 Open Access
Open Access
REVIEW
Reservoir of human immunodeficiency virus in the brain: New insights into the role of T cells
1 Department of Neurosurgery, The Second Affiliated Hospital, School of Medicine, Southern University of Science and Technology, Shenzhen, 518055, China
2 School of Medicine, The Second Affiliated Hospital, Shenzhen, 518000, China
3 Department of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Shenzhen Third People’s Hospital, Shenzhen, 518000, China
* Corresponding Authors: MING CHU. Email: ; HONGZHOU LU. Email:
(This article belongs to the Special Issue: Neuroimmune Interactions at the Crossroads of Health and Disease)
BIOCELL 2023, 47(12), 2591-2595. https://doi.org/10.32604/biocell.2023.030331
Received 31 March 2023; Accepted 31 July 2023; Issue published 27 December 2023
Abstract
Human immunodeficiency virus (HIV) infection of the central nervous system (CNS) has attracted significant attention because it contributes to severe complications of acquired immunodeficiency syndrome (AIDS) and seriously impairs the life quality of infected patients. In this review, we briefly describe the latent infection of HIV in CNS and focus on the role of the important immune cells, such as T cells, in the formation and maintenance of the HIV reservoir in CNS. This review explores the mechanisms by which T cells enter CNS and establish latent infection of HIV in the CNS. In conclusion, we summarize the role of these cells in the interaction between HIV and CNS. With our better understanding of the underlying mechanisms, we propose future directions for the development of novel strategies to eliminate HIV reservoirs in the CNS based on cellular components.Graphic Abstract
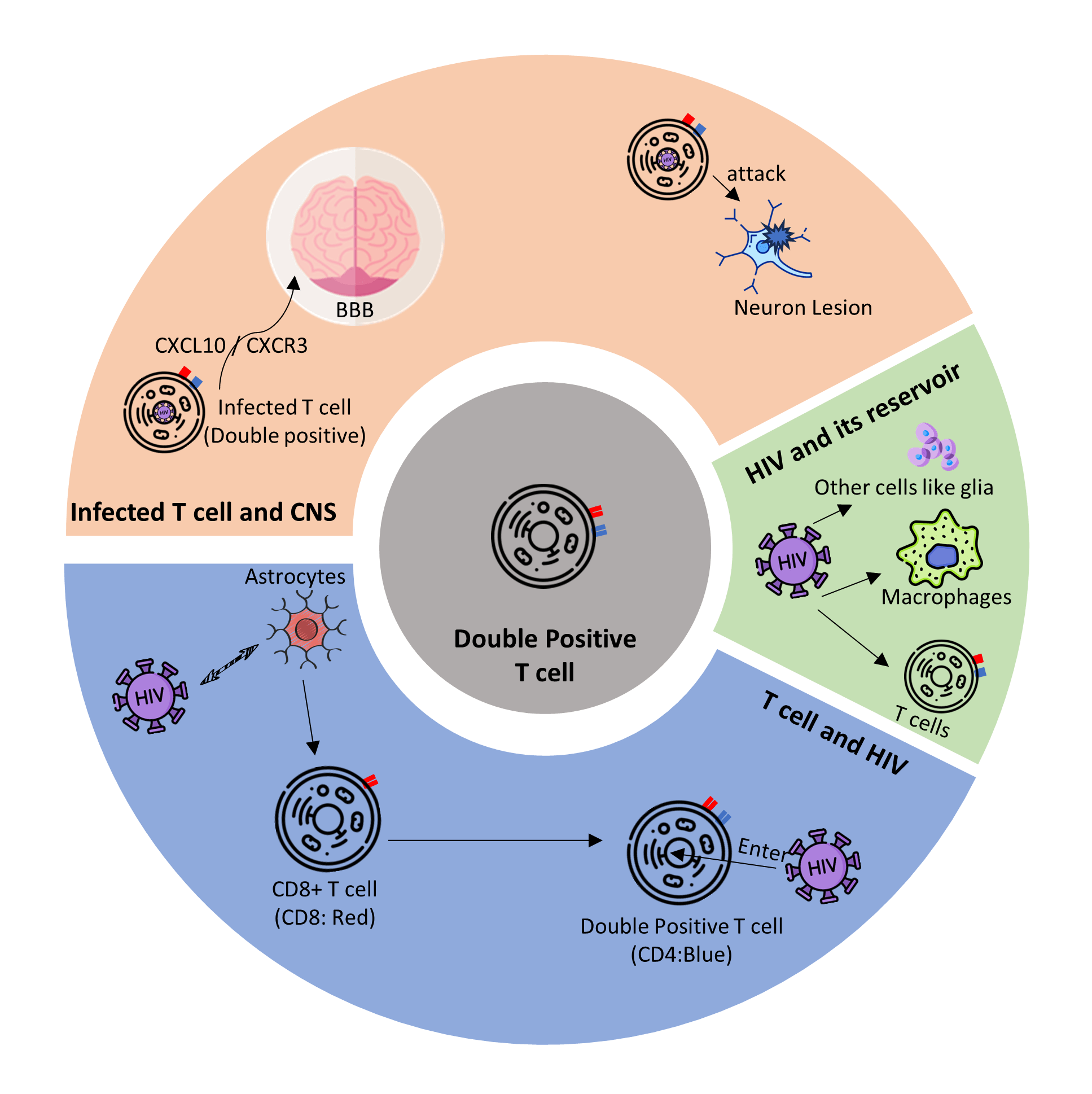
Keywords
In 2021, approximately 37.7 million people in the world had human immunodeficiency virus (HIV) infection, including 5.8 million in the Asia-Pacific region. In 2020, 1.5 million adults and children were newly infected with HIV globally, including 240,000 in the Asia-Pacific region (WHO, 2022). Due to the wide application of combined antiretroviral therapy (cART), new HIV infections and AIDS-related deaths have decreased in the past 10 years. However, it remains a challenge for the eradication of HIV infection and cure of HIV-induced acquired immunodeficiency syndrome (AIDS).
With the prolongation of the lifespan of people infected by HIV and AIDS patients, some complications related to chronic HIV infection have been paid more attention. One of the most important complications is HIV-associated neurocognitive disorders (HAND), closely related to HIV latency in the central nervous system (CNS). Similar to the latent infection reservoir in the peripheral environment, the HIV reservoir in CNS is based on the immune cells such as T cells and macrophages, and HIV in CNS interreacts closely with the components of CNS, leading to HAND and other HIV-related brain diseases. Understanding the mechanism of the interaction between HIV and CNS is important to develop novel strategies to eliminate latent infection of HIV in CNS and achieve the final goal of curing AIDS.
In this review, we briefly describe the latent infection of HIV in CNS and focus on the role of the important immune cells, such as T cells, in the formation and maintenance of HIV reservoir in CNS. In particular, we explore the mechanisms by which T cells enter the CNS and establish latent infection of HIV in CNS.
Human Immunodeficiency Virus in Latency and Its Reservoir
The viral reservoir formed by latent HIV has become the primary obstacle to curing AIDS. The latent HIV has been defined as replicant competent HIV DNA integrated into cell populations of patients under ART. The integration and latency of HIV involve multiple mechanisms, including the stability of the latent infection and the replication of the cells with HIV DNA integrated. HIV DNA in the integrated host usually has only one copy and keeps silent in the infected cells (Josefsson et al., 2011; Josefsson et al., 2013a). The infected cells make a stable and replicant-competent HIV reservoir (Josefsson et al., 2013b).
HIV reservoirs are established in peripheral blood and special anatomical sites with resident immune cells. Peripheral blood and lymph node reservoir of HIV mainly include CD4+ T cells (García et al., 2018; Barton et al., 2016). In addition to the viral reservoir in the peripheral tissues, one of the most important reservoirs is a viral reservoir in the CNS because the replicant-competent HIV spreads the virus in CNS and around the body and causes nervous disorders such as HAND and HIV encephalitis.
Latent Infection of Human Immunodeficiency Virus in the Central Nervous System
Central nervous system and human immunodeficiency
According to recent studies in Africa, among HIV hosts, up to 33% will finally develop HAND, and this ratio is up to 50% for older adults living with HIV/AIDS (PLWHA) (Belete et al., 2017; Flatt et al., 2021). HAND is a common neurological complication associated with HIV replication in the CNS. HAND is caused mainly by inflammatory responses mediated by infected non-neuron cells such as macrophages and microglial cells (Honeycutt et al., 2016; Letendre, 2011; NIH 2022). On the other hand, the T cells are even more important in this process. Upon infection by HIV, these cells could establish a reservoir of latent infection of HIV in the brain.
Normally, the brain is free from foreign pathogens. The blood-brain barrier (BBB) protects the CNS from harmful foreign substances and controls the entry of immune cells into the brain. However, in PLWH, the tight junction of BBB is disrupted by HIV protein Tat (András et al., 2003; Xu et al., 2012; Woollard et al., 2014; Killingsworth and Spudich, 2022). Consequently, the infected cells, including macrophages and T cells, as well as viral particles, enter the brain tissue to establish a latent reservoir in the CNS (Sturdevant et al., 2015). Moreover, although T cells entering CNS protect the CNS against HIV infection, T cells could activate inflammation responses that further disturb the tight junction of BBB and contribute to the deterioration of HAND (Killingsworth and Spudich, 2022; Subra and Trautmann, 2019). As a result of the early responses, HIV succeeds in keeping its latency in the CNS. Samples from six donors showed reactivation of the HIV reservoir in CNS 53 days after ART had been interrupted, and contributed to the virus spreading around the whole body (Chaillon et al., 2020).
In the following sections, we will focus on the role of T cells, which are one of the most important cell-sets in the response of the body to HIV infection and in the establishment and maintenance of the latent HIV reservoir in CNS. In addition, we will discuss the interaction between ART and other treatments and HIV reservoir in CNS.
T cells and human immunodeficiency virus reservoir in the central nervous system
CD4+ T cells are regarded as the largest reservoir in the circulation of PLWH, and these cells are intricately connected to inflammation in the brain (Subra and Trautmann, 2019). HIV-infected T cells also suffer from high transcript level of HIV RNA, and they can establish reservoirs in other sites, such as brain tissue and lymph nodes (García et al., 2018; Barton et al., 2016; Suzuki et al., 2022). Since it is almost impossible to figure out the real cell population and infection state of HIV in living people through repetitive biopsy or imaging, cerebrospinal fluid (CSF) has become the only available approach to examine HIV status in CNS. A recent study showed that CSF may reflect various resources, including both CNS and blood, so the time of sampling is quite important (Chan and Spudich, 2022).
Although macrophages were considered the primary cellular reservoir of HIV-1 in CNS (Saro et al., 2021; Farhadian et al., 2022) found that a considerable number of central memory CD4+ T cells in CNS released HIV-1 RNA based on single-cell RNA sequencing of freshly drawn CSF from PLWH. In acute HIV infection, more than 90% of cells in the CSF of PLWH are T cells, and the primary activated T cells in CSF are CD8+ T cells that cannot be found in the CSF of HIV-negative populations, showing the efforts of the body of PLWH to clear infected CD4+ T cells in CNS (Sadagopal et al., 2008a, 2008b). However, the CD8+ T cell is the predominant cell type contributing to lymphocytic pleocytosis in symptomatic HIV escape (Chan and Spudich, 2022). These findings show that CD8+ T cells also play a significant role in supporting or establishing HIV reservoirs in the CNS.
In fact, CD8+ T cell is the primary cell type in CSF that participates in the acute inflammatory response in PLWH (Killingsworth and Spudich, 2022). Autopsy of patients with HIV-CD8-associated encephalitis showed diffuse cerebral infiltration by CD8+ T cells in the white matter (Lucas et al., 2021). CD8+ T cells also express a unique TCR V-beta repertoire, suggesting a local expansion and delayed differentiation of T cells in CNS (Takata et al., 2017). However, upregulated CD8+ T cells can also activate neuropathogenic and persistent inflammatory responses, such as HIV CD8+ encephalitis reported in a Thailand cohort (Subra and Trautmann, 2019; Sailasuta et al., 2012). Therefore, CD8+ T cells could promote the damage of BBB and facilitate the entry of HIV into CNS.
Interestingly, a special type of CD8+ T cell, which has low CD4 expression on the cell surface (CD4dim CD8bright T cell), is upregulated after HIV infection. This double-positive expression is activated by astrocytes (Fig. 1A). Different from the premature cells, which also express both CD4 and CD8, this group of CD8+ T cells are highly activated mature CD8+ T cells that upregulate CD4 expression de novo on the surface and also highly express CD32 (Sullivan et al., 2001; Virdi et al., 2020). Due to the CD8 expression on the cell surface, CD4dim CD8bright T cell may help prevent HAND (Richards et al., 2016). A study suggested that this kind of T cell contributes to the adaptive immune reaction in the defense against viral infections (Marrero et al., 2021). Suni et al. demonstrated that these hybrid cells upregulated the cytolytic response in HIV infection, and the reaction probably favored the existence of these cells in the brain (Clénet et al., 2017). However, the CD4 receptors on the double-positive T cells have a critical impact on the susceptibility of these cells to HIV, making these cells a potential vehicle for the virus to enter other tissues like CNS (Choi et al., 2018). Swanstorm et al. (2014) reported a special HIV subtype that prefers cells with relatively low level of CD4 expression on their surface, like macrophages (‘M tropic’) (Joseph et al., 2014). Since the double-positive T cell has low CD4 expression on their surface, these cells should be susceptible to the M tropic HIV subtype. This subtype can also replicate in infected cells like their high-CD4 tropic kind, making their hosts serve as a ‘Trojan horse’ for HIV into deep tissues like the brain to establish a viral reservoir that can hardly be eliminated (Zhu et al., 2002). Since the CD8 T cell is the major lymph cell in CSF circulation and brain inflammation, and the double-positive T cell is still a special subtype of CD8 T cell, it is likely to become an aggressive virus reservoir that breaks down the defense barrier of the brain.
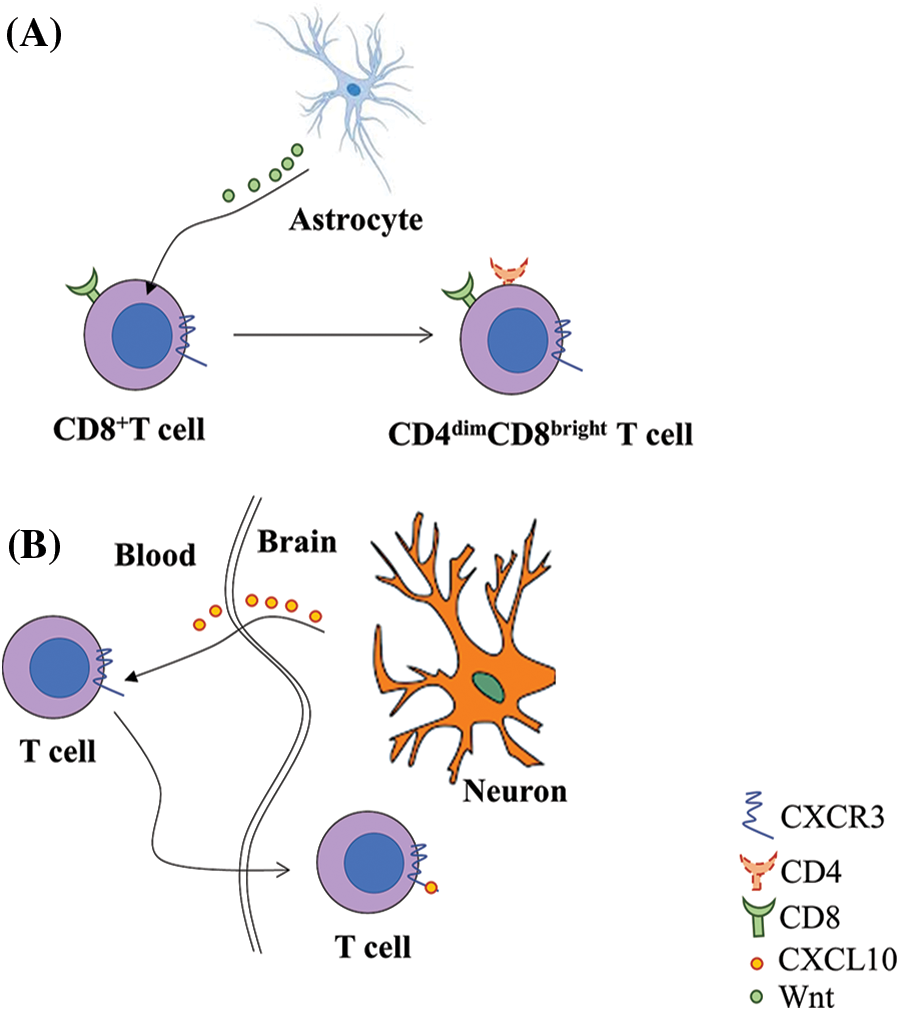
Figure 1: CD4dim CD8bright T cell serves as a reservoir for Human immunodeficiency virus (HIV) in the central nervous system. A. Single positive CD8+ T cells differentiate into CD4 cells through Wnt/β-catenin pathway with Wnt from astrocytes in the brain after HIV infection. B. The increase in chemokine receptor 3 (CXCR3) on the cell surface induces the cells to cross the blood-brain barrier by binding with C-X-C motif chemokine ligand 10 (CXCL10) from neurons.
As the life span of people infected with HIV extends, the HIV reservoir in CNS has become important because of the viral escape and related symptoms like HAND. The CNS latent reservoir remains the most primary barrier between the existing functional cure and HIV eradication. Myeloid cells and lymphocytes play important roles in establishing and maintaining this reservoir. During the establishment of HIV latent reservoir in CNS, one the most important cells is the T cell, which consists of the most favorable target of HIV and the major viral reservoir in AIDs patients.
Although T cell is a less important HIV latent reservoir than macrophages in CNS, it is essential for the interaction between HIV and CNS. T cells contribute to the invasion of HIV into the CNS, especially CD4+ T cells, which function as a ‘bridge’ for HIV between peripheral circulation and potential cells for the central reservoir; while CD8+ T cells have double effects. On one hand, CD8+ T cells clear the infected CD4+ T cells. On the other hand, CD8+ T cells secrete proinflammatory cytokines, which may lead to a fatal inflammatory response in the brain. Interestingly, a special type of CD8+ T cell (CD4dim CD8bright T cell), is both important in the immune reaction in HIV infection and susceptible to HIV. A study on animal models showed that in humanized mice, these double-positive cells would home to the brain through CXCL10/Wnt pathway with HIV loaded (Fig. 1B) (Albalawi et al., 2022).
However, because of the ethical constraints and poor accessibility of human brain tissue with active virus from autopsy, only a few studies directly based on human samples or human patients. Moreover, novel methods for the treatment of AIDS mainly focus on ART and major viral reservoirs such as macrophages and CD4 T cells, while few studies aim to develop therapies that directly target CD8 T cells and double-positive T cell, which may be the key to eradicating HIV from patients.
In conclusion, CD8 T cells and the double-positive mature T cells derived from them probably play a more important role in the progression of AIDS than inflammatory and defense response. The potential ability to deliver latent HIV into deep tissues like the brain leads to HIV escape in AIDs patients and neural complications of AIDs like HANDs. We should pay more attention to these special T cells and explore the mechanism by which these cells interact with HIV to develop novel strategies to eliminate HIV reservoir in CNS based on cellular components.
Acknowledgement: We thank Professor Lanlan Wei for constructive suggestions. We also thank the support of the Brain Science Base of the Southern University of Science and Technology.
Funding Statement: This study was funded by the Shenzhen Science and Technology Innovation Program (Nos. JCYJ20210324131607019, KCXFZ20211020163544002).
Author Contributions: ZY performed literature search and prepared the draft. CM and LH reviewed the draft. All authors wrote and approved the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest.
References
Albalawi YA, Narasipura SD, Olivares LJ, Al-Harthi L (2022). CD4dim CD8bright T Cells home to the brain and mediate HIV neuroinvasion. Journal of Virology 96: e00804–e00822. [Google Scholar] [PubMed]
András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M (2003). HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. Journal of Neuroscience Research 74: 255–265. [Google Scholar]
Barton K, Winckelmann A, Palmer S (2016). HIV-1 reservoirs during suppressive therapy. Trends in Microbiology 24: 345–355. [Google Scholar] [PubMed]
Belete T, Medfu G, Yemiyamrew E (2017). Prevalence of HIV associated neurocognitive deficit among HIV positive people in Ethiopia: A cross sectional study at Ayder referral hospital. Ethiopian Journal of Health Sciences 27: 67–76. [Google Scholar] [PubMed]
Chaillon A, Gianella S, Dellicour S, Rawlings SA, Schlub TE, de Oliveira MF, Ignacio C, Porrachia M, Vrancken B, Smith DM (2020). HIV persists throughout deep tissues with repopulation from multiple anatomical sources. Journal of Clinical Investigation 130: 1699–1712. [Google Scholar] [PubMed]
Chan P, Spudich S (2022). HIV Compartmentalization in the CNS and Its impact in treatment outcomes and cure strategies. Current HIV/AIDS Reports 19: 207–216. [Google Scholar] [PubMed]
Choi YJ, Park HJ, Park HJ, Jung KC, Lee JI (2018). CD4hiCD8low double-positive T cells are associated with graft rejection in a nonhuman primate model of islet transplantation. Journal of Immunology Research 2018: 1–11. [Google Scholar]
Clénet ML, Gagnon F, Moratalla AC, Viel EC, Arbour N (2017). Peripheral human CD4+CD8+ T lymphocytes exhibit a memory phenotype and enhanced responses to IL-2, IL-7 and IL-15. Scientific Reports 7: 11612. [Google Scholar]
Farhadian SF, Lindenbaum O, Zhao J, Corley MJ, Im Y et al. (2022). HIV viral transcription and immune perturbations in the CNS of people with HIV despite ART. JCI Insight 7: 2–16. [Google Scholar]
Flatt A, Gentry T, Kellett-Wright J, Eaton P, Joseph M, Urasa S, Howlett W, Dekker M, Kisoli A, Rogathe J (2021). Prevalence and 1-year incidence of HIV-associated neurocognitive disorder (HAND) in adults aged ≥50 years attending standard HIV clinical care in Kilimanjaro, Tanzania. International Psychogeriatrics 35: 1–12. [Google Scholar]
García M, Buzón MJ, Benito JM, Rallón N (2018). Peering into the HIV reservoir. Reviews in Medical Virology 28: e1981. [Google Scholar]
Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J et al. (2016). Macrophages sustain HIV replication in vivo independently of T cells. Journal of Clinical Investigation 126: 1353–1366. [Google Scholar] [PubMed]
Josefsson L, King MS, Makitalo B, Brännström J, Shao W et al. (2011). Majority of CD4+ T cells from peripheral blood of HIV-1-infected individuals contain only one HIV DNA molecule. Proceedings of the National Academy of Sciences of the United States of America 108: 11199–11204. [Google Scholar] [PubMed]
Josefsson L, Palmer S, Faria NR, Lemey P, Casazza J et al. (2013a). Single cell analysis of lymph node tissue from HIV-1 infected patients reveals that the majority of CD4+ T-cells contain one HIV-1 DNA molecule. PLoS Pathogens 9: e1003432. [Google Scholar] [PubMed]
Josefsson L, von Stockenstrom S, Faria NR, Sinclair E, Bacchetti P et al. (2013b). The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proceedings of the National Academy of Sciences of the United States of America 110: E4987–4996. [Google Scholar] [PubMed]
Joseph SB, Arrildt KT, Swanstrom AE, Schnell G, Lee B, Hoxie JA, Swanstrom R (2014). Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. Journal of Virology 88: 1858–1869. [Google Scholar] [PubMed]
Killingsworth L, Spudich S (2022). Neuropathogenesis of HIV-1: Insights from across the spectrum of acute through long-term treated infection. In: Seminars in Immunopathology. Germany: Springer. [Google Scholar]
Letendre S (2011). Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Topics in Antiviral Medicine 19: 137–142. [Google Scholar] [PubMed]
Lucas SB, Wong KT, Nightingale S, Miller RF (2021). HIV-associated CD8 encephalitis: A UK case series and review of histopathologically confirmed cases. Frontiers in Neurology 12: 628296. [Google Scholar] [PubMed]
Marrero YT, Suárez VM, Abraham CMM, Hernández IC, Ramos EH, Domínguez GD, Pérez YD, Zamora MCR, Guerra LFH (2021). Immunophenotypic characterization of double positive T lymphocytes in Cuban older adults. Experimental Gerontology 152: 111450. [Google Scholar] [PubMed]
NIH (2022). Neurological complications of HIV and AIDS fact sheet. https://www.ninds.nih.gov/neurological-complications-hiv-and-aids-fact-sheet#3042_4 (accessed on 13/08/2022) [Google Scholar]
Richards MH, Narasipura SD, Seaton MS, Lutgen V, Al-Harthi L (2016). Migration of CD8+ T cells into the central nervous system gives rise to highly potent anti-HIV CD4dimCD8bright T cells in a Wnt signaling-dependent manner. Journal of Immunology 196: 317–327. [Google Scholar]
Sadagopal S, Amara RR, Kannanganat S, Sharma S, Chennareddi L, Robinson HL (2008a). Expansion and exhaustion of T-cell responses during mutational escape from long-term viral control in two DNA/modified vaccinia virus Ankara-vaccinated and simian-human immunodeficiency virus SHIV-89.6P-challenged macaques. Journal of Virology 82: 4149–4153. [Google Scholar] [PubMed]
Sadagopal S, Lorey SL, Barnett L, Basham R, Lebo L et al. (2008b). Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. Journal of Virology 82: 10418–10428. [Google Scholar] [PubMed]
Sailasuta N, Ross W, Ananworanich J, Chalermchai T, DeGruttola V et al. (2012). Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One 7: e49272. [Google Scholar] [PubMed]
Saro A, Gao Z, Kambey PA, Pielnaa P, Marcellin DFH et al. (2021). HIV-proteins-associated CNS neurotoxicity, their mediators, and alternative treatments. Cellular and Molecular Neurobiology 42: 1–17. [Google Scholar]
Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S (2015). Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathogens 11: e1004720. [Google Scholar] [PubMed]
Subra C, Trautmann L (2019). Role of T lymphocytes in HIV neuropathogenesis. Current HIV/AIDS Reports 16: 236–243. [Google Scholar] [PubMed]
Sullivan YB, Landay AL, Zack JA, Kitchen SG, Al-Harthi L (2001). Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology 103: 270–280. [Google Scholar] [PubMed]
Suzuki K, Zaunders J, Gates TM, Levert A, Butterly S et al. (2022). Elevation of cell-associated HIV-1 transcripts in CSF CD4+ T cells, despite effective antiretroviral therapy, is linked to brain injury. Proceedings of the National Academy of Sciences of the United States of America 119: e2210584119. [Google Scholar] [PubMed]
Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R et al. (2017). Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Science Translational Medicine 9: eaag1809. [Google Scholar] [PubMed]
Virdi AK, Wallace J, Barbian H, Richards MH, Ritz EM, Sha B, Al-Harthi L (2020). CD32 is enriched on CD4dimCD8bright T cells. PLoS One 15: e0239157. [Google Scholar] [PubMed]
WHO (2022). UNAIDS Data 2021. Switzerland: UNAIDS. [Google Scholar]
Woollard SM, Bhargavan B, Yu F, Kanmogne GD (2014). Differential effects of Tat proteins derived from HIV-1 subtypes B and recombinant CRF02_AG on human brain microvascular endothelial cells: Implications for blood-brain barrier dysfunction. Journal of Cerebral Blood Flow and Metabolism 34: 1047–1059. [Google Scholar] [PubMed]
Xu R, Feng X, Xie X, Zhang J, Wu D, Xu L (2012). HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Research 1436: 13–19. [Google Scholar] [PubMed]
Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L (2002). Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. Journal of Virology 76: 707–716. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools