 Open Access
Open Access
REVIEW
Strengthening Tomato Resilience: Harnessing Microbial Consortia to Overcome Biotic and Abiotic Stress
1 Phytopathology Unit, Department of Plant Protection, Ecole Nationale d’Agriculture de Meknès, Meknès, 50000, Morocco
2 Laboratory of Functional Ecology and Environmental Engineering, Sidi Mohamed Ben Abdellah University, Fez, 30000, Morocco
3 Plant Protection Laboratory, Regional Center of Agricultural Research of Meknes, National Institute of Agricultural Research, Meknès, 50000, Morocco
4 Environment and Valorization of Microbial and Plant Resources Unit, Faculty of Sciences, Moulay Ismail University, Zitoune, Meknès, 50000, Morocco
* Corresponding Author: Rachid Lahlali. Email:
(This article belongs to the Special Issue: Plants Abiotic and Biotic Stresses: from Characterization to Development of Sustainable Control Strategies)
Phyton-International Journal of Experimental Botany 2025, 94(5), 1453-1495. https://doi.org/10.32604/phyton.2025.064598
Received 19 February 2025; Accepted 29 April 2025; Issue published 29 May 2025
Abstract
Tomato cultivation faces formidable challenges from both biotic and abiotic stressors, necessitating innovative and sustainable strategies to ensure crop resilience and yield stability. This comprehensive review delves into the evolving landscape of employing microbial consortia as a dynamic tool for the integrated management of biotic and abiotic stresses in tomato plants. The microbial consortium, comprising an intricate network of bacteria, fungi, and other beneficial microorganisms, plays a pivotal role in promoting plant health and bolstering defense mechanisms. Against biotic stressors, the consortium exhibits multifaceted actions, including the suppression of pathogenic organisms through antagonistic interactions and the induction of systemic resistance in tomato plants. On the abiotic front, the microbial consortium enhances nutrient availability, optimizes water retention, and ameliorates soil structure, thus mitigating the adverse effects of factors such as drought, salinity, and nutrient imbalances. This review synthesizes current research findings, highlighting the diverse mechanisms through which microbial consortia positively influence the physiological and molecular responses of tomato plants to stress. Furthermore, it explores the adaptability of microbial consortia to various agroecosystems, offering a versatile and sustainable approach to stress management. As a promising avenue for eco-friendly agriculture, the utilization of microbial consortia in tomato cultivation emerges not only as a tool for stress mitigation but also as a transformative strategy to foster long-term sustainability, reduce reliance on synthetic inputs, and enhance overall crop productivity in the face of changing environmental conditions.Keywords
Tomato (Solanum lycopersicum L.) is a globally significant crop within the Solanaceae family, widely appreciated for its economic and nutritional value. Tomatoes are extensively cultivated, with annual production reaching 182.3 million tons globally, making them the second most important vegetable crop after potatoes [1]. Despite this prominence, tomato cultivation faces significant challenges due to biotic and abiotic stresses, which reduce yields and compromise quality [2]. Tomato occupies a central position in world agriculture, both economically and nutritionally. Appreciated for their many culinary applications and rich nutrients, tomatoes have become a staple in diets around the world [3]. Belonging to the Solanaceae family, which encompasses economically significant crops like potato, eggplant, and pepper, the tomato represents a key member of one of the most valuable plant families for vegetable and fruit cultivation [4]. The global distribution of tomato production reveals that Asia contributes 61.1%, while Europe, America, and Africa contribute 13.5%, 13.4%, and 11.8%, respectively [5]. Tomato yields exhibit significant variability across different countries. For instance, in 2022, the global average tomato yield was approximately 36.97 tonnes per hectare. In contrast, certain regions experienced notably lower yields; for example, in 2022, some areas reported average yields of only 85 tonnes per hectare, a decrease from 95 tonnes per hectare in 2021 [6]. This disparity highlights the influence of regional agricultural practices, climate conditions, and resource availability on tomato production efficiency. The primary regions for tomato consumption include China, India, North Africa, the Middle East, the US, and Brazil, with per capita consumption ranging from 61.9 to 198.9 kg [1].
Although tomatoes are generally considered a temperate crop, their cultivation extends over different climatic zones, presenting increased challenges [7]. Crop growth and productivity are often hampered by fluctuating environmental conditions and abiotic stresses. Abiotic stress factors, such as extreme water and temperature conditions, elemental toxicity, and high salinity, are the main limitations to tomato production [8,9]. Alongside these abiotic challenges, tomato plants face a series of biotic challenges that affect both yield and quality. Fungal diseases, exemplified by early blight (Alternaria solani) and late blight (Phytophthora infestans), bacterial wilt (Ralstonia solanacearum), and viral infections such as tomato yellow leaf curl virus (TYLCV), pose substantial threats to global tomato production [7,10]. The tomato brown rugose fruit virus (ToBRFV) has grown to be a serious worldwide danger to tomato production since its discovery in Jordan in 2015, impacting important regions such as Europe, the Middle East, North America, and Asia. It infects almost all cultivated tomato varieties, even those resistant to other tobamoviruses like Tomato mosaic virus (ToMV) [11]. These pathogens not only reduce yields but also compromise the economic viability of this crop [6].
Hence, there is an imperative requirement for a sustainable approach to managing plant stresses. A promising avenue in biological control involves the utilization of microbial consortia [12,13]. Microorganisms, including beneficial fungi and bacteria, establish intricate communities that synergistically interact to enhance plant health and resilience. Serving as biological treatments, these microbial consortia provide comprehensive protection against both biotic and abiotic stressors [14]. The complex interplay between these microbes and the natural defense mechanisms of tomato plants contributes to a holistic and sustainable strategy for managing pests and diseases [13,15,16].
In this review, we highlight the two facets of stress (biotic and abiotic) by focusing on the various challenges faced by tomato plants. It also explores the use of microbial consortia as a promising avenue for plant protection. The complex mechanisms underlying the interactions between microorganisms and tomato plants, including the secretion of bioactive compounds and the induction of systemic tolerance, will be closely examined. In addition, we discuss the practical applications of microbial consortia in the context of sustainable agriculture, with an emphasis on integrating these biocontrol strategies into integrated crop management practices.
This review employed specific keywords, including “tomato” AND “biotic stress” OR “abiotic stress” OR “drought stress” OR “salinity” OR “diseases” AND “biocontrol” OR “microbial consortium,” to collect bibliometric data from the SCOPUS database (https://www.scopus.com/, accessed on 20 December 2023). The bibliometric analysis was conducted using VOSviewer processing software (v1.6.9, Leiden University, Leiden, The Netherlands). The resulting network analysis illustrated the global distribution of articles related to the abiotic and biotic stresses of tomatoes and the application of microbial consortia as a management strategy. Fig. 1 highlights the relationship between the collected keywords and the prevailing perspectives in current research within this field of study. The spatial arrangement of nodes in Fig. 1 provides critical insights into the degree of association between keywords, countries, and research themes. Larger nodes represent terms or countries with higher scientific activity, indicating areas of significant research focus. For example, keywords such as “biotic stress” and “abiotic stress” are prominently featured, reflecting their central role in studies on tomato resilience. Similarly, countries like China, India, and the United States have larger nodes, signifying their substantial contributions to this field. The proximity between nodes reflects the strength of association or collaboration. Closely positioned nodes indicate strong thematic or collaborative links. For instance, keywords related to abiotic stresses (“drought” and “salinity”) are often linked to microbial consortia studies, suggesting a concentrated effort to address these challenges through biological approaches. Colors represent clusters of related terms or regions, highlighting thematic groupings within the research landscape. For example, one cluster may focus on biotic stress management, including terms like “pathogens,” “fungal diseases,” and “biocontrol.” Another cluster might emphasize abiotic stress mitigation, featuring terms like “drought resistance,” “salinity tolerance,” and “microbial interactions.” The connections between nodes signify co-authorship or thematic relationships. Strong links between countries suggest active international collaborations. For example, partnerships between China and the United States are evident, reflecting joint efforts in advancing microbial solutions for tomato stress management.
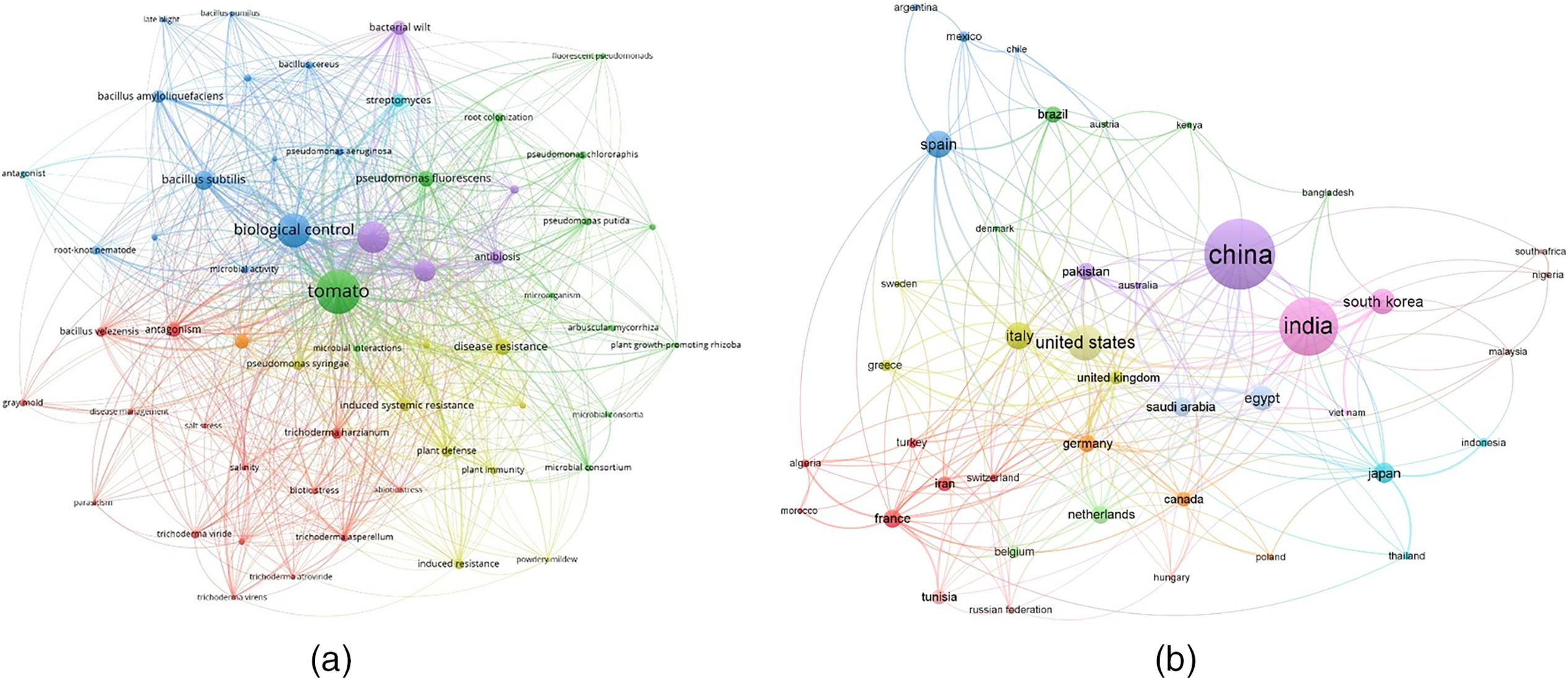
Figure 1: Bibliometric and network analysis of research on the management of biotic and abiotic stresses in tomatoes. A bibliometric analysis was conducted on 1546 published articles addressing the management of biotic and abiotic stress in tomatoes. The analysis utilized the Scopus database and employed relevant keywords such as “Tomato” AND “biotic stress” OR “abiotic stress” OR “drought stress” OR “salinity” OR “diseases” AND “biocontrol” OR “microbial consortium” (a). Additionally, a network analysis was performed to examine the global distribution of these articles (b). In the network analysis, larger circles indicate more intense scientific activity. Colors represent groups of linked terms, with label size indicating the number of publications associated with a term. The distance between two terms reflects the degree of their association. Each node in the network represents a country, and the links between countries signify co-authorship relationships
2 Biotic and Abiotic Stresses in Tomato Cultivation
Numerous abiotic and biotic factors that are detrimental to plant development and productivity are present in crops cultivated in open fields. The current climate change, which is accelerated by rising CO2 levels in the atmosphere [17,18], manifests itself as rising average temperatures and falling precipitation, especially in regions with a warmer climate [18]. Responses to mixtures of biotic and abiotic stressors can be influenced by the degree of abiotic stress [19]. Higher soil salt concentrations make tomatoes and other crop species more susceptible to soil-borne diseases; comparable patterns are noted when there is a water deficit [20,21]. Although research indicates that the effects on pathogenicity are often specific to particular pathosystems [22], there is a consensus that rising temperatures will lead to the geographic expansion of pathogens and increased fecundity. This, in turn, heightens the likelihood of host range expansion and the emergence of more virulent strains [22,23]. Tomato plants are particularly vulnerable to biotic stresses caused by pathogens, pests, and nematodes. Diseases such as early blight (A. solani), late blight (P. infestans), and bacterial speck (Pseudomonas syringae pv. tomato) pose major threats to crop productivity. Viral infections, including the TYLCV and ToMV, further exacerbate these challenges by causing stunted growth and reduced fruit quality [24,25]. Abiotic stresses, such as drought, salinity, extreme temperatures, and heavy metal toxicity, disrupt tomato physiology and limit productivity. For instance, drought reduces water uptake and photosynthesis, while salinity causes ion toxicity and nutrient imbalance. Extremes temperature adversely affect fruit setting, and heavy metals interfere with enzymatic processes, causing growth retardation [26–28]. The combination of these biotic and abiotic stresses significantly challenges tomato production, necessitating innovative and sustainable solutions to ensure stable yields and quality.
Biotic stress is considered a major threat to global food security [29,30]. Among the common pathogens affecting tomatoes, fungal diseases are a major threat. Including pathogens, pests, and nematodes, which collectively threaten the productivity and vitality of tomato plants, in addition, bacterial diseases and viral infections also contribute to biotic stress in tomato cultivation, with bacteria such as Xanthomonas causing bacterial spot disease and viruses such as TYLCV causing leaf yellowing and curling. Pathogens such as A. solani, which causes early blight, and P. infestans, which causes late blight, are major concerns [31]. At the same time, pests such as aphids, whiteflies, thrips, and tomato worms cause considerable damage [32]. Tomato plants are also attacked by the destructive moth Tuta absoluta, which damages the leaves, stems, and fruits, and it spreads quickly and is resistant to many pesticides [33]. In addition, plant-parasitic nematodes, particularly root-knot nematodes (Meloidogyne spp.) can compromise the plant’s root system, leading to reduced nutrient uptake and stunted growth [34]. To meet these challenges, researchers and farmers are using a multi-faceted approach. This includes the development and use of tomato varieties resistant to specific pathogens and pests, the implementation of Integrated Pest Management (IPM) strategies that incorporate the pests’ natural enemies, and soil management practices to mitigate the impact of nematodes [35]. Managing these pathogens is crucial to maintaining healthy tomato crops, and understanding the dynamics of their interactions is essential to developing effective strategies for mitigating biotic stress in tomato plants [2,36].
Early Blight (A. solani): The primary disease symptom brought on by A. solani (Ellis & Martin) Sorauer is early blight. This disease is especially harmful to tomatoes in areas with high humidity, heavy rainfall, and relatively high temperatures (24°C–29°C). In extreme situations, it can result in total defoliation. In semiarid regions with frequent and protracted nocturnal dews, epidemics can also happen [37]. In semiarid regions with frequent and prolonged nightly dews, epidemics can also occur, in addition to the leaf symptoms known as early blight (EB), A. solani can cause less economically significant symptoms on tomatoes, such as fruit rot [38] stem lesions on the adult plant, and collar rot (basal stem lesions at the seedling stage) [39]. The pathogen primarily infects leaves, stems, and fruits, leading to reduced yields and poor fruit quality [40]. Symptoms typically begin as small, dark, and irregular spots on older leaves, which expand into concentric rings resembling a “bullseye” pattern, a hallmark of the disease [37]. As the infection progresses, affected leaves yellow, wither, and drop prematurely, exposing fruits to sunscald and reducing photosynthetic capacity. On stems and fruits, dark, sunken lesions with similar concentric patterns may appear, further compromising the plant’s health [38]. A. solani thrives under warm, humid conditions, and its spores are dispersed by wind, rain splash, or contaminated tools. The disease is exacerbated by stress factors such as nutrient deficiencies, poor drainage, or crowded planting, which increase plant susceptibility [41]. Effective management strategies include the use of resistant tomato varieties, crop rotation with non-susceptible hosts, maintaining proper spacing for air circulation, and the application of fungicides. Integrated approaches combining cultural practices and chemical controls are essential for minimizing losses caused by early blight [41].
Late Blight (P. infestans): Phytophthora infestans, an oomycete pathogen, is the source of late blight, causing leaf necrosis and fruit rot. It spreads rapidly under moist conditions and can result in total crop loss [42], a devastating disease that affects tomatoes and potatoes all over the world. In the majority of the United States and Canada, late blight outbreaks were rare before 1992 [43]. However, serious late blight outbreaks on tomatoes and potatoes were documented in the United States and Canada in 1992 and 1993 [44]. Since then, late blight has been recorded yearly. It is defined as having lesions with a distinct edge and a rough surface [43]. Tomato fruits that come into contact with the soil develop brown to black lesions at first, which grow and acquire dark zonate “buckeye” bands in the afflicted region. This pathogen infects all above-ground parts of the plant, including leaves, stems, and fruits, leading to significant yield losses if not properly managed [45]. Initial symptoms include irregular, water-soaked lesions on leaves, which rapidly enlarge, turning brown with a characteristic whitish fungal growth under humid conditions. Infected stems develop dark lesions, and fruits exhibit brown, firm rot with a leathery texture, rendering them unmarketable [46]. The pathogen’s life cycle is facilitated by sporangia that disperse through water splashes or wind, germinate, and penetrate plant tissues directly or through natural openings. Under favorable conditions [46], the disease can spread rapidly, devastating entire crops within days. Effective management includes the use of resistant cultivars, proper crop rotation, and timely application of fungicides [45]. The global significance of late blight underscores the need for integrated disease management strategies to mitigate its impact on tomato production [47].
Fusarium Wilt (Fusarium oxysporum): Tomato wilt is one of the most significant diseases affecting tomatoes, caused by F. oxysporum f. sp. lycopersici (FOL) [48]. FOL infects the root epidermis, spreads through the vascular tissue, and colonizes the xylem vessels of the plant. This leads to vessel blockage and severe water stress, ultimately causing wilt-like symptoms [49]. The disease is characterized morphologically by wilted plants with yellowing leaves and little to no crop yield. Dormant chlamydospores of FOL can persist in infested soil indefinitely, even in the absence of a host [50]. The infection of vascular tissue by FOL is a complex process involving the following steps: (a) recognition of the host root through specific host-pathogen signaling, (b) attachment to root hair surfaces and hyphal propagation, (c) invasion of the root cortex and vascular tissue, followed by differentiation within xylem vessels, and (d) release of toxins and virulence factors. The colonization of xylem vessels triggers disease development, resulting in the characteristic wilting of the host plant [48]. Given the significant impact of fungal diseases on tomato production, further research into integrated management strategies, including the use of microbial consortia, is essential for ensuring sustainable crop yields [51].
Bacterial Speck (Pseudomonas syringae pv. tomato): This pathogen may infect Brassica and Arabidopsis species in addition to causing bacterial speck disease on tomatoes, this disease manifests as small dark spots on leaves and fruits, which can significantly reduce marketable yield, these spots, often surrounded by yellow halos, can coalesce, causing extensive leaf damage and defoliation [52]. Bacterial speck, caused by P. syringae pv. tomato (Pst), is a significant bacterial disease affecting tomato plants, especially under cool, moist conditions [53]. On fruits, lesions appear as small, raised, black specks that penetrate the epidermis, rendering the produce unmarketable. The pathogen is seed-borne and can survive in crop debris or soil, spreading through rain splashes, irrigation water, and contaminated tools [54]. Infection typically occurs via stomata or wounds, where the bacteria multiply and produce phytotoxins like coronatine, which contribute to symptom development by disrupting the plant’s immune response [53]. Management strategies include using pathogen-free seeds, crop rotation, and resistant tomato varieties. Cultural practices such as avoiding overhead irrigation, proper plant spacing, and prompt removal of infected plant debris can reduce disease incidence. In severe cases, copper-based bactericides can be applied, though their efficacy is limited and may lead to resistance [55]. An integrated disease management approach is essential for effectively controlling bacterial specks and minimizing their impact on tomato production [56].
Bacterial Wilt (R. solanacearum): It affects the plant’s vascular system, leading to wilting and eventual plant death. It is among the most damaging diseases found to date because it causes host plants to wilt quickly and fatally [57]. It has been identified as the second most significant bacterial pathogen and causes vascular wilt. The bacteria persist in soil and water, making it challenging to manage [58]. The disease is found all over the world, has a vast host range (more than 200 species), and has a detrimental economic impact [59]. Depending on the host, cultivar, temperature, soil type, cropping pattern, and strain, R. solanacearum can cause direct yield losses that vary greatly. The pathogen infects plants through the roots, often via wounds or natural openings, and colonizes the xylem vessels. This leads to vascular blockage, disrupting water transport and causing rapid wilting of the plant even under adequate soil moisture conditions [58]. The disease is often characterized by sudden and irreversible wilting of leaves, initially during the hottest part of the day, followed by yellowing and eventual death of the plant. A diagnostic sign is the exudation of milky bacterial ooze from cut stems or roots when immersed in water [60]. R. solanacearum persists in the soil for extended periods as saprophytic populations or as latent infections in alternate hosts. It thrives under warm, moist conditions, making its spread more prevalent during the rainy season [61]. The pathogen’s wide host range and ability to survive in diverse environmental conditions contribute to its status as a significant agricultural problem. Despite all the effective management that involves using traditional methods or biological control agents, bacterial wilt remains challenging to control, emphasizing the importance of continued research into sustainable management practices [62]. Due to the destructive nature of bacterial diseases, an integrated strategy that incorporates resistant varieties, efficient cultural methods, and the use of biological control agents is essential for sustainable tomato production [63].
Tomato Yellow Leaf Curl Virus (TYLCV) is spread by Bemisia tabaci (Homoptera: Aleyrodidae) and is one of the most well-known begomoviruses that infect tomatoes [64]. Tomato plants infected with TYLCV exhibit severe symptoms such as yellowing, leaf bending, and stunting, which significantly reduce tomato output, the disease’s symptoms include stunting, flower abortion, a more or less noticeable upward curling of the leaflet edges, a loss in the leaflet area, and yellowing of the young leaves. Infection generally slows down plant development and yields; if an infection strikes a plant in its early stages, productivity is nearly completely lost [65]. Symptoms typically appear 10–15 days after infection, with young leaves showing curling, distortion, and interveinal chlorosis. The virus can spread rapidly under warm and dry conditions that favor whitefly proliferation. TYLCV has a wide host range, including several solanaceous crops and weeds, which can act as reservoirs for both the virus and its vector, further complicating management efforts [64]. Effective management requires integrated strategies, including the use of resistant or tolerant tomato varieties, regular monitoring of whitefly populations, and cultural practices such as removing infected plants and using physical barriers like insect-proof nets. Biological control agents and judicious use of insecticides targeting whiteflies can also help reduce the incidence of TYLCV. Given the virus’s economic impact and adaptability, ongoing research into resistant cultivars and vector management remains crucial [66].
Tomato Mosaic Virus (ToMV) is a highly contagious and economically significant pathogen of tomatoes, caused by a member of the Tobamovirus genus. It is transmitted mechanically through contaminated tools, hands, or infected plant debris, and occasionally via seeds, which can harbor the virus on their coat or within the embryo [67]. ToMV infects plants systemically, causing a wide range of symptoms that vary depending on the strain, environmental conditions, and host susceptibility [67,68]. Common symptoms include mottling or mosaic patterns of light and dark green on leaves, leaf distortion, and stunted growth. Infected fruits may exhibit uneven ripening, discoloration, or surface lesions, reducing their marketability [69]. The virus spreads rapidly in densely planted crops, particularly in greenhouses, where frequent handling of plants increases the risk of mechanical transmission [70]. Managing ToMV relies primarily on preventative measures, as there is no cure for infected plants. Effective strategies include using resistant tomato varieties, ensuring proper sanitation by disinfecting tools and hands, and removing infected plant debris from fields or greenhouses. Seed treatments, such as hot water or chemical disinfectants, can also reduce seed-borne transmission [71]. Crop rotation and the use of physical barriers like plastic mulches can further limit the spread of ToMV. Despite these measures, the virus remains a persistent challenge, necessitating continued research and development of resistant cultivars. Effective management of these biotic challenges is crucial for maintaining healthy tomato crops, necessitating the development of integrated and sustainable strategies. To effectively combat viral diseases in tomato crops, an integrated approach combining resistant varieties, vector control, and innovative biotechnological solutions is essential [72].
Whiteflies and Aphids: Two commercially significant pests that impact tomato production, both in protected and field settings, are obligate phloem-feeding insects called whiteflies (B. tabaci) and aphids (Myzus persicae, Homoptera: Aphidae) [73]. These insects’ adults feed on the cell contents of afflicted plants by piercing the phloem and releasing copious amounts of honeydew, which eventually encourages the growth of sooty mold and lowers the plant’s photosynthetic efficiency [74]. However, their most significant impact is as vectors for viral diseases, such as TYLCV, which can cause severe yield losses. Whiteflies excrete honeydew, a sticky substance that promotes the growth of sooty mold, further reducing photosynthesis and fruit quality [75].
Root-Knot Nematodes (Meloidogyne spp.): Root-knot nematodes (Meloidogyne spp.) are microscopic, soilborne pests that cause significant damage to tomato crops worldwide [76]. The most common species affecting tomatoes include Meloidogyne incognita, M. javanica, and M. arenaria. These nematodes invade the roots of tomato plants, inducing the formation of characteristic galls or knots that disrupt water and nutrient uptake [77]. Symptoms of infection include stunted growth, yellowing of leaves, wilting, and reduced fruit yield. Severe infestations can lead to plant death, particularly under water stress [78]. The life cycle begins when second-stage juveniles (J2) hatch from eggs in the soil and penetrate root tissues. They establish feeding sites called giant cells, causing abnormal cell division and enlargement [76]. The nematodes complete their life cycle within the roots, laying eggs that are released into the soil to infect new plants [79]. The warm, moist conditions of tomato fields, especially in tropical and subtropical regions, favor their proliferation. Despite all the measures for managing, root-knot nematodes remain challenging, emphasizing the need for continued research into sustainable control methods [34]. Overall, integrated pest and nematode management strategies are crucial for minimizing crop losses and ensuring the long-term sustainability of tomato production systems. In conclusion, biotic stressors such as fungal diseases, bacterial infections, and viral pathogens pose significant threats to tomato cultivation worldwide. The complex interactions between these pathogens and environmental factors underscore the need for innovative management strategies. The next section will explore how microbial consortia can be leveraged to mitigate these biotic stresses, offering a promising avenue for sustainable crop protection.
Abiotic stress in tomato cultivation encompasses various environmental factors that significantly impact plant physiology and productivity. High temperatures, particularly during critical growth stages like flowering and fruit set, can induce heat stress, affecting pollination and resulting in poor fruit development [80]. Conversely, exposure to low temperatures may lead to cold injury, impeding overall plant growth. As far as water stress is concerned, drought and waterlogging can have adverse effects on tomato plants, drought can lead to reduced water uptake, affecting nutrient uptake and resulting in stunted growth and reduced yield [81]. On the other hand, waterlogged soils can damage roots, depriving them of oxygen and making them more susceptible to disease [82]. Soil salinity presents another challenge, as it interferes with water and nutrient uptake, ultimately impacting plant health. Nutritional imbalances, arising from deficiencies or excesses, also contribute to abiotic stress [83]. It is essential to understand the specific nutrient requirements of tomatoes and to maintain adequate soil fertility through balanced fertilization practices [80]. Finally, light intensity plays a key role in photosynthesis and fruit development. Inadequate or excessive light can impact plant growth and yield, requiring careful management of planting density and shading practices [2]. Addressing these abiotic stress factors through innovative strategies, such as the application of microbial consortia, is crucial for enhancing tomato resilience and ensuring sustainable crop production in the face of climate change.
Drought is one of the main environmental stresses hampering crop production, including tomatoes. Decreased water content interferes with many plant processes and leads to reduced plant growth [84]. Therefore, studies have focused on drought. It restricts plant development and production and is a significant issue for agriculture across the world [85]. Drought stress is a major challenge for tomato plants, as it has a profound impact on their growth, physiology, and overall productivity [86]. Being highly sensitive to water availability, tomatoes undergo a series of physiological reactions when confronted with water shortage [87]. The plant’s ability to absorb water is hampered, resulting in reduced water transport and nutrient uptake [84]. Stomatal closure, a common defensive mechanism, reduces water loss through transpiration but simultaneously hinders the crucial process of photosynthesis [86]. As a consequence, photosynthetic activity declines, and the plant grapples with osmotic stress, resulting in wilting and compromised cell expansion. The accumulation of reactive oxygen species (ROS) further exacerbates the challenge, causing oxidative damage to cellular structures [88]. Moreover, the effects of drought stress lead to a buildup of free radicals that affect how membranes function, proteins conform, lipids oxidize, and ultimately cause cell death [89]. Microbes that can withstand drought stress can improve plant development when there is a lack of water. To survive in low water potential, microbes have developed a tolerance mechanism through evolution, adaptation, or both [90]. Maintaining plant water status is crucial for sustaining tolerance to drought, achieved either by limiting water loss or by increasing water absorption capacity through osmotic adjustment [91]. Developing drought-resilient tomato varieties and implementing effective water management practices, potentially enhanced by microbial consortia, are essential for ensuring sustainable production in drought-prone regions.
Salinity stress represents a major challenge to the optimal growth of tomato plants (Fig. 2), as it disrupts various physiological processes and has an impact on overall crop productivity, high concentrations of salts in the soil, particularly sodium chloride, disturb the osmotic balance essential for water uptake [92]. This hampers the plant’s ability to absorb water, leading to water stress and subsequent difficulties in absorbing nutrients, the accumulation of sodium ions can induce ion toxicity, interfere with cellular processes, and damage cell structures [93]. Salinity stress also disrupts the balance of nutrients within the plant, competing with the uptake of essential elements such as potassium. In addition, salinity-induced osmotic stress can lead to symptoms such as leaf wilting, while excess salts can accumulate in leaf margins, causing burning and necrosis [93]. The overall consequence includes reduced photosynthetic activity, stunted growth, and lower fruit yield and quality [92]. Because of the harmful ion effects and osmotic stress caused by salinity, microbial activity is weak, which inhibits plant growth and development [94]. Under various circumstances, cations such as Na+ (sodium), Ca2+ (calcium), K+ (potassium), anions Cl (chloride), and NO3 (nitrate) are the main contributors to soil salinity, many elements of plant life, including agricultural production, seed germination, water, and nutrient intake, as well as physicochemical and ecological balance, are adversely affected by salinity stress [95]. Moreover, it harms the nodulation process, lowering crop yield and nitrogen fixation [92]. Employing integrated strategies, including salt-tolerant varieties and microbial interventions, is essential for mitigating the impact of salinity stress on tomato yields and ensuring sustainable production in affected areas.
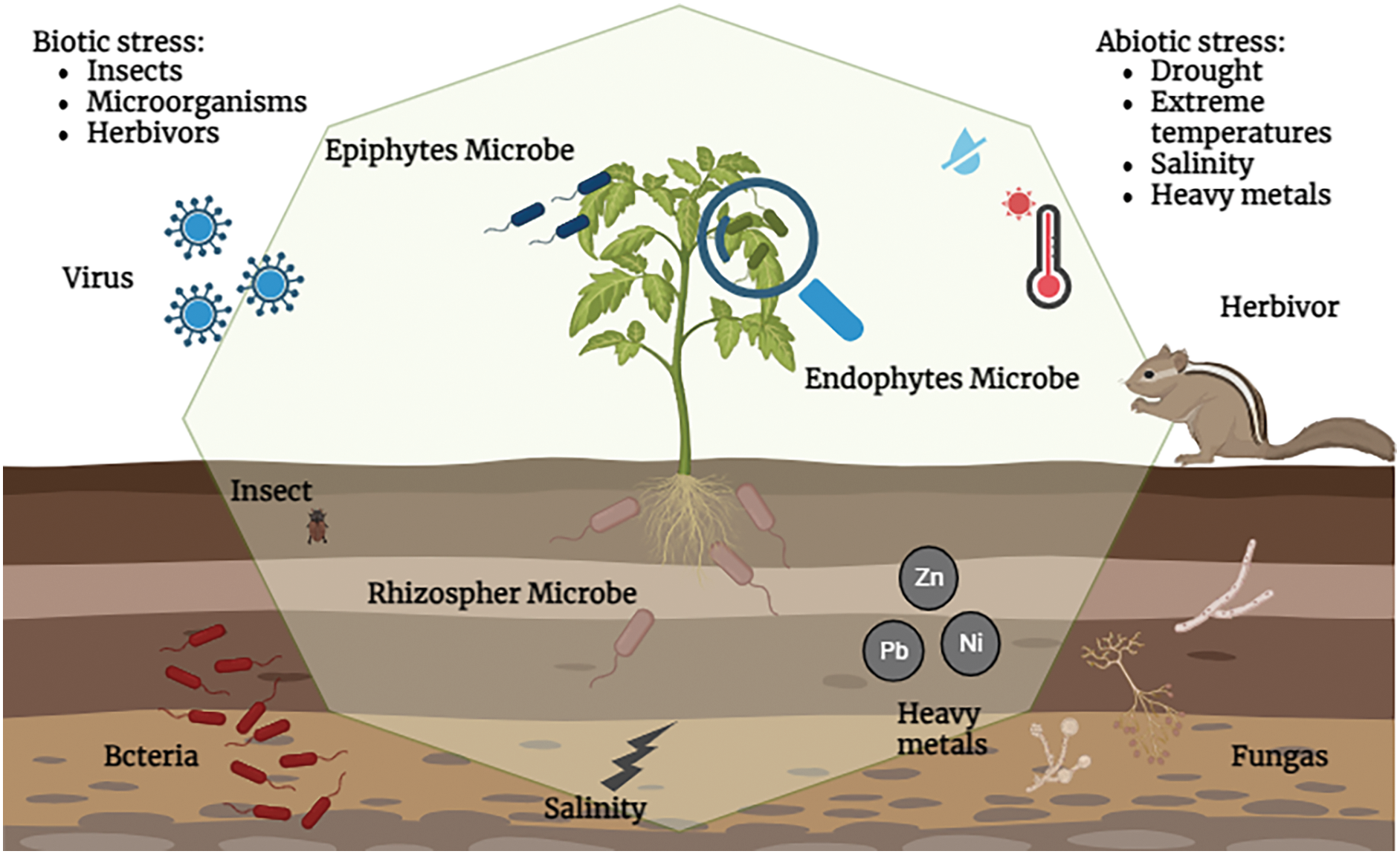
Figure 2: Tomato plant under different stresses and its association with Plant Growth Promoting Microorganisms
Due to their detrimental effects on crop production, human health, and the environment, heavy metal pollution of soil is one of the main issues for agricultural soils. The texture of the soil, crop productivity, growth, yield, nutrient availability, microbial community, and human health are all negatively impacted by excessive accumulation of heavy metals in the soil [96]. On tomato plants, Cu2+, Zn2+, and Cd2+ harmful effects included decreased vegetative seedling development, flowering, and fruiting (Fig. 2) [96,97]. The lasting negative impact of these metals on the surrounding environment is attributed to their non-biodegradable nature, long biological half-life ranging from 10 to 3000 years, and their potential for accumulation in various parts of the body, metal stress decreased tomato plant height by 24.6%, 43.8%, and 58.7% at seedling, vegetative, and flowering-fruiting stages, respectively. Growth retardation, a standard physical parameter for toxicity assessment, is evident [98]. In addition to negatively affecting helpful microbiological activities, high levels of certain heavy metals in soil can reduce crop production by accumulating in various plant sections [99]. Plant enzymes and proteins with high-affinity ligands efficiently chelate heavy metals to lessen their toxicity [99]. Heavy metal interactions with plant proteins and enzymes cause a loss of bioactivity, which manifests as chlorosis, stunted development, browning of the roots, and a decrease in the activity of the photosynthetic system [100]. The presence of heavy metals in soil is also known to induce oxidative stress by producing ROS which negatively affects the two important plant physiological processes i.e., photosynthesis and respiration causing an overall reduction in plant productivity [101].
Temperature stress has become more frequent and intense as a result of climate change. It is well-known that heat stress can occur in tomatoes at average daily temperatures of 28°C–29°C, which are just a few degrees above the optimum temperature range of 21°C–24°C [102]. Temperature stress has a significant impact on plasma membranes, water content (transpiration), photosynthetic activity, enzyme performance, cell division, and plant development. The impact of heat stress depends strongly on the intensity, duration, and rate of temperature change. The water status of the plant is of primary importance in case of temperature change [103]. In addition, higher temperatures are often accompanied by water stress, especially in tropical and subtropical environments, temperature significantly influences various physiological processes in tomatoes, both vegetative and reproductive, independently or in conjunction with other environmental factors such as light, nutrition, and humidity [102]. High temperatures before anthesis induce critical stress, causing developmental abnormalities in the anthers, including irregularities in the epidermis and endothemis, non-opening of the stomium, and poor pollen formation [103]. Manifestations of high-temperature stress resulting in fruit set failure in tomatoes encompass a range of issues such as bud drop, undeveloped flowers, persistent flowers or calyxes, division of the antheridial cone, absence of anther dehiscence, low pollen production, pollen sterility, degeneration of the embryo sac, browning and desiccation of the stigma, reduced stigma receptivity, elongation of the style, underdeveloped ovary, poor fertilization, slow pollen tube growth, low pollen viability, low pollen germination, low ovule viability, ovule abortion, endosperm degeneration, disruption of meiosis, impaired sugar metabolism, reduced carbohydrate availability to fruit, reduced total soluble protein content, developmental abnormalities, low fruit set, reduced fruit and seed size per fruit, and inhibition of pathogen-induced resistance mechanisms [104,105].
A microbial consortium refers to a community or group of microorganisms that interact with each other in a specific environment, often collaborating to perform various functions and eventually protecting surrounding plants from biotic and abiotic stresses (Fig. 2) [106]. These communities are essential for ecosystem functioning, biogeochemical cycling, and various industrial processes [107]. The composition of a microbial consortium is quite complex and may include bacteria, archaea, fungi, and other microorganisms [106]. Their composition is influenced by factors such as environmental conditions, nutrient availability, and interspecies interactions [108]. The members of microbial consortia often work synergistically, forming intricate networks of metabolic interactions. Each microorganism within the consortium contributes to the overall community function through its unique metabolic capabilities [109]. Developing heat- and cold-tolerant tomato varieties and implementing protective measures are crucial for ensuring stable yields and quality under challenging temperature conditions. In summary, abiotic stress factors like drought, salinity, and extreme temperatures severely impact tomato productivity. Understanding these stressors is crucial for developing effective mitigation strategies. The use of microbial consortia, discussed in the following section, presents a novel approach to enhancing plant resilience against these abiotic challenges.
3 The Toxic Effects of Pesticides on Tomatoes
The toxic effects of pesticides on tomatoes can be categorized into two main areas, which are the phytotoxic effects on the plants and health risks associated with pesticide residues [110]. Pesticides, particularly herbicides and, to a lesser extent, insecticides and fungicides, can cause significant damage to tomatoes. These phytotoxic effects include Yellowing in spots or diffuse yellowing of leaves, which can evolve into tissue necrosis. Yellowing of veins, young leaves, or the entire leaf blade, sometimes progressing to bleaching or dryness between veins [110]. Pesticide residues on tomatoes pose health risks to consumers. Studies have shown that a significant percentage of tomato samples contain pesticide residues, often exceeding maximum residue levels (MRLs) [111]. The common pesticides detected are acetamiprid, chlorpyrifos, lambda-cyhalothrin, and DDT, which are frequently found in tomato samples. Chlorpyrifos and DDT are known to be highly toxic to human health [111]. Many samples have pesticide concentrations above EU MRLs, though fewer exceed Codex Alimentarius Commission (CAC) MRLs. The exposure to these residues can lead to various health problems, including acute poisoning risks, especially if withdrawal periods are not observed [112]. Overall, while pesticides are crucial for controlling pests and increasing crop yields, their misuse can lead to both plant damage and consumer health risks. Proper application and adherence to safety guidelines are essential to minimize these effects [110].
The mechanism of action of microbial consortia in mitigating pesticide toxicity
In this regard, microbial consortia can play a crucial role in mitigating pesticide toxicity through several mechanisms, starting with the synergistic metabolic degradation where members of the bacterial consortium work together to degrade pesticides by breaking them down into less toxic or non-toxic compounds [111]. This process involves the sequential degradation of intermediate compounds produced by other members, enhancing the overall metabolic pathways for biodegradation [113]. This synergy increases the efficiency of pesticide degradation, reducing the accumulation of harmful intermediates [114]. Another mechanism is the production of biosurfactants, where Certain strains within the consortium produce biosurfactants, which increase the solubility and bioavailability of pesticides, making them more accessible for degradation. The enhanced solubility and bioavailability improve the biodegradability of pollutants [113]. There is also the self-regulation and adaptation that microbial consortia use to adapt to changing environmental conditions during degradation, ensuring optimal performance even in unstable environments. This adaptability allows consortia to maintain their degradation efficiency under various conditions. After degrading pollutants, consortia can utilize metabolites to promote the growth of other strains within the consortium [115]. This promotes a sustainable and resilient microbial community capable of continuous degradation. There are diverse degradation pathways where consortia often employ diverse bacterial species, each contributing unique enzymatic capabilities, such as oxidative and hydrolytic reactions, to degrade pesticides. This diversity ensures comprehensive degradation of a wide range of pesticides [116].
Overall, microbial consortia are generally more efficient than individual microbial strains in degrading pollutants due to their synergistic interactions [115]. They can thrive in various environmental conditions, making them suitable for different types of contaminated sites. And also, by converting toxic pesticides into less harmful metabolites, microbial consortia help mitigate environmental and health risks associated with pesticide residues [117].
4 Microbial Consortia: Definition and Functionality
4.1 Definition and Composition
Biological Control Agents (BCAs) are a promising substitute for the misuse of agrochemicals in the management of pests and diseases [109]. Numerous soil-borne microbes coexist with plant roots; while some of them are harmful, others offer significant advantages to the host plant, such as enhanced nutrition via growth and defense against a variety of biotic and abiotic challenges [118]. It is commonly known that microorganisms can manage diseases and pests, but one of the main obstacles to their broader use in agriculture is the unpredictability of the results that are frequently observed in natural environments [119]. Research on biocontrol initially concentrated on using individual microbes [120]. Their intricate interactions with the environment and soil microbes have a significant impact on the inoculant’s functionality and permanence [121–123]. Limited competitiveness against native bacteria and fluctuating environmental conditions may be the cause of single-strain inoculants’ inconsistent or inadequate performance [109]. A better understanding of plant microbiota, biotic and abiotic stresses, plant genotypes, and environmental factors enables us to identify more suitable microbial candidates or inoculation strategies adapted to specific environments [123]. Depending on the locally adapted resident microbiota, edaphic factors, host genotype, root architecture, tissue-specific expression, invader density and timing of invasion events, and the bioinoculant’s innate ability to get past these obstacles and establish itself in the microbiome, the bioinoculant’s ability to engraft, or successfully invade a microbial community, will vary. The microbiota of plants includes a variety of species, such as bacteria, fungi, and archaea [124–128]. Plant Growth Promoting Rhizobacteria, Trichoderma spp., and arbuscular mycorrhiza fungi (AMF) are important contributions to biocontrol mechanisms, which protect plants either directly through microbial antagonism (antibiosis, competition, parasitism) or indirectly through plant-mediated effects (better nutrition, induced systemic resistance, ISR) [129]. For instance, the study by Rana et al. (2025) investigates the field efficacy of Trichoderma viride, Pseudomonas fluorescens, and consortia of these microbial agents against tomato late blight caused by Phytophthora infestans. The research likely explores how these biocontrol agents individually and in combination can manage this significant disease, which is a major threat to tomato crops worldwide. Trichoderma viride is known for its ability to control soil-borne pathogens, while Pseudomonas fluorescens is recognized for inducing systemic resistance in plants [130]. Synthetic microbial communities (SynComs) that combine fungus and bacteria can produce more robust and efficient biocontrol solutions by utilizing complementary modes of action and adjusting to a variety of environmental conditions [131]. To provide an environmentally friendly alternative to chemical pesticides in agriculture, the change in crop protection is centered on creating microbial consortia that include several beneficial bacteria and fungi with complementing biocontrol modes of action [132].
The application of bacterial consortia in plant protection has emerged as a promising alternative to traditional chemical pesticides, offering several advantages in terms of sustainability and disease control. Bacterial consortia, which involve the combination of multiple beneficial bacterial strains, provide enhanced protection against a broad range of plant pathogens through synergistic interactions [133]. The creation of bacterial consortia has gained interest as a sustainable method of food production since they can improve the positive properties demonstrated by these bacteria. In a synergistic or additive interaction, two or more compatible bacteria from different species may form a bacterial consortium [132,134,135]. Key components of a microbial consortium include bacteria, which often dominate these communities and play crucial roles in nutrient cycling, organic matter decomposition, and other ecological processes [136,137]. Different bacterial species may specialize in the degradation of specific compounds or have complementary metabolic functions. Plant growth-promoting bacteria (PGPBs) such as Bacillus, Pseudomonas, Enterobacter, and Streptomyces are well-known members of microbial consortia, contributing to plant health and growth [32]. One of the primary benefits of bacterial consortia is their ability to combat plant diseases through various mechanisms. These include antimicrobial compound production, where bacteria produce natural antibiotics that inhibit pathogen growth, and competition for nutrients and space, which reduces the available resources for harmful microorganisms. Additionally, some bacterial consortia are known to trigger ISR in plants, enhancing their overall defense mechanisms against pathogens [138,139]. The versatility of bacterial consortia is particularly valuable in managing both soil-borne and foliar diseases, as these consortia can be tailored to target a wide range of pathogens [140]. Moreover, bacterial consortia often exhibit plant growth-promoting properties, enhancing crop yield and resilience. They can improve soil health by solubilizing nutrients, fixing nitrogen, and producing growth-promoting substances such as indole acetic acid (IAA) [141]. These benefits not only help protect crops from diseases but also support plant growth, particularly under stress conditions, thus contributing to overall agricultural productivity [142]. Research has shown that bacterial consortia can be more effective than single-strain inoculants, especially when dealing with the diversity of environmental challenges encountered in field conditions [143]. In addition to disease suppression, bacterial consortia contribute to sustainable agricultural practices [144]. By reducing reliance on chemical pesticides, which can have negative environmental impacts, these microbial solutions offer a more eco-friendly alternative [139]. Their use aligns with the principles of IPM, where multiple biological, physical, and chemical methods are combined to manage pests and diseases in an environmentally conscious manner [145]. Based on their modes of action, interactions with plants, and functions in promoting plant development and thwarting pathogens, bacterial strains employed in plant protection can be divided into several categories [146].
Numerous, if not all, plant species are linked to PGPB, which are frequently found in a variety of settings. The most extensively researched group of PGPB are plant growth-promoting rhizobacteria (PGPR) that colonize the rhizosphere, the closely adhering soil interface, and the root surfaces [137]. To promote plant development, PGPB can reduce environmental stress and enhance plant tolerance [147]. One of the most widely recognized groups of antagonistic bacteria are species from the genus Bacillus, such as Bacillus subtilis and Bacillus amyloliquefaciens [133]. The use of Bacillus species as biocontrol agents represents a promising and environmentally friendly alternative to chemical pesticides in agriculture [148]. These microorganisms exhibit diverse capabilities, including the production of antimicrobial compounds, promotion of plant growth, and ISR [148]. For instance, Bacillus thuringiensis produces Cry proteins, which are highly specific and effective against insect pests, reducing reliance on synthetic insecticides [149]. Other species, such as B. subtilis and B. amyloliquefaciens, are effective biofungicides, controlling through the production of lipopeptides, such as iturins and fengycins, which disrupt fungal cell membranes [150]. Additionally, Bacillus species contribute to soil health by solubilizing phosphorus and producing phytohormones like auxins and cytokinins, which enhance nutrient uptake and plant growth [151]. These multifaceted benefits position Bacillus as a cornerstone in sustainable agriculture, offering eco-friendly solutions for plant protection and growth stimulation while minimizing environmental and health risks associated with chemical inputs [148]. Additionally, Pseudomonas spp. are known for producing siderophores, which sequester iron and prevent pathogens from acquiring this critical nutrient, thereby inhibiting their growth [152]. Many PGPBs cause ISR under biotic stress, which is a physical and/or chemical alteration associated with plant defense [153]. Researchers have observed effective control of plant diseases brought on by a variety of pathogens in both greenhouse and field conditions through ISR elicited by PGPB [154]. Furthermore, induced systemic tolerance under abiotic stress has been used to characterize the physical and chemical changes caused by PGPB in plants that increase their tolerance to salt, drought, and other conditions [155]. The responses of plants to abiotic stress induced by PGPB are known as Induced Systemic Tolerance (IST) [147]. Plant biologists and microbiologists have focused a lot of their attention recently on the role of bacteria in reducing abiotic stressors on plants [156]. Many bacteria’s inherent metabolic and genetic capacity aids in the tolerance of and mitigation of the detrimental effects of abiotic stressors on plants. Superoxide dismutase (SOD), catalase, and peroxidase, the primary enzymes that scavenge excessive ROS, are controlled by PGPB during the management of salt stress in plants [157,158]. Some glycine betaine-producing or osmotolerant bacteria work in concert with glycine betaine generated by plants to increase the ability of plants to withstand drought stress [159].
4.2.2 Plant Growth-Promoting Rhizobacteria (PGPR)
PGPR are beneficial rhizospheric bacteria that enhance plant growth and suppress diseases in crops. The term “PGPR” was introduced by Kloepper and Schroth in 1978 to describe microbes that promote plant development by colonizing roots effectively [160]. These bacteria play a crucial role in agricultural sustainability by improving growth parameters and providing biological disease control. The rhizosphere supports the formation, development, and operation of a variety of microbial communities, including PGPRs [132]. It provides plants with protection against phytoparasites [161] while enhancing their tolerance to abiotic stresses [162]. PGPR modulates auxin/cytokinin ratios and stimulates phytohormonal signaling for plant defense. Plants also recruit beneficial microorganisms to the rhizosphere when facing pathogens [132]. PGPR offers plants both direct and indirect protection against pests and diseases. Species like Bacillus, Pseudomonas, Rhizobium, Azotobacter, Enterobacter, and Azospirillum have been shown to improve plant health through various mechanisms. These include the production of plant hormones such as ethylene, indole-3-acetic acid (IAA), cytokinins, and gibberellic acid, which promote growth by enhancing root development and nutrient and water uptake [132]. Additionally, PGPR can fix nitrogen, enriching soil fertility. Indirectly, they protect plants by producing antimicrobial compounds, including antibiotics and siderophores, which suppress harmful phytopathogens [163]. However, while PGPR have demonstrated success in controlled environments, their effectiveness often diminishes in field conditions due to unstable interactions with host plants, influenced by environmental factors such as soil pH, moisture, and temperature [133]. Moreover, for PGPR to be widely adopted, it is crucial to integrate them into sustainable agricultural practices, combining them with crop management techniques and cultural practices to maximize their effectiveness. PGPR can become a cornerstone of sustainable agriculture, reducing reliance on chemical pesticides and fostering healthier crop ecosystems [123,164].
Due to their effectiveness in managing plant diseases and infections, fungus antagonists are now being extensively researched and used as BCAs. Fungal consortia, which combine diverse fungal species with complementary traits, have emerged as a sustainable tool in modern agriculture. These consortia utilize the unique abilities of different fungi to simultaneously enhance plant growth, improve soil health, and suppress pathogens and pests [165]. Mycorrhizal fungi, such as Rhizophagus irregularis, contribute to better nutrient acquisition and increased plant resilience, while entomopathogenic fungi like Beauveria bassiana and Metarhizium robertsii target pest populations. The synergy between these fungi amplifies their individual effects, making fungal consortia a cornerstone of IPM strategies. Their ability to integrate pest control with soil fertility management makes them vital in sustainable crop production [166]. Remarkably, fungal inoculants, including all tested mycorrhizal strains, Trichoderma harzianum, the EPF (Entomopathogenic Fungi) Metarhizium robertsii, and the M2 SynCom (a fungal consortium composed of B. bassiana, M. robertsii, and R. irregularis), achieved significant reductions in the natural incidence of Tuta absoluta, a major pest in tomato crops [33]. R. irregularis and M. robertsii demonstrated exceptional effectiveness, reducing pest incidence by up to 90%. These findings highlight the potential of fungal consortia to enhance plant defenses and effectively protect crops from pests [33]. The dual role of fungal consortia in promoting plant health and directly suppressing pests showcases their importance in reducing reliance on chemical pesticides. Their integration into IPM practices offers a holistic approach to crop management, addressing both productivity and environmental sustainability [167]. Synergistic formulations, such as the M2 SynCom, underscore the advantages of combining multiple fungi, ensuring consistent performance across diverse agricultural conditions. These results reinforce the transformative role of fungal consortia in advancing sustainable agriculture and safeguarding essential crops [168].
Antibiosis is a crucial biological control system for protecting plants, wherein helpful microbes like fungi and bacteria create antimicrobial chemicals that stop plant diseases from growing [169]. These substances, which include volatile organic compounds (VOCs), antifungal proteins, and antibiotics, can directly inhibit the growth of pathogenic microorganisms by interfering with their biological functions or outcompeting them for resources [170]. For instance, Trichoderma spp. release enzymes like chitinases to break down fungal cell walls, while Bacillus spp. and Pseudomonas fluorescens generate antibiotics that target pathogens like Fusarium and Pythium (Table 1) [171]. In addition to assisting in the management of plant diseases, this mechanism lessens the demand for chemical pesticides, providing a more environmentally responsible and sustainable option for agricultural pest control [172].
The application of a microbial consortium, comprising effective strains for biological control, represents an effective strategy compared to the use of individual microbes for tomato plant disease management [140]. In addition, the application of microbes in a consortium can improve the efficacy, reliability, and consistency of microbes under various soil and environmental conditions [136]. One fundamental mechanism involves the suppression of plant pathogens through competitive exclusion, where beneficial microbes outcompete harmful pathogens for essential resources [121]. Additionally, certain members of the microbial consortium produce antimicrobial compounds, such as antibiotics and lytic enzymes, directly inhibiting the growth of plant pathogens [155]. Another crucial aspect is the ISR in plants, wherein the presence of specific microbial species triggers the plant’s immune response, fortifying it against future infections [12]. Nitrogen-fixing bacteria within the consortium contribute to nutrient availability, promoting plant growth [121]. Moreover, microbial communities enhance soil structure, water retention, and nutrient cycling, fostering a conducive environment for plant development [136]. These complex interactions within the microbial consortium exemplify nature’s ingenious strategy to bolster plant defenses and sustain agricultural ecosystems [152]. PGPB may modify plant gene expression to make the plant less susceptible to these stressors, even while they can offer some protection against the inhibitory effects of salt or drought stress [173]. For instance, it has been demonstrated that different PGPB promote plant production of the metabolites betaine, proline, and trehalose as well as the creation of enzymes like superoxide dismutase (SOD) and catalase (CAT) that may detoxify reactive oxygen species [174] (Table 1). Among these antagonistic microorganisms, which exert various mechanisms to combat the disease, namely inhibition of the pathogen by antimicrobial compounds (antibiosis), competition for iron through the production of siderophores; competition for colonization sites and nutrients supplied by seeds and roots; induction of plant resistance mechanisms, inactivation of pathogen germination factors present in seed or root exudates, or degradation of pathogenicity factors of the pathogens such as toxins and parasitism, which may involve the production of extracellular cell-wall-degrading enzymes such as chitinase and b-1,3 glucanase, which degrades the cell walls of pathogens [12,173,175]. Antibiotic production by bacteria, particularly Pseudomonads, appears to be tightly regulated by a two-component system involving an environmental sensor and a cytoplasmic response factor [173]. Using PAO1 mutants of Pseudomonas aeruginosa unable to produce hydrogen cyanide (HCN), it has been confirmed that cyanide poisoning is responsible for the death of the nematode Caenorhabditis elegans [176]. Pseudomonas species can synthesize enzymes that can also modulate plant hormone levels and limit available iron through the production of siderophores. In addition to killing the pathogen by producing antibiotics [177].
4.4.2 Induced Systemic Resistance (ISR)
Beneficial microorganisms, such as microbial consortia, can trigger the plant defense mechanism known as ISR (Fig. 3), which provides broad-spectrum resistance against a variety of diseases [178]. By producing elicitors like lipopeptides, siderophores, and volatile organic substances, microbial consortia, which are made up of synergistic mixtures of bacterial and fungal strains, improve ISR [179]. These elicitors cause the overexpression of genes linked to defense by initiating signaling pathways in plants, which mainly involve ethylene (ET) and jasmonic acid (JA). ISR offers a broader response against biotic stressors than systemic acquired resistance (SAR), which is pathogen-specific and reliant on salicylic acid (SA) [180]. For example, it has been demonstrated that consortia of Bacillus, Pseudomonas, and Trichoderma species can cause ISR by promoting the accumulation of phenolic substances (Table 1), secondary metabolites, and defense enzymes such as polyphenol oxidases and peroxidases [178]. Because systemic priming increases plant resistance to pathogen assault without causing growth penalties, ISR is a sustainable and environmentally beneficial crop protection method [181]. Further highlighting their function in integrated pest management (IPM) systems, studies have shown how effective microbial consortia are at activating ISR to fight diseases like bacterial specks, Rhizoctonia root rot, and Fusarium wilt in tomatoes [179]. Inducing systemic resistance through the application of microbial consortia offers a sustainable approach to enhancing tomato resistance against a broad spectrum of pathogens and pests, reducing the need for chemical interventions.
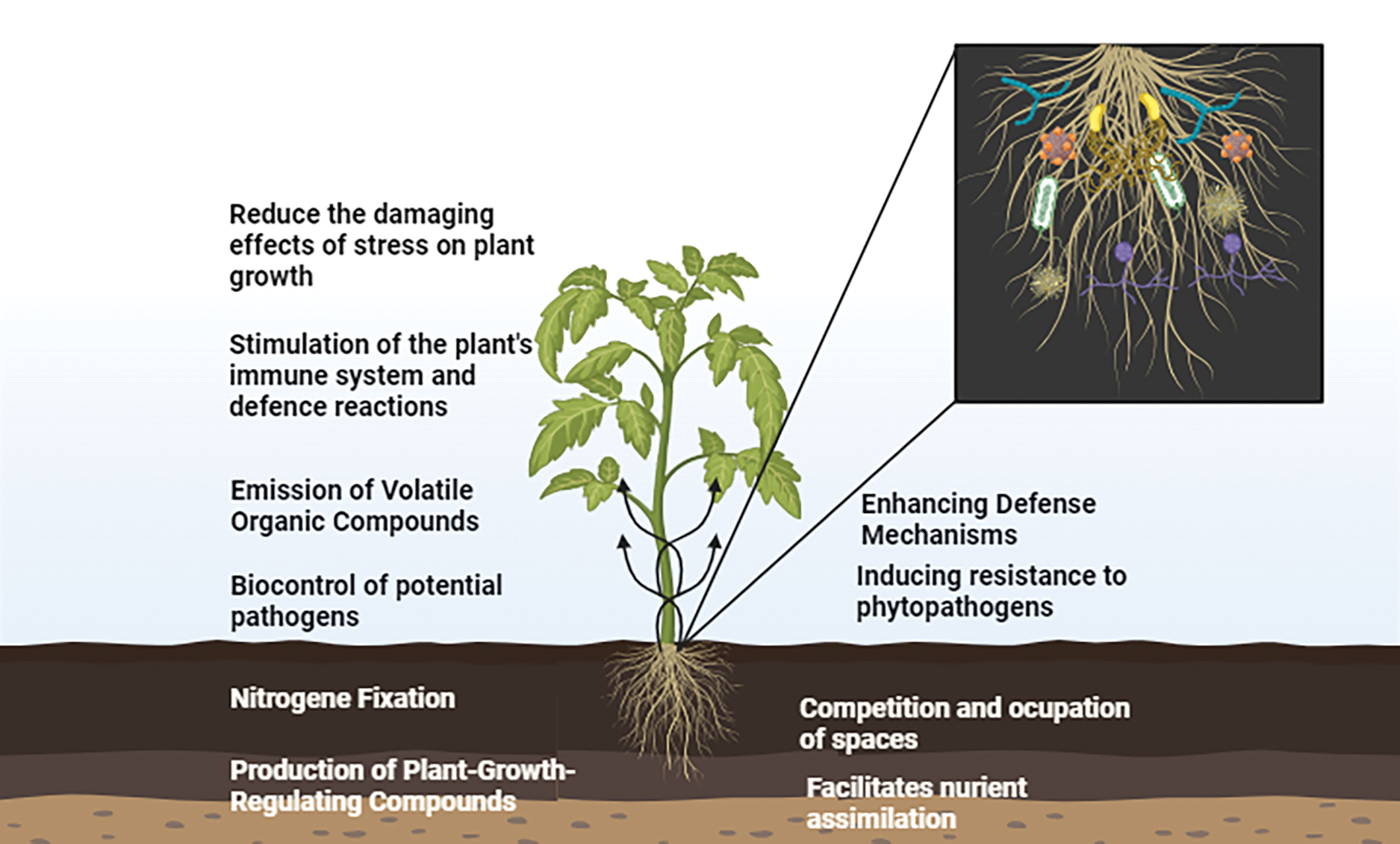
Figure 3: Diverse effects of microbial consortia on plant growth and health
4.4.3 Nutrient Solubilization and Cycling
Microorganisms play a pivotal role in nutrient solubilization and cycling (Fig. 3), which significantly enhances soil fertility and plant nutrient uptake [182]. Nitrogen fixation is a critical process performed by bacteria such as Azospirillum, which convert atmospheric nitrogen into bioavailable forms, ensuring an essential supply for plant growth [183]. Additionally, phosphorus, often present in insoluble forms in soil, is solubilized by beneficial microorganisms that produce organic acids, such as gluconic and citric acids [184]. These acids dissolve insoluble phosphate compounds, making phosphorus readily available for plant uptake [137]. The dual role of these microbes in nitrogen fixation and phosphorus solubilization underscores their importance in sustainable agriculture by reducing dependency on chemical fertilizers while maintaining soil health [185]. Enhancing nutrient uptake through the application of microbial consortia represents a sustainable strategy for improving tomato growth and productivity, particularly in nutrient-poor soils.
Through a variety of processes, microbial inoculants such as endophytic fungi and PGPR improve plant tolerance [181]. They generate phytohormones that regulate plant development and stress response, such as gibberellins and indole-3-acetic acid (IAA) [186]. Furthermore, by fixing atmospheric nitrogen and solubilizing phosphorus, these microbes enhance nutrient absorption and increase plant vigor under stress [187]. Beneficial microorganisms encourage the buildup of osmoprotectants such as proline, glycine betaine, and trehalose under salt and drought stress. These substances assist preserve cellular osmotic equilibrium and shield macromolecules from harm [188]. Additionally, these bacteria increase the activity of stress-related enzymes that detoxify ROS and stop oxidative damage brought on by environmental stressors, such as peroxidase, catalase, and superoxide dismutase (SOD) [156,189]. Under water-deficient situations, the formation of exopolysaccharides by specific bacteria also helps to improve soil structure and water retention, creating a more suitable environment for root development [190]. By lowering dependency on chemical inputs and increasing crop yield and sustainability, the incorporation of microbial solutions into agricultural methods presents a viable and environmentally responsible method of managing abiotic stress [191] (Table 1).
The mechanisms of action of microbial consortia in enhancing tomato resilience are complex and multifaceted, involving a combination of direct and indirect effects on plant physiology and defense responses. Further research is needed to fully elucidate these mechanisms and optimize the application of microbial consortia in tomato cultivation.
5 Applications in Plant Protection
5.1 Mitigation of Biotic Stress
To combat biotic stress, these consortia, which are made up of helpful bacteria, fungus, and other microorganisms, use two different strategies. First, by producing antimicrobial substances such as lipopeptides, antibiotics, and volatile chemical compounds, they directly inhibit infections. For instance, Bacillus species create lipopeptides called iturins and fengycins that break down the cell membranes of fungi, whereas antibiotics stop bacteria from growing [150]. Second, microbial consortia augment the plant’s innate immune system and induce systemic resistance (ISR), which improves plant defenses. Defense-related enzymes like peroxidase and polyphenol oxidase are produced as a result of signaling pathways being activated by the creation of elicitors such as surfactins and jasmonates. These enzymes reduce oxidative stress-induced damage and aid in the fight against invasive infections [167,203]. Furthermore, by increasing nutrient availability and generating phytohormones that boost general resistance to biotic stress, microbial consortia support plant health [151]. They are an essential part of sustainable agriculture since they may supplement or replace chemical pesticides, lowering pollution levels while preserving crop yield and health [148]. The utilization of microbial consortia represents a promising strategy for managing biotic stresses in tomato plants, offering a sustainable alternative to conventional chemical approaches [204].
5.2 Enhancing Resistance to Abiotic Stress
Abiotic stresses are major constraints of plant growth and development, which in turn affects crop yield, food quality, and global food security. Drought, salt, and heavy metal stress, as well as nutrition and temperature stress, are the major ones to be known. Drought is one of the most significant ones, followed by salt stress [205]. These two stresses have a maximum deleterious effect on extensive areas of land [206]. Under stress conditions, various parameters such as molecular biology, biochemistry, and physiology of plants are affected. Drought and salt stress are known to disrupt photosynthesis, leading to leaf senescence, excessive formation of ROS, nutritional deficiencies, and the breakdown of cellular organelles and metabolism, all of which contribute to reduced plant growth [14]. The resultant damage comprises both physiological and metabolically disrupted homeostasis of plants [205,206]. Another significant soil stressor is metal stress, which is getting worse due to a variety of human-caused reasons. The unchecked population growth and industrial revolution are causing organic wastes and toxic metals to accumulate in the soil, rendering it unsuitable for agricultural practices and harmful to all living organisms [207]. In this regard, the use of inorganic fertilizers and chemical pesticides degrades soil fertility and pollutes the ecosystem. Certain exudates released by the plant during the stress phase can serve as a signaling mechanism to change or establish a healthy rhizosphere soil community [208,209].
PGPB are a sustainable alternative for improving plant growth and soil fertility. They regulate crop growth, development, and productivity by improving the availability and biosynthesis of limiting macro- and micronutrients. Plant growth-promoting microorganisms (PGPMs), including bacteria, actinomycetes, fungi, and algae, have beneficial effects on plant growth through direct or indirect mechanisms. They play a significant role in sustainable agriculture by increasing crop production, improving soil fertility, promoting diversity, inhibiting pathogen growth, and maintaining system sustainability [12,209]. In the same context, applying microbial consortia to agricultural fields is a novel natural method that can improve plant development and help plants resist a variety of stressors since compost is composed of a variety of microbial consortia that can operate in a range of temperature variations [210].
Compared to bacteria, fungi are more susceptible to greater salt concentrations [211]. Their mechanisms and modes of operation differ. These processes result in an increase in root length, surface area, and root count, which facilitates nutrient absorption [212]. The main indirect process is a decrease in the incidence of plant pathogens that cause illness [213]. Rhizobacteria that colonize roots generate ACC (1-aminocyclopropane-1-carboxylate) deaminase, which breaks down ACC into ammonia and α-ketobutyrate while reducing ethylene [214]. The enzyme Rhizobitoxine increases nodulation and inhibits ethylene synthesis under stressful situations [215]. By possibly collecting osmolytes in their cytoplasm, which counteract osmotic stress and preserve cell turgor and plant development, plant growth-promoting bacteria (PGPB) may be able to reduce the effects of salt stress [211]. For plants and microorganisms, low and high osmotic potential make it difficult for them to absorb water from the soil [213].
5.2.1 Enhancing Drought Resilience in Plants
Low soil moisture or water content that is insufficient to meet plant needs is known as drought stress. Water deficit or stress is produced when a plant’s water content drops to the point that it interferes with regular plant functions or when water loss from metabolic processes and transpiration surpasses the amount of water available for absorption [14]. Reduced soil moisture brought on by decreased rainfall or additional irrigation may potentially be the cause. Numerous plant stages and metabolic systems have been revealed to be negatively impacted by water stress [216]. For instance, water stress lowers a plant cell’s water potential, which raises the concentration of solutes. This further prevents cell expansion, stem proliferation, and root elongation, all of which limit plant development [217]. But in stressful situations, rhizobacteria may be able to proliferate and help plants adapt to more stressful situations [218] even more PGPR changes the structure of the roots and increases the plant’s capacity to absorb nutrients and draw water [208,219]. ACC deaminase activity, production of exopolysaccharide (EPS) [220] and volatile organic compounds (VOCs), osmolyte and antioxidant production, enhanced mineral nutrient uptake, phytohormone production, and modulation are among the mechanisms proposed by PGPM to overcome drought stress in plants [207]. Additionally, through the production of osmoprotectants like proline and betaine, which stabilize cell structures, microbial consortia assist plants in maintaining water balance during dry circumstances [221]. For example, fungi such as R. irregularis work symbiotically with plant roots to enhance water absorption [222]. Through these pathways, the PGPRs are given to plants, either individually or in combination, to alleviate drought stress [223]. By improving physiological parameters like shoot length, root length, and fresh and dry weight of root and shoot, as well as by regulating defense enzymes like superoxide dismutase, catalase, and lipid peroxidase, microbial consortia of Pseudomonas putida NBRIRA + B. amyloliquefaciens NBRISN13 reduce drought stress in chickpeas. They also increase soil enzyme activity and microbial diversity in the rhizosphere area under drought stress [224].
5.2.2 Enhancing Salinity Stress Resilience in Plants
A salt concentration that is more than what plants need is referred to as soil salinity. The soil around the root zone turns saline when the electrical conductivity (EC) approaches 4 dS/m (40 mM NaCl) [225]. In addition to affecting the physicochemical characteristics of soil and interfering with nutrient absorption, excessive salt concentrations that result in limited water availability also produce drought-like conditions and make nutrients inaccessible to plants [14]. Plant growth, photosynthetic ability, CO2 absorption, and nitrogen content are all impacted by salt stress, which also causes ion toxicity and oxidative stress [226]. However, under salty conditions, the combination of distinct but compatible genera of microorganisms can significantly increase plant development and may help to mitigate salinity [227]. Under salinity, microbial consortia perform several vital functions, such as enhancing plant development, serving as osmoprotectants, antioxidants, and biocontrol agents, and lowering soil stress. For more details, the enzyme ACC deaminase, which is produced by bacteria in consortia, decreases ethylene levels in plants, increasing their ability to resist salt. Additionally, they release organic acids that decrease ion toxicity [213] (Fig. 4).
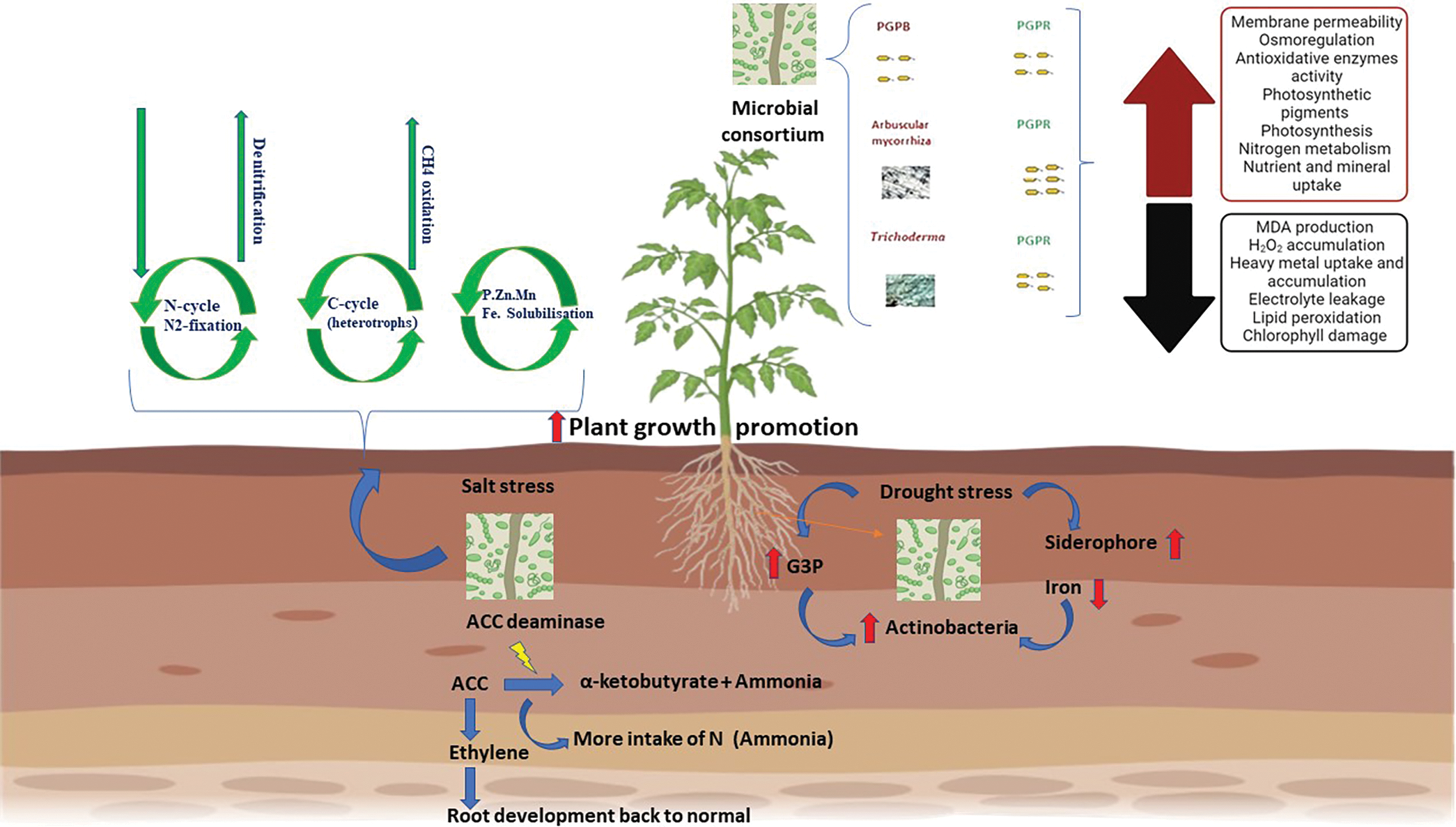
Figure 4: Mechanisms and role of microbial consortia to enhance resistance against abiotic factors
The interaction of halotolerant bacteria such as Enterobacter cloacae and Bacillus drentensis improved the growth of salt-stressed mung beans by enhancing water uptake and nutrient availability. These bacteria contribute to soil health through mechanisms like exopolysaccharide production, which helps retain moisture and nutrients [228]. One of the applications on tomato plant was the study of Kapadia C et al. who demonstrated that four rhizobacteria strains in microbial consortia Bacillus sp., Delftia sp., Enterobacter sp., and Achromobacter sp. can assist tomatoes in overcoming the effects of salt stress [229]. By improving plant development indicators, chlorophyll content, mineral absorption, accumulation, and translocation to a new region of the plant, consortiums help tomatoes that are under salt stress [227]. Other research on wheat, indicated that multi-strain bacterial consortia could effectively promote wheat growth under salinity stress. The co-inoculation of different PGPR strains was more beneficial than single-strain applications, as it harnessed a broader range of growth-promoting traits. This approach has shown promise in enhancing plant resilience to salinity, particularly in arid regions [230].
5.2.3 Heavy Metal Detoxification
Heavy metals (HMs) are defined as elements with metallic properties and a broad molecular weight range, including transition metals, which can be bioaccumulated easily in affected soils. Even more, they have increased significantly in soil due to the Industrial Revolution and human activities [231]. Excessive metals in soil hinder soil characteristics and fertility, making it unsuitable for agricultural purposes [207]. Plant metabolism and growth are also harmed by the abundant HM in soil, which is absorbed and translocated to numerous organs of plants [207].
In the soil, microorganisms have the potential to bind and sequester heavy metals, lowering their availability to plants. This enhances plant development and reduces oxidative stress. They also assist in the phytoremediation of polluted locations because of well-known plant growth-enhancing processes such as siderophore synthesis, nitrogen fixation, phosphate solubilization, and hormone secretion (IAA, GA) [232]. These types of PGPR are known as heavy metal-resistant bacteria in the rhizosphere and they have a major impact on plant tolerance and accumulation. Rhizobacteria that were isolated from mining and mining sites can resist perfectly the effects of heavy metal stress [233]. The mechanism behind it is the increasing dehydrogenase activity that reduces metal accumulation in plant parts and soil and controls the bioaccumulation factor (BAF) of heavy metals. The rhizobacteria consortia of Bacillus cereus MG257494.1, Alcaligenes faecalis MG966440.1, and Alcaligenes faecalis MG257493.1 demonstrated this type of mechanism of tolerance against heavy metals (Cu, Pb, Cd, and Zn) [14,234]. A study conducted in São Paulo, Brazil, utilized a heavy-metal resistant bacterial consortium derived from a contaminated river to design a fixed-bed bioreactor for copper removal. The consortium demonstrated a copper sorption capacity of 450 mg/g dry cells. In a controlled setup, the biofilm formed on granular activated carbon (GAC) retained 45% of the copper mass in the influent, significantly outperforming a control column with only GAC, which retained 17% [235], In another study, Bacillus marisflavi and Trichoderma longibrachiatum, two microbial strains, showed moderate tolerance to metals. Electrochemical screen-printed electrodes were used to determine metal concentrations before and after bioremediation. T. longibrachiatum showed higher bioremediation potential, removing 87% of Cr and 67% of Zn, while B. marisflavi removed 86% of Pb [236].
Microbial consortia, which are communities of two or more microbial species that interact symbiotically, have diverse applications in agriculture and environmental management [237]. Their use in seed treatments, root inoculants, and foliar sprays is gaining traction due to their ability to enhance plant growth, improve soil health, and facilitate the degradation of complex organic materials [133].
Coating seeds with microbial consortia promotes early colonization of the rhizosphere, increasing root health and early-stage resistance [238]. These treatments can enhance nutrient absorption and protect against disease. For example, specific microbial combinations have been demonstrated to improve the degeneration of paddy straw residues when employed with seeds, enabling better crop establishment and decreasing disease incidence. This strategy not only improves seedling performance but also adds to sustainable farming practices by reducing the demand for chemical pesticides and fertilizers [239].
The study conducted by Raja et al. (2019) used liquid microbial cultures to enhance germination and vigor in pigeonpea seeds [240]. The bacterial strains used were Methylobacterium, Rhizobium, and phosphobacteria, which were cultured in specific broths. After application, seeds were assessed for viability and vigor. The study also investigated the compatibility of microbial cultures with seed-treating chemicals. The results showed that pink pigmented facultative methylotroph (PPFM) performed the best in enhancing seed germination and vigor, particularly at a 1:100 dilution for 3 h. The use of microbial consortia improved germination and vigor. However, chemical treatments like carbendazim reduced microbial populations, but polymer coating mitigated this effect. The study concluded that liquid microbial cultures can effectively enhance seed germination and vigor in pigeonpea, provided the concentration and soaking duration are carefully managed [240]. Raza et al. (2024) used a synthetic consortium of beneficial Bacillus microbes to manage Fusarium wilt disease and improve seedling growth in pea seeds. The strains were B. subtilis, B. amyloliquefaciens, and Bacillus fortis, identified through 16S rRNA gene sequencing. They were combined into a seed coating consortium, which was prepared by preparing an aqueous suspension of the bacterial consortium [241]. The seeds were then coated with the consortium using gum arabica as a binding agent and talc as a filler material. The seeds were then wetted with the bacterial suspension, dried, and stored for future use. The study evaluated the performance of coated seeds in sterilized potting mix, revealing that they had higher germination rates, seedling length, and biomass compared to untreated seeds. When challenged with Fusarium wilt, coated seeds reduced disease index and improved growth. The coating also increased phenolic compounds and defense-related enzymes, enhancing disease resistance. This suggests that seed coating with beneficial Bacillus bacteria is effective for managing Fusarium wilt [241]. Sharma et al. (2003) experimented comparison between a cyanobacteria consortium (BF1-4) and a biofilm (AnTr biofilm) on maize inbreds (HKI323PV and HKI161PV). The cyanobacterial consortium included Anabaena torulosa (BF1), Nostoc carneum (BF2), Nostoc piscinale (BF3), and Anabaena doliolum (BF4). The study found that seed inoculation with microorganisms increased germination percentage and enzyme activities for both maize inbreds [242]. The highest germination values were found for HKI323PV seeds inoculated with An-Tr biofilm, while the greatest increases were found for HKI161PV. This improved mobilization of nutrients at the seed stage provided energy for seedling growth, with significant increases in root length, shoot length, fresh weight, and dry weight [243].
Mastouri (2010) investigated various abiotic stress conditions during tomato seed germination and assessed the efficacy of Trichoderma seed therapy as a way of maintaining high seedling performance even under such settings [244]. Trichoderma treatment had no impact in the absence of stress, but under osmotic, salt, or suboptimal temperature circumstances, it ensured a quicker and more uniform germination in comparison to the control. Trichoderma may provide physiological protection in stressed seedlings by reducing the buildup of lipid peroxides in the presence of oxidative damage [245]. Another coating inoculum composed of P. fluorescens and T. harzianum either in combination or singly induced a lower mean germination time (less than 2.5 days) of tomato seeds and a significantly higher germination rate (more than 48%); the combinations of inoculants were more effective than single-isolate treatments [246].
Applying consortia directly to the root zone enhances nutrient uptake, soil health, and long-term protection against stressors [247]. Root inoculants include the direct application of microbial consortia to plant root zones. These consortia can improve nutrient availability, notably nitrogen and phosphorus, while also improving soil structure through enhanced microbial activity. Studies have shown that microbial inoculants including strains such as Bacillus and Pseudomonas may greatly boost root development and nutrient absorption in diverse crops [239,248]. Furthermore, these inoculants may help in the bioremediation of polluted soils by degrading contaminants via the metabolic activities of the bacteria involved [248].
A study designed microbial consortia including B. amyloliquefaciens, Pseudomonas azotoformans, and T. harzianum to control root and shoot pathogens like F. oxysporum and Botrytis cinerea. The microorganisms were prepared and cultured in specific media, and their spores or cells were collected, washed, and resuspended in sterile water. The microbial suspensions were then applied to the seeds or roots of tomato plants (Fig. 5). Surface-sterilized tomato seeds were treated with microbial suspensions, and the seeds were sown in pre-infected soil to simulate a disease-prone environment. The microbial treatments were applied by pipetting the suspension onto the seeds and covering them with sterile vermiculite. The effectiveness of the treatments was evaluated by measuring plant survival rates and disease suppression after 15 days. The study also explored the root colonization of the applied microorganisms, quantifying colonization using colony-forming unit (cfu) counts after 15 days of growth. T. harzianum and the microbial consortia showed the highest efficacy in increasing plant survival and reducing disease symptoms [133].
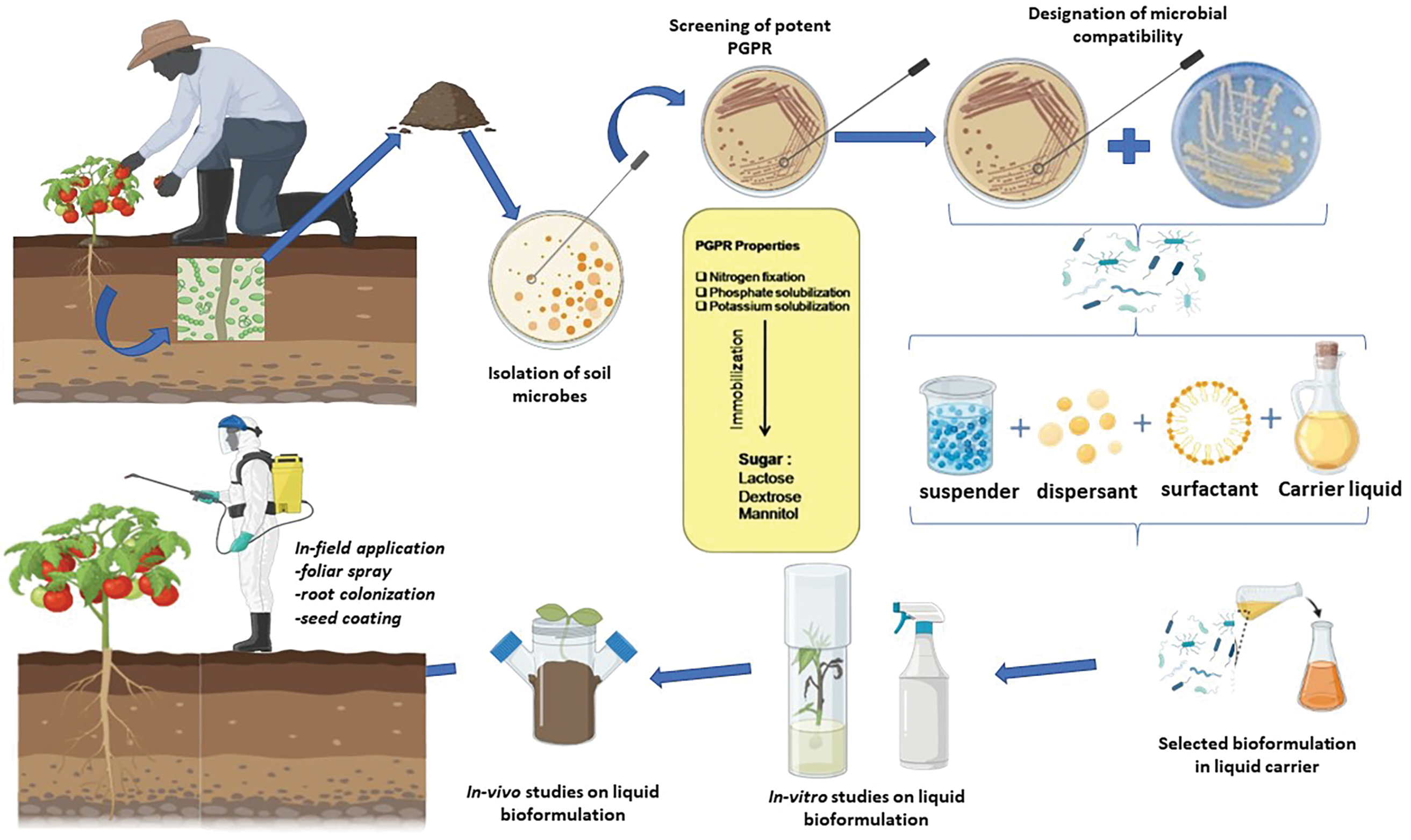
Figure 5: Preparation of microbial consortium and practical applications in plants and soil health
Another study investigated the effects of microbial consortia, B. amyloliquefaciens, and AMF + Pseudomonas brassicacearum on drought protection across various crops. The results indicated that specific combinations of fungal and bacterial inoculants significantly improved vegetative growth under drought conditions, showcasing the potential of microbial consortia to enhance resilience against abiotic stress [249]. This synergistic interaction enhances nutrient uptake and overall plant health when used as a root inoculant. It is also observed in other studies like the one made by Cirillo et al. (2023) which used the combination of Trichoderma strains with beneficial microorganisms such as Azotobacter and it has shown to improve tomato plant performance under varying environmental conditions [250]. Soil amendment with microbial consortia represents a sustainable strategy for improving soil health, enhancing nutrient availability, and promoting tomato growth and productivity.
Spraying microbial formulations on the leaves can lower the occurrence of foliar diseases and pests, work as a barrier of protection [133]. Foliar applications of microbial consortia offer helpful microorganisms straight to plant leaves. This method can help plants resist biotic pressures (such as pests and diseases) and abiotic difficulties (such as drought). For example, microbial sprays have been used to promote the suppression of organic wastes in-situ, allowing for greater nutrient cycling within the ecosystem [239]. The use of such sprays has been found to boost crop health and production while supporting sustainable agriculture practices. Sprayed directly onto tomato leaves, this consortium colonized foliar tissues and persisted for up to 4 weeks post-application. Upregulated defense genes (PR-1a, LoxD, GluB) linked to salicylic acid, jasmonic acid, and ethylene signaling pathways [133]. Trichoderma spp. degraded fungal cell walls via chitinases, while Bacillus and Pseudomonas spp. produced antimicrobial compounds (lipopeptides) and competitively excluded pathogens through biofilm formation [251]. Effectively controlled both foliar Botrytis cinerea (gray mold) and root Fusarium oxysporum, matching the efficacy of the best-performing individual strains [133]. Fusarium wilt diseases significantly impact crop growth and productivity. A consortium of antagonistic rhizospheric Bacillus strains and quercetin was evaluated as a potential remedy for managing tomato plant Fusarium wilt. The combination of Bacillus strains and quercetin reduced the disease index by 69% and increased plant growth attributes. The treatment also improved total phenolic contents and enzyme activities of the phenylpropanoid pathway enzymes. Non-targeted metabolomics analysis showed that the Bacillus consortium and quercetin effectively ameliorated the production of different metabolites in tomato plants [251]. Sidharthan et al. developed a microbial-based consortium to combat Fusarium wilt in tomatoes. The consortium, consisting of Chaetomium globosum, Pseudomonas putida, Bacillus subtilis, and Trichoderma harzianum, showed positive interactions in growth, antagonism, and gene expression. The combination was found to be most effective, reducing Fusarium wilt incidence by 71% and increasing plant growth by 135%. The consortium’s effectiveness is partly due to the upregulation of defense-related genes in tomato plants, suggesting a plant-mediated response. The consortium’s effectiveness was primarily applied via seed treatment and soil drenching, suggesting adaptability to foliar use [252]. Foliar spray application of microbial consortia offers a direct and effective approach for enhancing plant defense responses, mitigating stress, and improving overall tomato health. Optimizing application strategies for microbial consortia is crucial for maximizing their beneficial effects on tomato plants and ensuring their effective integration into sustainable crop management systems.
Apparently, within a plant environment and regarding its location, the microbial agents within a plant microbiome participate in tasks that are complicated in which they can coexist and communicate under so many environmental conditions [253,254]. Accordingly, when abiotic and biotic agents are presented in soil, they may negatively impact the beneficial properties of individual microbial strains, such as their disease-inhibiting and pollutant-eradicating effects, rendering them less effective under natural conditions compared to controlled environments like greenhouse experiments or laboratory settings [255]. This is why advanced associations of microbial agents have been developed to enhance the efficacy of microorganisms in improving soil health. Interestingly, recent studies have shown that physical and metabolic interactions within microbial associations help preserve beneficial properties that support soil health, in contrast to their individual components [256,257]. By adopting this type of research, we can explore the eventuality of using known industrial microorganisms together in a solid community to strengthened their desired functions in a numerous domains like biotechnology, dentistry, medicine and also food processing [258–260]. Likewise, for environmental protection, especially in the case of soil, this sort of association can become handy to alleviate contamination and control soil-borne pathogens [261,262].
6.1 Nutrient Cycling and Organic Matter Decomposition
The rhizosphere in complex regions helps in the control of physicochemical interactions of the soil, such as chemical signals that are exchanged between microbial communities and inhabiting plants, and also plant growth [263,264]. Nutrients consumed by microbial biomass are incorporated into soils in the form of useful organic matter linked with minerals, which are progressively released to improve soil quality [265,266]. There are microbial communities behind these processes that are crucial for the functioning of fertile soils. These processes are the basis for cycling minerals such as K, Fe, B, C, N, P, and S. Such microorganisms are often perceived as positive indicators of key soil functions in nutrient cycling because they can directly absorb minerals for slow and continuous release [267]. Vital soil enzymes that are produced by microbes are also employed as indications in various biogeochemical cycles for sufficient nutrient recycling [268]. Also, in soils that are naturally rich in nitrogen, microorganisms that are known for nitrogen cycling can be considered as an indicator for improving soil nitrogen and maintaining it. Additionally, in this regard, an association between fauna, flora, and microbial communities are important actors in biological nitrogen that makes it more available for plant roots by decomposing N-rich plant residues and releasing organic nitrogen [268]. These elements, together with soil fertility, are required for plant growth and development. Likewise, the chemical and physical functions of soils rely heavily on biological features, which are often covered by various microbial populations [269].
The study by Hassan et al. (2023) demonstrated that bacterial consortia containing Lysinibacillus sp. and Acinetobacter sp. significantly enhance nutrient uptake and biofortification in tomato fruits. These consortia alter the soil microbiome by increasing the abundance of beneficial bacteria such as Proteobacteria, which play a crucial role in nutrient cycling and plant growth promotion. The mechanisms of action involve enhancing nutrient availability through processes like nitrogen fixation, phosphorus solubilization, and the production of growth-promoting substances. Overall, this approach offers a sustainable method for improving crop nutrition, reducing reliance on synthetic fertilizers, and promoting environmental sustainability, thereby contributing to more sustainable agricultural practices [251]. The use of a microbial consortium comprising Azotobacter chroococcum 76A and Trichoderma afroharzianum T22 has been demonstrated to significantly enhance tomato yield, with increases of up to 48.5% under optimal water and nutrient conditions. This consortium not only boosts yield but also improves plant tolerance to water and nitrogen deficiencies by modulating the soil microbial community. The application of these microorganisms enhances nutrient availability and improves plant water relations, allowing tomatoes to adapt better to stress conditions. This approach offers a promising strategy for sustainable agriculture by improving crop resilience and productivity while reducing the need for chemical inputs [250]. Salinity significantly impacts crop growth, development, and reproductive biology. The accumulation of salts and chemicals reduces cultivable area, making it difficult to avoid abiotic stress factors. The addition of microbes, such as Bacillus sp., Delftia sp., Enterobacter sp., and Achromobacter sp., enriches soil without adverse effects. A study evaluated the effects of microbial consortia on tomato growth and mineral uptake under salt stress and normal soil conditions. Salinity treatments were established by mixing soil with seawater, and seedlings were inoculated with microbial consortia. The results showed that salt treatment significantly affected leaf, shoot, and root dry weight; shoot height; number of secondary roots; chlorophyll; and mineral contents in uninoculated seedlings. However, bacterized seedlings sown under saline soil increased leaf, shoot, and root dry weight; leaf number; shoot length; root length; secondary roots; and chlorophyll content. The inoculation of microbial consortia produced more secondary roots, accumulating more minerals and transporting substances to the plant, resulting in higher biomass and growth [270]. Microbial consortia appear to be the best alternative and cost-effective approach for managing soil salinity and improving plant growth under salt stress conditions [227]. This is a clear example demonstrating that the microbial population can directly contribute to soil fertility and crop yield. Hence, they serve as strong indicators of improved soil health, as they collectively drive decomposition processes [271].
6.2 Production of Phytohormones and Plant Growth Promotion
Microorganisms naturally create phytohormones such as gibberellins, indole acetic acid (IAA), cytokinins, and ethylene, which regulate plant growth and development [272]. Rhizobacteria and cyanobacteria are essential for plant growth, providing nitrogen, suppressing diseases, and producing vitamins and phytohormones. Cyanobacteria can photosynthesize, fix nitrogen, and stimulate rhizogenesis, aerial growth, and carbon supply. This study investigated the effectiveness of consortia using heterotrophic bacteria and cyanobacteria in developing tomato seedlings. The microbial collection consisted of three cyanobacteria (SAB-M612 and SAB-B866) and GS (unidentified cyanobacterium) and two phosphate and potassium solubilizing heterotrophic bacteria. The study found that the most compatible consortia were obtained by combining SAB-B866 and GS cyanobacteria with either of the two heterotrophic bacteria. Cyanobacteria GS promoted the growth of both rhizobacteria in vitro, while Cyanobacteria SAB-B866 stimulated tomato seedling growth in planta, leading to greater aerial development. The artificial formulation of microbial consortia can have positive synergistic effects on plant growth, making them of great agrobiotechnological interest [273].
GAs are also reported as regulators of plant growth by degrading DELLA proteins [178] and also as stimulators of elongation in plants and germination [274]. Adding to plant growth promotion properties. Plants treated with IAA-producing Azospirillum sp. showed improved host immunity against diseases [275]. As cytokinins are vital for cell division and defense responses against biotrophic plant diseases [178], it is widely reported that the increase in the level of cytokinins in plants can be microbe-mediated [276,277], thus, phytohormone-producing microbial communities are linked to plant growth promotion and can serve as indicators in plant health management. All this data about bacterial and fungal strain secretions has led the road to producing microbial inoculants for plant growth promotion, biological control of pests and pathogens, bioremediation of polluted soil locations, and the breakdown and degradation of agricultural wastes [268,272,278]. That is why eco-friendly agriculture promotes microbial biofertilizers, which perform specialized activities for fertile, productive, and sustainable soils, improving plant stress tolerance and agricultural yield [279–281].
As an example of studies utilizing microbial consortia for plant growth promotion. Additionally, plants inoculated with the bacterial consortium exhibited improved growth rates, increased flowering, and enhanced fruit. Another study by Cirillo et al. 2023, explored the combined inoculation of Azotobacter (a nitrogen-fixing bacterium) and Trichoderma on various crops and showed that this microbial consortium significantly enhanced the production of phytohormones such as IAA and gibberellins [250]. Additionally, crops treated with this combination demonstrated improved growth metrics, including root length, shoot height, and overall biomass compared to untreated controls [250].
In contemporary agriculture, microbial consortia provide a novel and sustainable strategy for enhancing plant resilience, production, and health. These consortia provide a potent substitute for chemical fertilizers and pesticides by utilizing the advantageous interactions between various microorganisms. These consortia offer several benefits, including improved soil structure, increased tolerance to abiotic stressors, enhanced nutrient availability, and pathogen suppression. Microbial consortia’s potential for sustainable crop management is highlighted by their capacity to stimulate plant defense mechanisms through ISR and SAR, as well as their capacity to enhance plant growth through nitrogen fixation, phosphorus solubilization, and phytohormone production.
The ability of microbial consortia to inhibit illness is among their most important contributions. Consortia like B. subtilis and T. harzianum or P. fluorescens and B. amyloliquefaciens have shown high efficiency against soil-borne diseases like F. oxysporum, R. solani, and P. infestans by competing with pathogens for nutrients and space, generating antimicrobial compounds, and triggering plant immune responses. Furthermore, microbial consortia contribute to improved soil health by fostering microbial biodiversity, enhancing soil aggregation, and increasing water retention capacity all of which are critical for maintaining soil fertility and structure over time. Additionally, microbial consortia are essential for reducing the effects of abiotic stressors such as salt, drought, and heavy metal toxicity. Beneficial bacteria help plants resist adverse climatic circumstances by generating antioxidant enzymes like catalase and superoxide dismutase, as well as osmoprotectants like proline and trehalose. In areas with harsh weather, this capacity to reduce stress is essential for stabilizing crop production and adjusting agricultural systems to climate change.
The agricultural industry may shift to more environmentally friendly and sustainable farming methods by incorporating microbial consortia into ICM systems. By lowering production costs and minimizing chemical pollution and soil degradation, the use of microbial-based treatments will lessen dependency on synthetic agrochemicals and promote environmental preservation. Microbial consortia will become more and more important in sustaining ecological balance while guaranteeing long-term agricultural production and food security as the world’s food demand rises. To sum up, microbial consortia provide a revolutionary approach to sustainable agriculture by striking a balance between environmental responsibility and production. Even if there are still obstacles to overcome, achieving their full potential in global farming systems will require more study, improved technology, and regulatory assistance.
Despite their advantages, there are several obstacles to overcome before microbial consortia may be successfully included into extensive agricultural systems. Their efficacy may be impacted by variations in soil composition, environmental factors, and native microbiota, necessitating the development of optimal formulations suited to particular crops and agroecosystems. To guarantee uniform outcomes, treatment techniques including foliar sprays, root inoculation, and seed coating must also be standardized. Adapting the application of microbial consortia to various agricultural contexts will need regular monitoring of microbial populations, soil health, and crop performance.
Improving the stability and effectiveness of microbial consortia in outdoor settings should be the main goal of future studies. Improvements in microbial formulations, their persistence in soil, and their capacity to respond to a variety of climatic settings can all be facilitated by developments in metagenomics, synthetic biology, and microbial engineering. Furthermore, to maximize their effectiveness and reduce any possible ecological disturbances, it will be essential to comprehend how microbial consortia interact with the natural soil microbiome.
Acknowledgement: This research was financially supported by the Phytopathology Unit of the Department of Plant Protection and Environment at the Ecole Nationale d’Agriculture de Meknès, Morocco.
Funding Statement: This research was funded by the Phytopathology Unit of the Department of Plant Pathology—Ecole Nationale d’Agriculture (Meknès). Financial support has been provided to SIRAM by PRIMA and MESRSI (Morocco), a program supported by H2020, the European Program for Research and Innovation.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Oumaima Benaissa, Ikram Legrifi; data collection: Oumaima Benaissa, Ikram Legrifi; analysis and interpretation of results: Mohammed Taoussi, Zineb Belabess, Ikram Legrifi, Rachid Lahlali, Abderrahim Lazraq; draft manuscript preparation: Oumaima Benaissa, Mohammed Taoussi, Ikram Legrifi; review and editing: Rachid Lahlali, Zineb Belabess; Supervision: Rachid Lahlali, Zineb Belabess, Abderrahim Lazraq. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No data was used for the research described in the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. FAO. Food and agriculture organization of the united nations [Internet]. 2023 [cited 2025 Apr 28]. Available from: http://www.fao.org/statistics/en/. [Google Scholar]
2. Cruz-López V, Granados-Echegoyen CA, Pérez-Pacheco R, Robles C, Álvarez-Lopeztello J, Morales I, et al. Plant diversity as a sustainable strategy for mitigating biotic and abiotic stresses in tomato cultivation. Front Sustain Food Syst. 2024;8:1336810. doi:10.3389/fsufs.2024.1336810. [Google Scholar] [CrossRef]
3. Ayvaz MÇ. Review of tomatoes unveiled: a comprehensive exploration from cultivation to culinary and nutritional significance. Qeios. 2024. doi:10.32388/CP4Z4W.2. [Google Scholar] [CrossRef]
4. Motti R. The Solanaceae family: botanical features and diversity. In: The wild solanums genomes. Cham, Switzerland: Springer International Publishing; 2021. doi:10.1007/978-3-030-30343-3_1. [Google Scholar] [CrossRef]
5. Quinet M, Angosto T, Yuste-Lisbona FJ, Blanchard-Gros R, Bigot S, Martinez JP, et al. Tomato fruit development and metabolism. Front Plant Sci. 2019;10:1554. doi:10.3389/fpls.2019.01554. [Google Scholar] [PubMed] [CrossRef]
6. Tiwari JK, Behera TK, Rai N, Yerasu SR, Singh MK, Singh PM. Tomato breeding for processing in India: current status and prospects. Veg Sci. 2023;49(2):123–32. doi:10.61180/vegsci.2022.v49.i2.01. [Google Scholar] [CrossRef]
7. Panno S, Davino S, Caruso AG, Bertacca S, Crnogorac A, Mandić A, et al. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the Mediterranean basin. Agronomy. 2021;11(11):2188. doi:10.3390/agronomy11112188. [Google Scholar] [CrossRef]
8. Rahim HU, Ali W, Uddin M, Ahmad S, Khan K, Bibi H, et al. Abiotic stresses in soils, their effects on plants, and mitigation strategies: a literature review. Chem Ecol. 2025;41(4):552–85. doi:10.1080/02757540.2024.2439830. [Google Scholar] [CrossRef]
9. Mittal U, Kumar V, Kukreja S, Singh B, Pandey NK, Goutam U. Role of beneficial elements in developing resilience to abiotic and biotic stresses in plants: present status and future prospects. J Plant Growth Regul. 2023;42(6):3789–813. doi:10.1007/s00344-022-10840-w. [Google Scholar] [CrossRef]
10. Chaerani R, Voorrips RE. Tomato early blight (Alternaria solanithe pathogen, genetics, and breeding for resistance. J Gen Plant Pathol. 2006;72(6):335–47. doi:10.1007/s10327-006-0299-3. [Google Scholar] [CrossRef]
11. Skelton A, Frew L, Ward R, Hodgson R, Forde S, McDonough S, et al. Tomato brown rugose fruit virus: survival and disinfection efficacy on common glasshouse surfaces. Viruses. 2023;15(10):2076. doi:10.3390/v15102076. [Google Scholar] [PubMed] [CrossRef]
12. Santoyo G, Guzmán-Guzmán P, Parra-Cota FI, de los Santos-Villalobos S, del Carmen Orozco-Mosqueda M, Glick BR. Plant growth stimulation by microbial consortia. Agronomy. 2021;11(2):219. doi:10.3390/agronomy11020219. [Google Scholar] [CrossRef]
13. Antonio Cruz AL, García Montalvo IA, Matías Pérez D, Pérez Santiago AD, Sánchez Medina MA. Use of microbial consortia in agriculture as an alternative for achieving sustainable agriculture. Ciencia Latina. 2025;9(2):872–87. doi:10.37811/cl_rcm.v9i2.16893. [Google Scholar] [CrossRef]
14. Sharma S, Rathod ZR, Jain R, Goswami D, Saraf M. Strategies to evaluate microbial consortia for mitigating abiotic stress in plants. In: Sustainable agrobiology. Singapore: Springer Nature Singapore; 2023. p. 177–203. doi:10.1007/978-981-19-9570-5_9. [Google Scholar] [CrossRef]
15. Vishwakarma K, Kumar N, Shandilya C, Mohapatra S, Bhayana S, Varma A. Revisiting plant-microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Front Microbiol. 2020;11:560406. doi:10.3389/fmicb.2020.560406. [Google Scholar] [PubMed] [CrossRef]
16. Fagnano FM, Ventorino V, Pasolli E, Romano I, Ambrosino P, Pepe O. From microbiome to biostimulants: unlocking the potential of tomato root endophytes. BMC Plant Biol. 2025;25(1):427. doi:10.1186/s12870-025-06447-4. [Google Scholar] [PubMed] [CrossRef]
17. Bacelar E, Pinto T, Anjos R, Morais MC, Oliveira I, Vilela A, et al. Impacts of climate change and mitigation strategies for some abiotic and biotic constraints influencing fruit growth and quality. Plants. 2024;13(14):1942. doi:10.3390/plants13141942. [Google Scholar] [PubMed] [CrossRef]
18. Lahlali R, Taoussi M, Laasli SE, Gachara G, Ezzouggari R, Belabess Z, et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024;3(3):159–70. doi:10.1016/j.crope.2024.05.003. [Google Scholar] [CrossRef]
19. Shiade SRG, Zand-Silakhoor A, Fathi A, Rahimi R, Minkina T, Rajput VD, et al. Plant metabolites and signaling pathways in response to biotic and abiotic stresses: exploring bio stimulant applications. Plant Stress. 2024;12:100454. doi:10.1016/j.stress.2024.100454. [Google Scholar] [CrossRef]
20. Zamora Re MI, Tomasek A, Hopkins BG, Sullivan DM, Brewer L. Managing salt-affected soils for crop production; 2022. [cited 2025 Apr 28]. Available from: https://extension.oregonstate.edu/catalog/pub/pnw-601-managing-salt-affected-soilscrop-production. [Google Scholar]
21. Beaulieu J. Characterizing the effects of deficit irrigation on soil borne diseases of processing tomatoes and evaluating tools for disease management under water scarcity [Ph.D. dissertation]. Davis, CA, USA: University of California; 2023. [Google Scholar]
22. Gurung SA, Rai AK, Sunar K, Das K. Plant-endophyte interactions: a driving phenomenon for boosting plant health under climate change conditions. In: Microbial symbionts and plant health: trends and applications for changing climate. Singapore: Springer Nature Singapore; 2023. p. 233–63. doi:10.1007/978-981-99-0030-5_10. [Google Scholar] [CrossRef]
23. Garrett KA, Jumpponen A, Toomajian C, Gomez-Montano L. Climate change and plant health: designing research spillover from plant genomics for understanding the role of microbial communities. Can J Plant Pathol. 2012;34(3):349–61. doi:10.1080/07060661.2012.706832. [Google Scholar] [CrossRef]
24. Prasad A, Sharma N, Hari-Gowthem G, Muthamilarasan M, Prasad M. Tomato yellow leaf curl virus: impact, challenges, and management. Trends Plant Sci. 2020;25(9):897–911. doi:10.1016/j.tplants.2020.03.015. [Google Scholar] [PubMed] [CrossRef]
25. Gupta R, Leibman-Markus M, Weiss D, Spiegelman Z, Bar M. Tobamovirus infection aggravates gray mold disease caused by Botrytis cinerea by manipulating the salicylic acid pathway in tomato. Front Plant Sci. 2023;14:1196456. doi:10.3389/fpls.2023.1196456. [Google Scholar] [CrossRef]
26. Manzoor MA, Xu Y, Lv Z, Xu J, Shah IH, Sabir IA, et al. Horticulture crop under pressure: unraveling the impact of climate change on nutrition and fruit cracking. J Environ Manage. 2024;357(1):120759. doi:10.1016/j.jenvman.2024.120759. [Google Scholar] [PubMed] [CrossRef]
27. Rachappanavar V, Padiyal A, Sharma JK, Gupta SK. Plant hormone-mediated stress regulation responses in fruit crops—a review. Sci Hortic. 2022;304(1):111302. doi:10.1016/j.scienta.2022.111302. [Google Scholar] [CrossRef]
28. Bhattacharya A. Plant growth hormones in plants under low-temperature stress: a review. In: Physiological processes in plants under low temperature stress. Singapore: Springer Singapore; 2022. p. 517–627. doi:10.1007/978-981-16-9037-2_6. [Google Scholar] [CrossRef]
29. Sandhu R, Bangarwa SK, Attri M, Tiwari S, Kohli S, Fayaz S, et al. Effects of biotic stresses and their mitigation strategies in legumes: a review. Legume Res Int J. 2025;48(2):193–202. doi:10.18805/lr-5160. [Google Scholar] [CrossRef]
30. Saha B, Biswas S, Datta S, Mojumdar A, Pal S, Mohanty PS, et al. Sustainable Nano solutions for global food security and biotic stress management. Plant Nano Biol. 2024;9(2):100090. doi:10.1016/j.plana.2024.100090. [Google Scholar] [CrossRef]
31. Gupta SK, Sharma M, Mukherjee S. Buckeye rot of tomato in India: present status, challenges, and future research perspectives. Plant Dis. 2022;106(4):1085–95. doi:10.1094/PDIS-04-21-0861-FE. [Google Scholar] [PubMed] [CrossRef]
32. Ngalimat MS, Mohd Hata E, Zulperi D, Ismail SI, Ismail MR, Mohd Zainudin NAI, et al. Plant growth-promoting bacteria as an emerging tool to manage bacterial rice pathogens. Microorganisms. 2021;9(4):682. doi:10.3390/microorganisms9040682. [Google Scholar] [PubMed] [CrossRef]
33. Aprile AM, Coppola M, Turrà D, Vitale S, Cascone P, Diretto G, et al. Combination of the Systemin peptide with the beneficial fungus Trichoderma afroharzianum T22 improves plant defense responses against pests and diseases. J Plant Interact. 2022;17(1):569–79. doi:10.1080/17429145.2022.2072528. [Google Scholar] [CrossRef]
34. Azlay L, El Mehdi El Boukhari M, Mayad EH, Barakate M. Biological management of root-knot nematodes (Meloidogyne spp.a review. Org Agric. 2023;13(1):99–117. doi:10.1007/s13165-022-00417-y. [Google Scholar] [CrossRef]
35. Karlsson Green K, Stenberg JA, Lankinen Å. Making sense of integrated pest management (IPM) in the light of evolution. Evol Appl. 2020;13(8):1791–805. doi:10.1111/eva.13067. [Google Scholar] [PubMed] [CrossRef]
36. Mendoza-Buenrostro E, Rangel-Vargas E, Gómez-Aldapa CA, Velázquez-Jiménez R, Torres-Vitela MR, Castro-Rosas J. Effective use of microbial and plant-based alternatives in tomato pathogen control: a comprehensive review. Plant Pathol. 2025. doi:10.1111/ppa.14085. [Google Scholar] [CrossRef]
37. Soni N. Status and factors affecting development of early blight of tomato incited by Alternaria solani (Ellis Martin) Jones & Grout [doctoral dissertation]. Jabalpur, India: Jawaharlal Nehru Krishi Vishwa Vidyalaya; 2020. [Google Scholar]
38. Alrudainy AM, Mshari A. Epidemiological study of early blight alternaria solani in tomato. Br J Glob Ecol Sustain Dev. 2022;7:11–8. [Google Scholar]
39. El-Baky NA, Amara AAAF. Recent approaches towards control of fungal diseases in plants: an updated review. J Fungi. 2021;7(11):900. doi:10.3390/jof7110900. [Google Scholar] [PubMed] [CrossRef]
40. Chaudhary AK, Yadav J, Gupta AK, Gupta K. Integrated disease management of early blight (Alternaria solani) of potato. Tropagrbio. 2021;2(2):77–81. doi:10.26480/trab.02.2021.77.81. [Google Scholar] [CrossRef]
41. Maurya S, Regar R, Kumar S, Dubey S. Management tactics for early blight of tomato caused by Alternaria solani: a review. J Plant Biol Crop Res. 2022;5(1):1062. [Google Scholar]
42. Singh V, Sharma N, Singh S. A review of imaging techniques for plant disease detection. Artif Intell Agric. 2020;4(4):229–42. doi:10.1016/j.aiia.2020.10.002. [Google Scholar] [CrossRef]
43. Maurya MK, Ram CN, Singh SK, Dalal PK, Singh V, Harshita, et al. Late blight of potato caused by Phytophthora infestans and its integrated management: a review. Agric Rev. 2025. doi:10.18805/ag.r-2863. [Google Scholar] [CrossRef]
44. Babarinde SO, Al-Mughrabi K, Burlakoti RR, Peters RD, Asiedu SK, Prithiviraj B. Current understanding and future perspectives on pathogen biology and management of potato and tomato late blight (Phytophthora infestans) in Canada. Can J Plant Pathol. 2025;2:1–21. doi:10.1080/07060661.2024.2448690. [Google Scholar] [CrossRef]
45. Cacaj I, Hasanaj N. The protection of tomatoes against the fruit blight and the sustainability of cultivars to the pathogen (Phytophthora infestans). Ecol Eng Environ Technol. 2024;4:292–300. doi:10.12912/27197050/184031. [Google Scholar] [CrossRef]
46. Miguel-Ferrer L, Romero-Arenas O, Andrade-Hoyos P, Sánchez-Morales P, Rivera-Tapia JA, Fernández-Pavía SP. Antifungal activity of Trichoderma harzianum and T. koningiopsis against Fusarium solani in seed germination and vigor of Miahuateco chili seedlings. Rev Mex De Fitopatología Mex J Phytopathol. 2021;39(2):228–47. doi:10.18781/r.mex.fit.2101-5. [Google Scholar] [CrossRef]
47. Ludwiczewska M, Janiszewska M, Yin Z, Śliwka J. Populations of Phytophthora infestans in northern and eastern Europe. Eur J Plant Pathol. 2025;171(1):81–95. doi:10.1007/s10658-024-02933-x. [Google Scholar] [CrossRef]
48. López-Zapata SP, García-Jaramillo DJ, López WR, Ceballos-Aguirre N. Interacción entre tomate (Solanum lycopersicum L.) y Fusarium oxysporum f. sp. lycopersici. Una revisión. Revista U.D.C.A Actualidad. Divulgación Científica. 2021;24(1). doi:10.31910/rudca.v24.n1.2021.1713. [Google Scholar] [CrossRef]
49. Alaux PL. Does the arbuscular mycorrhizal fungus Rhizophagus irregularis mitigate late blight in potato plants? Louvain-la-Neuve, Belgium: Presses universitaires de Louvain; 2020. [Google Scholar]
50. Sam SM, Okey EN, Okop IJ. Control of fungal diseases of tomato (Lycopersicum esculentum Mill) fruits using various plant extracts. Dujopas. 2024;10(3a):202–12. doi:10.4314/dujopas.v10i3a.19. [Google Scholar] [CrossRef]
51. Jamil A, Musheer N, Kumar M. Evaluation of biocontrol agents for management of wilt disease of tomato incited by Fusarium oxysporum f. sp. lycopersici. Arch Phytopathol Plant Prot. 2021;54(19–20):1722–37. doi:10.1080/03235408.2021.1938353. [Google Scholar] [CrossRef]
52. Shahid M, Singh UB, Ilyas T, Malviya D, Vishwakarma SK, Shafi Z, et al. Bacterial inoculants for control of fungal diseases in Solanum lycopersicum L. (tomatoesa comprehensive overview. In: Rhizosphere microbes. Singapore: Springer Nature Singapore; 2022. p. 311–39. doi:10.1007/978-981-19-5872-4_15. [Google Scholar] [CrossRef]
53. Córdova P, Rivera-González JP, Rojas-Martínez V, Fiore N, Bastías R, Zamorano A, et al. Phytopathogenic Pseudomonas syringae as a threat to agriculture: perspectives of a promising biological control using bacteriophages and microorganisms. Horticulturae. 2023;9(6):712. doi:10.3390/horticulturae9060712. [Google Scholar] [CrossRef]
54. Abou-Alhmd Soliman M. Pseudomonas syringae pv. tomato and severity of bacterial speck disease on tomato plants in Egypt. Nov Res Microbiol J. 2023;7(6):2210–28. doi:10.21608/nrmj.2023.326365. [Google Scholar] [CrossRef]
55. Yang P, Zhao L, Gao YG, Xia Y. Detection, diagnosis, and preventive management of the bacterial plant pathogen Pseudomonas syringae. Plants. 2023;12(9):1765. doi:10.3390/plants12091765. [Google Scholar] [PubMed] [CrossRef]
56. de la Vega-Camarillo E, Sotelo-Aguilar J, González-Silva A, Hernández-García JA, Mercado-Flores Y, Villa-Tanaca L, et al. Genomic insights into Pseudomonas protegens E1BL2 from giant jala maize: a novel bioresource for sustainable agriculture and efficient management of fungal phytopathogens. Int J Mol Sci. 2024;25(17):9508. doi:10.3390/ijms25179508. [Google Scholar] [PubMed] [CrossRef]
57. Manda RR, Addanki VA, Srivastava S. Bacterial wilt of solanaceous crops. Int J Chem Stud. 2020;8(6):1048–57. doi:10.22271/chemi.2020.v8.i6o.10903. [Google Scholar] [CrossRef]
58. Wang Z, Luo W, Cheng S, Zhang H, Zong J, Zhang Z. Ralstonia solanacearum—a soil borne hidden enemy of plants: research development in management strategies, their action mechanism and challenges. Front Plant Sci. 2023;14:1141902. doi:10.3389/fpls.2023.1141902. [Google Scholar] [CrossRef]
59. Paudel S, Dobhal S, Alvarez AM, Arif M. Taxonomy and phylogenetic research on Ralstonia solanacearum species complex: a complex pathogen with extraordinary economic consequences. Pathogens. 2020;9(11):886. doi:10.3390/pathogens9110886. [Google Scholar] [PubMed] [CrossRef]
60. Wang Y, Zhao A, Morcillo RJL, Yu G, Xue H, Rufian JS, et al. A bacterial effector protein uncovers a plant metabolic pathway involved in tolerance to bacterial wilt disease. Mol Plant. 2021;14(8):1281–96. doi:10.1016/j.molp.2021.04.014. [Google Scholar] [PubMed] [CrossRef]
61. Sharma S, Tiwari RK, Sagar V, Maharana C. Soil- and Tuber-borne diseases of potato. In: Approaches for potato crop improvement and stress management. Singapore: Springer Nature Singapore; 2024. p. 179–231. doi:10.1007/978-981-97-1223-6_7. [Google Scholar] [CrossRef]
62. Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, et al. Biological control of plant pathogens: a global perspective. Microorganisms. 2022;10(3):596. doi:10.3390/microorganisms10030596. [Google Scholar] [PubMed] [CrossRef]
63. Sheoran AR, Lakra N, Saharan BS, Luhach A, Kumar R, Seth CS, et al. Enhancing plant disease resistance: insights from biocontrol agent strategies. J Plant Growth Regul. 2025;44(2):436–59. doi:10.1007/s00344-024-11480-y. [Google Scholar] [CrossRef]
64. Murray L, Reeves E, Meadows I. Tomato yellow leaf curl virus. NC State Ext Publ [Internet]. 2023. [cited 2025 Apr 28]. Available from: https://content.ces.ncsu.edu/tomato-yellow-leaf-curl-virus. [Google Scholar]
65. Penicks AK, Hensley D, Wszelaki A, Hajimorad MR, Associate E, et al. Tomato yellow leaf curl virus: an emerging virus of tomatoes in tennessee. Real Life Solut. 2025:3–6. [Google Scholar]
66. Ma M, Taylor PWJ, Chen D, Vaghefi N, He JZ. Major soilborne pathogens of field processing tomatoes and management strategies. Microorganisms. 2023;11(2):263. doi:10.3390/microorganisms11020263. [Google Scholar] [PubMed] [CrossRef]
67. White L, Cooper A, Meadows I. Tobamoviruses that affect tomato (TMV, ToMV, ToBRFV). NC State Ext Publ. [Internet]. [cited 2025 Apr 29]. Available from: https://content.ces.ncsu.edu/tobamoviruses-that-affect-tomato-tmv-tomv-tobrfv. [Google Scholar]
68. Ishibashi K, Kubota K, Kano A, Ishikawa M. Tobamoviruses: old and new threats to tomato cultivation. J Gen Plant Pathol. 2023;89(6):305–21. doi:10.1007/s10327-023-01141-5. [Google Scholar] [CrossRef]
69. Sánchez-Sánchez M, Carrillo-Tripp J, Aispuro-Hernández E, Quintana-Obregón EA, Martínez-Téllez MÁ. Understanding tobamovirus-plant interactions: implications for breeding resistance to tomato brown rugose fruit virus. J Plant Pathol. 2023;105(1):83–94. doi:10.1007/s42161-022-01287-9. [Google Scholar] [CrossRef]
70. Zhang S, Griffiths JS, Marchand G, Bernards MA, Wang A. Tomato brown rugose fruit virus: an emerging and rapidly spreading plant RNA virus that threatens tomato production worldwide. Mol Plant Pathol. 2022;23(9):1262–77. doi:10.1111/mpp.13229. [Google Scholar] [PubMed] [CrossRef]
71. Chanda B, Shamimuzzaman M, Gilliard A, Ling KS. Effectiveness of disinfectants against the spread of tobamoviruses: tomato brown rugose fruit virus and cucumber green mottle mosaic virus. Virol J. 2021;18(1):7. doi:10.1186/s12985-020-01479-8. [Google Scholar] [PubMed] [CrossRef]
72. Fidan H, Ulusoy D, Albezirgan HN. Exploring effective strategies for ToBRFV management in tomato production: insights into seed transmission dynamics and innovative control approaches. Agriculture. 2024;14(1):108. doi:10.3390/agriculture14010108. [Google Scholar] [CrossRef]
73. Naalden D, van Kleeff PJM, Dangol S, Mastop M, Corkill R, Hogenhout SA, et al. Spotlight on the roles of whitefly effectors in insect-plant interactions. Front Plant Sci. 2021;12:661141. doi:10.3389/fpls.2021.661141. [Google Scholar] [PubMed] [CrossRef]
74. Singh H, Kaur T. Pathogenicity of entomopathogenic fungi against the aphid and the whitefly species on crops grown under greenhouse conditions in India. Egypt J Biol Pest Control. 2020;30(1):84. doi:10.1186/s41938-020-00287-0. [Google Scholar] [CrossRef]
75. Morin S, Atkinson PW, Walling LL. Whitefly-plant interactions: an integrated molecular perspective. Annu Rev Entomol. 2024;69(1):503–25. doi:10.1146/annurev-ento-120120-093940. [Google Scholar] [PubMed] [CrossRef]
76. Walia RK, Khan MR. Root-knot nematodes (Meloidogyne spp.). In: Root-galling disease of vegetable plants. Singapore: Springer Nature Singapore; 2023. p. 1–60. doi:10.1007/978-981-99-3892-6_1. [Google Scholar] [CrossRef]
77. Ferreira AL, de Faria JC, da Costa Moura M, de Mendonça Zaidem AL, Pizetta CSR, de Oliveira Freitas E, et al. Whitefly-tolerant transgenic common bean (Phaseolus vulgaris) line. Front Plant Sci. 2022;13:984804. doi:10.3389/fpls.2022.984804. [Google Scholar] [PubMed] [CrossRef]
78. Khan A, Khan A, Ali A, Fatima S, Ahmad Siddiqui M. Root-knot nematodes (Meloidogyne spp.biology, plant-nematode interactions and their environmentally benign management strategies. Gesunde Pflanz. 2023;75(6):2187–205. doi:10.1007/s10343-023-00886-5. [Google Scholar] [CrossRef]
79. Shilpa N, Sharma P, Thakur V, Sharma A, Rana RS, Kumar P. A status-quo review on management of root knot nematode in tomato. J Hortic Sci Biotechnol. 2022;97(4):403–16. doi:10.1080/14620316.2022.2034531. [Google Scholar] [CrossRef]
80. Kopecká R, Kameniarová M, Černý M, Brzobohatý B, Novák J. Abiotic stress in crop production. Int J Mol Sci. 2023;24(7):6603. doi:10.3390/ijms24076603. [Google Scholar] [PubMed] [CrossRef]
81. Tahir M, Ali MA, Khan A, Akram MZ, Ullah S, Farazet I, et al. Impact of various soil amendments on root knot nematodes and plant growth attributes of tomato (Solanum lycopersicum L.). J Pure Appl Agric. 2023;8(4):30–7. [Google Scholar]
82. Laxman RH, Ravishankar KV, Prasanna HC, Ramesh KV, Rashmi K, Kannan S, et al. Physiological, molecular and genetic analysis of abiotic stress tolerance in tomato. In: Genomic designing for abiotic stress resistant vegetable crops. Cham, Switzerland: Springer International Publishing; 2022. p. 1–47. doi:10.1007/978-3-031-03964-5_1. [Google Scholar] [CrossRef]
83. Abo Nouh F, Abu-Elsaoud A, Abdel-Azeem A. The role of endophytic fungi in combating abiotic stress on tomato. Microb Biosyst. 2021;6(1):35–48. doi:10.21608/mb.2021.91779.1037. [Google Scholar] [CrossRef]
84. Zhang H, Pan C, Gu S, Ma Q, Zhang Y, Li X, et al. Stomatal movements are involved in elevated CO2-mitigated high temperature stress in tomato. Physiol Plant. 2019;165(3):569–83. doi:10.1111/ppl.12752. [Google Scholar] [PubMed] [CrossRef]
85. Kissoudis C, Sunarti S, van de Wiel C, Visser RGF, van der Linden CG, Bai Y. Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J Exp Bot. 2016;67(17):5119–32. doi:10.1093/jxb/erw285. [Google Scholar] [PubMed] [CrossRef]
86. Gavassi MA, Monteiro CC, Campos ML, Melo HC, Carvalho RF. Phytochromes are key regulators of abiotic stress responses in tomato. Sci Hortic. 2017;222(3):126–35. doi:10.1016/j.scienta.2017.04.035. [Google Scholar] [CrossRef]
87. Mishra R, Shteinberg M, Shkolnik D, Anfoka G, Czosnek H, Gorovits R. Interplay between abiotic (drought) and biotic (virus) stresses in tomato plants. Mol Plant Pathol. 2022;23(4):475–88. doi:10.1111/mpp.13172. [Google Scholar] [PubMed] [CrossRef]
88. Virdi AS, Singh S, Singh P. Abiotic stress responses in plants: roles of calmodulin-regulated proteins. Front Plant Sci. 2015;6:809. doi:10.3389/fpls.2015.00809. [Google Scholar] [PubMed] [CrossRef]
89. Khan SH, Arsalan Khan AK, Uzma L, Shah AS, Khan MA, Bilal M, et al. Effect of drought stress on tomato cv. Bombino. J Food Process Technol. 2015;6(7):465–70. doi:10.4172/2157-7110.1000465. [Google Scholar] [CrossRef]
90. Visentin I, Vitali M, Ferrero M, Zhang Y, Ruyter-Spira C, Novák O, et al. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 2016;212(4):954–63. doi:10.1111/nph.14190. [Google Scholar] [PubMed] [CrossRef]
91. Ullah U, Ashraf M, Shahzad SM, Siddiqui AR. Growth behavior of tomato (Solanum lycopersicum L.) under drought stress in the presence of silicon and plant growth promoting rhizobacteria. Soil Env. 2016;35(1):65–75. [Google Scholar]
92. Roşca M, Mihalache G, Stoleru V. Tomato responses to salinity stress: from morphological traits to genetic changes. Front Plant Sci. 2023;14:1118383. doi:10.3389/fpls.2023.1118383. [Google Scholar] [PubMed] [CrossRef]
93. Kumari VV, Banerjee P, Verma VC, Sukumaran S, Chandran MAS, Gopinath KA, et al. Plant nutrition: an effective way to alleviate abiotic stress in agricultural crops. Int J Mol Sci. 2022;23(15):8519. doi:10.3390/ijms23158519. [Google Scholar] [PubMed] [CrossRef]
94. Alam MS, Tester M, Fiene G, Mousa MAA. Early growth stage characterization and the biochemical responses for salinity stress in tomato. Plants. 2021;10(4):712. doi:10.3390/plants10040712. [Google Scholar] [PubMed] [CrossRef]
95. Shahid MA, Sarkhosh A, Khan N, Balal RM, Ali S, Rossi L, et al. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy. 2020;10(7):938. doi:10.3390/agronomy10070938. [Google Scholar] [CrossRef]
96. Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ. Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics. 2021;9(3):42. doi:10.3390/toxics9030042. [Google Scholar] [PubMed] [CrossRef]
97. Zeng S, Wu X, Wu B, Zhou H, Wang M. Rapid determination of cadmium residues in tomato leaves by Vis-NIR hyperspectral and Synergy interval PLS coupled Monte Carlo method. Food Sci Technol. 2023;43(1):e113422. doi:10.1590/fst.113422. [Google Scholar] [CrossRef]
98. Goncharuk EA, Zagoskina NV. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium: review. Molecules. 2023;28(9):3921. doi:10.3390/molecules28093921. [Google Scholar] [PubMed] [CrossRef]
99. Shukla S, Das S, Phutela S, Triathi A, Kumari C, Shukla SK. Heavy metal stress in plants: causes, impact and effective management. In: Heavy metal toxicity. Cham, Switzerland: Springer Nature Switzerland; 2024. p. 187–215. doi:10.1007/978-3-031-56642-4_7. [Google Scholar] [CrossRef]
100. Kumar P, Goud EL, Devi P, Dey SR, Dwivedi P. Heavy metals: transport in plants and their physiological and toxicological effects. In: Plant metal and metalloid transporters. Singapore: Springer Nature Singapore; 2022. p. 23–54. doi:10.1007/978-981-19-6103-8_2. [Google Scholar] [CrossRef]
101. Kumar M, Seth A, Singh AK, Rajput MS, Sikandar M. Remediation strategies for heavy metals contaminated ecosystem: a review. Environ Sustain Indic. 2021;12(4):100155. doi:10.1016/j.indic.2021.100155. [Google Scholar] [CrossRef]
102. Alsamir M, Mahmood T, Trethowan R, Ahmad N. An overview of heat stress in tomato (Solanum lycopersicum L.). Saudi J Biol Sci. 2021;28(3):1654–63. doi:10.1016/j.sjbs.2020.11.088. [Google Scholar] [PubMed] [CrossRef]
103. Khan Q, Wang Y, Xia G, Yang H, Luo Z, Zhang Y. Deleterious effects of heat stress on the tomato, its innate responses, and potential preventive strategies in the realm of emerging technologies. Metabolites. 2024;14(5):283. doi:10.3390/metabo14050283. [Google Scholar] [PubMed] [CrossRef]
104. Alsamir M, Ahmad NM, Keitel C, Mahmood T, Trethowan R. Identification of high-temperature tolerant and agronomically viable tomato (S. lycopersicum) genotypes from a diverse germplasm collection. Adv Crop Sci Tech. 2017;5(4):1000299. doi:10.4172/2329-8863.1000299. [Google Scholar] [CrossRef]
105. Jahan MS, Guo S, Sun J, Shu S, Wang Y, El-Yazied AA, et al. Melatonin-mediated photosynthetic performance of toma to seedlings under high-temperature stress. Plant Physiol Biochem. 2021;167(6):309–20. doi:10.1016/j.plaphy.2021.08.002. [Google Scholar] [PubMed] [CrossRef]
106. Kouzuma A, Kato S, Watanabe K. Microbial interspecies interactions: recent findings in syntrophic consortia. Front Microbiol. 2015;6:477. doi:10.3389/fmicb.2015.00477. [Google Scholar] [PubMed] [CrossRef]
107. Bhatia SK, Bhatia RK, Choi YK, Kan E, Kim YG, Yang YH. Biotechnological potential of microbial consortia and future perspectives. Crit Rev Biotechnol. 2018;38(8):1209–29. doi:10.1080/07388551.2018.1471445. [Google Scholar] [PubMed] [CrossRef]
108. Shong J, Jimenez Diaz MR, Collins CH. Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotechnol. 2012;23(5):798–802. doi:10.1016/j.copbio.2012.02.001. [Google Scholar] [PubMed] [CrossRef]
109. Compant S, Samad A, Faist H, Sessitsch A. A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res. 2019;19(6):29–37. doi:10.1016/j.jare.2019.03.004. [Google Scholar] [PubMed] [CrossRef]
110. Dione MM, Djouaka R, Mbokou SF, Ilboudo GS, Ouedraogo AA, Dinede G, et al. Detection and quantification of pesticide residues in tomatoes sold in urban markets of Ouagadougou, Burkina Faso. Front Sustain Food Syst. 2023;7:1213085. doi:10.3389/fsufs.2023.1213085. [Google Scholar] [CrossRef]
111. Victoria K, Neema K, Martin K. Risk of exposures of pesticide residues from tomato in Tanzania. Afr J Food Sci. 2017;11(8):255–62. doi:10.5897/ajfs2016.1527. [Google Scholar] [CrossRef]
112. Lehel J, Vöröskői P, Palkovics A, Szabó C, Darnay L, Budai P, et al. Farm to table: residues of different pesticides in tomato and tomato juice-food safety aspects. Acta Vet Hung. 2022;70(3):236–44. doi:10.1556/004.2022.00025. [Google Scholar] [PubMed] [CrossRef]
113. Zhang T, Zhang H. Microbial consortia are needed to degrade soil pollutants. Microorganisms. 2022;10(2):261. doi:10.3390/microorganisms10020261. [Google Scholar] [PubMed] [CrossRef]
114. Guerrero Ramírez JR, Ibarra Muñoz LA, Balagurusamy N, Frías Ramírez JE, Alfaro Hernández L, Carrillo Campos J. Microbiology and biochemistry of pesticides biodegradation. Int J Mol Sci. 2023;24(21):15969. doi:10.3390/ijms242115969. [Google Scholar] [PubMed] [CrossRef]
115. Bhatt P, Bhatt K, Sharma A, Zhang W, Mishra S, Chen S. Biotechnological basis of microbial consortia for the removal of pesticides from the environment. Crit Rev Biotechnol. 2021;41(3):317–38. doi:10.1080/07388551.2020.1853032. [Google Scholar] [PubMed] [CrossRef]
116. Faridy N, Torabi E, Pourbabaee AA, Osdaghi E, Talebi K. Efficacy of novel bacterial consortia in degrading fipronil and thiobencarb in paddy soil: a survey for community structure and metabolic pathways. Front Microbiol. 2024;15:1366951. doi:10.3389/fmicb.2024.1366951. [Google Scholar] [PubMed] [CrossRef]
117. Pang S, Lin Z, Zhang Y, Zhang W, Alansary N, Mishra S, et al. Insights into the toxicity and degradation mechanisms of imidacloprid via physicochemical and microbial approaches. Toxics. 2020;8(3):65. doi:10.3390/toxics8030065. [Google Scholar] [PubMed] [CrossRef]
118. Syed Ab Rahman SF, Singh E, Pieterse CMJ, Schenk PM. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018;267:102–11. doi:10.1016/j.plantsci.2017.11.012. [Google Scholar] [PubMed] [CrossRef]
119. Bakker PAHM, Pieterse CMJ, de Jonge R, Berendsen RL. The soil-borne legacy. Cell. 2018;172(6):1178–80. doi:10.1016/j.cell.2018.02.024. [Google Scholar] [PubMed] [CrossRef]
120. Mitter B, Brader G, Pfaffenbichler N, Sessitsch A. Next generation microbiome applications for crop production-limitations and the need of knowledge-based solutions. Curr Opin Microbiol. 2019;49:59–65. doi:10.1016/j.mib.2019.10.006. [Google Scholar] [PubMed] [CrossRef]
121. Singh A, Sarma BK, Upadhyay RS, Singh HB. Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol Res. 2013;168(1):33–40. doi:10.1016/j.micres.2012.07.001. [Google Scholar] [PubMed] [CrossRef]
122. Barea JM. Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant-microbiome interactions. J Soil Sci Plant Nutr. 2015. doi:10.4067/s0718-95162015005000021. [Google Scholar] [CrossRef]
123. Trivedi P, Schenk PM, Wallenstein MD, Singh BK. Tiny microbes, big yields: enhancing food crop production with biological solutions. Microb Biotechnol. 2017;10(5):999–1003. doi:10.1111/1751-7915.12804. [Google Scholar] [PubMed] [CrossRef]
124. Trivedi P, Leach JE, Tringe SG, Sa T, Singh BK. Plant-microbiome interactions: from community assembly to plant health. Nat Rev Microbiol. 2020;18(11):607–21. doi:10.1038/s41579-020-0412-1. [Google Scholar] [PubMed] [CrossRef]
125. Kurkjian HM, Akbari MJ, Momeni B. The impact of interactions on invasion and colonization resistance in microbial communities. PLoS Comput Biol. 2021;17(1):e1008643. doi:10.1371/journal.pcbi.1008643. [Google Scholar] [PubMed] [CrossRef]
126. da Costa PB, van Elsas JD, Mallon C, dos Anjos Borges LG, Pereira Passaglia LM. Efficiency of probiotic traits in plant inoculation is determined by environmental constrains. Soil Biol Biochem. 2020;148:107893. doi:10.1016/j.soilbio.2020.107893. [Google Scholar] [CrossRef]
127. Qian JJ, Akçay E. The balance of interaction types determines the assembly and stability of ecological communities. Nat Ecol Evol. 2020;4(3):356–65. doi:10.1038/s41559-020-1121-x. [Google Scholar] [PubMed] [CrossRef]
128. Wippel K, Tao K, Niu Y, Zgadzaj R, Kiel N, Guan R, et al. Host preference and invasiveness of commensal bacteria in the Lotus and Arabidopsis root microbiota. Nat Microbiol. 2021;6(9):1150–62. doi:10.1038/s41564-021-00941-9. [Google Scholar] [PubMed] [CrossRef]
129. Birt HWG, Tharp CL, Custer GF, Dini-Andreote F. Root phenotypes as modulators of microbial microhabitats. Front Plant Sci. 2022;13:1003868. doi:10.3389/fpls.2022.1003868. [Google Scholar] [PubMed] [CrossRef]
130. Rana A, Kumar N, Roy R, Kumari A, Kumar R, Gautam A, et al. Field efficacy of Trichoderma viride, Pseudomonas fluorescens, and consortia of microbial agents against tomato late blight, Phytophthora infestans (Mont.) de Bary in Indo-Gangetic Plain of Bihar. Crop Prot. 2025;190(5):107128. doi:10.1016/j.cropro.2025.107128. [Google Scholar] [CrossRef]
131. Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001;52(suppl 1):487–511. doi:10.1093/jexbot/52.suppl_1.487. [Google Scholar] [PubMed] [CrossRef]
132. Sarma BK, Yadav SK, Singh S, Singh HB. Microbial consortium-mediated plant defense against phytopathogens: readdressing for enhancing efficacy. Soil Biol Biochem. 2015;87(7):25–33. doi:10.1016/j.soilbio.2015.04.001. [Google Scholar] [CrossRef]
133. Minchev Z, Kostenko O, Soler R, Pozo MJ. Microbial consortia for effective biocontrol of root and foliar diseases in tomato. Front Plant Sci. 2021;12:756368. doi:10.3389/fpls.2021.756368. [Google Scholar] [PubMed] [CrossRef]
134. Schoving C, Stöckle CO, Colombet C, Champolivier L, Debaeke P, Maury P. Combining simple phenotyping and photothermal algorithm for the prediction of soybean phenology: application to a range of common cultivars grown in Europe. Front Plant Sci. 2020;10:1755. doi:10.3389/fpls.2019.01755. [Google Scholar] [PubMed] [CrossRef]
135. Panwar M, Tewari R, Nayyar H. Microbial consortium of plant growth-promoting rhizobacteria improves the performance of plants growing in stressed soils: an overview. In: Phosphate solubilizing microorganisms. Cham, Switzerland: Springer International Publishing; 2014. p. 257–85. doi:10.1007/978-3-319-08216-5_11. [Google Scholar] [CrossRef]
136. Stockwell VO, Johnson KB, Sugar D, Loper JE. Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology. 2011;101(1):113–23. doi:10.1094/PHYTO-03-10-0098. [Google Scholar] [PubMed] [CrossRef]
137. Compant S, Duffy B, Nowak J, Clément C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol. 2005;71(9):4951–9. doi:10.1128/AEM.71.9.4951-4959.2005. [Google Scholar] [PubMed] [CrossRef]
138. Bashan Y, de-Bashan LE. BACTERIA | plant growth-promoting. In: Encyclopedia of soils in the environment. Amsterdam, The Netherland: Elsevier; 2005. p. 103–15. doi:10.1016/b0-12-348530-4/00513-0. [Google Scholar] [CrossRef]
139. Verbon EH, Liberman LM, Zhou J, Yin J, Pieterse CMJ, Benfey PN, et al. Cell-type-specific transcriptomics reveals that root hairs and endodermal barriers play important roles in beneficial plant-rhizobacterium interactions. Mol Plant. 2023;16(7):1160–77. doi:10.1016/j.molp.2023.06.001. [Google Scholar] [PubMed] [CrossRef]
140. Arkhipov A, Carvalhais LC, Schenk PM. PGPR control Phytophthora capsici in tomato through induced systemic resistance, early hypersensitive response and direct antagonism in a cultivar-specific manner. Eur J Plant Pathol. 2023;167(4):811–32. doi:10.1007/s10658-023-02734-8. [Google Scholar] [CrossRef]
141. Dickinson M. Fungal plant pathogens. C.R Lane, P.A Beales, K.J.D Hughes. 328pp. £39·95. ISBN 978 1 84593 668 6 and 978 1 84593 870 3. Wallingford, UK: cabi, 2012 (softcover). 2013 Sep 10. doi:10.1111/ppa.12005. [Google Scholar] [CrossRef]
142. Chang P, Gerhardt KE, Huang XD, Yu XM, Glick BR, Gerwing PD, et al. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: implications for phytoremediation of saline soils. Int J Phytoremediation. 2014;16(7–12):1133–47. doi:10.1080/15226514.2013.821447. [Google Scholar] [PubMed] [CrossRef]
143. de Graaff MA, Hornslein N, Throop HL, Kardol P, van Diepen LTA. Effects of agricultural intensification on soil biodiversity and implications for ecosystem functioning: a meta-analysis. In: Advances in agronomy. Amsterdam, The Netherland: Elsevier; 2019. p. 1–44. doi:10.1016/bs.agron.2019.01.001. [Google Scholar] [CrossRef]
144. Basu A, Chowdhury S, Ray Chaudhuri T, Kundu S. Differential behaviour of sheath blight pathogen Rhizoctonia solani in tolerant and susceptible rice varieties before and during infection. Plant Pathol. 2016;65(8):1333–46. doi:10.1111/ppa.12502. [Google Scholar] [CrossRef]
145. Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant Soil. 2014;383(1):3–41. doi:10.1007/s11104-014-2131-8. [Google Scholar] [CrossRef]
146. Zafar-ul-Hye M, Maqshoof Ahmad MA, Shahzad SM. Synergistic effect of rhizobia and plant growth promoting rhizobacteria on the growth and nodulation of lentil seedlings under axenic conditions. Proc Royal Soc B. 2013;32(1):79–86. [Google Scholar]
147. Bacilio M, Moreno M, Bashan Y. Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl Soil Ecol. 2016;107:394–404. doi:10.1016/j.apsoil.2016.04.012. [Google Scholar] [CrossRef]
148. Pérez-García A, Romero D, de Vicente A. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol. 2011;22(2):187–93. doi:10.1016/j.copbio.2010.12.003. [Google Scholar] [PubMed] [CrossRef]
149. Roh JY, Choi JY, Li MS, Jin BR, Je YH. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J Microbiol Biotechnol. 2007;17(4):547–59. [Google Scholar] [PubMed]
150. Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16(3):115–25. doi:10.1016/j.tim.2007.12.009. [Google Scholar] [PubMed] [CrossRef]
151. Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31(4):425–48. doi:10.1111/j.1574-6976.2007.00072.x. [Google Scholar] [PubMed] [CrossRef]
152. Maciag T, Kozieł E, Rusin P, Otulak-Kozieł K, Jafra S, Czajkowski R. Microbial consortia for plant protection against diseases: more than the sum of its parts. Int J Mol Sci. 2023;24(15):12227. doi:10.3390/ijms241512227. [Google Scholar] [PubMed] [CrossRef]
153. Vimal SR, Singh JS, Arora NK, Singh S. Soil-plant-microbe interactions in stressed agriculture management: a review. Pedosphere. 2017;27(2):177–92. doi:10.1016/S1002-0160(17)60309-6. [Google Scholar] [CrossRef]
154. Jetiyanon K. Defensive-related enzyme response in plants treated with a mixture of Bacillus strains (IN937a and IN937b) against different pathogens. Biol Control. 2007;42(2):178–85. doi:10.1016/j.biocontrol.2007.05.008. [Google Scholar] [CrossRef]
155. Harish Kumar K, Jagadeesh KS. Microbial consortia-mediated plant defense against phytopathogens and growth benefits. Sijbs. 2016;2(4):395. doi:10.22205/sijbs/2016/v2/i4/103445. [Google Scholar] [CrossRef]
156. Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv. 2014;32(2):429–48. doi:10.1016/j.biotechadv.2013.12.005. [Google Scholar] [PubMed] [CrossRef]
157. Jha Y, Subramanian RB. Effect of root-associated bacteria on soluble sugar metabolism in plant under environmental stress. In: Plant metabolites and regulation under environmental stress. Amsterdam, The Netherland: Elsevier; 2018. p. 231–40. doi:10.1016/b978-0-12-812689-9.00012-1. [Google Scholar] [CrossRef]
158. Ali S, Ganai BA, Kamili AN, Bhat AA, Mir ZA, Bhat JA, et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res. 2018;212–213(12):29–37. doi:10.1016/j.micres.2018.04.008. [Google Scholar] [PubMed] [CrossRef]
159. Dimkpa C, Weinand T, Asch F. Plant-rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009;32(12):1682–94. doi:10.1111/j.1365-3040.2009.02028.x. [Google Scholar] [PubMed] [CrossRef]
160. Mehmood U, Inam-ul-Haq M, Saeed M, Altaf A, Azam F, Hayat S. A brief review on plant growth promoting rhizobacteria (PGPRa key role in plant growth promotion. Plant Prot. 2018;2(2):77–82. [Google Scholar]
161. Lugtenberg B. Putting concerns for caution into perspective: microbial plant protection products are safe to use in agriculture. J Plant Dis Prot. 2018;125(2):127–9. doi:10.1007/s41348-018-0149-5. [Google Scholar] [CrossRef]
162. Liu F, Ma H, Liu B, Du Z, Ma B, Jing D. Effects of plant growth-promoting rhizobacteria on the physioecological characteristics and growth of walnut seedlings under drought stress. Agronomy. 2023;13(2):290. doi:10.3390/agronomy13020290. [Google Scholar] [CrossRef]
163. Friman J, Pineda A, van Loon JJA, Dicke M. Bidirectional plant-mediated interactions between rhizobacteria and shoot-feeding herbivorous insects: a community ecology perspective. Ecol Entomol. 2021;46(1):1–10. doi:10.1111/een.12966. [Google Scholar] [CrossRef]
164. Hashem A, Tabassum B, Fathi Abd Allah E. Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci. 2019;26(6):1291–7. doi:10.1016/j.sjbs.2019.05.004. [Google Scholar] [PubMed] [CrossRef]
165. Abdi M, Alemayehu C, Chris O, Mashilla D, Chemeda F, Amare A, et al. Integrated management of Aspergillus species and aflatoxin production in groundnut (Arachis hypogaea L.) through application of farm yard manure and seed treatments with fungicides and Trichoderma species. Afr J Plant Sci. 2018;12(9):196–207. doi:10.5897/ajps2018.1688. [Google Scholar] [CrossRef]
166. Shafiei F, Shahidi-Noghabi S, Sedaghati E. The impact of arbuscular mycorrhizal fungi on tomato plant resistance against Tuta absoluta (Meyrick) in greenhouse conditions. J Asia Pac Entomol. 2022;25(3):101971. doi:10.1016/j.aspen.2022.101971. [Google Scholar] [CrossRef]
167. Choudhary DK, Johri BN. Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol Res. 2009;164(5):493–513. doi:10.1016/j.micres.2008.08.007. [Google Scholar] [PubMed] [CrossRef]
168. Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol. 2014;52(1):347–75. doi:10.1146/annurev-phyto-082712-102340. [Google Scholar] [PubMed] [CrossRef]
169. Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol. 2005;3(4):307–19. doi:10.1038/nrmicro1129. [Google Scholar] [PubMed] [CrossRef]
170. Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2(1):43–56. doi:10.1038/nrmicro797. [Google Scholar] [PubMed] [CrossRef]
171. Guo F, Chen Q, Liang Q, Zhang M, Chen W, Chen H, et al. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front Microbiol. 2021;12:562094. doi:10.3389/fmicb.2021.562094. [Google Scholar] [CrossRef]
172. Kido K, Tanaka C, Mochizuki T, Kubota K, Ohki T, Ohnishi J, et al. High temperatures activate local viral multiplication and cell-to-cell movement of Melon necrotic spot virus but restrict expression of systemic symptoms. Phytopathology. 2008;98(2):181–6. doi:10.1094/PHYTO-98-2-0181. [Google Scholar] [PubMed] [CrossRef]
173. Jain A, Singh A, Singh BN, Singh S, Upadhyay RS, Sarma BK, et al. Biotic stress management in agricultural crops using microbial consortium. In: Bacteria in agrobiology: disease management. 1st ed. Berlin/Heidelberg, Germany: Springer; 2013. p. 427–48. [Google Scholar]
174. Nadeem SM, Naveed M, Zahir ZA, Asghar HN. Plant-microbe interactions for sustainable agriculture: fundamentals and recent advances. In: Plant microbe symbiosis: fundamentals and advances. New Delhi, India: Springer; 2013. p. 51–103. doi:10.1007/978-81-322-1287-4_2. [Google Scholar] [CrossRef]
175. Mehta CM, Gupta V, Singh S, Srivastava R, Sen E, Romantschuk M, et al. Role of microbiologically rich compost in reducing biotic and abiotic stresses. In: Microorganisms in environmental management. Dordrecht, The Netherland: Springer; 2011. p. 113–34. doi:10.1007/978-94-007-2229-3_5. [Google Scholar] [CrossRef]
176. Gallagher LA, Manoil C. Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol. 2001;183(21):6207–14. doi:10.1128/JB.183.21.6207-6214.2001. [Google Scholar] [CrossRef]
177. Khan MMA, Mujibur-Rahman M, Naeem M, Mohammad F, Siddiqui MH, Nasir M. Triacontanol-induced changes in the growth yield and quality of tomato (Lycopersicon esculentum Mill.). Electron J Environ Agric Food Chem. 2006;5(4):1492–9. [Google Scholar]
178. Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28(1):489–521. doi:10.1146/annurev-cellbio-092910-154055. [Google Scholar] [PubMed] [CrossRef]
179. Romera FJ, García MJ, Lucena C, Martínez-Medina A, Aparicio MA, Ramos J, et al. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front Plant Sci. 2019;10:287. doi:10.3389/fpls.2019.00287. [Google Scholar] [PubMed] [CrossRef]
180. Zhu L, Huang J, Lu X, Zhou C. Development of plant systemic resistance by beneficial rhizobacteria: recognition, initiation, elicitation and regulation. Front Plant Sci. 2022;13:952397. doi:10.3389/fpls.2022.952397. [Google Scholar] [PubMed] [CrossRef]
181. Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci. 2014;26(1):1–20. doi:10.1016/j.jksus.2013.05.001. [Google Scholar] [CrossRef]
182. Sindhu SS, Parmar P, Phour M. Nutrient cycling: potassium solubilization by microorganisms and improvement of crop growth. In: Geomicrobiology and biogeochemistry. Berlin/Heidelberg: Springer; 2013. p. 175–98. doi:10.1007/978-3-642-41837-2_10. [Google Scholar] [CrossRef]
183. Bashan Y, de-Bashan LE, Prabhu SR, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013). Plant Soil. 2014;378(1):1–33. doi:10.1007/s11104-013-1956-x. [Google Scholar] [CrossRef]
184. de-Bashan LE, Hernandez JP, Bashan Y. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation—a comprehensive evaluation. Appl Soil Ecol. 2012;61:171–89. doi:10.1016/j.apsoil.2011.09.003. [Google Scholar] [CrossRef]
185. Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63(1):541–56. doi:10.1146/annurev.micro.62.081307.162918. [Google Scholar] [PubMed] [CrossRef]
186. Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, et al. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact. 2014;9(1):673–82. doi:10.1080/17429145.2014.894587. [Google Scholar] [CrossRef]
187. Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 2016;184(10):13–24. doi:10.1016/j.micres.2015.12.003. [Google Scholar] [PubMed] [CrossRef]
188. Kanwal R, Maqsood MF, Shahbaz M, Naz N, Zulfiqar U, Ali MF, et al. Exogenous ascorbic acid as a potent regulator of antioxidants, osmo-protectants, and lipid peroxidation in pea under salt stress. BMC Plant Biol. 2024;24(1):247. doi:10.1186/s12870-024-04947-3. [Google Scholar] [PubMed] [CrossRef]
189. Masrahi AS, Alasmari A, Shahin MG, Qumsani AT, Oraby HF, Awad-Allah MMA. Role of arbuscular mycorrhizal fungi and phosphate solubilizing bacteria in improving yield, yield components, and nutrients uptake of barley under salinity soil. Agriculture. 2023;13(3):537. doi:10.3390/agriculture13030537. [Google Scholar] [CrossRef]
190. Naseem H, Ahsan M, Shahid MA, Khan N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J Basic Microbiol. 2018;58(12):1009–22. doi:10.1002/jobm.201800309. [Google Scholar] [PubMed] [CrossRef]
191. Shah LR, Sharma A, Nabi J, Rathore JP. Breeding approaches for abiotic stress management in vegetable crops. J Pharmacogn Phytochem. 2018;7(3):1023–8. [Google Scholar]
192. Liu X, Li Q, Li Y, Guan G, Chen S. Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. PeerJ. 2019;7(1):e7445. doi:10.7717/peerj.7445. [Google Scholar] [CrossRef]
193. Aksoy HM, Kaya Y, Ozturk M, Secgin Z, Onder H, Okumus A. Pseudomonas putida-induced response in phenolic profile of tomato seedlings (Solanum lycopersicum L.) infected by Clavibacter michiganensis subsp. michiganensis. Biol Control. 2017;105(3):6–12. doi:10.1016/j.biocontrol.2016.11.001. [Google Scholar] [CrossRef]
194. Dekkers LC, Mulders IH, Phoelich CC, Chin-A-Woeng TF, Wijfjes AH, Lugtenberg BJ. The sss colonization gene of the tomato-Fusarium oxysporum f. sp. radicis-lycopersici biocontrol strain Pseudomonas fluorescens WCS365 can improve root colonization of other wild-type Pseudomonas spp. bacteria. Mol Plant Microbe Interact. 2000;13(11):1177–83. doi:10.1094/MPMI.2000.13.11.1177. [Google Scholar] [PubMed] [CrossRef]
195. Kriaa M, Hammami I, Sahnoun M, Azebou MC, Triki MA, Kammoun R. Biocontrol of tomato plant diseases caused by Fusarium solani using a new isolated Aspergillus tubingensis CTM 507 glucose oxidase. C R Biol. 2015;338(10):666–77. doi:10.1016/j.crvi.2015.05.007. [Google Scholar] [PubMed] [CrossRef]
196. You J, Zhang J, Wu M, Yang L, Chen W, Li G. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biol Control. 2016;101(31):31–8. doi:10.1016/j.biocontrol.2016.06.006. [Google Scholar] [CrossRef]
197. Li Q, Ning P, Zheng L, Huang J, Li G, Hsiang T. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biol Control. 2012;61(2):113–20. doi:10.1016/j.biocontrol.2011.10.014. [Google Scholar] [CrossRef]
198. Saligkarias ID, Gravanis FT, Epton HAS. Biological control of Botrytis cinerea on tomato plants by the use of epiphytic yeasts Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I-182: II. a study on mode of action. Biol Control. 2002;25(2):151–61. doi:10.1016/S1049-9644(02)00052-X. [Google Scholar] [CrossRef]
199. Tsai SH, Chen YT, Yang YL, Lee BY, Huang CJ, Chen CY. The potential biocontrol agent Paenibacillus polymyxa TP3 produces fusaricidin-type compounds involved in the antagonism against gray mold pathogen Botrytis cinerea. Phytopathology. 2022;112(4):775–83. doi:10.1094/PHYTO-04-21-0178-R. [Google Scholar] [CrossRef]
200. Martínez-Hidalgo P, García JM, Pozo MJ. Induced systemic resistance against Botrytis cinerea by Micromonospora strains isolated from root nodules. Front Microbiol. 2015;6:922. doi:10.3389/fmicb.2015.00922. [Google Scholar] [PubMed] [CrossRef]
201. Romero FM, Marina M, Pieckenstain FL. Novel components of leaf bacterial communities of field-grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res Microbiol. 2016;167(3):222–33. doi:10.1016/j.resmic.2015.11.001. [Google Scholar] [PubMed] [CrossRef]
202. Bouchard-Rochette M. Bacillus pumilus et Bacillus subtilis pour lutter contre la pourriture grise chez la tomate et le concombre de serre [master’s thesis]. Québec, QC, Canada: Laval University; 2020. [Google Scholar]
203. Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, et al. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol. 2007;9(4):1084–90. doi:10.1111/j.1462-2920.2006.01202.x. [Google Scholar] [PubMed] [CrossRef]
204. Benaissa O, Leghrifi I, Laasli SE, Bourak K, Sagouti T, Belabess Z, et al. The role of microbial consortia in controlling soil-borne pathogens. In: Plant stress tolerance. Boca Raton, FL, USA: CRC Press; 2025. p. 318–38. doi:10.1201/9781003543237-16. [Google Scholar] [CrossRef]
205. Carmen B, Roberto D. Soil bacteria support and protect plants against abiotic stresses. In: Abiotic stress in plants-mechanisms and adaptations. Houston, TX, USA: InTech; 2011. doi:10.5772/23310. [Google Scholar] [CrossRef]
206. de Oliveira AB, Mendes Alencar NL, Gomes-Filho E. Comparison between the water and salt stress effects on plant growth and development. In: Responses of organisms to water stress. Houston, TX, USA: InTech; 2013. doi:10.5772/54223. [Google Scholar] [CrossRef]
207. Khan MS, Zaidi A, Musarrat J. Phosphate solubilizing microorganisms: Principles and application of microphos technology. 1st ed. Cham, Switzerland: Springer; 2014. [Google Scholar]
208. Shaikh NB, Shaikh N, Rochlani A, Dalwani A, Sharma S, Saraf SM. Rhizobacteria that promote plant growth and their impact on root system architecture, root development, and function. Act Scie Micro. 2022;5(4):53–62. doi:10.31080/asmi.2022.05.1035. [Google Scholar] [CrossRef]
209. Prajapati K, Prajapati M, Panchal R, Sharma S, Saraf SM. Application of microorganisms for bioremediation of heavy metals. Acta Sci Env Sci. 2022;1(1):1–9. [Google Scholar]
210. Sathiavelu A. A review on the role of compost microbes in the abiotic stress tolerance in plants. SGS-Eng Sci. 2021;1(1). [Google Scholar]
211. Kumar A, Verma JP. Does plant-Microbe interaction confer stress tolerance in plants: a review? Microbiol Res. 2018;207(2):41–52. doi:10.1016/j.micres.2017.11.004. [Google Scholar] [PubMed] [CrossRef]
212. Egamberdieva D, Lugtenberg B. Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. In: Use of microbes for the alleviation of soil stresses. New York, NY, USA: Springer New York; 2013. Vol. 1, p. 73–96. doi:10.1007/978-1-4614-9466-9_4. [Google Scholar] [CrossRef]
213. Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci. 2015;22(2):123–31. doi:10.1016/j.sjbs.2014.12.001. [Google Scholar] [PubMed] [CrossRef]
214. Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem. 2004;42(6):565–72. doi:10.1016/j.plaphy.2004.05.009. [Google Scholar] [PubMed] [CrossRef]
215. Vijayan K, Chakraborti SP, Doss SG, Ghosh P. Combining ability for morphological and biochemical characters in mulberry (Morns spp.) under salinity stress. Int J Ind Entomol. 2008;16(2):67–74. [Google Scholar]
216. Upadhyaya H, Panda SK. Abiotic stress responses in tea [Camellia sinensis L. (o) kuntze]: an overview. Rev Agric Sci. 2013;1:1–10. doi:10.7831/ras.1.1. [Google Scholar] [CrossRef]
217. Aknc E, Dorothy M. Plant water-stress response mechanisms. In: Water stress. Houston, TX, USA: InTech; 2012. doi:10.5772/29578. [Google Scholar] [CrossRef]
218. Jain R, Sharma S, Rathod ZR, Rupal KS, Lucie KM, Meenu SS. Analyzing the role of phosphate solubilizing bacteria isolated from Rhizospheric soil of Cyamopsis in improving agricultural productivity. Biosci Biotechn Res Commun. 2018;13(1):16–20. [Google Scholar]
219. Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J. Control of drought stress in wheat using plant-growth-promoting bacteria. J Plant Growth Regul. 2013;32(1):122–30. doi:10.1007/s00344-012-9283-7. [Google Scholar] [CrossRef]
220. Panchal R, Prajapati K, Prajapati M, Sharma S, Saraf SM. Bacterial exopolysaccharides: types, its biosynthesis and their application in different fields. Acta Sci Biotechnol. 2022;3(2):3–11. [Google Scholar]
221. Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11(1):15–9. doi:10.1016/j.tplants.2005.11.002. [Google Scholar] [PubMed] [CrossRef]
222. Calvo-Polanco M, Sánchez-Romera B, Aroca R, Asins MJ, Declerck S, Dodd IC, et al. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ Exp Bot. 2016;131:47–57. doi:10.1016/j.envexpbot.2016.06.015. [Google Scholar] [CrossRef]
223. Gontia-Mishra I, Sapre S, Deshmukh R, Sikdar S, Tiwari S. Microbe-mediated drought tolerance in plants: current developments and future challenges. In: Plant microbiomes for sustainable agriculture. Cham, Switzerland: Springer International Publishing; 2020. p. 351–79. doi:10.1007/978-3-030-38453-1_12. [Google Scholar] [CrossRef]
224. Kumar M, Mishra S, Dixit V, Kumar M, Agarwal L, Chauhan PS, et al. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal Behav. 2016;11(1):e1071004. doi:10.1080/15592324.2015.1071004. [Google Scholar] [PubMed] [CrossRef]
225. Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Liao H. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J Plant Interact. 2017;12(1):100–7. doi:10.1080/17429145.2017.1294212. [Google Scholar] [CrossRef]
226. Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015;168(1):343–56. doi:10.1104/pp.15.00030. [Google Scholar] [PubMed] [CrossRef]
227. Kapadia C, Sayyed RZ, Ali El Enshasy H, Vaidya H, Sharma D, Patel N, et al. Halotolerant microbial consortia for sustainable mitigation of salinity stress, growth promotion, and mineral uptake in tomato plants and soil nutrient enrichment. Sustainability. 2021;13(15):8369. doi:10.3390/su13158369. [Google Scholar] [CrossRef]
228. Ramasamy KP, Mahawar L. Coping with salt stress-interaction of halotolerant bacteria in crop plants: a mini review. Front Microbiol. 2023;14:1077561. doi:10.3389/fmicb.2023.1077561. [Google Scholar] [PubMed] [CrossRef]
229. Kapadia C, Patel N, Rana A, Vaidya H, Alfarraj S, Ansari MJ, et al. Evaluation of plant growth-promoting and salinity ameliorating potential of halophilic bacteria isolated from saline soil. Front Plant Sci. 2022;13:946217. doi:10.3389/fpls.2022.946217. [Google Scholar] [PubMed] [CrossRef]
230. Khan MY, Nadeem SM, Sohaib M, Waqas MR, Alotaibi F, Ali L, et al. Potential of plant growth promoting bacterial consortium for improving the growth and yield of wheat under saline conditions. Front Microbiol. 2022;13:958522. doi:10.3389/fmicb.2022.958522. [Google Scholar] [PubMed] [CrossRef]
231. Dabhi J, Solanki A, Patel R, Sharma S. Bioremediation of heavy metals: a brand new methodology to sustainable agriculture. Int J Innov Res Sci Eng Technol. 2021;10(6):6031–49. [Google Scholar]
232. Ojuederie OB, Babalola OO. Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health. 2017;14(12):1504. doi:10.3390/ijerph14121504. [Google Scholar] [PubMed] [CrossRef]
233. Sharma S, Saraf MS. Isolation, screening and biochemical characterizations with multiple traits of heavy metal tolerant rhizobacteria from mining area and landfill site. Adv Biores. 2022;13(1):147–56. doi:10.15515/abr.0976-4585.13.1.147156. [Google Scholar] [CrossRef]
234. Abou-Aly HE, Youssef AM, Tewfike TA, El-Alkshar EA, El-Meihy RM. Reduction of heavy metals bioaccumulation in sorghum and its rhizosphere by heavy metals-tolerant bacterial consortium. Biocatal Agric Biotechnol. 2021;31(1):101911. doi:10.1016/j.bcab.2021.101911. [Google Scholar] [CrossRef]
235. Carpio IE, Machado-Santelli G, Sakata SK, Ferreira Filho SS, Rodrigues DF. Copper removal using a heavy-metal resistant microbial consortium in a fixed-bed reactor. Water Res. 2014;62(1):156–66. doi:10.1016/j.watres.2014.05.043. [Google Scholar] [PubMed] [CrossRef]
236. Firincă C, Zamfir LG, Constantin M, Răut I, Capră L, Popa D, et al. Microbial removal of heavy metals from contaminated environments using metal-resistant indigenous strains. J Xenobiot. 2023;14(1):51–78. doi:10.3390/jox14010004. [Google Scholar] [PubMed] [CrossRef]
237. Cardarelli M, Woo SL, Rouphael Y, Colla G. Seed treatments with microorganisms can have a biostimulant effect by influencing germination and seedling growth of crops. Plants. 2022;11(3):259. doi:10.3390/plants11030259. [Google Scholar] [PubMed] [CrossRef]
238. Mathivanan N, Prabavathy VR, Vijayanandraj VR. Application of talc formulations of Pseudomonas fluorescens migula and Trichoderma viride pers. ex S.F. gray decrease the sheath blight disease and enhance the plant growth and yield in rice. J Phytopathol. 2005;153(11–12):697–701. doi:10.1111/j.1439-0434.2005.01042.x. [Google Scholar] [CrossRef]
239. Katyal P, Kocher GS, Bhardwaj RD, Kaur J, Sharma S, Alamri S, et al. Impact of microbial decomposers spray on in situ degradation of paddy straw stubble left in the field after paddy harvesting in Punjab. BioResources. 2024;19(4):8284–95. [Google Scholar]
240. Raja K, Anandham R, Sivasubramaniam K. Infusing microbial consortia for enhancing seed germination and vigour in pigeonpea (Cajanus Cajan (L.) millsp.). Curr Sci. 2019;117(12):2052. doi:10.18520/cs/v117/i12/2052-2058. [Google Scholar] [CrossRef]
241. Raza A, Hassan A, Akram W, Anjum T, Aftab ZEH, Ali B. Seed coating with the synthetic consortium of beneficial Bacillus microbes improves seedling growth and manages Fusarium wilt disease. Sci Hortic. 2024;325(1):112645. doi:10.1016/j.scienta.2023.112645. [Google Scholar] [CrossRef]
242. Sharma TR. Molecular diagnosis and application of DNA markers in the management of fungal and bacterial plant diseases. Indian J Biotechnol. 2003;2(1):99–109. [Google Scholar]
243. Sharma V, Prasanna R, Hossain F, Muthusamy V, Nain L, Das S, et al. Priming maize seeds with cyanobacteria enhances seed vigour and plant growth in elite maize inbreds. 3 Biotech. 2020;10(4):154. doi:10.1007/s13205-020-2141-6. [Google Scholar] [PubMed] [CrossRef]
244. Mastouri F. Use of Trichoderma spp. to improve plant performance under abiotic stresses [Ph.D. thesis]. Ithaca, NY, USA: Cornell University; 2010. [Google Scholar]
245. Mastouri F, Björkman T, Harman GE. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology. 2010;100(11):1213–21. doi:10.1094/PHYTO-03-10-0091. [Google Scholar] [PubMed] [CrossRef]
246. Srivastava R, Khalid A, Singh US, Sharma AK. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol Control. 2010;53(1):24–31. doi:10.1016/j.biocontrol.2009.11.012. [Google Scholar] [CrossRef]
247. Bradáčová K, Florea AS, Bar-Tal A, Minz D, Yermiyahu U, Shawahna R, et al. Microbial consortia versus single-strain inoculants: an advantage in PGPM-assisted tomato production? Agronomy. 2019;9(2):105. doi:10.3390/agronomy9020105. [Google Scholar] [CrossRef]
248. Cao Z, Yan W, Ding M, Yuan Y. Construction of microbial consortia for microbial degradation of complex compounds. Front Bioeng Biotechnol. 2022;10:1051233. doi:10.3389/fbioe.2022.1051233. [Google Scholar] [PubMed] [CrossRef]
249. Al Mamun A, Neumann G, Moradtalab N, Ahmed A, Dupuis B, Darbon G, et al. Microbial consortia versus single-strain inoculants as drought stress protectants in potato affected by the form of N supply. Horticulturae. 2024;10(1):102. doi:10.3390/horticulturae10010102. [Google Scholar] [CrossRef]
250. Cirillo V, Romano I, Woo SL, Di Stasio E, Lombardi N, Comite E, et al. Inoculation with a microbial consortium increases soil microbial diversity and improves agronomic traits of tomato under water and nitrogen deficiency. Front Plant Sci. 2023;14:1304627. doi:10.3389/fpls.2023.1304627. [Google Scholar] [PubMed] [CrossRef]
251. Hassan A, Akram W, Rizwana H, Aftab ZEH, Hanif S, Anjum T, et al. The imperative use of Bacillus consortium and quercetin contributes to suppress Fusarium wilt disease by direct antagonism and induced resistance. Microorganisms. 2023;11(10):2603. doi:10.3390/microorganisms11102603. [Google Scholar] [PubMed] [CrossRef]
252. Sidharthan VK, Pothiraj G, Suryaprakash V, Singh AK, Aggarwal R, Shanmugam V. A synergic and compatible microbial-based consortium for biocontrol of Fusarium wilt of tomato. Phytopathol Mediterr. 2023;60(2):183–97. doi:10.36253/phyto-13055. [Google Scholar] [CrossRef]
253. Büttner H, Niehs SP, Vandelannoote K, Cseresnyés Z, Dose B, Richter I, et al. Bacterial endosymbionts protect beneficial soil fungus from nematode attack. Proc Natl Acad Sci U S A. 2021;118(37):e2110669118. doi:10.1073/pnas.2110669118. [Google Scholar] [PubMed] [CrossRef]
254. Zhou X, Wang J, Liu F, Liang J, Zhao P, Tsui CKM, et al. Cross-Kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat Commun. 2022;13(1):7890. doi:10.1038/s41467-022-35452-6. [Google Scholar] [PubMed] [CrossRef]
255. Mazzola M, Freilich S. Prospects for biological soilborne disease control: application of indigenous versus synthetic microbiomes. Phytopathology. 2017;107(3):256–63. doi:10.1094/PHYTO-09-16-0330-RVW. [Google Scholar] [PubMed] [CrossRef]
256. Gu H, Chen Y, Liu X, Wang H, Jue ST, Wu L, et al. The effective migration of Massilia sp. WF1 by Phanerochaete chrysosporium and its phenanthrene biodegradation in soil. Sci Total Environ. 2017;593–594:695–703. doi:10.1016/j.scitotenv.2017.03.205. [Google Scholar] [PubMed] [CrossRef]
257. Ruan Z, Xu M, Xing Y, Jiang Q, Yang B, Jiang J, et al. Interspecies metabolic interactions in a synergistic consortium drive efficient degradation of the herbicide bromoxynil octanoate. J Agric Food Chem. 2022;70(37):11613–22. doi:10.1021/acs.jafc.2c03057. [Google Scholar] [PubMed] [CrossRef]
258. Gao J, Qin J, Ye F, Ding F, Liu G, Li A, et al. Constructing simplified microbial consortia to improve the key flavour compounds during strong aroma-type Baijiu fermentation. Int J Food Microbiol. 2022;369:109594. doi:10.1016/j.ijfoodmicro.2022.109594. [Google Scholar] [PubMed] [CrossRef]
259. Krom BP, Kidwai S, Ten Cate JM. Candida and other fungal species: forgotten players of healthy oral microbiota. J Dent Res. 2014;93(5):445–51. doi:10.1177/0022034514521814. [Google Scholar] [PubMed] [CrossRef]
260. Qian X, Chen L, Sui Y, Chen C, Zhang W, Zhou J, et al. Biotechnological potential and applications of microbial consortia. Biotechnol Adv. 2020;40:107500. doi:10.1016/j.biotechadv.2019.107500. [Google Scholar] [PubMed] [CrossRef]
261. Tao C, Wang Z, Liu S, Lv N, Deng X, Xiong W, et al. Additive fungal interactions drive biocontrol of Fusarium wilt disease. New Phytol. 2023;238(3):1198–214. doi:10.1111/nph.18793. [Google Scholar] [PubMed] [CrossRef]
262. Wu S, Li X, Fan H, Dong Y, Wang Y, Bai Z, et al. Engineering artificial microbial consortia based on division of labor promoted simultaneous removal of Cr(VI)-atrazine combined pollution. J Hazard Mater. 2023;443(Pt A):130221. doi:10.1016/j.jhazmat.2022.130221. [Google Scholar] [PubMed] [CrossRef]
263. Lareen A, Burton F, Schäfer P. Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol. 2016;90(6):575–87. doi:10.1007/s11103-015-0417-8. [Google Scholar] [PubMed] [CrossRef]
264. Halder S, Yadav KK, Sarkar R, Mukherjee S, Saha P, Haldar S, et al. Alteration of Zeta potential and membrane permeability in bacteria: a study with cationic agents. SpringerPlus. 2015;4(1):672. doi:10.1186/s40064-015-1476-7. [Google Scholar] [PubMed] [CrossRef]
265. van der Wal A, de Boer W. Dinner in the dark: illuminating drivers of soil organic matter decomposition. Soil Biol Biochem. 2017;105:45–8. doi:10.1016/j.soilbio.2016.11.006. [Google Scholar] [CrossRef]
266. Shahbaz M, Kuzyakov Y, Sanaullah M, Heitkamp F, Zelenev V, Kumar A, et al. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: mechanisms and thresholds. Biol Fertil Soils. 2017;53(3):287–301. doi:10.1007/s00374-016-1174-9. [Google Scholar] [CrossRef]
267. Johnston AE, Poulton PR, Coleman K. Soil organic matter: its importance in sustainable agriculture and carbon dioxide fluxes. Adv Agron. 2009;101:1–57. doi:10.1016/S0065-2113(08)00801-8. [Google Scholar] [CrossRef]
268. Wu D, Wang W, Yao Y, Li H, Wang Q, Niu B. Microbial interactions within beneficial consortia promote soil health. Sci Total Environ. 2023;900:165801. doi:10.1016/j.scitotenv.2023.165801. [Google Scholar] [PubMed] [CrossRef]
269. Krishnaprabu DS. Liquid microbial consortium: a potential tool for sustainable soil health. J Pharmacogn Phytochem. 2020;9(2):2191–9. doi:10.22271/phyto.2020.v9.i2aj.11182. [Google Scholar] [CrossRef]
270. Soltero-Rios MA, Medina-Orozco AY, Valenzuela-Yerena MA, Andrade-Robles NM, Mendoza-Flores AA, Morelia-Jiménez JAY, et al. Microbial consortia: an approach in plant growth promotion and plant diseases management. In: Microbial biocontrol techniques. Singapore: Springer Nature Singapore; 2024. p. 1–17. doi:10.1007/978-981-97-8739-5_1. [Google Scholar] [CrossRef]
271. Fabian J, Zlatanovic S, Mutz M, Premke K. Fungal-bacterial dynamics and their contribution to terrigenous carbon turnover in relation to organic matter quality. ISME J. 2017;11(2):415–25. doi:10.1038/ismej.2016.131. [Google Scholar] [PubMed] [CrossRef]
272. Sahu PK, Singh DP, Prabha R, Meena KK, Abhilash PC. Connecting microbial capabilities with the soil and plant health: options for agricultural sustainability. Ecol Indic. 2019;105(3):601–12. doi:10.1016/j.ecolind.2018.05.084. [Google Scholar] [CrossRef]
273. Toribio AJ, Suárez-Estrella F, Jurado MM, López-González JA, Martínez-Gallardo MR, López MJ. Design and validation of cyanobacteria-rhizobacteria consortia for tomato seedlings growth promotion. Sci Rep. 2022;12(1):13150. doi:10.1038/s41598-022-17547-8. [Google Scholar] [PubMed] [CrossRef]
274. King RW, Evans LT. Gibberellins and flowering of grasses and cereals: prizing open the lid of the “florigen” black box. Annu Rev Plant Biol. 2003;54(1):307–28. doi:10.1146/annurev.arplant.54.031902.135029. [Google Scholar] [PubMed] [CrossRef]
275. Spaepen S, Vanderleyden J. Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol. 2011;3(4):a001438. doi:10.1101/cshperspect.a001438. [Google Scholar] [PubMed] [CrossRef]
276. Arkhipova TN, Veselov SU, Melentiev AI, Martynenko EV, Kudoyarova GR. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil. 2005;272(1):201–9. doi:10.1007/s11104-004-5047-x. [Google Scholar] [CrossRef]
277. Ortíz-Castro R, Valencia-Cantero E, López-Bucio J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal Behav. 2008;3(4):263–5. doi:10.4161/psb.3.4.5204. [Google Scholar] [PubMed] [CrossRef]
278. Singh DP, Singh HB, Prabha R. Microbial inoculants in sustainable agricultural productivity. Cham, Switzerland: Springer; 2016. [Google Scholar]
279. Bhardwaj D, Ansari MW, Sahoo RK, Tuteja N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb Cell Fact. 2014;13(1):66. doi:10.1186/1475-2859-13-66. [Google Scholar] [PubMed] [CrossRef]
280. Saa S, Olivos-Del Rio A, Castro S, Brown PH. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Front Plant Sci. 2015;6:87. doi:10.3389/fpls.2015.00087. [Google Scholar] [PubMed] [CrossRef]
281. Alizadeh H, Behboudi K, Ahmadzadeh M, Javan-Nikkhah M, Zamioudis C, Pieterse CMJ, et al. Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol Control. 2013;65(1):14–23. doi:10.1016/j.biocontrol.2013.01.009. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF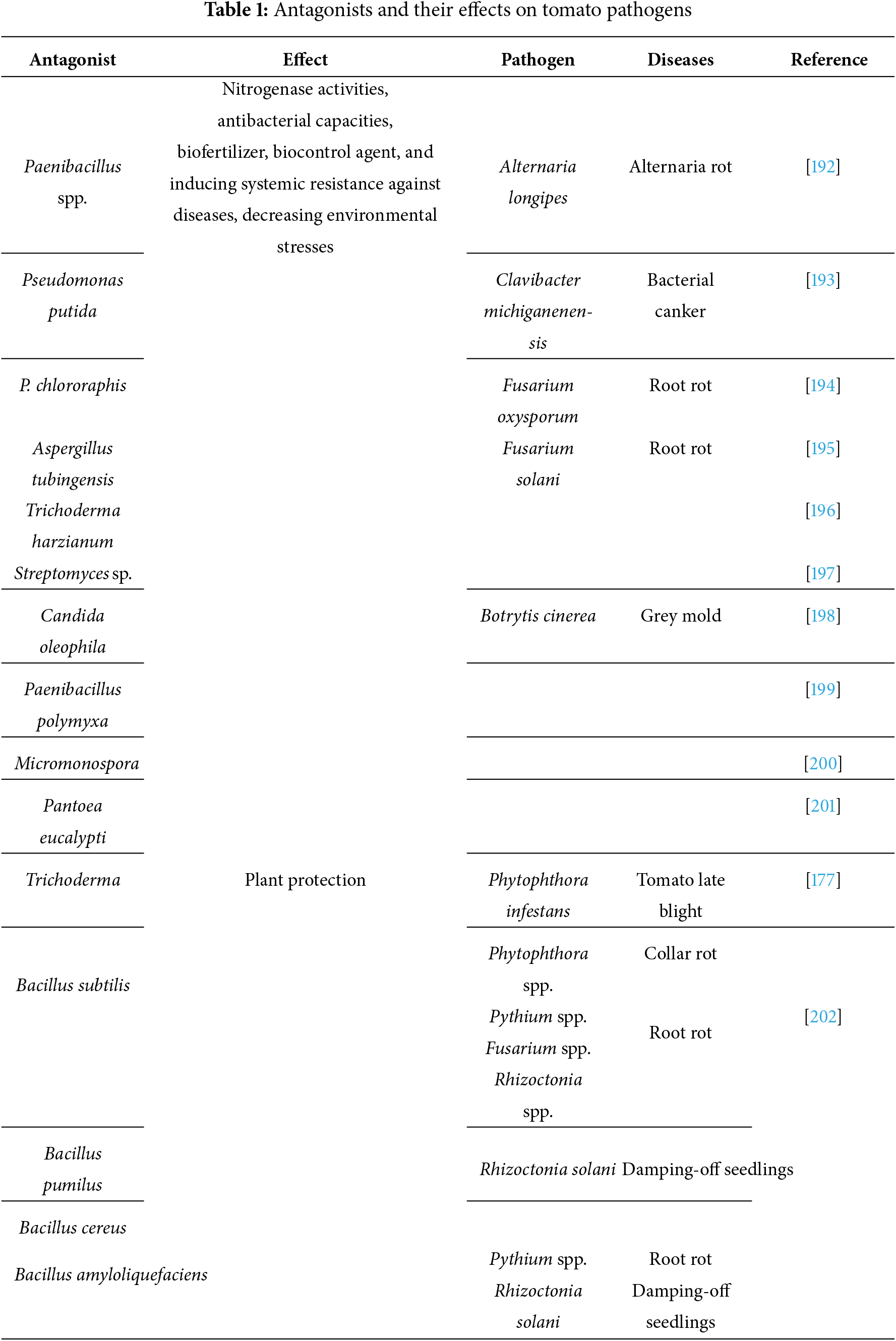
 Downloads
Downloads
 Citation Tools
Citation Tools