 Open Access
Open Access
ARTICLE
New Rigid Furan Biofoams Based on Hydrolysable Chesnut (Castanea sativa) Tannin by Chemical Expansion
1 UR 4370 USC 1445 LERMAB, INRAE, Faculté des Sciences et Technologies, Université de Lorraine, Nancy, 54506, France
2 GNanoAgro, Universidade Federal do Paraná, Curitiba, 82590, Brazil
* Corresponding Author: Christine Gérardin-Charbonnier. Email:
Journal of Renewable Materials 2025, 13(4), 687-697. https://doi.org/10.32604/jrm.2025.058902
Received 23 September 2024; Accepted 14 February 2025; Issue published 21 April 2025
Abstract
Tannins are polyphenols widely present in the plant kingdom, commonly divided into two groups: condensed and hydrolysable tannins. Sustainable furanic bio-foams based on condensed tannins have been largely studied, but little is described about the use of hydrolysable tannins for this material. This study examined the potential of hydrolysable chestnut tannin in comparison to condensed mimosa tannins to produce furanic foams by chemical expansion. Due to the low reactivity of the hydrolysable tannin, the use of an external source for its polymerization and curing was necessary. Through Fourier transform infrared spectroscopy (FTIR) chromatography, it was possible to observe that the new foams presented small differences in functional groups compared to the condensed tannin foams, presenting peaks related to carboxyl groups. In terms of physical properties, the chestnut foams showed an apparent density 36% higher than the conventional mimosa tannin foams and a superior hydrophilic character. In terms of thermal properties, both foams exhibit high thermal stability, with the acacia tannin foam being slightly superior. In summary, this research paves the way for new applications of hydrolysable tannins in bio-foams and materials science.Graphic Abstract
Keywords
Condensed tannins are natural polyphenols found in various plant sources and commonly extracted from the bark of acacia (Acacia sp.), pine (Pinus radiata), spruce (Picea abies L.) or quebracho (Schinopsis sp.) [1,2]. It can be copolymerized with furfuryl alcohol, a liquid organic compound issued from furfural, which is produced by acid hydrolyzing polysaccharides from agricultural waste [3,4] to create porous materials [5–11]. The properties and applications of these tannin-based cellular materials have been extensively investigated in previous studies [5,10,11]. They have been shown to be suitable for various purposes, such as thermal insulation [6–9], floral foams [12], flame retardant materials [13], adsorbents for heavy metal ions [14], polyurethane products [15] and other applications.
Furanic bio-foams have high environmental sustainability due to their composition consisting of 95% renewable plant-derived products [10,11], in contrast to commercial foams that are derived from petrochemicals [11]. The main component of these foams is a furanic-tannin resin, which is combined with various additives, such as a catalyst, crosslinker, surfactant, solvent, and foaming agent [11]. Different foaming methods can be applied to prepare these foams, including mechanical [8,16], chemical [7], or microwave-assisted techniques [17]. The chemical route involves either alkaline [18] or acid-catalyzed conditions [7]. Tannins from different sources, such as quebracho [19], acacia [20], pine [21,22], and spruce [23], are commonly used with furfuryl alcohol to react with different cross-linking compounds, such as formaldehyde, glyoxal, glutaraldehyde, and nanocellulose to synthesize cellular foams [5–13]. Tannins present natural phenolic structures similar to those of commercial polyphenols enabling thus their involvement in such applications [1].
Tannins are generally classified into two groups: condensed tannins, which are polymers based on catechin monomers, and hydrolysable tannins, based on complex structures consisting of sugar esters with different galloyl units [24,25]. Condensed tannins account for about 90% of the global tannin production [26], but the valorization of hydrolysable tannins is still relevant, as they constitute a large part of the tannins production in countries like France, Slovenia and Italy [27].
The raw materials used in the past for these bio foams were mostly condensed tannins, which are not available in all tree species. This tends to limit the use of other species to produce these foams. Few attempts have been made to use hydrolysable tannins to prepare cellular porous materials, either in the form of non-isocyanate polyurethane (NIPU) foams [28,29], as phenol-formaldehyde-chestnut tannin resins [18] or by producing tannin-furanic foams with diethylene glycol or other surfactants using the mechanical foaming method [30]. However, none of these studies have produced tannin-furanic foams under acidic conditions without surfactants. Therefore, this work aims to create and characterize a new foam based on the exothermic reaction of hydrolysable chestnut (Castanea sativa) tannins with furfuryl alcohol using acid catalyst to expand the range of applications of this type of tannins.
The commercial condensed tannin (CT) extract from Acacia bark (Acacia decurrens), known as FINTAN OP in the market, was utilized in this study and was provided by SilvaTeam from Italy. The hydrolysable tannin (HT) extract from chestnut (Castanea sativa) was supplied by KingTree from France. Furfuryl alcohol (≥97%, FA) and p-toluenesulfonic acid monohydrate (≥98%, p-TSA) were purchased from Sigma-Aldrich Co., Ltd. (Saint Louis, MO, USA). Formaldehyde (37 wt% solubilized in water, stabilized with 5%–15% methanol, FO) was acquired from Acros Organics Co., Ltd. (Strasbourg, France). Diethyl ether (Pure stabilized with BHT, DE) was obtained from Carlo Erba Co., Ltd. (Val de Reuil, France). All the chemicals were used without any further purification before application.
2.2 Preparation of Hydrolysable Tannin Bio-Based Foam and Condensed Tannin Bio-Based Foam
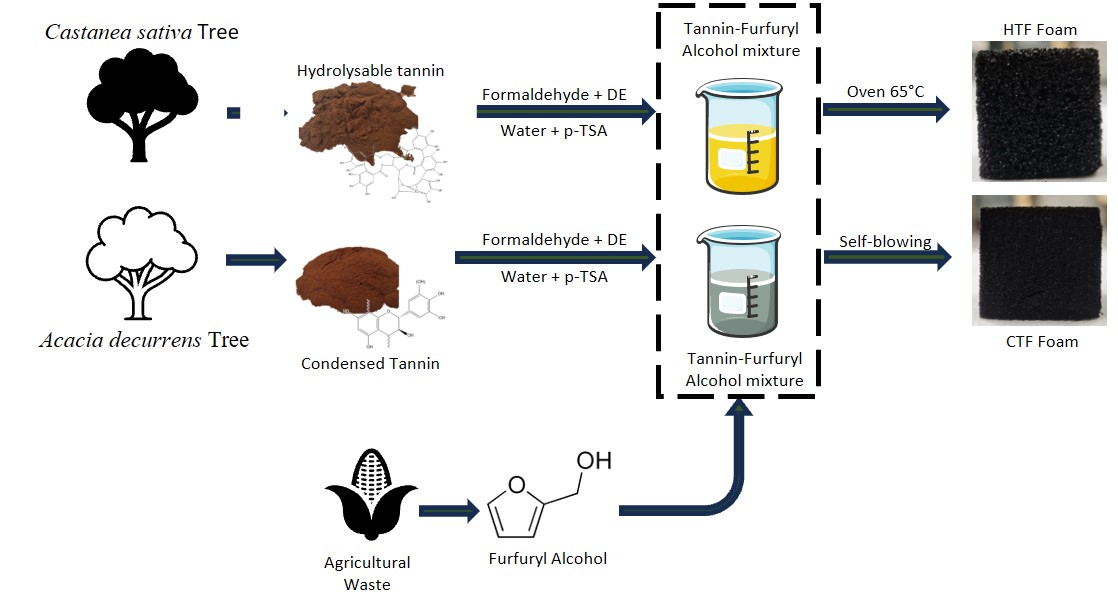
Two types of furanic foams were prepared, one using condensed tannins using the methodology presented in Meikleham et al’s work [31] and the other using hydrolysable tannins with an external heat source to form the foam. The applied methodology is summarized in Fig. 1. Comparison of the properties of the different foams obtained allowed to evaluate the interest of using hydrolysable tannins instead of condensed ones. The foam formulation is described in Table 1.
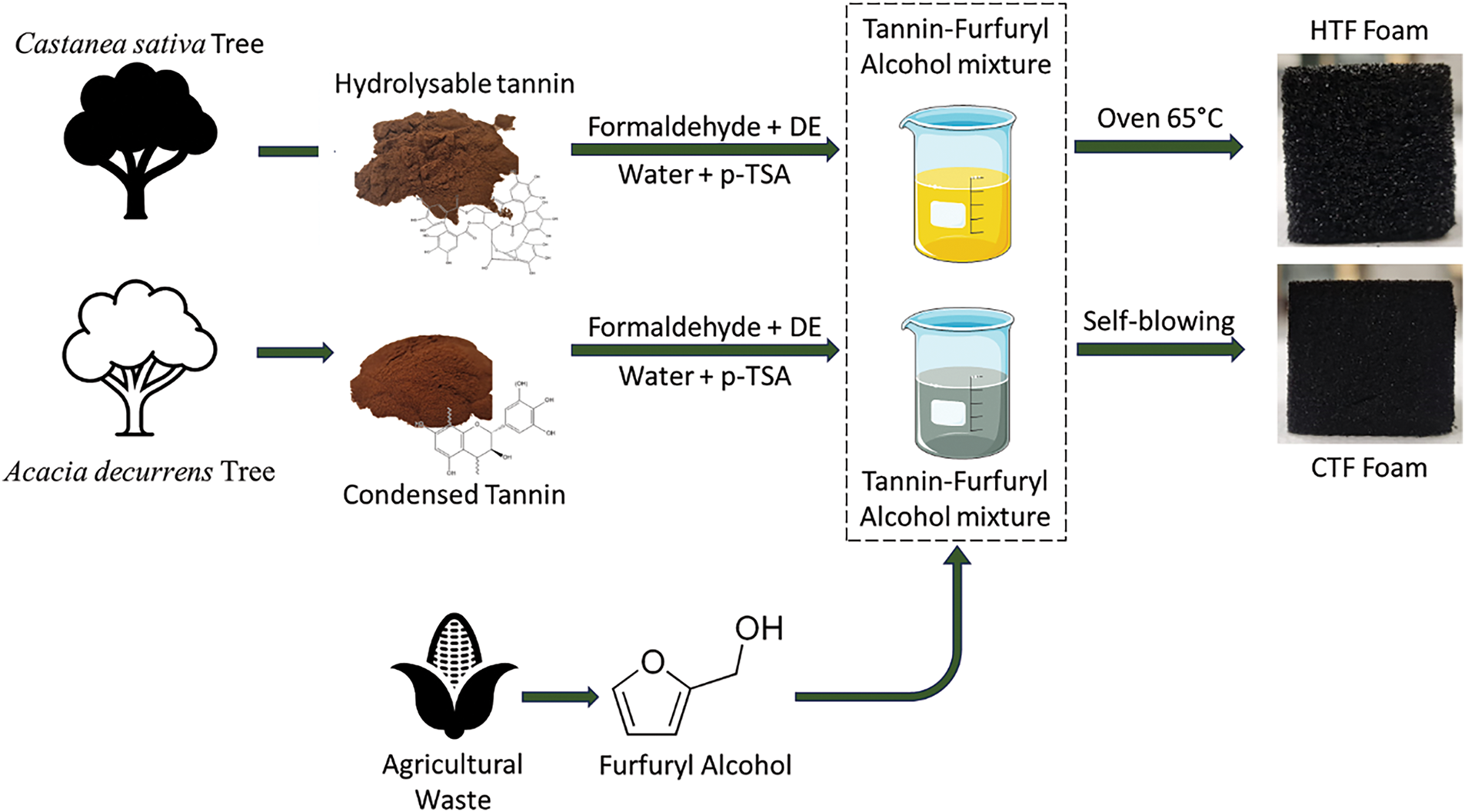
Figure 1: Methodology applied to produce CTF and HTF foam

To prepare foams based on condensed tannins, furfuryl alcohol, formaldehyde, and distilled water (DW) were combined in a beaker. Acacia tannins powder was then gradually incorporated into the solution and manually mixed. Once a uniform mixture was obtained, the blowing agent, diethyl ether, was added. The mixture was then manually stirred until uniformity was restored. The acid catalyst (p-TSA solution with a m/m concentration of 65% in water) was subsequently added, and the solution was continuously stirred manually for 10 to 15 s. The reaction began after a few seconds, leading to foam formation. The exothermic reaction between furfuryl alcohol and the acid catalyst generated enough heat to evaporate the blowing agent (diethyl ether), allowing expansion of the resin formed between furfuryl alcohol, tannins, and formaldehyde.
The same procedure was applied for the hydrolysable tannins foam, excepted for the polymerization of the foam, which need an external heat source (drying oven) set at 65°C, following the addition of the acid catalyst (initiation of reaction). This temperature was chosen by analogy with the exothermic reaction temperature recorded during the formation of furan condensed tannins foams [32].
2.3.1 Physical Characterization
For each type of foam, three cubes measuring 30 mm × 30 mm × 30 mm were cut and weighed on an analytical balance, in standard laboratory atmosphere at 20°C, their apparent density was calculated according to the ASTM D1622-03 standard. The density (ρ) of each obtained sample was calculated by measuring its length (l), width (w), height (h), and weight (m) and applying the equation ρ = m/(l × w × h). To ensure homogeneity for the density calculation, only the center of the foams was used. Thus, the outer part of the samples was discarded (about 1 cm).
To analyze the surface morphology of the material, numerous images of the surface of the foams were taken at a magnification of 50×, 100× and 250× using a Hitachi TM3000 scanning electron microscope (SEM). The samples did not undergo any type of surface treatment to obtain the images. The ImageJ program was used to estimate the thickness and width of the cells using several photos, with the diameter of each cell being calculated by the mean of these two measurements.
2.3.2 Thermal Properties Analysis
The foams were crushed using a pestle and mortar system until a fine and homogeneous powder was obtained. Then, 10 ± 1 mg of each foam, chestnut tannin or acacia tannins were used for thermogravimetric analysis using a Mettler Toledo brand differential gravimetric analyzer, model DSC 1 equipped with the STARe system. The tests were carried out between 30°C to 800°C with a temperature increase rate of 10°C/min.
2.3.3 Fourier Transform Infrared (FTIR) Spectroscopy
To study the functional groups of the condensed tannins (CT), hydrolysable tannins (HT), condensed tannins foam (CTF), hydrolysable tannins foam (HTF) infrared chromatography (FTIR) was performed using the Frontier IR chromatograph from Perkin Elmer, with a range of 650–4000 cm−1 and with 20 scans at a precision of 4 cm−1.
To analyze the hydrophilic character of the foams, 20 ± 1 mg of the homogeneous powder of each foam was analyzed using the dynamic vapor absorption instrument from Surface Measurement Systems, model DVS IntrisicPLUS. The Dynamic Vapor Sorption (DVS) technique was used to automatically generate different humidity levels for the measurement of the sorption isotherm. The instrument records the change in mass over time (dm/dt) to determine when equilibrium is reached. When dm/dt approaches zero, the next humidity level is automatically set. The test was carried out with air from 0% to 90% humidity at a constant temperature of 25°C, with control points at 0%, 15%, 25%, 45%, 60%, 75%, and 90% humidity. The analysis ended after more than 70 h.
3.1 Preparation of Hydrolysable Tannins Bio-Based Foams and Condensed Tannins Bio-Based Foams
The appearance of the different foams produced is shown in Fig. 2.
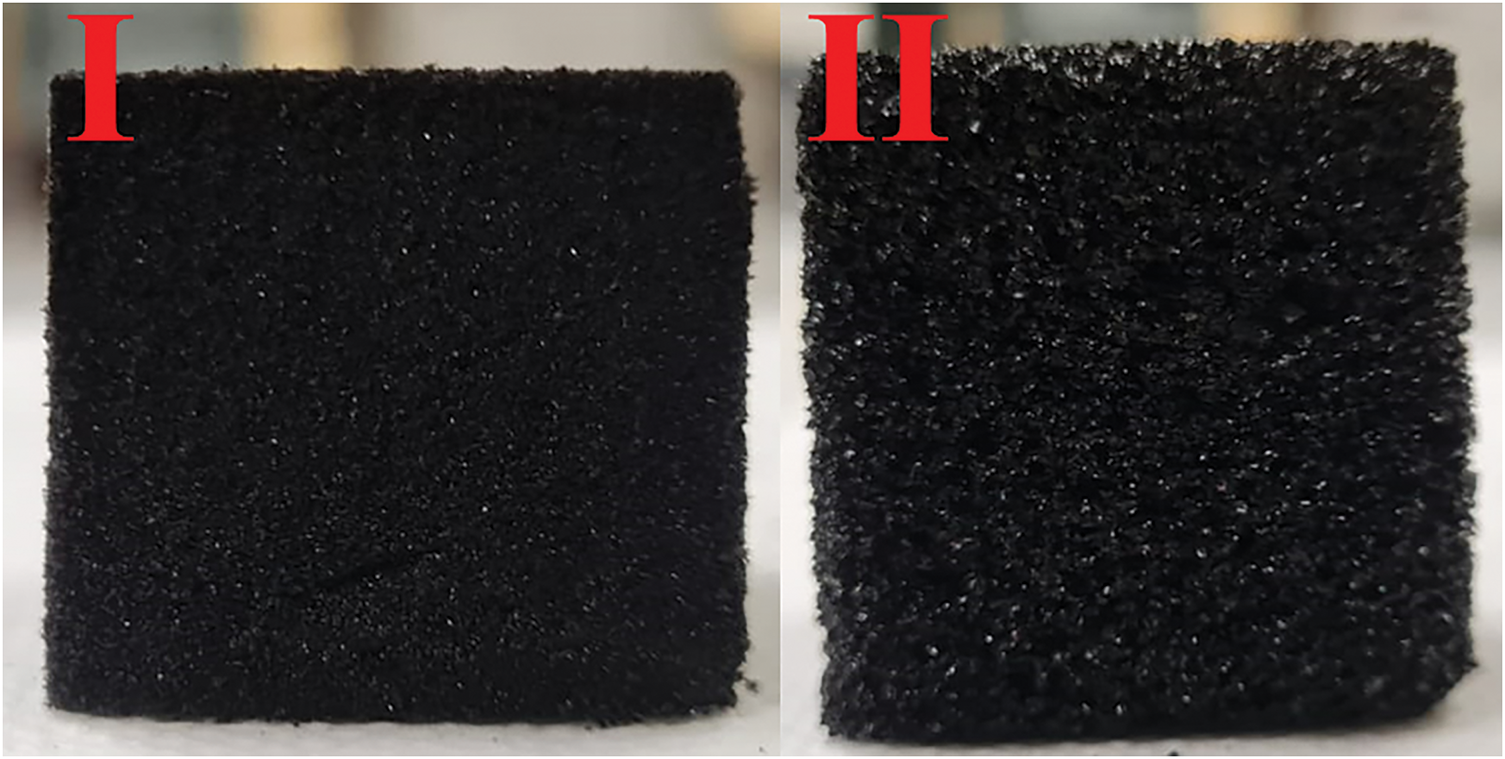
Figure 2: Furanic bio foams made from (I) condensed acacia tannin and (II) hydrolysable chestnut tannin
To produce HTF foams, an external heat source was essential. Without it, the foam formation reaction occurred but retracted completely within a few minutes, failing to form a cellular material. Heat improved the curing and hardening process, preventing retraction and allowing the foam to retain its expanded volume. This effect does not occur in traditional condensed tannins foams (CTF), for this reason, no external heat source was used to produce foams with condensed tannins.
Due to the different raw materials and processes of the CTF and HTF foams, the apparent density and morphological properties of the produced foams were significantly different. Average apparent density, cell length, thickness, diameter and orthotropicity (length/thickness ratio) measurements are given in Table 2.

The density obtained for the acacia tannins foams is similar to values reported in the literature. The density of HTF is 36% higher than the CTF foams, but the pores of the hydrolysable tannins foams are larger. A deeper analysis of the structure using SEM shows that a CTF foams present a greater number of pores and holes compared to HTF foam, thus explaining their lower density (Fig. 3). In both cases, the foams have irregular and heterogeneous structures and most of the cells in each foam are oval-shaped and have a similar orthotropism.
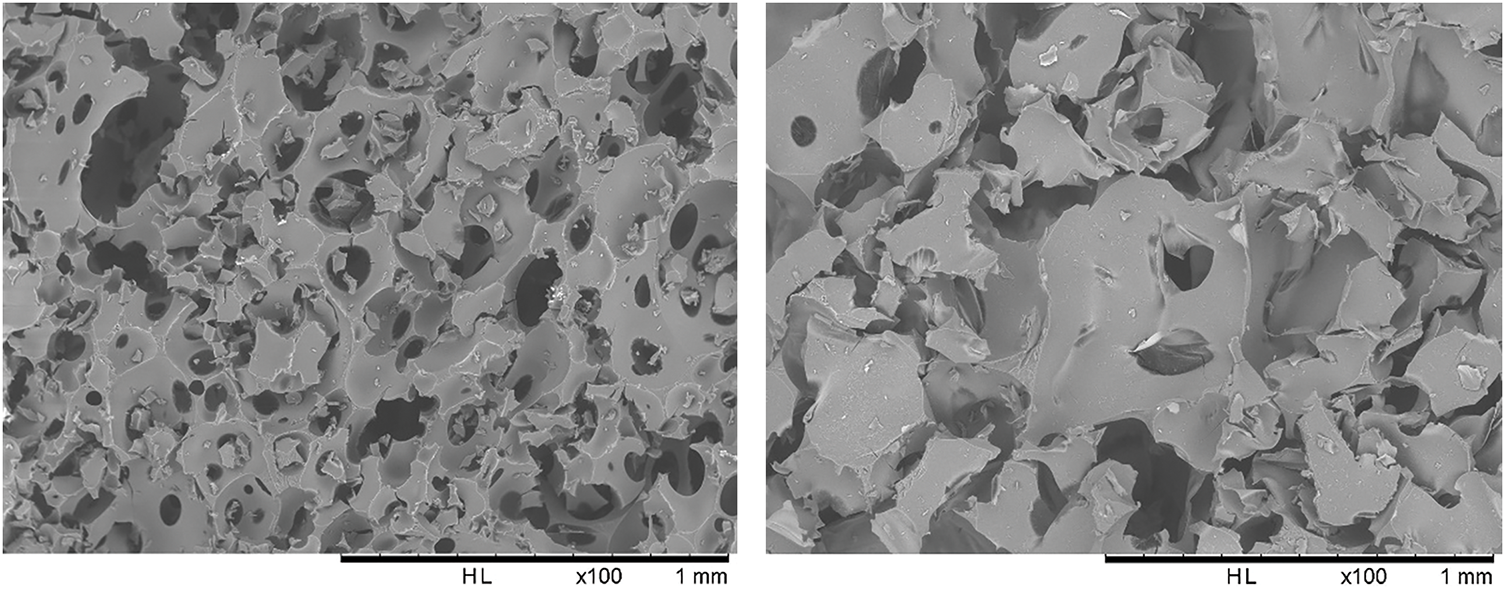
Figure 3: On the left the SEM image of the CTF foams and on the right that of the HTF foams at 100× magnification
3.3 Thermal Properties Analysis
Thermogravimetric analysis (TGA) was performed to analyze the thermal stability of the different foams and of the tannins involved in their preparation of CT, HT, CTF and HTF (Fig. 4).

Figure 4: (I) TGA and (II) DTG curves of HC, HT, CTF and HTF samples
Similarly to HT and CT tannins, the TGA curves obtained for both CTF and HTF foams exhibit similar behavior. By analyzing the peaks presented in the DTG curves of the samples, it is possible to determine that the first stage of mass loss of the tannins and foams occurs before and near 100°C; this is mainly due to the loss of moisture from the foams and tannins [9,20,33,34], as well as the evaporation of residues from the foaming agent and the cross-linker (formaldehyde) [34]. Immediately afterward, the HTF foams showed a degradation stage from 100°C to 200°C, linked to the thermal decomposition of gallic acid into pyrogallol [35]. This peak was not observed in the case of the DTG curve of HT. This is likely due to experimental conditions (acid catalyst, temperature and water) allowing the formation of gallic acid by hydrolysis during the polycondensation of furfuryl alcohol with the tannins. The second mass loss step of CTF occurs between 200°C–400°C, corresponding to the condensed tannins degradation [20] which is also observed in DTG curve of TC between 200°C–300°C. The third degradation step of the HTF occurred between 200°C–380°C, corresponding to hydrolysable tannins degradation observed in the same temperature range for HT. The last peak of mass loss of CTF and HTF occurred respectively between 450°C–550°C and 400°C–500°C, both caused by the breaking of polymer chains forming lower molecular weight compounds [9,20,34]. The CTF foams preserved about 46% of their mass when reaching 800°C, while the HTF foams preserved 40% of their mass at the same temperature. The lower residual mass of the HTF foams is due to the easier degradation of the carbohydrates skeletal chain of HT.
3.4 Fourier Transform Infrared (FTIR) Spectroscopy
FTIR chromatography was performed to compare the functional groups present in the HT and CT tannins as well as in the HTF and CTF foams (Fig. 5). Thus, in a general way, possible differences in the chemical structure between the materials analyzed can be observed.

Figure 5: FTIR spectrometry of CT, HT, CTF and HTF samples
The spectrum of both foams and of both tannins present quite similar absorption peaks indicating that there are not great differences in the chemical composition of the raw materials (CT and HT) and their respective foams (HTF and CTF). All the spectra presented the characteristic hydroxyl absorption peak between 3700–3000 cm−1, the C-H stretching peak between 2950–2800 cm−1, the C-C peaks of the aromatic ring skeleton at 1603, 1503, 1449 cm−1 and the aromatic C-H at 812 and 682 cm−1 as reported in previous studies [35].
The peak between 1740–1700 cm−1 of compounds corresponding to the carbonyl group is present in all the spectra except for the CT tannin. However, in each sample, the peak appears at a different position and with a different intensity compared to the nearest peak at ~1600 cm−1. In CTF, this peak appears in 1709 cm−1, represents a saturated and acyclic ketone group, formed by the opening of the furfuryl alcohol ring in its self-polymerization [36,37]. For the HT tannin the peak shifts to 1721 cm−1 corresponding to the carboxyl group present in gallic and digallic acids and/or esters groups present in the hydrolysable chestnut tannin [38]. For the HTF foams, these peaks appear at 1712 cm−1 with a higher intensity than the peak at 1603 cm−1, which may represent a sum of ketones created by the furfuryl alcohol, carboxylic acids and esters bonds present in chestnut tannins. In the case of spectra of foams, characteristic peaks of C-C aromatics or C-O bonds show different intensities compared to the spectra of CT or HT due to the combination of the band of the different polymers. A small band around 700 cm−1 appeared also in the two spectra of foams, which could be assigned to 2–5 disubstituted furan rings [39,40]. The intensity of the peaks at 3000–3600 cm−1 corresponding to O–H bond stretching decrease in the spectra of foams, compared to tannins spectra, indicated that polymerization has been effective.
Fig. 6 shows the result obtained in the dynamic vapor sorption (DVS) analysis of CTF and HTF foams.

Figure 6: Graph showing the cycle of the DVS analysis of CTF and HTF foams in (I) change in mass by time and (II) change in mass by target moisture
The two foams present hydrophilic character, but the HTF foam indicated an higher water absorption compared to CTF foam (mass increase of 28% compared to 23%). Thus, it is possible to observe that chestnut tannins foam are more hydrophilic than the acacia tannins foam, due to their higher carbohydrates content. To complete the cycle between 0% and 90% relative humidity, the two foams took approximately 73 h. The two foams show a hysteresis behavior, with the CTF foam having a more constant hysteresis, close to 2% among all the control points. Conversely, the HTF foam shows a hysteresis of 2.5% at humidities close to 10% relative humidity, which decreased to 1% at higher relative humidities, showing a greater ease in gaining or losing moisture at higher humidities compared to the CTF foam.
This difference between the two foams may be related to the differences in their tannins’ chemical structures. Being hydrolysable tannins, HT possess in their structure galloyl moiety as well as carbohydrate moiety, presenting both high affinity for water, increasing thus their hydrophilic character. Another possibility is the size of the cells present in the HTF foams, whose diameter is larger than the CTF cells, which could increase their water retention.
The Novel, environmentally friendly tannin-furanic rigid foams have been prepared with hydrolysable chestnut tannins using the same formulation of the classical furanic foam obtained with condensed tannins reported originally in the early 1990s. The methodology is quite similar, the only difference being a heating step to ensure that the HTF foams maintain their volumes and structures. This approach is an attractive way to valorize chestnut tannins by increasing the variety of raw materials that can be used to produce furanic foams.
In terms of physical properties, these new foams demonstrated a 36% increase in density and a more hydrophilic character than the CTF foams, being able to absorb up to 28% of their mass in water. This is an indication of its preferable use for floral foams, for example. The cells present in the HTF foams are considerably larger, with an average diameter of 373 µm, while the CTF foams have an average diameter of 225 µm. SEM images indicate more holes and pores in the CTF foams, which may explain their lower density.
The similarities of FTIR spectra of the different foams make it difficult to identify differences in the chemical structure of the two types of foams. FTIR analysis alone does not permit the accurate identification of the difference in the properties of foams according to the nature of the tannins used.
TGA indicates that the HTF foams exhibit similar behavior to the CTF foams, both presenting excellent thermal stability. However, the CTF foams demonstrated a slightly lower degradation at 800°C. Another significant difference is the early degradation of the HTF foams at a temperature of around 150°C due to the degradation of gallic acid resulting from the hydrolysis of HT.
In the future, mechanical characterizations of these new HT foams will be necessary to evaluate more deeply their potential applications. Evaluation of different sources of hydrolysable tannins could also be interesting to evaluate the effect of their structures on the properties of the resulting foams. Studies on the formulation of the foams could also be necessary to adapt their properties according to their final utilization and obtain smart materials.
Acknowledgement: The authors gratefully acknowledge the French National Research Agency (ANR), as part of the “Investissements d’Avenir” program, Laboratory of Excellence ARBRE for support of LERMAB and they thank “Lorraine Université d’Excellence” Master Grant, ORION program and the French Ministry of Foreign Office through EIFFEL program for the grant to Joao Vitor Dorini Falavinha. They acknowledge KING TREE firm for the samples of chesnut tannins.
Funding Statement: LERMAB is supported by a grant overseen by the French National Research Agency (ANR), as part of the “Investissements d’Avenir” program (ANR-11-LABX-0002-01, Lab of Excellence ARBRE). Joao Vitor Dorini Falavinha has been supported by “Lorraine Université d’Excellence” Master Grant, ORION program and by the French Ministry of Foreign Office through EIFFEL program.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Christine Gérardin-Charbonnier; methodology, Christine Gérardin-Charbonnier, Joao Vitor Dorini Falavinha; investigation, Joao Vitor Dorini Falavinha; writing—original draft preparation, Joao Vitor Dorini Falavinha; writing—review and editing, Christine Gérardin-Charbonnier, Joao Vitor Dorini Falavinha, Antonio Pizzi, Philippe Gérardin; supervision, Christine Gérardin-Charbonnier, Pedro Henrique Gonzales De Cademartori, Philippe Gérardin; project administration, Christine Gérardin-Charbonnier; funding acquisition, Christine Gérardin-Charbonnier. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data and materials are contained and presented within this article. The data that support the findings of this study are available from the corresponding author, CGC, upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Das AK, Islam MN, Faruk MO, Ashaduzzaman M, Dungani R. Review on tannins: extraction processes, applications and possibilities. S Afr N J Bot. 2020;135(6):58–70. doi:10.1016/j.sajb.2020.08.008. [Google Scholar] [CrossRef]
2. Bello A, Bergmann U, Vepsäläinen J, Leiviskä T. Effects of tree harvesting time and tannin cold/hot-water extraction procedures on the performance of spruce tannin biocoagulant for water treatment. Chem Eng J. 2022;449(1):137809. doi:10.1016/j.cej.2022.137809. [Google Scholar] [CrossRef]
3. Chen X, Yang Z, Yang F, Zhang J, Pizzi A, Essawy H, et al. Development of easy-handled, formaldehyde-free, high-bonding performance bio-sourced wood adhesives by co-reaction of furfuryl alcohol and wheat gluten protein. Chem Eng J. 2023;462(7):142161. doi:10.1016/j.cej.2023.142161. [Google Scholar] [CrossRef]
4. Mantanis GI. Chemical modification of wood by acetylation or furfurylation: a review of the present scaled-up technologies. BioResources. 2017;12(2):4478–89. doi:10.15376/biores.12.2.Mantanis. [Google Scholar] [CrossRef]
5. Li J, Liao J, Essawy H, Zhang J, Li T, Wu Z, et al. Preparation and characterization of novel cellular/nonporous foam structures derived from tannin furanic resin. Ind Crops Prod. 2021;162(1):113264. doi:10.1016/j.indcrop.2021.113264. [Google Scholar] [CrossRef]
6. Rodrigues MBB, Côrrea R, De Cademartori PHG, Ribeiro ACR, Coldebella R, Delucis RA, et al. Bio-based tannin foams: comparing their physical and thermal response to polyurethane foams in lightweight sandwich panels. Compounds. 2024;4(1):1–16. doi:10.3390/compounds4010001. [Google Scholar] [CrossRef]
7. Missio AL, Otoni CG, Zhao B, Beaumont M, Khakalo A, Kämäräinen T, et al. Nanocellulose removes the need for chemical crosslinking in tannin-based rigid foams and enhances their strength and fire retardancy. ACS Sustain Chem Eng. 2022;10(31):10303–10. doi:10.1021/acssuschemeng.2c02678. [Google Scholar] [PubMed] [CrossRef]
8. Zuo Z, Liu B, Essawy H, Huang Z, Tang J, Miao Z, et al. Preparation and characterization of biomass tannin-based flexible foam insoles for athletes. Polymers. 2023;15(16):3480. doi:10.3390/polym15163480. [Google Scholar] [PubMed] [CrossRef]
9. Wu X, Yan W, Zhou Y, Luo L, Yu X, Luo L, et al. Thermal, morphological, and mechanical characteristics of sustainable tannin bio-based foams reinforced with wood cellulosic fibers. Ind Crops Prod. 2020;158(67):113029. doi:10.1016/j.indcrop.2020.113029. [Google Scholar] [CrossRef]
10. Borrero-López AM, Nicolas V, Marie Z, Celzard A, Fierro V. A review of rigid polymeric cellular foams and their greener tannin-based alternatives. Polymers. 2022;14(19):3974. doi:10.3390/polym14193974. [Google Scholar] [PubMed] [CrossRef]
11. Pizzi A. Tannin-based biofoams—a review. J Renew Mater. 2019;7(5):477–92. doi:10.32604/jrm.2019.06511. [Google Scholar] [CrossRef]
12. DSouza GC, Dodangeh F, Venkata GB, Ray MB, Prakash A, Xu C. A comprehensive review of biobased polyurethane and phenol formaldehyde hydrophilic foams for environmental remediation, floral, and hydroponics applications. Biomass Bioenergy. 2025;192(20):107493. doi:10.1016/j.biombioe.2024.107493. [Google Scholar] [CrossRef]
13. Celzard A, Fierro V, Amaral-Labat G, Pizzi A, Torero J. Flammability assessment of tannin-based cellular materials. Polym Degrad Stab. 2011;96(4):477–82. doi:10.1016/j.polymdegradstab.2011.01.014. [Google Scholar] [CrossRef]
14. Tondi G, Oo CW, Pizzi A, Trosa A, Thevenon MF. Metal adsorption of tann in based rigid foams. Ind Crops Prod. 2009;29(2–3):336–40. doi:10.1016/j.indcrop.2008.06.006. [Google Scholar] [CrossRef]
15. Aristri MA, Lubis MAR, Laksana RPB, Sari RK, Iswanto AH, Kristak L, et al. Thermal and mechanical performance of ramie fibers modified with polyurethane resins derived from Acacia mangium bark tannin. J Mater Res Technol. 2022;18(3):2413–27. doi:10.1016/j.jmrt.2022.03.131. [Google Scholar] [CrossRef]
16. Sepperer T, Šket P, Petutschnigg A, Hüsing N. Tannin-furanic foams formed by mechanical agitation: influence of surfactant and ingredient ratios. Polymers. 2021;13(18):3058. doi:10.3390/polym13183058. [Google Scholar] [PubMed] [CrossRef]
17. Kolbitsch C, Link M, Petutschnigg A, Wieland S, Tondi G. Microwave produced tannin-furanic foams. J Mater Sci Res. 2012;1(3):84–91. doi:10.5539/jmsr.v1n3p84. [Google Scholar] [CrossRef]
18. Lagel MC, Pizzi A, Giovando S, Celzard A. Development and characterisation of phenolic foams with phenol-formaldehyde-chestnut tannins resin. J Renew Mater. 2014;2(3):220–9. doi:10.7569/JRM.2014.634113. [Google Scholar] [CrossRef]
19. Martinez de Yuso A, Lagel MC, Pizzi A, Fierro V, Celzard A. Structure and properties of rigid foams derived from quebracho tannin. Mater Des. 2014;63(3):208–12. doi:10.1016/j.matdes.2014.05.072. [Google Scholar] [CrossRef]
20. Yuan W, Xi X, Zhang J, Pizzi A, Essawy H, Du G, et al. A novel strategy inspired by steaming Chinese steamed bread for preparation of tannin-furanic rigid bio-foam. Constr Build Mater. 2023;376(1):131035. doi:10.1016/j.conbuildmat.2023.131035. [Google Scholar] [CrossRef]
21. Lacoste C, Basso MC, Pizzi A, Laborie MP, Celzard A, Fierro V. Pine tannin-based rigid foams: mechanical and thermal properties. Ind Crops Prod. 2013;43(1):245–50. doi:10.1016/j.indcrop.2012.07.039. [Google Scholar] [CrossRef]
22. Alonso-Esteban JI, Carocho M, Barros D, Velho MV, Heleno S, Barros L. Chemical composition and industrial applications of Maritime pine (Pinus pinaster Ait.) bark and other non-wood parts. Rev Environ Sci Bio/Technol. 2022;21(3):583–633. doi:10.1007/s11157-022-09624-1. [Google Scholar] [CrossRef]
23. Lacoste C, Čop M, Kemppainen K, Giovando S, Pizzi A, Laborie MP, et al. Biobased foams from condensed tannin extracts from Norway spruce (Picea abies) bark. Ind Crops Prod. 2015;73:144–53. doi:10.1016/j.indcrop.2015.03.089. [Google Scholar] [CrossRef]
24. Okuda T, Yoshida T, Hatano T. Classification of oligomeric hydrolysable tannins and specificity of their occurrence in plants. Phytochemistry. 1993;32(3):507–21. doi:10.1016/S0031-9422(00)95129-X. [Google Scholar] [CrossRef]
25. Ky I, Le Floch A, Zeng L, Pechamat L, Jourdes M, Teissedre PL. Tannins. In: Encyclopedia of food and health. 1st ed. Amsterdam, The Netherlands: Elsevier Inc.; 2015. p. 247–55. [Google Scholar]
26. Pizzi A. Recent developments in eco-efficient bio-based adhesives for wood bonding: opportunities and issues. J Adhes Sci Technol. 2006;20(8):829–46. doi:10.1163/156856106777638635. [Google Scholar] [CrossRef]
27. Pizzi A. Tannins: major sources, properties and applications. Monomers Polym Compos Renew Resour. 2008;85(2):179–99. doi:10.1016/B978-0-08-045316-3.00008-9. [Google Scholar] [CrossRef]
28. Azadeh E, Pizzi A, Gerardin-Charbonnier C, Gerardin P. Hydrolysable chestnut tannin extract chemical complexity in its reactions for non-isocyanate polyurethanes (NIPU) foams. J Renew Mater. 2023;11(6):2823–48. doi:10.32604/jrm.2023.027651. [Google Scholar] [CrossRef]
29. Azadeh E, Chen X, Pizzi A, Gérardin C, Gérardin P, Essawy H. Self-blowing non-isocyanate polyurethane foams based on hydrolysable tannins. J Renew Mater. 2022;10(12):3217–27. doi:10.32604/jrm.2022.022740. [Google Scholar] [CrossRef]
30. Eckardt J, Sepperer T, Cesprini E, Šket P, Tondi G. Comparing condensed and hydrolysable tannins for mechanical foaming of furanic foams: synthesis and characterization. Molecules. 2023;28(6):2799. doi:10.3390/molecules28062799. [Google Scholar] [PubMed] [CrossRef]
31. Meikleham NE, Pizzi A. Acid- and alkali-catalyzed tannin-based rigid foams. J Appl Polym Sci. 1994;53(11):1547–56. doi:10.1002/app.1994.070531117. [Google Scholar] [CrossRef]
32. Basso M, Pizzi A, Celzard A. Influence of formulation on the dynamics of preparation of tannin-based foams. Ind Crops Prod. 2013;51:396–400. doi:10.1016/j.indcrop.2013.09.013. [Google Scholar] [CrossRef]
33. Sebestyén Z, Jakab E, Badea E, Barta-Rajnai E, Şendrea C, Czégény Z. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J Anal Appl Pyrolysis. 2019;138(2):178–87. doi:10.1016/j.jaap.2018.12.022. [Google Scholar] [CrossRef]
34. Li J, Zhang A, Zhang S, Gao Q, Zhang W, Li J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos Part B Eng. 2019;156(6):368–77. doi:10.1016/j.compositesb.2018.09.005. [Google Scholar] [CrossRef]
35. Boles JS, Crerar DA, Grissom G, Key TC. Aqueous thermal degradation of Gallic acid. Geochim Cosmochim Acta. 1988;52(2):341–4. doi:10.1016/0016-7037(88)90089-0. [Google Scholar] [CrossRef]
36. Tondi G, Cefarin N, Sepperer T, D’Amico F, Berger RJF, Musso M, et al. Understanding the polymerization of polyfurfuryl alcohol: ring opening and Diels-alder reactions. Polymers. 2019;11(12):2126. doi:10.3390/polym11122126. [Google Scholar] [PubMed] [CrossRef]
37. Delliere P, Pizzi A, Guigo N. Structural variations in biobased polyfurfuryl alcohol induced by polymerization in water. Polymers. 2023;15(7):1745. doi:10.3390/polym15071745. [Google Scholar] [PubMed] [CrossRef]
38. Venter P, Causon T, Pasch H, de Villiers A. Comprehensive analysis of chestnut tannins by reversed phase and hydrophilic interaction chromatography coupled to ion mobility and high resolution mass spectrometry. Anal Chim Acta. 2019;1088:150–67. doi:10.1016/j.aca.2019.08.037. [Google Scholar] [PubMed] [CrossRef]
39. Ahmad EEM, Luyt AS, Djoković V. Thermal and dynamic mechanical properties of bio-based poly(furfuryl alcohol)/sisal whiskers nanocomposites. Polym Bull. 2013;70(4):1265–76. doi:10.1007/s00289-012-0847-2. [Google Scholar] [CrossRef]
40. Martha R, George B, Rahayu IS, Gérardin P, Darmawan W. Technological properties homogenization on sapwood and heartwood of short rotation teak wood by non-biocide method based on chemical and thermal treatments. Eur J Wood Wood Prod. 2024;82(2):371–86. doi:10.1007/s00107-023-01997-6. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools