 Open Access
Open Access
REVIEW
The genetics of pediatric inflammatory bowel disease: Towards precision medicine
Human Genome Centre, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, 16150, Malaysia
* Corresponding Author: MARAHAINI MUSA. Email:
BIOCELL 2025, 49(1), 149-160. https://doi.org/10.32604/biocell.2024.057352
Received 15 August 2024; Accepted 10 October 2024; Issue published 24 January 2025
Abstract
Pediatric inflammatory bowel disease (IBD) is a chronic and heterogeneous disease. IBD is commonly classified into Crohn’s disease and ulcerative colitis. It is linked to serious symptoms and complications. The onset of IBD commonly occurs during adolescence. Despite the significant number of cases globally (~5 million), the causes of pediatric IBD, which constitutes 25% of IBD patients, are not yet fully understood. Apart from environmental factors, genetic factors contribute to a higher risk of developing IBD. The predisposition risk of IBD can be investigated using genetic testing. Genetic mechanisms of pediatric IBD are highly complex which resulted in difficulty in selecting effective treatment or patient management. Genetic variation of IBD would serve as a basis for precision medicine and allow for the discovery of more robust treatment avenues for this condition in pediatric patients. This review aims to discuss the genetics of pediatric IBD, and current development in the screening, diagnosis, and treatment based on genetic profiling of pediatric IBD subjects toward more personalized management of this disease.Keywords
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder that can be classified into two predominant forms; Crohn’s disease (CD) and ulcerative colitis (UC) [1]. IBD causes significant physical and psychological burden on patients as it is an unpredictable condition and its onset in subjects at a young age. IBD commonly starts to develop during adolescence or young adulthood [2]. While IBD is predominantly observed in young adults, this condition can occur at any age and 10%–25% of IBD patients will develop this disease in their childhood or adolescence [3,4].
Pediatric IBD refers to cases where the IBD onset starts before 17 years of age. This is further divided into very early onset IBD (VEO-IBD) involving subjects diagnosed before the age of 6, infantile IBD for those that had the disorder before the age of 2, and neonatal-onset IBD for children diagnosed with IBD before reaching 28 days of life [5]. Early-onset IBD expresses different phenotypes than adult-onset IBD [6]. However, this is controversial, as earlier reports from genome-wide association studies (GWAS) showed similar results in both children and adults [7].
Pediatric IBD patients require close monitoring and care. Treatment for IBD is still largely empirical. The complex nature of this condition and evolving method of investigation as well as the discovery of more treatment avenues call for comprehensive input to warrant the optimal outcomes for patients on a long-term basis [8].
Pediatric IBD is a heterogeneous disorder, and the pathogenesis is multifactorial, encompassing a complex interplay of genetics, environmental factors, dysfunctional immune responses, and changes in the gut microbiome. Research has shed insights into the genetic mechanisms underlying the development and progression of pediatric.
IBD involves a significant number of genetic loci. Although pediatric IBD cases only account for 10%–25% of all IBD, in-depth studies into the genetic network of this disorder have unraveled exciting developments and associated molecular pathways [9,10]. Despite this, the genetic aspect of pediatric IBD is yet to be fully described due heterogeneous nature of this condition, in which multiple causes may contribute to the disease progression including the genetic factor. In this review, we will discuss the fundamentals of the genetics of pediatric IBD and the latest discoveries as well as developments in promoting precision medicine based on the genetic profiling of pediatric IBD patients.
There were approximately 4.9 million cases of IBD globally with the highest number of cases reported in China and the United States (66.9 and 245.3 cases per 100,000 people, respectively, in 2019 [11]. From 1990 to 2019, the global age-standardized incidence rate for IBD was 4.97 per 100,000 person-years while the age-standardized mortality rate during the same year was recorded at 0.54 per 100,000 person-years. The highest incidence rates of IBD were reported in high-income regions such as North America, Australasia, and Western Europe with age-standardized incidence rates of 24.51, 20.03, and 16.94 per 100,000 person-years, respectively, while emerging industrialized areas such as East Asia, the Middle East, and South America had rapid increases in IBD incidence, largely attributed to industrialization, westernized diets, and urbanization. Meanwhile, low-income regions reported the lowest incidence rates, with areas like Oceania and Southeast Asia reporting rates below 1 per 100,000 [12]. The IBD prevalence in 2023 was estimated to be 825 per 100,000 (410 per 100,000 for CD, and 414 per 100,000 for UD and other types [13]. Alarmingly, it is estimated by 2030, more than 7 million people in Europe and the United States will be diagnosed with IBD [14] and IBD incidence is estimated to be more than 0.3% in North America, Oceania, and various European countries by 2030 [15].
Similarly to adult-onset IBD, the prevalence of pediatric IBD is also rising globally, yet complete epidemiological data on children and adolescents are still lacking. More recent statistics by Long et al. in 2024 reported 25,659 new cases and 88,829 prevalent cases of IBD among children and adolescents reported worldwide, representing an increase of 22.8% and 18.5%, respectively, compared to 1990 [16]. A 2022 systematic review reported an increasing incidence and prevalence of pediatric IBD in 84% and 100% among 37 and 7 selected studies, respectively [17]. In the United Kingdom, approximately 2500 pediatric IBD cases were diagnosed between 2013 and 2022. A significant increase in the incidence of IBD in children was reported from 6.0 per 100,000 population per year (2013) to 12.4 per 100,000 population per year (2022) [18]. In Canada, it was proposed that the higher incidence of IBD is driven by pediatric-onset IBD, which is estimated to rise by 1.23% per year from 15.6 per 100,000 in 2023 to 18.0 per 100,000 in 2035 [13]. Intriguingly, pediatric IBD incidence has increased significantly during the last decade, more prominently in the regions where the low incidence was previously reported [17].
Classification of IBD is summarised in Fig. 1. IBD unclassified refers to the subjects lying between UC and CD groups. Characteristics that define UC and CD include disease location and histological appearance. According to the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; and Colitis Foundation of America, CD is manifested throughout the gastrointestinal tract and perianal region. It is characterized by transmural inflammation, patchy disease activity, alternate segments with inflammation and normal areas (skipped lesions), strictures, and/or fistula presence. The most common presenting phenotype of CD is the involvement limited to the ileocolic region. The presence of granulomas differentiates CD from UC, although this is not commonly found [19].
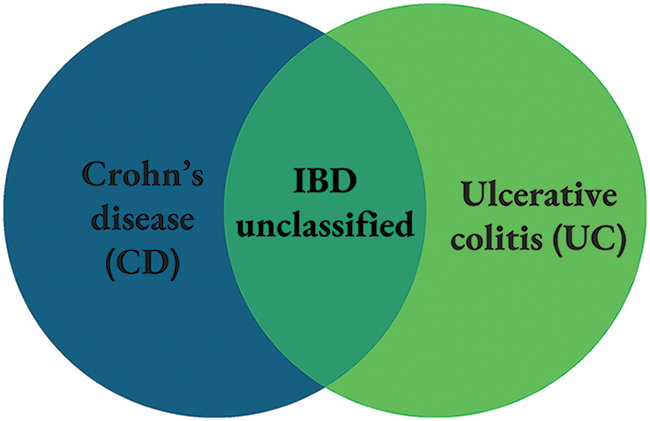
Figure 1: Classification of IBD.
UC is commonly confined to the colon. This condition is characterized by continuous rectal inflammation which extends proximally and results in proctitis, left-sided colitis, or pancolitis [20], where the latter is most common in pediatric patients [21]. Backwash ileitis and the involvement of the upper gastrointestinal tract may occur, but this is not common in pediatric UC [22].
Nevertheless, these characteristics are arbitrary where significant overlap is found in therapies, disease location, and genetic risk predisposition. Considering this, the IBD term used strategically captures all condition phenotypes. Further understanding of IBD would drive better risk stratification and treatment modality considering the severity of the disease, disease location, behavior, patient response to therapy, and other factors including disease recurrence. This subsequently will promote more personalized treatment and management of IBD patients [23].
Pediatric IBD vs. Adult-Onset IBD
Both pediatric IBD and adult-onset IBD present various similarities and differences in the mechanisms, patterns, symptoms, and treatment modalities, as shown in Table 1 [24–27].
The Genetic Basis of the Etiology and Pathogenesis of Pediatric IBD
The pathogenesis of IBD is multifactorial, involving a complex interplay of genetic predisposition, environmental factors, dysregulated immune responses, and alterations in the gut microbiome [28,29] although the exact cause and mechanisms of this disease are still not yet fully understood. Fig. 2 summarises the general mechanism of IBD.
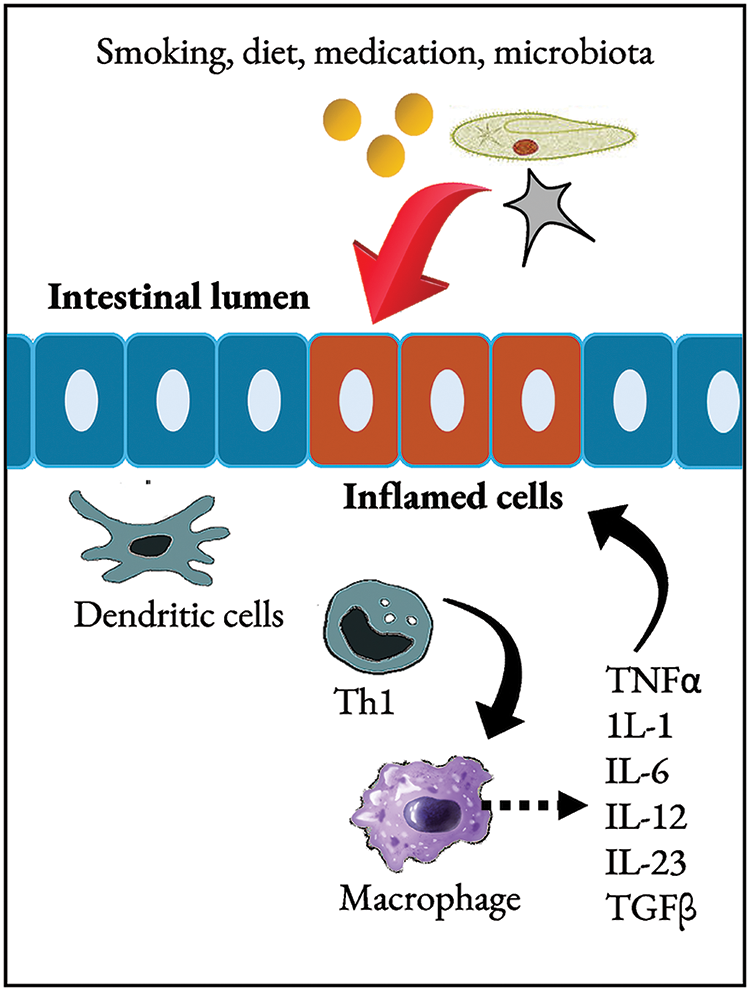
Figure 2: Mechanisms of IBD.
Upon exposure to external triggers such as smoking, diet, and medication, patients with IBD develop microbial dysbiosis. Mechanisms that maintain the intestinal barrier are also dysregulated in the mucosa of IBD patients. Activation of classic antigen-presenting cells including dendritic cells, or direct stimulation through pattern-recognition receptors drives the Th1 differentiation in CD patients (shown in the figure) or, possibly, atypical type 2 helper T cells in UC subjects. Products of Th1 promote self-activation with macrophages. Besides producing the vital cytokines that stimulate Th1 (IL-12 and macrophage migration inhibitor factor), macrophages secrete various inflammatory cytokines, including IL-1, IL-6, and TNF, which target a broad range of other cell types. IL: Interleukin; TGFβ: tumour growth factor beta; Th1: type 1 helper T cells; TNFα: tumour necrosis factor-alpha. Research has provided valuable insights into the mechanisms underlying the development and progression of chronic, immune-mediated intestinal inflammation in pediatric IBD. Extensive genetic studies suggested that IBD pathogenesis arises from dysfunction in interconnected and codependent molecular pathways that maintain homeostasis in the gastrointestinal tract [30].
A greater genetic component is implicated in pediatric onset IBD compared to adult patients due to a lower accumulation of exposure to external factors. Approximately 3%–6% of pediatric IBD cases are monogenic disorders [9,31]. Rare monogenic IBD and IBD-like syndromes possess the genetic extreme, where a single highly pathogenic variant leads to the IBD symptoms. Pediatric-onset IBD involves a wide range of spectrum from extreme monogenic variants to adolescent complex variants [32]. Fig. 3 depicts a comparison in the pathogenesis of polygenic (conventional) and monogenic IBD. Linkage studies have enabled the study of the inheritance pattern of polygenic and monogenic IBD, in which the latter is transmitted through Mendelian pattern as shown in the figure.
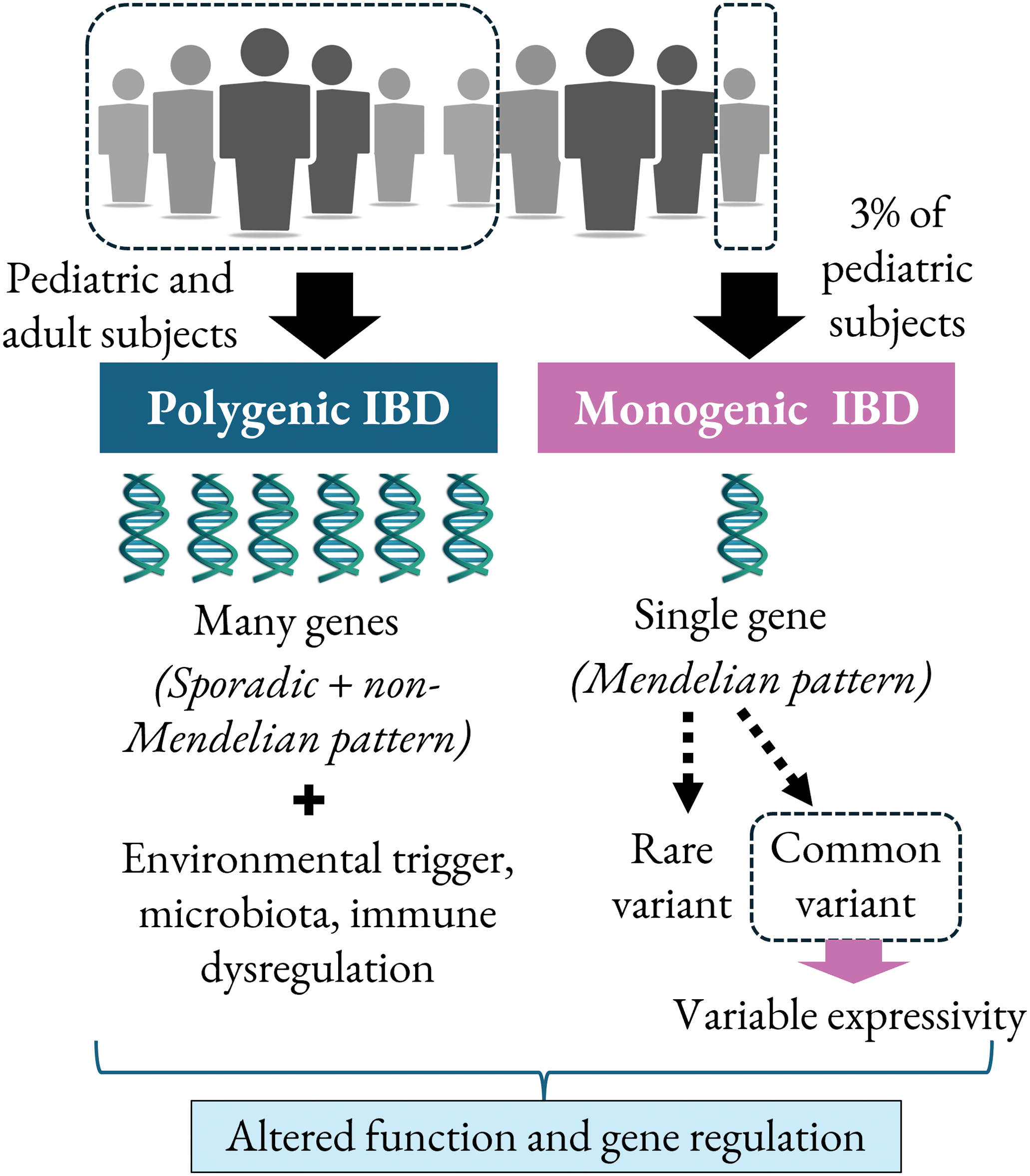
Figure 3: Pathogenesis of polygenic vs. monogenic IBD in adult and pediatric IBD cases.
GWAS has identified genetic variants associated with disease susceptibility, highlighting the importance of genetic predisposition in IBD development. Several genes that were widely reported in the pathogenesis of pediatric IBD are summarised in Table 2. Genes related to IBD pathogenesis mainly regulate the innate immune system response, bacterial clearance, and epithelial functions [33]. Recent GWAS have identified more than 200 risk loci connected to IBD, underscoring the polygenic nature of the condition [34]. Additionally, investigations into monogenic forms of IBD have unveiled approximately 60 causative genes to date, showcasing the diverse genetic landscape of the disease [35].
It is worth noting that variants shown in Table 2 were discovered using various methods on different populations of study subjects. These studies are limited by which a) exome sequencing (WES) may be performed instead of whole genome sequencing (WGS), which will generate larger data output; b) differences between ethnicity or race may be overlooked as the studies only involved subgroups of patients in selected countries, and c) lack of information on the association genetics variants with the severity of the disease.
A more recent report has shed light on various genes that may play an essential role in pediatric IBD [46]. TTC7A deficiency is the most common mutation resulting in intestinal epithelial dysfunction as it results in reduced cellular adhesion and induces early apoptosis. NF-κB essential modulator (NEMO) deficiency caused by IKBKG mutation can predispose to VEO IBD by both immune dysregulation and epithelial dysfunction. Other mutations such as ADAM17 and FERMT1 cause VEO IBD by disrupting epithelial adhesion. Mutations related to chronic diarrhoea such as GUCY2C and SLC26A3 mutations affect intestinal epithelial integrity by impairing fluid absorption and mucous production [46].
The role of epigenetics in the pathogenesis of IBD is still being explored. Epigenetic modification occurs by methylation, histone modification, or post-transcriptional regulation by non-coding ribonucleic acid (RNA). Global hypomethylation was found in the rectal mucosa of the patient with ulcerative colitis compared to normal healthy mucosa [47]. Hypomethylation of the RPS6KA2 gene which regulates the autophagy-associated mTOR pathway has been consistently demonstrated in IBD. There is also a significant correlation between methylation and gene expression, especially in the OSM gene, which was upregulated in IBD. Differential methylation in genes such as TESPA1, TAP1, and RPTOR is associated with the escalation of treatment in IBD. These findings might suggest the role of epigenetics in IBD pathogenesis [48].
Besides genetics, pediatric IBD is also linked to inflammatory reactions and the microbiome of the gut, which are briefly described as follows. Th17 cells, a subset of CD4+ T cells, are known for their pro-inflammatory properties and have been implicated in the pathogenesis of various autoimmune diseases, including IBD. In pediatric IBD, the balance between regulatory T cells, which are anti-inflammatory, and Th17 cells is crucial for maintaining immune homeostasis. Dysregulation of this balance, often seen in conditions like CD, can lead to excessive inflammation and tissue damage in the gastrointestinal tract. Studies have shown that despite the increase in the protective FOXP3+ regulatory T cells in intestinal mucosa in inflammatory bowel disease, it is still insufficient to suppress the inflammatory action by Th17 cells, highlighting the intricate interplay between different immune cell populations in disease pathogenesis [49].
The gut microbiome, which plays a crucial role in immune regulation and intestinal homeostasis, is implicated in the pathogenesis of pediatric IBD. Alterations in the gut microbiota, known as dysbiosis, have been associated with inflammatory responses in CD and UC [50]. Environmental factors such as diet, lifestyle, infections, smoking, and antibiotics can disrupt the protective normal intestinal flora resulting in the rise of pathogenic microorganisms and inflammation of intestinal mucosa. In genetically susceptible individuals with immune dysregulation and intestinal epithelial dysfunction, this leads to prolonged chronic inflammation and translocation of pathogenic microorganisms across the intestinal epithelial barrier, resulting in a vicious cycle of the inflammatory response which also causes extensive mucosal barrier disruption and more translocation of pathogens [51].
Diagnosis and Detection of Pediatric IBD
Recommendations for IBD diagnosis by the international guidelines involve the evaluation of the patient’s medical history and the incorporation of endoscopy, radiology, histology, and biochemical assessment [52]. There are 5 steps in the evaluation for the diagnosis of IBD, listed in Fig. 4 below.
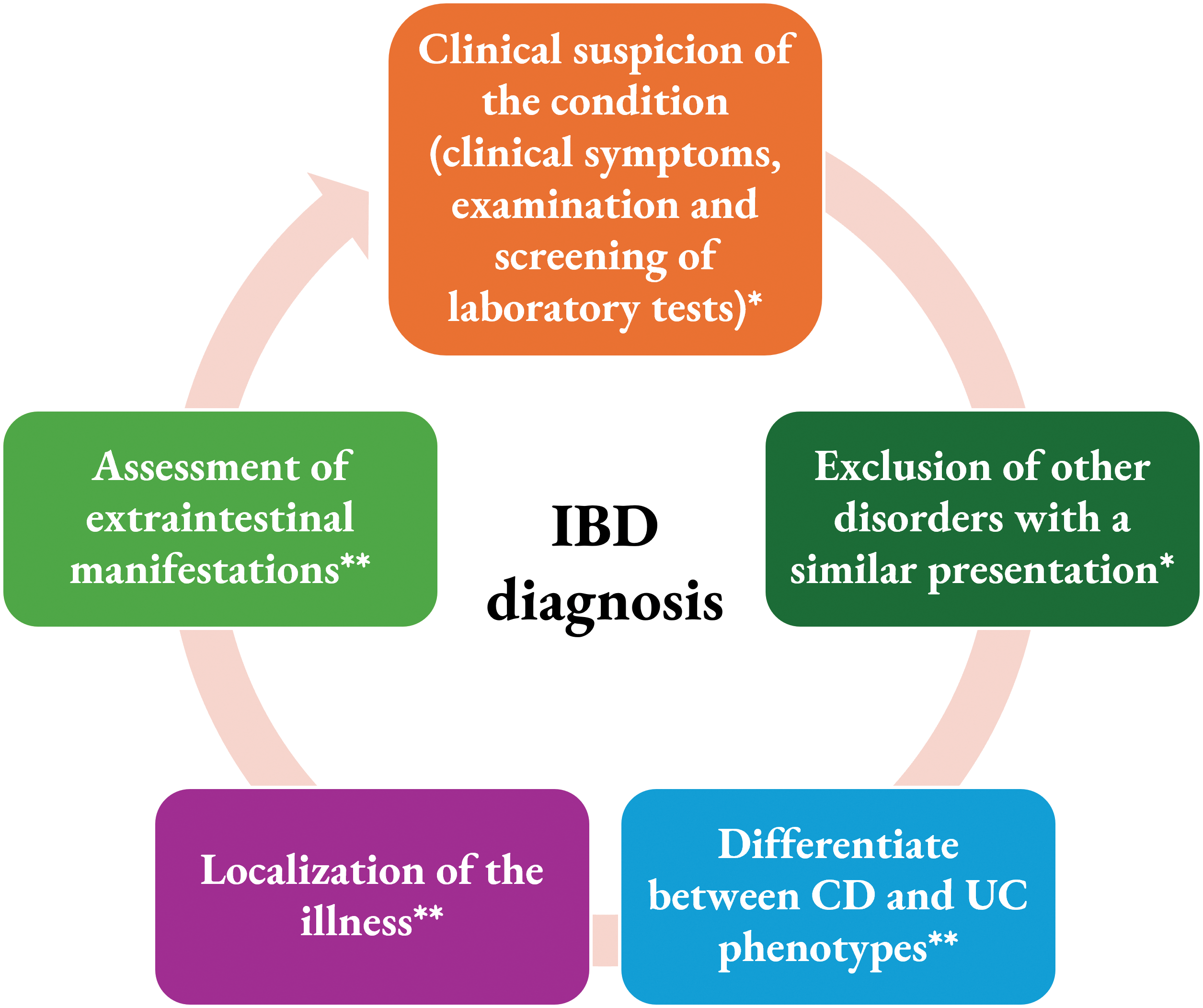
Figure 4: Steps in the evaluation for pediatric IBD diagnosis. The first two steps (*) are commonly conducted by the general pediatrician and the last three steps are performed by the pediatric gastroenterologist (**).
In a previously reported study in the Danish population, the majority of these subjects (more than 12,000) underwent endoscopy (84%), biopsy (84%), and/or radiological procedures (44%). A total of 7.5% of the subjects were diagnosed with IBD (6% for CD and 8% for UC) although not undergoing these clinical procedures. No registry was detected for these subjects to establish the diagnosis of IBD. These may be attributed to limited registry data or shortcomings in IBD management [53].
Diagnosing pediatric IBD involves a comprehensive approach that combines clinical evaluation, laboratory tests, imaging studies, endoscopic procedures, and genetic testing. The methods used to diagnose pediatric IBD aim to confirm the presence of the disease, determine its extent and severity, and guide appropriate treatment strategies. Several diagnostic modalities are employed in the evaluation of pediatric patients suspected of having IBD [52].
Clinical evaluation is a crucial initial step in diagnosing pediatric IBD. Healthcare providers assess the patient’s medical history, symptoms, and physical examination findings to identify potential signs of IBD. Symptoms such as abdominal pain, diarrhoea, weight loss, fatigue, and blood in the stool are common in pediatric IBD and can guide further diagnostic workup [54].
Laboratory tests play a significant role in the diagnostic process of pediatric IBD. Blood tests, including complete blood count (CBC), inflammatory markers (such as C-reactive protein and erythrocyte sedimentation rate), and tests for markers of inflammation and autoimmunity, can provide valuable information to support the diagnosis of IBD and monitor disease activity. Additionally, stool tests may be performed to assess the presence of blood, infectious agents, and markers of inflammation in the gastrointestinal tract such as faecal calprotectin [54].
Imaging studies are often utilized in the diagnosis and monitoring of pediatric IBD. Modalities such as ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT) scans can help visualize the gastrointestinal tract, assess for inflammation, and identify complications such as strictures, fistula, or abscesses [54]. These imaging studies provide valuable information about the extent and severity of the disease.
Endoscopic procedures, including upper endoscopy and colonoscopy, are essential for the diagnosis and evaluation of pediatric IBD. These procedures allow direct visualization of the gastrointestinal mucosa, collection of tissue samples for histological examination (biopsy), and assessment of disease activity and complications. Despite being invasive, endoscopy is considered the essential tool for diagnosing IBD and guiding treatment decisions in pediatric patients [55].
Limitations in the current methods of diagnosing IBD pose challenges in accurately identifying and managing the condition. Despite advancements in diagnostic techniques, several limitations persist in the current approaches to diagnosing IBD in pediatric patients.
One of the primary limitations is the lack of a definitive diagnostic test for IBD. The diagnosis of IBD which rely on a combination of clinical evaluation, laboratory tests, imaging studies, and endoscopic procedures, can be invasive, time-consuming, and costly. The absence of a single diagnostic test and the limited availability of special tests in the presence of non-specific presentation can lead to delays in diagnosis and treatment initiation, potentially impacting disease outcomes [56].
Another limitation is the overlap of symptoms between IBD and other gastrointestinal disorders. The nonspecific nature of symptoms such as abdominal pain, diarrhoea, growth failure, and rectal bleeding can make it challenging to differentiate IBD from other conditions, leading to diagnostic uncertainty and potential misdiagnosis [54,56]. This diagnostic ambiguity can delay appropriate management and result in suboptimal patient care.
Furthermore, the invasiveness of certain diagnostic procedures, such as endoscopy, can pose challenges in pediatric patients. Endoscopic procedures, while essential for diagnosing IBD, may be associated with discomfort, sedation risks, and potential complications, particularly in young patients. The need for repeated endoscopies for disease monitoring can also be burdensome for pediatric IBD patients and their families [55]. Parents and children alike both express discomfort with invasive imaging procedures such as endoscopy in the diagnosis and monitoring of IBD [57].
In addition, the reliance on subjective interpretation of histological findings from biopsy samples obtained during endoscopy can introduce variability in the diagnostic process. Variations in interpreting histopathological features of intestinal tissue samples can impact diagnostic accuracy and consistency, potentially leading to diagnostic errors. Standardizing histological assessment criteria and improving interobserver agreement are essential for enhancing the reliability of histopathological diagnosis in IBD [58].
VEO IBD subjects may also be referred for immunologic assessment and genetic sequencing to confirm monogenic forms of IBD [59]. Advances in genetic testing especially next-generation sequencing have enabled the identification of specific gene mutations associated with pediatric IBD, known as monogenic or Mendelian disorder-associated IBD (MD-IBD) [59]. Genetic testing can help confirm the diagnosis, guide treatment decisions, and provide valuable information about disease prognosis.
However, the limitations of genetic testing in diagnosing IBD, particularly in pediatric patients, should be acknowledged. While genetic testing can provide valuable information about disease susceptibility and pathogenesis, its utility in routine clinical practice for diagnosing IBD is still limited [60]. The complex interplay between genetic factors, environmental triggers, and disease phenotypes in IBD necessitates a comprehensive approach to genetic testing and interpretation.
Diagnosis of pediatric inflammatory bowel disease involves a multidisciplinary approach that integrates clinical evaluation, laboratory tests, imaging studies, endoscopic procedures, and genetic testing. By utilizing a combination of these diagnostic modalities, healthcare providers can accurately diagnose pediatric IBD, assess disease severity, and tailor treatment strategies to meet the individual needs of each patient.
Current Treatment Modalities for Pediatric IBD
Treatment for pediatric IBD involves a multidisciplinary approach aimed at inducing and maintaining disease remission, managing symptoms, and improving the quality of life for affected children. Several treatment modalities are utilized in pediatric IBD management, as outlined below.
Medical management plays a central role in the treatment of pediatric IBD. Therapeutic targets for both CD and UC in children are updated regularly to guide treatment decisions and optimize outcomes. Close monitoring of treatment response and timely adjustments in therapy is essential for achieving favorable outcomes in pediatric IBD management [61]. The therapeutic goal in treating pediatric IBD is to achieve biochemical or endoscopic remission rather than solely focusing on clinical remission. This approach is crucial as intestinal inflammation may persist despite the resolution of abdominal symptoms, emphasizing the importance of objective measures to assess treatment response [4].
Generally, current medical treatment of IBD aims to induce remission and prevent relapse instead of curing the disease. These medical treatments include glucocorticoids, anti-tumour necrosis factor antibodies, aminosalicylate, and exclusive enteral nutrition. Maintenance may include anti-TNF antibodies such as infliximab and adalimumab, immunomodulators such as azathioprine, 6-mercaptopurine, and methotrexate, or a combination of both anti-TNF antibodies and immunomodulators [62,63]. Exclusive enteral nutrition (EEN) is a dietary approach that may be used in pediatric IBD, particularly in CD, to induce remission and promote mucosal healing. EEN is effective in improving symptoms and reducing inflammation in the gut [63].
Surgical intervention may be necessary in cases of severe or refractory pediatric IBD. Surgical options include bowel resection, strictureplasty, and ostomy formation, aiming to alleviate symptoms, manage complications, and improve overall health outcomes. Psychosocial support and counseling are also integral components of pediatric IBD care. Addressing the emotional and psychological impact of the disease on children and their families is essential for holistic management and improving overall well-being [63].
Future Perspectives in the Diagnosis, Treatment, and Management of Pediatric IBD: Focus on the Genetics
Precision medicine in IBD is an ideal approach to provide tailored therapy to the right individual at the right time based on the patient’s biology and genetics [64]. Considering that pediatric IBD is a highly heterogeneous disease and involves different factors that influence its development, several initiatives have been proposed to promote more personalized diagnosis, treatment, and management of this condition, about its genetic aspect, as further discussed below.
Understanding the genetic basis of IBD in pediatrics can help to optimize the management of this disease. Comprehensive genetic profiling of pediatric IBD will assist in better phenotyping of the patients.
An emerging approach in pediatric IBD investigation is the implementation of genetic testing. Single-gene disorder masquerading as IBD represents a small percentage (lower than 0.5%) of pediatric-onset IBD patients [65]. These disorders typically involve primary immunodeficiencies, autoinflammatory conditions, and epithelial barrier dysfunction. More than 100 genes are being implicated with these conditions [66]. Recently, a test commissioned by the National Health Service able to screen approximately 70 monogenic IBD genes to establish causative variants, becomes available to subjects diagnosed below the age of 2 as well as to individuals older than this [67]. Despite a small number, this presents a potentially impactful approach in those patients receiving the diagnosis of monogenic IBD. Monogenic IBD is associated with high morbidity and mortality. The establishment of an early institution of genetic diagnosis will allow physicians to assess patient prognosis and dictate appropriate treatment modalities [68]. The utility of genetic testing can be explored further in various aspects of the disease such as risk prediction, novel biomarkers for screening, and pharmacogenomics. Genetic profiling of monogenic IBD also would help in genetic counseling for future pregnancy [8].
Although rare, VEO IBD has become recognized due to increased awareness among medical practitioners. To elucidate the complexity of IBD heterogeneity, the VEO IBD subgroup and Mendelian comorbidity should be further investigated as IBD and Mendelian disorders commonly share phenotype but involve variants of different sections of the spectrum in the same loci. Conducting GWAS using a larger number of subjects will provide insight into resolving any missing heritability. Mendelian loci contain variants that are responsible for risk predisposition to diseases including IBD. This elevates the importance of next-generation sequencing as a prime method to test the hypothesis [69].
Genetic testing is now considered a standard of care for children with IBD under the age of 6 years old. A study by El-Matary et al. in 2023, on 1000 Canadian children with VEO-IBD revealed that 7.8% of them have an identifiable monogenic cause [70]. There is also a benefit in testing adult patients with IBD since a minority of them may have undiagnosed monogenic causes [46]. Knowing the specific genetic defect in pediatric IBD may facilitate the discovery of more treatment avenues such as medication and haemopoietic stem cell transplantation as well as help in the genetic counseling process [8].
Until 2018, Japanese national health insurance has approved testing targeted panel sequences for 20 Mendelian disorder-associated IBD (MD-IBD) genes. This IBD gene panel which is partly an inborn error of immunity (IEI) panels cover up to 400 genes linked to monogenic primary immune deficiencies (PIDs) and autoinflammatory disorders. For patients for which responsible genes are unable to be detected using this IBD panel but with suspected IEI, 400 IEI-associated genes can be assessed as part of a national research initiative. If the responsible variants or genes cannot be detected using both IBD and IEI panels, then it is recommended that WES or WGS be performed. Functional testing for genetic variants is still presented as a challenge for physicians [35].
The genotype-phenotype association can differ according to ethnicity, age, and gender. For example, in Caucasians, NOD2/CARD15 was found to be a susceptible gene locus, but not in Asian groups, while ATG16L2 and IL17REL were shown as susceptible loci in Korean subjects [71]. IL10RA mutation was found to be the main mutation in the monogenic VEO IBD among the Chinese population [72]. In addition, Shim and Seo also reported on cases of IL10RA mutations in 7 out of 14 cases of infantile-onset IBD. Children with IL10RA mutations exhibited the phenotypes of CD, had anal fistulae, and required surgical resection as they showed resistance to medical treatment. Moreover, the patients also have recurrent infections and folliculitis [73].
Further exploration into the pharmacogenetics of pediatric IBD focusing on the actionable drug-gene interaction may also predict the action of specific medications on individuals, increasing the effectiveness while reducing the side effects of treatment. Variations between races and ethnicity also need to be accounted for as that may play a role in dictating IBD onset and progression. Additionally, as many molecular pathways involved in IBD are varied in their sensitivity to perturbation by particular external factors, this conceptual view of IBD pathogenesis may lead to personalized IBD prevention that is designed for a child’s genetic susceptibility [4].
Polygenic risk scores (PRS) have emerged as a valuable tool in predicting the risk, severity, and treatment response in pediatric IBD. A study incorporated the IBD susceptibility genes from the GWAS for the prediction of IBD and compared it to other methods of diagnosis. Although this method is not entirely accurate for diagnosis (area under the ROC curve [AUC] of up to 0.78 for CD and 0.70 for UC), it still outperformed other methods. Concurrently, it was also discovered that a higher score is associated with severe outcomes in terms of increased frequency of bowel resection and earlier onset of diseases. Earlier onset of IBD was found to be associated with a higher genetic burden [74].
In 2021, Bodea et al. devised the use of pathway-centered PRS to predict disease susceptibility, severity, and complication. This method is also able to stratify patients into treatment responses to anti-TNF antibody treatment [75]. Meanwhile, a study by Gettler et al. in 2021 explored the prediction and penetrance of IBD using polygenic risk scores in a large, multi-ethnic cohort and found that the incorporation of association data from diverse ethnic backgrounds and integration of both common and rare variants can improve risk prediction and maximize predictive accuracy [76].
PRS also shows promising benefits in identifying IBD comorbidities. In 2023, Wang et al. investigated the use of PRS in predicting the risk of primary sclerosing cholangitis (PSC) in patients with IBD. PRS was found to be a promising tool for identifying the risk of PSC in individuals with IBD and predicting the behavior of IBD-PSC, where those with UC-PSC are more likely to be associated with milder but more extensive disease and those with CD-PSC are more likely to develop colon cancer [77]. Meanwhile, In 2022, Li et al. utilized PRS to infer psychiatric comorbidity in IBD patients of European ancestry and found that a higher PRS is associated with a higher risk of developing comorbid psychiatric illness in patients with IBD, thus may help earlier intervention [78].
These studies collectively demonstrate the significance of PRS in pediatric IBD, providing valuable insights into disease risk, severity, treatment response, and personalised care strategies.
Emerging genetic, epigenetic, and multi-omics predictive biomarkers in pediatric IBD play a crucial role in understanding disease mechanisms, predicting treatment responses, and guiding personalized therapeutic interventions. DNA methylation, histone modification patterns, and long non-coding RNA show potential to be used as epigenetic biomarkers in screening and assessment of IBD. For example, hypermethylation of epithelial-mesenchymal transition-related genes such as CDH1 and CDX1 is associated with severe ulcerative colitis. Some long non-coding RNA (such as KIF9-AS1, LINC01272, and DIO3OS) and some micro-RNA (such as miR-21 and miR-92a) were found to be differentially expressed in the plasma of IBD patients and healthy individuals [79]. Hypermethylation of DEFA5 and hypomethylation of TNF can also be useful as non-invasive biomarkers for CD as it was found to be consistent regardless of disease activity [80].
Gene expression profiles such as FCGR1A, FCGR1B, and GBP1 have been demonstrated to be useful as non-invasive pharmacogenomic biomarkers for early response to anti-TNF drugs in pediatric IBD [81] (70). Another systematic review exhibited that variants in TLR2 (rs3804099), TLR4 (rs5030728), TNFRSF1A (rs4149570), IFNG (rs2430561), IL6 (rs10499563), and IL1B (rs4848306) influence the response to treatment with anti-TNF-α antibody in IBD. HLA-DQA1*05 polymorphism is also significantly associated with the rate of anti-drug antibody formation in the treatment of IBD with adalimumab and infliximab, thus affecting the efficacy of this medication in the long term [82].
Meanwhile, the determination of allelic variants in TPMT and NUDT15 genes can predict the possible toxicity during treatment with azathioprine and mercaptopurine in IBD. Both azathioprine and mercaptopurine are metabolized by thiopurine S-methyltransferase (encoded by TPMT) and nucleotide diphosphatase/Nudix hydrolase 15 (encoded by NUDT15) into thioguanine-nucleotides (6-TGNs). While an adequate level of 6-TGNs is required to achieve a therapeutic effect, some allelic variants in TMPT and NUDT15 can increase the metabolism of azathioprine and mercaptopurine thus increasing the level of 6-TGNs, leading to excessive immunosuppression [83].
These genetic markers can predict treatment responses and guide therapeutic decisions. These emerging genetic, epigenetic, and multi-omics predictive biomarkers offer promise in advancing the management of pediatric inflammatory bowel disease by providing insights into disease progression, treatment responses, and personalized care for pediatric patients with IBD.
Novel molecular therapies for pediatric IBD are rapidly evolving, aiming to target specific pathways involved in the pathogenesis of the disease. These therapies are designed to provide more effective and personalized treatment options with potentially fewer side effects compared to traditional therapies. Among the recently explored biological therapies are Janus Kinase (JAK) inhibitors, a family of small molecules that block intracellular tyrosine kinases, including JAK-1, JAK-2, JAK-3, and TYK-2. Despite the usage of JAK inhibitors such as tofacitinib and upadacitinib have been approved in adult IBD, there is still a lack of clear guidance on their usage in the treatment of pediatric IBD. A case report by Miller et al. noticed that upadacitinib can improve the outcome in refractory pediatric IBD and prevent colectomy [84].
Another emerging immunomodulator explored in IBD treatment is the sphingosine-1-phosphate receptor (S1PR) modulators such as ozanimod, estrasimod, and amiselimod. The binding of sphingosine-1-phosphate (S1P1-5) on the sphingolipid ligand of G-protein-coupled receptors regulates the release of leukocytes out of the lymphoid tissues [85]. S1P is one of the sphingosine-derived phospholipids. A metabolomic study of colon biopsies of IBD patients found that sphingolipid metabolism is highly affected in affected individuals. Increased levels of S1PR1 have also been observed in patients with UC. The binding of S1P to S1PR also triggered the regulation of the pro-inflammatory TNF-α in IBD. The main action of S1PR modulators is to antagonize the action of S1P1 on the receptors of T-lymphocytes, reducing the circulating lymphocytes and thus reducing inflammation [86].
Mesenchymal stem cell (MSC) therapy is currently being investigated as an alternative therapy to IBD, but it is still in the pre-clinical stage. The concept of MSC transplantation is to harvest the benefits of the MSC-derived exosomes which contain biological molecules that can promote healing and regulate immunity. MSC can reduce inflammation by stimulating the differentiation of dendritic cells into regulatory dendritic cells, increasing regulatory T cells, and inhibiting the proliferation of CD4+ T cells and the activation of Th1/Th17 cells. MSC also increases intestinal membrane integrity by promoting proliferation and inhibiting apoptosis of intestinal epithelial cells. Intestinal epithelial cells are responsible for regulating the contents of the intraluminal mucous layer and form a persistent intestinal barrier via the action of tight junctions [87]. Animal studies have demonstrated that MSC can regulate gut microbiota by reducing harmful pathogens and increasing beneficial flora. This action can occur by secretion of products with direct action on the microbiota, or indirectly by altering the intestinal immunity. However, the use of MSC transplant has its limitations such as the possibility of transplantation mismatch and immune rejection which may worsen the IBD, and the unpredictability of action of other products secreted by transplanted MSC [88]. Genetically modified MSC may be utilized to enrich the expression of beneficial products and tailor the MSC to specific patient requirements [87].
The Genetics of Pediatric IBD: Challenges in the Implementation of Personalised Care
Despite great excitement in the discoveries of the genetics of pediatric IBD within the past decade, there are challenges to be overcome in establishing personalized care for children with IBD. This article highlights the significance of genetic screening which would promote early detection, diagnosis, and ultimately more targeted treatment for pediatric IBD patients. However, genetic testing for pediatric IBD must be approached with care which cost-effectiveness of these tests has to be thoroughly investigated and proper guidelines must be proposed.
Regulation of genetic information proved to be extremely difficult and the law regarding genetic testing varied between different regions [89]. Most importantly, genetic testing must be accompanied by genetic counselling which would help the patients and their families to acquire information about the disease as well as necessary support. Worth noting that genetic counseling practice must be regulated to ensure proper integration into the management of pediatric IBD [90]. High cost and lack of availability as well as awareness of genetic testing especially in middle and low-income countries must be addressed to avoid disparity between regions [91]. A humongous task in implementing genetic testing, particularly in children warrants cooperation from various parties including healthcare providers, industry, insurance companies, and policymakers, which is not fully discussed in this review. The present work focuses more on the fundamental knowledge of the genetics of pediatric IBD which highlights the gap in translating information from research into clinical practice. Nevertheless, output from research has been tremendously useful in elucidating different gene variants and molecular pathways which are essential in the development of pediatric IBD, and these are currently being applied for the screening and diagnosis of this disease. However, the significance of genetic profiling on treatment efficacy is still yet fully determined and this demands a larger scale of investigation to be conducted.
The pathogenesis of pediatric IBD is a complex interplay of genetic, epigenetic, and environmental factors. Advances in genetic research have identified numerous susceptibility genes that contribute to the development and progression of IBD in children. Concurrently, epigenetic modifications have emerged as crucial regulators in the pathogenesis of IBD, influencing gene expression and immune responses. Understanding the roles of these genetic and epigenetic mechanisms is essential for the development of novel diagnostic tools and therapeutic strategies. Future research should focus on integrating not only genetics but also epigenetic and other multi-omics data to create comprehensive models of disease prediction and management, ultimately aiming to improve outcomes for pediatric patients with IBD. In the future, the concept of “precision medicine” in the clinical management of pediatric IBD driven by robust genetic profiling, application of PRS, identification of predictive biomarkers, and more tailored therapy will drive effective and more individualized care for the patients. However, the challenges in implementing personalized care for pediatric IBD must be addressed carefully, ideally to reduce morbidity and subsequently ensure optimum management of this disease.
Acknowledgement: The authors would like to acknowledge the support from the staff and students of the Human Genome Centre, Universiti Sains Malaysia.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Marahaini Musa, Ahmad Shahir Mohamad Nazri; draft manuscript preparation: Marahaini Musa, Ahmad Shahir Mohamad Nazri; review and editing: Marahaini Musa, Ahmad Shahir Mohamad Nazri, Nazihah Mohd Yunus; visualization: Marahaini Musa, Ahmad Shahir Mohamad Nazri; supervision: Marahaini Musa. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing does not apply to this article as no datasets were generated or analysed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. CDC. Inflammatory bowel disease (IBD). IBD Facts and Stats, Available from: https://www.cdc.gov/inflammatory-bowel-disease/php/facts-stats/index.html. [Accessed 2024]. [Google Scholar]
2. Weimers P, Munkholm P. The natural history of IBD: lessons learned. Curr Treat Options Gastro. 2018;16(1):101–11. doi:10.1007/s11938-018-0173-3. [Google Scholar] [PubMed] [CrossRef]
3. Borowitz SM. The epidemiology of inflammatory bowel disease: clues to pathogenesis? Front Pediatr. 2022;10:1103713. [Google Scholar] [PubMed]
4. Bouhuys M, Lexmond WS, Van Rheenen PF. Pediatric inflammatory bowel disease. Pediatrics. 2023;151(1):e2022058037. doi:10.1542/peds.2022-058037. [Google Scholar] [PubMed] [CrossRef]
5. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17(6):1314–21. doi:10.1002/ibd.21493. [Google Scholar] [PubMed] [CrossRef]
6. Shim JO. Recent advance in very early onset inflammatory bowel disease. Pediatr Gastroenterol Hepatol Nutr. 2019;22(1):41. doi:10.5223/pghn.2019.22.1.41. [Google Scholar] [PubMed] [CrossRef]
7. Essers JB, Lee JJ, Kugathasan S, Stevens CR, Grand RJ, Daly MJ. Established genetic risk factors do not distinguish early and later onset Crohn’s disease. Inflamm Bowel Dis. 2009;15(10):1508–14. doi:10.1002/ibd.20922. [Google Scholar] [PubMed] [CrossRef]
8. Ashton JJ, Beattie RM. Inflammatory bowel disease: recent developments. Arch Dis Child. 2024;109(5):370–6. doi:10.1136/archdischild-2023-325668. [Google Scholar] [PubMed] [CrossRef]
9. Ashton JJ, Mossotto E, Stafford IS, Haggarty R, Coelho TAF, Batra A, et al. Genetic sequencing of pediatric patients identifies mutations in monogenic inflammatory bowel disease genes that translate to distinct clinical phenotypes. Clin Transl Gastroenterol. 2020;11(2):e00129. doi:10.14309/ctg.0000000000000129. [Google Scholar] [PubMed] [CrossRef]
10. Nambu R, Muise AM. Advanced understanding of monogenic inflammatory bowel disease. Front Pediatr. 2021;22(8):618918. doi:10.3389/fped.2020.618918. [Google Scholar] [PubMed] [CrossRef]
11. Wang R, Li Z, Liu S, Zhang D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open. 2023;13(3):e065186. doi:10.1136/bmjopen-2022-065186. [Google Scholar] [PubMed] [CrossRef]
12. Zhou JL, Bao JC, Liao XY, Chen YJ, Wang LW, Fan YY, et al.Trends and projections of inflammatory bowel disease at the global, regional and national levels, 1990–2050: a Bayesian age-period-cohort modeling study. BMC Public Health. 2023;23(1):2507. doi:10.1186/s12889-023-17431-8. [Google Scholar] [PubMed] [CrossRef]
13. Coward S, Benchimol EI, Kuenzig ME, Windsor JW, Bernstein CN, Bitton A, et al. The 2023 impact of inflammatory bowel disease in Canada: epidemiology of IBD. J Can Assoc Gastroenterol. 2023;6(Suppl 2):S9–15. [Google Scholar] [PubMed]
14. Hammer T, Langholz E. The epidemiology of inflammatory bowel disease: balance between East and West? A narrative review. Dig Med Res. 2020;3:48. doi:10.21037/dmr. [Google Scholar] [CrossRef]
15. Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156(5):1345–53.e4. doi:10.1053/j.gastro.2019.01.002. [Google Scholar] [PubMed] [CrossRef]
16. Long D, Wang C, Huang Y, Mao C, Xu Y, Zhu Y. Changing epidemiology of inflammatory bowel disease in children and adolescents. Int J Colorectal Dis. 2024;39(1):73. doi:10.1007/s00384-024-04640-9. [Google Scholar] [PubMed] [CrossRef]
17. Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. 2022;162(4):1147–59.e4. doi:10.1053/j.gastro.2021.12.282. [Google Scholar] [PubMed] [CrossRef]
18. Green Z, Ashton JJ, Rodrigues A, Spray C, Howarth L, Mallikarjuna A, et al. OC6 Sustained increase in pediatric inflammatory bowel disease incidence across the South West United Kingdom over the last 10 years. Frontline Gastroenterol. 2024;15(Suppl 1):A5–6. [Google Scholar]
19. Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, et al. Differentiating ulcerative colitis from crohn’s disease in children and young adults: report of a working group of the North American society for pediatric gastroenterology, hepatology, and nutrition and the crohn’s and colitis foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653–74. doi:10.1097/MPG.0b013e31805563f3. [Google Scholar] [PubMed] [CrossRef]
20. Bharadwaj S, Narula N, Tandon P, Yaghoobi M. Role of endoscopy in inflammatory bowel disease. Gastroenterol Rep. 2018;6(2):75–82. doi:10.1093/gastro/goy006. [Google Scholar] [PubMed] [CrossRef]
21. Aziz DA, Moin M, Majeed A, Sadiq K, Biloo AG. Pediatric inflammatory bowel disease: clinical presentation and disease location. Pak J Med Sci. 2017;33(4):793–7. [Google Scholar] [PubMed]
22. Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: a pathological review. World J Gastroenterol. 2019;25(16):1928–35. doi:10.3748/wjg.v25.i16.1928. [Google Scholar] [PubMed] [CrossRef]
23. Ashton JJ, Mossotto E, Ennis S, Beattie RM. Personalising medicine in inflammatory bowel disease—current and future perspectives. Transl Pediatr. 2019;8(1):56–69. doi:10.21037/tp. [Google Scholar] [CrossRef]
24. Burgos K, Blanco A, Emanuelli N, Torres EA. A comparison of pediatric versus adult-onset IBD in Puerto Rican Hispanics: 2826. Am J Gastroenterol. 2018;113:S1568. doi:10.14309/00000434-201810001-02825. [Google Scholar] [CrossRef]
25. Fuller MK. Pediatric inflammatory bowel disease: special considerations. Surg Clin North Am. 2019;99(6):1177–83. doi:10.1016/j.suc.2019.08.008. [Google Scholar] [PubMed] [CrossRef]
26. Chan WM, Tinsley D, Crandall W, Du Y, Choong C, Hunter T. Treatment patterns of pediatric and adult patients with ulcerative colitis. Inflamm Bowel Dis. 2023;29(Suppl 1):S38–S9. [Google Scholar]
27. Silva ACV, Tumelero TJ, Yamamoto DR, Truppel SK, da Silva GS, Ribeiro LBM, et al. Biological therapy, surgery, and hospitalization rates for inflammatory bowel disease: an observational Latin American comparative study between adults and pediatric patients. Gastroent Hepatol. 2023. doi:10.1016/j.gastrohep.2023.10.006. [Google Scholar] [PubMed] [CrossRef]
28. Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94(1):155–65. doi:10.1016/j.mayocp.2018.09.013. [Google Scholar] [PubMed] [CrossRef]
29. Baydi Z, Limami Y, Khalki L, Zaid N, Naya A, Mtairag EM, et al. An update of research animal models of inflammatory bowel disease. Sci World J. 2021;2021:7479540. [Google Scholar]
30. Graham DB, Xavier RJ. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature. 2020;578(7796):527–39. doi:10.1038/s41586-020-2025-2. [Google Scholar] [PubMed] [CrossRef]
31. Crowley E, Warner N, Pan J, Khalouei S, Elkadri A, Fiedler K, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158(8):2208–20. doi:10.1053/j.gastro.2020.02.023. [Google Scholar] [PubMed] [CrossRef]
32. Jezernik G, Mičetić-Turk D, Potočnik U. Molecular genetic architecture of monogenic pediatric IBD differs from complex pediatric and adult IBD. J Pers Med. 2020;10(4):243. doi:10.3390/jpm10040243. [Google Scholar] [PubMed] [CrossRef]
33. Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Rev Gastroenterol Hepatol. 2006;3(7):390–407. doi:10.1038/ncpgasthep0528. [Google Scholar] [PubMed] [CrossRef]
34. Conrad MA, Kelsen JR. Genomic and immunologic drivers of very early-onset inflammatory bowel disease. Pediatr Dev Pathol. 2019;22(3):183–93. doi:10.1177/1093526619834807. [Google Scholar] [PubMed] [CrossRef]
35. Arai K. Very Early-onset inflammatory bowel disease: a challenging field for pediatric gastroenterologists. Pediatr Gastroenterol Hepatol Nutr. 2020;23(5):411. doi:10.5223/pghn.2020.23.5.411. [Google Scholar] [PubMed] [CrossRef]
36. Huang Z, Peng K, Li X, Zhao R, You J, Cheng X, et al. Mutations in interleukin-10 receptor and clinical phenotypes in patients with very early onset inflammatory bowel disease: a Chinese VEO-IBD collaboration group survey. Inflamm Bowel Dis. 2017;23(4):578–90. doi:10.1097/MIB.0000000000001058. [Google Scholar] [PubMed] [CrossRef]
37. Lin Z, Wang Z, Hegarty JP, Lin TR, Wang Y, Deiling S, et al. Genetic association and epistatic interaction of the interleukin-10 signaling pathway in pediatric inflammatory bowel disease. World J Gastroenterol. 2017;23(27):4897. doi:10.3748/wjg.v23.i27.4897. [Google Scholar] [PubMed] [CrossRef]
38. Konidari A, Dickens D, Pirmohamed M. Inflammatory bowel disease: a personalized approach. Front Pediatr. 2021;8:620545. doi:10.3389/fped.2020.620545. [Google Scholar] [PubMed] [CrossRef]
39. Ashton JJ, Boukas K, Stafford IS, Cheng G, Haggarty R, Coelho TAF, et al. Deleterious genetic variation across the NOD signaling pathway is associated with reduced NFKB signaling transcription and upregulation of alternative inflammatory transcripts in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2022;28(6):912–22. doi:10.1093/ibd/izab318. [Google Scholar] [PubMed] [CrossRef]
40. Schmid F, Chao CM, Däbritz J. Pathophysiological concepts and management of pulmonary manifestation of pediatric inflammatory bowel disease. Int J Mol Sci. 2022;23(13):7287. doi:10.3390/ijms23137287. [Google Scholar] [PubMed] [CrossRef]
41. Sewell GW, Kaser A. Interleukin-23 in the pathogenesis of inflammatory bowel disease and implications for therapeutic intervention. J Crohns Colitis. 2022;16(Suppl 2):ii3–19. [Google Scholar] [PubMed]
42. Schmitt H, Neurath MF, Atreya R. Role of the IL23/IL17 pathway in Crohn’s disease. Front Immunol. 2021;12:622934. doi:10.3389/fimmu.2021.622934. [Google Scholar] [PubMed] [CrossRef]
43. Bank S, Skytt Andersen P, Burisch J, Pedersen N, Roug S, Galsgaard J, et al. Polymorphisms in the inflammatory pathway genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG are associated with susceptibility of inflammatory bowel disease in a Danish cohort. PLoS One. 2014;9:e98815. doi:10.1371/journal.pone.0098815. [Google Scholar] [PubMed] [CrossRef]
44. Amre DK, Mack DR, Morgan K, Krupoves A, Costea I, Lambrette P, et al. Autophagy gene ATG16L1 but not IRGM is associated with Crohn’s disease in Canadian children. Inflamm Bowel Dis. 2009;15(4):501–7. doi:10.1002/ibd.20785. [Google Scholar] [PubMed] [CrossRef]
45. Noel DD, Marinella P, Mauro G, Tripodi SI, Pin A, Serena A, et al. Genetic variants assessing Crohn’s disease pattern in pediatric inflammatory bowel disease patients by a clinical exome survey. Bioinform Biol Insights. 2021;15. doi:10.1177/11779322211055285. [Google Scholar] [PubMed] [CrossRef]
46. Hall CHT, De Zoeten EF. Understanding very early onset inflammatory bowel disease (VEOIBD) in relation to inborn errors of immunity. Immunol Rev. 2024;322(1):329–38. doi:10.1111/imr.13302. [Google Scholar] [PubMed] [CrossRef]
47. El Hadad J, Schreiner P, Vavricka SR, Greuter T. The genetics of inflammatory bowel disease. Mol Diagn Ther. 2024;28(1):27–35. doi:10.1007/s40291-023-00678-7. [Google Scholar] [PubMed] [CrossRef]
48. Kalla R, Adams AT, Nowak JK, Bergemalm D, Vatn S, Ventham NT, et al. Analysis of systemic epigenetic alterations in inflammatory bowel disease: defining geographical, genetic and immune-inflammatory influences on the circulating methylome. J Crohns Colitis. 2023;17(2):170–84. doi:10.1093/ecco-jcc/jjac127. [Google Scholar] [PubMed] [CrossRef]
49. Cho J, Kim S, Yang DH, Lee J, Park KW, Go J, et al. Mucosal immunity related to FOXP3+ regulatory T cells, Th17 cells and cytokines in pediatric inflammatory bowel disease. J Korean Med Sci. 2018;33(52):e336. doi:10.3346/jkms.2018.33.e336. [Google Scholar] [PubMed] [CrossRef]
50. Santana PT, Rosas SLB, Ribeiro BE, Marinho Y, de Souza HSP. Dysbiosis in inflammatory bowel disease: pathogenic role and potential therapeutic targets. Int J Mol Sci. 2022;23(7):3464. doi:10.3390/ijms23073464. [Google Scholar] [PubMed] [CrossRef]
51. Abdulla M, Mohammed N. A review on inflammatory bowel diseases: recent molecular pathophysiology advances. Biologics. 2022;16:129–40. [Google Scholar] [PubMed]
52. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–64. doi:10.1093/ecco-jcc/jjy113. [Google Scholar] [PubMed] [CrossRef]
53. Rasmussen NF, Green A, Allin KH, Iversen AT, Madsen GI, Pedersen AK, et al. Clinical procedures used to diagnose inflammatory bowel disease: real-world evidence from a Danish nationwide population-based study. BMJ Open Gastroenterol. 2022;9(1):e000958. doi:10.1136/bmjgast-2022-000958. [Google Scholar] [PubMed] [CrossRef]
54. Higuchi LM, Bousvaros A. Clinical presentation and diagnosis ofinflammatory bowel disease in children. Available from: https://www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-inflammatory-bowel-disease-in-children. [Accessed 2024]. [Google Scholar]
55. Hong SM, Baek DH. Diagnostic procedures for inflammatory bowel disease: laboratory, endoscopy, pathology, imaging, and beyond. Diagnostics. 2024;14(13):1384. doi:10.3390/diagnostics14131384. [Google Scholar] [PubMed] [CrossRef]
56. Leiz M, Knorr M, Moon K, Tischler L, de Laffolie J, van den Berg N. Diagnostic delay in children with inflammatory bowel disease in the German-Austrian patient registry CEDATA-GPGE 2014–2018. Sci Rep. 2022;12(1):21162. doi:10.1038/s41598-022-25487-6. [Google Scholar] [PubMed] [CrossRef]
57. Van Wassenaer EA, Van Der Klift RR, Staphorst MS, Van Der Lee JH, Benninga MA, Koot BGP. The child’s perception on monitoring inflammatory bowel disease activity. Eur J Pediatr. 2022;181(3):1143–9. doi:10.1007/s00431-021-04315-5. [Google Scholar] [PubMed] [CrossRef]
58. Fabian O, Bajer L. Histopathological assessment of the microscopic activity in inflammatory bowel diseases: what are we looking for? World J Gastroenterol. 2022;28(36):5300–12. doi:10.3748/wjg.v28.i36.5300. [Google Scholar] [PubMed] [CrossRef]
59. Uhlig HH, Charbit-Henrion F, Kotlarz D, Shouval DS, Schwerd T, Strisciuglio C, et al. Clinical genomics for the diagnosis of monogenic forms of inflammatory bowel disease: a position paper from the pediatric ibd porto group of european society of pediatric gastroenterology hepatology and nutrition. J Pediatr Gastroenterol Nutr. 2021;72(3):456–73. doi:10.1097/MPG.0000000000003017. [Google Scholar] [PubMed] [CrossRef]
60. Baig A, Avlasevich SL, Torous DK, Bemis JC, Saubermann LJ, Lovell DP, et al. Assessment of systemic genetic damage in pediatric inflammatory bowel disease. Environ and Mol Mutagen. 2020;61(9):901–9. doi:10.1002/em.v61.9. [Google Scholar] [CrossRef]
61. Van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The medical management of pediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2021;15(2):171–94. doi:10.1093/ecco-jcc/jjaa161. [Google Scholar] [PubMed] [CrossRef]
62. Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56(6):489–526. doi:10.1007/s00535-021-01784-1. [Google Scholar] [PubMed] [CrossRef]
63. Lee WS, Arai K, Alex G, Treepongkaruna S, Kim KM, Choong CL, et al. Medical management of pediatric inflammatory bowel disease in the Asia-Pacific region: a position paper by the Asian Pan-Pacific Society for Pediatric Gastroenterology, Hepatology, and Nutrition (APPSPGHAN) PIBD Working Group. J Gastro and Hepatol. 2023;38(4):523–38. doi:10.1111/jgh.v38.4. [Google Scholar] [CrossRef]
64. Borg-Bartolo SP, Boyapati RK, Satsangi J, Kalla R. Precision medicine in inflammatory bowel disease: concept, progress and challenges. F1000Research. 2020;9:54. doi:10.12688/f1000research. [Google Scholar] [CrossRef]
65. Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014 Nov;147(5):990–1007.e3. doi:10.1053/j.gastro.2014.07.023. [Google Scholar] [PubMed] [CrossRef]
66. Bolton C, Smillie CS, Pandey S, Elmentaite R, Wei G, Argmann C, et al. An integrated taxonomy for monogenic inflammatory bowel disease. Gastroenterology. 2022;162(3):859–76. doi:10.1053/j.gastro.2021.11.014. [Google Scholar] [PubMed] [CrossRef]
67. Kammermeier J, Lamb CA, Jones KDJ, Anderson CA, Baple EL, Bolton C, et al. Genomic diagnosis and care co-ordination for monogenic inflammatory bowel disease in children and adults: consensus guideline on behalf of the british society of gastroenterology and british society of pediatric gastroenterology hepatology and nutrition. Lancet Gastroenterol Hepatol. 2023;8(3):271–86. doi:10.1016/S2468-1253(22)00337-5. [Google Scholar] [PubMed] [CrossRef]
68. Nameirakpam J, Rikhi R, Rawat SS, Sharma J, Suri D. Genetics on early onset inflammatory bowel disease: an update. Genes Dis. 2020;7(1):93–106. doi:10.1016/j.gendis.2019.10.003. [Google Scholar] [PubMed] [CrossRef]
69. Okou DT, Kugathasan S. Role of genetics in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(10):1878–84. doi:10.1097/MIB.0000000000000085. [Google Scholar] [PubMed] [CrossRef]
70. El-Matary W, Carroll MW, Deslandres C, Griffiths AM, Kuenzig ME, Mack DR, et al. The 2023 impact of inflammatory bowel disease in Canada: special populations-children and adolescents with IBD. J Can Assoc Gastroenterol. 2023;6(Suppl 2):S35–44. [Google Scholar] [PubMed]
71. Yang SK, Hong M, Zhao W, Jung Y, Baek J, Tayebi N, et al. Genome-wide association study of Crohn’s disease in Koreans revealed three new susceptibility loci and common attributes of genetic susceptibility across ethnic populations. Gut. 2014;63(1):80–7. doi:10.1136/gutjnl-2013-305193. [Google Scholar] [PubMed] [CrossRef]
72. Fang YH, Luo YY, Yu JD, Lou JG, Chen J. Phenotypic and genotypic characterization of inflammatory bowel disease in children under six years of age in China. World J Gastroentero. 2018;24(9):1035–45. doi:10.3748/wjg.v24.i9.1035. [Google Scholar] [PubMed] [CrossRef]
73. Shim JO, Seo JK. Very early-onset inflammatory bowel disease (IBD) in infancy is a different disease entity from adult-onset IBD; one form of interleukin-10 receptor mutations. J Hum Genet. 2014;59(6):337–41. doi:10.1038/jhg.2014.32. [Google Scholar] [PubMed] [CrossRef]
74. Hübenthal M, Löscher BS, Erdmann J, Franke A, Gola D, König IR, et al. Current developments of clinical sequencing and the clinical utility of polygenic risk scores in inflammatory diseases. Front Immunol. 2021;11:577677. doi:10.3389/fimmu.2020.577677. [Google Scholar] [PubMed] [CrossRef]
75. Bodea CA, Macoritto M, Liu Y, Zhang W, Karman J, King EA, et al. Pathway specific polygenic risk scores identify pathways and patient clusters associated with inflammatory bowel disease risk, severity and treatment response. medRxiv. 2011. doi:10.1101/2021.11.19.21266549. [Google Scholar] [CrossRef]
76. Gettler K, Levantovsky R, Moscati A, Giri M, Wu Y, Hsu NY, et al. Common and rare variant prediction and penetrance of IBD in a large, multi-ethnic, health system-based biobank cohort. Gastroenterology. 2021;160(5):1546–57. doi:10.1053/j.gastro.2020.12.034. [Google Scholar] [PubMed] [CrossRef]
77. Wang MH, Friton JJ, Raffals LE, Leighton JA, Pasha SF, Picco MF, et al. Polygenic risk score predicts risk of primary sclerosing cholangitis in inflammatory bowel disease. BMJ Open Gastroenterol. 2023;10(1):e001141. doi:10.1136/bmjgast-2023-001141. [Google Scholar] [PubMed] [CrossRef]
78. Li Y, Bernstein CN, Xu W, Hu P. Polygenic risk and causal inference of psychiatric comorbidity in inflammatory bowel disease among patients with European ancestry. J Transl Med. 2022;20(1):43. doi:10.1186/s12967-022-03242-9. [Google Scholar] [PubMed] [CrossRef]
79. Xu J, Xu H, Yang M, Liang Y, Peng Q, Zhang Y, et al. New insights into the epigenetic regulation of inflammatory bowel disease. Front Pharmacol. 2022;13:813659. doi:10.3389/fphar.2022.813659. [Google Scholar] [PubMed] [CrossRef]
80. Bastida G, Mínguez A, Nos P, Moret-Tatay I. Immunoepigenetic regulation of inflammatory bowel disease: current insights into novel epigenetic modulations of the systemic immune response. Genes. 2023;14(3):554. doi:10.3390/genes14030554. [Google Scholar] [PubMed] [CrossRef]
81. Salvador-Martín S, Kaczmarczyk B, Álvarez R, Navas-López VM, Gallego-Fernández C, Moreno-Álvarez A, et al. Whole transcription profile of responders to anti-TNF drugs in pediatric inflammatory bowel disease. Pharmaceutics. 2021;13(1):77. doi:10.3390/pharmaceutics13010077. [Google Scholar] [PubMed] [CrossRef]
82. Park SH, Park SH. Personalized medicine in inflammatory bowel disease: perspectives on Asia. J Gastro and Hepatol. 2022;37(8):1434–45. doi:10.1111/jgh.v37.8. [Google Scholar] [CrossRef]
83. Sandritter T, Chevalier R, Abt R, Shakhnovich V. Pharmacogenetic testing for the pediatric gastroenterologist: actionable drug-gene pairs to know. Pharmaceuticals. 2023;16(6):889. doi:10.3390/ph16060889. [Google Scholar] [PubMed] [CrossRef]
84. Miller M, Patel AS, Pasternak B. Rescue therapy with upadacitinib in medically refractory pediatric ulcerative colitis. JPGN Rep. 2024;5(2):197–9. doi:10.1002/jpr3.v5.2. [Google Scholar] [CrossRef]
85. Jefremow A, Neurath MF. Novel small molecules in IBD: current state and future perspectives. Cells. 2023;12(13):1730. doi:10.3390/cells12131730. [Google Scholar] [PubMed] [CrossRef]
86. Tourkochristou E, Mouzaki A, Triantos C. Unveiling the biological role of sphingosine-1-phosphate receptor modulators in inflammatory bowel diseases. World J Gastroenterol. 2023;29(1):110–25. doi:10.3748/wjg.v29.i1.110. [Google Scholar] [PubMed] [CrossRef]
87. Saadh MJ, Mikhailova MV, Rasoolzadegan S, Falaki M, Akhavanfar R, Gonzáles JLA, et al. Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy. Eur J Med Res. 2023;28(1):47. doi:10.1186/s40001-023-01008-7. [Google Scholar] [PubMed] [CrossRef]
88. Qiao Y, Tang X, Liu Z, Ocansey DKW, Zhou M, Shang A, et al. Therapeutic prospects of mesenchymal stem cell and their derived exosomes in the regulation of the gut microbiota in inflammatory bowel disease. Pharmaceuticals. 2024;17(5):607. doi:10.3390/ph17050607. [Google Scholar] [PubMed] [CrossRef]
89. Clayton EW, Evans BJ, Hazel JW, Rothstein MA. The law of genetic privacy: applications, implications, and limitations. J Law Biosci. 2019;6(1):1–36. doi:10.1093/jlb/lsz007. [Google Scholar] [PubMed] [CrossRef]
90. McCrary JM, Van Valckenborgh E, Poirel HA, de Putter R, van Rooij J, Horgan D, et al. Genetic counselling legislation and practice in cancer in EU Member States. Eur J Public Health. 2024;34(4):666–75. doi:10.1093/eurpub/ckae093. [Google Scholar] [PubMed] [CrossRef]
91. Adejumo PO, Aniagwu TIG, Awolude OA, Adedokun B, Kochheiser M, Sowunmi A, et al. Cancer genetic services in a low- to middle-income country: cross-sectional survey assessing willingness to undergo and pay for germline genetic testing. JCO Glob Oncol. 2023;9:e2100140. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF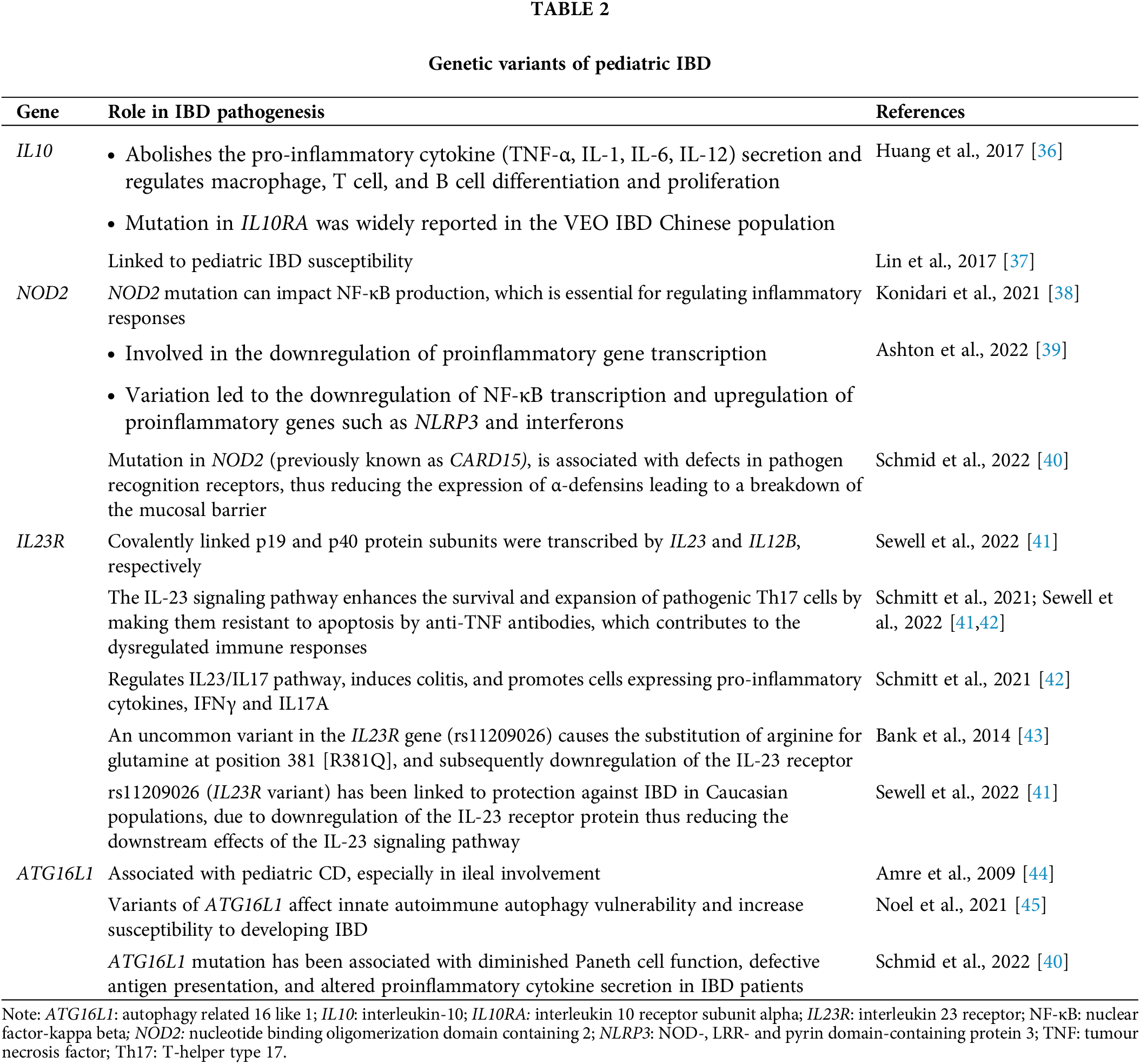
 Downloads
Downloads
 Citation Tools
Citation Tools