 Open Access
Open Access
CASE REPORT
Tumor-to-tumor metastasis: case report of a neuroendocrine tumor of the lung metastasizing to a benign oncocytoma of the kidney
1 Department of Urology, Emory University, Atlanta, GA 30322, USA
2 Winship Cancer Institute, Emory University, Atlanta, GA 30322, USA
3 Department of Pathology, Emory University, Atlanta, GA 30322, USA
* Corresponding Author: Siddharth Marthi. Email:
Canadian Journal of Urology 2025, 32(4), 349-353. https://doi.org/10.32604/cju.2025.063775
Received 23 January 2025; Accepted 23 April 2025; Issue published 29 August 2025
Abstract
Tumor-to-tumor metastasis (TTM) is a rare phenomenon in which a secondary tumor colonizes within a primary tumor of a different histogenesis. It is hypothesized that TTM is encouraged by conditions that promote increased cell growth and division in the primary tumor, such as hypervascularity and expression of oncogenic cytokines. However, the exact causes of TTM likely vary on a case-by-case basis and are dependent on the microenvironment of both the primary and secondary tumors. Herein, we present the first reported example of TTM in which a pulmonary neuroendocrine tumor (NET) metastasizes to a renal oncocytoma.Keywords
Tumor-to-tumor metastasis (TTM) is a rare variant of typical tumor metastasis and has been documented in several different patterns in prior case reports. Of the reported events of TTM, primary tumors of the lung are the most common donors of metastasis, and tumors of the kidney, particularly renal cell carcinoma (RCC), are the most common recipients of metastasis.1 TTM has also been documented to occur frequently in benign tumor recipients, such as meningioma, but few cases of TTM in benign renal tumors have been reported.2,3 Here, we present the first documented case of a pulmonary neuroendocrine tumor (NET) metastasizing to an oncocytoma. Understanding unique variants of TTM, such as this case, is critical in better understanding pathways of tumorigenesis and metastatic interactions between tumors. Further, we detail below the interdisciplinary discussion at our institution regarding this case to help inform treatment options and prognosis in TTM.
A 69-year-old male presented to the urology clinic with a renal mass incidentally found on CT after presentation to the emergency department with cholecystitis. Cross-sectional imaging identified an enhancing 3.3 × 2.6 cm exophytic mass of the lateral right kidney (Figure 1), and notably, lung adenopathy in the left hilum (Figure 2), initially concerning for metastasis from a renal primary. The patient was referred to interventional radiology and biopsy of the renal mass and hilar lymph nodes. The renal mass biopsy was completed successfully, but the hilar adenopathy was unable to be accessed percutaneously. Review of renal lesion pathology demonstrated a component of oncocytoma with a secondary component of low-grade spindle cell NET.
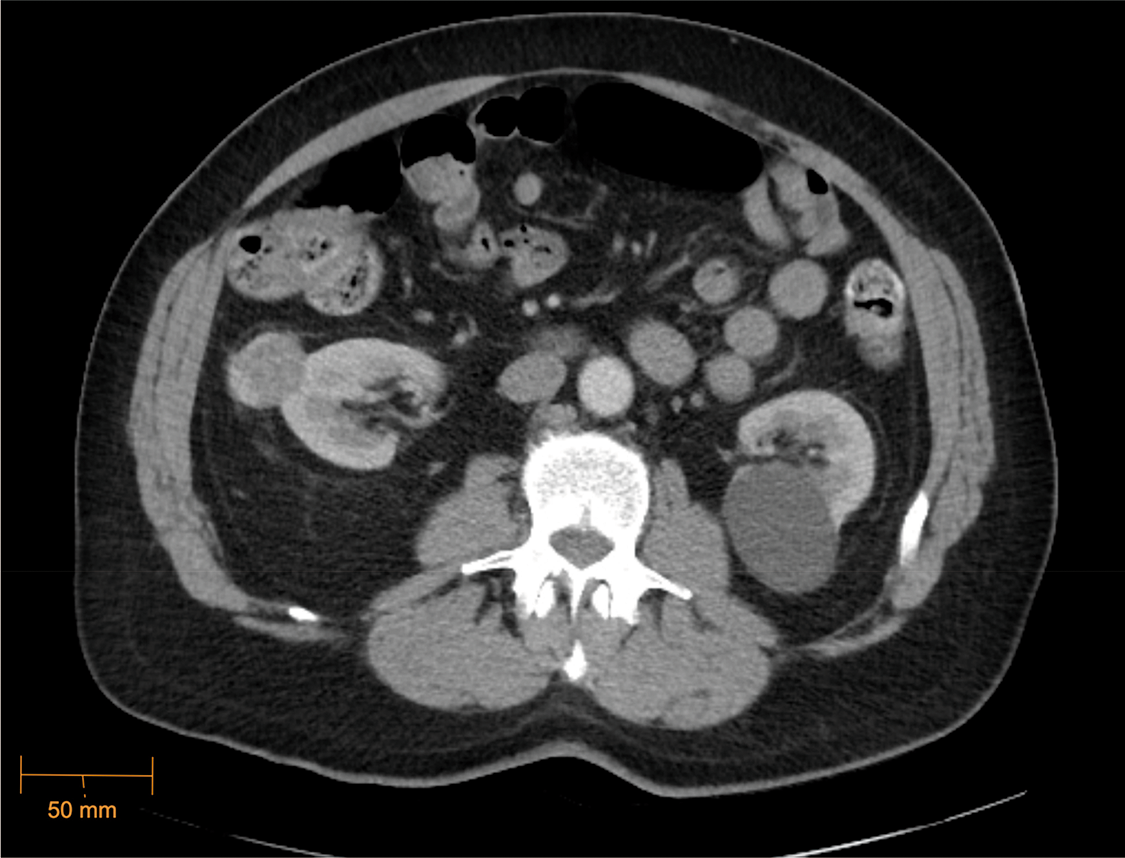
Figure 1: Enhancing right renal mass, CT Abdomen/Pelvis (axial) (5/21/2023)
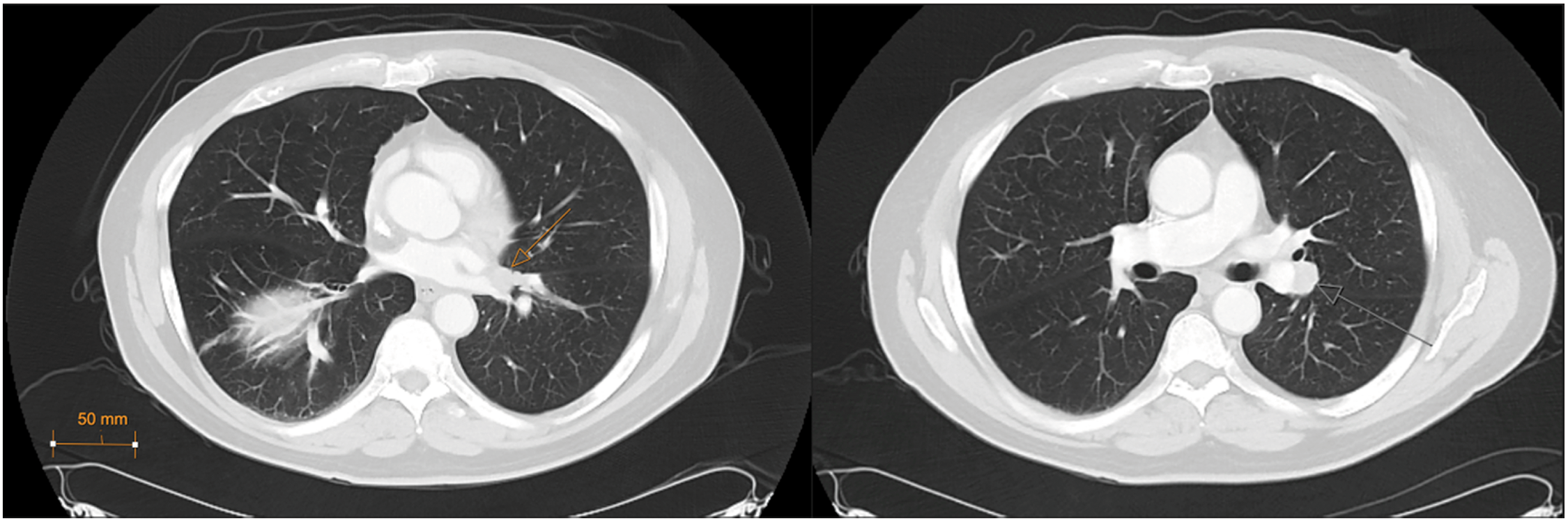
Figure 2: Left hilar lesions, CT Chest (axial, lung window) (7/14/2023)
Due to the unusual pathology, the case was discussed at our institution’s genitourinary tumor board, and consensus was to proceed with bronchoscopy to access the left hilar lesion. A fine needle aspiration of the 1.8 × 1.6 cm lymph node conglomerate surrounding the left hilar vasculature stained positive for synaptophysin and chromogranin A, identifying the mass as a well-differentiated NET. At this point, serum chromogranin A was obtained, which was mildly elevated at 132. A fluorodeoxyglucose (FDG) PET scan was subsequently performed to better evaluate the degree of metastatic disease, which did not demonstrate any additional foci of NET.
After the hilar node pathology and PET scan were reviewed with the genitourinary tumor board, it was determined that the hilar mass was, in fact, the primary tumor and had likely metastasized to the right kidney. He was referred to cardiothoracic surgery to discuss resection of the primary NET, but due to the central location between the upper and lower lobe bronchial bifurcations and concern for infiltration of disease past the interlobar fissure, the cardiothoracic team determined resection may require left pneumonectomy. He was therefore instead offered observation or radiation therapy to primary NET with subsequent right partial nephrectomy, and the patient elected to pursue treatment.
He completed radiotherapy with 60 Gy in 15 fractions, and repeat cross-sectional imaging was performed, demonstrating no local recurrence of disease in the left hilum and stability of the right renal lesion. Three months later, he underwent robotic right partial nephrectomy and had an uncomplicated post-operative recovery. Pathology was consistent with prior renal biopsy, demonstrating NET encapsulated by oncocytoma (Figure 3). Margins were negative for residual NET.
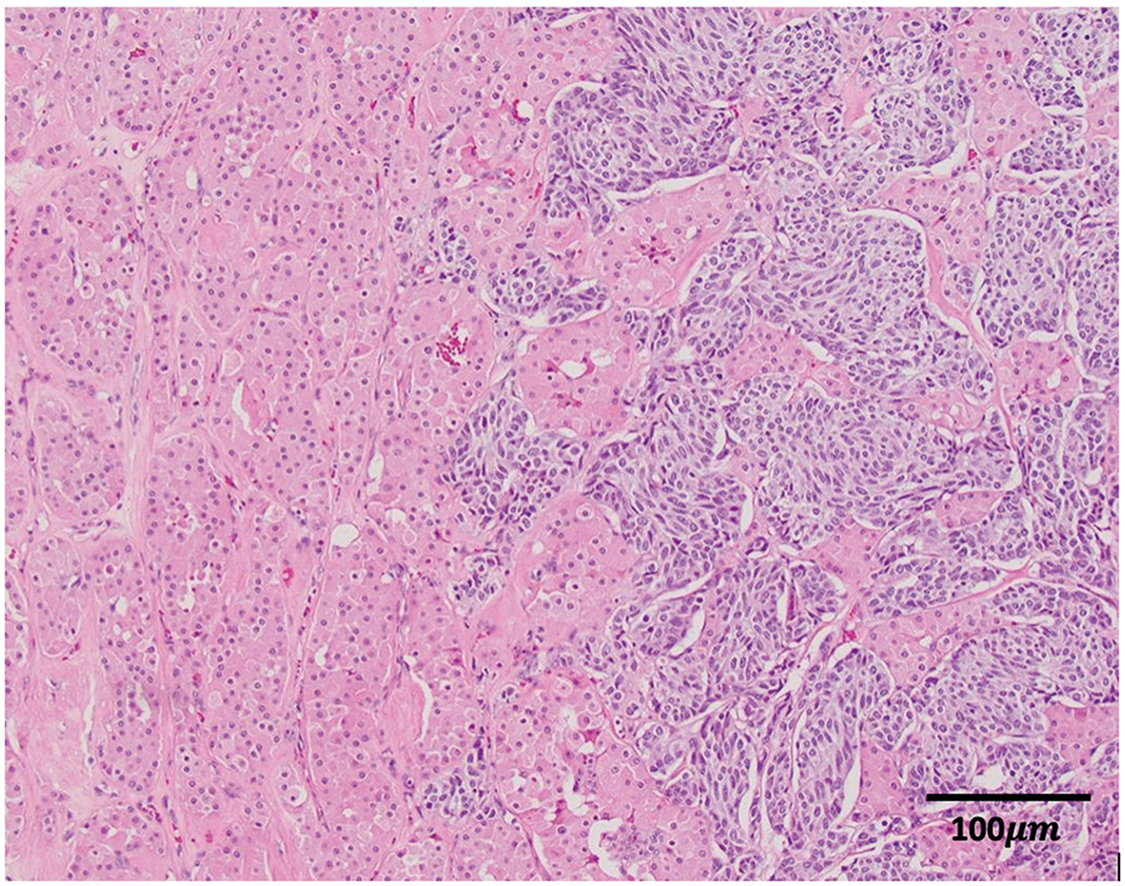
Figure 3: Surgical pathology: the oncocytic cells (with pink cytoplasm) interdigitate with the darker neuroendocrine nests on the right (H&E, 100×)
The rare phenomenon of tumor-to-tumor metastasis has challenged traditional cancer paradigms and complicates diagnosis and treatment strategies. Historically, each instance of TTM has been analyzed and managed on a case-by-case basis due to the unique properties of the donor and recipient tumor in each instance. Similarly, this previously undocumented occurrence of a pulmonary NET as a donor for TTM and an oncocytoma as the recipient required several interdisciplinary discussions to determine the course of management that balanced oncologic curative potential with morbidity. TTM can be challenging to diagnose, particularly when the tumors are histologically similar to each other, commonly seen in the setting of renal cell carcinoma metastasizing to parathyroid adenoma. In this case, the patient was not previously known to have a neuroendocrine tumor, and the differential diagnosis included a collision scenario between the oncocytoma and a primary renal neuroendocrine tumor, versus tumor to tumor metastasis from a metastatic neuroendocrine tumor, which was resolved through multi-disciplinary correlation, upon the discovery of the pulmonary NET.
When presenting on their own, NETs are rare, with an incidence rate of 5.86 per 100,000 patients worldwide, and management classically entails surgical resection and control of functional symptoms.4 However, due to the variety of presentations of NETs, tumor debulking strategies including radiation and embolization have been utilized in cases of metastatic disease where complete resection was not feasible.5 However, it is impossible to determine the presence of metastatic disease in cases of TTM with routine standard CT or MRI. In this case, partially due to the radiographic similarities between oncocytoma and malignant renal tumors, the pre-operative CT scan was unable to visualize the presence of the NET completely encapsulated within a benign lesion. Of note, positron emission tomography (PET) scans may also be used to identify primary and metastatic neuroendocrine tumors. Among radiotracers, FDG is generally preferred for the detection of aggressive and poorly differentiated NETs, 6 whereas 68Ga-radiolabelled and 64Cu-radiolabelled somatostatin analogues have been shown to detect well-differentiated NETs with high sensitivity and specificity in recent literature.7,8 Therefore, this report illustrates the importance of tissue sampling or appropriate PET imaging to ascertain the tumor components and evaluate the need for resection or alternative tumor debulking strategies.
Though RCC is the most common recipient tumor in TTM, there have been only six reported cases of oncocytoma as the recipient.9 Among intracranial neoplasms, however, benign meningiomas have been most frequently noted to act as TTM recipients. Though this is not fully understood, it is theorized that the microenvironment within meningiomas fosters angiogenesis, cell proliferation, and expansion of collagen and lipid content, creating a setting in which a metastasized donor tumor can easily grow.10 It is possible that the tumor characteristics of oncocytoma create an environment that encourages growth of micro-metastasis and TTM, though malignant renal tumors seem to do so to a greater degree. Of the reported cases of TTM with renal tumors acting as recipients, an overwhelming majority are clear cell RCC (Table 1). As pulmonary neuroendocrine tumors very infrequently metastasize to the kidney without concurrent implants in regional lymph nodes, this phenomenon may have overridden typical pulmonary lymphatic drainage to result in the presentation demonstrated in this report.11
Acknowledgement
Not applicable.
Funding Statement
No funding was required or used for the purposes of this manuscript.
Author Contributions
Siddharth Marthi contributed to the writing and conception of the study, drafting and revising the written and intellectual content, and final approval of the submitted version. Muhammad Mukarram contributed to the initial drafting and conception of the study. Fatemeh Ardeshir-Larijani contributed to the initial written and intellectual content of the study. Lara Rabih Harik contributed to the written and intellectual content of the study and provided revisions requested. Shreyas Subhash Joshi contributed to the conception of the study, revising and final approval of the submitted version. All of the aforementioned authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
All data and materials were readily available via our institutional electronic medical record. Access to other databases was not required.
Ethics Approval
Informed consent was obtained from the patient for this case report. Per the institutional review board at Emory University, this case report would not require IRB review because it is not “human subjects research” as defined in the federal regulations.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
References
1. Petraki C, Vaslamatzis M, Argyrakos T et al. Tumor to tumor metastasis: report of two cases and review of the literature. Int J Surg Pathol 2003;11(2):127–135. doi:10.1177/106689690301100214. [Google Scholar] [PubMed] [CrossRef]
2. Ricketts R, Tamboli P, Czerniak B, Guo CC. Tumor-to-tumor metastasis: report of 2 cases of metastatic carcinoma to angiomyolipoma of the kidney. Arch Pathol Lab Med 2008;132(6):1016–1020. doi:10.5858/2008-132-1016-TMROCO. [Google Scholar] [PubMed] [CrossRef]
3. Altinok GGG. Tumor metastasis to an oncocytoma. Scand J Urol Nephrol 1999;33(6):416–417. doi:10.1080/003655999750017121. [Google Scholar] [PubMed] [CrossRef]
4. Hallet J, Law CHL, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121(4):589–597. doi:10.1002/cncr.29099. [Google Scholar] [PubMed] [CrossRef]
5. Tsoli M, Chatzellis E, Koumarianou A, Kolomodi D, Kaltsas G. Current best practice in the management of neuroendocrine tumors. Ther Adv Endocrinol Metab 2019;10:204201881880469. doi:10.1177/2042018818804698. [Google Scholar] [PubMed] [CrossRef]
6. Alevroudis E, Spei ME, Chatziioannou SN et al. Clinical utility of 18F-FDG PET in neuroendocrine tumors prior to peptide receptor radionuclide therapy: a systematic review and meta-analysis. Cancers 2021;13(8):1813. doi:10.3390/cancers13081813. [Google Scholar] [PubMed] [CrossRef]
7. Subramaniam RM, Bradshaw ML, Lewis K, Pinho D, Shah C, Walker RC. ACR practice parameter for the performance of gallium-68 DOTATATE PET/CT for neuroendocrine tumors. Clin Nucl Med 2018;43(12):899–908. doi:10.1097/RLU.0000000000002309. [Google Scholar] [PubMed] [CrossRef]
8. Delpassand ES, Ranganathan D, Wagh N et al. 64Cu-DOTATATE PET/CT for imaging patients with known or suspected somatostatin receptor-positive neuroendocrine tumors: results of the first U.S. prospective, reader-masked clinical tria. J Nucl Med 2020;61(6):890–896. [Google Scholar] [PubMed]
9. Petts G, Rashid T, Hrouda D, Ngo NT. Tumour-to-tumour metastases: prostate carcinoma metastasising to a renal oncocytoma. BMJ Case Rep 2013;2013:bcr2012007366. doi:10.1136/bcr-2012-007366. [Google Scholar] [PubMed] [CrossRef]
10. Moody P, Murtagh K, Piduru S, Brem S, Murtagh R, Rojiani AM. Tumor-to-tumor metastasis: pathology and neuroimaging considerations. Int J Clin Exp Pathol 2012;5(4):367–373. [Google Scholar] [PubMed]
11. Lee GM, Stowell JT, Pope K, Carter BW, Walker CM. Lymphatic pathways of the thorax: predictable patterns of spread. Am J Roentgenol. 2021;216(3):649–658. doi:10.2214/AJR.20.23523. [Google Scholar] [PubMed] [CrossRef]
12. Baston C, Parosanu AI, Mihai M, Moldoveanu O, Stanciu IM, Nitipir C. Tumor-to-tumor metastasis of lung cancer to kidney cancer: a review of the literature and our experience. Diagnostics 2024;14(5):553. doi:10.3390/diagnostics14050553. [Google Scholar] [PubMed] [CrossRef]
13. Aggarwal N, Amin RM, Chung D, Parwani AV. Tumor-to-tumor metastasis: case report of a pulmonary adenocarcinoma metastatic to a clear cell renal cell carcinoma. Pathol Res Pract 2012;208(1):50–52. doi:10.1016/j.prp.2011.10.003. [Google Scholar] [PubMed] [CrossRef]
14. Bartoš V. Tumor-to-tumor metastasis—a unique case of clear cell renal cell carcinoma harboring metastasis of adenocarcinoma of unknown origin. Klin Onkol 2018;31(5):366–370. [Google Scholar] [PubMed]
15. Hau J, Briosa Neves J, Varia M. Tumour-to-tumour metastasis of breast cancer (donor) and clear cell renal cell carcinoma (recipienta case report. J Clin Pathol 2024;77(4):255–256. doi:10.1136/jcp-2023-209183. [Google Scholar] [PubMed] [CrossRef]
16. Manini C, Provenza C, Andrés L et al. Tumor-to-tumor metastases involving clear cell renal cell carcinomas: a diagnostic challenge for pathologists needing clinical correlation. Clin Pract 2023;13(1):288–296. doi:10.3390/clinpract13010026. [Google Scholar] [PubMed] [CrossRef]
17. Song JS, Taylor SM, Trites J et al. Tumor-to-tumor metastases: papillary thyroid carcinoma into a clear cell renal cell carcinoma. J Otolaryngol Head Neck Surg 2017;46(1):17. doi:10.1186/s40463-017-0193-3. [Google Scholar] [PubMed] [CrossRef]
18. Shin T, Kan T, Sato F, Mimata H. Tumor-to-tumor metastasis to chromophobe renal cell carcinoma: a first report. Case Rep Urol 2011;2011(3):520839. doi:10.1155/2011/520839. [Google Scholar] [PubMed] [CrossRef]
19. Sakai S, Ohba K, Migita K, Sekine I, Yamazaki Y. Intratumoral metastasis of sigmoid colon cancer to chromophobe renal cell carcinoma: a case report. Int Cancer Conf J 2024;13(2):134–138. doi:10.1007/s13691-023-00651-5. [Google Scholar] [PubMed] [CrossRef]
20. Wu PS, Pan CC. Lung adenocarcinoma metastasizing into a renal angiomyolipoma. Int J Surg Pathol 2015;23(3):230–233. doi:10.1177/1066896915572224. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF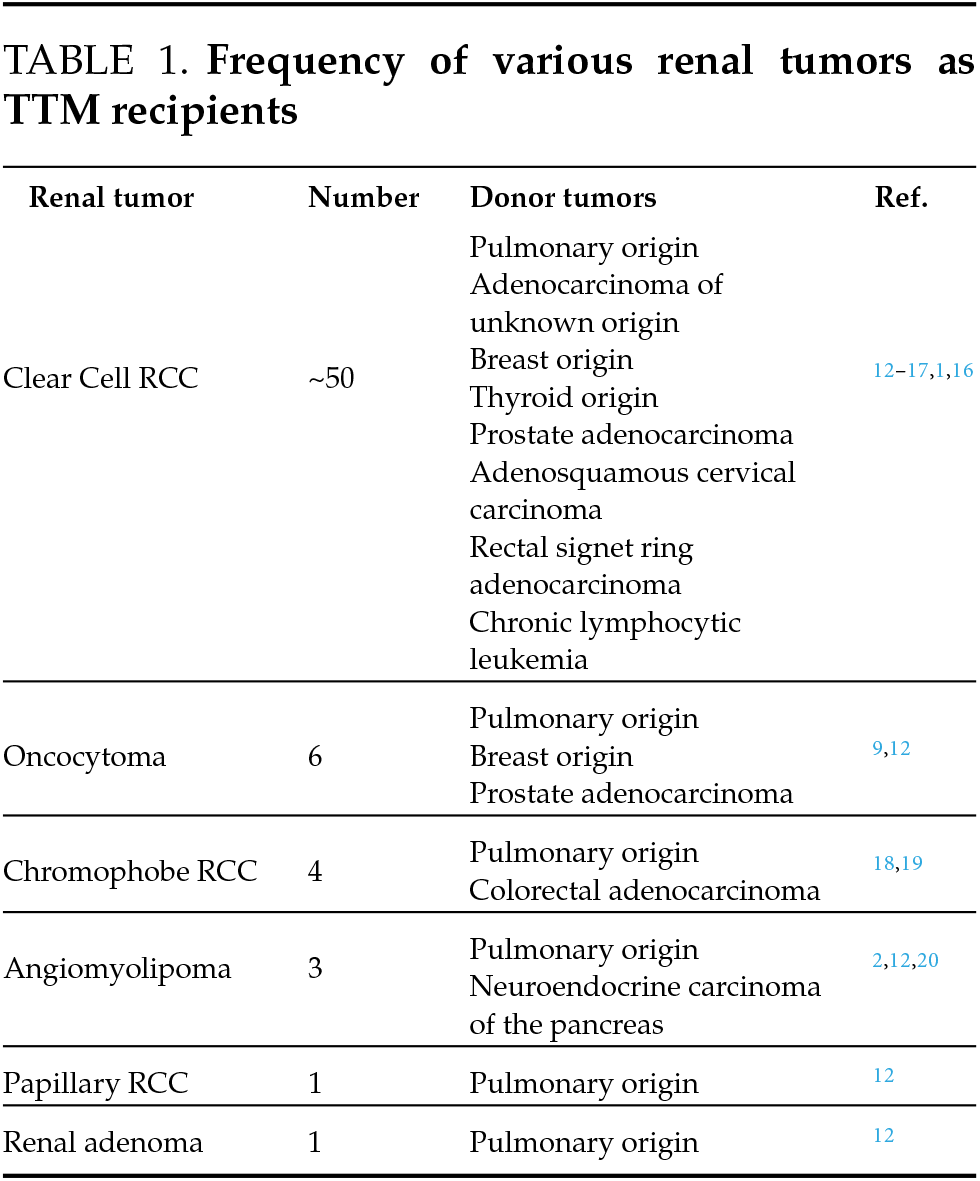
 Downloads
Downloads
 Citation Tools
Citation Tools