 Open Access
Open Access
ARTICLE
Impact of metabolic syndrome on combination therapy efficacy in LUTS due to BPH: a prospective study
1 Department of Surgery (Urology), University College of Medical Sciences (University of Delhi) & GTB Hospital, Delhi, 110095, India
2 Department of Surgery, University College of Medical Sciences (University of Delhi) & GTB Hospital, Delhi, 110095, India
3 Department of Medicine, University College of Medical Sciences (University of Delhi) & GTB Hospital, Delhi, 110095, India
* Corresponding Author: Iqbal Singh. Email:
Canadian Journal of Urology 2025, 32(4), 299-308. https://doi.org/10.32604/cju.2025.064827
Received 25 February 2025; Accepted 10 June 2025; Issue published 29 August 2025
Abstract
Objectives: Benign prostatic hyperplasia (BPH) is a common benign tumor in men, with an age-related prevalence of multifactorial etiology. The present study aimed to accurately assess and predict the effect of co-existing metabolic syndrome (MtS) upon treatment outcomes of combination medical therapy in select patients of lower urinary tract symptoms (LUTS) due to BPH. Methods: After obtaining informed consent from the patients, 70 eligible patients with LUTS due to BPH with and without MtS were enrolled in this study from September 2022 to January 2024 from the outpatient clinic at the University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi and were treated with a combination of Tamsulosin and Dutasteride, for two months, as per the protocol. The outcomes measured were a change in the International Prostate Symptom Score (IPSS), mean flow rate (MFR), and peak urine flow (Qmax) rates. Data was analysed using SPSS version 23. Results: The reduction in IPSS was higher in the control group than in the case group (p < 0.001), and the difference in MFR between the groups was also statistically significant (p < 0.001). Although there was a significant change in Qmax in both groups, the difference in the improvement in Qmax between the two groups was not significant (p < 0.829). The control group appeared to have achieved better symptomatic relief after treatment than did the case group. Conclusion: Metabolic syndrome had a negative adverse impact on medical treatment outcomes in selected patients of LUTS due to BPH. The study suggests that urologists should actively consider and appropriately counsel patients with LUTS-BPH and co-existing metabolic syndrome before selecting such patients for combination medical therapy.Keywords
Benign prostatic hyperplasia (BPH) is the most common benign tumor in men, with an age-linked incidence of 42% (51–60 years) and 85% in men older than 80 years. BPH arises from the non-malignant and unregulated proliferation of smooth muscles and epithelial cells in the prostatic transition zone. The majority of the risk factors associated with BPH appear to be age-linked and related to dihydrotestosterone (DHT) levels, as the prostate may become more sensitive to androgens in men with increasing age and this process may be multifactorial. Although multiple factors such as hormones (especially steroids such as androgens and estrogens), inflammation, lifestyle, and environmental factors have been known to play a role in the development of BPH, the exact etiology is unknown. The concomitant presence of metabolic syndrome (MtS) may determine the development, progression, growth rate, and severity of lower urinary tract symptoms (LUTS) due to BPH and may also be an additional major predictor of the clinical outcome of the disease. It has been hypothesized in published literature that hyperinsulinemia increases sympathetic activity, which may increase prostatic smooth muscle tone and consequently result in the worsening of LUTS.1 Selective alpha-1 blockers help reduce the effect of increased sympathetic tone and are therefore generally acknowledged as the first line of pharmacological management of LUTS-BPH unless indicated otherwise.2
Many published studies investigating the clinical response of BPH associated with metabolic syndrome to selective alpha-1 blockers have reported conflicting or equivocal results.3 However, more robust studies, especially from the Indian subcontinent, are missing to assess the same due to the high prevalence of MtS in the Indian population. Hence, the present study was conducted to evaluate the impact of MtS on treatment outcomes of combination medical therapy in patients with LUTS due to BPH. It also aimed to investigate the interference of MtS, if any, upon the nature of responsiveness to medical treatment of LUTS-BPH to guide clinicians for better selection of patients and appropriate counseling in those opting for medical management of LUTS-BPH.
Inclusion and exclusion criteria of patients
After obtaining the institutional ethics committee (IEC) and informed consent, this prospective experimental study was conducted at the University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi from September 2022 to January 2024. Patients aged 40–80 years with LUTS/International Prostate Symptom Score (IPSS) 8–19 (moderate category) due to BPH without an absolute indication for surgery, including non-operative patients with prostate volume >30 cm3 assessed by screening ultrasound, were included in this study. Patients with mental disorders or illnesses who could not understand or comply with the study protocol, BPH patients with complications like (recurrent Urinary Tract Infections [UTI], haematuria, chronic renal failure, refractory urinary retention etc., due to BPH), acute bacterial prostatitis, urethral stricture, urolithiasis, post-void residual urine >200 mL, bladder outlet obstruction/LUTS due to disorders other than BPH, known drug allergy/contraindications to tamsulosin/dutasteride, prior prostate or urethral surgery/diagnosed/suspicious case of cancer prostate, short-term weight changes (less than a month), severe cardiac, hepatic, or renal insufficiency, postural hypotension, uncontrolled severe hypertension, and history of concomitant drug treatment with androgens, anti-androgens, diuretics, cholinergic, anticholinergics, phytotherapy, metformin, statins, and insulin in the past 3 months were excluded from the current study.
The institutional ethics committee of the University College of Medical Sciences, Delhi had approved this study vide No. IECHR-2022-56-55-R1 dated 30th August’ 2022 from the ethical angle. All men participating in the study gave written and informed consent to participate in this study.
The working definition of MtS employed in the current study was as per the consensus definition for adult Asian Indians for MtS,2 which was the presence of three or more of the following: (i) waist circumference more than equal to 90 cm; (ii) triglycerides more than equal to 1.7 mM (150 mg/dL) or on drug treatment for triglycerides; (iii) reduced high-density lipoprotein (HDL)-cholesterol less than equal to 1 mM (40 mg/100 mL); (iv) systolic blood pressure (SBP) ≥ 130 mmHg and/or diastolic blood pressure (DBP) ≥ 85 mmHg or treated; (v) elevated fasting glucose more than equal to 5.6 mM (100 mg/100 mL) or on drug treatment for elevated glucose.
Enrolled patients were divided into two groups, Group 1—Case (BPH with MtS) and Group 2—Controls (BPH without MtS); both groups were treated with a combination of medical therapy, tamsulosin (0.4 mg) and dutasteride (0.5 mg) at bedtime, for two months and followed for 2 months. The allocation sequence was concealed, meaning that the investigator enrolling the patients was unaware of the group assignment until after enrolment and treatment initiation to reduce selection bias. The study was single-blind, in which patients were unaware of their group assignment, but the investigators were aware of the group allocation. To mitigate the possible bias in outcome assessment measures such as IPSS, peak urine flow (Qmax), and mean flow rate (MFR) were evaluated independently using standardized protocols.
The primary outcome measures assessed were a change in the IPSS score and, secondarily, an improvement in the MFR and Qmax in both groups, as per the flow of the study protocol depicted in Figure 1.
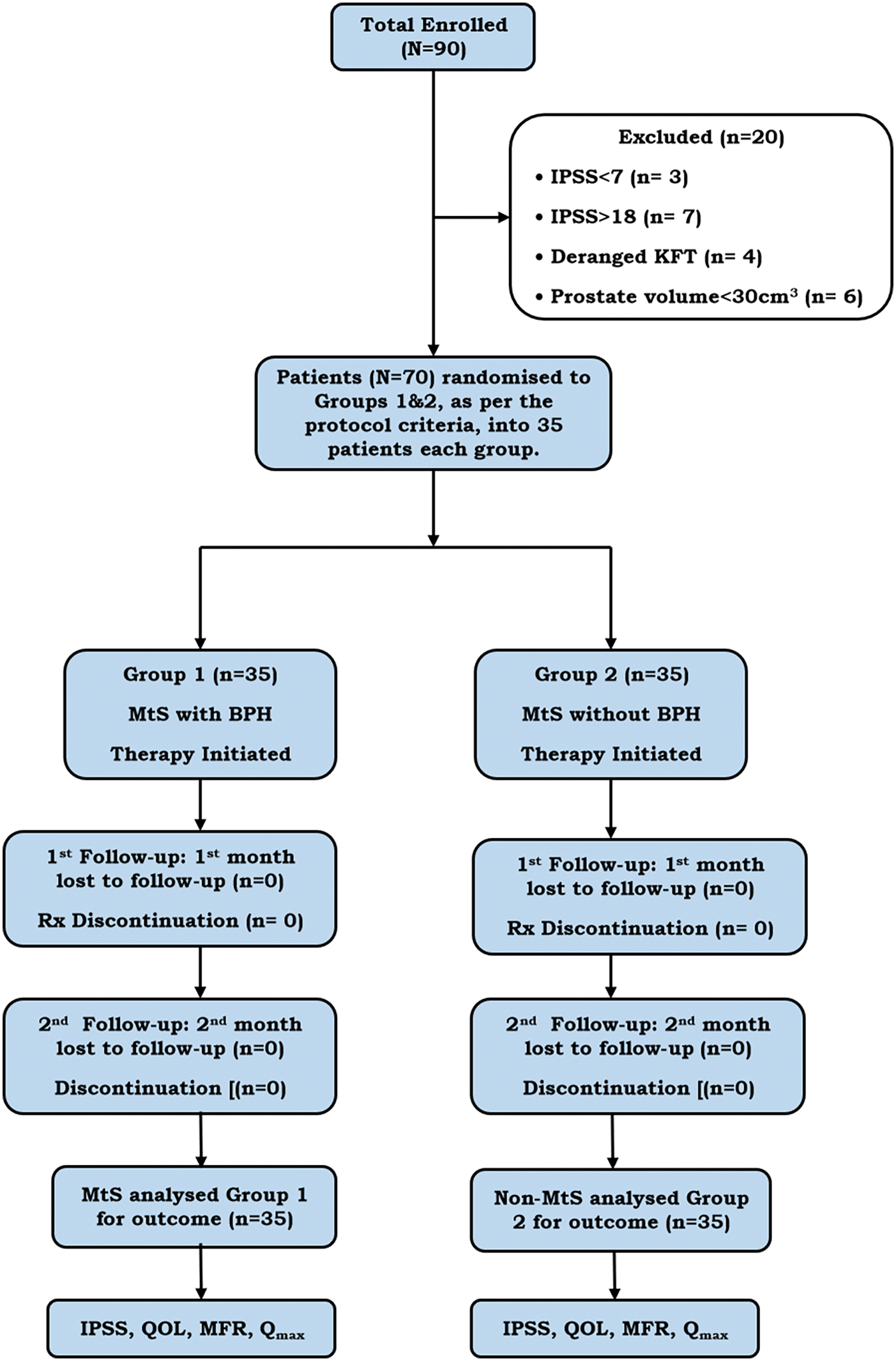
Figure 1: The flow box diagram depicting the flow of the study protocol of the present study. Note: IPSS, International Prostate Symptom Score; MtS, Metabolic Syndrome; QOL, Quality of Life; MFR, Mean flow rate; Qmax, Peak max flow rate; KFT, Kidney Function Test; Rx, Treatment.
The sample size for the study was based on a study by Pashootan et al.1 which reported mean IPSS scores of 9.62 ± 3 in the MtS group and 7.45 ± 3 in the non-MtS group. The sample size required for each arm of the study was calculated according to the formula given by Althubaiti.4
where σ is the standard deviation (SD), set to 3; δ is the expected difference in means, set to 2.17; α is the Type I error rate, set at 0.05, with corresponding Zα = 1.96; β is the Type II error rate, set at 0.20 (i.e., power = 80%), with corresponding Z1-β = 0.842.
Based on the above formula and mentioned values, the sample size (N) required was 31.01, to 32 per group. Thus, assuming 80% power and a 95% confidence interval, the minimum calculated sample size for each arm was 32 (total = 64). To compensate for the 5% loss to follow-up, 70 patients were enrolled in this study.
In the current study, data for various outcome variables, including age, IPSS, Qmax, and MFR, were initially assessed for normality. The results indicated that the data were not normally distributed for certain variables, particularly for age and flow rates (Qmax and MFR). To address the issue of non-normally distributed data, the study employed statistical methods tailored to the nature of the dataset. For skewed variables such as age and flow rates, non-parametric tests such as the Wilcoxon-Mann-Whitney U test (WMW) were used to compare the two patient groups, as these tests do not assume a normal distribution. For repeated measures (IPSS, Qmax, and MFR over time), the study applied the generalized estimating equations (GEE) method, a robust method capable of handling non-normal data, while accounting for correlations within subjects over time. In cases of severe skewness, data transformations (e.g., log transformation) were considered to approximate normality, although non-parametric methods were primarily relied upon, rendering transformations unnecessary in most instances.
The acquired data were analyzed using SPSS version 23 (IBM Corp., Armonk, NY, USA). Continuously distributed data variables were compared using the independent t-test and Wilcoxon test, as appropriate. Categorical data were compared using the chi-square test, as appropriate. To detect baseline differences and to minimize selection bias, the GEE method was used to compare changes in IPSS, Qmax, and MFR over time. Baseline values were included in the analyses to isolate treatment effects. Future research could benefit from propensity score matching to further balance the groups before treatment.
Descriptive statistics of patients
70 eligible patients were enrolled in the current study as per the protocol’s inclusion and exclusion criteria. 35 patients each were randomized and allotted to the case Group 1, BPH-LUTS with MtS, and Group 2, control BPH-LUTS without MtS. The flow chart of the current study protocol is depicted in Figure 1. The means (SDs) of age (in years) were 54.40 (6.52) and 54.20 (5.97) in Groups 1 and 2, respectively. The medians (interquartile ranges [IQRs]) of age (in years) were 54 (48–60) and 54 (50–58) in Groups 1 and 2, respectively. The age (in years) ranged from 42–65 years and 43–65 in Groups 1 and 2, respectively.
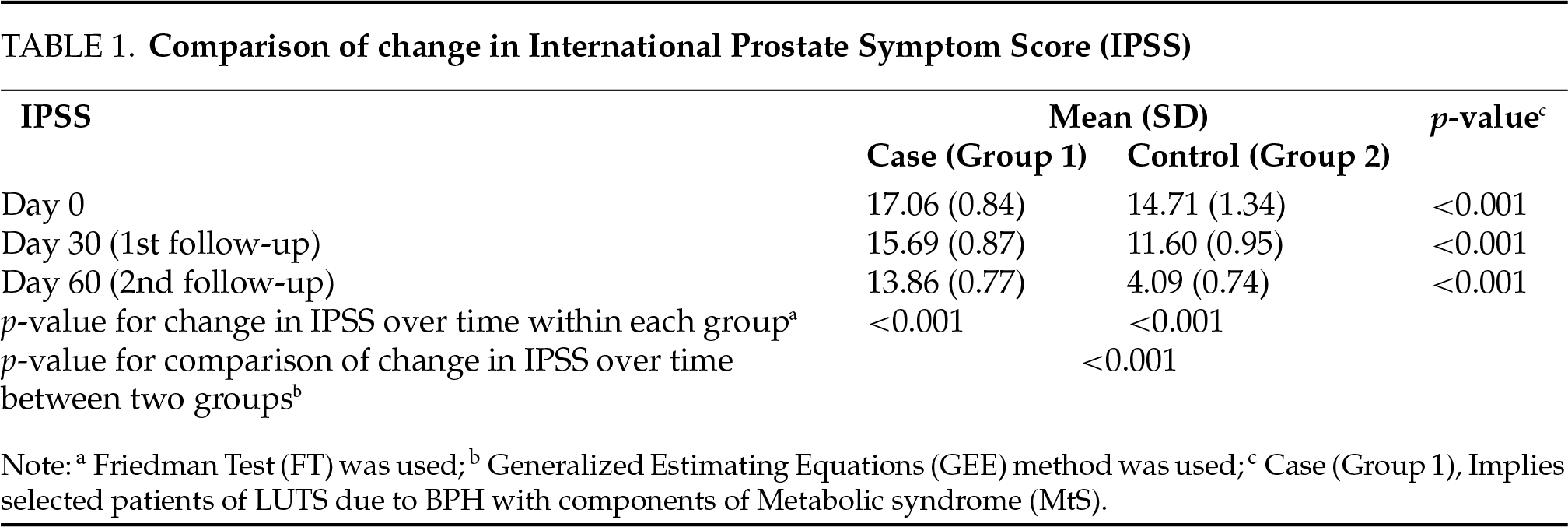
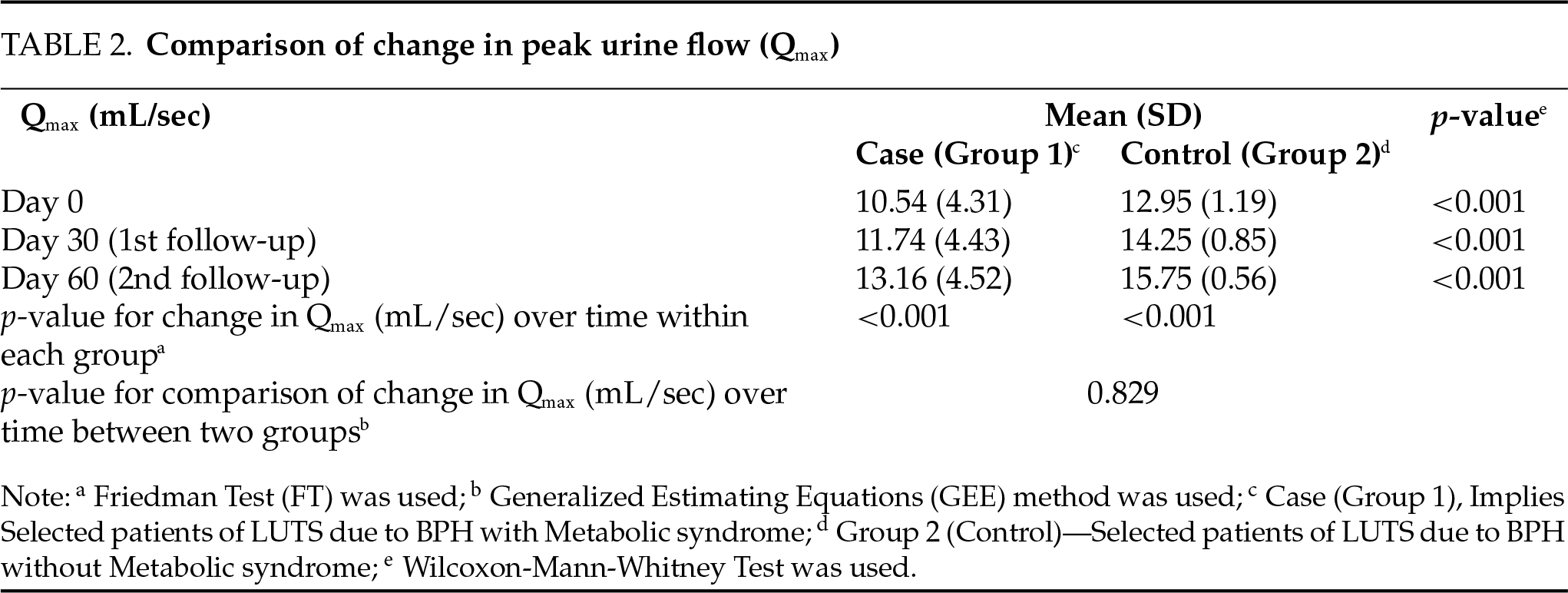
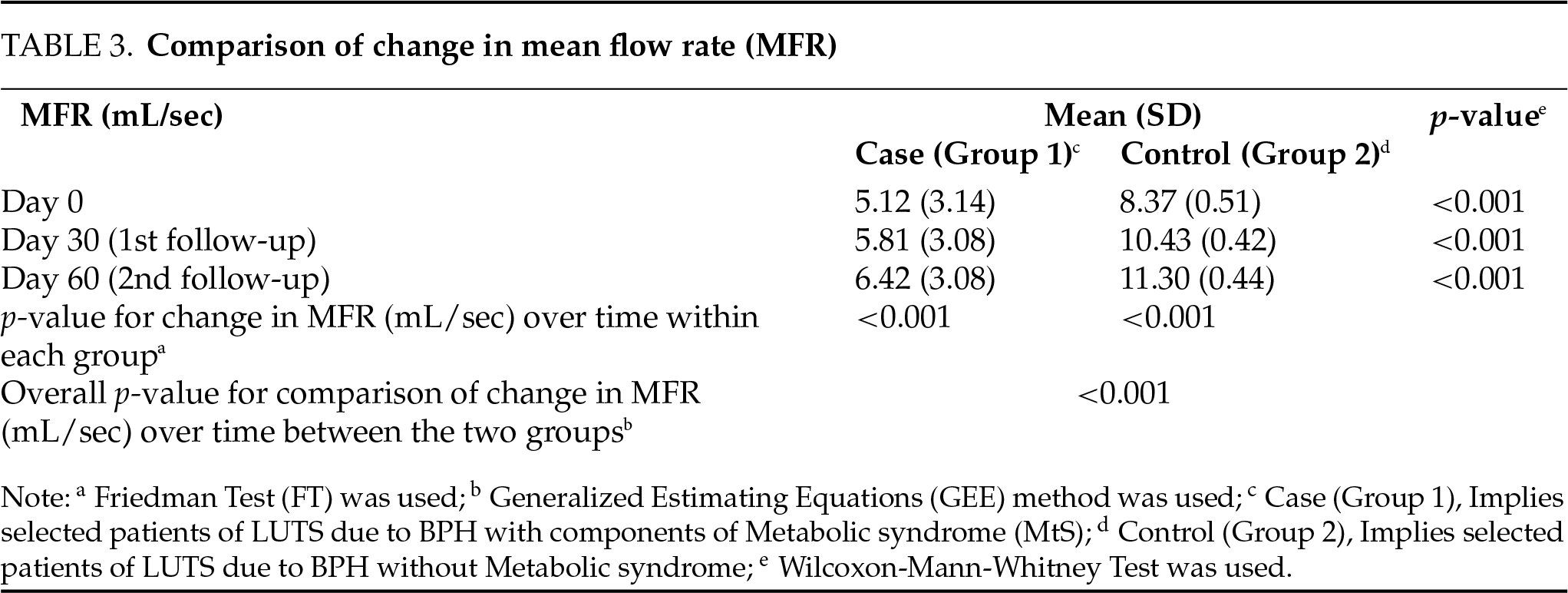
The overall change in IPSS from baseline over time was compared between the two groups using the GEE method. There was a significant difference in the trend of IPSS over time between the two groups (p < 0.001), as shown in Table 1. The overall change in Qmax (mL/sec) from baseline over time was compared between the two groups using the GEE method. There was no significant difference in the trend of Qmax (mL/sec) over time between the two groups (p = 0.829), as depicted in Table 2. The overall change in MFR (mL/sec) from baseline over time was compared between the two groups using the GEE method. There was a significant difference in the trend of MFR (mL/sec) over time between the two groups (p < 0.001) depicted in Table 3.
No treatment-emergent adverse events were encountered in the present study; all patients were fully compliant with the administered therapy and completed the study protocol, and no patient was lost to follow-up.
MtS is a diverse cluster spectrum of metabolic abnormalities associated with the central type of adiposity and insulin resistance with or without morbid obesity, dyslipidemia, and hypertension. According to Fu et al.,5 components of MtS, like insulin resistance, obesity, hypertension, and dyslipidemia, may be vital in contributing to prostatic volumetric enlargement and bladder outlet obstruction in patients of LUTS due to BPH. The impact of MtS on medical therapy for LUTS-BPH is scarcely documented in Indian men with BPH as well as in the globally published English literature.
In this study, we evaluated the impact of medical therapy on BPH-LUTSs with coexisting MtS. In the present study, our initial observations suggest that the combination of tamsulosin and dutasteride was significantly more efficacious in patients with BPH without MtS in terms of reduction in IPSS and improved MFR vs. BPH patients with co-existing MtS. While the reduction in IPSS was statistically significant in both groups, the same was significantly higher with greater symptomatic relief in Group 2 (p < 0.001) based on the reduced IPSS score. Although the improved MFR was statistically significant in both groups, it was significantly higher (p < 0.001) in patients with BPH without MtS. The intergroup comparison of the overall change in Qmax (mL/sec) over time suggested that the difference in the trend of peak flow rate over time between the two groups was not significant (p = 0.829).
In this study, both groups (BPH with/without MtS) showed improvements in the Qmax after receiving combination therapy. However, the difference in improvement between the two groups was not statistically significant (p = 0.829). This result may seem counterintuitive, especially when other outcomes such as IPSS and MFR showed more substantial improvements in the control group. Several factors could explain this, including baseline differences in the flow characteristics. Patients with MtS often have additional co-morbidities that may not immediately affect urine flow, as seen in the IPSS or MFR. Furthermore, the impact of MtS on bladder function may be gradual, requiring longer treatment duration to induce significant changes. Additionally, Qmax could be influenced by other metabolic factors such as blood pressure and glucose levels, which could affect the expected therapeutic response.
According to Pan et al.,6 in a retrospective study on Chinese men of varying age groups, they compared the association of MtS-BPH and demonstrated an increased risk of higher prostate volume and growth. a cross-sectional study (NHANES III) on 2372 males examined the association between different components of MtS and the severity of LUTS and demonstrated that LUTS severity was higher in patients with three or more components of MTS and that the presence of any component of MtS was positively associated with LUTS.7 Kupelian et al.8 also demonstrated that patients of BPH-LUTS with MtS had a statistically significant association between MtS and a voiding symptom score of 5 or greater (OR 1.73, 95% CI 1.06–2.80) versus storage symptom score (OR 0.94, 95% CI 0.66–1.33). Dibello et al.9 conducted a cross-sectional study comparing men with clinical BPH and matched controls without clinical BPH and found that men with clinical BPH were more likely to have MtS.
Cyrus et al.,10 a cohort study of 100 patients with BPH, with 47 vs. 53 patients with/without MtS in a multivariate analysis of covariance comparing the response in BPH patients with and without MtS before and after medical treatment with prazosin and finasteride for 3 months, demonstrated a significantly (p < 0.001) higher prostate volume in the metabolic group vs. in the non-metabolic group (57 ± 32.65 mL compared with 46.00 ± 20.19 mL, p = 0.036), with a significantly higher reduction in the IPSS by 11 vs. 6 in the control vs. case group with metabolic syndrome. The impact of the various components of MtS separately, such as serum triglyceride (p < 0.001), fasting blood sugar (p = 0.001), and waist circumference (p = 0.028), significantly affected the clinical progression of BPH in this study, which demonstrated that patients with BPH and coexisting MtS hurt the response to medical therapy.
Pashootan et al.1 another observational study of 4666 men aged 55–100 years with LUTS, reported MtS in 51.5% of their patients, of whom 47% were treated for LUTS, demonstrated a significant link between MtS and treated LUTS (p < 0.001) and concluded that the presence of MtS was associated with a higher IPSS score, and the same appeared to be higher by at least two points in BPH men with MtS who had more severe LUTS.
Lee et al.11 in their observational study on 109 men with a prostate volume of 20 cm3 or greater with moderate to severe LUTS-BPH who had been allocated to medical therapy with daily oral doxazosin 4 mg for 12 weeks demonstrated that MtS was an independent factor for drug non-responders (OR = 4.26, p = 0.002) and rate of response/IPSS improvements in patients with MtS decreased significantly as the number of MtScomponents increased (p = 0.012, 0.026) and amongst their MtS components, abnormal fasting blood glucose was the most significantly independent factor for drug non-responders (p = 0.020) and concluded that the presence of MtS had a significantly negative impact on the response to α1-blocker in men with BPH/LUTS. Ozden et al.,12 in a prospective study examining the correlation between MtS annual prostatic growth rate in BPH patients, demonstrated that the same appeared to be significantly higher in BPH patients with MtS. Tewari et al.13 conducted a case-control study of patients with high triglyceride levels, low HDL levels, and a high waist-to-hip ratio and demonstrated the same association with a higher incidence of BPH. Park et al.14 conducted an observational study on Korean men with LUTS-BPH with and without MtSand and suggested that there was a difference in the total IPSS/Qmax, in which the maximum scores of the two patient groups were not significant.
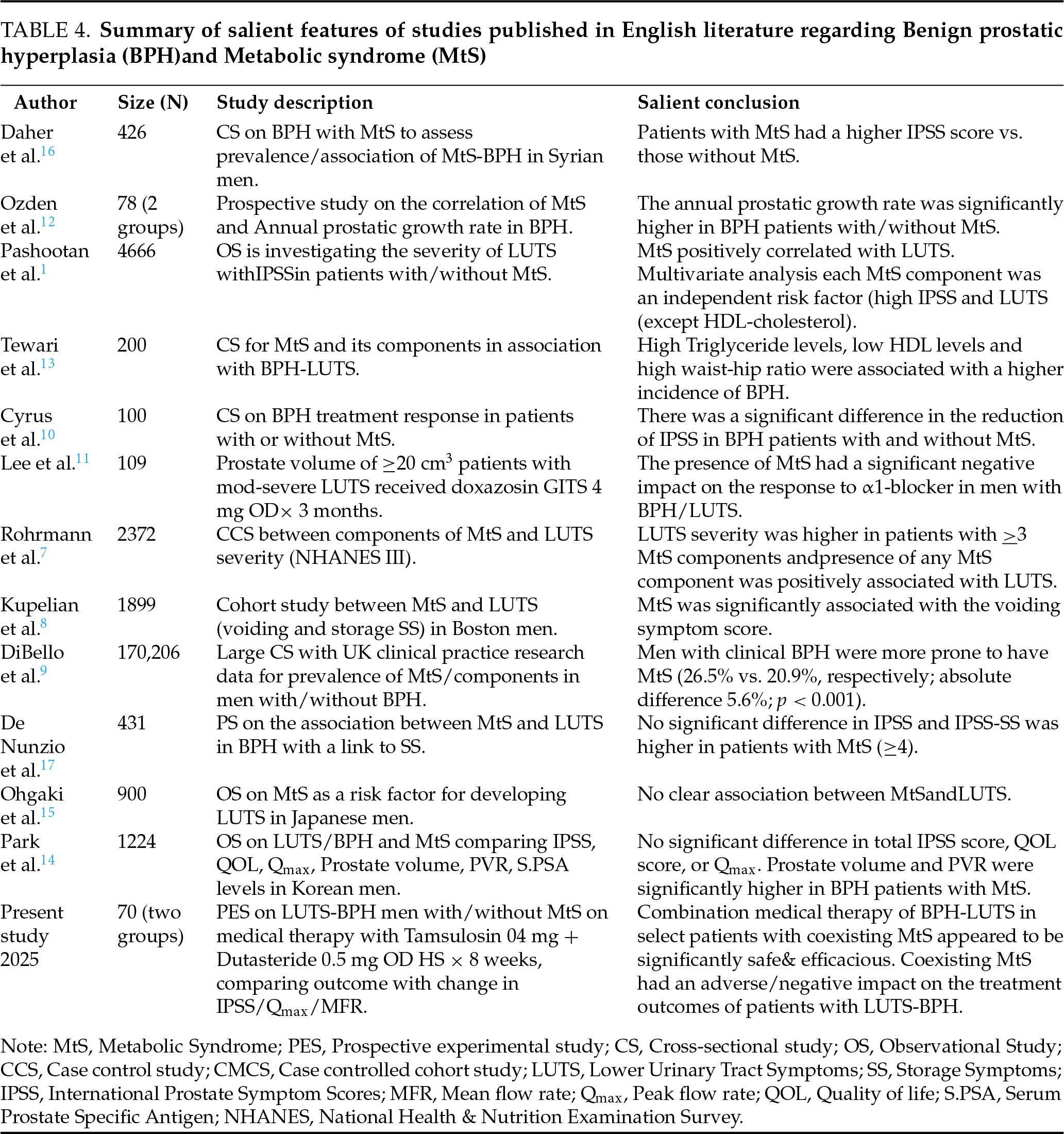
Ohgaki et al.15 in a prospective observational study, examined the association between MtS and LUTS in Japanese males using three sets of criteria for MtS and IPSS and concluded that LUTS was age-linked with no clear association between MtS and LUTS. Daher et al.16 in a cross-sectional study on Syrian men, assessed the prevalence and association between MtS-BPH and concluded that BPH patients with MtS had higher IPSS scores. De Nunzio et al.17 in a prospective observational study examining the association between MtS and LUTS-BPH, demonstrated that the IPSS of both groups was not significant, although they demonstrated a higher IPSS storage sub-score in their patients with MtS. Table 4 depicts the salient features of some landmark published studies on the impact of MtS upon LUTS-BPH in patients undergoing medical management for the same and its comparison with the present study.
The lack of a significant difference in Qmax despite improvements in other parameters highlights the need for individualized treatment plans for BPH patients with co-existing metabolic syndrome MtS. Although combination therapy may provide symptom relief, patients with MtS may require more intensive monitoring and longer follow-up, as improvements in flow rates may be gradual and unsustained. Healthcare providers should set forth realistic expectations and inform patients that improvements in their uroflow rates may require longer periods of medication Patients with MtS ought to be specifically and elaborately counselled at length about their likelihood of encountering either a delayed and or incomplete response and possibly even a prolonged course of medical therapy for symptomatic relief of LUTS-BPH. This should align with their realistic expectations for symptomatic improvement for same accordingly. Long-term management strategies to address the root causes of MtS should complement BPH treatments and research could examine the effects of extended medical therapy on their outcome in BPH patients with MtS.
Ngai et al.18 reported a 26.5%–55.6% prevalence of MtS in men with LUTS in worldwide studies with varying definitions of MtS asper (WHO, EGIR, NCEP-ATP III, and IDF definitions) and suggested that it was not unusual to encounter significantly different body fat compositions between different ethnic populations for a given body mass index (BMI) and concluded that LUTS-BPH were chronic inflammatory conditions with MtS playing a vital role in the pathogenesis of men with LUTS, alerting the clinician to identify MtS with associated cardiovascular risks. Xiong et al.,19 in a propensity match scoring, demonstrated that aging Chinese men with MtS had a greater likelihood of BPH-LUTS, excluding hypertensive patients. Varying working definitions of MtS could have been another limiting factor that was not evaluated in the current study due to the varying diversity of different components of MtS in individual patients, which could not be generalized. A case in this point is also supported in another cross-sectional study by Tarim et al.20 in which the authors found that while the occurrence of symptomatic BPH was no different to individual MtS components alone however when the same occurred with synergy and augmented as a full-blown metabolic syndrome the occurrence of BPH amplified. Omran et al.21 also, in a recent meta-analysis of 30 studies, showed a vital relationship amidst an overall detrimental effect of MtS that tended to frequently co-exist in a symbiotic relation with obesity-BPH, with higher prostatic volumes, though it was without any noteworthy projecting factor for its size effects. Future research could focus on some recently published research results suggesting that certain pro-inflammatory mediators and suppressors of inflammation could be involved in the pathogenesis of BPH with metabolic syndrome, whose exact impact, if any, remains to be ascertained.22
Although the present study was adequately powered to draw reliable conclusions, we acknowledge certain limitations. The study was limited by the small sample size, which warrants a larger multicenter long-term study. In the present study, the impact of various components of MtS was not compared separately, and the duration of MtS was not taken into consideration, as this was a short-term study. Further, the effect of other medications, if any, by the patients (insulin injection/oral hypoglycaemics, antihypertensives, among others) was not evaluated in this study as per protocol. The IPSS scores, Qmax, and MFR of the two groups were not comparable, even though the IPSS of both groups was moderately severe and patients with MtS appeared to be more symptomatic to start with, which could have had a bearing on our conclusions. Although we had consciously enrolled BPH patients with ultrasound-proven grade II prostate enlargement (≥30 cm3), the effect of intravesical prostate protrusion on treatment outcomes was not specifically evaluated, as the same was beyond the current protocol, which could have affected the study outcomes. The primary focus of this study was to study the impact of metabolic syndrome on symptom relief, urine flow, and Qmax rather than on prostate structure and volume. As per protocol criteria, we excluded patients with an absolute indication for surgical intervention, and including intravesical protrusion of the prostate (IPP) and prostate volume would have broadened the scope of this study, potentially diluting the main research question. Additionally, detailed imaging data for assessing these factors, such as transrectal ultrasound or MRI, were not included in the study protocol due to resource constraints.
While both IPP and prostate volume are important anatomical factors affecting urinary flow and symptom severity in BPH, their exclusion could have impacted our findings. Larger prostate volumes could typically be linked to more severe LUTS, which could have influenced the baseline severity and treatment responses, though this is not without exceptions. The control group might have had different prostate volume distributions than the MtS group, possibly due to confounding urinary flow characteristics and treatment outcomes. Moreover, IPP could play a role in bladder outlet obstruction and could have worsened symptoms, especially in the MtS group, affecting treatment responses. Without IPP data, it is unclear whether anatomical factors, in addition to metabolic dysfunction, had contributed to the lack of improvement in uroflow. While this study focused on short-term outcomes, we admit that prostate volume and IPP could play a significant role in long-term treatment outcomes. Future studies should include comprehensive evaluations of these factors alongside metabolic syndrome to better understand their combined effects on treatment outcomes, particularly longitudinal studies with extended follow-up periods. Nevertheless, we do admit to these limitations as above and our conclusions should be seen and read in the light of these limitations.
This study demonstrated that MtS negatively affects treatment outcomes of combination medical therapy for BPH-related LUTS, as shown by lower improvement in IPSS and maximum flow rate (MFR) compared to the control group. While these findings were statistically significant, limitations such as a small sample size and short follow-up duration reduce the generalisability of the results. Larger, multicentre studies with extended follow-up are needed to better understand the long-term impact of MtS on BPH treatment. Future research should also include detailed assessments of prostate volume and intravesical prostate protrusion to explore anatomical factors influencing treatment response. Despite these limitations, our findings suggest that co-existing metabolic syndrome may adversely affect treatment outcomes in men with LUTS due to BPH, and patients should be counseled accordingly. These results provide a strong rationale for future, large-scale studies incorporating additional variables.
Acknowledgement
None.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design & supervision: Iqbal Singh, Sanjay Gupta, Alpana Raizada; data collection: Vidhi Maurya, Himanshu Agrawal, Sanjay Gupta, Iqbal Singh, Alpana Raizada; draft manuscript preparation: Himanshu Agrawal, Iqbal Singh, Vidhi Maurya. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author, Iqbal Singh, upon reasonable request.
Ethics Approval
The study included human subjects. The Institutional Ethics Committee of the University College of Medical Sciences, Delhi had approved this study vide No. IECHR-2022-56-55-R1 dated 30th August 2022 from the ethical angle.
Informed Consent
All men participating in the study gave written and informed consent to participate in this study.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
References
1. Pashootan P, Ploussard G, Cocaul A, de Gouvello A, Desgrandchamps F. Association between metabolic syndrome and severity of lower urinary tract symptoms (LUTSan observational study in a 4666 European men cohort. BJU Int 2015 Jul;116(1):124–130. [Google Scholar]
2. Yuan Q, Zhou X, Ma L et al. The association between solid fuel use and lower urinary tract symptoms suggestive of benign prostatic hyperplasia in Sichuan, China: cross-sectional study. JMIR Public Health Surveill 2024 Oct 31;10:e53673. doi:10.2196/53673. [Google Scholar] [PubMed] [CrossRef]
3. Abdollah F, Briganti A, Suardi N et al. Metabolic syndrome and benign prostatic hyperplasia: evidence of a potential relationship, hypothesized etiology, and prevention. Korean J Urol 2011 Aug 22;52(8):507. [Google Scholar]
4. Althubaiti A. Sample size determination: a practical guide for health researchers. J Gen Fam Med 2022 Dec 14;24(2):72–78. doi:10.1002/jgf2.600. [Google Scholar] [PubMed] [CrossRef]
5. Fu X, Wang Y, Lu Y, Liu J, Li H. Association between metabolic syndrome and benign prostatic hyperplasia: the underlying molecular connection. Life Sci 2024 Oct 31;358:123192. doi:10.1016/j.lfs.2024.123192. [Google Scholar] [PubMed] [CrossRef]
6. Pan JG, Jiang C, Luo R, Zhou X. Association of metabolic syndrome and benign prostatic hyperplasia in Chinese patients of different age decades. Urol Int 2014 Nov 13;93(1):10–16. [Google Scholar]
7. Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the third national health and nutrition examination survey (NHANES III). Int J Obes 2005 Mar;29:310–316. [Google Scholar]
8. Kupelian V, McVary KT, Kaplan SA et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston area community health survey. J Urol 2013 Jan;189(1 Suppl):S107–S114; discussion S115–S16. [Google Scholar]
9. DiBello JR, Ioannou C, Rees J et al. Prevalence of metabolic syndrome and its components among men with and without clinical benign prostatic hyperplasia: a large, cross-sectional, UK epidemiological study. BJU Int 2016 May;117:801–808. [Google Scholar]
10. Cyrus A, Kabir A, Goodarzi D et al. Impact of metabolic syndrome on response to medical treatment of benign prostatic hyperplasia. Korean J Urol 2014 Dec 1;55(12):814–820. [Google Scholar]
11. Lee YC, Liu CC, Juan YS et al. The impact of metabolic syndrome on the responsiveness to α1-blocker in men with BPH/LUTS. Int J Clin Pract 2013 Apr;67(4):356–362. [Google Scholar]
12. Ozden C, Ozdal OL, Urgancioglu G, Koyuncu H, Gokkaya S, Memis A. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol 2007 Jan 1;51(1):199–206. [Google Scholar]
13. Tewari R, Prabhat P, Natu SM et al. Association of benign prostatic hyperplasia (BPH) with the metabolic syndrome (MS) and its components–‘a growing dilemma’. J Men’s Health 2011 Mar;8(1):66–71. [Google Scholar]
14. Park YW, Min SK, Lee JH. Relationship between lower urinary tract symptoms/benign prostatic hyperplasia and metabolic syndrome in Korean men. World J Men’s Health 2012 Dec 27;30(3):183. [Google Scholar]
15. Ohgaki K, Hikima N, Horiuchi K, Kondo Y. Association between metabolic syndrome and male lower urinary tract symptoms in Japanese subjects using three sets of criteria for metabolic syndrome and International Prostate Symptom Score. Urology 2011 Jun 1;77(6):1432–1438. [Google Scholar]
16. Daher M, Saqer T, Jabr M, Al-Mousa S. Benign prostatic hyperplasia and metabolic syndrome; prevalence and association: a cross-sectional study in Syria. BMC Urol 2023 Nov 16;23(1):187. [Google Scholar]
17. De Nunzio C, Cindolo L, Gacci M et al. Metabolic syndrome and lower urinary tract symptoms in patients with benign prostatic enlargement: a possible link to storage symptoms. Urology 2014 Nov 1;84(5):1181–1187. [Google Scholar]
18. Ngai HY, Yuen KK, Ng CM, Cheng CH, Chu SK. Metabolic syndrome and benign prostatic hyperplasia: an update. Asian J Urol 2017 Jul 1;4(3):164–173. [Google Scholar]
19. Xiong Y, Zhang Y, Tan J, Qin F, Yuan J. The association between metabolic syndrome and lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males: evidence based on propensity score matching. Transl Androl Urol 2021 Jan;10(1):384. [Google Scholar]
20. Tarım BA, Çamur E, Kavukoğlu Ö, Kösemen M, Özgür Y, Narter KF. Metabolic syndrome and benign prostatic hyperplasia/which component of metabolic syndrome is related to benign prostatic hyperplasia? J Urol Surg 2023;10(3):194–198. doi:10.4274/jus.galenos.2023.2022.0081. [Google Scholar] [CrossRef]
21. Omran A, Leca BM, Oštarijaš E et al. Metabolic syndrome is associated with prostate enlargement: a systematic review, meta-analysis, and meta-regression on patients with lower urinary tract symptom factors. Ther Adv Endocrinol Metab 2021 Dec;12:20420188211066210. [Google Scholar]
22. Ratajczak W, Walczakiewicz K, Laszczyńska M et al. The profile of oxidative stress markers (arachidonic and linoleic acid derivatives) in patients with benign prostatic hyperplasia in relation to metabolic syndrome. Aging 2025 Jan 6;17(1):116. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools