 Open Access
Open Access
REVIEW
Therapeutic actions of terpenes in neurodegenerative disorders and their correlations with the regulation and remodeling of the extracellular matrix
1 Laboratory of Science in Innovation and Technology-LACITEC, Department of Biophysics and Physiology, Federal University of Piauí, Teresina, 64049, Brazil
2 Laboratory of Natural Products and Bioprospecting, Natural Sciences Center, State University of Piauí, Teresina, 64018, Brazil
3 Laboratory of Experimental Neurochemistry Research-LAPNEX, Department of Biophysics and Physiology, Federal University of Piauí, Teresina, 64049, Brazil
* Corresponding Author: ANDERSON NOGUEIRA MENDES. Email:
(This article belongs to the Special Issue: Extracellular Matrix in Development and Disease)
BIOCELL 2025, 49(1), 109-125. https://doi.org/10.32604/biocell.2024.058405
Received 11 September 2024; Accepted 27 November 2024; Issue published 24 January 2025
Abstract
Neurodegenerative diseases are a major public health challenge, mainly affecting the elderly population and compromising their cognitive, sensory, and motor functions. Currently, available therapies focus on alleviating symptoms and slowing the progression of these conditions, but they do not yet offer a definitive cure. In this scenario, terpenes emerge as promising natural alternatives due to their neuroprotective properties. These compounds can reduce the formation of protein aggregates, neutralize free radicals, and inhibit pro-inflammatory enzymes, which are crucial factors in the development of neurodegenerative diseases. In addition, terpenes also play an important role in the regulation and remodeling of the extracellular matrix, a key target for improving neuronal functions. Substances such as linalool, pinene, and eugenol, among others, have potential therapeutic effects by modulating inflammatory and oxidative stress processes, the main factors that contribute to the progression of these diseases. Studies suggest that these compounds act on signaling pathways that regulate the extracellular matrix, improving neuronal integrity and, consequently, cognitive and motor function. This work aims to review the potential of terpenes in the treatment of neurodegenerative disorders, with emphasis on their ability to regulate oxidative stress and inflammation, as well as to remodel the extracellular matrix. The interaction between these mechanisms points to the promising use of terpenes as an innovative and natural therapeutic approach to combat these diseases.Keywords
Neurodegenerative diseases are debilitating pathophysiological conditions that result in progressive degeneration and/or death of nerve cells. Neurodegenerative diseases (NDs) represent a group of neuronal disorders in which the degeneration of nerve cells leads to the loss of motor, sensory, and neurological functions [1–3]. The progression of neurodegenerative diseases can lead to motor dysfunctions (ataxia) or mental functioning (dementia). The mechanisms of progression of these diseases show that neurodegeneration may be associated with changes in protein structure, oxidative stress, and neuroinflammatory processes [4,5]. Mainly related to aging, the appearance of NDs is also associated with external stimuli such as smoking, and exposure to pesticides and heavy metals [4].
More than 3 billion people live with some neurological disorder [6], and there are projections of 75 million new cases of Alzheimer’s Disease (AD) by 2030 [7]. In addition to AD, other neuronal disorders such as Parkinson’s Disease (PD), Huntington’s Disease (HD), and Amyotrophic Lateral Sclerosis (ALS) represent a major public health problem. There is still no cure for these diseases and treatments currently include medications and non-drug approaches that aim to reduce symptoms and progression of neuronal damage [8].
Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s share biochemical pathways, especially those related to protein homeostasis, oxidative stress, and inflammation. In Alzheimer’s, the deposition of beta-amyloid plaques and the formation of neurofibrillary tangles of hyperphosphorylated tau protein are central mechanisms in neuronal dysfunction, resulting in synaptic damage and cell death [9]. In Parkinson’s, the affected pathway involves the accumulation of alpha-synuclein, which leads to the formation of Lewy bodies and the progressive loss of dopaminergic neurons in the substantia nigra [10]. In Huntington’s disease, the mutation in the HTT gene results in the production of mutant huntingtin, forming toxic protein aggregates that affect axonal transport and mitochondrial function, exacerbating neurodegeneration [11,12].
The extracellular matrix (ECM) in the brain plays a key role in regulating synaptic plasticity and maintaining tissue homeostasis. In Alzheimer’s disease, there is remodeling of the ECM, with excessive deposition of proteoglycans, which bind to beta-amyloid, favoring its aggregation and deposition in the brain parenchyma, exacerbating disease progression [13]. Furthermore, ECM degradation affects synapse integrity and neuronal plasticity, promoting the cognitive decline associated with the disease [13–15]. These changes suggest that the ECM is not only a structural support but also an active mediator in the pathogenesis of Alzheimer’s disease.
In Parkinson’s disease, changes in the ECM include a decrease in structural components such as collagen and laminin, which directly affects the survival of dopaminergic neurons [16]. Inadequate regulation of matrix metalloproteinases (MMPs), which degrade the ECM, is another critical factor, leading to a pro-inflammatory environment that aggravates neuronal degeneration [17]. The interaction of alpha-synuclein with ECM components, such as laminin, promotes its aggregation, which accelerates the degenerative process in Parkinson’s [18–21].
In Huntington’s disease, the ECM also undergoes changes that compromise synaptic signaling and increase excitotoxicity, one of the causes of neuronal death exacerbated by the disease. Proteoglycans such as chondroitin sulfate play a significant role in the progression of synaptic dysfunction, influencing neuronal plasticity and regeneration [22–24]. Furthermore, the ECM modulates the activity of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), which is crucial for neuronal survival and is decreased in Huntington’s disease [25–27]. Thus, changes in the ECM are directly linked to the acceleration of neuronal degeneration in these diseases.
In the search for safe and effective substances that complement or replace traditional treatments and help improve patients’ quality of life, terpenes, secondary metabolites found in medicinal plants, stand out because they have a neuroprotective function and are capable of interacting with neurotransmitters and signaling molecules considered therapeutic targets in the treatment of neurological disorders [28]. Terpenes such as pinene, linalool, and eugenol are substances with high antioxidant and anti-inflammatory potential and can be used as a therapeutic strategy in neurodegenerative diseases [29,30].
The extracellular matrix (ECM) also emerges as an important target for the development of therapies that aim to preserve brain function and slow the progression of these conditions, since this complex network of proteins and polysaccharides is responsible for the structural integrity of brain tissue and regulation of cell signaling [31]. In this article, we discuss data on the investigation of terpenes as potential preventive and therapeutic agents in neurodegenerative diseases, focusing on their mechanisms of antioxidant and anti-inflammatory action and modulation of ECM composition.
Major neurodegenerative diseases
The major neurodegenerative diseases include Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Huntington’s disease (HD), which are characterized by progressive and irreversible degeneration of the nervous system, resulting in loss of neuronal function [32–34].
Alzheimer’s disease, the most common of all, is characterized by the accumulation of extracellular plaques of beta-amyloid and intracellular neurofibrillary tangles of hyperphosphorylated tau protein [35]. These abnormalities lead to synaptic dysfunction, inflammation, and neuronal death, particularly in brain regions associated with memory and cognition, such as the hippocampus and cerebral cortex [36]. Approximately 50 million people live with dementia, with AD accounting for 60%–70% of cases [37]. The incidence increases with age, affecting approximately 5%–10% of people over 65 years of age and up to 50% of people over 85 years of age [38–42].
PD, marked by the progressive loss of dopaminergic neurons in the substantia nigra of the brainstem, results in a reduction in dopamine levels in the striatum, with the accumulation of Lewy bodies [43]. Lewy bodies are clumps of alpha-synuclein protein that normally performs several cellular functions, but in PD, it aggregates abnormally, forming these accumulations within the cytoplasm of neurons, a hallmark pathological feature that is used for the postmortem diagnosis of PD. In addition to Lewy bodies, this neurological condition involves other pathological processes, such as mitochondrial dysfunction, oxidative stress, and inflammation. These processes contribute to neuronal death and disease progression [44]. The annual incidence rate of Parkinson’s disease is about 13.5/100,000 and may affect 2%–3% of the population over 65 years of age [45,46].
ALS involves the progressive degeneration of motor neurons in the brain and spinal cord, but the exact cause is unknown, as it involves a combination of genetic and environmental factors [47]. Proposed mechanisms include oxidative stress, mitochondrial dysfunction, accumulation of misfolded proteins, and excitotoxicity [48]. The worldwide prevalence of this disease is 2 to 5 people per 100,000 population and the incidence is approximately 2 cases per 100,000 people per year [49]. HD is caused by a mutation in the HTT gene, which results in an abnormal expansion of CAG trinucleotides [50]. This leads to the production of a mutant huntingtin protein, which accumulates in neurons, causing dysfunction and cell death, especially in the striatum and cerebral cortex. Proteostatic dysfunction and neuroinflammation are also important features [51].
Table 1 highlights the main cellular mechanisms associated with major neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, Huntington’s, and Amyotrophic Lateral Sclerosis (ALS). These diseases are characterized by the aggregation of specific proteins that accumulate in neurons, resulting in cellular dysfunction and, eventually, neuronal death. In Alzheimer’s, for example, beta-amyloid and tau proteins accumulate in areas of the brain related to memory, causing synaptic dysfunction and inflammation [35,36].
In Parkinson’s disease, the aggregation of alpha-synuclein in dopaminergic neurons of the substantia nigra leads to oxidative stress and mitochondrial dysfunction, which results in the death of these cells, affecting the motor control of patients [43,52]. In Huntington’s disease, the mutant huntingtin protein accumulates in neurons of the striatum and cerebral cortex, promoting proteostatic dysfunction and neuroinflammation, and contributing to cognitive and motor decline [50,51].
Amyotrophic lateral sclerosis (ALS) involves the aggregation of several proteins, such as TDP-43 and SOD1, which affect motor neurons in the brain and spinal cord, causing oxidative stress, mitochondrial dysfunction, and excitotoxicity [47,48]. In all these cases, the accumulation of defective proteins and the resulting cellular damage are central to the pathology of neurodegenerative diseases, illustrating the importance of understanding these processes for the development of therapies.
Main terpenes used in neurodegenerative diseases
The treatment of neurodegenerative diseases currently involves the use of drugs that act as inhibitors of enzymes related to neurodegenerative processes. In the treatment of AD, for example, acetylcholinesterase inhibitors and glutamate receptor antagonists such as memantine are used, while in PD, drugs that increase the synaptic availability of dopamine are used [53]. These therapeutic approaches, however, do not yet cure the diseases, representing only symptom management.
In the search for substances that act in the prevention or treatment of neuronal disorders, medicinal plants, and their derivatives stand out as a choice that can overcome the limitations of conventional treatments [54]. Natural products have been gaining prominence because they contain phytochemical compounds capable of acting in the maintenance of chemical substances present in neurons and interacting with receptors or structures involved in antioxidant and anti-inflammatory processes [55].
Terpenes have been investigated in preclinical studies due to their therapeutic potential in neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and other conditions involving neuronal degeneration [56]. Studies with animal models have shown that compounds such as limonene, camphene, and borneol have significant neuroprotective effects [57,58]. These compounds act primarily through antioxidant and anti-inflammatory mechanisms, which are crucial to combat oxidative stress and chronic inflammation associated with the progression of these diseases [56–58].
The phytoconstituents of plants are secondary metabolites that have a protective action against herbivores, temperature conditions, and light. These active principles are the ones that exhibit therapeutic activity in the human body [59,60]. The bioactivity of substances present in medicinal plants, such as terpenes and flavonoids, justifies their widespread use in the production of medicines derived or synthesized from these natural products and with effective action in the treatment of neurodegenerative disorders [61].
Terpenes are secondary metabolites that contain more than 30,000 compounds derived from isoprene (C5H8) [62]. The inclusion of functional groups in the hydrocarbon chains of isoprene derivatives gives rise to terpenoids. Terpenes and terpenoids can be classified according to the number of carbons per molecule, with monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) being the most abundant in medicinal plants [63]. This class of substances has antimicrobial, anti-inflammatory, antioxidant, and neuroprotective activity [64,65].
Terpenes synthesized in the Cannabis sativa L. plant are notable for their broad neuroprotective activity and are considered in the development of medicinal formulations aimed at neurodegenerative diseases. In a study with terpenoids from C. sativa, Laws III and Smid in 2024 identified a significant neuroprotective effect of α-pinene and β-pinene against exposure to amyloid β, associated with an inhibition of Aβ1-42 fibrillization and density [66]. α-pinene, linalool, phytol, and trans-nerolidol isolated from the same plant also showed immunomodulatory activity, suggesting the possibility of using these active principles as anti-inflammatory substances in the treatment of neurodegenerative disorders [29].
In a study of an inflammatory model induced in animals by trimethyltin, the extract of C. longa showed neuroprotective activity. Doses of 200 mg/kg of this extract showed inhibitory activity of oxidative stress, reducing the levels of malondialdehyde (MDA) in the brain and blood plasma, increasing the levels of reduced glutathione (GSH) and enzymatic activity of superoxide dismutase (SOD) and glutathione peroxidase (GP×) [67]. Analysis of the aqueous and ethanolic extracts of C. longa shows the presence of terpenes such as carvotanacetone, thymol, eugenol, β-pinene, 2,8-epoxy-5-hydroxybisabola-3,10-dioen9-one and 2-methyl-hept-2-ene-4-one [68,69].
Other terpenes, isolated or associated with other constituents in plant extracts, exhibit anti-inflammatory, antioxidant, and neuroprotective activity. Table 2 summarizes the mechanisms involved. The table presents several terpenes and their respective neuroprotective properties, demonstrating how these compounds can play a crucial role in the prevention and treatment of neurodegenerative diseases. Each terpene has a distinct mechanism of action, ranging from anti-inflammatory activities, antioxidant activities, and modulation of oxidative stress biomarkers. These activities are particularly important because inflammation and oxidative stress are closely linked to neurodegeneration.
DPPH (2,2-diphenyl-1-picrylhydrazyl) method (2,2-diphenyl-1-picrylhydrazyl assay); NOD-like receptor protein 3 (NLRP3); NSC-34 cells (Motor Neuron-Like Cells); FRAP (Ferric Reducing Antioxidant Power), ABTS (2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) and CUPRAC (upric Reducing Antioxidant Power) test.
Diterpene and triterpene compounds extracted from Schefflera rubriflora demonstrate anti-inflammatory activity by moderating the pro-inflammatory cytokines TNFα and IL-6, which may reduce inflammation in the central nervous system [70]. Aucubin, an isolated compound, also stands out for its ability to reduce oxidative stress and inflammation, especially in models of induced brain injury [71]. These mechanisms are essential to attenuate the progression of neuronal damage that can lead to neurodegenerative diseases.
Lupeol and campesterol, both extracted from the stem bark of Lannea humilis, show significant antioxidant activity, reducing oxidative stress by 70% and 75%, respectively, by the DPPH method [64]. Additionally, 3β-trans-p-coumaroyl acid and its derivatives, extracted from Osmanthus fragrans leave, exhibit anti-inflammatory activities that inhibit nitric oxide production and reduce the levels of pro-inflammatory cytokines, such as TNFα, IL-6, and IL-8 [72]. These antioxidant and anti-inflammatory properties may protect neurons from degeneration caused by chronic inflammatory processes.
Ginkgolide B, derived from Ginkgo biloba, is notable for its ability to reduce neuroinflammation by blocking the activation of the NLRP3 inflammasome, a crucial component of the inflammatory response. In addition, it promotes the polarization of microglia to an M2 state, which is associated with inflammation resolution and neuroprotection [73]. Another important compound is β-caryophyllene, which has antioxidant and anti-inflammatory activity and negatively acts on the nuclear factor kappa B (NF-κB) pathway, a key regulator of the inflammatory response [77]. These effects suggest that these terpenes may modulate important cellular pathways involved in chronic inflammation associated with neurodegenerative disorders.
In addition to antioxidant and anti-inflammatory activities, other terpenes such as eugenol, linalool, carvacrol, and α-terpineol, isolated from various plant sources, exhibit multiple neuroprotective activities. These compounds inhibit enzymes related to oxidative stress and demonstrate properties that may protect neurons against the toxicity of amyloid β, a major causative agent of diseases such as Alzheimer’s [30]. The combined action of these compounds, regulating both inflammation and oxidative stress, is promising for the treatment of neurodegenerative diseases, in which these processes play critical roles.
Chronic inflammation and oxidative stress are central pathological processes in conditions such as Alzheimer’s, Parkinson’s, and ALS, and the ability of terpenes to modulate these processes suggests that they may be used as natural therapeutic agents. By inhibiting pro-inflammatory cytokines, reducing oxidative stress, and protecting neurons against toxins, these compounds offer a promising approach to mitigating the progression of these diseases.
Fig. 1 shows the therapeutic actions of terpenes in four neurodegenerative diseases. In PD, represented by a dopaminergic neuron with Lewy bodies, terpenes show neuroprotective activity, combating oxidative stress, reducing free radicals, and decreasing glial inflammation. In HD, which is characterized by neuronal loss in the striatum, terpenes increase neuronal survival, inhibit apoptosis, and neutralize oxidative stress, protecting brain tissue. In ALS, where motor neuron degeneration and muscle atrophy occur, terpenes preserve motor neurons, delay neurodegeneration, and reduce glial inflammation and oxidative stress. In AD, illustrated by beta-amyloid plaques and neurofibrillary tangles, terpenes prevent protein aggregation, exert strong antioxidant activity, and reduce microglial inflammation, combating disease progression.
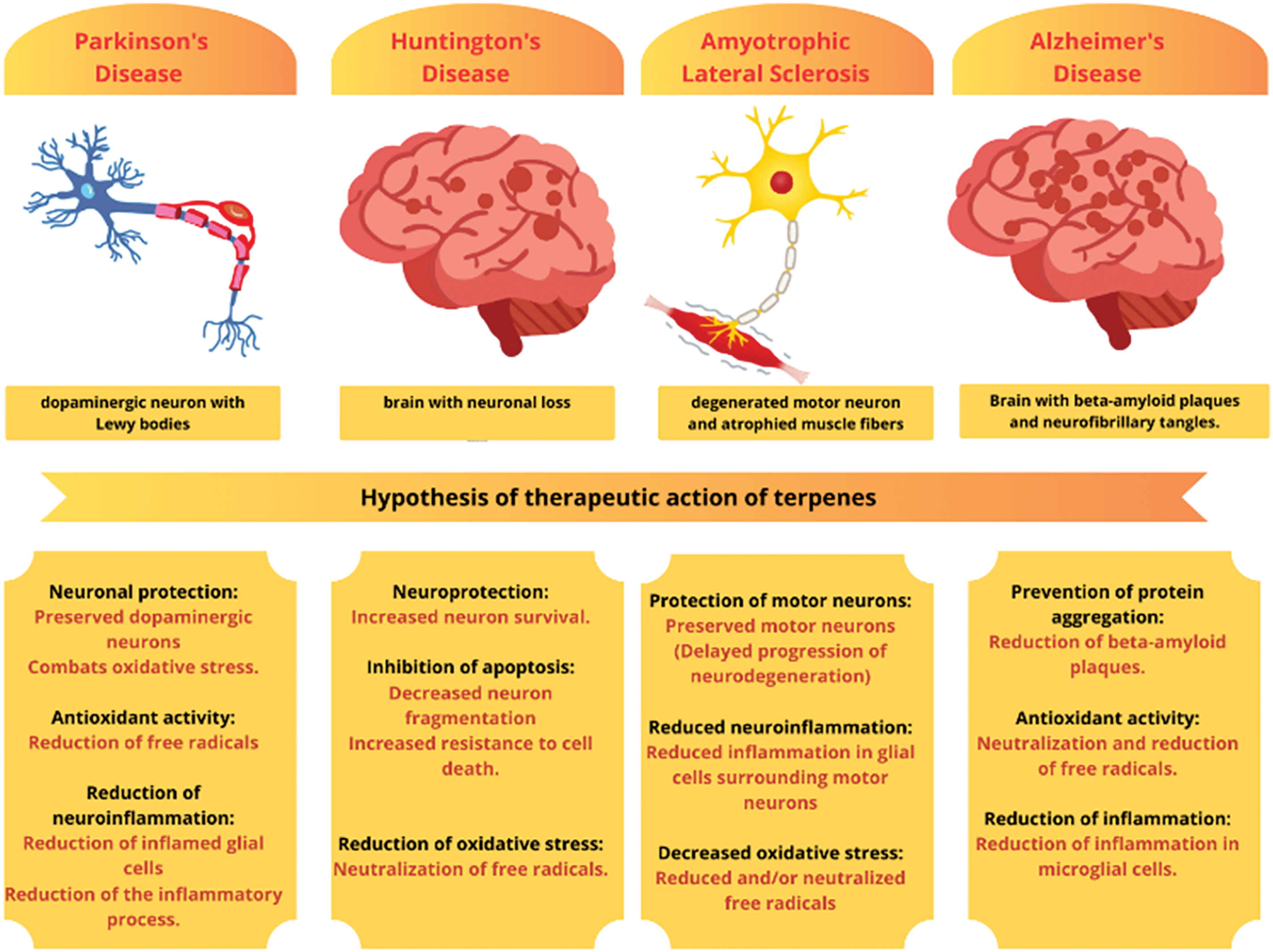
Figure 1: Therapeutic actions of terpenes in neurodegenerative diseases. By Canva.
According to the literature, terpenes can be considered potential antioxidant and anti-inflammatory agents in several neurodegenerative diseases, evidencing their multifaceted action in the brain and their possible therapeutic application [28,78,79]. These actions suggest that terpenes may be promising in attenuating neuronal damage and improving the quality of life of patients with neurodegenerative diseases.
Limonene, a terpene present in citrus fruit essential oils, has shown efficacy in reducing inflammation in animal models of lung injury and colitis, in addition to reducing brain inflammation associated with neurodegenerative diseases. In studies with Alzheimer’s models, limonene helped to reduce the accumulation of beta-amyloid plaques, one of the pathological characteristics of this disease [57,58]. Similarly, borneol has shown a potential to reduce neurotoxicity by modulating pathways such as NF-κB and mitogen-activated protein kinase (MAPK), which are central to the inflammatory process [80–82].
Neurodegenerative diseases are associated with aging or external and environmental factors. Studies with neuronal models indicate that increased levels of oxidative stress, chronic neuroinflammation, and accumulation of aggregated proteins lead to the onset of these diseases [83]. Considering the great influence that oxidative and inflammatory processes have on the worsening of neuronal disorders, a good strategy for the development of new therapies is the search for molecules that act as antioxidants and inhibitors of pro-inflammatory enzymes.
Fig. 2 summarizes the main therapeutic properties of terpenes, focusing on their antioxidant, anti-inflammatory, and neuroprotective activities. Terpenes demonstrate the ability to capture reactive oxygen species (ROS) and free radicals, in addition to inhibiting enzymes involved in oxidative stress, such as NADPH oxidase and xanthine oxidase [30,64,77]. This antioxidant effect is crucial to protect nerve cells from damage caused by oxidative stress, which is directly associated with cellular aging and several neurodegenerative diseases.
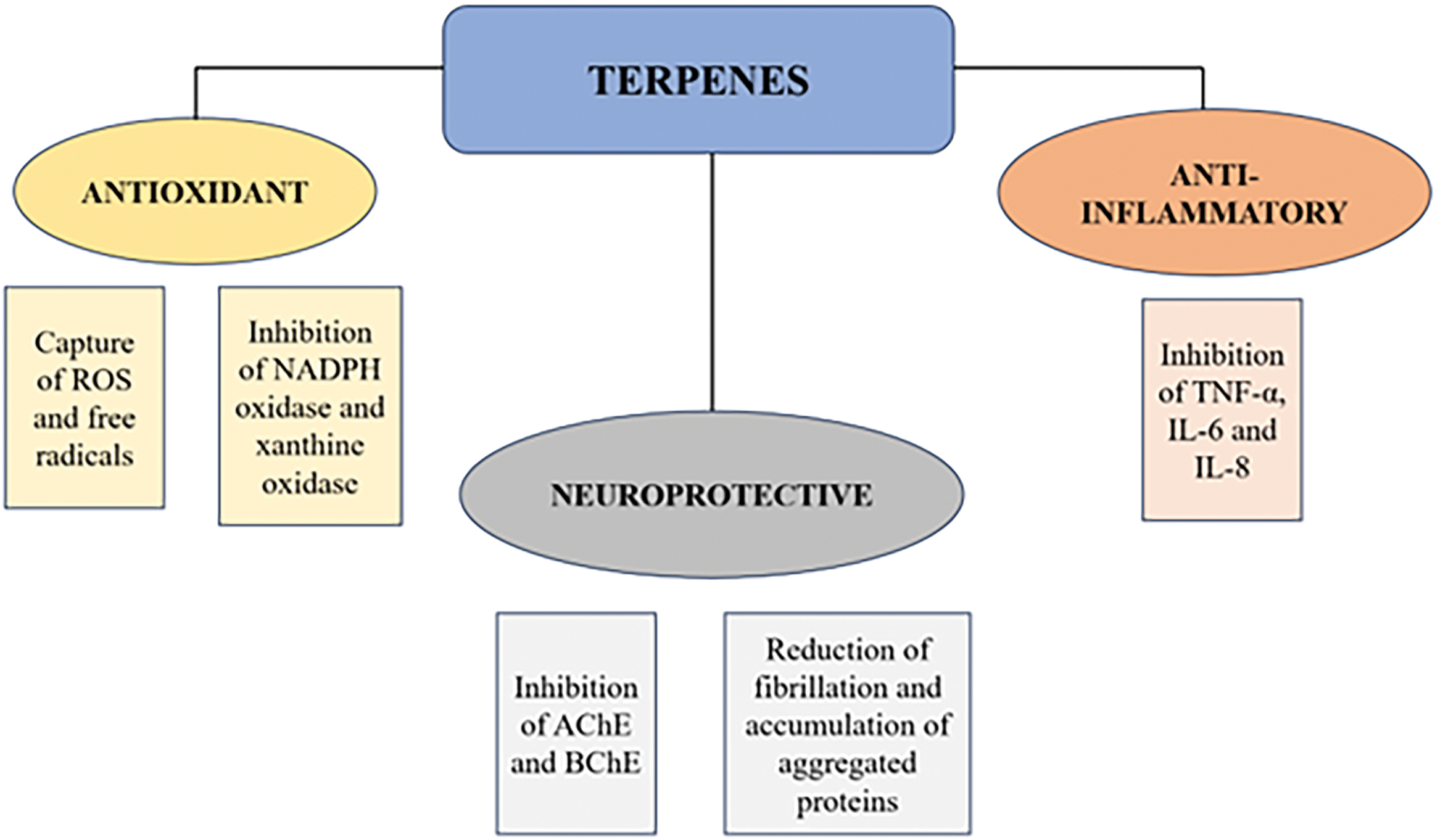
Figure 2: Therapeutic actions of terpenes: the role that terpenes play as well as the role of candidate molecules for neuroprotection.
Terpenes inhibit inflammatory cytokines such as TNF-α, IL-6, and IL-8, which are key mediators in the inflammatory process. Chronic inflammation in the central nervous system is a common feature in neurodegenerative diseases, and modulation of this response by terpenes may contribute to reducing disease progression [70,72,74,75,77]. The neuroprotective activity of terpenes involves the inhibition of the enzymes acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), in addition to the reduction of fibrillation and the accumulation of aggregated proteins, processes that are critical in the preservation of neuronal function and in the prevention of diseases such as Alzheimer’s and Parkinson’s.
The presence of reactive oxygen species, ROS, is important for the maintenance of physiological processes essential to human health, but when present in high concentrations, free radicals and these reactive species can trigger oxidative stress [84]. In oxidative stress, species such as superoxide, hydrogen peroxide, and hydroxyl and hydroperoxyl radicals can cause damage to DNA, proteins, and lipids, resulting in damage to motor and brain functions [85].
The in vitro antioxidant action of several classes of terpenes has been demonstrated by the ability of these phytoconstituents to eliminate free radicals and reactive oxygen species, in addition to inhibiting enzymes associated with the production of free radicals such as NADPH oxidase and xanthine oxidase, restoring the endogenous antioxidant system, highlighting the regulation of the Nuclear factor erythroid-2-related factor 2 (Nrf2) signaling pathway [86,87] (Fig. 2). Some terpenes have an unsaturated allylic portion in their structure that gives these substances a high potential for auto-oxidation, justifying their antioxidant capacity and free radical scavenging properties [88,89].
The antioxidant action of terpenes modulates the levels of ROS and free radicals, thus reducing oxidative stress (Fig. 3). Oxidative stress is associated with several pathological processes, such as lipid peroxidation (measured by increased MDA), autophagy, and cellular apoptosis, in addition to protein aggregation, factors that contribute to the progression of neurological diseases. By inhibiting these processes, terpenes demonstrate neuroprotective potential and may prevent or delay the development of neurological disorders caused by the accumulation of oxidative stress.
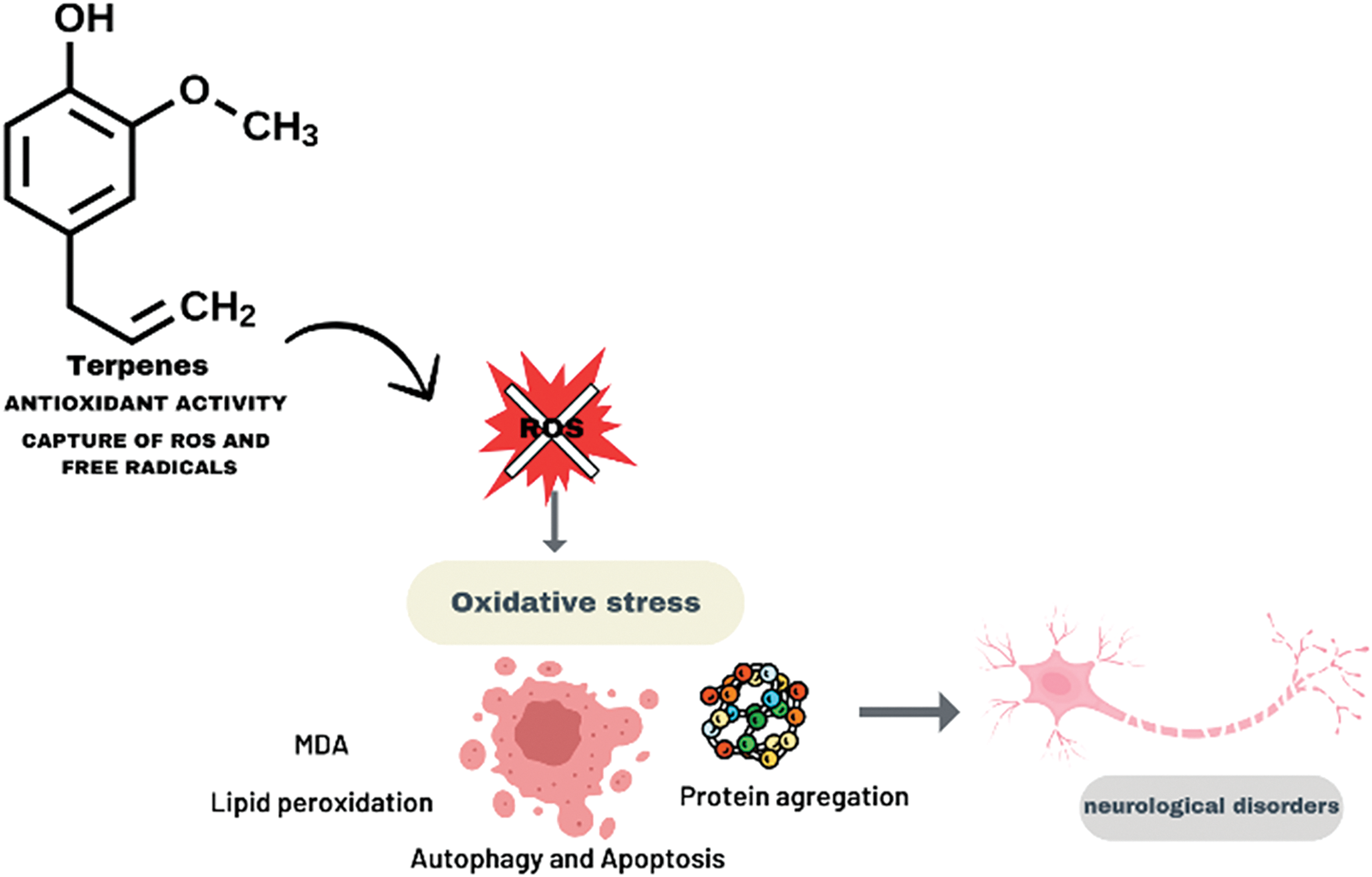
Figure 3: Antioxidant activity of terpenes: action in reducing oxidative stress in neurological diseases. By Canva.
Neuroinflammation is one of the three factors in the worsening of neurodegenerative diseases [90]. Although the inflammatory response is an effective strategy of the immune system in protecting the brain, the dysregulation of this process can result in significant impacts on the central nervous system. An example of this imbalance is the high levels of pro-inflammatory cytokines associated with the activation of microglia and the consequent increase in the permeability of the blood-brain barrier, facilitating the entry of toxins and accelerating the progression of diseases such as Alzheimer’s [90,91].
Terpenes can also act to reduce inflammatory processes, which are closely associated with the worsening of neurodegenerative diseases (Fig. 4). In the anti-inflammatory action of these phytoconstituents, inhibition of pro-inflammatory cytokines is observed through suppression of the MAPK pathway in neurons, negative regulation of inflammatory chemokines such as CXCL10 and CXCL1, in addition to influencing macrophage polarization and inflammasome modulation [92–94].
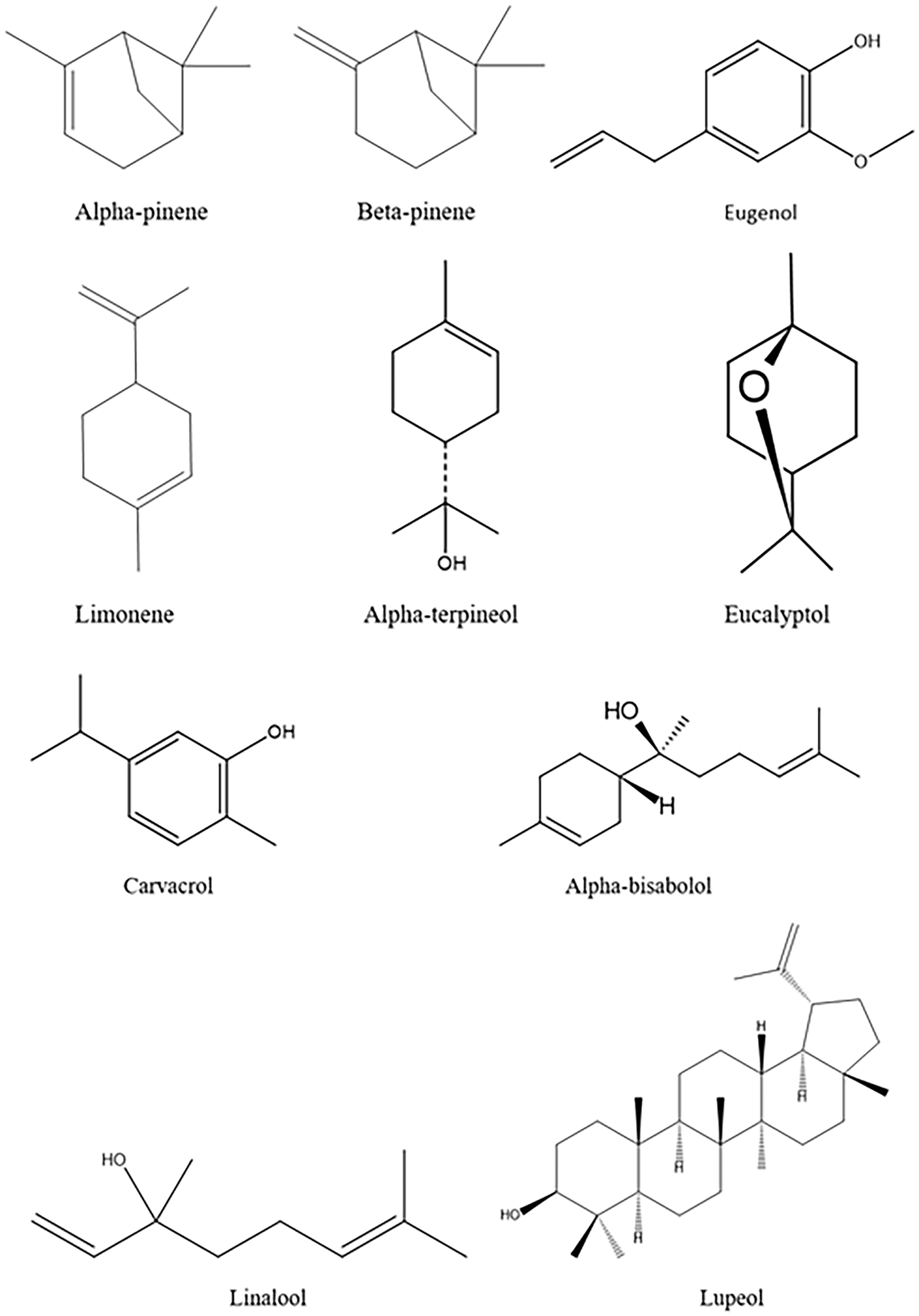
Figure 4: Chemical structure of some terpenes with antioxidant, anti-inflammatory, and neuroprotective actions.
Terpenes have been widely studied as alternative treatments for a variety of inflammatory conditions, including asthma, arthritis, and skin inflammation, as well as neuroinflammation associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s [79,95,96]. Inflammation is a key pathological factor in the progression of these diseases, driven by microglial cells, which release pro-inflammatory cytokines and ROS in response to injury or pathogens [97,98]. Studies have shown that terpenes such as Linalool and D-limonene are effective in suppressing this inflammatory response, exhibiting neuroprotective properties through the inhibition of inflammatory pathways such as NF-κB activation and nuclear translocation [78].
D-limonene, for example, has been associated with the reduction of inflammatory cytokines such as IL-1 and TNF-α, which are linked to neuroinflammation and depression [78]. Furthermore, studies in mice reveal that D-limonene has antidepressant effects due to the decrease in nitrite levels in the hippocampus, suggesting a direct impact on neuroinflammation [99]. Other terpenes, such as ginkgolides extracted from the Ginkgo biloba tree, also show promising antioxidant and anti-inflammatory effects, protecting neurons from synaptic damage and rescuing neuronal function in Alzheimer’s models, as evidenced by studies with ginkgolide A and B [100]. These compounds are especially effective in downregulating the Toll-like receptor 4/NF-κB pathway, a central mechanism in neuroprotection [78,100].
Extracellular matrix in neurodegenerative diseases
The extracellular matrix (ECM) is a complex network of proteins that fills the space between cells in multicellular tissues and is formed mainly by collagens, glycoproteins, and proteoglycans [101]. The ECM plays several roles such as structural support, regulation of cell functions, and signaling, and may be associated with the modulation and regulation of molecules in the brain [20].
The ECM in the central nervous system (CNS) plays a key role in the structural support and regulation of cellular processes such as synaptic adhesion, proliferation, and plasticity. The ECM is composed of glycoproteins such as laminin and tenascin, proteoglycans such as chondroitin sulfate proteoglycans (CSPGs), and agrin, collagen, and hyaluronan, forming a microenvironment that influences neuronal development and function [102,103]. These components are essential for maintaining synaptic stability and plasticity, with CSPGs, for example, playing critical roles in inhibiting axonal regeneration after injury [104].
Changes in the ECM and its components can affect the function and survival of neurons, influencing the pathology and progression of neurodegenerative diseases. In diseases such as Alzheimer’s, there is an increase in the expression of specific proteoglycans that can interfere with synaptic plasticity and neural regeneration. Heparan sulfate proteoglycans (HSPGs) have their expression patterns associated with neuroinflammation in diseases such as AD, where they have been identified in beta-amyloid plaques [105]. The chemical structure of proteoglycans explains their vast capacity to interact with other proteins since they contain molecules of variable length with different degrees and positions of sulfation [106].
ECM dysregulation is widely observed in neurodegenerative diseases such as Alzheimer’s and Parkinson’s. In Alzheimer’s disease, CSPGs accumulate around amyloid plaques, which prevents the effective clearance of these plaques, contributing to neurotoxicity and synaptic dysfunction [107]. Similarly, in Parkinson’s disease, abnormal ECM remodeling, including increased collagen production and laminin dysfunction, is associated with degeneration of the substantia nigra and loss of dopaminergic neurons [108–110]. These changes in the ECM impair synaptic plasticity and promote progressive neuronal loss.
The ECM also interacts directly with neuroinflammation processes, a crucial aspect in many neurodegenerative diseases. During neuroinflammation, immune cells such as microglia and astrocytes are activated and release matrix metalloproteinases (MMPs), which degrade ECM components [110–113]. In multiple sclerosis and Alzheimer’s disease, increased MMP activity results in ECM degradation, promoting a cycle of chronic inflammation and neurodegeneration [114–116]. These mechanisms show that the ECM is not only a structural support but also actively participates in disease progression by interacting with immune cells and contributing to the inflammatory environment.
Neuroinflammation, common in many neurodegenerative diseases, can lead to the degradation of the ECM through the activation of enzymes such as MMPs. Degradation of the ECM can result in loss of structural and functional support for neurons. A study showed that the metalloproteinases MMP-3 and MMP-9 influence the aggregation behavior of the tau protein, implying a potential role in neurodegeneration [117]. Modulation of MMPs, as well as interventions to restore the ECM, have shown the potential to reduce neuroinflammation and promote neuronal regeneration [118–121]. For example, inhibition of MMPs in animal models resulted in reduced ECM degradation and reduced neuroinflammation [102,110]. Furthermore, ECM-based biomaterials are being investigated as a strategy to provide structural support to damaged neural tissue, facilitating axonal regeneration [113,122].
The accumulation of misfolded and aggregated proteins in the extracellular spaces can also lead to cellular dysfunction and neuronal death, contributing to the progressive decline of motor and cognitive functions, and leading to the onset and worsening of neurodegenerative diseases. Misfolded protein variants are transferred from an affected brain region to a healthy region, inducing the incorrect folding of other proteins [85,106].
Inadequate folding of tau and beta-amyloid (Aβ) proteins and disorganization of the ECM, for example, characterize in part the typical pathology of AD [123], while the proteins a-synuclein and TDP-43 are associated with the development of PD and ALS, respectively [124]. Understanding the role of the ECM in neuronal disorders allows the development of new therapeutic strategies that seek to restore or alter its composition and function, potentially improving neuronal performance or slowing disease progression [106]. New substances are being investigated for the treatment of these conditions, as they include mechanisms for reducing oxidative stress and anti-inflammatory action, which suggests that they can preserve the integrity and stability of the ECM, in addition to acting indirectly to improve synaptic function [125].
The ECM is essential in neurodegeneration, influencing synaptic plasticity, neuronal regeneration, and neuroinflammation. Dysregulation of its components, such as CSPGs and collagen, is associated with Alzheimer’s and Parkinson’s. The interactions between the ECM and neuroinflammation remain a promising field of investigation, with studies suggesting that modulation of the ECM may provide novel therapeutic approaches to attenuate the progression of these diseases.
Action of terpenes in the regulation and remodeling of the extracellular matrix
The Extracellular Matrix (ECM) is a three-dimensional network composed of fibrous proteins, glycoproteins, and proteoglycans, which provide structural and functional support for cells. The ECM plays a very important role in the regulation of several cellular processes, including cell proliferation, migration, differentiation, and survival [102]. Dysfunctions in the ECM are associated with several pathologies, including neurodegenerative diseases such as Alzheimer’s and Parkinson’s [102].
Terpenes are a diverse class of natural compounds, known for their biological properties, including anti-inflammatory, antioxidant, and neuroprotective activities [126]. Terpenes, such as linalool and nerolidol, have been shown to have modulatory effects on the ECM, primarily through the regulation of enzymes that degrade matrix components, such as matrix metalloproteinases (MMPs). Dysregulation of these enzymes and proteins can lead to ECM imbalances, contributing to the progression of neurodegenerative diseases [127]. Linalool, for example, has been shown to inhibit the expression of MMPs, reducing ECM degradation and promoting a more stable and less inflammatory cellular environment [126].
In addition, terpenes can influence the expression of ECM proteins, such as collagen and laminin, which are essential for the structural and functional integrity of the matrix. The interaction between terpenes and the ECM can have both positive and negative effects on neurodegenerative diseases. On the positive side, modulation of the ECM by terpenes can reduce inflammation and oxidative stress, two critical factors in the pathogenesis of neurodegenerative diseases. For example, linalool has been shown to reduce the production of ROS and protect neuronal cells against oxidative damage [128]. This neuroprotective effect is particularly relevant for diseases such as Alzheimer’s, where oxidative stress plays a central role in disease progression [129].
Fig. 5 illustrates the interaction of three terpenes (linalool, limonene, and nerolidol) with ECM components, such as collagen and fibronectin, in addition to their modulatory actions on inflammatory factors and MMPs. According to the literature, linalool acts as an inhibitor of MMP-2 and MMP-9, which are enzymes responsible for the degradation of collagen and other ECM components. The inhibition of these enzymes suggests that linalool may help preserve the integrity of the ECM, preventing cellular damage and exacerbating inflammation, which is relevant in neurodegenerative conditions [127,130–132]. Limonene, in turn, is shown to stimulate the synthesis of collagen and fibronectin, both essential for the maintenance of ECM structure and function [99,133,134]. These protective effects on the ECM may be related to limonene’s ability to promote cell regeneration and healing [57,133–135].
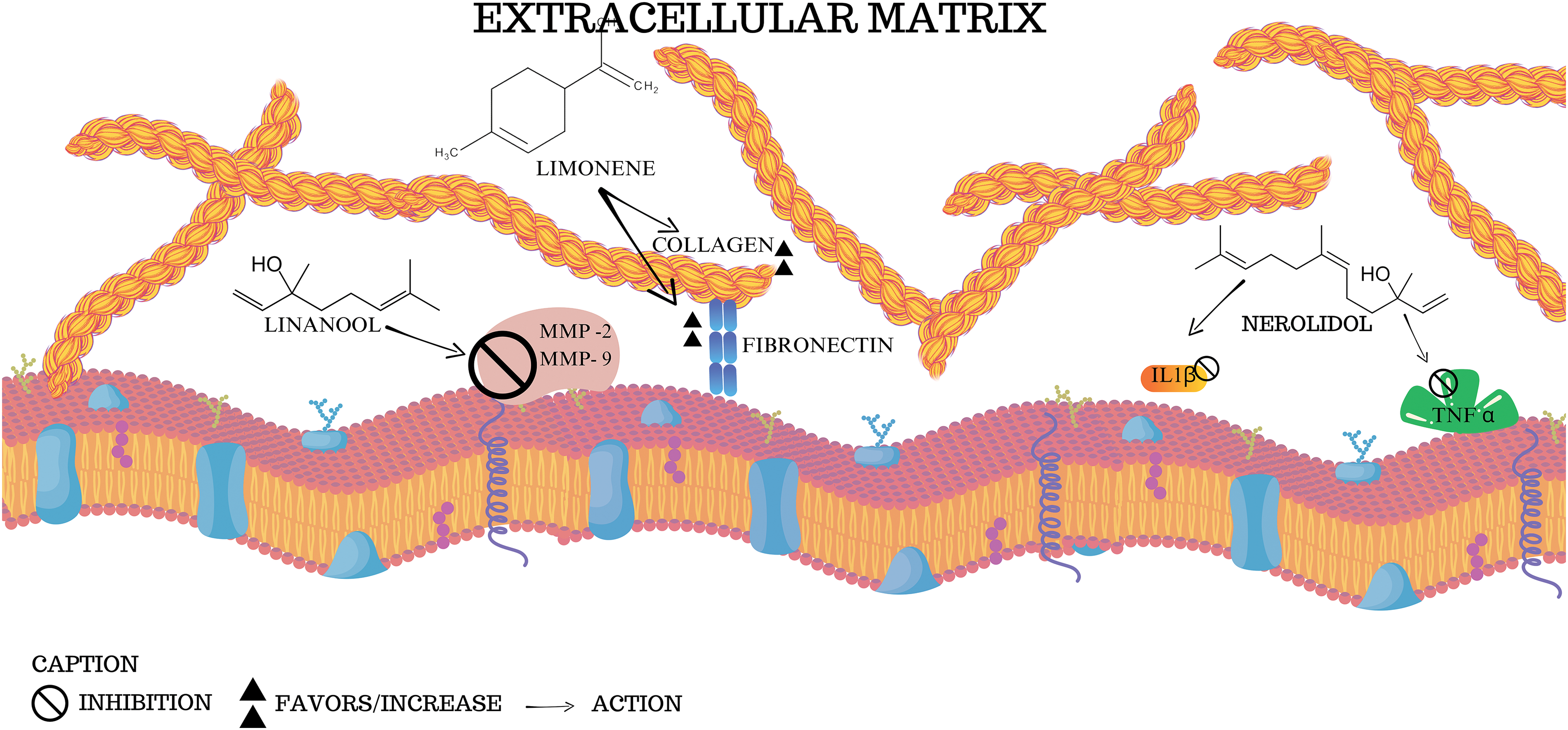
Figure 5: Interactions of terpenes with the extracellular matrix: effects on collagen, fibronectin and inflammation. The figure suggests the interactions represented by the compounds limonene, linalool, and nerolidol with components of the extracellular matrix, in addition to highlighting the effects on collagen, fibronectin, and inflammatory molecules (MMP, TNFα, and IL1β). By Canva.
Nerolidol, in turn, is shown to inhibit pro-inflammatory cytokines such as IL1β and TNFα, molecules that play critical roles in the exacerbated inflammatory response in several diseases, including neurodegenerative ones [29,136–138]. By reducing the activity of these cytokines, nerolidol can mitigate the inflammatory process that aggravates ECM degradation and contributes to neuronal dysfunction [127,139]. Thus, the figure summarizes the anti-inflammatory and protective effects of natural terpenes on the extracellular matrix and suggests their therapeutic potential to prevent the progression of neurodegenerative diseases.
The antioxidant effects of terpenes are crucial in protecting against oxidative stress, a key factor in neurodegenerative diseases. Oxidative stress results in cellular damage and ECM dysfunction, contributing to the progression of diseases such as Alzheimer’s and Parkinson’s [123]. Compounds such as (R)-(-)-linalool have been shown to significantly reduce the production of ROS, thereby protecting neuronal cells from oxidative damage [126].
Neuroinflammation is a central process in the pathogenesis of many neurodegenerative diseases. Terpenes such as nerolidol have been shown to modulate the inflammatory response by reducing glial cell activation and the production of pro-inflammatory cytokines [140,141]. This modulation of inflammation may result in a less damaging environment for neurons and, consequently, in the preservation of ECM integrity.
Terpenes can interact with several receptors and signaling pathways that regulate the ECM [131]. These interactions can alter the expression of ECM components, such as collagen and fibronectin, influencing the structure and function of the matrix. The use of terpenes as therapeutic agents in neurodegenerative diseases has shown promising results. Studies indicate that compounds such as limonene and linalool can not only protect against neurodegeneration but also promote neural regeneration and synaptic plasticity [57,58,126,128,129]. This suggests that terpenes may play a dual role in the protection and repair of damaged neural tissues.
Despite the potential benefits, the therapeutic application of terpenes faces several challenges [136]. Furthermore, the appropriate dosage and route of administration must be carefully determined to maximize therapeutic benefits and minimize potential adverse effects. Future research should focus on a detailed understanding of the molecular mechanisms by which terpenes modulate the ECM and their interaction with neural cells. Rigorous clinical trials are needed to validate the effects observed in preclinical models and establish effective and safe therapeutic protocols [126].
The integration of terpenes with other therapeutic approaches, such as antioxidants and anti-inflammatories, may enhance the beneficial effects on the ECM and neurons (Fig. 6). This multimodal approach may offer a more comprehensive and effective strategy for the treatment of neurodegenerative diseases [142]. Terpenes have significant potential in modulating the ECM and treating neurodegenerative diseases. The complexity of their interactions with the ECM and the underlying cellular processes requires a careful and evidence-based approach to their therapeutic application. Continued research in this field may provide new perspectives for the development of effective and safe treatments for neurodegenerative diseases.
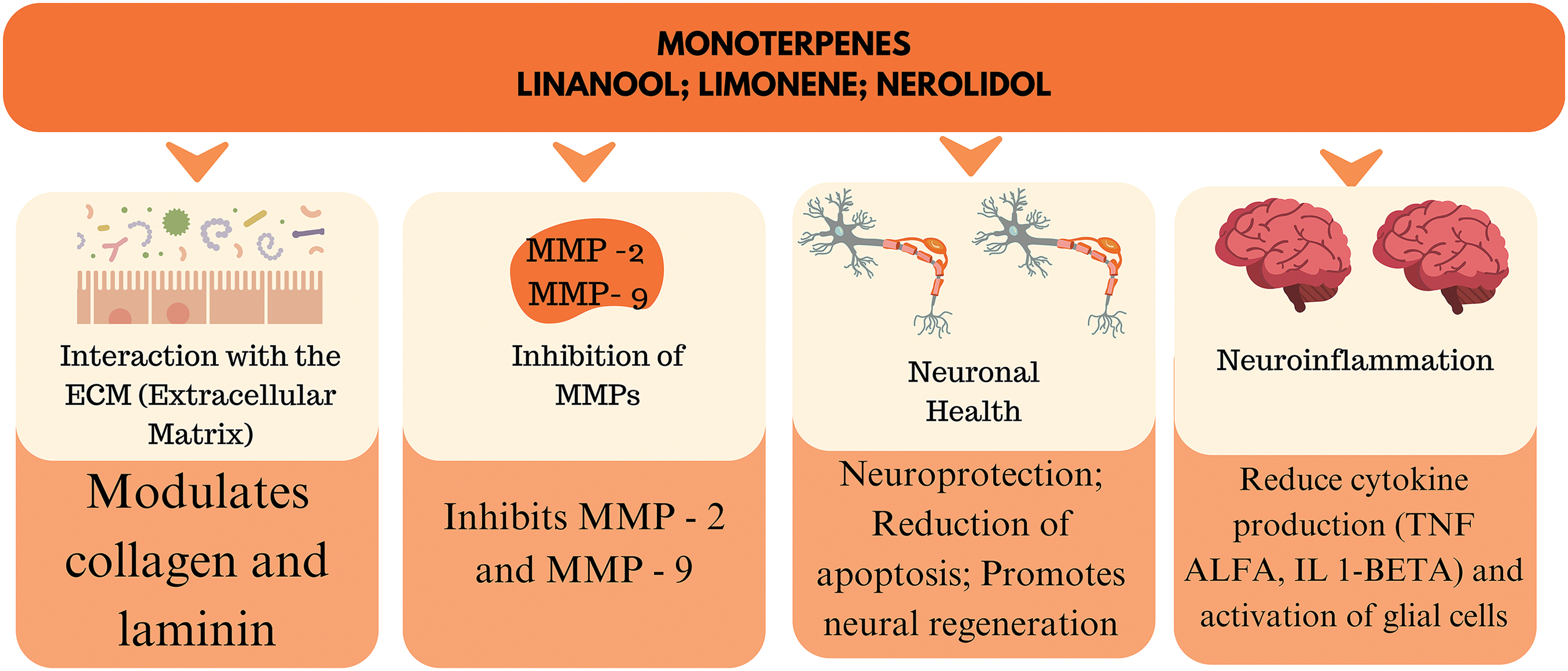
Figure 6: The action of the main terpenes in the ECM. By Canva.
The treatment of neurodegenerative diseases involves the use of drugs such as acetylcholinesterase inhibitors (in the case of Alzheimer’s) and levodopa (for Parkinson’s), which help control symptoms but do not stop the progression of the disease [143–146]. Furthermore, the low bioavailability of some drugs, due to their difficulty in crossing the blood-brain barrier, limits their effectiveness. In the search for more effective solutions, the use of terpenes, natural compounds found in plants, may be useful in combination therapy to improve the bioavailability and effectiveness of traditional treatments.
Terpenes are bioactive compounds known for their anti-inflammatory, antioxidant, and neuroprotective properties. β-Caryophyllene, for example, can modulate the NF-κB signaling pathway, which is involved in the inflammatory response in the brain, and the MAPK pathway, which plays a crucial role in regulating cell survival and apoptosis [77,147]. Other terpenes mentioned above may act in a similar way. These properties make terpenes promising for combination treatment, as they can not only enhance the efficacy of traditional drugs but also offer therapeutic benefits of their own.
Terpenes can improve the bioavailability of neuroprotective drugs. Due to their lipophilic nature, compounds such as limonene and myrcene can facilitate the passage of drugs across the blood-brain barrier, increasing the concentration of these drugs in the central nervous system. Limonene, when combined with donepezil (a drug commonly used in the treatment of Alzheimer’s), increased drug levels in the brain of animal models, improving neuroprotective efficacy [148]. This synergy can reduce the required dosage of traditional drugs, minimizing side effects (Fig. 7).
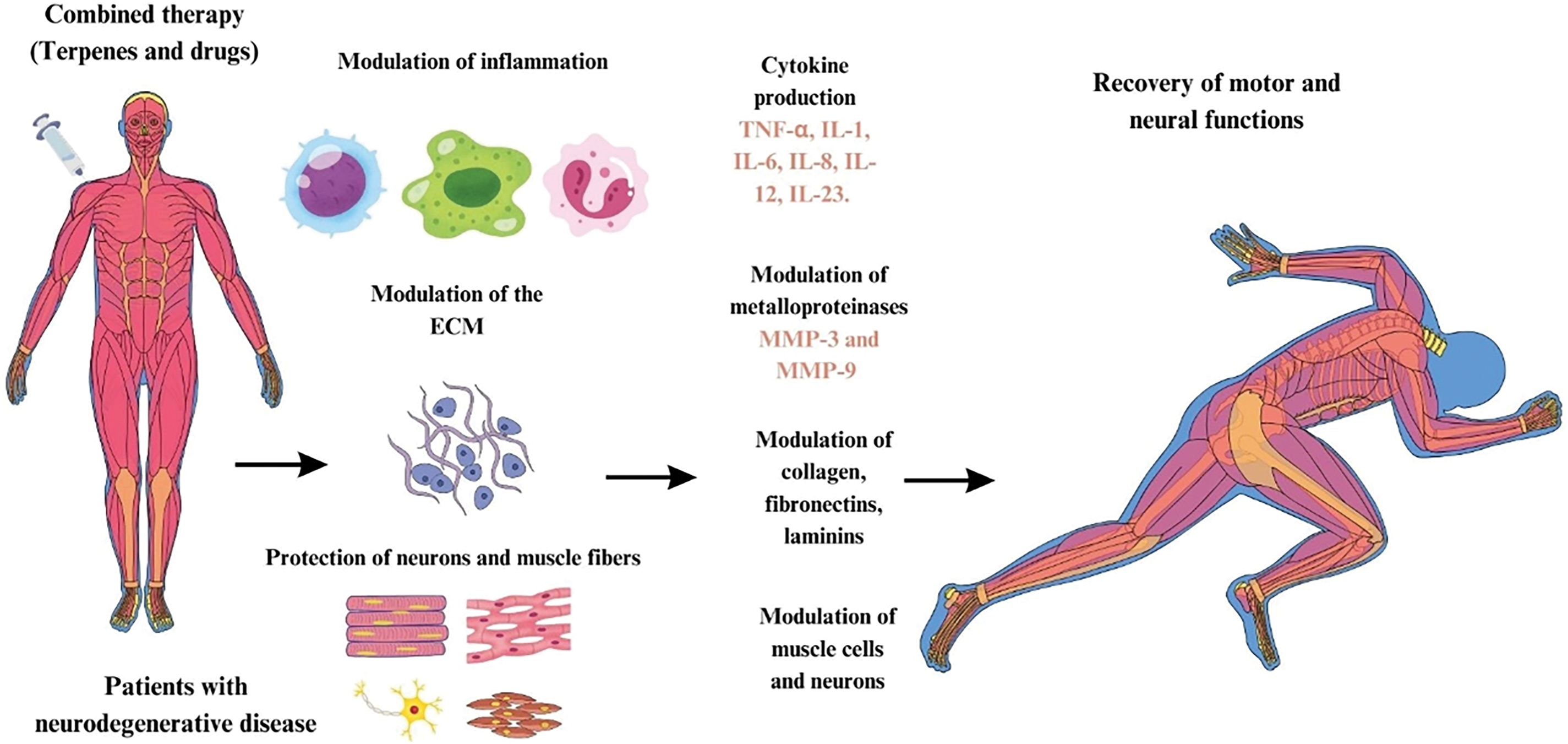
Figure 7: Combined therapy of terpenes and drugs for modulation of ECM and recovery of motor and neural functions in neurodegenerative diseases. By Canva.
Fig. 7 shows a diagram describing the action of combined therapies using terpenes and drugs in the treatment of neurodegenerative diseases. Based on the literature, we can suggest the possibility of combined therapies in patients with neurodegenerative diseases, considering different types of terpenes associated with drugs commonly used to control the progression of neurodegenerative diseases. These therapies can modulate inflammation, where the production of inflammatory cytokines, such as TNF-α, IL-1, IL-6, IL-8, IL-12 and IL-23, is regulated. This inflammatory modulation contributes to the reduction of the inflammatory process that aggravates neurodegeneration.
The modulation of the ECM occurs, with action on MMPs, specifically MMP-3 and MMP-9. These enzymes are responsible for the degradation and remodeling of the ECM, which is composed of collagen, fibronectins, and laminins, important structures for tissue integrity and communication between cells. This regulation promotes the protection of neurons and muscle fibers, preventing degeneration caused by inflammatory and oxidative damage. As a result, ECM modulation and neuronal and muscle protection lead to the recovery of motor and neural functions in patients, partially restoring the functionality lost due to neurodegenerative diseases.
In addition to modulating inflammation and improving bioavailability, terpenes have also demonstrated direct effects on pathological processes associated with neurodegenerative diseases. β-caryophyllene has been shown to reduce the accumulation of beta-amyloid plaques, a hallmark of the disease, and to promote neurogenesis through the activation of CB2 receptors linked to the endocannabinoid system [147,149,150]. In Parkinson’s, myrcene has been shown to reduce oxidative stress and protect dopaminergic neurons, suggesting a beneficial action in slowing disease progression [151].
Although preclinical evidence is promising, further clinical trials are needed to determine the efficacy and safety of the combined use of terpenes and conventional drugs in humans. The potential of terpenes as adjuvants in combination therapies opens a new perspective in the treatment of neurodegenerative diseases, offering a more comprehensive approach to address the multiple pathological mechanisms involved in these conditions.
Natural terpenes derived from plants have demonstrated significant therapeutic potential in modulating the ECM, a key component in cellular homeostasis and tissue integrity. The ECM plays a vital role in neurodegeneration since its degradation and remodeling are associated with inflammation and cellular dysfunction, especially in diseases such as Alzheimer’s and Parkinson’s. Alterations in the composition and organization of the ECM are associated with the progression of neurodegenerative diseases. Terpenes are substances that act in the regulation and remodeling of the matrix, inhibiting the expression of enzymes that degrade its components, in addition to influencing the expression of proteins such as collagen and laminin that contribute to the preservation of the structural and functional integrity of the ECM.
Terpenes such as β-caryophyllene and limonene have shown the ability to regulate the activity of enzymes responsible for ECM remodeling, such as MMPs, reducing inflammation and preventing the degradation of the blood-brain barrier. Furthermore, terpenes can interact with cellular receptors such as cannabinoid receptors, modulating signaling pathways that promote tissue regeneration and repair. These plant-derived compounds also exhibit potent antioxidant and anti-inflammatory activity, evidenced by their ability to inhibit pro-inflammatory cytokines and those associated with the production of free radicals. In this context, terpenes stand out as neuroprotective substances, which suggests their potential in the development of new drugs aimed at the prevention and progression of neurodegenerative diseases.
Future research directions include conducting clinical trials to test the efficacy of these compounds in humans and exploring combinations of terpenes with traditional neuroprotective drugs. Modulation of the ECM by terpenes offers a promising new therapeutic avenue that may not only protect neurons from degeneration but also promote cellular regeneration in damaged areas of the brain. If successful, these approaches could significantly impact clinical practice, enabling more effective management of neurodegenerative diseases, with less invasive therapies and reduced side effects, offering new hope for patients and improving their quality of life.
Acknowledgement: None.
Funding Statement: Anderson Nogueira Mendes (#302704/2023-0) is grateful to the public Brazilian agency “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) for their personal scholarships.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Renata da Silva Carneiro; Mateus Henrique de Almeida da Costa; José Zilton Lima Verde Santos; draft manuscript preparation Renata da Silva Carneiro; Mateus Henrique de Almeida da Costa; José Zilton Lima Verde Santos; Valdiléia Teixeira Uchôa; Luciano da Silva Lopes; Anderson Nogueira Mendes; review and editing: Renata da Silva Carneiro; Mateus Henrique de Almeida da Costa; José Zilton Lima Verde Santos; Anderson Nogueira Mendes; visualization: Renata da Silva Carneiro; Mateus Henrique de Almeida da Costa; José Zilton Lima Verde Santos; supervision: Anderson Nogueira Mendes. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Castro-Gomez S, Heneka MT. Innate immune activation in neurodegenerative diseases. Immunity. 2024;57(4):790–814. doi:10.1016/j.immuni.2024.03.010. [Google Scholar] [PubMed] [CrossRef]
2. Dejanovic B, Sheng M, Hanson JE. Targeting synapse function and loss for treatment of neurodegenerative diseases. Nat Rev Drug Discov. 2024;23(1):23–42. doi:10.1038/s41573-023-00823-1. [Google Scholar] [PubMed] [CrossRef]
3. Lefèvre-Arbogast S, Chaker J, Mercier F, Barouki R, Coumoul X, Miller GW, et al. Assessing the contribution of the chemical exposome to neurodegenerative disease. Nat Neurosci. 2024;27(5):812–21. doi:10.1038/s41593-024-01627-1. [Google Scholar] [PubMed] [CrossRef]
4. Appel SH, Beers DR, Zhao W. Chapter 23—the role of inflammation in neurodegenerative diseases. In: Zigmond MJ, Wiley CA, Chesselet M-F, editors. Neurobiology of brain disorders. 2nd ed. Academic Press; 2023. p. 403–21. doi:10.1016/B978-0-323-85654-6.00036-8. [Google Scholar] [CrossRef]
5. Scarian E, Viola C, Dragoni F, Di Gerlando R, Rizzo B, Diamanti L, et al. New insights into oxidative stress and inflammatory response in neurodegenerative diseases. Int J Mol Sci. 2024;25(5):2698. doi:10.3390/ijms25052698. [Google Scholar] [PubMed] [CrossRef]
6. Steinmetz JD, Seeher KM, Schiess N, Nichols E, Cao B, Servili C, et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. 2024;23(4):344–81. doi:10.1016/S1474-4422(24)00038-3. [Google Scholar] [PubMed] [CrossRef]
7. Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dementia. 2018;14(3):367–429. doi:10.1016/j.jalz.2018.02.001. [Google Scholar] [CrossRef]
8. da Silva Carneiro R, Nogueira TA, de Barros Sousa É, da Silva SDC, Mendes AN. Inflammatory modulation of compounds derived from turmeric (Curcuma longa) in neurodegenerative diseases. In: Rai M, Feitosa CM, editors. Curcumin and neurodegenerative diseases: from traditional to translational medicines. Singapore: Springer Nature Singapore; 2023. p. 437–52. doi:10.1007/978-981-99-7731-4_20. [Google Scholar] [CrossRef]
9. DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019;14(1):32. doi:10.1186/s13024-019-0333-5. [Google Scholar] [PubMed] [CrossRef]
10. Henderson MX, Sedor S, McGeary I, Cornblath EJ, Peng C, Riddle DM, et al. Glucocerebrosidase activity modulates neuronal susceptibility to pathological α-synuclein insult. Neuron. 2020;105(5):822–36. doi:10.1016/j.neuron.2019.12.004. [Google Scholar] [PubMed] [CrossRef]
11. Tabrizi SJ, Flower MD, Ross CA, Wild EJ. Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities. Nat Rev Neurol. 2020;16(10):529–46. doi:10.1038/s41582-020-0389-4. [Google Scholar] [PubMed] [CrossRef]
12. Tong H, Yang T, Xu S, Li X, Liu L, Zhou G, et al. Huntington’s disease: complex pathogenesis and therapeutic strategies. Int J Mol Sci. 2024;25(7):3845. doi:10.3390/ijms25073845. [Google Scholar] [PubMed] [CrossRef]
13. Soleman S, Filippov MA, Dityatev A, Fawcett JW. Targeting the neural extracellular matrix in neurological disorders. Neuroscience. 2013;253(Suppl. 3):194–213. doi:10.1016/j.neuroscience.2013.08.050. [Google Scholar] [PubMed] [CrossRef]
14. Ozsan McMillan I, Li J-P, Wang L. Heparan sulfate proteoglycan in Alzheimer’s disease: aberrant expression and functions in molecular pathways related to amyloid-β metabolism. Am J Physiol-Cell Physiol. 2023;324(4):C893–909. doi:10.1152/ajpcell.00247.2022. [Google Scholar] [PubMed] [CrossRef]
15. Zhang G, Zhang X, Wang X, Li J-P. Towards understanding the roles of heparan sulfate proteoglycans in Alzheimer’s disease. Biomed Res Int. 2014;2014(7):516028–9. doi:10.1155/2014/516028. [Google Scholar] [PubMed] [CrossRef]
16. Bonneh-Barkay D, Wiley CA. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009;19(4):573–85. doi:10.1111/j.1750-3639.2008.00195.x. [Google Scholar] [PubMed] [CrossRef]
17. Yun SP, Kam T-I, Panicker N, Kim S, Oh Y, Park J-S, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med. 2018;24(7):931–8. doi:10.1038/s41591-018-0051-5. [Google Scholar] [PubMed] [CrossRef]
18. Chapman MA, Sorg BA. A systematic review of extracellular matrix-related alterations in Parkinson’s disease. Brain Sci. 2024;14(6):522. doi:10.3390/brainsci14060522. [Google Scholar] [PubMed] [CrossRef]
19. Rike WA, Stern S. Proteins and transcriptional dysregulation of the brain extracellular matrix in Parkinson’s disease: a systematic review. Int J Mol Sci. 2023;24(8):7435. doi:10.3390/ijms24087435. [Google Scholar] [PubMed] [CrossRef]
20. Moretto E, Stuart S, Surana S, Vargas JNS, Schiavo G. The role of extracellular matrix components in the spreading of pathological protein aggregates. Front Cell Neurosci. 2022;16:844211. doi:10.3389/fncel.2022.844211. [Google Scholar] [PubMed] [CrossRef]
21. Rosh I, Tripathi U, Hussein Y, Rike WA, Djamus J, Shklyar B, et al. Synaptic dysfunction and extracellular matrix dysregulation in dopaminergic neurons from sporadic and E326K-GBA1 Parkinson’s disease patients. npj Parkinsons Dis. 2024;10(1):38. doi:10.1038/s41531-024-00653-x. [Google Scholar] [PubMed] [CrossRef]
22. Meem TM, Khan U, Mredul MBR, Awal MA, Rahman MH, Khan MS. A comprehensive bioinformatics approach to identify molecular signatures and key pathways for the Huntington disease. Bioinform Biol Insights. 2023;17:11779322231210098. doi:10.1177/11779322231210098. [Google Scholar] [PubMed] [CrossRef]
23. Paldino E, Migliorato G, Fusco FR. Neuroimmune pathways involvement in neurodegeneration of R6/2 mouse model of Huntington’s disease. Front Cell Neurosci. 2024;18:1360066. doi:10.3389/fncel.2024.1360066. [Google Scholar] [PubMed] [CrossRef]
24. Hernandez SJ, Lim RG, Onur T, Dane MA, Smith R, Wang K, et al. An altered extracellular matrix-integrin interface contributes to Huntington’s disease-associated CNS dysfunction in glial and vascular cells. Hum Mol Genet. 2023;32(9):1483–96. doi:10.1093/hmg/ddac303. [Google Scholar] [PubMed] [CrossRef]
25. Wang Y, Liang J, Xu B, Yang J, Wu Z, Cheng L. TrkB/BDNF signaling pathway and its small molecular agonists in CNS injury. Life Sci. 2024;336(19):122282. doi:10.1016/j.lfs.2023.122282. [Google Scholar] [PubMed] [CrossRef]
26. Antonijevic M, Dallemagne P, Rochais C. Indirect influence on the BDNF/TrkB receptor signaling pathway via GPCRs, an emerging strategy in the treatment of neurodegenerative disorders. Med Res Rev. 2024;110(5):167. doi:10.1002/med.22075. [Google Scholar] [PubMed] [CrossRef]
27. Speidell A, Bin Abid N, Yano H. Brain-derived neurotrophic factor dysregulation as an essential pathological feature in Huntington’s disease: mechanisms and potential therapeutics. Biomedicines. 2023;11(8):2275. doi:10.3390/biomedicines11082275. [Google Scholar] [PubMed] [CrossRef]
28. Masyita A, Mustika Sari R, Dwi Astuti A, Yasir B, Rahma Rumata N, Emran TB, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X. 2022;13:100217. doi:10.1016/j.fochx.2022.100217. [Google Scholar] [PubMed] [CrossRef]
29. Blevins LK, Bach AP, Crawford RB, Zhou J, Henriquez JE, Rizzo MD, et al. Evaluation of the anti-inflammatory effects of selected cannabinoids and terpenes from Cannabis Sativa employing human primary leukocytes. Food Chem Toxicol. 2022;170(1):113458. doi:10.1016/j.fct.2022.113458. [Google Scholar] [PubMed] [CrossRef]
30. Touhtouh J, Laghmari M, Benali T, Aanniz T, Lemhadri A, Akhazzane M, et al. Determination of the antioxidant and enzyme-inhibiting activities and evaluation of selected terpenes’ ADMET properties: in vitro and in silico approaches. Biochem Syst Ecol. 2023;111(5):104733. doi:10.1016/j.bse.2023.104733. [Google Scholar] [CrossRef]
31. Sethi MK, Zaia J. Extracellular matrix proteomics in schizophrenia and Alzheimer’s disease. Anal Bioanal Chem. 2017;409(2):379–94. doi:10.1007/s00216-016-9900-6. [Google Scholar] [PubMed] [CrossRef]
32. Ricci C. Neurodegenerative disease: from molecular basis to therapy. Int J Mol Sci. 2024;25(2):967. doi:10.3390/ijms25020967. [Google Scholar] [PubMed] [CrossRef]
33. Samanta S, Chakraborty S, Bagchi D. Pathogenesis of neurodegenerative diseases and the protective role of natural bioactive components. J Am Nutr Assoc. 2024;43(1):20–32. doi:10.1080/27697061.2023.2203235. [Google Scholar] [PubMed] [CrossRef]
34. Adamu A, Li S, Gao F, Xue G. The role of neuroinflammation in neurodegenerative diseases: current understanding and future therapeutic targets. Front Aging Neurosci. 2024;16:1347987. doi:10.3389/fnagi.2024.1347987. [Google Scholar] [PubMed] [CrossRef]
35. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi:10.15252/emmm.201606210. [Google Scholar] [PubMed] [CrossRef]
36. Henstridge CM, Hyman BT, Spires-Jones TL. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci. 2019;20(2):94–108. doi:10.1038/s41583-018-0113-1. [Google Scholar] [PubMed] [CrossRef]
37. World Health Organization. Dementia. 2023. Available from: https://wwwwhoint/news-room/fact-sheets/detail/dementia. [Accessed 2024]. [Google Scholar]
38. Liu M, Xie X, Xie J, Tian S, Du X, Feng H, et al. Early-onset Alzheimer’s disease with depression as the first symptom: a case report with literature review. Front Psychiatry. 2023;14:349. doi:10.3389/fpsyt.2023.1192562. [Google Scholar] [PubMed] [CrossRef]
39. Ciurea VA, Covache-Busuioc RA, Mohan AG, Costin HP, Voicu V. Alzheimer’s disease: 120 years of research and progress. J Med Life. 2023;2023(2):173–7. doi:10.25122/jml-2022-0111. [Google Scholar] [PubMed] [CrossRef]
40. Van Skike CE, DeRosa N, Galvan V, Hussong SA. Rapamycin restores peripheral blood flow in aged mice and in mouse models of atherosclerosis and Alzheimer’s disease. Geroscience. 2023;45(3):1987–96. doi:10.1007/s11357-023-00786-6. [Google Scholar] [PubMed] [CrossRef]
41. Edwards M, Corkill R. Disease-modifying treatments in Alzheimer’s disease. J Neurol. 2023;270(4):2342–4. doi:10.1007/s00415-023-11602-8. [Google Scholar] [PubMed] [CrossRef]
42. Kurkinen M, Fułek M, Fułek K, Beszłej JA, Kurpas D, Leszek J. The amyloid cascade hypothesis in Alzheimer’s disease: should we change our thinking? Biomolecules. 2023;13(3):453. doi:10.3390/biom13030453. [Google Scholar] [PubMed] [CrossRef]
43. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3(1):17013. doi:10.1038/nrdp.2017.13. [Google Scholar] [PubMed] [CrossRef]
44. Spillantini MG, Goedert M. Neurodegeneration and the ordered assembly of α-synuclein. Cell Tissue Res. 2018;373(1):137–48. doi:10.1007/s00441-017-2706-9. [Google Scholar] [PubMed] [CrossRef]
45. Zaib S, Javed H, Khan I, Jaber F, Sohail A, Zaib Z, et al. Neurodegenerative diseases: their onset, epidemiology, causes and treatment. ChemistrySelect. 2023;8(20):e202300225. doi:10.1002/slct.202300225. [Google Scholar] [CrossRef]
46. Cappelletti C, Henriksen SP, Geut H, Rozemuller AJM, van de Berg WDJ, Pihlstrøm L, et al. Transcriptomic profiling of Parkinson’s disease brains reveals disease stage specific gene expression changes. Acta Neuropathol. 2023;146(2):227–44. doi:10.1007/s00401-023-02597-7. [Google Scholar] [PubMed] [CrossRef]
47. Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. New Engl J Med. 2024;377(2):162–72. doi:10.1056/NEJMra1603471. [Google Scholar] [PubMed] [CrossRef]
48. Taylor JP, Brown RH, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539(7628):197–206. doi:10.1038/nature20413. [Google Scholar] [PubMed] [CrossRef]
49. Wolfson C, Gauvin D, Ishola F, Oskoui M. The global prevalence and incidence of amyotrophic lateral sclerosis: a systematic review (P2-5.023). Neurology. 2023;100(17_supplement_2):3346. doi:10.1212/WNL.0000000000203202. [Google Scholar] [CrossRef]
50. Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10(1):83–98. doi:10.1016/S1474-4422(10)70245-3. [Google Scholar] [PubMed] [CrossRef]
51. McColgan P, Tabrizi SJ. Huntington’s disease: a clinical review. Eur J Neurol. 2018;25(1):24–34. doi:10.1111/ene.13413. [Google Scholar] [PubMed] [CrossRef]
52. Zhan F, Lin G, Su L, Xue L, Duan K, Chen L, et al. The association between methylmalonic acid, a biomarker of mitochondrial dysfunction, and cause-specific mortality in Alzheimer’s disease and Parkinson’s disease. Heliyon. 2024;10(8):e29357. doi:10.1016/j.heliyon.2024.e29357. [Google Scholar] [PubMed] [CrossRef]
53. Oertel W, Schulz JB. Current and experimental treatments of Parkinson disease: a guide for neuroscientists. J Neurochem. 2016;139(S1):325–37. doi:10.1111/jnc.13750. [Google Scholar] [PubMed] [CrossRef]
54. Ayeni EA, Gong Y, Yuan H, Hu Y, Bai X, Liao X. Medicinal plants for anti-neurodegenerative diseases in West Africa. J Ethnopharmacol. 2022;285(S):114468. doi:10.1016/j.jep.2021.114468. [Google Scholar] [PubMed] [CrossRef]
55. Rabiei Z, Solati K, Amini-Khoei H. Phytotherapy in treatment of Parkinson’s disease: a review. Pharm Biol. 2019;57(1):355–62. doi:10.1080/13880209.2019.1618344. [Google Scholar] [PubMed] [CrossRef]
56. Mony TJ, Elahi F, Choi JW, Park SJ. Neuropharmacological effects of terpenoids on preclinical animal models of psychiatric disorders: a review. Antioxidants. 2022;11(9):1834. doi:10.3390/antiox11091834. [Google Scholar] [PubMed] [CrossRef]
57. Eddin LB, Jha NK, Meeran MFN, Kesari KK, Beiram R, Ojha S. Neuroprotective potential of limonene and limonene containing natural products. Molecules. 2021;26(15):4535. doi:10.3390/molecules26154535. [Google Scholar] [PubMed] [CrossRef]
58. Eddin LB, Azimullah S, Jha NK, Nagoor Meeran MF, Beiram R, Ojha S. Limonene, a monoterpene, mitigates rotenone-induced dopaminergic neurodegeneration by modulating neuroinflammation, hippo signaling and apoptosis in rats. Int J Mol Sci. 2023;24(6):5222. doi:10.3390/ijms24065222. [Google Scholar] [PubMed] [CrossRef]
59. Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6(12):81–90. doi:10.4103/0973-7847.99898. [Google Scholar] [PubMed] [CrossRef]
60. Kumar GP, Anilakumar KR, Naveen S. Phytochemicals having neuroprotective properties from dietary sources and medicinal herbs. Pharmacogn J. 2015;7(1):1–17. doi:10.5530/pj.2015.1.1. [Google Scholar] [CrossRef]
61. Li S, Wei Y, Liang Z, Guo L, Hao X, Zhang Y. Review on dietary supplements as an effective improvement of Alzheimer’s disease: focus on structures and mechanisms. Food Sci Hum Wellness. 2024;13(4):1787–805. doi:10.26599/FSHW.2022.9250150. [Google Scholar] [CrossRef]
62. Nguyen LAM, Pham TH, Ganeshalingam M, Thomas R. A multimodal analytical approach is important in accurately assessing terpene composition in edible essential oils. Food Chem. 2024;454(4):139792. doi:10.1016/j.foodchem.2024.139792. [Google Scholar] [PubMed] [CrossRef]
63. Guesmi F, Prasad S, Tyagi AK, Landoulsi A. Antinflammatory and anticancer effects of terpenes from oily fractions of teucruim alopecurus, blocker of IκBα kinase, through downregulation of NF-κB activation, potentiation of apoptosis and suppression of NF-κB-regulated gene expression. Biomed Pharmacother. 2017;95:1876–85. doi:10.1016/j.biopha.2017.09.115. [Google Scholar] [PubMed] [CrossRef]
64. Achika JI, Ayo RG, Habila JD, Oyewale AO. Terpenes with antimicrobial and antioxidant activities from Lannea humilis (Oliv.). Sci Afr. 2020;10(8):e00552. doi:10.1016/j.sciaf.2020.e00552. [Google Scholar] [CrossRef]
65. dos Santos LC, Álvarez-Rivera G, Sánchez-Martínez JD, Johner JCF, Barrales FM, de Oliveira AL, et al. Comparison of different extraction methods of Brazilian “pacová” (Renealmia petasites Gagnep.) oilseeds for the determination of lipid and terpene composition, antioxidant capacity, and inhibitory effect on neurodegenerative enzymes. Food Chem X. 2021;12(5):100140. doi:10.1016/j.fochx.2021.100140. [Google Scholar] [PubMed] [CrossRef]
66. Laws III JS, Smid SD. Characterizing cannabis-prevalent terpenes for neuroprotection reveal a role for α and β-pinenes in mitigating amyloid β-evoked neurotoxicity and aggregation in vitro. Neurotoxicology. 2024;100(5):16–24. doi:10.1016/j.neuro.2023.12.004. [Google Scholar] [PubMed] [CrossRef]
67. Pasciu V, Baralla E, Varoni MV, Demontis MP. Evaluation of curcuma and ginger mixture ability to prevent ROS production induced by bisphenol S: an in vitro study. Drug Chem Toxicol. 2022;45(1):324–30. doi:10.1080/01480545.2019.1690499. [Google Scholar] [PubMed] [CrossRef]
68. Zhang J, Ma X, Wang W, Wu C, Ma B, Yu C, et al. Three new sesquiterpenes from roots of Curcuma longa. Chin Herb Med. 2023;15(3):470–4. doi:10.1016/j.chmed.2022.11.007. [Google Scholar] [PubMed] [CrossRef]
69. Xu F-X, Zhang J-Y, Jin J, Li Z-G, She Y-B, Lee M-R. Microwave-assisted natural deep eutectic solvents pretreatment followed by hydrodistillation coupled with GC-MS for analysis of essential oil from turmeric (Curcuma longa L.). J Oleo Sci. 2021;70(10):1481–94. doi:10.5650/jos.ess20368. [Google Scholar] [PubMed] [CrossRef]
70. Li F, Zhang J, Lin M, Su X, Li C, Wang H, et al. Anti-inflammatory terpenes from Schefflera rubriflora C. J. Tseng & G. Hoo with their TNF-α and IL-6 inhibitory activities. Phytochemistry. 2019;163:23–32. doi:10.1016/j.phytochem.2019.03.021. [Google Scholar] [PubMed] [CrossRef]
71. Wang H, Zhou X-M, Wu L-Y, Liu G-J, Xu W-D, Zhang X-S, et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J Neuroinflammation. 2020;17(1):188. doi:10.1186/s12974-020-01863-9. [Google Scholar] [PubMed] [CrossRef]
72. Jeong DE, Shim S-Y, Lee M. Anti-inflammatory activity of phenylpropyl triterpenoids from Osmanthus fragrans var. aurantiacus leaves. Int Immunopharmacol. 2020;86:106576. doi:10.1016/j.intimp.2020.106576. [Google Scholar] [PubMed] [CrossRef]
73. Zhang Y, Zhao Y, Zhang J, Gao Y, Li S, Chang C, et al. Ginkgolide B inhibits NLRP3 inflammasome activation and promotes microglial M2 polarization in Aβ1-42-induced microglia cells. Neurosci Lett. 2021;764(2):136206. doi:10.1016/j.neulet.2021.136206. [Google Scholar] [PubMed] [CrossRef]
74. Rathinavel T, Ammashi S, Shanmugam G. Analgesic and anti-inflammatory potential of lupeol isolated from Indian traditional medicinal plant crateva adansonii screened through in vivo and in silico approaches. J Genet Eng Biotechnol. 2021;19(1):62. doi:10.1186/s43141-021-00167-6. [Google Scholar] [PubMed] [CrossRef]
75. Ayala-Ruiz LA, Ortega-Pérez LG, Piñón-Simental JS, Magaña-Rodriguez OR, Meléndez-Herrera E, Rios-Chavez P. Role of the major terpenes of Callistemon citrinus against the oxidative stress during a hypercaloric diet in rats. Biomed Pharmacother. 2022;153(30):113505. doi:10.1016/j.biopha.2022.113505. [Google Scholar] [PubMed] [CrossRef]
76. Laws JS III, Shrestha S, Smid SD. Cannabis terpenes display variable protective and anti-aggregatory actions against neurotoxic β amyloid in vitro: highlighting the protective bioactivity of α-bisabolol in motorneuronal-like NSC-34 cells. Neurotoxicol. 2022;90(1):81–7. doi:10.1016/j.neuro.2022.03.001. [Google Scholar] [PubMed] [CrossRef]
77. Rajab BS, Albukhari TA, Khan AA, Refaat B, Almehmadi SJ, Nasreldin N, et al. Antioxidative and anti-inflammatory protective effects of β-caryophyllene against amikacin-induced nephrotoxicity in rat by regulating the Nrf2/AMPK/AKT and NF-κB/TGF-β/KIM-1 molecular pathways. Oxid Med Cell Longev. 2022;2022(2):4212331–12. doi:10.1155/2022/4212331. [Google Scholar] [PubMed] [CrossRef]
78. Del Prado-Audelo ML, Cortés H, Caballero-Florán IH, González-Torres M, Escutia-Guadarrama L, Bernal-Chávez SA, et al. Therapeutic applications of terpenes on inflammatory diseases. Front Pharmacol. 2021;12:627. doi:10.3389/fphar.2021.704197. [Google Scholar] [PubMed] [CrossRef]
79. Kim T, Song B, Cho KS, Lee I-S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int J Mol Sci. 2020;21(6):2187. doi:10.3390/ijms21062187. [Google Scholar] [PubMed] [CrossRef]
80. Chang L, Yin C-Y, Wu H-Y, Tian B-B, Zhu Y, Luo C-X, et al. (+)-Borneol is neuroprotective against permanent cerebral ischemia in rats by suppressing production of proinflammatory cytokines. J Biomed Res. 2017;31(4):306–14. doi:10.7555/JBR.31.20160138. [Google Scholar] [PubMed] [CrossRef]
81. Rahman MdH, Bajgai J, Fadriquela A, Sharma S, Trinh TT, Akter R, et al. Therapeutic potential of natural products in treating neurodegenerative disorders and their future prospects and challenges. Molecules. 2021;26(17):5327. doi:10.3390/molecules26175327. [Google Scholar] [PubMed] [CrossRef]
82. Yu S, Wang X, Lv L, Liu T, Guan Q. Borneol-modified PEGylated graphene oxide as a nanocarrier for brain-targeted delivery of ginsenoside Rg1 against depression. Int J Pharm. 2023;643:123284. doi:10.1016/j.ijpharm.2023.123284. [Google Scholar] [PubMed] [CrossRef]
83. Srivastava G, Ganjewala D. An update on the emerging neuroprotective potential of moringa oleifera and its prospects in complimentary neurotherapy. Phytomed Plus. 2024;4(2):100532. doi:10.1016/j.phyplu.2024.100532. [Google Scholar] [CrossRef]
84. Chand Dakal T, Choudhary K, Tiwari I, Yadav V, Kumar Maurya P, Kumar Sharma N. Unraveling the triad: hypoxia, oxidative stress and inflammation in neurodegenerative disorders. Neuroscience. 2024;552(Suppl 2):126–41. doi:10.1016/j.neuroscience.2024.06.021. [Google Scholar] [PubMed] [CrossRef]
85. Gadhave DG, Sugandhi VV, Jha SK, Nangare SN, Gupta G, Singh SK, et al. Neurodegenerative disorders: mechanisms of degeneration and therapeutic approaches with their clinical relevance. Ageing Res Rev. 2024;99:102357. doi:10.1016/j.arr.2024.102357. [Google Scholar] [PubMed] [CrossRef]
86. Mussard E, Cesaro A, Lespessailles E, Legrain B, Berteina-Raboin S, Toumi H. A natural antioxidant: an update. Antioxidants. 2019;8(12):571. doi:10.3390/antiox8120571. [Google Scholar] [PubMed] [CrossRef]
87. Hilal B, Khan MM, Fariduddin Q. Recent advancements in deciphering the therapeutic properties of plant secondary metabolites: phenolics, terpenes, and alkaloids. Plant Physiol Biochem. 2024;211(8):108674. doi:10.1016/j.plaphy.2024.108674. [Google Scholar] [PubMed] [CrossRef]
88. Singh G, Kapoor IPS, Singh P, de Heluani CS, de Lampasona MP, Catalan CAN. Chemistry and antioxidant properties of essential oil and oleoresins extracted from the seeds of tomer (Zanthoxylum armatum DC)*. Int J Food Prop. 2013;16(2):288–300. doi:10.1080/10942912.2010.551311. [Google Scholar] [CrossRef]
89. Aprotosoaie AC, Hăncianu M, Costache I-I, Miron A. Linalool: a review on a key odorant molecule with valuable biological properties. Flavour Fragr J. 2014;29(4):193–219. doi:10.1002/ffj.3197. [Google Scholar] [CrossRef]
90. Cai Y, Zhang Y, Leng S, Ma Y, Jiang Q, Wen Q, et al. The relationship between inflammation, impaired glymphatic system, and neurodegenerative disorders: a vicious cycle. Neurobiol Dis. 2024;192(7767):106426. doi:10.1016/j.nbd.2024.106426. [Google Scholar] [CrossRef]
91. Knox EG, Aburto MR, Clarke G, Cryan JF, O’Driscoll CM. The blood-brain barrier in aging and neurodegeneration. Mol Psychiatry. 2022;27(6):2659–73. doi:10.1038/s41380-022-01511-z. [Google Scholar] [PubMed] [CrossRef]
92. Rivera Rodríguez R, Johnson JJ. Terpenes: modulating anti-inflammatory signaling in inflammatory bowel disease. Pharmacol Ther. 2023;248(8):108456. doi:10.1016/j.pharmthera.2023.108456. [Google Scholar] [PubMed] [CrossRef]
93. Mueller D, Triebel S, Rudakovski O, Richling E. Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD). Food Chem. 2013;139(1–4):339–46. doi:10.1016/j.foodchem.2013.01.101. [Google Scholar] [PubMed] [CrossRef]
94. Omar SH. Ginkgolides and neuroprotective effects. In: Ramawat KG, Mérillon J-M, editors. Natural products: phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Berlin, Heidelberg: Springer Berlin Heidelberg; 2013. p. 3697–741. doi:10.1007/978-3-642-22144-6_146. [Google Scholar] [CrossRef]
95. Lemanske Jr. RF, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125(2):S95–102. doi:10.1016/j.jaci.2009.10.047. [Google Scholar] [PubMed] [CrossRef]
96. Xin Q, Yuan R, Shi W, Zhu Z, Wang Y, Cong W. A review for the anti-inflammatory effects of paeoniflorin in inflammatory disorders. Life Sci. 2019;237:116925. doi:10.1016/j.lfs.2019.116925. [Google Scholar] [PubMed] [CrossRef]
97. Amor S, Peferoen LAN, Vogel DYS, Breur M, van der Valk P, Baker D, et al. Inflammation in neurodegenerative diseases—an update. Immunology. 2014;142(2):151–66. doi:10.1111/imm.12233. [Google Scholar] [PubMed] [CrossRef]
98. Fu Y, Yang J, Wang X, Yang P, Zhao Y, Li K, et al. Herbal compounds play a role in neuroprotection through the inhibition of microglial activation. J Immunol Res. 2018;2018:9348046–8. doi:10.1155/2018/9348046. [Google Scholar] [PubMed] [CrossRef]
99. Lorigooini Z, Boroujeni SN, Sayyadi-Shahraki M, Rahimi-Madiseh M, Bijad E, Amini-khoei H. Limonene through attenuation of neuroinflammation and nitrite level exerts antidepressant-like effect on mouse model of maternal separation stress. Behav Neurol. 2021;2021(9):8817309–8. doi:10.1155/2021/8817309. [Google Scholar] [PubMed] [CrossRef]
100. Gu J-H, Ge J-B, Li M, Wu F, Zhang W, Qin Z-H. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci. 2012;47(4):652–60. doi:10.1016/j.ejps.2012.07.016. [Google Scholar] [PubMed] [CrossRef]
101. Raghunathan R, Sethi MK, Klein JA, Zaia J. Proteomics, glycomics, and glycoproteomics of matrisome molecules. Mol Cell Proteom. 2019;18(11):2138–48. doi:10.1074/mcp.R119.001543. [Google Scholar] [PubMed] [CrossRef]
102. Pintér P, Alpár A. The role of extracellular matrix in human neurodegenerative diseases. Int J Mol Sci. 2022;23(19):11085. doi:10.3390/ijms231911085. [Google Scholar] [PubMed] [CrossRef]
103. Chelyshev YA, Kabdesh IM, Mukhamedshina YO. Extracellular matrix in neural plasticity and regeneration. Cell Mol Neurobiol. 2022;42(3):647–64. doi:10.1007/s10571-020-00986-0. [Google Scholar] [PubMed] [CrossRef]
104. Song I, Dityatev A. Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull. 2018;136(83):101–8. doi:10.1016/j.brainresbull.2017.03.003. [Google Scholar] [PubMed] [CrossRef]
105. Maïza A, Chantepie S, Vera C, Fifre A, Huynh MB, Stettler O, et al. The role of heparan sulfates in protein aggregation and their potential impact on neurodegeneration. FEBS Lett. 2018;592(23):3806–18. doi:10.1002/1873-3468.13082. [Google Scholar] [PubMed] [CrossRef]
106. Downs M, Sethi MK, Raghunathan R, Layne MD, Zaia J. Matrisome changes in Parkinson’s disease. Anal Bioanal Chem. 2022;414(9):3005–15. doi:10.1007/s00216-022-03929-4. [Google Scholar] [PubMed] [CrossRef]
107. Ferrer-Ferrer M, Dityatev A. Shaping synapses by the neural extracellular matrix. Front Neuroanat. 2018;12:25. doi:10.3389/fnana.2018.00040. [Google Scholar] [PubMed] [CrossRef]
108. Wilson MR, Satapathy S, Vendruscolo M. Extracellular protein homeostasis in neurodegenerative diseases. Nat Rev Neurol. 2023;19:235–45. doi:10.1038/s41582-023-00786-2. [Google Scholar] [PubMed] [CrossRef]
109. Wareham LK, Baratta RO, Del Buono BJ, Schlumpf E, Calkins DJ. Collagen in the central nervous system: contributions to neurodegeneration and promise as a therapeutic target. Mol Neurodegener. 2024;19(1):11. doi:10.1186/s13024-024-00704-0. [Google Scholar] [PubMed] [CrossRef]
110. Soles A, Selimovic A, Sbrocco K, Ghannoum F, Hamel K, Moncada EL, et al. Extracellular matrix regulation in physiology and in brain disease. Int J Mol Sci. 2023;24(8):7049. doi:10.3390/ijms24087049. [Google Scholar] [PubMed] [CrossRef]
111. Gardiner NJ. Integrins and the extracellular matrix: key mediators of development and regeneration of the sensory nervous system. Dev Neurobiol. 2011;71(11):1054–72. doi:10.1002/dneu.20950. [Google Scholar] [PubMed] [CrossRef]
112. Ortega JA, Soares de Aguiar GP, Chandravanshi P, Levy N, Engel E, Álvarez Z. Exploring the properties and potential of the neural extracellular matrix for next-generation regenerative therapies. WIREs Nanomed Nanobiotechnol. 2024;16(3):e1962. doi:10.1002/wnan.1962. [Google Scholar] [PubMed] [CrossRef]
113. Volpato FZ, Führmann T, Migliaresi C, Hutmacher DW, Dalton PD. Using extracellular matrix for regenerative medicine in the spinal cord. Biomaterials. 2013;34(21):4945–55. doi:10.1016/j.biomaterials.2013.03.057. [Google Scholar] [PubMed] [CrossRef]
114. Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17(1):120–7. doi:10.1016/j.conb.2006.09.004. [Google Scholar] [PubMed] [CrossRef]
115. Long KR, Huttner WB. How the extracellular matrix shapes neural development. Open Biol. 2019;9(1):180216. doi:10.1098/rsob.180216. [Google Scholar] [PubMed] [CrossRef]
116. Yang L, Wei M, Xing B, Zhang C. Extracellular matrix and synapse formation. Biosci Rep. 2023;43(1):BSR20212411. doi:10.1042/BSR20212411. [Google Scholar] [PubMed] [CrossRef]
117. Nübling G, Levin J, Bader B, Israel L, Bötzel K, Lorenzl S, et al. Limited cleavage of tau with matrix-metalloproteinase MMP-9, but not MMP-3, enhances tau oligomer formation. Exp Neurol. 2012;237(2):470–6. doi:10.1016/j.expneurol.2012.07.018. [Google Scholar] [PubMed] [CrossRef]
118. Fawcett JW, Oohashi T, Pizzorusso T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat Rev Neurosci. 2019;20(8):451–65. doi:10.1038/s41583-019-0196-3. [Google Scholar] [PubMed] [CrossRef]
119. Fawcett JW, Fyhn M, Jendelova P, Kwok JCF, Ruzicka J, Sorg BA. The extracellular matrix and perineuronal nets in memory. Mol Psychiat. 2022;27(8):3192–203. doi:10.1038/s41380-022-01634-3. [Google Scholar] [PubMed] [CrossRef]
120. Rowlands D, Lensjø KK, Dinh T, Yang S, Andrews MR, Hafting T, et al. Aggrecan directs extracellular matrix-mediated neuronal plasticity. J Neurosci. 2018;38(47):10102–13. doi:10.1523/JNEUROSCI.1122-18.2018. [Google Scholar] [PubMed] [CrossRef]
121. Carulli D, Verhaagen J. An extracellular perspective on CNS maturation: perineuronal nets and the control of plasticity. Int J Mol Sci. 2021;22(5):2434. doi:10.3390/ijms22052434. [Google Scholar] [PubMed] [CrossRef]
122. Kubinova S. Extracellular matrix based biomaterials for central nervous system tissue repair: the benefits and drawbacks. Neural Regen Res. 2017;12(9):1430–2. doi:10.4103/1673-5374.215249. [Google Scholar] [PubMed] [CrossRef]
123. Sun Y, Xu S, Jiang M, Liu X, Yang L, Bai Z, et al. Role of the extracellular matrix in Alzheimer’s disease. Front Aging Neurosci. 2021;13:1567. doi:10.3389/fnagi.2021.707466. [Google Scholar] [PubMed] [CrossRef]
124. Jo M, Lee S, Jeon Y-M, Kim S, Kwon Y, Kim H-J. The role of TDP-43 propagation in neurodegenerative diseases: integrating insights from clinical and experimental studies. Exp Mol Med. 2020;52(10):1652–62. doi:10.1038/s12276-020-00513-7. [Google Scholar] [PubMed] [CrossRef]
125. Jin S, Zhang L, Wang L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: from chemistry to medicine. Biomed Pharmacother. 2023;165(7):115215. doi:10.1016/j.biopha.2023.115215. [Google Scholar] [PubMed] [CrossRef]
126. Migheli R, Lostia G, Galleri G, Rocchitta G, Serra PA, Bassareo V, et al. Neuroprotective effect of (R)-(-)-linalool on oxidative stress in PC12 cells. Phytomed Plus. 2021;1(4):100073. doi:10.1016/j.phyplu.2021.100073. [Google Scholar] [CrossRef]
127. Lopez-Navarro ER, Gutierrez J. Metalloproteinases and their inhibitors in neurological disease. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(1):27–38. doi:10.1007/s00210-021-02188-x. [Google Scholar] [PubMed] [CrossRef]
128. Sabogal-Guáqueta AM, Hobbie F, Keerthi A, Oun A, Kortholt A, Boddeke E, et al. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed Pharmacother. 2019;118:109295. doi:10.1016/j.biopha.2019.109295. [Google Scholar] [PubMed] [CrossRef]
129. Yuan C, Shin M, Park Y, Choi B, Jang S, Lim C, et al. Linalool alleviates Aβ42-induced neurodegeneration via suppressing ROS production and inflammation in fly and rat models of Alzheimer’s disease. Oxid Med Cell Longev. 2021;2021(1):8887716. doi:10.1155/2021/8887716. [Google Scholar] [PubMed] [CrossRef]
130. Kotb EA, El-Shiekh RA, Abd-Elsalam WH, El Sayed NSED, El Tanbouly N, El Senousy AS. Protective potential of frankincense essential oil and its loaded solid lipid nanoparticles against UVB-induced photodamage in rats via MAPK and PI3K/AKT signaling pathways; a promising anti-aging therapy. PLoS One. 2023;18:e0294067. [Google Scholar] [PubMed]
131. Gunaseelan S, Balupillai A, Govindasamy K, Ramasamy K, Muthusamy G, Shanmugam M, et al. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS One. 2017;12:e0176699. doi:10.1371/journal.pone.0176699. [Google Scholar] [PubMed] [CrossRef]
132. Fraternale D, Flamini G, Ascrizzi R. In vitro anticollagenase and antielastase activities of essential oil of helichrysum italicum subsp. italicum (Roth) G Don. J Med Food. 2019;22(10):1041–6. doi:10.1089/jmf.2019.0054. [Google Scholar] [PubMed] [CrossRef]
133. Keskin I, Gunal Y, Ayla S, Kolbasi B, Sakul A, Kilic U, et al. Effects of foeniculum vulgare essential oil compounds, fenchone and limonene, on experimental wound healing. Biotech Histochem. 2017;92(4):274–82. doi:10.1080/10520295.2017.1306882. [Google Scholar] [PubMed] [CrossRef]
134. Yu S, Long Y, Li D, Shi A, Deng J, Ma Y, et al. Natural essential oils efficacious in internal organs fibrosis treatment: mechanisms of action and application perspectives. Pharmacol Res. 2022;182(7835):106339. doi:10.1016/j.phrs.2022.106339. [Google Scholar] [PubMed] [CrossRef]
135. Moraes TM, Rozza AL, Kushima H, Pellizzon CH, Rocha LRM, Hiruma-Lima CA. Healing actions of essential oils from citrus aurantium and d-limonene in the gastric mucosa: the roles of VEGF, PCNA, and COX-2 in cell proliferation. J Med Food. 2013;16(12):1162–7. doi:10.1089/jmf.2012.0259. [Google Scholar] [PubMed] [CrossRef]
136. Akhter S, Irfan HM, Alamgeer, Jahan S, Shahzad M, Latif MB. Nerolidol: a potential approach in rheumatoid arthritis through reduction of TNF-α, IL-1β, IL-6, NF-kB, COX-2 and antioxidant effect in CFA-induced arthritic model. Inflammopharmacol. 2022;30(2):537–48. doi:10.1007/s10787-022-00930-2. [Google Scholar] [PubMed] [CrossRef]
137. Fonsêca DV, Salgado PRR, de Carvalho FL, Salvadori MGSS, Penha ARS, Leite FC, et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: involvement of the GABAergic system and proinflammatory cytokines. Fundam Clin Pharmacol. 2016;30(1):14–22. doi:10.1111/fcp.12166. [Google Scholar] [PubMed] [CrossRef]
138. Batista PA, de Paula Werner MF, Oliveira EC, Burgos L, Pereira P, da Silva Brum LF, et al. The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J Pain. 2010;11(11):1222–9. doi:10.1016/j.jpain.2010.02.022. [Google Scholar] [PubMed] [CrossRef]
139. Zhang J, Li Y, Zhou T. Nerolidol attenuates cerebral ischemic injury in middle cerebral artery occlusion-induced rats via regulation of inflammation, apoptosis, and oxidative stress markers. Pharmacogn Mag. 2023;19(1):186–96. doi:10.1177/09731296221137380. [Google Scholar] [CrossRef]
140. Javed H, Azimullah S, Abul Khair SB, Ojha S, Haque ME. Neuroprotective effect of nerolidol against neuroinflammation and oxidative stress induced by rotenone. BMC Neurosci. 2016;17(1):58. doi:10.1186/s12868-016-0293-4. [Google Scholar] [PubMed] [CrossRef]
141. Kaur A, Jaiswal G, Brar J, Kumar P. Neuroprotective effect of nerolidol in traumatic brain injury associated behavioural comorbidities in rats. Toxicol Res. 2021;10(1):40–50. doi:10.1093/toxres/tfaa100. [Google Scholar] [PubMed] [CrossRef]
142. Rodenak-Kladniew B, Castro A, Stärkel P, De Saeger C, García de Bravo M, Crespo R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018;199:48–59. doi:10.1016/j.lfs.2018.03.006. [Google Scholar] [PubMed] [CrossRef]
143. Véronneau-Veilleux F, Ursino M, Robaey P, Lévesque D, Nekka F. Nonlinear pharmacodynamics of levodopa through Parkinson’s disease progression. Chaos. 2020;30(9):093146. doi:10.1063/5.0014800. [Google Scholar] [PubMed] [CrossRef]
144. Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacol. 2021;190(118):108352. doi:10.1016/j.neuropharm.2020.108352. [Google Scholar] [PubMed] [CrossRef]
145. Moss DE. Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: are irreversible inhibitors the future? Int J Mol Sci. 2020;21(10):3438. doi:10.3390/ijms21103438. [Google Scholar] [PubMed] [CrossRef]
146. Urso D, Chaudhuri KR, Qamar MA, Jenner P. Improving the delivery of levodopa in Parkinson’s disease: a review of approved and emerging therapies. CNS Drugs. 2020;34(11):1149–63. doi:10.1007/s40263-020-00769-7. [Google Scholar] [PubMed] [CrossRef]
147. Zhao H, Deng L, Chen S, Wang X, Dong Z. Neuroprotection of β-caryophyllene against cerebral ischemia/reperfusion injury by inhibiting P38 MAPK/NLRP3 signaling pathway. Neuroreport. 2023;34(12):617–23. doi:10.1097/WNR.0000000000001932. [Google Scholar] [PubMed] [CrossRef]
148. Galipoğlu M, Erdal MS, Güngör S. Biopolymer-based transdermal films of donepezil as an alternative delivery approach in Alzheimer’s disease treatment. AAPS PharmSciTech. 2015;16(2):284–92. doi:10.1208/s12249-014-0224-6. [Google Scholar] [PubMed] [CrossRef]
149. Iorio R, Celenza G, Petricca S. Multi-target effects of ß-caryophyllene and carnosic acid at the crossroads of mitochondrial dysfunction and neurodegeneration: from oxidative stress to microglia-mediated neuroinflammation. Antioxidants. 2022;11(6):1199. doi:10.3390/antiox11061199. [Google Scholar] [PubMed] [CrossRef]
150. Viveros-Paredes JM, González-Castañeda RE, Gertsch J, Chaparro-Huerta V, López-Roa RI, Vázquez-Valls E, et al. Neuroprotective effects of β-caryophyllene against dopaminergic neuron injury in a murine model of Parkinson’s disease induced by MPTP. Pharmaceuticals. 2017;10(3):60. doi:10.3390/ph10030060. [Google Scholar] [PubMed] [CrossRef]
151. Azimullah S, Jayaraj RL, Meeran MFN, Jalal FY, Adem A, Ojha S, et al. Myrcene salvages rotenone-induced loss of dopaminergic neurons by inhibiting oxidative stress, inflammation, apoptosis, and autophagy. Molecules. 2023;28(2):685. doi:10.3390/molecules28020685. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF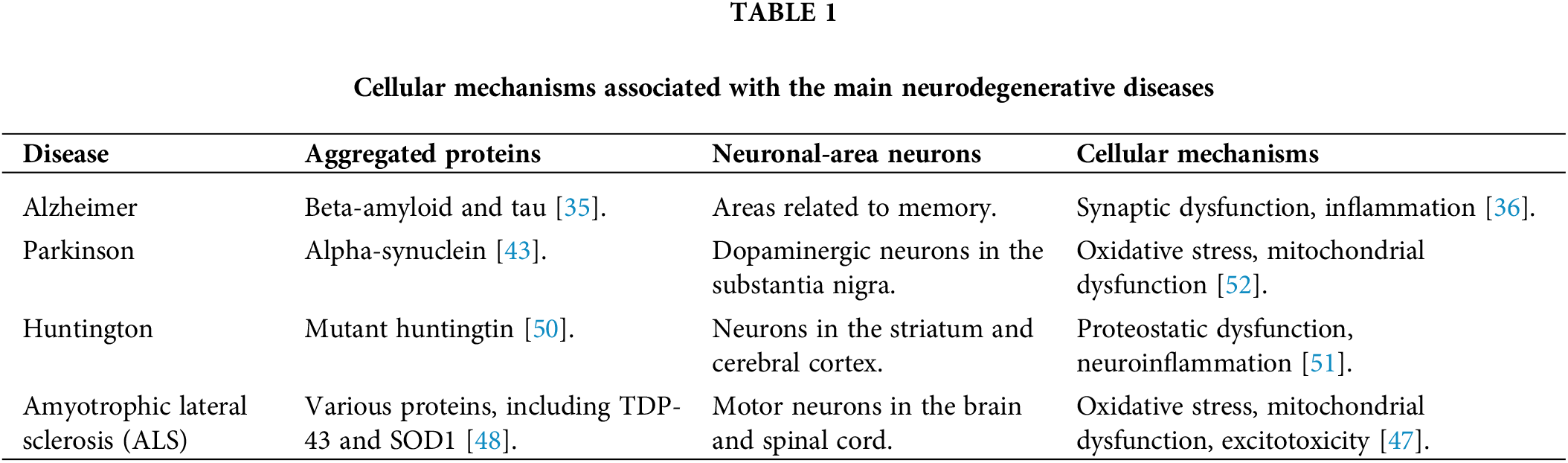
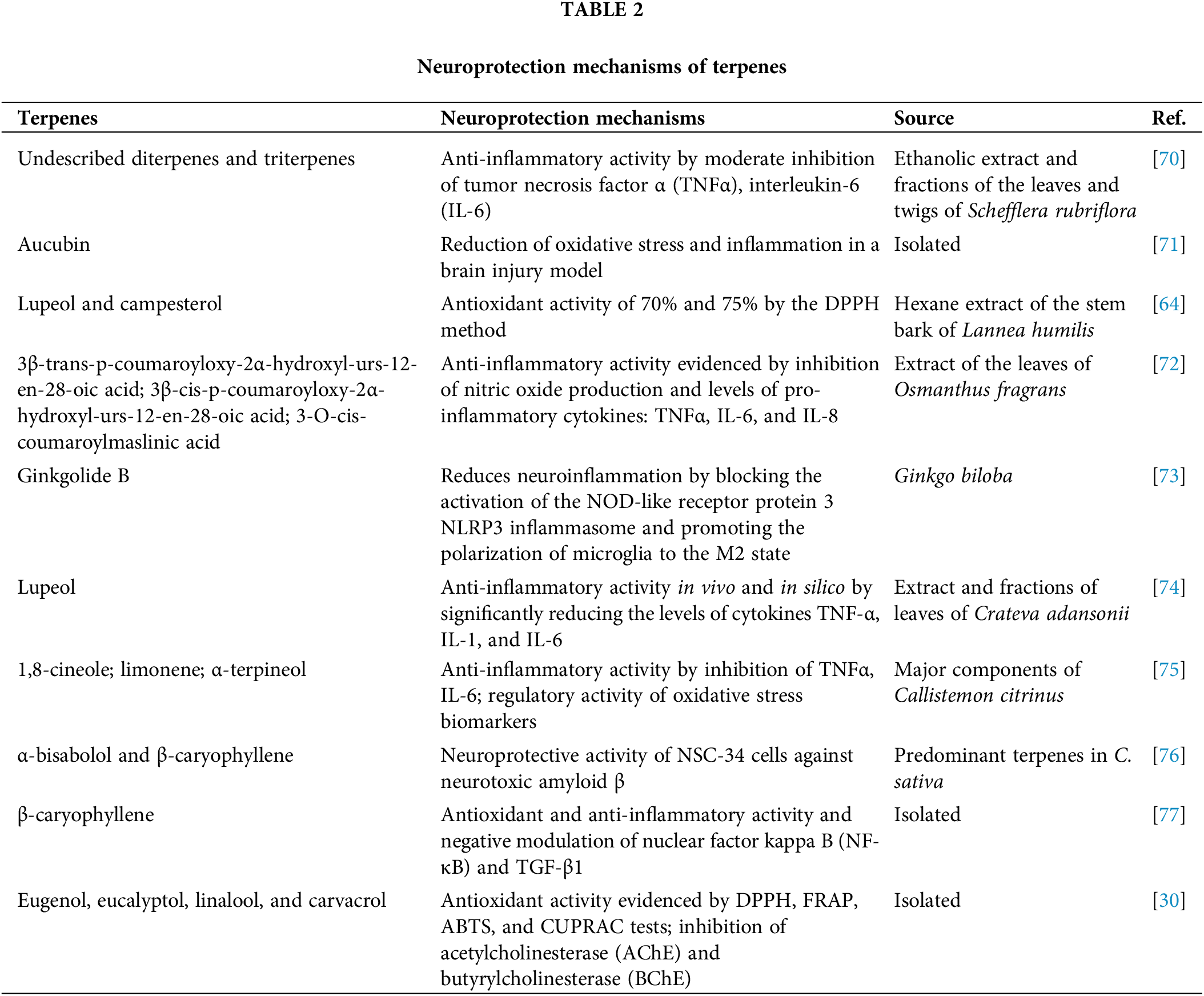
 Downloads
Downloads
 Citation Tools
Citation Tools