 Open Access
Open Access
REVIEW
Methyl Ester Type Produced by Catalytic Transesterification: From Various Oil Feedstock to Biodiesel Products
Department of Chemistry, Universitas Negeri Malang, Malang, 65145, Indonesia
* Corresponding Author: Aman Santoso. Email:
Energy Engineering 2022, 119(6), 2255-2276. https://doi.org/10.32604/ee.2022.021596
Received 22 January 2022; Accepted 17 May 2022; Issue published 14 September 2022
Abstract
Biodiesel research has been carried out via transesterification. However, biodiesel products (methyl esters) have not encountered new insights, because feedstocks have been explored and studied. Various optimum conditions on transesterification reaction could produce different methyl ester type with different compound. So, this review describes various oil feedstock that were to find new insights about methyl ester type. The review took the results of study that has been published with experience for 10 years. The results of the study reviewed on the transesterification method, characterization of methyl esters, and its components. The component reviewed and correlated to the literature, structure, and GC-MS analysis. The review can provide challenges for methyl ester research in future research.Graphic Abstract
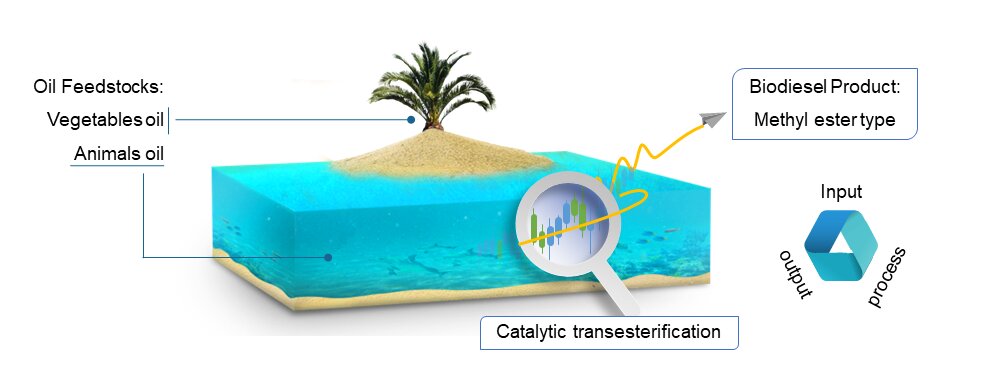
Keywords
Nomenclature
| SNI | Standar Nasional Indonesia |
Biodiesel is known as an alternative renewable fuel. In developing countries, biodiesel is a promising alternative fuel thanks to its largely economical manufacturing process, and abundant source of feedstocks [1]. Feedstocks in the form of triglycerides could come from oil [2]. The used feedstock can reduce both pollution and waste. Interest in biodiesels have been influencedby rising global energy demand and growing environmental concern to find green renewable alternative fuels [3].
Oil is divided into two types: e.g., vegetable oil and animal oil [4]. Vegetable oils are commonly used in biodiesel, including palm oil, coconut oil, castor oil, thumba oil, and used cooking oil. Animal oils as feedstock for biodiesel including chicken oil, beef oil, and fish oil. However, these two oil types always have different levels of triglyceride components [5]. This difference has caused the production of biodiesel fuels with under 100% yield; in fact, this happens quite naturally.
One of the most common processes of converting triglycerides into biodiesel is called catalytic transesterification, because it is widely applied in industrial parameters [6]. Prior to the transesterification reaction, many studies applied pretreatment (such as extraction, refining, cavitation, and esterification) to improve the quality of feedstocks [7]. The previous studies have evaluated the importance of pretreatment of biodiesel feedstocks because transesterification requires triglycerides, not other types of compounds often contained in the feedstocks. For this reason, the experiment needs the right technique in terms of initial pretreatment of biodiesel feedstocks, such as the use of catalysts, adsorbents, microwave, and cavitation techniques [8–10].
On the other hand, catalysts played a role in the development of transesterification. Theoretically, the catalyst accelerates the rate of the transesterification reaction to produce biodiesel. The previous studies, the categories of catalyst in transesterification were homogeneous base-catalyst, homogeneous acid-catalyst, heterogeneous base-catalyst, heterogeneous acid-catalyst, and enzyme. Another process was non-catalytic supercritical transesterification [11]. As a long review and development, transesterification catalysts essentially required a catalyst with a high degree of basicity (strong base) [12]. Two types of known catalysts were homogeneous catalysts and heterogeneous catalysts. Homogeneous catalysts have caused product separation problems, and they are not environmentally friendly. An example of a homogeneous catalyst was NaOH [13] and KOH [14]. Furthermore, heterogeneous catalysts have given rise to the problem of suboptimal catalytic activity. Examples of heterogeneous catalysts that have been applied were CaO, MgO, Al2O3, K2O, ZnO, and various other catalyst modifications [15]. So, until now, heterogeneous catalytic transesterification is still being explored because the catalyst has affected the transesterification results of biodiesel products [10]. Another study reported that the transesterification catalyst in a hydrodynamic cavitation reactor was not an important parameter of reaction [16]. However, the developed reactor influenced by the benefit of transesterification in recent years [17]. The technology for biodiesel production required cheap, simple, operator-friendly, and efficient [18].
A problem with biodiesel products produced from catalytic heterogeneous catalytic transesterification is inconsistency in the yield and characterization of the alkyl ester. In this review, the observed biodiesel product is methyl ester. The methyl ester is obtained from the reaction between methanol and triglycerides. It has also been well nown that methanol has a better reaction activity compared to other types of alcohol, such as ethanol and propanol [19]. Methyl ester products have long been characterized in order to obtain information on the quality of methyl esters, such as the characterization of density, viscosity, acid number, and refractive index. Then, the characterization was compared with biodiesel quality standards, such as SNI 7182:2015 (in Indonesia). So, the biodiesel with various methyl esters needs to be reviewed.
2 Transesterification of Various Oil Feedstock
Transesterification can be applied to various oil feedstock. Theoretically, transesterification is a chemical reaction between 1 mole of triglycerides and 3 moles of alcohol which can produce alkyl esters and glycerol. As reactants, methanol and ethanol are good alcohol based on their activity [19], but methanol is better than ethanol. In real experimental conditions, the ratio of triglycerides (oil) and alcohol is not equal to 1:3. The oil: Alcohol ratios commonly used in transesterification research are 1:10, 1:12, 1:15, and 1:16. Some of the optimum transesterification conditions from the reported studies are shown in Table 1.

Based on Table 1, various oil feedstocks have affected transesterification performance. So, before transesterification, the study requires pretreatment. The pretreatment aims to control oil quality so that it does not contain impurities. Although the used oil cannot be 100% pure, examples of the pretreatments were heating, filtration, degumming, extraction, and esterification. In development, esterification in hydrodynamic cavitation reactors [57], esterification in ultrasonic cavitation reactors [58], and microwave pretreatment [9] are profitable techniques. Without pretreatment, the transesterification product will vary greatly, and the obtained alkyl ester is not optimal [59].
Pretreatment is always adjusted to the condition of the feedstocks. Refining is applied to feedstocks such as oil, especially waste oil. Extraction is applied to feedstocks from animal oils and vegetable oils. Esterification has been widely applied to oils to reduce free fatty acid levels to < 2%. Free fatty acids will affect the transesterification, so the alkyl ester product is not optimal [60].
The transesterification conditions played an important process in the production of alkyl esters [2]. Alkyl esters have been defined as biodiesel with applicable product standards, such as EN 14214:2003 (Europe), ASTM D6751:2011 (America), ANP N° 42 (Brazil), IRAM 6515–13 (Argentina), DE 100–04 (Colombia), PNA 16 018 (Paraguay) UNI 1100 (Uruguay), JIS K2390 (Japan), GB/T20828:2007 (China), CNS 15072 (China Taipei), SNI 7182:2015 (Indonesia), IS 15607:2005 (India), MS 2008:2008 (Malaysia), PNS/DOE QS 002:2007 (Philippines), and TCVN 7717:2007 (Vietnam). The transesterification reaction conditions are always influenced by temperature, amount of catalyst, time, and oil: Alcohol ratio [10]. During the transesterification process, stirring has been applied in experiments (note: Some studies do not report details). In addition, the presence of additional devices such as microwave, ultrasonic cavitation, and hydrodynamic cavitation have affected biodiesel synthesis [31]. Also as important, is the used reactor in pretreatment/transesterification, as it affects the results and stability of the process [61]. With this understood, the results will give different characteristics of alkyl esters and yields.
In various oil feedstocks, the categories of feedstocks for biodiesel are three categories: (1) edible oil, (2) non-edible oil, (3) waste oil, (4) animal fat, and (5) microbial oil [17]. As known, edible oils will compete with the food feedstock. non-edible oil was not requiredas a food source, but the low conversion was a problem. The most potential feedstocks were waste oil and microbial oil. For waste oil, the production requires the available sources. Microbial oil, is an excellent source as well [62], but the difficulty of the source has been a problem in recent years because of both sensitivity and sustainability [63]. Also, it included a different process with other oil feedstocks [64,65].
3 Characterization of Methyl Ester Products
Characterization of biodiesel in the previous study was chosen methyl ester products, because this review controls the input-process-output of the previous study in the same culture. The characterization included density, viscosity, acid number, refractive index and, yield percent. These characterizations can be listed in Table 2.

Based on Table 2, the transesterification conditions state the differences in the characterization results. The difference still meets SNI 7182:2015 standard. In general, the density and viscosity characteristics have complied with SNI, but not all of the acid number and refractive index have complied with SNI. Then, the yields of methyl ester were all below standard (96.5%). Furthermore, the used catalysts, such as KOH and CaO, proved that the yield percent of biodiesel was different from different types of feedstocks. This means that the catalytic activity of a catalyst is still inconsistent, due to the differences in the components of the biodiesel feedstock. On the other hand, the mixing of catalysts into a reactor needs to be considered because the catalyst is required to be perfectly distributed. The used catalyst in different feedstock also produces different characteristics of biodiesel. In addition, the reactor serves an important role that affects chemical processes, like that of the microwave [26], the ultrasonic cavitation [22], and the hydrodynamic cavitation [31]. So, the transesterification process can be stated that the ongoing process has missing observations. This review will try to evaluate the missing observations in terms of the produced methyl ester. Methyl esters were reanalyzed in depth from the results of the GC-MS test. Methyl ester types will give information about characteristics of the catalyst via inverse direct.
4 Methyl Ester Analysis of Trans-Esterified Various Oil Feedstock
Methyl Esters have been known as biodiesel products from transesterification. Methyl esters have been commonly tested by Gas Chromatography-Mass Spectrophotometry (GC-MS). GC results were in the form of a chromatogram, and MS results were in the form of a mass spectrum according to the retention time of the GC. Various components of methyl esters from trans-esterified various oil feedstock are shown in Table 3.

Based on Table 3, various oil feedstocks have shown various component results of the trans-esterified methyl ester. There are always two main components in various oil feedstock whose levels are more than 20% in various oil feedstock. It shows certain peculiarities. However, in many feedstocks, methyl palmitate (C17H34O2) and methyl oleate (C19H36O2) are always found. Although the retention time value is different, the mass spectrum can show methyl ester specifically.
On the other hand, the retention time possessed by the spectrum of a methyl ester shows a significant difference. It also shows the various database has a high rate of similarity (∼95%–99%). Besides that, the presence of impurities in the methyl ester was caused by the complexity of heterogeneous catalytic transesterification, affected the results of GC-MS analysis. Also, the resulted methyl esters showed the complexity of structure, so it affected the similarity level of the identified methyl esters based on the database. The process of GC-MS analysis requires control to get good information data.
For example, a mass spectrum of methyl ester from used cooking oil with a retention time of 10.176 min is shown in Fig. 1. The peak of the mass spectrum of methyl palmitate has fragments with m/z 43, 74, 101, 143, 185, 227, 270, 297, 327, 355, and 401.

Figure 1: Mass spectrum of methyl palmitate of used cooking oil
Based on Fig. 1, the results were referred to the NIST17.LIB library with a 99% similarity percentage. The compound was methyl palmitate, about 30.90%, with a retention time of 10.176 min. Furthermore, the fragmentation pattern is shown in Fig. 2. The fragmentation for the molecular ion of methyl palmitate was at m/z 270, m/z 143, and m/z 227. Meanwhile, m/z 43 and m/z 74 were obtained from the fragmentation pattern through McLafferty rearrangement. One of the fatty acids of used cooking oil is palmitic acid, and one of the methyl esters is methyl palmitate.

Figure 2: Fragmentation pattern of methyl palmitate of used cooking oil
Furthermore, the mass spectrum of the methyl ester of off grade crude palm oil with a retention time (tR) of 9.990 min is shown in Fig. 3.

Figure 3: Mass spectrum of methyl palmitate of off grade crude palm oil
The mass spectrum in Fig. 2 was compared with standard peaks which are relatively the same as the mass spectrum listed in the W10N14.L library. The compound in Fig. 2 has a 98% similarity with library W10N14.L reference number 434884. Furthermore, the fragmentation pattern is shown in Fig. 4. Fragmentation for molecular ion of methyl palmitate was at m/z 270, m/z 143, and m/z 227. Meanwhile, m/z 43 and m/z 74 are obtained from the fragmentation pattern through the Mc Lafferty rearrangement. Based on the fragmentation pattern that appears at m/z 43, 74, 143, 227, and 270 in the mass spectrum, it proves that the compound formed is methyl palmitate.


Figure 4: Fragmentation pattern of methyl palmitate of Off grade crude palm oil
Transesterification research in optimization efforts is considered unwise if it focuses on modifying the transesterification reactor only. The methyl esters turned out to have peculiarities and characteristics that were always varied and not always the same. The notion is the existence of defect product of methyl ester, like in an immiscible mixture of palm oil: Ethanol [66]. It has been proved that in research by GC-MS analysis. As a notion, the defected product occurred because of a defect in the catalyst material. From the results of GC-MS, the retention time and mass spectrum values obtained from various oil feedstock are always different, so the fragmentation analysis and library data are also different. However, the components that can be found in high levels are methyl palmitate and methyl oleate in the transesterification of triglycerides. For evaluation, the feedstock of transesterification needs to have feedstock component/compound standards. The challenge of further studies is how to prevent transesterification from producing excessive by-products.
Acknowledgement: On this occasion, the authors would like to thank to Lembaga Penelitian dan Pengabdian Masyarakat Universitas Negeri Malang (LPPM UM) by supporting this study. Sincerely, the authors thank to Prof. Effendy, Ph.D, Prof. Hadi Nur, Prof. Dr. Subandi, M.Si, Dr. Evi Susanti, M.Si who have been inspirational. Also, the authors thank to colleagues of Universitas Negeri Malang (UM) and MA Integratif NU Al-Hikmah (YPPPI Jeru).
Funding Statement: The study have been supported by a funding of LPPM Universitas Negeri Malang. The funding has been received by Dr. Aman Santoso, M.Si.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Sánchez-Faba, E. M., Ferrero, G. O., Dias, J. M., Eimer, G. A. (2019). Alternative raw materials to produce biodiesel through alkaline heterogeneous catalysis. Catalysts, 9(8), 690. DOI 10.3390/catal9080690. [Google Scholar] [CrossRef]
2. Zahan, K. A., Kano, M. (2018). Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies, 11(8), 2132. DOI 10.3390/en11082132. [Google Scholar] [CrossRef]
3. Manaf, I. S. A., Embong, N. H., Khazaai, S. N. M., Rahim, M. H. A., Yusoff, M. M. et al. (2019). A review for key challenges of the development of biodiesel industry. Energy Conversion and Management, 185, 508–517. DOI 10.1016/j.enconman.2019.02.019. [Google Scholar] [CrossRef]
4. Avhad, M. R., Marchetti, J. M. (2015). A review on recent advancement in catalytic materials for biodiesel production. Renewable and Sustainable Energy Reviews, 50, 696–718. DOI 10.1016/j.rser.2015.05.038. [Google Scholar] [CrossRef]
5. Huang, D., Zhou, H., Lin, L. (2012). Biodiesel: An alternative to conventional fuel. Energy Procedia, 16, 1874–1885. DOI 10.1016/j.egypro.2012.01.287. [Google Scholar] [CrossRef]
6. Su, F., Guo, Y. (2014). Advancements in solid acid catalysts for biodiesel production. Green Chemistry, 16(6), 2934–2957. DOI 10.1039/c3gc42333f. [Google Scholar] [CrossRef]
7. Zhang, Q., Zhang, Y., Deng, T., Wei, F., Jin, J. et al. (2019). Sustainable production of biodiesel over heterogeneous acid catalysts. In: Saravanamurugan, S., Pandey, A., Li, H., Riisager, A. (Eds.Biomass, biofuels, biochemicals: Recent advances in development of platform chemicals, pp. 407–432. Netherlands: Elsevier B.V. DOI 10.1016/B978-0-444-64307-0.00016-0. [Google Scholar] [CrossRef]
8. Patil, A. D., Baral, S. S., Dhanke, P. B., Dharaskar, S. A. (2022). Cleaner production of catalytic thumba methyl ester (Biodiesel) from thumba seed oil (Citrullus colocyntis) using TiO2 nanoparticles under intensified hydrodynamic cavitation. Fuel, 313, 123021. DOI 10.1016/j.fuel.2021.123021. [Google Scholar] [CrossRef]
9. Cheng, S. F., Nor, L., Chuah, M., H, C. (2011). Microwave pretreatment: A clean and dry method for palm oil production. Industrial Crops and Products, 34(1), 967–971. DOI 10.1016/j.indcrop.2011.03.002. [Google Scholar] [CrossRef]
10. Sumari, S., Santoso, A., Asrori, M. R. (2021). A review: Synthesis of biodiesel from low/off grade crude palm oil on pretreatment, transesterification, and characteristics. Orbital: The Electronic Journal of Chemistry, 13(4), 385–391. DOI 10.17807/orbital.v13i4.1632. [Google Scholar] [CrossRef]
11. Helwani, Z., Othman, M. R., Aziz, N., Kim, J., Fernando, W. J. N. (2009). Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Applied Catalysis A: General, 363(1), 1–10. DOI 10.1016/j.apcata.2009.05.021. [Google Scholar] [CrossRef]
12. Lourinho, G., Brito, P. (2015). Advanced biodiesel production technologies: Novel developments. Reviews in Environmental Science and Bio/Technology, 14(2), 287–316. DOI 10.1007/s11157-014-9359-x. [Google Scholar] [CrossRef]
13. Kurniasih, E., Pardi, P. (2020). Estimating the opportunities of ester content improvement through variation of NaOH, KI and KIO3 developed impregnators on activated natural zeolite catalyst for methyl ester synthesis. IOP Conference Series: Materials Science and Engineering, 725(1), 012045. DOI 10.1088/1757-899X/725/1/012045. [Google Scholar] [CrossRef]
14. Abdullah, S., Ariyani, R. N. R., Nata, D., F, I. (2017). Conversion of palm oil sludge to biodiesel using alum and KOH as catalysts. Sustainable Environment Research, 27(6), 291–295. DOI 10.1016/j.serj.2017.07.002. [Google Scholar] [CrossRef]
15. Semwal, S., Arora, A. K., Badoni, R. P., Tuli, D. K. (2011). Biodiesel production using heterogeneous catalysts. Bioresource Technology, 102(3), 2151–2161. DOI 10.1016/j.biortech.2010.10.080. [Google Scholar] [CrossRef]
16. Patil, A. D., Baral, S. S. (2021). Process intensification of thumba methyl ester (biodiesel) production using hydrodynamic cavitation. Chemical Engineering Research and Design, 171, 277–292. DOI 10.1016/j.cherd.2021.05.007. [Google Scholar] [CrossRef]
17. Zulqarnain, A. M., Yusoff, M. H., Nazir, M. H., Zahid, I. et al. (2021). A comprehensive review on oil extraction and biodiesel production technologies. Sustainability, 13(2), 788. DOI 10.3390/su13020788. [Google Scholar] [CrossRef]
18. Helwani, Z., Othman, M. R., Aziz, N., Fernando, W. J. N., Kim, J. (2009). Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Processing Technology, 90, 1502–1514. DOI 10.1016/j.fuproc.2009.07.016. [Google Scholar] [CrossRef]
19. Musa, I. A. (2016). The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egyptian Journal of Petroleum, 25(1), 21–31. DOI 10.1016/j.ejpe.2015.06.007. [Google Scholar] [CrossRef]
20. Santoso, A., Wijaya, A. R., Rahmadani, A., Sukarianingsih, D., Putri, D. E. K. et al. (2021). Effect of CaO-K2O heterogeneous catalyst concentration and reaction temperature on trans-esterification of waste cooking oil with ultrasonic wave. AIP Conference Proceedings, 2330(1), 70009. DOI 10.1063/5.0043404. [Google Scholar] [CrossRef]
21. Santoso, A., Putri, D. E. K., Rusdi, M., Sumari, S., Wijaya, A. R. et al. (2021). The effect of basic catalyst concentration on tobacco oil transesterification (voor-oogst) using ultra-sonic wave and its potential as renewable energy. AIP Conference Proceedings, 2330(1), 070008. DOI 10.1063/5.0043406. [Google Scholar] [CrossRef]
22. Santoso, A., Sukarianingsih, D., Sumari, S., Wijaya, A. R., Retnosari, R. et al. (2021). Synthesis of MgO-K2O compound and its application as a heterogen catalyst for making biodiesel from crude palm oil off grade using ultrasonic waves. AIP Conference Proceedings, 2353(1), 30107. DOI 10.1063/5.0052698. [Google Scholar] [CrossRef]
23. Santoso, A., Rizky, M., Sumari, S., Wijaya, A. R., Retnosari, R. et al. (2021). Pengaruh jenis alkohol pada sintesis alkil ester dari CPO melalui reaksi transesterifikasi menggunakan katalis heterogen CaO-MgO. Jurnal Rekayasa Bahan Alam Dan Energi Berkelanjutan, 5(1), 1–9. [Google Scholar]
24. Santoso, A., Nadia, F., Retnosari, R., Wijaya, A. R., Sumari, S. et al. (2020). Konsentrasi katalis dan suhu optimum pada transesterifikasi minyak biji pepaya (Carica papaya) sebagai bahan Baku biodiesel. Jurnal Kimia dan Terapannya, 4(1), 29–36. DOI 10.17977/um0260v4i12020p029. [Google Scholar] [CrossRef]
25. Santoso, A., Sumari, S., Zakiyya, U. U., Nur, A. T. (2019). Methyl ester synthesis of crude palm oil off grade using the K2O/Al2O3 catalyst and its potential as biodiesel. IOP Conference Series: Materials Science and Engineering, 515(1), 012042. DOI 10.1088/1757-899X/515/1/012042. [Google Scholar] [CrossRef]
26. Santoso, A., Sumari, S., Sukarianingsih, D., Sari, R. M. (2018). Optimization of synthesis of biodiesel from Jatropha curcas L. with heterogeneous catalyst of CaO and Mgo by transesterification reaction using microwave. Journal of Physics: Conference Series, 1093, 12047. DOI 10.1088/1742-6596/1093/1/012047. [Google Scholar] [CrossRef]
27. Primadi, T. R., Fajaroh, F., Santoso, A., Nazriati Ciptawati, E. (2020). Synthesis of CaO@CoFe2O4nanoparticles and its application as a catalyst for biodiesel production from used cooking oil. Key Engineering Materials, 851, 184–193. [Google Scholar]
28. Santoso, A., Hanindita, C. F. A., Sumari, S., Budi Rachman, I. (2019). Synthesis of biodiesel from low-quality crude palm oil with heterogeneous catalyst CaO-ZnO. IOP Conference Series: Materials Science and Engineering, 515, 12082. DOI 10.1088/1757-899x/515/1/012082. [Google Scholar] [CrossRef]
29. Santoso, A., Sumari, S., Marfu’ah, A., S. (2018). Synthesis of methyl ester from chicken oil and methanol using heterogeneous catalyst of CaO-MgO as well as characterization its potential as a biodiesel fuel. Journal of Physic: Conference Series, 1093, 12035. DOI 10.1088/1742-6596/1093/1/012035. [Google Scholar] [CrossRef]
30. Santoso, A., Abdurrohman, W., Sukarianingsih, A. R., Sumari, D., Sumari et al. (2020). Synthesis of methyl ester from rice bran oil through the esterification reaction. Key Engineering Materials, 851, 164–171. DOI 10.4028/www.scientific.net/kem.851.164. [Google Scholar] [CrossRef]
31. Patil, A., Baral, S., Dhanke, P. (2021). Hydrodynamic cavitation for process intensification of biodiesel synthesis--A review. Current Research in Green and Sustainable Chemistry, 4, 100144. DOI 10.1016/j.crgsc.2021.100144. [Google Scholar] [CrossRef]
32. Patil, A., Baral, S. S., Dhanke, P., Kore, V. (2020). Biodiesel production using prepared novel surface functionalised TiO2 nano-catalyst in hydrodynamic cavitation reactor. Materials Today: Proceedings, 27, 198–203. DOI 10.1016/j.matpr.2019.10.009. [Google Scholar] [CrossRef]
33. Patil, A. D., Baral, S. S., Dhanke, P. B., Madankar, C. S., Patil, U. S. et al. (2018). Parametric studies of methyl esters synthesis from thumba seed oil using heterogeneous catalyst under conventional stirring and ultrasonic cavitation. Materials Science for Energy Technologies, 1(2), 106–116. DOI 10.1016/j.mset.2018.06.004. [Google Scholar] [CrossRef]
34. Abdullah, A., Ria, D. E., Santoso, U. T., Rosyidah, K. (2010). Penentuan waktu reaksi dan jumlah katalis (H2SO4 dan KOH) optimum pada pembuatan biodiesel dari minyak goreng bekas. Info Teknik, 10(1), 1–10. [Google Scholar]
35. Adhani, L., Aziz, I., Nurbayti, S., Octavia, C. A., Oktaviana, C. O. (2016). Pembuatan biodiesel dengan cara adsorpsi dan transesterifikasi dari minyak goreng bekas. Jurnal Kimia Valensi, 2(1), 71–80. DOI 10.15408/jkv.v2i1.3107. [Google Scholar] [CrossRef]
36. Afrielyanda, B., Khairat, S. (2015). Pembuatan biodiesel dari biji kapuk (Ceiba pentandra) dengan katalis padat H-zeolit. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 2(2), 1–5. [Google Scholar]
37. Arifin, A., Latifah, L. (2015). Sintesis biodiesel dari minyak goreng bekas dengan menggunakan katalis zeolit alam termodifikasi. Indonesian Journal of Chemical Science, 4(2), 138–143. [Google Scholar]
38. Aziz, I., Nurbayti, S., Hakim, A. R. (2012). Uji karakteristik biodiesel yang dihasilkan dari minyak goreng bekas menggunakan katalis zeolit alam (H-zeolit) dan KOH. Jurnal Kimia Valensi, 2(5), 541–547. DOI 10.15408/jkv.v2i5.296. [Google Scholar] [CrossRef]
39. Becker, J., Bahri, S., Herman, S. (2016). Pembuatan biodiesel dari biji saga (Adhenantera pavonina) dengan katalis padat H-zeolit. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 3(1), 1–5. [Google Scholar]
40. Ernes, A., Sari, P. D., Hartati, R. S., Winaya, N. S., Winaya, I. N. S. (2019). Biodiesel production from sardine flour used cooking oil using one step transesterification techniques. Journal of Applied Agricultural Science and Technology, 3(2), 289–298. DOI 10.32530/jaast.v3i2.109. [Google Scholar] [CrossRef]
41. Hernanda, S., Bahri, S., Yusnimar, Y. (2014). Pembuatan biodiesel dari limbah ikan baung dengan katalis padat lempung. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 1(1), 1–7. [Google Scholar]
42. Juliana, I., Widihati, I. A. G., Ratnayani, O. (2016). Karakterisasi katalis batu padas ledgestone teraktivasi asam dan aplikasinya pada pembuatan biodiesel dari minyak jelantah. Jurnal Kimia, 10(1), 32–42. DOI 10.24843/JCHEM.2016.v10.i01.p05. [Google Scholar] [CrossRef]
43. Kusyanto, K., Hasmara, P. A. (2017). Pemanfaatan abu sekam padi menjadi katalis heterogen dalam pembuatan biodiesel dari minyak sawit. Journal of Tropical Pharmacy and Chemistry, 4(1), 14–21. DOI 10.25026/jtpc.v4i1.127. [Google Scholar] [CrossRef]
44. Latupeirissa, J., Sutapa, I. W., Rego, H. P. (2012). Aplikasi katalis CaO dalam pembuatan biodiesel dari minyak goreng bekas. Molluca Journal of Chemistry Education, 2(1), 54–61. DOI 10.30598/MJoCEvol2iss1pp54-61. [Google Scholar] [CrossRef]
45. Malia, A., Suarya, P., Asih, I. A. R. A., Putra, I. M. W. A. (2016). Pengaruh rasio molar minyak jelantah dengan metanol dan suhu reaksi dalam reaksi transesterifikasi terkatalis CaO/zeolit alam terhadap yield biodiesel. Jurnal Kimia, 10(1), 49–57. DOI 10.24843/JCHEM.2016.v10.i01.p07. [Google Scholar] [CrossRef]
46. Ningtyas, D. P., Budhiyanti, S. A., Sahubawa, L. (2013). Pengaruh katalis basa (NaOH) pada tahap reaksi transesterifikasi terhadap kualitas biofuel dari minyak tepung ikan sardin. Jurnal Teknosains, 2(2), 71–158. DOI 10.22146/teknosains.6000. [Google Scholar] [CrossRef]
47. Nopriza, F., Bahri, S., Yusnimar. (2015). Pembuatan biodiesel dari minyak kelapa dengan katalis H-zeolit melalui proses metanolisis. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 2(2), 1–5. [Google Scholar]
48. Nurlis, N., Bahri, S., Saputra, E. (2017). Pembuatan biodiesel dari minyak biji kapuk (Ceiba pentandra) dengan katalis lempung teraktivasi; pengaruh waktu reaksi terhadap yield biodiesel. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 4(2), 1–6. [Google Scholar]
49. Padmaningsih, A. T., Trisunaryanti, W., Tahir, I. (2006). Study on the concentration effect of Nb2O5-ZAA catalyst towards total conversion of biodiesel in transesterification of wasted cooking oil. Indonesian Journal of Chemistry, 6(3), 268–274. DOI 10.22146/ijc.21730. [Google Scholar] [CrossRef]
50. Rahmawati, D. A., Intaningrum, D., Istadi, I. (2013). Pembuatan dan karakterisasi katalis heterogen SO4-ZnO dan SO42-/ZnO dengan metode kopresipitasi dan impregnasi untuk produksi biodiesel dari minyak kedelai. Jurnal Teknologi Kimia Dan Industri, 2(4), 243–252. [Google Scholar]
51. Rosmawaty, R., Bandjar, A., Gunoroso, S. (2015). Optimation transesterification reaction conditions on biodiesel production from beef tallow. Indonesian Journal of Chemical Research, 2(2), 213–222. [Google Scholar]
52. Salim, I. (2020). Transesterifikasi minyak jelantah menjadi biodiesel menggunakan katalis zeolit gismondin hasil sintesis dari lempung asal merauke. AVOGADRO Jurnal Kimia, 4(1), 12–23. [Google Scholar]
53. Sartoni, H., Bahri, S., Sunarno, S. (2014). Biodiesel dari limbah ikan baung (Mystus nemurus) dengan katalis padat H-zeolit. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 1(1), 1–6. [Google Scholar]
54. Silalahi, A., Bahri, S., Yusnimar. (2016). Perbandingan biodiesel hasil transesterifikasi minyak biji kepayang (Pangium edule reinw) dengan katalis NaOH dan H-zeolit. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 3(1), 1–6. [Google Scholar]
55. Sinaga, T. M. N., Bahri, S., Saputra, E. (2017). Biodiesel dari biji buta-buta (Hura crepitans Linn) dengan katalis Ni/NZA. Jurnal Online Mahasiswa (JOM) Bidang Teknik Dan Sains, 4(1), 1–6. [Google Scholar]
56. Zuhra, Z., Husin, H., Hasfita, F., Rinaldi, W. (2015). Preparasi katalis abu kulit kerang untuk transesterifikasi minyak nyamplung menjadi biodiesel. AgriTECH, 35(1), 69–77. DOI 10.22146/agritech.9421. [Google Scholar] [CrossRef]
57. Bokhari, A., Chuah, L. F., Yusup, S., Klemeš, J. J., Kamil, R. N. M. (2016). Optimisation on pretreatment of rubber seed (Hevea brasiliensis) oil via esterification reaction in a hydrodynamic cavitation reactor. Bioresource Technology, 199, 414–422. DOI 10.1016/j.biortech.2015.08.013. [Google Scholar] [CrossRef]
58. Chuah, L. F., Bokhari, A., Yusup, S., Klemeš, J. J., Akbar, M. M. et al. (2017). Optimisation on pretreatment of kapok seed (Ceiba pentandra) oil via esterification reaction in an ultrasonic cavitation reactor. Biomass Conversion and Biorefinery, 7(1), 91–99. DOI 10.1007/s13399-016-0207-9. [Google Scholar] [CrossRef]
59. Vincent, C. J., Shamsudin, R., Baharuddin, A. S. (2014). Pre-treatment of oil palm fruits: A review. Journal of Food Engineering, 143, 123–131. DOI 10.1016/j.jfoodeng.2014.06.022. [Google Scholar] [CrossRef]
60. Santoso, A., Wijaya, A. R., Purwaningtyas, C. F., Sukarianingsih, D., Retnosari, R. et al. (2020). The effect of K2O concentration in K2O/Al2O3 catalyst on methyl ester (Biodiesel) synthesis from CPO off grade with ultrasonic wave. IOP Conference Series: Materials Science and Engineering, 833, 012043. DOI 10.1088/1757-899X/833/1/012043. [Google Scholar] [CrossRef]
61. Chipurici, P., Vlaicu, A., Calinescu, I., Vinatoru, M., Vasilescu, M. et al. (2019). Ultrasonic, hydrodynamic and microwave biodiesel synthesis –A comparative study for continuous process. Ultrasonics Sonochemistry, 57, 38–47. DOI 10.1016/j.ultsonch.2019.05.011. [Google Scholar] [CrossRef]
62. Nan, Y., Liu, J., Lin, R., Tavlarides, L. L. (2015). Production of biodiesel from microalgae oil (Chlorella protothecoides) by non-catalytic transesterification in supercritical methanol and ethanol: Process optimization. Journal of Supercritical Fluids, 97, 174–182. DOI 10.1016/j.supflu.2014.08.025. [Google Scholar] [CrossRef]
63. Tang, D. Y. Y., Yew, G. Y., Koyande, A. K., Chew, K. W., Vo, D. V. N. et al. (2020). Green technology for the industrial production of biofuels and bioproducts from microalgae: A review. Environmental Chemistry Letters, 18, 1967–1985. DOI 10.1007/s10311-020-01052-3. [Google Scholar] [CrossRef]
64. Nautiyal, P., Subramanian, K., Dastidar, M. (2014). Kinetic and thermodynamic studies on biodiesel production from spirulina platensis algae biomass using single stage extraction–transesterification process. Fuel, 135, 228–234. DOI 10.1016/j.fuel.2014.06.063. [Google Scholar] [CrossRef]
65. Loh, S. H., Chen, M. K., Fauzi, N. S., Aziz, A., Cha, T. S. (2021). Enhanced fatty acid methyl esters recovery through a simple and rapid direct transesterification of freshly harvested biomass of Chlorella vulgaris and Messastrum gracile. Scientific Reports, 11(1), 2720. DOI 10.1038/s41598-021-81609-6. [Google Scholar] [CrossRef]
66. Asrori, M. R., Santoso, A., Sumari, S. (2022). Initial defect product on immiscible mixture of palm oil: Ethanol by amphiphilic chitosan/Zeolite LTA as optimization of microemulsion fuel. Industrial Crops and Products, 180, 114727. DOI 10.1016/j.indcrop.2022.114727. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools