 Open Access
Open Access
ARTICLE
An Evaluation on Physical Characteristics of Konjac Polysaccharides-Based Film Coating and Its Application for Strawberries Preservation
1 Department of Agricultural Industrial Technology, Faculty of Agro-Industrial Technology, Universitas Padjadjaran, Jatinangor, 45363, Indonesia
2 Research Center for Biomass and Bioproducts, National and Innovation Agency, Cibinong, 1691, Indonesia
3 Research Collaboration Center for Biomass and Biorefinery between BRIN and Universitas Padjadjaran, Jatinangor, 45363, Indonesia
4 Research Center for Appropriate Technology, Universitas Padjadjaran, Jatinangor, 45363, Indonesia
5 Department of Food Industrial Technology, Faculty of Agro-Industrial Technology, Universitas Padjadjaran, Jatinangor, 45363, Indonesia
6 Department of Forest Products, Faculty of Forestry and Environment, IPB University, Bogor, 16680, Indonesia
7 Institute of Wood Technology and Renewable Materials, Department of Material Sciences and Process Engineering, University of Natural Resources and Life Sciences, Vienna (BOKU), Vienna, 1180, Austria
* Corresponding Authors: Roni Kastaman. Email: ; Lukmanul Hakim Zaini. Email:
Journal of Renewable Materials 2025, 13(1), 181-197. https://doi.org/10.32604/jrm.2024.056475
Received 23 July 2024; Accepted 10 October 2024; Issue published 20 January 2025
Abstract
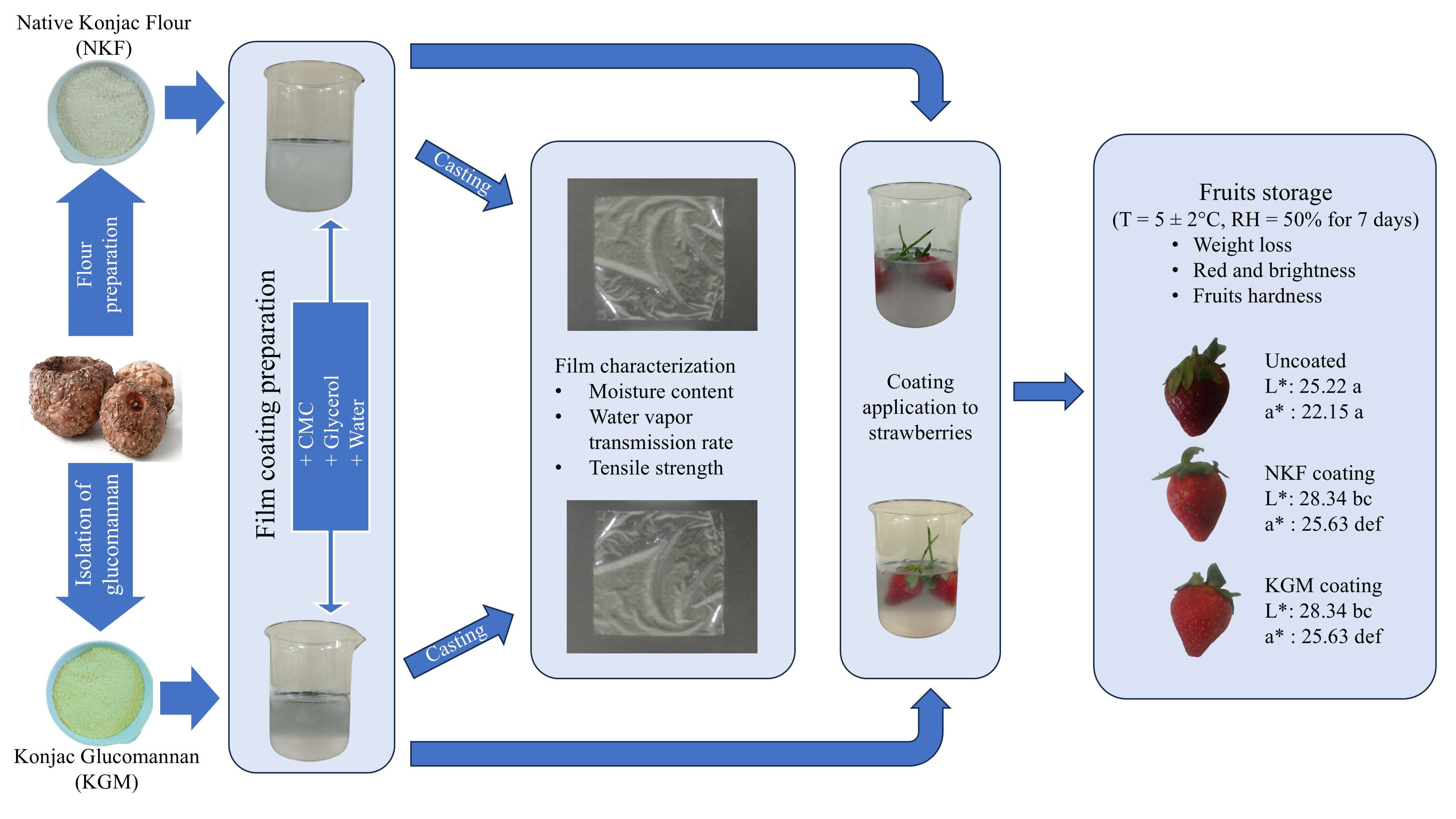
Konjac is an ideal candidate for edible coatings on fruits due to its hydrophilic properties, film-forming ability, barrier properties, safety, and biodegradability. Meanwhile, the high market demand for strawberries necessitates post-harvest treatment to extend their shelf life and preserve their quality, as strawberries are known for their fragile skin and soft texture. To fully utilize konjac and develop high-quality coating films, native konjac flour (NKF) and konjac glucomannan (KGM) were extracted from its corm and used as a coating film for strawberries in the present study. Therefore, this study aimed to compare the physical properties of the film coatings between NKF and KGM, and evaluate their effects on strawberries preservation over 7 days of storage. A multistage extraction process was employed to isolate NKF and KGM, after which the glucomannan content was measured. NKF yield was 31.81%, exceeding KGM yield of 26.42%, and the glucomannan content obtained of NKF (25.93%) was higher than KGM (21.41%). Nuclear magnetic resonance spectroscopy confirmed that both NKF and KGM contain glucomannan in their structure. Furthermore, both NKF and KGM were combined with carboxymethyl cellulose (CMC) and glycerol to produce eight thin-layer films to assess their physical and mechanical properties. Compared to the KGM variant, the NKF variant generally exhibited higher moisture content, water vapor transmission rate, and tensile strength. However, NKF was less effective than KGM in extending strawberry storage life, leading to faster color changes and greater weight loss, despite maintaining similar hardness values. Nonetheless, konjac-based coatings were generally effective at maintaining the freshness and quality of strawberries compared to uncoated samples. Konjac shows promise as an edible coating, improving fresh produce shelf life and appeal, aligning with consumer preferences for natural and sustainable products.Graphic Abstract
Keywords
Cellular respiration and transpiration are critical metabolic processes that greatly influence to fruit freshness and senescence. Respiration can damage the cellular structure of fruit by breaking down sugars as an energy source, a process that is further aggravated by transpiration, which includes cellular stress due to dehydration [1,2]. Managing these two physiological activities is essential to preserving fresh fruit, especially those with a short shelf life, such as strawberries [3]. The extremely thin skin, soft flesh, and high macro- and micro-nutrient content of strawberries contribute to high rates of respiration and transpiration [4–6]. This, in turn, accelerates fruit senescence, making it challenging to maintain strawberries in fresh condition during distribution.
Despite the rapid onset of fruit senescence and physiological deterioration, the market demand for fresh strawberries has continued to rise. Globally, the fresh strawberry market exceeded 14 billion USD in 2020 and is projected to grow by 80% over the next decade [7]. Asian countries are the largest consumers and producers of strawberries [4,8]. In Indonesia specifically, new challenges have emerged in maintaining strawberry freshness due to the tropical climate, which accelerates the physiological activity of this fruit, leading to faster senescence and decay [9,10]. Improper postharvest handling of strawberries has resulted in losses of 10%–20% of total production, amounting to billions of USD each year [11,12]. Addressing this issue is crucial to ensuring strawberries remain fresh and their quality is preserved.
A novel solution that has emerged to address the challenge of strawberry senescence, particularly in tropical countries, is innovation in fruit storage and packaging technology. Previous studies have shown that implementing various traditional and advanced storage techniques remains difficult in Indonesia [13–15]. This has led to the development of packaging technologies, with bio-based film coatings offering a promising alternative. These coatings can be made from natural materials such as polysaccharides, proteins, and lipids and are used to reduce the respiration and transpiration rates of strawberries [16,17]. Additionally, bio-based film coatings help maintain the fruit’s moisture content, slow down the ripening process, and provide protection against mechanical and biological damage, all of which contribute to delaying fruit senescence [18].
Recent studies have shown that polysaccharides are the most commonly used natural materials in bio-based film coatings for strawberries. Some examples include chitosan, cellulose nanofibrils (CNF), alginate, and polylactic acid (PLA). The use of chitosan and CNF can slow oxidation and preserve the vitamin C content in strawberries [19,20]. Meanwhile, the alginate-based film coatings enhance moisture barrier properties, which are associated with reduced fruit weight loss [21,22]. Unlike the other polysaccharides, PLA, a fermented polysaccharide, offers superior moisture resistance and helps maintain the freshness of strawberries [23]. Recent advances have introduced the use of polysaccharides from konjac (Amorphophallus sp.), a plant widely cultivated in Indonesia but underutilized in industry. This has opened up opportunities for utilizing konjac, which is abundant in Indonesia, as a promising polysaccharide for film coating materials that can be applied to strawberries.
Konjac polysaccharides possess unique physicochemical properties that make them ideal for use as bio-based film coatings. Their high gel-forming ability and strong water-binding capacity are particularly advantageous for applications such as strawberry coatings [24]. Previous studies have shown that combining konjac with pullulan significantly reduces weight loss and maintains strawberry color for up to 14 days, outperforming synthetic film-coating materials [25]. Additionally, a film-coating composite based on konjac polysaccharides in nanoparticle form, combined with k-carrageenan, has been proven to enhance the mechanical properties and provide excellent barrier protection for strawberries [26]. Moreover, the combination of konjac polysaccharides with polyvinyl alcohol and citric acid effectively minimized strawberry weight loss during 10 days of storage [27]. Using konjac polysaccharides offers a promising solution for developing environmentally friendly bio-based film coatings that preserve strawberry quality, particularly by reducing color changes, weight loss, and maintaining fruit hardness, as demonstrated in previous studies.
The development of konjac polysaccharide-based films faces several natural limitations that may reduce their effectiveness in preserving strawberry freshness. Konjac polysaccharides tend to be brittle, have relatively low mechanical strength, and are prone to breaking. A ruptured film coating can increase strawberries’ respiration and transpiration rates, accelerating fruit senescence. These issues can be mitigated by incorporating additives such as carboxymethyl cellulose (CMC) and glycerol, commonly used in recent studies [28,29]. Adding CMC enhances flexibility, film strength, and adhesion to the fruit, thereby improving moisture barrier properties. Meanwhile, glycerol acts as a plasticizer in konjac polysaccharide-based films, making the film more elastic and less rigid. Combining these additives with konjac polysaccharides has been shown to produce films with improved mechanical properties, moisture barriers, and biodegradability [30,31]. In this way, the freshness and quality of strawberries can be maintained, helping to minimize post-harvest losses.
Previous studies have demonstrated that konjac polysaccharides have great potential for maintaining the freshness of strawberries. However, the further isolation of konjac polysaccharides to obtain konjac glucomannan (KGM) is time-consuming and costly. As a result, using non-isolated konjac polysaccharides in the form of native konjac flour (NKF) presents a promising alternative for film-coating materials. Additionally, using Indonesian konjac, specifically the species Amorphophallus oncophyllus, offers further potential for this application. Therefore, this study aimed to compare the physical properties of NKF- and KGM-based film coatings and evaluate their effects on the physical quality of strawberries during seven days of storage.
2.1 Materials and Instrumentation
Two main materials used in this study were Java konjac corms (Amorphophallus oncophyllus) purchased from local farmers in Garut, West Java, Indonesia and strawberries (Fragaria x ananassa cv. Mencir, harvested at 60 days) purchased from local farmers in Bandung, West Java, Indonesia). The chemicals used were sodium metabisulfite, ethanol anhydrous, D-glucose, 3,5-dinitrosalicyclic acid, phenol, sodium hydroxyde, potassium bromide, carboxymethyl cellulose and glycerol obtained from Merck (Singapore, Singapore), ethanol anhydrous from Merck (Darmstadt, Germany), pottasium sodium tartrate and deuterium oxide from Scientific Laboratory Supplies (Nottingham, UK), deuterated acetone from Sigma-Aldrich (Singapore, Singapore), technical grade ethanol from Dwilab Mandiri Scientific (Bandung, Indonesia), and distilled water from Laboratory of Postharvest Technology Universitas Padjadjaran (Sumedang, Indonesia).
Several instrumentation have been used consist of Ohauss Mass Balance ME 204 220 g (Shanghai, China), Digital Hotplate Stirrer Thermo Scientific CIMAREC SP88850105 (Waltham, USA), Ball Miller Xmq 150 × 50 JKWKD (Ganzhou, China), Vibration Sieve Shaker VTSS-200-9 (Ahmedabad, India), DLAB Centrifuge T21-M (Beijing, China), IKA Laboratories Rotary Evaporator RV8-V (Selangor, Malaysia), Hywell Tray Dryer CT-C 8419399090 (Changzhou, China), UV-Vis Spectrophotometer Agilent Technologies 8453 (Santa Clara, CA, USA), Fourier-Transform Infrared (FTIR) Shimadzu Prestige-21 (Kyoto, Japan), Nuclear Magnetic Resonance (NMR) Agilent 500 MHz, Universal Testing Machine 100kN Servo Fully Automatic (Jinan, China), SHARP Refrigerant SJ-236MG-GB/GR (Karawang, Indonesia), Minolta Chromameter CR-310 (Ramsey, MN, USA), and Fruit Penetrometer GY-2 (Jakarta, Indonesia).
2.2 Konjac Polysaccharides Preparation
2.2.1 Native Konjac Flour (NKF) Preparation
The konjac corms were washed, cleaned, manually sliced to a thickness of approximately 2–3 mm, and soaked in 1% sodium metabisulfite (wt.%) solution. Konjac chips were dried at 120°C for 40–60 min and milled into a powder. Konjac powder was sieved through a 40-mesh sieve to obtain native konjac flour (NKF). The yield and moisture content were subsequently analyzed using standard methods from the AOAC [32].
2.2.2 Konjac Glucomannan (KGM) Preaparation
The glucomannan isolation stage followed a previous study with slight modification [33]. NKF was soaked in 200 mL of 70% ethanol and stirred at 200 rpm for 90 min. The solution was then centrifuged (5000× g, 30 min) to separate the precipitate from the ethanol. The precipitate was diluted in 200 mL of distilled water and stirred at 200 rpm for 3 h. After further dilution to 400 mL with distilled water, the solution was centrifuged (9000× g, 30 min) to remove the insoluble material. The supernatant was evaporated until 1/3 of its initial volume, then precipitated with 95% ethanol and centrifuged (9000× g, 40 min) to obtain glucomannan mucilage. The mucilage was washed with anhydrous ethanol, vacuum filtered, and dried at 80°C for 3 h before milling into konjac glucomannan (KGM). The yield and moisture content were subsequently analyzed using standard methods from the AOAC [32].
2.2.3 Glucomannan Content Quantification
Glucomannan content was measured using the 3,5-dinitrosalicyclic acid (DNS) colorimetric assay [34]. A calibration curve was constructed using a standard solution of D-glucose (16–80 μg/mL) and providing the equation y = 0.015x + 0.006 (R2 = 0.998). NKF and KGM (50 mg) were soaked in 40 mL of distilled water and stirred at 150 rpm for 4 h before being diluted to a final volume of 50 mL. The solution was centrifuged (4000 × g, 40 min) to separate the supernatant. A 3 mL aliquot of the supernatant was pipetted and diluted with 47 mL of distilled water to prepare the sample solution. The sample was quickly mixed with 5 mL of 1% phenol (wt.%) and 5 mL of sulfuric acid, and kept at room temperature for 10 min to prepare the sample hydrolysate. The sample was then incubated at room temperature for 20 min and measured using a UV-Vis spectrophotometer at 490 nm. The glucomannan content was calculated using Eq. (1).
where f = correction factor; T = glucose content of sample hydrolysate (mg); T0 = glucose content of sample solution (mg); m = weight of sample (mg).
2.2.4 Fourier-Transform Infrared (FTIR)
NKF and KGM were analyzed by Fourier infrared (FTIR) spectroscopy to determine their functional groups and chemical structures. The FTIR analysis was conducted with a Shimadzu Prestige-21 (4000 to 400 cm−1), employing the potassium bromide pellet technique to identify the presence of functional groups in the samples.
2.2.5 Nuclear Magnetic Resonance (NMR)
NKF and KGM samples were dissolved in 0.5 N sodium hydroxide/deuterium oxide at a 5–20 g/L concentration at 303 K. The stock sample solutions were then injected into the Nuclear Magnetic Resonance (NMR) spectrometer (Agilent 500 MHz) with a console system, operating at frequencies of 500 MHz (1H) and 125 MHz (13C), using deuterated acetone as the solvent.
2.3 Konjac-Based Film Coatings
2.3.1 Film Coating Preparation
Film coating preparation followed a procedure adapted from a prior study, as illustrated in Fig. 1 [35]. Either extracted NKF or KGM was combined with carboxymethyl cellulose (CMC) and glycerol to produce eight thin-layer thin films, which were then assessed for their physical and mechanical properties. NKF and KGM were dissolved in distilled water at concentrations of 0.4%, 0.6%, 0.8%, and 1% (wt.%) and stirred at 200 rpm until temperature reached 60°C. The solution was mixed with 50 mL of 0.3% CMC (wt.%) and stirred at 200 rpm until it reached 100°C. Next, 1 mL of glycerin was added, and the mixture was stirred for 90 min until the bubbles disappeared. After cooling to 60°C, the solution was poured onto a 16 × 16 cm glass plate. The resulting film was air dried at room temperature for 24 h (average RH 80%) before moisture content analysis (AOAC standard methods) and further characterization.

Figure 1: NKF- and KGM-based film preparation steps
2.3.2 Water Vapor Transmission Rate (WVTR) Determination
The gravimetric method was used to determine the moisture barrier properties of the konjac polysaccharide-based film coatings, following the ASTM F1249-20 procedure [36]. The coating film samples were placed in constant humidity cups within a desiccator (at room temperature and RH 80%) for 5 days. The samples were weighed daily to measure the increase in weight due to water vapor absorption from the environment. The measurement results were calculated using Eq. (2), and plotted as a time function to obtain each sample’s WVTR value, expressed in g/m2/24 h.
where mn = weight of film coating in day of n (g); m0 = weight of film coating on initial day (g); t = time (day); A = film coating areas (m2).
2.3.3 Mechanical Properties of Film Coatings
The procedure for determining the tensile strength of the coating film, as one of its mechanical properties, followed the standard procedure outlined in the Universal Testing Machine instrument manual. The film sample was clamped at both ends, and a gradually increasing load was applied to one end of the film until it ruptured.
2.4 Application of Konjac-Based Film Coatings on Strawberries
2.4.1 Strawberries Coating Application
Fruit coating applications were performed using film coating, which performed best based on characterization results. Strawberries were dipped in the film solution for 10 s and then dried in an air-conditioned room at 25°C–26°C [37]. The coated strawberries were stored in a refrigerator (T = 5 ± 2°C and RH = 50%) for 7 days, and their physical quality was evaluated daily.
2.4.2 Color Changes Determination
Color changes in strawberries were measured using a Minolta Chromameter CR-310. Samples were prepared and placed into the instrument measuring chamber, which assessed the brightness (L*) and red-green color (a*) parameters.
2.4.3 Weight Loss Determination
Weight loss was assessed by recording the weight of strawberries on the first day of storage and then daily for 7 consecutive days [38]. The measurement results were calculated using Eq. (3).
where m0 = initial weight of sample (g); mn = weight of sample on day-n.
2.4.4 Fruit Hardness Determination
The fruit hardness test was conducted according to the procedure outlined in the fruit hardness tester manual. The strawberry sample was pressed with a needle for 5 s. Measurements were taken at three different points on each strawberry, and the results were expressed in Pascals (Pa).
All sample measured in this study were replicated in three times. The collected data were analyzed using the independent t-test (p < 0.05) and analysis of variances (ANOVA), followed by the Duncan Multiple Range Test (p < 0.05).
3.1 Konjac Polysaccharides Characteristics
The extraction of 1 kg of Konjac yielded NKF at 31.81 ± 0.24%. Further purification of glucomannan from NKF resulted in 26.42% ± 0.53% KGM. This result was higher compared to an earlier study, which reported 18.05% KGM [39]. The moisture content for both NKF and KGM was similar (10.91 ± 0.97% and 10.67 ± 0.23%, respectively), and lower than maximum value of 13% allowed by Indonesian National Standard [40]. Despite using a simple isolation method, the moisture level of KGM in this study was lower than that of various glucomannan isolation procedures, which range from 11.3 to 12.9% [41]. As shown in Table 1, the glucomannan content of NKF reached 25.93 ± 0.68%, while KGM exhibited a lower content of 21.41 ± 0.03% (on a wet basis). Adjusting the stirring duration during the isolation process could further increase glucomannan content [42]. Moreover, several factors such as isolation time and ethanol concentration can the glucomannan percentage. This highlights the potential for further research, including optimizing these parameteres or utilizing modern techniques such as employing microwave-assisted extraction [43].

FTIR spectroscopy was employed to observe the characteristics of both NKF and KGM. The IR spectra are shown in in Fig. 2, with detailed values presented in Table 2. A large peak at 3415 cm−1 for NKF and 3404 cm−1 for KGM indicates O–H stretching in glucomannan [44]. The peak at 2926–2927 cm−1 corresponds to the stretching vibration of –CH2–. Two notable peaks at around 1740 and 1630 cm−1 correspond to C=O stretching in acetyl and amide groups, respectively [45,46]. The intensity at 1741 cm−1 was higher in NKF than KGM, suggesting a greater amount of acetyl groups in NKF [47]. Similarly, the peak intensity at 1643 cm−1 was observed to be higher in NKF than KGM, indicating a higher protein content in NKF (amide I peak) [48]. The peak at 1640 cm−1 revealed the presence of functional molecules in protein amide groups (–CONH–), associated with carbonyl (C=O) stretch vibration. The greater intesinty of peaks in NKF suggests that the protein contains multiple overlapping secondary structures of the polypeptide chain. The wave number 1381–1384 cm−1 is associated with C–H bending, and a peak at 1060 cm−1 in NKF indicates the presence of similar groups not found in KGM. C–O–C stretching was observed at wave numbers 1155–1153, 1060, and 1026 cm−1. A peak at 2926–2927 cm−1 suggests the presence of C=O group stretching, with the intensity decreasing due to reduced calcium oxalate content from NKF to KGM [43]. Additionally, the FTIR results identified β-pyranose structures, consisting of mannose and glucose, at wave numbers 875–802 cm−1 [46].

Figure 2: Comparison between the FTIR spectra of NKF and KGM

The shift in the 1H-NMR spectrum indicated structural changes based on the resonance signals emitted by the anomeric hydrogen, as shown in Fig. 3. Due to the complexity of the polysaccharide structure, the hydrogen bonds at C-2 to C-6 in glucomannan, composed of glucose and mannose, could not be distinctly separated. However, the signals for the anomeric hydrogen at C-1 from glucose (4.37 ppm) and mannose (4.68 ppm) were distinguishable, allowing for precisely determining the chemical shift. NKF exhibited a shift (δ) of 4.37 ppm for glucose and 4.61 for mannose, while KGM showed a shift (δ) of 4.38 and 4.68 ppm for glucose and mannose, respectively.

Figure 3: Comparison between 1H-NMR spectra of NKF and KGM
Fig. 4 compares 13C-NMR spectra of NKF and KGM. Anomeric signals were detected at 102.89–102.85 ppm for C-1 resonance of D-glucose and 100.13 ppm for D-mannose. The C-4 shift corresponding to the glucosyl and mannosyl units involved in the glycosidic bond was observed at 78.43–78.35 ppm. The resonance signals for mannose at C-5, C-3, and C-2 appeared at 76.25, 75.25, and 71.61 ppm, respectively. The C-5, C-3, and C-2 shifts for glucose were 76.31–76.26 ppm, 75.27, and 70.23 ppm, respectively. The signals at 60.56–60.54 ppm and 60.52 ppm indicated the C-6 resonance of unsubstituted glucose from the glucosyl and mannosyl.

Figure 4: Comparison between 13C-NMR spectra of NKF and KGM
Based on these results, it can be confirmed that glucomannan has a linear structure composed of 1,4-D-mannosyl and D-glucosyl bonds. The lower field shift observed for the C-1 anomer, which is the only carbon directly bonded to two oxygen atoms, indicates it is more deshielded compared to the other five carbons [49]. The C-4 carbon, involved in the glycosidic bond, exhibited a chemical shift of 78.00–76.30 ppm. Meanwhile, the chemical shifts for C-2, C-3, and C-5 occurred in the upfield region, likely due to the influence of the hydroxyl groups. For C-6, carbon showed a more shielded chemical shift, as no substitution occurred at this position.
3.2 Konjac Polysaccharides-Based Film Coating Performances
The moisture content in edible films plays crucial role in determining the physical properties of food packaging, particularly due to its impact on the activity of microorganisms. Good-quality edible films should have a low moisture content [50]. The results of this study revealed that increasing the amounts of NKF and KGM led to a reduction in moisture content, as depicted in Fig. 5a. This can be attributed to the higher total solid concentrations at elevated levels of NKF and KGM, which results in reduced water content. Consequently, the water content of the edible film decreases after the drying process. Given this, the lower moisture content observed in these films is an indictor of their improved quality [51].
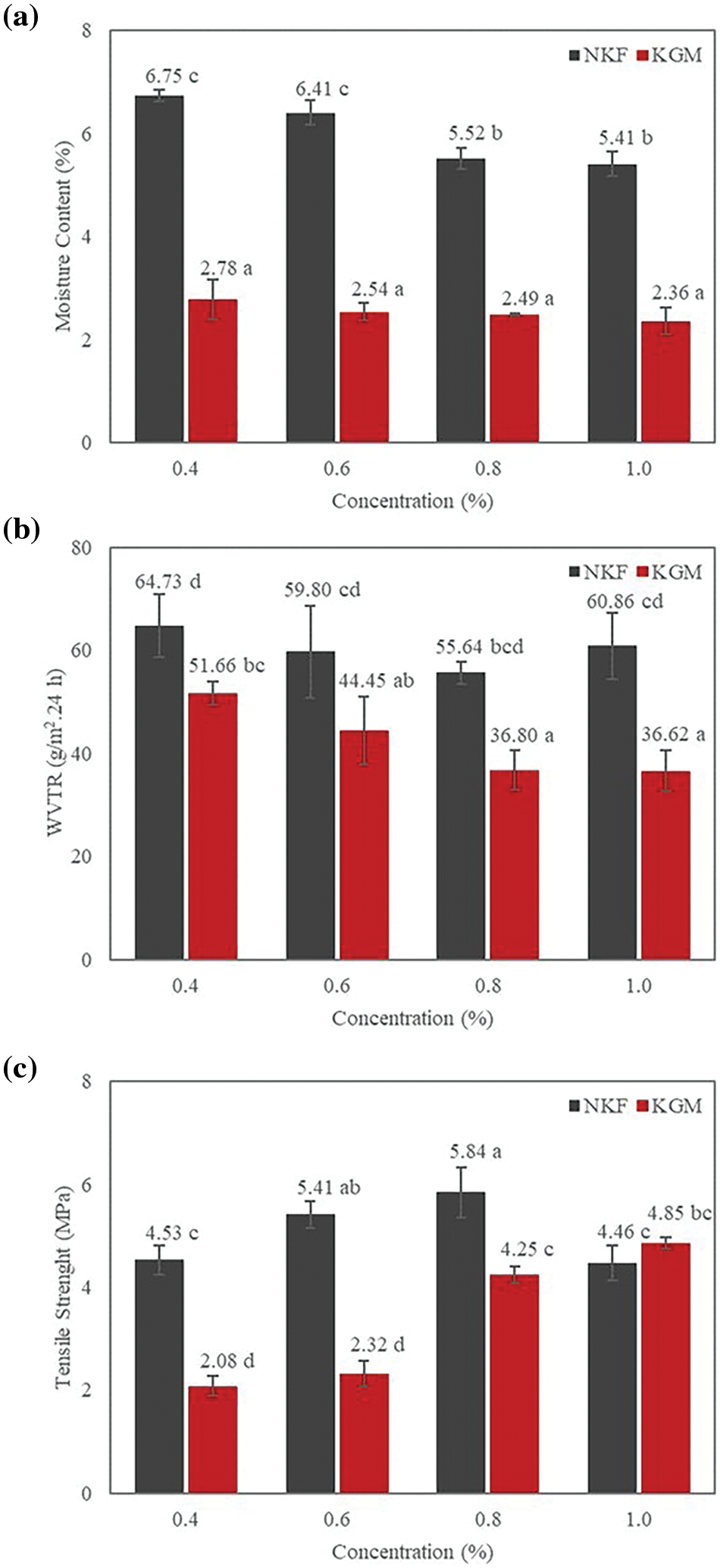
Figure 5: Physical and mechanical properties of konjac based coatings, (a) moisture content, (b) water vapor transimission rate (WVTR), and (c) tensile strength. Different symbols indicate significant differences as determined by the Duncan Multiple Range Test (p < 0.05)
While moisture content indicates the amout of water bound within films, WVTR reflects the amount of water vapor that can pass through a film per unit area over a specific time. WVTR is a critical parameter for assessing the permeability of films to water vapor [52]. As shown in Fig. 5b, increasing the concentrations of NKF and KGM led to a corresponding decrease in WVTR. This reduction can be attributed to the addition of glycerol, which acts as a lubricating agent that minimizes gaps and pores within the film’s matrix, thereby limiting the absorption of water vapor [53]. Furthermore, glycerol disrupts the hydrogen bond interactions between glucomannan and CMC, reducing the water uptake capacity of the film by weakening the hydrogen bonding network between glucomannan, CMC, and glycerol [54].
Fig. 5c illustrates the tensile strength of films with varying concentrations of NKF and KGM. The analysis reveals that increasing the amount of glucomannan flour results in higher tensile strength. This enhancement in tensile strength is attributed to the interaction between hydrogen bonds formed by glucomannan and CMC molecules. As the concentration of glucomannan rises, more hydrogen bonds are established within the polymer chains, strengthening the intermolecular forces. Consequently, the increased hydrogen bonding leads to stronger fil structures, thus raising the tensile strength values [55].
Based on the physical and mechanical characteristics of the films, the optimal formulation was achieved with 0.8% (wt.%) NKF and 1% (wt.%) KGM. These concentrations were selected as the best-performing formulations for film coatings. Both NKF and KGM-based films were then applied to strawberries, and their effectiveness was evaluated according to the predetermined parameters.
3.3 Film Coating Application on Strawberries
Color is a critical parameter for assessing fruit quaility, particularly for strawberries, as their color changes during storage [56]. The brightness (L*) and red color (a*) of uncoated, NKF-coated, and KGM-coated strawberries were measured over 7 days at a temperature of 5 ± 2°C. As shown in Fig. 6, uncoated strawberries began to deteriorate significantly after just one day. In contrast, strawberries coated with NKF or KGM maintained their color better, with noticeable quality decline occuring after 3 and 4 days of storage, respectively.

Figure 6: Brightness (L*) and red color (a*) changes on strawberries during storage. Different symbols indicate significant differences as determined by the Duncan Multiple Range Test (p < 0.05)
Color change in strawberries is primarily driven by a decrease in pigment content. The bright red color of strawberries results from high anthocyanin content and the degradation of chlorophyll [57]. This red coloration serves as an indicator of fruit maturity and senescence. However, anthocyanins are sensitive to pH changes during storage, leading to reduced sour taste and gradual loss of red color due to compound degradation [58]. The NKF- and KGM-based strawberry coatings, which incorporate glucomannan, CMC, and glycerol, effectively preserve fruit brightness. This preservation is attributed to the coating’ ability to shield the fruit from external damage, suppress oxidation, and maintain surface moisture, thereby slowing down senescence and keeping the fruit looking fresh [28,59]. Additionally, previous studies have shown that polysaccharide-based coatings with added fat components can better maintain fruit quality compared to those using polysaccharides alone [60].
A further variable considered during strawberry storage was fruit weight loss, as shown in Table 3. The weight loss of the uncoated strawberries was significantly lower compared to that of coated strawberries, demonstrating that coated fruits, particularly strawberries, can better maintain quality by delaying senescence. However, the NKF-based strawberry coating was less effective than the KGM-based coating in preserving fruit quality. This can be attributed to the presence of other starch substances in NKF, which are more susceptible to degradation by water vapor in the environment. Additionally, inclusion of CMC to the coating system, due to its hydrophilic properties, allows more water vapor to migrate [61]. The high content of hydrophilic materials, such as starch and CMC, facilitates water vapor migration and suggests the need for further evaluation in future studies [62].

Table 4 presents the results of fruit hardness testing, which initially ranged from 0.79–0.80 Pa. Both NKF- and KGM-based coatings were effective in maintaining fruit hardness better than uncoated strawberries, although the differences were not statistically significant. Uncoated strawberries experienced a significant decline in hardness, reaching a minimum value of 0.37 ± 0.09 Pa by the seventh day of storage, representing a decrease of over 50%. This decline is attrivuted to catabolic reactions that soften the fruit tissue, particularly the breakdown of pectin due to natural enzymes such as pectin esterase [63]. Pectin, which acts as an adhesive in cell walls, is weakened, leading to reduced fruit hardness. In contrast, coated strawberries better retained their quality because the coatings impact respiration and transpiration. Coating fruits can inhibit respiration and reduce the formation of natural enzymes that break down polysaccharides, while also lowering the rate of transpiration, which is directly related to the fruit’s water content [64].

Native flour (NKF) and glucomannan (KGM) were successfully extracted from konjac and used to coat strawberries. The yields of NKF and KGM were 31.81 ± 0.24% and 26.4 ± 0.53%, respectively, with their moisture content exceeding the Indonesian National Standard. The glucomannan content was up to 25.93 ± 0.68% in NKF and 21.41 ± 0.03% in KGM. Based on physical and mechanical properties, the best performances of NKF and KGM in film formulations were 0.8% and 1%, respectively. Both NKF- and KGM-based coatings effectively maintained the strawberries’ color, reduced weight loss, and preserved fruit hardness. These findings suggest that such coatings can extend the shelf life and preserve the quality of strawberries during transportation. Further study is needed to explore the effectiveness of these coatings with other additives and across different strawberry varieties.
Acknowledgement: We would like to acknowledge to Laboratory of Chemical and Agro-Industrial Process Technology, Faculty of Agro-Industrial Technology, Universitas Padjadjaran for their facilities; Research Center for Biomass and Bioproducts, National Research and Innovation Agency (BRIN) for the provision; and the farmers in Garut and Bandung, who provided the konjac corms and strawberries for this study.
Funding Statement: This study was funded by the Academic Leadership Grant of Universitas Padjadjaran, Bandung, Indonesia, with grant number 1540/UN6.3.1/PT.00/2024, and the Research Collaboration Center for Biomass and Biorefinery, Bandung, Indonesia, with grant number B-1723/II.7/HK.01.00/4/2024.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Desy Nurliasari, Roni Kastaman, Mohamad Djali, Efri Mardawati, and Lukmanul Hakim Zaini; data collection: Desy Nurliasari, Awaly Ilham Dewantoro, and Devi Maulida Rahmah; analysis and intepretation of results: Desy Nurliasari, Roni Kastaman, Muhammad Adly Rahandi Lubis, Devi Maulida Rahmah, Siti Nurhasanah, Akbar Hanif Dawam Abdullah, and Lukmanul Hakim Zaini; draft manuscript preparation: Desy Nurliasari, Awaly Ilham Dewantoro, Muhammad Adly Rahandi Lubis, Roni Kastaman, and Devi Maulida Rahmah. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data presented in this study are available on request from the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Brizzolara S, Manganaris GA, Fotopoulos V, Watkins CB, Tonutti P. Primary metabolism in fresh fruits during storage. Front Plant Sci. 2020;11:1–16. [Google Scholar]
2. Hou X, Li H, Zhang W, Yao Z, Wang Y, Du T. Water transport in fleshy fruits: research advances, methodologies, and future directions. Physiol Plant. 2021;172(4):2203–16. doi:10.1111/ppl.v172.4. [Google Scholar] [CrossRef]
3. Ansarifar E, Moradinezhad F. Preservation of strawberry fruit quality via the use of active packaging with encapsulated thyme essential oil in zein nanofiber film. Int J Food Sci Technol. 2021;56(9):4239–47. doi:10.1111/ijfs.v56.9. [Google Scholar] [CrossRef]
4. Ikegaya A, Ohba S, Toyoizumi T, Arai E. Quality evaluation of strawberries grown in various regions by Singaporeans and Japanese. Int J Fruit Sci. 2021;21(1):883–95. doi:10.1080/15538362.2021.1939832. [Google Scholar] [CrossRef]
5. Yahia EM, Gardea-Béjar A, de Ornelas-Paz JJ, Maya-Meraz IO, Rodríguez-Roque MJ, Rios-Velasco C, et al. Preharvest factors affecting postharvest quality. In: Yahia EM, editor. Postharvest technology of perishable horticultural commodities. Cambridge: Woodhead Publishing; 2019. p. 99–128. [Google Scholar]
6. Kwak H, Shin S, Kim J, Kim J, Lee D, Lee H, et al. Protective coating of strawberries with cellulose nanofibers. Carbohydr Polym. 2021;258:117688. doi:10.1016/j.carbpol.2021.117688. [Google Scholar] [PubMed] [CrossRef]
7. Hernández-Martínez NR, Blanchard C, Wells D, Salazar-Gutiérrez MR. Current state and future perspectives of commercial strawberry production: a review. Sci Hortic. 2023;312:111893. doi:10.1016/j.scienta.2023.111893. [Google Scholar] [CrossRef]
8. Dwiastuti ME, Soesanto L, Aji TG, Devy NF, Hardiyanto. Biological control strategy for postharvest diseases of citrus, apples, grapes and strawberries fruits and application in Indonesia. Egypt J Biol Pest Control. 2021;31(1):1–12. doi:10.1186/s41938-021-00488-1. [Google Scholar] [CrossRef]
9. Iturralde-García RD, Cinco-Moroyoqui FJ, Martínez-Cruz O, Ruiz-Cruz S, Wong-Corral FJ, Borboa-Flores J, et al. Emerging technologies for prolonging fresh-cut fruits’ quality and safety during storage. Horticulturae. 2022;8(8):731–29. doi:10.3390/horticulturae8080731. [Google Scholar] [CrossRef]
10. Iswahyudi I, Darmawati E, Mardjan S, Garfansa MP. Color and firmness quality changes of java apple during postharvest transportation and storage. Curr Appl Sci Technol. 2024;24(4):1–19. [Google Scholar]
11. Hussein Z, Fawole OA, Opara UL. Harvest and postharvest factors affecting bruise damage of fresh fruits. Hortic Plant J. 2020;6(1):1–13. doi:10.1016/j.hpj.2019.07.006. [Google Scholar] [CrossRef]
12. Guthman J, Jiménez-Soto E. Socioeconomic challenges of california strawberry production and disease resistant cultivars. Front Sustain Food Syst. 2021;5:764743. doi:10.3389/fsufs.2021.764743. [Google Scholar] [CrossRef]
13. Harmayani E, Anal AK, Wichienchot S, Bhat R, Gardjito M, Santoso U, et al. Healthy food traditions of Asia: exploratory case studies from Indonesia, Thailand, Malaysia, and Nepal. J Ethn Foods. 2019;6(1):1–18. doi:10.1186/s42779-019-0002-x. [Google Scholar] [CrossRef]
14. Achmad B, Sanudin B, Siarudin M, Widiyanto A, Diniyati D, Sudomo A, et al. Traditional subsistence farming of smallholder agroforestry systems in Indonesia: a review. Sustainability. 2022;14(14):8631–33. doi:10.3390/su14148631. [Google Scholar] [CrossRef]
15. Munir A, Fadhilah. Climate change and food insecurities: the importance of food loss and waste reduction in Indonesia. IOP Conf Ser: Earth Environ Sci. 2023;1134(1):012040. doi:10.1088/1755-1315/1134/1/012040. [Google Scholar] [CrossRef]
16. Iñiguez-moreno M, Ragazzo-Sánchez JA, Calderón-Santoyo M. An extensive review of natural polymers used as coatings for postharvest shelf-life extension: trends and challenges. Polymers. 2021;13:3271. doi:10.3390/polym13193271. [Google Scholar] [PubMed] [CrossRef]
17. Ungureanu C, Tihan G, Zgârian R, Pandelea G. Bio-coatings for preservation of fresh fruits and vegetables. Coatings. 2023;13(8):1420. doi:10.3390/coatings13081420. [Google Scholar] [CrossRef]
18. Amin U, Khan MU, Majeed Y, Rebezov M, Khayrullin M, Bobkova E, et al. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int J Biol Macromol. 2021;183(1):2184–98. doi:10.1016/j.ijbiomac.2021.05.182. [Google Scholar] [PubMed] [CrossRef]
19. Resende NS, Gonçalves GAS, Reis KC, Tonoli GHD, Boas EVBV. Chitosan/Cellulose nanofibril nanocomposite and its effect on quality of coated strawberries. J Food Qual. 2018;2018(1):1–13. doi:10.1155/2018/1727426. [Google Scholar] [CrossRef]
20. Jafarzadeh S, Nafchi AM, Salehabadi A, Oladzad-abbasabadi N, Jafari SM. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv Colloid Interface Sci. 2021;291:102405. doi:10.1016/j.cis.2021.102405. [Google Scholar] [PubMed] [CrossRef]
21. Zhang X, Zhang R, Zhao S, Wang T, Zhang B, Zhao H. Development, characterization and functional properties of sodium alginate-based films incorporated with Schisandra chinensis extract-natamycin complex. Int J Biol Macromol. 2023;253:127435. doi:10.1016/j.ijbiomac.2023.127435. [Google Scholar] [PubMed] [CrossRef]
22. Li Y, Wu Y, Li C. Development of CO2-sensitive antimicrobial bilayer films based on gellan gum and sodium alginate/sodium carboxymethyl cellulose and its application in strawberries. Int J Biol Macromol. 2024;264(P2):130572. [Google Scholar] [PubMed]
23. da Silva LRC, Rios A de O, Santana RMC. Polymer blends of poly(lactic acid) and starch for the production of films applied in food packaging: a brief review. Polym Renew Resour. 2023;14(2):108–53. [Google Scholar]
24. Sun Y, Xu X, Wu Z, Zhou H, Xie X, Zhang Q, et al. Structure, merits, gel formation, gel preparation and functions of konjac glucomannan and its application in aquatic food preservation. Foods. 2023;12(6):1215–8. doi:10.3390/foods12061215. [Google Scholar] [PubMed] [CrossRef]
25. Yan Y, Duan S, Zhang H, Liu Y, Li C, Hu B, et al. Preparation and characterization of Konjac glucomannan and pullulan composite films for strawberry preservation. Carbohydr Polym. 2020;243:116446–9. doi:10.1016/j.carbpol.2020.116446. [Google Scholar] [PubMed] [CrossRef]
26. Duan N, Li Q, Meng X, Wang Z, Wu S. Preparation and characterization of k-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 2021;364:130441–9. doi:10.1016/j.foodchem.2021.130441. [Google Scholar] [PubMed] [CrossRef]
27. Li K, Li Y, Jin H, Feng B, Jiang G. Konjac glucomannan/polyvinyl alcohol/citric acid-based active food-packaging films containing Polygonatum sibiricum polysaccharide. Food Chem Adv. 2024;4:100660. doi:10.1016/j.focha.2024.100660. [Google Scholar] [CrossRef]
28. Armghan Khalid M, Niaz B, Saeed F, Afzaal M, Islam F, Hussain M, et al. Edible coatings for enhancing safety and quality attributes of fresh produce: a comprehensive review. Int J Food Prop. 2022;25(1):1817–47. doi:10.1080/10942912.2022.2107005. [Google Scholar] [CrossRef]
29. Liyanapathiranage A, Dassanayake RS, Gamage A, Karri RR, Manamperi A, Evon P, et al. Recent developments in edible films and coatings for fruits and vegetables. Coatings. 2023;13(7):1–34. [Google Scholar]
30. You P, Wang L, Zhou N, Yang Y, Pang J. A pH-intelligent response fish packaging film: konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int J Biol Macromol. 2022;204:386–96. doi:10.1016/j.ijbiomac.2022.02.027. [Google Scholar] [PubMed] [CrossRef]
31. Ganesan AR, Shanmugam M, Ilansuriyan P, Anandhakumar R, Balasubramanian B. Composite film for edible oil packaging from carrageenan derivative and konjac glucomannan: application and quality evaluation. Polym Test. 2019;78:105936. doi:10.1016/j.polymertesting.2019.105936. [Google Scholar] [CrossRef]
32. AOAC. Determination of moisture, ash protein and fat. In: Official method of analysis of the association of analytical chemists. 18th edWashington, DC, USA: AOAC; 2005. [Google Scholar]
33. Nurlela N, Ariesta N, Laksono DS, Santosa E, Muhandri T. Characterization of glucomannan extracted from fresh porang tubers using ethanol technical grade. Molekul. 2021;16(1):1–8. doi:10.20884/1.jm.2021.16.1.632. [Google Scholar] [CrossRef]
34. Chua M, Chan K, Hocking TJ, Williams PA, Perry CJ, Baldwin TC. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr Polym. 2012;87(3):2202–10. doi:10.1016/j.carbpol.2011.10.053. [Google Scholar] [CrossRef]
35. Li C, Wu K, Su Y, Riffat SB, Ni X, Jiang F. Effect of drying temperature on structural and thermomechanical properties of konjac glucomannan-zein blend films. Int J Biol Macromol. 2019;138(1):135–43. doi:10.1016/j.ijbiomac.2019.07.007. [Google Scholar] [PubMed] [CrossRef]
36. ASTM. ASTM F1249-20: standard test method for water vapor transmission rate through plastic film and sheeting using a modulated infrared sensor; 2020. Available from: https://www.astm.org/f1249-20.html. [Accessed 2024]. [Google Scholar]
37. Wibowo C, Wicaksono R, Haryanti P, Irawan DM, Sulistyo SB, Fatoni A. Application of starch-based edible coating on tomato and its effect during storage. IOP Conf Ser: Earth Environ Sci. 2023;1155:012014. doi:10.1088/1755-1315/1155/1/012014. [Google Scholar] [CrossRef]
38. Muley AB, Kedia P, Pegu K, Kausley SB, Rai B. Analyzing the physical and biochemical changes in strawberries during storage at different temperatures and the development of kinetic models. J Food Meas Charact. 2022;16(1):222–47. doi:10.1007/s11694-021-01146-8. [Google Scholar] [CrossRef]
39. Harmayani E, Aprilia V, Marsono Y. Characterization of glucomannan from Amorphophallus oncophyllus and its prebiotic activity in vivo. Carbohydr Polym. 2014;112:475–9. doi:10.1016/j.carbpol.2014.06.019. [Google Scholar] [PubMed] [CrossRef]
40. Budiastra IW, Noviyanti AA. Determination of chemical content of porang flour (Amorphophallus muelleri blume) by near infrared spectroscopy. IOP Conf Ser: Earth Environ Sci. 2023;1187(1):1–8. [Google Scholar]
41. Zainuri, Sukmawaty, Basuki E, Handayani BR, Sulastri Y, Paramartha DNA, et al. Optimization process to increase the quality of lombok porang flour. IOP Conf Ser: Earth Environ Sci. 2021;913(1):012037. doi:10.1088/1755-1315/913/1/012037. [Google Scholar] [CrossRef]
42. Xu W, Wang S, Ye T, Jin W, Liu J, Lei J, et al. A simple and feasible approach to purify konjac glucomannan from konjac flour–temperature effect. Food Chem. 2014;158:171–6. doi:10.1016/j.foodchem.2014.02.093. [Google Scholar] [PubMed] [CrossRef]
43. Azhar B, Gunawan S, Febriana Setyadi ER, Majidah L, Taufany F, Atmaja L, et al. Purification and separation of glucomannan from porang tuber flour (Amorphophallus muelleri) using microwave assisted extraction as an innovative gelatine substituent. Heliyon. 2023;9(11):e21972. doi:10.1016/j.heliyon.2023.e21972. [Google Scholar] [PubMed] [CrossRef]
44. Azhar B, Angkawijaya AE, Santoso SP, Gunarto C, Ayucitra A, Go AW, et al. Aqueous synthesis of highly adsorptive copper-gallic acid metal-organic framework. Sci Rep. 2020;10(1):1–12. [Google Scholar]
45. Rahayu I, Wahyuningtyas I, Zaini L, Darmawan W, Maddu A, Prihatini A. Physical properties of impregnated ganitri wood by furfuryl alcohol and nano-SiO2. IOP Conf Ser: Earth Environ Sci. 2021;891(1):012012. [Google Scholar]
46. da Silva D Felix, Ogawa CYL, Sato F, Neto AM, Larsen FH, Matumoto-Pintro PT. Chemical and physical characterization of Konjac glucomannan-based powders by FTIR and 13C MAS NMR. Powder Technol. 2020;361:610–6. doi:10.1016/j.powtec.2019.11.071. [Google Scholar] [CrossRef]
47. Du X, Li J, Chen J, Li B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res Int. 2012;46(1):270–8. doi:10.1016/j.foodres.2011.12.015. [Google Scholar] [CrossRef]
48. Widjanarko SB, Nugroho A, Estiasih T. Functional intercation components of protein isolates and glucomannan in food bars by FTIR and SEM studies. Afr J Food Sci. 2011;5(1):12–21. [Google Scholar]
49. Nguyen AT, Hoa NT, Thien DT, Hue VT, Minh Thu VT, Thu Trang LT. Characterization of glucomannan from amorphophallus panomensis in Vietnam. Vietnam J Sci Technol. 2016;54(2):224. doi:10.15625/0866-708X/54/2/6384. [Google Scholar] [CrossRef]
50. Basiak E, Lenart A, Debeaufort F. Effect of starch type on the physico-chemical properties of edible films. Int J Biol Macromol. 2017;98(3):348–56. doi:10.1016/j.ijbiomac.2017.01.122. [Google Scholar] [PubMed] [CrossRef]
51. Lei Y, Wu H, Jiao C, Jiang Y, Liu R, Xiao D, et al. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019;94:128–35. doi:10.1016/j.foodhyd.2019.03.011. [Google Scholar] [CrossRef]
52. Donhowe IG, Fennema O. Edible films and coatings: characteristics, formation, definitions, and testing methods. In: Krochta JM, Baldwin EA, Nisperos-Carriedo MO, editors. Edible coatings amd films to improve food quality. Lancester, PA, USA: Technomic Publishing Co., Inc.; 1994. [Google Scholar]
53. Saberi B, Vuong QV, Chockchaisawasdee S, Golding JB, Scarlett CJ, Stathopoulos CE. Mechanical and physical properties of pea starch edible films in the presence of glycerol. J Food Process Preserv. 2016;40(6):1339–51. doi:10.1111/jfpp.2016.40.issue-6. [Google Scholar] [CrossRef]
54. Li W, Liu J, Liang B, Shu Y, Wang J. Small molecule hydrogen-bonded toughen nacre-inspired montmorillonite-konjac glucomannan-glycerin film with superior mechanical, transparent and UV-blocking properties. Compos B: Eng. 2021;204:108492. doi:10.1016/j.compositesb.2020.108492. [Google Scholar] [CrossRef]
55. Khan B, Niazi MBK, Samin G, Jahan Z. Thermoplastic starch: a possible biodegradable food packaging material—a review. J Food Process Eng. 2017;40(3):e12447. doi:10.1111/jfpe.2017.40.issue-3. [Google Scholar] [CrossRef]
56. Dong F, Wang X. Effects of carboxymethyl cellulose incorporated with garlic essential oil composite coatings for improving quality of strawberries. Int J Biol Macromol. 2017;104:821–6. doi:10.1016/j.ijbiomac.2017.06.091. [Google Scholar] [PubMed] [CrossRef]
57. Kapoor L, Simkin AJ, Priya Doss CG, Siva R. Fruit ripening: dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022;22(27):1–22. doi:10.1186/s12870-021-03411-w. [Google Scholar] [PubMed] [CrossRef]
58. Marszałek K, Woźniak Ł., Kruszewski B, Skapska S. The effect of high pressure techniques on the stability of anthocyanins in fruit and vegetables. Int J Mol Sci. 2017;18(2):1–23. doi:10.3390/ijms18020277. [Google Scholar] [PubMed] [CrossRef]
59. Biswas A, Ahmed T, Rana MR, Hoque MM, Ahmed MF, Sharma M, et al. Fabrication and characterization of ZnO nanoparticles-based biocomposite films prepared using carboxymethyl cellulose, taro mucilage, and black cumin seed oil for evaluation of antioxidant and antimicrobial activities. Agronomy. 2023;13(1):147. doi:10.3390/agronomy13010147. [Google Scholar] [CrossRef]
60. Tavassoli-Kafrania E, Gamageb MV, Duméea LF, Konga L, Zhaoa S. Edible films and coatings for shelf life extension of mango: a review. Crit Rev Food Sci Nutr. 2022;62(9):2432–59. doi:10.1080/10408398.2020.1853038. [Google Scholar] [PubMed] [CrossRef]
61. Tavares KM, de Campos A, Mitsuyuki MC, Luchesi BR, Marconcini JM. Corn and cassava starch with carboxymethyl cellulose films and its mechanical and hydrophobic properties. Carbohydr Polym. 2019;223:115055. doi:10.1016/j.carbpol.2019.115055. [Google Scholar] [PubMed] [CrossRef]
62. Youssef AM, El-Sayed SM. Bionanocomposites materials for food packaging applications: concepts and future outlook. Carbohydr Polym. 2018;193:19–27. doi:10.1016/j.carbpol.2018.03.088. [Google Scholar] [PubMed] [CrossRef]
63. Sharma P, Vishvakarma R, Gautam K, Vimal A, Gaur VK, Farooqui A, et al. Valorization of citrus peel waste for the sustainable production of value-added products. Bioresour Technol. 2022;351:127064. doi:10.1016/j.biortech.2022.127064. [Google Scholar] [PubMed] [CrossRef]
64. Chavan P, Lata K, Kaur T, Jambrak AR, Sharma S, Roy S, et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: a comprehensive review. Food Chem. 2023;418:135916. doi:10.1016/j.foodchem.2023.135916. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools