 Open Access
Open Access
REVIEW
Polymeric Nanofiber Scaffolds for Diabetic Wound Healing: A Review
1 Department of Pharmaceutical Technology, Faculty of Pharmacy, Universiti Malaya, Kuala Lumpur, 50603, Malaysia
2 Faculty of Medicine, Universiti Malaya Research Centre for Biopharmaceuticals and Advanced Therapeutics (UBAT), Universiti Malaya, Kuala Lumpur, 50603, Malaysia
3 Centre of Advanced Materials (CAM), Faculty of Engineering, Universiti Malaya, Kuala Lumpur, 50603, Malaysia
4 Faculty of Pharmaceutical Sciences, Chulalongkorn University, Pathum Wan, Bangkok, 10330, Thailand
* Corresponding Authors: Shaik Nyamathulla. Email: ; Syed Mahmood. Email:
(This article belongs to the Special Issue: Polymer Materials in Controlled Drug Delivery)
Journal of Polymer Materials 2025, 42(4), 959-992. https://doi.org/10.32604/jpm.2025.072005
Received 17 August 2025; Accepted 10 October 2025; Issue published 26 December 2025
Abstract
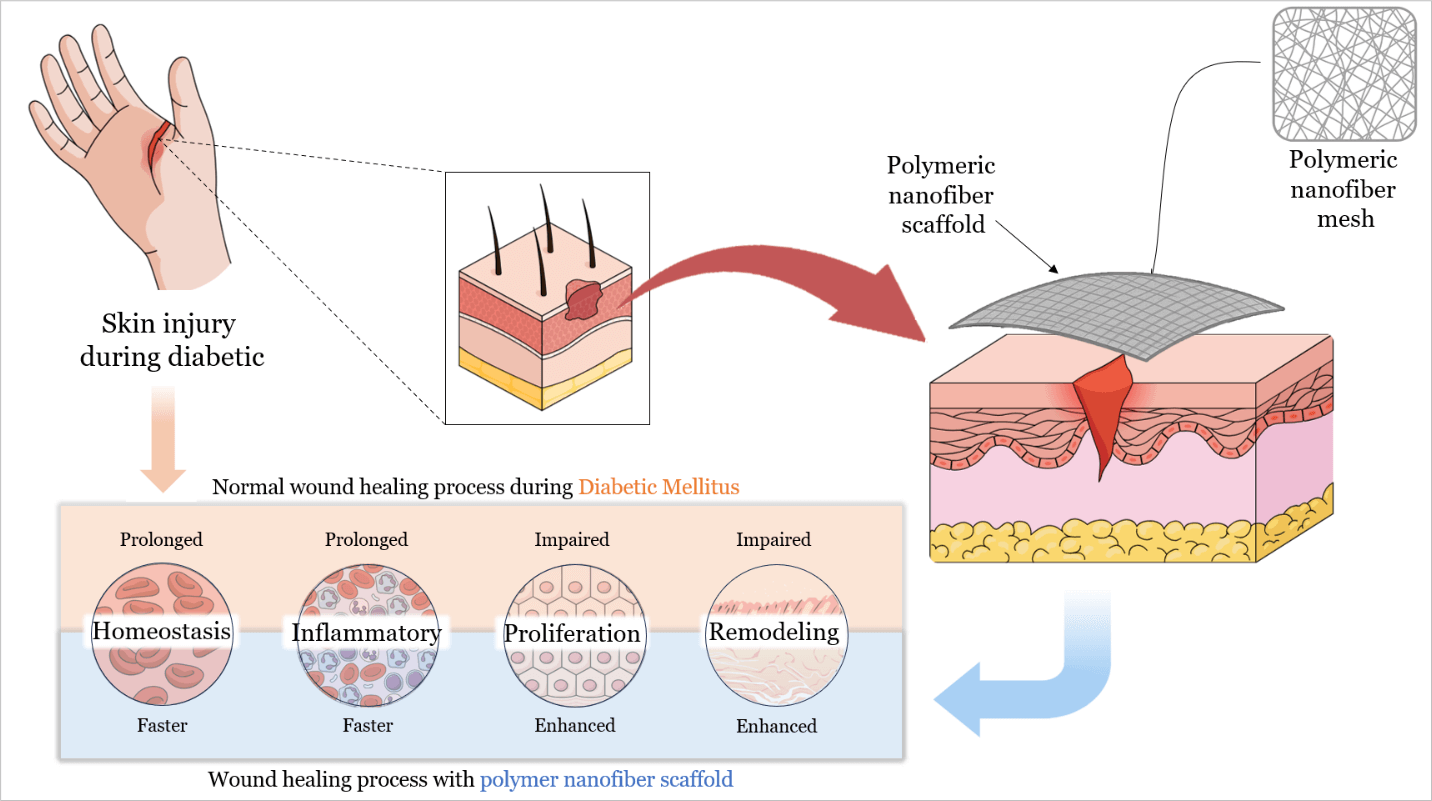
With the global diabetes epidemic, diabetic foot ulcers (DFUs) have become a major health burden, affecting approximately 18 million people worldwide each year, and account for about 80% of diabetes-related amputations. Five-year mortality among DFU patients approaches 30%, which is comparable to that of many malignancies. Yet despite standard wound care, only about 30%–40% of chronic DFUs achieve complete healing within 12 weeks. This persistent failure shows that conventional dressings remain passive supports. They do not counteract underlying pathologies such as ischemia, prolonged inflammation, and infection. Recent advances in polymeric nanofiber scaffolds, particularly electrospun matrices, provide bioactive wound dressings designed to overcome these limitations. By mimicking extracellular matrix architecture (ECM) and delivering therapeutic biomolecules, polymeric nanofiber scaffolds can promote tissue regeneration and angiogenesis. They also modulate the wound immune response and combat infection through embedded antimicrobial agents. Innovative scaffold architectures further enhance healing outcomes. Core–shell and multilayer nanofibers enable sequential or sustained release of multiple factors. Biomimetic “basketweave” fiber layouts improve cell alignment, neovascularization, and wound closure, and stimuli-responsive scaffolds release therapeutics in response to wound pH or oxidative stress. Preclinical diabetic wound models and early clinical trials show that these engineered scaffolds accelerate wound closure, increase re-epithelialization, and reduce chronic inflammation relative to standard care. Notably, a recent clinical trial in patients with DFU reported 74% wound closure by 12 weeks with an electrospun scaffold vs. 33% with conventional therapy. However, translational challenges persist, including stability, sterilization compatibility, and scalable manufacturing of nanofiber scaffolds. This review discusses these hurdles and highlights future directions, including the development of smart biosensor-integrated scaffolds for responsive drug delivery, personalized patient-specific dressings, and AI-assisted design of polymer nanofibers to further optimize DFU healing outcomes.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools