 Open Access
Open Access
ARTICLE
Antibacterial, Antioxidant, and Anti-Inflammatory Activities of Artemisia dracunculus Essential Oil: Estragole as the Major Bioactive Compound
Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, 21955, Saudi Arabia
* Corresponding Author: Ammar Attar. Email:
(This article belongs to the Special Issue: Innovative Approaches in Experimental Botany: Essential Oils as Natural Therapeutics)
Phyton-International Journal of Experimental Botany 2025, 94(4), 1225-1237. https://doi.org/10.32604/phyton.2025.063207
Received 08 January 2025; Accepted 27 March 2025; Issue published 30 April 2025
Abstract
Artemisia dracunculus L., or tarragon, is a perennial herb from the Asteraceae family that is extensively cultivated for its aromatic leaves, which are valued for its preventative and therapeutic properties in both cookery and traditional medicine. This study aims to investigate the antibacterial, antioxidant, and anti-inflammatory properties of A. dracunculus (tarragon) essential oil (ADEO), with estragole (57.23%) identified as the major compound through gas chromatography-mass spectrometry (GC-MS) analysis. ADEO exhibited varying degrees of antibacterial activity, with Escherichia coli showing higher resistance (inhibition zone (IZ) = 14.7 ± 0.58 mm, minimum inhibitory concentration (MIC) = 2% and minimum bactericidal concentration (MBC) = 4%), while Bacillus subtilis (IZ = 24.05 ± 2.11 mm and MIC = MBC = 0.125%) and Staphylococcus aureus (IZ = 18.69 ± 1.45 mm, MIC = 0.0612% and MBC = 0.125%) were more sensitive to its actions. Antioxidant ability was assessed using 1,1-diphenyl-2-picrylhydrazil (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) (ABTS), xanthine oxidase inhibition, and beta-carotene bleaching assays. ADEO showed remarkable antiradical effect on DPPH (IC50 = 127.05 ± 3.47 μg/mL) and ABTS radical (IC50 = 89.60 ± 8.73 μg/mL) as well as significant inhibition of xanthine oxidase (IC50 = 47.9 ± 2.04 μg/mL) and lipid peroxidation (IC50 = 231.63 ± 5.21 μg/mL). ADEO also showed significant anti-inflammatory activity by inhibiting the enzymes COX-1 (IC50 = 59.2 ± 2.43 μg/mL), Cyclo-oxygenase-2 (COX-2) (IC50 = 74.68 ± 1.34 μg/mL) and 5-lipooxygense (5-LOX) (IC50 = 93.18 ± 1.87 μg/mL), which are involved in the inflammatory pathway. These findings suggest that ADEO, with its high estragole content, holds promising potential as a natural antibacterial, antioxidant, and anti-inflammatory agent for preventive and therapeutic applications. Further research is needed to explore its safety and efficacy in clinical settings.Keywords
The historical usage of medicinal plants by various cultures and civilizations, such as the Egyptians, Greeks, Chinese, and Indians, has significantly contributed to the foundation of modern pharmacology. Many modern pharmaceuticals, including aspirin (derived from Willow Bark) and quinine (from Cinchona bark), can trace origins in traditional plant-based remedies [1–3]. Recently, there is a rising interest in the therapeutic potential of medicinal plants, driven by the search for natural, sustainable, and side-effect-free treatments. According to the World Health Organization (WHO), around 75% of the global population still depends on plant-based folk medicine for primary healthcare, emphasizing the lasting relevance of these remedies in worldwide health [4]. Indeed, medicinal plants have demonstrated their efficacy on various ailments, including infections, wounds, and chronic conditions. The therapeutic use of medicinal plants continues to hold significant importance today, as they serve as a rich source of bioactive compounds, proposing natural alternatives to conventional drugs in combating microbes, oxidative stress, and inflammation [5,6].
The antimicrobial properties of medicinal plants have been acknowledged for centuries, with many plant extracts being used to treat infections caused by bacteria, fungi, and viruses. Historically, plants like Thyme, oregano, rosemary, and lavender have been employed to boost immunity and fight off infections [7,8]. The discovery of bioactive compounds in these plants, especially essential oils, has provided scientific backing for their use. These volatile compounds work through multiple mechanisms, including disrupting microbial cell walls, inhibiting enzymes necessary for microbial survival, and preventing microbial replication. In an era of rising antibiotic resistance, medicinal plants offer a valuable resource for developing alternative antimicrobial therapies, demonstrating effective antibacterial and antifungal actions [9–11].
Oxidative stress and inflammation are closely associated with biological processes that play a significant role in the development of numerous chronic diseases, including cardiovascular disorders, diabetes, cancer, and neurodegenerative conditions [12]. Oxidative stress happens when there is a discrepancy between the production of free radicals; unstable molecules that can damage cell, and the body’s ability to neutralize them with antioxidants. This excess of free radicals leads to cellular and tissue damage, prompting inflammatory responses [13]. Although inflammation is a protective mechanism aimed at combating damage or infection, when it becomes chronic, it can worsen oxidative damage and contribute to disease progression [14]. In this context, medicinal plants are well-known for their potential to mitigate oxidative stress and inflammation owing to their high content of bioactive compounds such as flavonoids, phenolics, and EOs. These natural substances act via neutralizing free radicals, enhancing the body’s antioxidant systems, and regulating inflammatory pathways [15,16].
Artemisia dracunculus L., or tarragon, is a perennial herb belonging to the family of Asteraceae, native to regions of Europe, Asia, and North America, and is widely grown for its fragrant leaves, which are esteemed uses in both cooking and folk medicine [17]. Traditionally, tarragon has been used as preventive agent to alleviate digestive issues such as indigestion and bloating due to its carminative nature, and to ease menstrual cramps because of its antispasmodic effects. Besides, tarragon has been used to treat insomnia, eliminate intestinal parasites, and underscoring its long-standing therapeutic significance [18]. The rich chemical composition and wide-ranging biological activities of A. dracunculus EO (ADEO) make it a valuable resource in both traditional and contemporary therapeutic practices. The ADEO is typically obtained via steam distillation from the aerial parts and has a sweet, anise-like scent. Its major compounds include estragole, D-limonene, ocimene and myrcene, with estragole often being the main compound responsible for the oil’s distinct sweet-spicy aroma. These bioactive molecules are responsible for the biological properties of ADEO, such as antimicrobial, antioxidant, anti-inflammatory, and analgesic effects [19].
The objective of the current exploration is to examine the phytochemical profile, antibacterial, antioxidant, and anti-inflammatory activities of ADEO. Specifically, the research aims to assess the differential antibacterial efficacy of the oil against both Gram-negative and Gram-positive bacteria. Moreover, the study seeks to quantify the oil’s antioxidant activity through DPPH, ABTS, xanthine oxidase inhibition, and beta-carotene bleaching tests and to evaluate its anti-inflammatory potential by analyzing its inhibition of COX-1, COX-2, and 5-LOX enzymes.
An original aspect of this research lies in its comprehensive investigation of the ADEO multi-functional bioactivities, coupled with the comprehensive chemical profiling of its volatile compounds, contributing valuable insights into its potential for therapeutic and industrial applications.
2.1 Plant Material and Extraction
A. dracunculus L. aerial parts were collected in March 2023 from Taza region (34°13′26″ N, 4°00′ 24″ W) (Morocco). This region has a Mediterranean climate characterized by an average annual rainfall of approximately 600 mm, temperatures ranging from 10°C in winter to 30°C in summer, and calcareous-clay soil with moderate organic matter content [20]. The name of this species was identified and confirmed by the botanists at the Botany Department of the Scientific Institute of Rabat, Morocco, and a Voucher specimen (RAB18236) was deposited in the herbarium. In brief, the collected materials (a mixture of leaves, flowers, and stems) were cleaned to remove dust and impurities, then air-dried in a well-ventilated, shaded area at room temperature (25 ± 2°C) for 10 days to preserve their volatile compounds and prevent photodegradation. Once fully dried and brittle to the touch, the plant materials were ground into a coarse powder using a mechanical grinder.
Briefly, 150 g of dried plant material were submerged in 1150 mL of distilled water, maintaining a plant-to-water ratio of 1:1 (w/v). The mixture was heated to 200°C, and hydrodistillation was carried out for 190 min to ensure maximum recovery of volatile compounds. EOs were separately isolated and dehydrated using anhydrous sodium sulphate (Na2SO4) to remove any residual moisture. After the separation of A. dracunculus EO (ADEO) from the aqueous layer, the oils were carefully recovered from the burette and stored in amber glass vials at 4°C until further experimentation to protect them from light and oxidation.
The chemical characterization of ADEO components was established by the technique of GC-MS. The apparatus used is well-appointed with a split/splitless injector, an apolar capillary column HP-5MS (5% phenyl polymethyl siloxane) (30 m × 0.25 mm, film thickness i.d. 0.25 μm) and a Mass Detector type Agilent 5975C. The gas used is helium, while the temperature is maintained at 250°C as the injector temperature and 300°C as the detector temperature. The transfer line was heated at 280°C. The mass spectra adopted a scanning mode (70 eV) in the range 50–550 m/z. The samples were diluted with hexane and injected with a volume of 2 mL. The chemical representation was reported in terms of the relative concentration of the total peak area. Normalization-based relative peak concentrations were then estimated using the equation below:
where Y and P is the percentage and peak areas, respectively [21]. The compounds were separated on the column and each chromatogram was characterized by comparing its mass spectrum with those described in the NIST library.
2.3.1 Bacteria Strains and Growth Conditions
To assess the antibacterial potential of ADEO, we have chosen the following bacteria: Bacillus subtilis ATCC 6633, Micrococcus luteus ATCC 14452, Escherichia coli ATCC 27853, Staphylococcus aureus ATCC 29213, Klebsiella aerogenes ATCC 13048 which provided by the laboratory of microbiology, University Mohamed V. These bacteria were chosen based on their clinical significance and their ability to represent a broad spectrum of pathogenic bacteria. Bacteria were kept at inclined Luria-Bertani (LB) agar medium at 4°C. Prior to testing, the bacteria were reactivated by subculturing in an appropriate medium, specifically LB broth, at 35°C for 20–24 h. For the antibacterial screening, a final bacterial concentration of approximately 106 CFU/mL was used, following the guidelines set by the National Committee for Clinical Laboratory Standards.
2.3.2 Agar-Disc Diffusion Assessment
The antibacterial screening of ADEO was performed using the agar disc-diffusion assessment, with minor modifications to a previously published protocol [22]. In short, sterile 6 mm discs containing 8 μL of ADEO were placed on the surface of Muller-Hinton agar, which had been inoculated with bacteria at a concentration of 1 × 108 to 2 × 108 CFU/mL. Chloramphenicol (10 μg/disc) was served as the standard antibiotic. After incubating at 37°C for 18–24 h, the antibacterial activity was reported by quantifying the inhibition zone diameter in millimeters. The experiment was repeated three times independently.
MICs were performed in sterile 96 well-microplate using micro-dilution technique as reported by Nouioura et al. [23]. A 0.15% (w/v) bacteriological agar solution was used to emulsify the tested oil in the culture medium. Initially, 50 μL of Muller-Hinton broth was added to all test wells, except the first, which contained 100 μL of ADEO at a concentration of 16% v/v. ADEO was then serially diluted by transferring 50 μL from the first well to the 10th well, creating concentrations ranging from 0.194 to 100 mg/mL. The 11th well served as the growth control, while the 12th acted as the sterility control (negative control). Subsequently, 50 μL of inoculum (approximately 106 CFU/mL) was added to each well from the 1st to the 11th. After incubating at 37°C for 18–24 h, 20 μL of p-iodonitrotetrazolium chloride (TTC) was introduced to each well to detect bacterial growth. The microplate was incubated for an additional hour at 25°C, with bacterial growth indicated by a color change from clear to red. MBCs were then determined by transferring 5 μL from colorless wells (no bacterial growth) onto LB plates, followed by incubation at 37°C for 20 h.
The antioxidant ability of ADEO was determined adopting four known complementary assay, including ABTS, DPPH, xanthine oxidase and β-carotene/linoleic acid tests as indicated in the literature [24,25]. The three independent replicates (n = 3) were used for the trials, and IC50 values were determined based on inhibition curves and established as means ± SD. Allopurinol was used as positive control for xanthine oxidase test, while BHT was serve as standard antioxidant in three other assays.
2.5 Evaluation of the Enzyme Inhibitory Effects
The anti-inflammatory potential of ADEO have focused on their capacity to inhibit the cyclooxygenases 1 and 2 (COX-1 and COX-2) and 5-lipoxygenase (5-LOX) enzymes. Quercetin and indomethacin were used as standard drugs.
All results of this investigation were organized and analyzed statistically by adopted ANOVA-one way (Tukey test) using SPSS software. Except the analysis of chemical compounds, all other tests were performed in triplicate. p < 0.05 was considered statistically significant.
The phytochemical content of ADEO was analyzed by GC-MS. The identified molecules are presented in Table 1, along with their molecular formula, retention index (RI) and relative peak area percentage of each compound. A total of 11 constituents were detected, representing 97.04% of the total oil composition. The analysis showed that phenylpropanoids and monoterpene hydrocarbons were dominant compounds at 62.99% and 34.05%, respectively. Estragole was the major component, representing 57.23% of the total, followed by ß-ocimene (14.56%), α-ocimene (7.3%) and D-limonene (5.66%).
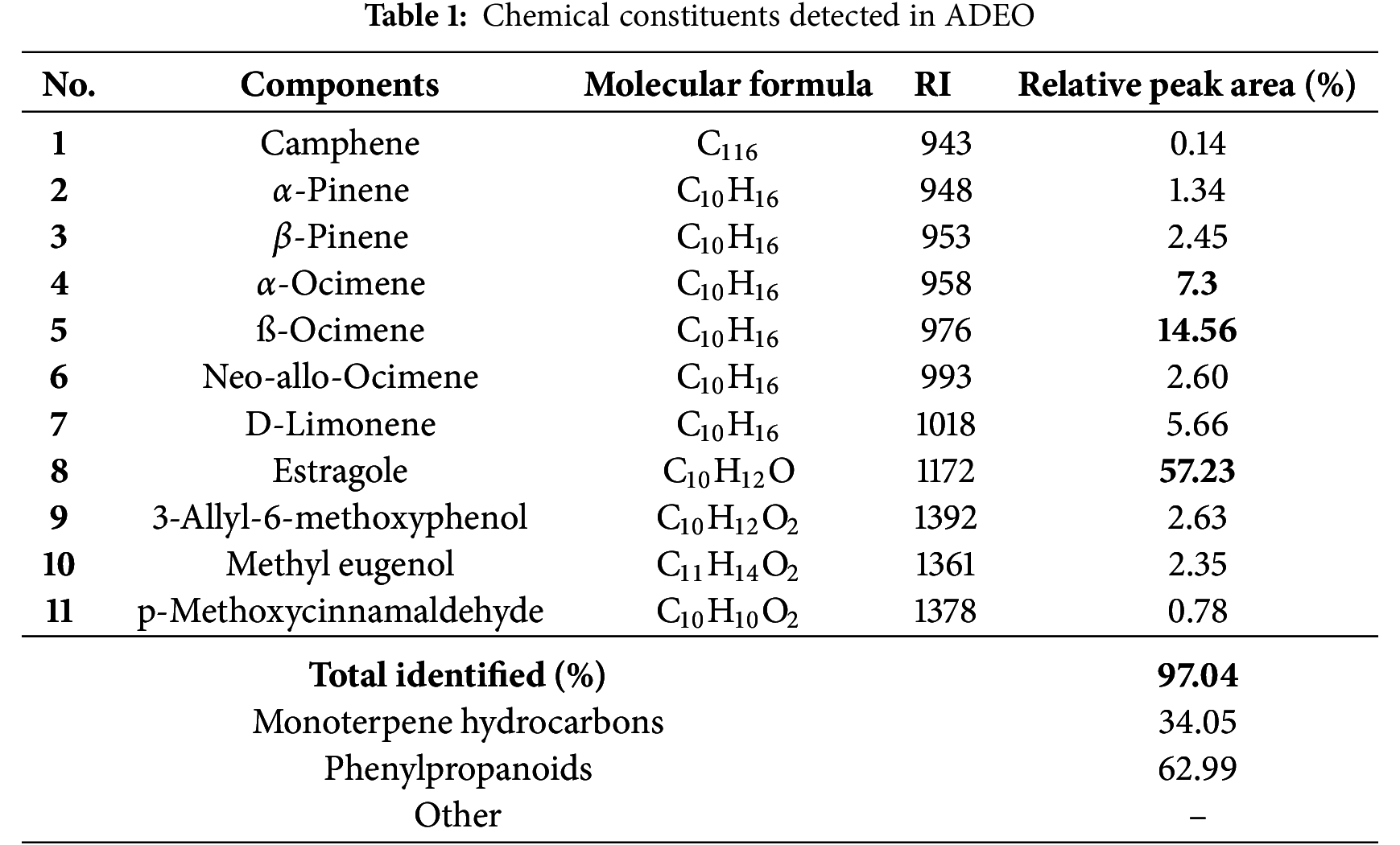
Our work is in line with other studies in the literature. A study carried out in Turkey on A. dracunculus L. showed that the main compound was estragole, with a percentage of 69.34% [26]. Similarly, another study carried out in Morocco on A. herba-alba and A. dracunculus also showed that the main compound of A. dracunculus was estragole (69.23%) [27]. In addition, analysis of the chemical profile of A. dracunculus L. grown in Armenia revealed a high content of estragole (84.9%), followed by linalool (5.09%) and β-ocimene (4.04%) [28]. Egypt highlighted a higher estragole concentration of 60.3%, with climate playing a significant role in the composition [29]. However, Ali Karimi and colleagues, after studying 26 genotypes of A. dracunculus cultivated in Iran, revealed a chemical profile dominated by methylchavicol (68.2%–81.1%), followed by D-limonene (7.2%–16.7%), terpinolene (0.01%–7.68%), (Z)-β-ocimene (0.89%–4.99%) and (E)-β-ocimene (0.81%–4.5%) [30]. Other studies have also shown a more distinct chemical profile. In a study investigating the effect of extraction method on the chemical profile of A. dracunculus, Fatemeh Abdollahnejad and colleagues found that the essential oil obtained was rich in anethole (53.74%) according to the different methods of extraction studied; hydrodistillation and empirical modified steam distillation [31]. In addition, a study carried out in Tajikistan on the chemical composition of A. dracunculus showed that this plant contained mainly sabinene (29.1%), estragole (24.6%) and limonene (7.8%) [32]. Another paper reported that the chemical composition of this species was characterized by sabinene (42.38%) and β-ocimene (6.46%) as the main compounds [33]. Similarly, sabinene (19.19%) was noticed as the key compound in ADEO [34].
This regional variability suggests that environmental conditions play a crucial role in determining the chemical profile of ADEO.
In summary, multiple parameters and circumstances, including geographical origin, climatic variations, harvesting and processing conditions, could explain the differences in ADEO composition. Ecological factors, genetic variability, environment variance, location and season of crop harvest can all affect the chemical content [35,36]. In fact, the latitude and altitude of the harvest site can have an effect on temperature, exposure to solar radiation and phylogenetic stage, all of which can alter the composition of EO [9,37].
The antibacterial efficacy of ADEO was explored using two complementary assays, including disc-diffusion and broth micro-dilution tests on Gram positive (Gram+) and Gram-negative (Gram−) bacteria. Indeed, ADEO has demonstrated potent antibacterial action against all tested strains. The results of disc-diffusion assessment showed that Gram + bacteria B. subtilis and S. aureus were highly susceptible to ADEO with mean inhibition zones (IZ) of 24.05 ± 2.11 and 18.69 ± 1.45 mm, respectively. E. coli, one of Gram-bacteria appeared to be resistant to ADEO with IZ of 14.7 ± 0.58 mm (Fig. 1). This outcome may be attributed to the dissimilarities in their cell wall composition among Gram+ and Gram– bacteria [38–40]. In fact, Gram+ bacteria possess a thick but permeable peptidoglycan layer, which allows EOs, particularly their fat-soluble components, to penetrate more easily and disrupt cell membranes. Whereas Gram-bacteria are characterized by an additional outer membrane rich in lipopolysaccharides (LPS), acting as a protective barrier, thus stopping the entry of EOs and therefore making these bacteria more resistant to their actions. The MIC and MBC assays confirmed the results obtained by IZ test [41].
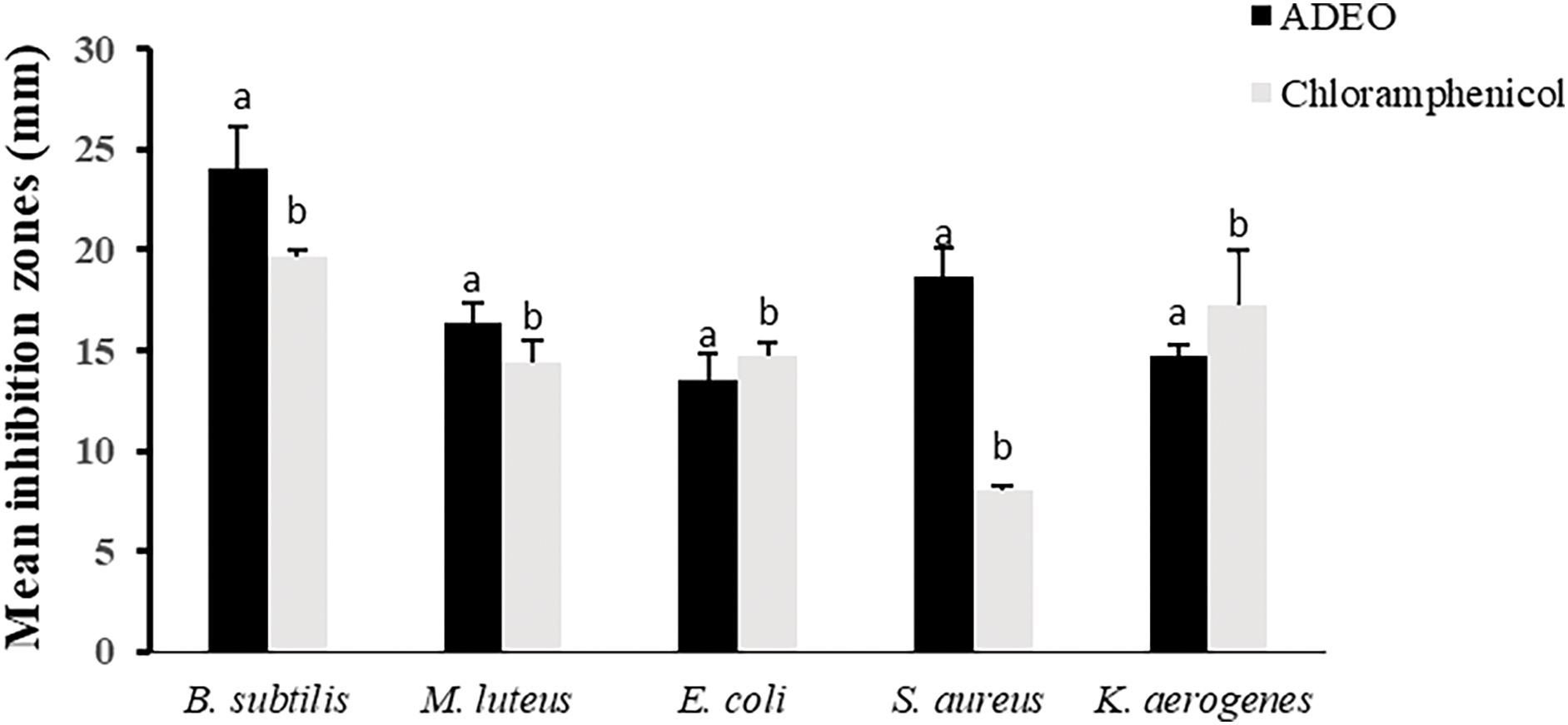
Figure 1: Antibacterial activity of ADEO through disc diffusion method. records with the same letter indicate non-significant differences by Tukey’s multiple range test (ANOVA, p < 0.05)
The findings showed that the lowest MIC and MBC values were noted with S. aureus and B. subtilis, K. aerogenes, fluctuating from 0.0612% to 0.5%, while, E. coli tend to be more resistant to ADEO, with the respective MIC and MBC values of 2 and 4% v/v (Table 2). These findings are competitive when compared to standard antibiotic Chloramphenicol with the MIC = MBC = 256 μg/mL. This highlights ADEO’s potential as an alternative treatment, particularly for infections caused by Gram-positive bacteria. However, given the variability in effectiveness and the need for higher concentrations, further studies are needed to optimize its use and assess its safety profile, especially when compared to synthetic antibiotics.
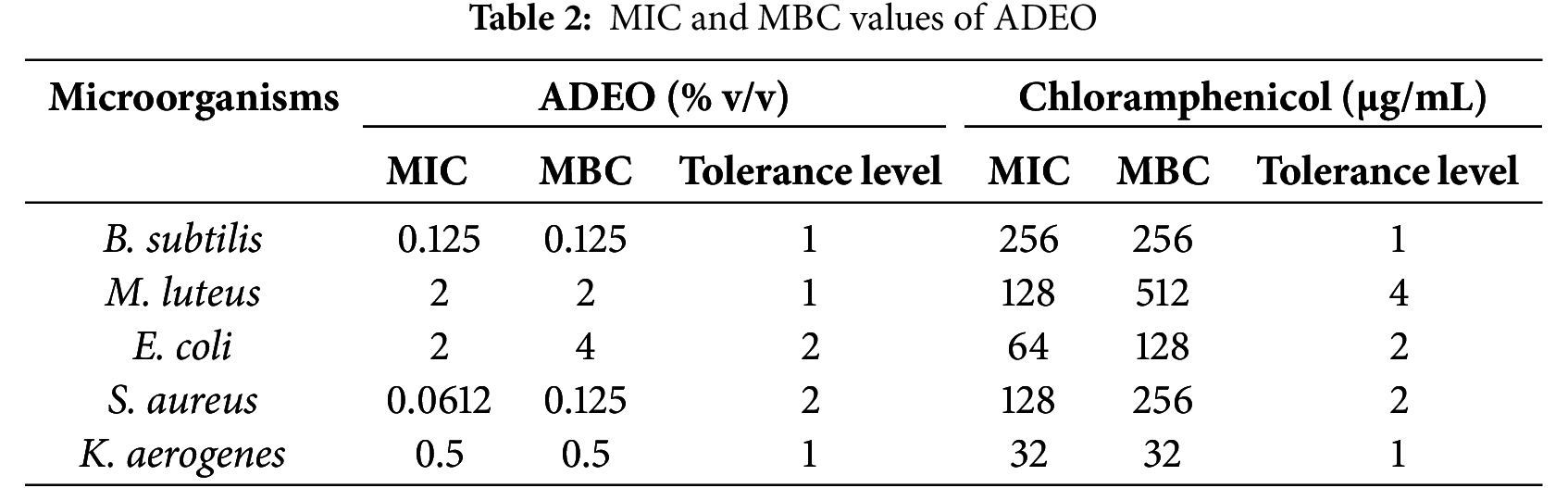
MIC ratios (tolerance level) provide important insights into whether an EO works by killing microorganisms or simply inhibiting their growth. When the fraction of MBC/MIC is 4.0 or lower, it shows that the oil possesses bactericidal action, meaning it can efficiently kill bacteria. In this case, the antimicrobial agent can reach a concentration sufficient to eliminate 99.9% of the microorganisms being treated [42]. This suggests the agent’s ability to cause microbial death rather than just inhibit growth. Conversely, if the ratio exceeds 4.0, it implies that a much higher concentration is required to kill the microorganisms, which may not always be achievable in practice. In such cases, the EO is classified as bacteriostatic, inhibiting the growth of the microorganisms rather than directly killing them. In the present investigation the MIC ratios of ADEO is 4.0 or lower against all studied strains, demonstrating its bactericidal mechanism [43].
Previous study carried out on ADEO obtained from different plant parts (aerial parts, leaves, steams) and with different extraction methods have reported the antibacterial efficacy of this natural compound against a panel of bacterial strains. ADEO has demonstrated potent antibacterial effect against S. aureus, Staphylococcus epidermidis, E. coli, M. luteus, B. subtilis, B. cereus, Streptococcus pyogenes, S. typhimurium, E. coli, Listeria monocytogenes, Shigella flexneri, S. marcescens, Pseudomonas aeruginosa, Klebsiella pneumonia, Salmonella typhimurium, Proteus spp. and Corynebacterium diphtheria [44].
Interestingly, Mohammadi Pelarti et al. [45] have reported the antibacterial and antibiofilm potential of ADEO against S. typhimurium and S. aureus. The MIC values were equal to 2.5 and 1.25 μL/mL for S. typhimurium and S. aureus, respectively and a microtiter-plate and scanning electron microscopy examinations revealed remarkable inhibitory effect of ADEO on biofilm formation by both bacteria at MIC/2 concentration.
In addition to estragole, ADEO contains multiple other components, such as α-pinene, camphene, 1,8-cineole, and sabinene, which may contribute to its overall pharmacological properties. The potential synergistic effects of these compounds, in combination with estragole, could enhance the therapeutic efficacy of ADEO in various applications, including antibacterial, antioxidant, and anti-inflammatory treatments.
For instance, α-pinene, a monoterpene, has been shown to possess antimicrobial properties and may enhance the antimicrobial activity of other essential oils when used synergistically. A study by Allenspach et al. [46] demonstrated that α-pinene exhibited synergistic antibacterial effects when combined with other compounds, leading to increased bactericidal activity against both Gram-positive and Gram-negative bacteria [46]. Additionally, 1,8-cineole has proved both anti-inflammatory and antioxidant activities, and its combination with other compounds like α-pinene has been shown to improve anti-inflammatory effects [47]. A study by Ben Akacha et al. [48] reported that 1,8-cineole exhibited significant antioxidant and anti-inflammatory effects, particularly when combined with other compounds like α-pinene andα-terpineol, enhancing its overall bioactivity. Furthermore, synergistic effects have been observed between the monoterpenes and sesquiterpenes in EOs, improving antimicrobial potency and reducing resistance [49].
The antibacterial effect of ADEO holds significant potential for practical applications in food preservation and healthcare. In the food industry, ADEO could be used as a natural antibacterial agent to reduce bacterial contamination, extend shelf life, and serve as an alternative to synthetic preservatives in products like meats, dairy, and fresh produce. In healthcare, ADEO’s antibacterial properties could contribute to developing natural therapies for antibiotic-resistant infections, as well as be incorporated into disinfectants, wound treatments, and skin care products for inflammatory conditions.
3.3 Anti-Inflammatory Activity
Inflammation is a complex biological response that the body activates as a defense mechanism against harmful stimuli such as pathogens, toxins, or tissue injury. Although critical for immune defense, chronic inflammation contribute to various health issues, including cardiovascular disease, cancer, and autoimmune disorders [50,51]. A key player in the inflammatory process is the enzymes lipoxygenase (LOX) and cyclooxygenases (COX), which act as a regulator of inflammation through catalyzing the oxidation of arachidonic acid, leading to the production of lipid mediators that regulate inflammation. COX-1 is a constitutive enzyme promotes normal physiological functions, by protecting the gastric lining and supporting blood clotting, while COX-2 is inducible and primarily responsible for producing pro-inflammatory prostaglandins during injury or infection, contributing to pain, fever, and swelling [52,53].
Furthermore, LOX enzymes, particularly 5-LOX, convert arachidonic acid into hydroperoxyeicosatetraenoic acids (HPETEs), which are further metabolized into leukotrienes. These lipid mediators are crucial signaling molecules that promote various inflammatory responses [54]. Understanding COX-1, COX-2, and 5-LOX functions has provided critical insights into the mechanisms underlying major inflammatory diseases. This fact has led to the development of therapeutic approaches aimed at inhibiting the activity of these enzymes. By blocking 5-LOX enzymes, it is possible to reduce the formation of leukotrienes, thereby preventing the inflammatory signaling pathways that contribute to chronic conditions like asthma, arthritis, and inflammatory bowel disease (IBD).
Natural inhibitors isolated from medicinal floras, such as EOs, show promise in modulating both COX and LOX pathways, offering potential for safer, more holistic anti-inflammatory therapies. In this study, ADEO revealed significant inhibition of the COX-1, COX-2 and 5-LOX enzyme, with IC50 values of 59.2 ± 2.43, 74.68 ± 1.34 and 93.18 ± 1.87 μg/mL, respectively (Table 3). Although less effective than the standard drug quercetin (IC50 = 26.22 ± 0.03 μg/mL) and indomethacin (14.72 ± 0.09 and 11.08 ± 0.03 μg/mL), these results highlight ADEO potential as an anti-inflammatory agent.
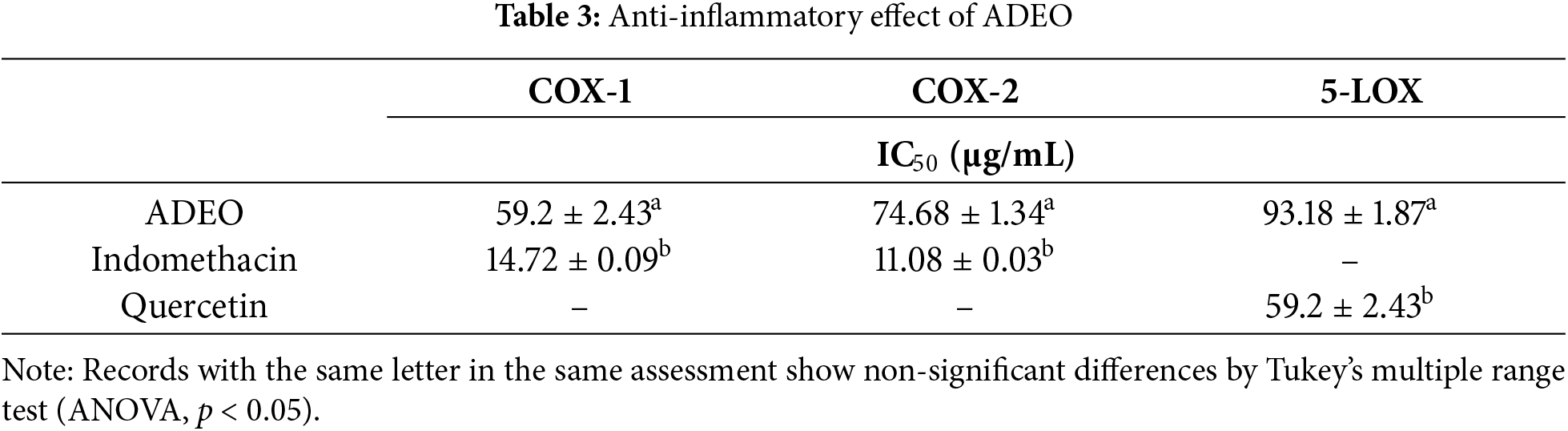
While ADEO has demonstrated potent antimicrobial and antioxidant properties, emerging research suggests its potential anti-inflammatory benefits. This EO contains high levels of estragole renowned for its anti-inflammatory effects [55,56]. This natural molecule can inhibit inflammatory events, by down-regulating nuclear factor kappa B, and MAPK pathways and up-regulating of Nrf-2/heme oxygenase (HO)-1 pathways [57].
Schepetkin et al. [58] reported that pretreatment with ADEO and its major compound farnesene modulate inflammatory responses through blocking human neutrophil chemotaxis triggered by fMLF. Moreover, recent investigation showed that ADEO exert important anti-inflammatory activity by reducing the serum level of nuclear factor-kB-p65 (NfkB-p65) IL-1b, IL-18, and gasdermin D [59].
The results shown in the Table 4 represent the IC50 value of ADEO in different antioxidant activity assays, compared with the positive controls, BHT and allopurinol. The 50% inhibitory concentration (IC50) is a measure of antioxidant efficiency; the lower IC50 value represent the higher antioxidant action. The antioxidant capacity of ADEO was moderate in the various tests performed, although generally less than that of the positive controls tested. In the DPPH test, ADEO has an IC50 of 127.05 μg/mL, suggesting moderately strong antioxidant activity, but significantly lower than that of BHT (IC50 = 57.18 μg/mL). the same pattern was observed in the ABTS test, where ADEO had an IC50 of 89.60 μg/mL, which was again lower than that of BHT (45.02 μg/mL). On the other hand, when tested for xanthine oxidase, ADEO showed an IC50 of 47.9 μg/mL, which indicates better inhibition of this enzyme than in the other antioxidant assays, although this value is slightly higher than that of allopurinol (IC50 = 27.36 μg/mL), a reference inhibitor for this assay. However, the antioxidant potency of ADEO was significantly less pronounced in the beta-carotene test, with an IC50 of 231.63 μg/mL, compared to that of BHT (IC50 = 31.65 μg/mL), indicating a relatively low ability to prevent lipid oxidation in this context.
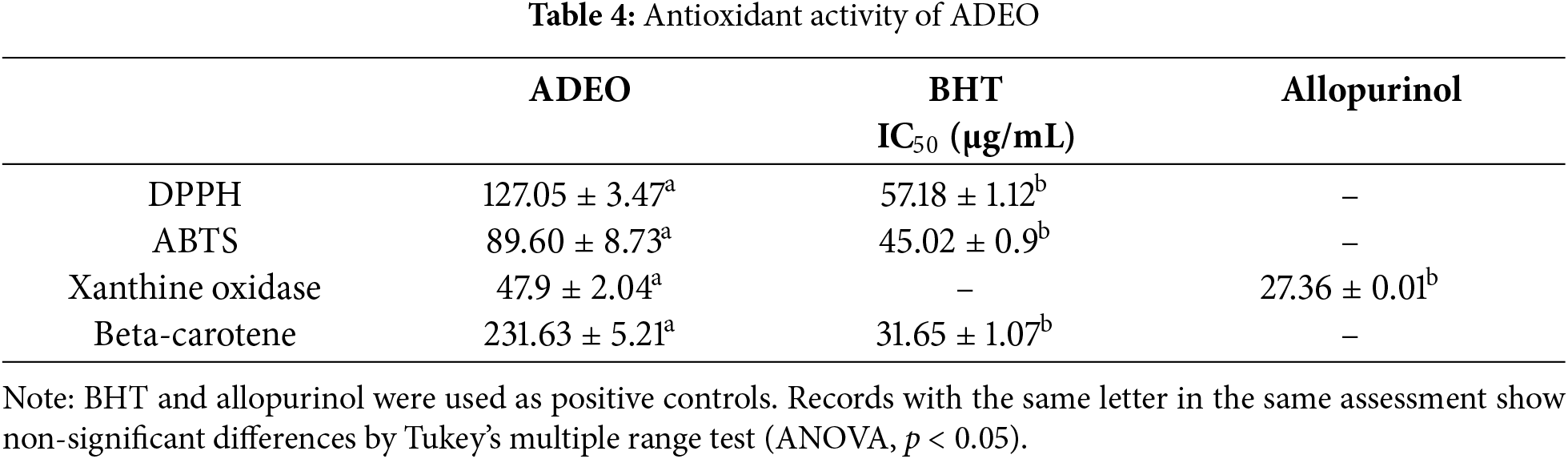
Previous research on the antioxidant activity of ADEO broadly confirms the present results, while highlighting interesting variations according to geographical origin and the assessment methods employed. In study carried out by [33], the free radical scavenging activity of ADEO, assessed in vitro DPPH test, was found to be relatively low (0.730 ± 0.213 μg/mL). Moreover, research conducted in Morocco by [27], explored the anti-radical action of ADEO using the DPPH and ABTS tests. Their results, with IC50 of 84.44 ± 5.98 μg/mL and 96.71 ± 1.52 μg/mL, respectively, are closer to the present observations, while remaining slightly lower. In contrast, a study of Armenian ADEO showed a significant anti-DPPH radical, with an IC50 of 94.2 μg/mL. This value, which is lower than the one we obtained, indicates a potentially higher antioxidant activity, possibly due to environmental or genetic factors specific to this region (ref). Another study showed that ADEO had a strong inhibitory effect on DPPH and ABTS radicals, with a remarkably low IC50 of 65.4 μg/mL [44]. This value suggests a significant variability in the antioxidant efficacy of ADEO depending on its origin or method of preparation, and is significantly lower than the current results and those of the other studies cited [32].
The beta-carotene bleaching assay, while useful for assessing antioxidant activity, has limitations in fully capturing ADEO antioxidant profile. It primarily measures the ability to prevent lipid peroxidation, but doesn’t account for all antioxidant mechanisms, such as radical scavenging or enzyme inhibition. The assay is sensitive to experimental conditions and may not reflect the contributions of individual compounds in ADEO. To obtain a more comprehensive assessment of ADEO’s antioxidant capacity, results from the beta-carotene test should be combined with other research using cellular and/or in vivo models
4 Conclusion and Future Direction
A. dracunculus EO, with estragole as its major compound, displays important antibacterial, antioxidant, and anti-inflammatory activities, highlighting its therapeutic potential. ADEO demonstrated potent antibacterial action, making it a promising candidate for combating bacterial infections. Its antioxidant ability, evaluated through DPPH, ABTS, xanthine oxidase inhibition, and the beta-carotene bleaching assays, further supports its role in neutralizing oxidative stress, which is crucial in preventing cellular damage and the related diseases. In addition, ADEO inhibition of enzymes endorses its effective anti-inflammatory potential, proposing a natural alternative for managing inflammation-related conditions.
Future research on ADEO should focus on several key areas to fully assess its therapeutic potential and safety. Investigating its synergistic effects with other antimicrobial and antioxidant agents could enhance its bioactivity and improve treatment outcomes. In vivo studies are essential to evaluate ADEO’s pharmacokinetics, bioavailability, and therapeutic efficacy, especially for chronic diseases linked to oxidative stress and inflammation. Additionally, due to the high estragole content in ADEO, further investigation into its toxicity, including genotoxicity and carcinogenicity, is crucial to determine safe usage levels. These studies will provide a deeper understanding of ADEO’s clinical applicability and its safety profile, ensuring its potential as a natural therapeutic agent. Furthermore, this investigation provides a strong foundation for the continued exploration of ADEO as a natural, multifunctional bioactive agent in various therapeutic and industrial applications.
Acknowledgement: Not applicable.
Funding Statement: The author received no specific funding for this study.
Availability of Data and Materials: No datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The author declares no conflicts of interest to report regarding the present study.
References
1. Petrovska BB. Historical review of medicinal plants’ usage. Pharmacogn Rev. 2012;6(11):1–5. doi:10.4103/0973-7847.95849. [Google Scholar] [PubMed] [CrossRef]
2. Giannenas I, Sidiropoulou E, Bonos E, Christaki E, Florou-Paneri P. The history of herbs, medicinal and aromatic plants, and their extracts: past, current situation and future perspectives. In: Feed additives: aromatic plants and herbs in animal nutrition and health. Amsterdam, The Netherlands: Elsevier; 2020. p. 1–18. [Google Scholar]
3. Halberstein RA. Medicinal plants: historical and cross-cultural usage patterns. Ann Epidemiol. 2005;15(9):686–99. doi:10.1016/j.annepidem.2005.02.004. [Google Scholar] [PubMed] [CrossRef]
4. Al-Mijalli SH, Mrabti HN, Elbouzidi A, Ashmawy NS, Batbat A, Abdallah EM, et al. Thymus serpyllum L. essential oil: phytochemistry and in vitro and in silico screening of its antimicrobial, antioxidant and anti-inflammatory properties. Phyton. 2025;94(1):209–27. [Google Scholar]
5. Abdallah EM, Alhatlani BY, de Paula Menezes R, Martins CHG. Back to nature: medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants. 2023;12(17):3077. doi:10.3390/plants12173077. [Google Scholar] [PubMed] [CrossRef]
6. Alam SM, Qureshi M, Jahan N. Antimicrobial screening of some medicinal plants of Pakistan. Pak J Bot. 2010;42:4281–4. [Google Scholar]
7. Angane M, Swift S, Huang K, Butts CA, Quek SY. Essential oils and their major components: an updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods. 2022;11(3):464. doi:10.3390/foods11030464. [Google Scholar] [PubMed] [CrossRef]
8. Boren K, Crown A, Carlson R. Multidrug and pan-antibiotic resistance—the role of antimicrobial and synergistic essential oils: a review. Nat Prod Commun. 2020;15(10):1934578X20962595. doi:10.1177/1934578X20962595. [Google Scholar] [CrossRef]
9. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46(2):446–75. doi:10.1016/j.fct.2007.09.106. [Google Scholar] [PubMed] [CrossRef]
10. Baptista-Silva S, Borges S, Ramos OL, Pintado M, Sarmento B. The progress of essential oils as potential therapeutic agents: a review. J Essent Oil Res. 2020;32(4):279–95. doi:10.1080/10412905.2020.1746698. [Google Scholar] [CrossRef]
11. Langeveld WT, Veldhuizen EJA, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014;40(1):76–94. doi:10.3109/1040841X.2013.763219. [Google Scholar] [PubMed] [CrossRef]
12. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20(9):689–709. doi:10.1038/s41573-021-00233-1. [Google Scholar] [PubMed] [CrossRef]
13. Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longev. 2020;2020:8609213. doi:10.1155/2020/8609213. [Google Scholar] [PubMed] [CrossRef]
14. Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S53–69. doi:10.1016/j.ajog.2015.08.041. [Google Scholar] [PubMed] [CrossRef]
15. Jayasena DD, Jo C. Potential application of essential oils as natural antioxidants in meat and meat products: a review. Food Rev Int. 2014;30(1):71–90. doi:10.1080/87559129.2013.853776. [Google Scholar] [CrossRef]
16. Ayoub Z, Mehta A. Medicinal plants as potential source of antioxidant agents: a review. Asian J Pharm Clin Res. 2018;11(6):50. doi:10.22159/ajpcr.2018.v11i6.24725. [Google Scholar] [CrossRef]
17. Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M. Artemisia dracunculus L. (tarragona critical review of its traditional use, chemical composition, pharmacology, and safety. J Agric Food Chem. 2011;59(21):11367–84. doi:10.1021/jf202277w. [Google Scholar] [PubMed] [CrossRef]
18. Ekiert H, Świątkowska J, Knut E, Klin P, Rzepiela A, Tomczyk M, et al. Artemisia dracunculus (tarragona review of its traditional uses, phytochemistry and pharmacology. Front Pharmacol. 2021;12:653993. doi:10.3389/fphar.2021.653993. [Google Scholar] [PubMed] [CrossRef]
19. Aglarova AM, Zilfikarov IN, Severtseva OV. Biological characteristics and useful properties of tarragon (Artemisia dracunculus L.) (review). Pharm Chem J. 2008;42(2):81–6. doi:10.1007/s11094-008-0064-3. [Google Scholar] [CrossRef]
20. Rezouki S, Allali A, Louasté B, Eloutassi N, Fadli M. Physico-chemical evaluation of soil resources in different regions of Taza–Taounate, Morocco. Mediterr J Chem. 2020;10(9):836. doi:10.13171/mjc10902011111537sr. [Google Scholar] [CrossRef]
21. Almas I, Innocent E, Machumi F, Kisinza W. Chemical composition of essential oils from Eucalyptus globulus and Eucalyptus maculata grown in Tanzania. Sci Afr. 2021;12:e00758. doi:10.1016/j.sciaf.2021.e00758. [Google Scholar] [CrossRef]
22. Boutabia L, Telailia S, Guenadil F, Chefrour A. Chemical composition and antibacterial activity of essential oils from Mentha pulegium L. and Mentha suaveolens Ehrh. growing in North-East of Algeria. Analele Univ Din Oradea Fasc Biol. 2020;2:143–8. [Google Scholar]
23. Nouioura G, El Fadili M, El Hachlafi N, Abuelizz HA, Elidrissi AE, Ferioun M, et al. Petroselinum crispum L., essential oil as promising source of bioactive compounds, antioxidant, antimicrobial activities: in vitro and in silico predictions. Heliyon. 2024;10(8):e29520. doi:10.1016/j.heliyon.2024.e29520. [Google Scholar] [PubMed] [CrossRef]
24. Assaggaf H, Jeddi M, Mrabti HN, Ez-Zoubi A, Qasem A, Attar A, et al. Design of three-component essential oil extract mixture from Cymbopogon flexuosus, Carum carvi, and Acorus calamus with enhanced antioxidant activity. Sci Rep. 2024;14(1):9195. doi:10.1038/s41598-024-59708-x. [Google Scholar] [PubMed] [CrossRef]
25. Nesrine B, Naoufal EH, Mohamed EF, Mohamed J, Rhizlan A, Mohamed B, et al. Unveiling the phytochemical profile, in vitro bioactivities evaluation, in silico molecular docking and ADMET study of essential oil from Clinopodium nepeta grown in Middle Atlas of Morocco. Biocatal Agric Biotechnol. 2023;54:102923. [Google Scholar]
26. Gıdık B. Comparison of essential oil components and yield parameters of Artemisia dracunculus and Artemisia dracunculoides. Res Agric Sci. 2024;55(2):58–66. doi:10.17097/agricultureatauni.1445748. [Google Scholar] [CrossRef]
27. Mrabti HN, El Hachlafi N, Al-Mijalli SH, Jeddi M, Elbouzidi A, Abdallah EM, et al. Phytochemical profile, assessment of antimicrobial and antioxidant properties of essential oils of Artemisia herba-alba Asso., and Artemisia dracunculus L. experimental and computational approaches. J Mol Struct. 2023;1294:136479. doi:10.1016/j.molstruc.2023.136479. [Google Scholar] [CrossRef]
28. Sahakyan N, Andreoletti P, Cherkaoui-Malki M, Petrosyan M, Trchounian A. Artemisia dracunculus L. essential oil phytochemical components trigger the activity of cellular antioxidant enzymes. J Food Biochem. 2021;45(4):e13691. doi:10.1111/jfbc.13691. [Google Scholar] [PubMed] [CrossRef]
29. Shalaby AS, Hendawy SF, Khalil MY. Evaluation of some types of fennel (Foeniculum vulgareMill.) newly introduced and adapted in Egypt. J Essent Oil Res. 2011;23(4):35–42. doi:10.1080/10412905.2011.9700466. [Google Scholar] [CrossRef]
30. Karimi A, Hadian J, Farzaneh M, Khadivi-Khub A. Phenotypic diversity and volatile composition of Iranian Artemisia dracunculus. Ind Crops Prod. 2015;65:315–23. doi:10.1016/j.indcrop.2014.12.003. [Google Scholar] [CrossRef]
31. Abdollahnejad F, Kobarfard F, Kamalinejad M, Mehrgan H, Babaeian M. Yield, chemical composition and antibacterial activity of Artemisia dracunculus L. essential oils obtained by two different methods. J Essent Oil Bear Plants. 2016;19(3):574–81. doi:10.1080/0972060x.2014.963167. [Google Scholar] [CrossRef]
32. Sharopov FS, Salimov A, Numonov S, Bakri M, Sangov Z, Habasi M, et al. Phytochemical study on the essential oils of tarragon (Аrtemisia dracunculus L.) growing in Tajikistan and its comparison with the essential oil of the species in the rest of the world. Nat Prod Commun. 2020;15(12):1934578X20977394. doi:10.1177/1934578x20977394. [Google Scholar] [CrossRef]
33. Fildan AP, Pet I, Stoin D, Bujanca G, Lukinich-Gruia AT, Jianu C, et al. Artemisia dracunculus essential oil chemical composition and antioxidant properties. Rev Chim. 2019;70(12):59–62. doi:10.37358/rc.19.1.6851. [Google Scholar] [CrossRef]
34. Liu T, Lin P, Bao T, Ding Y, Lha Q, Nan P, et al. Essential oil composition and antimicrobial activity of Artemisia dracunculus L. var. qinghaiensis Y. R. Ling (Asteraceae) from Qinghai-Tibet Plateau. Ind Crops Prod. 2018;125:1–4. doi:10.1016/j.indcrop.2018.08.085. [Google Scholar] [CrossRef]
35. El Hachlafi N, Elbouzidi A, Batbat A, Taibi M, Jeddi M, Addi M, et al. Chemical composition and assessment of the anti-inflammatory, antioxidant, cytotoxic and skin enzyme inhibitory activities of Citrus sinensis (L.) osbeck essential oil and its major compound limonene. Pharmaceuticals. 2024;17(12):1652. doi:10.3390/ph17121652. [Google Scholar] [PubMed] [CrossRef]
36. El Hachlafi N, Kandsi F, Elbouzidi A, Lafdil FZ, Nouioura G, Abdallah EM, et al. Extraction of bioactive compound-rich essential oil from Cistus ladanifer L. by microwave-assisted hydrodistillation: gc-MS characterization, in vitro pharmacological activities, and molecular docking. Separations. 2024;11(7):199. doi:10.3390/separations11070199. [Google Scholar] [CrossRef]
37. Bakha M, El Mtili N, Machon N, Aboukhalid K, Amchra FZ, Khiraoui A, et al. Intraspecific chemical variability of the essential oils of Moroccan endemic Origanum elongatum L. (Lamiaceae) from its whole natural habitats. Arab J Chem. 2020;13(1):3070–81. doi:10.1016/j.arabjc.2018.08.015. [Google Scholar] [CrossRef]
38. Ahmady-Asbchin S, Nasrolahi Omran A, Jafari N, Mostafapour M, Kia SM. Antibacterial effects of lavandula stoechas essential oil, on gram positive and negative bacteria. mljgoums. 2012;6(2):35–41. [Google Scholar]
39. Faiq MST, Ahmed Abdullah S, Ibrahim Ali S, Kamal Khder D. Antibacterial effect of fixed and volatile oils against gram-positive and gram-negative bacteria. Kurd J Appl Res. 2019:74–87. doi:10.24017/science.2019.ichms.8. [Google Scholar] [CrossRef]
40. Tavares TD, Antunes JC, Padrão J, Ribeiro AI, Zille A, Teresa PAM, et al. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics. 2020;9(6):314. doi:10.3390/antibiotics9060314. [Google Scholar] [PubMed] [CrossRef]
41. Semeniuc CA, Pop CR, Rotar AM. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J Food Drug Anal. 2017;25(2):403–8. doi:10.1016/j.jfda.2016.06.002. [Google Scholar] [PubMed] [CrossRef]
42. Tato M, López Y, Morosini MI, Moreno-Bofarull A, Garcia-Alonso F, Gargallo-Viola D, et al. Characterization of variables that may influence ozenoxacin in susceptibility testing, including MIC and MBC values. Diagn Microbiol Infect Dis. 2014;78(3):263–7. doi:10.1016/j.diagmicrobio.2013.11.010. [Google Scholar] [PubMed] [CrossRef]
43. Al-Mijalli SH, Mrabti HN, El Hachlafi N, El Kamili T, Elbouzidi A, Abdallah EM, et al. Integrated analysis of antimicrobial, antioxidant, and phytochemical properties of Cinnamomum verum: a comprehensive in vitro and in silico study. Biochem Syst Ecol. 2023;110:104700. doi:10.1016/j.bse.2023.104700. [Google Scholar] [CrossRef]
44. Behbahani BA, Shahidi F, Yazdi FT, Ali Mortazavi S, Mohebbi M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J Food Meas Charact. 2017;11(2):847–63. doi:10.1007/s11694-016-9456-3. [Google Scholar] [CrossRef]
45. Mohammadi Pelarti S, Karimi Zarehshuran L, Babaeekhou L, Ghane M. Antibacterial, anti-biofilm and anti-quorum sensing activities of Artemisia dracunculus essential oil (EOa study against Salmonella enterica serovar Typhimurium and Staphylococcus aureus. Arch Microbiol. 2021;203(4):1529–37. doi:10.1007/s00203-020-02138-w. [Google Scholar] [PubMed] [CrossRef]
46. Allenspach M, Steuer C. α-pinene: a never-ending story. Phytochemistry. 2021;190:112857. doi:10.1016/j.phytochem.2021.112857. [Google Scholar] [PubMed] [CrossRef]
47. Cai ZM, Peng JQ, Chen Y, Tao L, Zhang YY, Fu LY, et al. 1,8-cineole: a review of source, biological activities, and application. J Asian Nat Prod Res. 2021;23(10):938–54. doi:10.1080/10286020.2020.1839432. [Google Scholar] [PubMed] [CrossRef]
48. Ben Akacha B, Michalak M, Generalić Mekinić I, Kačániová M, Chaari M, Brini F, et al. Mixture design of α-pinene, α-terpineol, and 1,8-cineole: a multiobjective response followed by chemometric approaches to optimize the antibacterial effect against various bacteria and antioxidant activity. Food Sci Nutr. 2024;12(1):574–89. doi:10.1002/fsn3.3780. [Google Scholar] [PubMed] [CrossRef]
49. Bassolé IHN, Juliani HR. Essential oils in combination and their antimicrobial properties. Molecules. 2012;17(4):3989–4006. doi:10.3390/molecules17043989. [Google Scholar] [PubMed] [CrossRef]
50. Chatterjee S. Oxidative stress, inflammation, and disease. Oxidative stress and biomaterials. Amsterdam, The Netherlands: Elsevier; 2016. p. 35–58. [Google Scholar]
51. Ouadja B, Katawa G, Toudji GA, Layland L, Gbekley EH, Ritter M, et al. Anti-inflammatory, antibacterial and antioxidant activities of Chenopodium ambrosioides L. (Chenopodiaceae) extracts. J Appl Biosci. 2021;162:16764–94. doi:10.35759/jabs.162.7. [Google Scholar] [CrossRef]
52. Drikvandi P, Bahramikia S, Alirezaei M. Modulation of the antioxidant defense system in liver, kidney, and pancreas tissues of alloxan-induced diabetic rats by camphor. J Food Biochem. 2020;44(12):e13527. doi:10.1111/jfbc.13527. [Google Scholar] [PubMed] [CrossRef]
53. Jaismy JP, Manju SL, Ethiraj KR, Elias G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: a structure-based approach. Eur J Pharm Sci. 2018;121:356–81. doi:10.1016/j.ejps.2018.06.003. [Google Scholar] [PubMed] [CrossRef]
54. Biltekin SN, Karadaǧ AE, Demirci B, Demirci F. ACE2 and LOX enzyme inhibitions of different lavender essential oils and major components linalool and camphor. ACS Omega. 2022;7(41):36561–6. doi:10.1021/acsomega.2c04518. [Google Scholar] [PubMed] [CrossRef]
55. Ponte EL, Sousa PL, Rocha MVAP, Soares PMG, Coelho-de-Souza AN, Leal-Cardoso JH, et al. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol Rep. 2012;64(4):984–90. doi:10.1016/s1734-1140(12)70895-2. [Google Scholar] [PubMed] [CrossRef]
56. Rodrigues LB, Oliveira Brito Pereira Bezerra Martins A, Cesário FRAS, Ferreira ECF, de Albuquerque TR, Martins Fernandes MN, et al. Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: in vivo mouse models. Chem Biol Interact. 2016;257:14–25. doi:10.1016/j.cbi.2016.07.026. [Google Scholar] [PubMed] [CrossRef]
57. Roy A, Park HJ, Jung HA, Choi JS. Estragole exhibits anti-inflammatory activity with the regulation of NF-κB and nrf-2 signaling pathways in LPS-induced RAW 264.7 cells. Nat Prod Sci. 2018;24(1):13. doi:10.20307/nps.2018.24.1.13. [Google Scholar] [CrossRef]
58. Schepetkin IA, Özek G, Özek T, Kirpotina LN, Khlebnikov AI, Klein RA, et al. Neutrophil immunomodulatory activity of farnesene, a component of Artemisia dracunculus essential oils. Pharmaceuticals. 2022;15(5):642. doi:10.3390/ph15050642. [Google Scholar] [PubMed] [CrossRef]
59. Ticolea M, Pop RM, Pârvu M, Usatiuc LO, Uifălean A, Ranga F, et al. Phytochemical composition antioxidant and anti-inflammatory activity of Artemisia dracunculus and Artemisia abrotanum. Antioxidants. 2024;13(8):1016. doi:10.3390/antiox13081016. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools