 Open Access
Open Access
ARTICLE
Feasibility and short-term outcomes of robotic distal ureteroureterostomy for benign obstruction
Department of Urology, University of Arizona College of Medicine, Tucson, AZ 85724, USA
* Corresponding Author: Jonathan A. Seaman. Email:
Canadian Journal of Urology 2025, 32(3), 181-187. https://doi.org/10.32604/cju.2025.064047
Received 03 February 2025; Accepted 19 May 2025; Issue published 27 June 2025
Abstract
Introduction: Distal ureteral obstruction has classically been managed by ureteroneocystostomy (UNC). The feasibility and success of robotic primary ureteroureterostomy (UU) for benign obstruction appears promising with several benefits over UNC but is poorly studied. Robotic repair offers superior visualization and precision, allowing for minimal ureteral dissection. Here we report on our experience and short-term outcomes. Materials and Methods: We identified patients who underwent robotic distal ureteroureterostomy for benign distal ureteral obstruction at our institution from 2020–2024. Etiology, stricture length, and post-operative outcomes were recorded. All patients had renal ultrasound (US), diuretic renography, or cross-sectional imaging within 6 months of repair. Results: Seven patients underwent distal UU from 2020–2024, with one case of bilateral repair for a total of 8 anastomoses. Iatrogenic injury from hysterectomy represented 5/8 injuries. The mean time between injury and repair was 3.5 months. All defects were 1–1.5 cm in length. At follow-up imaging, there was no evidence of obstruction in any patient with a median follow-up of 10 months, including diuretic renography in 5 of 7 patients. One patient had mild hydronephrosis on their initial renal US but with normal drainage on subsequent diuretic renography. All patients reported no flank pain at follow-up. Conclusions: Robotic UU is feasible for short, benign distal ureteral obstruction in carefully selected patients. Advantages over traditional UNC include a significantly shorter catheter time, no risk of vesicoureteral reflux, no effect on bladder capacity or function, and the ability to retain the native ureteral orifice. Continued research will elucidate the long-term efficacy of this approach.Keywords
Ureteral obstruction is a common problem encountered by urologists. These obstructions occur most commonly from pelvic surgery, endoscopic surgery, infection, and radiation. Prior pelvic surgery is a common reason for distal ureteral obstruction in the developed world.1,2 Hysterectomy poses a significant risk due to the proximity of the distal ureter to the uterine artery, which is necessarily divided during hysterectomy.
The rate of iatrogenic ureteral injury is estimated to occur during 0.3%–1% of cases across gynecologic, colorectal, and general surgery cases according to modern series.3–6 However, with approximately 600,000 hysterectomies performed each year in the United States alone,7 this remains a pathology that urologists must be familiar with. Classically, distal ureteral obstruction has been managed with ureteroneocystostomy (UNC). This was felt to be not only technically easier but also associated with a theoretically lower risk of failure due to a more robust blood supply afforded by the bladder side of the anastomosis. Based on very minimal autopsy studies from the 1950s,8 it was proposed that the distal ureteral blood supply is more tenuous than that of the more proximal and abdominal ureter. However, there have been no clinical studies demonstrating this as it relates to ureteral repair. Rather, it seems to stem more from the dogma of the open surgical era, when achieving high-quality ureteroureterostomy (UU) in the deep pelvis was technically challenging and at high risk for failure.
The advent of robotic surgery has revolutionized pelvic procedures, including urological surgery, by providing superior visualization of the deep pelvis and enabling precise, stabilized movements. Patient-related factors are also superior, with many studies demonstrating decreased blood loss, shorter hospitalization, and lower pain scores.2–4 In response to these improvements, there has been a significant increase in the usage of robotic surgery within urology as a whole and specifically within reconstructive urology internationally.9,10 As just one example, Flegar et al. reported an increase in robotic pyeloplasty utilization in Germany from 0.3% in 2006 to 36% in 2022.9 In addition, robotic surgery has enabled new approaches and improved the surgeon’s experience and ergonomics in previously difficult-to-access areas. In the field of reconstructive urology, this is demonstrated with the advent of such approaches as transvesical bladder neck reconstruction, retroperitoneal ureteral surgery, and a myriad of ureteral reconstruction techniques, including UU of the mid-and proximal ureter, which has proven itself feasible with efficacy and complications similar to that of the open approach.11–13
With robotic-assisted laparoscopic surgery, we postulate that ureteroureterostomy for benign distal ureteral obstruction can be equally as effective as that for more proximal injury, owing to the improvements in visualization and precision of this platform. Investigation into this approach has been performed previously with an early 7-patient series in 201313 and a follow-up study including 22 patients who underwent robotic distal UU for benign ureteral stricture with a success rate of 91% at a mean follow-up of 54 months.14 Here we present our early experience and outcomes at our institution using this approach.
This is a single-institution, retrospective study of a prospectively collected robotic reconstructive database that included patients who underwent robotic-assisted distal UU from 2020–2024. Data was gathered from our the University of Arizona Institutional Review Board (IRB Number: STUDY00001823) approved database of robotic urological reconstruction cases. This study obtained the informed consent of all participants. The distal ureter is defined as either radiographically below the border of the sacrum or anatomically below the crossing of the common iliac vessels in the true pelvis. Inclusion criteria included patients >18 years old with benign, distal ureteral obstruction or injury who underwent robotic UU. Given that the UU is an accepted technique for mid or proximal ureteral obstruction, we excluded any patients that had obstruction at the junction of the mid to distal ureter and included only those clearly within the distal ureter definition. We also excluded patients with long (>2 cm) strictures, those with a history of radiation to the abdomen or pelvis, or malignant obstruction of the ureter.
We were primarily concerned with the success of the repair, defined as improved or resolved flank pain at post-operative visits, and either the absence of hydronephrosis on post-operative imaging or adequate functional drainage on diuretic renography (mercaptoacetyltriglycine, MAG-3). Secondary outcomes included operative time, intraoperative estimated blood loss (EBL), change in creatinine and GFR post-operatively, and major or minor complications. Baseline patient and injury characteristics were obtained, including injury length, time from injury, mechanism of injury, preoperative creatinine, and GFR if available. Preoperative assessment included retrograde and antegrade pyelography as well as diagnostic ureteroscopy to fully delineate the injury, except for those repairs performed immediately at the time of injury.
All surgeries were performed using the da Vinci Xi robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA) by two surgeons experienced with robot-assisted laparoscopic surgery, SK and JF. Patients were positioned in the modified dorsal lithotomy or supine position, with the table in Trendelenburg. The robot was docked at the side to allow for urethral/bladder access as needed.
Upon achieving intraperitoneal access, we employ indocyanine green with Firefly® fluorescence imaging combined with retrograde ureteroscopy to facilitate ureteral dissection and identification (Figure 1A). The proximal and distal ureteral segments are carefully identified and isolated, with meticulous attention to avoid direct grasping of the ureter. Following excision of the diseased segment, both ends are trimmed back to healthy ureteral mucosa, confirmed by visualization of pink, bleeding mucosal edges. The ureteral segments are then spatulated-posteriorly for the proximal segment and anteriorly for the distal segment (Figure 1B) and anastomosed using a 4-0 polyglactin suture. The surgical sequence involves: (1) approximating the posterior wall with interrupted sutures, (2) placing a double-J ureteral stent antegrade across the repair, and (3) closing the anterior wall with either running or interrupted sutures (Figure 1C demonstrates the completed anastomosis). A Jackson-Pratt (JP) drain is routinely placed in the pelvis. For elective cases, our standard postoperative protocol includes: Foley catheter removal on postoperative day 1; JP drain removal before discharge after confirming creatinine levels match serum values; removal of any nephrostomy tubes before discharge; and ureteral stent removal at 6 weeks. Patients undergo follow-up evaluations at 6 weeks and 6 months postoperatively, with assessments including a MAG-3 renal scan (our preferred modality for evaluating renal drainage), or CT scan/renal ultrasound to assess for hydronephrosis. Some patients had additional imaging performed >1 year postoperatively for unrelated indications, which were included when available for long-term outcome assessment.
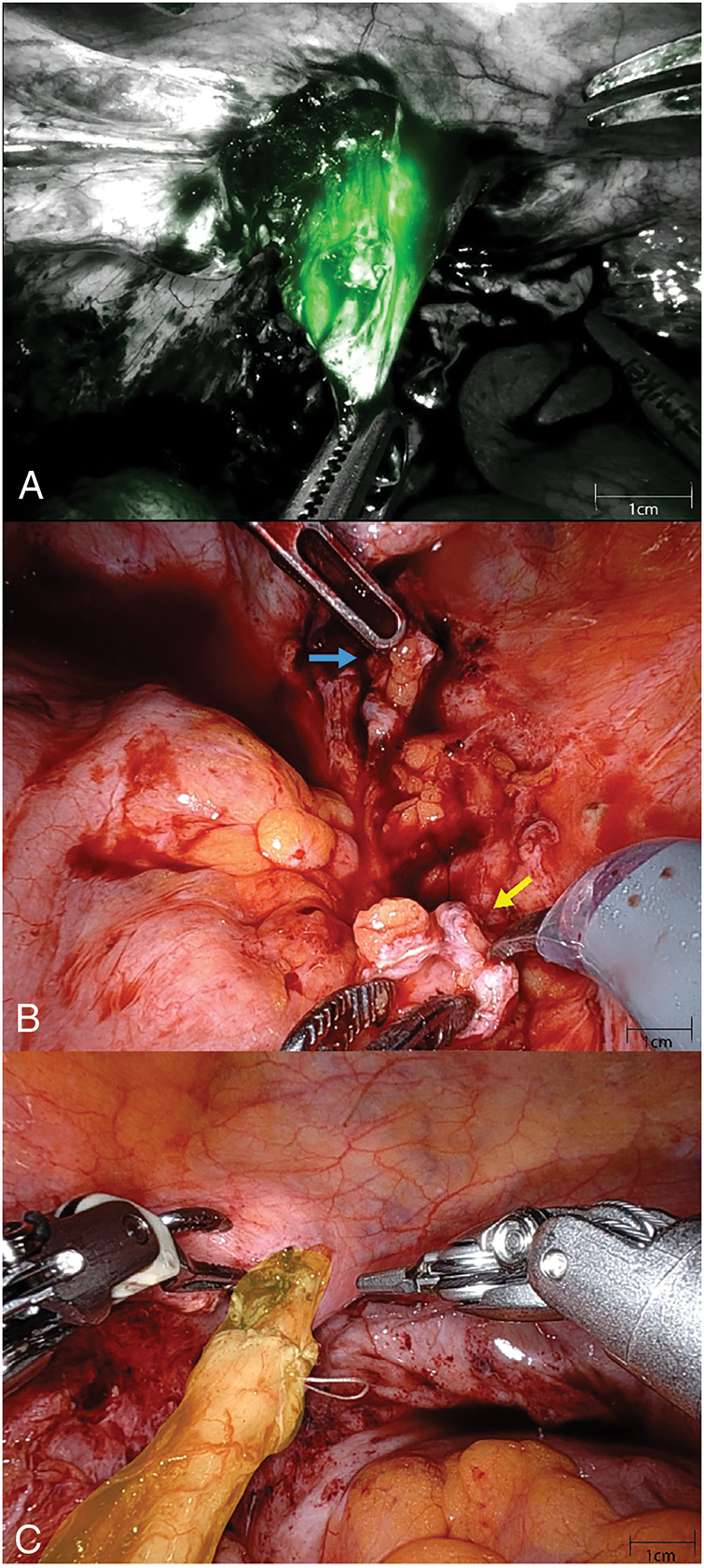
FIGURE 1. (A) With a digital flexible ureteroscope in the distal ureteral segment, the structure is easily identified using intraoperative firefly® imaging. (B) The distal ureteral segment (blue arrow) is spatulated along the anterior surface while the proximal segment (yellow arrow) is spatulated posteriorly. (C) Completed left-sided anastomosis can be seen highlighted in yellow
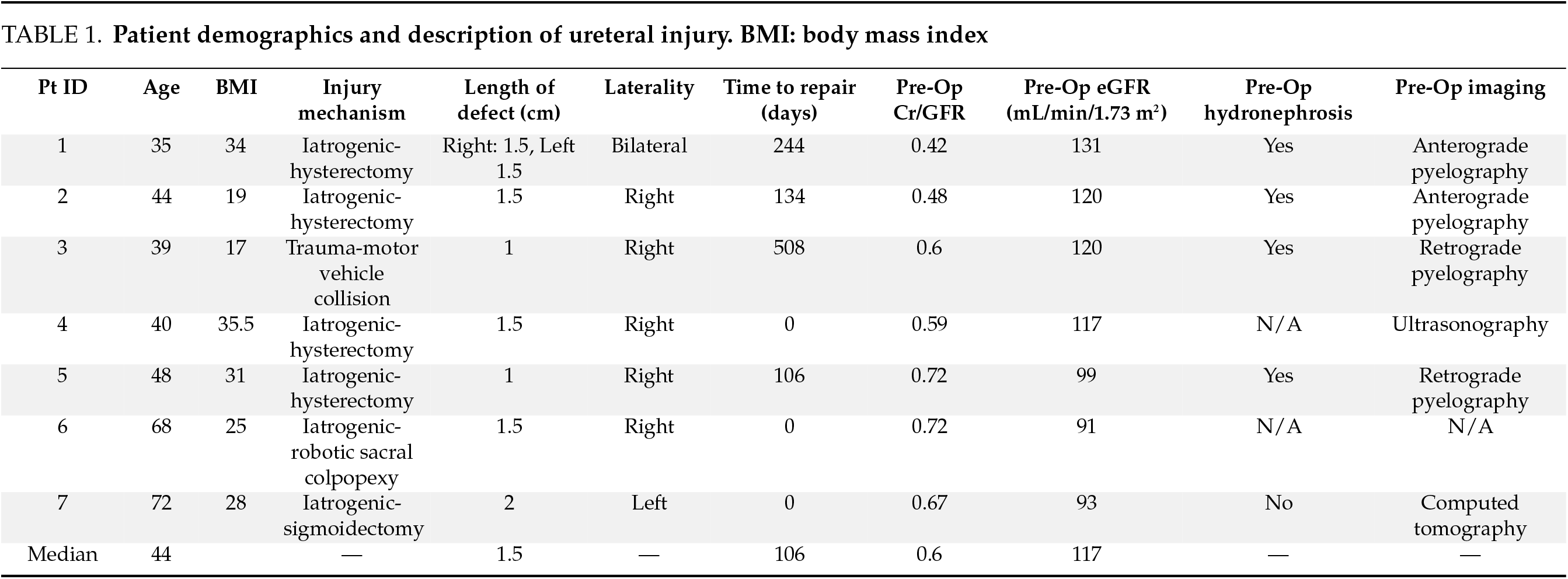
Between 2020 and 2024, we performed robotic-assisted laparoscopic ureteroureterostomy (RAL-UU) in seven patients, including one bilateral case, resulting in a total of eight anastomoses. As shown in Table 1, the median patient age was 44 years (range: 35–72), with a female predominance (86%, 6/7). The etiology was iatrogenic in six cases (86%), including four post-hysterectomy injuries, one following robotic sigmoidectomy, and one after robotic sacrocolpopexy. The median time from injury to repair was 3.5 months (IQR 6.3 months), with three cases (43%) repaired immediately during the index operation. The median stricture length was 1.5 cm (range: 1–2 cm).
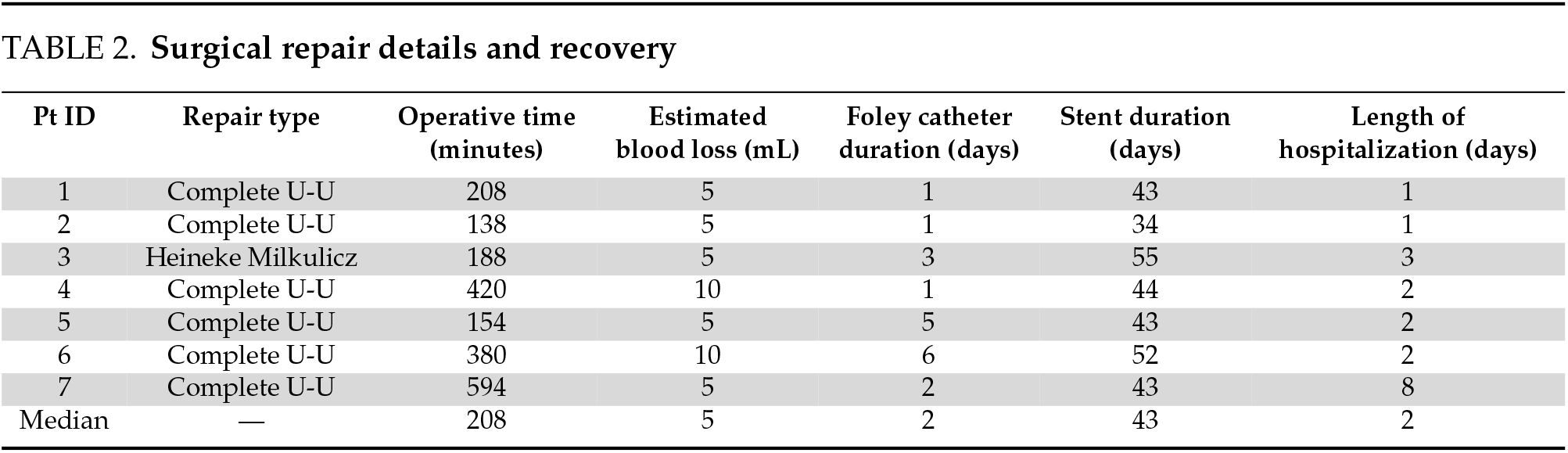
The median total operative time was 208 min (including those with intraoperative repair) with a minimal EBL (Table 2). The median hospital stay for all patients was 2 days. There were no readmissions for urologic-related concerns or complaints and no grade II or higher Clavien-Dindo complications within 30 days of surgery.
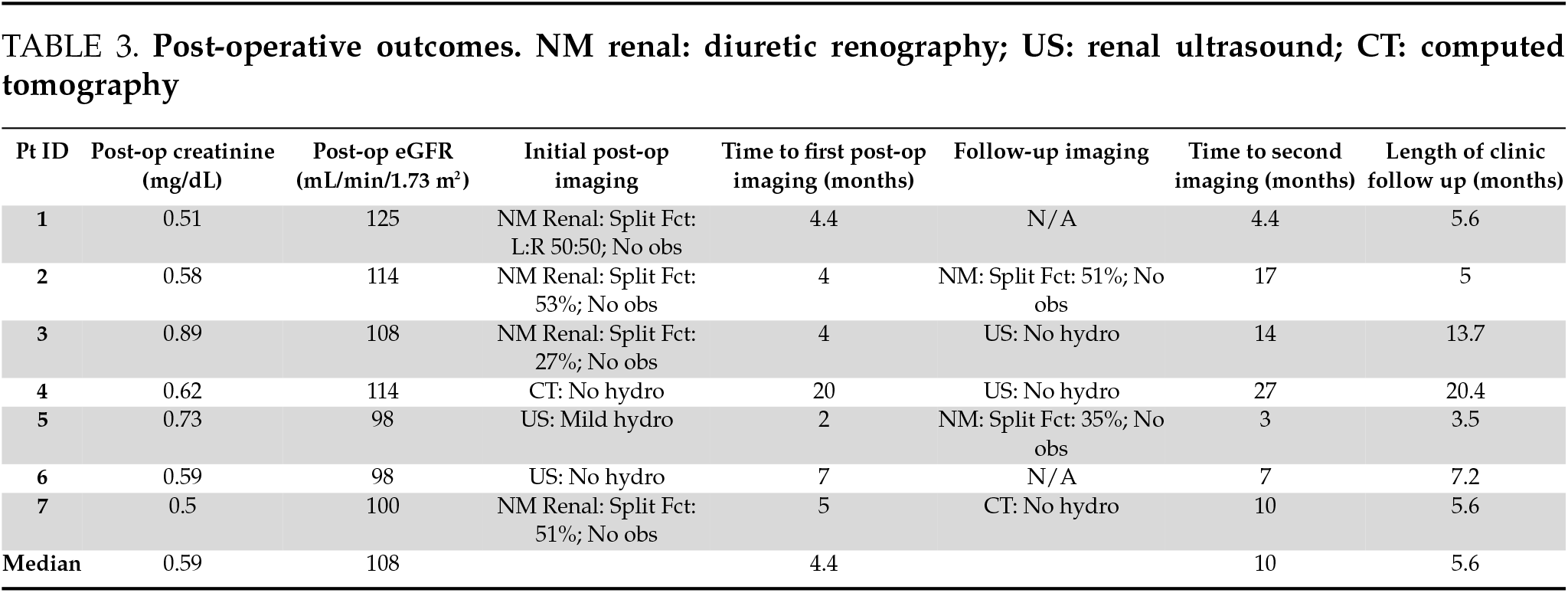
All patients presented to their 6-week post-operative visit for stent removal. Two patients reported bladder pain, 1 patient reported penile pain, and 1 patient reported vaginal pain, related to the indwelling ureteral stent. No patients reported flank pain on the side of the repair at their 6-month follow-up. All patients underwent follow-up imaging consisting of either renal US, diuretic renography, or cross-sectional imaging, with 5 of 7 patients having a second post-operative imaging modality done at a median follow-up of 10 months (Table 3). There was no radiographic evidence of obstruction on any patient at the most recent follow-up imaging. One patient had mild hydronephrosis on their initial 2-month renal US but no functional obstruction on subsequent diuretic renography.
Distal U-U has been historically avoided in the open area, but instead, the technically simpler UNC has been utilized. This has led to a paucity of data on its true clinical success rate—hesitancy has stemmed more from the theoretical fear of poor success rates rather than clinical evidence. There exists very little clinical data comparing UNC to UU for distal ureteral obstruction. Wang et al. performed a retrospective analysis of 60 patients with distal ureteral obstruction secondary to gynecological surgery.15 In their cohort, 34 patients were repaired using laparoscopic UNC and 26 with laparoscopic UU. They reported 1 and 3 cases of recurrent obstruction in the UU and UNC groups, respectively, with a mean follow-up of 36 months.
Within the renal transplant world, there are several studies demonstrating similar complication rates between UNC and UU, with an expected lower rate of VUR and comparable or lower post-operative urinary tract infection in the UU group,16,17 albeit in some cases a higher risk of urine leak. In contrast to our current study, these are typically done in an open approach during renal transplant and involve an anastomosis between a native and recipient ureter, with presumably poorer ureteral blood supply from the donor side. One would expect poorer outcomes in this setting compared with native repair for distal ureteral injury, and yet it appears to be feasible with acceptable outcomes in the transplant setting.
While UNC has a proven track record of a high success rate of >90% with low complications, several distinct advantages of UU warrant exploration of its feasibility. UNC requires cystotomy, and prolonged catheterization, and results in a refluxing system. Vesicoureteral reflux (VUR) in the adult patient, while seemingly less damaging in the renal unit than in the pediatric patient, does increase the risk of ascending urinary tract infection, pyelonephritis, and VUR-related pain,18,19 potentially increasing the rate of hospitalization and health care costs. By avoiding entrance into the bladder with the UU approach, patients experience a shorter catheter duration, a more rapid return to their usual routine, and decreased discomfort. The natural trigonal anatomy is preserved, thereby maintaining the anti-reflux mechanism and avoiding post-operative VUR.
This study builds upon the experience of other robotic reconstructive urologists. With the advent of robotic surgery, several groups have investigated the viability of UU for ureteral reconstruction with the precision that this platform allows. Yang et. al14 reported their experience with 21 patients in a single-surgeon retrospective review of robotic UU for benign distal ureteral obstruction. They reported durable repairs in 20/22 (91%) of patients with a median follow-up of 54 months. Only 2 patients required ureteroneocystostomy at a later date. Their patient population similarly consisted of primarily iatrogenic injury in middle-aged female patients. Here we are repeating their approach with a similar technique and comparable population. Our data validates their findings with short-term follow-up demonstrating good efficacy and low complication rates. We have even demonstrated the feasibility of bilateral repair with no recurrence at 5 months.
Robotic ureteroureterostomy (UU) for distal ureteral obstruction requires careful patient selection, with ideal candidates having short (1–2 cm) defects, no history of pelvic radiation, and adequate healthy distal ureteral tissue. Preoperative evaluation should include antegrade/retrograde pyelography and ureteroscopy to assess anatomy. Surgeons must be prepared to convert to ureteroneocystostomy (UNC) if intraoperative findings preclude tension-free, watertight anastomosis, as this adjustment requires no changes in the robotic setup. Comprehensive preoperative counseling about potential approach modifications is essential.
Our study has several limitations as a small (n = 7), single-institution case series with two surgeons and a retrospective design. The currently limited short-term follow-up and lack of direct UNC comparisons constrain data interpretation. While our series primarily involved iatrogenic pelvic surgery injuries (the population likely best suited for this approach), this limits generalizability to other stricture etiologies. The slow case accumulation reflects the narrow indications for distal UU, paralleling early experience of Lee et al. (2013).13
Moving forward, we will continue longitudinal follow-up with MAG-3 renography and patient-reported outcomes while expanding our case series for qualifying patients. This incremental approach will better establish the procedure’s durability while maintaining strict selection criteria to optimize outcomes. The technical reproducibility and favorable short-term results in our initial experience suggest robotic distal UU warrants further investigation as a viable alternative to UNC in appropriately selected patients.
The surgical options for ureteral reconstruction continue to expand with the advent and progression of robotic-assisted laparoscopic surgery. Our experience builds upon the current literature exploring distal UU for benign ureteral obstruction in the properly selected patient. Continued long-term data collection on these patients will further reveal the durability of this repair.
Acknowledgement
There are no separate acknowledgements outside of the below listed author contributions.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
The authors confirm contribution to the paper as follows: Study conception and design: Sunchin Kim, Jonathan A. Seaman; Analysis and interpretation of results: Jonathan A. Seaman, Rita Palanjian, John Fitzgerald, Sunchin Kim; Draft manuscript preparation: Jonathan A. Seaman, Rita Palanjian, Sunchin Kim; Review and editing: Jonathan A. Seaman, Kyle McCormick, Joel Funk, Sunchin Kim. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval
This study was reviewed and approved by the University of Arizona Institutional Review Board. IRB Number: STUDY00001823. Date: 2 May 2023. This study obtained the informed consent of all participants.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| CT | Computed tomography |
| EBL | Estimated blood loss |
| GFR | Glomerular filtration rate |
| JP | Jackson-Pratt drain, |
| RAL-UU | Robotic-assisted laparoscopic ureteroureterostomy |
| UNC | Ureteroneocystostomy |
| US | Ultrasound |
| UU | Ureteroureterostomy |
| VUR | Vesicoureteral reflux |
References
1. Engel O, Rink M, Fisch M. Management of iatrogenic ureteral injury and techniques for ureteral reconstruction. Curr Opin Urol 2015;25(4):331–335. doi:10.1097/MOU.0000000000000175. [Google Scholar] [PubMed] [CrossRef]
2. Elbers JR, Rodríguez Socarrás M, Rivas JG et al. Robotic repair of ureteral strictures: techniques and review. Curr Urol Rep 2021;22(8):39. doi:10.1007/s11934-021-01056-8. [Google Scholar] [PubMed] [CrossRef]
3. Wong JMK, Bortoletto P, Tolentino J, Jung MJ, Milad MP. Urinary tract injury in gynecologic laparoscopy for benign indication: a systematic review. Obstet Gynecol 2018;131(1):100–108. doi:10.1097/AOG.0000000000002414. [Google Scholar] [PubMed] [CrossRef]
4. Al-Awadi K, Kehinde EO, Al-Hunayan A, Al-Khayat A. Iatrogenic ureteric injuries: incidence, aetiological factors and the effect of early management on subsequent outcome. Int Urol Nephrol 2005;37(2):235–241. doi:10.1007/s11255-004-7970-4. [Google Scholar] [PubMed] [CrossRef]
5. Eswara JR, Raup VT, Potretzke AM, Hunt SR, Brandes SB. Outcomes of iatrogenic genitourinary injuries during colorectal surgery. Urology 2015;86(6):1228–1234. doi:10.1016/j.urology.2015.06.065. [Google Scholar] [PubMed] [CrossRef]
6. Marcelissen TAT, Den Hollander PP, Tuytten TRAH, Sosef MN. Incidence of iatrogenic ureteral injury during open and laparoscopic colorectal surgery: a single center experience and review of the literature. Surg Laparosc Endosc Percutan Tech 2016;26(6):513. doi:10.1097/SLE.0000000000000335. [Google Scholar] [PubMed] [CrossRef]
7. Simms KT, Yuill S, Killen J et al. Historical and projected hysterectomy rates in the USA: implications for future observed cervical cancer rates and evaluating prevention interventions. Gynecol Oncol 2020;158(3):710–718. doi:10.1016/j.ygyno.2020.05.030. [Google Scholar] [PubMed] [CrossRef]
8. Daniel O, Shackman R. The blood supply of the human ureter in relation to ureterocolic anastomosis. Br J Urol 1952;24(4):334–343. doi:10.1111/j.1464-410x.1952.tb06167.x. [Google Scholar] [PubMed] [CrossRef]
9. Flegar L, Kipfer F, Durmus T et al. Pyeloplasty and ureteral reconstruction surgery trends: a total population analysis in germany from 2006 to 2022. Eur Urol Open Sci 2024;70:116–123. doi:10.1016/j.euros.2024.10.011. [Google Scholar] [PubMed] [CrossRef]
10. Franco A, Ditonno F, Manfredi C et al. Robot-assisted surgery in the field of urology: the most pioneering approaches 2015–2023. Res Rep Urol 2023;15:453–470. doi:10.2147/RRU.S386025. [Google Scholar] [PubMed] [CrossRef]
11. Sun G, Yan L, Ouyang W et al. Management for ureteral stenosis: a comparison of robot-assisted laparoscopic ureteroureterostomy and conventional laparoscopic ureteroureterostomy. J Laparoendosc Adv Surg Tech A 2019;29(9):1111–1115. doi:10.1089/lap.2019.0357. [Google Scholar] [PubMed] [CrossRef]
12. Wang Q, Lu Y, Hu H et al. Management of recurrent ureteral stricture: a retrospectively comparative study with robot-assisted laparoscopic surgery versus open approach. PeerJ 2019;7(6):e8166. doi:10.7717/peerj.8166. [Google Scholar] [PubMed] [CrossRef]
13. Lee Z, Llukani E, Reilly CE et al. Single surgeon experience with robot-assisted ureteroureterostomy for pathologies at the proximal, middle, and distal ureter in adults. J Endourol 2013;27(8):994–999. doi:10.1089/end.2013.0075. [Google Scholar] [PubMed] [CrossRef]
14. Yang KK, Asghar AM, Lee RA et al. Robot-assisted laparoscopic distal ureteroureterostomy for distal benign ureteral strictures with long-term follow-up. J Endourol 2022;36(2):203–208. doi:10.1089/end.2021.0315. [Google Scholar] [PubMed] [CrossRef]
15. Wang Z, Chen Z, He Y, Li B, Wen Z, Chen X. Laparoscopic ureteroureterostomy with an intraoperative retrograde ureteroscopy-assisted technique for distal ureteral injury secondary to gynecological surgery: a retrospective comparison with laparoscopic ureteroneocystostomy. Scand J Urol 2017;51(4):329–334. doi:10.1080/21681805.2017.1304989. [Google Scholar] [PubMed] [CrossRef]
16. Suttle T, Fumo D, Baghmanli Z et al. Comparison of urologic complications between ureteroneocystostomy and ureteroureterostomy in renal transplant: a meta-analysis. Exp Clin Transplant 2016;14(3):276–281. doi:10.6002/ect.2015.0161. [Google Scholar] [PubMed] [CrossRef]
17. Nie Z, Zhang K, Huo W et al. Comparison of urological complications with primary ureteroureterostomy versus conventional ureteroneocystostomy. Clin Transplant 2010;24(5):615–619. doi:10.1111/j.1399-0012.2009.01134.x. [Google Scholar] [PubMed] [CrossRef]
18. Köhler J, Thysell H, Tencer J et al. Conservative treatment and anti-reflux surgery in adults with vesico-ureteral reflux: effect on urinary-tract infections, renal function and loin pain in a long-term follow-up study. Nephrol Dial Transplant 2001;16(1):52–60. doi:10.1093/ndt/16.1.52. [Google Scholar] [PubMed] [CrossRef]
19. Rosenfeld J, Boehm D, Raikar A et al. A review of complications after ureteral reconstruction. Asian J Urol 2024;11(3):348–356. doi:10.1016/j.ajur.2024.02.007. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools