 Open Access
Open Access
ARTICLE
Dynamics of serum cytokines in preeclampsia
1
Department of Clinical Disciplines, Al-Farabi Kazakh National University, 71 al-Farabi Ave., 050040 Almaty, Republic of Kazakhstan
2
Department of Science and Strategic Development, Scientific Center of Obstetrics, Gynecology and Perinatology, 125 Dostyk Ave., 050020
Almaty, Republic of Kazakhstan
3
Scientific Center of Obstetrics, Gynecology and Perinatology, 125 Dostyk Ave., 050020 Almaty, Republic of Kazakhstan
4
International School, Asfendiyarov Kazakh National Medical University, 94 Tole Bi Str., 050012 Almaty, Republic of Kazakhstan
* Corresponding Author: A. Kurmanova,
European Cytokine Network 2024, 35(2), 21-27. https://doi.org/10.1684/ecn.2024.0497
Accepted 14 June 2024;
Abstract
The aim of the present study was to evaluate the diagnostic significance of the dynamics of cytokines and growth factors during pregnancy with and without preeclampsia. The study included 168 pregnant women at risk of hypertensive disorders. The levels of biomarkers of all pregnant women were studied at 12-16 weeks, 28-30 weeks and 36-38 weeks. These included cytokines (tumour necrosis factor-α, interferon-γ, interleukin-4) and growth factors (placental growth factor, vascular endothelial growth factor). All pregnant women were divided into two groups: 124 patients with preeclampsia and 44 without preeclampsia (control group). In patients with preeclampsia, an increase in the level of tumour necrosis factor-α was observed, compared with the control group: a 6.1-fold increase at 12-16 weeks and a 5.9-fold increase at 36-38 weeks. The level of interferon-γ was also increased, by 44.3% in the first trimester of pregnancy and by 46.8% at 28-30 weeks, compared to the control group. The level of interleukin-4 did not significantly differ between the studied groups. The level of placental growth factor was reduced in pregnant women with preeclampsia at all stages of gestation, and at 28-30 weeks was reduced by 67.9% compared to the control group. The level of vascular endothelial growth factor was also reduced, by 75%, compared with the control group. An increase in the level of pro-inflammatory cytokines and decrease in growth factors may therefore be considered as potential predictors of the development of preeclampsia, and evaluation of these factors may be advocated in pregnant women with risk factors of preeclampsia.Keywords
Preeclampsia (PE) is the most common complication of pregnancy, however, its underlying mechanism not yet fully understood [1, 2]. Due to the complex pathogenetic mechanisms involved in the development of PE, routine clinical or laboratory research methods are often insufficient for early diagnosis. It is therefore necessary to search for available and diagnostically significant markers, which increase even before the onset of clinical symptoms. Abnormal placentation is one of the key links with PE pathogenesis, which provokes systemic endothelial dysfunction and leads to an imbalance of angiogenic and antiangiogenic factors [3]. Another important PE mechanism is pathological migration of the trophoblast, which leads to insufficient remodelling of the spiral arteries and hypoxia in the uteroplacental space [4]. In addition, in PE, an imbalance between pro- and anti-inflammatory cytokines is observed, as a manifestation of systemic inflammation with involvement of the vascular endothelium in the process [5, 6]. Biomarkers that reflect PE pathogenetic mechanisms therefore have diagnostic value. Thus, the use of markers of endothelial dysfunction and angiogenesis in pregnant women may make it possible to detect PE at the initial stages, before the development of arterial hypertension and proteinuria, which may prevent the development of complications. Recent studies confirm that an increase in the levels of certain biomarkers in the blood of pregnant women occurs long before PE clinical manifestation, therefore early examination of pregnant women at risk is important [7, 8].
Although early PE complicates only 0.38% of pregnancies [9], late PE is seven-fold more frequent, thus predictive markers of late PE would make a significant contribution to the management of global maternal and perinatal mortality and morbidity [10]. According to previous studies, the most informative early predictors of PE are growth factors: placental growth factor (PGF), vascular endothelial growth factor (VEGF), and soluble fms-like tyrosine kinase-1. Perdigao et al. [11] demonstrated that newly diagnosed hypertension in pregnant women and an imbalance of angiogenic factors may indicate PE development at the preclinical level. In this study, pregnant women with PE were shown to have an increase in the ratio of soluble fms-like tyrosine kinase-1 to PGF.
Proinflammatory cytokines that have diagnostic value, according to data from other studies, include: tumour necrosis factor α (TNF-α), interferon-γ (IFN-γ), and interleukin-4 (IL-4). TNF-α is a cytokine involved in vascular endothelial dysfunction, and is thus considered as a vasoactive cytokine [12, 13]. Weel et al. [14] confirmed that the increase in TNF-α associated with PE occurred both in blood serum and placental tissues. TNF-α and VEGF, together with the extracellular matrix, regulate the migration of the extravillous cytotrophoblast [4]. Most researchers have focussed on PGF and the ratio of soluble fms-like tyrosine kinase-1/PGF as early markers of PE [5, 15]. However, assessment of the level of growth factors alone is insufficient to characterise the entire cascade of processes, including the proliferation of endothelial cells and the reorganisation of blood vessels at the basement membrane. A comprehensive assessment of the level of several serum cytokines and growth factors over time may be of greater value for PE diagnosis and prevention of further complications.
The purpose of the study was to examine the dynamic levels of serum cytokines and growth factors in pregnant women with PE, with a view to improving the diagnosis of PE.
MATERIALS AND METHODS
This study was carried out at the Scientific Center of Obstetrics, Gynecology and Perinatology in Almaty (the Republic of Kazakhstan). The study included 168 pregnant women (aged 27.2±4.4 years, body mass index [BMI]: 23.2±2.6, arterial pressure [AP] at the time of inclusion: 126±3.2/72.1±2.3 mmHg). All pregnant women included in the study had risk factors for the development of hypertensive disorders (hypertensive disorders during previous pregnancies, elevated BMI, family anamnesis of hypertension, multiple pregnancy, extragenital diseases). Pregnant women with hypertension, thyroid disease, diabetes mellitus, and a history of kidney disease were excluded from the study, since the presence of these diseases could affect the levels of the studied markers. The gestational age of the groups was calculated based on menstrual cycle data and confirmed by early ultrasonography (<12 weeks of pregnancy). Pregnant women were included in the study at gestational age ≤12 weeks. The diagnosis of PE was determined based on the presence of the following features: AP at 140/90 mm Hg, measured twice, with an interval of two hours after 20 weeks of gestation; and proteinuria ≥0.3 g/L based on a single or daily urine sample from women without a history of arterial hypertension based on anamnesis. Women with PE were classified as patients with early- or late-onset PE, depending on whether disease manifestation had occurred before or after 34 weeks of gestation, respectively. All patients provided written consent to participate in the study.
Examination of pregnant women included the following: assessment of complaints, study of anamnesis of disease and lifestyle, physical examination, and study of the level of biomarkers in the blood. Blood sampling was performed in the morning on an empty stomach using a sample collection tube. For the study, blood serum was obtained by centrifuging 2 mL of venous blood from the patient. The sample was frozen at <-70 °C; and the storage period of samples did not exceed six months from the date of blood sampling. The sample was delivered to the laboratory within one hour without changing the temperature regime.
The serum level of cytokines (TNF-α, γ-interferon, IL-4) and growth factors (PGF, VEGF) was determined by enzyme immunoassay using the automatic analyser “Cobas e411” (Hitachi). The examination was conducted at 12-16 weeks, 28-30 weeks and 36-38 weeks of gestation.
Depending on the development of PE, all pregnant women were divided into two groups: the PE group consisting of 124 patients with a pregnancy complicated by PE, and control group consisting of 44 pregnant women without PE.
The results obtained were processed using biometric analysis with the aid of the Statistica program. Parametric and non-parametric statistical methods were used. After checking the normality of distribution of the quantitative indicators using the Shapiro-Wilk test, the significance of differences of the average values was assessed for quantitative traits with a normal distribution according to the Student’s test (t), and for quantitative traits with non-normal distribution according to the Mann-Whitney test (U). Significant differences between the compared groups and values were considered based on probability of error of p<0.05.
RESULTS
Analysis of the obtained data demonstrated that the two groups of pregnant women during the first visit were comparable with regards to age and gestational age at the time of inclusion in the study. Pregnancy was complicated by PE in 73.8% of the examined women, and 26.2% (n=44) of pregnant women were normotensive throughout the entire observation period. Analysis of the risk factors in the examined pregnant women revealed that pregnant women with PE had a significantly higher BMI level compared to that of normotensive pregnant women. Moreover, at the time of inclusion in the study, the level of systolic and diastolic AP in pregnant women with PE was significantly higher compared to that among pregnant women in the control group. Based on individual analysis, pregnant women in the PE group had significantly more risk factors associated with the development of hypertension (hypertensive disorders in previous pregnancies, elevated BMI, family anamnesis of hypertension), compared to pregnant women in the control group (table 1).
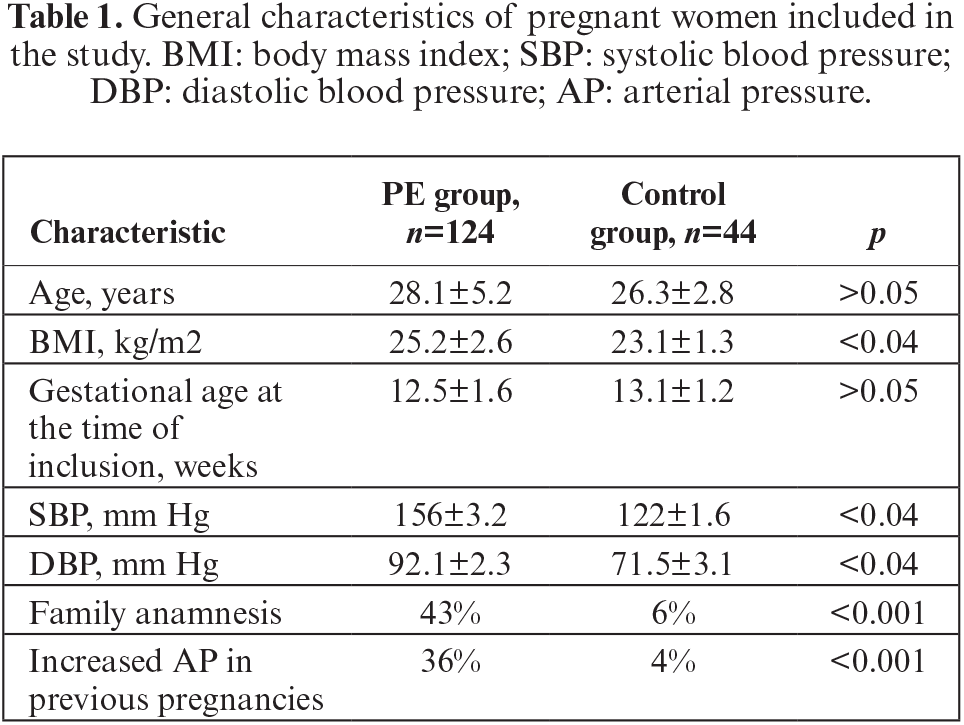
Data on serum cytokines and growth factors in pregnant women with PE (PE group) and women with uncomplicated pregnancy (control group) at different gestation periods are presented in table 2.
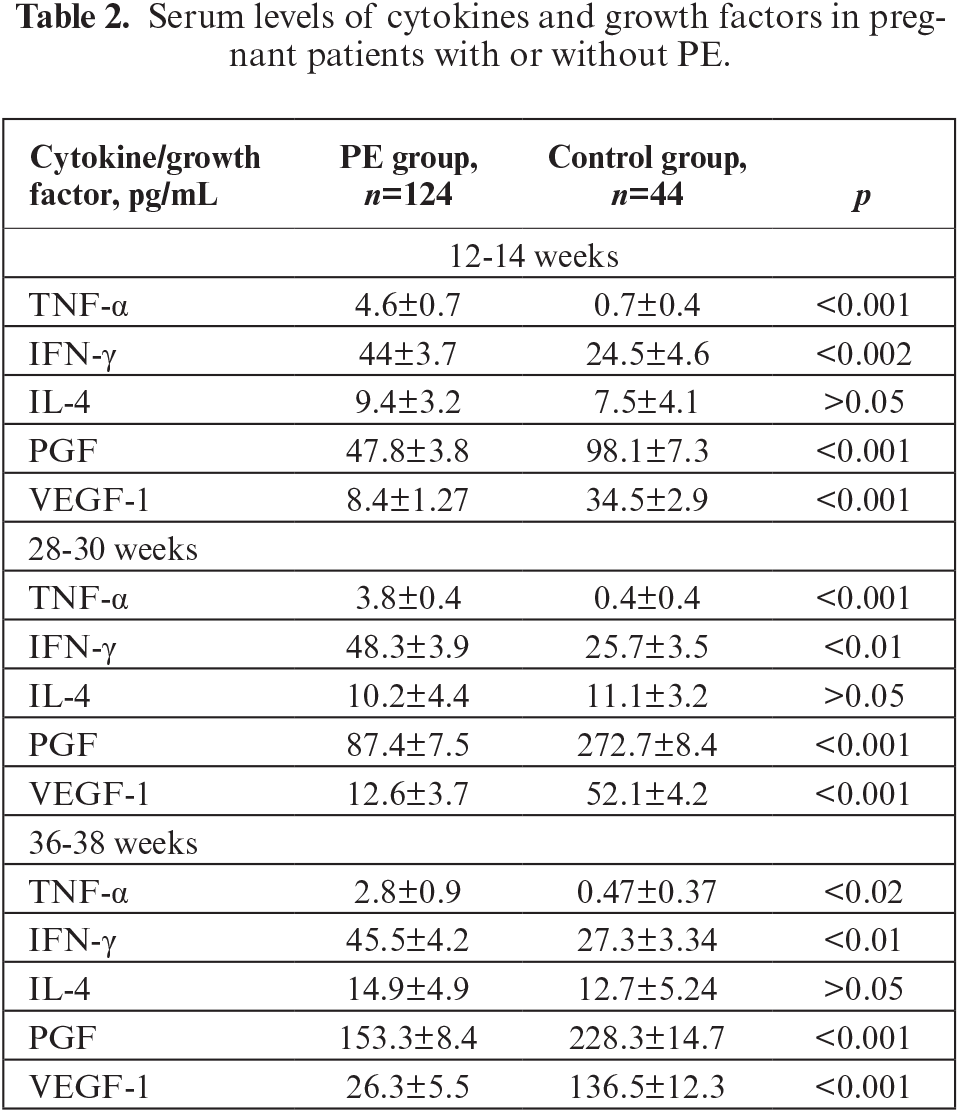
Based on analysis of the levels of studied biomarkers, a significant increase in pro-inflammatory cytokines was observed at all stages of gestation (except for IL-4) in the examined pregnant women with PE. Thus, the level of TNF-α was significantly higher in pregnant women with PE compared with that in pregnant women of the control group. The hyperproduction of TNF-α in pregnant women of the PE group was noted from the first trimester of pregnancy and persisted throughout pregnancy; the level of TNF-α was 6.1-fold higher at 12-16 weeks and 9.5-fold higher at 28-30 weeks compared to that in the control group. At gestational age of 36-38 weeks, the level of TNF-α was 5.9-fold higher than that in the control group. The increase in level of TNF-α in pregnant women with PE (compared with normotensive pregnant women) may thterfore indicate the possible use of this cytokine as a predictor of PE development.
An important aspect of determining the prognostic value of PE markers is how their levels change over time. Analysis of the dynamics of TNF-α level revealed that this pro-inflammatory cytokine gradually decreased from 12-14 weeks of gestation to 36-38 weeks in both groups, but remained elevated compared to the level in pregnant women of the control group. Over the entire period of observation, the level of TNF-α decreased by almost a half, both in the PE group and control group. In the PE group, a statistically significant decrease in TNF-α between visits 1 (12-14 weeks) and 3 (28-30 weeks) (p<0.05) was observed, while in the control group, the decrease in cytokine level was not significant during the observation period (p>0.05). This lack of change in TNF-α level over time is probably related to the fact that the cytokine level in healthy pregnant women did not increase and remained stable throughout pregnancy. Thus, determining the level of TNF-α in pregnant women may be informative as a predictor of PE, with regards to the dynamics of this pro-inflammatory cytokine at different gestational ages (figure 1).
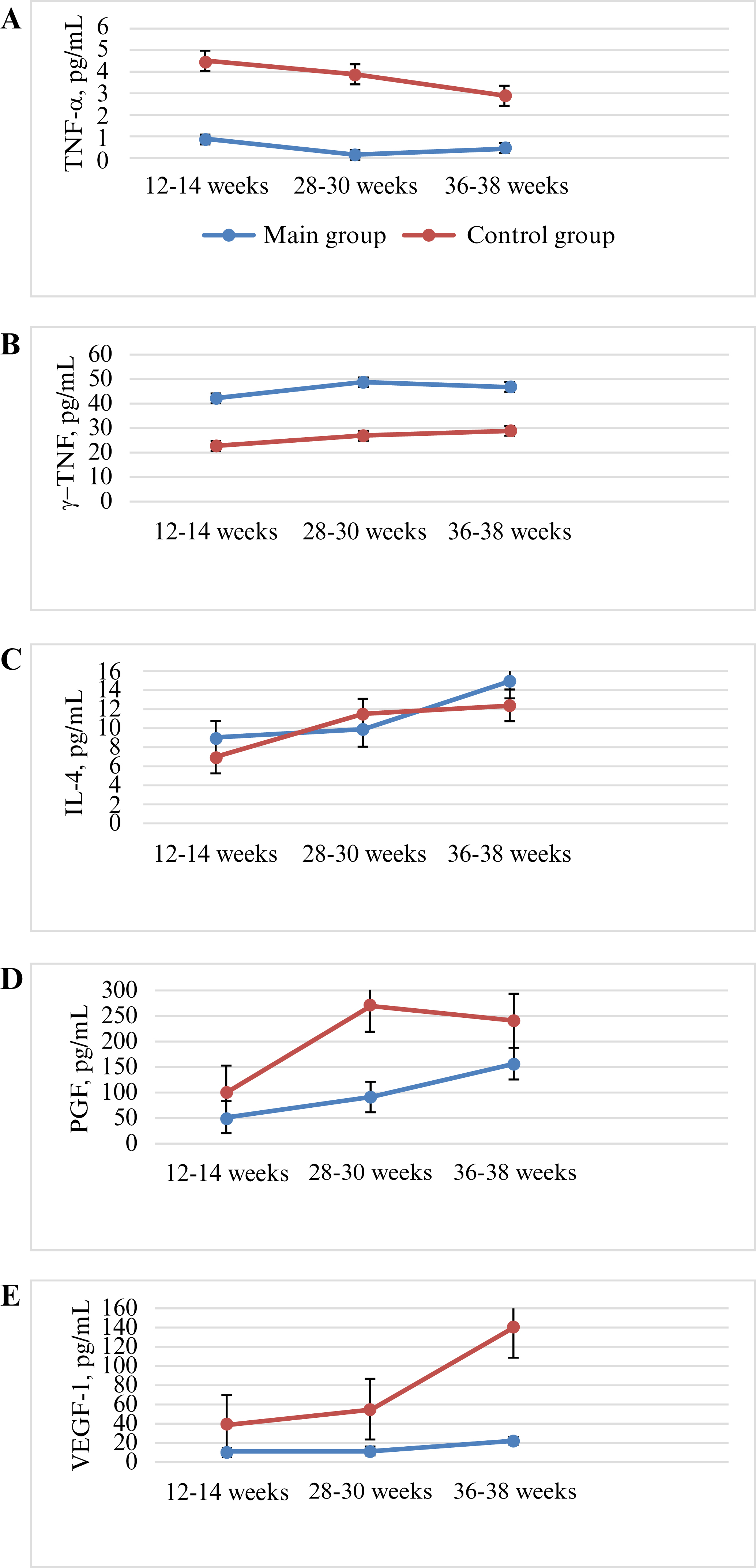
Figure 1.
Dynamics of cytokine and growth factor levels in pregnant women with and without PE. A) TNF-α; B) IFN-γ; C) IL-4; D) PGF; E) VEGF-1.
As well as TNF-α, another pro-inflammatory cytokine, IFN-γ, was also shown to be increased in pregnant patients with PE. During all visits, IFN-γ level was twice as high in the PE group relative to the control group. In the PE group, the level of IFN-γ was higher by 44.3% in the first trimester, 46.8% at 28-30 weeks of pregnancy, and 39.9% closer to birth, compared to the control group. In contrast to TNF-α, the level of IFN-γ did not differ between visits in both groups (p>0.05). Thus, according to the obtained data, an increased level of IFN-γ may indicate development of PE, however, the dynamics of this cytokine in pregnant women were not diagnostically significant (figure 1).
There was no statistically significant difference in IL-4 level between the PE group and control group over all periods of gestation (p>0.05). Blood IL-4 was 9.4±3.2 pg/mL (vs 7.5±4.1 pg/mL in the control group) at 12-14 weeks of pregnancy, 10.2±4.4 pg/mL (vs 11.1±3.2 pg/mL in the control group) at 28-30 weeks, and 14.9±4.9 pg/mL (vs 12.7±5.24 pg/mL in the control group) at 36-38 weeks. The level of IL-4 over time increased from the first to third trimester of pregnancy, however, this difference was not significant (figure 1). Based on the current study, therefore, the dynamics of IL-4 level do not appear to be of diagnostic value for pregnant women with PE relative to normotensive pregnant women.
Regarding serum PGF level, in the PE group, PGF was decreased by 51.3% from the end of the first trimester of pregnancy (p<0.001), 67.9% at 28-30 weeks during pregnancy, and 32.8% closer to birth compared to the control group (table 2). Thus, the level of PGF remained sharply reduced throughout the entire period of gestation in the PE group, in contrast to the control group (p<0.001). It is important to note that there was an increase in PGF level during the second trimester, in contrast to that in the control group, as well as a peak at 28-30 weeks of gestation (figure 1). In the PE group, the level of PGF did not reach that of the control group (although it had a tendency to increase at 28-30 weeks and 36-38 weeks of gestation). The level of PGF in the control group for the period between the first and second trimester increased three-fold, but was increased two-fold in the PE group, and this difference may serve as a marker of the development of PE.
The level of serumVEGF-1 decreased during pregnancy in the PE group, from 12-16 weeks of gestation (table 2). In the PE group, VEGF-1 was reduced by 75.5% in the first trimester of pregnancy, by 75.8% at 28-30 weeks of pregnancy, and 5.2-fold closer to birth, compared to the control group. Thus, the level of VEGF-1 remained low throughout the entire period of gestation in the PE group, in contrast to the control group (p<0.001). Despite the fact that VEGF-1 was significantly lower in the PE group than in the control group, the increase in VEGF-1 over time was significant in the PE group, however, not as significant as in the control group; during the period from 28-30 weeks to 36-38 weeks, the level of VEGF-1 doubled in the PE group and tripled in the control group (figure 1).
Analysis of the obtained data showed that VEGF-1 and PGF levels in pregnant women with PE were significantly reduced, with a weak tendency to increase over all gestation periods, while there was a persistent increase in the growth factors in the control group. In contrast, the levels of pro-inflammatory cytokines were significantly increased throughout pregnancy in women with PE, with a tendency to decrease closer to childbirth. Our data therefore show that the combined data on level of pro-inflammatory cytokines (TNF-α and IFN-γ) over time and level of growth factors may serve as a predictor of PE development, thus providing diagnostic value to determining late PE. Thus, an increase in the level of pro-inflammatory cytokines (TNF-α, IFN-γ) and low level of PGF and VEGF-1 may indicate the development of PE before clinical signs in pregnant women at any gestation period. The dynamic changes in level of TNF-α, PGF and VEGF-1 showed the greatest diagnostic potential for early diagnosis of PE based on the current study. The biomarkers reported here may thus be recommended for pregnant women in routine clinical practice, which may contribute to the early diagnosis of PE and prevention of complications.
DISCUSSION
Identifying biomarkers that indicate the pathogenetic aspects of PE may lead to individualised therapy for pregnant women with PE. The literature contains much information about growth factors and PE, but there is a lack of information on the level of growth factors and pro-inflammatory cytokines in pregnant women with PE. The novelty of this study is the simultaneous determination and comparison of levels of inflammation markers and growth factors in pregnant women with and without PE. The comprehensive assessment of serum levels of cytokines over time revealed an increased level of pro-inflammatory cytokines (TNF-α and IFN-γ) and reduced level of growth factors (PGF and VEGF-1) during all gestation periods (p<0.001) in pregnant women with PE.
Hyperproduction of Th1 cytokines (type 1 T helper) (TNF-α and IFN-γ) can limit the process of trophoblast invasion and disturb remodelling of the spiral arteries of the uterus by causing placental ischemia, clinically leading to PE. An increase in pro-inflammatory cytokines in patients with PE has been confirmed in many studies [12–14, 16]. Our data on pro-inflammatory cytokines are consistent with the results obtained by Spence et al. [17], who showed that the level of TNF-α and IFN-γ increases in pregnant women with PE compared to healthy pregnant women, whereas the level of IL-4 does not change over time. There is much information in the literature on the increase in serum level of IL-2, IL-8, and IL-6 in pregnant women with PE, but there is a lack of data on the level of IL-4 in pregnant women with PE, confirming the need to further study the level IL-4. Moreover, the data of the present study does not support the diagnostic potential of this cytokine in patients with PE. This is perhaps due to the fact that IL-4 mediates differentiation of naive T cells into Th2 cells and acts as anti-inflammatory cytokine [15]. According to the data of Stokkeland et al. [18], the concentration of IL-4 in serum of women with a healthy pregnancy does not change throughout the pregnancy, consistent with the results of this study.
According to the results of the current study, TNF-α is the most informative as a predictor of PE among the pro-inflammatory cytokines tested. It is important to note that TNF-α is of diagnostic significance both as a single measurement and over time, and may aid in the diagnosis of late PE. Mihu et al. [19] showed a significant increase (p<0.01) in the level of circulating TNF-α in the last trimester of pregnancy compared to that in non-pregnant women. Moreover, the authors demonstrated significantly elevated concentrations of TNF-α in the blood serum (p<0.001) of pregnant women with PE compared to healthy pregnant women with normal AP, consistent with our data. The dynamics of TNF-α are of diagnostic value, as TNF-α was significantly increased in women with PE and decreased over time, compared to normotensive pregnant women. This pro-inflammatory cytokine may thus be a potential marker for severity of PE.
Regarding IFN-γ, the data of Nurzadeh et al. [20] are consistent with the data of the current study and confirm the increase in IFN-γ in pregnant women with PE. The role of IFN-γ in the pathogenesis of PE was further confirmed by Sheibak et al. [13], who reported that IFN-γ immunoexpression was significantly increased in placental tissue samples in the PE group compared to samples of normotensive pregnant women. IFN-γ is suggested to play an important role in various mechanisms associated with the progression of PE. Travis et al. [21], based on an animal study, reported that a decrease in the level of IFN-γ in pregnant rats with placental ischaemia leads to a decrease in AP and oxidative stress, underlying the importance of IFN-γ in pregnant women and its possible use as a therapeutic target in the future. Another study showed that the average concentrations of IFN-γ increased significantly between the first, second and third trimesters of healthy pregnancy (108 pg/mL, 153.01 pg/mL, and 172.89 pg/mL, respectively) (in contrast to our study) [12], although in this study, the dynamics of this marker were not evaluated. The above-mentioned studies confirm the important role of IFN-γ in the pathogenesis of PE. Determining the level of this pro-inflammatory cytokine in serum is possible in routine clinical practice and may indicate the development of PE.
Many studies indicate that PE is accompanied by an antiangiogenic state, that is, a decrease in proangiogenic factors and an increase in antiangiogenic factors [6, 15]. Myers et al. [22] reported a significant increase in the levels of soluble tyrosine kinase and a significant decrease in the levels of PGF and VEGF-1 in the blood of pregnant women, 4-5 weeks before clinical manifestations. In the current study, the level of tyrosine kinase was not determined, however, the data on the level of PGF and VEGF-1 are consistent with previous studies and show a decrease in the level of these growth factors in pregnant women with PE. The level of PGF in women with PE was significantly reduced compared to healthy pregnant women. A low level of PGF may therefore be a predictor of PE, a few weeks before the development of clinical signs [8]. The study of Jardim et al. [5] also confirms the diagnostic value of PGF for PE. Reddy et al. [23] reported that PE is clinically characterized by a low serum concentration of PGF, compared to that in normotensive pregnant women. The current study confirms a decrease in the level of growth factors (PGF and VEGF-1) from the beginning of pregnancy, characterised by significantly lower levels during the entire gestation period. When these markers were studied over time, there was no increase during the second trimester but a pronounced peak at 28-30 weeks. According to the data from other studies, a decrease in PGF and VEGF may be an early prognostic marker that indicates the initial signs of a disturbance of trophoblast invasion, which occurs long before the clinical manifestation of PE.
Similar results of PGF levels were reported by Atakul [24]. According to these data, the serum levels of PGF were significantly higher in healthy pregnant women compared to women with PE (p<0.001), however, levels did not differ in women with mild and severe PE. Duhig et al. [25] illustrated that based on a group of 289 pregnant women with PE, a PGF cut-off point of <100 was identified as an important prognostic value. PGF<100 pg/mL demonstrated high sensitivity (87.5%, 95% confidence intervals [CI]: 67.6-97.3%) and was shown to have a high predictive value (97.7%, 95% CI: 93.5-99.5%). It is important to note that in this study, the PGF level was also <100 pg/mL in pregnant women with PE at 12-14 weeks and 28-30 weeks, while in the normotensive pregnant women, the PGF level had already reached 100 pg/ml in the first trimester, and this was significantly higher compared to pregnant women with PE. In the study by Duhig et al. [25], the authors confirmed the need to determine the level of PGF over time, which may be clinically important in order to stratify risk with PE symptoms. These data are consistent with the results of the current study on the informative value of determining both the level of TNF-α in pregnant women (as a likely predictor of PE) and the study of the dynamics of this pro-inflammatory cytokine at different gestational ages [26–29].
Thus, the pro-inflammatory cytokines and growth factors studied here may be considered as diagnostic markers for the development of PE. However, as the aetiology of PE is yet to be fully elucidated, the search for predictors of PE should continue with emphasis on sensitivity and specificity [30–32]. The levels of the studied cytokines showed significant differences during the development of PE both in the early stages and late stages of pregnancy [33–35]. The levels of these cytokines in combination may thus be considered as a potential predictor of the development of late PE.
CONCLUSIONS
In pregnant women with PE, the levels of pro-inflammatory cytokines (TNF-α, IFN-γ) are significantly increased compared to healthy pregnant women at all gestational ages (p<0.001). However, the level of IL-4 in pregnant women with PE is of low diagnostic significance, as the level of this cytokine did not differ relative to healthy pregnant women, and did not change significantly over time. At all visits, the level of IFN-γ was twice as high in pregnant women with PE, compared to the level in normotensive pregnant women, however, there was no change in IFN-γ between visits in both groups (p>0.05). IFN-γ may therefore be a marker for the development of PE in both early and late pregnancy.
The level of TNF-α over time gradually decreased from 12-14 weeks to 36-38 weeks of gestation in both groups, and this was significantly higher compared to the levels in pregnant women of the control group at all gestation periods. This data point to TNF-α as the cytokine with the greatest diagnostic potential among the studied pro-inflammatory cytokines. The dynamics of TNF-α level may thus be used to diagnose late PE. The levels of PGF and VEGF in pregnant women with PE were significantly lower than those in healthy pregnant women, and over time, the levels of these growth factors remained significantly lower compared to those of healthy pregnant women. In pregnant women with PE, there was no increase in the level of PGF or VEGF during the second trimester, and there was no pronounced peak at 28-30 weeks. In clinical practice, it may be recommended to measure the levels of IFN-γ, TNF-α, PGF and VEGF-1 over time in order to support the early diagnosis of PE. A high level of TNF-α and IFN-γ and low level of growth factors may indicate PE development before the onset of clinical signs in pregnant women at any gestational age.
DISCLOSURE
Financial support: This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP19678324 and APOR12165486). Conflict of interest: none.
REFERENCES
1. Gestational hypertension and preeclampsia. ACOG Practice Bulletin, Number 222. Obstet Gynecol 2020;135(6)e237-e260. [Google Scholar]
2. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet 2021;398(10297)341-54. [Google Scholar]
3. Dröge LA, Höller A, Ehrlich L, Verlohren S, Henrich W, Perschel FH. Diagnosis of preeclampsia and fetal growth restriction with the sFlt-1/PlGF ratio: Diagnostic accuracy of the automated immunoassay Kryptor. Pregnancy Hypertens 2017;8:31-6. [Google Scholar]
4. Hutabarat M, Wibowo N, Huppertz B. The trophoblast survival capacity in preeclampsia. PLoS One 2017;12(11):e0186909. [Google Scholar]
5. Jardim LL, Rios DRA, Perucci LO, de Sousa LP, Gomes KB, Dusse LMS. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clin Chim Acta 2015;20(447)34-8. [Google Scholar]
6. Dymara-Konopka W, Laskowska M, Błażewicz A. Angiogenic imbalance as a contributor of preeclampsia. Curr Pharm Biotechnol 2018;19(10)797-815. [Google Scholar]
7. Tanner MS, Davey MA, Mol BW, Rolnik DL. The evolution of the diagnostic criteria of preeclampsia-eclampsia. Am J Obstet Gynecol 2022;226(2)835-43. [Google Scholar]
8. Stepan H, Hund M, Andraczek T. Combining biomarkers to predict pregnancy complications and redefine preeclampsia: The angiogenic-placental syndrome. Hypertension 2020;75(4)918-26. [Google Scholar]
9. MacDonald TM, Walker SP, Hannan NJ, Tong S, Kaitu’u-Lino TJ. Clinical tools and biomarkers to predict preeclampsia. EBioMedicine 2022;75:103780. [Google Scholar]
10. Kallela J, Jääskeläinen T, Kortelainen E, et al. The diagnosis of pre-eclampsia using two revised classifications in the Finnish Pre-eclampsia Consortium (FINNPEC) cohort. BMC Pregnancy Childbirth 2016;16:221. [Google Scholar]
11. Perdigao JL, Chinthala S, Mueller A, et al. Angiogenic factor estimation as a warning sign of preeclampsia-related peripartum morbidity among hospitalized patients. Hypertension 2019;73(4)868-77. [Google Scholar]
12. Siwetz M, Blaschitz A, El-Heliebi A, et al. TNF-α alters the inflammatory secretion profile of human first trimester placenta. Lab Invest 2016;96(4)428-38. [Google Scholar]
13. Sheibak N, Mahmoudzadeh-Sagheb H, Moudi B, Heidari Z. Elevated immunoexpression of interferon-gamma in placenta tissue samples from pregnancies complicated with preeclampsia compared to the placenta previa. Pregnancy Hypertens 2020;22:175-80. [Google Scholar]
14. Weel IC, Baergen RN, Romao-Veiga M, et al. Association between placental lesions, cytokines and angiogenic factors in pregnant women with preeclampsia. PLoS One 2016;11(6):e0157584. [Google Scholar]
15. Chatterjee P, Valorie LC, Kelsey RB, Brett MM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol 2014;5:253. [Google Scholar]
16. Turbeville HR, Sasser JM. Preeclampsia beyond pregnancy: Long-term consequences for mother and child. Am J Physiol Renal Psysiol 2020;318(6)F1315-F1326. [Google Scholar]
17. Spence T, Philip JA, Alison JY, Mulhern MS, Strain JJ, McSorley EM. Maternal serum cytokine concentrations in healthy pregnancy and preeclampsia. J Pregnancy 2021;2021:6649608. [Google Scholar]
18. Stokkeland LMT, Giskeodegard GF, Stridsklev S, et al. Serum cytokine patterns in first half of pregnancy. Cytokine 2019;119:188-96. [Google Scholar]
19. Mihu D, Costin N, Blaga LD, Ciuchina S, Pop RB. Implication of tumor necrosis factor-alpha in preeclampsia. Appl Med Inform 2008;23(3-4)11-8. [Google Scholar]
20. Nurzadeh M, Ghalandarpoor-Attar SM, Ghalandarpoor-Attar SN, Rabiei M. The role of interferon (IFN)-γ in extravillous trophoblast cell (EVT) invasion and preeclampsia progression. Reprod Sci 2023;30(5)1462-9. [Google Scholar]
21. Travis OK, Tardo GA, Giachelli C, et al. Interferon γ neutralization reduces blood pressure, uterine artery resistance index, and placental oxidative stress in placental ischemic rats. Am J Physiol Regul Integr Comp Physiol 2021;321(2)R112-R124. [Google Scholar]
22. Myers JE, Kenny LC, McCowan LM, et al. SCOPE consortium. Angiogenic factors combined with clinical risk factors to predict preterm preeclampsia in nulliparous women: A predictive test accuracy study. BJOG 2013;120(10)1215-23. [Google Scholar]
23. Reddy A, Suri S, Sargent IL, Redman CW, Muttukrishna S. Maternal circulating levels of activin A, inhibin A, sFlt 1 and endoglinat parturition in normal pregnancy and preeclampsia. PLoS One 2009;4(2):e4453. [Google Scholar]
24. Atakul T. Serum levels of angiogenic factors distinguish between women with preeclampsia and normotensive pregnant women but not severity of preeclampsia in an obstetric center in Turkey. Med Sci Monit 2019;25:6924-31. [Google Scholar]
25. Duhig KE, Webster ML, Sharp A, et al. Diagnostic accuracy of repeat placental growth factor measurements in women with suspected preeclampsial. Acta Obstet Gynecol Scand 2020;99(8)994-1002. [Google Scholar]
26. Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016;374(1)13-22. [Google Scholar]
27. Poon LC, Galindo A, Surbek D, et al. From first-trimester screening to risk stratification of evolving pre-eclampsia in the second and third trimesters of pregnancy: A comprehensive approach. Ultrasound Obstet Gynecol 2020;55(1)5-12. [Google Scholar]
28. Chaemsaithong P, Sahota DS, Poon LC. First trimester preeclampsia screening and prediction. Am J Obstet Gynecol 2022;226(2S)S1071-S1097. [Google Scholar]
29. Garovic VD, White WM, Vaughan L. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol 2020;75(18)2323-34. [Google Scholar]
30. Phipps EA, Thadhani R, Benzing T, Karumanchi SA. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol 2019;15(5)275-89. [Google Scholar]
31. Mirzakhmetova DD, Svyatova GS, Berezina GM, Murtazaliyeva AV. Clinical and diagnostic significance of genetic predisposition to preeclampsia in the Kazakh population. Akush i Ginek (Russ Fed) 2020;3:58-63. [Google Scholar]
32. Gaponenko YY, Korda MM. Potentiation of the herbicide glyphosate negative effect on the cytokine profile by zinc oxide nanoparticles. Bull of Med and Biol Res 2023;15(1)5-9. [Google Scholar]
33. Khmil Doswald АS, Malanchuk LM. Hysteroscopic and morphological evaluation of endometrium in reproductive age women with comorbid polycystic ovarian syndrome and chronic endometritis in the protocols of in vitro fertilization. Bull of Med and Biol Res 2022;4(1)103-9. [Google Scholar]
34. Ivankiv V, Malanchyn I, Tkachuk N. Microbiota of vagina and mammary glands skin in the pregnant women with preeclampsia. Intern J of Med and Med Res 2018;4(2)44-9. [Google Scholar]
35. Boichuk OH, Hulii DY. Diagnostic peculiarities of benign ovarian tumors during pregnancy. Reprod Endoc 2021;56:38-42. [Google Scholar]
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools