 Open Access
Open Access
ARTICLE
Outcomes of Transcatheter Closure in Outlet-Type Ventricular Septal Defect after 1 Year
1 Department of Paediatrics, Division of Paediatric Cardiology, Faculty of Medicine, Prince of Songkla University, Songkhla, 90110, Thailand
2 Department of Medical Services, Paediatric Heart Centre, Queen Sirikit National Institute of Child Health, Ministry of Public Health, Bangkok, 10400, Thailand
3 College of Medicine, Rangsit University, Bangkok, 10200, Thailand
* Corresponding Authors: Supaporn Roymanee. Email: ; Worakan Promphan. Email:
Congenital Heart Disease 2023, 18(2), 169-181. https://doi.org/10.32604/chd.2023.021238
Received 04 April 2022; Accepted 14 August 2022; Issue published 15 March 2023
Abstract
Background: Ventricular septal defect (VSD) is the most common congenital heart disease. Transcatheter VSD closure is an effective treatment for patients with muscular and perimembranous VSD. However, there is a limit data for outlet VSD, especially impact to the aortic valve leaflet after transcatheter closure. This study aims to assess the outcomes of transcatheter closure of the outlet-type ventricular septal defect (OVSD) after 1 postoperative year. Methods: A retrospective study was performed including 50 patients who underwent transcatheter (n = 25) and surgical (n = 25) OVSD closure during the exact time frame at two medical centres. Results: The median age and body weight of patients in the transcatheter group were significantly higher than those of patients in the surgical group (7.0 vs. 2.8 years; 27.0 vs. 11.4 kg; p < 0.01). The defect size in the surgical group was significantly larger than that in the transcatheter group (5.0 vs. 3.0 mm; p < 0.01). All OVSD patients have successful transcatheter closure (100%) as effective as surgical closure. Less than small residual shunt was present 20% and 8% immediately after the procedure in the transcatheter and surgical groups (p = 0.50), which decreased to 12% and 4% at the 1-year follow-up (p = 0.61), respectively. No incidence of complete atrioventricular block and other complications was observed in both groups, and no significant differences were noted in the new onset or worsening of the aortic regurgitation in both groups (p = 1.0). Conclusions: Transcatheter treatment could be effectively and safely achieved for OVSD closure at 1-year follow-up.Graphic Abstract
Keywords
Ventricular septal defect (VSD) is the most common congenital heart disease. Transcatheter VSD closure is an effective treatment for patients with muscular and perimembranous VSD. However, in outlet-type VSD (OVSD) surgical closure is still preferred rather than transcatheter closure due to less study on aortic valve injury after transcatheter closure. The OVSD contributes 5%–10% of the total cardiovascular disease burden in western countries [1], whereas the incidence rate is approximately 19%–29% in Thailand and Asian countries [2,3]. This study aimed to evaluate the feasibility and early outcomes of transcatheter closure in selected OVSD patients and to compare them with those of conventional open heart OVSD closure surgery.
This was a retrospective study comprising 104 patients diagnosed with OVSD from 2 centres, Prince of Songkla University and Queen Sirikit National Institute of Child Health, between 01 January 2012 to 31 August 2020.
2.1 Indications and Treatment Selection for OVSD Closure
OVSD closure is indicated for patients any age with evidence of left heart enlargement on transthoracic echocardiogram (TTE) or new onset of the aortic valve prolapse (AVP) or new onset of the aortic regurgitation (AR). Transcatheter treatment is considered in patients with 1) body weight >15 kg; 2) VSD diameter from TTE at the left ventricular entry ≤8 mm and at the right ventricular exit ≤6 mm; 3) AVP/AR severity is less than mild; and 4) no malalignment of the outlet septum. Otherwise, surgical closure is considered. In addition, OVSD patients having irreversible pulmonary arterial hypertension, associated cardiac complications, or associated metabolic/systemic disease were excluded.
From 104 cases of OVSDs patients, fifty-four OVSDs were excluded from the study. Forty-four patients had associated lesions requiring surgical therapy. Three patients had minimal shunt with no aortic valve prolapse (AVP) or aortic regurgitation (AR). Six patients were lost to follow-up and one patient had spontaneous closure of the defect. As a result, 50 cases were enrolled into the study; 25 cases underwent transcatheter OVSD closure and 25 cases underwent surgical closure. Medical records of the patients were retrospectively reviewed for clinical assessment, chest radiograph, electrocardiogram (ECG), TTE, and transoesophageal echocardiography (TOE) at pre-procedure, during the procedure, immediate post-procedure, at 1–3 months, at 6–9 months, and 12 months post-procedure. The device position, presence of the residual shunt, the severity of AVP and AR were assessed. For the patients required cardiac catheterization, hemodynamic parameters, details of device implanted and technique of device deployment (if applicable) were recorded. The procedure time, length of hospital stays, and complications associated with the procedures were evaluated. This study was approved by the Ethics Committees of both institutions (Approval numbers: REC.63-455-1-3 and No. 64-014). All the research steps were performed according to the ethical standards and principles under the 1975 Declaration of Helsinki and its later Amendments (2008). Written informed consent was obtained from the parents or the guardians of all patients included in the study.
2.3 Defining and Measuring the OVSD
The OVSD was diagnosed by demonstrating the dropout of the interventricular septum with aortic-pulmonic valve fibrous continuity and the presence of a colour flow jet around the 12–2 o’clock position in the parasternal short-axis view of TTE [4] or around the 6–8 o’clock position in the short-axis with the mid-oesophageal view (40°–60° rotation) of TOE [5]. In addition, from the cardiac catheterization, the left-to-right shunt underneath the aortic and the pulmonary valves pointing up toward the right ventricular outflow tract (RVOT) from the left ventriculogram in the right anterior oblique (RAO) and true lateral projections confirmed the diagnosis of OVSD (Fig. 1). The VSD diameters and length from echocardiogram were the average of the measurements in 2 orthogonal image planes whilst from left ventricular (LV) angiogram were the most visualized numbers in lateral and/or RAO projections during systole. Nonetheless, for those with aortic valve prolapse, repeated angiogram may require after passing the delivery sheath to interrogate the true defect diameter after pushing the aortic cusp away. The largest right ventricular (RV) orifice diameter will be used for device size selection.
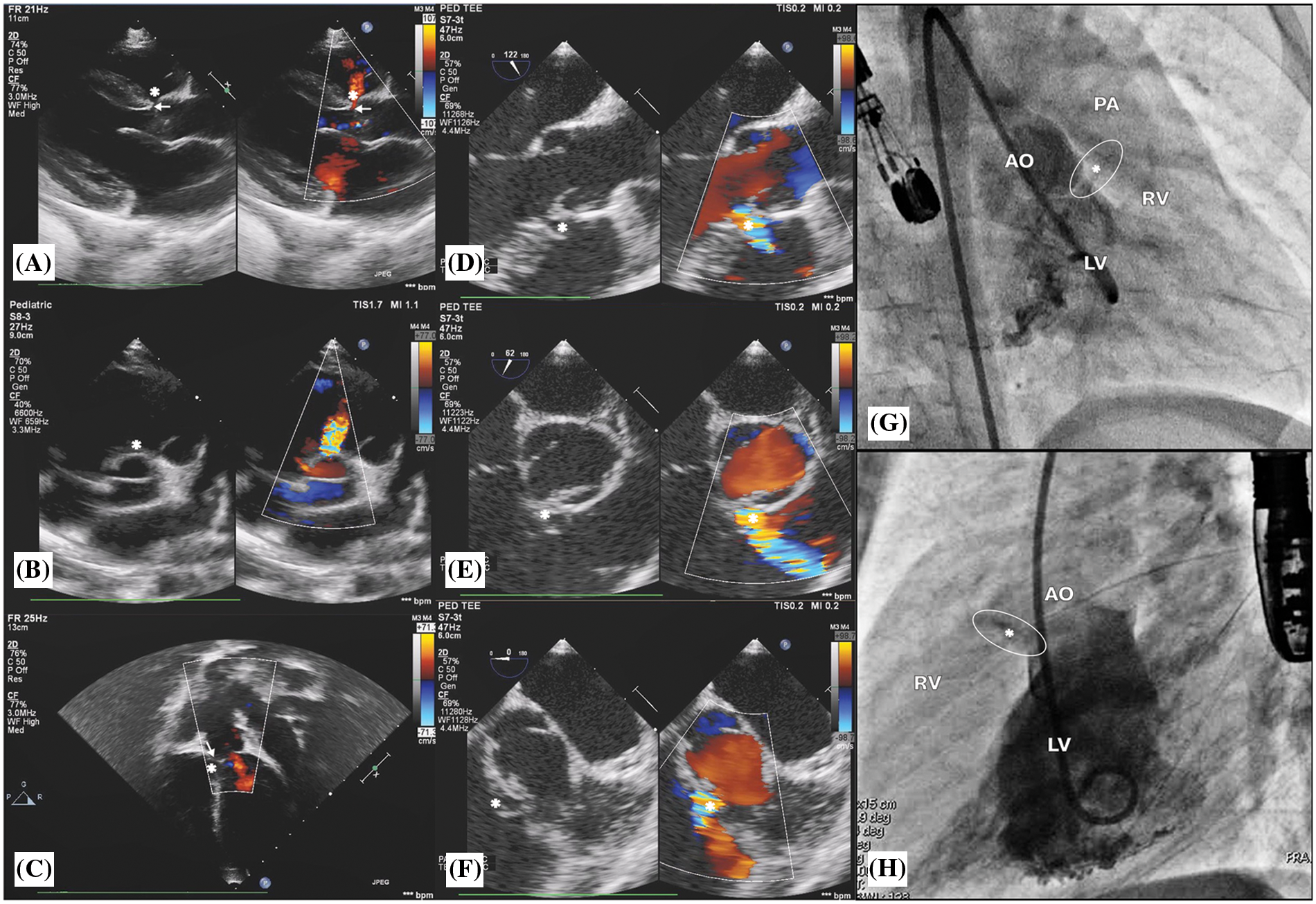
Figure 1: Transthoracic echocardiogram findings: (A), (B), and (C) show outlet ventricular septal defect, OVSD (*) and aortic valve prolapse (arrow) with mild AR. Transoesophageal echocardiogram findings: (D), (E), and (F) show OVSD (*) in the 120°, 60°, and 0° of the mid-oesophageal view. Left ventricle (LV) angiogram findings: (G) and (H) show a contrast jet beneath the aortic valve (AO) from the LV to the right ventricle (RV) in right anterior oblique 30° and true lateral views, respectively
AVP was classified as 1) mild: buckling of the aortic cusp down the left ventricular outflow tract (LVOT) with minimal herniation into the VSD; 2) moderate: aortic cusp prolapsing with the sinus of Valsalva, in which the aortic cusp has evident herniation and sinus into the VSD; or 3) severe: prolapse of the aortic cusp and its sinus through the defect into the right ventricular outflow tract (RVOT) [6].
The ratio of the jet width to the LVOT diameter was used to classify AR as 1) trivial: jet width/LVOT diameter < 10%; 2) mild: jet width/LVOT diameter ≥ 10%–24%; 3) moderate: jet width/LVOT diameter ≥ 25%–49%; and 4) severe: jet width/LVOT diameter ≥ 50% [7].
2.6 Transcatheter Closure Procedure
This procedure was performed under general anaesthesia. TOE was routinely evaluated at 0°, 60°, 90°, and 120° to reassess VSD morphology, AVP and AR degree, and guidance for deployment. Heparin 50–100 IU/kg and cefazolin 50 mg/kg were administered intravenously after obtaining the femoral artery and venous access. Hemodynamic were assessed. Left ventriculography and aortic root angiography on the RAO 30°–40° and lateral 90° projections were performed. VSD was measured from the LV angiogram and TOE in systole, including the diameter at the LV entry and RV exit and length from the LV entry to RV exit.
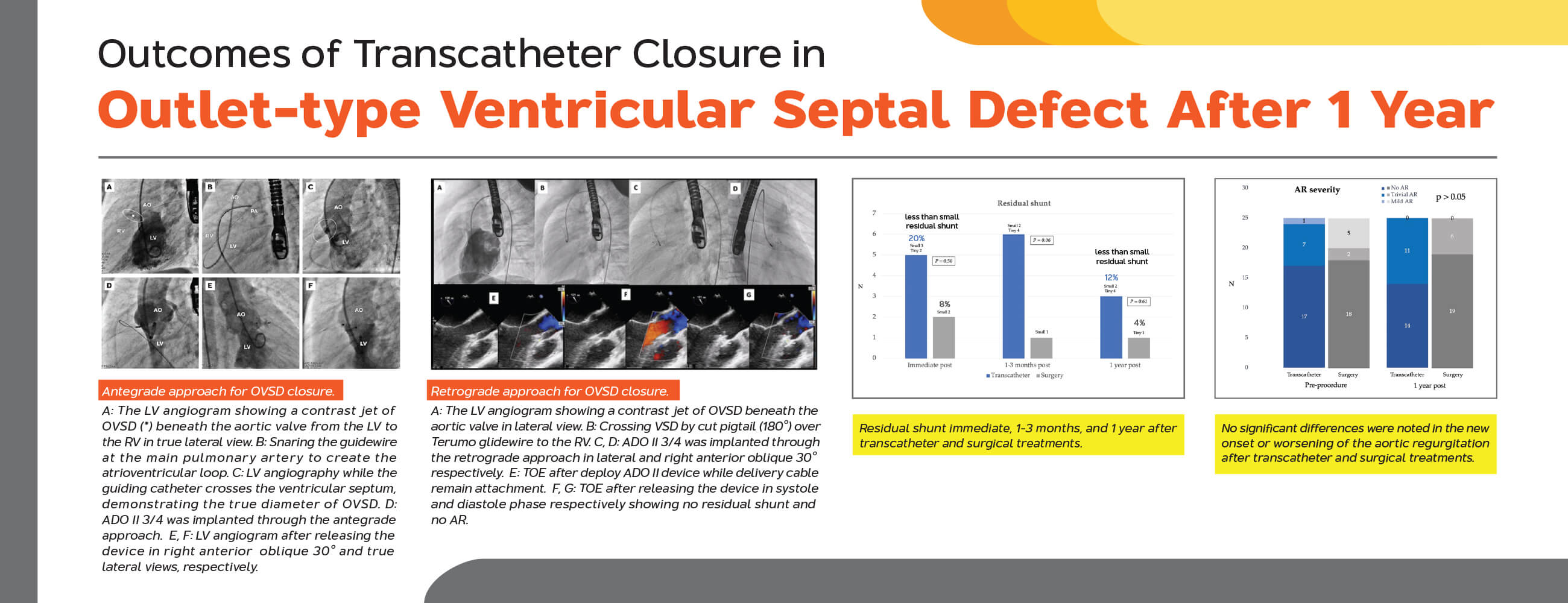
For antegrade approach (Figs. 2 and 3), the VSD was crossed from the LV using cut pigtail catheter (180°) introducing over the 0.035-inch J-Tip GLIDEWIRE® (Terumo, Tokyo, Japan) at 260 cm. The tip of cut pigtail was pointed anteriorly to ease crossing the defect. After placing the guidewire at the main pulmonary artery, it was snared via the femoral venous catheter to create the arterio-venous (AV) circuit. The 5–7 Fr delivery sheath was advanced from the femoral vein through the circuit placed at the descending aorta. VSD and degree of shunting were reassessed using repeat LV angiogram. Different occlusion devices were used for transcatheter OVSD closure as follows: AMPLATZER Duct Occluder I, II (ADO I, II; Abbott Medical, MN, USA), AMPLATZER Piccolo Occluder (APO; Abbott Medical, MN, USA), KONAR-MFTM Occluder (MFO; Lifetech Scientific, Shenzhen, China)], Nit-Occlud® Lê VSD coil (PFM coil; PFM Medical, Cologne, Germany). Generally, double-disc devices or the coil was selected for most OVSD, especially when the RV exit diameter was ≤5.0 mm (depending on device availability on shelf), whilst the ADO I was used for large defects (5.1–6.0 mm) with torrential flow. The selected device, which is 0.5–2 mm larger than the largest RV exit diameter, was introduced through the delivery sheath. The LV disc and part of the connecting waist were deployed in the ascending aorta. Subsequently, the whole system was steadily and gently pulled back across the aortic valve up to the device body seated inside the defect. Then, the RV disc was deployed by pushing the delivery cable.
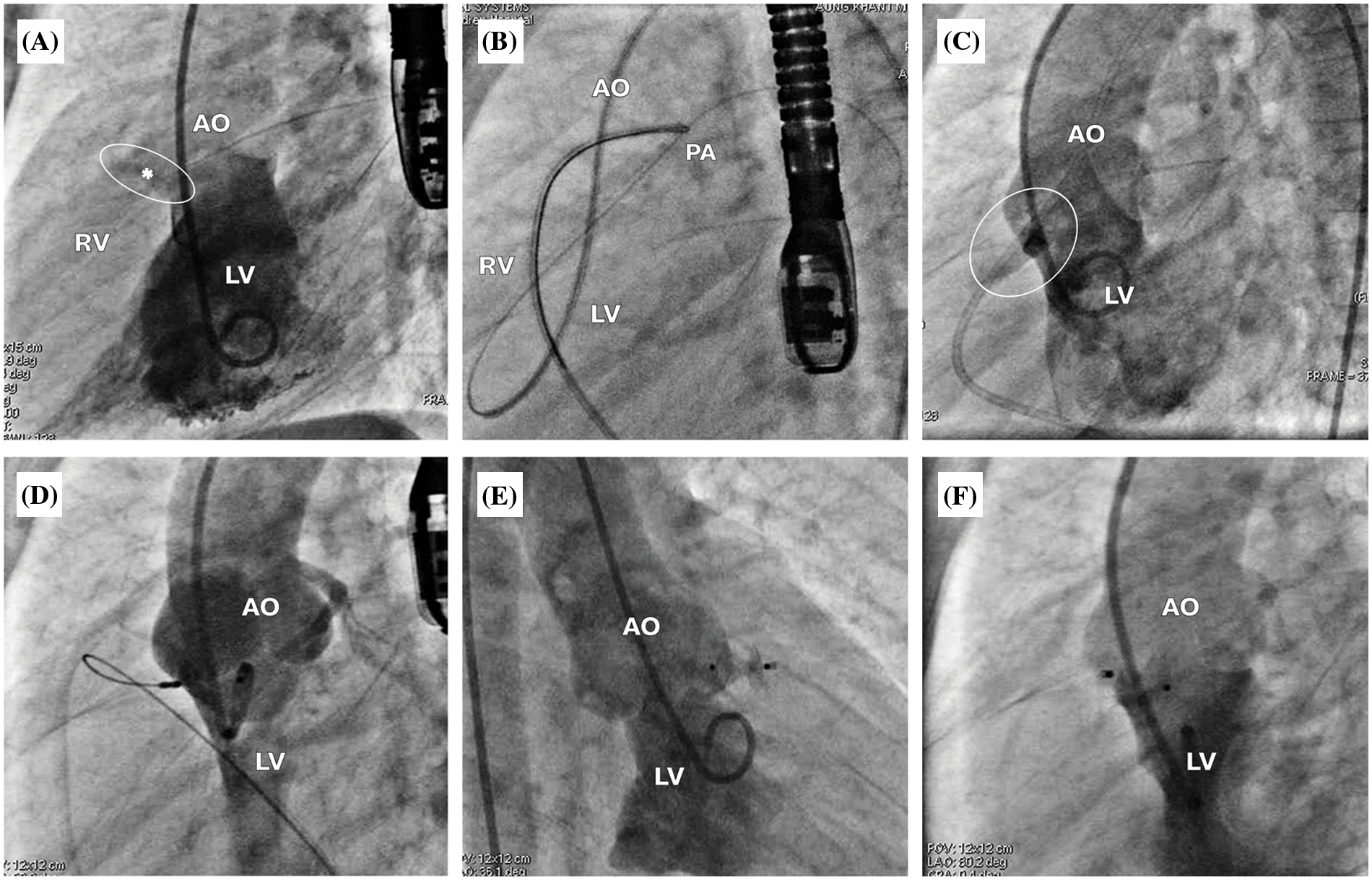
Figure 2: Antegrade approach for OVSD closure. (A) The LV angiogram showing a contrast jet of OVSD (*) beneath the aortic valve from the LV to the RV in true lateral view. (B) Snaring the guidewire at the main pulmonary artery to create the atrioventricular loop. (C) LV angiography while the guiding catheter crosses the ventricular septum, demonstrating the true diameter of OVSD. (D) ADO II 3/4 was implanted through the antegrade approach. (E), (F) LV angiogram after releasing the device in right anterior oblique 30° and true lateral views, respectively
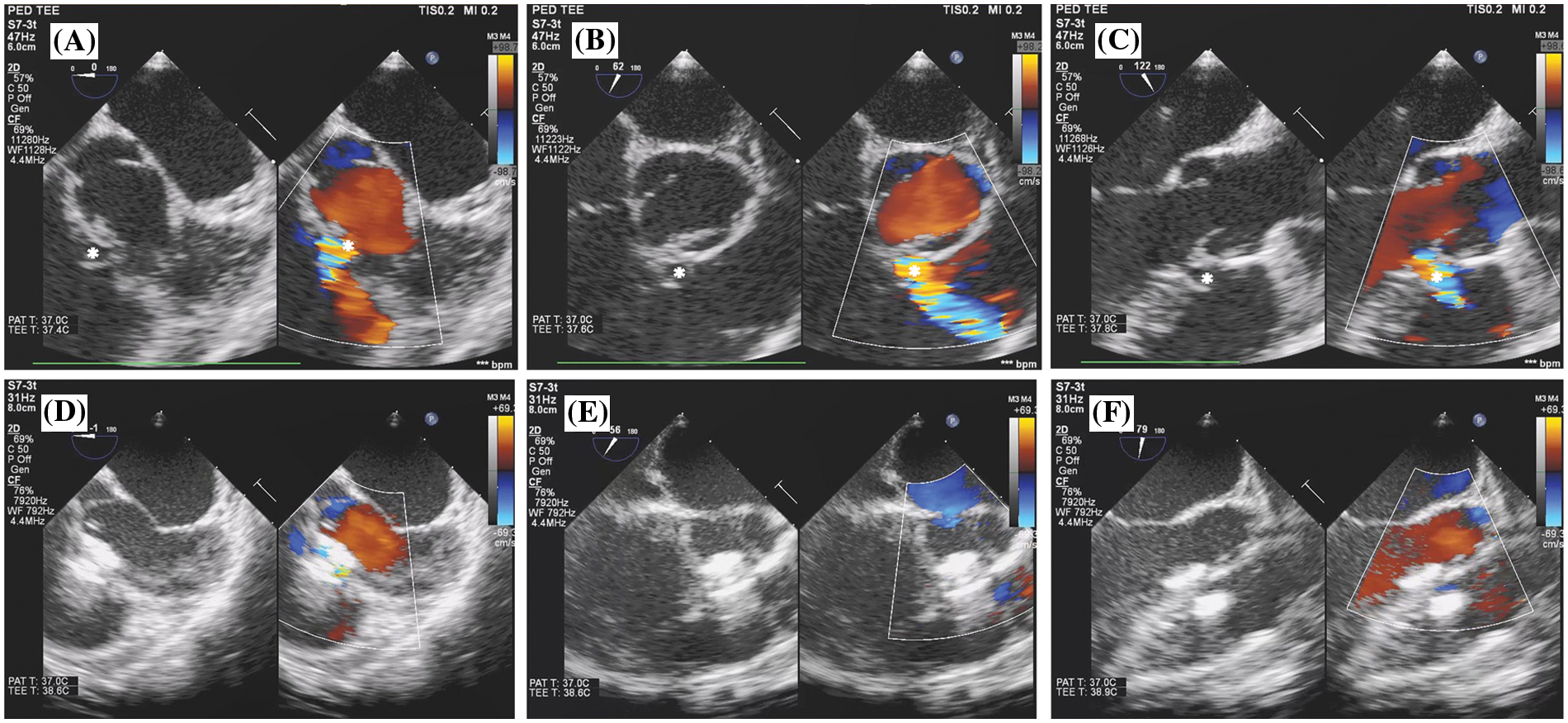
Figure 3: Transoesophageal echocardiogram findings of antegrade OVSD closure. (A), (B), and (C) show OVSD (*) in 0°, 60°, and 120° of the mid-oesophageal view before device implantation. (D), (E), and (F) show OVSD in 0°, 60°, and 120° of the mid-oesophageal view after device implantation
In later era, technique was slightly modified. Retrograde deployment was a preferential approach, without creating AV loop, to reduce procedural time and cost for the OVSDs with preexisting no more than trivial AR.
For retrograde approach (Fig. 4), after successful VSD crossing, the guidewire tip shall be placed as distal as possible to the right or left pulmonary artery branch. Subsequently, with the support from deeply seated guidewire, the 5 Fr Torque LP or 6 Fr Mullin delivery sheath was gently introduced across the defect to place at the mid RV chamber. The device was loaded and advanced to the tip of the sheath and deployed the RV disc in the RV. With echocardiographic and fluoroscopic guidances, the whole system was meticulously pulled back toward the ventricular septum. Once the RV disc well opposed to the VSD, the body and the LV disc were deployed. In order to minimize the tension to the aortic valve causing AR, the delivery system was pulled back into the ascending aorta and the delivery cable was gently pushed to coaxially align the LV disk to the VSD.
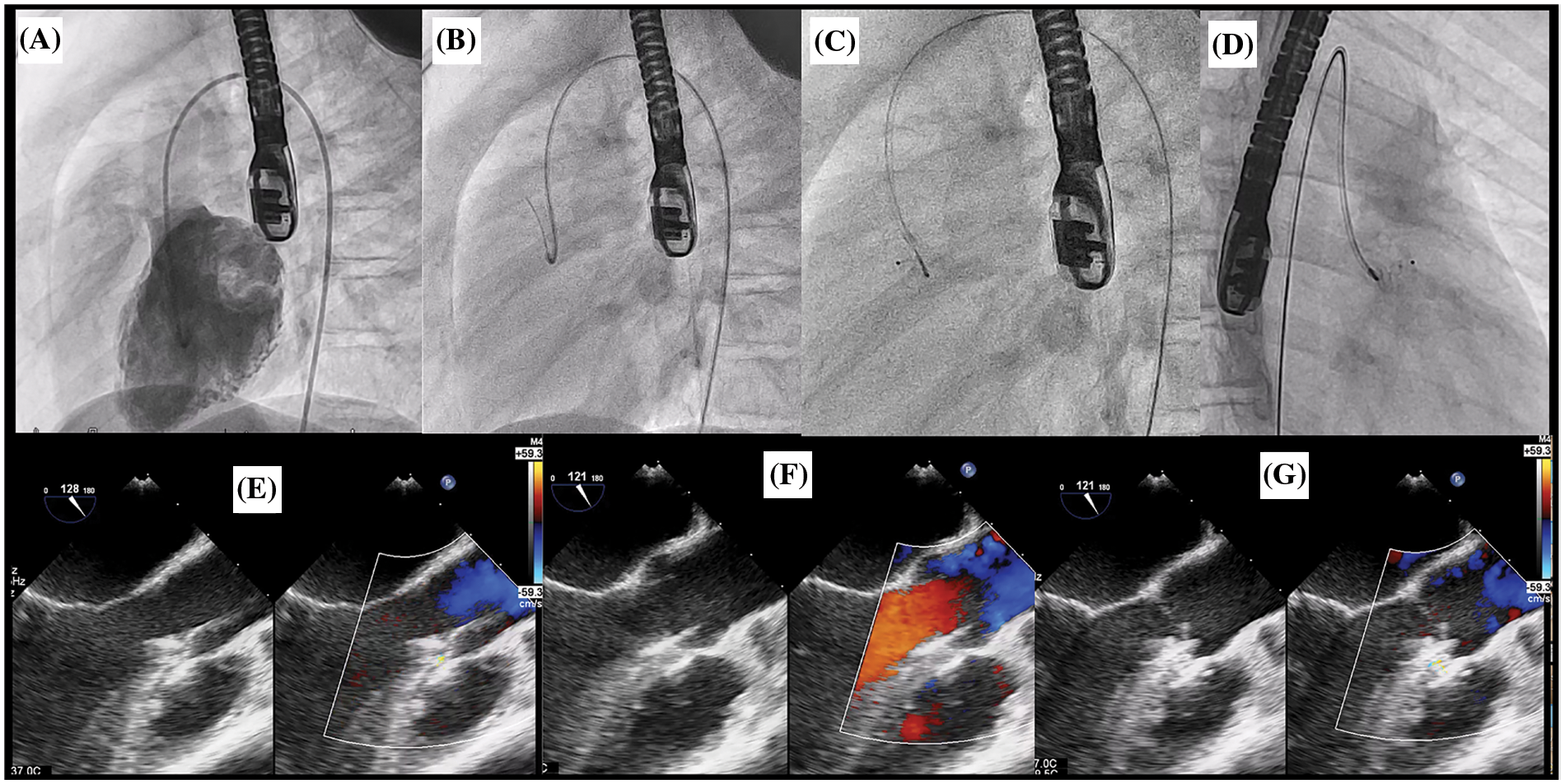
Figure 4: Retrograde approach for OVSD closure. (A) The LV angiogram showing a contrast jet of OVSD beneath the aortic valve in lateral view. (B) Crossing VSD by cut pigtail (180°) over terumo glidewire to the RV. (C), (D) ADO II 3/4 was implanted through the retrograde grade approach in lateral and right anterior oblique 30°, respectively. E: TOE after deploy ADO II device while delivery cable remain attachment. (F), (G) TOE after releasing the device in systole and diastole phase respectively showing no residual shunt and no AR
Repeated LV angiography and TOE (in 30°–45° and 75°–120°) were performed after deployment to evaluate residual shunt, device stability, evidence of RVOT/LVOT obstructions, and disturbance of the aortic and pulmonic valve function. In retrograde approach, trivial AR may be visualised from TOE since the delivery cable was still attached to the device. Nonetheless, with sleek design of the delivery cable, the disturbance of aortic valve function was minimal. Therefore, AR severity more than trivial at this stage was unacceptable. After obtaining satisfactory results, the device was released. Then, the LV angiography and TOE were conducted for final assessment.
Open heart surgery was performed through the median sternotomy under general anaesthesia and cardiopulmonary bypass. The transpulmonary approach was used to identify VSD and assess the AVP degree by visualising through the defect while infusing a cardioplegic solution into the coronary sinus. A transverse aortotomy was performed before inspecting the aortic valve. OVSD was closed with a prosthetic patch. Aortic valvuloplasty was performed if the aortic cusp was prolapsed and AR severity was moderate to severe.
Procedure time was defined as the duration from insertion of the introducer sheath to its removal for transcatheter OVSD closure and that from incision to chest wall closure in open OVSD closure surgery.
Treatment success was defined as successful implantation of the devices for OVSD closure without re-intervention and with less or negligible residual shunt. For open OVSD closure surgery, treatment success was defined as satisfactory patching of OVSD without redoing the surgery or absence of significant residual VSD. The residual shunt was routinely assessed in all patients using TTE in the apical five-chamber and parasternal long-axis views and categorised as tiny (<1-mm colour jet width), small (1–2 mm), moderate-to-large (>2–4 mm), or large (>4 mm). Major complications included Mobitz II atrioventricular block, complete heart block, new onset greater than moderate AR or tricuspid regurgitation (TR), re-intervention required within 24 h, neurovascular events, infective endocarditis, and cardiac arrest.
Data were described as mean ± standard deviation or median and interquartile range. These data were compared between groups using chi-square tests, Fisher exact tests, and two-sample t-test. Statistical significance was set at p < 0.05. All statistical analyses were performed on R (R version 4.0.4 R Foundation for Statistical Computing, Vienna, Austria).
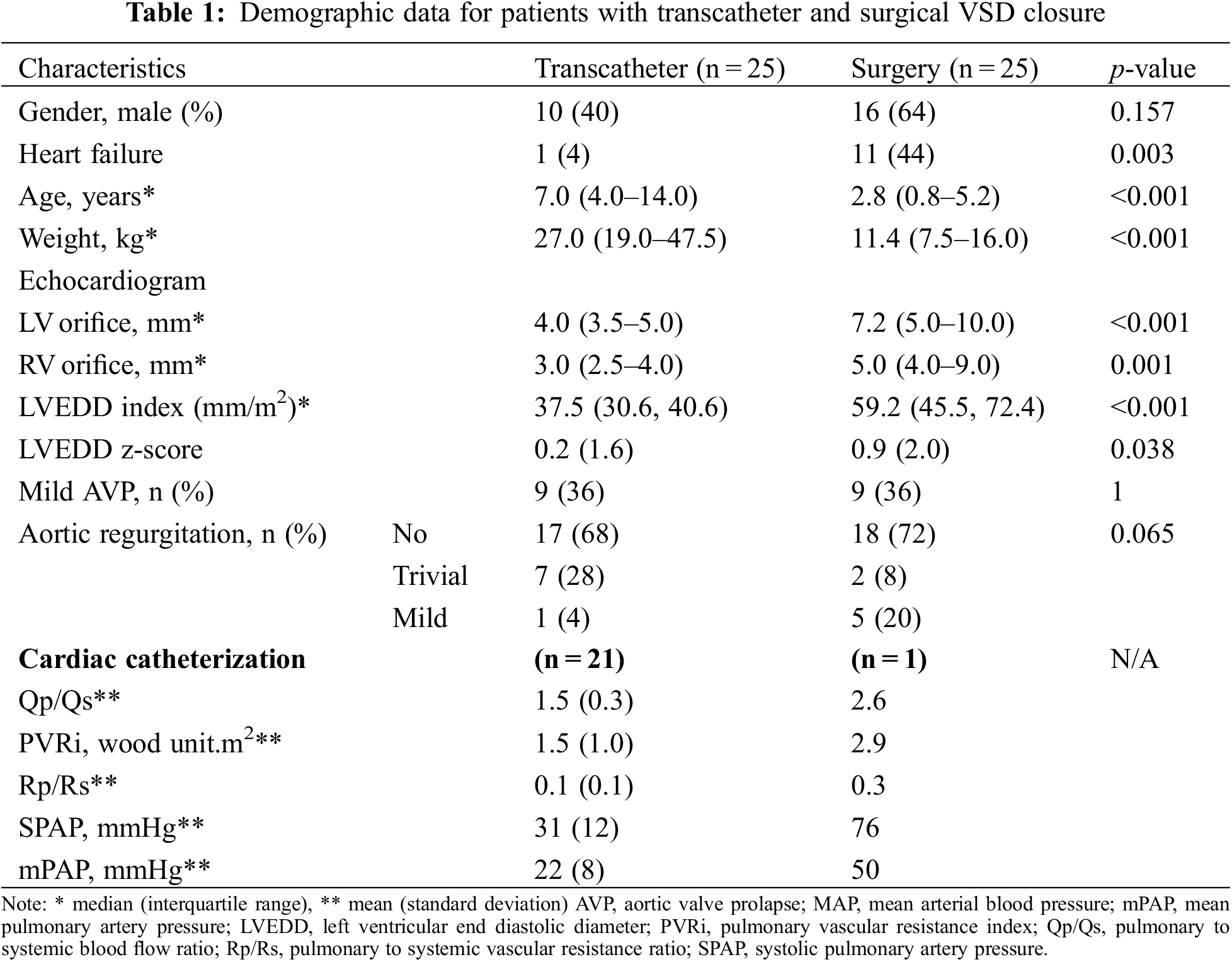
Table 1 presents the demographic, electrocardiographic, echocardiography, and hemodynamic data of the 50 patients. The transcatheter group had a median age of 7.0 years (range 4.0–14.0 years) and median weight of 27.0 kg (range 19.0–47.5 kg), which were significantly higher than those of the surgical group (median age: 2.8; range 0.8–5.2 years; median weight: 11.4 kg, range 7.5–16.0 kg; p < 0.001). Symptoms of heart failure were significantly more commonly noted in the surgical group (44% of patients) than in the transcatheter group (4%; p = 0.003). Similarly, the VSD size (5, 4–9 vs. 3, 2.5–4 mm, respectively), LVEDD index (37.5 vs. 59.2) and LVEDD Z score (0.2 vs. 0.9) were significantly larger in the surgical group than in the transcatheter group (p < 0.05). Prior to the procedure, nine patients (36%) in each group presented with mild AVP, the rest had no AVP. Eight patients (32%) in the transcatheter group and seven (28%) in the surgical group had pre-procedure trivial-to-mild AR (p = 0.065). Hemodynamic data were collected from 22 patients (21, transcatheter group; 1, surgical group), which were missing in four patients of the transcatheter group; however, these patients underwent the treatment: three had mild AVP and one had LV enlargement. In the transcatheter group, the mean pulmonary to systemic blood flow ratio (Qp/Qs) was 1.5 ± 0.3, pulmonary vascular resistant index (PVRi) was 1.5 ± 1.0 WU.m2, and mean pulmonary artery pressure (mPAP) was 21 + 8 mmHg. One patient in the surgical group underwent diagnostic cardiac catheterisation to assess PVRi in the presence of severe pulmonary hypertension; therefore, hemodynamic data could not be compared between the groups.
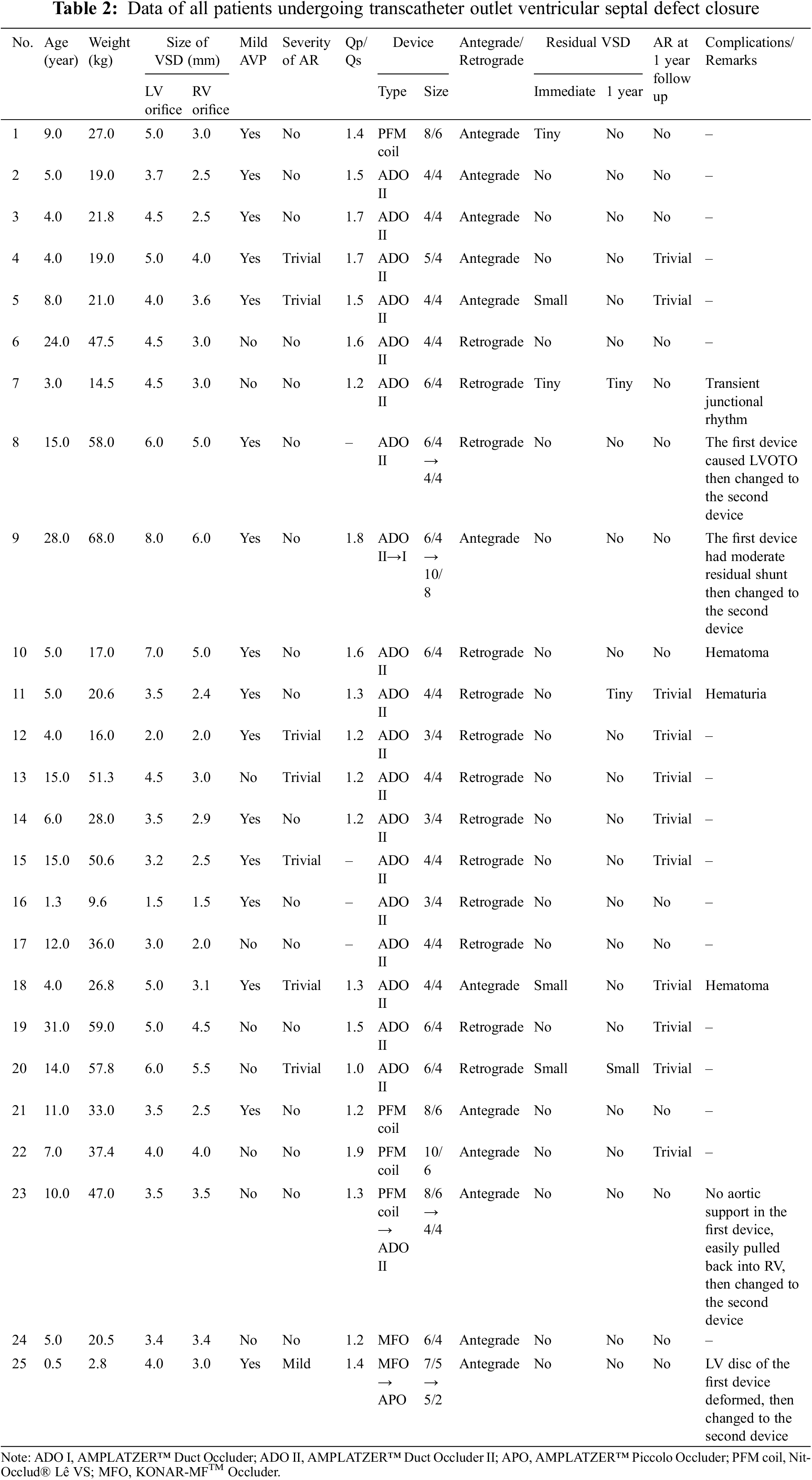
All OVSD closures were successfully performed via percutaneous and surgical approaches. The details of patient, device, and procedure of the transcatheter cases were summarized in Table 2. The ADO II was a major implanted device (19 cases, 76%). Deployment by the antegrade approach was performed in 12 patients (48%) and by the retrograde approach in 13 (52%). Four OVSDs (patient 8, 9, 23, 25) required second device replacement (Table 2). In patient 8, the first double disc device (ADO II 6/4) caused LVOT obstruction, which was abolished after replacing with smaller ADO II. In patient 9, the first device (ADO II) caused moderate residual shunt, which was considerably improved after changing to larger ADO II. In patient 23, due to unstable position of the Pfm coil, the device was changed to double disc device. Patient 25, small infant with relatively large OVSD who suffered from intractable CHF after primary repair of severe coarctation of the aorta, had deformation of the first device (MFO 7/5) with LVOT and RVOT obstructions. Smaller profile double disc device (APO 5/2) was successful implanted in this patient with satisfying result. The median procedure time and hospital stay length were significantly shorter in the transcatheter group (78 min, range 55–109 min and 2.1 days, range 2.0–2.1 days, respectively) than in the surgical group (200, 110–235 min and 8, 7–10 days, respectively).
Complete closure rate was not significantly different between transcatheter and surgical cases immediate and 12 months after the procedures. (80% and 88% vs. 92% and 96%; p > 0.05). The severity of AR after transcatheter OVSD closure remained unchanged in most patients (80%). Nonetheless, AR increased in 4 cases (16%) and disappeared in 1 case (4%) whilst in the surgical group, AR remained unchanged in 15 cases (60%), increased in 3 cases (12%), and disappeared in 7 cases (28%). The AR severity immediate and 12 months after the treatments were not significantly different between the groups. Furthermore, new onset or worsening of AR was not significantly different (p = 1) (Figs. 5 and 6).
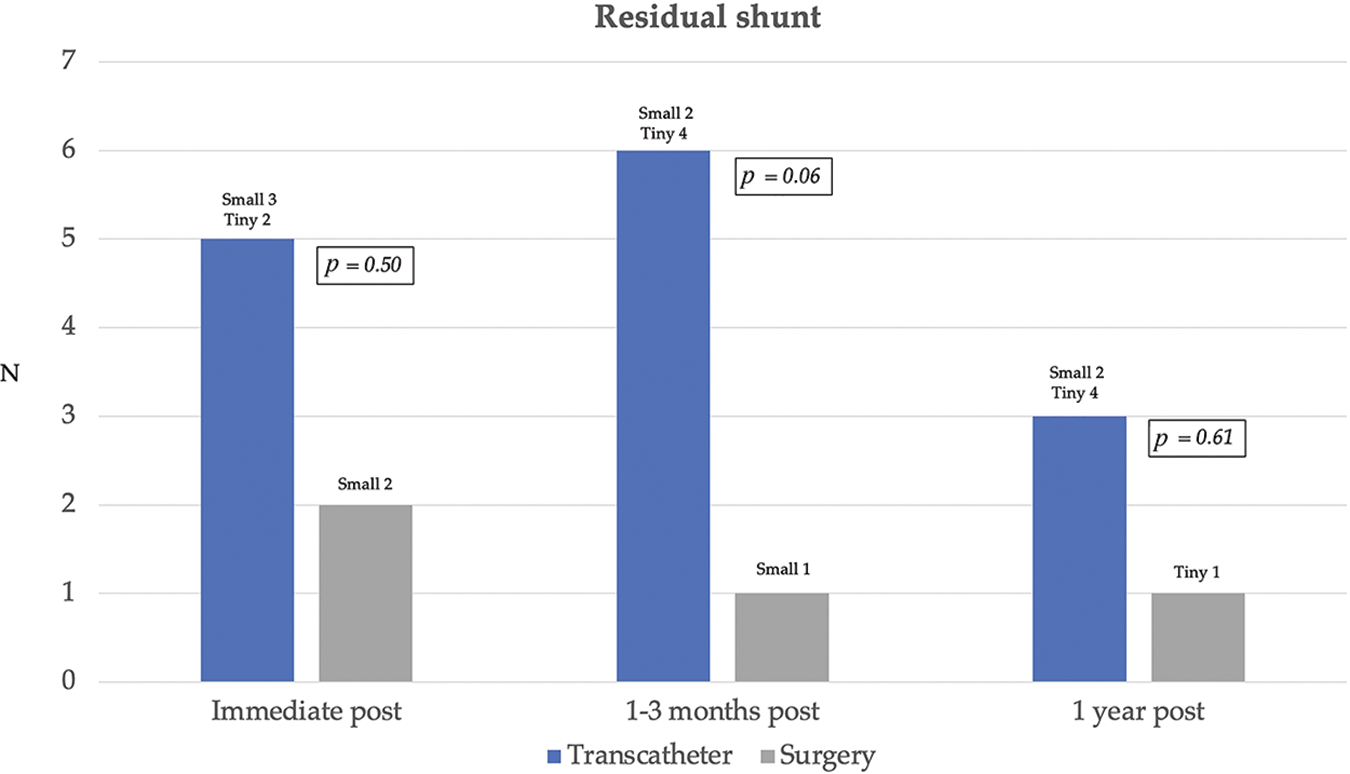
Figure 5: Residual shunt immediate, 1–3 months, and 1 year after transcatheter and surgical treatments
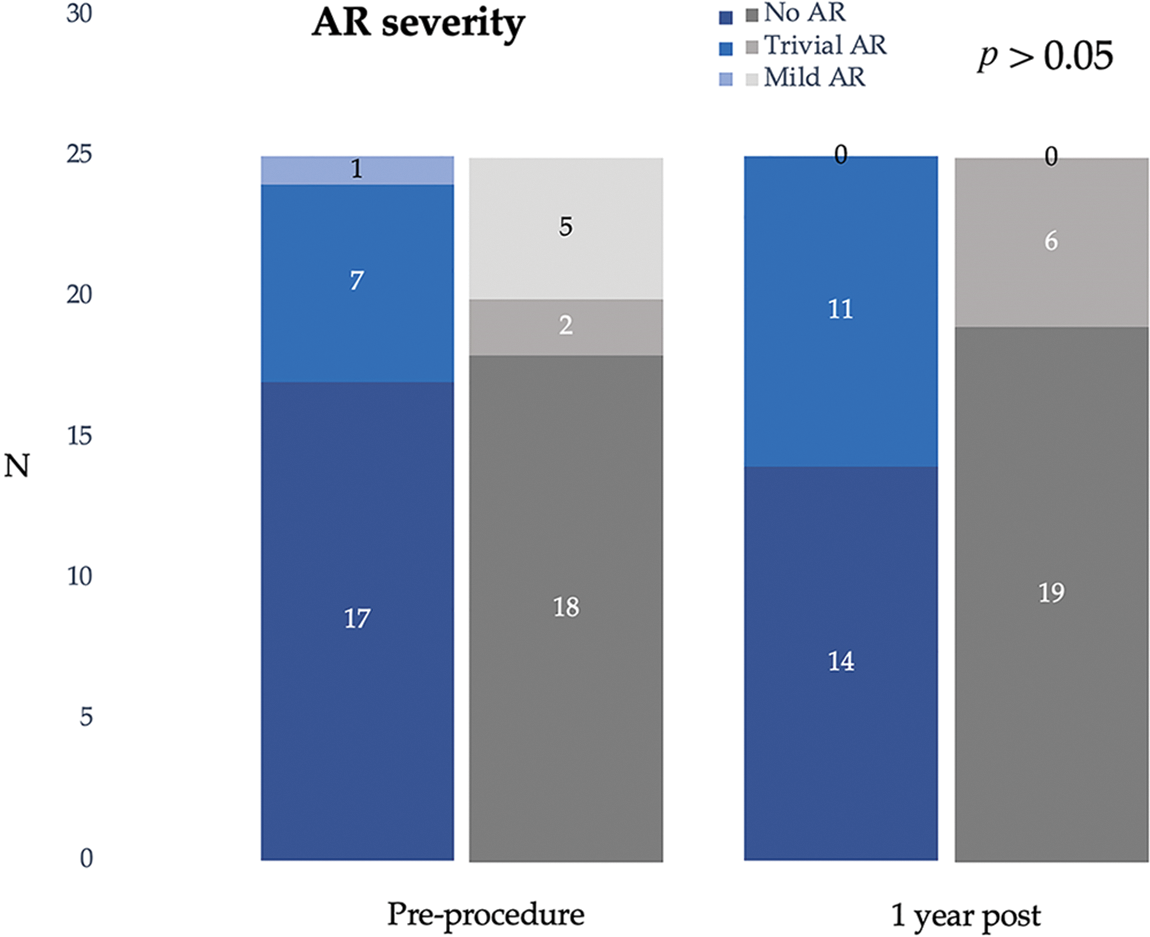
Figure 6: Severity of aortic regurgitation pre- and post-transcatheter and surgical treatments
There were no major complications (such as atrioventricular block, infective endocarditis, neurological events, or death) after transcatheter therapy. However, one patient had cardiac arrest from pulmonary hypertensive crisis within 24 h after surgery with return of spontaneous circulation. In the transcatheter group, three patients had groin hematoma and 1 patient had transient junctional rhythm during device deployment, which recovered spontaneously in the catheterisation lab. Post-procedure right bundle branch block was observed in 3 cases (12%) and six (24%) cases after transcatheter and surgical treatments, respectively (p = 0.46). All the surgical patients required post-operative inotropic support whilst none of the transcatheter patients required inotropic treatment after the procedure.
Transcatheter closure of the OVSD is an alternative treatment with high success rate. Overall procedural success is higher than 90% from previous publications [8,9]. In our study, all selected OVSDs were successfully treated by non-surgical approach. However, in the early phase of this alternative approach, as compare to standard surgical therapy, we intended to close small OVSDs which prone to have progression of aortic valve complications, not the large OVSDs with profound aortic valve distortion. As a result, the patient characteristic of transcatheter and surgical treatments are significantly different.
The efficacy of transcatheter treatment is comparable to surgery. In our study, although the number of residual shunts after device implantation was slightly higher than surgery, this difference did not reach statistical significance. We ascribed that residual shunt can be better controlled in all directions during surgical closure by suturing the superior rim of VSD through the base of pulmonic valve, while implanting the device to straddle the defect loses control of the superior rim throughout the cardiac cycle. Moreover, dynamic AV movement may delay device endothelialisation in this location. Innovative devices with occlusive internal material may provide better probability to overcome residual shunt.
Device type and size selection is the main factor for successful and effective procedure. With prolapsing of the aortic valve cusp, partially covered the OVSD, interrogation of the true defect diameter is challenging. As a result, the information from echocardiogram and LV angiogram is essential for decision making. Echocardiogram provides the details of OVSD orifice, degree of AVP, and severity of the AR. However, it cannot demonstrate true OVSD diameter in all cases. Therefore, LV angiogram is a complement modality, especially when the delivery sheath was placed through the defect pushing the prolapse aortic cusp upward.
In our early stage of transcatheter OVSD closure, despite reported of high incidence of residual shunt, the PFM coil was a favourable device due to its flexibility and ability to adjust itself to the defect [10]. However, this device is not available in our centres in recent years. Nowadays, double-disc devices are our workhorses for OVSD closure. They are flexible devices with two flat symmetrical retention discs connected by central cylindrical centre that can straddle the defect, support the aortic valve leaflets, and can be deployed either antegradely or retrogradely [11]. In this study, the ADO II was the most used device (76%), which is similar to other reports. For all double disc devices, we preferred to use the device with shortest length. This approach improves the device stability in the space with limited supporting tissue and reduces the risk of device protrusion onto the left and right ventricular outflows.
The closure device should have an appropriate diameter. An oversized device reduces the possibility of embolization but may further damage the aortic leaflets in the short- and long-term. We selected a device with diameter 0.5–2 mm larger than the maximum VSD diameter, either echocardiographically or angiographically, at the RV exit. With this approach, the incidence of device embolization, residual shunt, and aortic valve complications immediate and 1 year post-procedure are comparable to surgery and less than previous reports of OVSD transcatheter treatment [12,13]. This study confirmed that although the device is inevitably touching and interacting with aortic valve movement, in short term follow-up the aortic valve function post transcatheter treatment mostly remains intact. However, new-onset AR during follow up period was noted in few patients, and it could not be confirmed whether they were due to the transcatheter or surgical procedure. All of them have no pre-existing AVP, suggesting that AR progression in VSD needs close monitoring even if the shunt was abolished. Jung et al. reported that 86.4% of patients aged <4 years who underwent OVSD closure showed AR progression in only 0.98%, which suggests that early closure of OVSD before aortic valve deformity can prevent the development of aortic valve complications [14]. Zhang et al. suggested that once AVP appears, VSD should be closed immediately and the treatment of mild-to-moderate AVP can have a high success rate with fewer complications, which is safe and effective in long-term follow-up [15]. There were no other major complications in this study, right bundle branch block occurred in 12% after transcatheter therapy and 24% after surgery. One patient had transient junctional rhythm during device deployment, which recovered spontaneously after successful device deployment.
This retrospective study provides the result after procedure in both group of treatments but compare in different kind of patient and large variation of device. Prospective trial in the same selected patient, single device, large study size is warranted to compare their long-term outcomes.
Although this is the early phase of transcatheter OVSD closure, we would propose that in patients weighing ≥15 kg with the defect diameter ≤6 mm at the RV exit and trivial/negligible AVP/AR severity and no malalignment of the outlet septum are feasible and safe with comparable result to surgery in short term period.
Acknowledgement: We wish to thank Suppalak Puttarak and Phimphun Otongkum at the Division of Paediatric Cardiology, Department of Paediatrics. Faculty of Medicine, under the Prince of Songkla University, as well as Mr. Wiwat Wirajalarbha at the Queen Sirikit National Institute of Child Health, Department of Medical Services, Ministry of Public Health for their assistance in gathering medical records.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Nantawan Su-angka, Supaporn Roymanee, Worakan Promphan; data collection: Nantawan Su-angka, Kanjarut Wongwaitaweewong, Pimpak Prachasilchai; analysis and interpretation of results: Jirayut Jarutach, Rujira Buntharikpornpun; draft manuscript preparation: Nantawan Su-angka, Supaporn Roymanee, Worakan Promphan. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: We do not have online data for the reader to access however readers can access the data used in the study via contact the corresponding author via email. We are more than happy to share.
Ethics Approval: This study was approved by the Ethics Committees of both institutions (Approval numbers: REC.63-455-1-3 and No. 64-014).
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Devlin, P. J., Russell, H. M., Mongé, M. C., Patel, A., Costello, J. M. et al. (2014). Doubly committed and juxta arterial ventricular septal defect: Outcomes of the aortic and pulmonary valves. The Annals of Thoracic Surgery, 97, 2134–2140. https://doi.org/10.1016/j.athoracsur.2014.01.059 [Google Scholar] [PubMed] [CrossRef]
2. Layangool, T., Kirawittaya, T., Sangtawesin, C., Kojaranjit, V., Makarapong, P. et al. (2008). Natural aortic valve complications of ventricular septal defect: A prospective cohort study. Journal of the Medical Association of Thailand, 91, S53–59. [Google Scholar] [PubMed]
3. Tatsuno, K., Ando, M., Takao, A., Hatsune, K., Konno, S. (1975). Diagnostic importance of aortography in conal ventricular-septal defect. American Heart Journal, 89, 171–177. https://doi.org/10.1016/0002-8703(75)90042-3 [Google Scholar] [PubMed] [CrossRef]
4. Helmcke, F., de Souza, A., Nanda, N. C., Villacosta, I., Gatewood, R. et al. (1989). Two-dimensional and color Doppler assessment of ventricular septal defect of congenital origin. American Journal of Cardiology, 63, 1112–1116. https://doi.org/10.1016/0002-9149(89)90088-X [Google Scholar] [PubMed] [CrossRef]
5. Puchalski, M. D., Lui, G. K., Miller-Hance, W. C., Brook, M. M., Young, L. T. et al. (2019). Guidelines for performing a comprehensive transesophageal echocardiographic: Examination in children and all patients with congenital heart disease: Recommendations from the American society of echocardiography. Journal of American Society of Echocardiograph, 32, 173–215. https://doi.org/10.1016/j.echo.2018.08.016 [Google Scholar] [PubMed] [CrossRef]
6. Leung, M. P., Chau, K. T., Chiu, C., Yung, T. C., Mok, C. K. (1996). Intraoperative TEE assessment of ventricular septal defect with aortic regurgitation. The Annals of Thoracic Surgery, 61, 854–860. https://doi.org/10.1016/0003-4975(95)01133-1 [Google Scholar] [PubMed] [CrossRef]
7. Singh, J. P., Evans, J. C., Levy, D., Larson, M. G., Freed, L. A. et al. (1999). Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham heart study). American Journal of Cardiology, 83, 897–902. https://doi.org/10.1016/S0002-9149(98)01064-9 [Google Scholar] [PubMed] [CrossRef]
8. Yang, L., Tai, B. C., Khin, L. W., Quek, S. C. (2014). A systematic review on the efficacy and safety of transcatheter device closure of ventricular septal defects (VSD). Journal of Interventional Cardiology, 27(3), 260–272. https://doi.org/10.1111/joic.12121 [Google Scholar] [PubMed] [CrossRef]
9. Kuswiyanto, R. B., Rahayuningsih, S. E., Apandi, P. R., Hilmanto, D., Bashari, M. H. (2021). Transcatheter closure of doubly committed subarterial ventricular septal defect: Early to one-year outcome. International Journal of Cardiology Congenital Heart Disease, 2, 100081. https://doi.org/10.1016/j.ijcchd.2021.100081 [Google Scholar] [CrossRef]
10. Chungsomprasong, P., Durongpisitkul, K., Vijarnsorn, C., Soongswang, J., Lê, T. P. (2011). The results of transcatheter closure of VSD using Amplatzer® device and Nit Occlud®. Catheterization and Cardiovascular Interventions, 78, 1032–1040. https://doi.org/10.1002/ccd.23084 [Google Scholar] [PubMed] [CrossRef]
11. Wongwaitaweewong, K., Promphan, W., Roymanee, S., Prachasilchai, P. (2021). Effect of transcatheter closure by AmplatzerTM Duct Occluder II in patients with small ventricular septal defect. Cardiovascular Intervention and Therapeutics, 36, 375–383. https://doi.org/10.1007/s12928-020-00677-z [Google Scholar] [PubMed] [CrossRef]
12. Lin, H. C., Lin, M. T., Chen, C. A., Hsu, J. Y., Lin, S. M. et al. (2021). Safety and efficacy of transcatheter closure of outlet-type ventricular septal defects in children and adults with amplatzer duct occluder II. Journal of Formosan Medical Association, 120, 180–188. https://doi.org/10.1016/j.jfma.2020.04.015 [Google Scholar] [PubMed] [CrossRef]
13. Nguyen, H. L., Phan, Q. T., Dinh, L. H., Tran, H. B., Won, H. et al. (2018). Nit-Occlud Le VSD coil versus duct occluders for percutaneous perimembranous ventricular septal defect closure. Congenital Heart Disease, 13, 584–593. https://doi.org/10.1111/chd.12613 [Google Scholar] [PubMed] [CrossRef]
14. Jung, H., Cho, J. Y., Lee, Y. (2109). Progression of aortic regurgitation after subarterial ventricular septal defect repair: Optimal timing of the operation. Pediatric Cardiology, 40, 1696–1702. https://doi.org/10.1007/s00246-019-02206-z [Google Scholar] [PubMed] [CrossRef]
15. Zhang, W., Wang, C., Liu, S., Zhou, L., Li, J. et al. (2021). Safety and efficacy of transcatheter occlusion of perimembranous ventricular septal defect with aortic valve prolapse: A six-year follow-up study. Journal of Interventional Cardiology, 2021, 100081. https://doi.org/10.1155/2021/6634667 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools