 Open Access
Open Access
ARTICLE
Early Development and Phosphorus Use Efficiency Response to Phosphate Fertilizer Rates Associated with Phosphate-Solubilizing Bacteria in Contrasting Corn Hybrids
1 Department of Crop Science, State University of Mato Grosso do Sul (UEMS), Aquidauana, MS 79200-000, Brazil
2 Department of Agronomy, State University of Mato Grosso do Sul (UEMS), Cassilândia, MS 79540-000, Brazil
3 Department of Agronomy, State University of Maranhão (UEMA), Balsas, MA 65800-000, Brazil
4 Department of Sustainable Agricultural Innovation, Tecnológico Nacional de México (TecNM), Instituto Tecnológico del Valle del Yaqui, Bácum, 85276, Sonora, México
5 Research Institutes Office, National Intercultural University of Quillabamba (UNIQ), Cusco, 08741, Peru
* Corresponding Author: Jorge González Aguilera. Email:
(This article belongs to the Special Issue: Plant and Environments)
Phyton-International Journal of Experimental Botany 2025, 94(8), 2347-2363. https://doi.org/10.32604/phyton.2025.066264
Received 03 April 2025; Accepted 09 July 2025; Issue published 29 August 2025
Abstract
Corn (Zea mays L.) is a very sensitive crop to phosphorus (P) deficiency during the early development phase, which may be a limiting factor for the sustainable production of this crop in P-deficient tropical soils. However, scientific evidence indicates that inoculation with phosphate-solubilizing bacteria can improve the development, uptake, and P-use efficiency of corn plants. In the present study, two contrasting corn hybrids were investigated for their responsiveness to multiple inoculations of Bacillus subtilis, B. megaterium, B. velezencis, and Pseudomonas fluorescens and application of phosphate fertilizer rates in the sandy soil of the Brazilian Cerrado. Plants from stable (DKB 360 PRO3) and responsive (DKB 255 PRO3) corn hybrids were inoculated with 0 and 2 mL of inoculant containing multiple phosphate-solubilizing bacteria and fertilized with low (0 mg P·kg−1), medium (40 mg P·kg−1) and high (80 mg P·kg−1) levels of phosphate fertilizer using triple superphosphate (46% of P2O5). Treatments were distributed in a randomized block design using 2 × 2 × 3 factorial scheme, with four replicates. Plants were grown in 8-L pots for 70 days under greenhouse conditions. Morphological characteristics, leaf P concentration, and P use efficiency of corn plants were evaluated. Our results showed that the multiple inoculations of Bacillus subtilis, B. megaterium, B. velezencis, and Pseudomonas fluorescens are a promising sustainable agricultural practice to be recommended for corn cultivation, especially because it improves the development and P use efficiency of plants fertilized with medium P levels, which reduces the costs associated with mineral phosphate fertilization, a non-renewable fertilizer source. In sandy tropical soil with low P availability, applying intermediate rates of highly soluble phosphate fertilizer, such as triple superphosphate, is sufficient to maximize plant development and the nutritional status of corn crops for sustainable production with low environmental impact.Keywords
Corn (Zea mays L.) is the world’s main cereal crop. Brazil is currently the world’s third largest corn producer, with total production estimated at 122.8 million tons of grains for the 2024/2025 harvest. Corn production is important in Brazilian agribusiness, and approximately two-thirds of national production has been produced in the Cerrado tropical soils [1]. However, Cerrado soils are generally acidic and deficient in many essential plant nutrients, such as phosphorus (P) [2–4], which has limited the increase in yield of this crop in the Brazilian Cerrado region.
P deficiency in tropical soils is one of the main factors limiting crop development and productivity [4–6]. P is an essential element for the growth and development of corn plants, playing an important role in signal transduction, water absorption, photosynthesis, respiration, root development, flowering, and seed production [7–9]. P is also a structural component of many biomolecules, including phospholipids, nucleic acids (DNA and RNA), and adenosine triphosphate (ATP), which guide protein synthesis and corn development [8]. Therefore, P deficiency results in corn growth retardation and young plants are dwarfed and thin, with dark green leaves and the leaf margins, veins, and stems showing purple tints which may spread over the whole leaf blade [10,11]. In short, corn is susceptible to P at the early seedling stage, and the application of this nutrient is indispensable to maximize the growth and yield potential of the crop.
In soil, P is in the form of inorganic P or organic P. Although many tropical soils contain large amounts of total P (50 to 1800 mg·kg−1 P), only a minimal portion (<1%) is available to plants [12,13]. This is because in acidic soils (soil pH < 7.0), phosphate ions in the form of H2PO4– and HPO42– have high reactivity and high adsorption rate to soil colloids, especially to iron (Fe) and aluminum (Al) oxides and hydroxides associated with 1:1 clays (kaolinite) and 2:1 clays (montmorillonite) [14,15]. Nonetheless, plants of intense and short-cycle development, such as corn, require high levels of P in soil solution and faster replenishment of adsorbed-P compared to perennial crops [5,16].
Consequently, to satisfy the elevated P requirements of corn crops, substantial quantities of phosphate fertilizers have been utilized in agricultural production systems inside the Brazilian Cerrado region, which has been increasing production costs and decreasing the profitability of Brazilian farmers [5,17]. However, most of the phosphate fertilizer used in crop fertilization is lost in the environment due to the low P-use efficiency in cereal crops [18]. Furthermore, phosphate ore is a nonrenewable resource. Half of the world’s existing P reserves are predicted to be depleted within 50–100 years [19]. Therefore, improving soil P utilization efficiency is crucial for promoting plant growth, reducing environmental pollution, and improving resource management [13].
Over the last decade, some agroecological alternatives have been suggested to increase P use efficiency, increase grain yield, and reduce corn production costs in tropical agriculture. Among these agroecological and sustainable practices, the use of plant growth-promoting bacteria with the ability to solubilize phosphate has stood out [5,20]. The main phosphate-solubilizing bacteria used in tropical agriculture belong to the genera Azospirillum sp., Bacillus sp., and Pseudomonas sp. [20–23].
These bacteria can stimulate plant growth through a series of phosphate solubilization mechanisms, including especially the excretion of organic acids and the production of siderophores and indolic compounds [24,25]. The excretion of organic acids into the soil, such as malic, oxalic, and glutamic acid, can complex the Al3+ (accompanying ion) of aluminum phosphate (AlPO4), releasing the phosphate ion (PO4–3) into the soil solution, while siderophores are chelating agents with high affinity for metals such as aluminum (Al3+) and iron (Fe2+) present in insoluble Al and Fe oxides in the soil [26].
Some research has demonstrated the effectiveness of inoculation with phosphate-solubilizing bacteria, including Azospirillum sp., Bacillus sp., and Pseudomonas sp. in improving the development and yield of cereal crops [5,20]. Zarei et al. [27] reported that inoculation with Pseudomonas fluorescens (strains P1, P3, P8, and P14) improved plant growth and yield in corn due to its ability to solubilize phosphate and produce siderophores. Pereira et al. [5] showed that inoculation with Bacillus subtilis or Azospirillum brasilense resulted in higher P uptake efficiency in corn plants, which improved P nutritional status and grain yield. Galindo et al. [20] also reported positive effects of A. brasilense inoculation on corn development and yield. However, the effects of how the inoculation of multiple phosphate-solubilizing bacteria interacts with the application of soluble phosphate fertilizer rates to improve corn plant growth are still incipient and inconclusive. In this research, we tested the hypothesis that the combination of multiple phosphate-solubilizing bacteria and soluble phosphate fertilization could lead to a synergistic effect, resulting in higher P availability and higher plant biomass production compared to the use of phosphate-solubilizing bacteria or fertilizer alone. Additionally, we examined the interaction between varying rates of soluble phosphate fertilizer and specific strains of phosphate-solubilizing bacteria to optimize plant development and reduce environmental effects.
This study investigated the effect of multiple inoculations of Bacillus subtilis, B. megaterium, B. velezencis, and Pseudomonas fluorescens and mineral phosphate fertilization on the early development and P use efficiency of contrasting corn hybrids grown in a sandy soil of the Brazilian Cerrado.
The trial was conducted under greenhouse conditions in Cassilândia, Mato Grosso do Sul, Brazil (19°05′20 S, 51°48′50″ W, and an altitude of 540 m), from February to May 2024, in 8-L plastic pots. The minimum and maximum temperatures during the trial were 20.8°C and 31.2°C, respectively, and the average relative humidity was 76% (±7%).
The soil used in the trial was a P-deficient typical Quartzipsamment (sandy Entisol), collected from a Cerrado agricultural area, with 120 g·kg−1 of clay and 850 g·kg−1 of sand. The collected soil was initially acidic with a pH value of 4.5. Before starting the experiment, soil acidity was corrected by applying 0.85 g·kg−1 of limestone [30% CaO, 18% MgO, and 85% calcium carbonate equivalent (CCE)]. At 50 days after liming application, the soil had the following chemical properties: pH (water) of 6.4, 8.0 g·kg−1 organic matter, 6.8 mg kg−1·P (Mellich-1), 2.9 cmolc·kg−1 Ca2+, 0.8 cmolc·kg−1 Mg2+, 0.2 cmolc·kg−1 K+, 1.0 cmolc·kg−1 H + Al, 0.5 mg·kg−1 Cu2+, 1.0 mg·kg−1 Zn2+, 49 mg·kg−1 Fe2+, 8.7 mg·kg−1 Mn2+, 3.9 cmolc·kg−1 of CEC, 80% of soil base saturation. Tropical sandy soils with an availability of less than 12 mg·kg−1 P are considered deficient in this nutrient [28]. All the soil chemical properties were analyzed according to standard procedures.
The soil was fertilized with 30 mg·kg−1 N (urea), 100 mg·kg−1 K (potassium chloride), 10 mg·kg−1 S (gypsum), 1.3 mg·kg−1 Cu (copper sulfate), 1.3 mg·kg−1 Zn (zinc sulfate), and 0.7 mg·kg−1 B (boric acid). The pots were filled with 12 kg of soil (±8 dm3) sieved in a 5.0-mm mesh. The fertilizers were incorporated into the entire soil volume of the pots two days before planting the corn hybrids. At 30 and 50 days after corn planting, 30 mg·kg−1 of N was also applied using urea (45% N) previously diluted in pure water.
2.2 Corn Hybrids, Fertilization, Inoculation, and Experiment Management
The treatments were distributed in a randomized completely block design (RCBD), using two corn hybrids [one hybrid stable to the growing environment (DKB 360 PRO3) and another hybrid-responsive to the growing environment (DKB 255 PRO3), two inoculation treatments with phosphate-solubilizing bacteria [control (non-inoculated plants) and plants inoculated with Bacillus subtilis, B. megaterium, B. velezencis and Pseudomonas fluorescens] and three phosphate fertilization levels [0 mg·kg−1 P (low level), 40 mg·kg−1 P (medium level), and 80 mg·kg−1 P (high level)], in a factorial arrangement (2 × 2 × 3) with four replicates. Each experimental unit consisted of an 8-L pot containing two corn plants, totaling 48 pots in the experiment.
2.3 Experimental Design and Treatments
Corn seeds from two high-yielding single-hybrids (Table 1) widely cultivated in the Brazilian Cerrado region were purchased in the municipality of Chapadão do Sul, Mato Grosso do Sul, Brazil. The DKB 360 PRO3 is a stable hybrid with high stability and adaptability to various production environments. The DKB 255 PRO3 is a responsive hybrid with high production yields and excellent response to the best production environments.
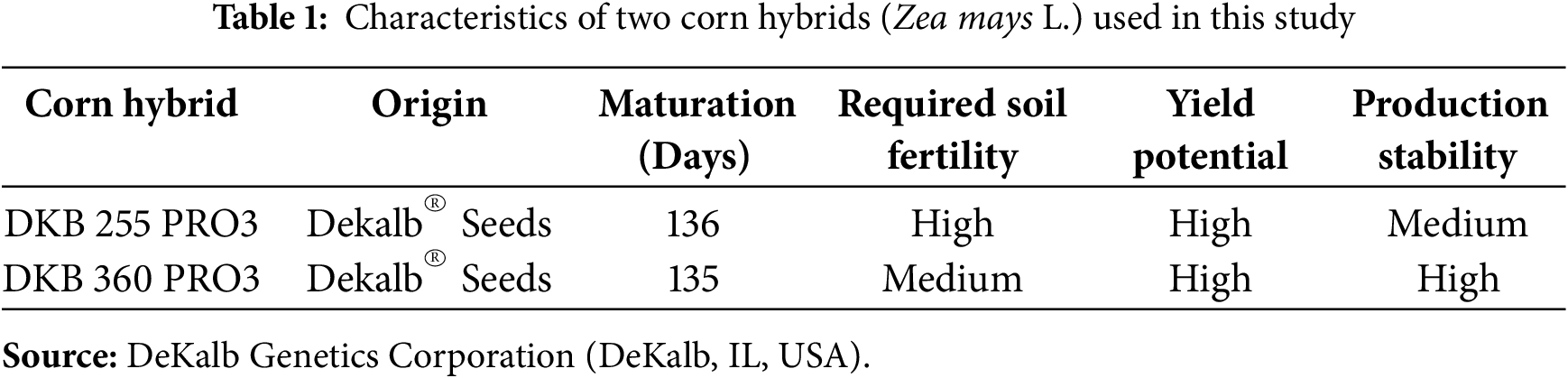
P levels were applied using triple superphosphate (46% of P2O5), and phosphate fertilizer amounts were incorporated into the entire soil volume of the pot two days before corn planting. Inoculation of multiple phosphate-solubilizing bacteria was performed in the sowing furrow using a pipette graduated in millimeters. A 2.0 mL aliquot of the commercial inoculant Acta Force® (Acta Bio, Jaboticabal, São Paulo, Brazil) containing strains of Bacillus subtilis, Bacillus megaterium, Bacillus velezencis, and Pseudomonas fluorescens was inoculated in each pot in 3.0 cm deep seeding furrows. All bacterial strains were at a minimum concentration of 1 × 109 colony-forming units per milliliter of product. The bacterial inoculant was kept refrigerated at 18°C until used in the experiment. The temperature range between 12°C and 25°C is considered adequate to maintain the viability of bacterial inoculants.
Two plants from the two corn hybrids were grown in individual pots and randomly distributed in the greenhouse. Soil moisture content was maintained close to the field capacity with daily irrigations using an automated micro-sprinkler system. The pest and disease control during the experiment was carried out according to need and the technical recommendations for the corn crops.
2.4 Determination of P Concentration
At 70 days after planting, corn leaves were collected from all pots. In the laboratory, the leaves were washed to remove any surface impurities, oven-dried at 65°C for 72 h, and finely ground in a Willey mill. A 500 mg sample was digested in a digestion block, with a nitric acid (HNO3) and perchloric acid (HClO4) mixture (3:1; v:v) in Pyrex tubes. After cooling, they were diluted to a final volume of 25 mL with deionized water. P concentration was analyzed by colorimetry method at 725 nm wavelength using a UV-visible spectrophotometer (Perkin Elmer-Lambda 3B, PerkinElmer, Shelton, CT, USA), as previously described by Malavolta et al. [29]. All analyses were processed in triplicate. Subsequently, the amount of accumulated P (g/plant) in the aboveground biomass was calculated.
2.5 Determination of Plant Morphological Traits
At 70 days after planting, the plants were harvested, and plant height (PH), stalk diameter (SD), leaf area (LA), root length (RL), root volume (RV), shoot dry matter (SDM), root dry matter (RDM), and total dry matter (TDM). Plants were divided into leaves, stalks, and roots, oven-dried for three days at 65°C, and then weighed. The SDM was obtained from the sum of the dry matter of the leaves and stalks. Total dry matter (TDM) was obtained from the sum of all the plant parts (leaves, stalks, and roots). The PH was determined using a metal tape measure, considering the distance from the soil surface to the +1 leaf. The SD was measured at 5 cm from the stalk collar using a digital caliper. The RL was determined using a metal tape measure, considering the length of the longest root. The RV was determined by the water displacement method using a 1000 mL graduated cylinder.
2.6 Determination of P Use Efficiency
The P-use efficiency (PUE) of corn hybrids at each P fertilizer level (medium or high) was calculated according to Eq. (1), adapted from Craswell and Godwin [30]:
where TDMFERTILIZED is the total dry matter production (in g/pot) of corn plants fertilized with medium or high levels of phosphate fertilizer; TDMUNFERTILIZED is the total dry matter production (in g/pot) of non-fertilized plants with soluble phosphate (low P level); and PAPPLIED is the amount of phosphate fertilizer applied at medium and high P levels (in g/pot) compared to low P level (not fertilized with phosphate fertilizer).
The data were previously tested for homoscedasticity of variances (Levene test; p = 0.05) and normality of residues (Kolmogorov–Smirnov test; p = 0.05). Data were submitted to analysis of variance (ANOVA), and the means were compared by the Tukey test at the 0.05 level of confidence. The analyses were performed using Sisvar® software (version 5.9) for Windows (Statistical Analysis Software, UFLA, Lavras, Minas Gerais, Brazil).
The results of the analysis of variance showed significant effects (p ≤ 0.05) for the main effects of hybrids, phosphate fertilization levels, and inoculation of phosphate-solubilizing bacteria, as well as for the triple interaction, for all morphological traits of corn plants (Table 2). This significant interaction for all plant morphological traits indicates that corn hybrids (stable or responsive) have distinct responses when exposed to phosphate fertilization levels or inoculated with phosphate-solubilizing bacteria. Therefore, the results of the statistical analyses were unfolded, and the morphological responses of each corn hybrid (stable or responsive) were presented individually and independently.
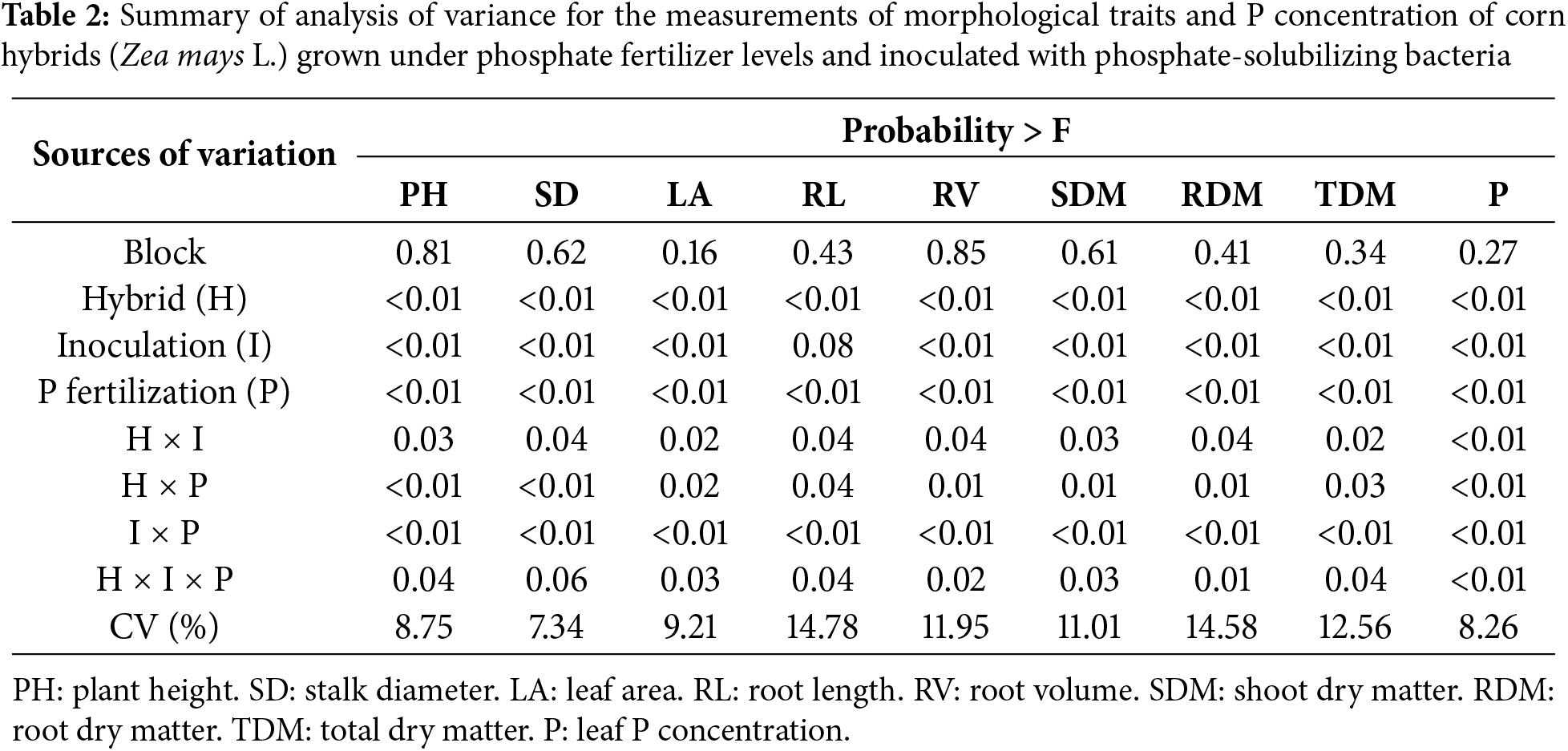
The coefficient of variation (CV) values obtained in the experimental trial were less than 15% for all morphological characteristics of corn plants, a value considered low for agricultural experiments (Table 2). The CV is a measure of dispersion used to estimate the precision of the experimental trial, and when the values are less than 20% it indicates that the results of the experiment have excellent precision.
Phosphate fertilization levels and inoculation of phosphate-solubilizing bacteria significantly affected (p < 0.05) plant height, stalk diameter, and leaf area of stable and responsive corn hybrids (Fig. 1). Both corn hybrids responded positively to phosphate fertilizer application; however, the greatest shoot growth response was observed in the responsive hybrid. Plants of the stable hybrid inoculated with phosphate-solubilizing bacteria have greater shoot height when fertilized with medium P levels (Fig. 1A). In contrast, in the responsive hybrid, inoculation with phosphate-solubilizing bacteria resulted in greater plant height with the application of low and medium P levels (Fig. 1B).
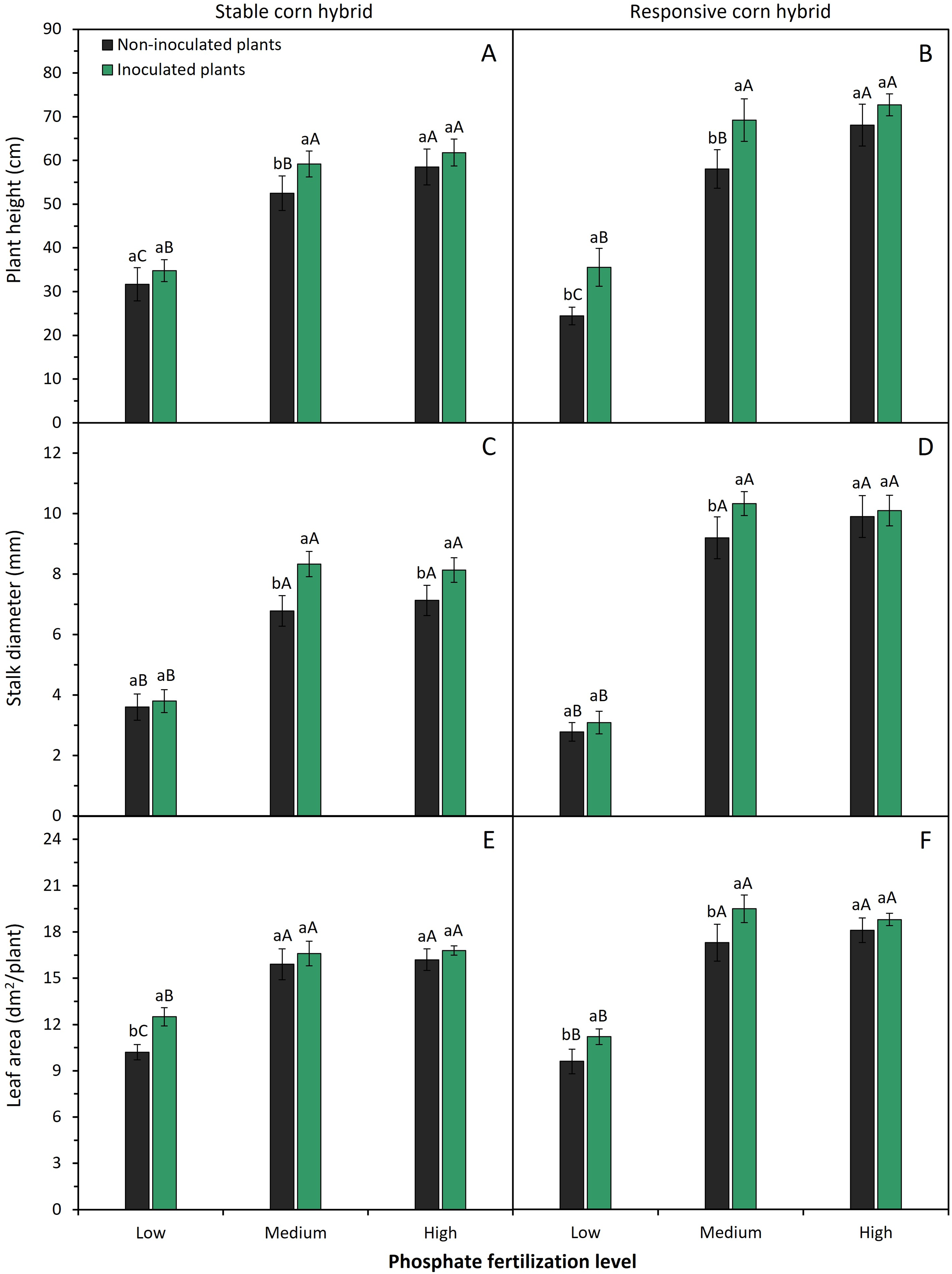
Figure 1: Effect of phosphate fertilizer level and inoculation of phosphate-solubilizing bacteria on shoot height (A,B), stalk diameter (C,D), and leaf area (E,F) of corn plants from the stable hybrid DKB 360 PRO3 (A,C,D) and the responsive hybrid DKB 255 PRO3 (B,D,E). Bars followed by distinct lowercase letters (a, b) for the inoculation of phosphate-solubilizing bacteria or distinct uppercase letters (A, B, C) for the phosphate fertilization levels show significant differences by the Tukey test (p = 0.05). Data refers to mean values (n = 4) ± standard error of the mean
The stable corn hybrid inoculated with phosphate-solubilizing bacteria has a larger stalk diameter when fertilized with medium and high P levels (Fig. 1C). In turn, the stalk diameter of the responsive hybrid was significantly larger with the inoculation of phosphate-solubilizing bacteria only with the application of the medium P level (Fig. 1D). In the stable hybrid, inoculation of phosphate-solubilizing bacteria resulted in the largest leaf area of plants only at the low P level (Fig. 1E). In contrast, plants of the responsive hybrid inoculated with phosphate-solubilizing bacteria have greater leaf area when fertilized with low and medium P levels (Fig. 1F). In general, the results presented here show that the positive response of corn to inoculation with phosphate-solubilizing bacteria is dependent on the level of phosphate fertilization, with the greatest responses being obtained when corn was exposed to low and medium P levels.
Phosphate fertilization levels and inoculation of phosphate-solubilizing bacteria significantly affected (p < 0.05) root length and root volume of stable and responsive corn hybrids (Fig. 2).
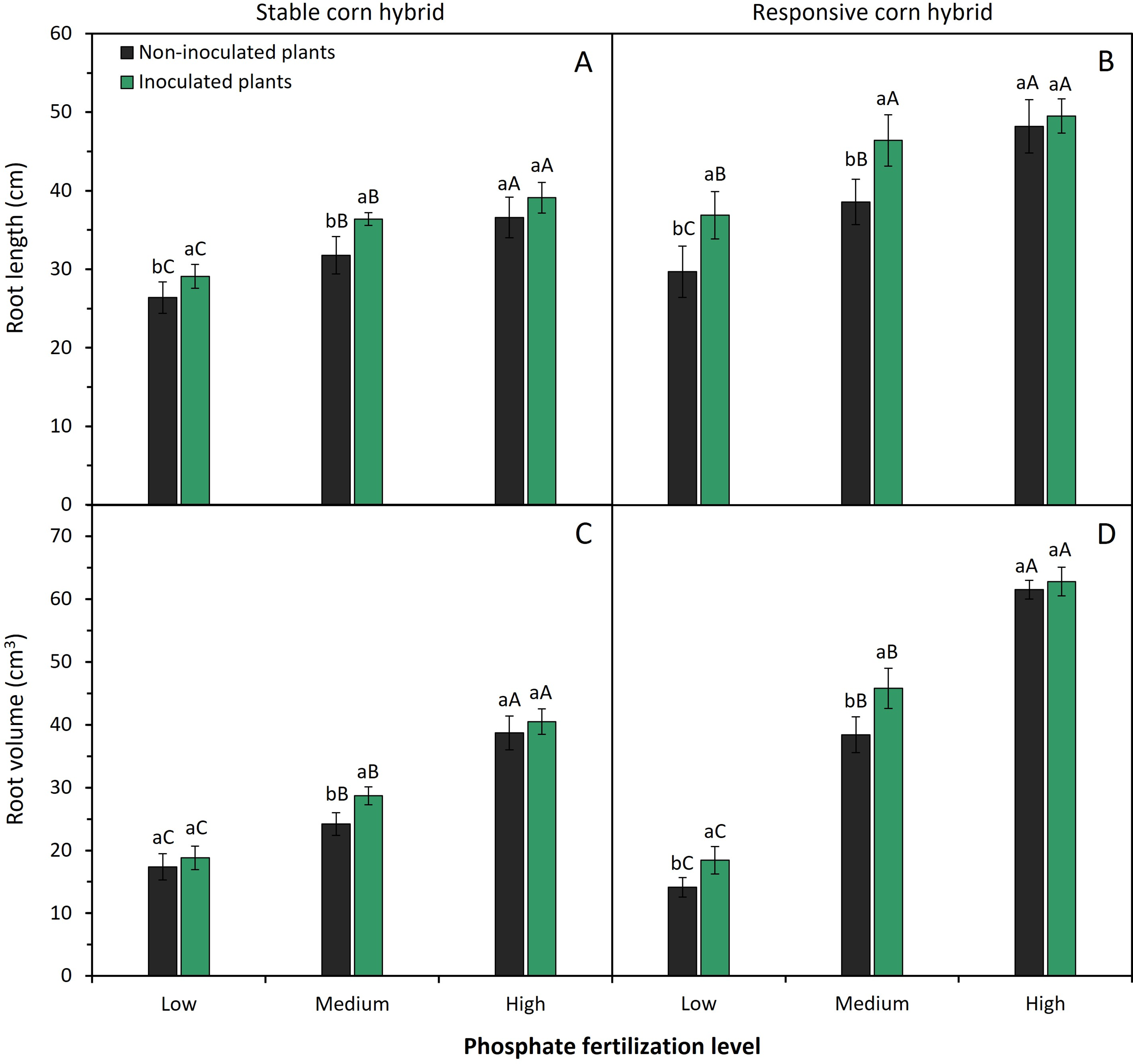
Figure 2: Effect of phosphate fertilizer level and inoculation of phosphate-solubilizing bacteria on root length (A,B) and root volume (C,D) of corn plants from the stable hybrid DKB 360 PRO3 (A,C) and the responsive hybrid DKB 255 PRO3 (B,D). Bars followed by distinct lowercase letters (a, b) for the inoculation of phosphate-solubilizing bacteria or distinct uppercase letters (A, B, C) for the phosphate fertilization levels show significant differences by the Tukey test (p = 0.05). Data refers to mean values (n = 4) ± standard error of the mean
Increasing phosphate fertilizer levels resulted in a progressive increase in root length and root volume of plants of both corn hybrids. In both corn hybrids, inoculation of phosphate-solubilizing bacteria increased root length when plants were grown under low and medium P levels (Fig. 2A,B). In the stable hybrid, plants inoculated with phosphate-solubilizing bacteria had greater root volume when fertilized with a medium P level (Fig. 2C). In contrast, in the responsive hybrid, inoculation with phosphate-solubilizing bacteria resulted in greater root volume with the application of low and medium P levels (Fig. 2D). In plants fertilized with a high level of P fertilizer, inoculation of phosphate-solubilizing bacteria has no significant effect on the root length and root volume of corn plants.
Phosphate fertilization levels and inoculation of phosphate-solubilizing bacteria significantly (p < 0.05) affected the shoot, root, and total dry matter production of corn plants, especially the responsive corn hybrid (Fig. 3). Medium and high levels of phosphate fertilizer application resulted in higher shoot, root, and total dry matter production of stable and responsive corn hybrids. Plants of stable and responsive corn hybrids fertilized with high P levels have an average increase of 205% and 670% in shoot dry matter production and an average increase of 80% and 140% in root dry matter production when compared to plants grown under low levels of phosphate fertilizer, respectively (Fig. 3).
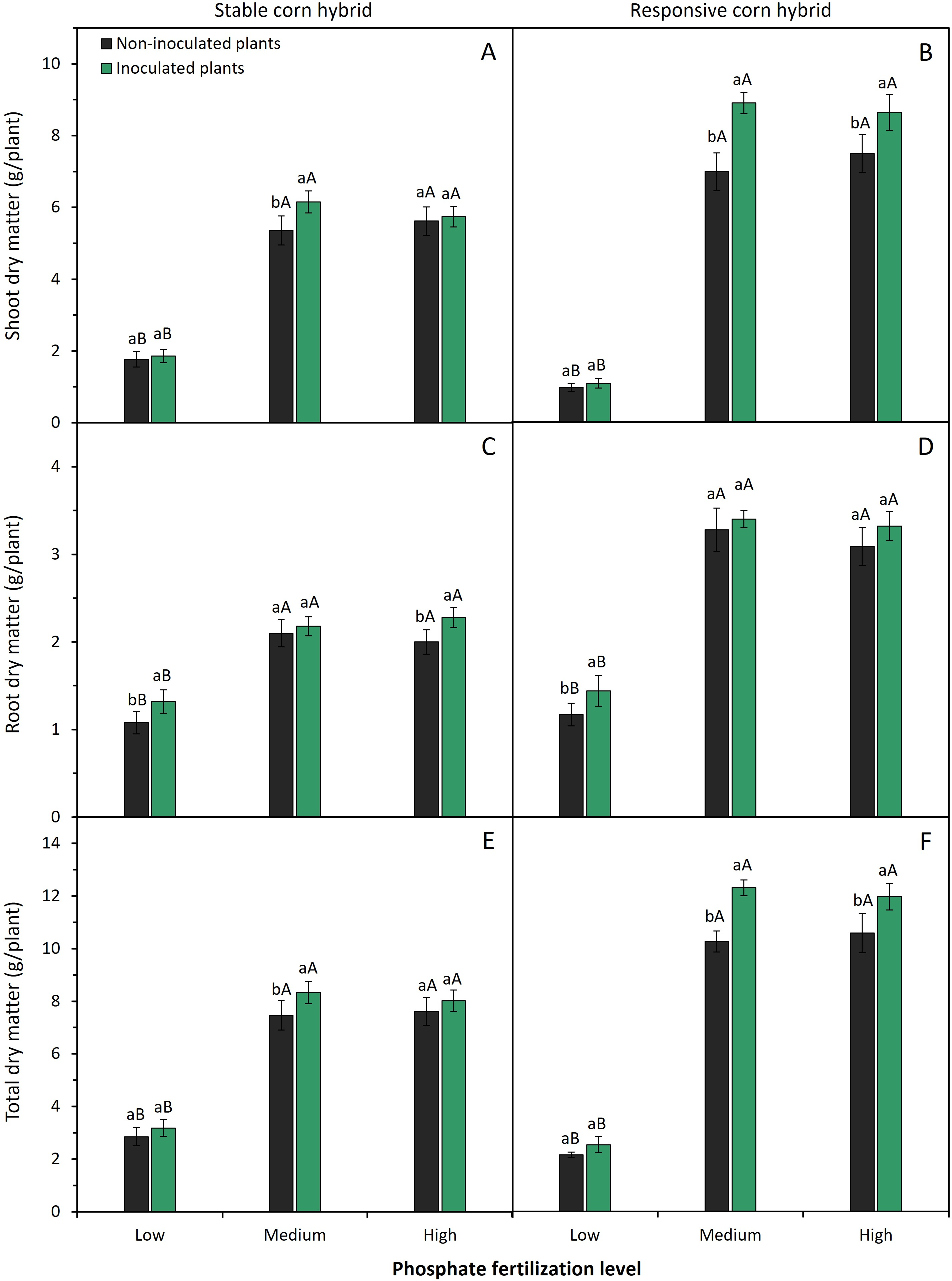
Figure 3: Effect of phosphate fertilizer level and inoculation of phosphate-solubilizing bacteria on shoot dry matter (A,B), root dry matter (C,D), and total dry matter (E,F) of corn plants from the stable hybrid DKB 360 PRO3 (A,C,E) and the responsive hybrid DKB 255 PRO3 (B,D,F). Bars followed by distinct lowercase letters (a, b) for the inoculation of phosphate-solubilizing bacteria or distinct uppercase letters (A, B) for the phosphate fertilization levels show significant differences by the Tukey test (p = 0.05). Data refers to mean values (n = 4) ± standard error of the mean
Inoculation of phosphate-solubilizing bacteria resulted in higher shoot dry matter production of the stable corn hybrid only when fertilized with medium P level (Fig. 3A). In the responsive hybrid, inoculation of phosphate-solubilizing bacteria resulted in greater shoot dry matter production with the application of medium to high P levels (Fig. 3B). Stable and responsive corn hybrids have greater root dry matter accumulation when grown under low P (Fig. 3C,D). Furthermore, root dry matter accumulation of stable corn hybrid is also enhanced by inoculation of phosphate-solubilizing bacteria (Fig. 3C). Finally, plants of both corn hybrids have higher total dry matter when inoculated with phosphate-solubilizing bacteria and grown under medium P levels (Fig. 3E,F).
Leaf P concentration under low P levels was 2.0 to 2.2 g P·kg−1 in the stable hybrid (Fig. 4A) and 1.4 to 1.9 g P·kg−1 in the responsive corn hybrid (Fig. 4B). These values are lower than the leaf P concentration considered optimal for adequate corn crop growth, which is between 2.5 and 3.5 g P·kg−1. However, under medium and high levels of P fertilization, leaf P concentration ranged from 2.9 to 3.6 g P·kg−1, with an average value of 3.2 g P·kg−1 in the stable hybrid (Fig. 4A) and 3.5 g P·kg−1 in the respective corn hybrid (Fig. 4B). These values indicate that the P concentration in the leaf tissue of corn plants was within the range considered adequate for the crop. Inoculation with phosphate-solubilizing bacteria increased the foliar P concentration of stable and responsive corn hybrids fertilized with medium and low levels of P, respectively (Fig. 4A,B).
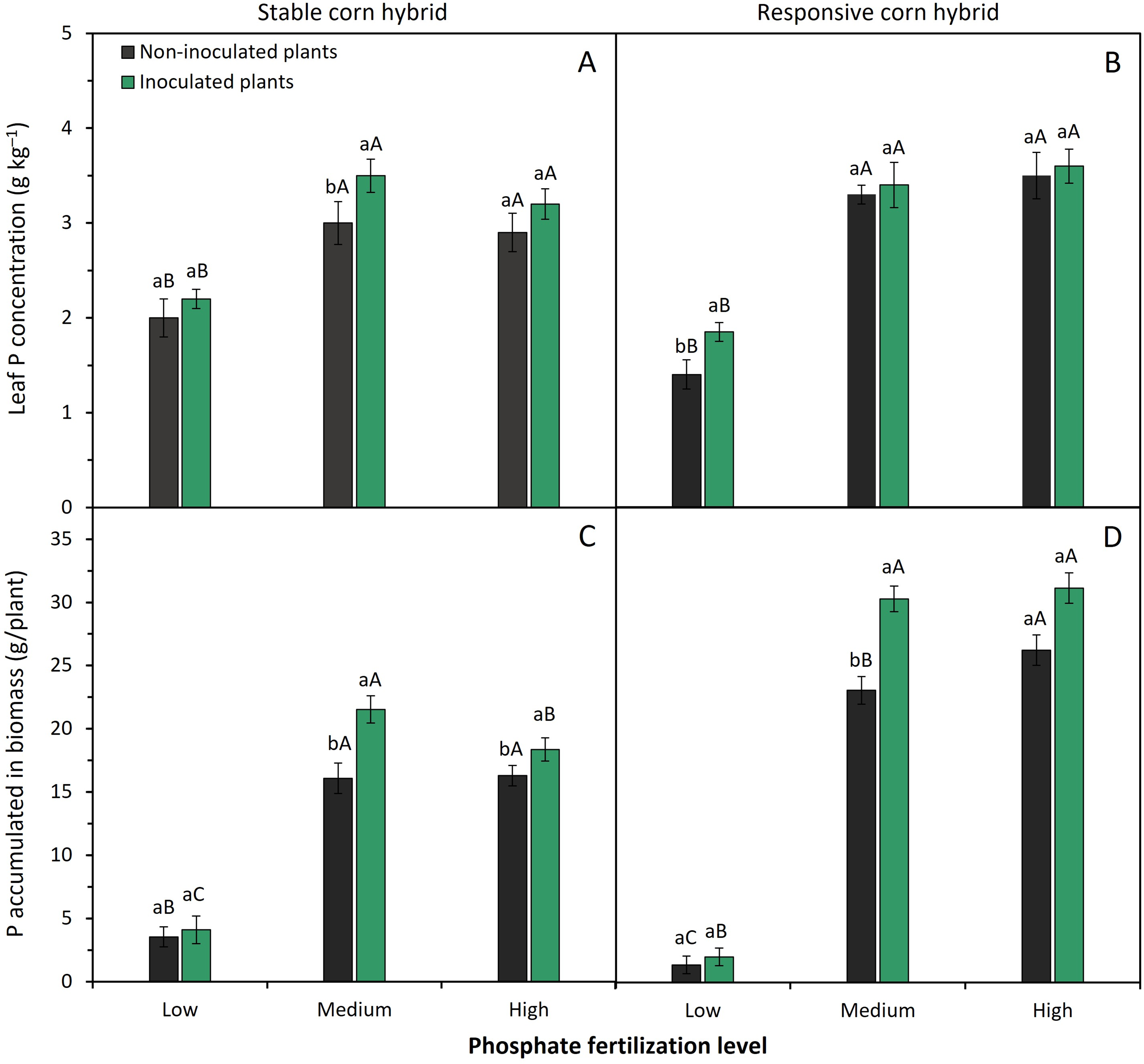
Figure 4: Effect of phosphate fertilizer level and inoculation of phosphate-solubilizing bacteria on leaf P concentration (A,B) and amount of P accumulated of biomass (C,D) of corn plants from the stable hybrid DKB 360 PRO3 (A,C) and the responsive hybrid DKB 255 PRO3 (B,D). Bars followed by distinct lowercase letters (a, b) for the inoculation of phosphate-solubilizing bacteria or distinct uppercase letters (A, B, C) for the phosphate fertilization levels show significant differences by the Tukey test (p = 0.05). Data refers to mean values (n = 4) ± standard error of the mean
The amount of P accumulated in the aboveground biomass was significantly higher in plants fertilized with medium and high levels of P in both corn hybrids. Furthermore, inoculation with phosphate-solubilizing bacteria resulted in greater P accumulation in the biomass of both corn hybrids grown under medium and high levels of P fertilizer (Fig. 4C,D). Responsive corn hybrids have a greater potential for P accumulation in biomass when fertilized with medium and high levels of P fertilization. However, under low P levels, the stable hybrid has greater adaptation to this nutrient-restrictive condition and a greater potential for P accumulation in plant biomass (Fig. 4C,D).
P use efficiency (PUE) ranged from 19.2 to 40.7 kg·kg−1 and from 9.9 to 19.6 kg·kg−1 with the application of medium and high levels of phosphate fertilizer, respectively (Fig. 5). Inoculation with phosphate-solubilizing bacteria improved PUE only in the responsive corn hybrid fertilized with a medium level of phosphate fertilizer. In addition, the responsive corn hybrid has higher PUE when compared to the stable hybrid (Fig. 5). These results show that the responsive hybrid has a greater absorption capacity and greater P-use efficiency when compared to the stable corn hybrid.
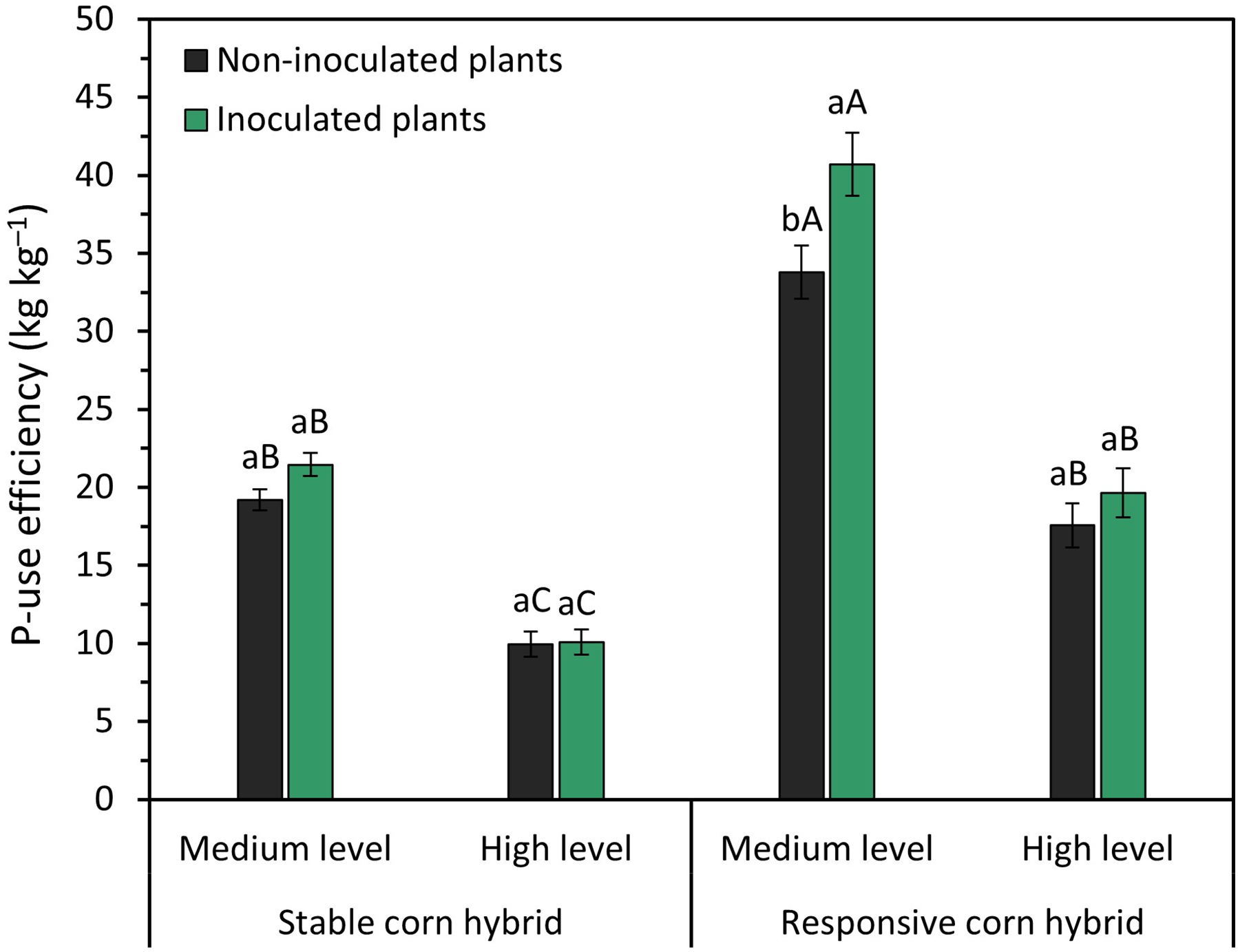
Figure 5: Effect of phosphate fertilizer level and inoculation of phosphate-solubilizing bacteria on P-use efficiency of stable (DKB 360 PRO3) and responsive (DKB 255 PRO3) corn hybrids. Bars followed by distinct lowercase letters (a, b) for the inoculation of phosphate-solubilizing bacteria or distinct uppercase letters (A, B, C) for the phosphate fertilizer levels and corn hybrids show significant differences by the Tukey test (p = 0.05). Data refers to mean values (n = 4) ± standard error of the mean
The application of medium and high levels of phosphate fertilizer improved shoot and root growth, P acquisition, and P accumulation of both corn hybrids (Figs. 1–4). These results highlight the importance of an adequate supply of phosphate fertilizer to enhance the development and P acquisition of corn plants grown in tropical soils of the Cerrado. The greater development and greater P uptake of plants fertilized with medium or high rates of phosphate fertilizer is associated with the low initial concentration of P available in the soil (6.8 mg·kg−1 P), which was not sufficient to meet all the requirements of this nutrient by plants grown under low P level (i.e., unfertilized plants with P). Indeed, the minimum concentration (critical level) of P in the sandy soils of the Cerrado to ensure agricultural production with maximum economic efficiency is 12 to 14 mg kg−1 P (P Melhich-1) [28]. The low availability of P in tropical Cerrado soils caused by the high reactivity and high adsorption capacity of phosphate ions (H2PO4– and HPO42–) to soil colloids, especially Fe and Al oxides and hydroxides [14,15], has been one of the main abiotic factors limiting the development of corn plants [5,17]. Therefore, high rates of phosphate fertilizers have been recommended and applied to obtain high levels of grain yield, which increases production costs and reduces farmers’ profitability.
Plant height (Fig. 1A,B), stalk diameter (Fig. 1C,D), root volume (Fig. 2C,D), shoot dry matter (Fig. 3A,B) and root dry matter (Fig. 3C,D) were the morphological traits of corn plants most impacted by the application of high rates of phosphate fertilizer. Pereira et al. [5] documented an enhancement in plant height, stem diameter, and shoot dry matter of corn plants fertilized with phosphate fertilizer rates. Similarly, Li et al. [18] showed that the application of P rates increased root volume and root dry matter production. P plays an important role in the corn plant’s growth and physiological metabolism, acting in the processes of signal transduction, respiration, photosynthesis, water uptake, and root development [7–9]. P is also a structural component of many biomolecules, including phospholipids, nucleic acids, and adenosine triphosphate (ATP), which guide plant development and protein synthesis [8]. Therefore, P deficiency results in corn growth retardation, young plants are dwarfed and thin, with dark green leaves, and the leaf margins, veins, and stems show purple tints which may spread over the whole leaf blade [10,11].
Plants of the responsive corn hybrid have a higher response potential to phosphate fertilization when compared to the stable hybrid (Figs. 1–3). For example, plants of the responsive hybrid grown under high P fertilization had an average increase in plant height (140%), stalk diameter (240%), leaf area (78%), root length (48%), root volume (288%), shoot dry matter (670%), root dry matter (139%), and leaf P concentration (120%) when compared to plants under low P fertilization. In turn, the average increase in plants of the stable hybrid grown under high P fertilization was 81% in plant height, 107% in stalk diameter, 46% in leaf area, 36% in root length, 118% in root volume, 205% in shoot dry matter, 80% in root dry matter, and 45% in leaf P concentration compared to plants grown under low P fertilization. The greater response to phosphate fertilization of the responsive hybrid is due to the plants of this hybrid having a high capacity to respond positively to the application of fertilizer rates. In turn, the stable hybrid has greater adaptability and greater development potential in conditions of low soil P availability, being an efficient hybrid in the use of nutrients, such as P. Therefore, the responsive hybrid should be recommended for cultivation by farmers who use high levels of phosphate fertilization, while the stable hybrid, which is efficient and non-responsive to phosphate fertilization, should be recommended for cultivation in agricultural areas with low soil P availability or, in situations where farmers only have conditions to apply low levels of phosphate fertilizer. These results are relevant and highlight the importance of studies that aim to provide information about new corn hybrids that can be increasingly productive and highly stable, to make them available to farmers in the Brazilian Cerrado region, confirming the findings of Lima et al. [31]. Kendal et al. [32] emphasized that a perfect corn hybrid should demonstrate consistent performance and reliability across various growing conditions to secure substantial grain output in diverse, difficult, and unfavorable farming situations.
Multiple inoculations of Bacillus subtilis, B. megaterium, B. velezencis, and Pseudomonas fluorescens improved shoot and root development of corn hybrids, especially under medium levels of phosphate fertilization (Figs. 1–3). Under low levels of phosphate fertilization, the inoculation of these phosphate-solubilizing bacteria had a greater beneficial effect on the plant development of the responsive corn hybrid. Other studies have also demonstrated the effectiveness of inoculating bacteria of the Bacillus and Pseudomonas genus in cereal crops, resulting in greater plant development, greater P use efficiency, and higher grain yield [5,27]. Zarei et al. [27] reported that inoculation of Pseudomonas fluorescens improved the development and yield of corn crops. Pereira et al. [5] showed that inoculation of B. subtilis, P. fluorescens, and Azospirillum brasilense improved the development and P acquisition of corn plants. Ahmad et al. [33] also showed that inoculation of B. aryabhattai S10 and B. subtilis ZM63 increased the growth and P uptake of corn plants. Rodrigues et al. [34] reported that inoculation with B. megaterium BRM 119 and B. subtilus BMF 2484 improved plant development due to the high P solubilization and mineralization capacity of these bacterial strains. Therefore, these results highlight the importance of inoculation with phosphate-solubilizing bacteria, especially Bacillus sp., in enhancing the early development and P acquisition of corn plants.
Based on previous research, the potential of these bacteria to solubilize inorganic phosphate and mineralize organic phosphate from the soil and stimulate plant growth is related to the mechanisms of excretion of organic acids and production of siderophores adopted by these microorganisms [24–26]. Indeed, studies by Velloso et al. [35] and Silva et al. [22] showed that some Bacillus species, including B. megaterium, B. subtilis, and B cereus, can solubilize phosphate (via the release of organic and inorganic acid and proton extrusion), mineralize phosphate (via phosphatase production) and produce chelating agents with high affinity for aluminum (Al3+) and iron (Fe2+) metals present in insoluble Al and Fe oxides in tropical soils (via siderophore production). Similarly, Oliveira-Paiva et al. [36] showed that strains of B. megaterium B119 and B. subtilis B2084 have relevant properties to improving soil P dynamics, including phosphate solubilization and mineralization, production of indole-3-acetic acid (IAA)-like molecules, siderophores, exopolysaccharides (EPS), biofilms, and phosphatases.
The greater P accumulation in plants inoculated with multiple phosphate-solubilizing bacteria and grown under medium and high rates of phosphate fertilizer indicates that the use of these bacteria improved the P acquisition from the soil by both corn hybrids (Fig. 4C,D). Pereira et al. [5] also reported that the inoculation of B. subtilis resulted in higher P uptake by corn plants. Similarly, Oliveira-Paiva et al. [36] showed that inoculation with B. megaterium and B. subtilis improved P acquisition by corn plants. Zarei et al. [27] also showed that corn plants inoculated with Pseudomonas fluorescens have a greater P uptake capacity compared to non-inoculated plants. These previous studies show that bacteria of the Bacillus and Pseudomonas genera have a high potential to solubilize and mineralize soil phosphate, and, therefore, have potential for use as biofertilizers in tropical soils. For example, these bacteria can produce and excrete organic acids into the soil, such as malic, oxalic, and glutamic acids, which can complex the Al3+ (accompanying ion) of aluminum phosphate (AlPO4), releasing the phosphate ion (PO4–3) into the soil solution, which can be absorbed by plant roots [26,36,37]. Therefore, the use of phosphate-solubilizing bacteria (Bacillus sp. and Pseudomonas fluorescens) allows Brazilian farmers to adopt a sustainable and low-cost technology and thus obtain an increase in the development and acquisition of P by corn plants in Cerrado soils.
Inoculation with multiple phosphate-solubilizing bacteria improved P use efficiency (PUE) only in the responsive corn hybrid grown under medium P fertilizer application (Fig. 5). Our results showed that the PUE of responsive hybrid plants inoculated with multiple phosphate-solubilizing bacteria increased by 20% when compared to uninoculated plants grown under a medium P rate (Fig. 1). Pereira et al. [5] also reported that inoculation of B. subtilis improved PUE in corn plants, which resulted in greater P acquisition, crop development, and grain yield. The greater PUE of plants inoculated with multiple phosphate-solubilizing bacteria is associated with the multifunctional mechanisms of these bacteria, especially those related to soil phosphate solubilization and mineralization [36,37], which play a crucial role in increasing P uptake and use efficiency, especially in soils with high P adsorption capacity [24,25].
Indeed, many studies have demonstrated the effectiveness of several mechanisms of phosphate solubilization, and mineralization exhibited by strains of Bacillus and Pseudomonas in mitigating P adsorption in tropical soils, where soluble P reacts with clay minerals and metal ions (Ca, Fe, Al) to form poorly soluble complexes that are unavailable to plants [12,25,36,37]. However, our results showed that these mechanisms of phosphate solubilization and mineralization by these bacteria are still inefficient when high rates of phosphate mineral fertilizers are applied to the soil. Therefore, future research should be conducted to elucidate how the various physicochemical and molecular mechanisms (e.g., root exudates, extracellular polysaccharides, organic acids, phosphatases, and phosphate-specific transport systems) utilized by phosphate-solubilizing bacteria are impacted when high rates of mineral phosphate fertilizers are applied to the soil.
The lower PUE with the application of high rates of phosphate fertilizer is related to Mitscherlich’s law of diminishing returns. Mitscherlich’s law of diminishing returns states that when increasing rates of nutrients are added, the greatest increase in development and productivity is obtained with the lowest rate applied, and with successive applications of nutrients, the productivity increases, and the nutrient use efficiency will be gradually lower [29]. Therefore, the application of high rates of phosphate fertilizer (80 mg P·kg−1) resulted in lower PUE when compared with the medium rate of phosphate fertilizer (40 mg P·kg−1) (Fig. 5).
Overall, our results reinforce the importance of using these multiple phosphate-solubilizing bacteria (Bacillus subtilis, B. megaterium, B. velezencis, and Pseudomonas fluorescens) to stimulate plant development and improve P use efficiency in corn hybrids grown in tropical soils, which reduces the costs associated with mineral P fertilization and increases the farmer’s profitability. In conclusion, we firmly believe that phosphate solubilizing bacteria represent a highly significant biotechnological tool to improve the development and grain yield of corn crops, as well as to promote the development of modern and sustainable agriculture for future generations. However, further research must be conducted under field conditions to prove the beneficial effects of these phosphate-solubilizing bacteria, when inoculated alone or in combination, in corn crops.
Multiple inoculation of Bacillus subtilis, B. megaterium, B. velezencis, and Pseudomonas fluorescens is a promising sustainable agricultural practice to be recommended for corn cultivation, especially because it improves the development and P use efficiency of plants fertilized with medium P levels, which reduces the costs associated with mineral phosphate fertilization, a non-renewable fertilizer source. However, these effects should be validated in field trials conducted in future agricultural research.
In sandy tropical soil with low P availability, applying intermediate rates of highly soluble phosphate fertilizer, such as triple superphosphate, is sufficient to maximize plant development and the nutritional status of corn crops for sustainable production with low environmental impact.
Acknowledgement: The authors thank the Plant Ecophysiology Laboratory, State University of Mato Grosso do Sul (UEMS).
Funding Statement: This research was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001. This article was translated with financial resources from the Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul—FUNDECT (Termo de Outorga: 133/2023/SIAFEM: 33108).
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Gilciany Ribeiro Soares and Fábio Steiner; methodology, Gilciany Ribeiro Soares, Fábio Steiner, Jorge González Aguilera and Alan Mario Zuffo; validation, Fábio Steiner, Jorge González Aguilera, Wellingthon da Silva Guimarães Júnnyor, Leandris Argentel-Martínez and Luis Morales-Aranibar; formal analysis, Fábio Steiner, Jorge González Aguilera, José Vitor Marçal do Prado, Wellingthon da Silva Guimarães Júnnyor, Leandris Argentel-Martínez and Luis Morales-Aranibar; investigation, Gilciany Ribeiro Soares, Jiovana Kamila Vilas Boas and Fábio Steiner; data curation, Fábio Steiner, Jorge González Aguilera and Alan M. Zuffo; writing—original draft preparation, Gilciany Ribeiro Soares, Jiovana Kamila Vilas Boas and José Vitor Marçal do Prado; writing—review and editing, Fábio Steiner, Jorge González Aguilera, Alan Mario Zuffo, Wellingthon da Silva Guimarães Júnnyor, Leandris Argentel-Martínez and Luis Morales-Aranibar; supervision, Fábio Steiner, Jorge González Aguilera, Alan Mario Zuffo, Wellingthon da Silva Guimarães Júnnyor, Leandris Argentel-Martínez and Luis Morales-Aranibar; funding acquisition, Gilciany Ribeiro Soares and Fábio Steiner. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the Corresponding Author, Jorge González Aguilera, upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Vilela GF, Farias AR, Paim FAP, Castro GSA, Oshiro OT, Carvalho CA. Cerrado: agricultural production and areas designated for environmental preservation registered in the Brazilian rural environmental registry (Cadastro Ambiental Rural). J Environ Sci Eng B. 2020;9(3):87–107. doi:10.17265/2162-5263/2020.03.001. [Google Scholar] [CrossRef]
2. Gomes L, Simões SJC, Dalla Nora EL, Sousa-Neto ER, Forti MC, Ometto JPHB. Agricultural expansion in the Brazilian Cerrado: increased soil and nutrient losses and decreased agricultural productivity. Land. 2019;8(1):e12. doi:10.3390/land8010012. [Google Scholar] [CrossRef]
3. Cassol CJ, Arruda EJ, Alovisi AMT, Lourente ERP, Abrão CMR. Natural fertility and intrinsic fragility of soils in the Brazilian Cerrado. Rev Agron Meio Amb. 2023;16(2):e10087. doi:10.17765/2176-9168.2023v16n2e10087. [Google Scholar] [CrossRef]
4. Marques ALR, Oliveira IS, Guedes JVFL, Aguilera JG, Zuffo AM, Steiner F. Use efficiency and response to the application of phosphorus from cotton cultivars in the tropical soil of the Cerrado. Ens E Ciência. 2024;28(2):149–58. (In Portuguese). doi:10.17921/1415-6938.2024v28n2p149-158. [Google Scholar] [CrossRef]
5. Pereira NCM, Galindo FS, Gazola RPD, Dupas E, Rosa PAL, Mortinho ES, et al. Corn yield and phosphorus use efficiency response to phosphorus rates associated with plant growth-promoting bacteria. Front Environ Sci. 2020;8:e40. doi:10.3389/fenvs.2020.00040. [Google Scholar] [CrossRef]
6. Ardon HJV, Steiner F, Rosa L, Zuffo AM, Bardiviesso DM. Classification of soybean genotypes for phosphorus use efficiency and response in sandy soil of the Brazilian Cerrado. Rev De Ciências Agrárias. 2022;45(3):94–104. (In Portuguese). doi:10.19084/rca.27469. [Google Scholar] [CrossRef]
7. Zhang W, Chen XX, Liu YM, Liu DY, Du YF, Chen XP, et al. The role of phosphorus supply in maximizing the leaf area, photosynthetic rate, coordinated to grain yield of summer maize. Field Crops Res. 2018;219:113–9. doi:10.1016/j.fcr.2018.01.031. [Google Scholar] [CrossRef]
8. Ma J, Chen T, Lin J, Fu W, Feng B, Li G, et al. Functions of nitrogen, phosphorus and potassium in energy status and their influences on rice growth and development. Rice Sci. 2022;29(2):166–78. doi:10.1016/j.rsci.2022.01.005. [Google Scholar] [CrossRef]
9. Khan F, Siddique AB, Shabala S, Zhou M, Zhao C. Phosphorus plays key roles in regulating plants’ physiological responses to abiotic stresses. Plants. 2023;12:e2861. doi:10.3390/plants12152861. [Google Scholar] [PubMed] [CrossRef]
10. Wang LY, Hou BC, Zhang DS, Lyu Y, Zhang K, Li HG. The niche complementarity driven by rhizosphere interactions enhances phosphorus-use efficiency in maize/alfalfa mixture. Food Energy Secur. 2020;9:e252. doi:10.1002/fes3.252. [Google Scholar] [CrossRef]
11. Xiao ZD, Chen ZY, Lin YH, Liang KG, Wang X, Huang SB, et al. Phosphorus deficiency promotes root: shoot ratio and carbon accumulation via modulating sucrose utilization in maize. J Plant Physiol. 2024;303:e154349. doi:10.1016/j.jplph.2024.154349. [Google Scholar] [PubMed] [CrossRef]
12. Pavinato PS, Rocha GC, Cherubin MR, Harris I, Jones DL, Withers PJA. Map of total phosphorus content in native soils of Brazil. Sci Agric. 2021;78(6):e20200077. doi:10.1590/1678-992X-2020-0077. [Google Scholar] [CrossRef]
13. Pang F, Li Q, Solanki MK, Wang Z, Xing YX, Dong DF. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front Microbiol. 2024;15:e1383813. doi:10.3389/fmicb.2024.1383813. [Google Scholar] [PubMed] [CrossRef]
14. Lizcano-Toledo R, Reyes-Martín MP, Celi L, Fernández-Ondoño E. Phosphorus dynamics in the soil-plant-environment relationship in cropping systems: a review. Applied Sci. 2021;11(23):e11133. doi:10.3390/app112311133. [Google Scholar] [CrossRef]
15. Soares AAVL, Prado RM, Caione G, Rodrigues M, Pavinato PS, Campos CNS. Phosphorus dynamics in sugarcane fertilized with filter cake and mineral phosphate sources. Front Soil Sci. 2021;1:e719651. doi:10.3389/fsoil.2021.719651. [Google Scholar] [CrossRef]
16. Lino ACM, Buzetti S, Teixeira-Filho MCM, Galindo FS, Maestrelo PR, Rodrigues MAC. Effect of phosphorus applied as monoammonium phosphate-coated polymers in corn culture under no-tillage system. Semina Cien Agrar. 2018;39:99–112. doi:10.5433/1679-0359.2018v39n1p99. [Google Scholar] [CrossRef]
17. Damaceno JBD, Lobato CAN, Gama RT, Silva CA, Martins JKD, Oliveira DM, et al. Agronomic efficiency of bone meal under acidification in Brachiaria ruziziensis dry matter production in Western Amazon. J Experimen Agric Int. 2019;34(4):1–7. doi:10.9734/jeai/2019/v34i430182. [Google Scholar] [CrossRef]
18. Li H, Mollier A, Ziadi N, Shi Y, Parent LÉ, Morel C. The long-term effects of tillage practice and phosphorus fertilization on the distribution and morphology of corn root. Plant Soil. 2017;412:97–114. doi:10.1007/s11104-016-2925-y. [Google Scholar] [CrossRef]
19. Zhu J, Li M, Whelan M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci Total Environ. 2018;612:522–37. doi:10.1016/j.scitotenv.2017.08.095. [Google Scholar] [PubMed] [CrossRef]
20. Galindo FS, Teixeira-Filho MCM, Buzetti S, Pagliari PH, Santini JMK, Alves CJ, et al. Maize yield response to nitrogen rates and sources associated with Azospirillum brasilense. Agronomy J. 2019;111:1985–97. doi:10.2134/agronj2018.07.0481. [Google Scholar] [CrossRef]
21. Gonçalves MC, Silva KC, Oliveira CES, Steiner F. Nitrogen and Azospirillum brasilense in the early development of sugarcane. Colloq Agrar. 2020;16(2):72–81. (In Portuguese). doi:10.5747/ca.2020.v16.n2.a360. [Google Scholar] [CrossRef]
22. Silva LI, Pereira MC, Carvalho AMX, Buttrós VH, Pasqual M, Dória J. Phosphorus-solubilizing microorganisms: a key to sustainable agriculture. Agriculture. 2023;13(2):e462. doi:10.3390/agriculture13020462. [Google Scholar] [CrossRef]
23. Ibanhes-Neto HF, Freiria GH, Silva AC, Ponce RM, Takahashi LSA. Snap bean production from seeds treated with Bacillus subtilis. Pesq Agropec Trop. 2023;53:e76324. doi:10.1590/1983-40632023v5376324. [Google Scholar] [CrossRef]
24. Kumar A, Kumar A, Patel H. Role of microbes in phosphorus availability and acquisition by plants. Int J Current Microbiol Applied Sci. 2018;7(5):1344–7. [Google Scholar]
25. Kalayu G. Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int J Agron. 2019;2019(1):e4917256. doi:10.1155/2019/4917256. [Google Scholar] [CrossRef]
26. Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA. Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2:1–14. doi: 10.1186/2193-1801-2-587. [Google Scholar] [PubMed] [CrossRef]
27. Zarei T, Moradi A, Kazemeini SA, Farajee H, Yadavi A. Improving sweet corn (Zea mays L. var saccharata) growth and yield using Pseudomonas fluorescens inoculation under varied watering regimes. Agric Water Manag. 2019;226:e105757. doi:10.1016/j.agwat.2019.105757. [Google Scholar] [CrossRef]
28. Sousa DMG, Lobato E. Cerrado: soil correction and fertilization. 2nd ed. Brasilia, Brazil: Embrapa Informação Tecnológica; 2004. 416 p. [Google Scholar]
29. Malavolta E, Vitti GC, Oliveira SA. Assessment of the nutritional status of plants: principles and applications. 2nd ed. Piracicaba, Brazil: POTAFOS; 1997. 319 p. (In Portuguese). [Google Scholar]
30. Craswell ET, Godwin DC. The efficiency of nitrogen fertilizers applied to cereals in different climates. In: Tinker PB, Lauchli A, editors. Advances in plant nutrition. New York, NY, USA: Praeger Scientific; 1984. p. 1–55. [Google Scholar]
31. Lima JA, Rossi AAB, Santos TO, Penna GF, Tardin FD, Trindade RS, et al. Adaptability and stability of corn hybrids for the south of the Amazon biome via GGE biplot. Pesq Agrop Bras. 2023;58:e02931. doi:10.1590/S1678-3921.pab2023.v58.02931. [Google Scholar] [CrossRef]
32. Kendal E, Tekdal S, Karaman M. Proficiency of biplot methods (AMMI and GGE) in the appraisal of triticale genotypes in multiple environments. Applied Ecol Environ Res. 2019;17:5995–6007. doi:10.15666/aeer/1703_59956007. [Google Scholar] [CrossRef]
33. Ahmad M, Adil Z, Hussain A, Mumtaz MZ, Nafees M, Ahmad I, et al. Potential of phosphate solubilizing Bacillus strains for improving growth and nutrient uptake in mungbean and maize crops. Pak J Agric Sci. 2019;56:283–9. doi:10.21162/PAKJAS/19.7285. [Google Scholar] [CrossRef]
34. Rodrigues DHS, Abes SS, Steiner F, Viana RS, Alves RS. Use of phosphate-solubilizing bacteria to enhance the growth of sugarcane varieties. Ens E Ciência. 2023;27(3):1–7. (In Portuguese). doi:10.17921/1415-6938.2023v27n3p254-260. [Google Scholar] [CrossRef]
35. Velloso CC, Oliveira CA, Gomes EA, Lana UGP, Carvalho CG, Guimarães LJM, et al. Genome-guided insights of tropical Bacillus strains efficient in maize growth promotion. FEMS Microbiol Ecol. 2020;96(9):e157. doi:10.1093/femsec/fiaa157. [Google Scholar] [PubMed] [CrossRef]
36. Oliveira-Paiva CA, Bini D, Sousa SM, Ribeiro VP, Santos FC, Paula-Lana UG, et al. Inoculation with Bacillus megaterium CNPMS B119 and Bacillus subtilis CNPMS B2084 improve P-acquisition and maize yield in Brazil. Front Microbiol. 2024;15:e1426166. doi:10.3389/fmicb.2024.1426166. [Google Scholar] [PubMed] [CrossRef]
37. Fukami J, Cerezini P, Hungria M. Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Express. 2018;8:1–12. doi:10.1186/s13568-018-0608-1. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools