 Open Access
Open Access
REVIEW
A Greener Future: Carbon Nanomaterials from Lignocellulose
Cellulose and Paper Department, National Research Centre, Dokki, Giza, 12622, Egypt
* Corresponding Author: Hebat-Allah S. Tohamy. Email:
(This article belongs to the Special Issue: Recent Advances in Biochar and Carbon-Based Materials Characteristics and Environment Applications)
Journal of Renewable Materials 2025, 13(1), 21-47. https://doi.org/10.32604/jrm.2024.058603
Received 16 September 2024; Accepted 18 November 2024; Issue published 20 January 2025
Abstract
Lignocellulosic materials (LCMs), abundant biomass residues, pose significant environmental challenges when improperly disposed of. LCMs, such as sugarcane bagasse, rice straw, saw dust and agricultural residues, are abundant but often burned, contributing to air pollution and greenhouse gas emissions. This review explores the potential of transforming these materials into high-value carbon nanomaterials (CNMs). We explore the potential of transforming these materials into high-value CNMs. By employing techniques like carbonization and activation, LCMs can be converted into various CNMs, including carbon nanotubes (CNTs), graphene (G), graphene oxide (GO), carbon quantum dots (CQDs), nanodiamonds (NDs), fullerenes (F), carbon nanofibers (CNFs), and others. Hybridizing different carbon allotropes further enhances their properties. CNMs derived from cellulose, lignin, and hemicellulose exhibit promising applications in diverse fields. For instance, CNTs can be used in energy storage devices like batteries and supercapacitors due to their exceptional electrical conductivity and mechanical strength. Additionally, CNTs can be incorporated into recycled paper as a fire retardant additive, enhancing its flame resistance. G, renowned for its high surface area and excellent electrical conductivity, finds applications in electronics, sensors, catalysis, and water treatment, where it can be used to adsorb heavy metal ions. CQDs, owing to their unique optical properties, are used in bioimaging, drug delivery, and optoelectronic devices. By harnessing the potential of LCMs, we can not only mitigate environmental concerns but also contribute to a sustainable future. Continued research is essential to optimize synthesis methods, explore novel applications, and unlock the full potential of these versatile materials.Graphic Abstract
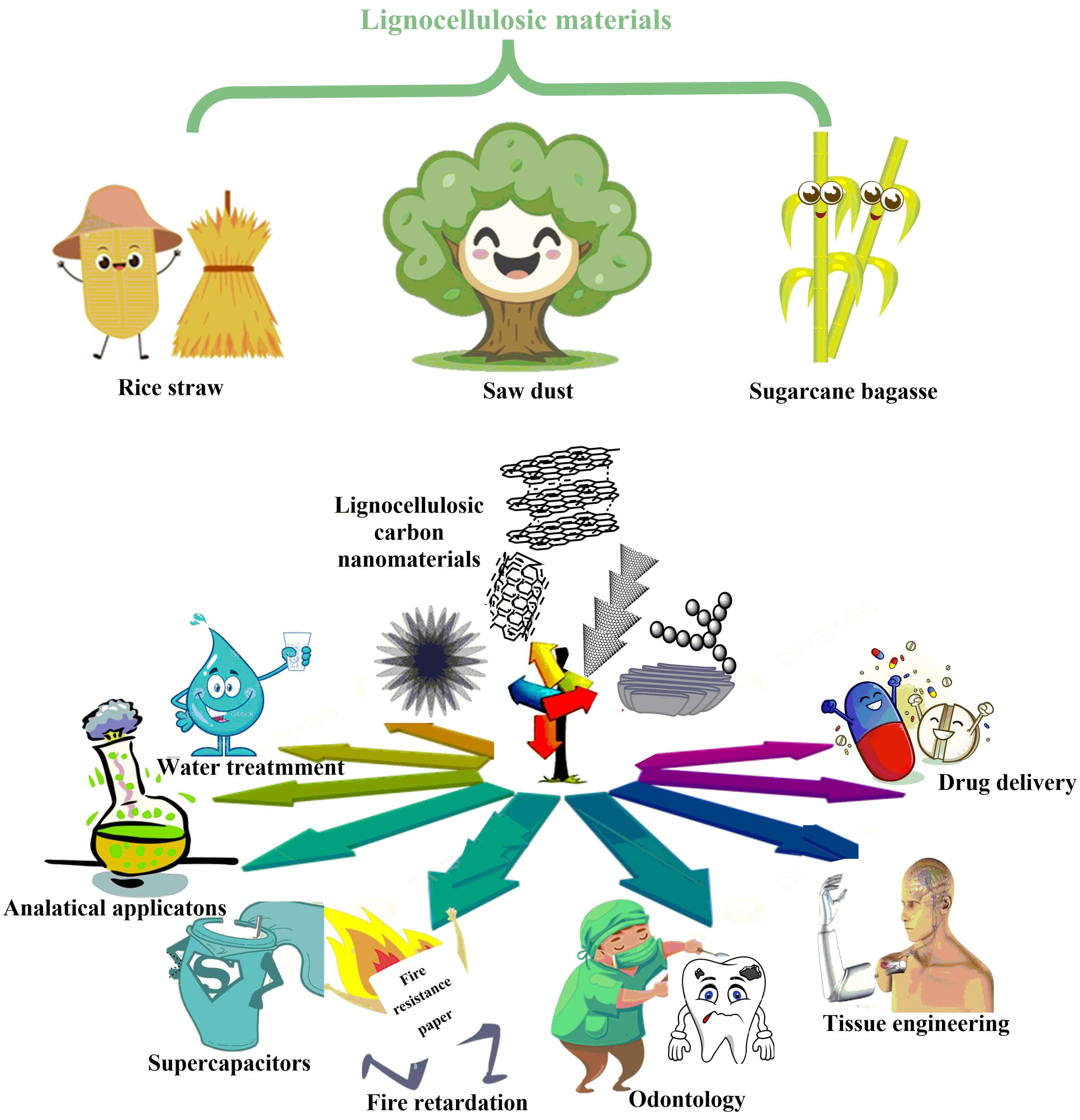
Keywords
Glossary/Nomenclature/Abbreviations
| LCMs | Lignocellulosic materials |
| CNFs | Carbon nanofibers |
| AGU | Anhydroglucose unit |
| CNMs | Carbon nanomaterials |
| CNTs | Carbon nanotubes |
| MWCNTs | Multi-walled carbon nanotubes |
| SWCNTs | Single-walled carbon nanotubes |
| G | Graphene |
| GO | Graphene oxide |
| FLG | Few-layer-graphene |
| GNP | Graphene nanoplatelets |
| NDs | Nanodiamonds |
| CNFs | Carbon nanofibers |
| N-CQDs | Nitrogen co-doped CQDs |
| GQDs | Graphene quantum dots |
| 2-D | Two-dimensional |
| F | Fullerenes |
| rGO | Reduced graphene oxide |
| NC | Nanocellulose |
| NCC | Nanocrystalline cellulose |
| OLCs | Onion-like carbons |
| CB | Carbon black |
| CQDs | Carbon quantum dots |
| 0-D | Zero-dimensional |
| 1-D | One-dimensional |
| 2-D | Two-dimensional |
| CNWs | Carbon nanowalls |
| CS | Spherical carbons |
| P-CQDs | Phosphorus co-doped CQDs |
Lignocellulosic materials (LCMs) are abundant, renewable, and inexpensive resources that, when combusted, contribute to increased CO2 emissions and air pollution. As non-edible plant components, LCMs do not compete with food sources. Moreover, the accumulation of LCM waste poses significant environmental challenges [1–5]. Carbon is the 6th most abundant element in the universe, capable of forming stable bonds with itself and other elements to create a wide range of compounds [6]. Carbon’s ability to form bonds with itself, resulting in oligomers and polymers, is crucial for life processes. With four valence electrons, carbon can create diverse structures, including those with C=C (e.g., graphite) and C≡C (e.g., acetylene) bonds. The potential to form carbon nanomaterials (CNMs), especially those with C=C bonds, has significantly impacted the field of nanotechnology [7]. CNMs comprise different low-dimension allotropes of carbon such as graphite, activated carbon, carbon nanotubes (CNTs), fullerenes (F), graphene (G), and its derivatives (graphene oxide (GO)), nanodiamonds (NDs), and carbon-based quantum dots (CQDs), as shown in Fig. 1 [4,6,8]. CNMs are suitable for many applications due to their excellent directionality, high surface area, and flexibility [6,9]. Low-dimensional CNMs can be divided into different dimensionality ranging from zero-dimensional (0-D) to one-dimensional (1-D) and two-dimensional (2-D) depending on their nanoscale range (<100 nm) [10,11]. This review outlines the types of shaped carbons that simple synthetic procedures can produce. Their mechanisms of formation and uses are also described.
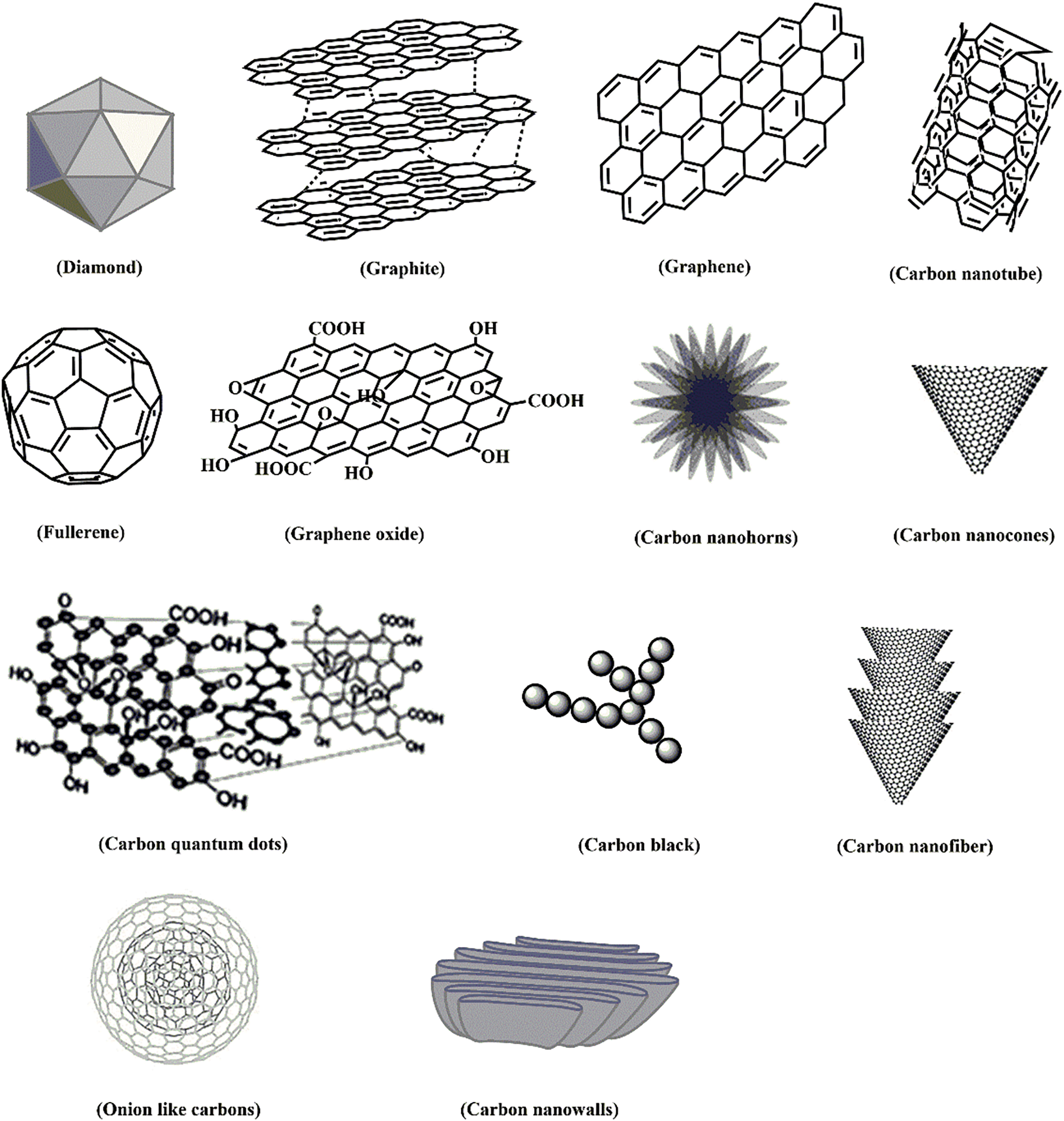
Figure 1: Some significant carbon allotropes
LCMs, including lignified fibers in the cell wall tissues [12–14], are produced by shrubs and trees, from the plant roots, stems, branches, and leaves [15]. These materials mainly composed of three polymers: cellulose, amorphous polysaccharides, and lignin, have a wide range of practical applications in the fields of biology, botany, and materials science [2]. Table 1 provides a detailed summary of certain types of LCMs and their chemical composition, inspiring further research and innovation [2].

LCMs can be utilized in two primary ways: direct consumption or conversion into valuable products. Currently, many agricultural wastes serve as animal feed, while LCMs are also used for heat generation. Additionally, LCMs can be employed as feedstock for producing energy carriers and chemicals. Various approaches are used to convert LCMs into valuable products, with cellulose, the component possessing the desired properties, being of particular importance. Purifying cellulose from its complex matrix in LCMs significantly enhances its value and expands its range of applications (Fig. 2) [16]. The following section gives short notice on the composition of LCMs.
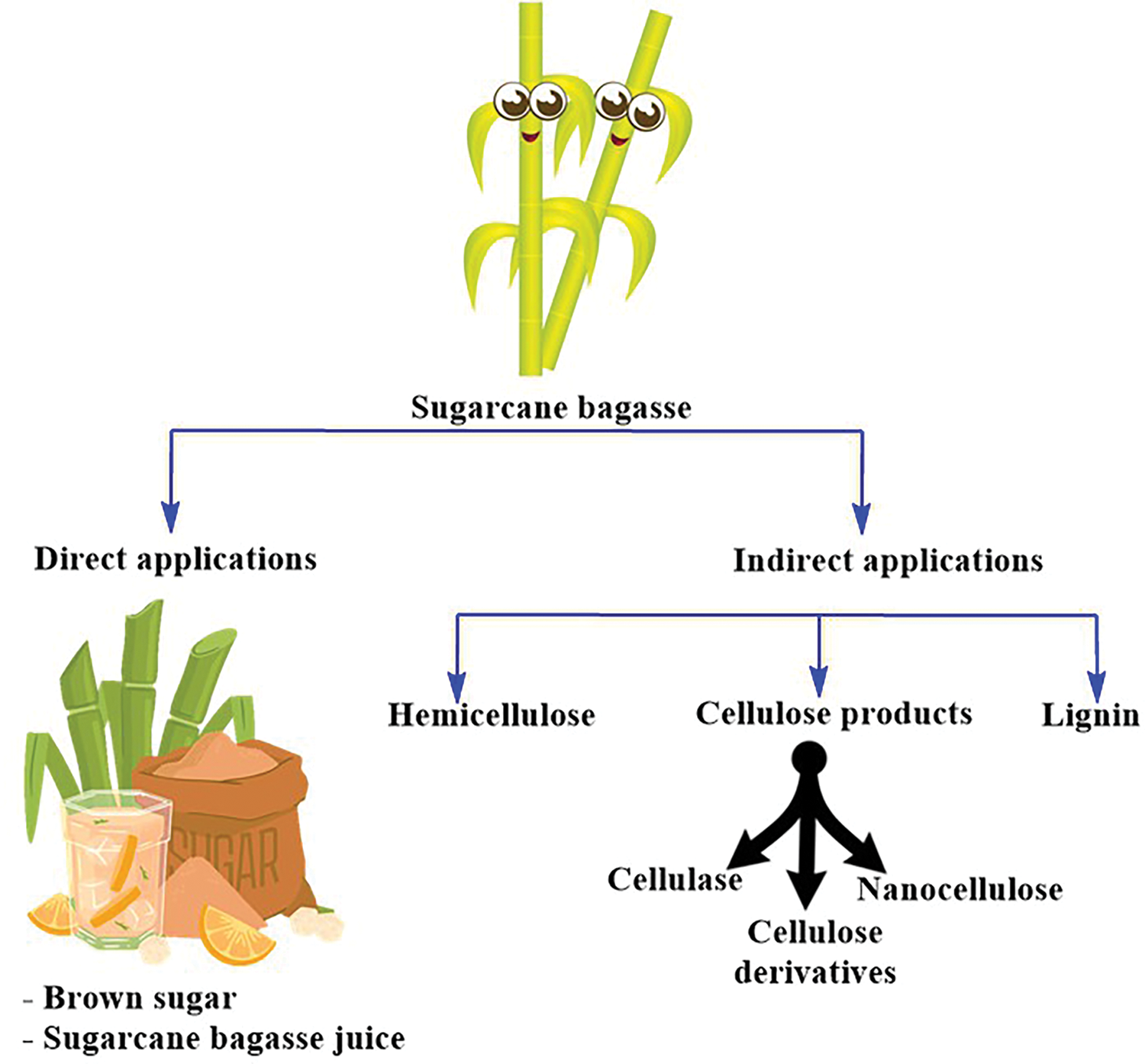
Figure 2: Direct and indirect products obtained from LCMs
2 Chemical Composition of Lignocellulosic Materials
LCMs comprise three different chemically structural polymers: cellulose, hemicellulose, and lignin. The distribution and proportions of these polymers within the cell wall vary across plant species, tissues, and maturity stages. Typically, LCMs contain 35%–50% cellulose, 20%–35% hemicellulose, 10%–25% lignin, and minor amounts of proteins, oils, and ash [17–19].
Cellulose is a linear biopolymer named (1,4)-ß-D-glucopyranose, (C6H10O5)n with repeating unit cellobiose. It is a significant component of LCMs, and its structure consists of intra- and inter-molecular H-bonding, which binds the glucose units tightly [20–23]. Since about half of the organic carbon in the biosphere is present in cellulose, converting cellulose into valuable chemicals is paramount [2]. Each anhydroglucose unit (AGU) has three OH groups at C2, C3 (secondary), and C6 (primary). OH groups can be functionalized with different polymers [24,25]. The three free OH groups share cellulose derivatization like alcohols [11,26]. The primary OH group is more reactive than the secondary OH group [27]. The three free OH groups, the oxygen atoms of the AGU ring, and the glycosidic linkage can form intra-molecular and inter-molecular hydrogen bonds, leading to the formation of secondary valence bonds between cellulose chains [21,22]. These bonds produce chemically stable and tightly packed cellulose fibrils with some crystalline regions (Fig. 3) [28].
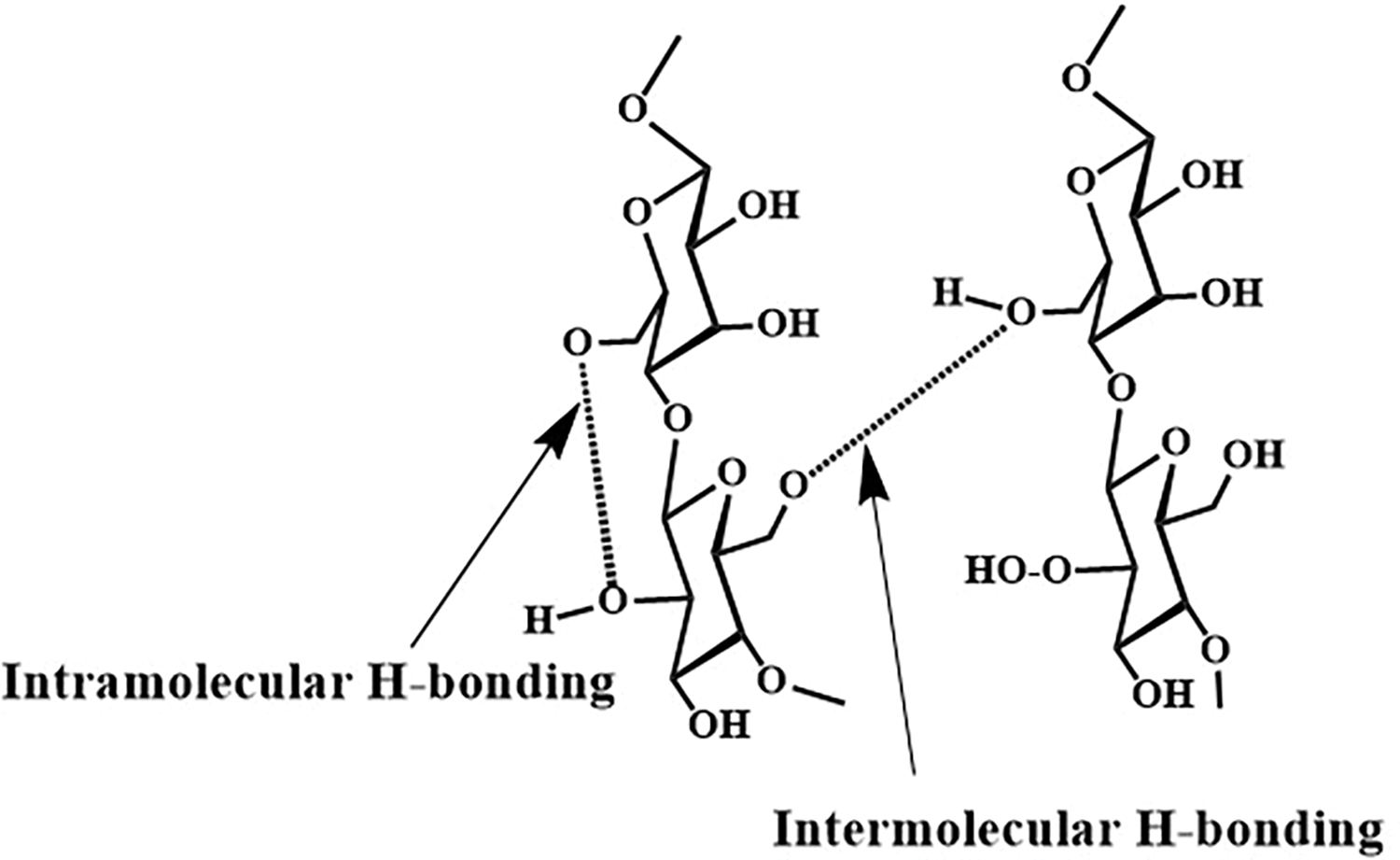
Figure 3: Intermolecular and intramolecular hydrogen bonding between and within two adjacent cellulose molecules
The crystalline regions within cellulose fibrils contribute to the strength and stiffness of plant cells, while the amorphous regions provide flexibility. Nanocellulose (NC), a form of cellulose composed of fibers or crystals less than 100 nm in length, can be extracted from natural cellulose fibers (Fig. 4). It is biodegradable, lightweight, and possesses a high tensile strength of 10 GPa. NC is a promising renewable, green material for various applications, including food packaging, coatings, and industrial fillers. Various techniques, such as de-structuring methods, are employed to isolate nanocrystalline cellulose (NCC) [28,29].
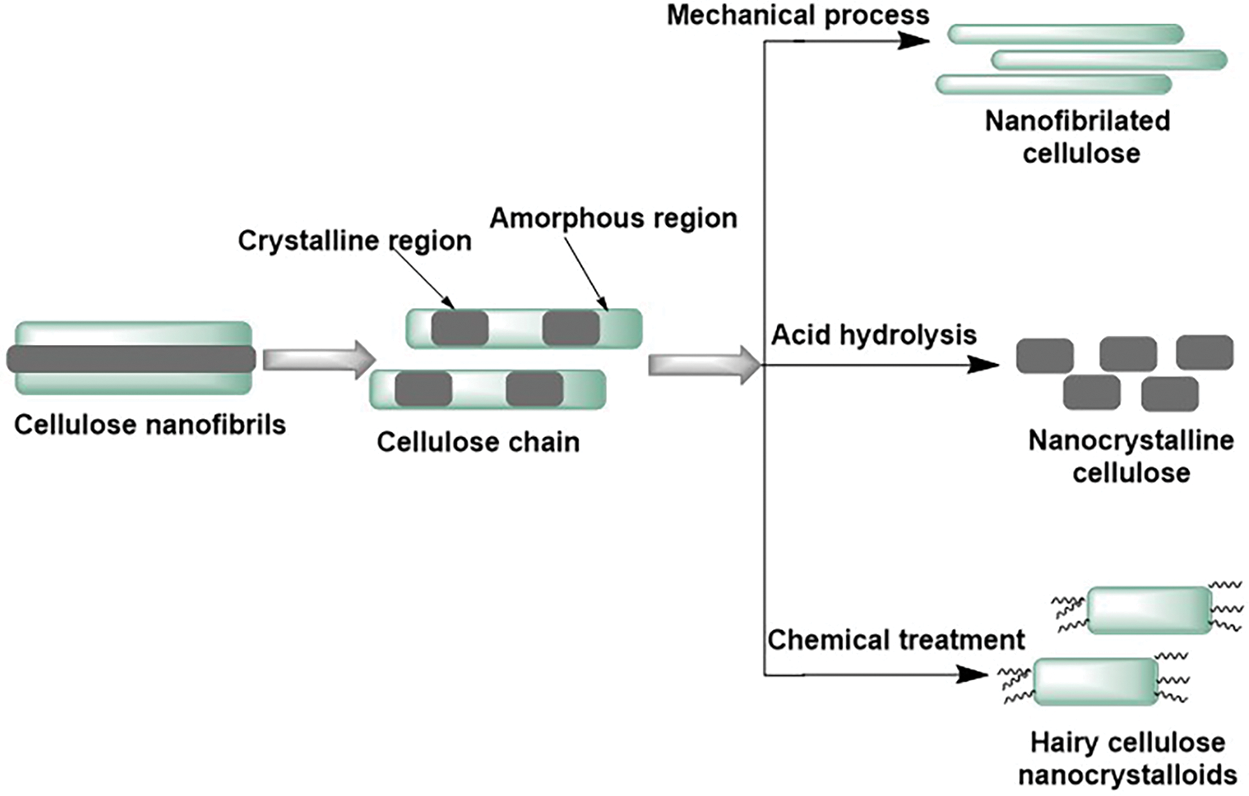
Figure 4: Schematic representation of extraction of NC from LCMs
Hemicellulose is the 2nd widespread LCMs polymer, following cellulose. It accounts for 30% of the dry mass of LCMs. It is found in the plant’s cell wall and is associated with cellulose [30]. Unlike the uniform, linear chains of cellulose, hemicellulose chains are characterized by their irregular branching and amorphous nature. These chains are composed of a mixture of 5-carbon (pentose) and 6-carbon (hexose) sugars. Common monosaccharides found in hemicellulose include xylose, arabinose, mannose, glucose, galactose, and acetylated sugars [31].
Lignin is the most complex polymer among the other LCMs polymers. Lignin, a complex three-dimensional polymer, is composed of phenylpropanoid units. It acts as a structural support system within plant cell walls, providing rigidity and compressive strength. Additionally, lignin serves as a natural defense mechanism, deterring attacks from insects and pathogens [32].
3 Hybridization in Carbon Allotropes
Hybridization means mixing the valence electronic orbitals. Generally, a molecular orbital binds with the same type of orbital (Fig. 5) [33,34].
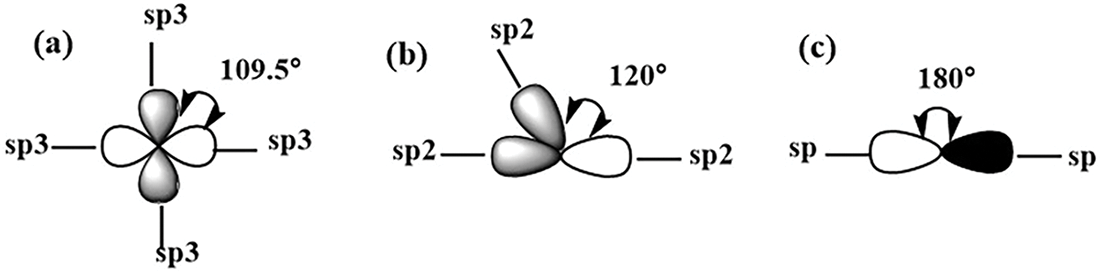
Figure 5: A schematic plot (a) sp3, (b) sp2, and (c) other hybridization of carbon atoms
Carbon possesses six electrons: two core electrons tightly bound within the 1s orbital and four valence electrons distributed across the 2s and 2p orbitals. The subtle energy difference between the 2s and 2p orbitals facilitates the excitation of an electron from the 2s to the 2p orbital in the presence of external influences, such as neighboring hydrogen atoms. This excitation triggers orbital hybridization, resulting in the formation of four new hybrid orbitals: one s-orbital and three p-orbitals (px, py, and pz). The mixing of the hybrid orbitals results in a tetrahedral configuration, a spatial arrangement with an angle of ≈109.5° between each orbital [34].
It mixes one 2s-orbital with two 2p orbitals (i.e., px and py orbitals), forming hybridized sp2 bonds [34,35]. The sp2 orbitals produce a planar structure with a characteristic angle of ≈120°, creating a σ-bond [34]. The 4th valence electron occupies the pz orbital perpendicular to the plane of G, forming a π-bond [24]. These levels overlap because the 2s level increases energy, and the 2p level decreases energy. Thus, the 2s and 2p electrons have the same power, and the valence state is increased from two to four (Fig. 5b) [36,37].
In sp-hybridization, one s- and one p-orbital hybridize to form two sp hybrid orbitals oriented at an angle of approximately 180°, resulting in a linear molecular geometry. Moreover, mixing d-orbitals with s- and p-orbitals is possible. For example, the sp3d2 hybridization combines one 2s-orbital with three 2p and two 3d orbitals with an angle of ≈90°, forming an octahedral configuration [35].
4 Classification of Carbon Nanomaterials Based on Their Dimensions and Their Preparations
Carbon is the 6th denoted element in the periodic table, and it can form stable bonds with other carbon atoms, oxygen, nitrogen, and other elements. It is one of the most plentiful materials on earth [38].
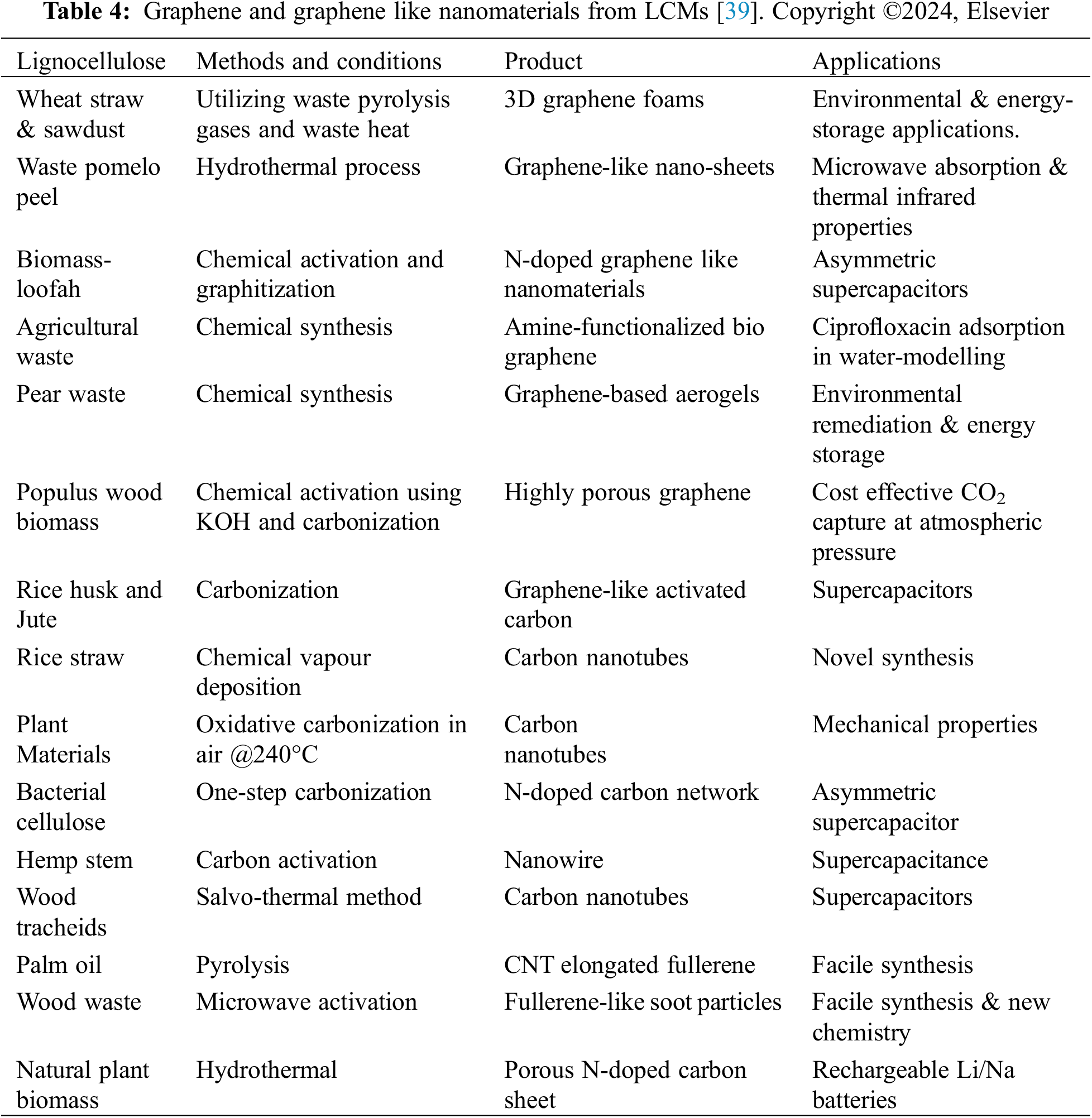
The potential applications of various CNMs derived from LCMs, such as CNTs, CQDs, porous nanocarbon, and other nanostructured carbon, are a source of inspiration for our research [59]. The synthesis of these materials from LCMs and their diverse applications, as evidenced by numerous research studies and reviews, represent a promising field. Table 2 summarizes previous research related to the synthesis and application of CNTs from LCMs, providing a roadmap for future investigations [39].
The diversity of carbon allotropes, each formed from carbon orbitals’ hybridization in sp, sp2, and sp3 configurations [6], is a fascinating aspect of our research. These allotropes can be classified according to the chemical bond related to hybridization: sp (e.g., carbyne), sp2 (e.g., fullerenes (F)), sp2 honeycomb lattice (e.g., G and GO) or sp3 (e.g., diamond), as well as several mixed sp2–sp3 shapes (e.g., CQDs or amorphous carbon). At the same time, allotropes could be classified according to their dimensionality, ranging from 0-D to 1-D and 2-D depending on their nanoscale range (<100 nm) in different spatial directions [6,60,61].
4.1 Zero-Dimensional Carbon Nanomaterials (0-DCNs)
4.1.1 Onion-Like Carbons (OLCs)
OLC, a member of the F family, is a unique carbon structure consisting of quasi-spherical- and polyhedral-shaped graphitic layers close to one another. It stands out from other carbon representatives like graphite, fullerenes (F), and nanotubes [2]. OLCs possess 3 to 8 closed graphitic shell structures with hollow cores of 20–100 nm outer diameters. Various methods have been developed for synthesizing OLCs, including arc discharge, high-electron irradiation, chemical vapor deposition, radio frequency plasma, and thermal annealing of diamond nanoparticles. However, research on OLCs remains limited due to challenges such as uncontrollable reactions, byproduct formation, complex equipment, and high costs. Currently, most OLCs are synthesized using vacuum annealing of NDs particles [13]. The transformation between the NDs and the OLCs was a reversal process that required a significant amount of energy [62]. One example of OLCs preparation from LCMs is the carbonization of Japanese cedar at 700°C under an argon atmosphere for 30 min, followed by natural cooling [63]. Porous carbon nano-onions (CNOs) can be synthesized from rice husks through a facile nickel-assisted graphitization process followed by activation. These CNOs exhibit exceptional electrochemical performance and good cycling stability [64].
NDs are spherical particles with a diamond-like structure, typically 5 nm in diameter. They consist of a diamond core (1–10 nm) composed of sp3-hybridized carbon atoms, surrounded by a carbon cover containing sp2-hybridized carbon atoms. This unique structure allows for flexibility, enabling the NDs to adopt different geometrical forms (sp3 state protected by a carbon cover containing carbon atoms in the sp2 state) [63]. The sp2/sp3 bonds in NDs are quite flexible, allowing the ability to assume two geometrical forms, i.e., the stretched face can behave as a G plane, and the puckered G may become a surface [8]. NDs are particularly promising due to their high surface area, ease of functionalization, and doping capabilities. Their optical properties are attributed to the sp3 hybridization of carbon atoms and nitrogen vacancy defects in the core, while their mechanical properties are associated with sp2 hybridization [65]. Two primary methods for synthesizing NDs include high-temperature, high-pressure transformation of graphite and detonation of carbon-based explosives [8].
Seed growth involves the chemical synthesis of an organic seed molecule containing one or more diamond lattice units. By incorporating dopant atoms into this seed molecule, specific color centers can be created. A reactive carbon source, derived from a hydrocarbon that decomposes at a lower temperature than the diamond seed, is then introduced. The subsequent growth of diamond around the seed molecule enables precise control over the placement of desired color centers, ensuring at least one fluorescent emitter per ND, regardless of size (Fig. 6a) [66,67].
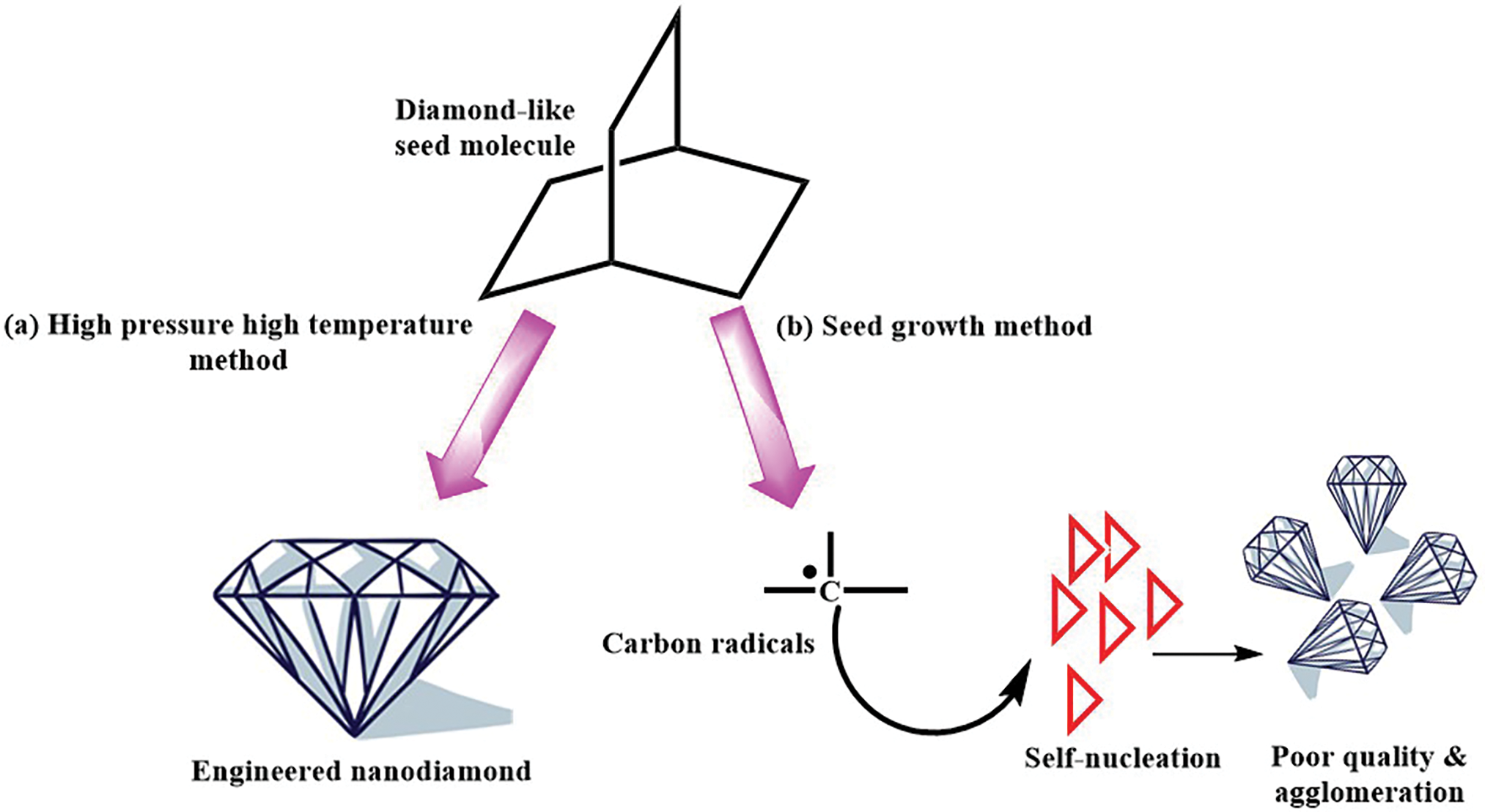
Figure 6: Basic concept of engineered fluorescent NDs via molecule seeded growth, (a) high pressure high temperature method, and (b) seeded growth method
Conventional methods of ND growth from organic precursors involve a thermal decomposition process, followed by spontaneous nucleation (i.e., high pressure high temperature method). This approach requires high concentrations of reactive carbon species to initiate nucleation and subsequent rapid growth, often resulting in lower-quality ND. In contrast, seeded growth allows for a more controlled process by maintaining lower concentrations of reactive carbon species. This enables slower, more precise growth, leading to higher-quality diamonds with enhanced crystal perfection and chemical purity (Fig. 6b) [67].
Fullerenes (F) are spherical, look like a soccer ball (a buckyball), and are caged molecules with pentagons and hexagons at the corner of a polyhedral structure [4]. The term “fullerene” refers to domes of buildings. The smallest fullerene member is C20 [38]. Most F (e.g., C60) are spheroid in shape, although oblong shapes like a rugby ball also exist (e.g., C70) [8]. F are stable (except small F) but not unreactive [38]. The F molecule requires that the C-C bonds interact through bent sp2 hybridized carbon atoms, leading to a strained structure with good reactivity, acting as an electron acceptor. C70 can be seen as a C60 molecule with a belt of five hexagons around the equatorial plane and exhibits a more oval shape. The main properties of C60 are Young’s modulus ≈of 14 GPa, electrical resistivity ≈of 1014 Ω m, thermal conductivity ≈of 0.4 W/mK, and band gap of 1.7 eV. The other fullerene types show similar properties to C60 [68]. C60 and C70 could be synthesized by pyrolysis of naphthalene at 1000°C. C60 can also be synthesized from the reduction of CO2 via metallic lithium or MgCO3 at 700°C [10].
The primary growth mechanism for carbon molecules involves reactions with intermediate-sized rings. Planar polycyclic and monocyclic ring compounds they observe react much faster than the fullerene isomers. Responses between the planar ring types create new polycyclic compounds, which can grow further through ring coalescence or anneal to give a F or monocyclic ring. A proposed mechanism for fullerene formation involves a two-step process. Initially, a Bergman cyclization of an enediyne occurs, followed by a “zipping up” of the resulting spiraling polyyne chain, leading to the coalescence of the structure into a fullerene cage (Fig. 7). Monocyclic rings, once formed, can further grow through a process known as coalescence, in which they combine with other planar ring structures. F, on the other hand, typically grows through the addition of smaller carbon-based particles [69].
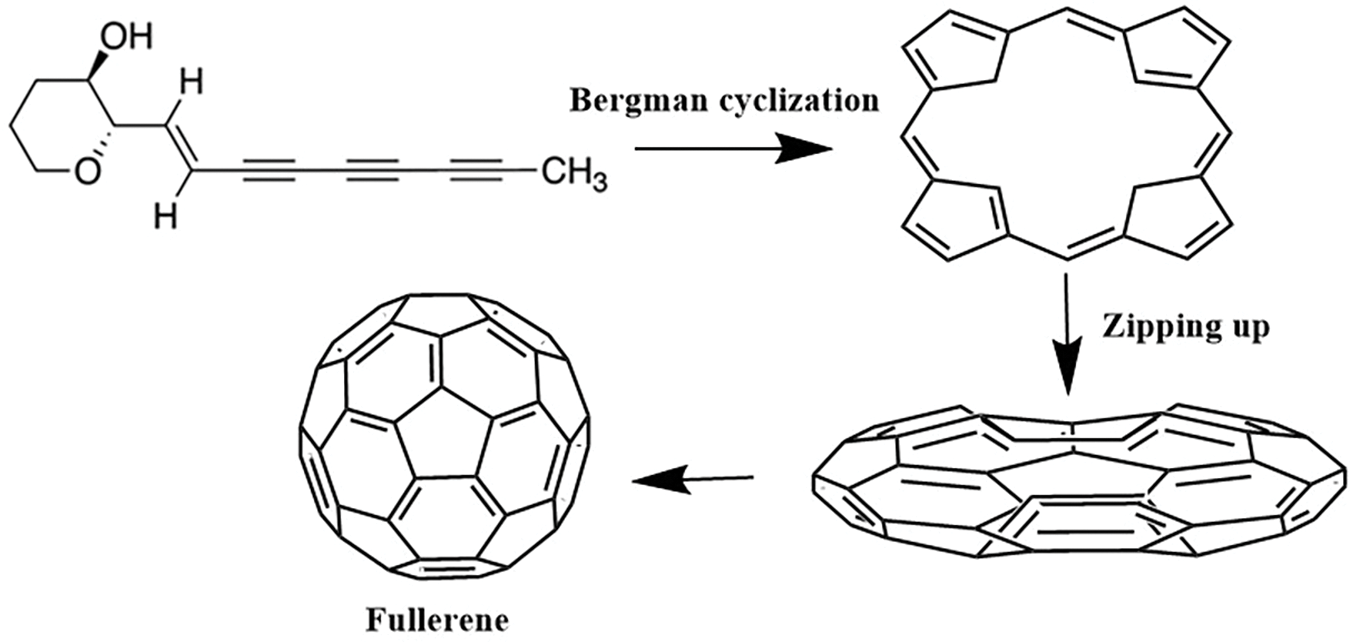
Figure 7: The proposed mechanism for initiation of the possible route for a spiraling polyyne chain to “zip up” to form buckminster fullerene
Carbon black (CB) is one of the spherical carbons (CS) [7,8]. CS can be synthesized with carbon flakes arranged parallel to the central carbon core [69]. The specific arrangement of these flakes significantly impacts the chemical and physical properties of the resulting CSs. The sp2 carbon at the edges of these flakes can react with other atoms, such as H and O, to form OH and COOH groups, thereby fulfilling their valence requirements [7]. CB is produced mainly by hydrocarbons’ partial combustion and thermal decomposition of LCMs. Various synthetic methods can be employed, including arc discharge, laser ablation and plasma processes, shock compression techniques, chemical vapor deposition, autoclave processes, and carbonization routes [7]. During CB growth, a carbon source is converted into C, H, and O radicals. The radicals subsequently react to form the CB. Three potential pathways for carbon particle formation have been proposed: polyacetylenes, polyaromatic hydrocarbons, and the formation of carbon vapor [38]. In one study, CB was synthesized by carbonizing the ground-frozen hydrolysis lignin in a horizontal tube furnace ranging from 600°C to 900°C for 6 h under N [70].
4.1.5 Carbon Quantum Dots (CQDs)
CQDs are less than 10 nm in size. They can be synthesized from diverse organic precursors such as natural polymers, amino acids, apple juice, grape peel, and vegetables. Several straightforward methods have been developed for CQD synthesis, including laser ablation, electrochemical oxidation, combustion/thermal microwave heating, chemical oxidation, hydrothermal carbonization, and pyrolysis [71–73].
The proposed methods to prepare CQDs can be classified into “Top-down” by breaking down larger carbon materials into smaller CQDs and “Bottom-up” by building CQDs from smaller molecular precursors. Several challenges exist in CQD synthesis. One major challenge is aggregation, where CQD particles tend to clump together. Electrochemical synthesis is one method to mitigate this issue. Another challenge is controlling the size and uniformity of CQDs. Post-treatment processes can help address this. Additionally, optimizing surface properties for specific applications, such as solubility and functionalization, is crucial and can be achieved through post-treatment techniques. LCMs, like those from the paper (WP), can serve as a sustainable source for CQDs synthesis [74]. Table 3 demonstrates CQDs’ preparation methods, properties, and applications prepared from lignocellulosic materials [39].

CQDs with diverse properties, including crystallinity, and surface properties can be derived from various biomass waste sources such as sugarcane bagasse, garlic peels, and taro peels, using ultrasonic-assisted oxidation of carbonized biomass. CQDs are widely utilized due to their photoluminescence combined with biocompatibility for biomedical applications. The high fraction of α-cellulose is necessary for a high graphitization degree and outstanding CQDs properties [75]. Hydrothermal carbonization of pine wood produced CQDs with excellent fluorescence characteristics, which selectively detected Fe(III) in an aqueous phase [76].
Green hydrothermal techniques have been employed to produce CQDs from corn stalk shells and corncob residues were developed. These CQDs exhibit excellent fluorescence properties, making them promising candidates for applications in detection and bioimaging. Specifically, CQDs derived from corncob residues have shown potential for the detection of Fe(III) ions [77,78].
Aloe peel-derived CQDs were prepared by hydrothermal treatment as accelerants to increase cumulative methane production and total chemical oxygen demand removal efficiency [79]. CQDs were derived from waste acorn cups by a hydrothermal carbonization process and applied as an attractive solution to functionalize PVA material [80].
Lignin-based CQDs have emerged as promising fluorescent nanomaterials due to their excellent luminescent performance, long-term stability, and water solubility. These CQDs can be synthesized from natural lignin via hydrothermal treatment. CQDs efficiently serve as fluorescence-switchable nanoprobes for sensitively detecting common metal ions and for real-time and visual self-detection of formaldehyde gas [81,82]. The prepared CQDs doped with N and Mg elements onto their framework show strong turquoise fluorescence [82].
Nitrogen and phosphorus co-doped CQDs (N, P-CQDs) have been synthesized through a simple one-pot hydrothermal method using cellulose pulp. These N, P-CQDs have demonstrated potential applications in metal ion detection, pH sensing, and cell imaging [83].
Fluorescent CQDs were synthesized through a straightforward one-step microwave heating process involving urea and various biomass sources, including bagasse, cellulose, or carboxymethyl cellulose. These CQDs have found application in the adsorption of Pb(II) ions from aqueous solutions [84].
Graphene Quantum Dots (GQDs) have garnered significant attention due to their exceptional properties, including high surface area, sustainable bright fluorescence, long-term photostability, excellent electrochemical properties, biocompatibility, high chemical stability, high water solubility, and unique drug delivery capacity. These properties make GQDs highly desirable for various applications such as bioimaging, biosensors, photocatalysis, solar cells, light-emitting diodes, optoelectronics, and chemical sensing [85]. GQDs can be synthesized from dispersed lignin in nitric acid solution through an ultrasonication process, followed by hydrothermal treatment in an autoclave at 180°C for 12 h [86]. GQDs were fabricated from Miscanthus (silver grass) biorefinery waste via fractionation, followed by hydrothermal carbonization. The as-fabricated GQDs exhibit a linear fluorescent response towards trace amounts of Fe(III) in real-environment water [87]. GQDs prepared from bagasse were used in composites with activated carbon and embedded with titanium dioxide to enhance activated carbon’s adsorption and photocatalytic efficiency in removing methylene blue dye pollutants [9,84].
The G sheet can be transformed into a cone shape by removing and resealing a wedge. Nanocones can be produced through various techniques, including CO2 laser ablation of graphite at room temperature and plasma-enhanced chemical vapor deposition using a mixture of Ar + H2 + CH4. Due to their high-temperature stability, nanocones are promising candidates for scanning probe applications in harsh environments. They also find use in nanocage applications, such as gas storage and drug delivery [7]. Nanocones form through a unique process involving the growth of a G-sheet on a nickel catalyst anchored to a substrate. This G-sheet then develops into a nearly cylindrical nanostructure with equal base and top radii. As the lateral surface area increases, carbon atoms attach to the edges of the hydrogen-terminated G-sheet, causing the nanocone to grow. The height of the nanocone increases as a new layer forms on the catalyzed surface. The nanocone widens due to the attachment of carbon atoms to the edges of parallel carbon platelets [88,89].
4.2 One-Dimensional Carbon Nanomaterials (1-DCNs)
4.2.1 Carbon Nanofibers (CNFs)
CNFs are 1-D filaments composed of stacked, curved G layers. They exhibit a conical nanostructures with diameter ranged from nanometers to hundreds of nanometers and lengths spanning from sub-micrometer to millimeter scales. The morphology of CNFs varies depending on the angle between the G layers and the fiber axis. This can result in different types, such as plate-like, ribbon-like, or herringbone CNFs [10].
CNFs can be synthesized through two primary methods. The first method, the seeded catalyst method, involves depositing catalyst particles onto a substrate within a reactor. The second method, the floating catalyst method, utilizes evaporation techniques to deposit catalyst particles onto a substrate (Fig. 8) [10].
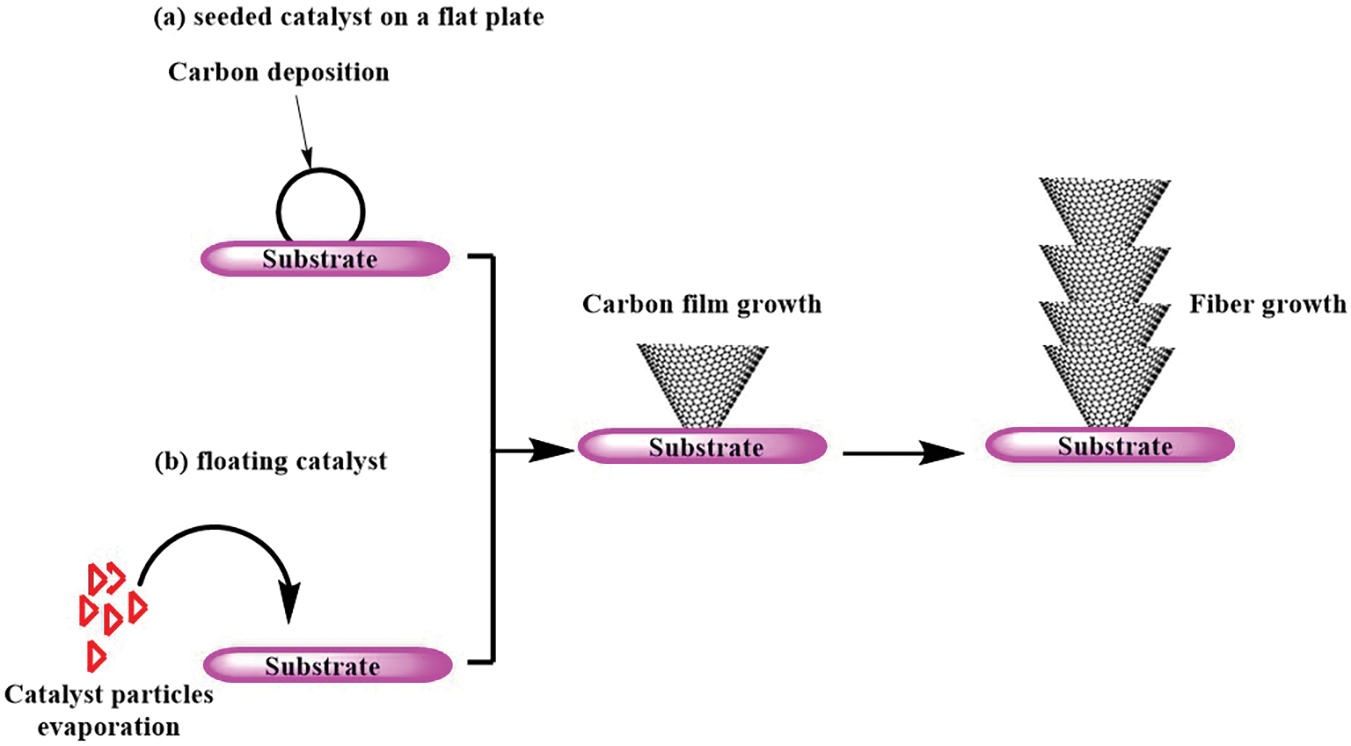
Figure 8: Methods of synthesizing CNFs: (a) seeded catalyst on a flat plate and (b) floating catalyst
Microwave pyrolysis of LCMs palm kernel shells at 500°C and 600°C resulted in the formation of hotspots, which acted as nucleation sites for the growth of hollow CNFs. Additionally, bamboo-shaped CNFs were formed due to secondary pyrolysis or a lower temperature at the outer regions compared to the core of the active site [90].
Fractionated sugarcane bagasse lignin, characterized by high molecular weight, narrow polydispersity index, and a linear structure, was obtained through ethanol/water solution fractionation. Electrospinning of this lignin with polyacrylonitrile, followed by thermostabilization and carbonization, yielded high-quality lignin-based CNFs suitable for supercapacitor applications. These CNFs exhibited a large specific surface area, independent filamentous morphology networks, and excellent electrochemical performance [91].
CNT are 1-D rolled G. They can be single-walled (SWCNT) and multi-walled (MWCNT) based on the number of G layers [92]. Chemical bonding involves an entirely sp2-hybrid state with outstanding properties [38]. The diameter varies from 0.4 to 2.5 nm and a few nanometers to 100 nm for SWCNTs and MWCNTs, respectively. Each layer in MWCNT interacts through Vander Waals forces [8,38]. CNTs exhibit remarkable properties, including tensile strength of ≈37 GPa, strain to failure of ≈6%, Young’s modulus of ≈0.62–1.25 TPa, electrical resistivity of ≈1 μΩ cm, thermal conductivity ≈3000 W/mK and density ≈1.33–1.4 g/cm3 (which is half of the density of aluminum, making them very attractive for lightweight applications [22].
There are two primary mechanisms for the growth of SWCNTs. The first mechanism, known as the tip-growth model (Fig. 9a), occurs when the interaction between the catalyst and the substrate is weak. In this process, hydrocarbon decomposition takes place on the top surface of the metal catalyst. The carbon atoms then diffuse through the metal and precipitate at the bottom, pushing the entire metal particle away from the substrate. As the metal particle is lifted, its top surface becomes exposed to new hydrocarbon molecules, allowing for further carbon diffusion and CNT growth. This process continues until the metal particle becomes saturated with carbon, halting the growth of the CNT [93].
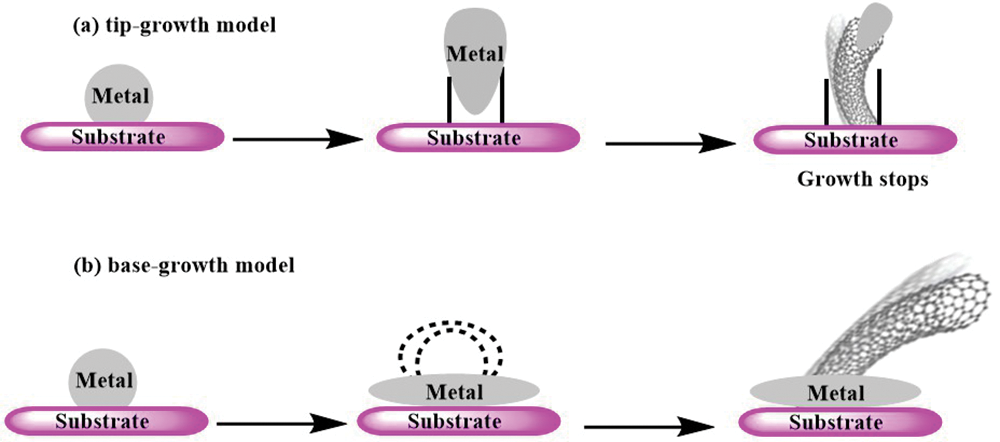
Figure 9: Growth mechanisms for CNTs: (a) tip-growth model, and (b) base-growth model
The second mechanism for SWCNT growth is the base-growth model (Fig. 9b). In this model, a strong interaction exists between the catalyst and the substrate. Similar to the tip-growth model, hydrocarbon decomposition and carbon diffusion occur initially. However, the CNT precipitation fails to displace the metal particle, leading to the emergence of the CNT from the metal’s apex. Initially, carbon crystallizes as a hemispherical dome, which then extends into a seamless graphitic cylinder. Subsequent hydrocarbon decomposition occurs on the lower peripheral surface of the metal, and the dissolved carbon diffuses upward, resulting in the growth of the CNT with the catalyst particle remaining rooted at its base. For MWCNT growth from acetylene decomposition on Ni particles at 480°C, the Ni cluster had a round shape, which transformed into an elongated shape surrounded by a thin carbon layer. The Ni cluster left the substrate contracted upward, taking a round shape and leaving a hollow CNTs [93,94].
CNTs derived from LCMs possess a diverse array of functional groups, minerals, and a large specific surface area, making them effective adsorbents. Additionally, they can be incorporated as fillers in polymer composites to enhance the polymers’ toughness, stiffness, and electrical conductivity [95].
CNTs were synthesized from wood sawdust through a process involving the mixing of sawdust, Fe (catalyst), zinc (reducing agent), calcite (bed material), and clay. This mixture was arranged in a stainless steel column reactor with perforated plates at both ends. The reactor was then heated to 750°C for 3 h in the absence of air. To prevent the release of CO2 and CO, KOH was placed at the top of the column, converting these gases into potassium carbonate salt. The CNTs grew in the lower part of the column where the catalysts were located. This process can be summarized as a combination of wood pyrolysis and carbon vapor deposition on the bed material and reactor walls [96].
CNTs were synthesized through a two-step carbonization process involving α-cellulose. Initially, the α-cellulose was carbonized in air at 240°C for 8 h. Subsequently, the temperature was increased to 400°C, and the carbonization process continued for another 8 h at ambient pressure. The resulting carbon samples were then subjected to a purification process. This involved washing the samples with a 15% HCl solution using sonic pulsation, followed by rinsing with deionized water and centrifugation. Finally, the samples were dried at 110°C for 12 h [97]. In another study, CNTs were prepared from sugarcane bagasse, black liquor, and mature beech pinewood sawdust wastes by hydrothermal treatment of raw agricultural waste, followed by hydrolysis of hydrochar product. Multiwalled carbon nanotubes were attained and used efficiency for Ni(II) adsorption [92]. CNTs were prepared from lignin, using iron powders as a catalyst, after thermal treatment at 1000°C for 105 min. The microwave-enhanced pyrolysis method was applied to treat gumwood at 500°C to produce CNTs that are shaped on the surface of biochar [85]. Also, the production of CNTs in lignocellulose materials was achieved through the ablation of cellulose microfibrils within the lignin matrix of the intact secondary plant cell walls during low-temperature carbonization [98].
4.3 Two-Dimensional Carbon Nanomaterials (2-DCNs)
G is one layer of sp2 carbon atoms arranged in a honeycomb lattice and is a separate graphite plane [68]. The C–C bond length is about 0.142 nm. Each carbon atom forms four bonds; s, px, and py orbitals constitute the σ-bond while pz electron makes the π-bond [48]. G is the mother of all graphitic forms like graphite, carbon nanotubes, carbon nanofibers, and F [4,5,11]. G can be stacked up to form a 3D graphite crystal, rolled up along a given direction to create a SWCNTs or a MWCNT, depending on the number of G layers, or wrapped up into C60 buckyball, creating F [4,66]. G with a few layers is often referred to as few-layer graphene (FLG), while G with a slightly larger number of layers is classified as ultrathin graphite. Graphene oxide (GO) is a form of G that contains oxygen-containing functional groups, such as hydroxyl, epoxy, and carboxyl groups. Reduced graphene oxide (rGO) is obtained by partially removing these oxygen-containing groups from GO. Graphene nanoplatelets (GNPs) are G flakes with a lateral size in the micrometer range and a thickness of a few atomic layers [99].
G and rGO are easily obtained through the reduction of GO. Besides, G can also be functionalized into its oxidized form (i.e., GO) through the Hummers’ method, which shows a high density of oxygen functional groups [100,101]. This GO can be synthesized from simple and low-cost methods such as chemical oxidation of graphite to GO followed by exfoliation in ultra-sonication [100]. Due to its hydrophilic nature, high specific surface area, and high functional group density, it can be used as an adsorbent and a catalyst for removing organic and inorganic pollutants [102]. The GO’s surface is decorated with oxygen-containing functional groups, including hydroxyl and epoxy groups on sp3 hybridized on the basal carbon plane [3].
Unlike pristine G, GO possesses carbonyl and carboxyl groups attached to the edges of its sp2 hybridized carbon-structure. This allows GO to disperse well in polar organic solvents through sonication, forming stable dispersions of single-layer GO sheets. The main advantage of GO over pristine G is its improved dispersibility in various solvents, including organic solvents, water, and inorganic solvents [3,103].
A catalytic graphitization process was employed to fabricate G using lignin as the carbon source in the presence of Fe(III) as the catalyst. The process was conducted at 1000°C using different carrier gases. It was observed that methane and natural gas, as carrier gases, accelerated the formation of multilayer G with a range of 2–30 layers [85]. High-quality G sheets were prepared by initially converting dried monocotyledonous and dicotyledonous materials into biochar through a pyrolysis process at 350°C for 1 h. The biochar was then acidified at room temperature for 14 days. Following acid pretreatment, the biochar was dried at 105°C and subsequently heated at temperatures ranged between 500°C–1500°C under vacuum conditions [104]. G sheet-like porous activated carbons were prepared by mixing a fine powder of Bougainvillea spectabilis flowers with 10% ZnCl2, stirring at 60°C under an N2 atmosphere for 24 h. This was followed by carbonization in a tubular furnace at different temperatures for 3 h in the N2 atmosphere. Finally, the carbonized powder was washed with 1.0 M HCl and hot distilled water until neutral and dried [105].
The synthesis of GO from Indonesian lignocelluloses (coconut shell, rice husk, bagasse) was established through a modified Hummers method employing the KMnO4 as an oxidant and concentrated H2SO4 to exfoliate several layers from the graphite flakes. The hydrothermal pyrolysis of agave fiber was successfully used to synthesize carbon fibers and subsequently potentiate the production of graphite sheets and GO [106]. Also, GO was prepared from different agricultural wastes such as sugarcane bagasse, rice straw, Mature pinewood sawdust, and lignin by separately oxidizing with ferrocene at 300°C in muffled for 10 min under atmospheric conditions [3,5].
G can be synthesized through thermal processes, where carbon radicals from decomposed precursors nucleate and grow into G structures (Fig. 10a); stripping methods, which involve physically exfoliating graphene layers from bulk graphite (Fig. 10b); and chemical vapor deposition (CVD), where carbon atoms from a gas phase adsorb onto a substrate and form G nuclei that coalesce into a continuous film (Fig. 10c) [107]. Table 4 provides a comprehensive overview of various methods and conditions employed for the conversion of different LCMs into G and CNMs.
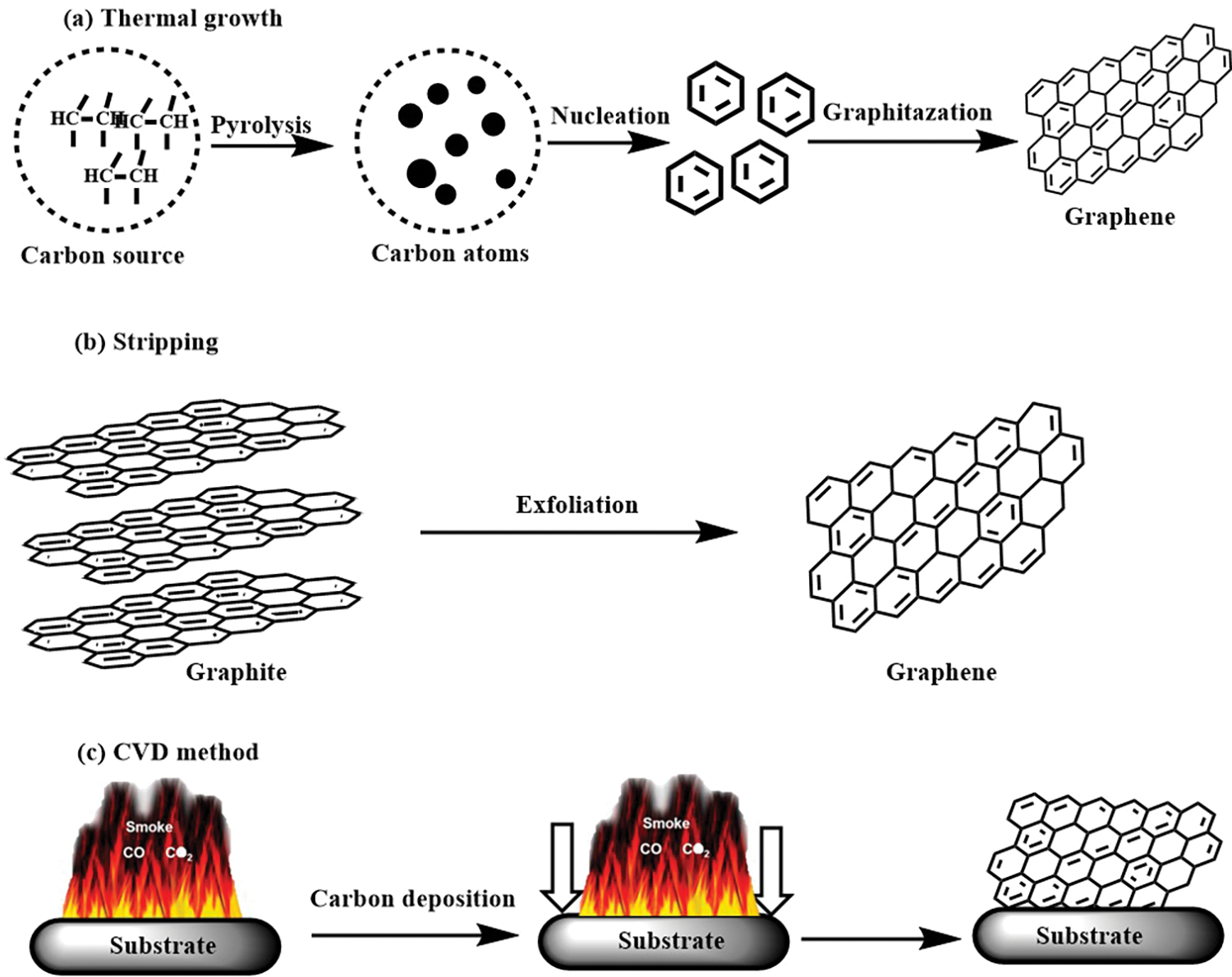
Figure 10: (a) Thermal, (b) stripping growth mechanisms of graphene, and (c) mechanism of CVD growth graphene on Cu substrate
CNWs, consisting of vertically aligned G sheets forming a 2-D wall structure, possess a large surface area and sharp edges. Their thickness ranges from a few nanometers to a few tens of nanometers. Various synthesis methods have been explored for CNWs, including microwave plasma-enhanced CVD, radio-frequency plasma-enhanced CVD, hot-wire CVD, and electron beam-excited plasma CVD [10]. CNWs can grow both without and with the presence of O2 gas. Without O2 gas, carbon nanoislands nucleate on the Si surface at a lower density compared to the oxygen-free case. This is attributed to the cleaning and etching effect of oxygen on the silicon surface, which suppresses the nucleation of amorphous carbon nanoflakes. With O2 gas, carbon nanoislands were nucleated on the Si surface with lower density than the densities of nanoislands in the case without O2. This is because of the O2, which cleans the surface of the Si substrate and etches the amorphous carbon. So, carbon nanoflake nucleation was suppressed to some degree. Isolated carbon nanoislands still exist, and the CNWs grow on them. Each CNW grows without an interface layer. We can say that the nucleation will be controlled by varying the O radical injection [108].
4.4 Three-Dimensional Carbon Nanomaterials (3-DCNs)
Graphite is the most stable allotrope of carbon. It is composed of stacked parallel G layers with sp2 hybridization [34,104]. One carbon atom covalently bonded to three other carbon atoms with a short length of 0.141 nm, forming a hexagonal lattice. The hybridized fourth valence electron is paired with another delocalized electron of the adjacent layer by Vander Waals bonds, giving electric conductive properties to graphite [37]. Methane is first decomposed at high temperatures for the synthesis of G from methane, and the decomposed carbon atoms are then integrated into the Ni substrate. After cooling, the carbon atoms precipitate from the substrate and form G on both sides of the Ni substrate. A new G layer is formed between the G and the Ni substrate. G layers gradually increase, creating the graphite-like film [109].
Porous graphitic carbons were produced from various lignocellulosic biomass sources such as softwood sawdust, bamboo, seed husks, and nut shells. Lignocellulosic biomass is milled, sieved to produce fine powders, and converted to porous graphitic carbons by iron-catalyzed graphitization [110]. Palm kernel shell waste is utilized to produce graphite via the direct impregnation of a catalyst into raw material, followed by a thermal treatment [111].
Diamond is a carbon allotrope in which the carbon atoms are arranged in a face-centered cubic crystal structure. Atoms are bonded covalently with stable 3-D structures [38]. Diamond thin films have optical, electrical, mechanical, and thermal properties, which make these attractive for applications in different fields [112].
The initial stage of diamond growth involves anisotropic etching, which leads to the formation of graphite islands. Disordered and defective carbon regions around the microcrystalline graphite are etched off by H, O, and OH radicals and ions. The created graphite aggregates on the silicon oxide surface, forming various carbon clusters. Some of these clusters then convert into precursors for diamond nucleation, enabling the growth of diamond particles on the carbide intermediate layer [112].
The diamond-like carbon nanofoam (DCNs) was prepared from sucrose by heating sucrose solution with naphthalene at 130°C in a 130 mL stainless steel autoclave for 72 h. After filtering the product with hot water and drying, DCNs was obtained. A control experiment without naphthalene did not yield DCNs, indicating that naphthalene acts as a nucleation seed, initiating the growth of DCNs. These experimental results suggest that these intermediates may lead to DCNs formation [113].
5 Applications of Carbon Based-Nanomaterials
• Carbon-based nanomaterials such as F, NDs, and CNTs have been used in analytical applications [114].
• Due to their attractive properties, diamonds are ideal semiconductors for applications in current and future electronics [112].
• Graphite has potential applications in metal surface protection, flexible conduction, heating, and electromagnetic shielding [109].
• CNWs are a promising material for various applications, including catalyst support electrochemistry, Field electron emitters, electronic devices, hydrophobic coatings, filters, and nanoimprints [108].
• G is mainly used as a template in batteries, flexible and transparent conductors, supercapacitors, transistors, fuel cells, solar cells, hydrogen storage, electrochemical devices, catalysis, electrochemical resonators, sensors, and wastewater purification. It can also be used in bio-medicinal engineering, such as drug delivery, gene transfection, tissue engineering, neural network regeneration, cancer cell imaging, targeting, and therapy [5,11,92].
• GO could be an efficient sorbent for inorganic metals [115].
• CNTs are also used in chemical nanowires, conductive films, electric motor brushes, magnets, and optical motion [92].
• CB was mainly used to write letters on papyrus in ancient Egypt and bamboo strips in ancient China. It is also used in the black pigment of the newspaper’s inks [7].
• These NDs are used in many areas, mainly biosensors, coating, bioimaging, and drug delivery [63].
• OLCs are used in many areas, mainly in electronics, optical limiting, catalysis, energy conversion and storage, Li-ion electrochemical energy-storage devices, hyperlubricants, sensors, and biological and environmental applications [31].
• The cellulose was examined, along with their potential as raw materials for producing bioethanol, biobutanol, and biodiesel (i.e., biofuels) [116].
• Numerous studies have explored the application of CNCs in biological fields, including dental restoration (i.e., odontology) [117].
• GO could be an efficient hepatoprotective agent [5].
• The use of lithium-ion batteries (LIBs) made from sugarcane bagasse-derived LCMs presents an alternative energy source for numerous nations. The integration of nanomaterials into these LCMs could potentially improve their performance and overall energy storage capacity [118].
• CNTs were used as fire retardants for the recycled paper sheets and showed excellent fire retardation and enhanced mechanical properties [119].
The accumulation of LCMs poses significant environmental challenges. However, this review highlights the potential of transforming these abundant, renewable resources into valuable CNMs. By exploring the diverse range of CNMs, including CNTs, F, G, GO, rGO, CNFs, OLCs, CQDs, etc., this review underscores the significant advancements in the field. While substantial progress has been made, future research should focus on addressing key challenges such as the development of efficient and cost-effective synthesis methods, the enhancement of CNM properties, and the exploration of novel applications. By further advancing the field of CNM research, we can not only mitigate the environmental impact of LCMs accumulation but also contribute to a more sustainable and technologically advanced future.
This review delves into the intricate details of CNMs’ synthesis techniques, highlighting the importance of optimizing these processes to achieve high-quality and scalable production. Moreover, it emphasizes the potential applications of CNMs derived from LCMs in numerous fields, including energy storage, catalysis, sensors, fire retardation, and environmental remediation. While substantial progress has been made, future research should focus on addressing key challenges such as the development of efficient and cost-effective synthesis methods, the enhancement of CNM properties, and the exploration of various applications. By further advancing the field of CNMs’ research, we can unlock the full potential of LCMs and contribute to a more sustainable and technologically advanced future.
Acknowledgement: The authors thank the National Research Centre, Egypt, for the facilities’ support.
Funding Statement: The authors acknowledge the financial support of this paper by the Science, Technology & Innovation Funding Authority (STDF) under grant (45892).
Author Contributions: Hebat-Allah S. Tohamy: Idea, visualization, conceptualization, drawings, writing—original draft, and reviewing. Mohamed El-Sakhawy: Supervision, preparing tables, review & editing. Samir Kamel: Supervision, preparing tables, review & editing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Sugiarto S, Pong RR, Tan YC, Leow Y, Sathasivam T, Zhu Q, et al. Advances in sustainable polymeric materials from lignocellulosic biomass. Mater Today Chem. 2022;26:101022. doi:10.1016/j.mtchem.2022.101022. [Google Scholar] [CrossRef]
2. Isikgor FH, Becer CR. Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem. 2015;6(25):4497–559. doi:10.1039/C5PY00263J. [Google Scholar] [CrossRef]
3. Song M, Cai D. Graphene functionalization: a review. Polym-Graph Nanocomposit. 2012;26(26):1–51. [Google Scholar]
4. Tohamy H-AS, El-Sakhawy M, Kamel S. Fullerenes and tree-shaped/fingerprinted carbon quantum dots for chromium adsorption via microwave-assisted synthesis. RSC Adv. 2024;14(35):25785–92. doi:10.1039/D4RA04527K. [Google Scholar] [PubMed] [CrossRef]
5. Razaq A, Bibi F, Zheng X, Papadakis R, Jafri SHM, Li H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: from fabrication to applications. Materials. 2022;15(3):1012. doi:10.3390/ma15031012. [Google Scholar] [PubMed] [CrossRef]
6. Nasir S, Hussein MZ, Zainal Z, Yusof NA. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials. 2018;11(2):295. doi:10.3390/ma11020295. [Google Scholar] [PubMed] [CrossRef]
7. Korczeniewski E, Bryk P, Szymański GS, Kowalczyk P, Zięba M, Zięba W, et al. Open sensu shaped graphene oxide and modern carbon nanomaterials in translucent hydrophobic and omniphobic surfaces-insight into wetting mechanisms. Chem Eng J. 2023;462:142237. doi:10.1016/j.cej.2023.142237. [Google Scholar] [CrossRef]
8. Wang MW, Fan W, Li X, Liu Y, Li Z, Jiang W, et al. Molecular carbons: how far can we go? ACS Nano. 2023;17(21):20734–52. doi:10.1021/acsnano.3c07970. [Google Scholar] [PubMed] [CrossRef]
9. Tohamy H-AS. Fluorescence ‘turn-on’ probe for chromium reduction, adsorption and detection based on cellulosic nitrogen-doped carbon quantum dots hydrogels. Gels. 2024;10(5):296. doi:10.3390/gels10050296. [Google Scholar] [PubMed] [CrossRef]
10. Vaka M, Ramakrishna TRB, Mohammad K, Walvekar R. Low-dimensional carbon nanomaterials: synthesis, properties, and applications related to heat transfer, energy harvesting, and energy storage. In: Spectroscopy and characterization of nanomaterials and novel materials: experiments, modeling, simulations, and applications; 2022. p. 33–53. doi:10.1002/9783527833689.ch2. [Google Scholar] [CrossRef]
11. Chen L, Li N, Yu X, Zhang S, Liu C, Song Y, et al. A general way to manipulate electrical conductivity of graphene. Chem Eng J. 2023;462:142139. doi:10.1016/j.cej.2023.142139. [Google Scholar] [CrossRef]
12. Thejas R, Naveen CS, Khan MI, Prasanna GD, Reddy S, Oreijah M, et al. A review on electrical and gas-sensing properties of reduced graphene oxide-metal oxide nanocomposites. Biomass Convers Bioref. 2024;14(12):12625–35. doi:10.1007/s13399-022-03258-7. [Google Scholar] [CrossRef]
13. Ha TJ, Hedau B, Park SJ. Electronic properties of zero-dimensional carbon-based nanomaterials. In: Zero-dimensional carbon nanomaterials. 2024. p. 185–248. [Google Scholar]
14. Sayyed AJ, Pinjari DV, Sonawane SH, Bhanvase BA, Sheikh J, Sillanpää M. Cellulose-based nanomaterials for water and wastewater treatments: a review. J Environ Chem Eng. 2021;9(6):106626. doi:10.1016/j.jece.2021.106626. [Google Scholar] [CrossRef]
15. Mohamad Amini MH. Forest and agricultural biomass. In: Plant biomass derived materials: sources, extractions, and applications. 2024. p. 271–90. [Google Scholar]
16. Blasi A, Verardi A, Lopresto CG, Siciliano S, Sangiorgio P. Lignocellulosic agricultural waste valorization to obtain valuable products: an overview. Recycling. 2023;8(4):61. doi:10.3390/recycling8040061. [Google Scholar] [CrossRef]
17. Martin AF, Tobimatsu Y, Lam PY, Matsumoto N, Tanaka T, Suzuki S, et al. Lignocellulose molecular assembly and deconstruction properties of lignin-altered rice mutants. Plant Physiol. 2023;191(1):70–86. doi:10.1093/plphys/kiac432. [Google Scholar] [PubMed] [CrossRef]
18. Yan J, Oyedeji O, Leal JH, Donohoe BS, Semelsberger TA, Li C, et al. Characterizing variability in lignocellulosic biomass: a review. ACS Sustain Chem Eng. 2020;8(22):8059–85. doi:10.1021/acssuschemeng.9b06263. [Google Scholar] [CrossRef]
19. Xiao W, Sun R, Hu S, Meng C, Xie B, Yi M, et al. Recent advances and future perspective on lignocellulose-based materials as adsorbents in diverse water treatment applications. Int J Biol Macromol. 2023;253:126984. doi:10.1016/j.ijbiomac.2023.126984. [Google Scholar] [PubMed] [CrossRef]
20. Hassan MM, Wang XY, Bristi AA, Yang R, Li X, Lu Q. Composite scaffold of electrospun nano-porous cellulose acetate membrane casted with chitosan for flexible solid-state sodium-ion batteries. Nano Energy. 2024;128:109971. doi:10.1016/j.nanoen.2024.109971. [Google Scholar] [CrossRef]
21. Fredricks JL, Jimenez AM, Grandgeorge P, Meidl R, Law E, Fan J, et al. Hierarchical biopolymer-based materials and composites. J Polym Sci. 2023;61(21):2585–632. doi:10.1002/pola.v61.21. [Google Scholar] [CrossRef]
22. Nasrollahzadeh M, Sajjadi M, Iravani S, Starch RS. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: a review. Carbohydr Polym. 2021;251:116986. doi:10.1016/j.carbpol.2020.116986. [Google Scholar] [PubMed] [CrossRef]
23. Nawaz H, Zhang X, Chen S, You T, Xu F. Recent studies on cellulose-based fluorescent smart materials and their applications: a comprehensive review. Carbohydr Polym. 2021;267:118135. doi:10.1016/j.carbpol.2021.118135. [Google Scholar] [PubMed] [CrossRef]
24. Fahmy TY, Fahmy Y, Mobarak F, El-Sakhawy M, Abou-Zeid RE. Biomass pyrolysis: past, present, and future. Environ Develop Sustain. 2020;22:17–32. doi:10.1007/s10668-018-0200-5. [Google Scholar] [CrossRef]
25. Muir VG, Burdick JA. Chemically modified biopolymers for the formation of biomedical hydrogels. Chem Rev. 2020;121(18):10908–49. [Google Scholar] [PubMed]
26. Yao T, Song J, Hong Y, Gan Y, Ren X, Du K. Application of cellulose to chromatographic media: cellulose dissolution, and media fabrication and derivatization. J Chromatogr A. 2023;1705:464202. doi:10.1016/j.chroma.2023.464202. [Google Scholar] [PubMed] [CrossRef]
27. Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon LC, Bacabac RG, et al. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28(4):1893–931. doi:10.1007/s10570-020-03674-w. [Google Scholar] [CrossRef]
28. Nugroho RWN, Tardy BL, Eldin SM, Ilyas RA, Mahardika M, Masruchin N. Controlling the critical parameters of ultrasonication to affect the dispersion state, isolation, and structural color of cellulose nanocrystals. Ultrason Sonochem. 2023;106581. doi:10.1016/j.ultsonch.2023.106581. [Google Scholar] [PubMed] [CrossRef]
29. Thapliyal D, Verma S, Sen P, Kumar R, Thakur A, Tiwari AK, et al. Natural fibers composites: origin, importance, consumption pattern, and challenges. J Composit Sci. 2023;7(12):506. doi:10.3390/jcs7120506. [Google Scholar] [CrossRef]
30. Rahaman T, Biswas S, Ghorai S, Bera S, Dey S, Guha S, et al. Integrated application of morphological, anatomical, biochemical and physico-chemical methods to identify superior, lignocellulosic grass feedstocks for bioenergy purposes. Renew Sustain Energ Rev. 2023;187:113738. doi:10.1016/j.rser.2023.113738. [Google Scholar] [CrossRef]
31. Rao J, Lv Z, Chen G, Peng F. Hemicellulose: structure, chemical modification, and application. Prog Polym Sci. 2023;140:101675. doi:10.1016/j.progpolymsci.2023.101675. [Google Scholar] [CrossRef]
32. Ali S, Rani A, Dar MA, Qaisrani MM, Noman M, Yoganathan K, et al. Recent advances in characterization and valorization of lignin and its value-added products: challenges and future perspectives. Biomass. 2023;4(3):947–77. [Google Scholar]
33. Jia XB, Wang J, Liu YF, Zhu YF, Li JY, Li YJ, et al. Facilitating layered oxide cathodes based on orbital hybridization for sodium-ion batteries: marvelous air stability, controllable high voltage, and anion redox chemistry. Adv Mater Deerfield. 2024;36(15):2307938. doi:10.1002/adma.v36.15. [Google Scholar] [CrossRef]
34. Goesten MG, Schoop LM. Diradicals as topological charge carriers in metal-organic toy model Pt3(HIB)2. J Am Chem Soc. 2024;146(43):29599–608. doi:10.1021/jacs.4c09993. [Google Scholar] [PubMed] [CrossRef]
35. Liu Y, Xu D, Wang Y, Hu K, Yao Z. Superhard BN allotropes with tunable hybridization sp2/sp3 ratios by compressed nanotubes. Diam Relat Mater. 2023;139:110313. doi:10.1016/j.diamond.2023.110313. [Google Scholar] [CrossRef]
36. Wang C, Jia L, Jin Y, Qin S. Study on regeneration mechanism of composite adsorbent by Mg-MOF-74-based modified biochar. Sci Total Environ. 2024;946:173944. doi:10.1016/j.scitotenv.2024.173944. [Google Scholar] [PubMed] [CrossRef]
37. Wang Y, Yang P, Zheng L, Shi X, Zheng H. Carbon nanomaterials with sp2 or/and sp hybridization in energy conversion and storage applications: a review. Energy Storage Mater. 2020;26:349–70. doi:10.1016/j.ensm.2019.11.006. [Google Scholar] [CrossRef]
38. Börner A, Zeidler J. The periodic table of elements and basic consequences for the structure of natural substances and the course of biochemical processes. In: The chemistry of biology: basis and origin of evolution. Berlin, Heidelberg: Springer Berlin Heidelberg; 2023. p. 1–79. [Google Scholar]
39. Tiwari SK, Bystrzejewski M, De Adhikari A, Huczko A, Wang N. Methods for the conversion of biomass waste into value-added carbon nanomaterials: recent progress and applications. Prog Energy Combust Sci. 2022;92:101023. doi:10.1016/j.pecs.2022.101023. [Google Scholar] [CrossRef]
40. Somyanonthanakun W, Greszta A, Roberts AJ, Thongmee S. Sugarcane bagasse-derived activated carbon as a potential material for lead ions removal from aqueous solution and supercapacitor energy storage application. Sustainability. 2023;15(6):5566. doi:10.3390/su15065566. [Google Scholar] [CrossRef]
41. Zhang H, Sun H, Huang S, Lan J, Li H, Yue H. Biomass-derived carbon materials for electrochemical sensing: recent advances and future perspectives. Crit Rev Anal Chem. 2024;1–26. doi:10.1080/10408347.2024.2401504. [Google Scholar] [PubMed] [CrossRef]
42. Li Y, Xia D, Tao L, Xu Z, Yu D, Jin Q, et al. Hydrothermally assisted conversion of switchgrass into hard carbon as anode materials for sodium-ion batteries. ACS Appl Mater Interf. 2024;16(22):28461–72. doi:10.1021/acsami.4c02734. [Google Scholar] [PubMed] [CrossRef]
43. Patwary MAM, Rahman MA, Haque SR, Ghos BC, Rahman MR, Matin MM, et al. Biosynthetic and natural nanocarbon production. In: Advanced nanocarbon polymer biocomposites. UK: Woodhead Publishing; 2024. p. 105–84. [Google Scholar]
44. Qasim M, Clarkson AN, Hinkley SF. Green synthesis of carbon nanoparticles (CNPs) from biomass for biomedical applications. Int J Mol Sci. 2023;24(2):1023. doi:10.3390/ijms24021023. [Google Scholar] [PubMed] [CrossRef]
45. Tang Y, He J, Peng J, Yang J, Wu Z, Liu P, et al. Electrochemical behavior of the biomass hard carbon derived from waste corncob as a sodium-ion battery anode. Energy Fuels. 2024;38(8):7389–98. doi:10.1021/acs.energyfuels.4c00564. [Google Scholar] [CrossRef]
46. Alwi MMA, Singh J, Choudhury A, Hossain SS, Butt AN. Improvement in electrochemical performance of waste sugarcane bagasse-derived carbon via hybridization with SiO2 nanospheres. Molecules. 2024;29(7):1569. doi:10.3390/molecules29071569. [Google Scholar] [PubMed] [CrossRef]
47. Saji VS. Nanocarbons-based trifunctional electrocatalysts for overall water splitting and metal-air batteries: metal-free and hybrid electrocatalysts. Chem-Asian J. 2024;e202400712. doi:10.1002/asia.202400712. [Google Scholar] [PubMed] [CrossRef]
48. Stepacheva AA, Markova ME, Lugovoy YV, Kosivtsov YY, Matveeva VG, Sulman MG. Plant-biomass-derived carbon materials as catalyst support, a brief review. Catalysts. 2023;13(4):655. doi:10.3390/catal13040655. [Google Scholar] [CrossRef]
49. Nazir G, Rehman A, Hussain S, Mahmood Q, Fteiti M, Heo K, et al. Towards a sustainable conversion of biomass/biowaste to porous carbons for CO2 adsorption: recent advances, current challenges, and future directions. Green Chem. 2023;25(13):4941–80. doi:10.1039/D3GC00636K. [Google Scholar] [CrossRef]
50. Liu H, Huang X, Zhou M, Gu J, Xu M, Jiang L, et al. Efficient conversion of biomass waste to N/O co-doped hierarchical porous carbon for high performance supercapacitors. J Anal Appl Pyrol. 2023;169:105844. doi:10.1016/j.jaap.2022.105844. [Google Scholar] [CrossRef]
51. Verma J. Preparation and characterization of activated carbon from agro biomass (Doctoral Dissertation). Haryana Agricultural University Hisar: India; 2023. [Google Scholar]
52. Singh P, Kumar S, Kumar P, Kataria N, Bhankar V, Kumar K, et al. Assessment of biomass-derived carbon dots as highly sensitive and selective templates for the sensing of hazardous ions. Nanoscale. 2023;15(40):16241–67. doi:10.1039/D3NR01966G. [Google Scholar] [PubMed] [CrossRef]
53. Alvira D, Antorán D, Manyà JJ. Plant-derived hard carbon as anode for sodium-ion batteries: a comprehensive review to guide interdisciplinary research. Chem Eng J. 2022;447:137468. doi:10.1016/j.cej.2022.137468. [Google Scholar] [CrossRef]
54. Zhuo C, Alves JO, Tenorio JA, Levendis YA. Synthesis of carbon nanomaterials through up-cycling agricultural and municipal solid wastes. Ind Eng Chem Res. 2012;51(7):2922–30. doi:10.1021/ie202711h. [Google Scholar] [CrossRef]
55. Al Masud MA, Shin WS, Kim DG. Fe-doped kelp biochar-assisted peroxymonosulfate activation for ciprofloxacin degradation: multiple active site-triggered radical and non-radical mechanisms. Chem Eng J. 2023;471:144519. doi:10.1016/j.cej.2023.144519. [Google Scholar] [CrossRef]
56. Chen C, Qiu S, Ling H, Zhao J, Fan D, Zhu J. Effect of transition metal oxide on microwave co-pyrolysis of sugarcane bagasse and Chlorella vulgaris for producing bio-oil. Ind Crops Prod. 2023;199:116756. doi:10.1016/j.indcrop.2023.116756. [Google Scholar] [CrossRef]
57. Hughes MA. An investigation of the reduction of molten carbonate salts for the formation of electrochemically active supercapacitor materials (Doctoral Dissertation). University of Newcastle: Australia; 2019. [Google Scholar]
58. Ferdous AR, Shah SS, Shaikh MN, Barai HR, Marwat MA, Oyama M, et al. Advancements in biomass-derived activated carbon for sustainable hydrogen storage: a comprehensive review. Chem-Asian J. 2024;19(16):e202300780. doi:10.1002/asia.v19.16. [Google Scholar] [CrossRef]
59. Solikhin A, Syamani FA, Hastati DY, Budiman I, Purnawati R, Mubarok M, et al. Review on lignocellulose valorization for nanocarbon and its composites: starting from laboratory studies to business application. Int J Biol Macromol. 2023;239:124082. doi:10.1016/j.ijbiomac.2023.124082. [Google Scholar] [PubMed] [CrossRef]
60. Cui L, Ren X, Sun M, Liu H, Xia L. Carbon dots: synthesis, properties and applications. Nanomaterials. 2021;11(12):3419. doi:10.3390/nano11123419. [Google Scholar] [PubMed] [CrossRef]
61. Viscusi G, Mottola S, Tohamy HAS, Gorrasi G, De Marco I. Design of cellulose acetate electrospun membranes loaded with N-doped carbon quantum dots for water remediation. In: IWA Regional Membrane Technology Conference, 2024 Jun; Cham: Springer Nature Switzerland; p. 133–7. [Google Scholar]
62. Bydzovska I, Shagieva E, Gordeev I, Romanyuk O, Nemeckova Z, Henych J, et al. Laser-induced modification of hydrogenated detonation nanodiamonds in ethanol. Nanomaterials. 2021;11(9):2251. doi:10.3390/nano11092251. [Google Scholar] [PubMed] [CrossRef]
63. Castaño JA, Betancourth JG, Caicedo DL, Visbal R, Chaur MN. Synthesis and electrochemical applications of carbon nano-onions. Curr Nanosci. 2024;20(1):47–73. doi:10.2174/1573413719666230329134840. [Google Scholar] [CrossRef]
64. Pande PM, Pandey SP, Shirsat SP, Wagh SM. Zero-, one-, two-, and three-dimensional carbon nanostructures derived from bio-based material. In: Bio-derived carbon nanostructures. The Netherlands: Elsevier; 2024. p. 83–107. [Google Scholar]
65. Qin JX, Yang XG, Lv CF, Li YZ, Liu KK, Zang JH, et al. Nanodiamonds: synthesis, properties, and applications in nanomedicine. Mater Des. 2021;210:110091. doi:10.1016/j.matdes.2021.110091. [Google Scholar] [CrossRef]
66. Boruah A, Saikia BK. Synthesis, characterization, properties, and novel applications of fluorescent nanodiamonds. J Fluoresc. 2022;32(3):863–85. doi:10.1007/s10895-022-02898-2. [Google Scholar] [PubMed] [CrossRef]
67. Ghasemlou M, Pn N, Alexander K, Zavabeti A, Sherrell PC, Ivanova EP, et al. Fluorescent nanocarbons: from synthesis and structure to cancer imaging and therapy. Adv Mater Deerfield. 2024;36(19):2312474. doi:10.1002/adma.v36.19. [Google Scholar] [CrossRef]
68. Mandal S. Nucleation of diamond films on heterogeneous substrates: a review. RSC Adv. 2021;11(17):10159–82. doi:10.1039/D1RA00397F. [Google Scholar] [PubMed] [CrossRef]
69. Hunter RD, Ramírez-Rico J, Schnepp Z. Iron-catalyzed graphitization for the synthesis of nanostructured graphitic carbons. J Mater Chem A. 2022;10(9):4489–516. doi:10.1039/D1TA09654K. [Google Scholar] [CrossRef]
70. Jiang C, Bo J, Xiao X, Zhang S, Wang Z, Yan G, et al. Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manag. 2020;102:732–42. doi:10.1016/j.wasman.2019.11.019. [Google Scholar] [PubMed] [CrossRef]
71. Lai C, Lin S, Huang X, Jin Y. Synthesis and properties of carbon quantum dots and their research progress in cancer treatment. Dyes Pigm. 2021;196:109766. doi:10.1016/j.dyepig.2021.109766. [Google Scholar] [CrossRef]
72. Tohamy HAS. Novel, speedy, and eco-friendly carboxymethyl cellulose-nitrogen doped carbon dots biosensors with DFT calculations, molecular docking, and experimental validation. Gels. 2024;10(11):686. doi:10.3390/gels10110686. [Google Scholar] [PubMed] [CrossRef]
73. Tian L, Li Z, Wang P, Zhai X, Wang X, Li T. Carbon quantum dots for advanced electrocatalysis. J Energy Chem. 2021;55:279–94. doi:10.1016/j.jechem.2020.06.057. [Google Scholar] [CrossRef]
74. Rodriguez-Padron D, Algarra M, Tarelho LA, Frade J, Franco A, de Miguel G, et al. Catalyzed microwave-assisted preparation of carbon quantum dots from lignocellulosic residues. ACS Sustain Chem Eng. 2018;6(6):7200–5. doi:10.1021/acssuschemeng.7b03848. [Google Scholar] [CrossRef]
75. Matveeva VG, Bronstein LM. From renewable biomass to nanomaterials: does biomass origin matter? Prog Mater Sci. 2022;130:100999. doi:10.1016/j.pmatsci.2022.100999. [Google Scholar] [CrossRef]
76. Zhao S, Song X, Chai X, Zhao P, He H, Liu Z. Green production of fluorescent carbon quantum dots based on pine wood and its application in the detection of Fe3+. J Clean Prod. 2020;263:121561. doi:10.1016/j.jclepro.2020.121561. [Google Scholar] [CrossRef]
77. Dhariwal J, Rao GK, Vaya D. Recent advancements towards the green synthesis of carbon quantum dots as an innovative and eco-friendly solution for metal ion sensing and monitoring. RSC Sustain. 2024;(1):11–36. [Google Scholar]
78. Li Z, Wang Q, Zhou Z, Zhao S, Zhong S, Xu L, et al. Green synthesis of carbon quantum dots from corn stalk shell by hydrothermal approach in near-critical water and applications in detecting and bioimaging. Microchem J. 2021;166:106250. doi:10.1016/j.microc.2021.106250. [Google Scholar] [CrossRef]
79. Liu L, Yun S, Ke T, Wang K, An J, Liu J. Dual utilization of aloe peel: aloe peel-derived carbon quantum dots enhanced anaerobic co-digestion of aloe peel. Waste Manag. 2023;159:163–73. doi:10.1016/j.wasman.2023.01.036. [Google Scholar] [PubMed] [CrossRef]
80. Xu N, Gao S, Xu C, Fang Y, Xu L, Zhang W. Carbon quantum dots derived from waste acorn cups and its application as an ultraviolet absorbent for polyvinyl alcohol film. Appl Surf Sci. 2021;556:149774. doi:10.1016/j.apsusc.2021.149774. [Google Scholar] [CrossRef]
81. Xu J, Zhou P, Yuan L, Liu X, Ma J, Zhang C. Dual lignin valorization enabled by carbon quantum dots and lithium-sulfur cathode. Ind Crops Prod. 2021;170:113801. doi:10.1016/j.indcrop.2021.113801. [Google Scholar] [CrossRef]
82. Tao X, Liao M, Wu F, Jiang Y, Sun J, Shi S. Designing of biomass-derived carbon quantum dots@polyvinyl alcohol film with excellent fluorescent performance and pH-responsiveness for intelligent detection. Chem Eng J. 2022;443:136442. doi:10.1016/j.cej.2022.136442. [Google Scholar] [CrossRef]
83. Liu W, Jiang C, Zhang L, Li X, Hou Q, Ni Y. Valorization of cellulose pulp derived carbon quantum dots by controllable fractionation. Ind Crops Prod. 2022;188:115560. doi:10.1016/j.indcrop.2022.115560. [Google Scholar] [CrossRef]
84. Zhang H, Li S, Xu L, Momen R, Deng W, Hu J, et al. High-yield carbon dots interlayer for ultra-stable zinc batteries. Adv Energy Mater. 2022;12(26):2200665. doi:10.1002/aenm.v12.26. [Google Scholar] [CrossRef]
85. Kaushal G, Kaur B, Kumar P, Dhiman M, Khanra P. A short review: synthesis methods and application of biomolecule derived graphene quantum-dots. ECS Trans. 2022;107(1):6297. doi:10.1149/10701.6297ecst. [Google Scholar] [PubMed] [CrossRef]
86. Saud A, Oves M, Shahadat M, Arshad M, Adnan R, Qureshi MA. Graphene-based organic-inorganic hybrid quantum dots for organic pollutants treatment. In: Graphene quantum dots. The Netherlands: Elsevier, Woodhead Publishing; 2023. p. 133–55. [Google Scholar]
87. Wang R, Jiao L, Zhou X, Guo Z, Bian H, Dai H. Highly fluorescent graphene quantum dots from biorefinery waste for tri-channel sensitive detection of Fe3+ ions. J Hazard Mater. 2021;412:125096. doi:10.1016/j.jhazmat.2021.125096. [Google Scholar] [PubMed] [CrossRef]
88. Boustani I. One-dimensional nanotubes. In: Molecular modelling and synthesis of nanomaterials: applications in carbon-and boron-based nanotechnology. Cham: Springer International Publishing; 2020. p. 363–413. [Google Scholar]
89. Guerrero GP. Development of new probes based on carbon nanocones for near-field microscopies. Université Paul Sabatier-Toulouse III: France; 2020. [Google Scholar]
90. Adeoye AO, Yelwa JM, Imam N, Quadri RO, Lawal OS, Malomo D, et al. Pyrolysis of plastic wastes towards achieving a circular economy: an advanced chemistry and technical approach. In: Dynamics of transportation ecosystem, modeling, and control. Singapore: Springer Nature Singapore; 2024. p. 215–59. [Google Scholar]
91. Du B, Chai L, Zhu H, Cheng J, Wang X, Chen X, et al. Effective fractionation strategy of sugarcane bagasse lignin to fabricate quality lignin-based carbon nanofibers supercapacitors. Int J Biol Macromol. 2021;184:604–17. doi:10.1016/j.ijbiomac.2021.06.061. [Google Scholar] [PubMed] [CrossRef]
92. Abdulhameed A, Wahab NZA, Mohtar MN, Hamidon MN, Shafie S, Halin IA. Methods and applications of electrical conductivity enhancement of materials using carbon nanotubes. J Electron Mater. 2021;50:3207–21. doi:10.1007/s11664-021-08928-2. [Google Scholar] [CrossRef]
93. Chen X, Pang X, Fauteux-Lefebvre C. The base versus tip growth mode of carbon nanotubes by catalytic hydrocarbon cracking: review, challenges and opportunities. Carbon Trends. 2023;12:100273. doi:10.1016/j.cartre.2023.100273. [Google Scholar] [CrossRef]
94. Reizer E, Viskolcz B, Fiser B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: a mini-review. Chemosphere. 2022;291:132793. doi:10.1016/j.chemosphere.2021.132793. [Google Scholar] [PubMed] [CrossRef]
95. Soffian MS, Halim FZA, Aziz F, Rahman MA, Amin MAM, Chee DNA. Carbon-based material derived from biomass waste for wastewater treatment. Environ Adv. 2022;9:100259. doi:10.1016/j.envadv.2022.100259. [Google Scholar] [CrossRef]
96. Omoriyekomwan JE, Tahmasebi A, Dou J, Wang R, Yu J. A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process Technol. 2021;214:106686. doi:10.1016/j.fuproc.2020.106686. [Google Scholar] [CrossRef]
97. Janas D. From bio to nano: a review of sustainable methods of synthesis of carbon nanotubes. Sustainability. 2020;12(10):4115. doi:10.3390/su12104115. [Google Scholar] [CrossRef]
98. Song D, Zheng D, Li Z, Wang C, Li J, Zhang M. Research advances in wood composites in applications of industrial wastewater purification and solar-driven seawater desalination. Polymers. 2023;15(24):4712. doi:10.3390/polym15244712. [Google Scholar] [PubMed] [CrossRef]
99. Ojrzyńska M, Daniewski AR, Wilczyński K, Jamroz J, Dużyńska A, Zdrojek M. High-quality graphene nanoplatelets production with 100% yield based on popular fertilizer industry feedstock. J Phys Chem C. 2023;128(1):516–24. [Google Scholar]
100. Liu Z, Navik R, Tan H, Xiang Q, Goto M, Ibarra RM, et al. Graphene-based materials prepared by supercritical fluid technology and its application in energy storage. J Supercrit Fluids. 2022;188:105672. doi:10.1016/j.supflu.2022.105672. [Google Scholar] [CrossRef]
101. Yu W, Sisi L, Haiyan Y, Jie L. Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv. 2020;10(26):15328–45. doi:10.1039/D0RA01068E. [Google Scholar] [PubMed] [CrossRef]
102. Madima N, Mishra SB, Inamuddin I, Mishra AK. Carbon-based nanomaterials for remediation of organic and inorganic pollutants from wastewater. A review. Environ Chem Lett. 2020;18:1169–91. doi:10.1007/s10311-020-01001-0. [Google Scholar] [CrossRef]
103. Mohammed S. Graphene oxide: a mini-review on the versatility and challenges as a membrane material for solvent-based separation. Chem Eng J Adv. 2022;12:100392. doi:10.1016/j.ceja.2022.100392. [Google Scholar] [CrossRef]
104. Liou Y-J, Huang W-J. A process for preparing high graphene sheet content carbon materials from biochar materials. Electroplat Nanostruct. 2015. doi:10.5772/61200. [Google Scholar] [CrossRef]
105. Veeramani V, Sivakumar M, Chen SM, Madhu R, Alamri HR, Alothman ZA, et al. Lignocellulosic biomass-derived, graphene sheet-like porous activated carbon for electrochemical supercapacitor and catechin sensing. RSC Adv. 2017;7(72):45668–75. doi:10.1039/C7RA07810B. [Google Scholar] [CrossRef]
106. Guevara-Martínez SJ, Espino-Valencia J, del Carmen Chávez-Parga M, Arroyo-Albiter M. Synthesis of graphene oxide from agave fiber Tequilana Weber by hydrothermal method. Nanotechnology. 2021;32(45):455704. doi:10.1088/1361-6528/ac1752. [Google Scholar] [PubMed] [CrossRef]
107. Wu Y, Wang S, Komvopoulos K. A review of graphene synthesis by indirect and direct deposition methods. J Mater Res. 2020;35(1):76–89. doi:10.1557/jmr.2019.377. [Google Scholar] [CrossRef]
108. Astle MA, Weilhard A, Rance GA, LeMercier TM, Stoppiello CT, Norman LT, et al. Defect etching in carbon nanotube walls for porous carbon nanoreactors: implications for CO2 sorption and the hydrosilylation of phenylacetylene. ACS Appl Nano Mater. 2022;5(2):2075–86. doi:10.1021/acsanm.1c03803. [Google Scholar] [PubMed] [CrossRef]
109. Zhang Y, Shu H, Chen Z, Mu G, Sui Y, Liang Y, et al. Chemical vapor deposition growth and characterization of graphite-like film. Mater Res Express. 2020;7(1):015609. doi:10.1088/2053-1591/ab664b. [Google Scholar] [CrossRef]
110. Hunter RD, Davies J, Hérou SJA, Kulak A, Schnepp Z. Milling as a route to porous graphitic carbons from biomass. Philosoph Trans Royal Soc A. 2021;379(2209):20200336. doi:10.1098/rsta.2020.0336. [Google Scholar] [PubMed] [CrossRef]
111. Jabarullah NH, Kamal AS, Othman R. A modification of palm waste lignocellulosic materials into biographite using iron and nickel catalyst. Processes. 2021;9(6):1079. doi:10.3390/pr9061079. [Google Scholar] [CrossRef]
112. Riley PR, Joshi P, Narayan J, Narayan RJ. Enhanced nucleation and large-scale growth of CVD diamond via surface-modification of silicon-incorporated diamond-like carbon thin films. Diam Relat Mater. 2021;120:108630. doi:10.1016/j.diamond.2021.108630. [Google Scholar] [CrossRef]
113. Frese N, Taylor Mitchell S, Bowers A, Gölzhäuser A, Sattler K. Diamond-like carbon nanofoam from low-temperature hydrothermal carbonization of a sucrose/naphthalene precursor solution. C. 2017;3(3):23. doi:10.3390/c3030023. [Google Scholar] [CrossRef]
114. Mallakpour S, Hussain CM. Environmental applications of carbon nanomaterials-based devices. Germany: Wiley-VCH; 2021. [Google Scholar]
115. Abd-Elhamid AI, Elgoud EA, Emam SS, Aly HF. Superior adsorption performance of citrate modified graphene oxide as nano material for removal organic and inorganic pollutants from aqueous solution. Sci Rep. 2022;12(1):9204. doi:10.1038/s41598-022-13111-6. [Google Scholar] [PubMed] [CrossRef]
116. Srivastava RK, Shetti NP, Reddy KR, Kwon EE, Nadagouda MN, Aminabhavi TM. Biomass utilization and production of biofuels from carbon neutral materials. Environ Pollut. 2021;276(10):116731. doi:10.1016/j.envpol.2021.116731. [Google Scholar] [PubMed] [CrossRef]
117. Zamel D, Khan AU, Khan AN, Waris A, Ilyas M, Ali A, et al. Regenerated cellulose and composites for biomedical applications. In: Regenerated cellulose and composites: morphology-property relationship. Singapore: Springer; 2023. p. 265–311. doi:10.1007/978-981-99-1655-9_10. [Google Scholar] [CrossRef]
118. Dwiyaniti M, Krisnawati L, Pramono AE, Subhan A, Setiabudy R, Hudaya C. Electrochemical characteristics of sugarcane bagasse-activated carbon as cathode material of lithium-ion capacitors. J Appl Res Technol. 2023;21(4):571–80. doi:10.22201/icat.24486736e.2023.21.4.1976. [Google Scholar] [CrossRef]
119. Tohamy HAS. Greener, safer packaging: carbon nanotubes/gelatin-enhanced recycled paper for fire retardation with DFT calculations. J Renew Mater. 2024. doi:10.32604/jrm.2024.054977. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools