 Open Access
Open Access
ARTICLE
Taxonomic Status of the Neglected Ophrys sphegodes subsp. grammica in the Balkan Peninsula
1 Department of Biology and Ecology, Faculty of Sciences, University of Novi Sad, Novi Sad, 21000, Serbia
2 Department of Agricultural Botany, Faculty of Agriculture, University of Zagreb, Zagreb, 10000, Croatia
* Corresponding Author: Jovan Peškanov. Email:
(This article belongs to the Special Issue: Taxonomy, Phytogeography and Ecology of Mediterranean Flora)
Phyton-International Journal of Experimental Botany 2025, 94(6), 1769-1786. https://doi.org/10.32604/phyton.2025.065536
Received 15 March 2025; Accepted 23 May 2025; Issue published 27 June 2025
Abstract
Since its description, the taxon Ophrys sphegodes subsp. grammica has been considered endemic to Greece. The morphological and chorological data of this taxon have been overlooked because the name has been used as a synonym for O. sphegodes subsp. taurica in most publications and online databases. Recently discovered Ophrys populations in Serbia were identified as O. sphegodes subsp. grammica. As these populations represent the northernmost point of distribution of this taxon, we provided data on the morphology, flowering season, and ecology. To determine the taxonomic status of this taxon, we performed comparative morphological analyses, comparing them to other populations of affined taxa (O. sphegodes subsp. grammica, O. sphegodes subsp. taurica, and O. sphegodes subsp. sphegodes). The analysis included 23 morphometric characters, performed on a total of 120 individuals. Examined populations of O. sphegodes subsp. grammica are closely associated with populations of O. sphegodes subsp. sphegodes, with near overlap in the values of the characteristics evaluated, indicating that this subspecies is more morphologically similar to the typical one than to O. sphegodes subsp. taurica. Although O. sphegodes subsp. grammica and O. sphegodes subsp. sphegodes are morphologically similar, the flowers of the first taxon are smaller, as evidenced by the length and width of the stigmatic cavity, as well as the length of the petals and labellum. The labellum of O. sphegodes subsp. grammica is shorter than the dorsal sepal, and this feature distinguishes it from both the typical subspecies and O. sphegodes subsp. taurica, where it is longer. The results of this study clearly show that the name O. sphegodes subsp. grammica should not be equated with O. sphegodes subsp. taurica, since it is a morphologically distinct and well-defined taxon.Keywords
Given its unique relationship with pollinators, the genus Ophrys L., a member of the Orchidaceae family, has caught the interest of numerous scientists, including Darwin himself [1], as a model organism for the study of floral evolution. The species of this genus are perennial herbaceous geophytes with a predominant distribution in the Mediterranean area, and one of the centers of diversity is the Balkan Peninsula [2]. The genus Ophrys is one of the genera that have had several taxonomic treatments and the most significant increase in the number of species in recent decades [3–6]. Depending on the methodology applied in the circumscription of the genus and the applied species concepts, the number of species varies significantly. Molecular methods, primarily sequencing different parts of the nuclear and plastid genomes, recognize up to ten species [6–9]. In contrast, there are 350 species [5], of which a considerable number have been recognized and described mostly based only on morphology. Some researchers have argued that the interactions between Ophrys species and their pollinators offer more valuable insights than neutral markers due to their crucial role in the process of speciation [10,11]. Ophrys species are known to attract a limited number of pollinator species [12] by emitting floral scents that mimic sex pheromones [13,14]. Variations in these floral scents can lead to shifts in pollinator species, potentially facilitating reproductive isolation among populations of the same species and promoting speciation [15]. Taxonomic confusion arising from conflicting views regarding which criteria should be used to delimit species in this genus could have significant consequences for conservation [16]. Ophrys sphegodes complex is one of the most taxonomically difficult groups within the genus, with the number of species varying from 3 to 48 [4,17]. Many of the newly proposed taxa seem to be artificial and merely extremes in the cline of variation [18]. On the other hand, several recently recognized taxa do appear to be isolated by flowering time and pollinator specificity [18]. Since the distribution and morphology of these species are not sufficiently documented, some authors have decided to treat this entire complex of species as several subspecies of O. sphegodes [19,20]. Ophrys sphegodes subsp. grammica (B. Willing & E. Willing) Kreutz was described as a subspecies of O. mammosa Desf. [21]. Delforge et al. [22] elevated it to the species status. Antonopoulos [23] and Delforge [4] also recognized this taxon as a species. In the Catalogue of European Orchids, Kreutz [24] introduced a new nomenclatural combination: O. sphegodes subsp. grammica. However, this name is more commonly used as a synonym for O. sphegodes subsp. mammosa (Desf.) Soo ex E. Nelson [19,20]. In Niketić et al. [25], the name O. sphegodes subsp. grammica is treated as a synonym of O. sphegodes subsp. taurica (Aggeenko) Soó ex Niketić & Djordjevic, which is a new nomenclatural combination for O. sphegodes subsp. mammosa. In Serbia seven taxa have been recorded from the genus Ophrys [26], including two from the O. sphegodes complex. Morphometric studies are traditionally used in species delimitation of orchids [27–30] as well as in genus Ophrys [31–34]. Phenotypic differentiation among taxa is often more significant than genetic data [35], but using morphometric characters for taxonomic purposes is relevant in the genus Ophrys [35]. This may be attributed to the fact that morphometric traits, which are likely subject to selection pressures to align with the morphology of pollinators [36], may undergo more rapid evolutionary changes and be less affected by hybridization compared to neutral genetic markers [15].
The aims of the study were: a) to clarify the taxonomic status of Ophrys sphegodes subsp. grammica based on comparative morphological and morphometric analyses, and to compare it with O. sphegodes subsp. taurica and the type subspecies, b) to provide a morphological description and habitat preferences for O. sphegodes subsp. grammica from the northernmost point of distribution (Serbia) and c) to estimate the IUCN threatened status of this taxon, new to Serbia.
Ophrys sphegodes subsp. grammica was discovered for the first time in Serbia in April 2023, on Svrljiške Mts., and data on its distribution, habitat preference, flowering period, population size, and morphology were obtained. One specimen from each site, without underground organs for vegetative propagation, was collected and deposited in the general collection of the Herbarium of the University of Novi Sad (BUNS) [37].
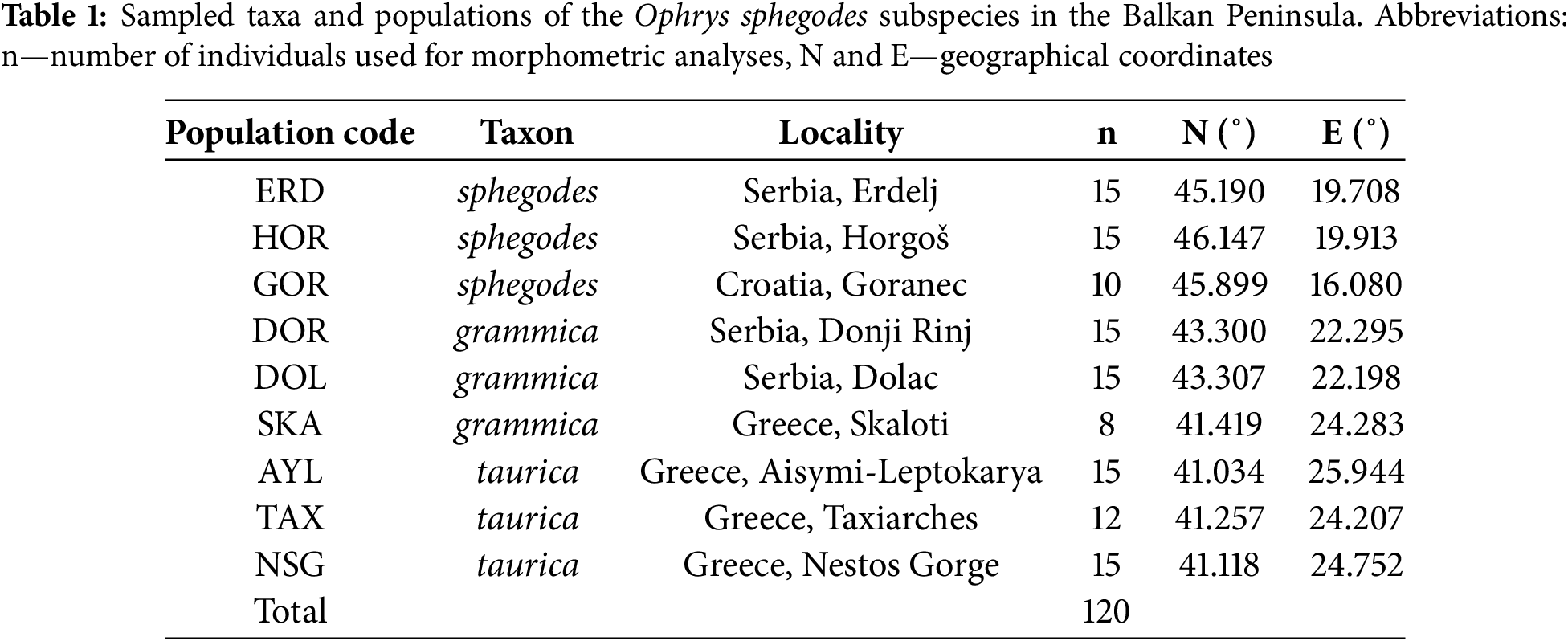
Morphological analyses were carried out on a total of nine populations and 120 individuals (Table 1). The location of the populations is given with less precise coordinates due to the high risk of endangerment of these species in nature and to prevent their exploitation. Aboveground vegetative organs of 8–15 plants from each population were measured in the field, and one well-developed flower was removed from each of them and placed in an alcohol:glycerol:water solution (4:4:2) for morphological and morphometric description. The flowers were dissected and photographed with a Leica DFC 290 HD camera (Leica Microsystems GmbH, Wetzlar, Germany). To preserve the three-dimensional shape of the dissected flowers, they were measured using a calibrated sliding scale immediately after dissection. After photographing, finer details were measured using Leica LASV software (Leica Microsystems GmbH, Wetzlar, Germany). Character selection and measurement methodology followed Bateman [6] and Paušić [38] with the exclusion of speculum characters due to a different method of flower preservation, as well as the additions of new characters made by the authors of this study. In total, 23 floral traits were measured with an accuracy of 0.1 mm.
Material identification was done according to Willing and Willing [21] and Delforge [4]. The material was also compared with the digitized holotype specimen in the herbarium of the Botanic Garden and Botanical Museum Berlin-Dahlem (B) (http://herbarium.bgbm.org/object/B100178004) (accessed on 22 May 2025) [39]. The morphological characteristics of the O. sphegodes subsp. grammica populations from Serbia were compared with literature data [4,17,19,20,23] for O. sphegodes subsp. grammica, O. sphegodes subsp. sphegodes, and O. sphegodes subsp. taurica (Table 2). The morphological description of O. sphegodes subsp. grammica from Serbia is based on our study and obtained results.
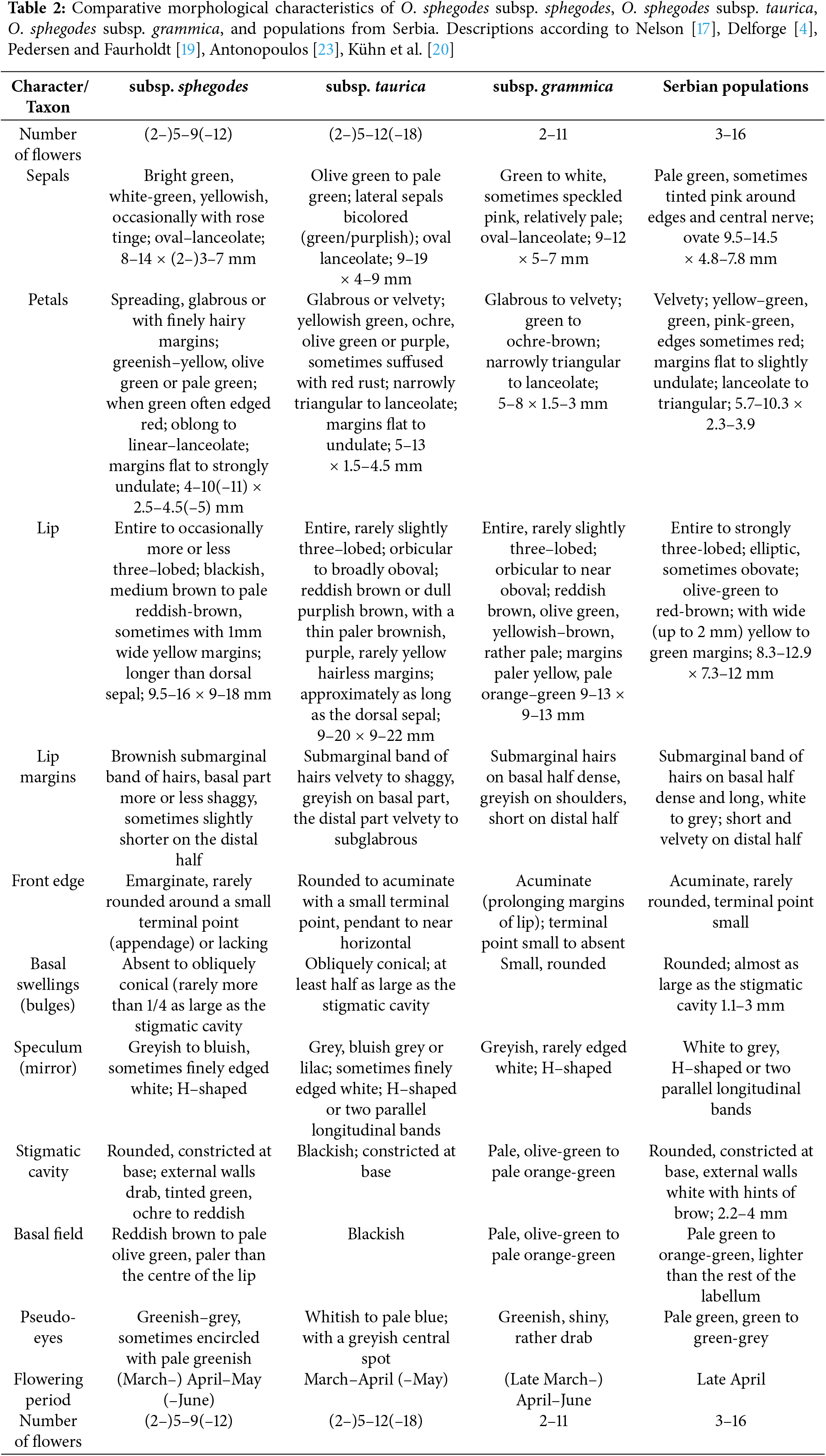
Measurement data for each plant (Table 1) were summarized in an Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA). The data collected were analysed using the software Statistica for Windows, version 13 (TIBCO Software Inc., Santa Clara, CA, USA). The mean, standard deviation, and coefficient of variation values were obtained for each morphometric trait. Statistical analyses included descriptive statistics, testing of statistical significance (ANOVA), and multivariate analyses (discriminant and principal component analysis).
The distribution of the new taxon for Serbia is presented on a map generated in QGIS 3.42 (QGIS Association, Bern, Switzerland) [40] with a 10 × 10 km grid using the Universal Transverse Mercator (UTM) projection.
The geological substrate was determined using a 1:100,000 scale geological map of the study area. Habitat type was determined using the EUNIS habitat classification [41]. The total number of individuals at each site was used to assess population size. The IUCN Red List categories and criteria [42] were used to determine the endangerment status of O. sphegodes subsp. grammica in Serbia.
3.1 Ophrys sphegodes subsp. grammica in Serbia
Ophrys sphegodes subsp. grammica (B. Willing & E. Willing) Kreutz, Kompend. Eur. Orchid.:116 (2004), Fig. 1.

Figure 1: Ophrys sphegodes subsp. grammica–Svrljiške Mts. (Eastern Serbia, 23 April 2023). Photo B. Radak
Homotypic Synonyms: Ophrys mammosa subsp. grammica B. Willing & E. Willing, Mitteilungsbl. Arbeitskreis Heimische Orchid. Baden-Württemberg 17(4):523 (1985); Ophrys grammica (B. Willing & E. Willing) Devillers-Terschuren & Devillers, Naturalistes Belges 72(3):101 (1991).
Holotype: GREECE, Kastoria, 2,8 km O Ptelea (Kas 87), Erodierte, fast bewuchsfreie Sand-steinhänge, Sa, 740 m, UTM:EK 0879, 18 May 1985, leg. B. & E. Willing 1694 (B 10 0178004!).
3.1.1 Morphological Description of Ophrys sphegodes subsp. grammica from Serbia
Plants are slender, 13–63 cm tall, with 3–16 small flowers in a loose inflorescence. Sepals pale green, lateral sepals sometimes tinged pink at margin and central nerve, ovate, 9.5–14.5 × 4.8–7.8 mm. Dorsal sepal longer than the labellum. Petals velvety, yellow–green, green to pinkish–green, margins sometimes red, lanceolate to triangular, margins flat to slightly undulate, 5.7–10.3 × 2.3–3.9 mm. Lip entire to strongly trilobed, elliptic to obovate; olive green to reddish brown; with broad yellow to green margin, acuminate, rarely rounded, terminal point small, 8.3–12.9 × 7.3–12 mm. Basal part of the lip and basal swelling with long and dense grey to white hairs. Basal swellings rounded; almost as large as the stigmatic cavity, 1.1–3.0 × 2.2–4.1 mm. Speculum white to grey, H–shaped or two parallel longitudinal bands. Stigmatic cavity rounded, constricted at base, external walls white with a hint of brown. Basal field pale green to orange–green, lighter than the rest of the lip. Pseudo–eyes pale green, green to green–grey (Table 2).
3.1.2 Habitat and Ecology of Ophrys sphegodes subsp. grammica from Serbia
In Serbia, this taxon was found on a substrate predominantly composed of layered limestones, between 294 and 884 m a.s.l. At the Donji Rinj site, the herbaceous layer covered up to 95%, dominated by the following species: Artemisia alba Turra, Carex humilis Leyss., Chamaecytisus hirsutus (L.) Link, Festuca valesiaca Schleich. ex Gaudin, Fragaria vesca L., Globularia cordifolia L., Hieracium pilosella L. Plantago media L., Potentilla incana P. Gaertn., B. Mey. & Scherb., and Satureja kitiaibelli Wierzb (ass. Potentilleto-Caricetum humilis R. Jov. 1955). Individual dwarf specimens of Crataegus monogyna Jacq and Juniperus communis L. have been recorded. According to the EUNIS classification, this habitat type belongs to Continental dry grassland (true steppe) (Code R1B). The herbaceous layer cover at sites Dolac and Ljubatovica was less substantial than the previous location (about 30%), and according to EUNIS this habitat belongs to Temperate and submediterranean thorn scrub (Code S35). The following species were identified at the site: Acer monspessulanum L., Carpinus orientalis Mill, Cornus sanguinea L., Genista tinctoria L., J. communis, Pyrus communis L., Prunus spinosa L., Quercus pubescens Willd., and Rosa canina L. The herbaceous layer consisted of: Agrimonia eupatoria L., Campanula lingulata Rchb., Clematis vitalba L, Clinopodium vulgare L., Euphorbia cyparissias L., Galium verum L., G. cordifolia, H. pilosella, and Teucrium chamaedrys L (ass. Carpinetum orientalis serbicum Rudski 1940 emend. B. Jovanović 1953).
3.1.3 Distribution of Ophrys Sphegodes subsp. grammica in Serbia
Ophrys sphegodes subsp. grammica has been recorded at three locations in the Svrljiške Mts. in Eastern Serbia (Fig. 2): Dolac, N 43.317, E 22.194, UTM EN99, limestone, 294 m a.s.l., Exp E 26°, 23 April 2023, leg. B. Radak, A.Vlku, J. Peškanov s.n. (BUNS 2-0043); Donji Rinj, N 43.300, E 22.295, UTM FN09, limestone, 865 m a.s.l., Exp S 24°, 23 April 2023, leg. B. Radak, A. Vlku, J. Peškanov s.n. (BUNS 2-0044); Ljubatovica, N 43.237, E 22.394, UTM FN18, limestone and dolomite, 350 m a.s.l., Exp S 25°, 24 April 2023, leg. B. Radak, A. Vlku, J. Peškanov s.n. (BUNS 2-0045).
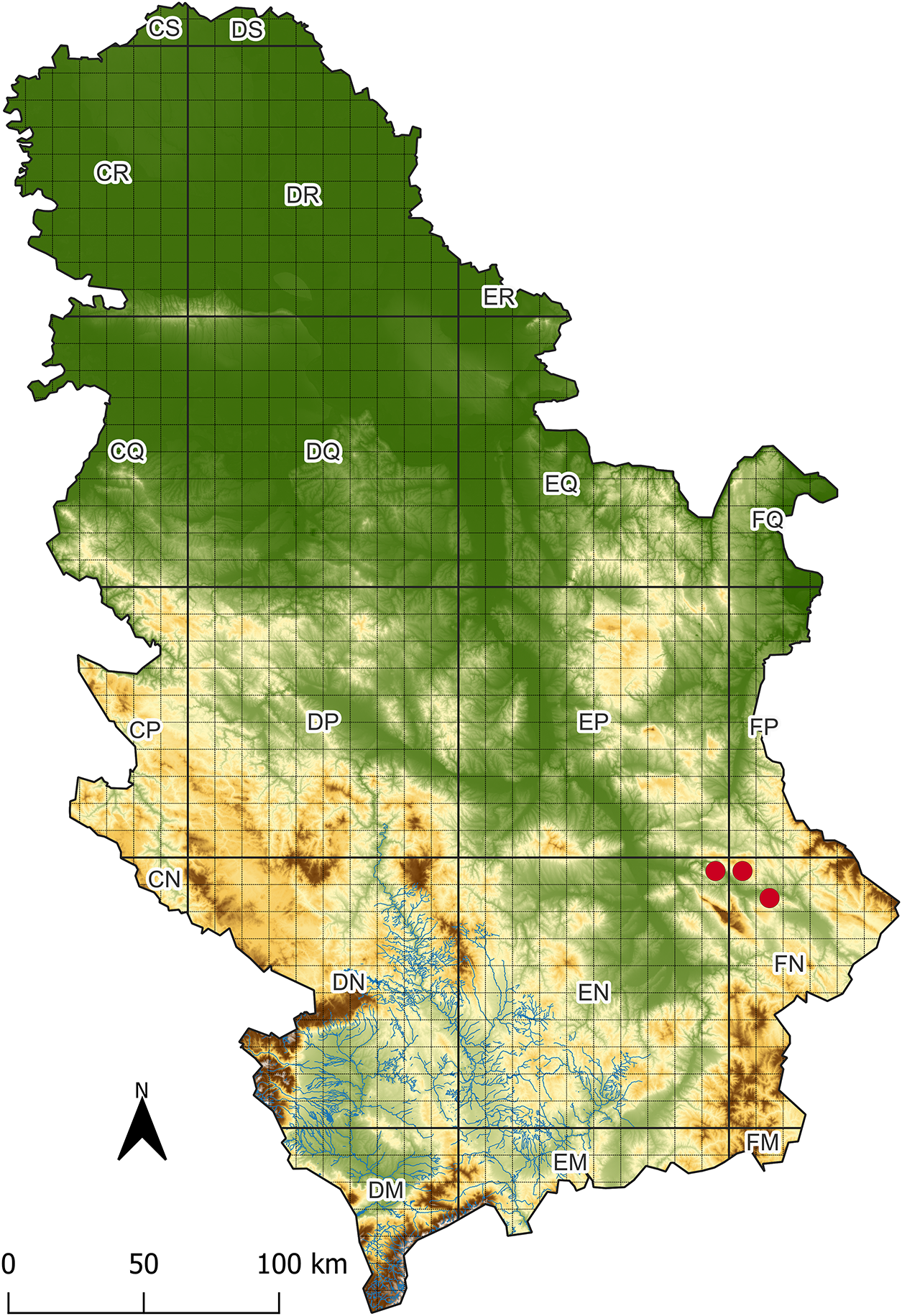
Figure 2: Distribution of Ophrys sphegodes subsp. grammica in Serbia. Map created using the Free and Open Source QGIS [40]
3.1.4 Population Size and Conservation Status of Ophrys sphegodes subsp. grammica from Serbia
In April 2023, 265 specimens of O. sphegodes subsp. grammica were found in full bloom on an area of 100 m2 near the village of Dolac. Another smaller group of 38 specimens was found not far from the previous population. Between the villages of Donji Rinj and Gornji Rinj, a total of 134 specimens were found, 34 of which had just begun to flower. The remaining specimens were found in rosettes. At the Ljubatovica site, only two specimens were found, and they were in full bloom. At all sites combined, 339 flowering specimens were found. Applying the categories and criteria of the IUCN Red List, the current threatened status of O. sphegodes subsp. grammica on the territory of Serbia is classified as Endangered (EN): B2ac(iv)+C2b. Based on the number of mature individuals and the fact that individuals of this species were found in only three locations, its area of occupancy (AOO) is estimated to be less than 500 km2. As far as we know, the threatened status of O. sphegodes subsp. grammica in Greece has not yet been determined. At all three sites, the expansion of the surrounding shrub vegetation, occurring to varying degrees, poses a potential threat to the continued existence of this species’ habitat. There is a quarry near the Dolac site, and its expansion, as well as the related operations (construction and traffic), could completely degrade the habitat at this site.
Basic statistical parameters were processed at both the taxon and population levels (Table 1). The coefficient of variation analysis reveals that the entire sample at the taxon level exhibits moderate variability (CV = 10%–30%) for the majority of the examined morphometric features (Table 3).
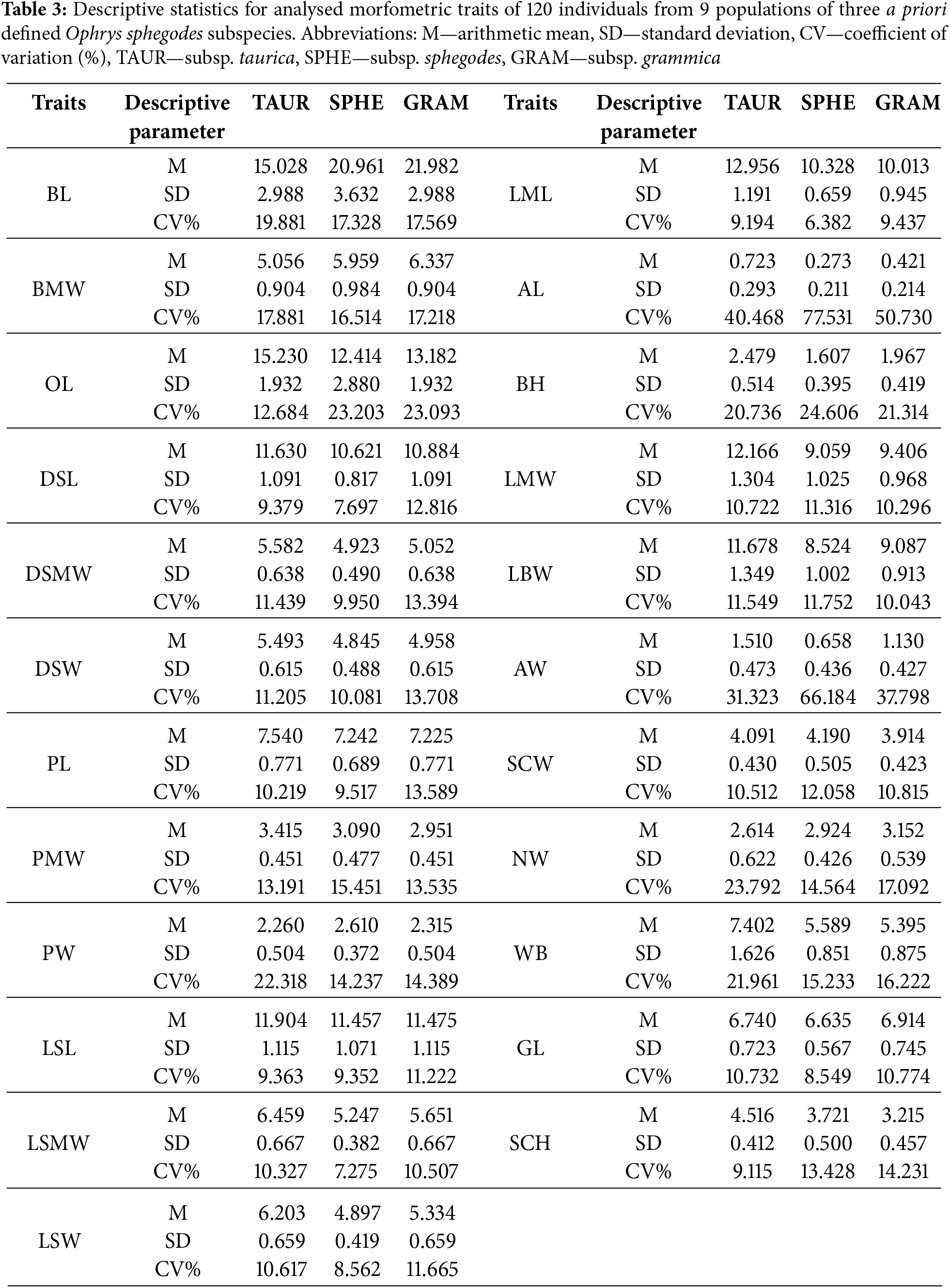
The apical appendage features are the only variables in the zone of increased variability (CV = 30%–50%). Individual populations of each species exhibit a similar pattern of variability in the examined morphometric features, with the majority of characters falling into the zone of moderate variability. Populations of O. sphegodes subsp. sphegodes has a somewhat higher proportion of characters in the low variability zone than the other two. According to the ANOVA results, the most statistically significant differences between the examined taxa occur for four characters: lip maximum length (LML), labellum width at the level of the bulges (LBW), lip maximum width (LMW), and stigmatic cavity height (SCH). For all four characters, O. sphegodes subsp. taurica has higher mean values, on average 1.5 to 3 mm higher than the other two subspecies. In comparison to O. sphegodes subsp. sphegodes, O. sphegodes subsp. grammica populations (SKA, DOR, DOL) have lower mean values for lip maximum length and stigmatic cavity height, but greater values for labellum width (Table 3). Comparing the height of bulges to stigmatic cavity height, we observed that in O. sphegodes subsp. taurica populations, bulges are always larger than half the height of the stigmatic cavity, whereas in O. sphegodes subsp. sphegodes populations, they are always smaller (Fig. 3A). Ophrys sphegodes subsp. grammica populations (SKA, DOR, DOL) have bulges that are two-thirds the height of the stigmatic cavity, with lower mean values compared to O. sphegodes subsp. taurica. Comparing the length of the labellum and the dorsal sepal, O. sphegodes subsp. taurica shows a longer labellum than the dorsal sepal; instead, O. sphegodes subsp. grammica shows the opposite pattern. The labellum length in O. sphegodes subsp. sphegodes populations have slightly higher mean values than the dorsal sepal (Fig. 3B).
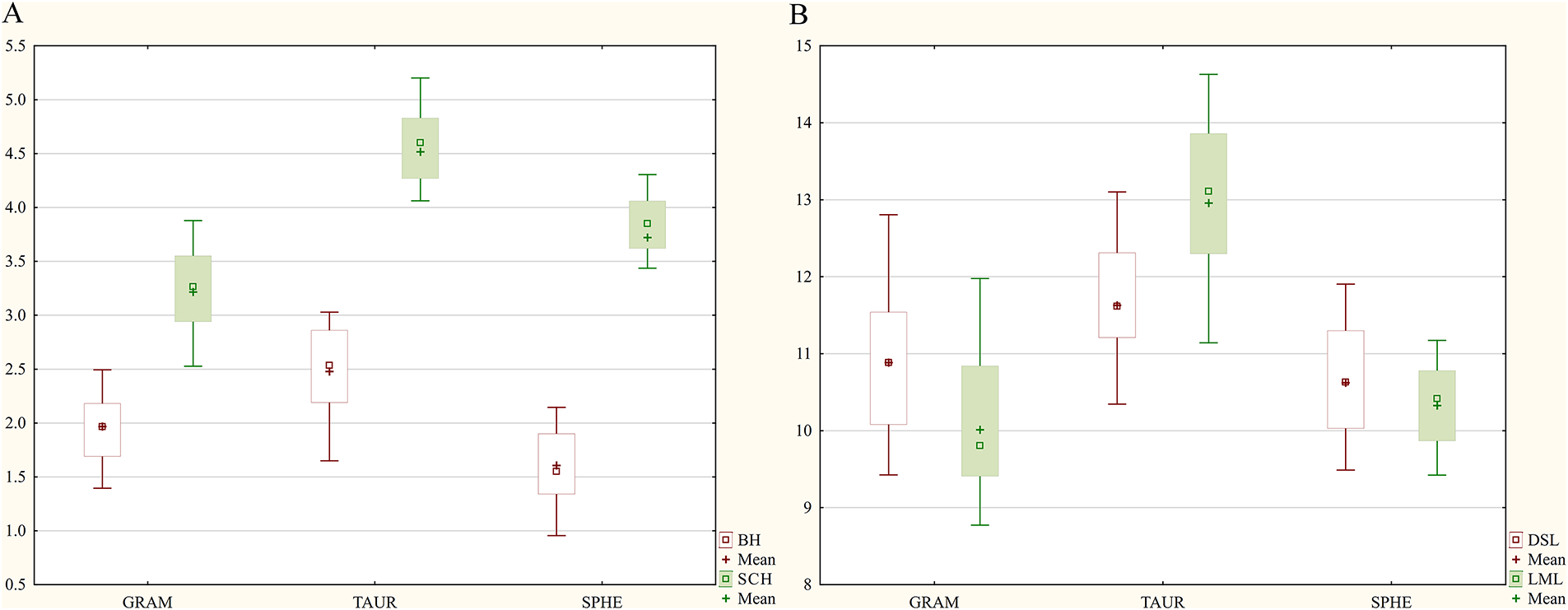
Figure 3: Box Plot of multiple variables grouped by taxon, based on 120 individuals from 9 populations of three a priori defined Ophrys sphegodes subspecies. (A) Comparative overview of morphometric characters; SCH—stigmatic cavity height and BH—bulges height; (B) Comparative overview of morphometric characters; DSL—dorsal sepal length and LML—labellum maximum length. Abbreviations: GRAM—subsp. grammica, TAUR—subsp. taurica, SPHE—subsp. sphegodes
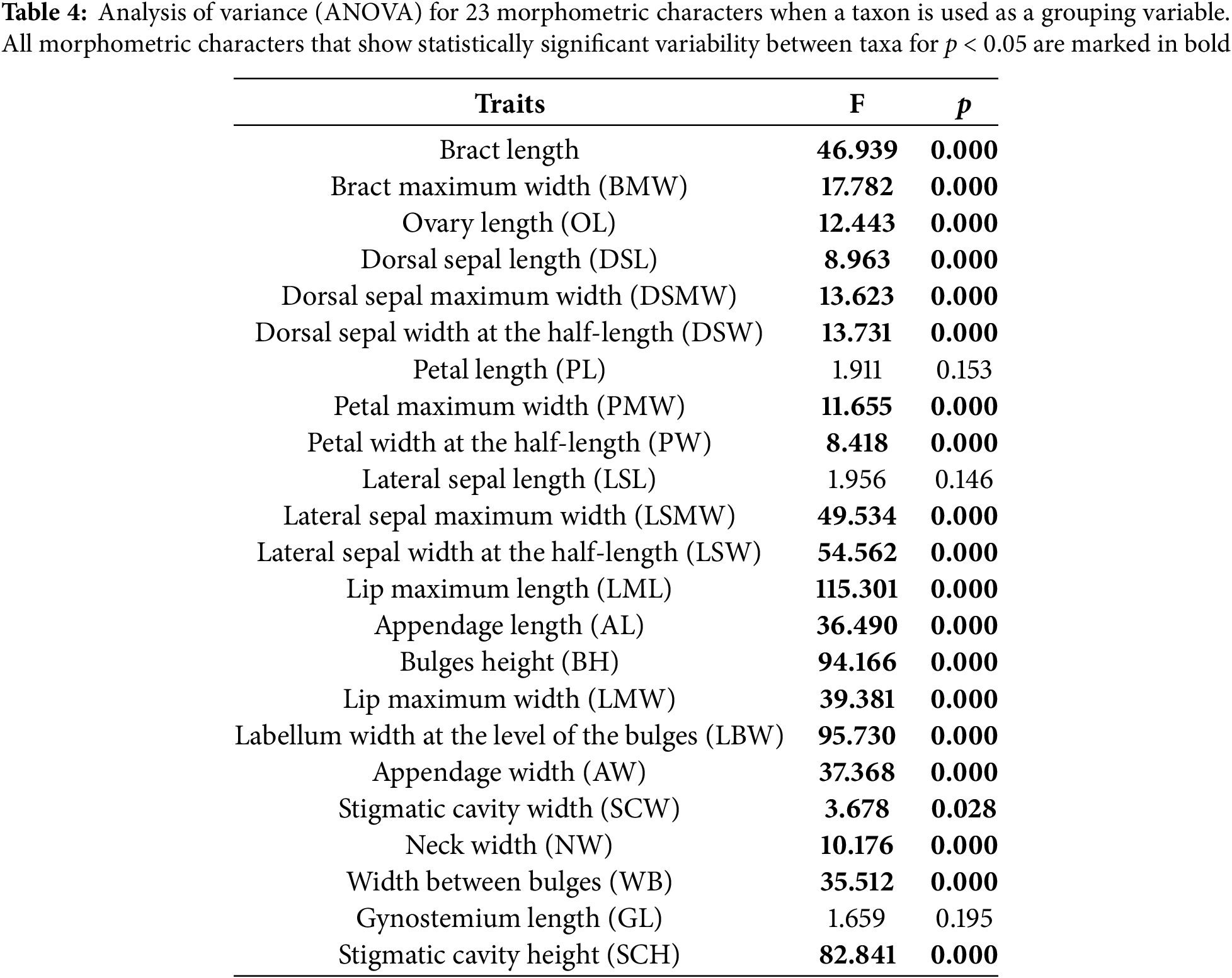
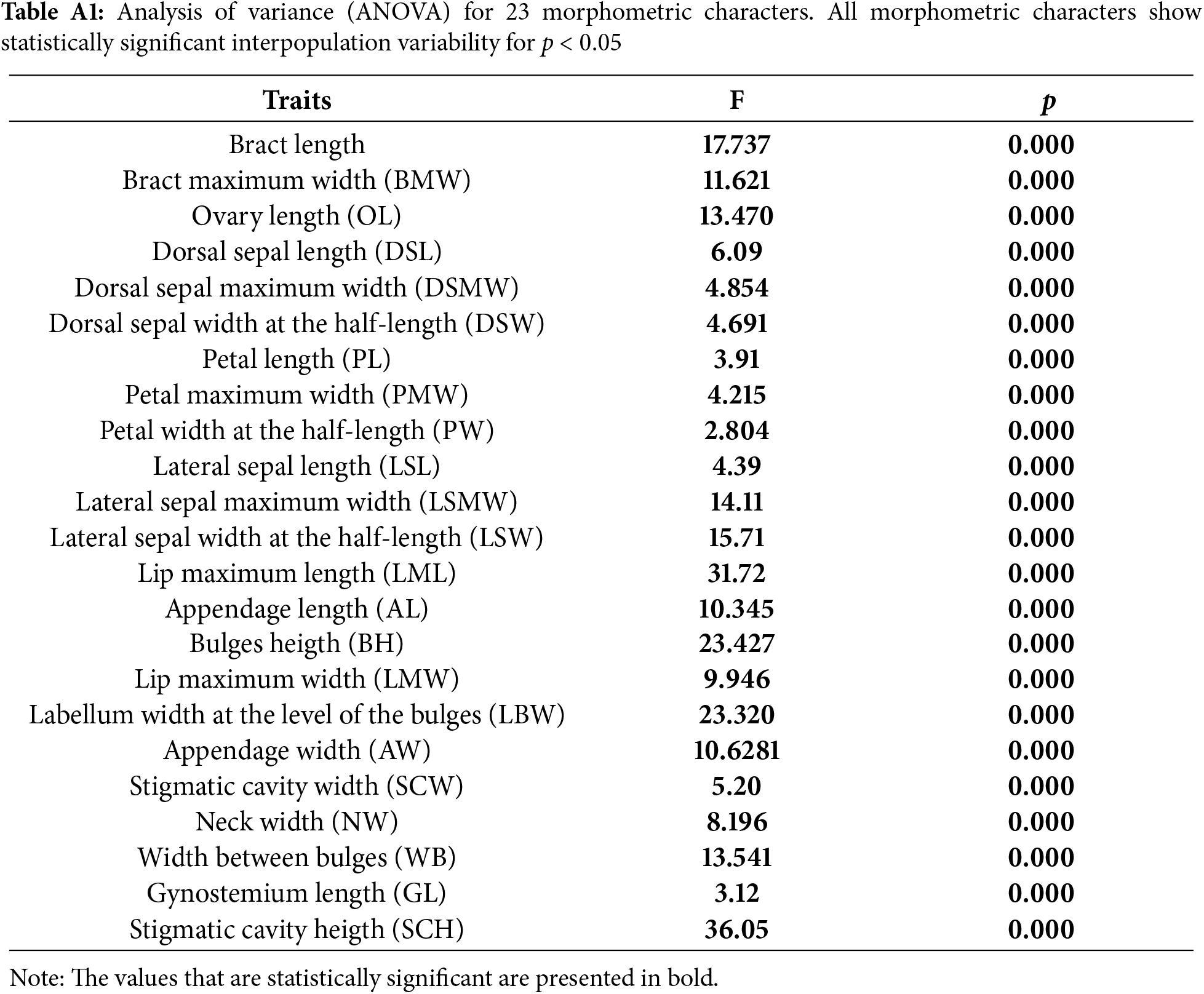
A one-factor analysis of variance was conducted on two levels: between all sampled populations at the whole sample level and between three a priori defined taxa. Each morphometric feature examined revealed considerable interpopulation variability among all populations in the total samples (Table A1). The characters that statistically differ the most between the analysed populations are: lip maximum length (LML), labellum width at the level of the bulges (LBW), lip maximum width (LMW), and stigmatic cavity height (SCH). At the taxa level, a large number of characters show statistically significant differences between the observed subspecies. The same characteristics between taxa, as at the population level, exhibit high F values. Furthermore, characters that differ statistically among the investigated taxa are: lateral sepal maximum width (LSMW), appendage length (AL), appendage width (AW), bract length (BL), and bulges height (BH) (Table 4).
3.2.3 Canonical Discriminant Analysis (CDA)
In the a priori classification, when a taxon is used as a grouping variable, only three individuals (2.50%) were determined differently; that number is slightly greater when the population is utilized and is 11.67%, i.e., 14 individuals. The first two axes defined more than 79.64% of the group discrimination (CDA 1, 65.17% and CDA 2, 14.47%) (Table 5).
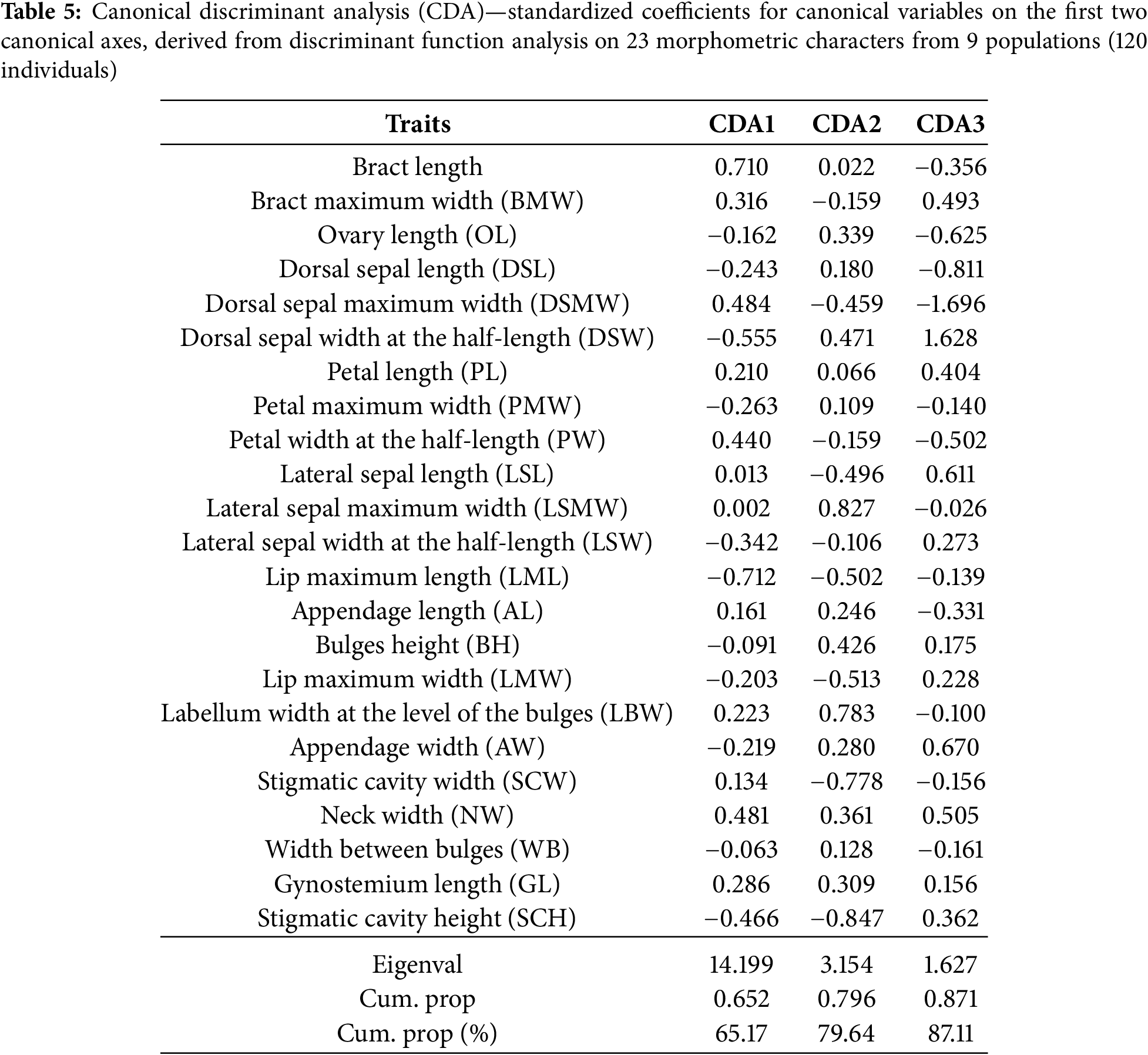
The morphological space of the first two CDA axes clearly separates the three investigated taxa. Ophrys sphegodes subsp. taurica (Aisymi-Leptokarya, Greece (AYL), Taxiarches, Greece (TAX), Nestos Gorge (NSG)) and populations of the other two taxa separate along the first axis (Fig. 4A). The following characteristics contribute the most to differentiation with respect to the first axis: lip maximum length (LML), bract length (BL), dorsal sepal width at the half-length (DSW), neck width (NW), and dorsal sepal maximum width (DSMW). The features that contribute the most to discriminating along the second axis are stigmatic cavity height (SCH), lateral sepal maximum width (LSMW), labellum width at the level of the bulges (LBW), stigmatic cavity width (SCW), and lip maximum width (LMW). Regarding this axis, the populations of O. sphegodes subsp. grammica (Donji Rinj, Serbia (DOR), Dolac, Serbia (DOL), Skaloti, Greece (SKA)) restricted to the positive space of this axis are distinguished from the populations of O. sphegodes subsp. sphegodes (Erdelj, Serbia (ERD), Horgoš, Serbia (HOR), Goranec, Croatia (GOR)). Separation between O. sphegodes subsp. sphegodes and O. sphegodes subsp. grammica was considerably more evident when a taxon was employed as the grouping variable. However, in the analysis of populations, the mixing of individuals of these two taxa appears to be restricted to a narrow zone (Fig. 4B).
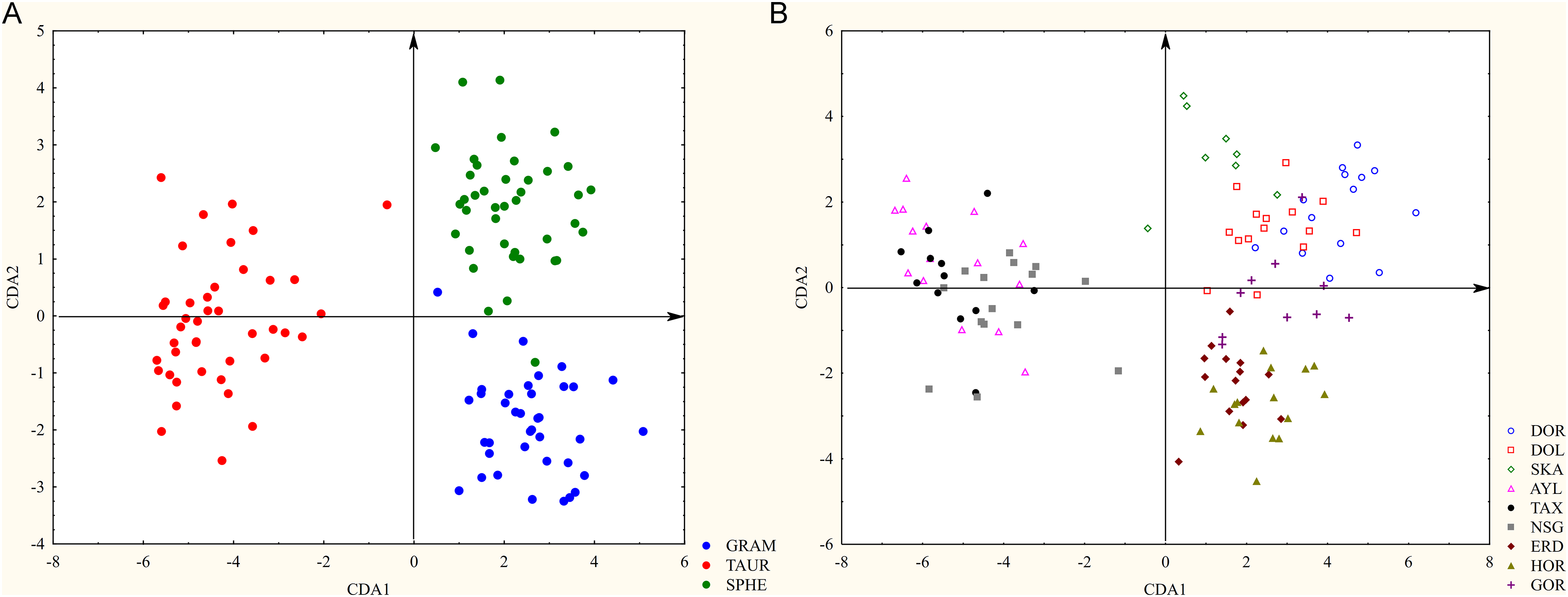
Figure 4: Canonical discriminant analyses of 120 individuals from 9 populations belonging to three Ophrys sphegodes subspecies, based on 23 morphometric characters. (A) The first two discriminant axes when a taxon is applied as a grouping variable; (B) the first two discriminant axes when a population is applied as a grouping variable. Abbreviations: GRAM—subsp. grammica, TAUR—subsp. taurica, SPHE—subsp. sphegodes
The taxon Ophrys mammosa subsp. grammica was discovered in the area of Kastoria Prefecture in northwestern Greece by Willing and Willing [21], and since then it has been considered endemic to northwestern Macedonia, with Mount Grammos as the center of its range [23]. More recently, populations of this taxon have also been recorded in other regions of Greece, on mountain ranges in eastern Macedonia [43] and in southern Pindos, the Ionian Islands, and Crete [2,23]. Although Delforge [4] suggests that this species may also occur in Albania, to our knowledge, its occurrence has not been documented in Albania, North Macedonia, or Bulgaria. Its range outside Greece has been found only in the territory of Montenegro [44,45]. The finding of the subspecies O. sphegodes subsp. grammica in the Svrljiške Mts. is its first record for Serbia. Moreover, this record represents the northernmost point of range for this taxon. According to Antonopoulos [23], O. sphegodes subsp. grammica flowers throughout May and the first half of June, where Delforge [4] gives the flowering period from late March to June. All three populations in Serbia were found in late April (23rd and 24th). The populations at Dolac and Ljubatovica, located at lower altitudes(294–350 m a.s.l.), were in full bloom, while the population at Donji Rinj (884 m a.s.l.) was just beginning to flower. The habitats where this subspecies has been discovered in Serbia are usually warm and dry, with substrates that have a slightly acidic to slightly basic reaction and are low in nutrients. The occurrence of O. sphegodes subsp. grammica in such habitats is consistent with the statements in the literature about this taxon and the genus in general. In Greece, O. sphegodes subsp. grammica grows mainly on skeletal soils formed by erosion of limestone or serpentine, It can occur on short grasslands, in shrublands, oak forests, pine forests, pine forest clearings, and alpine meadows at elevations up to 1200 m above sea level [4,23,43]. Despite having somewhat differing perspectives on the O. mammosa complex, Devillers and Devillers-Terschuren [3] and Delforge [4] both identify O. sphegodes subsp. grammica as a species within it. Although some authors split O. mammosa into several species, Pedersen and Faurholdt [19] threat the whole complex as one subspecies of O. sphegodes. Various forms of data, such as molecular, morphometric, and chemical, often do not align [35], resulting in differing interpretations of species boundaries. Methods based on genetic data are then judged as more reliable; however, speciation sometimes leaves no signature at the level of neutral markers, and in that case, neutral marker-based methods may fail to detect species boundaries by excluding the data that are the most informative [35]. Although some floral features may resemble the body forms of female wasps, other floral features most likely serve other purposes, such as effective pollinium transfer [46]. Rakosy et al. [47] proposed that there is strong pollinator-mediated selection for the labellum’s morphological characteristics, which pollinators use for functional interaction. On the other hand, relaxed selection and other stochastic factors are more likely to have an impact on features that are not utilized in effective interaction. Collecting data on the range of morphological variability, as well as their pattern of variability, is crucial to understand which traits are involved in population divergence and to establish the evolutionary scenario underlying the speciation process [48]. According to Delforge [4] pattern of coloration of O. grammica (basal field green-orange, lighter than centre of lip) could define a group consisting of O. grammica, O. herae M. Hirth & H. Spaeth, O. cretensis (H. Baumann & Künkele) Paulus, and O. caucasica Woronow ex Grossh. There is confusion in identification between O. grammica and O. herae, especially in the costal parts of Greece [23]. The main difference according to Paulus [49] between these two species is their pollinators, O. grammica is pollinated by Andrena nigroaenea, while O. herae is pollinated by Andrena thoracica. Identification of O. grammica is more certain in higher altitudes of continental Greece [2]. Apart from the different pollinators, O. herae has a larger and darker labellum and flowers earlier than O. grammica [23]. Ophrys cretensis is considered endemic to the Greek islands, occurring mainly in Crete [19]. According to Antonopoulos [23], this species flowers later than O. herae and has a different pollinator (Andreana vachali subsp. creticola). On the other hand, the presence of O. grammica in Crete was also confirmed. This calls into question the separation of these taxa, and suggests the possibility of a single widespread taxon. From the results of this study, it is evident that the populations of O. sphegodes subsp. taurica are morphologically significantly different from those of O. sphegodes subsp. grammica, and it is clear that the names of these two taxa should not be used as synonyms. Despite their geographical proximity, no O. sphegodes subsp. grammica populations from our sample, including one from Greece, were grouped with O. sphegodes subsp. taurica in the morphospace of CD analysis. The fact that a greater number of studied characters fall into the zone of intermediate variability, with values closer to the lower limits of the zone, suggests that certain morphological characteristics are rather stable. The height of O. sphegodes subsp. grammica specimens from Serbia varies considerably, as does the number of flowers, and differ from the values given in the literature for this taxon. The lateral sepals are never distinctly bicoloured, as in O. sphegodes subsp. taurica, although pink pigment occasionally occurs along the margins and central nerve, which also occurs in the type subspecies. The flower of O. sphegodes subsp. grammica are smaller than those of the other two taxa. It has a pale, predominantly olive-green lip and long, dense grey to white hairs on the shoulders and basal swellings (Fig. 1). The lip has a broad yellow-green margin and is shorter than the dorsal sepal, both characteristics that distinguish it from the other two taxa. In addition, this subspecies differs from the type subspecies by the front edge, which is acuminate. The general coloration of the flower differs markedly from O. sphegodes subsp. taurica, both in the colour of the sepal and lip, and in the colour of the basal field and pseudoeyes. The basal field is pale green or orange-green and always lighter than the rest of the lip, while the colour of the pseudoeyes is often the same as the basal field.
The results of this study show that the O. sphegodes subsp. grammica is a morphologically well-defined taxon, which is best seen from the size and shape of the labellum, as well as the colour of the basal field and labellum. This taxon should not be equatd with O. sphegodes subsp. taurica, since there is a greater number of morphological characters that clearly separate them. The near overlap in the values of analysed morphological characters with O. sphegodes subsp. sphegodes indicates that this taxon should not be treated as a species. This study provides a foundation for future research on the distribution, ecology, and morphology of the Ophrys sphegodes complex in the Balkan Peninsula. A more thorough analysis of the morphology of these taxa should reveal additional features that distinguish similar taxa in this region and help to clarify the taxonomy of this complex.
Acknowledgement: Not applicable.
Funding Statement: This research was funded by Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grants Nos. 451-03-137/2025-03/200125 & 451-03-136/2025-03/200125).
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization and methodology, Jovan Peškanov, Boris Radak; Resources, Jovan Peškanov, Sandro Bogdanović, Aleksa Vlku, Boris Radak; Formal analysis, Jovan Peškanov, Boris Radak; Writing—original draft, Jovan Peškanov, Boris Radak; Writing—review and editing, Jovan Peškanov, Sandro Bogdanović, Aleksa Vlku, Goran Anačkov, Boris Radak; Visualization, Jovan Peškanov, Aleksa Vlku; Supervision, Boris Radak. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data available within the article or its supplementary materials.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Appendix A
References
1. Darwin C. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: Murray; 1859. [Google Scholar]
2. Antonopoulos Z, Tsiftsis S. Atlas of the Greek orchids. Rethymno: Mediterraneo Editions; 2017. [Google Scholar]
3. Devillers P, Devillers-Terschuren J. Essai d’analyse systématique du genre Ophrys. Nat Belges. 1994;75:273–400. (In French). [Google Scholar]
4. Delforge P. Orchidees d’ Europe, North Africa and the Middle East. London: A&C Black Publishers Ltd.; 2006. [Google Scholar]
5. Delforge P. Orchidées d’ Europe, d’ Afrique du Nord et du Proche-Orient. Paris: Delachaux et Niestlé; 2016. [Google Scholar]
6. Bateman RM, Rudall PJ. Morphological continua make poor species: genus-wide morphometric survey of the European bee orchids (Ophrys L.). Biology. 2023;12(1):136. doi:10.3390/biology12010136. [Google Scholar] [PubMed] [CrossRef]
7. Soliva M, Kocyan A, Widmer A. Molecular phylogenetics of the sexually deceptive orchid genus Ophrys (Orchidaceae) based on nuclear and chloroplast DNA sequences. Mol Phylogenet Evol. 2001;20(1):78–88. doi:10.1006/mpev.2001.0953. [Google Scholar] [PubMed] [CrossRef]
8. Devey DS, Bateman RM, Fay MF, Hawkins JA. Friends or relatives? Phylogenetics and species delimitation in the controversial European orchid genus Ophrys. Ann Bot. 2008;101(3):385–402. doi:10.1093/aob/mcm299. [Google Scholar] [PubMed] [CrossRef]
9. Bateman RM. Two bees or not two bees? An overview of Ophrys systematics. Berichte Aus Den Arbeitskreisen Heim Orchid. 2018;35:5–46. [Google Scholar]
10. Vereecken NJ, Streinzer M, Ayasse M, Spaethe J, Paulus HF, Stökl J, et al. Integrating past and present studies on Ophrys pollination-a comment on Bradshaw et al. Bot J Linn Soc. 2011;165(4):329–35. doi:10.1111/j.1095-8339.2011.01112.x. [Google Scholar] [CrossRef]
11. Baguette M, Bertrand JAM, Stevens VM, Schatz B. Why are there so many bee-orchid species? Adaptive radiation by intra-specific competition for mnesic pollinators. Biol Rev. 2020;95(6):1630–63. doi:10.1111/brv.12633. [Google Scholar] [PubMed] [CrossRef]
12. Schatz B, Genoud D, Claessens J, Kleynen J. Orchid-pollinator network in Euro-Mediterranean region: what we know, what we think we know, and what remains to be done. Acta Oecologica. 2020;107(6):103605. doi:10.1016/j.actao.2020.103605. [Google Scholar] [CrossRef]
13. Schiestl FP, Ayasse M, Paulus HF, Lofstedt C, Hansson BS, Ibarra F, et al. Orchid pollination by sexual swindle. Nature. 1999;399(6735):421. doi:10.1038/20829. [Google Scholar] [CrossRef]
14. Ayasse M, Schiestl FP, Paulus HF, Ibarra F, Francke W. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. In: Proceedings of the Royal Society B: Biological Sciences. Royal Society; 2003. p. 517–22. doi:10.1098/rspb.2002.2271. [Google Scholar] [PubMed] [CrossRef]
15. Sedeek KEM, Scopece G, Staedler YM, Schönenberger J, Cozzolino S, Schiestl FP, et al. Genic rather than genome-wide differences between sexually deceptive Ophrys orchids with different pollinators. Mol Ecol. 2014;23(24):6192–205. doi:10.1111/mec.12992. [Google Scholar] [PubMed] [CrossRef]
16. Bertrand J, Baguette M, Joffard N, Schatz B. Challenges inherent in the systematics and taxonomy of genera that have recently experienced explosive radiation: the case of orchids of the genus Ophrys. In: Systematics and the exploration of life. New Jersey: Wiley Blackwell; 2021. p. 113–34. doi:10.1002/9781119476870.c. [Google Scholar] [CrossRef]
17. Nelson E. Gestaltwandel und Artbildung erörtert am Beispiel der Orchidaceen Europas und der Mittelmeerländer, insbersondere der Gattung Ophrys. Chernex: E. Nelson; 1962. (In Germany). [cited 2025 Jan 10]. Available from: https://books.google.rs/books/about/Gestaltwandel_und_Artbildung_Er%C3%B6rtert_a.html?id=wVEJPwAACAAJ&redir_esc=y. [Google Scholar]
18. Pridgeon AM. Genera orchidacearum. London: OUP Oxford; 1999. [Google Scholar]
19. Pedersen HÆ., Faurholdt N. Ophrys: the bee orchids of Europe. London: Royal Botanic Gardens Kew; 2007. [Google Scholar]
20. Kühn R, Pedersen H, Cribb P. Field guide to the orchids of Europe and the mediterranean. London: Kew Publishing, Royal Botanic Gardens; 2019. [Google Scholar]
21. Willing B, Willing E. Beitrag zur Orchideenflora NWGriechenlands—Kartierungsergebnisse 1984/85. Mitt Bl Arbeitskr Heim Orch Baden-Württ. 1985;17(4):508–628. (In Germany). [Google Scholar]
22. Delforge P, Devillers-Terschuren J, Devillers P. Contributions taxonomiques et nomenclaturales aux Orchidées d’Europe (Orchidaceae). Nat Belges. 1991;72(3):99–101. [Google Scholar]
23. Antonopoulos Z. The bee orchids of Greece. The genus Ophrys. Rethymno: Mediterraneo Editions; 2009. [Google Scholar]
24. Kreutz CAJ. Catalogue of European orchids. Landgraaf: Kreutz Publishers; 2004. [Google Scholar]
25. Niketic M, Tomović G, Perić R, Zlatković B, Anačkov G, Djordjević V, et al. Material on the annotated checklist of vascular flora of serbia. Nomencl, Taxono Florist Notes I. 2018;11(16):101–80. doi:10.5937/bnhmb2316057N. [Google Scholar] [CrossRef]
26. Djordjević V, Niketic M, Tomović G. Liliopsida: orchidaceae. In: Niketic M, Tomović G, editors. Kritička lista vrsta vaskularne flore Srbije 1, Lycopodiopsida, Polypodiopsida, Gnetopsida, Pinopsida i Liliopsida. Beograd: Srpska akademija nauka i umetnosti; 2018. p. 102–10. [Google Scholar]
27. Cruz-Lustre G, Batista JAN, Radins JA, González A, Borba EL. Morphometric analysis of the Habenaria parviflora complex (Orchidaceae). Plant Syst Evol. 2020;306(2):37. doi:10.1007/s00606-020-01634-2. [Google Scholar] [CrossRef]
28. de Melo MC, Borba EL. Morphological variability in rupicolous species of the Acianthera prolifera complex (Orchidaceae) occurring in southeastern Brazil. Plant Syst Evol. 2011;293(1–4):135–45. doi:10.1007/s00606-011-0435-1. [Google Scholar] [CrossRef]
29. Sumbembayev AA, Abugalieva SI, Danilova AN, Matveyeva EV, Szlachetko DL. Flower morphometry of members of the genus Dactylorhiza Necker ex Nevski (Orchidaceae) from the altai mountains of Kazakhstan. Biodiversitas. 2021;22(8):3545–55. doi:10.13057/biodiv/d220855. [Google Scholar] [CrossRef]
30. Radak BĐ, Vlku AZ, Peškanov JM, Matevski VS, Anačkov GT. Morphological characterization of three natural hybrid orchid taxa, new for Serbia, Montenegro and North Macedonia. Arch Biol Sci. 2019;71(4):596–607. doi:10.2298/ABS190520042R. [Google Scholar] [CrossRef]
31. Turco A, Medagli P, Wagensommer RP, D’Emerico S, Gennaio R, Albano A. A morphometric study on Ophrys sect. Pseudophrys in Apulia (Italy) and discovery of Ophrys japigiae sp. nov. (Orchidaceae). Plant Biosyst. 2022;156(2):560–71. doi:10.1080/11263504.2021.1897702. [Google Scholar] [CrossRef]
32. Amich F, García-Barriuso M, Crespí A, Bernardos S. Taxonomy, morphometric circumscription and karyology of the Mediterranean African representatives of Ophrys sect. Pseudophrys (Orchidaceae). Plant Biosyst. 2009;143(1):47–61. doi:10.1080/11263500802633485. [Google Scholar] [CrossRef]
33. Gölz P, Reinhard HR. Reinhard. Biostatistische Untersuchungen über Ophrys bertoloniiformis O. et E. Danesch Ber Schweiz Bot Ges. 1975;85(1):31–56. [Google Scholar]
34. Bernardos S, Crespí A, Del Rey F, Amich F. The section Pseudophrys (Ophrys, Orchidaceae) in the Iberian Peninsula: a morphometric and molecular analysis. Bot J Linn Soc. 2005;148(3):359–75. doi:10.1111/j.1095-8339.2005.00403.x. [Google Scholar] [CrossRef]
35. Joffard N, Buatois B, Arnal V, Véla E, Montgelard C, Schatz B. Delimiting species in the taxonomically challenging orchid section Pseudophrys: bayesian analyses of genetic and phenotypic data. Front Ecol Evol. 2022;10:1058550. doi:10.3389/fevo.2022.1058550. [Google Scholar] [CrossRef]
36. Triponez Y, Arrigo N, Pellissier L, Schatz B, Alvarez N. Morphological, ecological and genetic aspects associated with endemism in the Fly Orchid group. Mol Ecol. 2013;22(5):1431–46. doi:10.1111/mec.12169. [Google Scholar] [PubMed] [CrossRef]
37. Index Herbariorum [Internet]. New York, USA: New York Botanical Garden; 2016 [cited 2025 Jan 10]. Available from: https://sweetgum.nybg.org/science/ih/. [Google Scholar]
38. Paušić I, Ivajnsic D, Pipenbaher N, Bester M. Intraspecific morphological variability in the genus Ophrys (Orchidaceae) is driven by changing environments. Wulfenia. 2022;29:47–60. [Google Scholar]
39. Virtual herbaria website [Internet]. Vienna, Austria: JACQ consortium; 2004 [cited 2024 Dec 10]. Available from: https://jacq.org/. [Google Scholar]
40. QGIS Geographic Information System [Internet]. Open source geospatial foundation project. 2016 [cited 2025 Jan 12]. Available from: http://qgis.osgeo.org/en/site/. [Google Scholar]
41. Chytrtfytf M, Tichtfytf L, Hennekens SM, Knollová I, Janssen JAM, Rodwell JS, et al. EUNIS Habitat Classification: expert system, characteristic species combinations and distribution maps of European habitats. Appl Veg Sci. 2020;23(4):648–75. doi:10.1111/avsc.12519. [Google Scholar] [CrossRef]
42. IUCN Standards and Petitions Committee [Internet]. International union for conservation of nature; 2022 [cited 2024 Dec 10]. Available from: https://www.iucnredlist.org/documents/RedListGuidelines.pdf. [Google Scholar]
43. Tsiftsis S. The orchids (Orchidaceae) of E. Macedonia: distribution, ecology, and high conservation value areas [dissertation]. Thessaloniki, GR: Aristotle University of Thessalonniki; 2009. [Google Scholar]
44. Radak B, Peškanov J, Bokić B, Miljković P, Beloica J, Anačkov G. New orchid taxa for Montenegro. In: 2 Symposium Treći vek botanike u Vojvodini. Novi Sad: Matica srpska; 2023. p. 73–5. [Google Scholar]
45. Radak BĐ, Peškanov JM, Bokić BS, Anačkov GT. Exploring the orchid flora of montenegro: ten newly identified taxa. Diversity. 2025;17(5):337. doi:10.3390/d17050337. [Google Scholar] [CrossRef]
46. Gaskett AC. Floral shape mimicry and variation in sexually deceptive orchids with a shared pollinator. Biol J Linn Soc. 2012;106(3):469–81. doi:10.1111/j.1095-8312.2012.01902.x. [Google Scholar] [CrossRef]
47. Rakosy D, Cuervo M, Paulus HF, Ayasse M. Looks matter: changes in flower form affect pollination effectiveness in a sexually deceptive orchid. J Evol Biol. 2017;30(11):1978–93. doi:10.5061/dryad.q7p5j. [Google Scholar] [CrossRef]
48. Gibert A, Louty F, Buscail R, Baguette M, Schatz B, Bertrand JAM. Extracting quantitative information from images taken in the wild: a case study of two vicariants of the Ophrys aveyronensis species complex. Diversity. 2022;14(5):400. doi:10.3390/d14050400. [Google Scholar] [CrossRef]
49. Paulus HF. Deceived males-pollination biology of the Mediterranean orchid genus Ophrys (Orchidaceae). J Eur Orchideen. 2006;38:303–53. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools