 Open Access
Open Access
ARTICLE
Adaptive Responses of Secale Cereale to Moderate Soil Drought: Role of Phytohormones, Free Amino Acids, and Phenolic Compounds
1 Department of Phytohormonology, M.G. Kholodny Institute of Botany National Academy of Sciences of Ukraine, Kyiv, 01004, Ukraine
2 Educational and Scientific Center “Institute of Biology and Medicine” of Taras Shevchenko Kyiv National University, Kyiv, 03127, Ukraine
3 Institute of Plant Physiology and Genetics, National Academy of Sciences of Ukraine, Kyiv, 02000, Ukraine
* Corresponding Author: Lesya Voytenko. Email:
(This article belongs to the Special Issue: Stress Metabolites of Plants: Protective and Regulatory Functions)
Phyton-International Journal of Experimental Botany 2025, 94(7), 2195-2214. https://doi.org/10.32604/phyton.2025.067772
Received 12 May 2025; Accepted 07 July 2025; Issue published 31 July 2025
Abstract
Prolonged lack of rain and high-temperature lead to soil water deficits, inhibiting cereal crop growth in early ontogenesis and reducing grain quality and yield. Rye (Secale cereale L.) is a key grain crop, particularly in regions where wheat cultivation is challenging or unfeasible. To clarify its drought adaptation mechanisms, we analyzed the effects of moderate soil drought on growth, hormonal homeostasis, and the dynamics and distribution of free amino acids and phenolic compounds in rye at early vegetative stages and post-recovery. Drought triggered both general and organ-specific changes in endogenous phytohormones. A nonspecific response involved the accumulation of stress hormones abscisic acid (ABA) and salicylic acid (SA), alongside the suppression of growth hormones indole-3-acetic acid (IAA) and gibberellins. However, hormone dynamics and localization varied across plant organs. ABA and SA levels significantly increased in shoots of drought-stressed and recovered plants, corresponding with inhibited growth. Prolonged drought further enhanced ABA accumulation in both shoots and roots of recovered plants, while SA levels declined in roots but remained elevated in shoots. Drought also caused a substantial reduction in IAA, particularly in shoots, while gibberellins (GA3 + GA4) significantly decreased in roots. GA3 was predominant in most samples, except in the shoots of 2-day-old control plants. Post-recovery, IAA levels increased but remained below control values, while GA4 accumulation in roots led to a rise in total gibberellin levels. In contrast, shoot GA3 + GA4 levels declined, primarily due to GA3 reduction. The dominant free amino acids: aspartic acid, glutamic acid, glycine, alanine, and leucinedecreased significantly, underscoring their key role in stress adaptation. Increased flavonoid accumulation, especially in roots, suggests their involvement in antioxidant defense against oxidative stress. A significant increase in ABA and SA levels, along with a marked reduction in IAA and GA content in stressed rye plants occurred alongside a reduction in free amino acid content, accumulation of phenolic compounds, and an increase in flavonoid levels. These findings indicate distinct adaptation strategies in rye shoots and roots under moderate soil drought. They provide a foundation for further research on drought resistance mechanisms in cereals and the development of strategies to enhance their adaptive potential.Keywords
The prolonged absence of precipitation and rising environmental temperatures due to global warming led to soil water shortages, limiting the availability of moisture for plant root systems. These adverse conditions inhibit the early growth and development of cereal crops, reducing grain quality and yield [1–4]. Drought slows shoot growth, restricts carbon dioxide supply to chloroplasts, disrupts photosynthesis, reduces chlorophyll and relative water content in leaves, and alters protein and sugar composition [5,6]. To compensate for water scarcity, plants develop additional roots, increasing the root-to-shoot ratio [7].
Winter rye (Secale cereale L.) is an important cereal crop, particularly in regions where wheat cultivation is difficult or impractical [8]. It possesses a variety of physiological and morphological adaptations that contribute to its notable drought tolerance, with root system architecture playing a central role. Under water-limited conditions, rye develops deeper and more highly branched root systems, enabling it to access moisture from deeper soil layers [9]. This characteristic makes rye especially well-suited for cultivation on sandy, nutrient-poor soils with low water retention capacity [2]. Traditionally grown in northern latitudes, rye is distinguished by its exceptional frost tolerance—the highest among winter cereals [10].
Nevertheless, climate change has emerged as a significant threat to rye production. Despite its resilience to freezing temperatures, rye is vulnerable to temperature fluctuations, changing precipitation patterns, and extreme weather events [11]. In particular, the increasing frequency and severity of droughts due to climate change are projected to reduce rye yields in Central and Eastern Europe significantly [2]. These challenges highlight the need for further research into the physiological and biochemical traits that could serve as reliable indicators of drought tolerance.
Phytohormones regulate adaptive responses, enabling plants to cope with water deficits [12]. Abscisic acid is particularly crucial, as it governs root elongation and branching [13]. ABA interacts with other hormones to modulate root architecture, enhancing drought resilience [14]. Stomatal regulation is vital in drought response, as stomatal closure minimizes transpiration and conserves water. ABA plays a primary role in this process by signaling guard cells to close stomatal pores in response to water stress [15]. Cereals respond to drought stress by accumulating osmolytes such as proline and soluble sugars, which contribute to stomatal regulation [16]. Increased stomatal closure during drought is linked to the synergistic interaction between nitric oxide and ABA [17]. Proline and glycine-betaine aid osmotic regulation by stabilizing proteins and cellular structures, with their synthesis stimulated by ABA and other hormones [18]. ABA also plays a long-term role in stress response by regulating gene expression, leading to the production of protective compounds such as dehydrins, antioxidants (glutathione and ascorbate), and proline [19].
Besides ABA, auxins, particularly indole-3-acetic acid, contribute to drought tolerance by regulating root elongation and lateral root formation, improving water uptake [20]. Gibberellins, which primarily promote growth, influence drought response by modulating the balance of other hormones, including ABA and IAA, thereby indirectly enhancing drought tolerance [21,22]. One key effect of GA is stimulating water uptake by increasing the concentration of osmotically active substances [23]. Additionally, salicylic acid has been found to mitigate drought effects in wheat by boosting antioxidant enzyme activity and stress protein biosynthesis [24,25].
The interactions between phytohormones involve complex feedback mechanisms. For instance, high ABA levels can suppress gibberellin synthesis, affecting plant growth under drought conditions [26]. The interplay between ABA and IAA is crucial in regulating root growth and stomatal function, optimizing water use efficiency [27]. Overall, phytohormonal regulation is a highly dynamic and hierarchical process, enabling plants to adjust to environmental stressors. By coordinating root development, stomatal closure, and osmotic balance, phytohormones play a crucial role in drought adaptation. A deeper understanding of these interactions is essential for developing drought-resistant cereal varieties through genetic and agronomic strategies.
Water shortage-induced stress triggers significant metabolic changes in plants, primarily due to the accumulation of reactive oxygen species (ROS), which lead to lipid membrane peroxidation and the degradation of nucleic acids and proteins. To counteract oxidative stress and maintain cellular homeostasis, plants activate antioxidant defense mechanisms composed of both enzymatic and non-enzymatic components. Free amino acids and phenolic compounds play a crucial role in this response, with their qualitative and quantitative profiles undergoing notable changes under stress conditions. These shifts make them valuable markers for assessing the intensity and severity of stress responses. Under drought conditions, plants actively accumulate free amino acids, which help mitigate oxidative stress and maintain cellular homeostasis [28]. Cereals like wheat and maize exhibit increased levels of specific free amino acids, notably proline, which functions as an osmoprotectant and antioxidant [29]. A significant increase in proline content, particularly in shoots, aids in osmotic balance and protection against oxidative damage [30]. Drought-tolerant genotypes exhibit higher free AA levels and accumulate them more effectively than sensitive varieties [31]. Drought conditions induced an increase in both free and bound phenolic acids with a notable rise in the flavonoid isoorientin, enhancing the plant’s antioxidant capacity [32]. Phenolic compounds activate the absorption of reactive oxygen species, stabilize membranes, and prevent lipid peroxidation, protein denaturation, and DNA damage [33]. The shifts in AA and phenolic compound dynamics reflect metabolic adjustments aimed at enhancing plant survival under drought stress.
Studies on the influence of endogenous phytohormones on the accumulation of secondary metabolites in cereals are lacking. However, there are a few isolated reports on the effects of exogenous growth regulators on the accumulation of free amino acids, phenols, and flavonoids. For instance, the application of abscisic acid (ABA) and benzyladenine has been reported to promote proline accumulation and support osmotic adjustment in wheat [34]. In barley, exogenous melatonin (MEL) application under water stress was found to enhance antioxidant capacity [35]. Similarly, exogenous application of MEL and indole-3-acetic acid (IAA) increased total phenolic content in wheat, thereby enhancing antioxidant activity and stress tolerance [36]. Treatment with the strigolactone analog GR24 under drought stress also elevated total phenolic content in maize leaves, strengthening the antioxidant defense system [37].
Winter rye was selected due to its active secondary metabolism and the limited understanding of its stress adaptation mechanisms compared to other cereals [38,39]. This study aimed to examine the impact of moderate soil drought on rye growth, hormonal balance, and the dynamics of free amino acids and phenolic compounds during early vegetative stages and recovery, with the goal of clarifying their roles in drought adaptation.
2.1 Plant Material and Experimental Design
We examined 18 and 21-day-old plants of winter rye (Secale cereale L. cv. ‘Boghuslavka’), a domestically developed Ukrainian genotype. ‘Boghuslavka’ was created by researchers at the Institute of Plant Physiology and Genetics of the National Academy of Sciences of Ukraine and the Nosivka Breeding and Research Station of the Chernihiv Institute of Agroindustrial Production of the National Academy of Agrarian Sciences of Ukraine. It has been officially registered and introduced into agricultural production. This mid-season variety exhibits moderate drought, frost, and disease resistance and is widely cultivated across Ukraine. Given the current challenges posed by land contamination and degradation due to Russian aggression, studying the biological characteristics of ‘Boghuslavka’ as a resilient grain crop is highly relevant.
Calibrated seeds were sterilized in 70% ethanol for no more than 1–2 min and rinsed with distilled water. They were then soaked in water for 24 h at 21°C in a thermostat. Germinated seeds were sown in plastic containers containing 2 kg of coarse-grained white calcined river sand. Plants were grown under controlled conditions: 20°C, light intensity 190 μmol·m−2·s−1, 16/8 h (day/night) photoperiod, 65 ± 5% relative humidity, and 60% substrate moisture capacity. They were watered daily with 50 mL of Knop’s solution per vessel.
2.2 Drought Stress Simulation and Sampling
As a control, we served unstressed 18-day-old and 21-day-old plants. To simulate drought conditions, watering was withheld from 14-day-old plants for four days, leading to all leaves wilting and a 50% reduction in substrate moisture content. Watering resumed on day 18 (2–3 leaf stage), and plants continued to grow under the same conditions until day 21 (3–4 leaf stage). Shoots and roots from 18 and 21-day-old unstressed and stressed plants were analyzed.
2.3 Phytohormone Extraction and Analysis
Shoots and roots of fresh weight (two g of crude substance each) were frozen in liquid nitrogen, homogenized, and extracted in 10 mL of methanol: distilled water: formic acid (15:4:1) for 24 h at +4°C in the dark. Extracts were centrifuged at 15,000 rpm for 30 min at 0°C and evaporated to 5 mL using a vacuum evaporator at +40°C. Further purification was performed using a C18 Sep-Pak Plus cartridge (Waters, Framingham, MA, USA) to remove lipophilic substances, proteins, and pigments. Phytohormones were adsorbed with an Oasis MCX cartridge (6 cc/150 mg, Waters) and eluted in 100% methanol (acidic fraction) following Kosakivska et al. [40].
Phytohormone quantification was performed using high-performance liquid chromatography (HPLC) on an Agilent 1200 LC/MS system (Agilent, Santa Clara, CA, USA) equipped with a G1315B diode array detector and an Agilent G6120A single quadrupole mass spectrometer. For IAA, ABA, GA3, and cytokinins, chromatographic separation was achieved using an Agilent ZORBAX Eclipse Plus C18 (4.6 mm × 250 mm, 5 μm) column. For salicylic acid (SA) quantification, an Agilent ZORBAX Eclipse Plus C18 SS (3.0 mm × 150 mm, 3.5 μm) column was used [40]. Calibration curves were constructed using unlabeled ABA, SA, IAA, GA3, and GA4 standards (Sigma-Aldrich, St. Louis, MO, USA). The mass spectrometer operated in a negative electrospray ionization mode. Data analysis and hormone quantification were performed using Agilent OpenLAB CDS ChemStation Edition (rev. C.01.09).
2.4 Free Amino Acid Determination
Free amino acid (AA) content was determined in shoot and root samples of 18-day-old rye seedlings, which were previously oven-dried at 60°C until a constant weight was achieved. One gram of dry tissue was homogenized in a 3% (w/v) sulfosalicylic acid solution, followed by incubation and centrifugation at 4000 rpm for 30 min at 4°C to obtain the supernatant containing free AAs. The extraction procedure and subsequent ion-exchange liquid chromatography with post-column derivatization using ninhydrin were carried out according to the methods described by Hare et al. [41] and Ng et al. [42]. Amino acid detection was performed using a T 339 amino acid analyzer (Mikrotechna Praha, Prague, Czech Republic). Results are expressed in micromoles per gram of dry weight (μmol·g−1 DW).
2.5 Total Phenol and Flavonoid Determination
One gram of absolutely dried shoots and roots was used to determine the total phenolic and flavonoid contents. The total phenolic content was assessed using the Folin-Ciocalteu reagent according to the method described by Bobo-García et al. [43]. Extraction was performed using 80% methanol solution. The concentration of phenolics was calculated using a gallic acid calibration curve, and the results were expressed as mg of gallic acid equivalent per gram of dry weight (mg GAE/g). Total flavonoid content was determined based on its reaction with zirconyl (IV) nitrate hydrate, following the method of Smirnov et al. [44], and quantified using a rutin calibration curve, expressed as mg rutin equivalent per gram of dry weight (mg RE/g DW). The optical density of the samples was measured using a Jenway UV-6850 spectrophotometer (Gransmore Green, UK) at wavelengths of 750 nm for phenolics and 397.6 nm for flavonoids.
All experiments were conducted in three biological replicates, each independently repeated three times. Data were visualized using Microsoft Excel in Microsoft Office 2021 (Redmond, WA, USA), and statistical analysis was performed with SPSS 16.0 (IBM SPSS Statistics, Chicago, IL, USA). The effect of treatment on measurements was analyzed using one-way ANOVA, with p ≤ 0.05 (Tukey’s test) considered statistically significant. Results are presented as mean ± standard error (±SE).
3.1 Growth Parameters of Winter Rye after Soil Drought
Moderate soil drought suppressed the growth of winter rye. In 18-day-old plants, shoot height decreased by 13.8%, while root length remained unchanged. After irrigation resumed, shoot height increased by 32% but remained 7.5% lower than in control plants. Root length increased by 9.3% during recovery and remained within control values. Drought reduced FW by 24.6% in shoots and 22.5% in roots. DW decreased by 6.7% only in roots, while shoot DW remained at control levels. After rewatering, FW and DW accumulation in shoots increased by 45.6% and 17.0%, respectively, but were still 7.4% and 9.9% lower than in controls. In contrast, root FW and DW increased by 35.5% and 15.9%, reaching control values (Fig. 1). These results indicate that shoots of young ‘Boghuslavka’ rye plants were more vulnerable to drought, while roots exhibited greater drought resistance, as seen in their similarity to control plants.
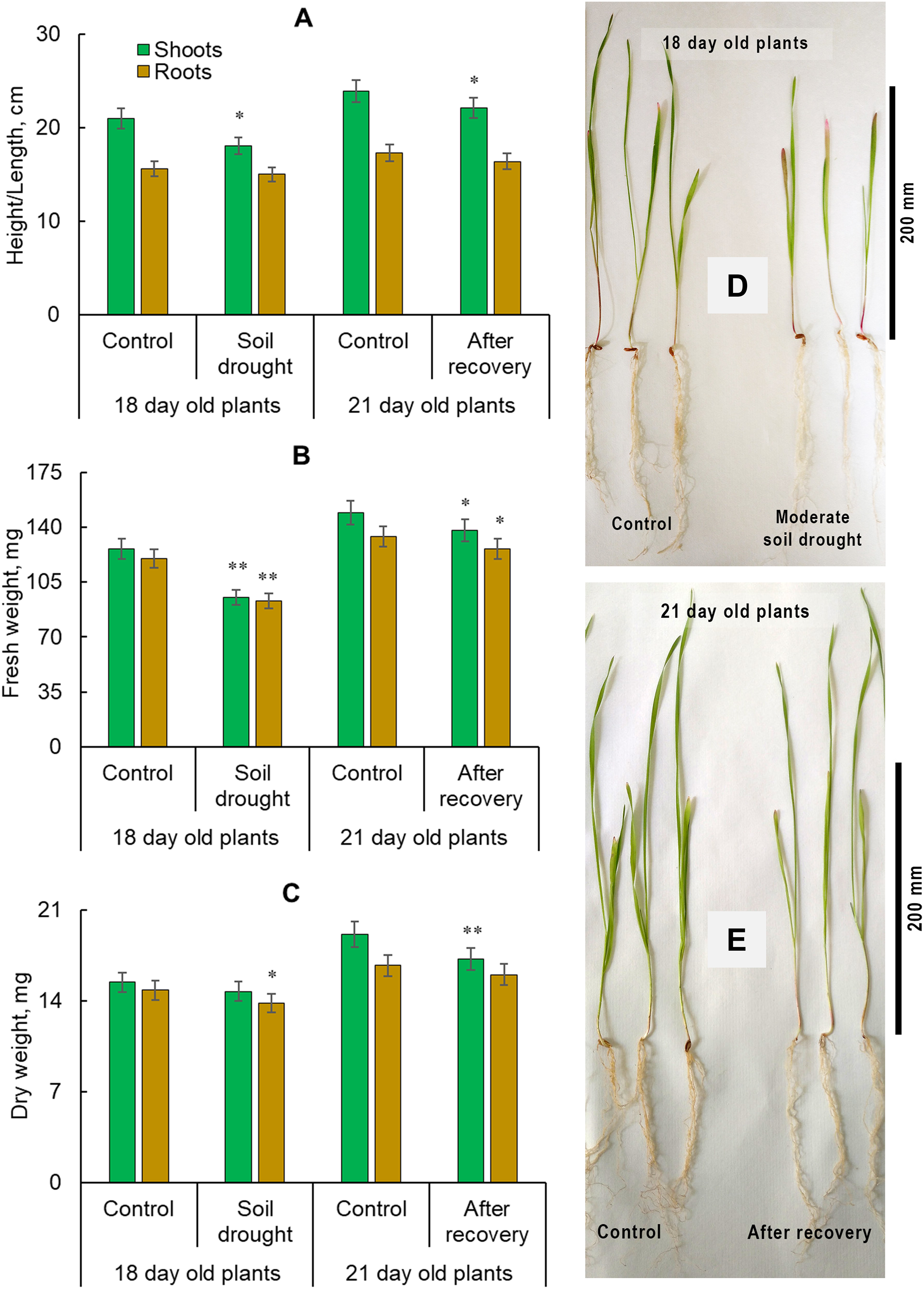
Figure 1: Effect of moderate soil drought (four days without watering) on the growth of 18-day-old Secale cereale L. cv. ‘Boghuslavka’ and on 21-day-old plants after recovery. (A) shoot height and root length, (B) shoot and root fresh weight, (C) shoot and root dry weight, (D) control and drought-stressed 18-day-old plants, (E) control and recovered 21-day-old plants. Significance at *p < 0.05 and **p < 0.01 compared with the control for each group of plants; n = 90; x ± standard error (SE)
3.2 Accumulation and Distribution of Endogenous Phytohormones in Winter Rye after Soil Drought
Abscisic Acid. Under control conditions, ABA was primarily found in shoots of 18-day-old plants, but by day 21, its accumulation shifted to roots. ABA content in 21-day-old control shoots was 161.7 ng·g−1 DW, 1.6 times lower than in 18-day-old plants, while root ABA content was 272.1 ng·g−1 DW, 1.2 times lower than at day 18. The total ABA content decreased from 483.3 ng·g−1 DW (day 18) to 433.8 ng·g−1 DW (day 21). Moderate soil drought significantly increased ABA levels by 75.8% in shoots and 36.1% in roots in 18-day-old plants, with the shoots being the primary site of accumulation. After rewatering, ABA levels in 21-day-old plants remained higher than in controls, exceeding control values by 2.3 times in shoots and 1.1 times in roots, indicating a prolonged drought effect. While shoots retained high ABA levels, root ABA content returned closer to control values (Fig. 2A). The total ABA content in 18-day-old stressed plants was 829.8 ng·g−1 DW, decreasing to 651.4 ng·g−1 DW in 21-day-old recovered plants. These results confirm that ABA accumulation is a key drought response, with more pronounced changes occurring in shoots.
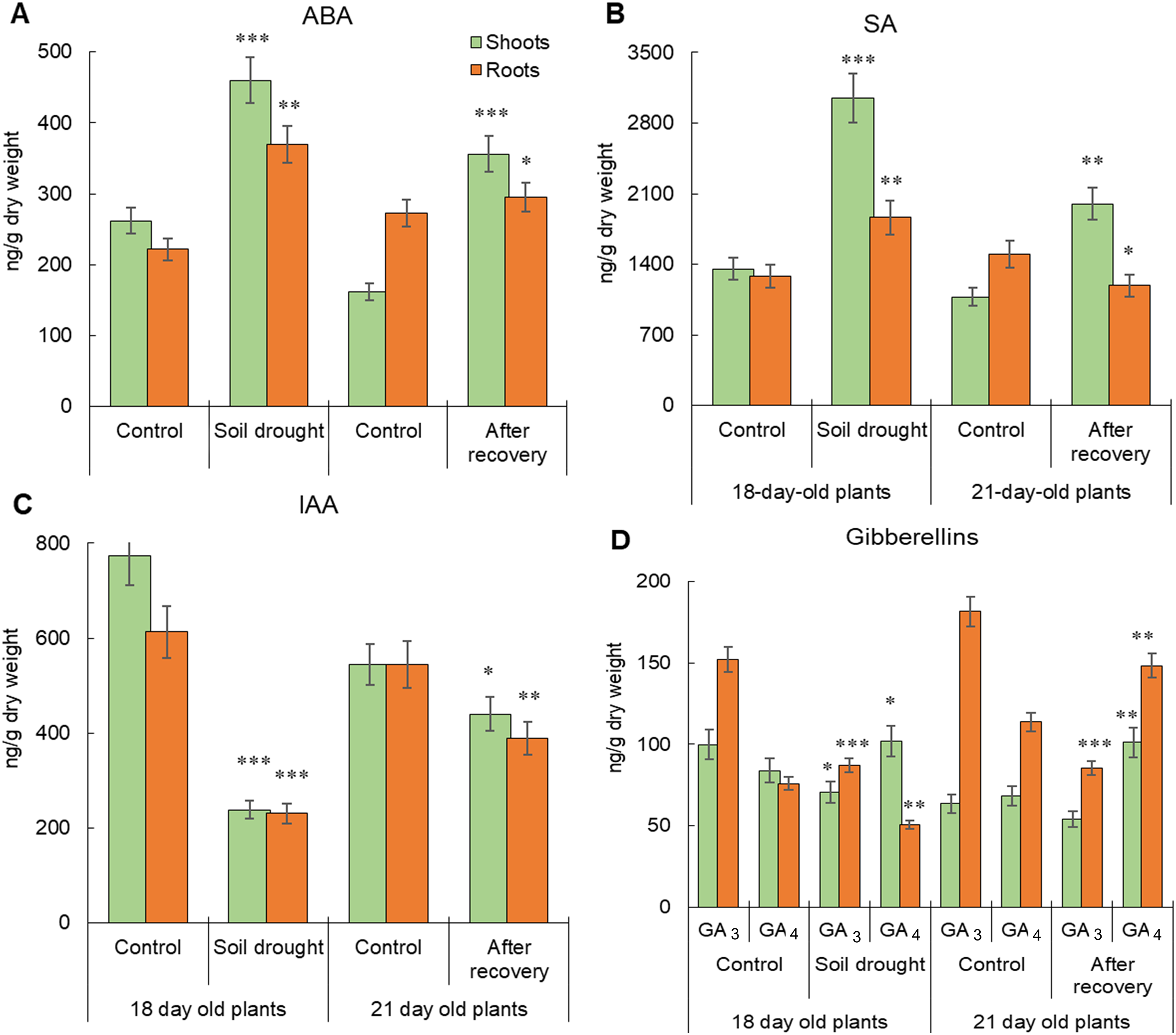
Figure 2: Accumulation and distribution of endogenous phytohormones in 18-day-old Secale cereale L. cv. ‘Boghuslavka’ after moderate soil drought and in 21-day-old plants after recovery (ng·g−1 DW). Significance at *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the control; n = 3; x ± standard error (SE)
Salicylic Acid. SA was present in high concentrations in both control and stressed plants, with shoots being the primary site of accumulation in 18-day-old control plants. Under drought, SA levels increased by 2.3 times in shoots and 1.5 times in roots. The maximum SA concentration in stressed shoots was 3051.0 ng·g−1 DW, 1.6 times higher than in roots. In 21-day-old control plants, SA accumulation shifted to roots, reaching 1502.4 ng·g−1 DW, 1.4 times higher than in shoots. After rewatering, SA levels in 21-day-old shoots nearly doubled, while root SA decreased by 1.3 times. However, SA content in recovered plants remained lower than in 18-day-old stressed plants by 34.5% in shoots and 36.2% in roots (Fig. 2B). The total SA content in 18-day-old stressed plants was 4885.2 ng·g−1 dry weight. In 21-day-old recovered plants, the hormone content decreased to 3188.6 ng·g−1 dry weight. These findings suggest that drought-induced SA accumulation primarily occurs in shoots, with an organ-specific response during recovery: shoots continue accumulating SA, whereas root SA levels decline.
Indole-3-Acetic Acid. Under control conditions, IAA was most abundant in shoots of 18-day-old plants (773.3 ng·g−1 DW), 20.4% higher than in roots. By day 21, IAA levels declined in both shoots and roots, equalizing at 544.0 ng·g−1 DW. Under the influence of drought, the level of IAA decreased by 3.3 times in shoots and by 2.3 times in roots of 18-day-old plants, which amounted to 237.9 ng·g−1 dry weight and 230.9 ng·g−1 dry weight, respectively. After recovery, IAA levels increased but remained lower than in control plants, with shoot IAA 19.1% lower and root IAA 28.6% lower than in 21-day-old controls (Fig. 2C). The total IAA content in control 18-day-old plants was 1386.1 ng·g−1 DW, while in stressed plants it was three times lower (468.8 ng·g−1 DW). By day 21, the total IAA in recovered plants (828.9 ng·g−1 DW) remained 1.3 times lower than in control 21-day-old plants (1088.2 ng·g−1 DW). These results indicate that drought significantly reduces IAA accumulation, especially in shoots, and full recovery does not occur by day 21.
Gibberellins. Under control conditions, gibberellins were predominantly accumulated in the roots of both 18 and 21-day-old plants. GA3 was dominant, except in 21-day old control shoots. Drought decreased total GA3 + GA4 levels by 1.1 times in shoots and 1.7 times in roots. Specifically, GA3 content dropped by 29.4% in shoots and 42.8% in roots, while GA4 increased by 21.5% in shoots and decreased by 34.0% in roots. Unlike controls, where GA3 + GA4 accumulation was primarily in roots, drought caused higher gibberellin levels in shoots. By day 21, GA3 + GA4 increased by 70.5% in roots, but only due to GA4. In contrast, shoot GA3 + GA4 levels declined by 10.1%, mainly due to GA3 reduction (Fig. 2D). Overall, post-recovery, GA3 + GA4 content in roots was 20.9% lower, while in shoots, it was 17.8% higher than in controls. The changes in roots were driven by GA3 reduction and GA4 accumulation, while in shoots, GA4 was the primary contributor.
Thus, moderate soil drought induced the accumulation of stress-related phytohormones ABA and SA in both shoots and roots of 18-day-old plants, while growth-promoting hormones (IAA and gibberellins) declined. During recovery, IAA and gibberellin levels increased but did not reach control values. Conversely, ABA and SA levels decreased but remained elevated compared to controls, highlighting the prolonged impact of drought stress.
3.3 Dynamics and Distribution of Free Amino Acids in Winter Rye after Soil Drought
Eighteen free amino acids were identified in the shoots and roots of 18-day-old rye plants, including 17 major proteinogenic AAs and the non-protein γ-aminobutyric acid (GABA). The total free AA content was higher in shoots than in roots under both control and drought conditions. Moderate soil drought reduced total free AA content by 31% in shoots and 41% in roots. Under both control and drought conditions, aspartic acid, glutamic acid, glycine, alanine, and leucine were predominant in shoots, while glutamic acid and alanine dominated in roots. Under drought stress, most free AAs in shoots decreased by 30%–60%, with notable reductions in GABA (61%), isoleucine (49%), methionine and lysine (47%), leucine (46%), and threonine (43%). However, arginine and histidine levels remained unchanged (Fig. 3A).
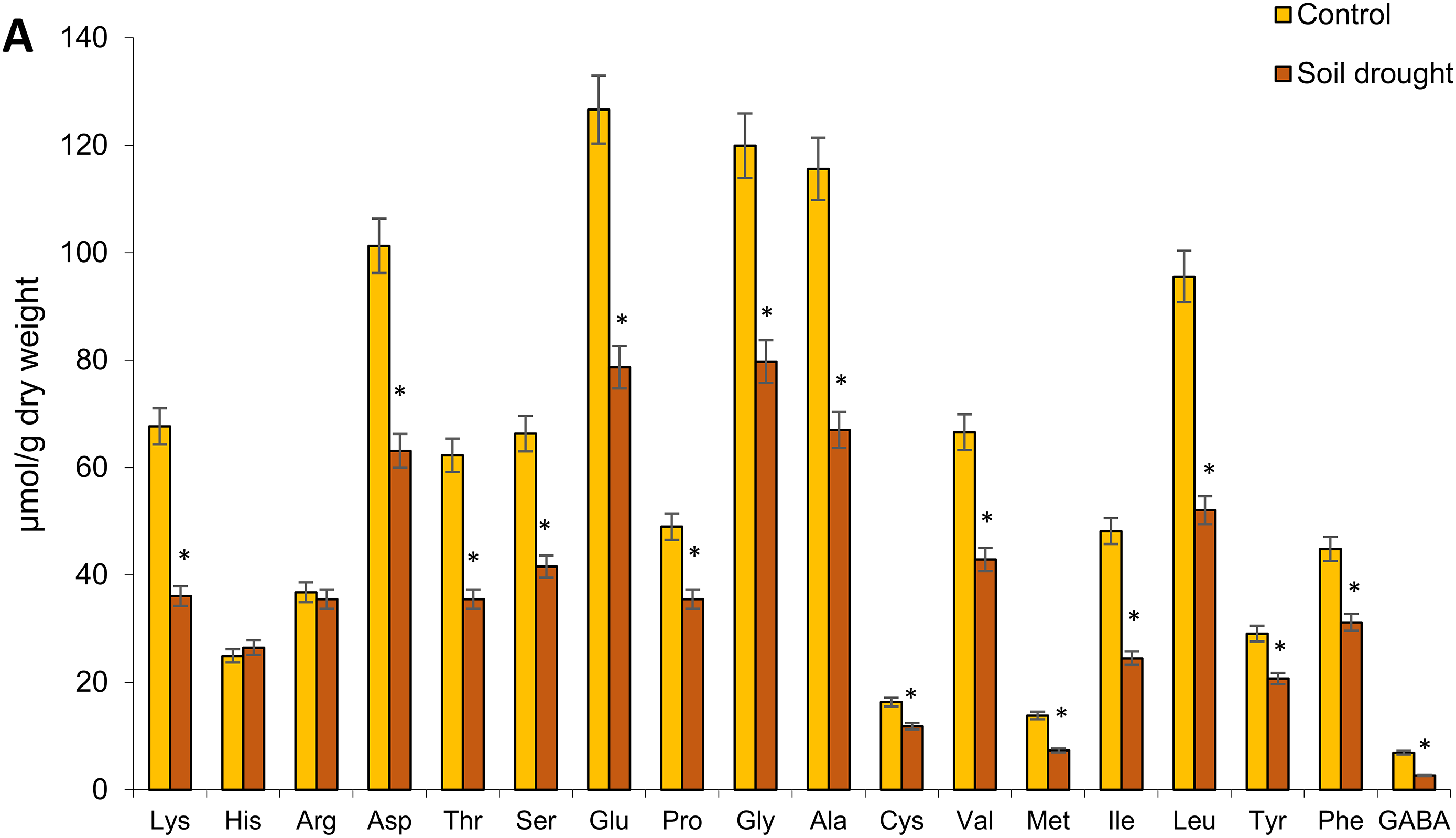
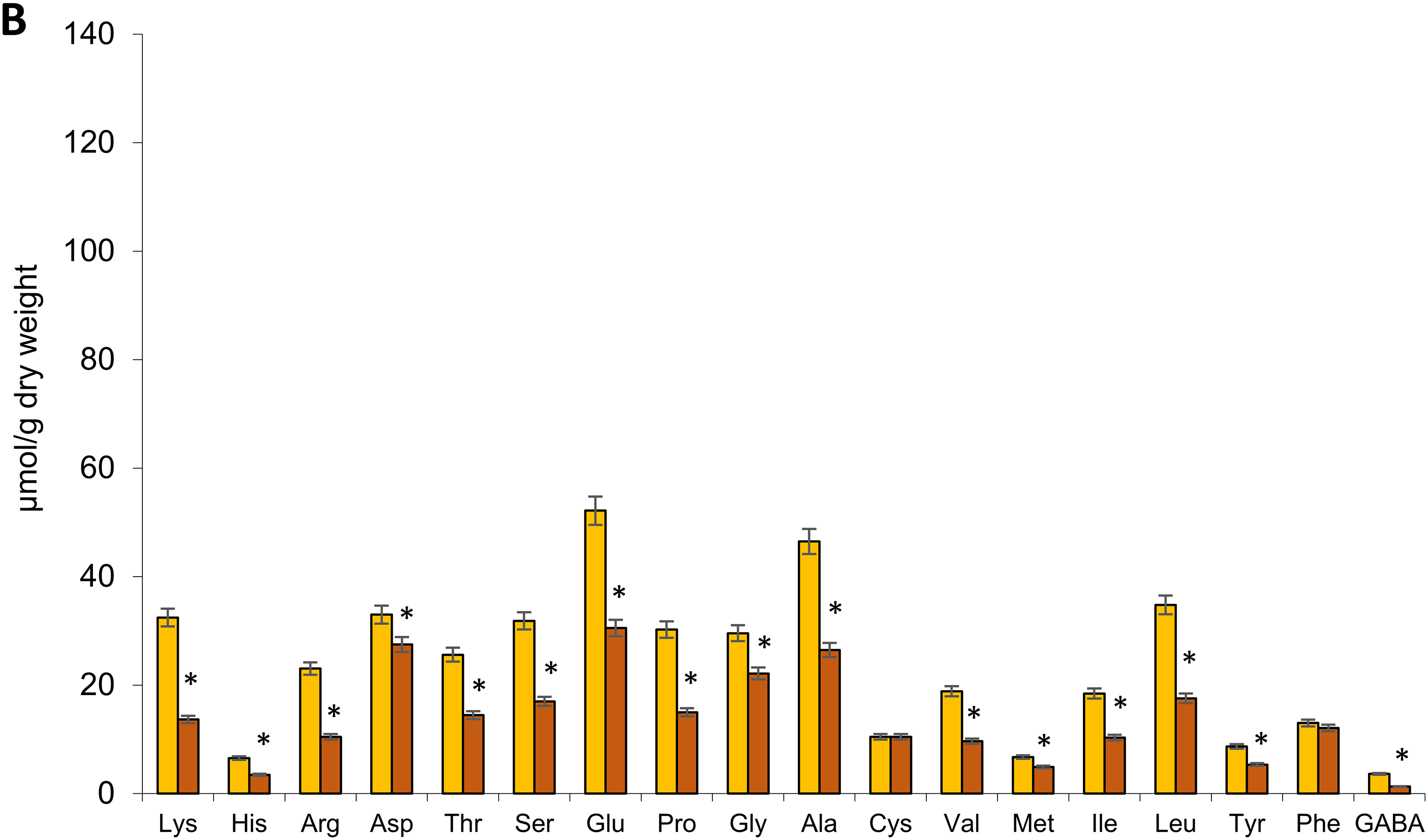
Figure 3: Effect of moderate soil drought on certain AA content in the shoots (A) and roots (B) of 18-day-old plants of Secale cereale cv. ‘Boghuslavka’. Abbreviations for AAs: Lys, lysine; His, histidine; Arg, arginine; Asp, aspartic acid; Thr, threonine; Ser, serine; Glu, glutamic acid; Pro, proline; Gly, glycine; Ala, alanine; Cys, cysteine; Val, valine; Met, methionine; Ile, isoleucine; Leu, leucine; Tyr, tyrosine; Phe, phenylalanine; GABA, γ-aminobutyric acid. The data are mean values (n = 3), the bars represent standard deviation (SD), and the asterisk * shows significant differences at p < 0.05 vs. control. The absence of an asterisk means there is no statistically significant difference between the “soil drought” group and the control (p > 0.05)
In roots, drought had a more pronounced effect, decreasing the content of most free AAs by 17%–64%. GABA levels declined by 64%, lysine by 58%, arginine by 55%, leucine and proline by 50%, valine by 49%, and serine by 47%. Only cysteine and phenylalanine remained stable under drought stress (Fig. 3B). Overall, moderate soil drought significantly reduced free AA accumulation in 18-day-old rye plants, with a greater impact on roots. The most substantial decline was observed in GABA levels.
3.4 Dynamics and Distribution of Total Phenols and Flavonoids in Winter Rye after Soil Drought
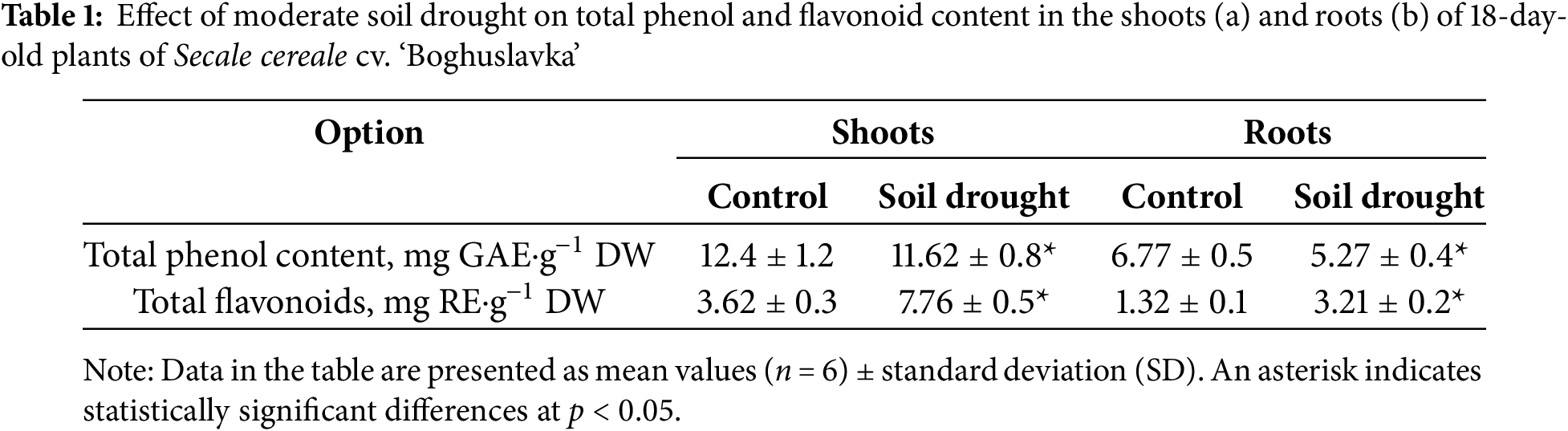
Under moderate soil drought, total phenol (TPH) content decreased by 6% in shoots and 22% in roots. In contrast, total flavonoid (TF) content increased by 111% in shoots and 143% in roots (Table 1). Under control conditions, the TPH shoot-to-root ratio was 1.8, increasing to 2.2 under drought, indicating a greater decline in root phenol content. In contrast, the TF ratio decreased from 2.7 to 2.4 under drought, reflecting an increased flavonoid concentration in roots relative to shoots.
Drought is one of the most detrimental abiotic stressors threatening plant survival [45]. Plants employ various adaptive strategies to counteract drought, including stress avoidance (by modifying their developmental program to complete the reproductive cycle before drought onset), stress prevention (through morphological and physiological adaptations that maintain water balance), and resistance acquisition at the cellular and molecular levels [46]. The formation and nature of plant responses to drought are influenced by multiple factors, including species, age, developmental stage, and the duration and intensity of stress [47].
Drought triggers stomatal closure, reduces carbon dioxide influx into chloroplasts, inhibits photosynthetic activity [48], and slows the growth of aboveground biomass [49]. Simultaneously, additional root growth occurs in search of water sources, increasing the root-to-shoot ratio [7]. Given the limited potential for expanding agricultural land, enhancing productivity becomes essential. One approach to addressing this challenge is mitigating drought effects and developing stress-resistant crop varieties. Our study demonstrated that moderate soil drought inhibited shoot growth in 18-day-old rye plants, whereas the root system exhibited greater resilience. The root-to-shoot length ratio increased under stress from 0.74 to 0.8. Upon cessation of stress, root growth recovery was more pronounced (Fig. 1). These morphometric changes coincided with hormonal balance adjustments in rye organs. Specifically, shoot growth inhibition was accompanied by increased accumulation of abscisic acid and salicylic acid, along with a significant reduction in indole-3-acetic acid and gibberellin levels in 18-day-old plants. Conversely, IAA and GA levels increased in the roots of 21-day-old recovered plants (Fig. 2).
Phytohormones play a crucial role in shaping drought tolerance in crops [50–53]. During drought, ABA and SA function as chemical messengers, activating physiological processes such as osmolyte accumulation, stomatal closure, and root growth stimulation [54,55]. Kuromori et al. [56] demonstrated that ABA is transported from its biosynthetic sites—roots and vascular leaf tissues—to other plant organs. The identification of ABA transmembrane transporters suggests that their movement is tightly regulated within the intercellular network. The increased ABA content in the shoots and roots of the rye ‘Boghuslavka’ under moderate soil drought suggests enhanced biosynthesis of the hormone (Fig. 2A), which plays a protective role by regulating transpiration intensity and root system growth. Previous reports indicate that ABA synthesized in roots under drought stress is transported to leaves, where it serves as a trigger for adaptation mechanisms that inhibit growth and induce stomatal closure [57].
Khan et al. [58] highlighted that SA’s signaling role depends on multiple factors, including endogenous hormone levels and stress type. Exogenous SA application has been shown to enhance drought tolerance in wheat by activating the antioxidant defense system, improving growth, increasing photosynthetic efficiency, enhancing water potential, and boosting stress protein and osmolyte levels [59–61]. Our study revealed that under moderate soil drought, SA levels in 18-day-old rye plant cv. ‘Boghuslavka’ increased more significantly in shoots than in roots. At 21 days post-rehydration, SA levels declined, but shoot concentrations remained higher than root levels. The response to post-drought recovery in 21-day-old plants was organ-specific: shoots continued accumulating SA, while root SA levels decreased (Fig. 2B). These findings suggest an organ-specific protective role of SA in young rye plants, particularly in aboveground tissues, in response to soil drought.
Under drought conditions, the total IAA content in 18-day-old rye plants cv. ‘Boghuslavka’ decreased by 66.2% (Fig. 2C). Similarly, a 72% reduction in IAA levels after three days of drought was reported in rice plants [62]. IAA plays a crucial role in growth regulation and long-term morphological adaptations due to its biosynthesis, transport, and signaling mechanisms [20,63,64]. Under the influence of drought, ABA accumulation is observed in the tissues of the root tip. The interaction between IAA and ABA optimizes root growth and branching under sufficient soil moisture but restricts it under drought [65]. Our findings suggest that the rye root system exhibited significant drought acclimation, which correlated with ABA accumulation and reduced IAA levels (Fig. 2). Similarly, Sadok and Schoppach [66] found that auxin accumulation in the root system reduces water use during the day and night and modulates the hydraulic properties of plants. Other studies indicate that young leaves, characterized by high IAA levels and active hormone biosynthesis [67], demonstrate increased stress resistance [68]. Thus, drought-induced a substantial decrease in IAA, particularly in the shoots of 18-day-old rye plants. By the 21st day, post-stress plants still had lower IAA levels than control plants of the same age, indicating prolonged stress effects.
We also found that drought reduced total GA content in rye shoots and roots, with gibberellins dominating in shoots (Fig. 2D). Omena-Garcia et al. [69] reported that under stress, declining GA levels promote the accumulation of free amino acids, such as proline, which accelerates carbon transport to the roots, maintains leaf turgor, and enhances drought tolerance. GA, along with ABA, regulates stomatal conductance under drought. Deactivation of GA in stomatal guard cells contributes to their closure, while inhibition of GA synthesis in leaves suppresses crown growth, limiting the transpiration zone [70]. A decline in biologically active GAs under drought has also been observed in maize leaves [71].
A reduction in endogenous GA levels and activity enhances drought tolerance by promoting the accumulation of DELLA proteins, key growth inhibitors, thereby facilitating adaptation to abiotic stresses such as drought [72,73]. Exogenous GA application has demonstrated stress-mitigating effects. Al-Huqail et al. [74] found that foliar treatment of wheat with GA alleviated the toxic effects of ZnO nanoparticles by increasing antioxidant enzyme activity. Under salt stress, exogenous GA3 induced chloroplast lipid biosynthesis in rice [75] and enhanced proline accumulation, maintaining membrane permeability and improving nutrient levels in maize [76]. GA3 also improved gas exchange under salt stress and stimulated antioxidant enzyme activity and flavonoid accumulation [77]. By the 21st day post-irrigation, under GA4 dominance, total GA content in rye shoots exceeded control levels but remained half as high in roots (Fig. 2D).
Comparing drought responses across cereal genotypes, we found that the drought-tolerant winter wheat cv. ‘Podolyanka’ exhibited the most active ABA accumulation in the roots of 18-day-old stressed and 21-day-old recovered plants. In contrast, the environmentally plastic spelled wheat cv. ‘Frankenkorn’ showed a significant increase in SA levels in the roots of both stressed and recovered plants. In the cold-resistant, moderately drought-resistant winter rye cv. ‘Boghuslavka’, drought suppressed IAA accumulation in both shoots and roots. GA3 accumulation was also inhibited in the roots of 18-day-old stressed spelt wheat plants and 21-day-old recovered plants [78]. Overall, the general drought response across the studied cereals involved ABA and SA accumulation and reduced auxin and gibberellin levels. However, species- and organ-specific variations in hormone dynamics and localization were evident.
We found that moderate soil drought triggered a specific response in 18-day-old rye plants cv. ‘Boghuslavka’ is characterized by the differentiated accumulation and distribution of low-molecular-weight protectants—amino acids, total phenols, and flavonoids. Aspartic acid, glutamic acid, glycine, alanine, and leucine dominated the roots and shoots of both control and drought-stressed plants. However, drought caused a significant reduction in most AAs in the shoots (by 30%–60%) and roots (by 17%–64%), suggesting their utilization in metabolic processes that support acclimation. Additionally, the extent of AA depletion may reflect the genotype’s sensitivity to water deficit, as more drought-tolerant cultivars tend to maintain or even accumulate certain AAs (e.g., proline) to support osmoprotection and redox balance. In contrast, the pronounced reduction in AAs observed in the ‘Boghuslavka’ variety may indicate insufficient metabolic compensation of AAs or a limited capacity for stress mitigation and may also reflect cultivar-specific characteristics.
Aspartic acid participates in the biosynthesis of other AAs and serves as a precursor for biomolecules involved in cellular homeostasis and antioxidant defense [31]. Glutamic acid plays a central role in nitrogen metabolism and contributes to drought tolerance in cereals [79], acting as a nitrogen donor for AA biosynthesis and other nitrogen-containing compounds while also maintaining redox balance and protecting cells from oxidative stress [80]. Glycine and alanine are crucial for drought protection; glycine supports antioxidant synthesis (including glutathione), osmoregulation, and photosynthetic activity, while alanine functions as an osmoprotectant and plays a role in nitrogen transport and energy metabolism [81,82]. Leucine serves as an energy source via catabolic pathways, which is especially vital during drought stress [83]. The preservation of histidine levels in rye shoots under drought suggests its role in metabolic adaptations aimed at mitigating water deficit effects [84]. Meanwhile, the stable arginine content in roots and shoots indicates a balance between its biosynthesis and utilization in adaptation processes. Typically, drought-tolerant cereals accumulate proline, tryptophan, and branched-chain AAs (BCAAs) [85], but our results showed a decline in these AAs in ‘Boghuslavka’ rye, highlighting its sensitivity to drought in early ontogenesis. However, the stable levels of cysteine and phenylalanine in roots may indicate their protective roles, correlating with greater root system resilience. Cysteine is a precursor to glutathione, a key antioxidant that neutralizes reactive oxygen species generated under drought stress [86], while phenylalanine serves as a substrate for the synthesis of phenolic compounds that reinforce root cell walls, enhancing dehydration resistance and water balance maintenance [87,88].
A significant decrease in the levels of GABA and proline was observed in both the shoots and roots of 18-day-old rye seedlings. These amino acids typically accumulate under stress conditions like drought, aiding in osmoregulation, antioxidant defense, and cell signaling [89]. GABA levels under drought stress may follow a biphasic pattern—initially increasing, then decreasing as it is used in the tricarboxylic acid cycle to meet energy demands [90]. Drought also activates GABA transporters (ProTs, AAP3) and affects the expression of GABA biosynthesis genes, particularly GAD, which encodes glutamate decarboxylase [91,92]. Studies show that drought-tolerant chickpea varieties exhibit dynamic GAD expression [93], while GAD knockout in Arabidopsis results in reduced GABA levels and heightened drought sensitivity [94]. Thus, the decrease in GABA in the ‘Boghuslavka’ rye variety may reflect both its varietal sensitivity to water deficit and the intensive use of GABA as an energy source under drought stress.
The reduction in proline levels may be linked to the low GABA content, as GABA is a precursor for proline and regulates its metabolism [89]. Elevated GABA enhances proline synthesis, improving drought tolerance through osmotic adjustment and reduced ROS, while GABA deficiency limits proline production and increases tissue damage [90,95–97]. Drought also influences the expression of proline biosynthesis genes such as P5CS and P5CR [98,99]. The decrease in proline content in our study in the shoots and roots of rye may also be associated with a decrease in the expression of these genes. Regulatory mechanisms controlling proline biosynthesis may be suppressed under certain drought stress conditions, potentially leading to a decrease in its overall synthesis [100,101]. Therefore, the decline in GABA and proline levels in rye seedlings likely results from imbalances in synthesis and utilization driven by both physiological stress responses and varietal traits. The decrease in proline content in rye under drought conditions may be attributed to its relatively high constitutive levels—approximately 30 μmol/g in roots and 50 μmol/g in leaves—which significantly exceed those found in wheat and spelt (up to 10 μmol/g) [28]. This elevated baseline likely reduces the need for additional proline accumulation during drought and may even allow its utilization as a metabolic pool for synthesizing other compounds essential for stress adaptation.
In contrast to rye, spelt wheat (‘Frankenkorn’) exhibited a significant increase in total AA content in shoots under drought, while winter wheat (‘Podolyanka’) showed no significant changes. Spelt wheat shoots were dominated by arginine, proline, phenylalanine, cysteine, and valine, whereas winter wheat shoots primarily accumulated phenylalanine and tyrosine [28]. Drought also induced the synthesis of total phenols and flavonoids), which help prevent oxidative damage [33,102]. The accumulation pattern of TF depends on the genotype’s stress tolerance, stress intensity, duration, and the efficiency of ROS neutralization [28]. Our results showed a differential response in the phenolic compound distribution in 18-day-old ‘Boghuslavka’ rye plants under drought. Under control conditions, total phenols were more abundant in shoots. Drought stress reduced phenol content in both shoots and roots, with more pronounced declines in roots. The increase in the shoot-to-root ratio for total phenols suggests that phenol biosynthesis in roots was inhibited or degradation accelerated in response to stress.
Conversely, drought-enhanced flavonoid biosynthesis, particularly in roots, correlated with phenotypic signs of increased drought tolerance. The decreased shoot-to-root ratio for flavonoids suggests that protective mechanisms were activated in the roots, potentially through increased synthesis or reduced degradation. When comparing phenolic compound dynamics in cereals with varying stress tolerance [26], we found that moderate drought-induced phenol and flavonoid accumulation in the shoots of drought-resistant winter wheat (‘Podolyanka’) and the roots of the ecologically plastic spelt wheat (‘Frankenkorn’).
Thus, the general drought response across the studied cereals involved flavonoid accumulation. However, ‘Boghuslavka’ rye exhibited a specific response, characterized by a decrease in free AAs, total phenols, and proline content, indicating its sensitivity to drought at the early growth stage.
Drought-induced both nonspecific and organ-specific changes in the accumulation and distribution of endogenous phytohormones. A general response to drought was the accumulation of stress hormones ABA and SA, along with the suppression of growth hormones IAA and gibberellins. However, hormonal changes vary between plant organs. A significant increase in ABA and SA levels was observed in the shoots of both stressed and recovered plants, correlating with inhibited growth. The prolonged impact of drought was evident in the sustained accumulation of ABA in both shoots and roots of recovered plants. In contrast, SA levels in the roots decreased, while the shoots continued to accumulate this hormone.
Drought also led to a marked reduction in IAA content, particularly in rye shoots, while GA3 + GA4 levels significantly decreased in the roots. GA3 was dominant in all samples except in the shoots of 21-day-old control plants. After irrigation was restored, IAA levels increased but did not return to control values. In the roots, GA3 + GA4 levels increased due to GA4, whereas in the shoots, GA3 + GA4 levels decreased, primarily due to GA3 reduction.
The study also revealed distinct patterns in the changes and distribution of low-molecular-weight protective compounds, including free amino acids and phenolic compounds, under drought conditions. The overall decrease in most free AAs suggests their involvement in energy metabolism defense and antioxidants. The reduced phenol content in the roots suggests either suppressed synthesis or increased degradation, whereas the increased flavonoid content, particularly in the roots, indicates their role in mitigating oxidative stress. Overall, rye shoots and roots adopt different acclimation strategies in response to moderate soil drought. The reduction of key AAs and dynamic changes in flavonoid distribution highlight the sensitivity of the Boghuslavka variety to drought in the early stages of development.
In drought-stressed rye plants, a substantial increase in ABA and SA levels was observed, accompanied by a marked decline in IAA and gibberellins levels. These hormonal changes occurred alongside a reduction in free amino acid content, accumulation of phenolic compounds, and an increase in flavonoid levels.
This study provides a foundation for further research on the mechanisms of drought resistance in cereal crops and the development of strategies to enhance their adaptive potential.
Acknowledgement: Not applicable.
Funding Statement: This publication presents findings from research conducted under Project No. III-99-24.489, Natural Growth Regulators in the Induction of Resistance of Cereal Plants to Heavy Metals (2024-2028), funded by the National Academy of Sciences of Ukraine.
Author Contributions: Conceptualization, Iryna Kosakivska; methodology, Valentyna Vasyuk, Lesya Voytenko, Mykola Shcherbatiuk, Kateryna Romanenko and Oleksandr Smirnov; investigation, Lesya Voytenko, Mykola Shcherbatiuk, Valentyna Vasyuk, Kateryna Romanenko, Lidiya Babenko and Oleksandr Smirnov; data curation, Lesya Voytenko, Mykola Shcherbatiuk, Kateryna Romanenko and Iryna Kosakivska; writing—original draft preparation, Lesya Voytenko, Mykola Shcherbatiuk and Kateryna Romanenko; writing—review and editing, Iryna Kosakivska; supervision, Lesya Voytenko. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| AA | Amino acid |
| ABA | Abscisic acid |
| Ala | Alanine |
| Arg | Arginine |
| Asp | Aspartic acid |
| Cys | Cysteine |
| DW | Dry weight |
| FW | Fresh weight |
| GA3 | GA4 Gibberellic acids |
| GABA | γ-Aminobutyric acid |
| Gln | Glutamine |
| Glu | Glutamic acid |
| Gly | Glycine |
| His | Histidine |
| HPLC-MS | High-performance liquid chromatography-mass spectrometry |
| IAA | Indole-3-acetic acid |
| Ill | Isoleucine |
| Leu | Leucine |
| Lys | Lysine |
| Met | Methionine |
| Phe | Phenylalanine |
| Pro | Proline |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| Ser | Serine |
| TF | Total Flavonoids |
| Thr | Threonine |
| TPH | Total Phenols |
| Tyr | Tyrosine |
| Val | Valine |
References
1. Chéour F, Kaddachi I, Achouri D, Bannour S, Zorgui L. Effects of water stress on relative water, chlorophylls and proline contents in barley (Hordeum vulgare L.) leaves. IOSR J Agric Vet Sci. 2014;7:13–6. doi:10.6084/M9.FIGSHARE.1226297. [Google Scholar] [CrossRef]
2. Kottmann L, Wilde P, Schittenhelm S. How do timing, duration, and intensity of drought stress affect the agronomic performance of winter rye? Eur J Agron. 2016;75(5):25–32. doi:10.1016/j.eja.2015.12.010. [Google Scholar] [CrossRef]
3. Mariey SA, Khedr RA. Evaluation of some Egyptian Barley cultivars under water stress conditions using drought tolerance indices and multivariate analysis. J Sustain Agric Sci. 2017;43(2):105–14. doi:10.21608/JSAS.2017.1061.1005. [Google Scholar] [CrossRef]
4. Rzepka-Plevneš D, Krupa-Małkiewicz M, Kurek J, Smolik M. Effects of water deficits on development and yield of rye varieties differing in tolerance to drought at seedling stage. J Food Agric Environ. 2009;7(3&4):492–95. [Google Scholar]
5. Bao G, Pan X, Yan B, Chang Y, Tang W, Qu Y, et al. Resistance of rye seedlings to drought and freeze-thaw stress. Pol J Environ Stud. 2022;31(2):1559–68. doi:10.15244/pjoes/142608. [Google Scholar] [CrossRef]
6. Qiao M, Hong C, Jiao Y, Hou S, Gao H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants. 2024;13(13):1808. doi:10.3390/plants13131808. [Google Scholar] [PubMed] [CrossRef]
7. Dietrich D. Hydrotropism: how roots search for water. J Exp Bot. 2018;69(11):2759–71. doi:10.1093/jxb/ery034. [Google Scholar] [PubMed] [CrossRef]
8. Bushuk W. Rye: production, chemistry, and technology. 2nd ed., St. Paul, MN, USA: American Association of Cereal Chemistry; 2001. 239 p. [Google Scholar]
9. Haffke S, Wilde P, Schmiedchen B, Hackauf B, Roux S, Gottwald M, et al. Toward a selection of broadly adapted germplasm for yield stability of hybrid rye under normal and managed drought stress conditions. Crop Sci. 2015;55(3):1026–34. doi:10.2135/cropsci2014.08.0532. [Google Scholar] [CrossRef]
10. Halford NG, Curtis TY, Chen Z, Huang J. Effects of abiotic stress and crop management on cereal grain composition: implications for food quality and safety. J Exp Bot. 2015;66(5):1145–56. doi:10.1093/jxb/eru473. [Google Scholar] [PubMed] [CrossRef]
11. Ghafoor AZ, Karim H, Studnicki M, Raza A, Javed HH, Asghar MA. Climate change and rye (Secale cereale L.) production: challenges, opportunities and adaptations. J Agron Crop Sci. 2024;210(4):e12725. doi:10.1111/jac.12725. [Google Scholar] [CrossRef]
12. Abdelaal KAA, Attia KA, Alamery SF, El-Afry MM, Ghazy AI, Tantawy DS, et al. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability. 2020;12(5):1736. doi:10.3390/su12051736. [Google Scholar] [CrossRef]
13. Kaya C, Uğurlar F, Adamakis IS. Epigenetic modifications of hormonal signaling pathways in plant drought response and tolerance for sustainable food security. Int J Mol Sci. 2024;25(15):8229. doi:10.3390/ijms25158229. [Google Scholar] [PubMed] [CrossRef]
14. Huang P, Xu Z, He W, Yang H, Li B, Ding W, et al. The cooperation regulation of antioxidative system and hormone contents on physiological responses of Wedelia trilobata and Wedelia chinensis under simulated drought environment. Plants. 2024;13(4):472. doi:10.3390/plants13040472. [Google Scholar] [PubMed] [CrossRef]
15. Cui XY, Du YT, Fu JD, Yu TF, Wang CT, Chen M, et al. Wheat CBL-interacting protein kinase 23 positively regulates drought stress and ABA responses. BMC Plant Biol. 2018;18(1):93. doi:10.1186/s12870-018-1306-5. [Google Scholar] [PubMed] [CrossRef]
16. Nawaz M, Wang Z. Abscisic acid and glycine betaine mediated tolerance mechanisms under drought stress and recovery in Axonopus compressus: a new insight. Sci Rep. 2020;10(1):6942. doi:10.1038/s41598-020-63447-0. [Google Scholar] [PubMed] [CrossRef]
17. Shi H, Ye T, Zhu JK, Chan Z. Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J Exp Bot. 2014;65(15):4119–31. doi:10.1093/jxb/eru184. [Google Scholar] [PubMed] [CrossRef]
18. Zhang S, He C, Wei L, Jian S, Liu N. Transcriptome and metabolome analysis reveals key genes and secondary metabolites of Casuarina equisetifolia ssp. incana in response to drought stress. BMC Plant Biol. 2023;23(1):200. doi:10.1186/s12870-023-04206-x. [Google Scholar] [PubMed] [CrossRef]
19. Zhang Y, Zhao Y, Li T, Ni C, Han L, Du P, et al. TaPYL4, an ABA receptor gene of wheat, positively regulates plant drought adaptation through modulating the osmotic stress-associated processes. BMC Plant Biol. 2022;22(1):423. doi:10.1186/s12870-022-03799-z. [Google Scholar] [PubMed] [CrossRef]
20. Sharma A, Gupta A, Ramakrishnan M, Ha CV, Zheng B, Bhardwaj M, et al. Roles of abscisic acid and auxin in plants during drought: a molecular point of view. Plant Physiol Biochem. 2023;204:108129. doi:10.1016/j.plaphy.2023.108129. [Google Scholar] [PubMed] [CrossRef]
21. Li P, Yang H, Wang L, Liu H, Huo H, Zhang C, et al. Physiological and transcriptome analyses reveal short-term responses and formation of memory under drought stress in rice. Front Genet. 2019;10:55. doi:10.3389/fgene.2019.00055. [Google Scholar] [PubMed] [CrossRef]
22. Liu Y, Wei X. Dark septate endophyte improves drought tolerance of Ormosia hosiei Hemsley & E. H. Wilson by modulating root morphology, ultrastructure, and the ratio of root hormones. Forests. 2019;10(10):830. doi:10.3390/f10100830. [Google Scholar] [CrossRef]
23. Gantait S, Sinniah UR, Ali N, Sahu NC. Gibberellins—a multifaceted hormone in plant growth regulatory network. Curr Protein Pept Sci. 2015;16(5):406–12. doi:10.2174/1389203716666150330125439. [Google Scholar] [PubMed] [CrossRef]
24. Awadalla RA, Sallam A, Börner A, Elshamy MM, Heikal YM. The role of salicylic acid in modulating phenotyping in spring wheat varieties for mitigating drought stress. BMC Plant Biol. 2024;24(1):948. doi:10.1186/s12870-024-05620-5. [Google Scholar] [PubMed] [CrossRef]
25. Liu J, Qiu G, Liu C, Li H, Chen X, Fu Q, et al. Salicylic acid, a multifaceted hormone, combats abiotic stresses in plants. Life. 2022;12(6):886. doi:10.3390/life12060886. [Google Scholar] [PubMed] [CrossRef]
26. Khan I, Awan SA, Ikram R, Rizwan M, Akhtar N, Yasmin H, et al. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol Plant. 2021;172(2):696–706. doi:10.1111/ppl.13237. [Google Scholar] [PubMed] [CrossRef]
27. Zhao MR, Han YY, Feng YN, Li F, Wang W. Expansins are involved in cell growth mediated by abscisic acid and indole-3-acetic acid under drought stress in wheat. Plant Cell Rep. 2012;31(4):671–85. doi:10.1007/s00299-011-1185-9. [Google Scholar] [PubMed] [CrossRef]
28. Romanenko KO, Babenko LM, Smirnov OE, Kosakivska IV. Impact of moderate soil drought on the dynamics and distribution of low molecular weight protectors in Triticum aestivum and Triticum spelta. J Crop Health. 2025;77(1):32. doi:10.1007/s10343-024-01097-2. [Google Scholar] [CrossRef]
29. Guo X, Xin Z, Yang T, Ma X, Zhang Y, Wang Z, et al. Metabolomics response for drought stress tolerance in Chinese wheat genotypes (Triticum aestivum). Plants. 2020;9(4):520. doi:10.3390/plants9040520. [Google Scholar] [PubMed] [CrossRef]
30. Gregorová Z, Kováčik J, Klejdus B, Maglovski M, Kuna R, Hauptvogel P, et al. Drought-induced responses of physiology, metabolites, and PR proteins in Triticum aestivum. J Agric Food Chem. 2015;63(37):8125–33. doi:10.1021/acs.jafc.5b02951. [Google Scholar] [PubMed] [CrossRef]
31. Ali Q, Athar HR, Haider MZ, Shahid S, Aslam N, Shehzad F, et al. Role of amino acids in improving abiotic stress tolerance to plants. In: Hasanuzzaman M, Fujita M, Oku H, Islam MT, editors. Plant tolerance to environmental stress, role of phytoprotectants. Boca Raton, FL, USA: CRC Press; 2019. p. 175–203. doi:10.1201/9780203705315-12. [Google Scholar] [CrossRef]
32. Zhou G, Wu S, Chen D, Wu X, Cai Q. Polyphenols and phytohormones profiling of pre-harvest sprouting resistant and susceptible wheat genotypes. SN Appl Sci. 2023;5(9):249. doi:10.1007/s42452-023-05464-y. [Google Scholar] [CrossRef]
33. Babenko LM, Smirnov OE, Romanenko KO, Trunova OK, Kosakіvskа IV. Phenolic compounds in plants: biogenesis and functions. Ukr Biochem J. 2019;91(3):5–18. doi:10.15407/ubj91.03.005. [Google Scholar] [CrossRef]
34. Bano A, Yasmeen S. Role of phytohormones under induced drought stress in wheat. Pak J Bot. 2010;42(4):2579–87. [Google Scholar]
35. Talaat NB. Drought stress alleviator melatonin reconfigures water-stressed barley (Hordeum vulgare L.) plants’ photosynthetic efficiency, antioxidant capacity, and endogenous phytohormone profile. Int J Mol Sci. 2023;24(22):16228. doi:10.3390/ijms242216228. [Google Scholar] [PubMed] [CrossRef]
36. Zafar S, Akhtar M, Perveen S, Hasnain Z, Khalil A. Attenuating the adverse aspects of water stress on wheat genotypes by foliar spray of melatonin and indole-3-acetic acid. Physiol Mol Biol Plants. 2020;26(9):1751–62. doi:10.1007/s12298-020-00855-6. [Google Scholar] [PubMed] [CrossRef]
37. Luqman M, Shahbaz M, Maqsood MF, Farhat F, Zulfiqar U, Siddiqui MH, et al. Effect of strigolactone on growth, photosynthetic efficiency, antioxidant activity, and osmolytes accumulation in different maize (Zea mays L.) hybrids grown under drought stress. Plant Signal Behav. 2023;18(1):2262795. doi:10.1080/15592324.2023.2262795. [Google Scholar] [PubMed] [CrossRef]
38. Fu P, Wilen RW, Robertson AJ, Low NH, Tyler RT, Gusta LV. Heat tolerance of cold acclimated puma winter rye seedlings and the effect of a heat shock on freezing tolerance. Plant Cell Physiol. 1998;39(9):942–49. doi:10.1093/oxfordjournals.pcp.a029458. [Google Scholar] [CrossRef]
39. Kolupaev YE, Mаkaova BE, Ryabchun NI, Kokorev AI, Sakhno TV, Sakhno Y, et al. Adaptation of cereal seedlings to oxidative stress induced by hyperthermia. Agric For. 2022;68(4):7–18. doi:10.17707/AgricultForest.68.4.01. [Google Scholar] [CrossRef]
40. Kosakivska IV, Shcherbatiuk MM, Voytenko LV. Profiling of hormones in plant tissues: history, modern approaches, use in biotechnology. Biotechnol Acta. 2020;13(4):14–25. doi:10.15407/biotech13.04.014. [Google Scholar] [CrossRef]
41. Hare PE, John SPA, Engel MH. Ion-exchange separation of amino acids. In: Barrett GC, editor. Chemistry and biochemistry of the amino acids. Dordrecht, The Netherlands: Springer; 1985. p. 415–25. doi:10.1007/978-94-009-4832-7_14. [Google Scholar] [CrossRef]
42. Ng LT, Wong DY, Francis T, Anderson GH. Ion-exchange chromatography for physiological fluid amino acid analysis. J Nutr Biochem. 1991;2(12):671–79. doi:10.1016/0955-2863(91)90066-E. [Google Scholar] [CrossRef]
43. Bobo-García G, Davidov-Pardo G, Arroqui C, Vírseda P, Marín-Arroyo MR, Navarro M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J Sci Food Agric. 2015;95(1):204–9. doi:10.1002/jsfa.6706. [Google Scholar] [PubMed] [CrossRef]
44. Smirnov OE, Kosyan AM, Pryimak YV, Kosyk OI, Taran NY. Organo-specific accumulation of phenolic compounds in a buckwheat seedling under aluminium-acid stress. Ukr Biochem J. 2021;93(1):75–81. doi:10.15407/ubj93.01.075. [Google Scholar] [CrossRef]
45. Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14. doi:10.1007/s00425-003-1105-5. [Google Scholar] [PubMed] [CrossRef]
46. Dolferus R. To grow or not to grow: a stressful decision for plants. Plant Sci. 2014;229:247–61. doi:10.1016/j.plantsci.2014.10.002. [Google Scholar] [PubMed] [CrossRef]
47. Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, et al. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021;10(2):259. doi:10.3390/plants10020259. [Google Scholar] [PubMed] [CrossRef]
48. Hu W, Ren T, Meng F, Cong R, Li X, White PJ, et al. Leaf photosynthetic capacity is regulated by the interaction of nitrogen and potassium through coordination of CO2 diffusion and carboxylation. Physiol Plant. 2019;167(3):418–32. doi:10.1111/ppl.12919. [Google Scholar] [PubMed] [CrossRef]
49. Lind C, Dreyer I, López-Sanjurjo EJ, von Meyer K, Ishizaki K, Kohchi T, et al. Stomatal guard cells co-opted an ancient ABA-dependent desiccation survival system to regulate stomatal closure. Curr Biol. 2015;25(7):928–35. doi:10.1016/j.cub.2015.01.067. [Google Scholar] [PubMed] [CrossRef]
50. Chhaya, Yadav B, Jogawat A, Gnanasekaran P, Kumari P, Lakra N, et al. An overview of recent advancement in phytohormones-mediated stress management and drought tolerance in crop plants. Plant Gene. 2021;25:100264. doi:10.1016/j.plgene.2020.100264. [Google Scholar] [CrossRef]
51. Liao Z, Chen B, Boubakri H, Farooq M, Mur LAJ, Urano D, et al. The regulatory role of phytohormones in plant drought tolerance. Planta. 2025;261(5):98. doi:10.1007/s00425-025-04671-8. [Google Scholar] [PubMed] [CrossRef]
52. Takahashi F, Kuromori T, Urano K, Yamaguchi-Shinozaki K, Shinozaki K. Drought stress responses and resistance in plants: from cellular responses to long-distance intercellular communication. Front Plant Sci. 2020;11:556972. doi:10.3389/fpls.2020.556972. [Google Scholar] [PubMed] [CrossRef]
53. Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, Schroeder JI. Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol. 2022;23(10):680–94. doi:10.1038/s41580-022-00479-6. [Google Scholar] [PubMed] [CrossRef]
54. Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, et al. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi:10.3390/biom9070285. [Google Scholar] [PubMed] [CrossRef]
55. Ullah A, Manghwar H, Shaban M, Khan AH, Akbar A, Ali U, et al. Phytohormones enhanced drought tolerance in plants: a coping strategy. Environ Sci Pollut Res Int. 2018;25(33):33103–18. doi:10.1007/s11356-018-3364-5. [Google Scholar] [PubMed] [CrossRef]
56. Kuromori T, Seo M, Shinozaki K. ABA transport and plant water stress responses. Trends Plant Sci. 2018;23(6):513–22. doi:10.1016/j.tplants.2018.04.001. [Google Scholar] [PubMed] [CrossRef]
57. Qi J, Song CP, Wang B, Zhou J, Kangasjärvi J, Zhu JK, et al. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol. 2018;60(9):805–26. doi:10.1111/jipb.12654. [Google Scholar] [PubMed] [CrossRef]
58. Khan MI, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:462. doi:10.3389/fpls.2015.00462. [Google Scholar] [PubMed] [CrossRef]
59. Ilyas N, Gull R, Mazhar R, Saeed M, Kanwal S, Shabir S, et al. Influence of salicylic acid and jasmonic acid on wheat under drought stress. Commun Soil Sci Plant Anal. 2017;48(22):2715–23. doi:10.1080/00103624.2017.1418370. [Google Scholar] [CrossRef]
60. Khalvandi M, Siosemardeh A, Roohi E, Keramati S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon. 2021;7(1):e05908. doi:10.1016/j.heliyon.2021.e05908. [Google Scholar] [PubMed] [CrossRef]
61. Sharma M, Gupta SK, Majumder B, Maurya VK, Deeba F, Alam A, et al. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J Proteomics. 2017;163(2009):28–51. doi:10.1016/j.jprot.2017.05.011. [Google Scholar] [PubMed] [CrossRef]
62. Du H, Liu H, Xiong L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci. 2013;4:397. doi:10.3389/fpls.2013.00397. [Google Scholar] [PubMed] [CrossRef]
63. Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 2006;7(11):847–59. doi:10.1038/nrm2020. [Google Scholar] [PubMed] [CrossRef]
64. Voytenko LV, Kosakivska IV. Auxins as regulators of growth and development of cereal crops under abiotic stresses: a review. Adv Biol Earth Sci. 2025;10(1):5–31. doi:10.62476/abes.1015. [Google Scholar] [CrossRef]
65. Leftley N, Banda J, Pandey B, Bennett M, Voß U. Uncovering how auxin optimizes root systems architecture in response to environmental stresses. Cold Spring Harb Perspect Biol. 2021;13(11):a040014. doi:10.1101/cshperspect.a040014. [Google Scholar] [PubMed] [CrossRef]
66. Sadok W, Schoppach R. Potential involvement of root auxins in drought tolerance by modulating nocturnal and daytime water use in wheat. Ann Bot. 2019;124(6):969–78. doi:10.1093/aob/mcz023. [Google Scholar] [PubMed] [CrossRef]
67. Ljung K, Bhalerao RP, Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001;28(4):465–74. doi:10.1046/j.1365-313x.2001.01173.x. [Google Scholar] [PubMed] [CrossRef]
68. Mühlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mateo A, Mullineaux PM, et al. Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell. 2008;20(9):2339–56. doi:10.1105/tpc.108.059618. [Google Scholar] [PubMed] [CrossRef]
69. Omena-Garcia RP, Martins AO, Medeiros DB, Vallarino JG, Ribeiro DM, Fernie AR, et al. Growth and metabolic adjustments in response to gibberellin deficiency in drought stressed tomato plants. Environ Exp Bot. 2019;159:95–107. doi:10.1016/J.ENVEXPBOT.2018.12.011. [Google Scholar] [CrossRef]
70. Shohat H, Cheriker H, Kilambi HV, Illouz Eliaz N, Blum S, Amsellem Z, et al. Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’ responses in tomato. New Phytol. 2021;232(5):1985–98. doi:10.1111/nph.17709. [Google Scholar] [PubMed] [CrossRef]
71. Nelissen H, Sun XH, Rymen B, Jikumaru Y, Kojima M, Takebayashi Y, et al. The reduction in maize leaf growth under mild drought affects the transition between cell division and cell expansion and cannot be restored by elevated gibberellic acid levels. Plant Biotechnol J. 2018;16(2):615–27. doi:10.1111/pbi.12801. [Google Scholar] [PubMed] [CrossRef]
72. Colebrook EH, Thomas SG, Phillips AL, Hedden P. The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol. 2014;217(Pt 1):67–75. doi:10.1242/jeb.089938. [Google Scholar] [PubMed] [CrossRef]
73. Kosakivska IV, Vasyuk VA. Gibberelins in regulation of plant growth and development under abiotic stresses. Biotechnol Acta. 2021;14(2):5–18. doi:10.15407/biotech14.02.005. [Google Scholar] [CrossRef]
74. Al-Huqail AA, Alshehri D, Nawaz R, Irshad MA, Iftikhar A, Hussaini KM, et al. The effect of gibberellic acid on wheat growth and nutrient uptake under combined stress of cerium, zinc and titanium dioxide nanoparticles. Chemosphere. 2023;336(23):139199. doi:10.1016/j.chemosphere.2023.139199. [Google Scholar] [PubMed] [CrossRef]
75. Liu X, Wang X, Yin L, Deng X, Wang S. Exogenous application of gibberellic acid participates in up-regulation of lipid biosynthesis under salt stress in rice. Theor Exp Plant Physiol. 2018;30(4):335–45. doi:10.1007/s40626-018-0129-y. [Google Scholar] [CrossRef]
76. Tuna AL, Kaya C, Dikilitas M, Higgs D. The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ Exp Bot. 2008;62(1):1–9. doi:10.1016/j.envexpbot.2007.06.007. [Google Scholar] [CrossRef]
77. Ahmad F, Kamal A, Singh A, Ashfaque F, Alamri S, Siddiqui MH, et al. Seed priming with gibberellic acid induces high salinity tolerance in Pisum sativum through antioxidants, secondary metabolites and up-regulation of antiporter genes. Plant Biol. 2020;23(S1):113–21. doi:10.1111/plb.13187. [Google Scholar] [PubMed] [CrossRef]
78. Kosakivska IV, Vasyuk VA, Voytenko LV, Shcherbatiuk MM. Changes in hormonal status of winter wheat (Triticum aestivum L.) and spelt wheat (Triticum spelta L.) after moderate soil drought and in recovery period. Cereal Res Commun. 2022;50(4):821–30. doi:10.1007/s42976-022-00332-8. [Google Scholar] [CrossRef]
79. Li G, Wei J, Li C, Fu K, Li C, Li C. Amino acid metabolism response to post-anthesis drought stress during critical periods of elite wheat (Triticum aestivum L.) endosperm development. Environ Exp Bot. 2024;218(11):105577. doi:10.1016/j.envexpbot.2023.105577. [Google Scholar] [CrossRef]
80. Liao HS, Chung YH, Hsieh MH. Glutamate: a multifunctional amino acid in plants. Plant Sci. 2022;318:111238. doi:10.1016/j.plantsci.2022.111238. [Google Scholar] [PubMed] [CrossRef]
81. Hildebrandt TM, Nunes Nesi A, Araújo WL, Braun HP. Amino acid catabolism in plants. Mol Plant. 2015;8(11):1563–79. doi:10.1016/j.molp.2015.09.005. [Google Scholar] [PubMed] [CrossRef]
82. Rai V. Role of amino acids in plant responses to stresses. Biol Plant. 2002;45(4):481–7. doi:10.1023/A:1022308229759. [Google Scholar] [CrossRef]
83. Heinemann B, Hildebrandt TM. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J Exp Bot. 2021;72(13):4634–45. doi:10.1093/jxb/erab182. [Google Scholar] [PubMed] [CrossRef]
84. Stepansky A, Leustek T. Histidine biosynthesis in plants. Amino Acids. 2006;30(2):127–42. doi:10.1007/s00726-005-0247-0. [Google Scholar] [PubMed] [CrossRef]
85. Romanenko KO, Babenko LM, Kosakivska IV. Amino acids in regulation of abiotic stress tolerance in cereal crops: a review. Cereal Res Commun. 2024;52(2):333–56. doi:10.1007/s42976-023-00418-x. [Google Scholar] [CrossRef]
86. Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35(2):454–84. doi:10.1111/j.1365-3040.2011.02400.x. [Google Scholar] [PubMed] [CrossRef]
87. Thakur S, Pandit K, Kumar A, Kaur J, Kaur S. Phenylpropanoid biosynthesis and its protective effects against plants stress. In: Kaur T, Arora S, editors. Environmental stress physiology of plants and crop productivity. Sharjah, United Arab Emirates: Bentham Science; 2021. p. 144–57. doi:10.2174/9781681087900121010013. [Google Scholar] [CrossRef]
88. Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010;3(1):2–20. doi:10.1093/mp/ssp106. [Google Scholar] [PubMed] [CrossRef]
89. Signorelli S, Tarkowski ŁP, O’Leary B, Tabares-da Rosa S, Borsani O, Monza J. GABA and proline metabolism in response to stress. In: Gupta DK, Corpas FJ, editors. Hormones and plant response: plant in challenging environments. Vol. 2. Berlin/Heidelberg, Germany: Springer; 2021. p. 291–314. doi:10.1007/978-3-030-77477-6_12f. [Google Scholar] [CrossRef]
90. Yong B, Xie H, Li Z, Li YP, Zhang Y, Nie G, et al. Exogenous application of GABA improves peg-induced drought tolerance positively associated with GABA-shunt, polyamines, and proline metabolism in white clover. Front Physiol. 2017;8:1107. doi:10.3389/fphys.2017.01107. [Google Scholar] [PubMed] [CrossRef]
91. AL-Quraan N, Al-Ajlouni Z, Qawasma N. Physiological and biochemical characterization of the GABA shunt pathway in pea (Pisum sativum L.) seedlings under drought stress. Horticulturae. 2021;7(6):125. doi:10.3390/horticulturae7060125. [Google Scholar] [CrossRef]
92. Hu Y, Huang X, Xiao Q, Wu X, Tian Q, Ma W, et al. Advances in plant GABA research: biological functions, synthesis mechanisms and regulatory pathways. Plants. 2024;13(20):2891. doi:10.3390/plants13202891. [Google Scholar] [PubMed] [CrossRef]
93. Seifikalhor M, Niknam V, Aliniaeifard S, Didaran F, Tsaniklidis G, Fanourakis D, et al. The regulatory role of γ-aminobutyric acid in chickpea plants depends on drought tolerance and water scarcity level. Sci Rep. 2022;12(1):7034. doi:10.1038/s41598-022-10571-8. [Google Scholar] [PubMed] [CrossRef]
94. Mekonnen DW, Flugge UI, Ludewig F. γ-aminobutyric acid depletion affects stomata closure and drought tolerance of Arabidopsis thaliana. Plant Sci. 2016;245(2):25–34. doi:10.1016/j.plantsci.2016.01.005. [Google Scholar] [PubMed] [CrossRef]
95. Ashraf U, Anjum SA, Naseer S, Abbas A, Abrar M, Nawaz M, et al. Gamma amino butyric acid (GABA) application modulated the morpho-physiological and yield traits of fragrant rice under well-watered and drought conditions. BMC Plant Biol. 2024;24(1):569. doi:10.1186/s12870-024-05272-5. [Google Scholar] [PubMed] [CrossRef]
96. Iqbal B, Hussain F, Khan MS, Iqbal T, Shah W, Ali B, et al. Physiology of gamma-aminobutyric acid treated Capsicum annuum L. (Sweet pepper) under induced drought stress. PLoS One. 2023;18(8):e0289900. doi:10.1371/journal.pone.0289900. [Google Scholar] [PubMed] [CrossRef]
97. Jurkonienė S, Gavelienė V, Mockevičiūtė R, Jankovska-Bortkevič E, Šveikauskas V, Jankauskienė J, et al. GABA and proline application induce drought resistance in oilseed rape. Plants. 2025;14(6):860. doi:10.3390/plants14060860. [Google Scholar] [PubMed] [CrossRef]
98. Liu C, Zhao L, Yu G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in Nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J Integr Plant Biol. 2011;53(8):608–18. doi:10.1111/j.1744-7909.2011.01049.x. [Google Scholar] [PubMed] [CrossRef]
99. Parida AK, Dagaonkar VS, Phalak MS, Aurangabadkar LP. Differential responses of the enzymes involved in proline biosynthesis and degradation in drought tolerant and sensitive cotton genotypes during drought stress and recovery. Acta Physiol Plant. 2008;30(5):619–27. doi:10.1007/s11738-008-0157-3. [Google Scholar] [CrossRef]
100. Maghsoudi K, Emam Y, Niazi A, Pessarakli M, Arvin MJ. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J Plant Interact. 2018;13(1):461–71. doi:10.1080/17429145.2018.1506516. [Google Scholar] [CrossRef]
101. Zhang Y, Zhang R, Song Z, Fu W, Yun L, Gao J, et al. Iris lactea var. chinensis plant drought tolerance depends on the response of proline metabolism, transcription factors, transporters and the ROS-scavenging system. BMC Plant Biol. 2023;23(1):17. doi:10.1186/s12870-022-04019-4. [Google Scholar] [PubMed] [CrossRef]
102. Naikoo MI, Dar MI, Raghib F, Jaleel H, Ahmad B, Raina A, et al. Chapter 9—role and regulation of plants phenolics in abiotic stress tolerance: an overview. In: Khan MIR, Reddy PS, Ferrante A, Khan NA, editors. Plant signaling molecules. Cambridge, UK: Woodhead Publishing; 2019. p. 157–68. doi:10.1016/B978-0-12-816451-8.00009-5. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools