 Open Access
Open Access
ARTICLE
Investigating Drought Resilience in Fig Cultivars: A Comprehensive Study of Leaf Structural and Functional Characteristics
1 Fruit Trees and Vine Research Program, National Institute for Agricultural Research (INRA), Regional Center of Meknes, P.O. Box 578, Meknes, 50000, Morocco
2 Laboratory of Biotechnology, Conservation and Valorisation of Natural Resources (LBCVNR), Faculty of Sciences Dhar El Mehraz, Sidi Mohamed Ben Abdellah University, P.O. Box 1796, Atlas, Fez, 30003, Morocco
* Corresponding Author: Rachid Razouk. Email:
(This article belongs to the Special Issue: Vegetable Resources, Sustainable Plant Protection and Adaptation Strategies to Climate Change)
Phyton-International Journal of Experimental Botany 2025, 94(6), 1857-1877. https://doi.org/10.32604/phyton.2025.065116
Received 04 March 2025; Accepted 04 June 2025; Issue published 27 June 2025
Abstract
This study was carried out to assess plasticity to drought of 30 adult fig cultivars, based on a screening of leaf structural and functional traits under sustained deficit irrigation, corresponding to 60% of crop evapotranspiration. All trees, three per cultivar, are planted in an ex-situ collection in Sais plain, northern Morocco. The measurements concerned leaf area, blade thickness, trichomes density, trichome hair length, stomatal density, stomatal dimensions, stomatal area index, chlorophyll concentration index, relative water content, stomatal conductance, leaf temperature, water loss in detached leaves, cuticular wax content, proline content, total phenolic compounds, and total soluble sugars. The ranking of cultivars regarding drought tolerance was established based on a two-level clustering approach, primarily relying on chlorophyll concentration index and secondarily on water status traits. Results showed significant genotypic variations for all measured traits, except phenolic compounds content. Correlations between structural and functional traits have pinpointed blade thickness and trichome hair length as the key indicators of fig drought tolerance, owing to their involvement in maintaining chlorophyll content under water stress conditions. The extent of the variations shows that fig leaf is endowed with a wide structural and functional diversity, which can give to the species potential for resilience to various environmental stresses, including drought. Among the cultivars assessed, two exotic varieties, “Kadota” and “Royal Blanck”, as well as four local cultivars, namely, “Ferqouch Jmel”, “El Qoti Labied”, “Hamra” and “Fassi” showed the highest drought plasticity level.Keywords
Ficus is a diverse genus of the Moraceae family with over 800 species, of which Ficus carica is particularly most studied [1,2]. It is native to the Middle East and Asia Minor and grows in all warm regions of the globe. Nowadays, Ficus carica is a pillar of the fruit crops sector in Morocco with an annual production level of 119,166.59 t (3rd rank worldwide) on a total area of about 69,737 ha (1st rank worldwide) [3]. Fig leaves are a source of bioactive compounds that play an important role in reducing the complications of obesity and type 2 diabetes. Several studies have demonstrated the bioactivity of Ficus carica leaf extracts, which can be used as anti-diabetic and anti-obesity dietary supplements [4]. The Moroccan fig orchard is spread over different regions, with contrasting environmental conditions, ranging from the northeast to the southwest of the country. The fig tree is known as a resilient plant, which can grow under less favorable conditions, but with the intensification of its cultivation in drip-irrigated orchards, its performance remains more compromised by environmental stress, mainly drought [5].
Effectively, between 2018 and 2024, Morocco experienced a series of exceptionally dry years, marked by annual water deficits of −54%, −71%, −59%, −85%, −66%, −72% and −75%, respectively. Dam storage levels have fallen to critical levels (from 50% (2014) to 23% (2024)). The Souss-Massa, Draa-Tafilalet, and Tangier-Tetouan-Al Hoceima regions are among the worst affected. Drought stress is a significant environmental constraint that affects the physiological, biochemical, morphological, ecological, and even molecular processes of plants [6]. The changes induced by drought in plant behavior impede its vegetative growth and limit its productivity [7,8]. In recent years, drought frequency and severity have increased in many regions, including Morocco, leading to concerns about the role of climate change in exacerbating this phenomenon. Water stress induced by prolonged severe and consecutive drought periods constitutes a major obstacle for fig production in Morocco. The vulnerability of the fig tree to drought, which would be more alarming in coming years as predicted by climate scenarios, therefore requires sustainable adaptation strategies [9].
Studies on tree response to drought are important, particularly in Mediterranean regions threatened by water scarcity and high temperatures. These studies are particularly more justified on crops known to be drought tolerant in their wild state, such as fig trees [10]. Selecting drought-tolerant fig cultivars, especially in arid and semiarid areas, requires research on water stress impacts and how the trees recover from it [11]. For this reason, it is essential to identify and analyze the processes involved by trees to escape water stress and to predict their potential effects in the long term [12,13]. In this sense, various physiological parameters have been used in previous studies to examine plant response to drought stress, including photosynthetic activity, stem water potential, stomatal traits, trichomes, and the accumulation of biochemical compounds in leaves. Among the latter, particular interest is given to proline and cuticular waxes as crucial substances that regulate gas and water exchange and protect plants from damage induced by dehydration [14,15]. The development of efficient breeding selection techniques to obtain highly drought-resistant varieties is necessary to maintain a sustainable production and meet future demand in figs [16]. In addition, the mechanisms underpinning its drought resilience remain poorly understood, especially at the level of leaf structure and function, which play a central role in water regulation, gas exchange, and overall plant performance under stress. The present study is a contribution in this sense, aiming to (1) assess water stress tolerance of local and exotic cultivars of fig, and (2) identify the structural and functional leaf traits that can be used as morpho-physiological markers of drought resistance. The findings will provide valuable information for developing sustainable cultivation strategies, improving crop productivity and enhancing the resilience of fig production systems in water-scarce environments.
The genotypic variation was significant for all leaf morphological traits assessed (Table 1). The Student-Newman-Keuls (SNK) test highlighted four distinct groups of cultivars with regard to mean leaf area (LA). The highest LA values were recorded in “Nabout” (202.11 cm2) and “Aboucharchaou” (334.14 cm2). However, the lowest values were shown by “Brown Turkey” (99.27 cm2), “Jeblia” (110.62 cm2), “Bousbati” (113.01 cm2), “Kadota” (114.89 cm2), and “Chetoui” (115.75 cm2). Several studies affirmed that a small leaf has long been considered a typical mechanism for drought adaptation in many woody species, due to better transpiration regulation [17]. Conversely, a large leaf is generally a sign of sensitivity to water stress. In addition, in previous work on olives, a low LA was singled as a relevant marker to screen cultivars for drought tolerance [18]. According to these findings, low leaf area observed in certain fig cultivars constitutes an asset for them to be drought tolerant.
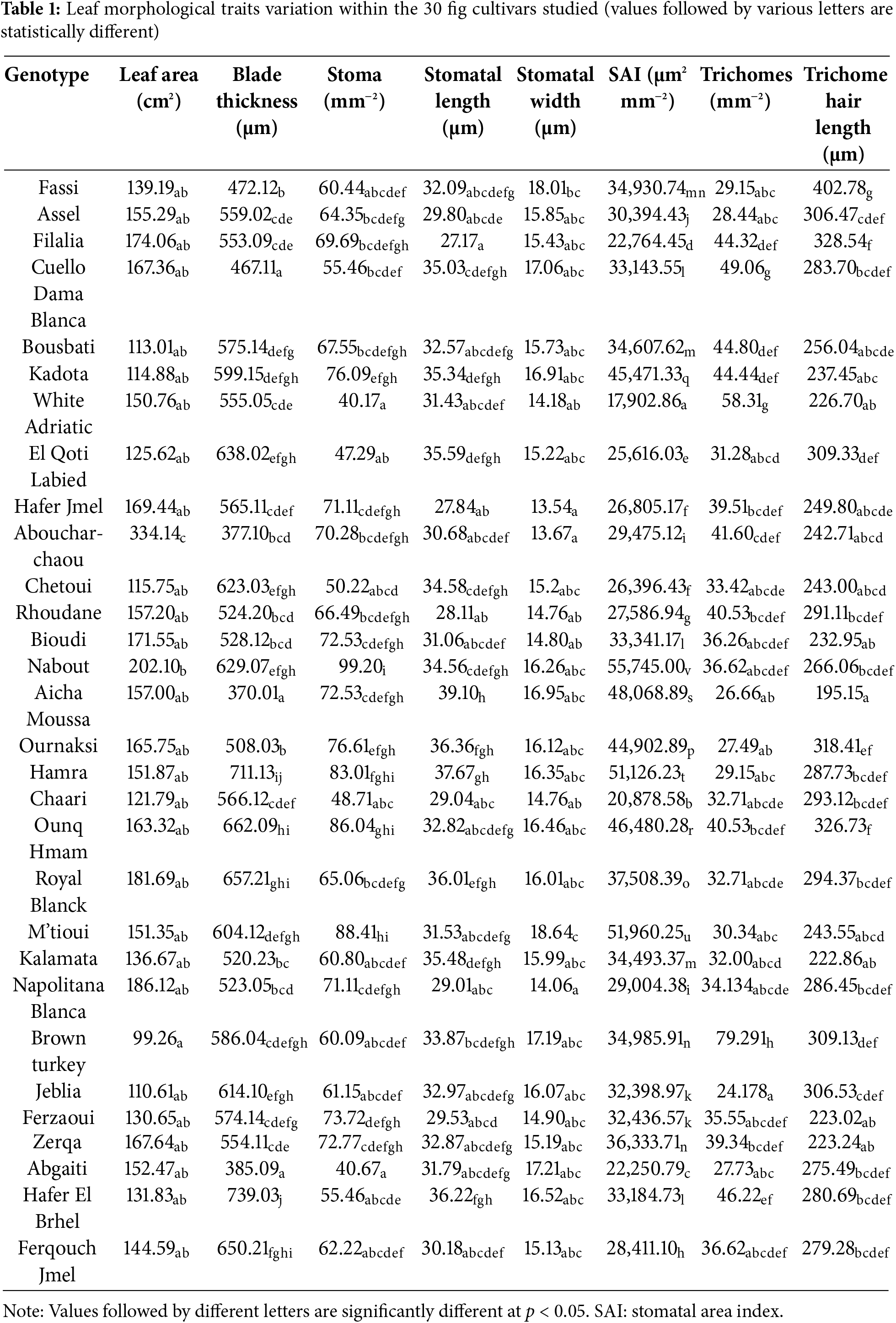
Leaf blade thickness (LBT) varied from 370.33 to 739.33 µm, with 15 dissimilar groups revealed by the SNK test. Among the fig cultivars studied, “Aicha Moussa”, “Abgaiti”, and “Cuello Dama Blanca” presented the thinnest leaves (less than 470 µm), while the thickest ones were observed in “Hamra” and “Hafer El Brhel” (more than 700 µm). Plants with small and thick leaves are associated with low transpiration, which constitutes an advantage for their adaptation to drought [19]. Within the fig collection studied, the two cultivars, “Chetoui” and “Jeblia” are the closest to meeting this condition. However, some studies highlighted that the thickness of the leaf blade is more related to water loss than its area while segregating between cultivars about drought tolerance. The thicker the blade, the thicker its palisade parenchyma, making dehydration caused by water stress reversible [20,21].
Stomata are the seat of gas exchange, including water vapor and by this role, their characteristics are relevant in discrimination between the genotypes for drought tolerance. Indeed, there is a consensus that a low stomatal density (SD) is associated with a high water-use efficiency under drought conditions [22]. This can be the case of the two fig cultivars, “White Adriatic” and “Abgaiti”, which showed the lowest SD of around 40 stomata mm2 in average. On the other hand, the cultivar “Nabout”, “M’tioui”, and “Ounq Hmam” can be less efficient as they showed the highest SD of 99.20, 88.41 and 86.05 stomata mm−2, respectively. The values for these latter cultivars are in the interval reported by Bercu and Popoviciu [23] for the majority of the fig cultivars of 70–100 stomata mm−2. However, it was reported that drought tolerance is rather associated with smaller stomata, owing to their rapid closure in response to water stress, compared to large stomata [24]. Within the fig collection studied, stomatal size varied widely, from 376.95 µm2 in “Hafer Jmel” to 662.74 µm2 in “Aicha Moussa”, thereby making this stomatal trait a relevant descriptor of drought-tolerant cultivars.
Leaf trichomes constitute a physical barrier to water loss through transpiration, and therefore, they are influential in plant performance under drought conditions [25]. In general, leaves containing large and numerous trichomes constitute an advantage for plant drought tolerance [26]. Among the fig genotypes studied, “Brown Turkey” fulfilled this condition well since it showed the highest trichome density of 79.29 trichomes mm−2, with an average hair length of 309.13 µm. The lowest trichome densities were found in “Jeblia”, “Aicha Moussa”, and “Ournaksi”, containing less than 30 trichomes mm−2. The hair trichome varied from 195.16 in the genotype “Aicha Moussa” to 402.78 µm in “Fassi”. The value ranges are in agreement with those reported in previous similar studies [27,28].
2.2 Physiological Traits Related to Water Status
Relative water content (RWC) is a relevant parameter for assessing plant response to water stress [29]. Within the fig collection studied, the differences were significant in RWC, with range values of 50.23%–76.14% in June and 42.36%–73.97% in July (Table 2). The highest RWC was shown in the genotype “Fassi”, with averages of more than 70% in June and July, while the lowest value was observed in “El Qoti Labied” with an average of 50.23% in June, which decreased to 42.36% in July. Indeed, many genotypes exhibited a decrease in RWC from June to July. Alongside “El Qoti Labied”, a noticeable decrease in RWC between the two measurement dates was shown in the genotypes “Assel”, “Filalia”, “Cuello Dama Blanca”, “Bousbati”, “Kadota”, “Aboucharchaou”, and “Ournaksi”. Contrary, other genotypes such as “White Adriatic”, “Chaari”, and “Hafer El Brhel” maintained a relatively stable RWC. This suggests that there were genotypic differences in leaves’ ability to regulate transpiration and in resistance mechanisms to water stress damages [30,31]. However, in the two genotypes, “Hafer El Brhel” and “Ferqouch Jmel”, an increase in RWC was observed instead. This finding could be explained by leaf blade thickness in this genotype, which was the highest in the collection studied, giving it resistance to water loss [32].
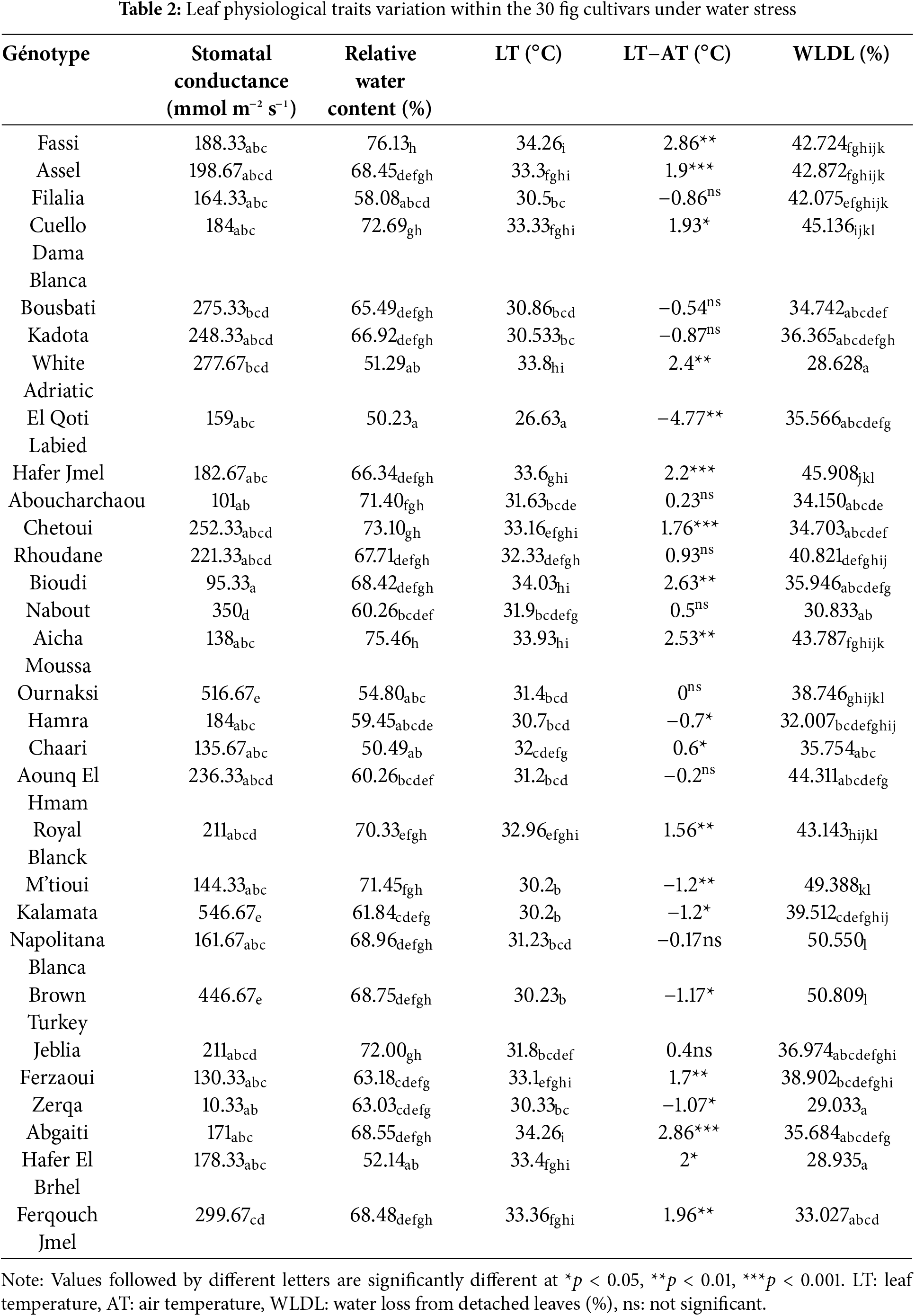
Stomatal conductance (gs) is an important indicator of plant water status, which corresponds to the amount of gas that can be exchanged through the total open stomata [33]. High values generally indicate a sensitivity to drought and vice versa [34–36]. Accordingly, the three cultivars “Bioudi”, “Aboucharchaou” and “Zerqa” could present a high plasticity to drought as they displayed the lowest gs value of around 100 mmol m−2s−1 in average. However, in similar studies, it was reported that a low stomatal conductance is often associated with a low photosynthesis rate, thereby compromising the water use efficiency of the genotype [37]. Low gs is generally attributed to rapid stomatal closure in response to water deficit, representing a drought tolerance mechanism. On the other hand, the highest gs values were shown by “Kalamata”, “Ournaksi”, and “Brown Turkey” with an average of 503 mmol m−2 s−1. The maintenance of high levels of stomatal conductance under moderate water stress could confirm that these cultivars are sensitive to drought.
Leaf temperature is a key factor in triggering mechanisms regulating gas exchange at the stomatal level [38]. In well-irrigated plants, leaf temperature may be 1°C to 2°C lower than air temperature. However, in response to drought, it becomes close to the air temperature, or even exceeds it, due to transpiration decrease [39]. Thus, based on leaf temperature values taken under air temperature of 31.4°C, it appears that 70% of the fig genotypes were not able to ensure cooling of their leaf surfaces under water stress applied, displaying a leaf temperature of up to 3.3°C higher than the air temperature. This indicates a sensitivity of the genotypes to water stress, as they are unable to evacuate excess heat via transpiration. Among the other genotypes, only “El Qoti Labied” seems unaffected, with a leaf temperature 4.8°C lower than the air temperature.
Water loss from detached leaves (WLDL) was used in many studies assessing plant drought tolerance as a relevant physiological indicator, which is often linked with leaf cuticular resistance against dehydration and stomata closing speed in response to water stress [40,41]. WLDL was significant for all leaves samples after 1 h under ambient temperature (25°C). It was particularly important in “Brown Turkey” and “Napolitana Blanca”, which reached an average of 50% of leaf moisture. However, the three genotypes “White Adriatic”, “Hafer El Brhel”, and “Zerqa” stand out with a reduced water loss of less than 30%. This indicates that the leaves of these cultivars are better adapted to water retention, which could delay water stress damage on their tissues. Based on the leaf traits assessed herein, this adaptation may be related to their thick blade and high content in trichomes. According to Cominelli et al. [42], these leaf structural traits can confer high cuticular resistance to water loss.
Biochemical compound contents in leaves are among leaf functional traits, which express the magnitude of plant response to water stress. Chlorophyll is among the most vital of these compounds due to its direct involvement in photosynthesis, and its reduction constitutes an apparent sign of a plant’s exposure to water stress [43,44]. Within the fig collection, chlorophyll content index (CCI) varied widely in the range of 29.43%–47.50%, indicating the existence of genotypic differences in chlorophyll accumulation and photosynthetic capacity (Table 3). The highest CCI value was displayed by “Nabout”, while the lowest one was observed in “Aicha Moussa”. A high CCI level suggests that the genotype might have an adaptive behavior to ensure the efficient functioning of its leaves under stressful conditions. Based on the structural traits herein assessed, the thick leaves that characterize the genotype “Nabout” could partly explain this behavior. In fact, the thickest leaves may contain several layers of mesophyll, which increase chlorophyll concentration and improves light absorption efficiency. Note that similar studies reported that high CCI values are linked to full leaf expansion, while low values may be linked to the beginning of leaf senescence [32,45–47], added that variability in chlorophyll content can be generated by differences in nutrient uptake, especially nitrogen, iron, and magnesium.
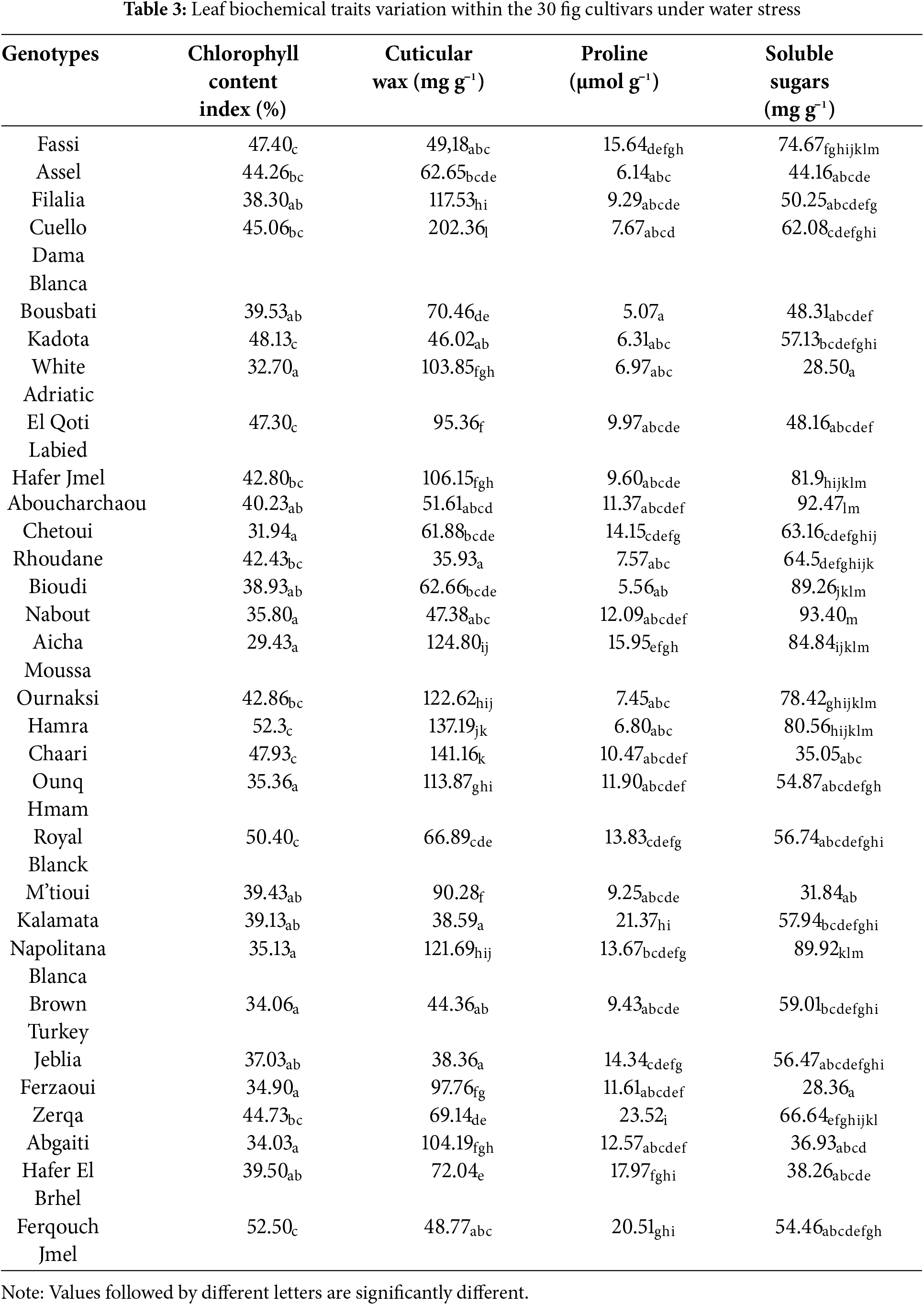
Cuticular wax accumulation in leaves constitutes a biochemical adaptation mechanism to drought. It is a complex hydrophobic substance that covers the leaf surface and forms a protective barrier between the plant and its environment, insulating plant tissues from harsh external conditions [48,49]. The waxy layer of the leaf cuticle is made up of very long-chain aliphatic lipids (alkanes, primary and secondary alcohols, esters, sterols, and flavonoids) [50]. This leaf waxy coating plays an essential role in plant tolerance to abiotic stress, including drought, through reduced transpiration and also in reducing attacks of pests and diseases [51]. Cuticular wax content (CWC) in fig leaves varied widely, from 38.60 mg g−1 in the genotype “Kalamata” to 202.37 mg g−1 in “Cuello Dama Blanca”. Cuticular wax biosynthesis occurs at the cuticle, which is generally not related to the leaf structural traits. It is regulated by complex genetic expressions linked to various waxy aliphatic compounds [52], which seem to be differently increased by water stress depending on fig genotypes. In general, high leaf CWC constitutes an advantage for plant drought tolerance by reducing water loss through transpiration, particularly in conditions of low atmospheric humidity or soil water deficit [50,53]. In a similar study on olive, it was singled as a relevant biochemical marker for drought tolerance alongside soluble sugar content [18]. The latter trait is often included in similar studies as a mechanism indicator of plant response to water stress. Soluble sugar content (SSC) in leaves generally increases in response to water stress, as an osmotic adjustment strategy to maintain cell turgor, which is induced by the expression of various specific genes [54,55]. Hence, an increased leaf SSC strengthens the plant’s plasticity to drought [56]. Within the fig collection studied, variation in SSC was highly significant, displaying 21 levels according to the SNK test. Maximum values were found in “Nabout” and “Aboucharchaou”, with 92.40 and 93.45 mg g−1, respectively, while the lowest ones were exhibited by “White Adriatic” (28.35 mg g−1) and “Ferzaoui” (28.50 mg g−1). Genotypes that tend to accumulate higher levels of SSC may have improved drought tolerance through more active osmotic adjustment mechanisms or more efficient sugar metabolism. SSC content is a reliable marker for assessing the adaptability of genotypes to water stress, and its variability among fig cultivars could be used in breeding programs for drought-tolerant varieties. Phenolic compounds are secondary metabolites whose biosynthesis becomes more activated in response to water stress as a defense mechanism. They are antioxidants that protect plant cells against oxidative damage caused by reactive oxygen species (ROS) [57]. Contrary to SSC, there was non-significant difference in total phenolic compounds (TPC) content between the fig genotypes, with an overall average of 7.57 mg g−1. This leaf biochemical trait, therefore, has no impact on discrimination between the fig genotypes herein studied with regard to drought tolerance. Note that similar studies reported significant differences in phenolic compounds, as found by Petruccelli et al. [58] on a collection of Italian fig varieties. The contradictory results concerning this trait could be explained by differences in the genetic pool studied, but also in the environmental conditions.
Proline accumulation in the leaf is considered among the adaptive strategies observed in fig trees to cope with water stress [59]. Proline is an amino acid that contributes in leaf structure stabilization under drought conditions [60]. In addition, it enables pH regulation and can serve as a carbon and nitrogen reserve for plants during a stress period [61]. There was a notable difference in leaf proline concentration among the fig genotypes studied. Some of them showed high proline levels, such as “Zerqa” and “Kalamata”, with respective average values of 23.52 and 21.37 µmol g−1. The lowest values were found in “Bousbati” and “Bioudi”, of 5.07 and 5.56 µmol g−1, respectively. The genotypes exhibiting high leaf proline accumulation may have a greater ability to withstand water stress than those with low proline levels [62]. Several previous studies have underlined that the increased biosynthesis of proline in plant cells enables the recovery of reactive oxygen species (ROS), which play an important role in the plant’s defense system against drought-induced oxidative damage [63].
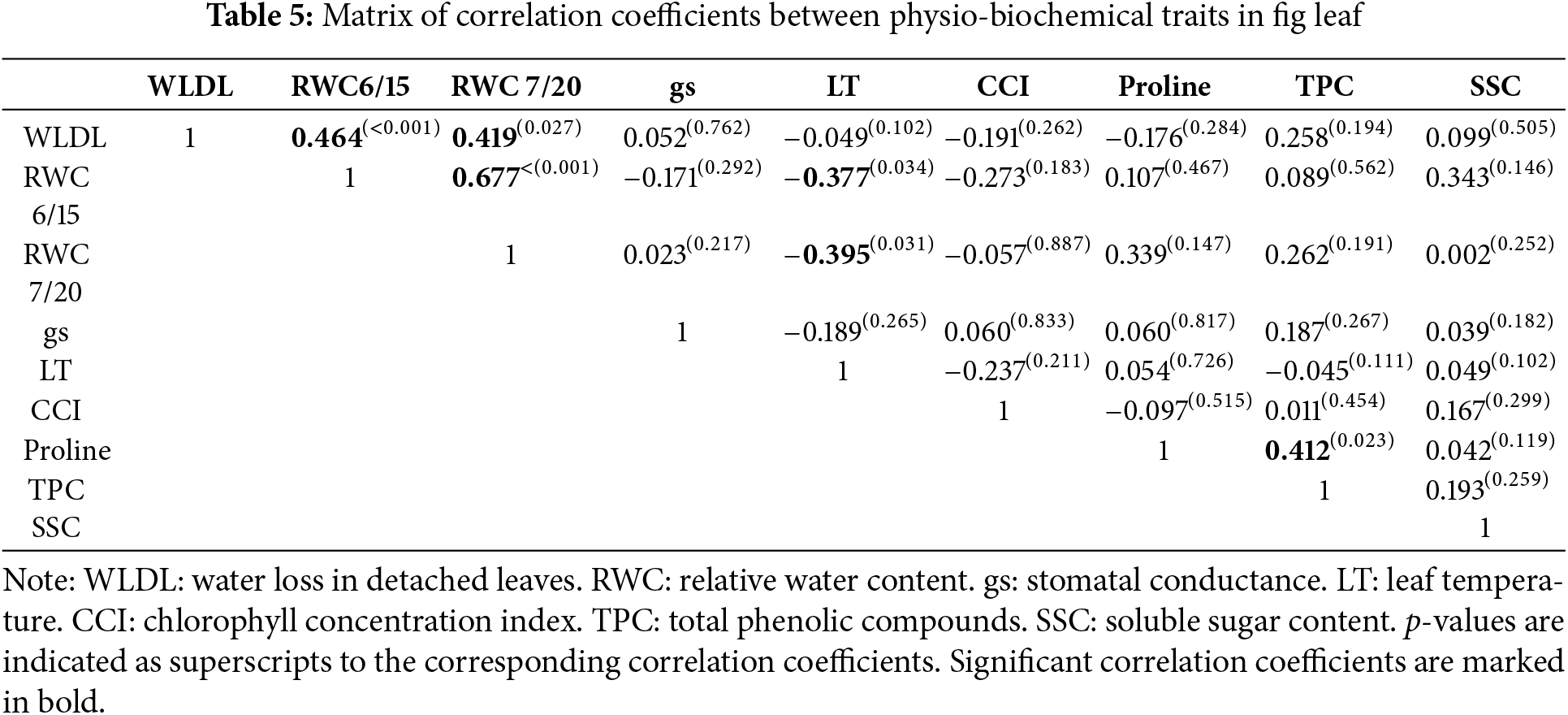
Correlations between variables allows to predict the relationship between them and to identify among them the traits that can be potentially used as drought tolerance markers. The bilateral correlations within leaf morphological traits and their connections with physio-biochemical traits are summarized in Table 4. It emerges that leaf area is negatively correlated with blade thickness, thereby indicating that smaller fig leaves are thicker and vice versa. The significant correlation between these two traits seems obvious in most plants. A reduced leaf area combined with increased leaf thickness constitutes a structural strategy for adapting plants to stressful situations [19]. This can be proven by the positive and significant correlation herein observed between leaf thickness and chlorophyll concentration (r = 0.387). However, fig genotypes with thicker leaves exhibited a significant decrease in leaf water status, more pronounced than genotypes with thinner leaves, as can be deduced from the negative correlation between leaf thickness and RWC (r = −0.415). It appears that a high blade thickness constitutes a favorable structural trait to maintain chlorophyll concentration relatively stable, even at low water potential levels. In this sense, Taratima et al. [64] reported that in thick leaves, chlorophyll concentration is high, the reduction of which under water stress does not severely affect the chloroplast activity. Based on these results, leaf thickness seems to be associated with plasticity to drought in fig trees.
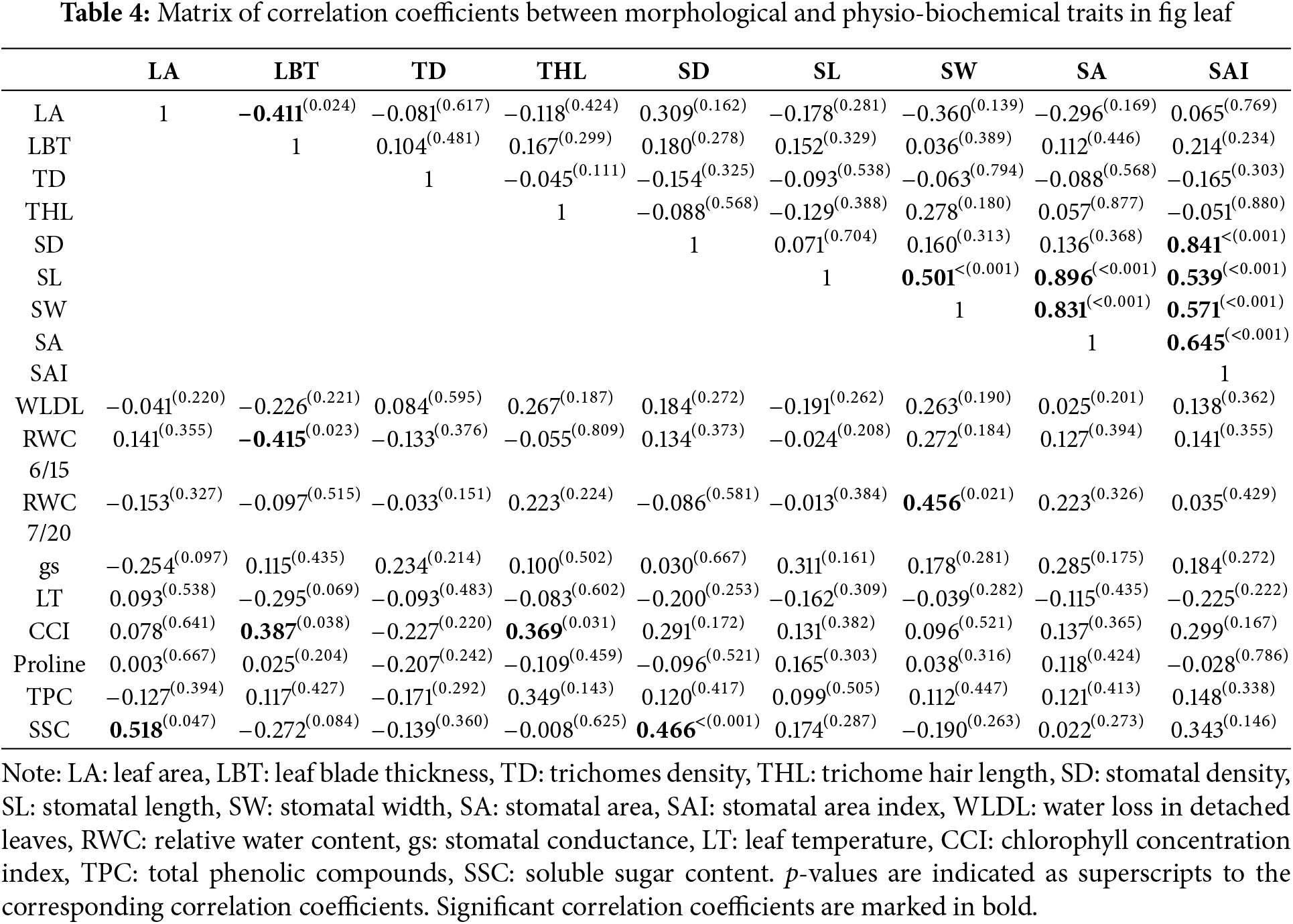
As for trichomes, the correlation matrix shows that their hair length is more related to drought tolerance degree than their density, as can be presumed by its positive and significant correlation with chlorophyll concentration (r = 0.369). It is known that a high coverage of leaf surface by trichomes is associated with a high drought tolerance level by regulating transpiration [65,66]. According to the result herein found on the fig tree, the leaf surface coverage rate by trichomes seems to be more determined by their hairs, especially when they are longer. Up to then, the trichome hair length, alongside the blade thickness appears to be leaf structural traits that are significantly involved in fig drought tolerance owing to their correlations with chlorophyll content. The involvement of the other leaf structural traits in drought tolerance could not be highlighted based on the results obtained. Among these, leaf area and stomatal density allow to predict richness of leaves in soluble sugars, with highly significant positive correlation coefficients. This indicates that soluble sugar accumulation is higher in leaves that are large or contain a high stomatal density. High leaf SSC may be linked to the requirements in metabolite translocation generated by fruit, as it may be an adaptive response of the genotype to stabilize leaf water potential under increased transpiration [67].
On the other hand, two important observations emerge from the correlation matrix between the physio-biochemical traits in Table 5. The first one concerns the non-significance of correlations between chlorophyll content and all of them. Therefore, water stress’s effect on leaf water status was not intense enough to induce a decrease in chlorophyll concentration. This functional behavior in fig leaf has been reported on other known drought-tolerant species, which are able to maintain their photosynthetic activity under severe water stress, such as olive [68], carob, eucalyptus, Moringa [69], and jujube [70]. The second observation is related to the significant negative correlation between relative water content and leaf temperature. This result suggests that leaf temperature can be used as a reliable indicator for easy and quick assessment of fig tree water status. It is, therefore, a typical functional relationship in fig leaf since similar studies on other species have not reported this relationship to be significant, as observed in peach, almond and plum [13].
Among the measured leaf traits, chlorophyll concentration emerges as a pivotal factor in clustering fig cultivars according to their drought tolerance level, given that its decline signifies an advanced stage of water stress effects on both physiological and biochemical characteristics [68,71]. Furthermore, these traits exhibited a comparable impact on discrimination among the cultivars, as shown by principal component analysis (PCA), 77.84% of the total variance is explained in seven principal components (Table 6). The first two principal components represent 33.31% (PC1 = 17.74% and PC2 = 15.57%). These results highlight the complex interaction of structural and functional traits in drought resilience. Generally, the obtained results reveal that stomatal traits, water status, trichome characteristics, and biochemical properties are the primary drivers of variation in the dataset. Thus, to effectively assess the drought tolerance of the 30 fig cultivars, they were initially grouped into four primary clusters according to the chlorophyll concentration index (CCI), which proved to be a discriminating primary criterion for the selection of water-stress tolerant genotypes. Subsequently, within each primary cluster, the cultivars underwent classification using the unweighted pair group method with arithmetic mean (UPGMA), relying on the average values of RWC, gs, SSC, and proline content (Fig. 1). These four traits were chosen given their significant correlations with the other physiological and biochemical parameters, as outlined in Table 7. These traits were distinguished as secondary discriminating indicators for genotype classification.
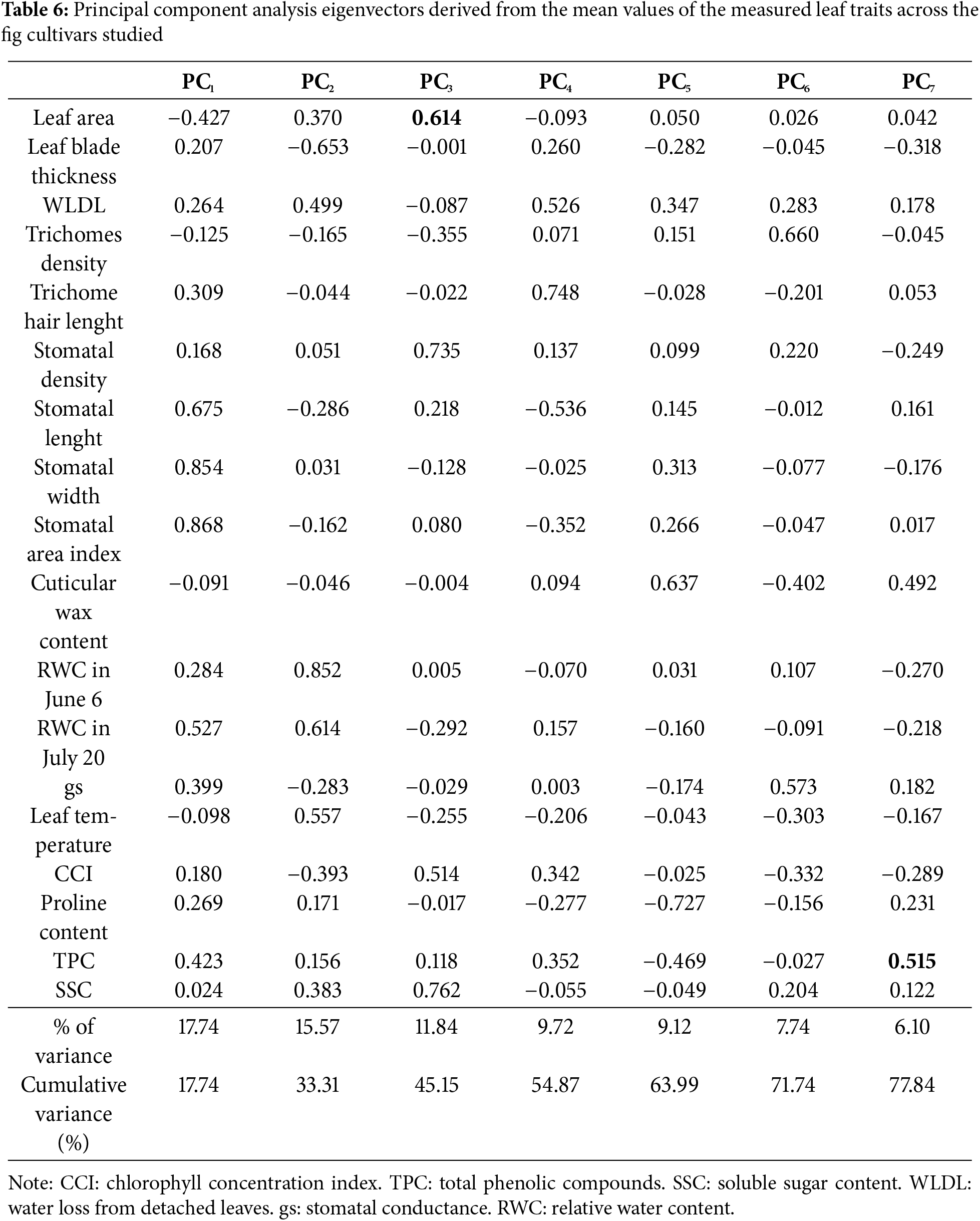
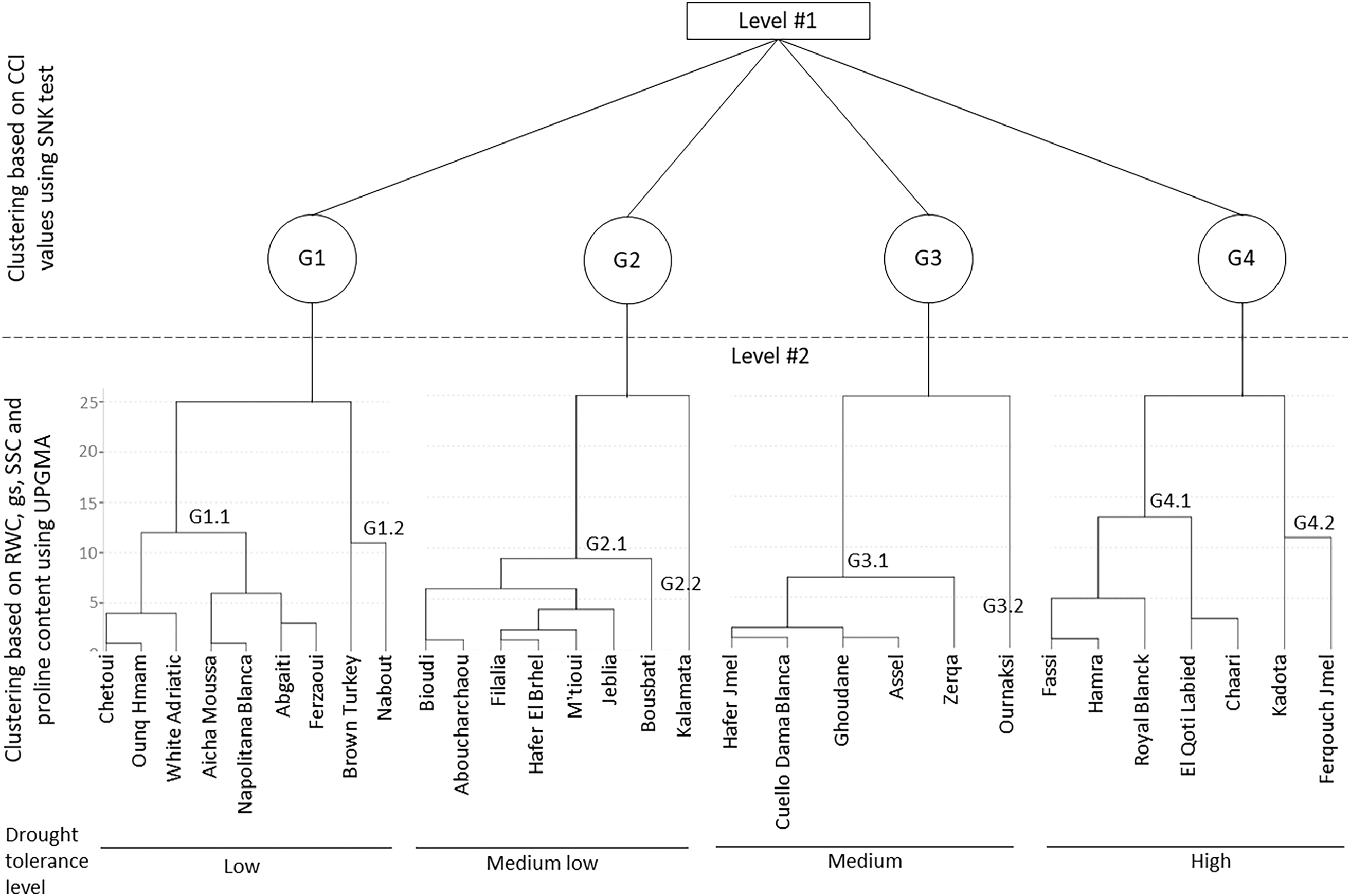
Figure 1: Two-level clustering of the 30 fig cultivars, initially stratified by CCI (level 1), with further refinement based on RWC, gs, SSC and proline content (level 2).
The first main group (G1), classified as the most sensitive to drought due to its low chlorophyll concentration, is subdivided into two subgroups. The first subgroup (G1.1) contains 7 cultivars, including “White Adriatic” and “Chetoui”, widely cultivated in the Mediterranean. Meanwhile, “Nabout” and “Brown Turkey”, also common cultivars, are classified in a distinct subgroup (G1.2), relatively less sensitive to water stress, compared to G1.1. The differentiation of these two cultivars is linked to their stomatal conductance, which was twice as high as that of subgroup G1.1, as well as their soluble sugar content, which was 38% higher. The second main group (G2) comprises 8 locally less widespread cultivars, classified as moderately sensitive to water stress. Within this group, the cultivar “Kalamata” is notably distinguished due to its superiority in terms of relative water content, stomatal conductance and proline accumulation. The third main group (G3), categorized as moderately tolerant to water deficit, comprises 6 cultivars divided into two subgroups. The first subgroup (G3.1) encompasses the two familiar cultivars, “Cuello Dama Blanca” and “Ghoudane”, along with 3 local cultivars. However, the second subgroup (G3.2) consists solely of the cultivar “Ournaksi”, distinguished notably by its significantly higher stomatal conductance, approximately three times greater than that of subgroup G3.1. Finally, the fourth main group (G4), the most drought-tolerant within the studied fig collection due to its chlorophyll concentration index being less affected under water stress, contains 7 cultivars. Among them, “Kadota” and “Royal Blanck” are two exotic varieties, ranked alongside five local cultivars, among which “El Qoti Labied”, “Chaari”, and “Fassi” are the most locally known. Within this group, the exotic variety “Kadota” and the local cultivar “Ferqouch Jmel” showed high stomatal conductance values, thereby classifying them into a distinct subgroup (G4.2) as the most drought-tolerant genotypes within the studied collection.
This ranking of cultivars is in line with that found by Oukabli et al. [10] for 7 cultivars herein studied, including “El Qoti Labied” and “Ferqouch Jmel”, classified as drought tolerant based on shoot growth decrease and leaf fall rates during the summer period. The results also align with those of Tapita et al., ranking “Kadota” variety as more drought-tolerant than “Brown Turkey”. The phenotypic clustering of the 30 fig tree cultivars is highly useful for deepening the analysis of functional and structural characteristics related to fig tree adaptation. It is of great interest in guiding fig variety selection and diversification in arid areas. In this regard, the cultivars of the group G4 could be suggested as a first step to enhance the resilience of the sector to climate change.
3.1 Plant Material and Experimental Conditions
The plant material consisted of 30 fig cultivars (Table 7), 12 years old, gathered in an ex-situ collection at the experimental station of the National Institute of Agricultural Research (INRA) in Ain Taoujdate, northern Morocco (33°56′ E, 5°13′ N, 499 m a.s.l.). Three individual trees represented each cultivar, which were planted in parallel lines, with a spacing of 5 × 3 m. The climate in the experimental station is of semiarid type, with hot and dry summer. The soil is of calcimagnesic type, sandy clay textured, with a useful water reserve of 1.7 mm cm−1, quite rich in organic matter (2.8% in the top layer). The experimental year, 2022, was particularly very dry, with annual rainfall of 300 mm and reference evapotranspiration (ET0) of 1128 mm, which were recorded from the weather station near the trial. The monthly distributions of these two climatic parameters are presented in Fig. 2, which clearly shows the occurrence of a pronounced rainfall deficit from April to November.
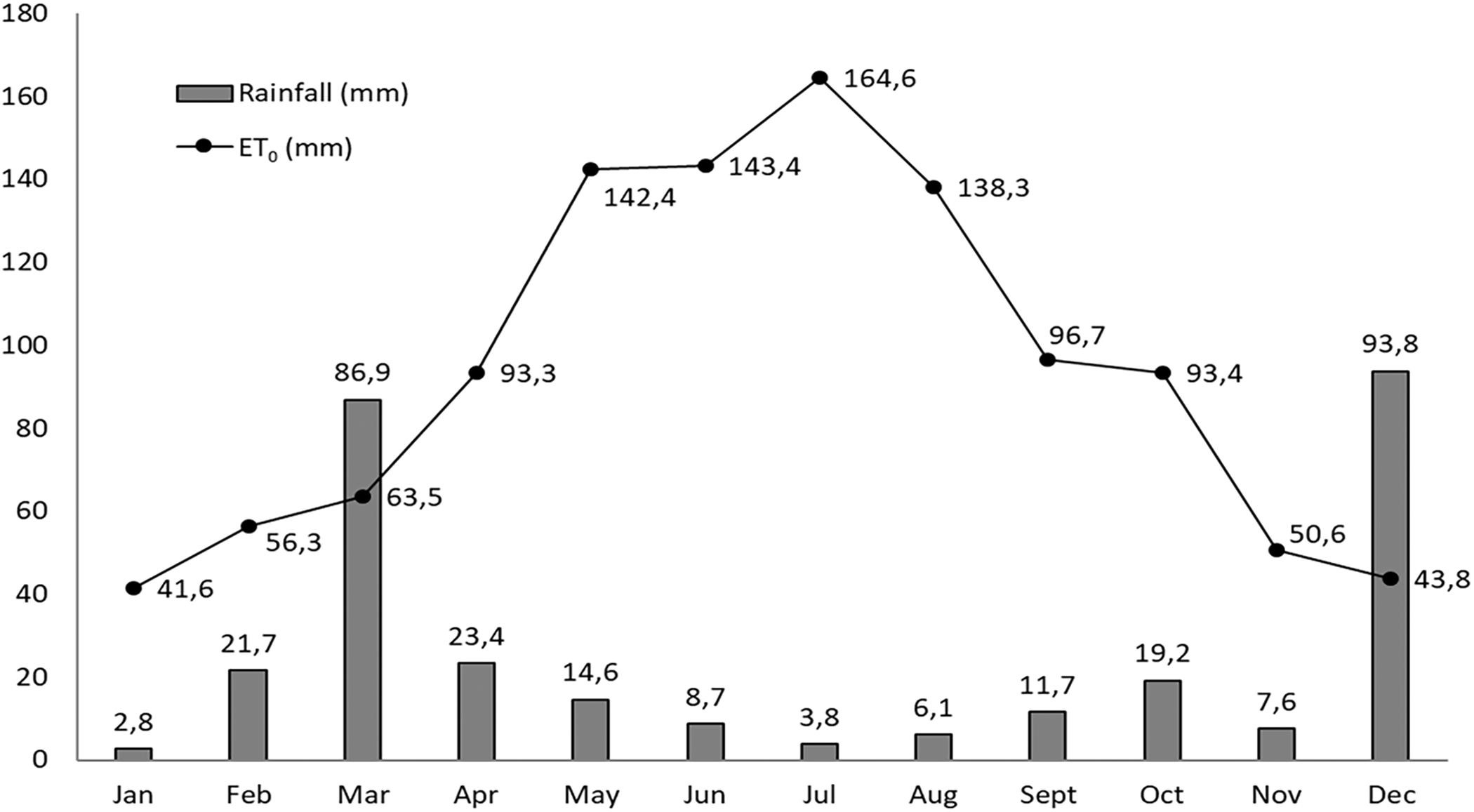
Figure 2: Monthly rainfall and reference evapotranspiration level in the experimental station during the study year
All trees were drip irrigated daily from early April (vegetative departure) to end October (3 months after fruit harvest), except during the rainy days. The daily amount of irrigation was determined to ensure a deficit water regime, corresponding to 60% of crop evapotranspiration (ETc). The daily ETc values were calculated using the formula [ETc = Kr x Kc x ET0−Reff], where Kr is the reduction coefficient, which was equal to 1 as the tree canopy cover was high, Kc is the crop coefficient of 0.6 as recommended by Andrade et al. (2014), and Reff is the effective rainfall, equivalent to 80% of the recorded rainfall. The monthly amounts of irrigation applied to produce a water deficit of 60% are presented in Table 8.
Measurements on the leaf were taken in mid-June when the rainfall deficit was severe, and the fig trees were in the fruit-set stage. During this period, the activity of the fig trees was intense to ensure their needs for rapid growth of fruits and shoots, while being under a marked water deficit. The samples consisted of 20 fully developed leaves, taken from the four sides of each fig tree (60 leaves per genotype).
3.2.1 Morphological Measurements
Morphological measurements concerned leaf area, blade thickness, stomata (density and pore dimensions), and trichomes (density and length). Leaf area was measured using a digital leaf area meter (ADC Bioscientific Ltd., Guildford, UK), and the thickness of its blade was measured by a digital caliper. Stomata and trichomes were observed microscopically on the abaxial side of six small leaf pieces cut from the central section of 12 leaves per genotype, approximately 1 cm2 each. First, the cuticle covering the leaf piece epidermis was wiped using transparent tape before being mounted on a glass slide and observed with a photomicroscope (Micros, Austria) connected to a digital camera (DeltaPix, Invenio 12EIII, Horsholm, Denmark), with a computer attachment. Stomata and trichomes were observable under a magnification of 600× and counted in ten places in the leaf piece of 0.25 mm2 each while measuring stomatal pore length (SL) and width (SW) as well as trichome hair length. Stomatal area index (SAI), corresponding to the portion of the leaf surface covered by stomatal pores, was calculated by the following equation [SAI = SL × SW × SD], where SD is the stomatal density [73].
3.2.2 Physiological Measurements
The leaf water status was assessed through measurement in the field of stomatal conductance (gs, mol m−2 s−1), leaf temperature (LT, °C), chlorophyll concentration, relative water content (RWC), and water loss in detached leaves (WLDL).LT, gs, and chlorophyll concentration were measured in the field at midday on 10 fully expanded leaves per tree using an infrared thermometer (Spectrum technologies, Aurora, IL, USA), a portable porometer (AP4, Delta-T-Devices, Cambridge, UK), and a chlorophyll meter (SPAD 502 Plus). The leaves sampled for LT, gs, and chlorophyll measurements were taken for RWC and WLDL determination in the laboratory. RWC was determined according to the method of Turner [74], using the formula [RWC = (FW-DW) × 100/(SW − DW)], where FW, DW, and SW designate fresh, dry, and saturation weights of leaf, respectively. The leaf was saturated by placing its petiole in water-soaked cotton for 24 h in a refrigerator set at 5°C, and afterward it was dried in an oven set at 80°C for 48 h. Note that RWC was measured during two dates, June 15 and July 20. On the other hand, WLDL was determined following the method of Schwabe et Lionakis [75], by quantifying water evaporated from detached leaves placed under ambient temperature (25°C) during 1 h.
3.2.3 Biochemical Measurements
The leaf biochemical traits of the fig cultivars studied concerned soluble sugars, proline, cuticular wax, and phenolic compounds.
Soluble sugar content (SSC) was analyzed following the phenol sulfuric acid method developed by DuBois et al. [76], using glucose for standard solutions. Indeed, 50 mg of leaf powder was mixed with 1 mL of 80% ethanol. The extract was then centrifuged at 2000 rpm for 40 min at 4°C. Following that, 0.5 mL of phenol and 1.5 mL of concentrated sulfuric acid were added and homogenized with 0.5 mL of the extract. The mixture obtained was placed in a water bath at 95°C for 5 min before measurement of the optical density at 485 nm by a spectrophotometer (6850 UV/VIS, Jenway, Staffordshire, UK). The method of Marcell and Beattie [77] was used to determine leaf cuticular wax content (CWC). First, the sampled leaf was washed with distilled water and weighed. Then, it was stirred in 20 mL of concentrated chloroform for 30 s using a brush. The extracted cuticular wax was separated by evaporating chloroform using a hot plate and weighed.
Leaf proline content was determined following the method of Monneveux and Nemmar [78]. Indeed, 100 mg of lyophilized leaf powder was added to 2 mL of 40% methanol and heated to 85°C in a water bath for 1 h. The extract obtained was cooled to room temperature before adding 1 mL of acetic acid, 25 mg of ninhydrin, and a mixture of 1 mL of distilled water, acetic acid, and orthophosphoric acid (120, 300, 80: v/v/v). The mixture was boiled for 30 min in water bath and then cooled. Following that, 5 mL of toluene was added. Then, a pinch of sodium sulfate was added after vortex agitation. The absorbance at 528 nm was measured using a spectrophotometer, using free proline solutions as standards.
Total phenolic compounds (TPC) were determined according to the method of Folin-Ciocalteu, described by Singleton and Rossi [79]. Thus, 40 μL of the extract is pipetted into a test tube to which 3160 μL of distillate water and 200 μL of reactive folin citrate were added. After 30 min, 600 μL of sodium carbonate solution 7.5% was added, and the mixture was then stirred in a vortex. The mixture was incubated for 30 min in darkness under ambient temperature. Then, the optical density was measured at 765 nm using a spectrophotometer and gallic acid for standard solutions.
Statistical analysis was carried out using SPSS v22 software. Analysis of variance (Two-Way ANOVA) followed by a post-hoc test using Student-Newman-Keuls (SNK) test was employed to ascertain significant differences among leaf samples for each trait. The difference between LT and AT was determined by Student test (t-test). To discern the leaf structural traits most associated with fig drought tolerance, a correlation test was performed using the Pearson model, examining relationships among all measured traits. Additionally, principal component analysis (PCA) and a two-level clustering approach based on relevant traits, were employed to rank cultivars with regard to drought tolerance.
The response of fig cultivars to water stress varies significantly. It triggers chlorophyll degradation, particularly pronounced in sensitive cultivars, whereas drought-tolerant cultivars display internal physio-biochemical alterations with minimal external manifestations. Among the leaf structural traits, blade thickness and trichome size emerge as phenotypic markers of drought tolerance, owing to their significant connections with chlorophyll content maintenance under water stress conditions. This latter parameter has been used for the initial categorization of the cultivars with respect to drought tolerance, subsequently refined based on discriminant leaf physio-biochemical characteristics. This classification has highlighted seven cultivars potentially tolerant to drought, including two exotic varieties, “Kadota” and “Royal Blanck”, as well as four local cultivars, namely, “Ferqouch Jmel”, “El Qoti Labied”, “Hamra”, and “Fassi”. This study provides essential information on the drought resistance of different fig cultivars by establishing a link between specific structural and functional characteristics of the leaves and their ability to withstand water stress. These results have direct practical applications for sustainable agriculture, particularly in arid and semiarid regions, by guiding the selection and cultivation of fig tree varieties best adapted to drought-prone environments. In addition, the results can inform breeding programs aimed at improving drought tolerance in fig crops, thereby contributing to food security and climate resilience in vulnerable farming systems.
Acknowledgement: This study was supported by the MCRDV research project under the Ministry of Agriculture of Morocco. We extend our gratitude to Khalfi Chemseddoha and Ouhoussa Houssam for their valuable technical assistance.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: Rachid Razouk, Jamila Bahhou; data collection: Nouha Haoudi, Hicham Aboumadane, Abderrahim Bentaibi; analysis and interpretation of results: Nouha Haoudi, Rachid Razouk, Jamila Bahhou, Lahcen Hssaini; draft manuscript preparation: Nouha Haoudi, Rachid Razouk, Lahcen Hssaini. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Yang Q, Liu Y, Guo Y, Jiang Y, Wen L, Yang B. New insights of fig (Ficus carica L.) as a potential function food. Trends Food Sci Technol. 2023;140(5):104146. doi:10.1016/j.tifs.2023.104146. [Google Scholar] [CrossRef]
2. Xu B, Song S, Yao L, Wang H, Sun M, Zhuang H, et al. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human gut microbiota of polysaccharides from Ficus carica Linn. Food Hydrocoll. 2024;146(10):109204. doi:10.1016/j.foodhyd.2023.109204. [Google Scholar] [CrossRef]
3. FAOSTAT [Internet]. 2024 [cited 2024 Sep 21]. Available from: https://www.fao.org/faostat/fr/#data/QCL/visualize. [Google Scholar]
4. Pucci M, Mandrone M, Chiocchio I, Sweeney EM, Tirelli E, Uberti D, et al. Different seasonal collections of Ficus carica L. leaves diversely modulate lipid metabolism and adipogenesis in 3T3-L1 adipocytes. Nutrients. 2022;14(14):2833. doi:10.3390/nu14142833. [Google Scholar] [PubMed] [CrossRef]
5. Yuan S, Yin T, He H, Liu X, Long X, Dong P, et al. Phenotypic, metabolic and genetic adaptations of the Ficus species to abiotic stress response: a comprehensive review. Int J Mol Sci. 2024;25(17):9520. doi:10.3390/ijms25179520. [Google Scholar] [PubMed] [CrossRef]
6. Naikwade PV. Plant responses to drought stress: morphological, physiological, molecular approaches, and drought resistance. In: Desai NM, Patil M, Pawar UR, editors. Plant metabolites under environmental stress. Palm Bay, FL, USA: Apple Academic Press; 2023. 35 p. [Google Scholar]
7. Habib MA, Azam MG, Haque MA, Hassan L, Khatun MS, Nayak S, et al. Climate-smart rice (Oryza sativa L.) genotypes identification using stability analysis, multi-trait selection index, and genotype-environment interaction at different irrigation regimes with adaptation to universal warming. Sci Rep. 2024;14(1):13836. doi:10.1038/s41598-024-64808-9. [Google Scholar] [PubMed] [CrossRef]
8. Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, et al. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021;10(2):259. doi:10.3390/plants10020259. [Google Scholar] [PubMed] [CrossRef]
9. Leng X, Feng X, Fu B. Driving forces of agricultural expansion and land degradation indicated by Vegetation Continuous Fields (VCF) data in drylands from 2000 to 2015. Glob Ecol Conserv. 2020;23(2):e01087. doi:10.1016/j.gecco.2020.e01087. [Google Scholar] [CrossRef]
10. Oukabli A, Mekaoui A, Ibnouali-El-Aloui M, Bari A. Contribution to identification of fig (Ficus carica) genotypes tolerant to drought. Acta Hortic. 2008;798(798):87–93. doi:10.17660/actahortic.2008.798.10. [Google Scholar] [CrossRef]
11. Rostami AA, Rahemi M. Screening drought tolerance in caprifig varieties in accordance to responses of antioxidant enzymes. World Appl Sci J. 2013;21(8):1213–9. doi:10.5829/idosi.wasj.2013.21.8.91. [Google Scholar] [CrossRef]
12. Intrigliolo DS, Castel JR. Performance of various water stress indicators for prediction of fruit size response to deficit irrigation in plum. Agric Water Manag. 2006;83(1):173–80. doi:10.1016/j.agwat.2005.12.005. [Google Scholar] [CrossRef]
13. Razouk R, Ibijbijen J, Kajji A, Karrou M. Response of peach, plum and almond to water restrictions applied during slowdown periods of fruit growth. Am J Plant Sci. 2013;4(3):561–70. doi:10.4236/ajps.2013.43073. [Google Scholar] [CrossRef]
14. Qiao M, Hong C, Jiao Y, Hou S, Gao H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants. 2024;13(13):1808. doi:10.3390/plants13131808. [Google Scholar] [PubMed] [CrossRef]
15. de Araújo Silva MM, Ferreira LT, de Vasconcelos FMT, Willadino L, Camara TR, dos Santos DYAC, et al. Water stress-induced responses in the growth, cuticular wax composition, chloroplast pigments and soluble protein content, and redox metabolism of two genotypes of Ricinus communis L. J Plant Growth Regul. 2021;40(1):342–52. doi:10.1007/s00344-020-10103-6. [Google Scholar] [CrossRef]
16. Abdolinejad R, Shekafandeh A, Jowkar A, Gharaghani A, Alemzadeh A. Indirect regeneration of Ficus carica by the TCL technique and genetic fidelity evaluation of the regenerated plants using flow cytometry and ISSR. Plant Cell Tiss Organ Cult. 2020;143(1):131–44. doi:10.1007/s11240-020-01903-5. [Google Scholar] [CrossRef]
17. Ackerly DD. Adaptation, niche conservatism, and convergence: comparative studies of leaf evolution in the California chaparral. Am Nat. 2004;163(5):654–71. doi:10.1086/383062. [Google Scholar] [PubMed] [CrossRef]
18. Razouk R, Hssaini L, Alghoum M, Adiba A, Hamdani A. Phenotyping olive cultivars for drought tolerance using leaf macro-characteristics. Horticulturae. 2022;8(10):939. doi:10.3390/horticulturae8100939. [Google Scholar] [CrossRef]
19. Trujillo I, Rivas M, Castrillo M. Leaf recovery responses during rehydration after water deficit in two bean (Phaseolus vulgaris L.) cultivars. J Plant Interact. 2013;8(4):360–9. doi:10.1080/17429145.2012.754959. [Google Scholar] [CrossRef]
20. Bartlett MK, Scoffoni C, Sack L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett. 2012 May;15(5):393–405. doi:10.1111/j.1461-0248.2012.01751.x. [Google Scholar] [PubMed] [CrossRef]
21. Bosabalidis AM, Kofidis G. Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Sci. 2002;163(2):375–9. doi:10.1016/s0168-9452(02)00135-8. [Google Scholar] [CrossRef]
22. Drake PL, Froend RH, Franks PJ. Smaller, faster stomata: scaling of stomatal size, rate of response, and stomatal conductance. J Exp Bot. 2013;64(2):495–505. doi:10.1093/jxb/ers347. [Google Scholar] [PubMed] [CrossRef]
23. Bercu R, Popoviciu D. Anatomical study of Ficus carica L. leaf. Ann Rom Soc Cell Biol. 2015;19(1):33–7. doi:10.ANN/RSCB-2014-0002:RSCB. [Google Scholar] [CrossRef]
24. Yadollahi A, Arzani K, Ebadi A, Wirthensohn M, Karimi S. The response of different almond genotypes to moderate and severe water stress in order to screen for drought tolerance. Sci Hortic. 2011;129(3):403–13. doi:10.1016/j.scienta.2011.04.007. [Google Scholar] [CrossRef]
25. Wang X, Shen C, Meng P, Tan G, Lv L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021;21(1):70. doi:10.1186/s12870-021-02840-x. [Google Scholar] [PubMed] [CrossRef]
26. Xiao K, Mao X, Lin Y. Trichome, a functional diversity phenotype in plant. Mol Biol. 2017;6:183. [Google Scholar]
27. Giordano C, Maleci L, Agati G, Petruccelli R. Ficus carica L. leaf anatomy: trichomes and solid inclusions. Ann Appl Biol. 2020;176(1):47–54. doi:10.1111/aab.12557. [Google Scholar] [CrossRef]
28. Baldini E, Facini O, Nerozzi F, Rossi F, Rotondi A. Leaf characteristics and optical properties of different woody species. Trees. 1997;12(2):73–81. doi:10.1007/s004680050124. [Google Scholar] [CrossRef]
29. An Y, Qi L, Wang L. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS One. 2016;11(1):e0147202. doi:10.1371/journal.pone.0147202. [Google Scholar] [PubMed] [CrossRef]
30. Guizani A, Askri H, Amenta ML, Defez R, Babay E, Bianco C, et al. Drought responsiveness in six wheat genotypes: identification of stress resistance indicators. Front Plant Sci. 2023;14:1232583. doi:10.3389/fpls.2023.1232583. [Google Scholar] [PubMed] [CrossRef]
31. Wood JD, Gu L, Hanson PJ, Frankenberg C, Sack L. The ecosystem wilting point defines drought response and recovery of a Quercus-Carya forest. Glob Chang Biol. 2023;29(7):2015–29. doi:10.1111/gcb.16582. [Google Scholar] [PubMed] [CrossRef]
32. Ammar A, Ben Aissa I, Mars M, Gouiaa M. Comportement physiologique comparatif des cultivars de figuier (Ficus carica L.) en réponse au stress hydrique et à la récupération. Sci Hortic. 2020;260:108881. (In French). doi:10.1016/j.scienta.2019.108881. [Google Scholar] [CrossRef]
33. Cheng KH, Sun Z, Zhong W, Wang Z, Visser M, Liu S, et al. Enhancing wheat crop physiology monitoring through spectroscopic analysis of stomatal conductance dynamics. Remote Sens Environ. 2024;312(5):114325. doi:10.1016/j.rse.2024.114325. [Google Scholar] [CrossRef]
34. Endres L. Daily and seasonal variation of water relationship in sugar apple (Annona squamosa L.) under different irrigation regimes at semi-arid Brazil. Sci Hortic. 2007;113(2):149–54. doi:10.1016/j.scienta.2007.03.007. [Google Scholar] [CrossRef]
35. Patanè C. Leaf area index, leaf transpiration and stomatal conductance as affected by soil water deficit and VPD in processing tomato in semi arid mediterranean climate. J Agron Crop Sci. 2011;197(3):165–76. doi:10.1111/j.1439-037x.2010.00454.x. [Google Scholar] [CrossRef]
36. Rodrigues BM, Souza BD, Nogueira RM, Santos MG. Tolerance to water deficit in young trees of jackfruit and sugar apple. Rev Ciênc Agron. 2010;41(2):245–52. doi:10.1590/s1806-66902010000200011. [Google Scholar] [CrossRef]
37. Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol. 2003;30(3):239–64. doi:10.1071/fp02076. [Google Scholar] [PubMed] [CrossRef]
38. Deans RM, Brodribb TJ, Busch FA, Farquhar GD. Optimization can provide the fundamental link between leaf photosynthesis, gas exchange and water relations. Nat Plants. 2020;6(9):1116–25. doi:10.1038/s41477-020-00760-6. [Google Scholar] [PubMed] [CrossRef]
39. Parkash V, Singh S. A review on potential plant-based water stress indicators for vegetable crops. Sustainability. 2020;12(10):3945. doi:10.3390/su12103945. [Google Scholar] [CrossRef]
40. Chen X, Wang T, Rehman AU, Wang Y, Qi J, Li Z, et al. Arabidopsis U-box E3 ubiquitin ligase PUB11 negatively regulates drought tolerance by degrading the receptor-like protein kinases LRR1 and KIN7. J Integr Plant Biol. 2021;63(3):494–509. doi:10.1111/jipb.13058. [Google Scholar] [PubMed] [CrossRef]
41. Haghpanah M, Hashemipetroudi S, Arzani A, Araniti F. Drought tolerance in plants: physiological and molecular responses. Plants. 2024;13(21):2962. doi:10.3390/plants13212962. [Google Scholar] [PubMed] [CrossRef]
42. Cominelli E, Galbiati M, Vavasseur A, Conti L, Sala T, Vuylsteke M, et al. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr Biol. 2005;15(13):1196–20. doi:10.1016/j.cub.2005.05.048. [Google Scholar] [PubMed] [CrossRef]
43. Dai Y, Shen Z, Liu Y, Wang L, Hannaway D, Lu H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ Exp Bot. 2009;65(2):177–82. doi:10.1016/j.envexpbot.2008.12.008. [Google Scholar] [CrossRef]
44. Zhang YJ, Xie ZK, Wang YJ, Su PX, An LP, Gao H. Effect of water stress on leaf photosynthesis, chlorophyll content, and growth of oriental lily. Russ J Plant Physiol. 2011;58(5):844–50. doi:10.1134/s1021443711050268. [Google Scholar] [CrossRef]
45. Mafakheri M, Kordrostami M, Al-Khayri JM. Abiotic stress in plants: socio-economic consequences and crops responses. In: Al-Khayri JM, Ansari MI, Singh AK, editors. Nanobiotechnology: mitigation of abiotic stress in plants. Cham, Switzerland: Springer International Publishing; 2021. p. 1–28. doi:10.1007/978-3-030-73606-4_1. [Google Scholar] [CrossRef]
46. Jung S. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol Biochem. 2004;42(3):225–31. doi:10.1016/j.plaphy.2004.01.001. [Google Scholar] [PubMed] [CrossRef]
47. Herbinger K, Tausz M, Wonisch A, Soja G, Sorger A, Grill D. Complex interactive effects of drought and ozone stress on the antioxidant defence systems of two wheat cultivars. Plant Physiol Biochem. 2002;40(6):691–6. doi:10.1016/s0981-9428(02)01410-9. [Google Scholar] [CrossRef]
48. de Araújo Silva MM, dos Santos DYAC, Melo-de-Pinna GFA, de Câmara TJR, Oliveira AFM. Chemical composition and ultrastructure of the foliar cuticular wax of two Brazilian cultivars of castor bean (Ricinus communis L.). Ind Crops Prod. 2017;95:558–63. doi:10.1016/j.indcrop.2016.11.010. [Google Scholar] [CrossRef]
49. Oliveira AP, Valentão P, Pereira JA, Silva BM, Tavares F, Andrade PB. Ficus carica L.: metabolic and biological screening. Food Chem Toxicol. 2009;47(11):2841–6. doi:10.1016/j.fct.2009.09.004. [Google Scholar] [PubMed] [CrossRef]
50. He J, Li C, Hu N, Zhu Y, He Z, Sun Y, et al. ECERIFERUM1-6A is required for the synthesis of cuticular wax alkanes and promotes drought tolerance in wheat. Plant Physiol. 2022;190(3):1640–57. doi:10.1093/plphys/kiac394. [Google Scholar] [PubMed] [CrossRef]
51. Shaheenuzzamn M, Shi S, Sohail K, Wu H, Liu T, An P, et al. Regulation of cuticular wax biosynthesis in plants under abiotic stress. Plant Biotechnol Rep. 2021;15(1):1–12. doi:10.1007/s11816-020-00656-z. [Google Scholar] [CrossRef]
52. Lee SB, Suh MC. Regulatory mechanisms underlying cuticular wax biosynthesis. J Exp Bot. 2022;73(9):2799–816. doi:10.1093/jxb/erab509. [Google Scholar] [PubMed] [CrossRef]
53. Negin B, Hen-Avivi S, Almekias-Siegl E, Shachar L, Aharoni A. Cuticular wax composition is essential for plant recovery following drought with little effect under optimal conditions. bioRxiv. 2021;43:807. doi:10.1101/2021.06.08.447487. [Google Scholar] [CrossRef]
54. Pommerrenig B, Ludewig F, Cvetkovic J, Trentmann O, Klemens PAW, Neuhaus HE. In concert: orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol. 2018;59(7):1290–9. doi:10.1093/pcp/pcy037. [Google Scholar] [PubMed] [CrossRef]
55. Živanović B, Milić KS, Tosti T, Vidović M, Prokić L, Veljović JS. Leaf soluble sugars and free amino acids as important components of abscisic acid-mediated drought response in tomato. Plants. 2020;9(9):1147. doi:10.3390/plants9091147. [Google Scholar] [PubMed] [CrossRef]
56. Falchi R, Bonghi C, Drincovich MF, Famiani F, Lara MV, Walker RP, et al. Sugar metabolism in stone fruit: source-sink relationships and environmental and agronomical effects. Front Plant Sci. 2020;11:573982. doi:10.3389/fpls.2020.573982. [Google Scholar] [PubMed] [CrossRef]
57. Nakabayashi R, Mori T, Saito K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal Behav. 2014;9(8):e29518. doi:10.4161/psb.29518. [Google Scholar] [PubMed] [CrossRef]
58. Petruccelli R, Ieri F, Ciaccheri L, Bonetti A. Polyphenolic profiling and chemometric analysis of leaves from Italian Ficus carica L. Varieties Eur J Hortic Sci. 2018;83(2):94–103. doi:10.17660/ejhs.2018/83.2.5. [Google Scholar] [CrossRef]
59. Ben Rejeb K, Abdelly C, Savouré A. La proline, un acide aminé multifonctionnel impliqué dans l’adaptation des plantes aux contraintes environnementales. Biol Aujourd’hui. 2012;206(4):291–9. doi:10.1051/jbio/2012030. [Google Scholar] [CrossRef]
60. Hare PD, Cress WA, Van Staden J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998;21(6):535–53. doi:10.1046/j.1365-3040.1998.00309.x. [Google Scholar] [CrossRef]
61. Verslues PE, Sharma S. Proline metabolism and its implications for plant-environment interaction. Arab Book. 2010;8:e0140. doi:10.1199/tab.0140. [Google Scholar] [PubMed] [CrossRef]
62. Mardinata Z, Edy Sabli T, Ulpah S. Biochemical responses and leaf gas exchange of fig (Ficus carica L.) to water stress, short-term elevated CO2 levels and brassinolide application. Horticulturae. 2021;7(4):73. doi:10.3390/horticulturae7040073. [Google Scholar] [CrossRef]
63. Desoky ESM, Mansour E, Ali MMA, Yasin MAT, Abdul-Hamid MIE, Rady MM, et al. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants. 2021;10(2):354. doi:10.3390/plants10020354. [Google Scholar] [PubMed] [CrossRef]
64. Taratima W, Sudjai S, Maneerattanarungroj P. Growth and anatomical adaptations in response to salinity stress in Cucurbita moschata Duchesne ‘Butternut’ (Cucurbitaceae). Sains Malays. 2022;51(5):1317–24. doi:10.17576/jsm-2022-5105-04. [Google Scholar] [CrossRef]
65. Mollick M, Khan SAKU, Sultana N. Influence of shading and anatomical structure of the stem cutting on rooting performance of fig (Ficus sp.). South Asian Res J Agri Fish. 2023;5(5):55–67. doi:10.36346/sarjaf.2023.v05i05.002. [Google Scholar] [CrossRef]
66. Abassi M, Mguis K, Nja RB, Albouchi A, Boujneh D, Béjaoui Z. Adaptations micromorphologiques foliaires développées par le peuplier blanc (Populus alba L.) face à la salinité. Acta Bot Gall. 2012 Mar;159(1):9–15. doi:10.1080/12538078.2012.671627. [Google Scholar] [CrossRef]
67. Hernandez FBT, Modesto JC, Suzuki MA, Corrêa LS, Reichardt K. Effects of irrigation and nitrogen levels on qualitative and nutritional aspects of fig-trees (Ficus carica L.). Sci Agric. 1994;51(2):292–7. doi:10.1590/s0103-90161994000200015. [Google Scholar] [CrossRef]
68. El Yamani M, Sakar EH, Boussakouran A, Rharrabti Y. Leaf water status, physiological behavior and biochemical mechanism involved in young olive plants under water deficit. Sci Hortic. 2020;261(2):108906. doi:10.1016/j.scienta.2019.108906. [Google Scholar] [CrossRef]
69. Ezzine H, Metougui ML, Boukcim H, Abbas Y. Physiological responses of three field-grown species (Ceratonia siliqua, Eucalyptus camaldulensis, and Moringa oleifera) to water deficits in a Mediterranean semi-arid climate. Sci Rep. 2023;13(1):4536. doi:10.1038/s41598-023-31664-y. [Google Scholar] [PubMed] [CrossRef]
70. Gao Z, Wang X. Spatial variability of leaf wetness under different soil water conditions in rainfed jujube (Ziziphus jujuba Mill.) in the loess hilly region, China. J Arid Land. 2022;14(1):70–81. doi:10.1007/s40333-022-0003-2. [Google Scholar] [CrossRef]
71. Wu J, Wang J, Hui W, Zhao F, Wang P, Su C, et al. Physiology of plant responses to water stress and related genes: a review. Forests. 2022;13(2):324. doi:10.3390/f13020324. [Google Scholar] [CrossRef]
72. de Andrade IPS, de Carvalho DF, de Almeida WS, Silva JBG, da Silva LDB. Water requirement and yield of fig trees under different drip irrigation management. Eng Agríc. 2014;34(1):17–27. doi:10.1590/s0100-69162014000100003. [Google Scholar] [CrossRef]
73. Hamdani A, Bouda S, Adiba A, Laaraj S, Bouhrim M, Herqash RN, et al. Agro-physiological and pomological characterization of plum trees in ex-situ collections: evaluation of their genetic potential in the saïss plain. Sustainability. 2025;17(6):2374. doi:10.3390/su17062374. [Google Scholar] [CrossRef]
74. Turner NC. Techniques and experimental approaches for the measurement of plant water status. Plant Soil. 1981 Feb;58(1–3):339–66. doi:10.1007/bf02180062. [Google Scholar] [CrossRef]
75. Schwabe WW, Lionakis SM. Leaf attitude in olive in relation to drought resistance. J Hortic Sci. 1996;71(1):157–66. doi:10.1080/14620316.1996.11515392. [Google Scholar] [CrossRef]
76. DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–6. doi:10.1021/ac60111a017. [Google Scholar] [CrossRef]
77. Marcell LM, Beattie GA. Effect of leaf surface waxes on leaf colonization by Pantoea agglomerans and Clavibacter michiganensis. Mol Plant Microbe Interact. 2002 Dec;15(12):1236–44. doi:10.1094/mpmi.2002.15.12.1236. [Google Scholar] [CrossRef]
78. Monneveux P, Nemmar M. Contribution à l’étude de la résistance à la sécheresse chez le blé tendre (Triticum aestivum L.) et chez le blé dur (Triticum durum Desf.étude de l’accumulation de la proline au cours du cycle de développement. Agronomie. 1986;6(6):583–90. (In French). doi:10.1051/agro:19860611. [Google Scholar] [CrossRef]
79. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–58. doi:10.5344/ajev.1965.16.3.144. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools