 Open Access
Open Access
ARTICLE
Amphiphilic Carboxymethyl Cellulose Stearate for Pickering Emulsions and Antimicrobial Activity of Chrysanthemum Essential Oil
1 Cellulose and Paper Department, National Research Centre, 33 El Bohouth St., Dokki, Giza, P.O. Box 12622, Egypt
2 Chemistry of Medicinal Plants Department, National Research Centre, Dokki, Giza, P.O. Box 12622, Egypt
3 Chemistry of Natural and Microbial Products, Pharmaceutical and Drug Institute, National Research Centre, Giza, P.O. Box 12622, Egypt
* Corresponding Author: Mohamed El-Sakhawy. Email:
Journal of Renewable Materials 2025, 13(5), 981-995. https://doi.org/10.32604/jrm.2025.02024-0024
Received 23 October 2024; Accepted 31 December 2024; Issue published 20 May 2025
Abstract
This study prepared and characterized amphiphilic carboxymethyl cellulose stearate (CMCS) recycled from sugarcane bagasse agro-waste (SB). The Fourier-transform infrared (FTIR) analysis confirmed cellulose, carboxymethyl cellulose (CMC), and CMCS structures, with CMCS showing increased H-bonding. X-ray diffraction analysis (XRD) revealed reduced crystallinity in CMC and CMCS. CMCS exhibited a hydrophobic nature but dispersed in water, enabling nanoemulsion formation. Optimal nanoemulsion was achieved with CMCS1, showing a particle size of 99 nm. Transmission electron microscopy (TEM) images revealed CMC’s honeycomb structure, transforming into spherical particles in CMCS1. Antimicrobial tests demonstrated strong activity of CMCS formulations against Escherichia coli and Staphylococcus aureus, with CMCS3 exhibiting the highest efficacy. These findings highlight the potential of CMCS-based nanoemulsions for antimicrobial applications and nanoemulsification.Keywords
The genus Chrysanthemum belongs to the family Asteraceae, most of which are cultivated as ornamentals worldwide because of their good adaptation to different environments [1]. The genus is a good source of secondary metabolites, represented by polyacetylenes, flavonoids, steroids, terpenoids, and essential oils constituting a wide variety of volatile terpene hydrocarbons and their corresponding oxygenated derivatives [2–4]. These plants exhibit antibacterial, antioxidant, anti-inflammatory, and immunomodulatory activities [5,6]. Chrysanthemum frutescens, also known as “Marguerite Daisy”, well-reputed in Egyptian folk medicine, is either cultivated as cut flowers or pot plants or both [7]. For this reason, we try to emulsify Chrysanthemum frutescens by dispersant polymers for medicinal applications.
Pickering emulsions, stabilized by solid particles rather than surfactants, offer a promising platform for dispersing oil materials due to their exceptional stability, low toxicity, and adaptability to various environments [8–10]. Nano-emulsions (NEMs) are tiny spheres, ranging from 10 to 100 nm in size, created by combining typically immiscible liquids like water and oil. Scientists use specialized chemicals called surfactants and co-surfactants to stabilize these droplets, acting as intermediaries between the oil and water to prevent them from recombining [11,12]. Traditional oil dispersants, often made from harmful organic solvents, pose a greater threat to the environment than the oil spills they aim to clean up. However, research into environmentally friendly alternatives, such as dispersants made from biodegradable agricultural waste, offers hope for a less toxic solution to this pressing environmental challenge. Agricultural wastes represent a readily available source of raw materials that can be transformed into valuable products for diverse applications, such as the production of surfactants [9].
Sugarcane bagasse agro-waste (SB), a byproduct of agricultural processing, contains cellulose, a chain-like molecule composed of sugar units known as D-glucose [13]. Cellulose, the building block of plants, exhibits both hydrophilic and hydrophobic characteristics due to the structure of its glucopyranose units. The axial orientation of C-H bonds within the ring contributes to hydrophobic properties, while the equatorial positioning of hydroxyl (OH) groups at carbons 6, 2, and 3 imparts a hydrophilic nature, allowing for interactions with water molecules [9]. Cellulose molecules possess a unique combination of hydrophilic and hydrophobic properties, making them amphiphilic. This dual nature significantly influences how cellulose interacts with both water-soluble and water-insoluble substances, impacting their behavior in aqueous environments [9]. Amphiphilic cellulosic polymers are created by chemically modifying cellulose derived from agricultural waste through a process called esterification, which imparts both water-loving and water-repelling properties to the resulting material [9]. Stearic acid, when combined with carbohydrates, forms a powerful emulsifier that is used in small quantities to effectively blend flavors and colors within a product [14]. In addition, cellulose carboxymethylation imparts a more hydrophilic nature to the cellulose backbone which makes it a good surfactant for Pickering emulsification of hydrophobic oil [9,13].
This research employed a gentle, chemical-free method to create a stable emulsion incorporating Plectranthus amboinicus extract. The study centered on examining the properties of SB-derived cell-stearate, assessing its role in enhancing emulsion stability and antimicrobial activity, and identifying the key constituents within the plant extract.
In this work, we presented a carboxymethyl cellulose stearate (CMCS) using the Pickering emulsification method to prepare an amphiphilic emulsion incorporating the essential oil of C. frutescens. The oil components were investigated via Gas chromatography/mass spectrometry (GC/MS) analysis.
The sugarcane bagasse (SB) used in this experiment was provided by Quena Company for Paper Industry in Quena, Egypt, and the paraffin oil was obtained from Sigma-Aldrich in St. Louis, MO, USA. Neither the SB nor the paraffin oil underwent any further purification before being used in the experiment.
2.2 Plant Material and Extraction
The aerial parts of C. frutescens were collected from the National Research Centre gardens in spring, according to the national institutional guidelines and regulations. The plant was authenticated at the Agricultural Museum, Dokki, Giza, Egypt, and a voucher sample was kept at the herbarium of the National Research Center (No. M218). The fresh plant (flower heads and leaves) was placed in a conical flask with distilled water, and the volatile oil was obtained by the hydro-distillation method using a Clevenger apparatus. The oil was stored in a clear glass vial for GC/MS analysis [15].
SB (150 g) underwent a multi-step process. Initially, it was hydrolyzed using dilute hydrochloric acid (1.5%) at 120°C for 2 h. Subsequently, the resulting material was converted into pulp by treating it with sodium hydroxide (20 g in 300 mL water) at 170°C for 2 h. The obtained pulp (80 g) was then subjected to a bleaching process involving chlorite (3%) and acetic acid (1.7 mL) at 80°C for 2 h to eliminate lignin, resulting in pure α-cellulose. Throughout the bleaching stage, acetic acid was continuously added to maintain a pH between 1 and 3, ensuring optimal reaction conditions [9,16].
2.4 Carboxymethyl Cellulose Preparation
Pure α-cellulose (2.5 g) was dissolved in isopropanol (75 mL) and reacted with sodium hydroxide (40%, 3 g in 7.5 mL water) and monochloroacetic acid (3 g) for 3.5 h at 55°C with constant stirring. The resulting carboxymethyl cellulose (CMC) was precipitated by adding 70% ethanol, filtered, and dried [13,16].
2.5 Amphiphilic Carboxymethyl Cellulose Stearate (CMCS)
The process involved treating CMC (1.6 g; DS = 0.7) with DMSO (35 mL) and stirring until complete dissolution. The stearic acid (3 g) was mixed with acetic anhydride (2 mL) and a few drops of sulfuric acid as a catalyst. This mixture was stirred at 90°C until it became viscous for 30 min, then filtered, washed with ethanol, and finally dried.
2.6 CMCS as a Dispersant in O/W Emulsion
The performance of amphiphilic CMC–Stearate as a dispersant was investigated by mixing CMC–Stearate (0.5 g) with water (1.5 mL). The oil (with different weight percentages of 2.5%, 5%, and 10%; denoted as CMCS1, CMCS2, and CMCS3, respectively) was added by mixing through an Ultrasonicator (700 W; Sigma-Aldrich) for 3 min in an ice water bath [17].
The Fourier-transform infrared (FTIR) spectroscopy was used to analyze the chemical composition of the samples. They employed a Mattson-5000 instrument (Unicam, Somerset, UK) and prepared the samples using the KBr disk method. The FTIR analysis covered a range of wavenumbers from 4000 to 400 cm−1, providing detailed information about the molecular vibrations within the samples.
The degree of substitution (DS) was calculated according to the following equation:
where AC=O and AC-O refer to the FTIR absorbance bands at C=O and C–O–C [17].
The hydrophilic-lipophilic balance (HLB) value can be calculated by using the following equation:
where
The empirical crystallinity index (LOI) and mean hydrogen bond strength (MHBS) were calculated according to the following equations:
where A1425 and A900 refer to the FTIR absorbance at 1425 and 900 cm−1. In addition, AOH and ACH refer to the FTIR absorbance for the OH and CH peaks [16].
XRD was determined by Bruker D8 Advance X-ray diffractometer (Karlsruhe, Germany) using copper (Kα) radiation (1.5406 Å) at a 40 kV voltage and a 40 mA current.
Crystallinity index Cr.I. (%) was determined using Eqs. (5).
where AC is the crystalline peak area and At is the total area [9].
where λ is the wavelength, β and θ are full widths at half maxima and Bragg’s angle of the XRD peak, respectively [9].
2.7.3 Size Distribution and Zeta Potential
A particle size analyzer (Nano-ZS, Malvern Instruments Ltd., Malvern, UK) was used to assess the average diameter, size distribution, and zeta potential of the samples. To ensure accurate measurement of size distribution and zeta potential, the samples were sonicated for 30–60 min before the analysis.
2.7.4 Morphological Properties
The morphology was established via transmission electron microscopy (TEM, JEM-1230, Gunma, Japan) with magnification 600 × 103, resolution 0.2 nm, and 120 kV.
Contact Angle (C.A.) was measured on glass using Ramé-Hart 500-F1 at 25°C, following DIN ISO 55660-1/2.
The qualitative tests were performed by the disc diffusion method using nutrient agar plates [20]. Inoculations of pathogenic microorganisms used in this study included the gram-positive bacteria Bacillus cereus, Micrococcus luteus, and Staphylococcus aureus; the gram-negative bacteria Escherichia coli and Helicobacter pylori; and the pathogenic yeast Candida albicans.
The analysis used Excel software for particle size distribution. To ensure accuracy, each experiment was repeated three times, and the reported results represent the average of three samples with less than 5% variation.
The yield of the volatile oil extracted from the aerial parts of C. frutescens using the hydro-distillation method was 0.11% v/w. Based on their mass spectrum data (MS), 36 metabolites were identified using the Wiley spectral library collection, GNPS, and NIST library database [20]. The total percentage of identified compounds was estimated to be 68.7%. The distribution of the main essential oil classes of C. frutescens investigated is shown in (Fig. 1).

Figure 1: The distribution of the major classes of C. frutescens volatile oil
Volatile oil constituents along with their respective retention times (Rt), experimental Kovats retention index reported in the investigation (KI*), Kovats retention index from the literature (KI**), and percentages are listed in (Table 1, Fig. 1).

As illustrated in Fig. 2, oxygenated monoterpenes (28.54%) and sesquiterpene hydrocarbons (27.23%) were the predominant classes, while alkanes, monoterpene hydrocarbons, and oxygenated sesquiterpenes were demonstrated at moderate levels (4.8%, 3.11%, and 2.49%, respectively). Contrariwise, benzenoids were present with a percentage of 0.71, and the percentage of oxygenated diterpenes was 1.82 under hydro-distillation conditions.

Figure 2: GC/MS chromatogram for C. frutescens volatile oil
Oxygenated monoterpenes were the predominant class in C. frutescens essential oil extracted (28.54%). The most privileged-identified compounds are dihydrocarveol and isopulegol acetate, which occurred with relative percentages of 12% and 5.5%, respectively. Dihydrocarveol is used as an important additive in the flavor industry and as a building block in the synthesis of natural products [21].
Also, the most abundant sesquiterpene hydrocarbons were β-caryophyllene (15.7%), followed by aromadendrene (6.85%). β-caryophyllene has antioxidant, anticancer, cardioprotective, hepatoprotective, nephroprotective, immunomodulatory, gastroprotective, and antimicrobial activities [8,22]. While aromadendrene is used to produce environmentally friendly insecticides against cereal pests, it also has anticholinesterase, antinociceptive, anti-hyperalgesic, and anti-mycobacterial activities [22]. 2-methyl decane was an abundant alkane present in the oil (4%). 1, 3, 8-p-menthatriene was the major monoterpene hydrocarbon (2.4%). Nerolidol (1.71%) was presented as a major oxygenated sesquiterpene. It possesses pharmacological and biological activities such as anti-biofilm, anti-microbial, anti-parasitic, antioxidant, anti-inflammatory, anti-ulcer, skin penetration enhancer, insect repellent, and anti-cancer effects. Nonetheless, verticiol (1.82%) was the only oxygenated diterpene detected; similarly, benzenoids were present at far lower quantities (0.17%). Notice that the antimicrobial activities of the essential oils of various Chrysanthemum species against a wide range of microbes were investigated, aiming to use these plants and their oils as natural spices and preservatives in food and pharmaceutical preparations, along with other various applications [3,23].
3.2 Characterizations of the Amphiphilic Cellulose Stearate
3.2.1 Fourier-Transform Infrared Spectra
Cellulose and CMC were identified through their characteristic infrared spectroscopy bands, with peaks between 3462–3418 cm−1 (O–H stretching), 2882–2902 cm−1 (C–H stretching), 1375–1377 cm−1 (CH2 bending and C–O–C of pyranose ring vibration), and 1040–1057 cm−1 (the β-glycosidic linkage between glucose units in the cellulose structure) [15,19]. The cellulose showed a peak at 1650 cm−1, which is attributed to OH bending of adsorbed water for cellulose, while CMC showed a peak at 1715 cm−1, which is attributed to the C=O group [13,16].
CMCS exhibited additional characteristic absorption bands at 2915 and 2848 cm−1, which are attributed to CH2 and CH3 groups, respectively. In addition, the C=O group was shifted to 1700 cm−1, and the OH group was shifted to 3336 cm−1, which means the strong H–bond after preparing CMC–Stearate. Moreover, 1471 (O=C–O), 1297 (C–O), and 1037 cm−1 (C–O–C) [9]. Moreover, a band at 717 cm−1 ((CH2 rocking), which is characteristic of at least four linearly connected CH2 groups), and at 894 cm−1 (related to the last carbon from the aliphatic chain) [24,25]. These findings prove that cellulose was esterified (Fig. 3a).

Figure 3: (a) FTIR spectra of cellulose, CMC, and CMCS, and (b) XRD spectra of cellulose, CMC, and CMCS
The esterification process transformed cellulose into CMCS, leading to several changes. Hydrogen bonding increased between stearate and cellulose functional groups, causing the MHBS value to rise from 0.61 to 1.21. In addition, the relative absorbance of the CH2 group for CMC and CMCS was 0.70 and 0.90, respectively, which proves the esterification reaction. The calculated DS for stearic acid was 1.05.
3.2.2 X-Ray Diffraction Analysis
The characteristic peaks for cellulose were identified at 11°, 20°, and 21°θ. Interestingly, the splitting of the peaks at 20° and 21° suggests the formation of cellulose II [16]. In addition, the CMC powder indicates reflections at 10° and 20°θ. The broadening of the 20.22°θ peak indicates the amorphous structure of CMC [9,13].
In the case of CMCS, slightly broader reflections may be observed at 15°, 21°, and 22°. The reflection for CMCS at 15° represents the patterns of the amorphous structure. However, CMCS exhibits the formation of a new reflection band at 34°, which is attributed to the stearate side chains (Fig. 3b) [9]. As calculated from the X-ray patterns, it is clear that the Cr.I (%) of CMCS (40%) was decreased compared to cellulose (61%), because of the esterification reactions that led to the cleavage of the H-bonds, which broke down the H-bonds, which minimized the Cr.I. (%) (Fig. 2b). The FTIR MHBS results do not correlate with the XRD Cr.I (%) readings, as a result of intramolecular H-bonding.
The decreased Cr.I. (%) observed in CMC (43%) and CMCS (40%), as evidenced by XRD analysis, has significant implications for their properties and emulsification behavior. Reduced Cr.I. (%) often leads to increased amorphous regions, which can enhance solubility, swelling, and interaction with solvents [17]. In the case of CMCS, the lower crystallinity may facilitate the formation of smaller, more stable nanoparticles. The increased amorphous character can also influence the mechanical properties of the material, potentially affecting its film-forming ability and stability under various conditions. Additionally, the reduced crystallinity may enhance the interaction between CMCS and the oil phase, promoting interfacial adsorption and stabilization of the emulsion.
3.3 Characterization of the Oil/Water Systems
The CMCS mixture exhibited a calculated HLB value of 3.02 and a DS of 1.05, suggesting predominantly hydrophobic characteristics. However, despite the low HLB, the mixture readily dispersed in water when combined with CMCS, likely due to the hydrophilic nature of CMCS. This aqueous dispersion allowed for the successful detection of antimicrobial compounds from the extract within the resulting nanoemulsions (NEMs).
3.3.2 Size Distribution and Zeta Potential
This study explored the impact of cell-Stearate on the size and polydispersity index (PDI) of PEE for the first time. Results (Fig. 4) indicate that a 2.5% w/v concentration of CMCS1 yielded the smallest particle size at 62.12 nm. This reduction in particle size and PDI, as depicted in Fig. 3, suggests that CMCS1 provides the most stable nanoemulsion (NEM) formulation compared to other concentrations tested.

Figure 4: Particle size distributions of the prepared NEMs (a–c)
TEM analysis revealed that CMC-Stearate formed irregular honeycomb structures with a radius of 0.12 μm (Fig. 5). However, upon nanoemulsification, CMCS1 transformed into discrete, rounded particles ranging from 2.46 to 3.51 nm in diameter, demonstrating its effective dispersion capabilities.

Figure 5: TEM for (a) CMC–stearate and (b) CMCS1
TEM analysis revealed a striking morphological transformation from the irregular honeycomb structures of CMC-Stearate to discrete, spherical nanoparticles in CMCS/2.5% oil (i.e., CMCS1). This transition is crucial for the formation of stable nanoemulsions. The spherical nanoparticles, with their high surface area and reduced particle size, effectively adsorb at the oil-water interface, acting as Pickering stabilizers. The compact and spherical morphology minimizes interfacial energy, enhancing the stability of the nanoemulsion against coalescence and Ostwald ripening. Additionally, the smaller particle size contributes to improved colloidal stability and prevents sedimentation. This morphological change, induced by the esterification of CMC with stearic acid, underscores the importance of molecular design in tailoring the properties of nanomaterials for nanoemulsification applications.
3.5 Antimicrobial Activity of the Prepared NEMs
The standard well diffusion assay was used to record the inhibition zone [16]. Table 2 and Fig. 6 show that the CMCS1, CMCS2, and CMCS3 had strong antimicrobial activity against Escherichia coli, with inhibition zones of 17, 18, and 21 mm, while only CMCS2 and CMCS3 had strong antimicrobial activity against Staphylococcus aureus, with inhibition zones of 20 and 22 mm. The CMCS1 didn’t give any inhibition zone against Staphylococcus aureus. This activity is likely due to a combination of volatile and non-volatile compounds in the material [26]. The CMCS3 exhibited a stronger antibacterial activity against Escherichia coli and Staphylococcus aureus than CMCS1 and CMCS2 due to their low content of C. fructescens.


Figure 6: Inhibition zone diameter (mm) of the prepared NEMs towards Escherichia coli, Helicobacter pylori, Bacillus cereus, Staphylococcus aureus, and Candida albicans
The standard well diffusion assay used in this study gave a higher inhibition diameter compared to the disc diffusion method.
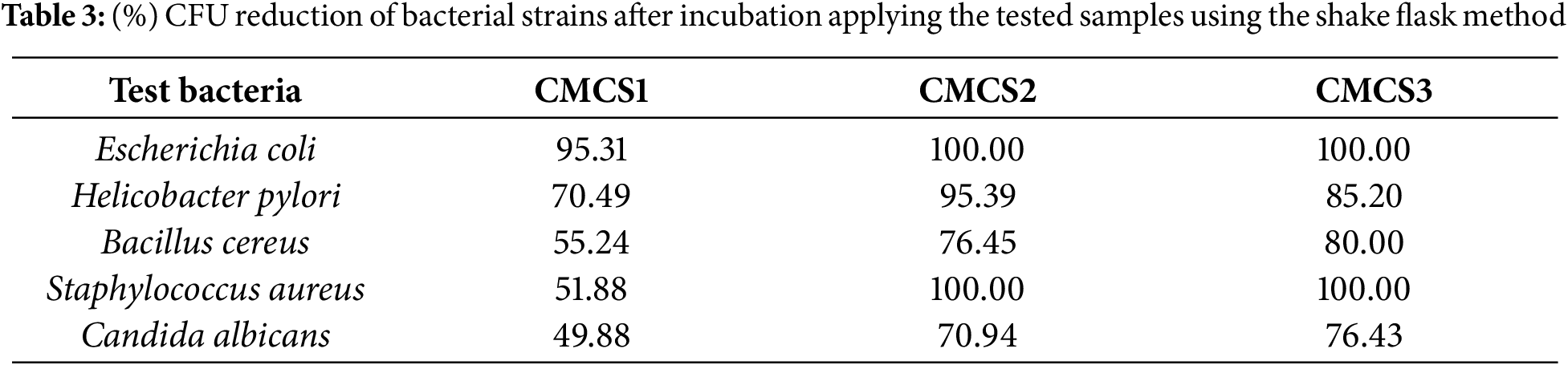
The activity profile for MICs of C. fructescens extracts (Table 3) appeared to give super antibacterial activity against all types of bacteria. In addition, CMCS3 gave the best antibacterial activity (i.e., 100.00%, 85.20%, 80.00%, 100.00%, and 76.43% against Escherichia coli, Helicobacter pylori, Bacillus cereus, Staphylococcus aureus, and Candida albicans) due to its high content of C. fructescens.
This research aimed to develop low-cost dispersants from the volatile oil of C. fructescens. They successfully created CMCS using a simple esterification process with stearic acid. The formation of this CMCS was confirmed by specific techniques. This eco-friendly method used cell-stearate to emulsify the plant oil through a straightforward ultrasonic technique, achieving a successful oil-in-water emulsion. Microscopic analysis showed oil-in-water emulsions were between 27 and 59 nm in size. As the oil content increased, the cell pores filled, blocking them. This was confirmed by contact angle analysis, which showed that the oil wet the cell wall perfectly up to a certain concentration. Further research is needed to find eco-friendly materials for these applications.
Acknowledgement: The authors of this paper are thankful to the National Research Centre, Egypt, for providing the infrastructure and research facilities.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Mohamed El-Sakhawy, Hebat-Allah S. Tohamy and Mona Mohamed AbdelMohsen conceived designed the experiments, analyzed and interpreted the results and reviewed the paper. Sally A. Abdel-Halim, Hebat-Allah S. Tohamy and Hossam M. El-Masry performed the experiment, interpreted the results and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Mabberley DJ. Mabberley’s plant-book: a portable dictionary of plants, their classification and uses. 4th ed. Cambridge, UK: Cambridge University Press; 2017. [Google Scholar]
2. Mostafa NM. Comparative analysis of rutin content in some Egyptian plants: a validated RP-HPLC-DAD approach. Eur J Med Plants. 2017;19(2):1–8. doi:10.9734/EJMP/2017/33760. [Google Scholar] [CrossRef]
3. Youssef FS, Eid SY, Alshammari E, Ashour ML, Wink M, El-Readi MZ. Chrysanthemum indicum and Chrysanthemum morifolium: chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities. Foods. 2020;9(10):1460. doi:10.3390/foods9101460. [Google Scholar] [PubMed] [CrossRef]
4. Kuang CL, Lv D, Shen GH, Li SS, Luo QY, Zhang ZQ. Chemical composition and antimicrobial activities of volatile oil extracted from Chrysanthemum morifolium Ramat. J Food Sci Technol. 2018;55(7):2786–94. doi:10.1007/s13197-018-3203-1. [Google Scholar] [PubMed] [CrossRef]
5. Cui H, Bai M, Sun Y, Abdel-Samie MAS, Lin L. Antibacterial activity and mechanism of Chuzhou chrysanthemum essential oil. J Funct Foods. 2018;48:159–66. doi:10.1016/j.jff.2018.07.021. [Google Scholar] [CrossRef]
6. Cheng W, Li J, You T, Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J Ethnopharmacol. 2005;101(1–3):334–7. doi:10.1016/j.jep.2005.04.035. [Google Scholar] [PubMed] [CrossRef]
7. Ammar NM, El-Hawary SS, El-Anssary AA, El-Desoky AH, Abdelaal TA, Abdelrahman RF, et al. Phytochemical study of the bioactive fractions of Chrysanthemum fructescens L. cultivated in Egypt. Int J Pharmacognosy Phytochem Res. 2016;8(8):1314–21. [Google Scholar]
8. Cui ZG, Shi KZ, Cui YZ, Binks BP. Double phase inversion of emulsions stabilized by a mixture of CaCO3 nanoparticles and sodium dodecyl sulphate. Coll Surf A Colloid Surf A Physicochem Eng Asp. 2008;329(1–2):67–74. doi:10.1016/j.colsurfa.2008.06.049. [Google Scholar] [CrossRef]
9. Tohamy HAS. Oil dispersing and adsorption by carboxymethyl cellulose-oxalate nanofibrils/nanocrystals and their kinetics. J Surfactants Deterg. 2023;27(1):147–60. doi:10.1002/jsde.12706. [Google Scholar] [CrossRef]
10. Wang H, Hobbie EK. Amphiphobic carbon nanotubes as macroemulsion surfactants. Langmuir. 2003;19(8):3091–3. doi:10.1021/la026883k. [Google Scholar] [CrossRef]
11. Barroso A, Mestre H, Ascenso A, Simões S, Reis C. Nanomaterials in wound healing: from material sciences to wound healing applications. Nano Select. 2020;1(5):443–60. doi:10.1002/nano.202000055. [Google Scholar] [CrossRef]
12. Dinshaw IJ, Ahmad N, Salim N, Leo BF. Nanoemulsions: a review on the conceptualization of treatment for psoriasis using a ‘green’surfactant with low-energy emulsification method. Pharmaceutics. 2021;13(7):1024. doi:10.3390/pharmaceutics13071024. [Google Scholar] [PubMed] [CrossRef]
13. Tohamy HAS, El-Sakhawy M, Elnasharty MM. Carboxymethyl cellulose membranes blended with carbon nanotubes/ag nanoparticles for eco-friendly safer lithium-ion batteries. Diam Relat Mater. 2023;138:110205. doi:10.1016/j.diamond.2023.110205. [Google Scholar] [CrossRef]
14. Jufri M, Namirah J, Suryadi H. Formulation and stability study of black cumin (Nigella sativa L.) seed oil emulsion using sucrose palmitate as emulsifier. Int J App Pharm. 2022;14(5):113–8. doi:10.22159/ijap.2022v14i5.44945. [Google Scholar] [CrossRef]
15. Hassanein H, Said-Al Ahl H, Abdelmohsen M. Antioxidant polyphenolic constituents of Satureja montana L. growing in Egypt. Int J Pharm Pharm Sci. 2014;6(4):578–81. [Google Scholar]
16. El-Sakhawy M, Tohamy HAS, AbdelMohsen MM, El-Missiry M. Biodegradable carboxymethyl cellulose based material for sustainable/active food packaging application. J Thermoplast Compos Mater. 2024;37(6):2035–50. doi:10.1177/08927057231211236. [Google Scholar] [CrossRef]
17. Tohamy HAS, El-Sakhawy M, Abdel-Halim SA, El-Masry HM, AbdelMohsen MM. Antimicrobial Plectranthus amboinicus emulsions prepared with amphiphilic cellulose stearate. Euro-Mediterr J Environ Integr. 2024;2024(1):1–12. doi:10.1007/s41207-024-00675-0. [Google Scholar] [CrossRef]
18. Fingas M. Oil spill science and technology. 2nd ed. Oxford, UK: Gulf Professional Publishing; 2016. [Google Scholar]
19. Jafarirad S. Innovative amphiphilic cellulose nanobiostructures: physicochemical, spectroscopic, morphological, and hydrophilic/lipophilic properties. J Disper Sci Technol. 2017;38(8):1187–95. doi:10.1080/01932691.2016.1225509. [Google Scholar] [CrossRef]
20. Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. 4th ed. Carol Stream, IL, USA: Allured Publ; 2007. [Google Scholar]
21. Guo J, Zhang R, Ouyang J, Zhang F, Qin F, Liu G, et al. Stereodivergent synthesis of carveol and dihydrocarveol through ketoreductases/ene-reductases catalyzed asymmetric reduction. ChemCatChem. 2018;10(23):5496–504. doi:10.1002/cctc.201801391. [Google Scholar] [CrossRef]
22. Gyrdymova YV, Rubtsova SA. Caryophyllene and caryophyllene oxide: a variety of chemical transformations and biological activities. Chem Pap. 2022;76(1):1–39. doi:10.1007/s11696-021-01865-8. [Google Scholar] [CrossRef]
23. Sadaf S, Munir N, Saeed A, Hassan K, Ahmad Z. Antimicrobial activity of comfort related properties of silk treated with herbal extracts in making of reusable masks. Bioscience J. 2023;39:e39035. doi:10.14393/BJ-v39n0a2023-65193. [Google Scholar] [CrossRef]
24. Huang F-Y. Thermal properties and thermal degradation of cellulose tri-stearate (CTs). Polymers. 2012;4(2):1012–24. doi:10.3390/polym4021012. [Google Scholar] [CrossRef]
25. Jayapal R. Synthesis and electrospinning of cellulose stearate [master’s thesis]. Tallinn, Estonia: Tallinn University of Technology; 2015. [Google Scholar]
26. Subha T, Srilatha M, Naveen P, Thirumalaisamy R. Green synthesis, characterization and optimization of silver nanoparticles from Carica papaya using Box Behnken design and its activity against dental caries causing Streptococcus sp. Chem Data Collect. 2024;51(4):101139. doi:10.1016/j.cdc.2024.101139. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools