 Open Access
Open Access
ARTICLE
Preparation of Film Based on Polyvinyl Alcohol Modified by Alkaline Starch and Lignin Fiber
1
Yunnan Provincial Key Laboratory of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming, 650224, China
2
Department of Polymers and Pigments, National Research Centre, Dokki, Cairo, 12622, Egypt
* Corresponding Authors: Xiaojian Zhou. Email: ; Jun Zhang. Email:
Journal of Renewable Materials 2023, 11(2), 837-852. https://doi.org/10.32604/jrm.2022.022792
Received 26 March 2022; Accepted 23 May 2022; Issue published 22 September 2022
Abstract
This study presents an easily prepared film based on alkaline starch-polyvinyl alcohol hybrid and lignin fiber as an additive (SPL film). The SPL film was prepared under acidic conditions through a polycondensation reaction of PVA and a mixture incorporating alkaline starch and lignin fiber from agriculture or forest source. The examination using scanning electron microscopy (SEM) showed that the surface of SPL film was smooth and the lignin fiber had good compatibility within the film hybrid. Electrospray ionization mass spectroscopy (ESI-MS) and fourier transform infrared (FTIR) investigations indicated that alkaline starch and lignin fiber reacted with PVA under acidic conditions and that –CH2–O– groups were involved in the cross-linking of the SPL system. In addition, the SPL film exhibited only 4% light transmittance, which effectively reduces the ultraviolet and visible light (UV-Vis) penetration, along with good performance when exposed to thermal degradation, in which the mass loss reached around 60% at 400°C. Moreover, the SPL film acquired excellent tensile strength, which is much higher than that of PVA-lignin (PL) composite film.Graphic Abstract
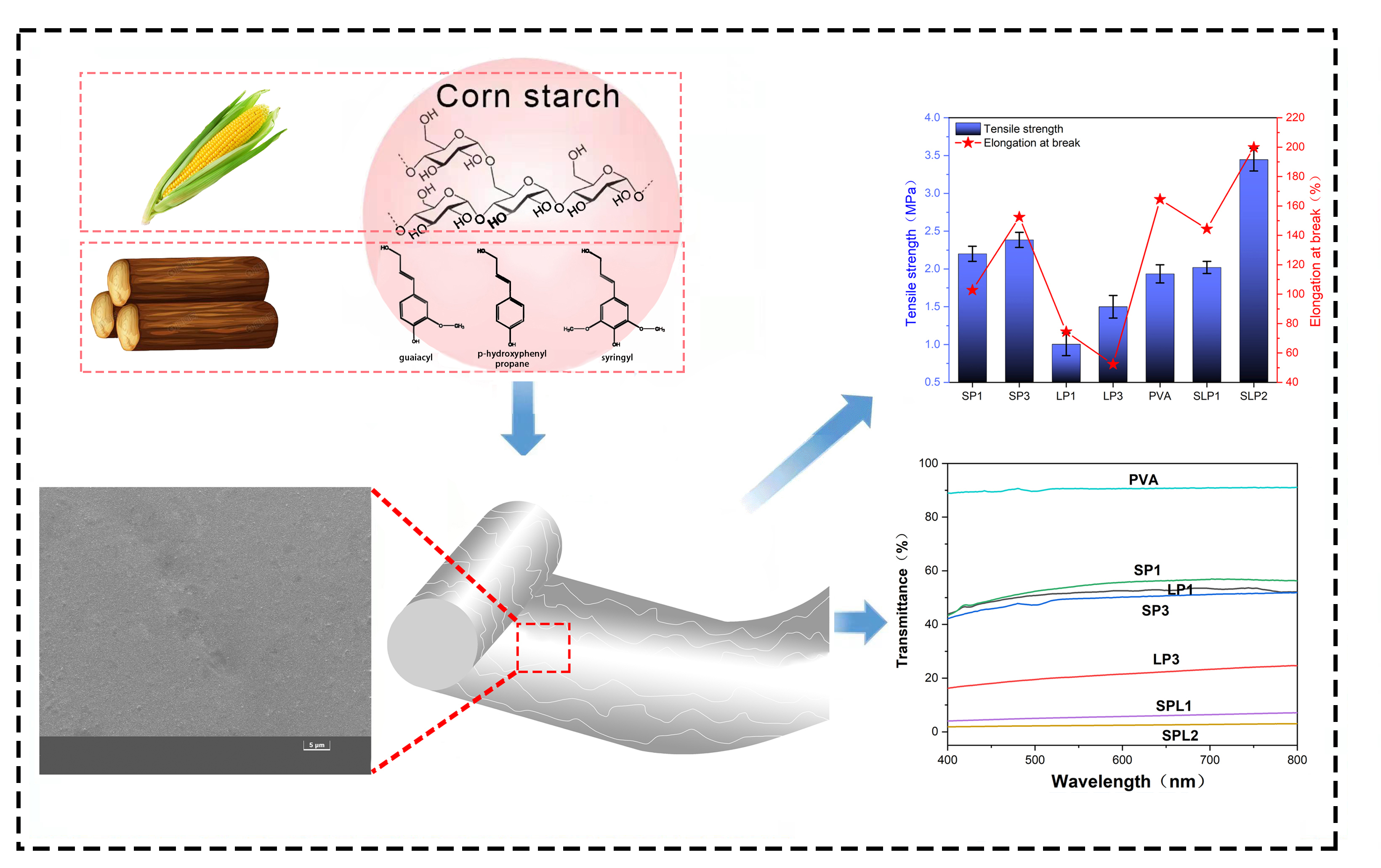
Keywords
Films have been widely used in electronic and electrical appliances, machinery, printing and other industries. They can also be divided into optical films of Abdelaziz et al. [1], composite films of Božanić et al. [2], superconducting films of Zhao et al. [3], polyester films of Zhong et al. [4], nylon films of Zhang et al. [5] and plastic films of Lin et al. [6]. In recent years, the problems of energy shortage and environmental pollution in the world have become increasingly prominent. Exploring and studying environment-friendly, renewable and degradable films to alleviate the environmental and energy aspects has become an important trend for the survival and development of the film industry. PVA film is a biodegradable film of Kochkina et al. [7–9]. In the past decades, it has been widely used to replace the traditional non-degradable films because of its good properties such as good adhesion, high transparency, oil resistance, corrosion resistance, solvent resistance and wear resistance. However, the monomeric units of PVA film contain a lot of hydrophilic hydroxyl groups, which leads to poor water resistance, low solid content and easy bonding after moisture absorption of Shi et al. [10]. Under the conditions of low temperature and low humidity, it becomes hard and brittle, which greatly limits its production, promotion and application. After that, modified PVA film with biomass constituents has been developed to improve the water resistance and solid content. Such constituents like starch and oleic acid were used via grafting or oxidation to starch-based nanocomposites (SNCs) using acid hydrolysis, whereas nanocomposite films were prepared by adding SNCs into PVA of Mittal et al. [11]. However, it was found that the elongation at break of the nanocomposite film was only 42.67%, which was caused by the lack of suitable fluidity of the modified starch during processing. This component becomes turbid upon cooling with the formation of a gel that is very liable to aging, resulting in crispness and poor flexibility of PVA film of Chen et al. [12–17]. Meanwhile, advanced polyvinyl alcohol-lignin (PVAL) films were prepared by blending lignin and PVA through the mechanical stirring of Korbag et al. [18]. In this way, the elongation at break of PVA film was effectively improved. However, the preparation process of the film became complicated, which is not favorable to industrialization. Based on that, it can be concluded that a separate use of either lignin or modified starch for blending with PVA yields films with improved properties. However, the preparation of modified starch is somewhat convoluted, and the cost of the derived composites is high. In addition, besides the complicated processing of lignin with PVA, it produces a composite film that shows a modest anti-UV-Vis effect, which makes it so difficult to achieve industrialization. Furthermore, In our previous study of Zhang et al. [19], it was found that the crosslinking reaction among starch under alkaline conditions to form the gelatinized starch could improve the substitution activity of molecular groups of starch via etherification and crosslinking with polyvinyl alcohol to reduce the hydroxyl content, which is in favor of the hydrophobicity of PVA and ensures improving the toughness and limiting the brittleness of starch-PVA film. Besides, lignin fiber which is the main byproduct of pulp and paper industries is an abundant, cheap, renewable, and nontoxic natural polymer of Cheng et al. [20–23]. Compared with lignin powder, the good toughness and dispersibility of lignin fiber makes its blending with the starch-PVA mixture much easier, which brings about enhancement of the elongation at break and resistance to UV radiation. Based on the aforementioned background, alkaline starch and lignin fibers will be considered to modify PVA in order to synthesize easily-prepared transparent films with reasonable water resistance, high elongation at break, good thermal stability and high resistance to UV radiation.
Corn (Zea mays L.) starch with a low content of linear amylose (30%) and 70% of branched amylopectin, was purchased from Tai’an Jingshan Starch Company (Shandong, China). Flocculent lignin fiber, extracted from Eucalyptus (Eucalyptus robusta Smith), with lignin content of 98%, and comprises p-hydroxyphenyl propane, guaiacyl, and syringyl, was a product of Sinopharm Group (Beijing, China). Polyvinyl alcohol (with a molecular weight of 250∼300 × 103 Da), glycerol, sodium hydroxide (analytical purity, 30%), and acetic acid (analytical purity, 15%) were all purchased from Sinopharm Group (Beijing, China).
2.2 Formulation and Processing of Various Films
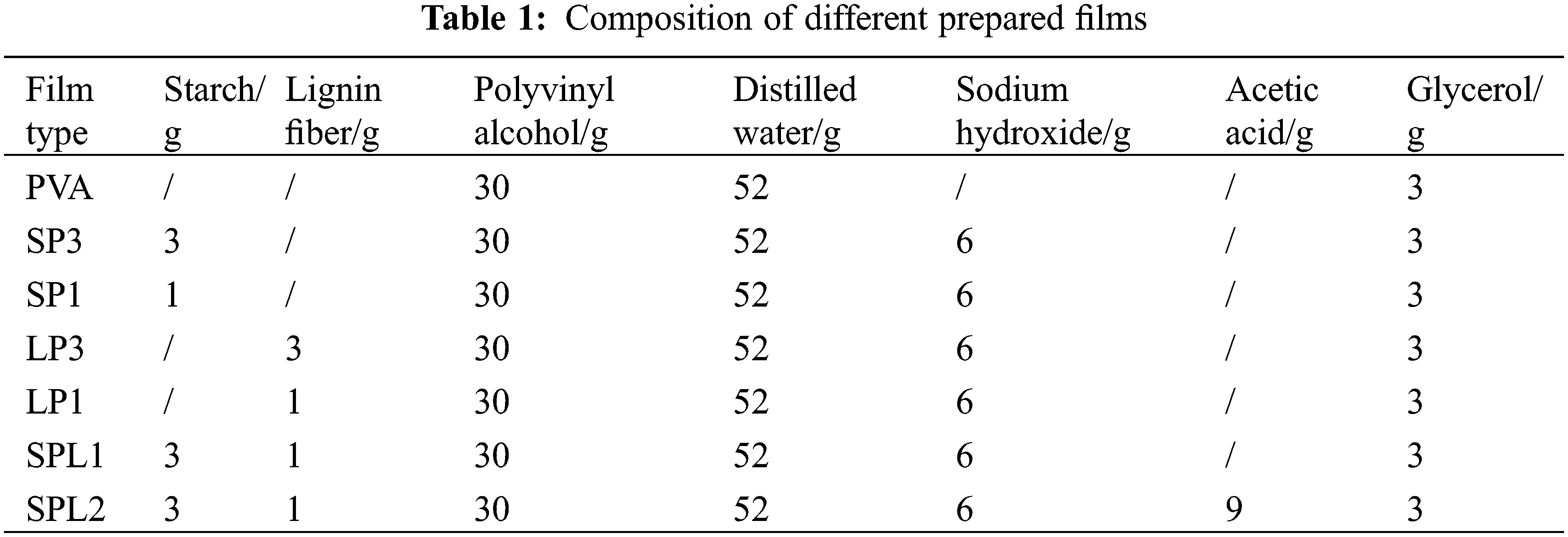
The preparation of PVA-based films was performed according to the literature of Abdelaziz et al. [1,2,8]. Thus, PVA was put into a three-port flask and dissolved at 90°C. Then, starch was mixed with distilled water in a beaker under stirring for 2 min using an electromagnetic stirrer (JJ-1, Haojie, China) to form a solution, and then sodium hydroxide was added into the solution under stirring for 4 min to prepare the alkalized starch solution. After that, lignin fiber was put into the alkalized starch solution under stirring for 1 min. Then, the PVA and glycerol as plasticizer were combined with the mixture and stirred altogether at 90°C for 1 h to form the SPL1 resin. Acetic acid (15%) was added into SPL1 resin under stirring for 1 min to form SPL2 resin. Starch-PVA (SP) and lignin-PVA (LP) resins were also formulated for comparison with SPL resin, and their preparation process was consistent with that of SPL1. Then, PVA, SP, LP, SPL1, SPL2 resins were laid separately on moulds with a size of 26 × 19 × 1.5 cm3 and dried at room temperature so that degradable films with size of 26 cm × 19 cm × 0.2 mm were produced. The formulations employed for the preparation of the various films are shown in Table 1.
A schematic illustration for the preparation of SPL2 film is shown in Scheme 1.

Scheme 1: Schematic illustration for the preparation of SPL2 film
The gelation (gel) time of SP, LP, SPL1 and SPL2 resins was determined according to the Chinese norm of GB/T 14074 9. Thus, 10 g of each resin was placed in a test tube, which was then immersed in a boiling water bath (Model HWS-26, YIHENG, Shanghai, China) at 100°C while gently and continuously stirred using a muddler via an upward-downward movement until gelation occurred. The gelation time was counted from the beginning of the resin immersion in boiling water until gelation was attained.
The developed interactions within the various films were followed using FTIR spectroscopy with a varian1000 infrared spectrometer (Varian, Palo Alto, CA, USA). One g of KBr and 0.01 g of the film powder were mixed to prepare the test sample, which was scanned over a wavenumber range from 400 to 4000 cm−1.
Electrospray ionization mass spectroscopy (ESI-MS) was also used to follow the ongoing interactions within films. A film was dissolved in chloroform (10 ug/mL), and the solution was then injected into the instrument with a syringe for recording in a positive ion mode. The ion energy was 0.3 eV at ambient temperature (22°C–25°C) and the relative humidity was ≤60%.
The morphology of each film was observed using a scanning electron microscope (S4800, Hitachi, Japan). Before testing, the films were frozen in liquid nitrogen, and then cut into samples with a size of 5 mm × 5 mm × 0.2 mm.
The contact angle measurements for the films were implemented by OCA-25 Video optical contact angle instrument (Dataphysics, Germany), in which each measurement was carried out on a water droplet of 2 μL size and the contact angle was obtained at 3 s after the water droplet falls on the films surface The values were recorded on six different places for each surface of the various films and the average of these readings was considered.
The opacity of the films was characterized by the ultraviolet (UV) spectrophotometer (A260, Mettler Toledo, USA). The film samples were cut into 4 cm × 1 cm × 0.2 mm, and the film samples were placed on the inner surface of the colorimetric cup to measure the absorbance at 600 nm. Three separate readings were performed on each film and the average value was calculated according to the following formula (1):
where A is the absorbance of the film at 600 nm and x is the film thickness (mm).
The thermal stability of the films was evaluated using a thermogravimetric analyzer (TG 209 F3, Netzsch, Germany). About 5–10 mg of each sample was put into a crucible for testing under the nitrogen atmosphere in the range 30°C–600°C while a heating rate of 15 °C/min was applied.
The physical damages to the films were evaluated on film samples with a size of 50 mm × 15 mm × 0.2 mm according to GB/T 30776-2014, after being exposed to tension by fixing both ends with two clips of the same mass, while one clip was fixed with a weight of 500 g, and the weight was hanged for 5 min.
The tensile strength and elongation at the break of the films were evaluated using a universal testing machine at ambient temperature (23°C) and 45%–55% humidity according to GB/T 1040.3-2006. Each film was cut into strips of 160 mm × 20 mm × 0.2 mm, and a test distance of 50 mm was set and the tensile rate during measurements was kept at 50 mm/min. The measurements were repeated on each sample for five times.
The prepared resins were characterized in terms of gel time (Fig. 1). It is interesting to note that the gel time of LP1 (97 s), LP3 (129 s) and SPL1 (84 s) resins are longer than that of SP1 (48 s) and SP3 (68 s), indicating that the reaction activity of alkaline starch and PVA is higher than that of lignin and PVA. Compared with the SP film system with a smaller molecular rigidity, the addition of lignin fiber in LP system makes the reaction more complex and requires a longer gel time. The gel time of SPL2, 73 s, is shorter with respect to SPL1 confirming that the reaction of the system comprising lignin fiber and alkaline starch with PVA is more reactive under acidic conditions, resulting in an increase in molecular weight and a decrease in the gap between chain segments, resulting in a shorter gel time. Moreover, the gel time of SPL2 is much lower than that of PVA (107 s), which indicates that alkaline starch and lignin can react with PVA to effectively shorten the gel time.
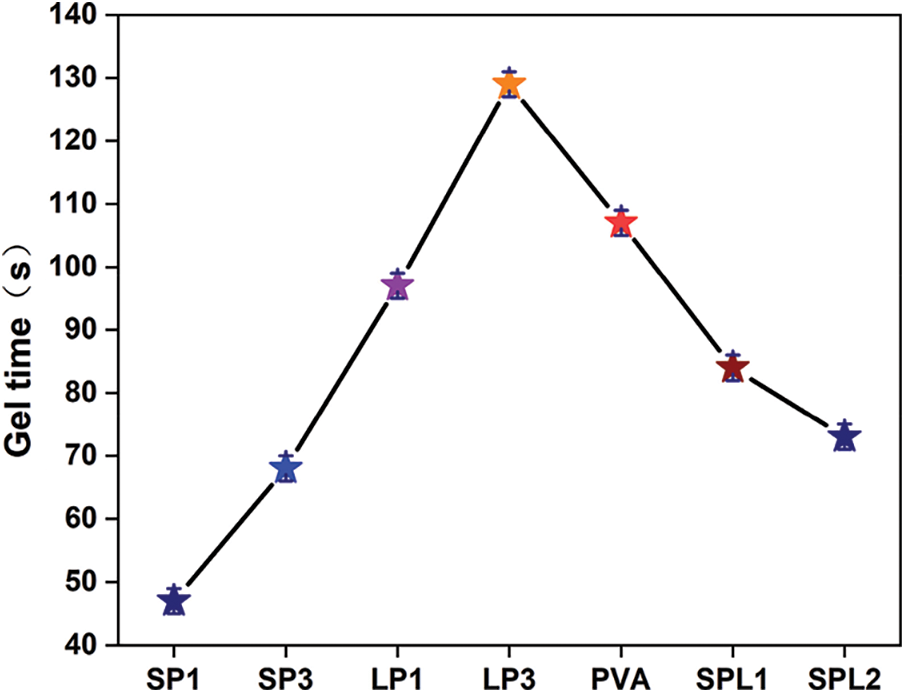
Figure 1: The gel time of the various resins
The FTIR spectra of SP3, LP1, PVA, SPL1 and SPL2 films are shown in Fig. 2. The absorption peaks at 3700–3200 cm−1 are attributed to hydroxyl groups, peaks at 3300–2700 cm−1 belong to C-H while those at 1636 and 1582 cm−1 are indicative of C=C of the aromatic skeleton. In addition, peaks at 1653 and 1571 cm−1 belong to adsorbed water. Meanwhile, the peaks at the range of 1412–566 cm−1 were produced by stretching vibration of C–C. In addition, the peaks at 1412, 1036, 1052 and 1058 cm−1 belong to –CH3. Further, the peak at 1075 cm−1 is characteristic of –C–O–C–because carbon sites at positions 2 and 4 of lignin react with the carbon site at position 6 of glucose monomer under acidic conditions to form a large conjugation. The etherification and crosslinking with polyvinyl alcohol renders the electron cloud in the conjugate system tend to average thereby the absorption of its –C–O–C–groups shifts to a lower wavenumber at 1041 cm−1. This peak of 1041 cm−1 is detected of various intensity in case of SPL2 but not in case of SPL1, indicating that lignin, alkaline starch and polyvinyl alcohol have reacted to varying degrees under acidic conditions.

Figure 2: FTIR spectra of the various films
The results of ESI-MS analysis revealed different species that are formed during the preparation of the SPL1 and SPL2 films, as a result of co-polycondensation reactions involving alkaline starch, PVA, and lignin fibers as presented in Figs. 3 and 4. The main fractions of the SPL1 and SPL2 films are collected in Table 2. All the peak values are based on the corresponding molecular weight of the different species +23 Da due to the presence of Na+ ion of the NaCl, which is used as matrix enhancer or +1 Da due to protonation with H+. It can be generally recognized that the obtained results suggest the formation of several possible products or intermediates.

Figure 3: ESI-MS spectrum of SPL1 film at different m/z intervals, (a) 50–550, (b) 650–1100
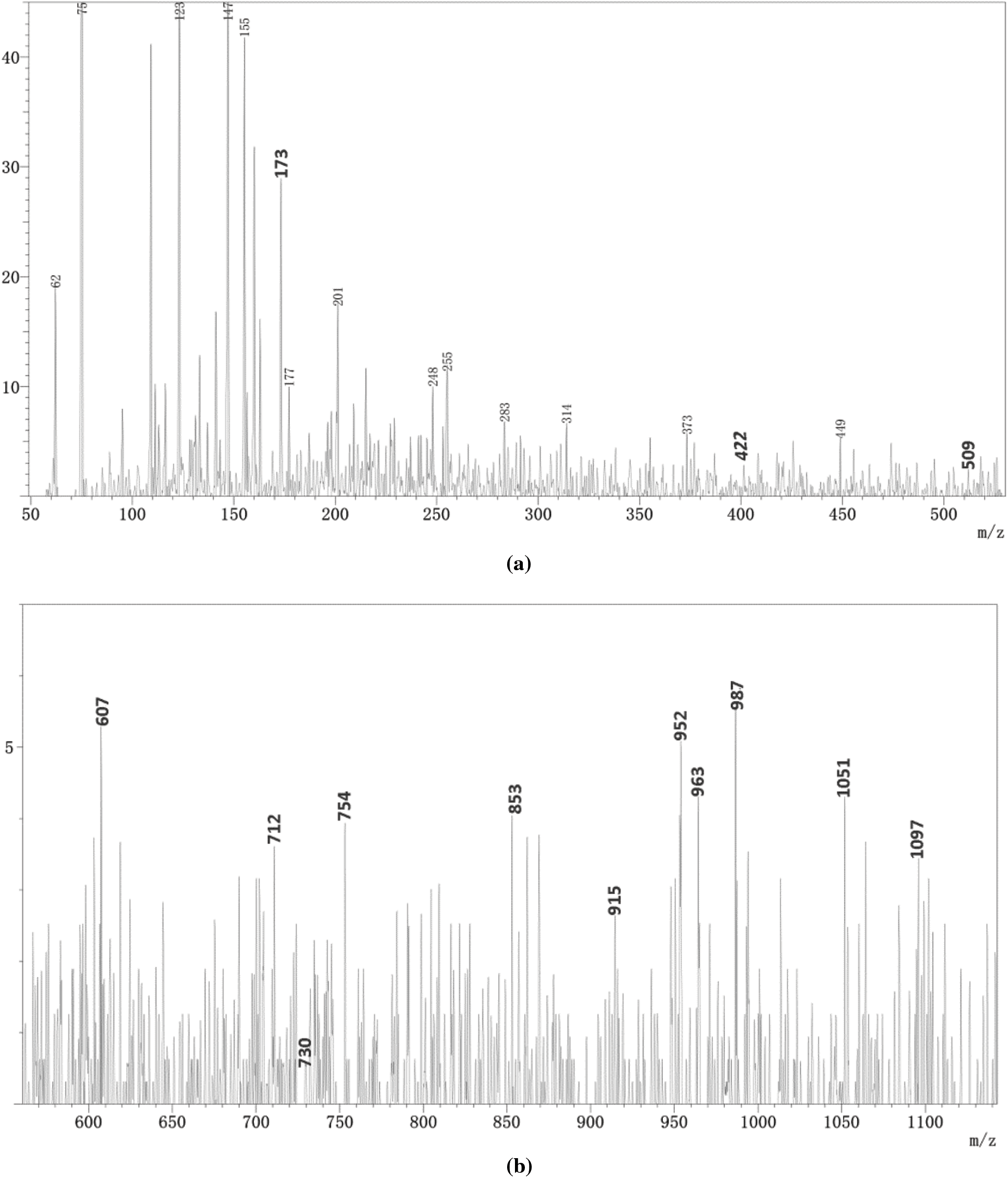
Figure 4: ESI-MS spectrum of SPL2 films at different m/z intervals, (a) 50–530, (b) 550–1140
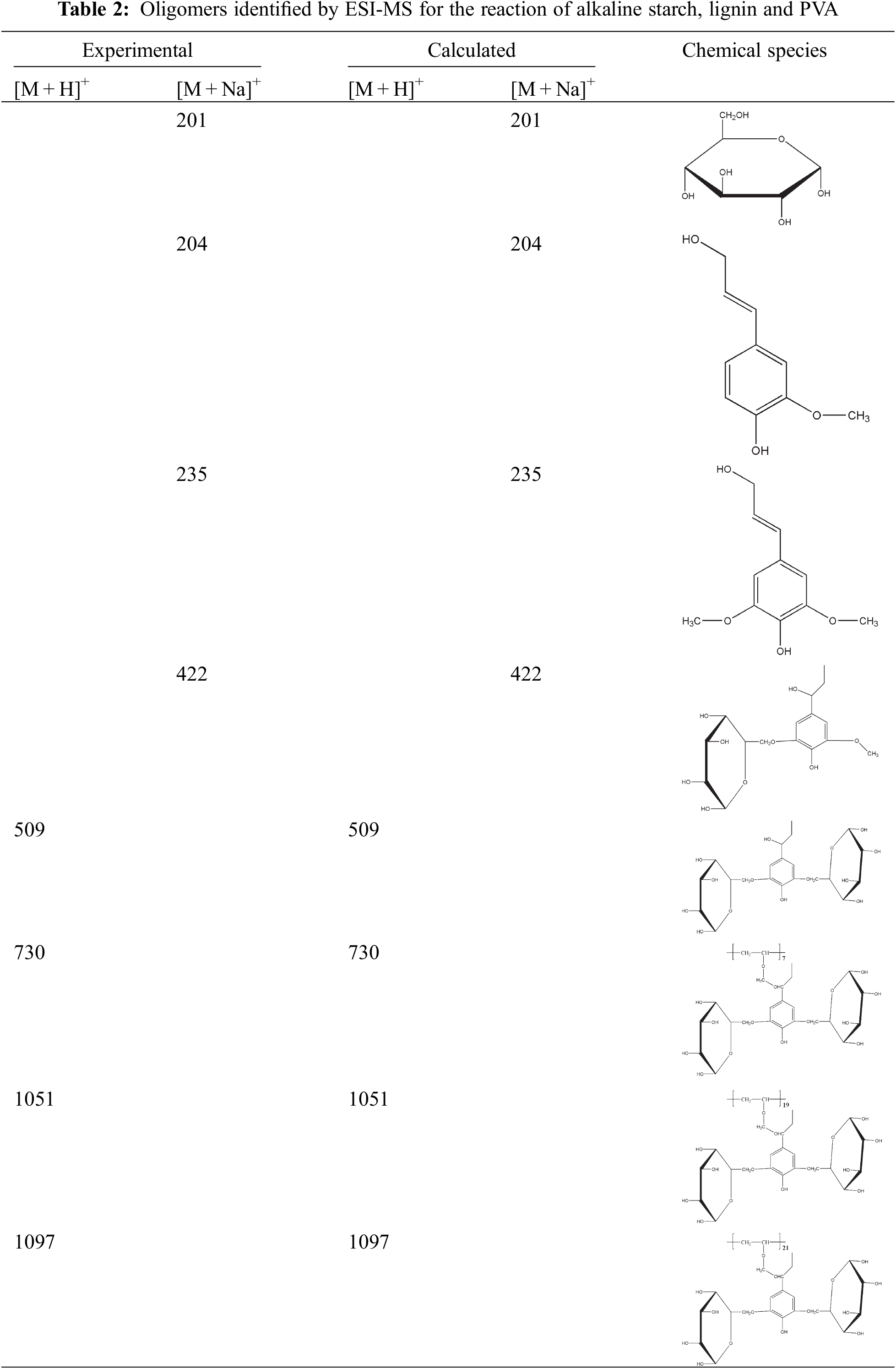
In case of SPL1 and SPL2, the peak at 201 Da belongs to glucose units, while the peaks at 204 and 235 Da can be identified to lignin structure, indicating that some alkaline starch and lignin are still free and not virtually involved in the condensation reaction.
Meanwhile, the peak at 422 Da can be attributed to the reaction between lignin and alkaline starch, while the peak at 730 Da is assumed to describe the reaction between lignin, alkaline starch and PVA, indicating that lignin, alkaline starch and polyvinyl alcohol react both under acidic and alkaline conditions. It is worth noting that the peaks at 1051 and 1097 Da in the spectra of SPL2 correspond to the reaction of alkaline starch, polyvinyl alcohol and lignin fiber. Nevertheless, they were not found in case of SPL1, indicating that the degree of etherification between the hydroxyl group at carbon position 6 of alkaline starch and lignin fiber under acidic conditions proceeds to a higher extent as compared to the alkaline conditions. Such result is consistent with the obtained gel time data.
From the results of FTIR and ESI-MS, it can be ensured that the high reactivity of carbon sites at the positions 1, 4 and 6 of glucose make them liable to easily react with the hydroxyl at the two ortho sites of the lignin precursor. Therefore, under acidic conditions, glucose and lignin precursor undergo etherification and cross-linking reactions to form low molecular weight species (Schemes 2a and 2b), then succeeding etherification reaction with the dissolved PVA takes place to form a cross-linked network structure, the proposed reaction routes are shown in Scheme 2c.

Scheme 2: Proposed reaction routes for formation of crosslinked network structure
Fig. 5 shows the appearance and SEM micrographs of the various films. The surface of SPL2 looks smoother than that of other films and is lacking of cracks or poles. On the contrary, the SEM of SP1 and SP3 films revealed that some alkaline starch was not dissolved in PVA, indicating that the reaction between alkaline starch and PVA is not totally favored under alkaline conditions. The SEM of LP1 and LP3 films appeared rough, bubbly, and fibrous elements could be obviously detected, which may be caused by the uneven distribution of the insoluble lignin fibers after blending with PVA under alkaline conditions, most likely due to compatibility issues. Similarly, the surface of SPL1 shows a low uniformity with a lot of agglomerations of lignin fiber, caused by the poor compatibility between lignin fiber and SP resin system. Besides, compared with SPL2 film, imaging using SEM showed that there are many tiny bubbles in PVA film, which shows that under the same curing conditions as for SPL2, a large number of bubbles appear in the cured film of PVA due to the moisture generated during the polycondensation reaction, which is consistent with the results of gel time data, indicating that the lignin fiber and SP resin system have high reactivity under acidic conditions, and this contributes to a smooth surface and improved mechanical properties of SPL2.
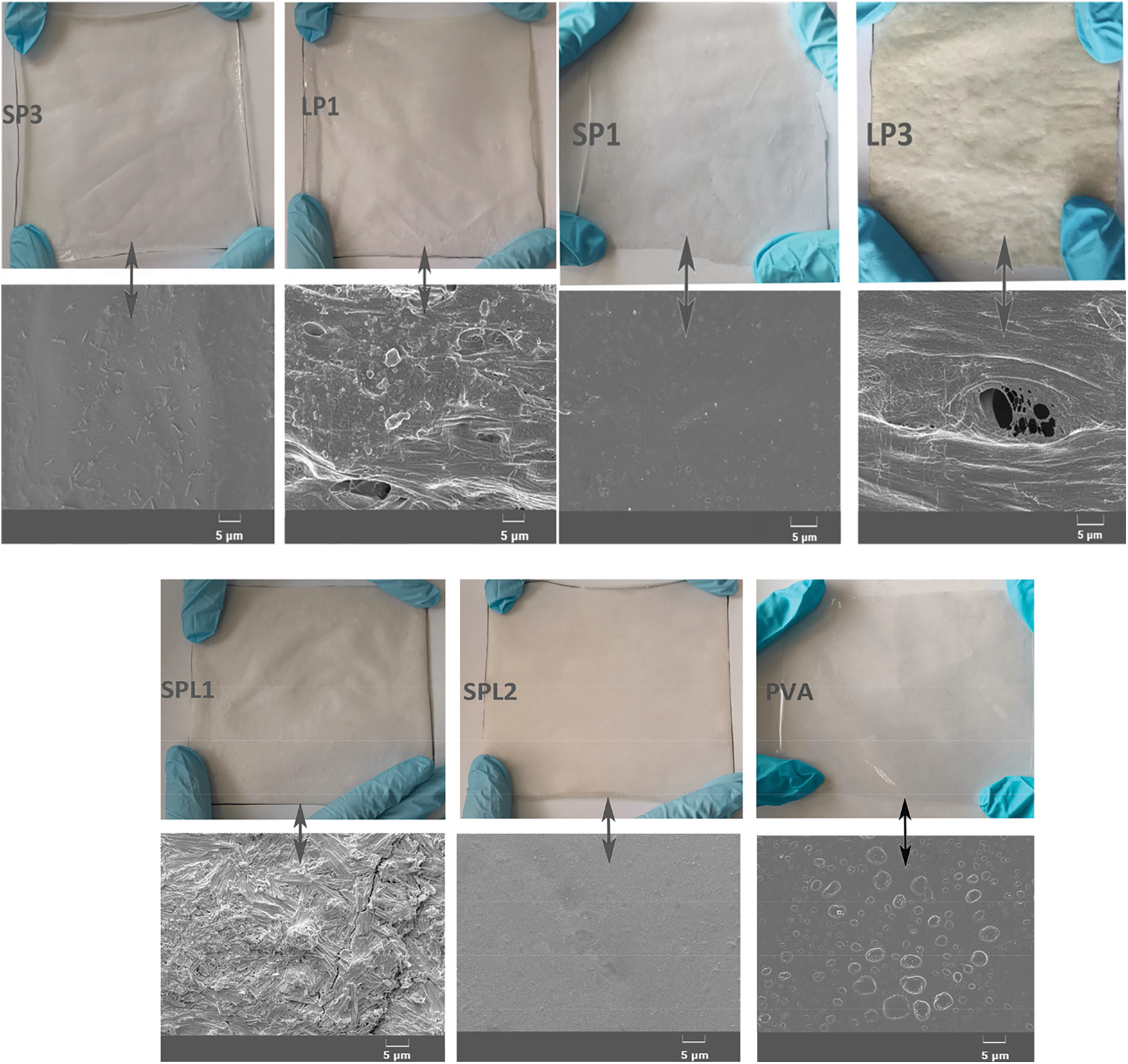
Figure 5: Digital as well as SEM micrographs of the various films
The opacity results of the films are presented in Fig. 6, from which we can see that the UV-Vis transmittance of PVA film is higher than that of other films. The highest transmittance exceeds 89% in the 400–800 nm range. Meanwhile, UV-Vis transmittance of SP1 film is higher than that of SP3. In the range of 400–800 nm, the highest transmittance of SP1 is more than 27%. Meanwhile, UV-Vis transmittance of LP1 film is higher than that of LP3. The UV-Vis transmittance of LP1 film at the highest point is about 21% in the range of 400–800 nm. These results indicated that with the increase of starch or lignin content, the anti UV absorption ability of the film was strengthened. Compared with PVA film, the addition of lignin fiber in PVA system can absorb the light at specific wavelength in the region of 400–800 because lignin contains many aromatic rings, which have the aptitude to absorb UV radiation. In addition, the UV-Vis transmittance of SPL1 (about 4%) and SPL2 (about 2%) is much lower than that of SP3 and LP3 films. This can be attributed to the cross-linking among alkaline starch, lignin and PVA that occurred after the addition of lignin fibers and alkaline starch, (proved using of FTIR and ESI-MS) which caused strengthening of PVA composite film and rendered it more resistive to UV-Vis. Moreover, The UV-Vis transmittance of SPL2 is lower than that of SPL1, and even lower than that of formaldehyde crosslinked lignin and polyvinyl alcohol film (about 16.12%) of Zhang et al. [24], indicating that the film prepared by alkaline starch, PVA and lignin fiber under acidic conditions has good resistance to the effect of UV-Vis light.

Figure 6: UV-Vis transmittance of the various films in the region of 400–800 nm
Fig. 7 shows the TG traces of PVA, SP1, SP3, LP1, LP3, SPL1 and SPL2 films. It can be seen from the figure that there is a slight mass loss before 100°C for all films, which may be caused by water and volatile substances in the films. From the temperatures at which the mass loss of 30%, 60% and 70%, were attained, Td30 (°C), Td60 (°C) and Td70 (°C), SPL2 showed the lowest thermal degradation rate. At 800°C, it still maintained about 28% of its original mass, which reflects its good thermal stability. This is accounted for by the presence of phenylpropane structural units within the structure of lignin fibers and the high cross-inking among lignin, alkaline starch and PVA under acidic conditions. For the lignin fiber, by the overlap of P orbitals of these aromatic structures, Π electrons can be completely delocalized, which improves its thermal stability of Ling et al. [25]. With the addition of lignin fiber, the residual mass of LP1, LP3, SPL1 and SPL2 films at temperatures above 400°C is higher than that of SP1 and SP3 films. For LP1 and LP3 films, before 300°C, its degradation rate is faster, indicating that under alkaline conditions the lignin has low reactivity and cannot be properly crosslinked with PVA from one side, and from another side it also hinders the self polycondensation of PVA, which results in a low degree of crosslinking for the prepared film. At the same time, the heat resistance of SPL2 film prepared under acidic conditions is superior to that of SPL1 film, which is prepared under alkaline conditions, indicating that the reaction within SPL resin system is rather privileged under acidic conditions than under alkaline conditions, which is consistent with the results of ESI-MS.

Figure 7: Thermogravimetric and derivative thermogravimetric curves of various films
The physical damages experienced for the samples after being exposed to tension forces are displayed in Fig. 8. Although all films showed varied extensibility, cracks were never developed on SPL2. On the contrary, cracks or bubbles appeared in case of SP1, SP3, PVA, LP1, LP3 and SPL1 films, especially SP1, LP1 and LP3.

Figure 8: Macroscopic change of the various films under the same tensile force
Meanwhile, Fig. 9 shows the tensile strength and elongation at break of the films. Compared with respect to PVA film, adding lignin fiber and alkaline starch under acidic conditions can effectively improve the tensile strength and elongation at break of the film. The SEM images show that the surface of SPL2 film is smooth without cracks. Thus, the tensile strength and elongation at break of SPL2 film reached 3.446 MPa and 193%, respectively, which is even higher than and lignin modified PVA composite film (T.S., 0.36 MPa and E.B., 180%) of Posoknistakul et al. [26]. However, the addition of lignin under alkaline conditions caused a drop in the tensile strength and elongation at break of the film. The tensile strength values of LP1 and LP3 are lower than that of PVA. Besides, the tensile strength of SPL1 film was only 2.0 MPa while the elongation at break was 142%. A large number of cracks appear on the surface of SPL1 (as illustrated from the SEM images in Fig. 5). According to the FTIR and ESI-MS investigations, under alkaline conditions, lignin rarely participates in the reaction of SP system. Its addition increases the rigidity of the film and makes the film rather brittle, which is the reason for the premature failure. Under acidic conditions, more prominent chemical interaction are developed between lignin and SP to form a complicated network structure, which improves the tensile strength and elongation at break of SPL2 film.

Figure 9: Tensile strength and elongation at break of the various films
Fig. 10 shows contact angles of water droplets on PVA, SP1, SP3, LP1, LP3, SPL1 and SPL2 films. Compared with PVA film, adding alkaline starch or a small amount of lignin fiber significantly improves the hydrophobicity of PVA film producing formulations, which is the case for SP1, SP3 and LP1. On the contrary, when the amount of lignin reaches a certain value, it reduces the hydrophobicity of PVA film resulting from the LP3 formulation. Moreover, the contact angle of SPL2 is greater than that of SP1. This corroborates that under alkaline conditions, the intermolecular force between alkaline starch and lignin fiber can be destroyed to form low molecular weight fragments, which can be arranged irregularly during film formation in such a way that more hydroxyl groups became exposed at the interface to interact effectively with water, resulting in low hydrophobicity of SP1 film, while under acidic conditions, due to etherification and crosslinking of alkaline starch, lignin fiber and the low molecular weight fragments of polyvinyl alcohol in SPL2 formulation, more robust network structure is formed, which promotes further the hydrophobicity of SPL2 film.

Figure 10: Contact angle measurements of water droplets on the different films
A bio-based film, based on PVA can be simply fabricated from a resin mixture with suitable viscosity, comprising renewable resources such as alkaline starch and lignin fiber. The reaction between components such as alkaline starch, lignin fiber and polyvinyl alcohol was proved to be more promoted under acidic conditions. Thus, the resulting films exhibited good appearance with no visual cracks or holes, in addition to other interesting features such as high thermal stability and resistance to UV-Vis light. Furthermore, it showed excellent tensile strength and elongation at break. The addition of lignin fiber and alkaline starch effectively improved the hydrophobicity of PVA film. Based on the low cost and green route of fabrication, it is pretty feasible to consider this material as a very promising bio-based film with a great development prospect.
Author Contributions: Formal analysis and writing—original draft, Jun Zhang; supervision, Yunxia zhou, Ai Liu, Chenyu Yang, Defa Hou and Guanben Du; review & editing, Hisham Essawy and Xiaojian Zhou.
Funding Statement: This work was supported by the Yunnan Provincial Natural Science Foundation (Grant No. 202101AT070038), Yunnan Agricultural Joint Fund (202101BD070001-105), China Scholarship Council, and, as well as the Yunnan Provincial Youth Top Talent Project (Grant No. YNWR-QNBJ-2020-166) and Middle-Age Reserve Talents of Academic and Technical Leaders (2019HB026), and the 111 Project (D21027). The authors would like to thank Bo-Chen and Shudi-Ren from Shiyanjia Lab (www.shiyanjia.com) for the partly measurements.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Abdelaziz, M., Ghannam, M. M. (2010). Influence of titanium chloride addition on the optical and dielectric properties of PVA films. Physica B: Condensed Matter, 405(3), 958–964. DOI 10.1016/j.physb.2009.10.030. [Google Scholar] [CrossRef]
2. Božanić, D. K., Dojčilović, R., Pajović, J. D., Tošić, D., Dudić, D. et al. (2022). Fluorescence microscopy and photodielectric characterization studies of the composite films of polyvinyl alcohol and tryptophan functionalized silver nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 634, 128050. DOI 10.1016/j.colsurfa.2021.128050. [Google Scholar] [CrossRef]
3. Zhao, W. L., Zhu, X., He, Z. H., Gao, K. H., Li, Z. Q. (2020). The vortex state in FeSe superconducting thin film. Superconductor Science and Technology, 33(10), 105010. DOI 10.1088/1361-6668/ababeb. [Google Scholar] [CrossRef]
4. Zhong, R., He, Z., Zhang, X., Han, D., Wang, H. et al. (2021). The strategy of modulation blood responses by surface modification with different functional groups on polyester film. Journal of Biomedical Materials Research Part A, 109(10), 1955–1966. DOI 10.1002/jbm.a.37188. [Google Scholar] [CrossRef]
5. Zhang, H., Wang, X., Li, Y., Zuo, K., Lyu, C. (2021). A novel MnOOH coated nylon membrane for efficient removal of 2, 4-dichlorophenol through peroxymonosulfate activation. Journal of Hazardous Materials, 414, 125526. DOI 10.1016/j.jhazmat.2021.125526. [Google Scholar] [CrossRef]
6. Lin, Y., Xie, J., Xiang, Q., Liu, Y., Wang, P. et al. (2022). Effect of propiconazole on plastic film microplastic degradation: Focusing on the change in microplastic morphology and heavy metal distribution. Science of the Total Environment, 822, 153609. DOI 10.1016/j.scitotenv.2022.153609. [Google Scholar] [CrossRef]
7. Kochkina, N. E., Butikova, O. A. (2019). Effect of fibrous TiO2 filler on the structural, mechanical, barrier and optical characteristics of biodegradable maize starch/PVA composite films. International Journal of Biological Macromolecules, 139, 431–439. DOI 10.1016/j.ijbiomac.2019.07.213. [Google Scholar] [CrossRef]
8. Monteiro, V. A. C., da Silva, K. T., da Silva, L. R. R., Mattos, A. L. A., de Freitas, R. M. et al. (2021). Selective acid precipitation of Kraft lignin: A tool for tailored biobased additives for enhancing PVA films properties for packaging applications. Reactive and Functional Polymers, 166, 104980. DOI 10.1016/j.reactfunctpolym.2021.104980. [Google Scholar] [CrossRef]
9. Zanela, J., Bilck, A. P., Casagrande, M., Grossmann, M. V. E., Yamashita, F. (2018). Polyvinyl alcohol (PVA) molecular weight and extrusion temperature in starch/PVA biodegradable sheets. Polímeros, 28, 256–265. DOI 10.1590/0104-1428.03417. [Google Scholar] [CrossRef]
10. Shi, M., Lei, B., Luo, H., Zhang, X. (2017). Structure and properties of carboxymethyl starch/poly (vinyl alcohol) films modified with magnnesium chloride and glycerol. Gaofenzi Cailiao Kexue Yu Gongcheng/Polymeric Materials Science and Engineering, 33(5), 61–65. DOI 10.16865/j.carolcarrollnki.1000-7555.2017.05.010. [Google Scholar] [CrossRef]
11. Mittal, A., Garg, S., Premi, A., Giri, A. S. (2021). Synthesis of polyvinyl alcohol/modified starch-based biodegradable nanocomposite films reinforced with starch nanocrystals for packaging applications. Polymers and Polymer Composites, 29(5), 405–416. DOI 10.1177/0967391120922429. [Google Scholar] [CrossRef]
12. Chen, C., Zong, L., Wang, J., Xie, J. (2021). Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbohydrate Polymers, 272, 118448. DOI 10.1016/j.carbpol.2021.118448. [Google Scholar] [CrossRef]
13. Lee, S. Y., Mohan, D., Kang, I. A., Doh, G. H., Lee, S. et al. (2009). Nanocellulose reinforced PVA composite films: Effects of acid treatment and filler loading. Fibers and Polymers, 10, 77–82. [Google Scholar]
14. Kong, R., Wang, J., Cheng, M., Lu, W., Chen, M. et al. (2020). Development and characterization of corn starch/PVA active films incorporated with carvacrol nanoemulsions. International Journal of Biological Macromolecules, 164, 1631–1639. DOI 10.1016/j.ijbiomac.2020.08.016. [Google Scholar] [CrossRef]
15. Lim, R., Kiew, P. L., Lam, M. K., Yeoh, W. M., Ho, M. Y. (2021). Corn starch/PVA bioplastics—The properties and biodegradability study using Chlorella vulgaris cultivation. Asia-Pacific Journal of Chemical Engineering, 16(3), e2622. DOI 10.1002/apj.2622. [Google Scholar] [CrossRef]
16. Li, H., Yang, J., Feng, X., Qin, Z. (2022). Cellulose nanofiber-assisted dispersion of halloysite nanotubes via silane coupling agent-reinforced starch–PVA biodegradable composite membrane. Membranes, 12(2), 169. DOI 10.3390/membranes12020169. [Google Scholar] [CrossRef]
17. Zong, L., Chen, C., Chen, Z., Xie, J. (2020). Research progress in starch/poly (vinyl alcohol) active packaging film and its application in food packaging. China Plastics, 34(8), 101–112. DOI 10.19491/j.issn.1001-9278.2020.08.016. [Google Scholar] [CrossRef]
18. Korbag, I., Mohamed Saleh, S. (2016). Studies on mechanical and biodegradability properties of PVA/lignin blend films. International Journal of Environmental Studies, 73(1), 18–24. DOI 10.1080/00207233.2015.1082249. [Google Scholar] [CrossRef]
19. Zhang, J., Liu, B., Zhou, Y., Essawy, H., Chen, Q. et al. (2021). Preparation of a starch-based adhesive cross-linked with furfural, furfuryl alcohol and epoxy resin. International Journal of Adhesion and Adhesives, 110, 102958. DOI 10.1016/j.ijadhadh.2021.102958. [Google Scholar] [CrossRef]
20. Cheng, M., Kong, R., Zhang, R., Wang, X., Wang, J. et al. (2021). Effect of glyoxal concentration on the properties of corn starch/poly (vinyl alcohol)/carvacrol nanoemulsion active films. Industrial Crops and Products, 171, 113864. DOI 10.1016/j.indcrop.2021.113864. [Google Scholar] [CrossRef]
21. Wang, X., Bian, H., Ni, S., Sun, S., Jiao, L. et al. (2020). BNNS/PVA bilayer composite film with multiple-improved properties by the synergistic actions of cellulose nanofibrils and lignin nanoparticles. International Journal of Biological Macromolecules, 157, 259–266. DOI 10.1016/j.ijbiomac.2020.04.178. [Google Scholar] [CrossRef]
22. Yang, M., Zhang, X., Guan, S., Dou, Y., Gao, X. (2020). Preparation of lignin containing cellulose nanofibers and its application in PVA nanocomposite films. International Journal of Biological Macromolecules, 158, 1259–1267. DOI 10.1016/j.ijbiomac.2020.05.044. [Google Scholar] [CrossRef]
23. Yang, W., Qi, G., Kenny, J. M., Puglia, D., Ma, P. (2020). Effect of cellulose nanocrystals and lignin nanoparticles on mechanical, antioxidant and water vapour barrier properties of glutaraldehyde crosslinked PVA films. Polymers, 12(6), 1364. DOI 10.3390/polym12061364. [Google Scholar] [CrossRef]
24. Zhang, Y., Remadevi, R., Hinestroza, J. P., Wang, X., Naebe, M. (2020). Transparent ultraviolet (UV)-shielding films made from waste hemp hurd and polyvinyl alcohol (PVA). Polymers, 12(5), 1190. DOI 10.3390/polym12051190. [Google Scholar] [CrossRef]
25. Ling, S. U., Jiang, G., Pang, J., Shi, J. (2018). Light transmittance and gas permeability properties of lignin-based polyelectrolyte reaction film. Gongneng Cailiao/Journal of Functional Materials, 49(6), 06097–06102. DOI 10.3969/j.issn.1001-9731.2018.06.015. [Google Scholar] [CrossRef]
26. Posoknistakul, P., Tangkrakul, C., Chaosuanphae, P., Deepentham, S., Techasawong, W. et al. (2020). Fabrication and characterization of lignin particles and their ultraviolet protection ability in PVA composite film. ACS Omega, 5(33), 20976–20982. DOI 10.1021/acsomega.0c02443. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools