 Open Access
Open Access
ARTICLE
Impact of COVID-19 care reorganization on the prognosis of patients with bladder urothelial carcinoma: a multicentric retrospective study
1 Department of Urology, University Hospital of Tours (CHRU Tours), 2 Boulevard Tonnellé, Tours Cedex 9, 37044, France
2 Department of Urology, Centre Hospitalier Régional d’Orléans (CHR Orléans), 14 Avenue de l’Hôpital, Orléans, 45100, France
* Corresponding Author: Ali Bourgi. Email:
Canadian Journal of Urology 2025, 32(4), 359-366. https://doi.org/10.32604/cju.2025.066470
Received 09 April 2025; Accepted 15 August 2025; Issue published 29 August 2025
Abstract
Background: The COVID-19 pandemic disrupted healthcare systems globally, raising concerns about delayed cancer diagnosis and treatment. In France, transurethral resection of bladder tumors (TURBT) was prioritized in national urology guidelines to ensure the timely management of urothelial carcinoma. This study aimed to assess the impact of care reorganization on tumor staging, recurrence, palliative care, and mortality in bladder cancer patients from the pre-pandemic through late-pandemic periods. Methods: We conducted a retrospective multicenter study including all patients who underwent TURBT with histologically confirmed urothelial carcinoma between April and December of 2019 (pre-pandemic), 2020 (early pandemic), 2021 (mid-pandemic), and 2022 (late pandemic) in two French institutions. TURBT indications were categorized as diagnostic, palliative, or staging. Clinical and pathological data were compared across the four periods. Statistical analyses included Chi-square tests, Estimated Annual Percentage Change (EAPC), and multivariable logistic regression adjusted for age, sex, ASA score, and center. Results: A total of 790 TURBT procedures were analyzed. The proportion of muscle-invasive bladder cancer (pT ≥ 2) declined over time (18.7% in 2019 to 13.2% in 2022; p = 0.63), while superficial tumors (pTa) increased (57.2% to 65.5%). All-cause mortality significantly decreased from 38.0% in 2019 to 22.0% in 2020, 20.5% in 2021, and 19.5% in 2022 (p = 0.006). EAPC showed a significant annual decline in mortality (–24.3%, p = 0.004). In multivariable analysis, 2020, 2021, and 2022 were each associated with significantly lower odds of mortality compared to 2019. Recurrence rates remained stable across all periods (p = 0.93). Inter-hospital variation persisted in mortality and recurrence. Conclusions: Despite the pandemic, urothelial bladder cancer outcomes did not worsen through 2022. On the contrary, timely reorganization, prioritization of TURBT, and triage strategies were associated with reduced mortality and palliative care needs, highlighting the resilience of cancer care when guided by adaptive health policies.Keywords
The COVID-19 outbreak in France in early 2020 led to substantial changes in hospital workflows. National and international urology societies issued guidelines for cancer care during this crisis, emphasizing the need to maintain timely management for high-risk malignancies.1–3 A decline in new bladder cancer diagnoses and consultations was reported,4–8 raising concerns about potential diagnostic delays and their impact on disease progression.9,10
During this epidemic, most different cancers saw their incidence decrease.11–15 The number of surgical procedures for bladder cancer decreased in France in 2020 (transurethral resection of bladder tumors [TURBT] −2%, radical cystectomy for bladder cancer −6.1%).16 The local stage of bladder cancer decreased by around 4%11,17 between 2019 and 2020, probably due to delays in care. Other studies suggest that higher stages of bladder carcinoma were found during TURBT after the beginning of the pandemic.18–21 It suggests that bladder carcinoma was the most impacted urological cancer during the COVID-19 pandemic.22,23
Since March 2020 (the start of the COVID-19 pandemic in France), several periods have followed one another. Many episodes of confinement and hospital tensions occurred, with some regional variations. Some of these French hospitals have had to restrict access to operating rooms to prioritize the opening of intensive care beds in the recovery room. Organizational differences between cities and the degree of saturation of their hospitals were noted. TURBT was designated as a priority by the French Association of Urology (AFU), which recommended it be performed within one month of diagnosis.1 Hence, this study aimed to assess whether the COVID-19 pandemic affected tumor staging and patient prognosis for those diagnosed with bladder urothelial carcinoma.
This retrospective multicentric study included all TURBTs performed between 1 April and 31 December of 2019 (Period 1: pre COVID), between 1 April and 31 December of 2020 (Period 2: early COVID), between 1 April and 31 December of 2021 (Period 3: mid COVID), and between April 1 and December 31 of 2022 (Period 4: after COVID) in two French hospitals: a university hospital (Center 1: CHRU Tours) and a regional hospital (Center 2: CHR Orleans). Only TURBTs with histologically confirmed urothelial carcinoma were included.
This study was approved by the Institutional Review Board of CHRU Tours (Ref. No. 2025_077). Given the retrospective design, informed consent was waived.
At Center 1, elective surgeries were postponed, but TURBT procedures and cystoscopies were largely maintained. In contrast, Center 2 experienced a more severe COVID-19 wave and had to repurpose its post-anesthesia care unit as an ICU, resulting in a sharp decline in urological surgeries. We evaluated tumor stage (pTa, pT1, pT2 or greater), tumor grade (high vs. low), tumor multiplicity, size, and location, history of prior TURBT (new vs. recurrent cases), delays from diagnosis to surgery, patient characteristics (age, sex, BMI, ASA score, smoking), and outcomes including recurrence, need for BCG/mitomycine, palliative care, cystectomy, radiotherapy or chemotherapy, and mortality.
Each TURBT procedure was retrospectively categorized based on clinical indication into one of three groups: diagnostic (new tumor diagnosis), palliative (relief of symptoms in advanced disease), or staging (confirming muscle invasiveness). Indications were identified through operative reports and clinical records.
To evaluate time trends, we calculated the Estimated Annual Percentage Change (EAPC) using Poisson regression as described by Li et al.24 In addition, we used multivariable logistic regression to assess the association between each year and key outcomes, adjusting for age, sex, ASA score, and treatment center.
Statistical analyses were performed using Chi-square or Fisher’s exact tests for categorical variables and Mann-Whitney/Welch tests for quantitative data. A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using R (version 4.4.0) within the RStudio environment (version 2024.04.1), utilizing the stats, dplyr, and ggplot2 packages. Results were independently validated using Medistica’s Pvalue.io, a certified web-based statistical calculator.
Patient characteristics and tumor features
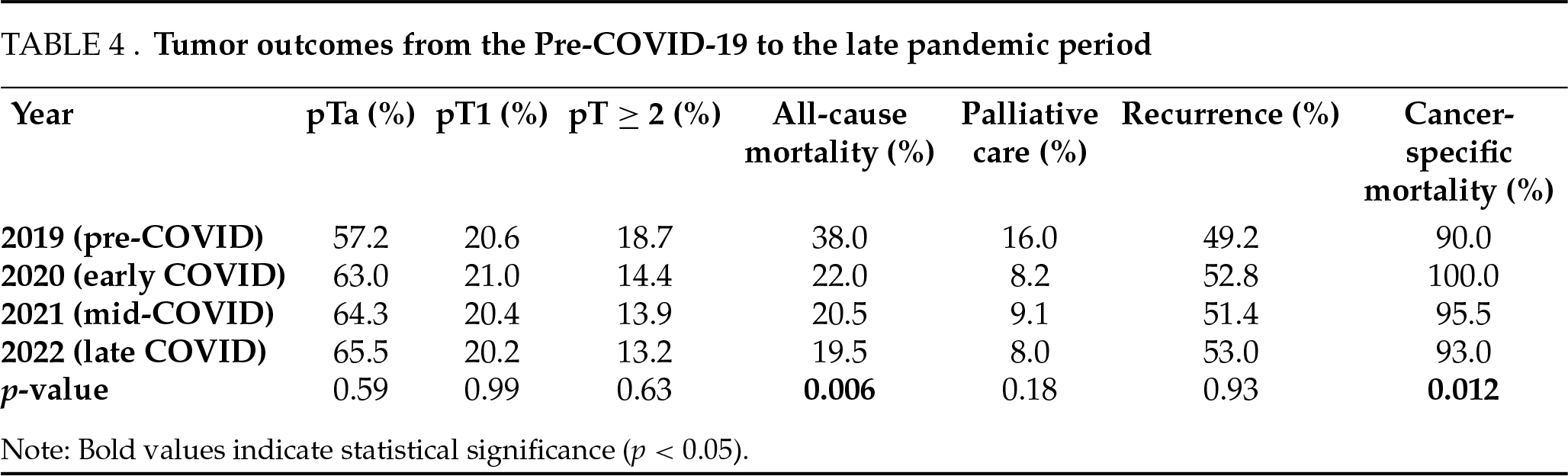
A total of 790 patients underwent TURBT between April and December of 2019 (n = 194), 2020 (n = 182), 2021 (n = 222), and 2022 (n = 192). The demographic characteristics of the patients studied were relatively comparable between the three periods studied (Table 1). No significant difference was shown over the years regarding the histological findings in initial TURBT procedures (Table 2) or in TURBT for tumor recurrence (Table 3), including tumor stage, grade, size, multiplicity, anatomical location, and estimated recurrence risk.



Overall, these tables provide insight into the consistency or variation in patient presentation and tumor characteristics from the pre-COVID-19 to the late pandemic period.
Pathological staging demonstrated a non-significant but progressive reduction in muscle-invasive bladder tumors (MIBT; pT ≥ 2) after the onset of COVID-19 (Tables 2 and 3). In 2019, MIBT either on primary resection or at recurrence accounted for 18.7% of tumors; this decreased to 14.4% in 2020, 13.9% in 2021, and 13.2% in 2022 (p = 0.63). The proportion of superficial tumors (pTa) increased from 57.2% in 2019 to 63.0% in 2020, 64.3% in 2021, and 65.5% in 2022 (p = 0.59). pT1 tumors remained stable (20.6% in 2019, 21.0% in 2020, 20.4% in 2021, and 20.2% in 2022; p = 0.99).
The EAPC for the proportion of muscle-invasive tumors (pT ≥ 2) showed a non-significant decline of −7.6% per year (95% CI: −15.4% to +1.1%; p = 0.08).
All-cause mortality significantly decreased from 38.0% in 2019 to 22.0% in 2020 (p = 0.029), 20.5% in 2021, and 19.5% in 2022 (p = 0.006 for trend). Palliative care needs also declined from 16.0% in 2019 to 8.2% in 2020, remained low at 9.1% in 2021, and further decreased slightly to 8.0% in 2022 (p = 0.18). Cancer-specific mortality was high and variable: 90.0% in 2019, 100.0% in 2020, 95.5% in 2021, and 93.0% in 2022 (p = 0.012). A significant decrease in mortality was observed, with an EAPC of −24.3% per year (95% CI: −37.5% to −8.1%; p = 0.004) (Table 4).
Multivariable logistic regression
To further account for potential confounding factors, we performed multivariable logistic regression analyses adjusting for age, sex, ASA score, and treatment center. Compared to 2019, the odds of all-cause mortality were significantly lower in:
– 2020 (odds ratio [OR] = 0.56; 95% CI: 0.34–0.94; p = 0.028)
– 2021 (OR = 0.51; 95% CI: 0.32–0.84; p = 0.007)
– 2022 (OR = 0.48; 95% CI: 0.30–0.81; p = 0.005)
These findings indicate that the observed decline in mortality was not solely due to fluctuating case volume but may reflect sustained prioritization and adaptation of care pathways during and after the pandemic.
Recurrence rates remained stable over time: 49.2% in 2019, 52.8% in 2020, 51.4% in 2021, and 53.0% in 2022 (p = 0.93), indicating preserved oncologic follow-up and care continuity across all periods (Table 4).
Among all TURBT procedures, 78% were performed for diagnostic purposes, 14% for palliative intent, and 8% for staging in suspected advanced cases. These proportions remained stable throughout the study period. Trimodal therapy (TMT) was administered in 9% of muscle-invasive bladder cancer cases, without significant variation between 2019 and 2022.
In 2019, all-cause mortality was higher at Center 2 (CHR Orleans: 47.5%) compared to Center 1 (CHRU Tours: 31.2%). In 2020, mortality decreased in both centers but remained higher at CHR Orleans (27.1%) than at CHRU Tours (19.5%). In 2021, mortality continued to decline (CHR Orleans: 23.3%, CHRU Tours: 18.0%), and this trend persisted in 2022 (CHR Orleans: 21.0%, CHRU Tours: 17.5%).
Recurrence remained more frequent at CHRU Tours in 2020 (56.2%), 2021 (54.7%), and 2022 (55.5%) compared to CHR Orleans (45.8% in 2020, 48.1% in 2021, and 49.0% in 2022). However, these inter-center differences in 2022 were not statistically significant (mortality: p = 0.49; recurrence: p = 0.43).
This multicentric retrospective analysis evaluated the impact of the COVID-19 pandemic on the oncologic outcomes of patients undergoing TURBT for bladder urothelial carcinoma. Contrary to initial concerns about delays in diagnosis and treatment, our findings suggest that the pandemic-related reorganization of care did not lead to more advanced tumor stages and may have even coincided with a reduction in all-cause mortality and the need for palliative care. Importantly, the inclusion of data from 2021 shows a continuation of these trends, providing a more comprehensive view of the mid-term consequences of the pandemic on bladder cancer management.
It is important to note that this study is descriptive in nature. Although we observed a temporal association between the COVID-19 pandemic and changes in tumor staging and mortality, the retrospective design does not allow us to establish causality. Multiple confounding factors, including healthcare access, patient selection, and regional variation in pandemic burden, may have influenced these outcomes.
To provide a more robust evaluation of trends, we performed EAPC and adjusted logistic regression models. These confirmed a significant annual reduction in mortality and a consistent, though non-significant, decrease in muscle-invasive tumors. While these trends may reflect the positive impact of care prioritization, causality cannot be established due to the retrospective design.
The main outcome assessed in our study—the proportion of muscle-invasive bladder cancer (MIBC) during the pre-COVID-19 period and the early phase of the pandemic. This finding is consistent with the literature, which typically reports approximately 20% MIBC among all bladder tumors.25,26 In the Austrian study conducted by Tulchiner et al.,27 however, a significantly higher proportion of pT1 and pT2 bladder tumors was observed in 2020 compared to 2019 (pT1: 4.6% in 2019 vs. 10.5% in 2020; pT2: 7.1% in 2019 vs. 10.5% in 2020; p = 0.008). These rates remain below the usual 20% MIBC found in the literature.25,26 This Austrian study was monocentric, but the center had a high annual TURBT volume (240 in 2019 and 209 in 2020). All TURBT procedures performed during the study period were included, regardless of the pathological nature of the lesion. No malignant tumor was found in 31.3% of TURBTs performed in 2019 and in 36.4% of those in 2020. Additionally, the study did not specify whether bladder cancers detected were urothelial carcinoma or of another type, nor whether second-look TURBTs were included.
In our cohort, the proportion of muscle-invasive bladder tumors (MIBT, pT ≥ 2) decreased from 18.7% in 2019 to 14.4% in 2020, and further to 13.9% in 2021 and 13.2% in 2022. While these differences did not reach statistical significance (p = 0.631, chi-square), they contrast with earlier studies suggesting a shift toward more advanced disease during pandemic restrictions. This discrepancy may reflect the prioritization of TURBT as an urgent surgical procedure, as recommended by the French Association of Urology and other international bodies. The stable or improving staging profile in our cohort supports the notion that clinical triage and protected surgical access were effective in minimizing diagnostic and therapeutic delays.
A significant decrease in all-cause mortality was observed, from 38.0% in 2019 to 22.0% in 2020 (p = 0.007, chi-square), with further declines to 20.5% in 2021 and 19.5% in 2022. Similarly, palliative care needs declined from 16.0% in 2019 to 8.2% in 2020, remaining low at 9.1% in 2021 and 8.0% in 2022 (p = 0.18). Several hypotheses may explain this finding. First, during the pandemic, oncology pathways may have been streamlined, with increased focus on high-priority cases, leading to more rapid and efficient interventions for patients with urothelial carcinoma. Second, it is possible that patients presenting with more aggressive disease or severe comorbidities in 2020 were either deferred or not offered surgical treatment, introducing a selection bias that favored better outcomes. Third, both hospitals implemented emergency protocols that prioritized essential surgeries, reduced hospital length of stay, and possibly enhanced follow-up and support for high-risk patients, which could have contributed to improved survival metrics.
This contrasts with the expected outcomes of a viral epidemic that disproportionately affects elderly, obese, chronically ill, and smoking individuals. Notably, risk factors for severe COVID-19 and high-risk urothelial carcinoma are quite similar.28
Despite the use of EAPC and multivariable adjustment, our observational study design precludes any conclusion of causality. Residual confounding and selection biases remain possible.
These results differ from early pandemic studies reporting increased cancer-related mortality and decreased access to care. However, they align with more recent analyses suggesting that timely cancer surgeries were feasible and effective when systems adapted quickly.
In addition to temporal trend analysis, our adjusted multivariable models demonstrated that the reduction in all-cause mortality remained statistically significant across all post-2019 years, even after controlling for baseline demographic and institutional factors. This supports the hypothesis that proactive care reorganization during the pandemic period contributed to sustained improvements in bladder cancer outcomes, rather than reflecting only a change in case mix or hospital volume.
The recurrence rates remained stable across both periods (49.2% in 2019, 52.8% in 2020, and 51.4% in 2021), indicating that short-term oncologic control was not compromised. This is an important marker of care continuity and reflects well on post-TURBT surveillance programs. Nevertheless, longer-term follow-up is necessary to determine whether COVID-related disruptions had any delayed oncologic consequences, especially for patients whose re-TURBTs, cystoscopies, or intravesical therapies may have been postponed.
Marked differences between the two participating centers were observed, particularly in mortality rates and recurrence frequencies. Center 2 (CHR Orleans), more severely affected by COVID-related ICU saturation, had higher all-cause mortality in 2019 but managed to reduce it substantially in 2020 and 2021. In contrast, Center 1 (CHRU Tours) maintained stable access to urological care throughout the crisis and recorded slightly higher recurrence rates. These inter-institutional discrepancies underline the importance of localized healthcare capacity, ICU availability, and intra-hospital coordination. Even within the same national framework, the pandemic’s impact on oncologic care was not homogeneous.
This study has several limitations. Its retrospective design is prone to selection and reporting biases. Tumor grade data were incomplete, limiting in-depth assessment of tumor aggressiveness. The follow-up duration remains relatively short, preventing thorough evaluation of outcomes such as cancer-specific survival or progression beyond one year. The lack of causal inference is an inherent limitation of the retrospective, non-randomized design. Our findings should be interpreted as associative trends rather than definitive effects of healthcare reorganization during the pandemic. Finally, changes in referral or triage policies over time may have introduced selection biases, particularly in 2020 and 2021, where healthier patients may have been preferentially operated.
In this multicentric study, the COVID-19 pandemic did not adversely impact tumor stage or recurrence among patients undergoing TURBT for urothelial bladder carcinoma. On the contrary, improved care prioritization during the crisis may have contributed to lower overall mortality and palliative care needs. Significant inter-hospital variability highlights the importance of resource allocation and surgical planning. A larger-scale, prospective study is warranted to assess long-term outcomes and refine strategies for cancer care delivery in future health crises.
Acknowledgement
We thank the clinical and administrative teams at CHRU Tours and CHR Orléans for their assistance in retrieving patient records and supporting the data collection process. We also extend our gratitude to the patients whose anonymized data were used for this retrospective study. Their indirect contribution to improving uro-oncologic care during the pandemic is sincerely acknowledged.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
The authors confirm contribution to the paper as follows: Study conception and design: Marie Chaumel, Franck Bruyère, Ali Bourgi; Data collection: Marie Chaumel, Nicolas Brichart; Analysis and interpretation of results: Ali Bourgi, Franck Bruyère, Marie Chaumel; Draft manuscript preparation: Marie Chaumel, Ali Bourgi. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Due to the sensitive nature of the data and institutional policies, data cannot be made publicly available.
Ethics Approval
This study involved human subjects and was approved by the Institutional Review Board of CHRU Tours (Approval reference number: 2025_077). The research was conducted in accordance with national regulations and the Declaration of Helsinki. Given the retrospective nature of the study, the requirement for informed consent was waived by the ethics committee.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
References
1. Méjean A, Rouprêt M, Rozet F et al. Recommendations CCAFU on the management of cancers of the urogenital system during an epidemic with Coronavirus COVID-19. Prog Urol 2020 Apr;30(5):221–231. [Google Scholar]
2. de la Peña E, Hernández V, Guijarro A et al. Recommendations on bladder cancer management during COVID-19 pandemia: lessons learned and future plans. Arch Esp Urol 2020 Jun;73(5):374–383. [Google Scholar]
3. Ribal MJ, Cornford P, Briganti A et al. European association of urology guidelines office rapid reaction group: an organisation-wide collaborative effort to adapt the European association of urology guidelines recommendations to the coronavirus disease 2019 era. Eur Urol 2020 Jul;78(1):21–28. [Google Scholar]
4. Maganty A, Yu M, Anyaeche VI et al. Referral pattern for urologic malignancies before and during the COVID-19 pandemic. Urol Oncol 2021 May;39(5):268–276. [Google Scholar]
5. Ferrara G, De Vincentiis L, Ambrosini-Spaltro A et al. Cancer diagnostic delay in northern and central Italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic. Am J Clin Pathol 2021 Jan 4;155(1):64–68. [Google Scholar]
6. De Vincentiis L, Carr RA, Mariani MP, Ferrara G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018–2019: an audit study from cellular pathology. J Clin Pathol. Mar. 2021;74(3):187–189. [Google Scholar]
7. Dogan C, Yazici CM, Akgül HM, Cinar O, Ateş H, Yaz İ. The delay in the diagnosis and treatment of newly diagnosed bladder cancer patients during COVID-19 pandemic. Afr Health Sci 2022 Sep;22(3):241–249. [Google Scholar]
8. Alexander CE, Nathan A, Light A et al. Understanding the long-term impact of the COVID-19 pandemic on non-muscle-invasive bladder cancer outcomes: 12-month follow-up data from the international, prospective COVIDSurg Cancer study. BJUI Compass 2024;5(11):1158–65. [Google Scholar]
9. Spencer-Bowdage S, Russell B, Rigby J et al. The experience of UK patients with bladder cancer during the COVID-19 pandemic: a survey-based snapshot. BJU Int 2021 Feb;127(2):179–181. [Google Scholar]
10. Rosenzweig B, Bex A, Dotan ZA et al. Trends in urologic oncology clinical practice and medical education under COVID-19 pandemic: an international survey of senior clinical and academic urologists. Urol Oncol Déc 2020;38(12):929.e1–929.e10. [Google Scholar]
11. Schafer EJ, Islami F, Han X et al. Changes in cancer incidence rates by stage during the COVID-19 pandemic in the US. Int J Cancer 2024 Mar 1;154(5):786–792. [Google Scholar]
12. Burus T, Lei F, Huang B et al. COVID-19 and rates of cancer diagnosis in the US. JAMA Netw Open 2024 sep 3;7(9):e2432288. [Google Scholar]
13. Semprini J, Pagedar NA, Boakye EA, Osazuwa-Peters N. Head and neck cancer incidence in the united states before and during the COVID-19 pandemic. JAMA Otolaryngol–Head Neck Surg 2024;150(3):193–200. [Google Scholar] [PubMed]
14. Han X, Yang NN, Nogueira L et al. Changes in cancer diagnoses and stage distribution during the first year of the COVID-19 pandemic in the USA: a cross-sectional nationwide assessment. Lancet Oncol 2023 Aug;24(8):855–867. [Google Scholar]
15. Peacock HM, Van Meensel M, Van Gool B et al. Cancer incidence, stage shift and survival during the 2020 COVID-19 pandemic: a population-based study in Belgium. Int J Cancer 2024 Oct;155(7):1212–1224. [Google Scholar]
16. Robert G, Bernhard JC, Capon G et al. Consequences of SARS-CoV-2 pandemic on urological surgery in France: a nationwide analysis of the healthcare system database. BMJ Open 2022 Nov 14;12(11):e066220. [Google Scholar]
17. Van Hoogstraten LMC, Kiemeney LA, Meijer RP et al. The impact of the COVID-19 pandemic on bladder cancer care in the netherlands. Bladder Cancer Amst Neth 2022;8(2):139–154. [Google Scholar]
18. Taheri D, Jahanshahi F, Khajavi A et al. The impact of COVID-19 pandemic on genitourinary cancers stage and grade. Clin Genitourin Cancer Févr 2023 Feb;21(1):84–90. doi:10.1016/j.clgc.2022.11.016. [Google Scholar] [PubMed] [CrossRef]
19. Anderson S, Rigney K, Hayes L et al. A retrospective cohort study of bladder cancer following the COVID-19 pandemic: are patients presenting with more aggressive disease? Ann Med Surg 2022 Sep;81:104430. [Google Scholar]
20. Vinícius Suartz C, Araújo Simões PA, Doratioto Serrano Faria Braz N et al. Surviving the storm: challenges of bladder cancer care during the COVID-19 pandemic. Clin Genitourin Cancer 2024 Oct;22(5):102129. [Google Scholar]
21. Yildiz AK, Ozgur BC, Bayraktar AS, Demir DO, Doluoglu OG. How has the COVID-19 pandemic affected patients with primary bladder cancer? Cir Cir 2023;91(2):204–211. [Google Scholar] [PubMed]
22. Culpan M, Keser F, Acar HC et al. Impact of delay in cystoscopic surveillance on recurrence and progression rates in patients with non-muscle-invasive bladder cancer during the COVID-19 pandemic. Int J Clin Pract 2021 Sep;75(9):e14490. [Google Scholar]
23. Wei Y, Yang X, Zhu H et al. Impact of the COVID-19 pandemic on bladder cancer patients: a multicenter real-world study. J Int Med Res 2023 Oct;51(10):3000605231204465. [Google Scholar]
24. Li J, Chen C, Nie J, Wang L, Zhang Z, Li Y. Changes in the disease burden of breast cancer along with attributable risk factors in China from 1990 to 2019 and its projections: an analysis of the global burden of disease study 2019. Cancer Med 2023;12(2):1888–1902. doi:10.1002/cam4.5006. [Google Scholar] [PubMed] [CrossRef]
25. Aldousari S, Kassouf W. Update on the management of non-muscle invasive bladder cancer. Can Urol Assoc J J Assoc Urol Can Févr 2010;4(1):56–64. [Google Scholar]
26. Heney NM. Natural history of superficial bladder cancer. Prognostic features and long-term disease course. Urol Clin North Am Août 1992;19(3):429–433. [Google Scholar]
27. Tulchiner G, Staudacher N, Fritz J et al. The COVID-19 pandemic gap and its influence on oncologic outcomes of bladder cancer. Cancers 2021 Apr;13(8):1754. [Google Scholar]
28. Busetto GM, Porreca A, Del Giudice F et al. SARS-CoV-2 infection and high-risk non-muscle-invasive bladder cancer: are there any common features? Urol Int 2020;104(7–8):510–522. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools