 Open Access
Open Access
REVIEW
The Effects of Physical Activity on Cognitive Function in People with Mild Cognitive Impairment: A Meta-Analysis
Department of Sport Science, Seoul National University of Science and Technology, Seoul, 01811, Republic of Korea
* Corresponding Author: Dojin An. Email:
(This article belongs to the Special Issue: Active Living, Active Minds: Promoting Mental Health through Physical Activity)
International Journal of Mental Health Promotion 2025, 27(3), 257-270. https://doi.org/10.32604/ijmhp.2025.061234
Received 20 November 2024; Accepted 10 February 2025; Issue published 31 March 2025
Abstract
Objectives: The current study aimed to perform a meta-analysis to comprehensively investigate effect of physical activity on cognitive function in people with Mild Cognitive Impairment. The findings of this study can offer an important basis for identifying the significance of physical activity as an important factor in designing and implementing strategies to enhance cognitive function in mild cognitive impairment. Methods: 21 articles were selected through academic databases (EBSCOhost, PubMed, ScienceDirect, Web of Science), and 20 Montreal Cognitive Assessment (MoCA) data and 15 Mini-Mental State Examination (MMSE) data were obtained. The study was conducted using the meta-analysis. To test the validity of each article included in this study, a funnel plot and Egger ’s regression analysis were carried out to check for publication bias. Results: The 95% confidence interval (CI) for the effect size was interpreted as a small effect size if the effect size was between 0.2 and 0.5, a moderate effect size if the effect size was between 0.5 and 0.8, and large if the effect size was greater than 0.8. First, the meta-analysis of MoCA data showed a large effect size of 0.96; second, the meta-analysis of MMSE data indicated a large effect size of 0.93; and third, the meta-analysis of MoCA and MMSE data together indicated a moderate effect size of 0.68. Conclusion: The current study demonstrates the significant effect of physical activity on cognitive function and provides a basis for developing programs to improve cognitive function. People diagnosed with mild cognitive impairment generally experience minimal disruption in daily living activities. However, as the severity of the condition progresses, significant challenges emerge, impacting the individual’s ability to carry out daily tasks. Research has demonstrated that physical activity can enhance cognitive function in individuals with MCI. Consequently, it is recommended that these individuals be motivated to participate in physical activity to optimize their cognitive function and enhance their overall quality of life.Keywords
Supplementary Material
Supplementary Material FileMild Cognitive Impairment (MCI) is a state distinguished by cognitive decline and other types of dementia [1]. MCI is a shifting condition from normal aging to dementia that does not substantially affect daily life [2]. Therefore, people with MCI are often unaware of it. However, 10%–15% of people over the age of 65 have cognitive disorders, and more than half of them are at serious risk of progressing to dementia within five years [3]. Cognitive impairment not only highly impacts the life satisfaction of life of older adults, but also places a severe economic burden on healthcare [4,5]. Therefore, there is a need to detect, prevent, and treat them before they lead to significant cognitive decline, such as dementia.
The MCI is generally diagnosed by the standardized Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) [6,7]. Both instruments can assess cognitive function in a relatively short time and without special tools or equipment, with 30 points. Higher points indicate higher cognitive function. However, the cutoff points for MCI are slightly different. Generally, a point of ≥26 for MoCA and ≥24 for MMSE is considered normal. However, since both tools are simple screening tests, it is important to have a detailed diagnosis and evaluation by a specialist to make a final diagnosis. The MoCA assesses visuospatial/executive, vocabulary, attention, sentence, abstract, recall, and passing, while the MMSE assesses time, place, memory registration, attention and computation, memory recall, language, and spatial organization. The MoCA was developed as a method for quick screening of MCI [8]. Therefore, it has been proven to have high specificity and sensitivity in confirming MCI [9]. In contrast, the MMSE is relatively not sensitive enough to confirm MCI, but it is more specific and appropriate for diagnosing dementia [10].
Physical activity on a regular basis is a significant strategy to improve cognitive performance [11,12]. Many studies have indicated that people with MCI can maintain or return to normal cognitive levels with proper strategies [13,14]. Some studies in the field of neuroimaging have argued that physical activity is significantly effective in reducing cognitive function in older adults with and without cognitive impairment [15,16]. Moreover, some longitudinal studies for healthy adults have also revealed that engaging in physical activity strategies has effects on improving cognitive function and enhanced brain volume in the brain [17–19]. However, other studies have reported little gap in cognitive function between physical activity strategy and health education programs in longitudinal studies [20], and very limited evidence that specific physical activity improves cognitive function in individuals with MCI [21], and the evidence for the benefits of physical activity in elderly people with MCI is unclear [22]. To integrate the results of these conflicting individual studies, we conducted a meta-analysis with a systematic literature review.
The current study aims to verify the effect of physical activity by providing comprehensive and objective evidence of the disseminative to which physical activity has an effect on cognitive function in MCI through meta-analysis. The findings of this study can offer a significant basis for identifying the importance of physical activity as an important factor in designing and implementing strategies to improve cognitive function in MCI.
The current study followed the PRISMA 2020 guideline [23]. We carried out a literature search for research publications between January 2014 and August 2024. EBSCOhost, PubMed, ScienceDirect, and Web of Science were used for the literature search. In each academic database, we searched for (“Montreal Cognitive Assessment (MCA)” OR “Mini-Mental State Examination (MMSE)”) AND (“physical activity” OR “exercise” OR “walking” OR “swimming” OR “cycling”).
The inclusion criteria for the literature were adopted and searched referring to PICOS [24]. First, participants were required to have mild cognitive impairment. Second, the treatment and program (strategy) of the experimental group was physical activity. Third, the control group did not apply physical activity as a treatment method and program. Fourth, the results were MoCA and MMSE. Fifth, the study with a randomized controlled trial (RCT) was adopted. Sixth, if a single article included more than one physical activity group, we included all of them. Seventh, we included all studies that examined both MoCA and MMSE in a single study. The characteristics of the studies selected according to PICOS are shown in Table 1.
In the initial stage of the data selection, 2078 articles from EBSCOhost, 1353 from PubMed, 317 from ScienceDirect, and 1245 from Web of Science were retrieved. Of these, we excluded 3371 duplicates, and 1581 were eliminated by reviewing the titles and abstracts; finally, the remaining 21 articles were chosen and used for the current study. The PRISMA flow chart of the literature selection process is shown in Fig. 1 [43].
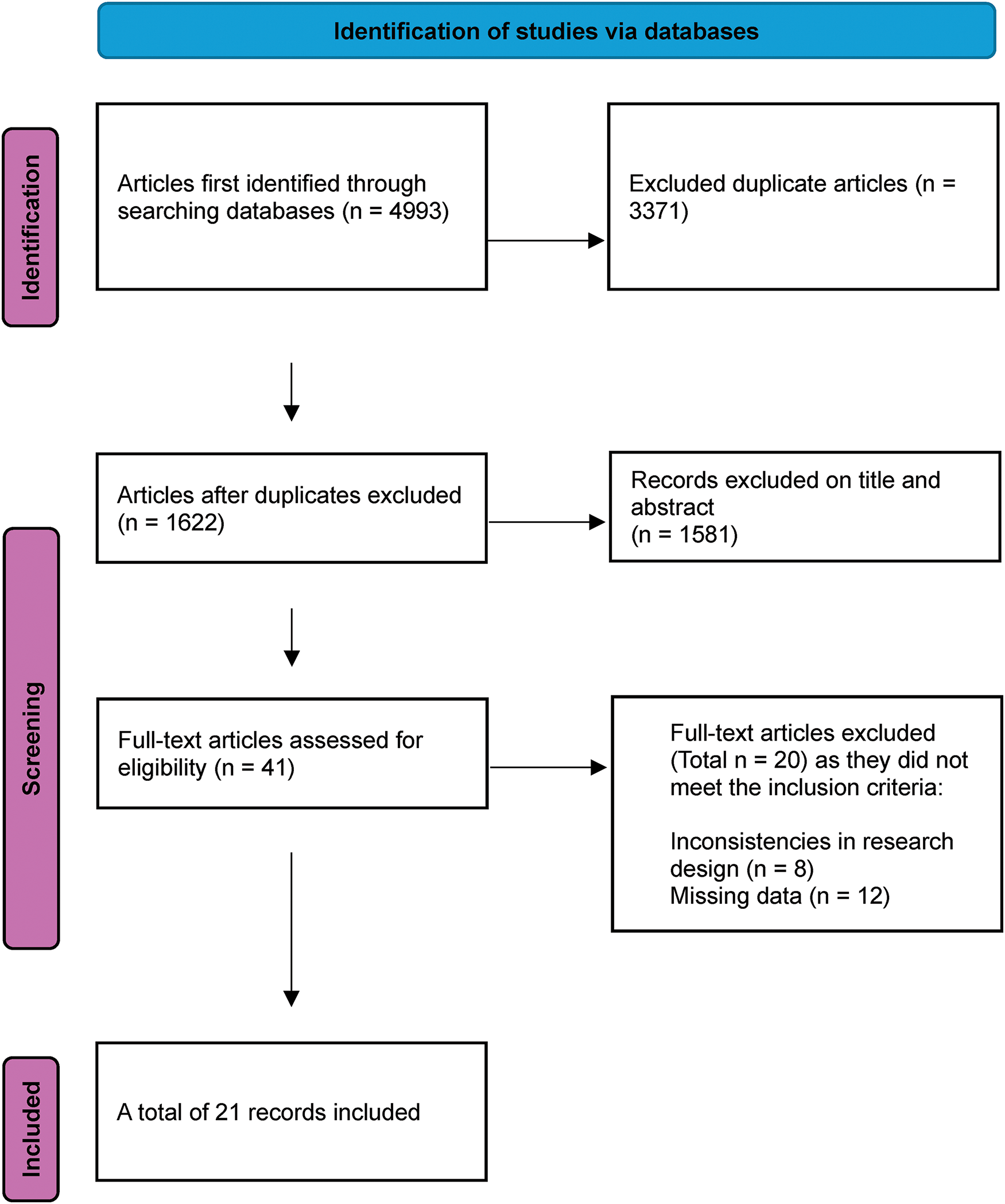
Figure 1: Flowchart outlining article selection process
Authors (year of publication), age of participants, exercise program (intervention), duration of treatment, and measurement were coded as shown in Table 1. If a study included more than one physical activity group, we coded them in separate rows. If a study assessed both MoCA and MMSE, we entered both instruments in the instrument field. The data was coded by two PhDs in sport psychology, and a professor of sports and exercise psychology was consulted in the event of disparities during the coding process.
The R program (ver. 4.3.3) was applied for the meta-analysis and the effect size was determined based on Cohen’s recommendation [44]. The I2 value was applied to test the heterogeneity of the selected articles and Schmitt et al. indicated the level of heterogeneity (I2 = 25%–50%: low heterogeneity, 50%–75%: moderate heterogeneity, 75% or more: high heterogeneity) [45]. Additionally, a random-effects model was chosen if high heterogeneity appeared among the included studies, and a fixed-effect model was chosen for low heterogeneity [45]. To test the validity of each article included in this study, a funnel plot and Egger’s regression analysis were carried out to confirm publication bias [46].
3.1 General Characteristics of the Selected Literature
21 articles were selected for analysis in this study. However, six studies included two physical activity groups in one study and eight studies examined both MoCA and MMSE. Therefore, a total of 35 studies were analyzed. The participants were aged 50 years or older with MCI. Participants participated in a physical activity program (intervention) of 6 to 36 weeks.
We performed funnel plots and Egger’s regression tests to check publication bias. The funnel plot for the MoCA data is presented in Fig. 2a, and the regression analysis reveals that there is no publication bias (=2.2829; t = 1.99, df = 18, p = 0.0616). The funnel plot for the MMSE data is indicated in Fig. 2b, and the regression reveals that there is no publication bias (=2.0506; t = 1.75, df = 13, p = 0.1044). In contrast, when MoCA data and MMSE data were regressed together (bias = 2.0513; t = 2.71, df = 33, p = 0.0106) indicated publication bias, so we adjusted the publication bias (=0.2837; t = 0.32, df = 42, p = 0.7537) by adding 9 articles using a trim-and-fill algorithm. The adjusted funnel plot is presented in Fig. 2c.
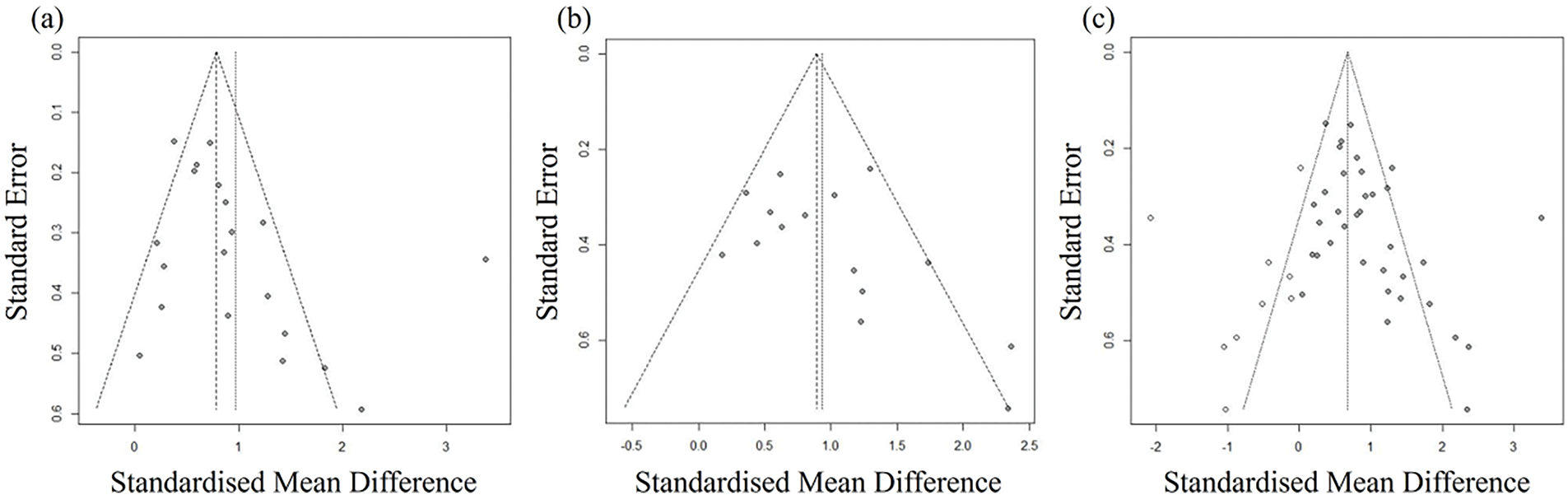
Figure 2: Funnel plots. (a) Funnel plot of MoCA; (b) Funnel plot of MMSE; (c) Adjusted funnel plot of MoCA and MMSE
The findings of the MoCA data analysis are shown in Fig. 3. The heterogeneity among the studies was considered statistically significant (I2 = 79.7%, p < 0.001) by analyzing with a random-effects model. The effect size was 0.9648 (95% CI = 0.6344, 1.2952), which revealed a large effect size and a significant difference (z = 5.72, p < 0.001). The findings of the MMSE data analysis are also shown in Fig. 3. Given the significant heterogeneity among the studies (I2 = 49.8%, p = 0.0148), a random effects model was applied. From this analysis, the effect size was 0.9317 (95% CI = 0.6700, 1.1933), which revealed a large effect size, and there was a statistically significant difference (z = 6.98, p < 0.001). In addition, the results of the MoCA and MMSE data analysis together are demonstrate in Fig. 3. As the heterogeneity between the included studies was significant (I2 = 72.2%, p < 0.001), a random effects model was applied. The effect size was 0.9605 (95% CI = 0.7383, 1.1828), which is a large effect size, and there was a statistically significant difference (z = 8.47, p < 0.001). However, regressing the MoCA and MMSE data together showed publication bias, so publication bias was adjusted using a trim-and-fill algorithm. Fig. 4 shows the findings of the meta-analysis after adjusting bias. The effect size was 0.6776 (95% CI = 0.4029, 0.9523), demonstrating a moderate effect size, and there was a statistically significant difference (z = 4.84, p < 0.001).
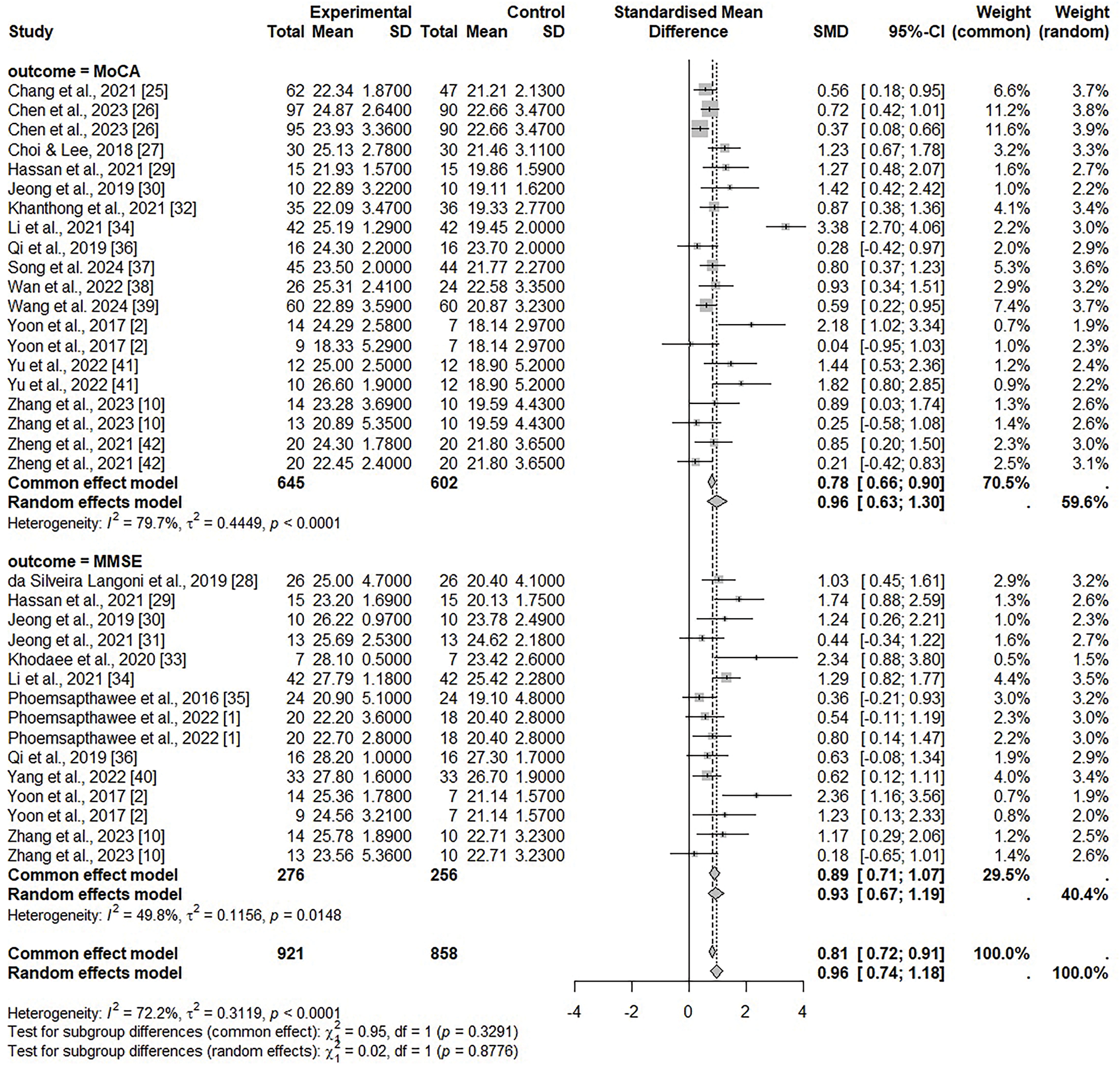
Figure 3: Forest plots of MoCA and MMSE [1,2,10,25–42]
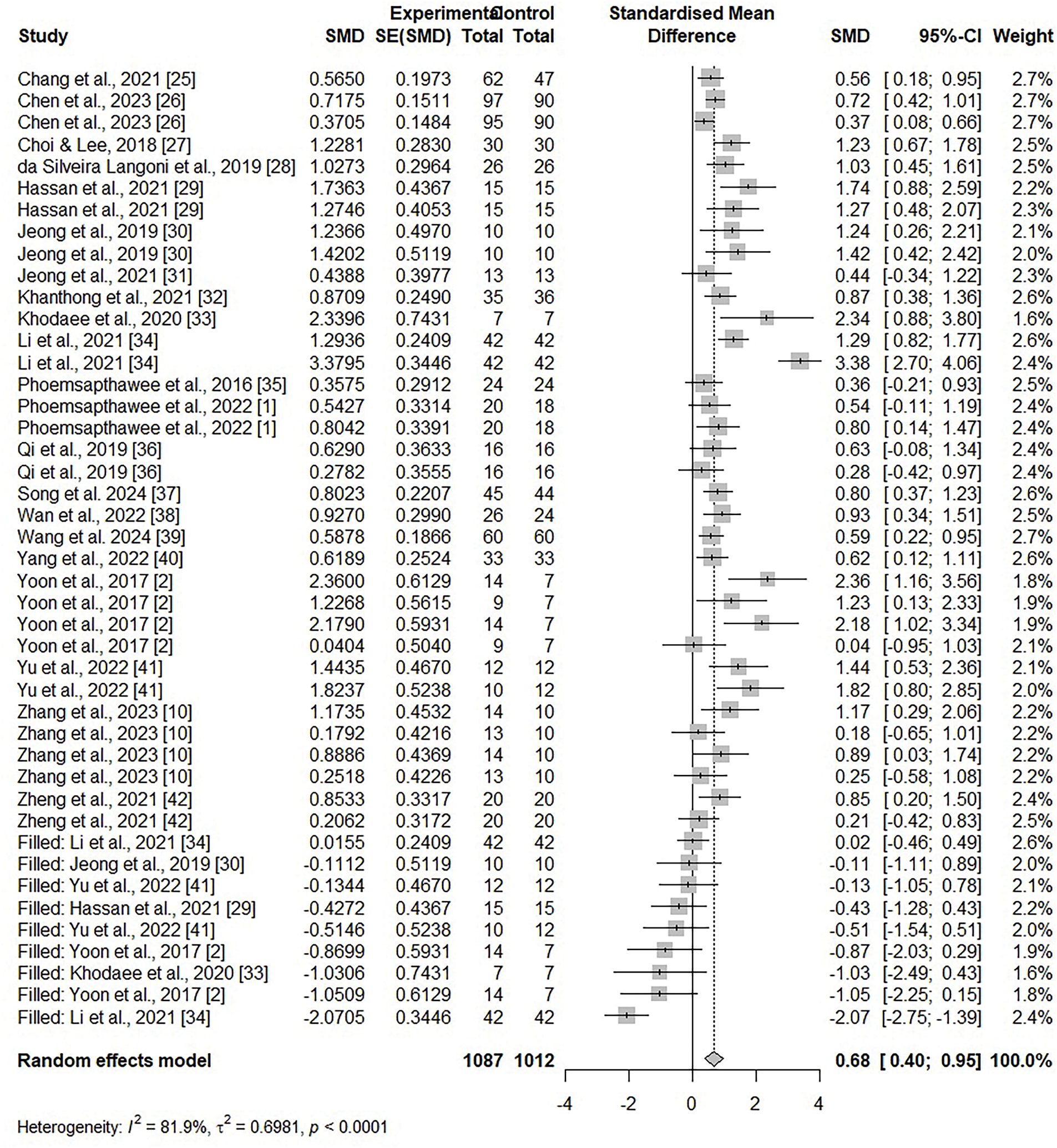
Figure 4: Adjusted forest plot of combined MoCA and MMSE [1,2,10,25–42]
A meta-analysis was performed to comprehensively analyze and confirm the effect of physical activity on MCI. As a result, the effect size of physical activity on the MoCA score of MCI was 0.96 and the MMSE score was 0.93, which both values were large. Moreover, when the MoCA and MMSE scores were analyzed together, the effect size before adjusting for publication bias was 0.96, while after adjusting the effect size was 0.68, which was a moderate effect size. The findings can be explained that physical activity has a significant effect on MCI. The publication bias is likely because the MoCA and MMSE tests have the same total score, but different assessment criteria and sensitiveness and specification of the cognitive function assessment tools.
The current findings indicated that physical activity significantly improves cognitive function demonstrating that cognitive function scores increased in all included studies. However, none of the studies showed statistical differences between groups. When analyzed by type of physical activity, the intervention effects of aerobic exercise [33], dance [25,37], and training with elastic bands did not show statistical differences in MoCA scores when trained at low speeds, although some studies showed statistical differences between walking and multicomponent or combined exercise [26,40,41], while others have not [10,31]. However, statistical differences were found for resistance exercise [29], Tai Chi [26,41], Baduanjin [38,39], and traditional Thai exercise [32]. A meta-analysis of studies examining the effects of exercise in individuals with MCI revealed a modest effect, with a 42% effect size for clinical outcomes and an 8% effect size for cognitive outcomes, which was statistically significant [21]. It can be inferred that different types of physical activity produce different effects on cognitive function.
Furthermore, when analyzed according to the duration of the physical activity program, walking did not show differences after 6 months of participation [42]. However, a longer intervention of 36 weeks showed a statistical difference even for relatively low-intensity exercises such as walking [26], and for multicomponent exercises, some studies found no statistical difference after 12 weeks of intervention [31], while others found a statistical difference [1]. When looking at resistance exercise programs, the studies that showed a statistical difference used three 80 min exercises per week, while the studies that did not show a statistical difference used two 90 min exercises per week. This suggests that the intensity of exercise may also be a crucial factor in cognitive improvement. In the case of resistance training and ground kayaking, there was a statistical difference between the two exercise programs, even after a short six-week period of participation. A meta-analysis of cognitive function studies in mildly cognitively impaired individuals also found significant improvements in short-term memory, attention, visual-spatial/executive function, and global cognitive function [47]. Taken together, these findings imply that the type of exercise, intensity, and duration of the intervention can affect cognitive function.
Previous research has reported that complex physical activity combined with strenuous mental activities can help enhance physical and cognitive function [48]. In recent, mind-body exercises such as Tai Chi [26,41], Baduanjin [38,39], and Ruesi Dadton have been studied for their positive effect on cognitive function [32]. The findings indicated that cognitive function improved with statistically significant differences, and that mind-body exercises were more effective in promoting cognitive function compared to walking [26,42]. Mind-body exercises include various activities such as skeletal muscle relaxation and stretching, as well as body coordination and regular breathing movements [49]. In addition, meditative states are also involved in mind-body exercises to regulate attention and consciousness [50]. In particular, mind-body exercises are comparatively mild intensity and slow pace compared to aerobic or resistance exercises, making them suitable for older people with MCI.
In recent, it has become plausible to non-invasively assess cortical brain activity using near-infrared light in human subjects [51–53]. Concurrently, extant studies employing optical neuroimaging techniques have demonstrated the capacity to assess various forms of cerebral activity, including physical activity [54,55], visual activation [56,57], and cognitive task performance [51,57,58]. This ability is predicated on the premise that heightened cortical brain activity is concomitant with elevated levels of oxygenated hemoglobin and diminished levels of deoxygenated hemoglobin, phenomena that manifest within seconds of the onset of augmented brain activity [59].
Based on these mechanisms, in neuroimaging, studies have reported a significant effect of regular exercise on cognitive function in older adults with and without MCI [15,16]. Some studies have indicated that sexercise interventions enhance the amount of gray and white matter in the prefrontal cortex, resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobe, and the function of the dorsolateral prefrontal, occipital parietal, and anterior cingulate cortex in the executive control network [60,61]. It has also been reported that the mind-body exercise intervention, Baduanjin, increases resting functional connectivity between the bilateral hippocampus and prefrontal cortex and may delay memory decline due to aging [62]. In summary, exercise has a positive and significant effects on brain structure [19,63] and brain function [64].
5 Limitations and Implications
The current study provides significant findings regarding effects of exercise on cognitive function. Nevertheless, this study has some limitations. The main outcome measures employed in the current study were MoCA and MMSE. A meta-analysis of the extant literature revealed significant heterogeneity among the findings. This result is hypothesized to have occurred because the environment, methods, and duration of physical activity interventions varied from study to study. Furthermore, the observed publication bias in combining the two measurement techniques is believed to result from differences in the susceptibility and specificity of the cognitive function assessment instruments, along with variations in evaluation criteria, even though the total scores on MoCA and MMSE assessments are equivalent.
MoCA is sensitive to MCI and early dementia; however, it carries a high risk of false positives among normal subjects. It evaluates various cognitive domains, including executive function, but its results are significantly influenced by education level and language proficiency. In comparison, MMSE demonstrates lower sensitivity than MoCA in detecting MCI but exhibits higher specificity in distinguishing normal from abnormal subjects. Nevertheless, its assessment of cognitive domains remains limited and is influenced by educational and linguistic factors. Therefore, selecting the appropriate instrument for a specific assessment or adopting a complementary approach is crucial to maximize each tool’s strengths while addressing their respective limitations.
The number of articles applied to the meta-analysis may not be sufficient. However, the number of studies applied for a meta-analysis is not depend on the researcher’s control, and it is technically feasible to performe a meta-analysis with more than two studies [65]. In addition, applying a general framework for MCI raises the problem that other potentially pathogenic processes and subtypes may be confounded in the MCI diagnosis. The training period was relatively short, and some training was not sufficiently intense to optimize neurophysiological or neuropsychological changes.
Future research should employ more effective measures and tests of cognitive function to assess longitudinal changes, in addition to the MoCA and MMSE. The most critical issue in this field is to expand findings to evaluate the impact of cognitive improvement on daily activities, quality of life, and psychological well-being. Furthermore, research is needed that considers various factors, such as frequency, intensity, time, and type of exercise, which may positively influence both activities of daily living and cognitive function in people with MCI. In spite of these limitations, the current study offers significant data on the effects of physical activity strategies on the cognitive function of individuals with MCI.
The current study carried out a meta-analysis to determine the effects of physical activity on cognitive function in people with MCI. The conclusions are as follows. Physical activity has significant effects on cognitive function in people with MCI. Based on these findings, it is concluded that the current study provides fundamental knowledge for developing physical activity interventions to improve cognitive function in MCI.
Acknowledgement: We acknowledge all participants involved in this research and those who helped in recruiting.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Jonghwa Lee and Dojin An designed the study. Youngho Kim and Jonghwa Lee collected data. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The raw data supporting the conclusions of this article will be made available by the author, without undue reservation.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at 10.32604/ijmhp.2025.061234.
References
1. Phoemsapthawee J, Ammawat W, Prasertsri P, Sathalalai P, Leelayuwat N. Does Gotu kola supplementation improve cognitive function, inflammation, and oxidative stress more than multicomponent exercise alone?—a randomized controlled study. J Exerc Rehabil. 2022;18(5):330–42. doi:10.12965/jer.2244388.194. [Google Scholar] [PubMed] [CrossRef]
2. Yoon DH, Kang D, Kim HJ, Kim JS, Song HS, Song W. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr Gerontol Int. 2017;17(5):765–72. doi:10.1111/ggi.12784. [Google Scholar] [PubMed] [CrossRef]
3. Anderson ND. State of the science on mild cognitive impairment (MCI). CNS Spectr. 2019;24(1):78–87. doi:10.1017/S1092852918001347. [Google Scholar] [PubMed] [CrossRef]
4. Duan J, Lv YB, Gao X, Zhou JH, Kraus VB, Zeng Y, et al. Association of cognitive impairment and elderly mortality: differences between two cohorts ascertained 6 years apart in China. BMC Geriatr. 2020;20(1):1–9. doi:10.1186/s12877-020-1424-4. [Google Scholar] [PubMed] [CrossRef]
5. Patterson C. World Alzheimer Report 2018—the state of the art of dementia research: new frontiers [Internet]. London, UK: Alzheimer’s Disease International (ADI); 2018 [cited 2025 Feb 09]. Available from: https://www.alzint.org/resource/world-alzheimer-report-2018/. [Google Scholar]
6. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi:10.1111/j.1532-5415.2005.53221.x. [Google Scholar] [PubMed] [CrossRef]
7. Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1133–42. doi:10.1212/WNL.56.9.1133. [Google Scholar] [PubMed] [CrossRef]
8. Freitas S, Simões MR, Alves L, Santana I. Montreal cognitive assessment: validation study for mild cognitive impairment and Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2013;27(1):37–43. doi:10.1097/WAD.0b013e3182420bfe. [Google Scholar] [PubMed] [CrossRef]
9. Luis CA, Keegan AP, Mullan M. Cross-validation of the Montreal Cognitive Assessment in community-dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24(2):197–201. doi:10.1002/gps.2101. [Google Scholar] [PubMed] [CrossRef]
10. Zhang Q, Zhu M, Huang L, Zhu M, Liu X, Zhou P, et al. A study on the effect of traditional Chinese exercise combined with rhythm training on the intervention of older adults with mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2023;38(4):15333175231190626. doi:10.1177/15333175231190626. [Google Scholar] [PubMed] [CrossRef]
11. Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013(9):657508. doi:10.1155/2013/657508. [Google Scholar] [PubMed] [CrossRef]
12. Angulo J, El Assar M, Álvarez-Bustos A, Rodríguez-Mañas L. Physical activity and exercise: strategies to manage frailty. Redox Biol. 2020;35(3):101513. doi:10.1016/j.redox.2020.101513. [Google Scholar] [PubMed] [CrossRef]
13. Jones JD, Kuhn TP, Szymkowicz SM. Reverters from PD-MCI to cognitively intact are at risk for future cognitive impairment: analysis of the PPMI cohort. Park Relat Disord. 2017;47(10):3–7. doi:10.1016/j.parkreldis.2017.12.006. [Google Scholar] [PubMed] [CrossRef]
14. Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN et al. Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: a population-based study. PLoS One. 2013;8(3):e59649. doi:10.1371/journal.pone.0059649. [Google Scholar] [PubMed] [CrossRef]
15. Li MY, Huang MM, Li SZ, Tao J, Zheng GH, Chen LD. The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. Int J Neurosci. 2017;127(7):634–49. doi:10.1080/00207454.2016.1212855. [Google Scholar] [PubMed] [CrossRef]
16. Song D, Yu DSF, Li PWC, Lei Y. The effectiveness of physical exercise on cognitive and psychological outcomes in individuals with mild cognitive impairment: a systematic review and meta-analysis. Int J Nurs Stud. 2018;79(16):155–64. doi:10.1016/j.ijnurstu.2018.01.002. [Google Scholar] [PubMed] [CrossRef]
17. Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33(8):1690–8. doi:10.1016/j.neurobiolaging.2011.05.010. [Google Scholar] [PubMed] [CrossRef]
18. Tamura M, Nemoto K, Kawaguchi A, Kato M, Arai T, Kakuma T, et al. Long-term mild-intensity exercise regimen preserves prefrontal cortical volume against aging. Int J Geriatr Psychiat. 2015;30(7):686–94. doi:10.1002/gps.4205. [Google Scholar] [PubMed] [CrossRef]
19. Ten-Brinke LF, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomized controlled trial. Br J Sports Med. 2015;49(4):248–54. doi:10.1136/bjsports-2013-093184. [Google Scholar] [PubMed] [CrossRef]
20. Sink KM, Espeland MA, Castro CM, Church T, Cohen R, Dodson JA, et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA. 2015;314(8):781–90. doi:10.1001/jama.2015.9617. [Google Scholar] [PubMed] [CrossRef]
21. Gates N, Singh MAF, Sachdev PS, Valenzuela M. The effects of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiat. 2013;21(11):1086–97. doi:10.1016/j.jagp.2013.02.018. [Google Scholar] [PubMed] [CrossRef]
22. Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, et al. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67(1):80–6. doi:10.1001/archneurol.2009.297. [Google Scholar] [PubMed] [CrossRef]
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA, 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71. [Google Scholar] [PubMed] [CrossRef]
24. Wood S, Mayo-Wilson E. School-based mentoring for adolescents: a systematic review and meta-analysis. Res Soc Work Pract. 2012;22(3):257–69. doi:10.1177/1049731511430836. [Google Scholar] [CrossRef]
25. Chang J, Zhu W, Zhang J, Yong L, Yang M, Wang J, et al. The effect of Chinese square dance exercise on cognitive function in older women with mild cognitive impairment: the mediating effect of mood status and quality of life. Front Psychiat. 2021;12:711079. doi:10.3389/fpsyt.2021.711079. [Google Scholar] [PubMed] [CrossRef]
26. Chen Y, Qin J, Tao L, Liu Z, Huang J, Liu W, et al. Effects of Tai Chi Chuan on cognitive function in adults 60 years or older with type 2 diabetes and mild cognitive impairment in China: a randomized clinical trial. JAMA Netw Open. 2023;6(4):e237004. doi:10.1001/jamanetworkopen.2023.7004. [Google Scholar] [PubMed] [CrossRef]
27. Choi WJ, Lee SW. Ground kayak paddling exercise improves postural balance, muscle performance, and cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. Med Sci Monit. 2018;24:3909–15. doi:10.12659/MSM.908248. [Google Scholar] [PubMed] [CrossRef]
28. Langoni CDS, Resende TL, Barcellos AB, Cecchele B, Knob MS, Silva TDN, et al. Effect of exercise on cognition, conditioning, muscle endurance, and balance in older adults with mild cognitive impairment: a randomized controlled trial. J Geriatr Phys Ther. 2019;42(2):E15–22. doi:10.1519/JPT.0000000000000191. [Google Scholar] [PubMed] [CrossRef]
29. Hanssan M, Rashid S, Khan R, Khalid M, Mansha H, Khalid H. Effects of structured resisted exercise on cognition level among patients with mild cognitive impairment. Pakistan J Med Health Sci. 2021;15(6):1876–8. doi:10.53350/pjmhs211561876. [Google Scholar] [CrossRef]
30. Jeong MK, Lee SH, Ryu JK, Kim YH, Kim EH, Hong GR et al. Effects of long-term multi-task exercise program on blood pressure, physical function and cognitive function in mild cognitive impairment elderly women with hypertension. Health Promot Prev. 2019;15:93–102. [Google Scholar]
31. Jeong MK, Park KW, Ryu JK, Kim GM, Jung HH, Park H. Multi-component intervention program on habitual physical activity parameters and cognitive function in patients with mild cognitive impairment: a randomized controlled trial. Int J Environ Res Public Health. 2021;18(12):6240. doi:10.3390/ijerph18126240. [Google Scholar] [PubMed] [CrossRef]
32. Khanthong P, Sriyakul K, Dechakhamphu A, Krajarng A, Kamalashiran C, Tungsukruthai P. Traditional Thai exercise (Ruesi Dadton) for improving motor and cognitive functions in mild cognitive impairment: a randomized controlled trial. J Exerc Rehabil. 2021;17(5):331–8. doi:10.12965/jer.2142542.271. [Google Scholar] [PubMed] [CrossRef]
33. Khodaee F, Nikbakht H, Gholami M, Babaee-Beigi MA, Ebrahim K. Effect of aerobic exercise on HbA1c and cognitive function in prediabetes patients with mild cognitive impairment. Iran J Diab Obes. 2020;11(4):218–25. doi:10.18502/ijdo.v11i4.2877. [Google Scholar] [CrossRef]
34. Li L, Liu M, Zeng H, Pan L. Multi-component exercise training improves the physical and cognitive function of the elderly with mild cognitive impairment: a six-month randomized controlled trial. Ann Palliat Med. 2021;10(8):8919–29. doi:10.21037/apm-21-1809. [Google Scholar] [PubMed] [CrossRef]
35. Phoemsapthawee J, Ammawat W, Leelayuwat N. The benefit of arm swing exercise on cognitive performance in older women with mild cognitive impairment. J Exerc Physiol. 2016;19(6):123–36. [Google Scholar]
36. Qi M, Zhu Y, Zhang L, Wu T, Wang J. The effect of aerobic dance intervention on brain spontaneous activity in older adults with mild cognitive impairment: a resting-state functional MRI study. Exp Ther Med. 2019;17:715–22. doi:10.3892/etm.2018.7006. [Google Scholar] [PubMed] [CrossRef]
37. Song D, Yu D, Liu T, Wang J. Effect of an aerobic dancing program on sleep quality for older adults with mild cognitive impairment and poor sleep: a randomized controlled trial. J Am Med Direct Assoc. 2024;25(3):494–9. doi:10.1016/j.jamda.2023.09.020. [Google Scholar] [PubMed] [CrossRef]
38. Wan M, Xia R, Lin H, Ye Y, Qiu P, Zheng G. Baduanjin exercise modulates the hippocampal subregion structure in community-dwelling older adults with cognitive frailty. Front Aging Neurosci. 2022;14:956273. doi:10.3389/fnagi.2022.956273. [Google Scholar] [PubMed] [CrossRef]
39. Wang X, Wu J, Zhang H, Zheng G. Effect of Baduanjin exercise on executive function in older adults with cognitive frailty: a randomized controlled trial. Clin Rehabil. 2024;38(4):510–9. doi:10.1177/02692155231215891. [Google Scholar] [PubMed] [CrossRef]
40. Yang JG, Thapa N, Park HJ, Bae S, Park KW, Park JH, et al. Virtual reality and exercise training enhance brain, cognitive, and physical health in older adults with mild cognitive impairment. Int J Environ Res Public Health. 2022;19(20):13300. doi:10.3390/ijerph192013300. [Google Scholar] [PubMed] [CrossRef]
41. Yu AP, Chin EC, Yu DJ, Fong DY, Cheng CP, Hu X et al. Tai Chi versus conventional exercise for improving cognitive function in older adults: a pilot randomized controlled trial. Sci Rep. 2022;12(1):8868. doi:10.1038/s41598-022-12526-5. [Google Scholar] [PubMed] [CrossRef]
42. Zheng G, Ye B, Xia R, Qiu P, Li M, Zheng Y, et al. Traditional Chinese mind-body exercise Baduanjin modulate gray matter and cognitive function in older adults with mild cognitive impairment: a brain imaging study. Brain Plast. 2021;7(2):131–42. doi:10.3233/BPL-210121. [Google Scholar] [PubMed] [CrossRef]
43. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097. [Google Scholar] [PubMed] [CrossRef]
44. Cohen J. Statistical power analysis for the behavioral sciences. New York, NY, USA: Routledge; 2013. doi:10.4324/9780203771587. [Google Scholar] [CrossRef]
45. Anderson V, Fontinha R. Research methods in human resources management. London, UK: Kogan Page GPSR; 2024. [Google Scholar]
46. Lin L. Graphical augmentations to sample-size-based funnel plot in meta-analysis. Res Synth Methods. 2019;10(3):379–88. doi:10.1002/jrsm.1340. [Google Scholar] [PubMed] [CrossRef]
47. Zou L, Loprinzi PD, Yeung AS, Zeng N, Huang T. The beneficial effects of mind-body exercises for people with mild cognitive impairment: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2019;100(8):1556–73. doi:10.1016/j.apmr.2019.03.009. [Google Scholar] [PubMed] [CrossRef]
48. Canli S, Ozyurda F. A multi-modal exercise intervention that improves cognitive function and physical performance, elderly with mobility-related disability: a randomized controlled trial. J Sports Med Phys Fitness. 2020;60(7):1027–33. doi:10.23736/S0022-4707.20.10286-X. [Google Scholar] [PubMed] [CrossRef]
49. Zhang S, Hu Q, Tang T, Liu C, Li C, Zang YY, et al. Changes in gray matter density, regional homogeneity, and functional connectivity in methamphetamine-associated psychosis: a resting-state functional magnetic resonance imaging (fMRI) study. Med Sci Monit. 2018;24:4020–30. doi:10.12659/MSM.905354. [Google Scholar] [PubMed] [CrossRef]
50. Zou L, Yeung A, Li C, Wei GX, Chen KW, Kinser PA, et al. Effects of meditative movements on major depressive disorder: a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2018;7(8):195. doi:10.3390/jcm7080195. [Google Scholar] [PubMed] [CrossRef]
51. Schroeter ML, Zysset S, Kupka T, Kruggel F, von Cramon DY. Near-infrared spectroscopy can detect brain activity during a color-word matching Stroop task in an event-related design. Hum Brain Mapp. 2002;17(1):61–71. doi:10.1002/hbm.10052. [Google Scholar] [PubMed] [CrossRef]
52. Schroeter ML, Zysset S, Kruggel F, von Cramon DY. Age dependency of the hemodynamic response as measured by functional near-infrared spectroscopy. NeuroImage. 2003;19(3):555–64. doi:10.1016/S1053-8119(03)00155-1. [Google Scholar] [PubMed] [CrossRef]
53. Boecker M, Buecheler MM, Schroeter ML, Gauggel S. Prefrontal brain activation during stop-signal response inhibition: an event-related functional near-infrared spectroscopy study. Behav Brain Res. 2007;176(2):259–66. doi:10.1016/j.bbr.2006.10.009. [Google Scholar] [PubMed] [CrossRef]
54. Mihara M, Miyai I, Hattori N, Hatakenaka M, Yagura H, Kawano T, et al. Neurofeedback using real-time near-infrared spectroscopy enhances motor imagery related cortical activation. PLoS One. 2012;7(3):e32234. doi:10.1371/journal.pone.0032234. [Google Scholar] [PubMed] [CrossRef]
55. Shoaib Z, Ahmad KM, Mannan MMN, Jeong MY. Approach to optimize 3-dimensional brain functional activation image with high resolution: a study on functional near-infrared spectroscopy. Biomed Opt Express. 2019;10(9):4684–710. doi:10.1364/BOE.10.004684. [Google Scholar] [PubMed] [CrossRef]
56. Ward LM, Aitchison RT, Tawse M, Simmers AJ, Shahani U. Reduced hemodynamic response in the ageing visual cortex measured by absolute fNIRS. PLoS One. 2015;10(4):e0125012. doi:10.1371/journal.pone.0125012. [Google Scholar] [PubMed] [CrossRef]
57. Karen T, Morren G, Haensse D, Bauschatz AS, Bucher HU, Wolf M. Hemodynamic response to visual stimulation in newborn infants using functional near-infrared spectroscopy. Hum Brain Mapp. 2008;29(4):453–60. doi:10.1002/hbm.20411. [Google Scholar] [PubMed] [CrossRef]
58. Richter MM, Zierhut KC, Dresler T, Plichta MM, Ehlis AC, Reiss K, et al. Changes in cortical blood oxygenation during arithmetical tasks measured by near-infrared spectroscopy. J Neural Transm. 2009;116(3):267–73. doi:10.1007/s00702-008-0168-7. [Google Scholar] [PubMed] [CrossRef]
59. Holper L, Muehlemann T, Scholkmann F, Eng K, Kiper D, Wolf M. Testing the potential of a virtual reality neurorehabilitation system during performance of observation, imagery and imitation of motor actions recorded by wireless functional near-infrared spectroscopy (fNIRS). J Neuroeng Rehabil. 2010;7(1):57. doi:10.1186/1743-0003-7-57. [Google Scholar] [PubMed] [CrossRef]
60. Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–70. doi:10.1093/gerona/61.11.1166. [Google Scholar] [PubMed] [CrossRef]
61. Li R, Zhu X, Yin S, Niu Y, Zheng Z, Huang X et al. Multimodal intervention in older adults improves resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Front Aging Neurosci. 2014;6:39. doi:10.3389/fnagi.2014.00039. [Google Scholar] [PubMed] [CrossRef]
62. Tao J, Liu J, Egorova N, Chen X, Sun S, Xue X, et al. Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front Aging Neurosci. 2016;8:25. doi:10.3389/fnagi.2016.00025. [Google Scholar] [PubMed] [CrossRef]
63. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases the size of the hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. doi:10.1073/pnas.1015950108. [Google Scholar] [PubMed] [CrossRef]
64. Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. doi:10.3389/fnagi.2010.00032. [Google Scholar] [PubMed] [CrossRef]
65. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35(2):215–47. doi:10.3102/1076998609346961. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF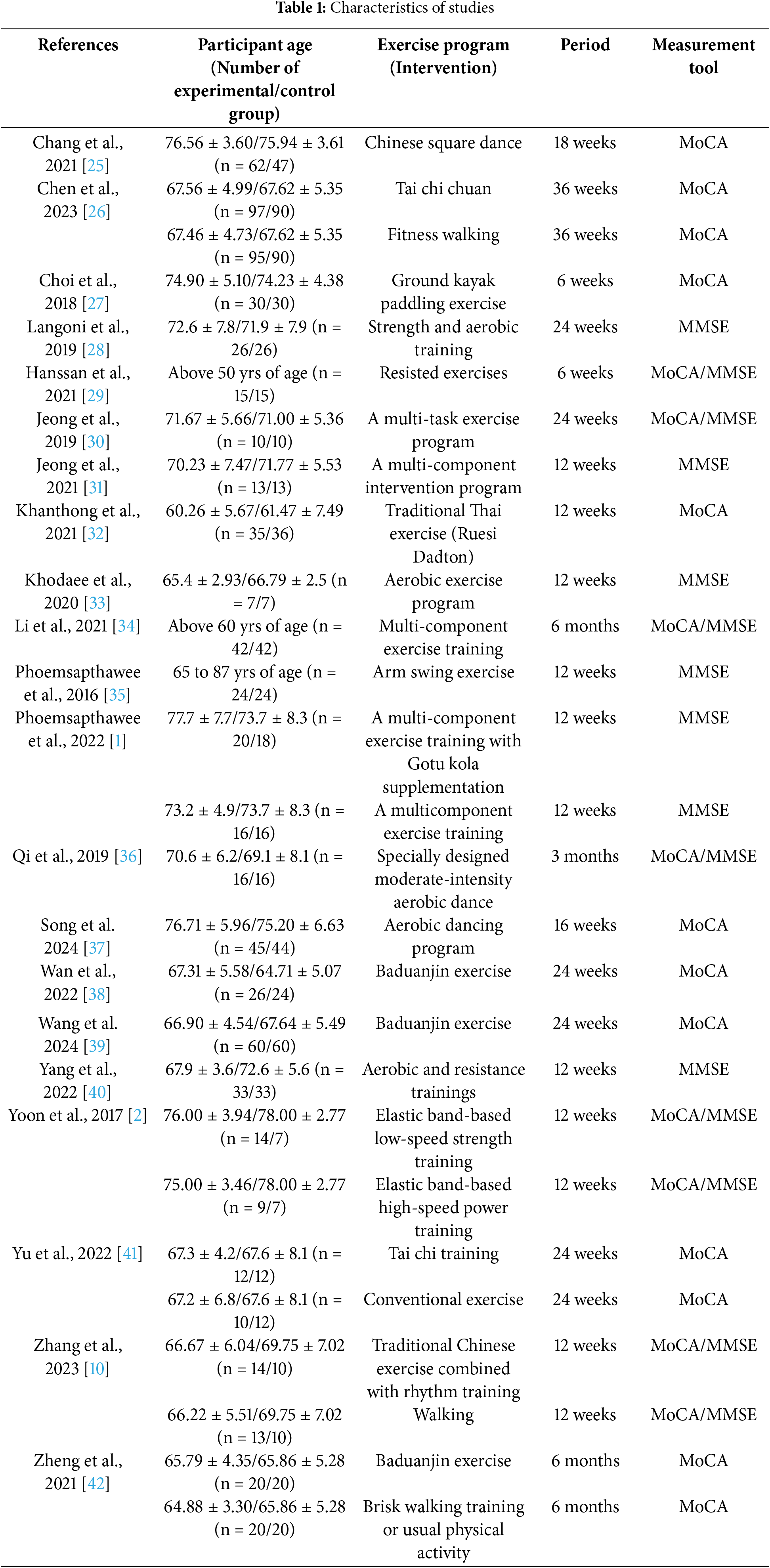
 Downloads
Downloads
 Citation Tools
Citation Tools