 Open Access
Open Access
ARTICLE
Dexamethasone Effects on Cell Composition and Myelin Content in the Mouse Brain
1 Institute of Molecular Biology and Biophysics FRC FTM, Novosibirsk, 630117, Russia
2 Laboratory of Cellular Biology, Novosibirsk State Medical University, Novosibirsk, 630091, Russia
3 V. Zelman Institute for Medicine and Psychology, Novosibirsk State University, Novosibirsk, 630090, Russia
* Corresponding Author: Stanislav Aladev. Email:
(This article belongs to the Special Issue: Advances in Cellular and Molecular Mechanisms and Therapeutic Strategies for Neurodegenerative Diseases)
BIOCELL 2025, 49(6), 1057-1069. https://doi.org/10.32604/biocell.2025.064100
Received 05 February 2025; Accepted 06 May 2025; Issue published 24 June 2025
Abstract
Background: Glucocorticoids are used as anti-inflammatory drugs for the treatment of various diseases, however, their side effects on normal brain tissue remain underinvestigated. Objectives: The study aimed to investigate dexamethasone (DXM) effects on cell composition and myelin content in the mouse brain tissue. Methods: C57Bl/6 male mice (n = 60) received single and ten multiple intraperitoneal DXM injections (2.5 mg/kg), and the studied parameters were analysed at 1, 3, 7, 10 days after a single DXM injection and 15, 30, 60, and 90 days after the multiple injections. Oligodendrocytes, microglia, and astrocytes were assayed by immunohistochemistry with specific antibodies (Olig2, CD68, and GFAP, respectively) in the corpus callosum of the normal brain tissue. The myelin content was estimated by staining with LuxolFastBlue. The presence of GFAP isoforms was determined by western blotting. Results: DXM administration did not affect oligodendrocytes in the mouse brain but temporarily significantly decreased myelin content (1.2-fold, p = 0.0058; 1.4-fold, p < 0.0001) at 3–15 days time points. At the same time, DXM significantly decreased the number of microglial cells (1.5–3.5-fold, p < 0.0001) and significantly increased astrocytes (1.8-fold, p < 0.0001). Prolonged administration of DXM resulted in the decrease of the main GFAP α-isoform (50 kDa) and the appearance of shorter GFAP isoforms (30 kDa, 42 kDa, 44 kDa) similar to that in some neurodegenerative animal models. Conclusion: DXM can modify the cell composition of the normal mouse brain tissue by decreasing microglial cells and increasing astrocytes. Long-term use of DXM results in the inhibition of myelin formation and the appearance of truncated GFAP isoforms, suggesting its ability to induce neurodegeneration-like changes in the normal mouse brain.Keywords
Glucocorticoids (GCs) are anti-inflammatory, anti-tumor, and anti-allergic drugs that are actively used in the treatment of many diseases, such as Alzheimer’s disease, COVID-19, and others [1–3]. Nevertheless, GCs have a large number of side effects, which can vary from a minor case of acne to Cushing syndrome that may result in diabetes mellitus [4,5]. Different side effects may appear in up to 90% of patients who took GCs for more than 60 days, and these side effects depend on the doses and route of GC administration [6,7].
GC’s involvement in the development of the side effects is realized through different molecular mechanisms. It was shown that neuroinflammatory mechanisms mediated by activated glial cells and cytokines (TNF-a, IL-1b) might contribute to neuronal degeneration leading to Alzheimer’s and Parkinson’s disease [8]. Dexamethasone (DXM) is capable of changing the plasticity of neurons, astrocytes, and astroglia [9], but it also has neuroprotective effects against neurodegenerative diseases [10].
However, the molecular mechanisms of DXM’s effect on myelin content and astrocyte activation in mouse brain tissue are still poorly understood. DXM administration (50 μM) for 4 days reduced proliferation of oligodendrocyte progenitor cells in both innately anxious and innately non-anxious cell cultures, but reduced differentiation and myelin production in innately anxious cultures isolated from C57Bl/6NCrl (innately non-anxious strain) and DBA/2NCrl (innately anxious strain) mice on the model of stress [11]. Since the molecular mechanisms of myelin plasticity in the modulation of neural networks are still poorly understood, this study provides the first results on GCs effect on oligodendrocyte progenitor cells and which genetic factors can additionally modulate it. It is also known that DXM is able to reduce neuropathic pain by upregulation of dynorphin A expression, a component of spinal microglia, via glucocorticoid receptor (GR)/p38 MAPK/cAMP signaling pathways in neuropathic rats [12]. DXM administration at 1 μM dosage regulated glutamine synthetase activity in astrocytes of 18-month-old mice glial cell cultures via GR-mediated pathways, in particular GR-α and GR-β isoforms [13]. It has also been shown that DXM as a stress factor affected the localization of astrocytic protein aquaporin-4 and its role in extracellular protein uptake, which is modulated by adenosine A2A receptors A2ARin primary cell cultures of astrocytes obtained from the cortex of postnatal Wistar rats [14]. Thus, molecular mechanisms of DXM on myelin content and astrocyte activation have been studied quite differently, but are still poorly investigated. At the moment, the most well-known is that DXM is realized through GR-signaling pathways such as transactivation and transrepression.
Mainly, GC’s effects towards various brain pathologies are investigated, whereas there are not so many studies that describe the effect of DXM administration on cell composition or myelin content in the normal brain tissue.
It is known that single low-dose DXM administration (0.5 mg/kg) to neonatal Sprague-Dawley rats (1–5 days of age) reduces myelin basic protein (MBP) mRNA level in the white matter of normal brain tissue. That fact demonstrates that even a single DXM injection can increase the risk of oligodendrocyte apoptosis, which leads to hypomyelination [15]. A similar study examines the single DXM administration (1 mg/kg) effect on the relative abundance of MBP and glial fibrillary acidic protein (GFAP) mRNA levels in the developing rat brain. It was shown that DXM administration suppresses expression of genes related to glial functions, such as myelination [16]. Single high-dose DXM administration (50 mg/kg) protects against experimental autoimmune encephalomyelitis (EAE)-induced motor deficiency but impairs neurocognitive abilities like learning and memory by reducing neuronal activity and enhancing some aspects of neuroinflammation in mice [17]. These data demonstrate that DXM is capable of affecting both MBP and GFAP expression in normal brain tissue, possibly associated with damage to astrocytes and probably inflammation.
At the cellular level, DXM administration results in abnormalities such as edema, neuronal vacuoles, astrocyte hyperplasia, and microglial cells with positive reaction for GFAP antibodies in the rat brain [18]. DXM administration reduces hippocampal cell proliferation and density of astrocytes in the hippocampus and corpus callosum in the normal brain of Long Evans rats in the postnatal period. These acute alterations in the neonate brain might underlie later functional disability in DXM-treated animals and humans [19]. The long-term effects of antenatal DXM treatment on the brain were shown in 3-month-old male Sprague-Dawley rats, whose mothers were treated with DXM. The significant reduction in the total astroglia primary process in CA1 and CA3 hippocampal subregions was detected, demonstrating a non-adaptive glial plasticity induced by DXM prenatally [20].
DXM treatment results in sustained elevation of macrophage content in isolated microglia cultures treated with lipopolysaccharide (LPS), possibly indicating persistence of microglia activation [21]. Single DXM administration in high-dose (3 mg/kg) decreases immunoreactivity of microglia in a Wistar rat brain on the Parkinson’s disease model induced by 6-hydroxydopamine [22].
Taken together, these results demonstrate the ability of GCs to affect normal brain tissue in terms of its cell composition, although there are still very few of them, and further studies are warranted.
In our previous work, DXM effects on brain extracellular matrix (ECM) were studied in a model system, in which single and multiple DXM administration was given to experimental mice with a 3–90 days period follow-up [23,24]. Due to the fact that ECM provides a cellular microenvironment and regulates various processes such as growth, differentiation, and homeostasis of the cells [25,26], it plays a significant role in cell composition and functionality in brain tissue. Brain ECM may be heavily implicated in many pathological states like cancer [27] and consists of complex polysaccharide molecules called proteoglycans (PGs) [28] and glycosaminoglycans (GAGs) [29]. It was shown that DXM administration affects expression of some PG core proteins in mouse brain in a dose-dependent manner, both at the mRNA and protein levels, as well as GAG content [30]. The obtained results demonstrate that even single/multiple DXM administration can reorganise the brain ECM in terms of its glycosylation pattern [24].
The aim of this study was to investigate the ability of GCs to affect some cell populations and myelin content in the same mouse experimental model.
All experiments were performed on 8-week-old male C57Bl/6 mice according to our previous work [24]. All the animals (total n = 54) were obtained from the Institute of Cytology and Genetics (Novosibirsk, Russia). The control group (n = 6) received a single injection of saline. The rest of mice (n = 48) received single intraperitoneal injection or ten intraperitoneal injections of DXM (KRKA Pharma, 1.3.1., Novo Mesto, Slovenia) at a dosage of 2.5 mg/kg (6 animals in each group—1, 3, 7, 10 days groups for single injection; 15, 30, 60, 90 days groups for multiple injections) during 1–90 days. The corpus callosum zone of brain tissue was studied. All procedures with experimental animals were conducted according to Directive of the Council of the European Community 2010/63/EU and approved by the Animal Care and Use Ethics Committees of the Institute of Molecular Biology and Biophysics FRC FTM (approval No. N4/2017 from 23.06.2017; Novosibirsk, Russia).
2.2 Manual Immunohistochemistry
For histological and immunohistochemical (IHC) analysis, we used the mouse cerebral hemisphere, which was fixed in a buffered 10% formalin solution and embedded in paraffin (BioOptica, 08-7910E, Milan, Italy). Paraffin sections 3–4 μm thick were mounted on glass slides and stained with hematoxylin and eosin (BioVitrum, 05-006, Saint-Petersburg, Russia). The corpus callosum was examined on the coronal sections obtained at a distance of 5 ± 0.5 mm from Bregma.
To unmask oligodendrocyte, macrophage and astrocyte antigens paraffin sections were deparaffinized and unmasked using Dewax and HIER Buffer H with pH = 6 (ThermoFisher Scientific, TA-999-DHBM, Waltham, MA, USA) for GFAP and CD68 and Dewax and HIER Buffer H with pH = 6 for Olig2 (ThermoFisher Scientific, TA-999-DHBL) for 60 min at 98°C and an indirect immunoperoxidase reaction was performed with primary antibodies against Olig2 (RTU, Cell Marque, 211F1.1, Rocklin, CA, USA), CD68 (RTU, ThermoFisher Scientific, PG-M1, Waltham, MA, USA), GFAP (RTU, ThermoFisher Scientific, ASTRO6) for 60 min at room temperature. Mouse and Rabbit Specific HRP/DAB (ABC) Detection IHC kit (PrimeBioMed, 78-310004-15, Moscow, Russia) was used to visualize the reaction products for 60 min at room temperature. The two-stage detection system includes an enhancer—a solution required for binding the polymer complex and the antigen-antibody complex; a poly-HRP conjugate, which contains conjugates of goat anti-mouse/anti-rabbit antibodies and horseradish peroxidase; a DAB substrate solution, and a chromogen. The nuclear staining was performed using hematoxylin and eosin (BioVitrum, 05-006, Saint-Petersburg, Russia) for 15 min at room temperature. All the stained slides were embedded under a cover medium.
The stained slides were analyzed using a Zeiss AxioScope. A1 microscope (Zeiss, 430035-9030-000, Reutlingen, Germany) with an AxioCam MRc5 camera (Zeiss, 000000-0450-354) with 400× magnification, and the obtained data were processed using Zen Blue 2.6 (Zeiss, 410138-1006-260).
LFB (Luxol Fast Blue) staining was used to visualize the myelin sheaths of the corpus callosum in the brain tissue with modification according to Viсtorov (use of an aqueous solution of sodium tetraborate during differentiation instead of an aqueous solution of lithium carbonate). Staining was carried out in several stages: deparaffinization, staining, dehydration, and embedding under a cover medium. Solvent blue 38 (Sigma Aldrich, CAS 1328-51-4, St. Louis, MO, USA) and Cresyl violet (Sigma Aldrich, CAS 10510-54-0) were used for staining to assess the myelin content.
The stained slides were analyzed using Zeiss AxioScope. A1 microscope (Zeiss, 430035-9030-000) with AxioCam MRc5 camera (Zeiss, 000000-0450-354), and the obtained data was processed using Zen Blue 2.6 (Zeiss, 410138-1006-260).
All the western blotting procedures were performed according to our previous work [24]. It was loaded 15 μg of protein for bands of 1, 3, 7, 10 days groups (10 mL of samples were diluted with 10 mL of SDS 1% and 10 mL of β-Mercaptoethanol; 5 mL of each probe was loaded), and 30 μg for bands of 15, 30, 60, 90 days groups (10 mL of samples were diluted with 10 mL of SDS 1% and 10 mL of β-Mercaptoethanol; 10 mL of each probe was loaded). The membranes were incubated with primary rabbit antibodies to GFAP (Cloud-Clone Corp., PAA068Mu01, Katy, TX, USA, 1:2000) or Gapdh (Abcam, ab181602, Cambridge, UK, 1:15,000) for 60 min at room temperature. After that, membranes were incubated with secondary rabbit antibodies (Abcam, ab3578, 1:10,000) for 60 min at room temperature.
Statistical analysis of the received data was conducted according to our previous work [24]. The normal distribution was calculated in Origin Pro 10.1 (OriginLab, GF3S4-3089-7909456, Northampton, MA, USA) using the Shapiro-Wilk test. The value of p < 0.05 was considered to indicate a statistically significant difference.
3.1 DXM Administration Decreased Myelin Content, but Did Not Affect the Number of Oligodendrocytes in the Normal Mouse Brain Tissue
To study potential DXM effects on brain cell composition and myelin content, the experimental animals received single or multiple (10 injections during 10 subsequent days) DXM injections (2.5 mg/kg). The studied parameters were detected at 1–10 and 15–90 days time-points, respectively. DXM effect on the number of oligodendrocytes and myelin content in normal brain tissue was investigated by immunohistochemical analysis for the specific oligodendroglia lineage marker Olig2 (Fig. 1A) or Luxol Fast Blue staining by Victorov (Fig. 1B).
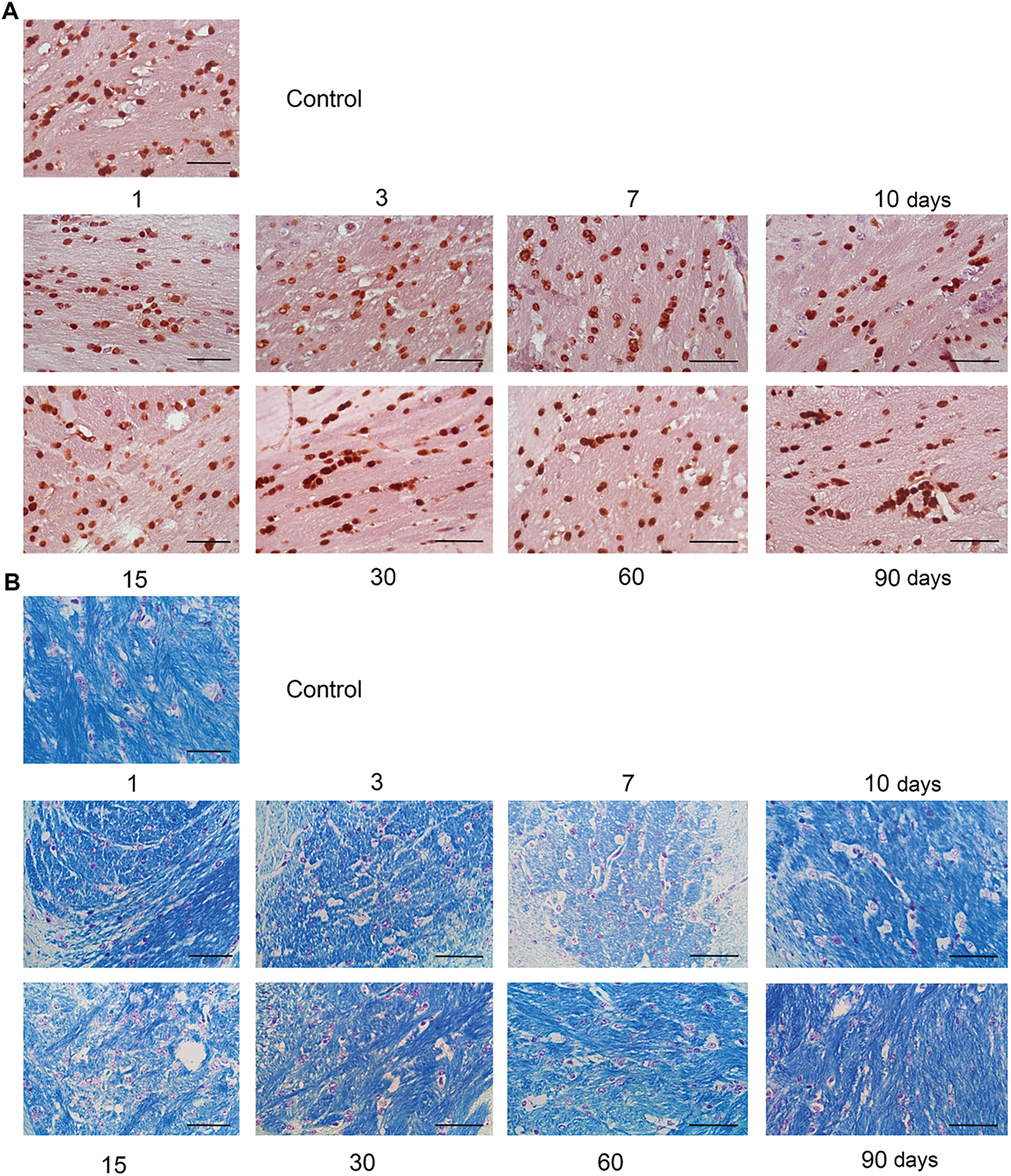
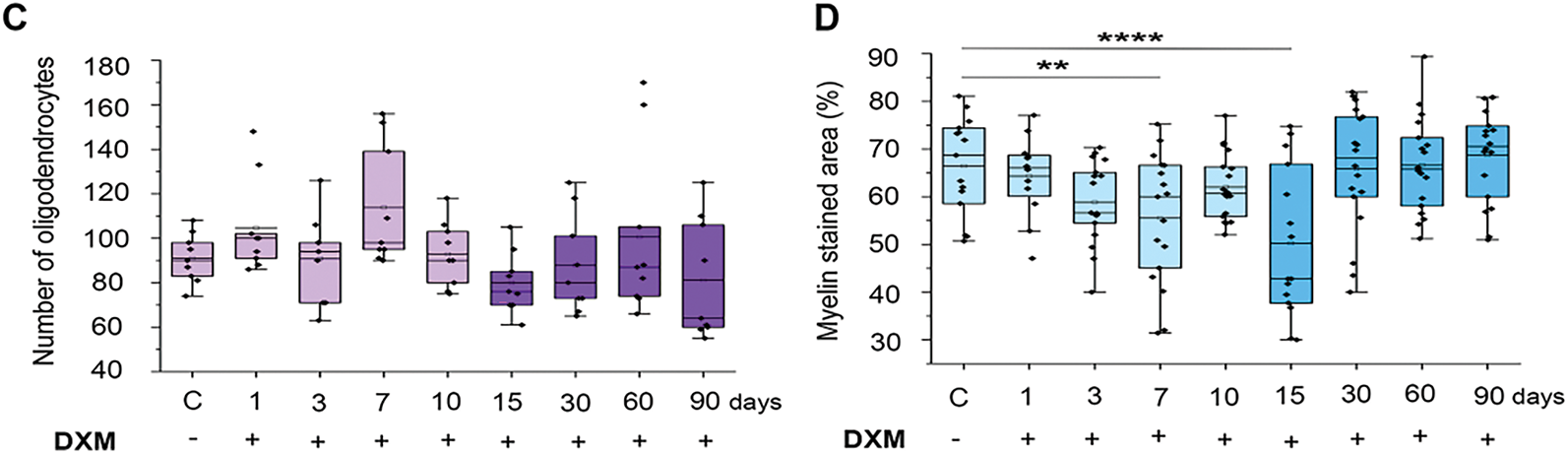
Figure 1: Dexamethasone’s effects on the number of oligodendrocytes and myelin content in the normal mouse brain tissue. (A) Microphotographs of IHC staining for oligodendrocytes with Olig2 antibodies. (B) Microphotographs of myelin staining with Luxol Fast Blue. (C) Quantitative analysis of the number of oligodendrocytes in the mouse brain. The number of cells was detected by the number of their nuclei in the stained area of the sample. N = 27; Control—3 mice, 1 day—3 mice, 3 days—3 mice, 7 days—3 mice, 10 days—3 mice, 15 days—3 mice, 30 days—3 mice, 60 days—3 mice, 90 days—3 mice. (D) Quantitative analysis of myelin content in the mouse brain. The content was detected by the percentage of the stained area in the sample. DXM (2.5 mg/kg) was administered as a single injection with follow-up at 1, 3, 7, or 10 days after the injection or multiple injections with follow-up at 15, 30, 60, or 90 days after the last injection. Control—mice treated with saline. N = 50; Control—5 mice, 1 day—4 mice, 3 days—6 mice, 7 days—6 mice, 10 days—6 mice, 15 days—5 mice, 30 days—6 mice, 60 days—6 mice, 90 days—6 mice. Medians, means, and interquartile ranges (IQRs) are presented. Statistical analysis—ANOVA + Fisher’s least significant difference test; **p < 0.01, ****p < 0.0001. Magnification 400×. Scale bar 50 μm
DXM administration did not affect the number of oligodendrocytes in the normal mouse brain tissue significantly, although a tendency to the increase of this parameter (especially on 1–7 days after DXM injection) was observed (Fig. 1C). At the same time, the myelin content responded to DXM administration by 1.2-fold significant decrease at 3–7 days (p = 0.0058, degrees of freedom (DF) = 8, standard deviation (SD) = 13.56, σ = 1, t = −2.8, F = 4.94, n = 6) upon single DXM injection and by 1.4-fold (p < 0.0001, DF = 8, SD = 15.57, σ = 1, t = −4.04, F = 4.94, n = 6) at 15th day after multiple DXM injections (Fig. 1D). These changes were temporary, and myelin content returned to the control values at 10 or 30–90 days, respectively.
These results demonstrate that the number of oligodendrocytes does not change upon DXM administration, but they seem to form fewer myelin sheaths. Although this effect is temporary, it needs to be borne in mind when using GCs.
3.2 DXM Administration Decreases the Number of Microglial Cells in the Normal Mouse Brain Tissue
DXM effect on microglia in normal brain tissue was studied by immunohistochemical analysis for the specific monocyte lineage marker CD68, highly expressed in microglia (Fig. 2A).
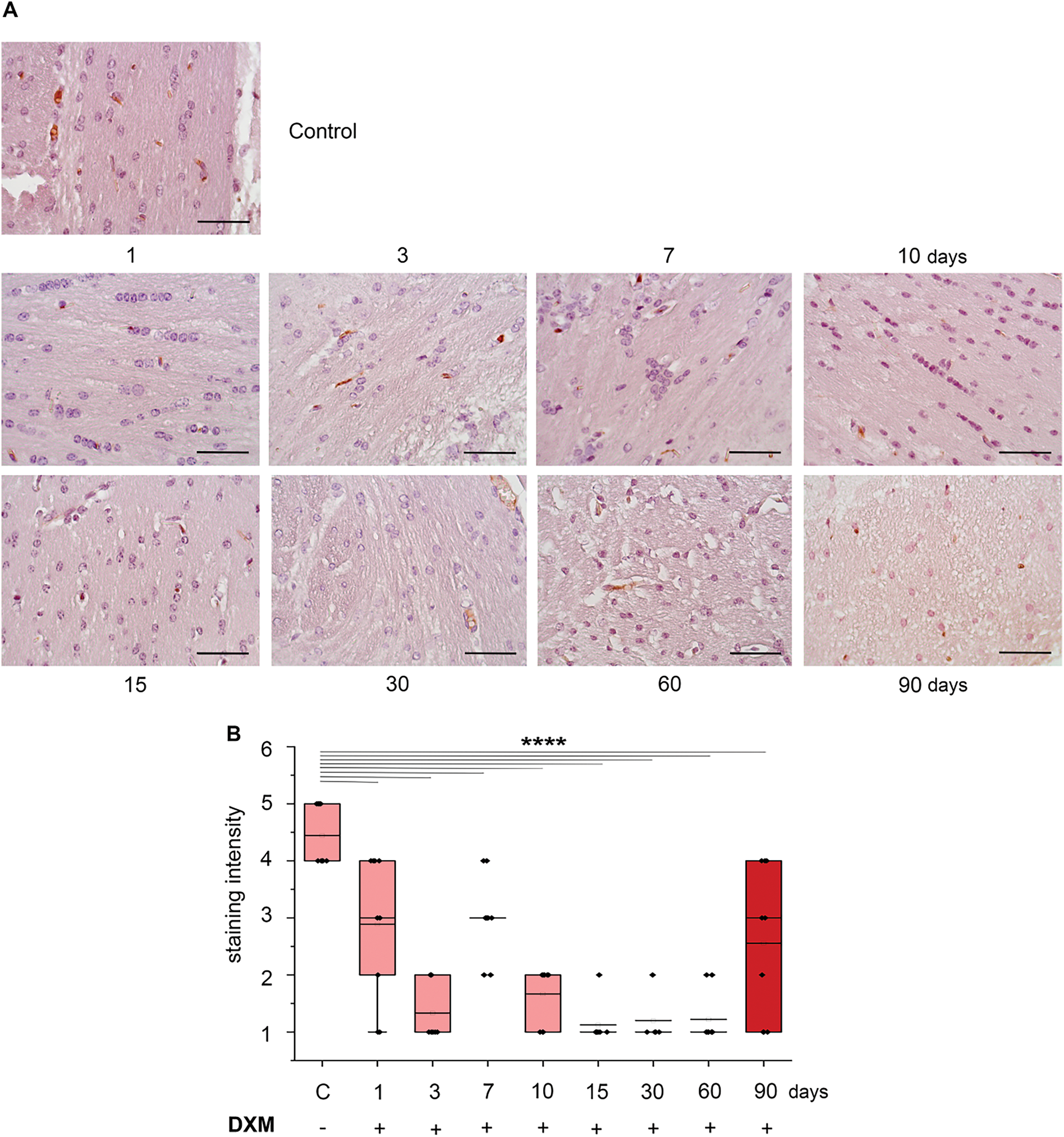
Figure 2: Dexamethasone effects on microglia in the normal mouse brain tissue. (A) Microphotographs of IHC staining for macrophages with CD68 antibodies. (B) Quantitative analysis of microglial cells in the mouse brain. DXM (2.5 mg/kg) was administered as a single injection with follow-up at 1, 3, 7, or 10 days after the injection or multiple injections with follow-up at 15, 30, 60, or 90 days after the last injection. Control—mice treated with saline. N = 27; Control—3 mice, 1 day—3 mice, 3 days—3 mice, 7 days—3 mice, 10 days—3 mice, 15 days—3 mice, 30 days—3 mice, 60 days—3 mice, 90 days—3 mice. Medians, means, and IQRs are presented. Statistical analysis—ANOVA + Fisher’s least significant difference test; ****p < 0.0001. Magnification 400×. Scale bar 50 μm
DXM administration significantly decreased number of microglial cells in the normal mouse brain tissue by 1.5–3.5-fold (p < 0.0001, DF = 8, SD = 0.5–1.2, σ = 1, t = −4.24–8.48, F = 18.3, n = 3) upon single DXM injection (1–10 days) and 2–3.5-fold (p < 0.0001, DF = 8, SD = 0.35–1.33, σ = 1, t = −5.15–8.78, F = 18.3, n = 3) upon multiple DXM injections (15–90 days) (Fig. 2B). This significant decrease of number of microglial cells might contribute to a potential reorganisation in brain tissue structure through the changes in the ratio of different cell types.
3.3 DXM Administration Affects Molecular Characteristics but Not the Number of Astrocytes in the Normal Mouse Brain Tissue
To study the DXM effect on the normal brain tissue, it is also important to evaluate the DXM effects on astrocytes, because these substantial glial cells are capable of supporting chronic inflammation and progressive neurodegeneration. DXM effect on astrocytes in normal brain tissue was investigated by immunohistochemical analysis for glial fibrillary acidic protein (GFAP), which is a major protein found in the astrocytic cells of the brain (Fig. 3A–C).
Figure 3: Dexamethasone effects on astrocytes in the normal mouse brain tissue. (A) Microphotographs of IHC staining for astrocytes with GFAP antibodies. (B) Quantitative analysis of the number of astrocytes in the mouse brain. (C) Quantitative analysis of the astrocyte outgrowth in the mouse brain was estimated as the stained area of the sample. (D) Original microphotographs of Western blotting. (E) Quantitative analysis of GFAP protein α isoform (50 kDa) and other GFAP protein isoforms (42, 44, 30 kDa) (F) in mouse brain. DXM (2.5 mg/kg) was administered as a single injection with follow-up at 1, 3, 7, or 10 days after the injection or multiple injections with follow-up at 15, 30, 60, or 90 days after the last injection. Control—mice treated with saline. N = 27; Control—3 mice, 1 day—3 mice, 3 days—3 mice, 7 days—3 mice, 10 days—3 mice, 15 days—3 mice, 30 days—3 mice, 60 days—3 mice, 90 days—3 mice. Medians, means, and IQRs are presented. Statistical analysis—ANOVA + Fisher’s least significant difference test; **p < 0.01, ****p < 0.0001. Magnification 400×. Scale bar 50 μm
Single DXM administration to the experimental animals resulted in the significant increase of the number of astrocytes in the mouse brain by 1.8-fold at the 7–10 days (p < 0.0001, DF = 8, SD = 9.3–12.2, σ = 1, t = 3.18–3.64, F = 4.2, n = 3) (Fig. 3B). However, after multiple DXM administration, there were no changes in this parameter, possibly reflecting an adaptation of the brain tissue to prolonged DXM exposure. Also, a tendency to the increase of astrocyte outgrowth was observed (especially upon multiple DXM administrations), although not statistically significant (Fig. 3C).
To investigate the demonstrated DXM effects toward astrocytes further, Western blotting of the DXM-treated brain tissues with anti-GFAP antibodies was performed (Fig. 3D). DXM administration increased the main GFAP protein isoform (a-isoform, 50 kDa) content by 1.5–4.5-fold in all experimental groups (Fig. 3E). At the same time, additional GFAP isoforms were detected, with a maximum 3 isoforms (42, 44, 30 kDa) observed on the 90th day after prolonged DXM administration (Fig. 3D,F). The appearance of these GFAP isoforms may indicate changes in some of the molecular characteristics of astrocytes, and this issue needs further investigation.
In this study, a complex investigation of DXM effects on different cells (oligodendrocytes, microglial cells, astrocytes), the normal brain tissue in the experimental system was performed. We have not found any other studies that would simultaneously investigate all these parameters. However, there is some data on the DXM effects on individual brain cell types, which will be discussed in this section.
Long-term DXM therapy is actively used in the clinic. High-dose DXM (40 mg/kg/day for 4 days every 28 days) and a usual dose of prednisone (1 mg/kg/day for 4 days every 28 days) administration were used for the first-line therapy for the treatment of adult immune thrombocytopenia (ITP) for 30 months. However, the side effects were serious. Out of 72 patients, 2 died, and 21 other patients had a side effect in the form of concomitant diseases such as hypertension and hyperglycemia [31]. In another study, DXM administration (40 mg/kg/day for 4 days every 28 days and 20 mg/kg/day with the same scheme) was used for multiple myeloma treatment for more than 30 months. It was shown that the DXM 40 mg group had lower toxicity than the 20 mg group, but at least 26 out of 96 patients with multiple myeloma had side effects such as gastrointestinal reactions and abnormal glucose tolerance [32]. Thus, our DXM administration regimens are consistent with pulse-therapy regimens, which are used in the clinic.
In our experimental system, DXM did not affect the number of oligodendrocytes but decreased myelin content in the affected brain tissue. This result stay in line with those obtained upon investigation of DXM effects to rat brain: single DXM administration at 0.5 mg/kg dosage to neonatal Sprague-Dawley rats (age of 1–5 days) significantly decreased myelin basic protein content by 83% (p < 0.05) in the white matter of normal brain tissue [15]; single DXM administration at 1 mg/kg dosage significantly decreased GFAP mRNA level by 5-fold (p < 0.05) in developing rat brain and suppressed myelination [16]. Totally, these two independent studies support the conclusion that even a single DXM administration is able to inhibit myelin formation, potentially creating a basis for some neurodegenerative conditions.
At the same time, DXM administration significantly decreased the number of microglial cells in the normal brain tissue. This result is quite logical because DXM is an anti-inflammatory drug. According to the literature, there are no publications describing DXM administration on microglial cell content in the normal brain. But in the transgenic mouse model of Alzheimer’s disease, DXM treatment in 1 μM dosage significantly increased CD68 content up to 209% (p < 0.05) by western blotting assay [33]. Our results about the decrease in the number of microglial cells in normal brain upon DXM administration can contribute to the research of inflammation in brain tissue.
As to DXM effects towards astrocytes, the demonstrated increase of the number of astrocytes in normal mouse brain corresponds to published data on the induction of astrocytic hyperplasia and positive GFAP antibody reaction in rat microglial cultures upon DXM administration [18]. On the other hand, DXM possesses the opposite effect on the developing brain. It was shown that DXM administration significantly decreased hippocampal cell proliferation by 1.2-fold (p < 0.05) in the corpus callosum of normal brain in Long-Evans rats on the 4th day of the postnatal period, but this effect was normalized by the 10th day. Also, DXM administration caused a significant decrease by 1.6-fold (p < 0.05) of astrocyte density in the corpus callosum of the hippocampus on the 10th day [19]. Long-term DXM administration (19 days) to pregnant Sprague-Dawley rats at a dosage of 0.5 mg/kg led to brain remodeling of their 3-month-old male offspring. Morphological analysis indicated a decrease of total primary process length by 32% in CA1 and by 50% in CA3 hippocampal region in normal brain tissue of rats [20]. Taken together, these results demonstrate that the complex influence of GCs on the normal brain seems to depend on the age of the experimental animals or different brain zones.
An interesting finding of this study is related to the appearance of minor GFAP isoforms upon DXM administration. This result perfectly fits and extends the very little existing data on this subject. Few GFAP isoforms with molecular masses of 35 to 50 kDa were detected in the spinal cord of the motor neuron degeneration (Mnd) mouse, a mutant that exhibits progressive degeneration of lower spinal motor neurons. During 9-month experiment, the 50 kDa intact GFAP isoform was continuously replaced by other GFAP isoforms with molecular masses of 35 to 48 kDa (lacked the head domains from the amino terminus), which became dominant at the time of the appearance of behavioral paralytic gait around 6–7 months of age. Appearance of these shorter GFAP isoforms were associated with progressive degenerative loss of motor neurons and a significant increase in the number of astrocytes in the ventral horn at 7–9 months of age and this process seems to precede the deterioration of motor activities in this animal model of amyotrophic lateral sclerosis (ALS) [34]. These results coincide with another study on high-volume training in Wistar rats. The rats were divided into 6 groups according to training period, followed or not by exhaustion test (ET)-control (C), control + ET (C-ET), moderate-volume (MV) training and MV-ET, high-volume (HV) training and HV-ET. It was shown that the GFAP isoforms 42 and 39 kDa content were reduced by 40% and 26%, respectively, in C-ET and MV-ET groups, while the GFAP isoform 50 kDa content was reduced by 40%–60%, and isoform 39 kDa increased by 7-fold in HV-ET group. Overall, extensive training of experimental Wistar rats modified the GFAP isoform profile, suggesting impaired astrocyte reactivity in the cerebellum [35]. These several GFAP isoforms were also detected in the spinal cords of patients with ALS compared with the non-ALS spinal cords. Immunohistochemical analysis showed a significant decrease of 50 kDa and 45 kDa GFAP isoforms and an increase of the content of truncated 36 and 37 kDa isoforms, increase of the number of astrocytes in the shrunken ventral horn with massive degeneration of motor neurons, providing new insight on the important role of astrocytes into the pathogenesis of ALS [36].
Our results for the first time demonstrate that DXM can induce changes in the number of astrocytes and presence of specific GFAP isoforms in the mouse brain similar to that in some neurodegenerative animal models. Keeping in mind the data on the principal possibility of DXM to affect off-spring of DXM-treated pregnant rats [20], the use of GCs during pregnancy needs further investigations.
In this study, we used C57Bl/6 male mice, because the administration of hormonal drugs (DXM) can affect the female mice’s sexual cycle. In addition, we used only one GC (DXM), because this study is a part of a larger work about DXM effects on normal brain tissues. In this particular investigation, we focused on the DXM effects on myelin content and cell composition of the normal brain tissue in mice. We used the minimum number of animals in each group of oligodendrocytes, microglial cells, and astrocytes for statistics (n = 3) and each group of myelin (n = 6), since not all sections were of good quality. In this case, we agree with the use of the research name as exploratory or pilot.
The obtained results demonstrate that even a single DXM administration results in fluctuations in the cell composition of normal mouse brain tissues, which consist of a relative increase in astrocytes and a decrease in microglial cells. In addition to changing the ratio of different cell types, these cells may undergo some molecular changes, which consist of a decrease in the myelin layers formed by oligodendrocytes and changes in the molecular characteristics of astrocytes. The appearance of minor GFAP isoforms upon DXM administration may indicate an ability of long-term use of GCs to induce neurodegeneration-like changes in the normal mouse brain.
Acknowledgement: The authors thank Luydmila Aksenova for the assistance with Western blotting experiments and Tatiana Kotyusheva for performing immunohistochemical staining. The work was performed using the equipment of the Center for Collective Use “Proteomic Analysis”, supported by the Ministry of Science and Higher Education of the Russian Federation (agreement No. 075-15-2021-691).
Funding Statement: This research was funded by the budgetary funding to FRC FTM for the project “Post-Genomic High-Tech Research on the Mechanisms of Development of Socially Significant Diseases and Stress-Induced Conditions” (Grant No. 125031203556-7).
Author Contributions: Study conception and design: Stanislav Aladev, Elvira Grigorieva. Data collection: Sveltana Aidagulova, Galina Kazanskaya, Maxim Politko. Analysis and interpretation of results: Stanislav Aladev, Svetlana Aidagulova, Dmitry Sokolov. Draft manuscript preparation: Stanislav Aladev, Elvira Grigorieva. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data generated or analyzed during the current study are included in this published article. Further inquiries can be directed to the corresponding author.
Ethics Approval: The study was approved by the Animal Care and Use Ethics Committees of the Institute of Molecular Biology and Biophysics FRC FTM (approval No. N4/2017 from 23.06.2017; Novosibirsk, Russia).
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Zhang H, Yuan Z, Yan Y, Chen L, Zhou Y, Zhang D, et al. The combination of acyclovir and dexamethasone protects against Alzheimer’s disease-related cognitive impairments in mice. Psychopharmacology. 2020;237(6):1851–60. doi:10.1007/s00213-020-05503-1. [Google Scholar] [PubMed] [CrossRef]
2. Meybodi SM, Rabori VS, Salkhorde D, Jafari N, Zeinaly M, Mojodi E, et al. Dexamethasone in COVID-19 treatment: analyzing monotherapy and combination therapy approaches. Cytokine. 2024;184:156794. doi:10.1016/j.cyto.2024.156794. [Google Scholar] [PubMed] [CrossRef]
3. Lu J, Zhang C, Lv J, Zhu X, Jiang X, Lu W, et al. Antiallergic drug desloratadine as a selective antagonist of 5HT2A receptor ameliorates pathology of Alzheimer’s disease model mice by improving microglial dysfunction. Aging Cell. 2021;20(1):e13286. doi:10.1111/acel.13286. [Google Scholar] [PubMed] [CrossRef]
4. Strokotova AV, Grigorieva EV. Glucocorticoid effects on proteoglycans and glycosaminoglycans. Int J Mol Sci. 2022;23(24):15678. doi:10.3390/ijms232415678. [Google Scholar] [PubMed] [CrossRef]
5. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–65. doi:10.1517/14740338.2016.1140743. [Google Scholar] [PubMed] [CrossRef]
6. Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55(3):420–6. doi:10.1002/art.21984. [Google Scholar] [PubMed] [CrossRef]
7. Pereira RMR, Carvalho JF, Canalis E. Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics. 2010;65(11):1197–205. doi:10.1590/s1807-59322010001100024. [Google Scholar] [PubMed] [CrossRef]
8. Singh R, Bansal R. Investigations on 16-arylideno steroids as a new class of neuroprotective agents for the treatment of Alzheimer’s and Parkinson’s diseases. ACS Chem Neurosci. 2017;8(1):186–200. doi:10.1021/acschemneuro.6b00313. [Google Scholar] [PubMed] [CrossRef]
9. Madalena KM, Lerch JK. The effect of glucocorticoid and glucocorticoid receptor interactions on brain, spinal cord, and glial cell plasticity. Neural Plast. 2017;2017:8640970. doi:10.1155/2017/8640970. [Google Scholar] [PubMed] [CrossRef]
10. Srinivasan M, Lahiri DK. Glucocorticoid-induced leucine zipper in central nervous system health and disease. Mol Neurobiol. 2017;54(10):8063–70. doi:10.1007/s12035-016-0277-5. [Google Scholar] [PubMed] [CrossRef]
11. Gigliotta A, Mingardi J, Cummings S, Alikhani V, Trontti K, Barbon A, et al. Genetic background modulates the effect of glucocorticoids on proliferation, differentiation and myelin formation of oligodendrocyte lineage cells. Eur J Neurosci. 2024;59(9):2276–92. doi:10.1111/ejn.16285. [Google Scholar] [PubMed] [CrossRef]
12. Deng MY, Cheng J, Gao N, Li XY, Liu H, Wang YX. Dexamethasone attenuates neuropathic pain through spinal microglial expression of dynorphin A via the cAMP/PKA/p38 MAPK/CREB signaling pathway. Brain Behav Immun. 2024;119:36–50. doi:10.1016/j.bbi.2024.03.047. [Google Scholar] [PubMed] [CrossRef]
13. Kazazoglou T, Panagiotou C, Mihailidou C, Kokkinopoulou I, Papadopoulou A, Moutsatsou P. Glutamine synthetase regulation by dexamethasone, RU486, and compound A in astrocytes derived from aged mouse cerebral hemispheres is mediated via glucocorticoid receptor. Mol Cell Biochem. 2021;476(12):4471–85. doi:10.1007/s11010-021-04236-9. [Google Scholar] [PubMed] [CrossRef]
14. Dias L, Nabais AM, Borges-Martins VPP, Canas PM, Cunha RA, Agostinho P. Impact of glucocorticoid-associated stress-like conditions on aquaporin-4 in cultured astrocytes and its modulation by adenosine A2A receptors. J Neurochem. 2025;169(1):e16299. doi:10.1111/jnc.16299. [Google Scholar] [PubMed] [CrossRef]
15. Kim JW, Kim YJ, Chang YP. Administration of dexamethasone to neonatal rats induces hypomyelination and changes in the morphology of oligodendrocyte precursors. Comp Med. 2013;63(1):48–54. [Google Scholar] [PubMed]
16. Tsuneishi S, Takada S, Motoike T, Ohashi T, Sano K, Nakamura H. Effects of dexamethasone on the expression of myelin basic protein, proteolipid protein, and glial fibrillary acidic protein genes in developing rat brain. Brain Res Dev Brain Res. 1991;61(1):117–23. doi:10.1016/0165-3806(91)90121-x. [Google Scholar] [PubMed] [CrossRef]
17. Dos Santos N, Novaes LS, Dragunas G, Rodrigues JR, Brandão W, Camarini R, et al. High dose of dexamethasone protects against EAE-induced motor deficits but impairs learning/memory in C57BL/6 mice. Sci Rep. 2019;9(1):6673. doi:10.1038/s41598-019-43217-3. [Google Scholar] [PubMed] [CrossRef]
18. Hamdi BA, Amin ZA, Shareef AMY, Al-Bustany HA. Diclofenac sodium and dexamethasone co-therapy restores brain neuron-specific enolase (NSES-100 Beta and glial fibrillary acid protein (GFAP) proteins in experimental rat’s model: a possible inhibition of P-glycoprotein. Cell Mol Biol. 2023;69(9):100–5. doi:10.14715/cmb/2023.69.9.14. [Google Scholar] [PubMed] [CrossRef]
19. Claessens SEF, Belanoff JK, Kanatsou S, Lucassen PJ, Champagne DL, de Kloet ER. Acute effects of neonatal dexamethasone treatment on proliferation and astrocyte immunoreactivity in hippocampus and corpus callosum: towards a rescue strategy. Brain Res. 2012;1482:1–12. doi:10.1016/j.brainres.2012.08.017. [Google Scholar] [PubMed] [CrossRef]
20. Shende VH, McArthur S, Gillies GE, Opacka-Juffry J. Astroglial plasticity is implicated in hippocampal remodelling in adult rats exposed to antenatal dexamethasone. Neural Plast. 2015;2015:694347. doi:10.1155/2015/694347. [Google Scholar] [PubMed] [CrossRef]
21. Koss K, Churchward MA, Tsui C, Todd KG. In vitro priming and hyper-activation of brain microglia: an assessment of phenotypes. Mol Neurobiol. 2019;56(9):6409–25. doi:10.1007/s12035-019-1529-y. [Google Scholar] [CrossRef]
22. Aono H, Choudhury ME, Higaki H, Miyanishi K, Kigami Y, Fujita K, et al. Microglia may compensate for dopaminergic neuron loss in experimental Parkinsonism through selective elimination of glutamatergic synapses from the subthalamic nucleus. GLIA. 2017;65(11):1833–47. doi:10.1002/glia.23199. [Google Scholar] [PubMed] [CrossRef]
23. Aladev SD, Sokolov DK, Strokotova AV, Kazanskaya GM, Volkov AM, Politko MO, et al. Dexamethasone effects on the expression and content of glycosylated components of mouse brain tissue. Usp Mol Onkol. 2023;10(1):25–39. (In Russian). doi:10.17650/2313-805x-2023-10-1-25-39. [Google Scholar] [CrossRef]
24. Aladev SD, Sokolov DK, Strokotova AV, Kazanskaya GM, Volkov AM, Aidagulova SV, et al. Multiple administration of dexamethasone possesses a deferred long-term effect to glycosylated components of mouse brain. Neurol Int. 2024;16(4):790–803. doi:10.3390/neurolint16040058. [Google Scholar] [PubMed] [CrossRef]
25. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123(Pt 24):4195–200. doi:10.1242/jcs.023820. [Google Scholar] [PubMed] [CrossRef]
26. Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Curr Opin Biotechnol. 2013;24(5):830–3. doi:10.1016/j.copbio.2013.04.011. [Google Scholar] [PubMed] [CrossRef]
27. Karamanos NK, Theocharis AD, Neill T, Iozzo RV. Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019;75-76:1–11. doi:10.1016/j.matbio.2018.08.007. [Google Scholar] [PubMed] [CrossRef]
28. Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288(24):6850–912. doi:10.1111/febs.15776. [Google Scholar] [PubMed] [CrossRef]
29. Soles A, Selimovic A, Sbrocco K, Ghannoum F, Hamel K, Moncada EL, et al. Extracellular matrix regulation in physiology and in brain disease. Int J Mol Sci. 2023;24(8):7049. doi:10.3390/ijms24087049. [Google Scholar] [PubMed] [CrossRef]
30. Tsidulko AY, Bezier C, Bourdonnaye GDL, Suhovskih AV, Pankova TM, Kazanskaya GM, et al. Conventional anti-glioblastoma chemotherapy affects proteoglycan composition of brain extracellular matrix in rat experimental model in vivo. Front Pharmacol. 2018;9:1104. doi:10.3389/fphar.2018.01104. [Google Scholar] [CrossRef]
31. Xu J, Zhang X, Feng S, Zhao N, Hu X, Cheng Y, et al. Clinical efficacy of high-dose dexamethasone with sequential prednisone maintenance therapy for newly diagnosed adult immune thrombocytopenia in a real-world setting. J Int Med Res. 2021;49(4):3000605211007322. doi:10.1177/03000605211007322. [Google Scholar] [PubMed] [CrossRef]
32. Hu SL, Liu M, Zhang JY. Comparing the efficacy of different dexamethasone regimens for maintenance treatment of multiple myeloma in standard-risk patients non-eligible for transplantation. World J Clin Cases. 2022;10(32):11712–25. doi:10.12998/wjcc.v10.i32.11712. [Google Scholar] [PubMed] [CrossRef]
33. Pedrazzoli M, Losurdo M, Paolone G, Medelin M, Jaupaj L, Cisterna B, et al. Glucocorticoid receptors modulate dendritic spine plasticity and microglia activity in an animal model of Alzheimer’s disease. Neurobiol Dis. 2019;132:104568. doi:10.1016/j.nbd.2019.104568. [Google Scholar] [PubMed] [CrossRef]
34. Fujita K, Yamauchi M, Matsui T, Titani K, Takahashi H, Kato T, et al. Increase of glial fibrillary acidic protein fragments in the spinal cord of motor neuron degeneration mutant mouse. Brain Res. 1998;785(1):31–40. doi:10.1016/s0006-8993(97)00612-4. [Google Scholar] [PubMed] [CrossRef]
35. de Souza RF, Augusto RL, de Moraes SRA, de Souza FB, Gonçalves LVDP, Pereira DD, et al. Ultra-endurance associated with moderate exercise in rats induces cerebellar oxidative stress and impairs reactive GFAP isoform profile. Front Mol Neurosci. 2020;13:157. doi:10.3389/fnmol.2020.00157. [Google Scholar] [PubMed] [CrossRef]
36. Fujita K, Kato T, Yamauchi M, Ando M, Honda M, Nagata Y. Increases in fragmented glial fibrillary acidic protein levels in the spinal cords of patients with amyotrophic lateral sclerosis. Neurochem Res. 1998;23(2):169–74. doi:10.1023/a:1022476724381. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools