30 min in a dark environment, select the top second leaf and use a chlorophyll fluorescence imager (Maxi Imaging PAM, Effeltrich Germany) to measure the primary light energy utilization rate Fv/Fm, non-photochemical quenching coefficient Y(NPQ), energy dissipation quantum yield Y(NO), and photosynthetic electron transfer rate ETR of live plant leaves.
2.4 Antioxidant Enzyme, SPS, and GS Activity Detection
Enzyme extraction was carried out according to the method described by Wang et al. [16]. 0.5 g of fresh leaves were mixed with 3 mL of cooled 50 mmol/L Tris-HCl solution (pH = 7.0) and a tiny bit of quartz sand. The leaves were then ground thoroughly on ice, transferred to a 10 mL centrifuge tube, rinsed with 2 mL of extraction solution, collected, centrifuged at 10,000× g at 4°C for 10 min, and the supernatant was collected for enzyme activity determination.
SOD activity was measured using the Nitro blue tetrazolium (NBT) method, POD activity was measured using the Guaiacol method [16], and SPS, GS, CAT activity, and enzyme protein were all measured using a kit manufactured by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Microsoft Excel 2016 and SPSS 16.0 software were used to standardize or normalize the raw data, perform data statistics, and variance analysis. Each dataset consisted of three biological replicates.
3.1 The Effect of Drought Stress on Photosynthetic Pigments in Tobacco Leaves
The results of pigment content in tobacco leaves under various drought stresses showed that chlorophyll a and b content steadily decreased during the vigorous growth to maturity period. The chlorophyll a and b levels in tobacco leaves did not significantly differ across treatments following ten days of drought stress. At 25 days of drought stress, the chlorophyll a content under severe drought stress (soil moisture content of 20%) was significantly lower than that of the control group (soil moisture content of 60%) (p < 0.05), while the chlorophyll b content was significantly higher than that under moderate drought stress and the control group. At 40 days of drought stress, the chlorophyll a and b contents under moderate drought stress (soil moisture content of 40%) and severe drought stress were significantly lower than those in the control group (p < 0.05). However, chlorophyll a and b content did not significantly differ among stress levels at 55 days of drought stress (Fig. 1A,B). There was no significant difference in chlorophyll a/b values across drought stress treatments (Fig. 1C). Furthermore, carotenoid content remained relatively stable with the development of tobacco plants, and there was little fluctuation in carotenoid content under various drought stresses [14]. The decrease in carotenoid content under severe and moderate drought stresses surpassed that of the control group only after 40 days of drought stress (p < 0.05). At 55 days of drought stress, however, there is no significant difference in ca-rotenoid content across drought stress treatments (Fig. 1D), indicating that drought stress does not generate substantial changes in carotenoid content in tobacco K326 leaves.
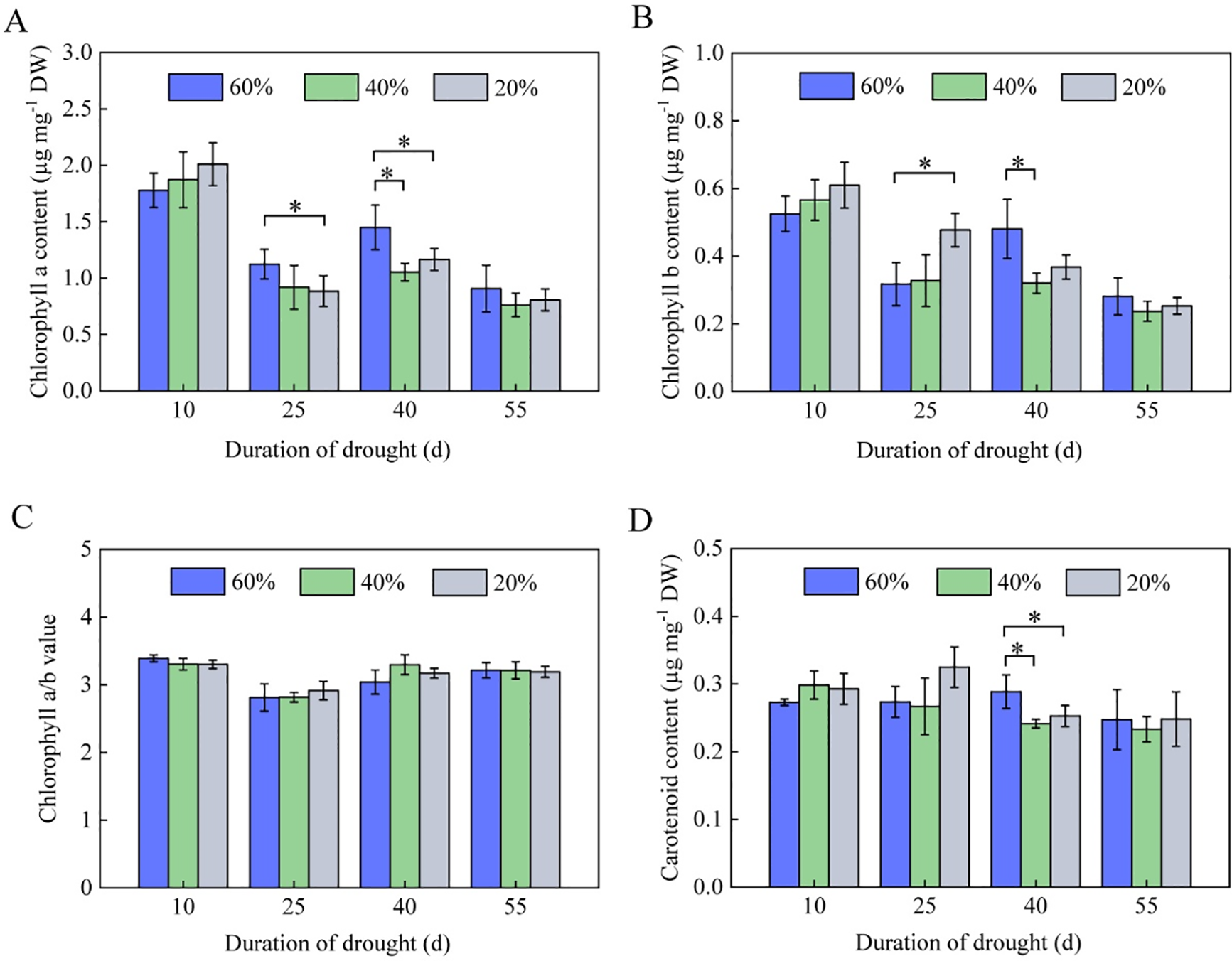
Figure 1: Pigment content in the leaves of flue-cured tobacco K326 under drought stress during the vigorous growing period. (A) Chlorophyll a content; (B) Chlorophyll b content; (C) Chlorophyll a/b value; and (D) Carotenoid content. The asterisks represent a significant difference between treatments (p < 0.05)
3.2 The Effect of Drought Stress on Chlorophyll Fluorescence Parameters in Tobacco Leaves
The Fv/Fm ratio represents the primary light energy utilization efficiency of PSII [17]. As shown in Fig. 2A, different drought stresses had no significant effect on the Fv/Fm of vigorous-growing tobacco leaves, suggesting that the primary light energy utilization efficiency of the tobacco leaf photosystem was not significantly inhibited by drought stresses.
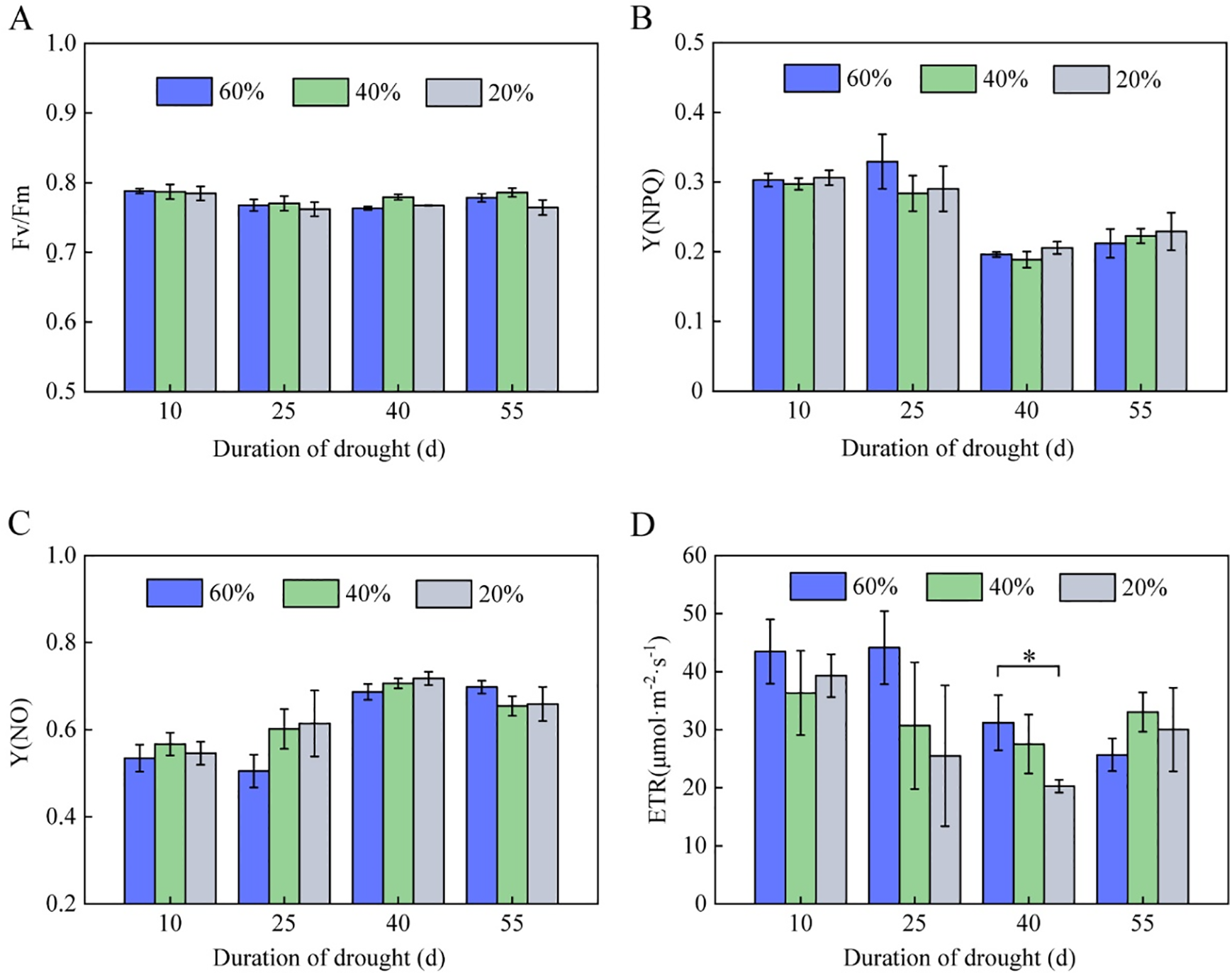
Figure 2: Chlorophyll fluorescence parameters in the leaves of flue-cured tobacco K326 under drought stress during the vigorous growing period. (A) Fv/Fm; (B) Y(NPQ); (C) Y(NO); and (D) ETR. The asterisks represent a significant difference between treatments (p < 0.05)
The non-photochemical quenching coefficient Y(NPQ) under all three water conditions began to decrease after 40 days of drought stress (Fig. 2B), however, the non-regulatory energy dissipation quantum yield Y(NO) started to increase after 25 days of drought stress and was higher than the control. After 40 days of drought stress, Y(NO) increased by nearly 30% in comparison to the initial stage of stress (Fig. 2C). These results suggested that drought stress weakened the tobacco plant’s ability to control energy dissipation and provide light protection, which caused damage to the photosynthetic system of tobacco leaves.
The photosynthetic electron transfer rate (ETR) value in tobacco leaves began to decline after 25 days of drought stress and eventually decreased over time. At 40 days of drought stress, the ETR value reached its lowest point, and tobacco leaves under severe drought stress had a significantly lower ETR value than the control (Fig. 2D, p < 0.05), indicating that drought stress decreased the photosynthetic electron transfer rate in tobacco tissue cells and inhibited tobacco leaves’ photosynthetic assimilation ability.
3.3 The Effect of Drought Stress on Antioxidant Enzyme Activity in Tobacco Leaves
At 10 days of drought stress, the SOD activity in tobacco leaves under drought stress was higher than that in the control group, with severe and moderate drought stress increasing by 17.53% and 9.08%, respectively, in comparison to the control group. Afterward, under severe drought stress, the SOD activity in tobacco leaves rapidly decreased and remained below that of the moderate drought stress and control group throughout the same period. Under moderate drought stress, SOD activity in tobacco leaves remained high within 30 days of stress, surpassing that of severe drought stress and the control group. After 40 days of drought stress, it fell to a level equal to severe drought stress, which was lower than in the control group (Fig. 3A).
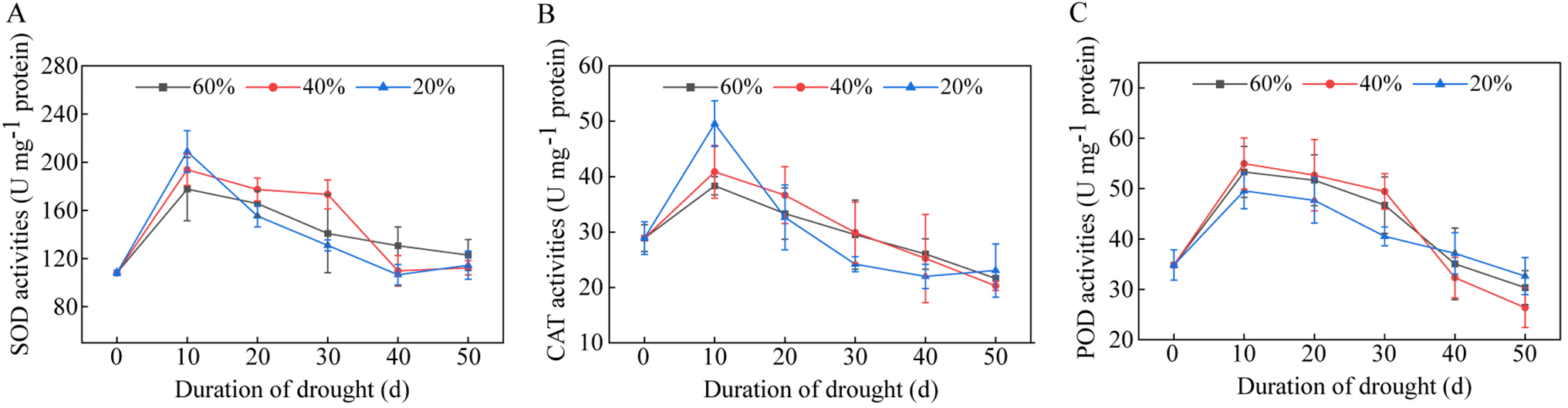
Figure 3: The activities of antioxidant enzymes in the leaves of flue-cured tobacco K326 under drought stress during the vigorous growing period. (A) SOD activities; (B) CAT activities; and (C) POD activities
The trend of CAT activity changes in each treatment of tobacco leaves is comparable to that of SOD activity (Fig. 3B). Among these, the CAT activity of tobacco leaves under severe drought stress exhibited the largest change amplitude; following 10 days of drought stress, it rapidly increased and was higher than that of the moderate drought stress and control group. However, within 20 days of moderate drought stress, the CAT activity in tobacco leaves was higher than that of the control group, and after that fell below the level of the control group.
Drought stress significantly affected POD activity in tobacco leaves. POD activity rapidly increased and remained high throughout the early stage of vigorous growth before gradually decreasing after 30 days of drought stress treatment. Within 30 days of drought stress, POD activity under severe drought stress was lower than that under moderate drought stress and the control group; after 30 days of drought stress, POD activity exhibited a declining trend under various drought conditions, with the degree of decline varying from moderate drought stress to control group and severe drought stress. POD activity under severe drought stress was lower than that under moderate drought stress and the control group. Under moderate drought stress, tobacco leaves’ POD activity varied significantly.
3.4 The Effect of Drought Stress on the Activities of Sucrose Phosphate Synthase (SPS) and Glutamine Synthetase (GS) in Tobacco Leaves
The SPS activity in tobacco leaves was measured under various drought stresses, and the results showed that drought stress inhibited the SPS activity in tobacco leaves. During the vigorous growing stage, SPS activity in tobacco leaves increased quickly, peaked in the late vigorous growing stage, and then decreased. Among these, SPS activity in tobacco leaves under moderate drought stress was lower than in the severe drought stress or control groups in the early vigorous growing stage. However, at 40 days of drought stress, the SPS activity in tobacco leaves under moderate drought stress was higher than in the other two treatments (Fig. 4A), indicating that under moderate stress, carbohydrate synthesis in tobacco leaves was lower in the early and middle stages of vigorous growing than it was under severe drought stress, but it was still higher in the late vigorous growing stage.
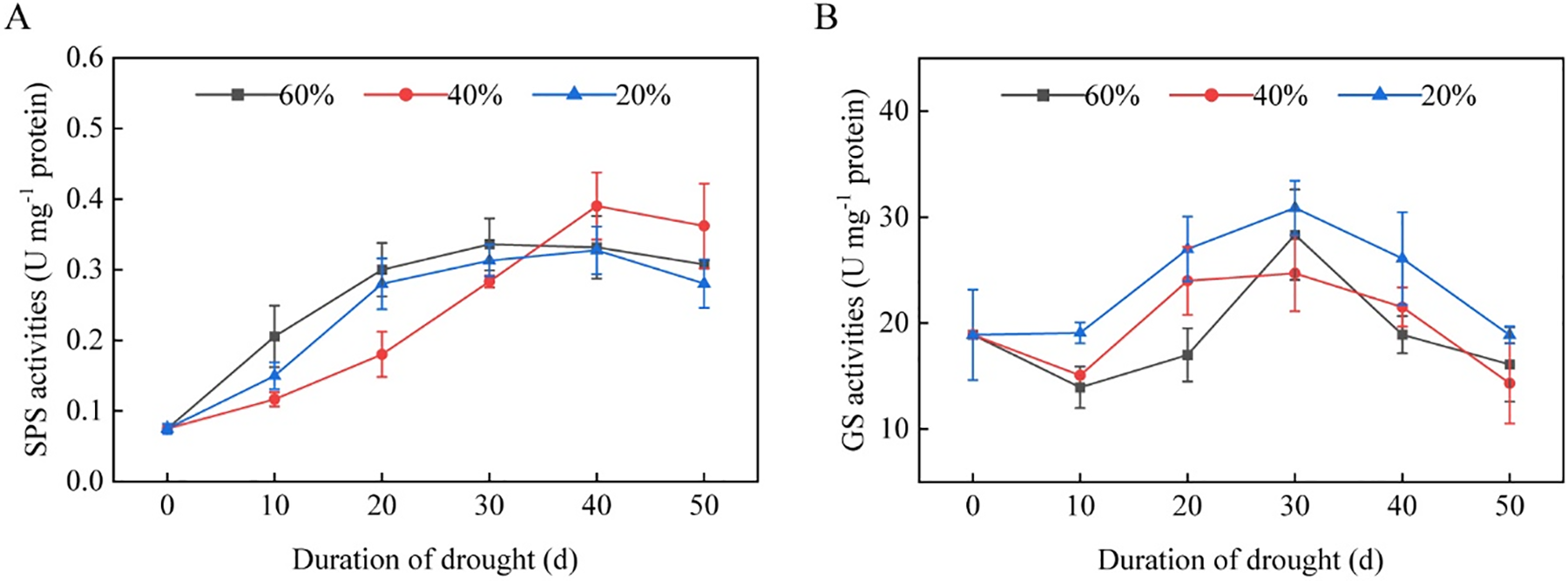
Figure 4: The activities of SPS and GS in the leaves of flue-cured tobacco K326 under drought stress during the vigorous growing period. (A) SPS activities; and (B) GS activities
The GS activity in tobacco leaves decreased to varying degrees in both the control group and the mediate drought stress group after 10 days of drought stress. However, both severe and moderate drought stresses resulted in an increase in GS activity after 10 days of stress. As the tobacco plant transitioned from vegetative to reproductive growth, GS activity peaked at 30 days of drought stress before gradually declining. GS activity under severe drought stress remained continuously higher than moderate drought stress (Fig. 4B). These findings suggested that drought stress can rapidly elevate GS activity in tobacco leaves, accelerating nitrogen metabolism.
During the vigorous growing period, the photosynthetic pigments in tobacco leaves are primarily regulated by adjusting photosynthesis to control tobacco plant growth, with the stability of chlorophyll levels being an important indicator of tobacco plants’ drought resistance [13–15]. In drought conditions, the chlorophyll content of tobacco leaf cells declines, and leaf stomata close, limiting water transpiration and loss [18]. Simultaneously, leaf stomata close hinders gas exchange between leaf mesophyll cells and the external environment, resulting in insufficient CO2 available for assimilation by leaf mesophyll cells, which leads to a decrease in tobacco leaves’ photosynthetic capacity and affects tobacco leaf extension, as well as the growth of tobacco plant tissues [19]. This study found that moderate and severe drought stresses for 25 days reduced chlorophyll a content in tobacco leaves during the vigorous growing period, and chlorophyll b and carotenoid content decreased significantly after 40 days of drought stress, suggesting that tobacco K326 is more susceptible to drought stress (Fig. 1A,B,D). The leaves photosynthetic pigments started to deteriorate and decrease after 25 days of moderate drought stress (Fig. 1A–D). In tobacco cultivation management, reducing pigment content in K326 tobacco leaves due to water restriction can be remedied by modifying cultivation agronomic measures or chemical control methods. According to Wang et al. [20], appropriate concentrations of exogenous 5-aminolevulinic acid treatment can slow down the decrease in photosynthetic pigment content in tobacco leaves caused by soil drought. Exogenous 2,4-brassinosteroid spraying can raise chlorophyll content in tobacco leaves, enhance photosynthetic rate, and dramatically boost tobacco growth under drought stress [21]. Furthermore, at 25 days of severe drought stress, chlorophyll b content was much higher than that of moderate drought stress and regular water supply, but later declined significantly (Fig. 1B), which may be attributed to the compensating effect of environmental ecological variables in tobacco. Reduced chlorophyll b content results from tobacco plants’ inability to completely compensate for chlorophyll b loss as severe drought stress continues [13,19].
Chlorophyll fluorescence parameters are crucial indicators for evaluating the photosynthetic physiology of plants because they may be used to depict photosystem II (PSII) capture, absorption, and electron transfer efficiency for solar energy in the thylakoid membrane of green tissue cells [21]. Moderate drought stress does not significantly affect tobacco leaves’ photosynthetic capacity, whereas severe drought stress reduces tobacco leaves’ net photosynthetic rate and leaf stomatal conductance by about 72.7% [22]. Soil drought stress mainly damages the electron acceptor of the photosystem in tobacco seedling leaves by reducing the openness of PSII reaction centers [23] and accumulating QA on the receptor of PSII reaction centers, resulting in excess light energy that deoxidizes QA, blocking the electron transfer pathway, and drastically lowers the photosynthetic electron transfer capacity. This leads to a considerable decrease in the Fv/Fm of tobacco leaves and a significant increase in the heat dissipation quantum ratio and heat dissipation [24]. This study found that the primary light energy utilization efficiency Fv/Fm of tobacco leaves was not significantly affected by drought stress during the vigorous growing period (Fig. 2A). However, after 40 days of drought stress, the non-photochemical quenching coefficient Y(NPQ) started to decrease under three moisture conditions, while Y(NO) increased (Fig. 2B,C). Still, there was no substantial difference between the various moisture treatments. This could be because the light system structure of tobacco plants during the vigorous growing period differed greatly from that of tobacco leaves at the seedling stage when drought stress was more likely to cause damage. These results also mean that K326 tobacco at the seedling stage is more sensitive to drought stress than that at the vigorous growing stage. Moreover, throughout the vigorous growing period, tobacco leaves’ light system structure was more influenced by their developmental dynamics than by soil water supply conditions. The initial light energy utilization rate Fv/Fm represents the capture and absorption ability of light reaction centers. The PSII structure of tobacco K326 leaves is relatively intact, enabling improved physical absorption of light energy in the beginning of drought stress. As the tobacco plant grows and develops, the photosynthetic system of leaves suffers physiological damage, Y(NO) increases, and its efficiency in converting light energy to electrical energy falls. This reduces the photochemical reaction of PSII, which results in a decrease in the non-photochemical quenching coefficient Y(NPQ) and the regulating ability of energy dissipation. Meanwhile, the physiological damage to the photosynthetic system lowers the photosynthetic electron transfer rate, as determined by ETR, and decreases tobacco leaves’ photosynthetic assimilation ability. In tobacco cultivation, appropriate selenium and water retaining agents can effectively alleviate the damage to PSII centers, partially compensate for the loss of PSII photochemical reaction function caused by drought stress, and increase the drought tolerance of tobacco leaves [24].
Plants under drought stress have more intracellular reactive oxygen species (ROS), which oxidatively damages the structure of the cell membrane. The antioxidant enzyme system, which consists mostly of SOD, CAT, and POD, is critical for the clearance of ROS [25,26]. SOD mainly removes superoxide anion radical (O2−) from plant cells, and its activity is closely associated with tobacco plants’ drought resistance. In the early stage of drought stress, SOD activity in tobacco varieties with high drought resistance rapidly increases, regulating the early adaptation of tobacco plant tissue cells to drought stress and preventing the accumulation of superoxide anion radical (O2−) in cells [24]. CAT and POD primarily remove the hydrogen peroxide H2O2 produced by superoxide anion radical (O2−) catalyzed by SOD. The activities of CAT and POD in tobacco leaves are higher in the early stage of drought stress but decline dramatically in the later stage of drought stress [27,28]. In this study, SOD and CAT activities in K326 tobacco leaves increased sharply over the first 10 days of drought stress before rapidly decreasing (Fig. 3A,B). POD activity was higher in the early stage of vigorous growing and started to decline in the late stage of vigorous growing (Fig. 3C). These results suggest that drought stress increased the cytoplasmic production of superoxide anion radical (O2−) in K326 tobacco leaves in the early stage of stress, induced the activity of SOD to catalyze the removal of excessive superoxide anion radical (O2−) in the cell, and caused a temporary buildup of hydrogen peroxide H2O2 in the cell, thereby inducing an increase in the activities of CAT and POD. However, tobacco leaves’ adaptability to drought stress declined as the drought stress continued and tobacco tissues grew and aged, and SOD activity started to decrease, while the accumulation of intracellular superoxide anion radical (O2−) increased. Excess superoxide anion radical (O2−) are not promptly removed, and as CAT and POD activity sharply decline, the generated H2O2 accumulates, exacerbating the peroxidation reaction of cell membrane lipids and causing oxidative damage to cell membrane structure [24,29].
The carbon metabolism of tobacco plants provides energy and a carbon skeleton for nitrogen metabolism. In contrast, nitrogen metabolism supplies the nitrogen-containing components and catalytic enzymes required for carbon metabolism, which operate together to regulate the growth and quality of tobacco plant tissues and organs [30]. When exposed to stresses, such as drought, tobacco plants respond by modulating the activity of enzymes involved in the metabolism of carbon and nitrogen and their products [31]. For example, tobacco plants that overexpress BnDREB1-5 show increased activity of important enzymes involved in carbon and nitrogen metabolism during drought stress, resulting in enhanced drought tolerance [32]. In this study, the activity of sucrose phosphate synthase (SPS), a crucial enzyme for starch synthesis in carbon metabolism, rapidly increased in tobacco after entering the vigorous growing stage, peaking at the bud stage (Fig. 4A). However, drought stress had an inhibitory effect on SPS activity. Under moderate drought stress, the increase in SPS activity in tobacco leaves was less than that under severe drought stress or in the control group. In the late stage of drought stress (40 days), as the tobacco plants reached maturity, SPS activity under moderate drought stress was higher than that under severe drought stress and the control group (Fig. 4A). These results suggest that moderate drought stress can enhance carbon metabolism in tobacco leaves during the vigorous growing stage. When tobacco plants move from vegetative to reproductive growth, glutamine synthetase (GS), an enzyme essential to nitrogen metabolism, reaches its highest activity. In contrast to severe drought stress, GS activity was decreased under moderate drought stress (Fig. 4B). Severe drought stress can accelerate nitrogen metabolism transformation and intensity in tobacco leaves, whereas moderate drought stress relatively decelerates nitrogen metabolism. The timely transition from nitrogen metabolism and carbon fixation to carbon accumulation metabolism of tobacco leaves at the appropriate growth stage is crucial to producing high-quality tobacco [33,34]. Tobacco leaves have higher nitrogen metabolism in the early stage of development, whereas lowering nitrogen metabolism in the later stage of development can enhance tobacco leaves’ carbon metabolism, encourage yellowing, and effectively improve their intrinsic chemical quality [35,36]. Thus, moderate drought stress during the late stage of tobacco plant growth may accelerate the transition of tobacco leaves from nitrogen metabolism to carbon metabolism, which has a favorable influence on improving tobacco quality.
Drought stress during the vigorous growing period reduced the content of chlorophyll a and chlorophyll b in tobacco K326, although the chlorophyll fluorescence parameter Fv/Fm remained mostly unchanged. Y(NPQ) decreased throughout the late vigorous growing period, but Y(NO) increased. Tobacco leaves’ photosynthetic system suffered physiological damage during the later growth period, reducing their ability to regulate energy dissipation. The antioxidant enzymes SOD and CAT are more active in the early stage of drought stress, allowing them to swiftly remove the accumulation of superoxide ions in tobacco cells generated by drought stress. However, SOD and CAT activities drastically decrease in the later stage of drought stress, whereas POD activity is unaffected by moderate drought stress but inhibited by severe drought stress. Additionally, moderate drought stress increases the SPS activity of tobacco leaves in the later stage of growth, inhibits GS activity, and promotes the conversion of nitrogen metabolism to carbon metabolism in tobacco leaves in the later stage of development, all of these would be beneficial for tobacco quality.
Acknowledgement: We would like to thank Instrumental Analysis Center of Fujian Agriculture and Forestry University for providing the facilities to determine the chlorophyll fluorescence parameters.
Funding Statement: This research was funded by the Science and Technology Project of China Tobacco Company (110202202016), Science and Technology Project of Science and Technology Department of Guizhou Province (QKHZC [2024]YB159 and QKHJC-ZK [2022]YB288), and Science and Technology Project of Guizhou Tobacco Company (2022XM17).
Author Contributions: Conceptualization: Zhaowei Li and Kesu Wei; validation: Zhaowei Li, Kesu Wei, and Bin Wei; formal analysis: Kesu Wei, Guangju Liu, and Qifang Zhang; investigation: Kesu Wei and Guangju Liu; data curation: Kesu Wei, Qifang Zhang, and Shengjiang Wu; writing—original draft preparation: Kesu Wei and Zhaowei Li; writing—review and editing: Zhaowei Li; supervision: Zhaowei Li; funding acquisition: Kesu Wei. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The raw data supporting the conclusions of this article will be made available by the authors on request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Wang YK, Li HL, Wang J, Wei JY. Progress of research on water stress in tobacco. Chin Tob Sci. 2005;26(4):33–6. doi:10.3969/j.issn.1007-5119.2005.04.011. [Google Scholar] [CrossRef]
2. Liu M, Liu X, Song Y, Hu Y, Yang C, Li J, et al. Tobacco production under global climate change: combined effects of heat and drought stress and coping strategies. Front Plant Sci. 2024;15:1489993. doi:10.3389/fpls.2024.1489993. [Google Scholar] [PubMed] [CrossRef]
3. Chaudhry S, Sidhu GPS. Climate change regulated abiotic stress mechanisms in plants: a comprehensive review. Plant Cell Rep. 2022;41(1):1–31. doi:10.1007/s00299-021-02759-5. [Google Scholar] [PubMed] [CrossRef]
4. Zia R, Nawaz MS, Siddique MJ, Hakim S, Imran A. Plant survival under drought stress: implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol Res. 2021;242:126626. doi:10.1016/j.micres.2020.126626. [Google Scholar] [PubMed] [CrossRef]
5. Mu Y, Qi SW, Li Y, Yang XH. Research progress of drought stress on tobacco. Crop Res. 2012;26(2):193–6. doi:10.3969/j.issn.1001-5280.2012.02.21. [Google Scholar] [CrossRef]
6. Yan W, Lu Y, Guo L, Liu Y, Li M, Zhang B, et al. Effects of drought stress on photosynthesis and chlorophyll fluorescence in blue honeysuckle. Plants. 2024;13(15):2115. doi:10.3390/plants13152115. [Google Scholar] [PubMed] [CrossRef]
7. Zhou CY, Wu GL, Duan ZQ, Wu LL, Gao YS, Chen KM. H2O2-NOX system: an important mechanism for developmental regulation and stress response in plants. Chin Bull Bot. 2010;45(5):615–31. doi:10.3969/j.issn.1674-3466.2010.05.012. [Google Scholar] [CrossRef]
8. Han JF, Yue CL, Jiang YF, Zhang XY. A study on photosynthetic characteristics add nitrogen metabolism in flue-cured tobacco under drought stress. Acta Agric Boreali Sin. 1994;9(2):39–45. doi:10.3321/j.issn:1000-7091.1994.02.008. [Google Scholar] [PubMed] [CrossRef]
9. Chen AG, Wang SS, Shen GM, Liang XF, Liu GL. Analysis of main material flows in carbon and nitrogen metabolism in maturing leaves in flue-cured tobacco. Acta Tabacaria Sin. 2010;16(4):30–4. doi:10.3969/j.issn.1004-5708.2010.04.007. [Google Scholar] [CrossRef]
10. Lei J, Lu YH, Li HY, Deng SY, Chen JJ. Advances on regulatory mechanism and index of carbon-nitrogen metabolism in flue-cured tobacco. Guangdong Agric Sci. 2018;45(12):20–6. doi:10.16768/j.issn.1004-874X.2018.12.004. [Google Scholar] [CrossRef]
11. Shi HZ, Han JF, Liu GS, Wang YT. Studies on the relationship of carbon and nitrogen metabolism to leaf flavor quality in flue-cured tobacco. Acta Tabacaria Sin. 1998;4(2):56–63. [Google Scholar]
12. Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M. The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant Cell Environ. 1999;22(10):1177–99. doi:10.1046/j.1365-3040.1999.00466.x. [Google Scholar] [CrossRef]
13. Zhao L, Zhou Y, Wang HY, Shi HZ, Zhao SM, Chang LK, et al. Research on the differential physiological responses in different flue-cured tobacco lines under drought stress. J Nucl Agric Sci. 2019;33(3):607–15. doi:10.11869/j.issn.100-8551.2019.03.0607. [Google Scholar] [CrossRef]
14. Li C, Wang C, Cheng Z, Li Y, Li W. Carotenoid biosynthesis genes LcLCYB, LcLCYE, and LcBCH from wolfberry confer increased carotenoid content and improved salt tolerance in tobacco. Sci Rep. 2024;14(1):10586. doi:10.1038/s41598-024-60848-3. [Google Scholar] [PubMed] [CrossRef]
15. Wang J, Zhang S, Fu Y, He T, Wang X. Analysis of dynamic global transcriptional atlas reveals common regulatory networks of hormones and photosynthesis across Nicotiana varieties in response to long-term drought. Front Plant Sci. 2020;11:672. doi:10.3389/fpls.2020.00672. [Google Scholar] [PubMed] [CrossRef]
16. Wang XK, Huang JL. Principles and techniques of plant physiology and biochemistry experiments. Beijing, China: Higher Education Press; 2015. 132 p. [Google Scholar]
17. Miyake C, Amako K, Shiraishi N, Sugimoto T. Acclimation of tobacco leaves to high light intensity drives the plastoquinone oxidation system—relationship among the fraction of open PSII centers, non-photochemical quenching of Chl fluorescence and the maximum quantum yield of PSII in the dark. Plant Cell Physiol. 2009;50(4):730–43. doi:10.1093/pcp/pcp032. [Google Scholar] [PubMed] [CrossRef]
18. Tan SL, Yang YJ, Liu T, Zhang SB, Huang W. Responses of photosystem I compared with photosystem II to combination of heat stress and fluctuating light in tobacco leaves. Plant Sci. 2020;292:110371. doi:10.1016/j.plantsci.2019.110371. [Google Scholar] [PubMed] [CrossRef]
19. Hu W, Kang J, Liu Y, Liu Y, Dong AW. The effect of drought stress on photosynthetic physiological characteristics of the different tobacco varieties. Chin Tob Sci. 2013;34(2):69–73. doi:10.3969/j.issn.1007-5119.2013.02.015. [Google Scholar] [CrossRef]
20. Wang FZ, Jin YN, Li ZW, Chen B, Xiong YN, Hao HH, et al. Effects of exogenous ALA (5-aminolevulinic acid) on photosynthesys and antioxidant system of flue-cured tobacco seedlings under drought stress. Chin Tob Sci. 2020;41(1):22–9. doi:10.13496/j.issn.1007-5119.2020.01.004. [Google Scholar] [CrossRef]
21. Ding DY, Zhang LX, Zhu ZW, Han D, Zhang YJ, Lu YT, et al. Effect of leaf spray 2, 4-epibrassinolide on drought resistance of tobacco. Chin Tob Sci. 2018;39(4):50–7. doi:10.13496/j.issn.1007-5119.2018.04.007. [Google Scholar] [CrossRef]
22. Li D, Shen HT, Wang YF, Wang LJ, Zhao SM, Liu L. Effect of exogenous hydrogen sulfide on photosynthetic fluorescence parameters and antioxidant system of flue-cured tobacco seedlings under drought stress. Acta Bot Boreali Occidentalia Sin. 2019;39(9):1609–17. doi:10.7606/j.issn.1000-4025.2019.09.1609. [Google Scholar] [CrossRef]
23. Chen B, Zhang J, Ma XH, Wang XD, Li JW, Xu ZC. Influences of exogenous selenium on the chlorophyll fluorescence characteristics and chemical composition in flue-cured tobacco under drought stress. J Agric Sci Tech. 2018;20(10):95–104. [Google Scholar]
24. Chen FQ, Shao HF, Jia GT, Xu ZC, Huang WX, Fan YK, et al. Effect of water retaining agent on photosynthetic characteristics and chlorophyll fluorescence parameters of tobacco. J Agric Sci Tech. 2016;18(5):157–63. [Google Scholar]
25. Yuan YB, Li JX, Ding FZ, Su XK. Effects of drought stress on activity of cell defense enzymes in flue-cured tobacco leaves. Chin Tob Sci. 2009;30(5):10–3. doi:10.3969/j.issn.1007-5119.2009.05.003. [Google Scholar] [CrossRef]
26. Liu Z, Liu S, Fei J, Wang P, Ma Y, Guan S, et al. The maize WRKY transcription factor ZmWRKY25 respond drought stress in transgenic tobacco. Phyton-Int J Exp Bot. 2024;93(12):3617–35. doi:10.32604/phyton.2024.052704. [Google Scholar] [CrossRef]
27. Devireddy AR, Tschaplinski TJ, Tuskan GA, Muchero W, Chen JG. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int J Mol Sci. 2021;22(16):8843. doi:10.3390/ijms22168843. [Google Scholar] [PubMed] [CrossRef]
28. Che Y, Wang H, Yao T, Wang Z, Bo L, Zhang H. Activation of the antioxidant system and transduction of the mediated by exogenous calcium improve drought resistance in tobacco. Plant Stress. 2024;13:100551. doi:10.1016/j.stress.2024.100551. [Google Scholar] [CrossRef]
29. Lin J, Wang YP, Zhao DF, Wen XX, Xu SF, Sun WD, et al. Effects of drought stress on photosynthesis and antioxidant properties of tobacco in budding stage. Acta Agric Boreali Occidentalis Sin. 2013;22(5):55–61. [Google Scholar]
30. Su J, Yao Y, Liu Y, Han Q, Zhang W. Function, structure and catalytic mechanism of sucrose phosphate synthase: a review. Chin J Biotechnol. 2021;37(6):1858–68. doi:10.13345/j.cjb.200743. [Google Scholar] [PubMed] [CrossRef]
31. Zhao HN, Gao SF, Chen P, Wu W, Gu Y, Zhang SN, et al. Research progress in tobacco carbon and nitrogen metabolism and its relationship with quality and stress resistance. Acta Agric Jiangxi. 2023;35(6):10–5. doi:10.19386/j.cnki.jxnyxb.2023.06.002. [Google Scholar] [CrossRef]
32. Hu YJ, Song K, Wang C, Liu WQ, Wei JY. Effects of drought stress on carbon and nitrogen metabolism and osmotic adjustment substances of transgenic tobacco with BnDREB1-5 gene. Chin Tob Sci. 2011;32(4):36–40. doi:10.3969/j.issn.1007-5119.2011.04.009. [Google Scholar] [CrossRef]
33. Bai WQ, Hu MY, Wang CP, Jiang XY, Lei KR, Wu H. Utilization of nitrogen in plants: a review. Jiangsu Agric Sci. 2020;48(4):1–11. [Google Scholar]
34. Zhang S, Xu ZC, Li JJ, Chen Z, Fang X. Advance on carbon and nitrogen metabolism and regulation of tobacco. Curr Biotechnol. 2016;6(5):312–8. doi:10.3969/j.issn.2095-2341.2016.05.02. [Google Scholar] [CrossRef]
35. Zhou LF, Guo N, Li FJ, Zeng FD, Lin JW, Chen JJ, et al. Effects of real-time and field precision nitrogen management on nitrogen metabolism of different parts of flue-cured tobacco. Guangdong Agric Sci. 2013;40(14):70–4. doi:10.3969/j.issn.1004-874X.2013.14.023. [Google Scholar] [CrossRef]
36. Deng SY, Xie J, Zhou LF, Li FJ, Zeng FD, Lin JW, et al. Effects of precise nitrogen application on carbon and nitrogen metabolism of flue-cured tobacco leaves at maturity stage. Tob Sci Technol. 2013;46(9):76–81. doi:10.3969/j.issn.1002-0861.2013.09.018. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools