 Open Access
Open Access
ARTICLE
Artificial Intelligence and Internet of Things Enabled Intelligent Framework for Active and Healthy Living
1 Department of Surgery, College of Medicine, Najran University Saudi Arabia, Najran, 61441, Saudi Arabia
2 Department of Computer Science, Edge Hill University, St Helens Rd, Ormskirk, L39 4QP, United Kingdom
3 Electrical Engineering Department, College of Engineering, Najran University Saudi Arabia, Najran, 61441, Saudi Arabia
4 Department of Electrical Engineering, Hamdard University, Islamabad, Pakistan
* Corresponding Author: Muhammad Awais. Email:
Computers, Materials & Continua 2023, 75(2), 3833-3848. https://doi.org/10.32604/cmc.2023.035686
Received 31 August 2022; Accepted 02 February 2023; Issue published 31 March 2023
Abstract
Obesity poses several challenges to healthcare and the well-being of individuals. It can be linked to several life-threatening diseases. Surgery is a viable option in some instances to reduce obesity-related risks and enable weight loss. State-of-the-art technologies have the potential for long-term benefits in post-surgery living. In this work, an Internet of Things (IoT) framework is proposed to effectively communicate the daily living data and exercise routine of surgery patients and patients with excessive weight. The proposed IoT framework aims to enable seamless communications from wearable sensors and body networks to the cloud to create an accurate profile of the patients. It also attempts to automate the data analysis and represent the facts about a patient. The IoT framework proposes a co-channel interference avoidance mechanism and the ability to communicate higher activity data with minimal impact on the bandwidth requirements of the system. The proposed IoT framework also benefits from machine learning based activity classification systems, with relatively high accuracy, which allow the communicated data to be translated into meaningful information.Keywords
Obesity is strongly linked with health-related issues and can increase the prevalence of several chronic diseases [1,2]. A surgical solution for obese individuals is to undergo bariatric surgery that can provide an immediate resolution. Although this surgery can result in significant weight loss, it is not a permanent and complete cure for obesity. Therefore, even after bariatric surgery, an active lifestyle is vital to prevent obesity. Patients undergoing weight loss surgery should ensure a balanced diet and regular exercise during and after surgery.
Moreover, this requires monitoring and tracking activity behaviors to promote health and active life routine where technology can assist. Postoperative surgery care and long-term support can be managed through the recent advancements in big data, information and communication technology (ICT), data analytics, artificial intelligence, machine learning, and the internet of things (IoT). This study focuses on developing an intelligent IoT-based framework that can classify and profile daily living activities via the cloud and send all the sensory information and data to the cloud. The aim is to promote healthy and active living in obese individuals and avoid weight gain.
Postoperative follow-up care is an essential aspect of any surgery. Patient recovery depends on the implementation of postoperative follow-up care. Some surgical patients can leave the hospital in 3–5 days, but it may take longer to return to normal activities. For example, a patient who has undergone weight loss surgery can be discharged from the hospital in one to three days. However, it will take four to six weeks to return to everyday life, and the patient will still follow a relatively rigorous exercise routine and maintains proper dietary habits [3,4]. In such scenarios, regular follow-up appointments may last up to two years [5]. Therefore, providing patients with the necessary care and appropriate resources during the postoperative period is critical to support their healthy recovery. Unfortunately, traditional monitoring systems have increased the burden on health services and discouraged them from taking the necessary steps for minor surgeries where most support is directed to life-threatening critical cases.
Novel technological solutions are needed to meet the enormous burden of healthcare. Developing intelligent healthcare systems using machine learning (ML) and the IoT has the potential to solve problems related to surgery and postoperative care.
This article presents an IoT-enabled, machine learning-based solution for monitoring vital signs and promoting healthy living for surgical patients. In postoperative scenarios, patients must monitor their cardiovascular system, fluid and electrolyte balance, prevalence and treatment of infections and excessive bleeding, major organ function, deep vein thrombosis, and anastomotic leaks. In addition, wearable sensors to measure other vitals and accelerometer readings to classify the physical activities performed are to be maintained along with the eating habits and logging food intake against the activity level. This paper proposes an extensive framework with IoT-enabled infrastructure to collect the necessary information from the users/patients and a cloud-based machine-learning solution to transform the collected data into actionable information. The main contributions of this work can be divided into three systems interlinked to give a technology-driven healthcare and monitoring framework. The main contributions of the work are as follows.
• An IoT-based solution is proposed to communicate patients’ (regular/surgery patients) vitals and activity information to the cloud.
• The proposed IoT framework offers a time-sensitive communications infrastructure that enables seamless data communications. It also allows adaptive channel resource allocation to accommodate more patients without causing notable delays.
• The proposed machine learning algorithm effectively labels the data collected from the patients using the IoT framework, which could be transformed into actionable plans using cloud-based AI-driven analysis.
• The proposed AI-based solution classifies the data from wearable devices to identify physical activities performed by the patients and keeps a record of the prescribed vs. performed activity levels.
• This activity classification framework also highlights a comprehensive framework to provide feedback to the patients on their physical activity accurately.
The rest of the paper is organized as follows: The literature review and existing works are covered in Section 2. A system model is presented in Section 3, whereas results and discussion are provided in Section 4. Finally, the concluding remarks are provided in Section 5.
Remote monitoring and IoT-enabled intelligent healthcare systems offer great potential to address health challenges [6–10]. IoT-enabled intelligent systems have the potential to be applied to almost every aspect of healthcare, solving many challenges and reducing the burden on healthcare workers and medical professionals. New and sophisticated monitoring and diagnostic systems can be developed with the help of IoT and artificial intelligence for patient care. One of the many application areas where IoT offers new and innovative solutions is postoperative patient monitoring [11]. Patient monitoring is not critical in most cases, except for a few isolated instances requiring urgent care. Providing adequate care for non-critical patients requires time and resources [12]. IoT provides an infrastructure that can remotely monitor such patients more effectively and with fewer resources. With patient vital signs collected every few seconds and alert systems detecting anomalies, IoT can provide a reliable and highly effective solution for patient care and post-surgical recovery. IoT is a great solution and has the potential to collect data from various sensors. However, some limitations require excessive attention to make the system reliable and safe when working with patients. Although studies are looking at some challenges of IoT to make it more suitable for healthcare scenarios [13–16], these studies do not address the issues related to prioritizing communications and resource allocation of vulnerable patients. One of these constraints is allocating adequate channel resources for each surgical patient to accommodate more patients while transmitting vital signs to trained personnel with relatively low latency. IoT infrastructure becomes even more complicated when different surgical patients in one department have additional resource requirements than in other departments. An IoT system based on a whale optimization algorithm is built by Sangia et al. [17] to allocate medical resources. However, the system provided a global solution for resource allocation and did not include healthcare infrastructure as an application scenario. Furthermore, there has not been adequate intervention with machine learning techniques to interpret the data. Baker et al. [18] proposed a system implemented in common healthcare scenarios with the ideology of naming everything as a resource. The characteristics analyzed were the allocation of resources in terms of capacity (calculation, resources consumed) and limits (who can and cannot use the resources). However, the work does not focus on automatic resource allocation mechanisms nor proposes a hospital paradigm that allows for a comprehensive picture that can be optimized. The authors of [19] suggest that orchestration and service management remain challenging issues in healthcare applications and services. Furthermore, existing systems cannot meet the demands and services required locally by the healthcare infrastructure. Another challenge is that existing systems [15,20,21] have focused less on developing IoT systems for postoperative patient monitoring of vital signs, recovery patterns, and activity levels. These were primarily aimed at general healthcare applications. Postoperative surveillance mainly includes the cardiovascular system, normal function of major organs, water and electrolyte balance, prevalence and treatment of infections and excessive bleeding, deep vein thrombosis, anastomosis leakage, nutritional needs, and progression [22]. Therefore, a suitable IoT framework is needed to enable timely communication between different surgical patients. It is also crucial that the proposed framework has the potential to handle remote monitoring of patients when discharged after surgery. It should also enable adequate tracking of the users/patients’ physical activity and eating habits to provide a machine learning-based analysis of their routine, as relying on human feedback adds a significant delay factor in the overall process and does fall in conventional technology-driven remote monitoring solution which is not very effective without human intervention.
Conventional remote monitoring of patients is challenging, primarily when the response to sensory data accumulated with sensor networks relies on human feedback. While traditional remote monitoring solutions offer limited functionality beyond managing extended records for medical experts to view before revising the course of action, it also lacks two-way communication and feedback to the patients. This work proposes an IoT-enabled intelligent monitoring framework that primarily targets healthy living. The proposed framework integrates three contributions to offer a comprehensive solution. These are as follows
• An IoT-based solution is proposed to offer seamless communications with the patients (regular/surgery patients) suffering from obesity-related issues. The IoT framework proposes a time-sensitive communications infrastructure to communicate the data gathered from wearable devices from obesity/obesity-surgery patients to the cloud. The proposed IoT framework also enables on-demand access to the network, thus facilitating a more significant number of users to be reduced by the network with limited resources.
• It also proposes cloud-based AI-driven analysis of the sensory data accumulated from the patients using the IoT framework. The AI-based solution classifies the data from wearable devices to identify physical activities performed by the patients and to keep a record of the prescribed vs. performed activity levels. Thus, AI solution offers insight into patients' daily routines and activity levels.
• In addition, a machine learning based obesity level prediction system based on dietary habits is proposed. This system, in connection with activity classification, offers an extended framework to accurately provide feedback to the patients on their physical activity and eating habits and nudge them towards a balanced and desirable healthy living/eating routine.
The graphical representation of the proposed framework is presented in Fig. 1.
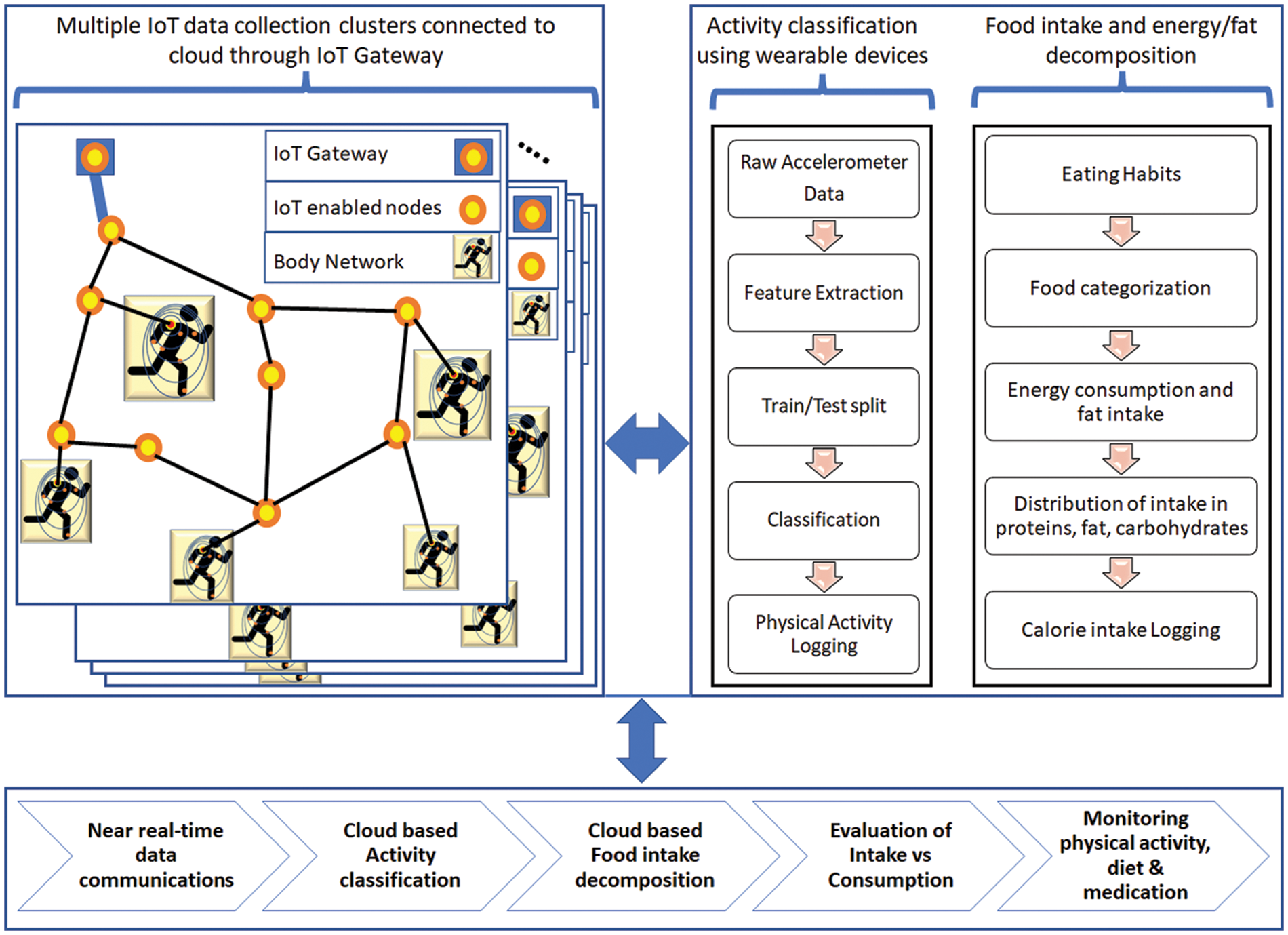
Figure 1: Smart monitoring framework for obesity and surgery patients
The work in this paper is divided into three sections, as represented in Fig. 1. An IoT-enabled data-gathering network is proposed. The proposed IoT infrastructure is responsible for collecting the vitals and accelerometer data from wearables and self-fed eating routines from the users. The proposed study also includes a motion sensor-based activity classification paradigm using machine learning methods to enable the logging of physical activity such as walking, jogging, climbing stairs, etc. The classification of physical activities is achieved using the accelerometer readings received from wearable devices. In addition, the paper also proposes an obesity predictor based on eating habits and recommendations for maintaining the desired level of activity. Collectively, the framework offers a prototype for healthy living and maintaining a nutritious diet, especially for post-surgery patients who need constant monitoring and feedback.
The proposed IoT infrastructure is established in clusters where hierarchical architecture is adopted. In the proposed infrastructure, the information is gathered in two-tier hierarchical architecture. System parameters and key terms are presented in Table 1.
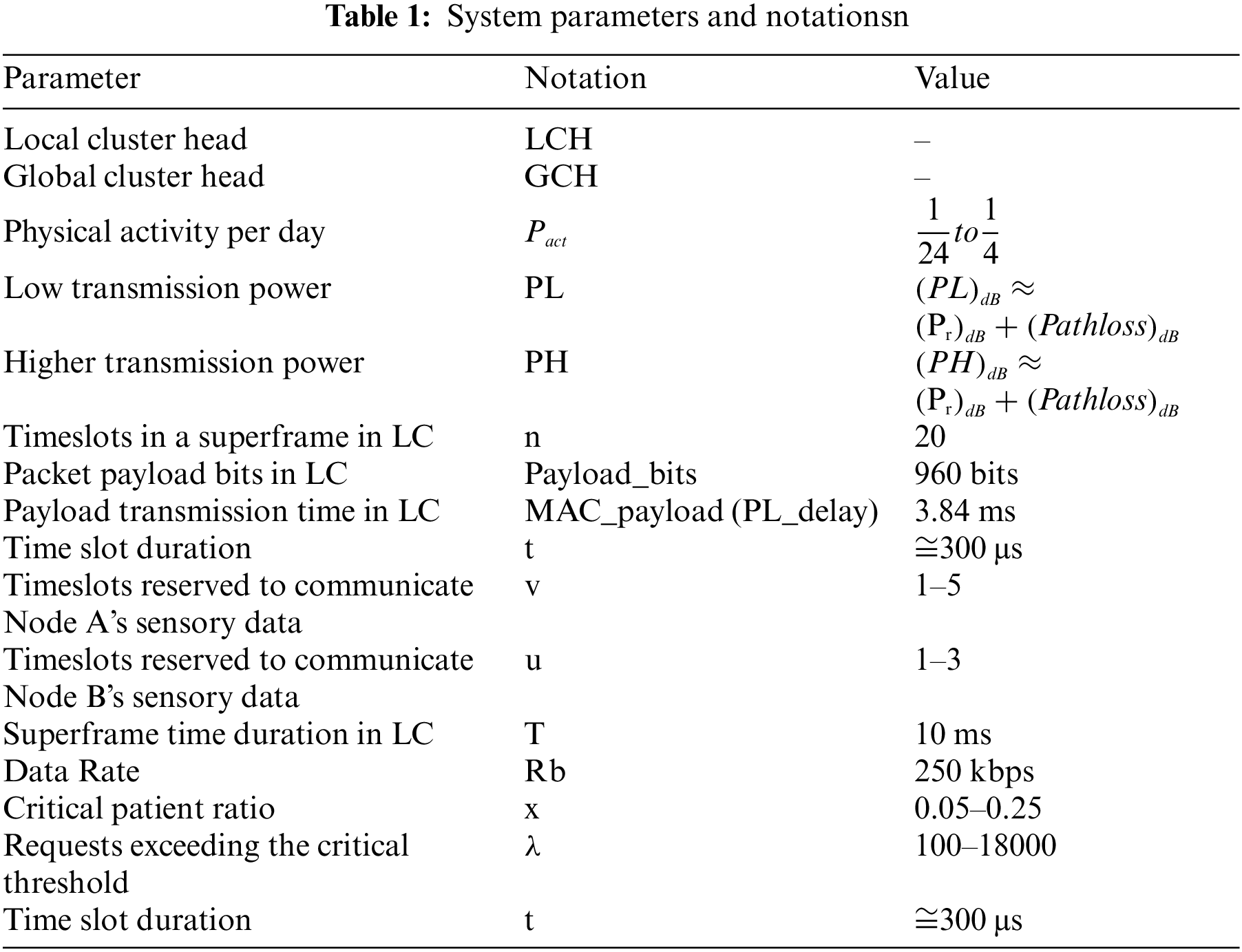
At the first tier, serving as the body area network, a cluster (LC) is formed with the IoT Hub at the center of this body area network to collect vitals from the patient/user. This data includes the information gathered from wearable gadgets for movement analysis and the vital information gathered with the help of wearable sensors. To enable guaranteed channel access to all the sensory elements in the body area network, IEEE802.15.4e is used as the base framework, with suitable interventions to support the desired network infrastructure. The information is collected in the first-tier cluster using a TDMA-based superframe with each timeslot specified for the individual sensory element data communication. The sensory data collected from the potential multisensory agents on the body are communicated to IoT-Hub (LCH) using the IoT-enabled body area network. The pictorial depiction of the on-body sensory network and body network is shown in Fig. 2.
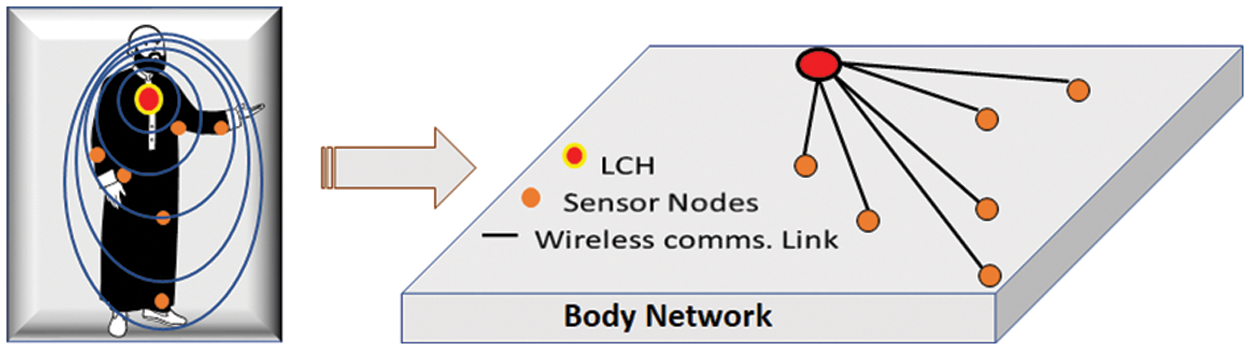
Figure 2: Body network for on-body sensory data collection
At the second tier, communication occurs from the IoT Hub to the IoT gateway, thus enabling multiple patients/users to be observed simultaneously. The second-tier communications take the vitals/sensory data collected at the IoT-Hub (which serves as the local cluster head (LCH)) for each of the patients/users to the IoT gateway (which serves as the global cluster-head (GCH)), thus forming second-tier cluster (GC). Each IoT gateway is connected to a backhaul network, thus providing access to the cloud services.
Two frequency channels facilitate seamless communication within the proposed IoT infrastructure. The first-tier communications at frequency channel (CL) are low-power transmissions (<PL) to avoid co-channel interference. Whereas the second-tier communications at frequency channel (CG) utilize the higher transmission power (PH).
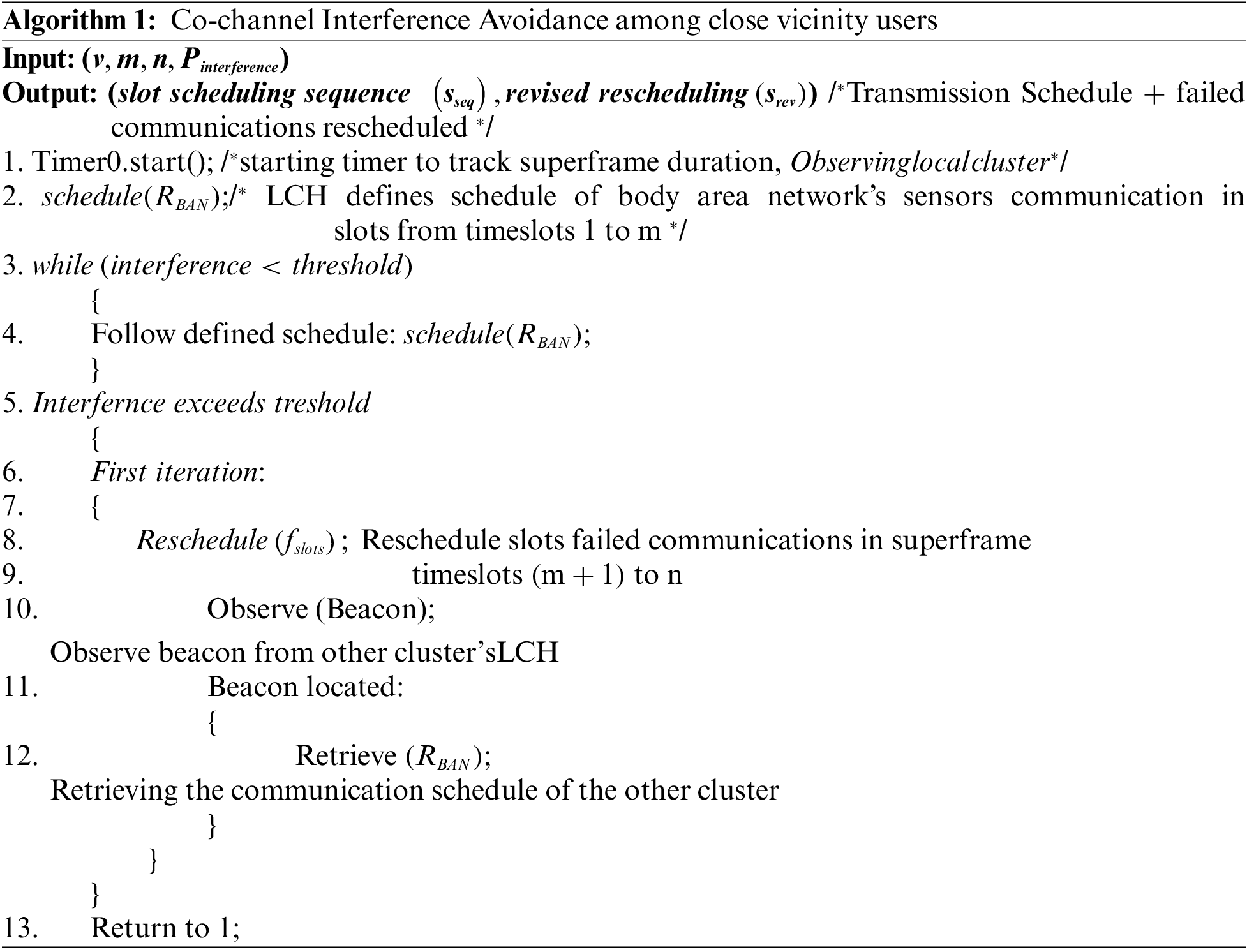
The communications in LC are limited due to a relatively low number of sensory elements and some information, such as food intake, very occasionally triggered. While the low transmission power in CL reduces co-channel interference in places such as hospitals and recovery centers, the sheer number of patients/users could contribute to the co-channel interference. Therefore, an enable/disable bit in the beacon frame from GCH is used to introduce a random transmitting schedule for LC. The communication in LC is carried out in a superframe. Each superframe consists of n timeslots, where each timeslot allows one communication. A total of v timeslots are reserved for communicating the sensory data (accelerometer, patient’s vitals). The first m timeslots (t1–tm) are used for randomly scheduling v timeslots to avoid collisions. In contrast, the remaining timeslots (t (m + 1) − n) are used for retransmission if the information communication fails due to interference.
The superframe with the random timeslot scheduling and rescheduling, along with an example scenario, is presented in Fig. 3. As shown in the example scenario in the figure, the communication taking place from User 1 and User 2 body network to LCH interferes with the timeslot seven which causes a failure in communication. Therefore, these communications are rescheduled randomly at timeslots 11–20.
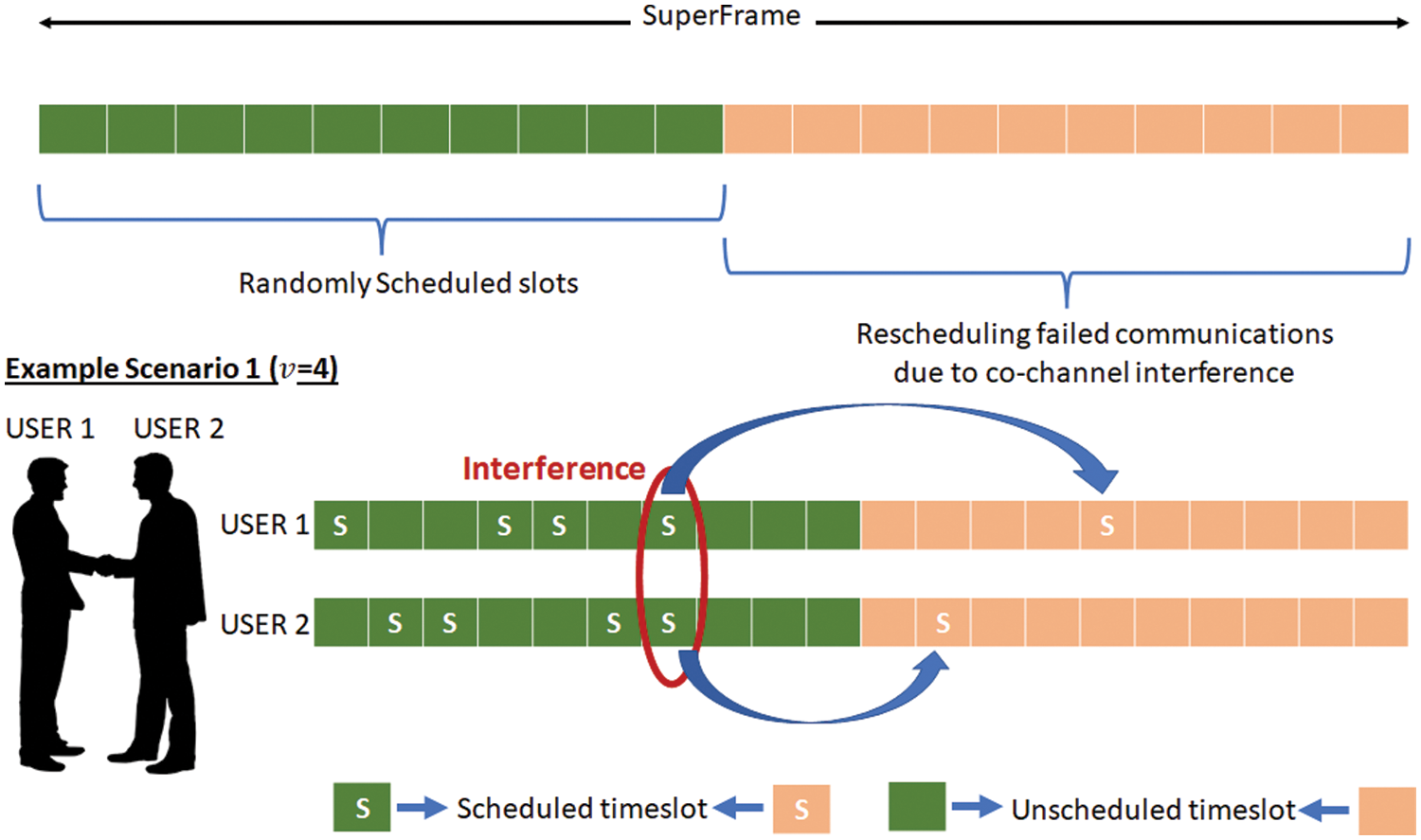
Figure 3: LC superframe structure and the example scenario for interference avoidance
To evaluate the effectiveness of such a scheme, given that the PL is chosen appropriately, only allowing at most two users to be in close vicinity to interfere with each other’s communications. The probability that at least one communication fails in t1-m due to co-channel interference is expressed as P_i (A_v |B_u). Where A_v defines the likelihood of v timeslots to be scheduled by user one, given user two has u slots. The two events where two communications from user one and user two are scheduled are independent as slots selected by one user are independent of the other. This has been described using Eq. (1),
A smaller value of
The communication in the upper tier in GC is scheduled in a larger superframe. Each superframe is expected to be 100 ms with the adaptive on-demand extension of the superframe duration. The data gathered by LCH is locally processed and evaluated before communication. The superframe in GC consists of 200 slots where the LCH could request additional timeslots on demand. A control channel is also introduced if any of the LCHs would like to request extra slots from the cluster head. If no information is needed to be communicated from the LCH, it only occupies one of the 200 timeslots, thus enabling up to 200 users to be facilitated at a given time. Each LCH corresponds to one patient/user. Therefore, if the GCH facilitates 200 LCH, 200 patients/users are accommodated by a single Gateway IoT (referred to as GCH). However, as the LCH could request anywhere from 1 to
3.2 Activity Classification Framework
The activity classification framework in this study is adopted from our earlier work [23] related to obesity. The machine learning based physical activity classification paradigm was developed using real-life datasets and exploited a variety of machine learning classifiers. The findings suggested that the support vector machine (SVM) classifier-based physical activity classification framework performs best among all proposed solutions with high performance.
The publicly available dataset [24] is composed of 30 participants in the age range of 19 to 48. The participants performed a variety of daily life activities. The activity patterns were captured using a smartphone mounted on the waistline to record triaxial (3D) accelerometer signals and triaxial (3D) gyroscope signals. Both signals are vital to record the physical patterns as the gyroscope captures angular velocity in all three directions while the accelerometer captures linear acceleration in all three directions. The signals were collected at a 50 Hz sampling rate, and various time and frequency domain features were computed using the windowing method. A total of 2.56 s time window (128 raw data samples) was used to calculate components. The computed features over the window of 2.56 s were comprised of time domain features (minimum value, maximum value, signal magnitude area), statistical features (mean, standard deviation, skewness, kurtosis, median, etc.), frequency domain features (band energy, etc.) and biomechanical features (angle between signals.). The signal collection resulted in 1722 walking window instances, 1544 walking upstairs instances, 1406 walking downstairs instances, and 1777 sitting instances. One thousand nine hundred six standing and 1944 lying instances, as reported in Table 2.
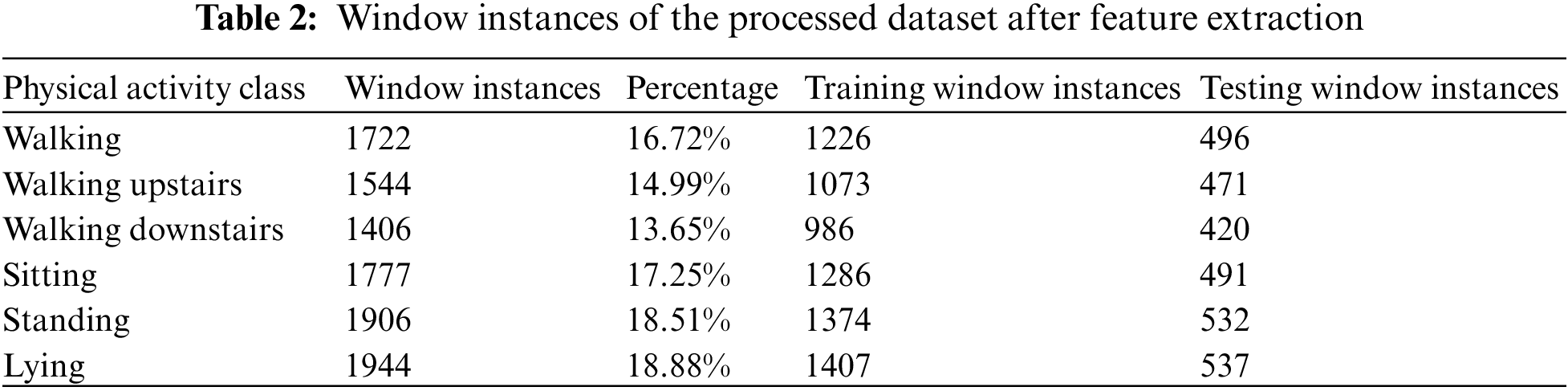
Further details about the extracted features and their implementation can be found in our earlier work on obesity [23]. The total dataset obtained after feature extraction is then split into training and testing using a 70/30 cross-validation method when 70% samples of the processed dataset (after feature extraction) are used to train the machine learning classifier, and the remaining 30% windows are used for validation and performance analysis. The processed data distribution in the training and testing stages after the 70/30 split is presented in Table 2. The characteristics of the dataset are shown in Table 2.
The findings of our obesity-related work [23] suggested that SVM performed the best among all other classifiers investigated for the given scenario. Therefore, the same classifier is implemented in this study. The SVM classifier is implemented in python using the scikit learn library, and the linear kernel is used with a balanced weight and complexity of 1.
Accuracy is used as a performance measure, as presented in Eq. (2).
where TP–True positive, TN–True Negative, FP–False positive, FN–False Negative.
The results in this section are divided into two categories: The proposed IoT framework and machine learning based analysis.
As discussed earlier, IoT-enabled communications are distributed in clusters in two tiers. In the first cluster, or LC (forming IoT-enabled body area network), co-channel interference avoidance challenges are addressed with v ranging from 1 to 5. In addition to adaptive communication scheduling within LC by LCH to minimize interference, low-power transmissions are also considered. Using the data gathered from a similar transceiver (CC2420) experimentally in our earlier works, received radio signal strength at LCH is suggested to be maintained slightly above desired received power (
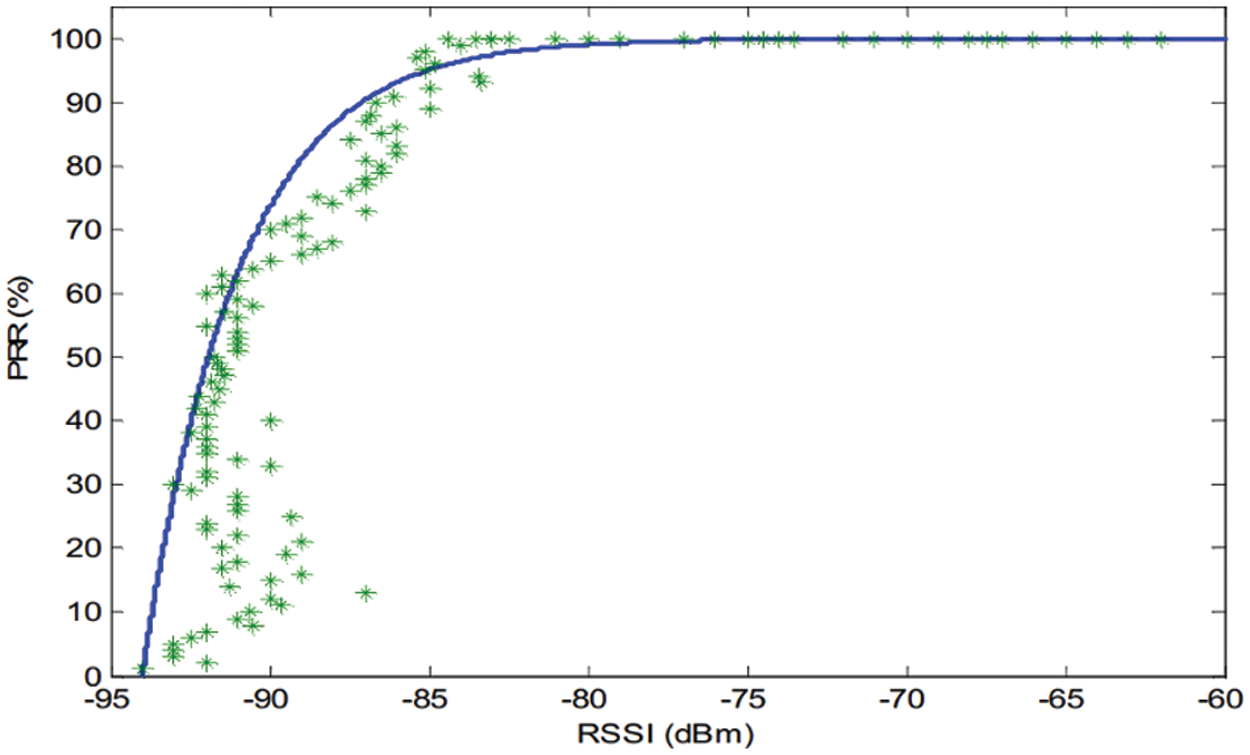
Figure 4: Packet Reception Rate (PRR) vs. Received Signal Strength (RSSI) (based on in-lab experimentation of CC2420 transceiver and Taken from our earlier work in [3])
Maintaining above −80 dBm signal strength allows a near 100% packet reception rate (PRR) with relatively lesser co-channel interference with the nearby clusters (LCs). Only in close contact does the interference becomes significant though Algorithm 1 offers a way out where the affected communications are rescheduled effectively to minimize the interference. This scenario presents only the case where only two users/patients come in close vicinity. The longer-duration superframe could address the problem to accommodate instances where more than two patients are in the immediate area. The proposed interference avoidance algorithm is scalable and could be used accordingly. As shown in Fig. 5, the first frame communications are represented where the increase in both
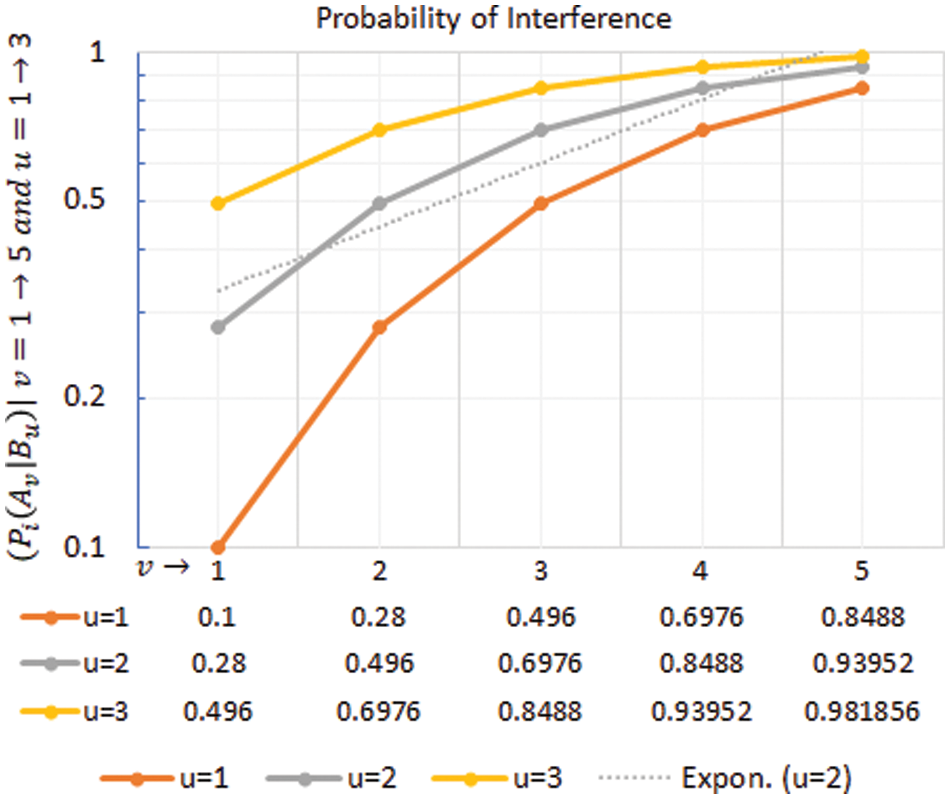
Figure 5: Probability of co-channel interference in example scenario 1 for different values of v and u
In the second tier cluster, referred to as GC, the communication takes place in a larger superframe of duration 100 ms. The GCH allows the LCH to register demands for timeslots for the next superframe using the control channel. Thus, the on-demand access requirements are dynamic and must be scheduled accordingly. When performed by the user/patient, the physical activity requires additional timeslots. If, on any day, a patient spends
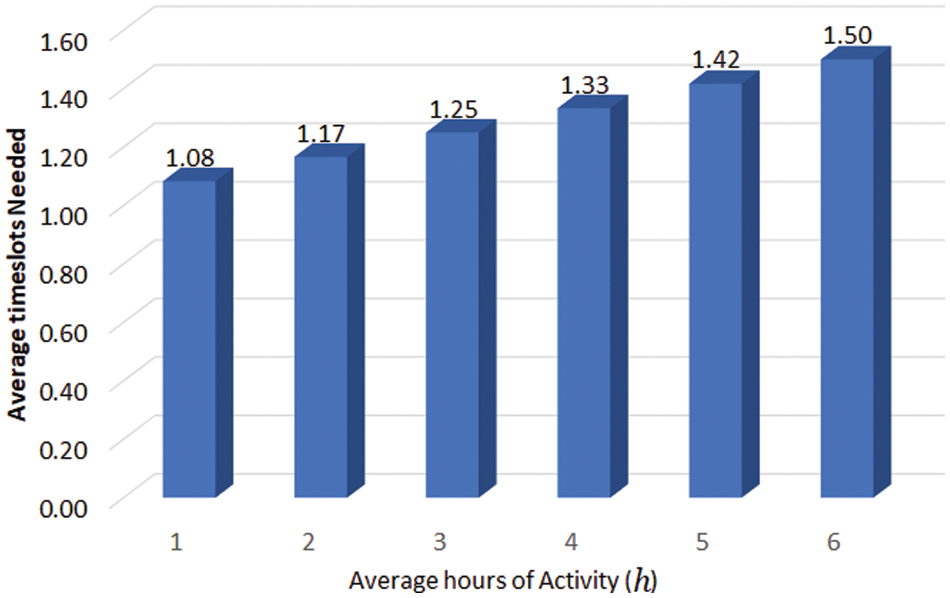
Figure 6: Average timeslots needed to communicate wearable sensors data from LCH to GCH
Similarly, it was also considered that the vitals rarely needed to be communicated within acceptable ranges but should be communicated if certain thresholds were exceeded. Given the sensitivity of some patients and allocating two additional timeslots for sharing vitals, the overall impact on the average timeslots per patient is highly dependent on how frequently the vitals need to be communicated and for what percentage of users within a cluster. To evaluate this, the critical patients (
In Fig. 7, where vitals exceeded the threshold (
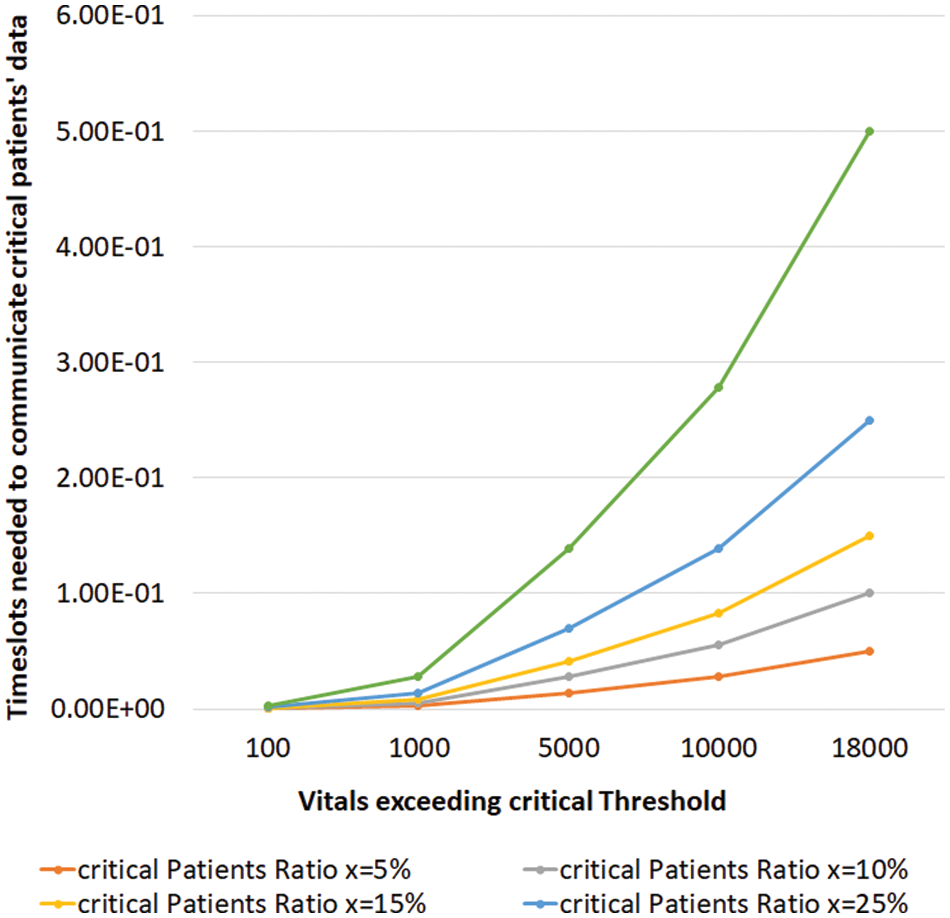
Figure 7: Vital sign monitoring and communication of critical patients data
4.2 Activity Classification Results
The findings of the proposed SVM-based physical activity classification framework are presented in Table 3 as a confusion matrix and in Fig. 8 as a performance by class. Table 2 suggests that the proposed activity classification system achieved a very high overall performance of 98.79%.
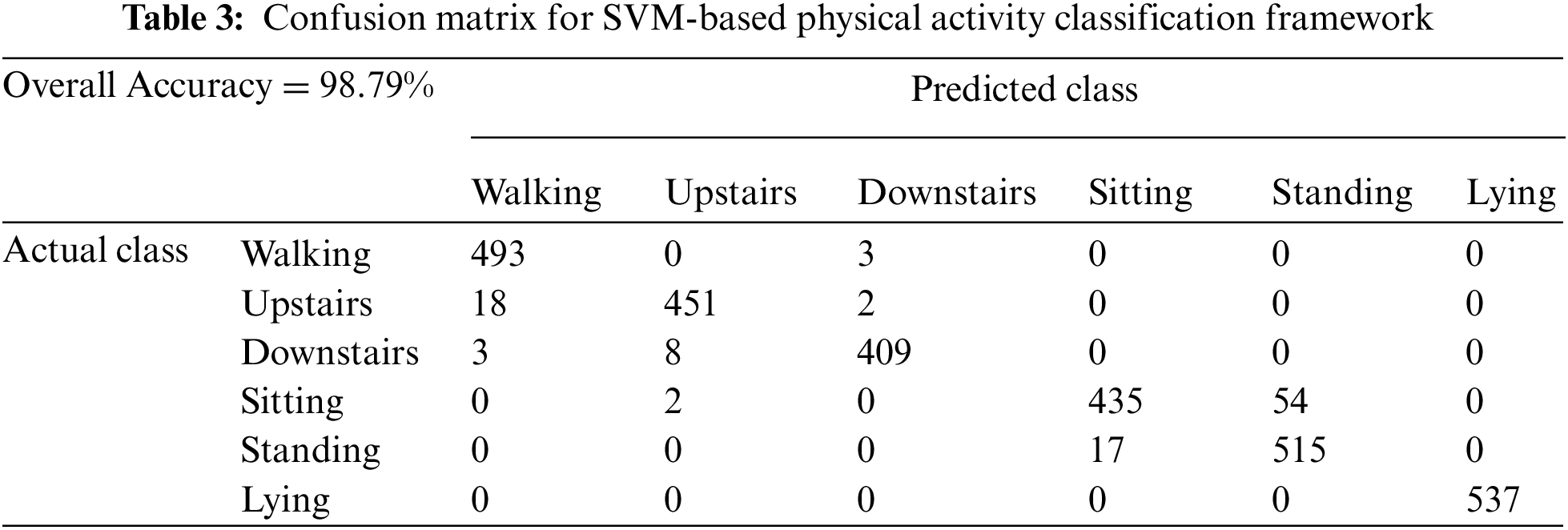
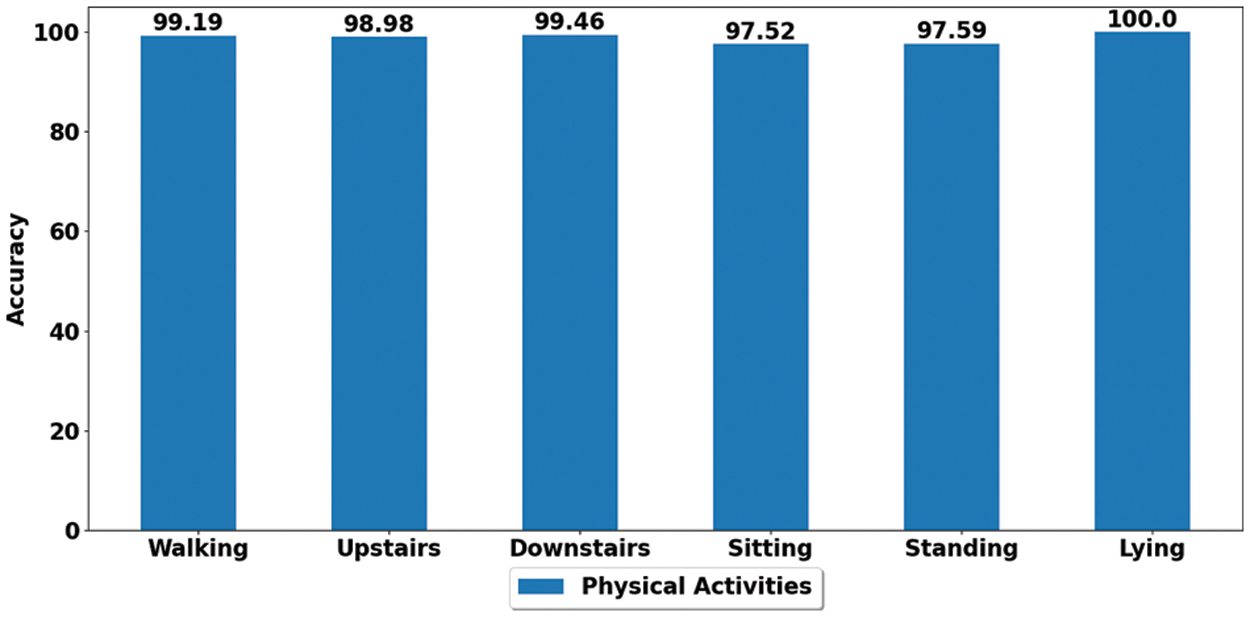
Figure 8: Performance by class of proposed for SVM-based physical activity classification framework
Fig. 8 depicts the activity by a class performance where each physical activity is profiled with a performance above 97%. The lying class achieved the highest accuracy of 100%, followed by the walking class with a performance of 99.1%, and even the least performing class, i.e., sitting, achieved an accuracy of 97.5%. These are very encouraging results and show the strength of the proposed system to accurately classify and profile the variety of daily living activities investigated (sit, stand, walk, lie, upstairs, downstairs) in real-life conditions.
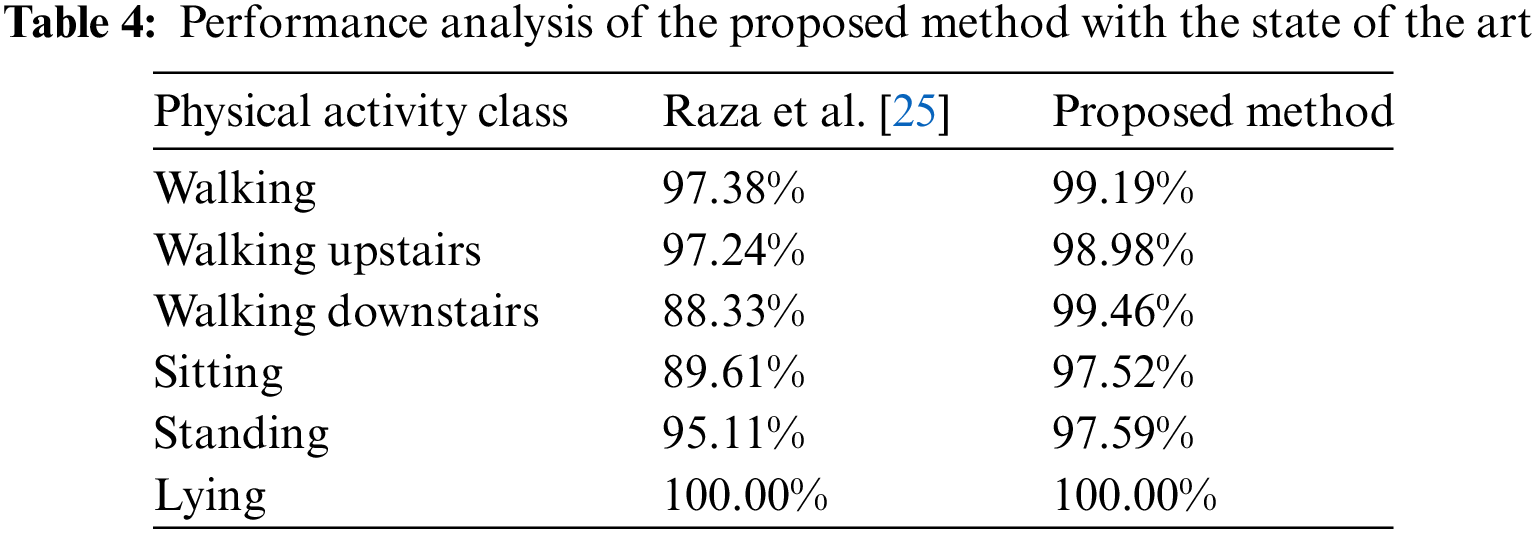
The comparison of the proposed method with state-of-the-art is presented in Table 4. The finding suggests that our proposed system has outperformed the work by Ullah et al. [26] in most of the activities classified. This indicates the strength of the proposed approach in classifying daily life activities.
The proposed IoT framework and machine learning techniques offer several benefits in terms of data communication and the ability to withstand a more significant number of users. The framework can also scale as per the needs and thus not only allows a higher number of patients accommodated by the network but also manages higher volumes of data expected from critical patients, allowing to accommodate severe patients more effectively. However, there are some limitations to work. The lack of appropriate priority establishment to distinguish between urgent and regular communications makes this work less effective in highly sensitive and critical medical cases. Similarly, the framework does not offer communication suppression from non-critical patients to optimize critical data communications. These are some of the aspects which could be improved in the future.
The proposed work offers an extensive framework to support both the communications infrastructure and the machine learning based analysis of physical activities to encourage healthy living and activity logging capabilities. The proposed framework offers two-tier clustered architecture to support communications, accommodating up to 100 patients per IoT gateway, which is highly suitable for healthcare setups and hospitals. Along with the proposed interference avoidance scheme, the number of users per cluster is carefully modeled to allow seamless communications within the network. In addition, the data collected from the IoT network is further processed where the machine learning based activity classification framework is proposed to evaluate the physical activities performed and promote healthy living effectively. The proposed IoT framework demonstrates the ability to manage plenty of patients within the network. The results also established that a higher critical patients’ ratio could be effectively managed in the proposed framework, thus, demonstrating scalable behavior.
This work while evaluates some aspects of the proposed framework yet. It can be extended further by including a machine learning based calorie intake against an activity-based calorie-burning analysis system. The ability to link any individual's food intake and activity levels could help formulate a precise weight predictor with a better impact on the patients. The work could also be extended to incorporate dynamic scheduling, thus, reducing the need to follow fixed schedules. The research could also benefit from real-life deployment of the IoT network and cloud-based activity classification and food intake analysis with future weight predictors and goal organizers.
Acknowledgement: The authors would like to acknowledge the support of the Deputy for Research and Innovation-Ministry of Education, Kingdom of Saudi Arabia, for this research through a grant (NU/IFC/ENT/01/020) under the institutional Funding Committee at Najran University, Kingdom of Saudi Arabia. The authors would like to acknowledge Saeed Saad Alahamri from Najran University for his valuable feedback on the draft to improve the flow and quality of the work.
Funding Statement: The authors would like to acknowledge the support of the Deputy for Research and Innovation-Ministry of Education, Kingdom of Saudi Arabia, for this research through a grant (NU/IFC/ENT/01/020) under the institutional Funding Committee at Najran University, Kingdom of Saudi Arabia
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Z. Ghaleb Al-Mekhlafi, E. Mohammed Senan, T. H. Rassem, B. Abdulkarem Mohammed, N. M. Makbol et al., “Deep learning and machine learning for early detection of stroke and haemorrhage,” Computers, Materials & Continua, vol. 72, no. 1, pp. 775–796, 2022. [Google Scholar]
2. V. Devadoss Ambeth Kumar, C. Swarup, I. Murugan, A. Kumar, K. Udham Singh et al., “Prediction of cardiovascular disease using machine learning technique—A modern approach,” Computers, Materials & Continua, vol. 71, no. 1, pp. 855–869, 2022. [Google Scholar]
3. M. Raza, G. Ahmed and N. M. Khan, “Experimental evaluation of transmission power control strategies in wireless sensor networks,” in Int. Conf. on Emerging Technologies, Islamabad, Pakistan, pp. 1–4, 2012. [Google Scholar]
4. CC2420: Active single-chip 2.4 GHz IEEE 802.15.4 compliant and ZigBee™ ready RF transceiver last accessed: [August 2022]. Available: https://www.ti.com/product/CC2420 [Google Scholar]
5. K. D. Hall and S. Kahan, “Maintenance of lost weight and long-term management of obesity,” Medical Clinics, vol. 102, no. 1, pp. 183–197, 2018. [Google Scholar] [PubMed]
6. M. Awais, M. Raza, N. Singh, K. Bashir, U. Manzooret et al., “LSTM based emotion detection using physiological signals: IoT framework for healthcare and distance learning in COVID-19,” IEEE Internet of Things, vol. 8, no. 23, pp. 16863–16871, 2020. [Google Scholar]
7. M. Raza, M. Awais, I. Haider, M. U. Hadi and E. Javed, “Overview of IoT and machine learning for e-healthcare in pandemics and health crises,” in Data Science Advancements in Pandemic and Outbreak Management. New York, USA: IGI Global, pp. 16–43, 2021. [Google Scholar]
8. M. Raza, N. Singh, M. Khalid, S. Khan, M. Awais et al., “Challenges and limitations of internet of things enabled Healthcare in COVID-19,” IEEE Internet of Things Magazine, vol. 4, no. 3, pp. 60–65, 2021. [Google Scholar]
9. S. S. Ullah, S. Hussain, A. Gumaei and H. AlSalman, “A secure NDN framework for internet of things enabled healthcare,” Computers, Materials and Continua, vol. 67, no. 1, pp. 223–240, 2021. [Google Scholar]
10. H. Abdulkareem, A. Mutlag, M. Dinar, J. Frnda, A. Mohammed et al., “Smart healthcare system for severity prediction and critical tasks management of COVID-19 patients in IoT fog computing environments,” Computational Intelligence and Neuroscience, vol. 1, no. 2, pp. 105–125, 2022. [Google Scholar]
11. M. McGillion, C. Ouellette, A. Good, M. Bird, S. Henry et al., “Postoperative remote automated monitoring and virtual hospital-to-home care system following cardiac and major vascular surgery: User testing study,” Journal of Medical Internet Research, vol. 22, no. 3, pp. 15548–15570, 2020. [Google Scholar]
12. F. Rubino, C. V. Ricardo, M. Geltrude, R. W. Carel, M. I. Jeffrey et al., “Bariatric and metabolic surgery during and after the COVID-19 pandemic: DSS recommendations for management of surgical candidates and postoperative patients and prioritisation of access to surgery,” The Lancet Diabetes & Endocrinology, vol. 8, no. 7, pp. 640–648, 2020. [Google Scholar]
13. M. Hassanalieragh, A. Page, T. Soyata, G. Sharma, M. Aktas et al., “Health monitoring and management using Internet of Things sensing with cloud-based processing: Opportunities and challenges,” in IEEE Int. Conf. on Services Computing, New York, USA, pp. 285–292, 2015. [Google Scholar]
14. S. Zahoor and R. N. Mir, “Resource management in pervasive internet of things: A survey,” Journal of King Saud University—Computer and Information Sciences, vol. 33, no. 8, pp. 921–935, 2021. [Google Scholar]
15. S. Zeadally, F. Siddiqui, Z. Baig and A. Ibrahim, “Smart healthcare: Challenges and potential solutions using internet of things and big data analytics,” PSU Research Review, vol. 10, no. 1, pp. 65–85, 2019. [Google Scholar]
16. N. Iqbal, S. Ahmad, R. Ahmad and D. -H. Kim, “A scheduling mechanism based on optimization using IoT tasks orchestration for efficient patient health monitoring,” Sensors, vol. 21, no. 16, pp. 5430–5455, 2021. [Google Scholar] [PubMed]
17. A. K. Sangaiah, A. A. R. Hosseinabadi, M. B. Shareh, S. Y. B. Rad, A. Zolfagharian et al., “IoT resource allocation and optimization based on heuristic algorithm,” Sensors, vol. 20, no. 2, pp. 539, 2020. [Google Scholar] [PubMed]
18. T. Baker, E. Ugljanin, N. Faci, M. Sellami, Z. Maamar et al., “Everything as a resource: Foundations and illustration through internet-of-things,” Computers in Industry, vol. 94, no. 2, pp. 62–74, 2018. [Google Scholar]
19. R. Mahmud, F. L. Koch and R. Buyya, “Cloud-fog interoperability in IoT-enabled healthcare solutions,” in Proc. of the 19th Int. Conf. on Distributed Computing and Networking, Varanasi, India, pp. 1–10, 2018. [Google Scholar]
20. N. S. M. Hadis, M. N. Amirnazarullah, M. M. Jafri and S. Abdullah, “IoT based patient monitoring system using sensors to detect, analyse and monitor two primary vital signs,” Journal of Physics, vol. 1535, no. 1, pp. 12004–12025, 2020. [Google Scholar]
21. S. Selvaraj and S. Sundaravaradhan, “Challenges and opportunities in IoT healthcare systems: A systematic review,” SN Applied Sciences, vol. 2, no. 1, pp. 1–8, 2020. [Google Scholar]
22. S. J. Concors, B. L. Ecker, R. Maduka, A. Furukawa, S. E. Raper et al., “Complications and surveillance after bariatric surgery,” Current Treatment Options Neurology, vol. 18, no. 5, pp. 56–75, 2016. [Google Scholar]
23. S. A. Alsareii, M. Awais, A. M. Alamri, M. Y. AlAsmari, M. Irfan et al., “Physical activity monitoring and classification using machine learning techniques,” Life, vol. 12, no. 8, pp. 1103–11023, 2022. [Google Scholar] [PubMed]
24. D. Anguita, A. Ghio, L. Oneto, X. Parra and J. L. Reyes-Ortiz, “A public domain dataset for human activity recognition using smartphones,” ESANN, vol. 3, no. 1, pp. 3–4, 2013. [Google Scholar]
25. M. Raza, H. Le-Minh, N. Aslam and S. Hussain, “A novel MAC proposal for critical and emergency communications in industrial wireless sensor networks,” Computers & Electrical Engineering, vol. 72, no. 2, pp. 976–989, 2018. [Google Scholar]
26. S. S. Ullah, S. Hussain, A. Gumaei and H. AlSalman, “A secure NDN framework for internet of things enabled healthcare,” Computers, Materials and Continua, vol. 67, no. 1, pp. 223–240, 2021. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools