 Open Access
Open Access
ARTICLE
Pulmonary Valve Preservation without Right Ventriculotomy in Biventricular Repair of Atrioventricular Septal Defect with Tetralogy of Fallot or Double-Outlet Right Ventricle
1 Department of Thoracic and Cardiovascular Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, 02841, Republic of Korea
2 Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, 03080, Republic of Korea
3 Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, 03080, Republic of Korea
4 Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, Seoul, 03080, Republic of Korea
5 JW Lee Center for Global Medicine, Seoul National University College of Medicine, Seoul, 03087, Republic of Korea
* Corresponding Author: Woong-Han Kim. Email:
Congenital Heart Disease 2025, 20(6), 647-658. https://doi.org/10.32604/chd.2025.075046
Received 23 October 2025; Accepted 30 December 2025; Issue published 10 February 2026
Abstract
Background: We evaluated surgical outcomes of biventricular repair for atrioventricular septal defect (AVSD) with tetralogy of Fallot (TOF) or double-outlet right ventricle (DORV). Methods: This retrospective pilot study included 12 patients who underwent biventricular repair of AVSD with TOF (n = 6) or DORV (n = 6) between 2004 and 2023. Right ventricular outflow tract (RVOT) reconstruction was performed using transannular patch (TAP, n = 4) or pulmonary valve preservation (PVP, n = 8). Clinical outcomes, including longitudinal pulmonary valve growth, RVOT obstruction, and pulmonary regurgitation, were reviewed descriptively, with particular focus on the feasibility of PVP. Results: The median age and body weight at the time of surgery were 11.7 (8.3–18.8) months and 8.6 (7.3–10.5) kg, respectively. The median follow-up duration was 67.9 (58.7–174.3) months. The two-patch technique (n = 10) was most commonly used for AVSD repair. There were no early mortalities and one late mortality. At discharge, significant (≥36 mmHg) RVOT obstruction was observed in two patients who underwent PVP. During follow-up, one patient required reoperation for significant (moderate or greater) atrioventricular valve regurgitation, and two patients in the PVP group underwent transcatheter intervention for significant RVOT obstruction. At the last follow-up, significant RVOT obstruction was present in two patients. Significant (moderate or greater) pulmonary regurgitation occurred in three patients in the TAP group. In patients who underwent PVP, the pulmonary valve annulus z-score remained within acceptable ranges, and the RVOT pressure gradient predominantly decreased to acceptable levels during follow-up. Conclusions: Mid-term outcomes of PVP in the biventricular repair of AVSD with TOF or DORV are acceptable. In selected patients, the PVP strategy may be considered a reasonable and feasible option for RVOT reconstruction.Keywords
Atrioventricular septal defect (AVSD) with tetralogy of Fallot (TOF) or double-outlet right ventricle (DORV) is a rare complex cardiac anomaly. Right ventricular (RV) outflow tract (RVOT) obstruction results from malalignment of the inlet and outlet portions of the ventricular septum, which may result in hypoplasia of the pulmonary valve (PV) annulus [1]. Secondary RV hypertrophy may further restrict the RVOT [2].
Despite reduced surgical mortality, biventricular repair of AVSD-TOF/DORV remains challenging owing to high reoperation rates [3,4,5,6,7]. Previous studies have reported reoperation rates ranging from approximately 10% to 40% after biventricular repair of AVSD-TOF/DORV [8,9,10,11,12], with common indications including residual ventricular septal defects, left atrioventricular valve (AVV) regurgitation, pulmonary regurgitation (PR), and RVOT obstruction [13,14,15,16,17].
The optimal surgical approach to prevent these complications has yet to be established, given that operative strategies vary across institutions and may require individualization based on patient-specific clinical and anatomic characteristics [1,2,3]. Previously, we applied the transatrial-transpulmonary approach with RVOT reconstruction using limited right ventriculotomy and a transannular patch (TAP) to avoid a long ventriculotomy incision and external conduits when-ever possible.
Over time, in addition to preserving PV function, we have increasingly focused on infundibular function to optimize long-term RV function. This was motivated by our observation that aneurysmal change frequently occurs in the infundibular area where right ventriculotomy was performed, with the widening of the TAP over time. Aneurysmal change in the infundibular area caused paradoxical contractions during RV systole that interrupted effective RV ejection and cardiac output. Moreover, limited right ventriculotomy was reported to have no long-term beneficial effects on RV volume and function compared with conventional right ventriculotomy in patients who underwent trans-annular repair of TOF [18]. Our rationale is supported by previous studies that have described infundibular dysfunction following right ventriculotomy [19].
Accordingly, since 2015, the pulmonary valve preservation (PVP) strategy has been applied in our institution. This approach aimed to minimize PR and avoid right ventriculotomy to preserve the function of the PV and the RV infundibulum, and then allow growth and functional restoration of the PV with forward pulmonary flow. We hypothesized that our revised strategy of preserving the PV and RV infundibular area might be helpful in ultimately preserving RV function in the long-term. Therefore, we reviewed surgical outcomes of biventricular repair for AVSD with TOF or DORV, with particular focus on evaluating the feasibility and potential benefits of PVP without right ventriculotomy.
The retrospective pilot study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Seoul National University Hospital (Approval date: 29 September 2020, Approval No.: H-2009-135-1159). Patient consent was waived due to the retrospective nature of the study and the use of de-identified data.
A total of 13 patients consecutively underwent biventricular repair for balanced AVSD with TOF or DORV from January 2004 to August 2023. One patient with pulmonary atresia was excluded. Finally, twelve patients were included in this pilot study. All data were obtained retrospectively. The median follow-up duration was 67.9 [58.7–174.3] months.
DORV was diagnosed if both great arteries originated predominantly from the right ventricle with the application of the “50% rule” [20]. Aorto-mitral discontinuity was confirmed for the diagnosis of DORV in all patients.
The repair was performed under standard cardiopulmonary bypass with bi-caval cannulation, mild-to-moderate hypothermia (32°C), and antegrade cardioplegia.
AVSD repair was performed using the one- or two-patch technique at the surgeon’s discretion through right atriotomy. Superior or inferior bridging leaflets were divided for better exposure of defects and appropriate partitioning, as required. A comma-shaped intraventricular patch was tailored to prevent left ventricular outflow tract (LVOT) obstruction, as appropriate. The AVV was repaired by cleft closure with the interrupted technique and annuloplasty.
We attempted to preserve the PV annulus with extensive pulmonary commissurotomy to widen the PV orifice and to provide sufficient motion of PV leaflets by releasing tethered PV leaflets while ensuring that the leaflets remained attached to the pulmonary artery wall. After extensive pulmonary commissurotomy, the RVOT was measured with sized Hegar dilators.
A longitudinal incision was made from just distal to the PV annulus to the bifurcation of the pulmonary artery. Before and after extensive pulmonary commissurotomy, resection of the hypertrophied right ventricular cavity muscle, including extensive infundibulectomy, was performed through right atriotomy and pulmonary arteriotomy. Relief of subvalvular stenosis is an important part of RVOT reconstruction, as any stenosis below the valve will mask successful PVP. Furthermore, slicing of sub-leaflet tissues might allow elongation of the leaflets and increase mobility. Finally, pulmonary arterioplasty was performed to widen the main pulmonary artery using a glutaraldehyde-treated autologous pericardial patch, which was appropriately prepared in a normal size (z-score: approximately zero) with a Hegar dilator.
After cardiopulmonary bypass weaning, RVOT reconstruction was considered acceptable when subvalvular and supravalvular obstructions were judged to be adequately relieved based on direct pressure measurements and intraoperative transesophageal echocardiography (TEE) after cardiopulmonary bypass weaning, including evaluation of Doppler-derived peak velocities at each RVOT level, regardless of the PV annulus z-score that was measured with a Hegar dilator after commissurotomy or the postoperative pressure ratio of the RV to the left ventricle (RV inlet/ascending aorta). The surgical procedures for RVOT obstruction relief were completed when TEE confirmed that all surgically correctable subvalvular and supravalvular lesions had been relieved and the patient’s hemodynamic status was stable, allowing a residual pressure gradient at the level of the PV.
2.4 Echocardiographic Evaluation
The perioperative and follow-up degree of RVOT obstruction and PR were evaluated by transthoracic echocardiography based on the following European Association of Echocardiography and the American Society of Echocardiography criteria: for RVOT obstruction, mild was defined as a peak velocity of <3.0 m/s; moderate as 3.0–4.0 m/s; and severe as >4.0 m/s; and for PR, 0 was none-to-trivial; 1, mild; 2, mild-to-moderate; 3, moderate; and 4, severe [21,22]. A significant grade was defined as a moderate or more than moderate grade. We defined RV dysfunction as a RV fractional area change of less than 35%. All preoperative and postoperative echocardiographic findings were obtained and subsequently reviewed by two cardiologists, who validated the final findings.
2.5 Evaluation of Clinical Outcomes
Early mortality was defined as death within 30 days postoperatively or during the same hospitalization. Late mortality was defined as any death that occurred >30 days after surgery and after hospital discharge. Primary endpoints were all-cause mortality and reoperation due to a cardiac cause, and secondary endpoints were the composite of surgical and transcatheter interventions due to native PV failure caused by significant stenosis and regurgitation. Regular postoperative follow-up was performed at 3–6-month intervals.
Continuous variables are expressed as median [interquartile range] and categorical variables as frequencies (%). Survival and event-free survival rates were estimated using the Kaplan-Meier method. Given the small sample size, all data were analyzed descriptively.
Statistical analyses were performed using IBM SPSS statistical software (version 25.0, IBM Inc., Armonk, NY).
3.1 Baseline Characteristics and Operative Data
Six patients diagnosed with AVSD with TOF and six patients with AVSD with DORV underwent biventricular repair. TAP widening was performed in four patients and PV was preserved in eight patients. The median age and body weight at the time of operation were 11.7 [8.3–18.8] months and 8.6 [7.3–10.5] kg, respectively.
There were no cases of heterotaxy syndrome or pulmonary vein anomalies. Two patients underwent palliative pulmonary artery banding due to aggravated heart failure, followed by biventricular repair of AVSD with DORV. Rastelli type C complete AVSD was the most common (9/12, 75.0%), and the two-patch technique (10/12, 83.3%) was mostly used for AVSD repair. Baseline characteristics and perioperative data are summarized in Table 1.
Table 1: Baseline characteristics and perioperative data of the study patients.
| Variable | Total (N = 12) | TAP (n = 4) | PVP (n = 8) |
|---|---|---|---|
| Female | 7 (58.3) | 2 (50.0) | 5 (62.5) |
| Age (month) | 11.7 [8.3–18.8] | 10.5 [9.2–28.5] | 14.0 [8.0–18.8] |
| Body weight (kg) | 8.6 [7.3–10.5] | 8.4 [6.9–11.2] | 8.6 [8.2–10.5] |
| Body surface area (m2) | 0.42 [0.37–0.49] | 0.40 [0.36–0.49] | 0.42 [0.39–0.49] |
| Diagnosis | |||
| AVSD-TOF | 6 (50.0) | 2 (50.0) | 4 (50.0) |
| AVSD-DORV | 6 (50.0) | 2 (50.0) | 4 (50.0) |
| Preterm | 2 (16.7) | 0 (0.0) | 2 (25.0) |
| Low birth weight (<2.5 kg) | 4 (33.3) | 2 (50.0) | 2 (25.0) |
| Down syndrome | 5 (41.7) | 3 (75.0) | 2 (25.0) |
| Prior palliative surgery | 2 (16.7) | 0 (0.0) | 2 (25.0) |
| Pulmonary valve annulus z-score | −1.89 [−3.41 to 0.38] | −2.93 [−3.41 to −1.67] | −1.08 [−3.54 to 0.84] |
| RVOT pressure gradient (mmHg) | 77.4 [60.1–89.4] | 72.3 [54.3–97.4] | 77.4 [60.1–89.4] |
| Significant AVVR | 3 (25.0) | 1 (25.0) | 2 (25.0) |
| The Type of AVSD | |||
| Intermediate | 2 (16.7) | 1 (25.0) | 1 (12.5) |
| Rastelli type A | 1 (8.3) | 0 (0.0) | 1 (12.5) |
| Rastelli type C | 9 (75.0) | 3 (75.0) | 6 (75.0) |
| AVSD Repair | |||
| One-patch technique | 2 (16.7) | 0 (0.0) | 2 (25.0) |
| Two-patch technique | 10 (83.3) | 4 (100.0) | 6 (75.0) |
| Pulmonary Valve | |||
| Tricuspid | 5 (41.7) | 1 (25.0) | 4 (50.0) |
| Bicuspid | 7 (58.3) | 3 (75.0) | 4 (57.1) |
| Cardiopulmonary bypass time (min) | 249.0 [214.5–299.8] | 282.0 [246.5–302.5] | 224.5 [200.8–286.3] |
| Aortic cross-clamp time (min) | 192.5 [173.5–216.0] | 211.0 [192.3–237.3] | 177.5 [149.8–210.3] |
There were no early mortalities or morbidities. Postoperative atrioventricular block, residual shunt, and cardiogenic shock requiring mechanical circulatory support did not occur in either group. At discharge, no patient had significant PR, left AVV regurgitation, right AVV regurgitation, or LVOT obstruction, whereas significant RVOT obstruction was identified in two patients from the PVP group. The median duration of mechanical ventilation and the median lengths of intensive care unit and hospital stay were 2.0 (1.0–4.8), 4.0 (2.0–13.3), and 13.5 (7.3–29.8) days, respectively.
Late mortality occurred in one patient who was diagnosed with Rastelli type C complete AVSD with TOF and had Down syndrome. This patient was the youngest (3.3 months) and lightest (3.5 kg) in this study at the time of operation. Owing to the progression of heart failure, surgery was performed earlier than in other patients. The patient died of infection 2.5 months after surgery, with no clinical evidence to suspect a cardiac cause at the time of death. The overall survival rate at 15 years was 91.7% (Fig. 1).
Three patients required surgical (n = 1) or transcatheter (n = 2) intervention after surgery. Of these, one patient in the PVP group required reoperation at 77.3 months postoperatively due to progression to significant left AVV regurgitation. The patient had undergone biventricular repair for intermediate AVSD with DORV using a two-patch technique. At discharge, the patient had mild left AVV regurgitation, which progressed to a moderate degree at 5.6 months after surgery. The regurgitation then remained stable without further deterioration until worsening to more than a moderate grade at 76.2 months. At the time of reoperation, both the significant left AVV regurgitation and the concomitant mild-to-moderate right AVV regurgitation were repaired. Other patients underwent transcatheter intervention due to significant pulmonary valvular stenosis; the RVOT pressure gradient was reduced to less than 36 mmHg (peak velocity < 3 m/s) upon cardiac catheterization after balloon pulmonary valvuloplasty. Freedom from reoperation and the composite of surgical and transcatheter interventions at 15 years were 80.0% and 64.6%, respectively (Fig. 1).
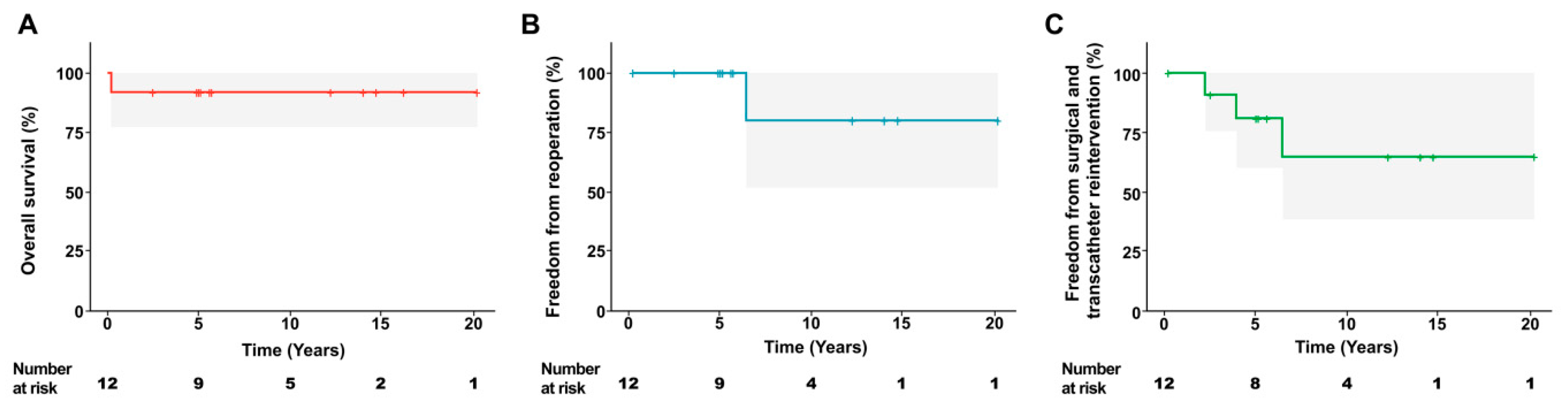
Figure 1: Freedom from reoperation and the composite of surgical and transcatheter interventions at 15 years. (A) Overall survival, (B) freedom from reoperation, and (C) freedom from surgical and transcatheter reintervention.
Of the two patients who had significant RVOT obstruction at discharge, one showed improvement after discharge, whereas the other continued to have significant RVOT obstruction. After discharge, significant RVOT obstruction was identified in three patients during follow-up; significant RVOT obstruction newly developed in one patient from the TAP group and in one additional patient from the PVP group, resulting in a total of three patients (two in the PVP group and one in the TAP group). In the PVP group, two patients (one with persistent significant RVOT obstruction from discharge and one who newly developed significant RVOT obstruction during follow-up) underwent balloon pulmonary valvuloplasty, which reduced the pressure gradient to <36 mmHg; however, significant RVOT obstruction subsequently recurred in one of these patients. At the last follow-up, significant RVOT obstruction remained in two patients (one in the PVP group and one in the TAP group), both of whom had no RVOT obstruction at discharge. Significant PR occurred in three patients from the TAP group, which persisted until the most recent follow-up. At the last follow-up, no patient exhibited significant right AVV regurgitation, LVOT obstruction, or RV dysfunction. During follow-up, significant left AVV regurgitation developed in two patients from the PVP group; one required reoperation, whereas the other improved to less than moderate grade (mild) with medical follow-up. At the last follow-up, there was no significant left AVV regurgitation.
3.3 Pulmonary Valve Preservation Approach
In the PVP group, throughout the follow-up period, the PV annulus z-score was maintained within an acceptable range, and the RVOT pressure gradient decreased to acceptable levels (Fig. 2). Significant RVOT obstruction was identified in two patients at discharge; one improved spontaneously during follow-up, whereas the other required balloon pulmonary valvuloplasty and subsequently showed improvement. However, one patient who had no significant RVOT obstruction at discharge developed new RVOT obstruction. Preoperatively, this patient had the lowest PV annulus z-score (−4.15) and the highest RVOT pressure gradient (92.2 mmHg), along with a dysplastic bicuspid PV. Although the PV annulus z-score and RVOT pressure gradient improved at discharge (z-score: −2.93 and 33.6 mmHg, respectively), the RVOT pressure gradient deteriorated during follow-up. Consequently, the patient underwent balloon pulmonary valvuloplasty for significant pulmonary valvular stenosis at 27.2 months postoperatively. At the last follow-up, the patient’s PV annulus z-score and RVOT pressure gradient were −0.3 and 41.0 mmHg, respectively. No significant PR was observed in the PVP group at the last follow-up. Additionally, the PR grade for all patients in this group remained below grade 1 with no further progression during the follow-up period.
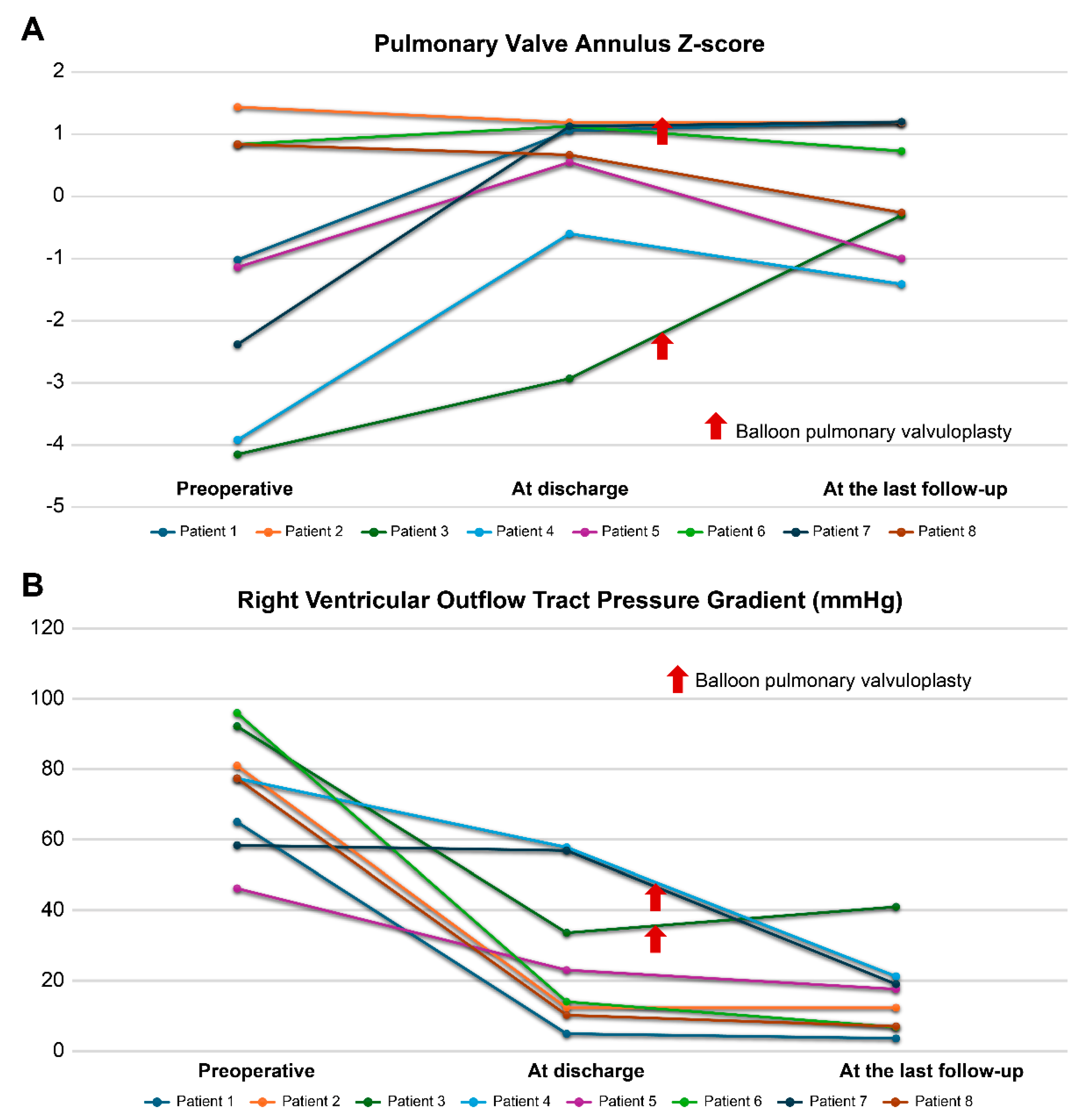
Figure 2: Changes in (A) pulmonary valve annulus z-score and (B) right ventricle outflow tract pressure gradient in the pulmonary valve preservation group. Patients 3 and 7 underwent balloon pulmonary valvuloplasty for right ventricular outflow tract obstruction during the follow-up.
Surgical outcomes of AVSD with TOF or DORV have improved over the decades [1,2,3,4,5]. Primary repair may be the ideal treatment approach, but it remains challenging. Tailored complex patches are required to prevent LVOT and RVOT obstruction, while ensuring the competency of the AVV. In addition to the complex anatomy, the presence of AVV regurgitation, other associated cardiac anomalies, and prior palliation can make successful repair difficult [3,7,16]. In biventricular repair of AVSD with TOF or DORV, preserving both AVV and PV function is critical. In particular, the competence of the left AVV is important in repaired AVSD with RVOT obstruction. In addition, effective RVOT reconstruction may play a key role in maintaining the long-term function of PV and RV.
A previous study in patients with repaired TOF showed that PR and RVOT aneurysm/akinesia following RVOT reconstruction, especially when using a trans-ventricular approach and a TAP, were associated with left as well as RV dysfunction [19]. Another study revealed that RVOT patch dysfunction related to akinetic or paradoxically contracting RVOT was responsible for biventricular systolic dysfunction when compared with pulmonary stenosis (treated with valvotomy) and comparable amounts of residual PR [23]. These studies suggest that measures to maintain or restore PV function and avoid RVOT aneurysm/akinesia are mandatory to preserve biventricular function after surgery. In addition, PR following RVOT reconstruction, particularly when using a trans-ventricular approach, is poorly tolerated in patients with AVSD-TOF/DORV who have residual AVV regurgitation [1].
In patients with AVSD with TOF/DORV, long-term PV and RV function may be influenced by the strategy used for RVOT reconstruction. Excessive PR and RVOT aneurysm or akinesia following trans-ventricular repair have been associated with impaired biventricular performance, ventricular arrhythmia, and sudden cardiac death [24,25]. These concerns provided the rationale for adopting a PVP strategy to preserve the PV annulus and avoid right ventriculotomy. By preserving the function of the PV and the RV infundibulum while minimizing PR, this approach is intended to promote forward pulmonary flow, facilitate PV growth, and reduce the long-term risk of RV failure and arrhythmia. To achieve satisfactory outcomes, maintaining the optimal balance between acceptable RVOT obstruction and minimal PR is a key objective. Within this framework, we evaluated our experience with biventricular repair of AVSD with TOF or DORV, focusing on the surgical outcomes of the PVP strategy, including residual RVOT obstruction, PR, and RV function.
4.1 Biventricular Repair of Atrioventricular Septal Defect with TOF or DORV
Our mid-term surgical outcomes were acceptable with favorable RVOT obstruction. In terms of overall survival, reoperation, and intervention, outcomes were acceptable compared to those of other recent studies [1,2,3,4,5]. Recent advances in surgical and perioperative management have markedly improved survival in patients undergoing biventricular repair for AVSD with TOF/DORV, and recent series have reported favorable mortality rates comparable to those in our cohort [6,7,8,9,10]. Nevertheless, despite improvements in survival, reoperation and reintervention rates in contemporary studies remain substantial [11,12,13], reflecting the inherent anatomic complexity of this population and the persistent long-term challenges associated with AVV- and RVOT-related issues [14,15,16,17].
Perioperative AVV regurgitation has been shown to be a risk factor for death and reoperation, and is associated with poor prognosis [3,7,10]. Additionally, heterotaxy syndrome with pulmonary vein anomalies has been reported as a risk factor for death or significant complications [3]. In our cohort, significant left AVV regurgitation occurred in two patients during follow-up; one patient, who had undergone surgery before 2015, required reoperation as mentioned above, while the other showed improvement without further progression under close follow-up. Furthermore, LVOT obstruction or right AVV regurgitation did not occur during follow-up. Although significant RVOT obstruction and PR each occurred in three patients during follow-up, these patients had acceptable functional statuses and did not require surgical intervention for native PV failure. Among the three patients who developed significant RVOT obstruction during follow-up, two underwent transcatheter intervention for RVOT obstruction, and significant RVOT obstruction was present in two patients at the last follow-up.
4.2 RVOT Reconstruction with Preservation of the Pulmonary Valve Annulus
In patients with surgically treated TOF, the severity of PR is significantly associated with RV function and ventricular arrhythmia, underscoring the importance of preserving PV function [24,25]. Consistent with this concept, several studies on preserving the PV annulus in TOF have demonstrated favorable outcomes [26,27,28,29]. This approach may lower the risks of PR, RV dilatation, and reoperations and reinterventions, including late PV implantation [30,31,32,33]. Furthermore, our previous study showed the encouraging early outcomes of PVP and the avoidance of right ventriculotomy in the total repair of TOF; the PV appeared to grow during short-term follow-up in patients after total correction of TOF with the PVP strategy [28]. However, to our knowledge, follow-up data on outcomes of RVOT reconstruction with preserved PV annulus in biventricular repair of AVSD with TOF or DORV remain limited [6,8,9,14].
In the present study, RVOT obstruction improved predominantly without progression of PR in the PVP group during follow-up. At the last follow-up, the PV annulus z-score remained within the normal range, and the RVOT pressure gradient decreased to acceptable levels. These findings can be explained by the active approach, provided that the near-complete elimination of obstruction at the subvalvular and supravalvular levels was accomplished with acceptable residual RVOT obstruction at the PV level.
Regarding the significant RVOT obstruction observed at discharge in two patients from the PVP group, one showed spontaneous improvement during follow-up, whereas the other required balloon pulmonary valvuloplasty and subsequently remained free of significant RVOT obstruction. However, significant RVOT obstruction was newly detected in one patient with a severely hypoplastic PV (z-score: −4.15) combined with severe preoperative RVOT obstruction (92.2 mmHg). Considering the preoperative PV annulus size, this finding may reflect aggravation of valvular pulmonary stenosis due to a small, restricted leaflet opening caused by tethering despite prior surgical valvotomy. During follow-up, the patient’s functional status was acceptable without clinical symptoms, and balloon pulmonary valvuloplasty was successfully performed while preserving the PV. At the last follow-up, the PV annulus z-score was within the normal range (−0.3), and the peak RVOT pressure gradient was 41 mmHg. Although further improvement in RVOT obstruction is expected with continued growth of the native PV, careful longitudinal follow-up is warranted. Although long-term data remain limited, we believe that RVOT obstruction will not worsen significantly in the long-term, considering the trends observed in the PV annulus z-score and RVOT pressure gradient in our cohort. In addition, the previous study showed a progressive drop in the RVOT pressure gradient over time [8]. Ultimately, we expect the RVOT, including the PV, to grow owing to forward pulmonary flow and sufficient elimination of multi-level obstruction. However, if severe RVOT obstruction persists postoperatively in cases with severely dysplastic or hypoplastic PV, additional preemptive and proactive treatment may be required.
In our study, most patients in the TAP group (3/4, 75%) showed significant progression of PR within 3 years postoperatively. Among them, one patient, after a follow-up period of 167.5 months, was scheduled for percutaneous PV replacement. Conversely, the PVP group showed no progression beyond grade 1 during follow-up. Previous studies showed that RVOT reconstruction with a TAP is associated with the development of significant PR [2,4,6,8,9]. In addition to the aforementioned advantages of PVP from surgically treated TOF, prior studies on AVSD with TOF have demonstrated that PVP in AVSD-TOF repair resulted in lower rates of PR and RVOT-related reintervention, as well as reduced RV dilatation [6,8,9]. These findings, which align with our results, further support the role of PVP in reducing long-term PR burden when RVOT obstruction can be maintained within an acceptable range.
Collectively, these findings suggest that PVP may offer a physiologic advantage in preventing PR over the TAP approach and could lead to improved long-term outcomes if RVOT obstruction remains stable over time. Accordingly, longitudinal assessment of PV function and RVOT obstruction is crucial. Dysplastic bicuspid or monocuspid PVs may deteriorate more rapidly, underscoring the need for regular and close monitoring, particularly for PV function, in these patients.
4.3 Feasibility of Pulmonary Valve Preservation Strategy
Our findings collectively support the feasibility of adopting a PVP strategy in biventricular repair for AVSD with TOF or DORV. Despite the anatomical complexity and technical challenges, PVP demonstrated encouraging early and mid-term outcomes, with acceptable RVOT function and preserved PV competence, suggesting that this approach can be a reasonable and feasible option in appropriately selected patients. Nevertheless, a non-negligible proportion of patients (2/8, 25%) required transcatheter reintervention for significant RVOT obstruction, indicating that PVP may carry a potential risk of residual or recurrent RVOT obstruction, even though it can minimize PR. Importantly, all RVOT-related reinterventions were managed exclusively with balloon pulmonary valvuloplasty while preserving the native PV. Considering these findings, while the potential risk of RVOT obstruction should be recognized, we believe this risk is clinically acceptable with close follow-up and timely intervention when indicated.
This study has some limitations. First, it was a non-randomized retrospective single-center pilot study. Second, the interpretation of results could be biased owing to the small sample size, which reflects the rare incidence of this disease, and the differences in the follow-up duration between the groups. Accordingly, the results were presented in a descriptive manner. This descriptive pilot design due to limited sample size results in insufficient statistical power, which limits causal inference and precludes formal comparative analyses between different RVOT reconstruction strategies. Third, there were no cases of severe PV anomalies, such as monocuspid PV, nor patients with previously known risk factors, including heterotaxy syndrome and pulmonary vein anomalies. Fourth, the difference in the periods when surgery was performed between the groups should be considered. Because the PVP and TAP procedures were performed during different surgical eras, not only the RVOT reconstruction technique itself but also contemporaneous advances in operative techniques, cardiopulmonary bypass management, and perioperative care may have influenced the results. In addition, although PVP may be effective in preventing PR, the need for transcatheter reintervention for RVOT obstruction in a notable proportion of patients represents a clinically important limitation. Given these limitations, the generalizability of our findings should be interpreted with caution, and further studies with larger cohorts and longer follow-up periods are warranted to more fully evaluate PR, RVOT obstruction, and RV function over time. Furthermore, future studies incorporating cardiac magnetic resonance imaging will be valuable for objectively assessing long-term RV size and function, and for further validating the durability and feasibility of PVP over time.
The mid-term outcomes of biventricular repair for AVSD with TOF or DORV were acceptable. In selected patients, the PVP strategy may be considered a reasonable and feasible option to potentially preserve PV and RV function, allow continued PV growth, and prevent PR. However, the high rate of transcatheter reintervention for RVOT obstruction suggests that careful patient selection and close long-term surveillance are essential.
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization: Jae Hong Lee and Woong-Han Kim; methodology: Jae Hong Lee; software: Jae Hong Lee; validation: Jae Hong Lee, Seung Min Baek and Hye Won Kwon; formal analysis: Jae Hong Lee; investigation: Jae Hong Lee, Seung Min Baek and Hye Won Kwon; resources: Seung Min Baek, Hye Won Kwon, Sungkyu Cho, Jae Gun Kwak and Woong-Han Kim; data curation: Jae Hong Lee, Seung Min Baek and Hye Won Kwon; writing—original draft preparation: Jae Hong Lee; writing—review and editing: Woong-Han Kim; visualization: Jae Hong Lee; supervision: Woong-Han Kim; project administration: Woong-Han Kim. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval: The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Seoul National University Hospital (Approval date: 29 September 2020, Approval No.: H-2009-135-1159). Patient consent was waived due to the retrospective nature of the study and the use of de-identified data.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Malm T , Karl TR , Mee RB . Transatrial-transpulmonary repair of atrioventricular septal defect with right ventricular outflow tract obstruction. J Card Surg. 1993; 8( 6): 622– 7. doi:10.1111/j.1540-8191.1993.tb00421.x. [Google Scholar] [CrossRef]
2. Brancaccio G , Michielon G , Filippelli S , Perri G , Di Carlo D , Iorio FS , et al. Transannular patching is a valid alternative for tetralogy of Fallot and complete atrioventricular septal defect repair. J Thorac Cardiovasc Surg. 2009; 137( 4): 919– 23. doi:10.1016/j.jtcvs.2008.09.055. [Google Scholar] [CrossRef]
3. Devaney EJ , Lee T , Gelehrter S , Hirsch JC , Ohye RG , Anderson RH , et al. Biventricular repair of atrioventricular septal defect with common atrioventricular valve and double-outlet right ventricle. Ann Thorac Surg. 2010; 89( 2): 537– 42 ;discussion542–3. doi:10.1016/j.athoracsur.2009.10.049. [Google Scholar] [CrossRef]
4. Ong J , Brizard CP , d’Udekem Y , Weintraub R , Robertson T , Cheung M , et al. Repair of atrioventricular septal defect associated with tetralogy of Fallot or double-outlet right ventricle: 30 years of experience. Ann Thorac Surg. 2012; 94( 1): 172– 8. doi:10.1016/j.athoracsur.2012.02.070. [Google Scholar] [CrossRef]
5. Shuhaiber JH , Robinson B , Gauvreau K , Breitbart R , Mayer JE , Del Nido PJ , et al. Outcome after repair of atrioventricular septal defect with tetralogy of Fallot. J Thorac Cardiovasc Surg. 2012; 143( 2): 338– 43. doi:10.1016/j.jtcvs.2011.05.031. [Google Scholar] [CrossRef]
6. Gupta U , Polimenakos AC , El-Zein C , Ilbawi MN . Tetralogy of Fallot with atrioventricular septal defect: surgical strategies for repair and midterm outcome of pulmonary valve-sparing approach. Pediatr Cardiol. 2013; 34( 4): 861– 71. doi:10.1007/s00246-012-0558-3. [Google Scholar] [CrossRef]
7. Raju V , Burkhart HM , Rigelman Hedberg N , Eidem BW , Li Z , Connolly H , et al. Surgical strategy for atrioventricular septal defect and tetralogy of Fallot or double-outlet right ventricle. Ann Thorac Surg. 2013; 95( 6): 2079– 84 ;discussion2084–5. doi:10.1016/j.athoracsur.2013.02.016. [Google Scholar] [CrossRef]
8. Kotani Y , Chetan D , Ono N , Mertens LL , Caldarone CA , Van Arsdell GS , et al. Late functional outcomes after repair of tetralogy of Fallot with atrioventricular septal defect: a double case-match control study. J Thorac Cardiovasc Surg. 2013; 145( 6): 1477– 84.e1–4. doi:10.1016/j.jtcvs.2013.01.007. [Google Scholar] [CrossRef]
9. Stephens EH , Tingo J , Najjar M , Yilmaz B , Levasseur S , Dayton JD , et al. Cardiac function after tetralogy of fallot/complete atrioventricular canal repair. World J Pediatr Congenit Heart Surg. 2017; 8( 2): 189– 95. doi:10.1177/2150135116682719. [Google Scholar] [CrossRef]
10. Vitanova K , Cleuziou J , Schreiber C , Günther T , Pabst von Ohain J , Hörer J , et al. Long-term outcome of patients with complete atrioventricular septal defect combined with the tetralogy of fallot: staged repair is not inferior to primary repair. Ann Thorac Surg. 2017; 103( 3): 876– 80. doi:10.1016/j.athoracsur.2016.07.038. [Google Scholar] [CrossRef]
11. Prifti E . Repair of complete atrioventricular septal defect with tetralogy of Fallot. Transl Pediatr. 2017; 6( 1): 1– 7. doi:10.21037/tp.2017.01.01. [Google Scholar] [CrossRef]
12. Alhawri KA , McMahon CJ , Alrih MM , Alzein Y , Khan AA , Mohammed SK , et al. Atrioventricular septal defect and tetralogy of Fallot—a single tertiary center experience: a retrospective review. Ann Pediatr Cardiol. 2019; 12( 2): 103– 9. doi:10.4103/apc.APC_87_18. [Google Scholar] [CrossRef]
13. Sugimoto A , Tachimori H , Hirata Y , Sakamoto K , Ota N , Shiraishi S , et al. Contemporary surgical management of complete atrioventricular septal defect with tetralogy of Fallot in Japan. Gen Thorac Cardiovasc Surg. 2022; 70( 10): 835– 41. doi:10.1007/s11748-022-01809-3. [Google Scholar] [CrossRef]
14. Fernandez-Cisneros A , Staffa SJ , Emani SM , Chávez M , Friedman KG , Hoganson DM , et al. Association of tetralogy of Fallot and complete atrioventricular canal: a single-centre 40-year experience. Eur J Cardiothorac Surg. 2024; 65( 2): ezae037. doi:10.1093/ejcts/ezae037. [Google Scholar] [CrossRef]
15. Schumacher K , Marin-Cuartas M , Meier S , Aydin MI , Borger MA , Dähnert I , et al. Long-term results following combined repair of atrioventricular septal defect with tetralogy of fallot. Pediatr Cardiol. 2025; 46( 1): 134– 8. doi:10.1007/s00246-023-03343-2. [Google Scholar] [CrossRef]
16. Callahan CP , Argo MB , McCrindle BW , Barron DJ , Jegatheeswaran A , Honjo O , et al. Early outcomes for management of atrioventricular septal defect-tetralogy of fallot in the last decade: a congenital heart surgeons' society study. World J Pediatr Congenit Heart Surg. 2025; 16( 2): 262– 72. doi:10.1177/21501351241293158. [Google Scholar] [CrossRef]
17. Mashali MH , Abdelmohsen GA , Baamer AS , Elhudairy MS , Alkhushi NA , Bahaidarah SA , et al. Clinical outcomes of tetralogy canal repair: a multidisciplinary perspective. Saudi Med J. 2025; 46( 9): 1039– 45. doi:10.15537/smj.2025.46.9.20250398. [Google Scholar] [CrossRef]
18. Lee C , Lee CH , Kwak JG , Kim SH , Shim WS , Lee SY , et al. Does limited right ventriculotomy prevent right ventricular dilatation and dysfunction in patients who undergo transannular repair of tetralogy of Fallot? Matched comparison of magnetic resonance imaging parameters with conventional right ventriculotomy long-term after repair. J Thorac Cardiovasc Surg. 2014; 147( 3): 889– 95. doi:10.1016/j.jtcvs.2013.11.019. [Google Scholar] [CrossRef]
19. Davlouros PA , Kilner PJ , Hornung TS , Li W , Francis JM , Moon JCC , et al. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J Am Coll Cardiol. 2002; 40( 11): 2044– 52. doi:10.1016/S0735-1097(02)02566-4. [Google Scholar] [CrossRef]
20. Ueda M , Becker AE . Classification of hearts with overriding aortic and pulmonary valves. Int J Cardiol. 1985; 9( 3): 357– 69. doi:10.1016/0167-5273(85)90033-6. [Google Scholar] [CrossRef]
21. Zoghbi WA , Enriquez-Sarano M , Foster E , Grayburn PA , Kraft CD , Levine RA , et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003; 16( 7): 777– 802. doi:10.1016/S0894-7317(03)00335-3. [Google Scholar] [CrossRef]
22. Baumgartner H , Hung J , Bermejo J , Chambers JB , Evangelista A , Griffin BP , et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009; 10( 1): 1– 25. doi:10.1093/ejechocard/jen303. [Google Scholar] [CrossRef]
23. Puranik R , Tsang V , Lurz P , Muthurangu V , Offen S , Frigiola A , et al. Long-term importance of right ventricular outflow tract patch function in patients with pulmonary regurgitation. J Thorac Cardiovasc Surg. 2012; 143( 5): 1103– 7. doi:10.1016/j.jtcvs.2011.09.039. [Google Scholar] [CrossRef]
24. Gatzoulis MA , Balaji S , Webber SA , Siu SC , Hokanson JS , Poile C , et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000; 356( 9234): 975– 81. doi:10.1016/S0140-6736(00)02714-8. [Google Scholar] [CrossRef]
25. Frigiola A , Redington AN , Cullen S , Vogel M . Pulmonary regurgitation is an important determinant of right ventricular contractile dysfunction in patients with surgically repaired tetralogy of Fallot. Circulation. 2004; 110( 11 Suppl 1): II153– 7. doi:10.1161/01.CIR.0000138397.60956.c2. [Google Scholar] [CrossRef]
26. Kim GS , Han S , Yun TJ . Pulmonary annulus preservation lowers the risk of late postoperative pulmonary valve implantation after the repair of tetralogy of Fallot. Pediatr Cardiol. 2015; 36( 2): 402– 8. doi:10.1007/s00246-014-1021-4. [Google Scholar] [CrossRef]
27. Hickey E , Pham-Hung E , Halvorsen F , Gritti M , Duong A , Wilder T , et al. Annulus-sparing tetralogy of fallot repair: low risk and benefits to right ventricular geometry. Ann Thorac Surg. 2018; 106( 3): 822– 9. doi:10.1016/j.athoracsur.2017.11.032. [Google Scholar] [CrossRef]
28. Kwak JG , Kim WH , Kim ER , Lim JH , Min J . One-year follow-up after tetralogy of fallot total repair preserving pulmonary valve and avoiding right ventriculotomy. Circ J. 2018; 82( 12): 3064– 8. doi:10.1253/circj.CJ-18-0690. [Google Scholar] [CrossRef]
29. Jiang X , Liu J , Peng B , Zhang H , Li S , Yan J , et al. Impact of annulus-sparing on surgical adequacy of pulmonary valve in complete repair of tetralogy of fallot with right ventricular outflow tract incision. Pediatr Cardiol. 2021; 42( 2): 379– 88. doi:10.1007/s00246-020-02493-x. [Google Scholar] [CrossRef]
30. Guariento A , Schiena CA , Cattapan C , Avesani M , Doulamis IP , Padalino MA , et al. Pulmonary valve preservation during tetralogy of Fallot repair: midterm functional outcomes and risk factors for pulmonary regurgitation. Eur J Cardiothorac Surg. 2022; 62( 2): ezac365. doi:10.1093/ejcts/ezac365. [Google Scholar] [CrossRef]
31. Martins RS , Fatimi AS , Mahmud O , Qureshi S , Nasim MT , Virani SS , et al. Comparing clinical and echocardiographic outcomes following valve-sparing versus transannular patch repair of tetralogy of Fallot: a systematic review and meta-analysis. Interdiscip Cardiovasc Thorac Surg. 2024; 39( 1): ivae124. doi:10.1093/icvts/ivae124. [Google Scholar] [CrossRef]
32. Raju V , Srinivasan N , Kadavanoor D , Moorthy R , Jothinath K , Gangadharan S , et al. Mid-term results of pulmonary valve-sparing repair for tetralogy of fallot with pulmonary stenosis. World J Pediatr Congenit Heart Surg. 2025; 16( 2): 237– 45. doi:10.1177/21501351241279519. [Google Scholar] [CrossRef]
33. John JD , Patel T , Kharat M , Patel JH , Al Hooti J , Syeda ZR , et al. Does valve-sparing repair improve outcomes in tetralogy of fallot? A systematic review. J Cardiothorac Surg. 2025; 20( 1): 343. doi:10.1186/s13019-025-03519-2. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools