 Open Access
Open Access
ARTICLE
Preliminary Study: Furfural Production from Oat Husks via Phosphorus-Containing Catalysts Catalyzed Hydrothermal Pretreatment in the Context of Biorefinery
Biorefinery Laboratory, Latvian State Institute of Wood Chemistry, Riga, LV-1001, Latvia
* Corresponding Author: Prans Brazdausks. Email:
(This article belongs to the Special Issue: Advances in Biorefinery Technologies and Products – 2024)
Journal of Renewable Materials 2025, 13(4), 719-730. https://doi.org/10.32604/jrm.2025.057944
Received 31 August 2024; Accepted 07 January 2025; Issue published 21 April 2025
Abstract
Oat husks, a byproduct of oat milling operations with limited economic value, present a promising feedstock for biorefinery processes due to their chemical composition. This study investigates the conversion of C5 carbohydrates in oat husks into furfural through hydrothermal pretreatment using various phosphate-based catalysts, including H3PO4, NH4H2PO4, NaH2PO4, KH2PO4, K2HPO4 and K3PO4 as catalyst. The catalysts’ effectiveness in promoting furfural production was evaluated under identical hydrothermal conditions (treatment time for 60 min at a constant temperature of 170°C and a catalyst amount). Continuous water steam was used to strip furfural from the reaction zone and minimize its degradation. Results indicated that H3PO4 was the most effective catalyst, achieving a furfural yield of 13.99 wt.%, which corresponds to approximately 57% of the theoretical yield. NH4H2PO4 also showed moderate effectiveness, while sodium and potassium phosphate salts were significantly less effective. A scanning electron microscope analysis shows that catalysts with lower pH may disrupt the oat husks external layer thus providing a higher C5 carbohydrates conversion rate into furfural. The chemical complexity of oat husk contributes to side reactions between its carbohydrates and lignin during the hydrothermal treatment. This results in an increase in acid-insoluble lignin and inorganic matter in the oat husk lignocellulosic residue, which can reduce the effectiveness of further cellulose saccharification by enzymatic hydrolysis.Graphic Abstract
Keywords
Today, as we gradually transition from a fossil-based economy, opportunities emerge for an industrial revolution to accelerate the adoption of clean technologies in the market. It aims to bring an end to the era of fossil-based products. Lignocellulosic biomass (LCB), a widely available resource, is a promising alternative to replace a fossil-based economy [1,2]. Its chemical composition offers a remarkable opportunity to produce a lot of high-valued platform chemicals and bioproducts (e.g., furfural, 5-HMF, succinic acid, biofuels, etc.) [3,4]. Despite its abundance and valuable chemical properties, current methods for converting LCB into bioproducts are limited. The root of this challenge lies in the structure of LCB, which consists of a matrix of cross-linked biopolymer networks (primarily of lignin, hemicellulose, and cellulose), with different chemical and physical properties [1,4]. Cellulose is a polysaccharide composed of long chains of β-1,4 linked glucan units, which form long crystalline regions. Hemicellulose (composed primarily of xylan) is a branched polysaccharide complex that wraps around the cellulose fibres, providing additional stability. Lignin, a complex polymer of aromatic compounds (aromatic alcohols of syringyl, guaiacyl, and p-hydroxyphenyl types), forms a network through ether and ester bindings [3,5,6]. Therefore, a highly selective approach is essential to effectively separate each LCB component without significant loss of initial mass and its original chemical properties.
In the biorefinery context, hemicellulose is the first component to be processed. Due to its amorphous and heterogeneous structure, hemicellulose is more sensitive to acids and bases and can be readily broken down at relatively low temperatures during chemical processing. Hemicellulose can be converted into a target product through dilute acid or alkaline pretreatment, implemented as either a one-step or two-step strategy [7,8]. Among these target products, furfural is considered one of the most valuable products that could be derived from the xylan-rich solution or the hemicellulose fraction of LCB. The direct, one-step production of furfural requires severe conditions and/or the use of more chemicals. In contrast, the two-step production requires gentle hydrolysis and separation of prehydrolysate, followed by more drastic hydrolysis conditions. Furfural itself is a reactive molecule with an aldehyde group attached to a furan ring, which allows it to undergo various chemical reactions, including condensation, hydrogenation and oxidation. This reactivity renders furfural a versatile chemical intermediate for industrial applications, particularly in the production of solvents, polymers, additives, resins, pharmaceuticals, chemicals and biofuels [6,9–11].
Since 1922, furfural has been produced on an industrial scale from the non-edible lignocellulosic biomass with a high C5 carbohydrate content (close to 30% of o.d.m.) such as sugarcane bagasse and corn cobs [12]. Today, most furfural is produced using the Chinese batch process, the “Quaker” batch process and the Rosenlew process technologies. To maintain the economic viability of these furfural production technologies, the C5 carbohydrate content in the LCB must be higher than 25 wt.%. The “Quaker” batch process, one of the earliest and most traditional technologies for furfural production, involves treating pentosan-rich LCB with acid hydrolysis at high temperatures (150°C–155°C) and pressure for approximately five hours, typically using sulfuric acid as a catalyst. To extract furfural from the hydrolysate steam is used, followed by steam distillation [13]. The Chinese batch process, a derivative of the “Quaker” batch process, operates on a similar principle. However, it uses slightly higher treatment temperatures (160°C–165°C) and a shorter residence time of about four hours, resulting in marginally higher furfural yields [13,14]. In contrast, the Rosenlew process is based on a continuous production method. Pentosan-rich LCB is continuously fed into a reactor, where it undergoes autohydrolysis to produce furfural. Acetic acid, formed during the treatment, serves as a catalyst. The furfural is then distilled using a steam distillation system. The LCB is treated for two hours at a temperature of 180°C. This method achieves higher productivity (around 55%–60%, calculated on the theoretically possible yield) and lower energy consumption per unit of product compared to the Chinese batch and “Quaker” batch process [13,14].
It is worth noting that the effectiveness of these technologies is only about 45%–60% of the theoretically possible furfural yield [12,13]. Additionally, these processes are energy-intensive, consume large amounts of water, and result in the irreversible degradation of over 50% of the initial cellulose during treatment [15]. This level of cellulose degradation limits the potential for using this lignocellulosic leftover in other bioproduct production and hinders its integration into modern biorefinery systems. For these reasons, increasing attention is being directed toward modern methods that are more energy-efficient, less polluting, and designed in compliance with contemporary environmental protection standards.
Studies on furfural production from LCB have been reviewed from various perspectives, including the influence of feedstock type, pretreatment conditions, reaction conditions, and process types [10,12,14]. Catalysts and solvents are considered to be two crucial factors in obtaining high furfural yields through the selective solubilization and conversion of LCB hemicellulose. So far, a lot of catalytic systems (mineral acids (H2SO4, HCl, H3PO4, etc.), organic acid (acetic acid, ionic liquids, etc.), metal salts (Al2(SO4)3, FeCl3, CrCl3, AlCl3, etc.) in water or aqueous biphasic (H2O/MIBK, H2O/GVL, H2O/MTHF, etc.) systems were studied [3,8–10,12,16,17]. The furfural yield can reach up to 96% of the theoretically possible yield [18,19]. Such high furfural yield is achieved in biphasic systems. From an economic point of view, efforts should focus on reducing the number of processing steps. Therefore, a simpler system should be sought for industrial applications.
The previous study [20] shows that under optimal hydrothermal conditions, using H3PO4 as a catalyst for the conversion of oat husks C5 carbohydrates into furfural, it is possible to produce 11.84 wt.% of oven-dried mass (60% of the theoretical amount possible). This approach enabled the preservation of 88.3 wt.% of the initial cellulose content in the lignocellulosic residue of oat husks. Another study [21] demonstrates that by partially replacing H3PO4 with a NaH2PO4 salt, it is possible to maintain an equivalent yield of furfural and retain even more cellulose (cellulose loss is no more than 2 wt.%) in the lignocellulosic residue after hydrothermal treatment. It is well known that not all LCBs can be treated with the same treatment method. Therefore, the current study aims to extend this research and identify the best phosphorus-containing salt catalyst that can be used to replace H3PO4 for selective furfural production from oat husks in the biorefinery context.
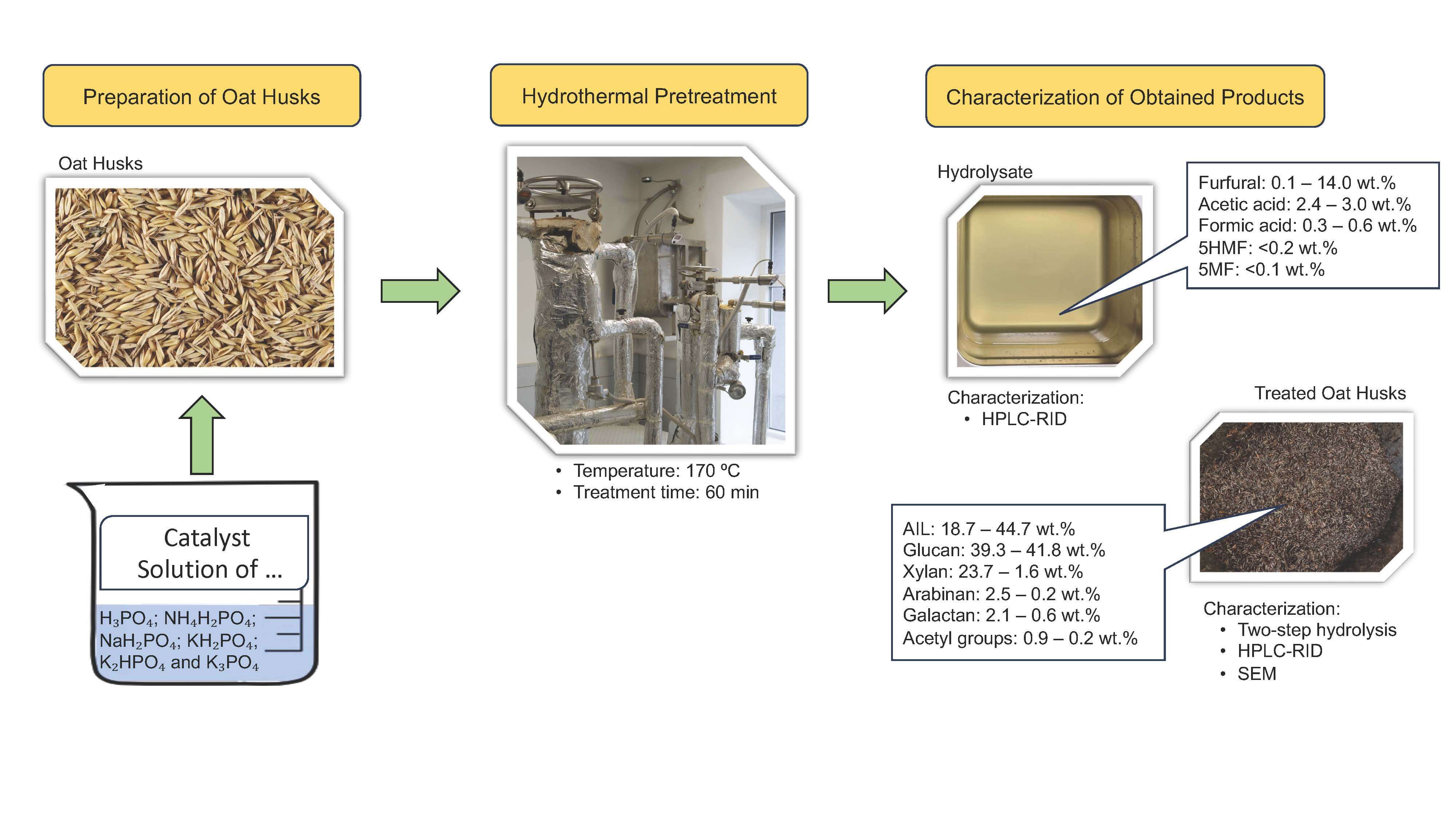
The oat husks were kindly provided by the local food producer J/S Dobeles Dzirnavnieks (Dobele, Latvia) and were used in their supplied form. The moisture content of the oat husks ranged from 8 to 9 wt.%, and their bulk density was 200 kg/m3. Analytical standards for the quantification of monosaccharides (glucose, xylose, galactose, arabinose, mannose), furans (5-hydroxymethylfurfural, furfural), organic acids (formic acid, acetic acid, levulinic acid) and other chemicals (sulfuric acid, barium carbonate, benzene) were purchased from Sigma-Aldrich. Ethanol was purchased from local grain alcohol producer J/S Kalsnavas elevators.
2.2.1 Chemical Compositional Analysis
The chemical composition of all solid fractions (untreated and treated oat husks) was analyzed using the methodology proposed by the National Renewable Energy Laboratory (NREL). Approximately 0.3 g of sample was hydrolyzed with 72% sulfuric acid for 60 min at 30°C. The resulting mixture was then diluted to a final concentration of 4% by adding distilled water. Next, the mixture was autoclaved at 121°C for another 60 min to complete the hydrolysis of polysaccharides. After cooling, the mixture was filtered through the porous-bottom porcelain crucible (7 μm) [22]. To determine the monosaccharide content (glucose, xylose, galactose, arabinose and mannose) in the oat husks, 10 mL aliquots of the resulting hydrolysate were taken and neutralized with 2.8 g of barium carbonate. This step was performed over two days in the refrigerator. After neutralization, the liquid fraction was filtered directly into HPLC vials using the nylon syringe filter with a porosity of 0.22 µm. Monosaccharide concentrations in the hydrolysate were analyzed using a Shimadzu LC20AD high-performance liquid chromatograph (HPLC) equipped with an RI detector (Shimadzu RID 10A) and a Thermo Scientific HyperREZ XP Carbohydrates Pb2+ column. The analysis was performed using Milli-Q water as the mobile phase at a flow rate of 0.6 mL/min, with the column oven temperature of 70°C. The total analysis time was 35 min. To analyze the degradation products of oat husks (formic acid, acetic acid, levulinic acid, 5-hydroxymethylfurfural, and furfural) unneutralized hydrolysate was filtered directly into HPLC vials using a nylon syringe filter with a porosity of 0.22 µm. The same HPLC system and detector were used, equipped this time with a Shodex Sugar SH 1821 column. The analysis was performed at 50°C using 5mM H2SO4 as the mobile phase with a flow rate of 0.5 mL/min. The total analysis time was 55 min. The acid-insoluble residue (Klason lignin) was determined following the NREL TP-510-42618 protocol [22], while acid-soluble lignin was quantified using the Perkin Elmer lambda 650 UV-spectrometer at a wavelength of 203 nm. The ash content in the oat husk samples was determined according to the NREL TP-510-42622 protocol [23]. Before determining carbohydrate content in the untreated oat husks, extractives were quantified following the NREL TP-510-42619 protocol [24]. The extraction process was organized using Knöfler-Böhm extractors with an ethanol–benzene solvent mixture in a 1:2 ratio. The extraction time was 5 h.
2.2.2 Hydrothermal Pretreatment
Before the hydrothermal pretreatment process, the oat husks were mixed with catalysts in the specially constructed paddle mixer. Six different catalysts (H3PO4, NH4H2PO4, NaH2PO4, KH2PO4, K2HPO4, K3PO4) were studied for their catalytic properties in the selective conversion of C5 carbohydrates into furfural. Prepared oat husks (approximately 2300 g of oven-dried material) were loaded into the reactor. The hydrothermal pretreatment experiments were conducted in a bench-scale reactor system designed to model the industrial furfural production process. The reactor had an internal diameter of 110 mm and a volume of 13.7 dm3. It was reactor was equipped with a steam jacket and an automatic control system to maintain constant temperature and pressure throughout the experiment. The material was treated in a continuous water steam flow (120 mL/min). The hydrolysis time (60 min), the treatment temperature (170°C) and the catalyst amount (4 wt.%) were constant. The effect of varying catalyst amounts (2, 3 and 5 wt.%) on the furfural production and cellulose degradation was studied for the two most effective catalysts (H3PO4 and NH4H2PO4). After the reactor, the steam was condensed and collected every 10 min. The obtained hydrolysates were filtered into HPLC vials using a nylon syringe filter with a porosity of 0.22 µm and analyzed by the Shimadzu LC20AD HPLC using Shodex Sugar SH1821. The lignocellulosic residue was dried to ambient moisture content and ground in a Retsch GmbH SM100 cutting mill for chemical analysis according to the NREL TP-510-42618 protocol [22]. The flowchart of the hydrothermal pretreatment process is available in our previous study [21]. All hydrolysis experiments were performed in duplicate under the identical conditions, and the average values, calculated from the oven-dried mass, were reported.
2.2.3 Scanning Electron Microscopy Analysis
A scanning electron microscope was used to analyze the oat husk microstructure and evaluate the impact of catalyzed hydrothermal treatment. The samples were placed in an Emitech K550X sputter coater (Emitech Ltd., Ashford, United Kingdom) and plated with gold plasma twice. The prepared samples were then examined using a VEGA TS 5136MM scanning electron microscope at a voltage of 15 kV and a magnification of 1000×. Images were taken using Vega TC software (version 2.9.9.21) (Tescan R&D, Brno, Czech Republic).
3.1 Characterization of Feedstock
Oat is one of the most widely produced cereals worldwide. Oat husks, a high-volume byproduct of oat milling, have a low monetary value. Based on an oven-dry basis, the oat husks consist of glucan (36.74 ± 0.10%), xylan (30.08 ± 0.54%), acid-insoluble lignin (15.63 ± 0.01%), ashes (4.43 ± 0.03%), arabinan (3.49 ± 0.01%), acid-soluble lignin (3.38 ± 0.07%), acetyl groups (2.24 ± 0.06%), galactan (2.05 ± 0.15%), acetone-soluble extractives (1.50 ± 0.03%), mannan (1.30 ± 0.04%). The chemical composition of oat husks aligns with previously reported data [20]. Minor differences may arise from the fact that the oat husks were sourced from a factory processing oats from a broad geographic area, leading to mixed hulls. Despite this variation, the chemical composition highlights oat husks as a valuable feedstock for biorefinery systems, particularly for furfural production, due to their high carbohydrate content (74 wt.%), of which 45% are C5 carbohydrates. Theoretically, based on the presented data, 24.41 wt.% of furfural can be obtained from oat husks.
3.2.1 Influence on the Yield of Furfural and Other by-Products
The hydrothermal process was used as a pretreatment to selectively remove C5 carbohydrates from the oat husks while simultaneously converting them to furfural. Six different types of catalysts (H3PO4, NH4H2PO4, NaH2PO4, KH2PO4, K2HPO4 and K3PO4) were tested to catalyze this process. In the first stage, the catalyst amount was set at 4 wt.%, based on the oven-dried mass of oat husks. Among the catalysts studied, H3PO4 and NH4H2PO demonstrated higher efficiency in converting C5 carbohydrates to furfural compared to the others. Therefore, additional experiments were conducted in which their amounts in the reaction zone were varied. To minimize furfural degradation, continuous water steam was used as a stripping agent to remove furfural from the reaction zone. All obtained hydrolysate samples were analyzed by HPLC. The yield of the main products is summarized in Fig. 1.

Figure 1: Products detected in the final hydrolysate
As shown in Fig. 1, furfural and acetic acid were the primary products obtained from the catalyzed conversion of oat husks through hydrothermal treatment. Formic acid, 5-HMF and 5-MF were detected as the minor compounds. The yield of acetic acid remained consistent across all catalysts studied, indicating that the catalysts exhibit similar catalytic activity in converting oat husk acetyl groups into acetic acid. However, the efficiency of C5 carbohydrate conversion to furfural varied depending on the catalyst used.
After the 60-min treatment process, the highest furfural yield was obtained using H3PO4 as a catalyst. When recalculated to the theoretically possible yield, this corresponds to 46%–57%. Therefore, it can be concluded that an equivalent amount of furfural was produced compared to today’s most commonly used furfural production technologies (the Chinese batch process, the “Quaker” batch process and the Rosenlew process), but in 2–5 times shorter time. When NH4H2PO4 was used as a catalyst, the furfural yield was 7.4–9.5 wt.%, which represents a 31%–35% lower conversion efficiency compared to H3PO4. In contrast, when sodium and potassium phosphate salts were used, the conversion efficiency of C5 carbohydrates into furfural was significantly lower than with the catalysts mentioned above. This suggests that sodium and potassium phosphate salts are ineffective catalysts for furfural production from oat husks.
Yemiş et al. [25] indicated that the pH level of the reaction medium is a crucial factor for furfural production from wheat straw by microwave-assisted process. In our study, during the first stage, the amount of catalyst in the reaction medium (4 wt.%) and treatment time (60 min) were identical, however, the initial pH levels varied depending on the catalyst used. The pH levels of the H3PO4, NH4H2PO4, NaH2PO4, KH2PO4, K2HPO4 and K3PO4 solutions, sprayed onto the oat husks before hydrothermal pretreatment, were 0.55, 3.50, 3.52, 3.78, 8.94, and 12.68, respectively. In this regard, our results align with the Yemiş et al. study–at lower pH levels, the conversion of C5 carbohydrates into furfural is more efficient. However, for catalysts with similar pH levels (NH4H2PO4, NaH2PO4, and KH2PO4), it is evident that the cation also plays a significant role. The main reason is that ammonium cation in water donates a proton to the water, forming NH3 and increasing the concentration of H3O+, leading to a slightly acidic environment that lowers the pH level during the hydrothermal treatment. In contrast, Na+ and K+ remain neutral.
Compared to the results obtained in the previous study [21], where H3PO4 and NaH2PO4 were used as catalysts to convert birch wood C5 carbohydrates into furfural, it can be concluded that these catalysts have a lower effect in the case of oat husks. In hydrothermal treatment catalyzed by H3PO4, the conversion efficiency of birch wood C5 carbohydrates to furfural was 64%, whereas for oat husks, it was approximately 55%. Similarly, hydrothermal treatment with NaH2PO4 resulted in conversion efficiencies of 39% for birch wood and only 13% for oat husks. These results indicate that the chemical composition and matrix of used LCB plays a crucial role. As a result, individual studies are required for each feedstock.
3.2.2 Influence on the Furfural Formation During the Pretreatment
One of the hydrothermal pretreatment technological parameters affecting cellulose degradation during furfural production is the treatment time of the LCB. Understanding the time period during which the highest furfural yield is achieved is essential. Therefore, the effect of treatment time on furfural formation dynamics at different catalyst doses was investigated. This analysis was conducted for the two most effective catalysts–H3PO4 and NH4H2PO4 (see Fig. 2). The results indicate that H3PO4 initiates the catalytic process significantly faster than NH4H2PO4, allowing a much higher amount of furfural to be obtained in the first 40 min. Notably, during the first 10 min of the process, furfural production with NH4H2PO4 is minimal, ranging between 0.07% and 0.33% of the oven-dried mass. Moreover, increasing the amount of NH4H2PO4 over the interval studied does not result in a significant increase in furfural amount during the first 10 min of the treatment process, in contrast to what is observed with H3PO4. This demonstrates that NH4H2PO4 is a weaker acid compared to H3PO4, so its catalytic activity is not as immediate. The initial hydrolysis of hemicellulose to release pentose is less efficient with NH4H2PO4 due to its milder acidity, resulting in slower hemicellulose degradation and delayed conversion of C5 sugars to furfural. As a result, it takes longer for NH4H2PO4 to reach an optimal catalytic state where sufficient H3O+ ions are generated to promote furfural formation. In contrast, H3PO4 creates a stronger acidic environment immediately, accelerating the catalytic process and producing higher furfural yields early in the reaction.

Figure 2: Produced furfural amount in each 10 min period at the different amounts of H3PO4 (A) and NH4H2PO4 (B)
Continuation of the oat husk treatment results in a significant increase in the amount of furfural produced in both cases. The positive effect of increasing catalyst dosage in the reaction zone is particularly evident during the next two 10-min intervals. However, the amount of furfural produced gradually decreases during the last 30 min of the treatment process. This can be explained by the fact that the amount of C5 carbohydrates in each of the next 10-min periods is lower than in the previous period. Thus, furfural production is enhanced by both the abundance of C5 sugars and the added catalyst in the first half of the treatment process. As the reaction progresses, the diminishing supply of C5 carbohydrates limits furfural formation, resulting in lower yields during the later stages. This highlights the dual importance of substrate availability and catalyst concentration in maximizing furfural output, particularly during the initial phase of the treatment.
3.2.3 Influence on the Oat Husk Microstructure
The untreated oat husk microstructure analysis shows (see Fig. 3), that it has a solid external layer on both sides, which seems almost non-porous, while the internal structure is much more disordered than birch wood [26]. This means that the matrix of oat husks is more recalcitrant than that of birch wood. In the context of hemicellulose, oat husk hemicellulose contains D-xylopyranose chains, where glucuronic acids and arabinose chains have been bonded [27].

Figure 3: Microstructure of untreated (A) and treated (B–with 4 wt.% of H3PO4, C–with 4 wt.% of NaH2PO4) oat husks
The presence of these side chains in hemicellulose makes it more branched, increasing the complexity of the hemicellulose structure and affecting its physical properties, such as solubility and availability for furfural production during the catalyzed hydrothermal treatment process. In contrast, the birch wood hemicellulose consists of linear xylan chains formed from D-xylopyranose but has fewer side chains [28]. As a result, it is less complex and more readily available for catalytic reactions, promoting more efficient furfural production compared to oat husk hemicellulose.
As seen in pictures B and C of Fig. 3, NaH2PO4 is not strong enough to break the external layer of the oat husk. In contrast, when H3PO4 is used, this layer breaks into fine pieces under the same hydrothermal treatment conditions. The same outcome occurs with the other catalysts used (pictures not shown). Therefore, if this external layer is more crashed, a higher conversion rate of C5 carbohydrates into furfural can be achieved. Otherwise, the catalyst cannot reach the C5 carbohydrates, and the chemical structure remains almost intact (see Fig. 4).

Figure 4: Chemical composition of oat husks before and after hydrothermal treatment
From the perspective of the biorefinery concept, it is essential to understand how the pretreatment affects the chemical composition of oat husks. Therefore, the impact of hydrothermal pretreatment catalyzed by H3PO4, NH4H2PO4, NaH2PO4, KH2PO4, K2HPO4 and K3PO4 on the chemical composition in lignocellulosic residue was analyzed. The chemical composition of the treated oat husks is summarized in Fig. 4.
The chemical composition of the resulting lignocellulosic residue indicates that its main components are acid-insoluble lignin, cellulose (expressed as glucan), and inorganic matter. The study highlights the critical role of catalyst and its dosage selection. For example, catalysts such as KH2PO4, K2HPO4, and K3PO4, which did not exhibit catalytic properties on the effective conversion of oat husk C5 carbohydrates into furfural, left a significant amount of xylan in the lignocellulosic residue. In contrast, when H3PO4 and NH4H2PO4 were used as catalysts, a very low amount of C5 carbohydrates remained in the lignocellulosic residue. Additionally, increasing the catalyst dosage resulted in a decrease in the amount of C5 carbohydrates remaining in the oat husk lignocellulosic residue. A similar observation can be made for acetyl groups. This demonstrates that the pH level in the reaction zone is an important factor in effectively separating C5 carbohydrates from the oat husk chemical structure and converting them into furfural.
Nevertheless, a notable disadvantage has been identified. After the furfural production stage, the acid-insoluble lignin (AIL) content in the oat husk lignocellulosic residue increased considerably, and a significant amount of the initial cellulose (20%–30%) was also lost during processing (see Fig. 5). This leads to the conclusion that side reactions involving oat husk carbohydrates and lignin occur during the hydrothermal treatment. Additionally, the formation of a significant amount of acid-insoluble fraction presents a major challenge for further cellulose saccharification, as it can deactivate cellulase enzymes and reduce the efficiency of the enzymatic process.

Figure 5: Catalyst impact on the glucan loss (% of initial content) in the oat husks after hydrothermal treatment
The study by Hu et al. [29] where the interaction of pure glucose, xylose and its mixture in an acidic environment studied, clearly demonstrates that these compounds react with each other and form a solid polymer. Therefore, assuming that the external layer of oat husks limits both the penetration of the catalyst into its inner layers and the removal of products generated during autohydrolysis, the cross-polymerization of the main oat husk components is the primary reason contributing to such an impressive increase in the acid-insoluble lignin. This is undesirable in the context of biorefining, where this lignocellulosic residue could be utilized. For example, lignin in its native form leads to low enzymatic saccharification by creating steric hindrance, causing non-productive adsorption of cellulase enzymes onto lignin and deactivating it [30]. Since, in the case of processed oat husks, the insoluble part is predominant, it may play a much greater role in the saccharification of the cellulose fraction through enzymatic hydrolysis. Therefore, we suggest that a two-step process may be more suitable for furfural production from oat husks. This process could involve initial solubilization of C5 carbohydrates in the first stage, followed by monosaccharide conversion in an appropriate catalytic environment.
In summary, oat husks have significant potential as a feedstock for a biorefinery system where furfural production is integrated because of their high carbohydrate content (74 wt.%) from which 45% is C5 carbohydrates. However, the chemical composition of the oat husk matrix greatly influences the C5 carbohydrate conversion efficiency into furfural by catalyzed hydrothermal treatment. The highest furfural yield (14.0 ± 0.6% of o.d.m.) was achieved using H3PO4 as a catalyst at its highest used dosage (5 wt.%). Among the used salts, NH4H2PO4 demonstrated the best catalytic properties for furfural production from oat husks. The highest furfural yield (9.5 ± 0.5% of o.d.m.) also was reached at catalyst dosage of 5 wt.%. Unfortunately, at these catalyst dosages significant degradation of the cellulose fraction was observed (almost 30% of the initial cellulose content), which, combined with C5 carbohydrate degradation products, led to an increase in the acid-insoluble fraction. The results highlight the need for a detailed study on how the severity of hydrothermal pretreatment affects the interaction of hemicellulose and cellulose degradation products with lignin. Finding a catalyst with the right properties to effectively convert C5 carbohydrates in oat husks into furfural without significant degradation of C6 carbohydrates by hydrothermal pretreatment remains one of the key challenges for efficient furfural production in the context of biorefinery.
Acknowledgement: The authors are grateful to M.Sc. Chem. Velta Fridrihsone from the Cellulose Laboratory of the Latvian State Institute of Wood Chemistry for providing SEM analysis.
Funding Statement: This study was funded by the Latvian State Institute of Wood Chemistry Bioeconomic Research Grant No. 09–24 titled “Selective Valorization of Lignocellulosic Biomass (SeVaLi)”.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Prans Brazdausks; data collection: Maris Puke, Guntis Sosins; analysis and data interpretation: Prans Brazdausks; validation: Maris Puke, Guntis Sosins; manuscript preparation: Prans Brazdausks, Maris Puke, Guntis Sosins; editing of the manuscript: Prans Brazdausks. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Segers B, Nimmegeers P, Spiller M, Tofani G, Jasiukaitytė-Grojzdek E, Dace E, et al. Lignocellulosic biomass valorisation: a review of feedstocks, processes and potential value chains and their implications for the decision-making process. RSC Sustain. 2024;2(12):3730–49. doi:10.1039/D4SU00342J. [Google Scholar] [CrossRef]
2. Mustafa A, Faisal S, Singh J, Rezki B, Kumar K, Moholkar VS, et al. Converting lignocellulosic biomass into valuable end products for decentralized energy solutions: a comprehensive overview. Sustain Energy Technol Assess. 2024 Dec 1;72. doi:10.1016/j.seta.2024.104065. [Google Scholar] [CrossRef]
3. Deng W, Feng Y, Fu J, Guo H, Guo Y, Han B, et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023 Feb;8(1):10–114. doi:10.1016/j.gee.2022.07.003. [Google Scholar] [CrossRef]
4. Nair LG, Verma P. Lignocellulosic biomass conversion into platform chemicals and biofuels using tetrahydrofuran-assisted pretreatment: a future for sustainable and bio-circular economy. Biomass Bioenergy. 2024 Dec 1;191. doi:10.1016/j.biombioe.2024.107454. [Google Scholar] [CrossRef]
5. Zoghlami A, Paës G. Lignocellulosic biomass: understanding recalcitrance and predicting hydrolysis. Front Chem. 2019 Dec 18;7. doi:10.3389/fchem.2019.00874. [Google Scholar] [PubMed] [CrossRef]
6. Yong KJ, Wu TY, Lee CBTL, Lee ZJ, Liu Q, Jahim JM, et al. Furfural production from biomass residues: current technologies, challenges and future prospects. Biomass Bioenergy. 2022 Jun 1;161. doi:10.1016/j.biombioe.2022.106458. [Google Scholar] [CrossRef]
7. Martins RP, Schmatz AA, de Freita LA, Mutton MJR, Brienzo M. Solubilization of hemicellulose and fermentable sugars from bagasse, stalks, and leaves of sweet sorghum. Ind Crops Prod. 2021 Oct 15;170. doi:10.1016/j.indcrop.2021.113813. [Google Scholar] [CrossRef]
8. Cousin E, Namhaed K, Pérès Y, Cognet P, Delmas M, Hermansyah H, et al. Towards efficient and greener processes for furfural production from biomass: a review of the recent trends. Sci Total Environ. 2022 Nov;847:157599. doi:10.1016/j.scitotenv.2022.157599. [Google Scholar] [PubMed] [CrossRef]
9. Li X, Jia P, Wang T. Furfural: a promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016 Nov 4;6(11):7621–40. doi:10.1021/acscatal.6b01838. [Google Scholar] [CrossRef]
10. Zhou Q, Ding A, Zhang L, Wang J, Gu J, Wu TY, et al. Furfural production from the lignocellulosic agro-forestry waste by solvolysis method—a technical review. Fuel Process Technol. 2024 May 1;255. doi:10.1016/j.fuproc.2024.108063. [Google Scholar] [CrossRef]
11. Zhao K, Wen B, Tang Q, Wang F, Liu X, Xu Q, et al. Recent catalytic innovations in furfural transformation. Green Chem. 2024;26(19):9957–92. doi:10.1039/D4GC01983K. [Google Scholar] [CrossRef]
12. Pierrat L, García-Triñanes P. Optimising furfural production from lignocellulosic biomass: feedstock selection, process enhancement, and techno-economic and environmental viability. Chem Eng Res Des. 2024 Dec 1;212:261–80. doi:10.1016/j.cherd.2024.10.035. [Google Scholar] [CrossRef]
13. Zeitsch KJ. The chemistry and technology of furfural and its many by-products. 1st ed. Sugar series. Amsterdam: Elsevier; 2000. Vol. 13, p. 1–358. [Google Scholar]
14. Edumujeze D, Fournier-Salaün MC, Leveneur S. Production of furfural: from kinetics to process assessment. Fuel. 2025 Feb 1;381. doi:10.1016/j.fuel.2024.133423. [Google Scholar] [CrossRef]
15. Avci A, Saha BC, Kennedy GJ, Cotta MA. High temperature dilute phosphoric acid pretreatment of corn stover for furfural and ethanol production. Ind Crops Prod. 2013 Oct;50:478–84. doi:10.1016/j.indcrop.2013.07.055. [Google Scholar] [CrossRef]
16. Adhami W, Richel A, Len C. A review of recent advances in the production of furfural in batch system. Mol Catal. 2023 Jul;545:113178. doi:10.1016/j.mcat.2023.113178. [Google Scholar] [CrossRef]
17. Luo Y, Li Z, Li X, Liu X, Fan J, Clark JH, et al. The production of furfural directly from hemicellulose in lignocellulosic biomass: a review. Catal Today. 2019 Jan;319:14–24. doi:10.1016/j.cattod.2018.06.042. [Google Scholar] [CrossRef]
18. Alonso DM, Wettstein SG, Mellmer MA, Gurbuz EI, Dumesic JA. Integrated conversion of hemicellulose and cellulose from lignocellulosic biomass. Energy Environ Sci. 2013;6(1):76–80. doi:10.1039/C2EE23617F. [Google Scholar] [CrossRef]
19. Li X, Liu Q, Luo C, Gu X, Lu L, Lu X. Kinetics of furfural production from corn cob in γ-valerolactone using dilute sulfuric acid as catalyst. ACS Sustain Chem Eng. 2017 Oct 2;5(10):8587–93. doi:10.1021/acssuschemeng.7b00950. [Google Scholar] [CrossRef]
20. Puke M, Godina D, Brazdausks P. Catalyzed hydrothermal pretreatment of oat husks for integrated production of furfural and lignocellulosic residue. Polymers. 2024 Mar 1;16(5). doi:10.3390/polym16050707. [Google Scholar] [PubMed] [CrossRef]
21. Brazdausks P, Godina D, Puke M. Direct furfural production from deciduous wood pentosans using different phosphorus-containing catalysts in the context of biorefining. Molecules. 2022 Oct 29;27(21):7353. doi:10.3390/molecules27217353. [Google Scholar] [PubMed] [CrossRef]
22. Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, et al. Laboratory analytical procedure: determination of carbohydrates in biomass by high-performance liquid chromatography. NREL/TP-510-42618; 2012 Aug [cited 2025 Jan 02]. Available from: https://www.nrel.gov/docs/gen/fy13/42618.pdf. [Google Scholar]
23. Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D. Laboratory analytical procedure: determination of ash in biomass. NREL/TP-510-42622; 2008 Jan [cited 2025 Jan 02]. Available from: https://www.nrel.gov/docs/gen/fy08/42622.pdf. [Google Scholar]
24. Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D. Laboratory analytical procedure: determination of extractives in biomass. NREL/TP-510-42619; 2008 Jan [cited 2025 Jan 02]. Available from: https://www.nrel.gov/docs/gen/fy13/42618.pdf. [Google Scholar]
25. Yemiş O, Mazza G. Optimization of furfural and 5-hydroxymethylfurfural production from wheat straw by a microwave-assisted process. Bioresour Technol. 2012 Apr;109:215–23. doi:10.1016/j.biortech.2012.01.031. [Google Scholar] [PubMed] [CrossRef]
26. Biziks V, Andersons B, Beļkova Ļ., Kapača E, Militz H. Changes in the microstructure of birch wood after hydrothermal treatment. Wood Sci Technol. 2013 Jul 22;47(4):717–35. doi:10.1007/s00226-013-0531-1. [Google Scholar] [CrossRef]
27. Anderson E, Krznarich PW. Hemicellulose from oat hulls. J Biol Chem. 1935 May 22;111:549–52. doi:10.1016/S0021-9258(18)75055-2. [Google Scholar] [CrossRef]
28. Pettersen RC. The chemical composition of wood. In: Roger R, editor. The chemistry of solid wood. Washington, DC, USA: American Chemical Society; 1984. p. 57–126. doi:10.1021/ba-1984-0207.ch002. [Google Scholar] [CrossRef]
29. Hu X, Wang S, Wu L, Dong D, Mahmudul Hasan M, Li CZ. Acid-treatment of C5 and C6 sugar monomers/oligomers: insight into their interactions. Fuel Process Technol. 2014;126:315–23. doi:10.1016/j.fuproc.2014.05.024. [Google Scholar] [CrossRef]
30. Yuan Y, Jiang B, Chen H, Wu W, Wu S, Jin Y, et al. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol Biofuels. 2021;14(1):205. doi:10.1186/s13068-021-02054-1. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools