 Open Access
Open Access
ARTICLE
ANLN Promotes Cervical Cancer Cell Proliferation, Migration and Invasion and Suppresses Apoptosis via the Wnt/β-Catenin Pathway
1 Graduate Department, Jiangxi Medical College, Nanchang University, Nanchang, 330006, China
2 Department of Oncology, Jiangxi Maternal and Child Health Hospital, Nanchang, 330006, China
3 Department of Gynecological Oncology, Jiangxi Cancer Hospital, Nanchang, 330006, China
4 Department of Oncology, The First Affiliated Hospital of Nanchang University, Nanchang, 330006, China
* Corresponding Authors: Ling Li. Email: ; Jianping Xiong. Email:
(This article belongs to the Special Issue: Genetic Biomarkers of Cancer: Insights into Molecular and Cellular Mechanisms)
BIOCELL 2025, 49(2), 253-267. https://doi.org/10.32604/biocell.2025.061585
Received 28 November 2024; Accepted 01 January 2025; Issue published 28 February 2025
Abstract
Objective: Anillin (ANLN) is considered an oncogene in various cancers, but its effect on cervical cancer remains poorly understood. Hence, this study aimed to describe the action of ANLN on cervical cancer development and investigate the potential mechanism. Methods: Analysis of ANLN expression and its association with survival in carcinoma and endocervical adenocarcinoma (CESC) patients based on GEO and UALCAN databases. The tumor and adjacent normal tissues of 100 cervical cancer cases were harvested to detect the ANLN expression and explore its relationship with patient survival. Cell proliferation, apoptosis, migration, and invasion were measured by utilizing 5-ethynyl-2′-deoxyuridine (EdU) staining, Flow cytometry, and Transwell assay, respectively. ANLN and Wnt expression were analyzed by RT-qPCR and Western Blot. Results: ANLN was significantly elevated in tumor tissues, and cervical cancer cases with high ANLN expression exhibited poor survival and high dead proportion. Besides, ANLN induced cervical cancer cell proliferation, migration, and invasion and restrained cell apoptosis. In addition, ANLN promoted Wnt/β-catenin pathway activation. Furthermore, ANLN accelerated cell aggressive behaviors and suppressed cell apoptosis via activating the Wnt/β-catenin signaling in cervical cancer. Conclusion: ANLN was enhanced in cervical cancer tissues and related to poor prognosis. ANLN accelerated cervical cancer cell aggressive behaviors and suppressed cell apoptosis via activating the Wnt/β-catenin pathway.Keywords
Cervical cancer is one of the most common gynecologic malignancies and ranks as the fourth leading cause of cancer-related deaths among women [1,2]. It is estimated that there are 604,127 new cases and 341,831 deaths globally of cervical cancer in 2020 [3,4]. At present, most early-stage cervical cancer cases are usually curable, and the 5-year overall survival at 5 years is about 92% [5]. However, approximately 50% of cases are diagnosed at the locally advanced stage [5]. The 5-year overall survival of cases at locally advanced stage and metastatic disease decreased to 65% and 17%, respectively [5]. Thus, it is prominent to elucidate the potential mechanism of cervical cancer progression and metastasis and explore valuable strategies for therapy.
Anillin (ANLN) is an actin-binding protein responsible for cell motility and muscle cell contraction [6]. It functions critically in various cellular processes, especially cytokinesis, growth, and migration [7–9]. Recent studies revealed that ANLN could serve as the oncogene and participate in tumorigenesis of various cancers, such as oral, colorectal, and breast cancers [6,10,11]. Wang et al. discovered that ANLN was enhanced in oral cancer cells, and ANLN knockdown decreased cell viability, colony formation, and invasion [6]. Liu et al. found that silencing of ANLN suppressed cell proliferation and tumor growth in colorectal cancer [10]. Zhou et al. revealed that silenced ANLN suppressed malignant behaviors in breast cancer [11]. Interestingly, the bioinformatic analysis performed by Xia et al. indicated that ANLN might serve as a potential tumor modulator in cervical cancer patients [12]. Nevertheless, the action of ANLN on cervical cancer and its potential mechanism remained elusive.
The Wnt/β-catenin pathway modulates various biological processes, including tissue homeostasis, regeneration, and oncogenesis [13]. The Wnt/β-catenin pathway usually presents as highly activated in various cancers [14]. Zhang et al. found that the knockdown of Homeo box B5 (HOXB5) suppressed aggressive behaviors of breast cancer by inhibiting the Wnt/β-catenin pathway [15]. Chen et al. demonstrated that preferentially expressed antigen in melanoma (PRAME) knockdown restrained cervical cancer growth and increased cell apoptosis via suppressing the Wnt/β-catenin pathway [16]. Furthermore, Pandi et al. proved that ANLN expression was correlated with the Wnt/β-catenin pathway in gastric cancer [17]. Therefore, we supposed that ANLN might modulate cervical cancer development through the Wnt/β-catenin pathway.
Thus, the research elucidated the function of ANLN on cervical cancer development and metastasis and ascertained the mechanism.
GEO (https://www.ncbi.nlm.nih.gov/geo) (accessed on 31 December 2024) is a data collection, processing, and normalization platform. The datasets GSE192897, GSE138080, and GSE166466 that contained normal and cervical cancer samples were downloaded from the GEO website and analyzed differentially expressed genes. UALCAN (http://ualcan.path.uab.edu/cgi-bin/ualcan-res.pl) (accessed on 31 December 2024) is a bioinformatics webserver that can analyze TCGA data [18]. The ANLN expression level in subgroups of cervical cancer was investigated using UALCAN. Besides, the associations of ANLN expression and patient survival of cervical cancer were explored using UALCAN with p < 0.05 as statistical significance.
One hundred diagnosed primary cervical cancer patients with different pathological Federation International of Gynecology and Obstetrics (FIGO) grades were enrolled in this research. Experienced pathologists verified all histologic diagnoses. Individuals who received chemotherapy or radiotherapy before tissue collection, as well as those with incomplete clinical information or follow-up data, were excluded from the study. For each patient, both the tumor and corresponding normal tissues were harvested and preserved in liquid nitrogen. In subsequent analyses, tumor tissues were directly matched with their paired normal tissues on a one-to-one basis. Written informed consent was obtained from the donor. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Medical Ethics Committee of Jiangxi Maternal and Child Health Hospital (Approval No: EC-KY-202320). All methods were performed in accordance with the relevant guidelines and regulations.
The fixed and embedded tissue samples were prepared into 5 µm sections. Antigen retrieval was carried out by heating cervical tissue sections in a 0.01 M citrate buffer at 100°C for 5 min. To inhibit endogenous peroxidase activity prior to immunohistochemical analysis, the cervical samples were treated with 3% hydrogen peroxide at 25°C for 10 min. Following this, a 5% bovine serum solution (Sigma, #V900933) (Shanghai, China) was applied, and the specimens were incubated for 30 min at ambient temperature. The slices were processed by dewaxing, rehydration, antigen retrieval, endogenous peroxidase obstruction, and non-specific binding blockade and probed to anti-ANLN antibody (1:200) (Thermo Fisher Scientific, #PA5-82206) (Waltham, MA, USA) overnight at 4°C. After the washing step, the slices were treated with secondary antibody (1:1000) (Thermo Fisher Scientific, #SA5-10198) (Waltham, MA, USA) for 30 min at 25°C and dyed with DAB reagent (Beyotime, #ST067) (Shanghai, China). The stained slices were analyzed utilizing the confocal microscope (Zeiss, #LSM700) (Gottingen, Germany). The integral optical density (IOD) was quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA). ImageJ 1.6.0 (National Institutes of Health, Bethesda, MD, USA) was used to analyze the data on Immunohistochemistry.
2.4 Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
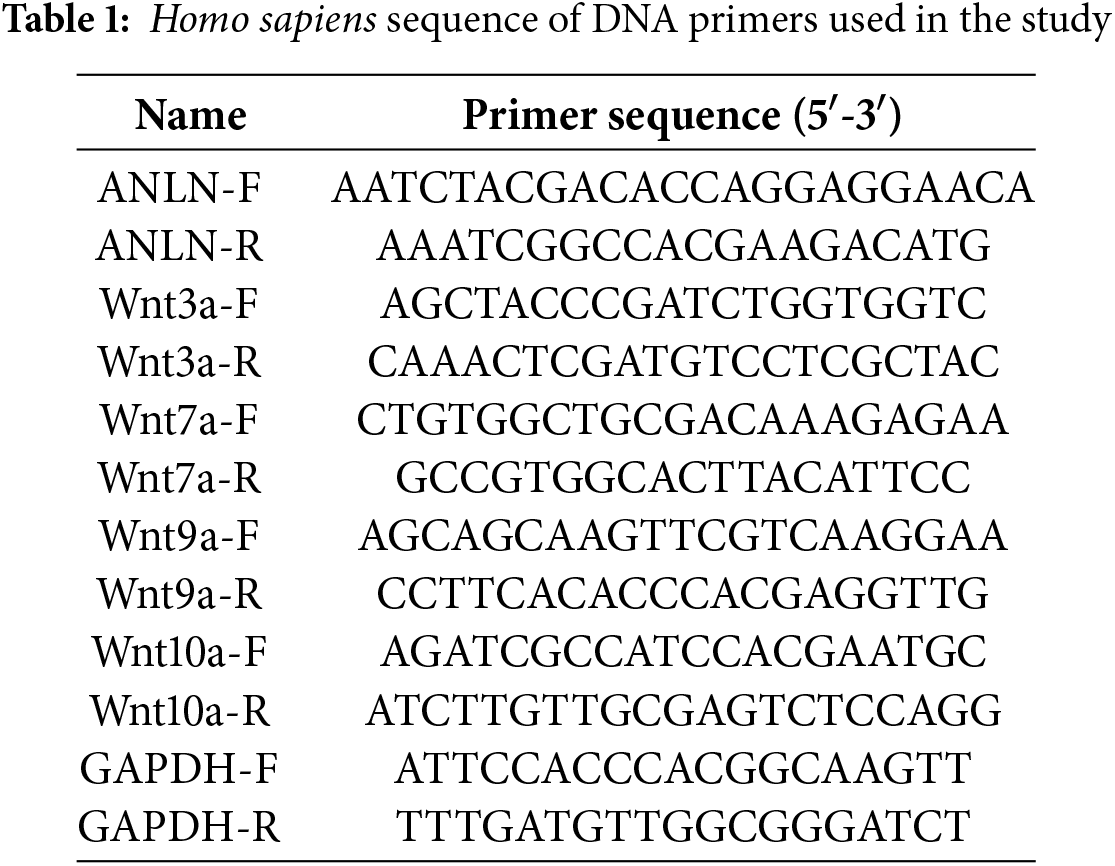
TRIzol (Invitrogen, #15596018CN) (Carlsbad, CA, USA) was used to extract RNA. cDNA Synthesis Kit (Beyotime, #D7168M) (Shanghai, China) was used to synthesize cDNA. qRT-PCR assay was carried out in the presence of the SYBR Green qPCR Mix (Beyotime, #D7260) (Shanghai, China). The 2−ΔΔCt method was used to calculate the relative mRNA levels. Table 1 shows these primer sequences.
Protein samples were obtained using RIPA (Beyotime, #P0013B) (Shanghai, China). Total proteins were then separated by 10% SDS-PAGE gel and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (Beyotime, #FFP39) (Shanghai, China). After obstructed non-specific binding using 5% nonfat dry milk for 1 h at 25°C, PVDF membranes were probed to anti-ANLN (1:1000) (Thermo Fisher Scientific, #PA5-82206) (Waltham, MA, USA), anti-Bad (1:1000) (Abcam, #ab62465) (Cambridge, MA, UK), anti-Bcl-2 (1:1000) (Abcam, #ab196495) (Cambridge, MA, UK), anti-Caspase-3 (1:500) (Abcam, #ab49822) (Cambridge, MA, UK), anti-E-cadherin (1:1000) (Abcam, #ab227639) (Cambridge, MA, UK), anti-N-cadherin (1:1000) (Abcam, #ab245117) (Cambridge, MA, UK), anti-Vimentin (1:2000) (Abcam, #ab137321) (Cambridge, MA, UK), anti-PCNA (1:2000) (Abcam, #ab18197) (Cambridge, MA, UK), anti-P21 (1:1000) (Abcam, #ab227443) (Cambridge, MA, UK), anti-CyclinD1 (1:1000) (Abcam, #ab226977) (Cambridge, MA, UK), anti-Wnt3a (1:1000) (Abcam, #ab28472) (Cambridge, MA, UK), anti-Wnt7a (1:1000) (Abcam, #ab100792) (Cambridge, MA, UK), anti-Wnt9a (1:1000) (Abcam, #ab176973) (Cambridge, MA, UK), anti-β Catenin (1:1000) (Abcam, #ab16051) (Cambridge, MA, UK), anti-p-β Catenin (1:1000) (Abcam, #ab75777) (Cambridge, MA, UK), anti-Tubulin (1:5000) (Abcam, # ab7291) (Cambridge, MA, UK) and anti-GAPDH (1:5000) (Abcam, #ab245355) (Cambridge, MA, UK) antibodies for 12 h at 4°C. The bands were visualized by applying the ECL chemiluminescence Kit (Beyotime, #P0018S) (Shanghai, China), and the data was quantitated by utilizing Image J (NIH, Bethesda, MD, USA).
Human cervical epithelial cells (HCEC) and human cervical cancer cells CaSki, C-33A, SiHa, HeLa-S3, and HeLa were provided by the BeNa Culture Collection (Beijing, China). All utilized cells in this study were free from mycoplasma contamination. Cells were kept in Dulbecco’s modification of Eagle’s medium (DMEM) (Gibco, #C11330500BT) (Grand Island, NY, USA) with the addition of 10% fetal bovine serum (FBS) (Gibco, #CB14808348) (Grand Island, NY, USA) with 1% streptomycin and penicillin, at 37°C with 5% CO2. In specific experiments, cells were incubated with Wnt pathway inhibitor IWP-2 (10 nM) (MCE, #HY-13912) for 24 h.
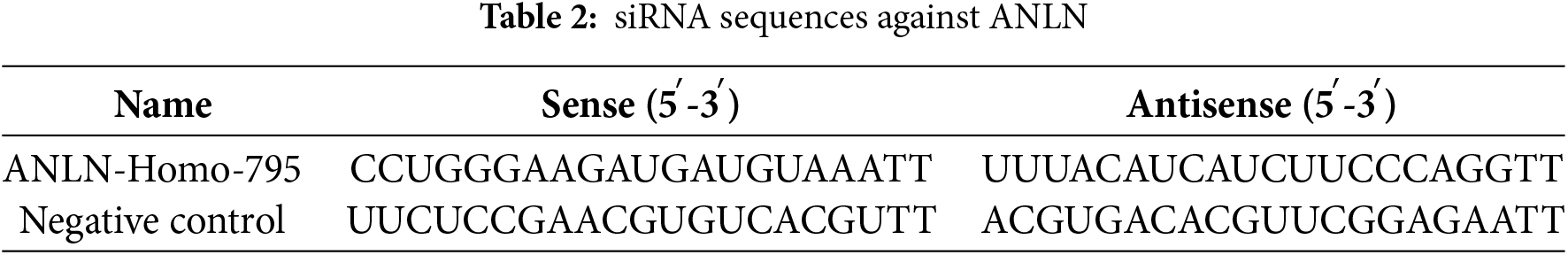
The siRNAs against ANLN (si-ANLN), ANLN overexpression plasmid (OV-ANLN), and corresponding negative controls were compounded at RiboBio (Guangzhou, China) (Table 2). Following the supplier’s protocol, these plasmids were introduced into CaSki or SiHa cells by applying Lipofectamine 3000 (Invitrogen, #L3000008) (MA, USA). Forty-eight hours later, transfected cells were obtained to conduct subsequent experiments. The transfection efficiency was verified using qRT-PCR.
EdU Cell Proliferation Kit with Alexa Fluor 488 (Beyotime, #P0176) (Shanghai, China) was used to detect the ability of cell proliferation. Cells were plated at a concentration of 1 × 105 cells per well in a 24-well plate and subsequently incubated with 10 µM EdU for 2 h. After the fixation step using 4% polyformaldehyde and the permeabilization step using 0.3% Triton X-100, cells were treated with Click Additive Solution for 30 min without light and labeled with DAPI (Beyotime, #C1002) (Shanghai, China) for 10 min at 25°C. The results were analyzed utilizing the confocal microscope (LSM700, Zeiss) (Gottingen, Germany).
The Annexin V-FITC Apoptosis Detection Kit (Beyotime, #C1062) (Shanghai, China) was applied to assess apoptosis following the supplier’s procedure. Cells at a concentration of 6 × 105 cells/mL were harvested and prepared for cell suspension. After the centrifugation step, cells were resuspended in Annexin V-FITC binding buffer. Afterward, cells were dyed with 5 μL of Annexin V-FITC and 10 μL PI for 15 min without light at room temperature. The apoptotic cells were determined utilizing the Flow Cytometer (BD Biosciences) (San Jose, CA, USA). The apoptosis rate was expressed as the total apoptosis, a sum of the early and late apoptosis rates.
Cells (1 × 105) were plated into the top chamber of Transwell with Matrigel (Solarbio, M8370, Beijing, China) coated (invasion assay) or without Matrigel (migration assay) and maintained in FBS-free DMEM. The bottom chamber was added to DMEM plus 10% FBS. The chambers were placed at 37°C with 5% CO2 for 24 h. After that, migrated and invaded cells were immobilized and dyed with 0.5% crystal violet for observation using the light microscope (Axiostar Plus, Zeiss) (Gottingen, Germany).
Data collected from triplicate were expressed as mean ± standard deviation (SD) and analysis was achieved by SPSS Statistics 22.0 (Chicago, IL, USA). The significance between the tumor and paired normal tissues was verified by a paired t-test. Differences among multiple groups were conducted by one-way ANOVA with Tukey’s post hoc test. The survival analysis of cervical cancer patients was performed by Kaplan-Meier analysis. p < 0.05 was identified as statistically significant.
3.1 High ANLN Expression Correlated with Poor Prognosis of Cervical Cancer
To clarify the action of ANLN in cervical cancer, the expression level of ANLN in cervical cancer was first determined. Based on the analysis of GSE192897, GSE138080, and GSE166466 data sets, ANLN was the overlapping gene in these three datasets, and its differential expression presented statistical significance (Fig. 1A). Besides, the ANLN expression in subgroups of cervical cancer was investigated by the UALCAN analysis. Results proved that ANLN was elevated in cervical cancer cases vs. normal control in sub-group analyses (Fig. 1B,E). UALCAN analysis also revealed that elevated ANLN correlated with poor survival of cervical cancer cases (Fig. 1F). To verify the results of bioinformatics analysis, the tumor and paired normal tissues of 100 cervical cancer cases were obtained for further analysis. Immunohistochemistry results presented the enhanced ANLN level in tumor tissues (p < 0.01) (Fig. 1G,H). Based on the median value of IOD as the cutoff, 100 cases were allocated into the high ANLN group and the low ANLN group. Cases with high ANLN levels exhibited poor survival and high dead proportion (Fig. 1I,J). Additionally, the ANLN mRNA and protein levels were enhanced in tumor tissues (p < 0.01) (Fig. 1K,L). Therefore, ANLN was enhanced and correlated with poor prognosis of cervical cancer cases.
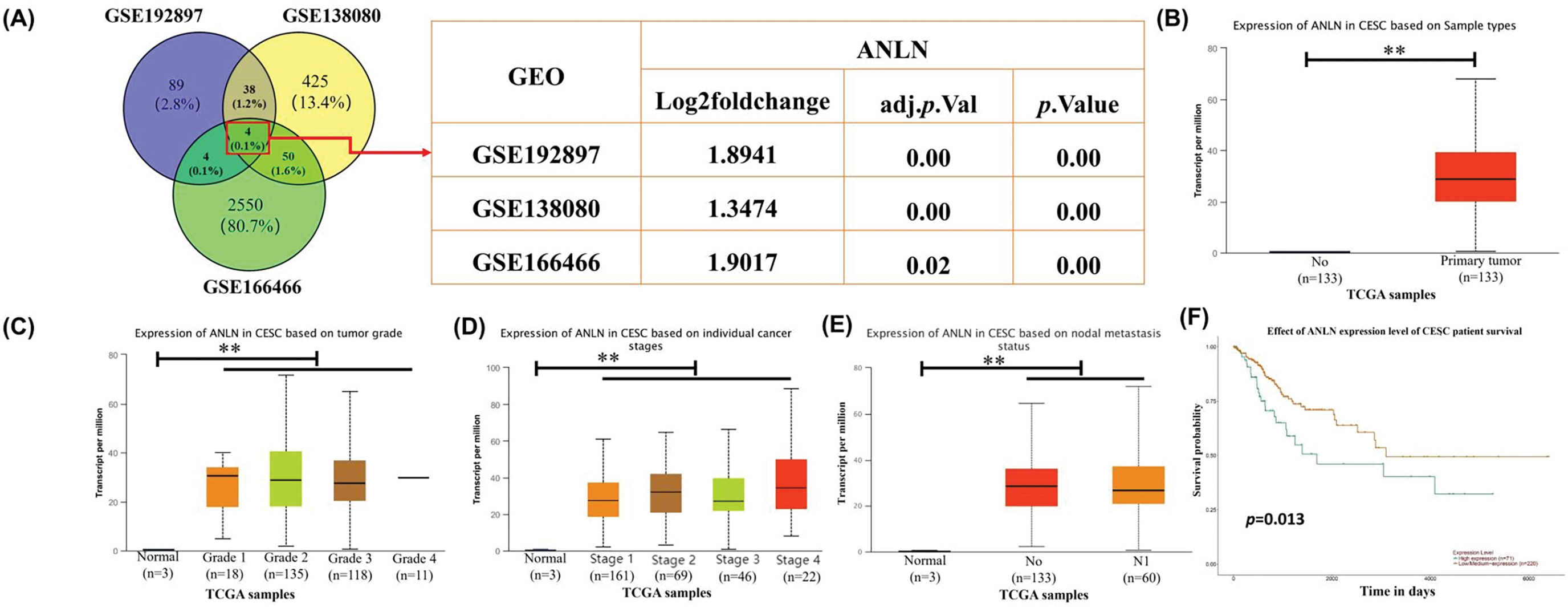
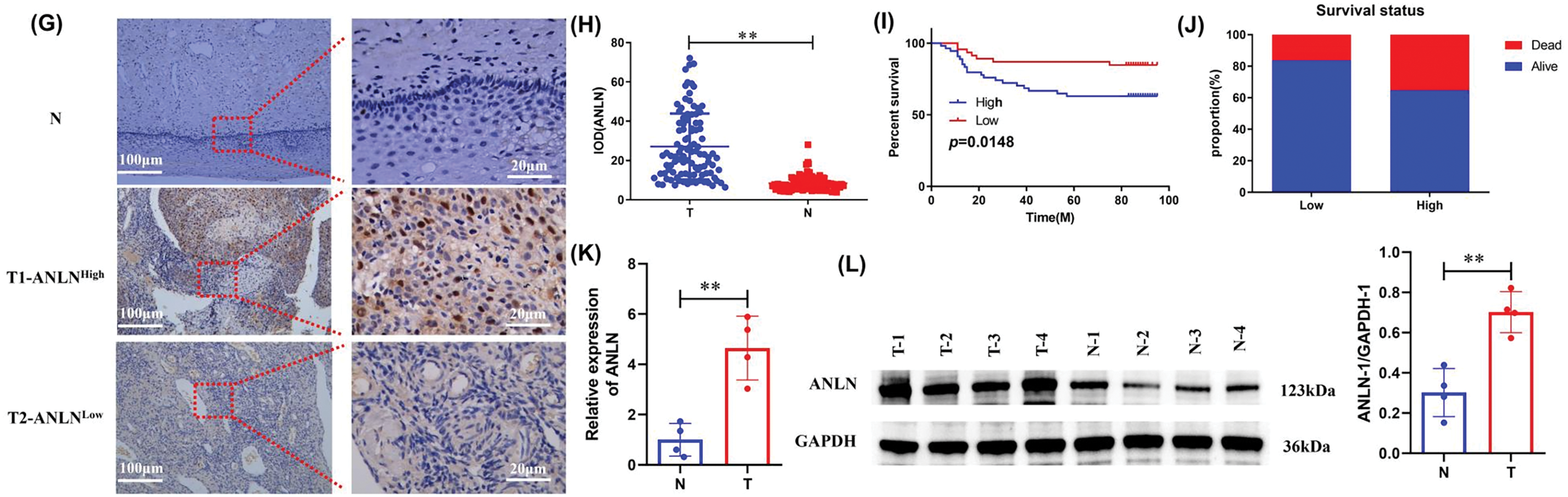
Figure 1: High ANLN expression is related to poor prognosis in cervical cancer patients. (A) Venn diagram of potential overlapping genes among GSE192897, GSE138080, and GSE166466 datasets and the fold change of ANLN within the three GEO datasets. (B–F) The ANLN expression in sub-group analyses of cervical cancer and the association of ANLN expression and patient survival of cervical cancer using UALCAN analysis. (G) Representative immunohistochemistry images of ANLN expression in cervical cancer cases. (H) The IOD of immunohistochemistry results on ANLN expression in 100 cervical cancer cases was presented. (I) The relation between ANLN level and the survival of cervical cancer cases was confirmed utilizing Kaplan-Meier analysis. (J) The survival status of 100 cervical cancer cases with different ANLN expressions was determined. (K) The ANLN mRNA level in cervical cancer patients was determined utilizing qRT-PCR. (L) Representative western blot results of ANLN in 4 cervical cancer patients were exhibited. **p < 0.01. N: paired normal tissues, T: tumor tissues
3.2 ANLN Promotes Cervical Cancer Cell Aggressive Behaviors and Restrains Cell Apoptosis
To validate the function of ANLN, we examined the expression of ANLN in five commonly used human cervical cancer cell lines (SiHa, HeLa, CaSki, C-33A, and HeLa-S3 cells) and human cervical epithelial cells (HCEC cell) to select the cells with the highest and lowest ANLN expression. ANLN exhibited the highest level in CaSki cells and the lowest in SiHa cells compared to C-33A, HeLa-S3, and HeLa cells (p < 0.05) (Fig. 2A,B). Therefore, CaSki and SiHa cells were chosen for follow-up experiments. CaSki cells were introduced into si-ANLN to knockdown ANLN, and SiHa cells were transfected with OV-ANLN to overexpress ANLN, which was verified using qRT-PCR and Western Blot (p < 0.01) (Fig. 2C,F). After that, the influences of altered ANLN on cervical cancer aggressive behaviors were explored. It was observed that silenced ANLN significantly restrained cell proliferation of CaSki cells, and overexpressed ANLN promoted cell proliferation of SiHa cells (p < 0.05) (Fig. 2G,H).
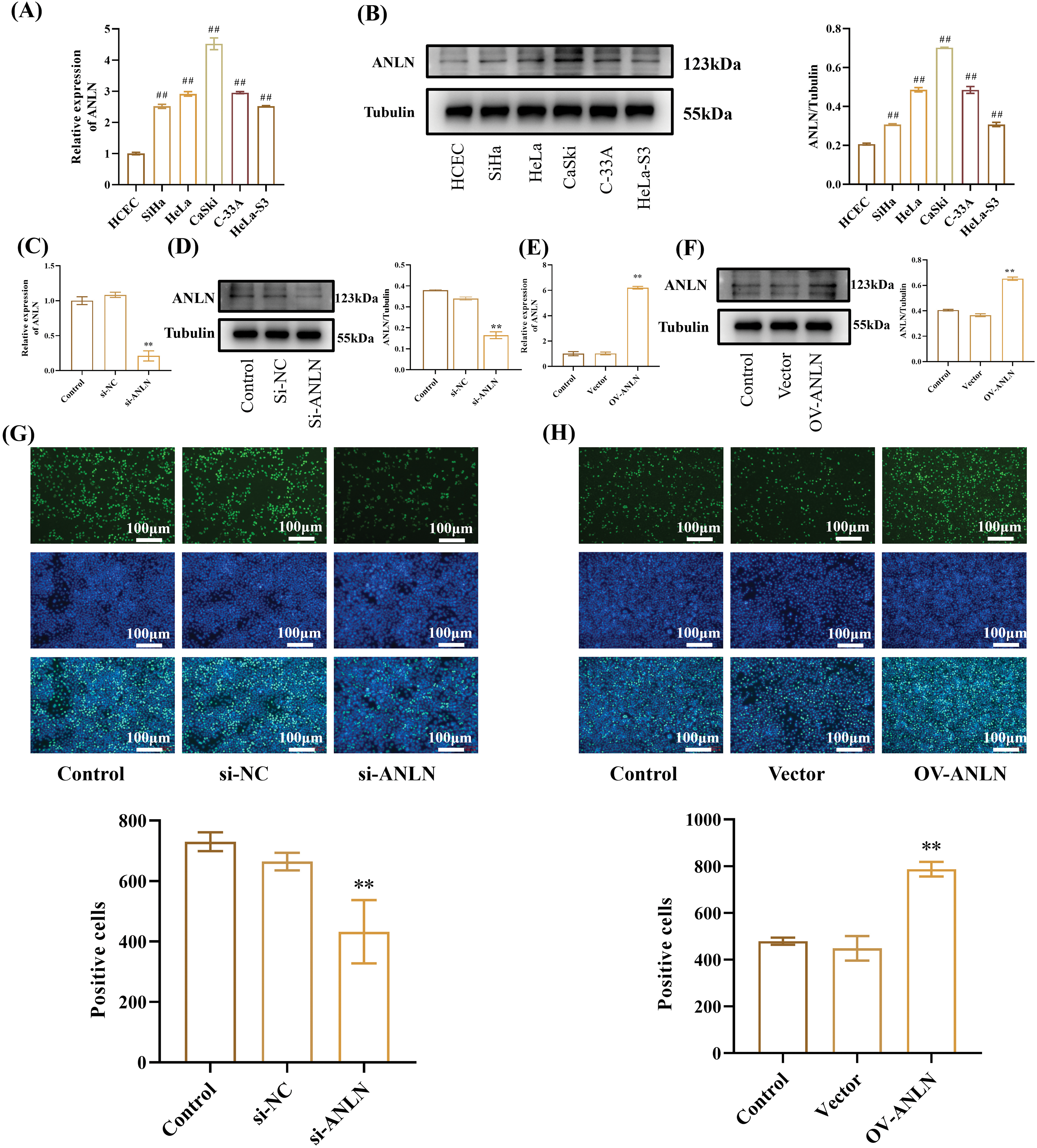
Figure 2: ANLN promotes cervical cancer cell proliferation. (A) The ANLN mRNA level in HCEC, SiHa, HeLa, CaSki, C-33A, and HeLa-S3 cells was tested utilizing qRT-PCR. GAPDH was chosen as the reference to obtain the relative expression. (B) The ANLN protein level in HCEC, SiHa, HeLa, CaSki, C-33A, and HeLa-S3 cells was measured utilizing Western Blot. (C and D) The ANLN expression was determined after transfected with si-ANLN using qRT-PCR and Western Blot in CaSki cell. (E and F) The ANLN expression was determined after transfected with OV-ANLN using qRT-PCR and Western Blot in SiHa cells. (G and H) The proliferation of CaSki and SiHa cells after being introduced with si-ANLN and OV-ANLN was assessed using EdU staining assay, respectively. ##p < 0.01 vs. HCEC group; **p < 0.01 vs. control group
Moreover, silenced ANLN induced CaSki cell apoptosis and enforced ANLN-suppressed SiHa cell apoptosis (p < 0.01) (Fig. 3A,B). Down-regulated ANLN restrained CaSki cell migration and invasion (p < 0.05) (Fig. 3C,F). In contrast, overexpressed ANLN enhanced SiHa migration and invasion (p < 0.01) (Fig. 3C,F). Furthermore, the influences of altered ANLN on the expressions of apoptosis-related proteins (Bad, Bcl-2, and Cleaved Caspase-3), invasion-related proteins (E-cadherin, N-cadherin, and vimentin), and proliferation-related proteins (PCNA, P21, and cyclinD1) were explored. Results proved that silenced ANLN enhanced the Bad, Caspase-3, E-cadherin, and p21 levels and restrained the Bcl-2, N-cadherin, Vimentin, PCNA, and CyclinD1 levels in CaSki cells (p < 0.01) (Fig. 3G,P). The opposite results were obtained in SiHa cells after overexpressing ANLN (p < 0.01) (Fig. 3G,P). Thus, ANLN accelerated cervical cancer aggressive behaviors and suppressed cell apoptosis.
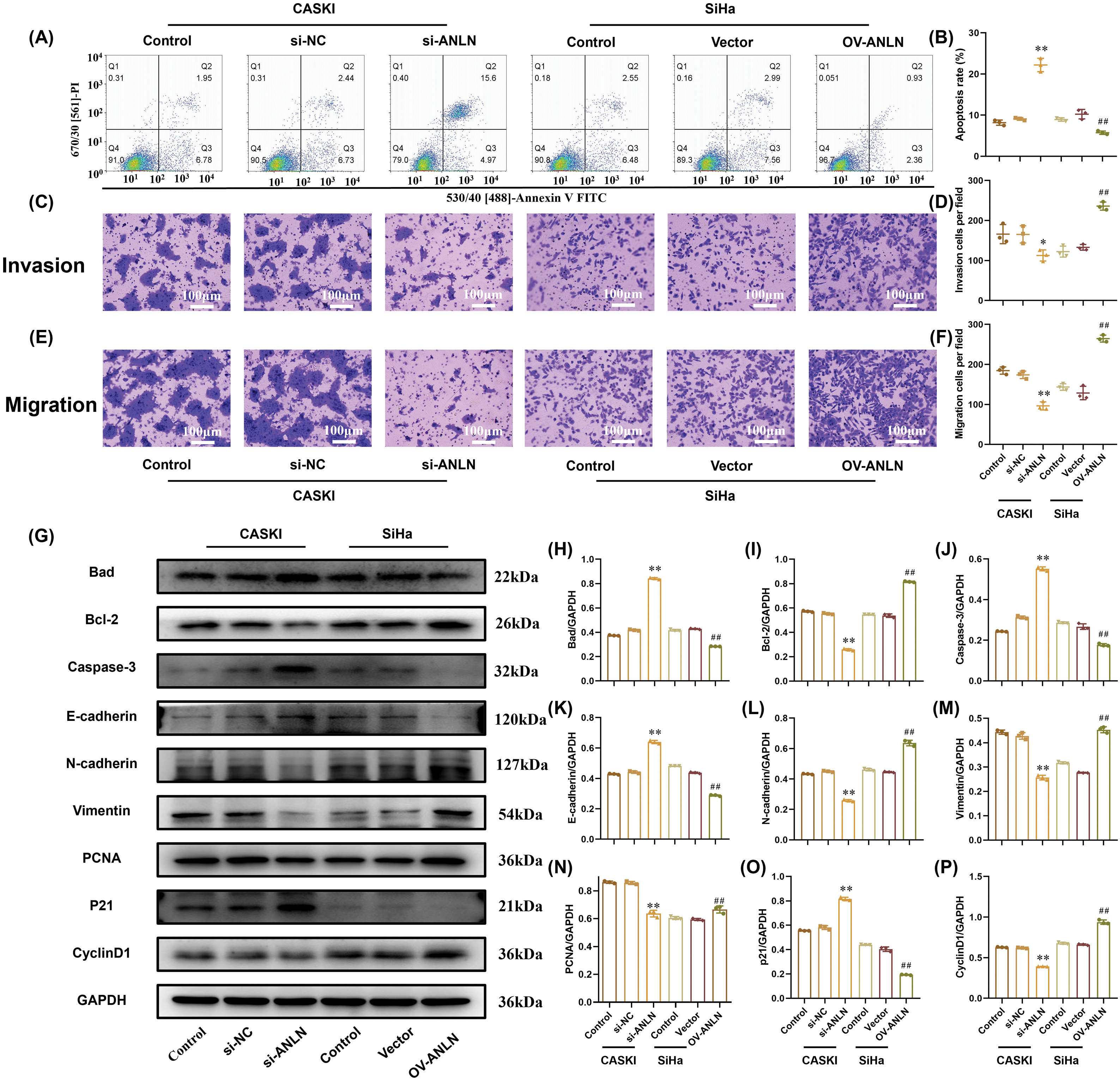
Figure 3: ANLN promotes cervical cancer aggressive behaviors and suppresses cell apoptosis. (A and B) Cell apoptosis of CaSki and SiHa cells after being introduced into si-ANLN and OV-ANLN were evaluated using flow cytometry, respectively. (C and D) Cell invasion ability of CaSki and SiHa after being introduced into si-ANLN and OV-ANLN, respectively, was assessed utilizing Transwell assay. (E and F) Cell migration of CaSki and SiHa after being introduced into si-ANLN and OV-ANLN was verified by Transwell assay, respectively. (G–P) The protein levels of Bad, Bcl-2, Caspase-3, E-cadherin, N-cadherin, Vimentin, PCNA, P21, CyclinD1 in CaSki and SiHa cells after being introduced into si-ANLN and OV-ANLN respectively was determined utilizing western blot. *p < 0.05, **p < 0.01 vs. si-NC group. ##p < 0.01 vs. vector group
3.3 ANLN Promotes Wnt/β-catenin Signaling Activation in Cervical Cancer
Given that the Wnt/β-catenin signaling was critical in cancer cell proliferation and invasions, the role of ANLN in activating the Wnt/β-catenin signaling was expounded after altering the ANLN expression. Results showed that down-regulated ANLN suppressed the Wnt3a, Wnt9a, and Wnt10a mRNA levels and promoted the Wnt7a level in CaSki cells (p < 0.01) (Fig. 4A,D). Instead, overexpressed ANLN enhanced the Wnt3a, Wnt9a, and Wnt10a mRNA levels and repressed the Wnt7a level in SiHa cells (p < 0.01) (Fig. 4A,D). Besides, silenced ANLN inhibited the Wnt3a, Wnt9a, and β-catenin phosphorylation protein levels and increased the Wnt7a level in CaSki cells (p < 0.01) (Fig. 4E,J). The opposite results were obtained in SiHa cells after overexpressed ANLN (p < 0.01) (Fig. 4E,J). Hence, ANLN promoted the Wnt/β-catenin signaling activation.
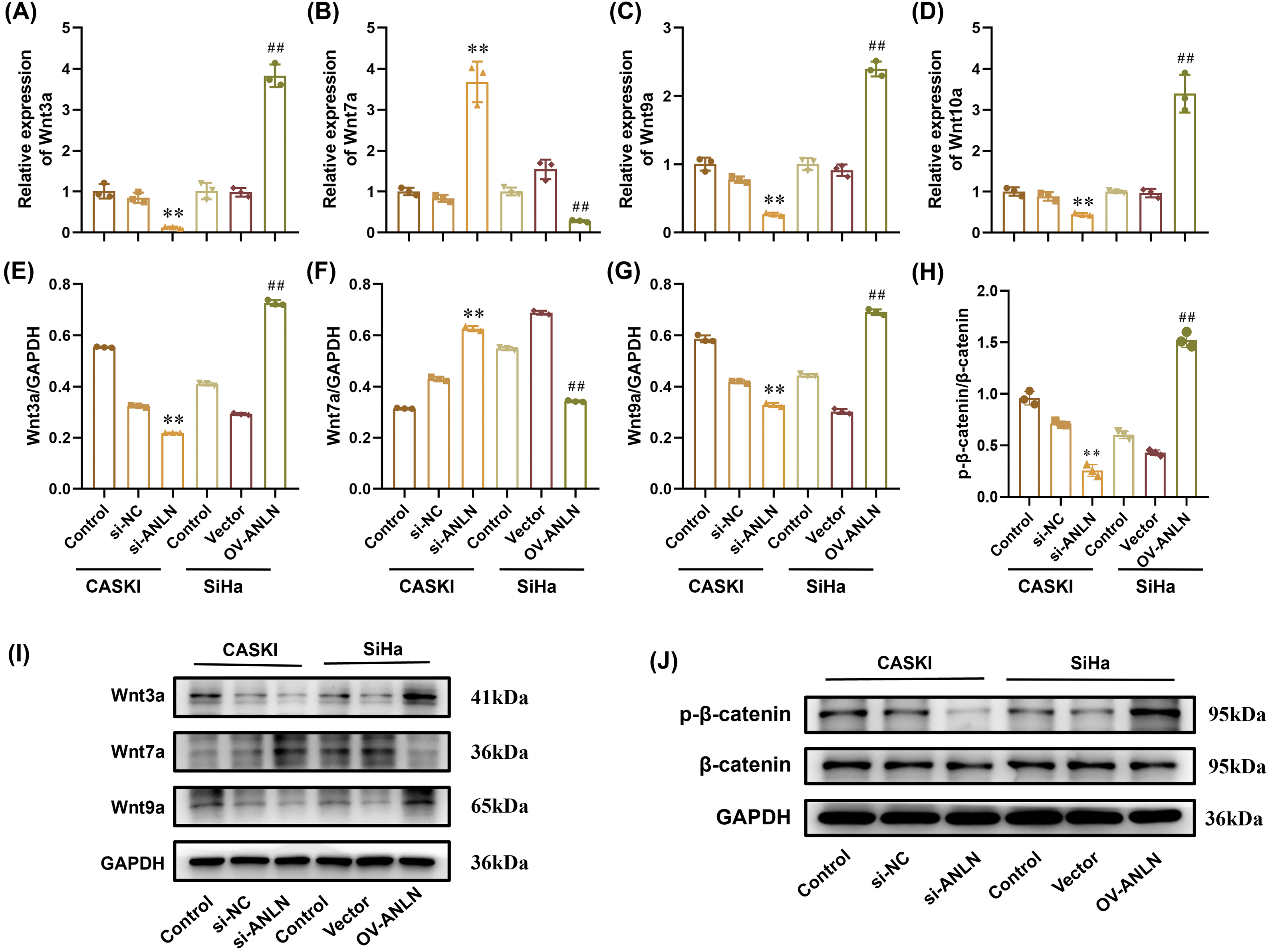
Figure 4: ANLN promotes Wnt/β-catenin pathway activation in cervical cancer. (A–D) The Wnt3a, Wnt7a, Wnt9a, and Wnt10a mRNA levels in CaSki and SiHa cells after being introduced into si-ANLN and OV-ANLN were assessed utilizing qRT-PCR, respectively. (E–J) The Wnt3a, Wnt7a, Wnt9, β-catenin, and β-catenin phosphorylation protein levels in CaSki and SiHa cells after being introduced into si-ANLN and OV-ANLN were evaluated utilizing western blot, respectively. **p < 0.01 vs. si-NC group. ##p < 0.01 vs. vector group
3.3.1 ANLN Promotes Cervical Cancer Cell Aggressive Behaviors and Suppresses Cell Apoptosis via Activating the Wnt/β-Catenin Pathway
To interpret the mechanism of ANLN on the modulation of cervical cancer progression, cervical cancer cells transfected with cervical cancer si-ANLN or OV-ANLN were treated with Wnt pathway inhibitor IWP-2. Results demonstrated that the inhibitory role of silenced ANLN in CaSki cell proliferation capacity was strengthened by IWP-2 (p < 0.05) (Fig. 5A,E). Conversely, the facilitation effect of overexpressed ANLN on SiHa cell proliferation was inhibited by IWP-2 (p < 0.05) (Fig. 5A,E). Besides, IWP-2 treatment enhanced the promotion role of silenced ANLN in the apoptosis of CaSki cells and reversed the inhibitory effect of overexpressed ANLN on apoptosis of SiHa cells (p < 0.05) (Fig. 5B,F). Unexpectedly, IWP-2 mildly abolished the inhibitory effect of silenced ANLN on CaSki cell invasion (p < 0.05) (Fig. 5C,G). Furthermore, the promotion effect of overexpressed ANLN on SiHa cell invasion was abrogated by IWP-2 (p < 0.01) (Fig. 5C,G). Similarly, overexpressed ANLN promoted SiHa cell migration, which was abolished by IWP-2 (p < 0.05) (Fig. 5D,H). However, IWP-2 had no effect on the inhibition role of silenced ANLN in CaSki cell migration (Fig. 5D,H). Collectively, ANLN facilitates cervical cancer cell aggressive behaviors and suppresses cell apoptosis via activating the Wnt/β-catenin signaling.
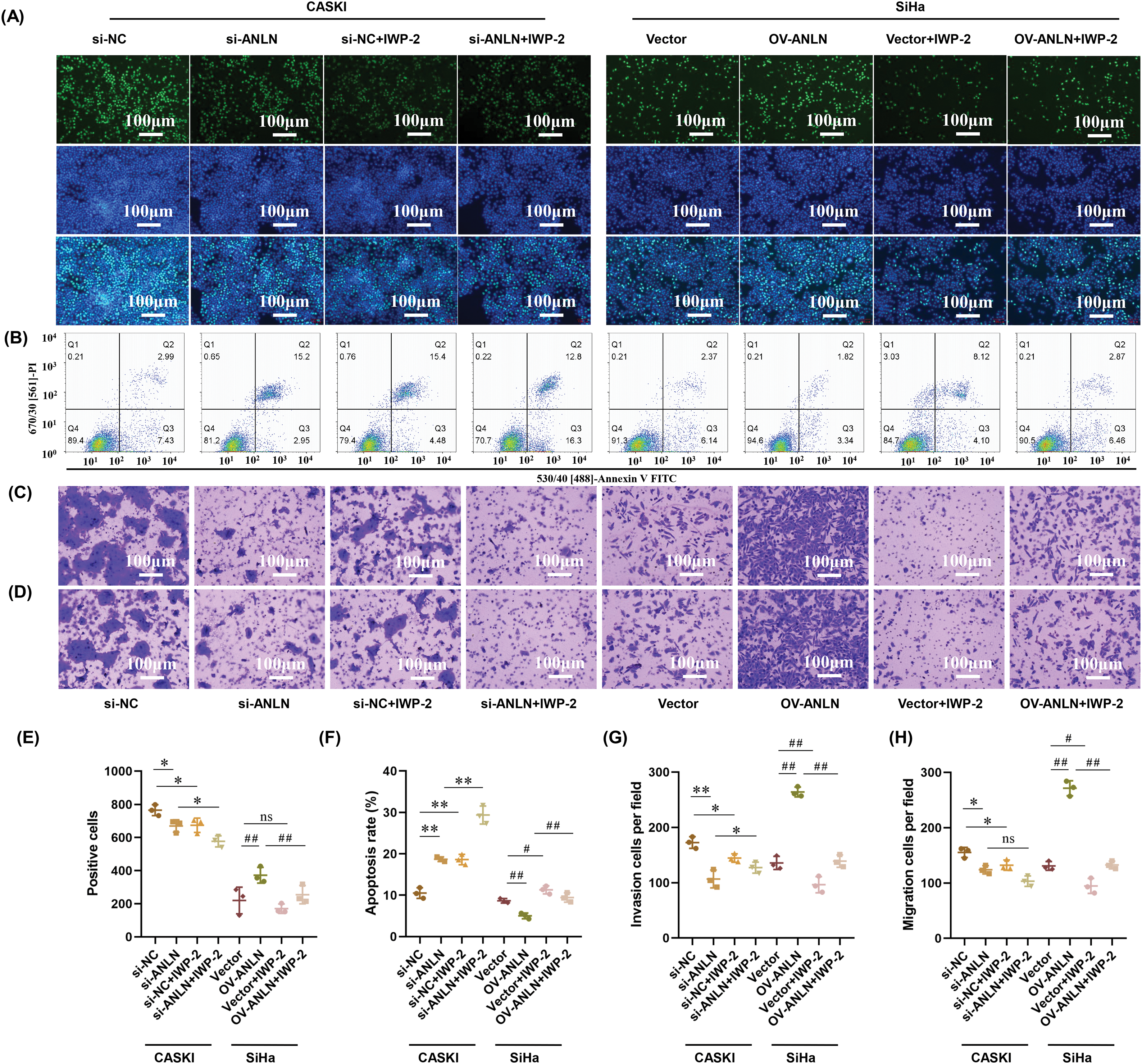
Figure 5: ANLN promotes cervical cancer cell aggressive behaviors and restrains cell apoptosis via the Wnt/β-catenin signaling. (A) The proliferation of CaSki and SiHa cells after being introduced with si-ANLN and OV-ANLN and treated with IWP-2 was measured utilizing EdU staining assay, respectively. (B) Cell apoptosis of CaSki and SiHa cells after being introduced into si-ANLN and OV-ANLN and treated with IWP-2 was evaluated using flow cytometry, respectively. (C) Cell invasion ability of CaSki and SiHa after being introduced into si-ANLN and OV-ANLN and treated with IWP-2 was assessed using Transwell assay, respectively. (D) Cell migration ability of CaSki and SiHa after being introduced into si-ANLN and OV-ANLN and treated with IWP-2 was assessed using Transwell assay, respectively. (E) Quantified results of EdU staining assay. (F) Quantified results of cell apoptosis. (G) Quantified results of Transwell invasion assay. (H) Quantified results of Transwell migration assay. *p < 0.05, **p < 0.01 vs. si-NC group or si-ANLN group. #p < 0.05, ##p < 0.01 vs. vector group or OV-ANLN group. ns: no significant differences
Cervical cancer is the growth of malignant cells in the cervix. However, the development of cervical cancer has not been elucidated clearly. ANLN was identified as a cancer modulator in various cancers, such as oral, colorectal, and breast cancers [6,10,11]. However, the action of ANLN on cervical cancer and its mechanism remained elusive. To explicate the effect of ANLN on cervical cancer, the ANLN expression in tumor tissues was evaluated. The findings proved that ANLN was highly expressed in cervical cancer tumor tissues, consistent with previous studies [19–21]. Sheng et al. proved that ANLN was strengthened in lung adenocarcinoma [19]. Wang et al. identified that ANLN was elevated in colorectal cancer [20]. Zhang et al. demonstrated that ANLN was enhanced in hepatocellular carcinoma [21]. Furthermore, we found that elevated ANLN correlated with poor prognosis in cervical cancer patients. Similarly, Xu et al. discovered that lung adenocarcinoma cases with elevated ANLN presented shorter survival [22]. Wang et al. proved that the highly expressed ANLN was correlated with poor prognosis in pancreatic cancer [23]. These findings indicated that ANLN was elevated in cervical cancer, and elevated ANLN was related to poor prognosis. Meanwhile, the expression of ANLN and its relationship with the prognosis of cases exhibit high consistency in various cancers, providing the possibility for ANLN to act as a potential therapeutic target.
Proliferation is a requisite feature of cancer cells and a vital part of cancer development and progression [24]. Proliferation is usually manifest by alteration of cell cycle-related protein levels such as PCNA, p21, and cyclinD1 [24–27]. In this study, cervical cancer cell proliferation was studied to explain the influence of ANLN on cancer progression. Results revealed that silenced ANLN inhibited cervical cancer cell proliferation ability, and suppressed the PCNA and CyclinD1 levels. The opposite results were obtained in SiHa cells after overexpression of ANLN. In other words, ANLN facilitated cervical cancer proliferation. The finding agreed with the published research [10,28]. Liu et al. revealed that silenced ANLN repressed colorectal carcinoma cell proliferation [10]. Wang et al. found that knockdown of ANLN restrained breast cancer proliferation [28]. Furthermore, migration and invasion are critical processes in cancer metastasis, which is a hallmark of malignancy [29]. Epithelial-mesenchymal transition (EMT), a process by which epithelial cells get invasive mesenchymal cell characteristics, is strongly related to cancer cell invasion and metastasis, and it is usually accompanied by expression changes of biomarkers such as E-cadherin, N-cadherin and vimentin [30]. ANLN was demonstrated to accelerate cancer cell migration and invasion in some cancers, such as lung adenocarcinoma, pancreatic cancer, and bladder urothelial carcinoma [22,23,31]. Therefore, we inferred that ANLN might regulate migration and invasion ability of cervical cancer. Indeed, results revealed that silenced ANLN restrained cervical cancer cell migration and invasion, enhanced the E-cadherin level, and suppressed the N-cadherin and Vimentin levels. Apoptosis is programmed cell death that exerts a prominent function in tissue development and homeostasis [32]. Cancer cells typically have impaired apoptotic signaling, which promotes tumor development and metastasis [32]. This research found that silenced ANLN induced cervical cancer cell apoptosis and overexpressed ANLN inhibited cell apoptosis. Similarly, the knockdown of ANLN increased cell apoptosis in oral cancer [6]. Silenced ANLN promoted cell apoptosis of nasopharyngeal carcinoma [33]. Taken together, these findings indicated that ANLN accelerated cervical cancer aggressive behaviors and suppressed cell apoptosis.
Wnt/β-catenin pathway usually presents highly activated in cancers and exerts critical functions in cancer cell proliferation, invasion, apoptosis, and angiogenesis [13,34]. Inhibition of the Wnt/β-catenin signaling is regarded as a valuable cancer therapy strategy [13]. Pandi et al. found that the ANLN expression was related to the Wnt/β-catenin signaling in gastric cancer [17]. Thus, we inferred that ANLN might affect the Wnt/β-catenin signaling in cervical cancer. As expected, silenced ANLN restrained the Wnt3a, Wnt9a, and Wnt10a levels and promoted the Wnt7a level. Among Wnt ligands, Wnt3a, Wnt9a, and Wnt10a are demonstrated to activate the Wnt/β-catenin pathway [35,36]. Wnt7a can trigger both the canonical and non-canonical pathways [37]. The canonical pathway includes Wnt binding to Frizzled (Fz), a negative modulator of β-catenin accumulation [37,38]. The non-canonical pathway contains the planar cell polarity pathway and JNK pathway [37,39]. However, unlike the increased expression of some other Wnt family members, Wnt7a is usually downregulated in some cancers, such as lung cancer [37,40].
In this study, the action of ANLN on Wnt7a was inconsistent with that on Wnt3a, Wnt9a, and Wnt10a, which may be due to the opposite basic expression of Wnt7a in cervical cancer, or possibly because Wnt7a triggers non-canonical pathway in cervical cancer. Furthermore, we investigated whether the Wnt/β-catenin signaling mediated the regulation function of ANLN on cervical cancer progression using the Wnt inhibitor. The findings revealed that overexpressed ANLN accelerated cervical cancer aggressive behaviors and suppressed cell apoptosis via the Wnt/β-catenin pathway. Up to now, this research reported that ANLN could regulate cervical cancer development via modulating the Wnt/β-catenin pathway (Fig. 6). Unexpectedly, the findings also revealed that the role of Wnt inhibitor IWP-2 in the regulation of silenced ANLN on cervical cell invasion and migration was opposite to that of cervical cell proliferation. We speculated that the action of Wnt7a may cause this phenomenon. Xu et al. discovered that Wnt7a exerted the opposite action on cell proliferation, migration, and invasion in non-small cell lung cancer (NSCLC) [37]. They revealed that Wnt7a overexpression strongly restrained cell proliferation but induced migration and invasion via activated the JNK pathway but not the β-catenin pathway in NSCLC [37]. The isoform of Wnts has 19 kinds, mainly including Wnt3a, Wnt5a, Wnt7a, Wnt8b, et al. The different subfamily of Wnts involved in ANLN-mediated cervical cancer makes it difficult to distinguish the specific isoform. From this study, we speculate the most possible subfamily of Wnts are Wnt3a and wnt9a involved in ANLN-mediated cervical cancer, which will be addressed in the subsequent research.
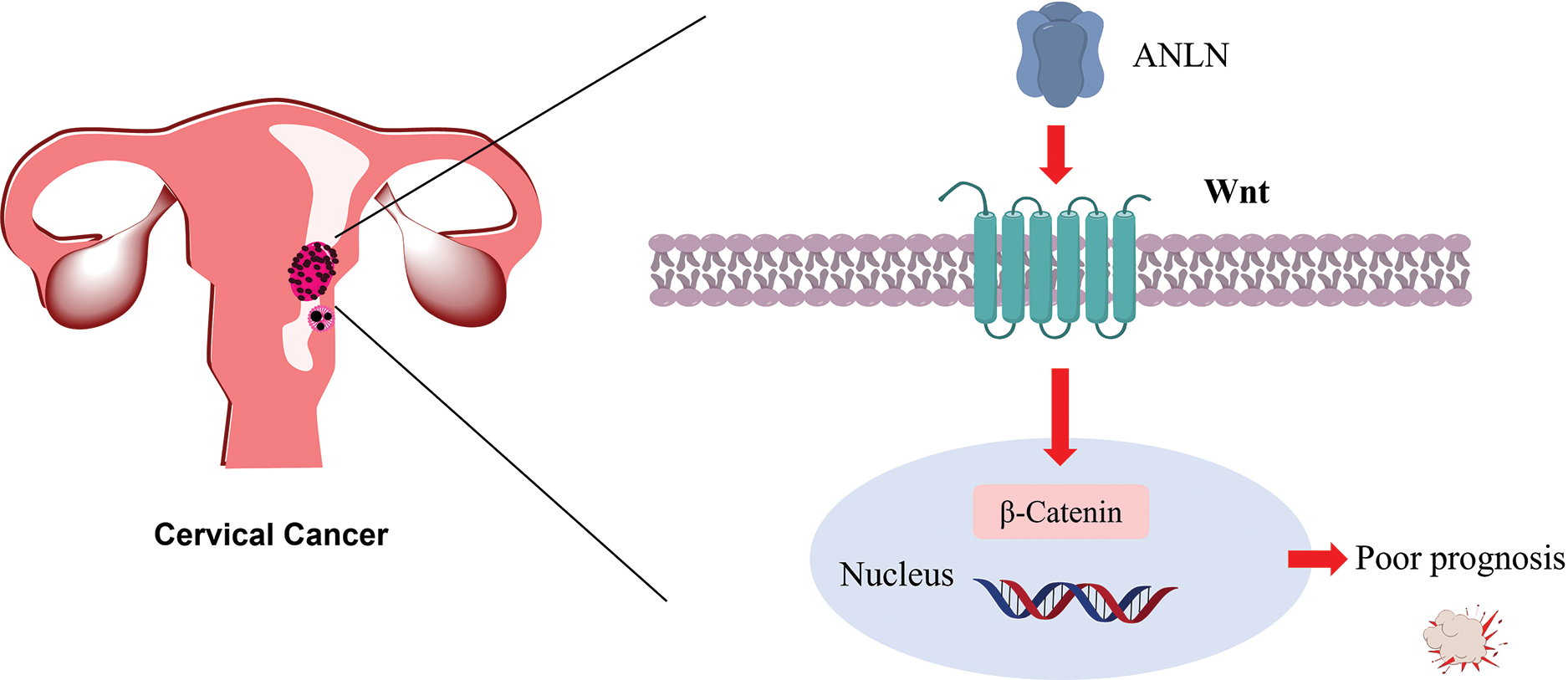
Figure 6: Diagram of ANLN promotes the poor prognosis of cervical cancer via the Wnt/β-catenin pathway
This research revealed that ANLN was enhanced in cervical cancer and related to poor prognosis. ANLN promoted cervical cancer cell proliferation, migration, and invasion and suppressed cell apoptosis via the Wnt/β-catenin pathway, which provides important clues for the future development of therapeutic strategies against ANLN from small molecule inhibitors and biomarkers. However, the study lacks studies at the animal level, which limits the persuasive power of ANLN to affect cervical cancer by regulating the Wnt/β-catenin pathway. In the future study, we will take more comprehensive studies to explore the effect of ANLN on cervical cancer.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Jianping Xiong and Ling Li; data collection: Lingling Zhang, Hualing Wang and Yawen Liu; analysis and interpretation of results: Lingling Zhang; draft manuscript preparation: Lingling Zhang and Jianping Xiong. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author, Jianping Xiong, upon reasonable request.
Ethics Approval: Written informed consent was obtained from the donor. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Committee of Jiangxi Maternal and Child Health Hospital (Approval No. EC-KY-202320). All methods were performed in accordance with the relevant guidelines and regulations.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [Google Scholar] [PubMed]
2. D’Oria O, Corrado G, Laganà AS, Chiantera V, Vizza E, Giannini A. New advances in cervical cancer: from bench to bedside. Int J Environ Res Public Health. 2022;19(12):7094. [Google Scholar] [PubMed]
3. Viveros-Carreño D, Fernandes A, Pareja R. Updates on cervical cancer prevention. Int J Gynecol Cancer. 2023;33(3):394–402. [Google Scholar]
4. Mustafa WA, Ismail S, Mokhtar FS, Alquran H, Al-Issa Y. Cervical cancer detection techniques: a chronological review. Diagnostics. 2023;13(10):1763. [Google Scholar] [PubMed]
5. Gennigens C, De Cuypere M, Hermesse J, Kridelka F, Jerusalem G. Optimal treatment in locally advanced cervical cancer. Expert Rev Anticancer Ther. 2021;21(6):657–71. [Google Scholar] [PubMed]
6. Wang B, Zhang XL, Li CX, Liu NN, Hu M, Gong ZC. ANLN promotes carcinogenesis in oral cancer by regulating the PI3K/mTOR signaling pathway. Head Face Med. 2021;17(1):18. [Google Scholar] [PubMed]
7. Gbadegesin RA, Hall G, Adeyemo A, Hanke N, Tossidou I, Burchette J, et al. Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J Am Soc Nephrol. 2014;25(9):1991–2002. [Google Scholar] [PubMed]
8. Piekny AJ, Maddox AS. The myriad roles of Anillin during cytokinesis. Semin Cell Dev Biol. 2010;21(9):881–91. [Google Scholar] [PubMed]
9. Zhang X, Li L, Huang S, Liao W, Li J, Huang Z, et al. Comprehensive analysis of ANLN in human tumors: a prognostic biomarker associated with cancer immunity. Oxid Med Cell Longev. 2022;2022:5322929. [Google Scholar] [PubMed]
10. Liu Y, Cao P, Cao F, Wang S, He Y, Xu Y, et al. ANLN, regulated by SP2, promotes colorectal carcinoma cell proliferation via PI3K/AKT and MAPK signaling pathway. J Invest Surg. 2022;35(2):268–77. [Google Scholar] [PubMed]
11. Zhou W, Wang Z, Shen N, Pi W, Jiang W, Huang J, et al. Knockdown of ANLN by lentivirus inhibits cell growth and migration in human breast cancer. Mol Cell Biochem. 2015;398(1–2):11–9. doi:10.1007/s11010-014-2200-6. [Google Scholar] [PubMed] [CrossRef]
12. Xia L, Su X, Shen J, Meng Q, Yan J, Zhang C, et al. ANLN functions as a key candidate gene in cervical cancer as determined by integrated bioinformatic analysis. Cancer Manag Res. 2018;10:663–70. doi:10.2147/CMAR. [Google Scholar] [CrossRef]
13. Jung YS, Park JI. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med. 2020;52(2):183–91. doi:10.1038/s12276-020-0380-6. [Google Scholar] [PubMed] [CrossRef]
14. Zhu F, He XG, Shuang F, Fang XM, Jiang JX. Wnt/β-catenin signaling activation by TIMP1 confers cisplatin-resistant gastric cancer cells to malignant behaviors and epithelial-mesenchymal transition. Oncologie. 2023;25(2):169–78. doi:10.1515/oncologie-2022-1028. [Google Scholar] [CrossRef]
15. Zhang J, Zhang S, Li X, Zhang F, Zhao L. HOXB5 promotes the progression of breast cancer through wnt/beta-catenin pathway. Pathol Res Pract. 2021;224:153117. doi:10.1016/j.prp.2020.153117. [Google Scholar] [PubMed] [CrossRef]
16. Chen X, Jiang M, Zhou S, Chen H, Song G, Wu Y, et al. PRAME promotes cervical cancer proliferation and migration via Wnt/β-catenin pathway regulation. Cancers. 2023;15(6):1801. [Google Scholar] [PubMed]
17. Pandi NS, Manimuthu M, Harunipriya P, Murugesan M, Asha GV, Rajendran S. In silico analysis of expression pattern of a Wnt/β-catenin responsive gene ANLN in gastric cancer. Gene. 2014;545(1):23–9. [Google Scholar] [PubMed]
18. Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–58. [Google Scholar] [PubMed]
19. Sheng L, Kang Y, Chen D, Shi L. Knockdown of ANLN inhibits the progression of lung adenocarcinoma via pyroptosis activation. Mol Med Rep. 2023;28(3):177. [Google Scholar] [PubMed]
20. Wang G, Shen W, Cui L, Chen W, Hu X, Fu J. Overexpression of Anillin (ANLN) is correlated with colorectal cancer progression and poor prognosis. Cancer Biomark: Sect A Dis Markers. 2016;16(3):459–65. [Google Scholar]
21. Zhang LH, Wang D, Li Z, Wang G, Chen DB, Cheng Q, et al. Overexpression of anillin is related to poor prognosis in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2021;20(4):337–44. [Google Scholar] [PubMed]
22. Xu J, Zheng H, Yuan S, Zhou B, Zhao W, Pan Y, et al. Overexpression of ANLN in lung adenocarcinoma is associated with metastasis. Thorac Cancer. 2019;10(8):1702–9. [Google Scholar] [PubMed]
23. Wang A, Dai H, Gong Y, Zhang C, Shu J, Luo Y, et al. ANLN-induced EZH2 upregulation promotes pancreatic cancer progression by mediating miR-218-5p/LASP1 signaling axis. J Exp Clin Cancer Res. 2019;38(1):347. [Google Scholar] [PubMed]
24. Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, et al. Sustained proliferation in cancer: mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35:S25–54. [Google Scholar] [PubMed]
25. Lu EM, Ratnayake J, Rich AM. Assessment of proliferating cell nuclear antigen (PCNA) expression at the invading front of oral squamous cell carcinoma. BMC Oral Health. 2019;19(1):233. [Google Scholar] [PubMed]
26. Shamloo B, Usluer S. p21 in cancer research. Cancers. 2019;11(8):1178. doi:10.3390/cancers11081178. [Google Scholar] [PubMed] [CrossRef]
27. Hao J, Zhang W, Lyu Y, Zou J, Zhang Y, Lyu J, et al. Combined use of cyclinD1 and Ki67 for prognosis of luminal-like breast cancer patients. Front Oncol. 2021;11:737794. doi:10.3389/fonc.2021.737794. [Google Scholar] [PubMed] [CrossRef]
28. Wang Z, Hu S, Li X, Liu Z, Han D, Wang Y, et al. MiR-16-5p suppresses breast cancer proliferation by targeting ANLN. BMC Cancer. 2021;21(1):1188. doi:10.1186/s12885-021-08914-1. [Google Scholar] [PubMed] [CrossRef]
29. Tahtamouni L, Ahram M, Koblinski J, Rolfo C. Molecular regulation of cancer cell migration, invasion, and metastasis. Anal Cell Pathol. 2019;2019:1356508. doi:10.1155/2019/1356508. [Google Scholar] [PubMed] [CrossRef]
30. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13(1):395–412. doi:10.1146/annurev-pathol-020117-043854. [Google Scholar] [PubMed] [CrossRef]
31. Chen S, Gao Y, Chen F, Wang TB. ANLN serves as an oncogene in bladder urothelial carcinoma via activating JNK signaling pathway. Urol Int. 2023;107(3):310–20. doi:10.1159/000524204. [Google Scholar] [PubMed] [CrossRef]
32. Melet A, Song K, Bucur O, Jagani Z, Grassian AR, Khosravi-Far R. Apoptotic pathways in tumor progression and therapy. Adv Exp Med Biol. 2008;615:47–79. doi:10.1007/978-1-4020-6554-5_4. [Google Scholar] [PubMed] [CrossRef]
33. Wang S, Mo Y, Midorikawa K, Zhang Z, Huang G, Ma N, et al. The potent tumor suppressor miR-497 inhibits cancer phenotypes in nasopharyngeal carcinoma by targeting ANLN and HSPA4L. Oncotarget. 2015;6(34):35893–907. doi:10.18632/oncotarget.5651. [Google Scholar] [PubMed] [CrossRef]
34. Vallée A, Lecarpentier Y, Vallée JN. The key role of the WNT/β-catenin pathway in metabolic reprogramming in cancers under normoxic conditions. Cancers. 2021;13(21):5557. doi:10.3390/cancers13215557. [Google Scholar] [PubMed] [CrossRef]
35. Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4(9):a007864. doi:10.1101/cshperspect.a007864. [Google Scholar] [PubMed] [CrossRef]
36. Kim HK, Bae J, Lee SH, Hwang SH, Kim MS, Kim MJ, et al. Blockers of Wnt3a, Wnt10a, or β-catenin prevent chemotherapy-induced neuropathic pain in vivo. Neurother. 2021;18(1):601–14. doi:10.1007/s13311-020-00956-w. [Google Scholar] [PubMed] [CrossRef]
37. Xu X, Xu S, Wei Z, Li J. Wnt7a inhibits transformed cell proliferation while promoting migration and invasion in non-small cell lung cancer. Transl Cancer Res. 2020;9(8):4666–75. doi:10.21037/tcr-20-215. [Google Scholar] [PubMed] [CrossRef]
38. MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi:10.1016/j.devcel.2009.06.016. [Google Scholar] [PubMed] [CrossRef]
39. Mazieres J, He B, You L, Xu Z, Jablons DM. Wnt signaling in lung cancer. Cancer Lett. 2005;222(1):1–10. doi:10.1016/j.canlet.2004.08.040. [Google Scholar] [PubMed] [CrossRef]
40. Calvo R, West J, Franklin W, Erickson P, Bemis L, Li E, et al. Altered HOX and WNT7A expression in human lung cancer. Proc Natl Acad Sci U S A. 2000;97(23):12776–81. doi:10.1073/pnas.97.23.12776. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools