 Open Access
Open Access
ARTICLE
Eco-Friendly Materials Composed of Cellulose Fibers from Agave Bagasse with Silver Nanoparticles and Shrimp Chitosan
1 Department of Water and Energy, University of Guadalajara Campus Tonalá, Tonalá, 45425, México
2 Department of Wood Cellulose and Paper, University of Guadalajara, Zapopan, 44430, Mexico
3 Department of Biomedical Sciences, University of Guadalajara Campus Tonalá, Tonalá, 45425, México
4 Laboratory of Polymer Science and Technology (POLIUNA), School of Chemistry, Universidad Nacional, Campus Omar Dengo, Heredia, 40101, Costa Rica
* Corresponding Author: Belkis Sulbarán-Rangel. Email:
Journal of Renewable Materials 2025, 13(5), 849-863. https://doi.org/10.32604/jrm.2025.02024-0061
Received 15 December 2024; Accepted 14 February 2025; Issue published 20 May 2025
Abstract
In this research, the antibacterial properties of a composite material prepared from agave bagasse cellulose fibers doped with silver nanoparticles and chitosan were studied. The development of composite materials with antibacterial properties and environmentally friendly based on cellulose fibers from agave bagasse with silver nanoparticles prepared by green synthesis and chitosan from shrimp waste enhances the value of these agro-industrial wastes and offers the opportunity for them to have biomedical applications since these raw materials have been poorly reported for this application. The antibacterial properties of chitosan and silver nanoparticles are already known. However, the combination of silver nanoparticles with cellulose fibers and chitosan has been studied poorly. Green synthesis of silver nanoparticles was carried out, and spherical shape nanoparticles with a size between 20 and 50 nm were obtained by ultraviolet-visible (UV-Vis) spectroscopy and transmission electron microscopy (TEM) analysis. Additionally, in this research, the cellulose obtained from agave bagasse, the chitosan extracted from shrimp shells, and the composite material were characterized by infrared spectroscopy, mechanical analysis, and antibacterial tests. A decrease in the growth of Escherichia coli bacteria with 100% growth inhibition on cellulose, chitosan, and silver nanoparticles composite material and an increase in mechanical properties from 13.67 MPa of cellulose pure to 110 MPa of composite material was observed. These findings support the idea that the composite material has potential use in wound care dressings for antibacterial care.Graphic Abstract
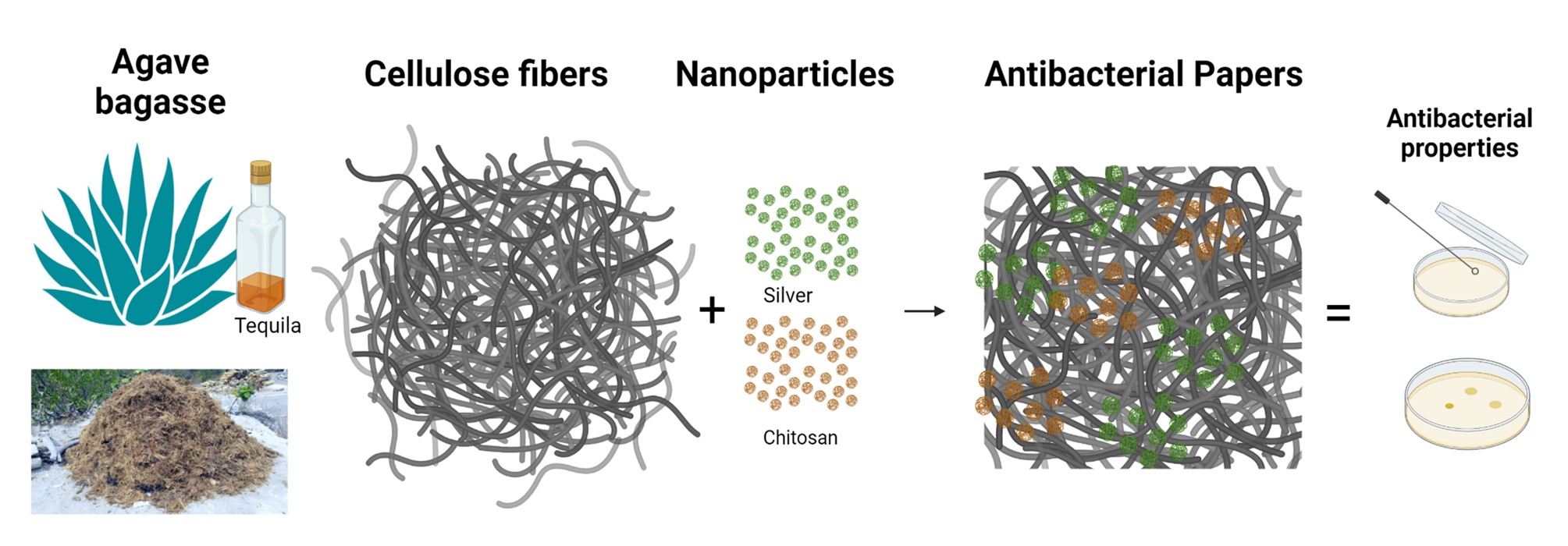
Keywords
In recent decades, industrial waste production and using non-renewable resources as raw materials for industries have gained significant importance due to the need to reduce the environmental impact of such activities and ensure that natural resources are available for future generations. Consequently, actions have been taken to guide the industry’s path toward a sustainable future [1]. Among these actions is the emergence of so-called green chemistry, which involves the synthesis of compounds or products by less polluting methods than conventional ones so that no harmful waste is generated or the least amount of it is produced. Additionally, non-renewable resources used by industry have been replaced with renewable raw materials, and there is even a focus on utilizing industrial waste to manufacture products or energy production [1,2].
In Jalisco, Mexico, due to the significant tequila industry expansion, one of the central agro-industrial residues is agave bagasse, obtained after preparing the alcoholic beverage called tequila. It has been reported that almost half of bagasse composition is cellulose, around one-fifth is hemicellulose, and one-sixth is lignin [3]. These bagasse components have recently been proposed to obtain new raw materials, with particular attention given to cellulose [3,4]. Cellulose can be defined as a biopolymer formed by a long chain of carbohydrates, composed exclusively of glucose molecules linked by glycosidic bonds (1-β4) and interconnected by hydrogen bonds [5,6]. This polymer can be obtained from natural sources such as cotton [7], trees [8], and bacteria [9,10].
Furthermore, cellulose can be isolated from agave bagasse and used for various purposes, such as bioplastics manufacture or composite materials [11,12]. Another critical residue is the crustacean shells, such as shrimp and lobsters, from which chitin can be extracted to obtain chitosan [13]. Chitosan is a natural polymer derived from chitin deacetylation with antimicrobial, anti-inflammatory, and antioxidant properties, used in pharmacy, food, and cosmetics [13,14]. The cellulose and chitosan combination has been extensively investigated due to their complementary properties and potential in various applications [9,15]. Cellulose has a fibrous structure, providing high mechanical strength and chemical stability, while chitosan contributes antimicrobial and biocompatibility properties [9]. This composite material has found applications in various fields, such as cellulose-chitosan films with improved oxygen and water vapor barrier properties for food packaging [16]. Other studies have demonstrated that cellulose-chitosan sponges are helpful in the removal of heavy metals and dyes from wastewater, leveraging the adsorption capacity of chitosan and the porous structure of cellulose [17–19]. In the biomedical field, studies have been reported using cellulose derivatives (carboxymethyl cellulose, cellulose acetate, and others) [20,21], nanocellulose [22], and bacterial cellulose combined with chitosan loaded with silver nanoparticles to enhance antibacterial activity [23], suggesting their potential use for applications in wound dressings and medical devices. Silver nanoparticles (AgNPs) are highly effective in biological tests for burn treatment or antisepsis, and they have been widely used for their antimicrobial properties in drug delivery vehicles development or other medical devices [24].
Research reporting the use of cellulose and chitosan for biomedical applications generally sources cellulose from natural sources such as cotton [7], trees [8], and bacteria [9,10]. In this research, the aim is to add value to agave bagasse residues by obtaining cellulose to develop eco-friendly composite materials made of agave bagasse cellulose fibers with silver nanoparticles prepared by green synthesis and shrimp chitosan to enhance their antibacterial properties and potential uses in biomedical applications, as this raw material has not been reported for this application. Green synthesis is defined as using processes that minimize or eliminate the generation of toxic byproducts through renewable raw materials, safe solvents, or more benign reaction conditions [25]. Therefore, the research aims to prepare a composite material with agave bagasse cellulose fibers, varying the chitosan amounts between 0 to 4% w/w and the size (20 and 100 nm) and concentration (0 to 4% w/w) from silver nanoparticles. In this study, the developed composite materials are prepared from agave bagasse fibers and chitosan derived from shrimp shells, two renewable and underutilized raw materials that, if not harnessed, could be disposed of inefficiently or polluting [26]. In addition, the silver nanoparticles were synthesized using glucose as a reducing agent, thus avoiding toxic chemicals used in conventional commercial methods [27]. The aim is to revalue agro-industrial and marine residues for biomedical applications. In medicine, there is a need for low-cost and accessible wound care materials with a broad range of antimicrobial activity. The synergy between agave cellulose fibers and chitosan will support silver nanoparticles to enhance their antibacterial activity, a characteristic that will be evaluated based on their concentration and size. Finally, agave fibers and chitosan are biodegradable, reducing their persistence in the environment after use. Promoting the production of this composite material highlights its contribution to sustainability, its alignment with the principles of the circular economy, and its ability to meet industrial demands with a lower ecological impact.
2.1 Isolation and Characterization of Cellulose from Agave Bagasse
Bagasse fibers from the agave tequilana Weber plant were obtained from a tequila company named “Grupo Internacional de Exportación, S.A de C.V.” located in Tlajomulco de Zúñiga Jalisco, Mexico. A total of 20 kg of agave bagasse was collected at the company and sun-dried to eliminate the excess moisture. The bagasse was passed through a Sprout Waldron mill with a Kopper’s feeder to undo the existing knots in the bagasse and separate the fiber from the pith (demented) to facilitate the pulping stage. Once the bagasse was depicted, alkaline cooking was carried out, for which a solution of 200 g of sodium hydroxide (NaOH) and 1 g of anthraquinone in 2 L of water was prepared. One kg of bagasse, NaOH, and anthraquinone solutions were introduced into a Jaime-type digester for 90 min at 165°C ± 5°C. After washing the pulp with water, it was purified in a scrubber and a Dutch pile to separate the fibers. The Kappa number was determined by the T 236 om-22 standard [28] to estimate the residual lignin amount and proceed to bleach the fibers. Then, the cellulose pulp was bleached by applying 80% w/v sodium hypochlorite (NaClO2) and 99% w/v acetic acid (CH3COOH) in a ratio of 1:25 at 75°C. Three doses of the bleaching solution were added every 12 h to remove lignin from agave bagasse. The fibers were then washed with distilled water.
The cellulose obtained was characterized according to the T 203 cm-22 standard to determine the percentage of alpha cellulose [29]. The morphology of cellulose fiber obtained from agave bagasse (fiber length, crimp index, twist index, and fiber width) was evaluated using a fiber quality analyzer (FQA) Op Test Equipment Inc. The FQA was calibrated according to the T 271 om-07 standard [30]. Additionally, images were obtained with a scanning electron microscope (SEM) model MIRA 3 LMU; TESCAN. For SEM images, the cellulose fiber samples were coated with a layer of gold. The inherent viscosity of cellulose was used to measure the degree of polymerization of agave bagasse cellulose, as defined in standard D1795-13 [31]. Fourier transform infrared spectroscopy (FT-IR) analysis identified the functional groups present in cellulose, which was carried out with a Perkin Elmer Spectrum GX spectrometer in the range of 4000 to 500 cm−1 with a resolution of 4 cm−1.
2.2 Preparation and Characterization of Chitosan
The shrimp shells were sourced from an aquaculture byproduct company on the Pacific coast of Costa Rica. The shells were crushed and treated with 5% w/w hydrochloric acid followed by 5% w/w sodium hydroxide at room temperature. After that, the material was rinsed to neutral pH and sun-dried using a solar oven. Finally, the material underwent deacetylation to transform chitin to chitosan with 50% w/w NaOH at 100°C for 2 h with continuous stirring at 200 rpm. The resulting mixture was filtered, washed to neutral pH, and then dried in an oven at 70°C. The chitosan’s viscosimetric molecular weight (Mv) was determined by viscosimetry using a buffer of 0.25 M acetic acid/0.25 M sodium acetate, applying the Mark–Houwink–Sakurada constants of K = 1.57 × 10−4 and a = 0.79 [32]. To estimate the degree of deacetylation was measured by conductometric titration with 0.1 M HCl as the solvent and 0.1 M NaOH as the titrant [33].
2.3 Synthesis and Characterization of Silver Nanoparticles
Silver nanoparticles were synthesized using the green carbohydrate reduction method described by Aguilar-Méndez et al. (2010). The synthesis consists of adding 10 mL of 0.1 M silver nitrate to a solution of glucose and gelatin in different proportions for 30 min under magnetic stirring and controlling the pH at 10 with NaOH to obtain silver nanoparticles in two size ranges as described in Table 1 [27].

An UV-Vis spectrophotometer (Shimadzu 1600 UV spectrometer, Kyoto, Japan) was used to monitor the reduction of silver nitrate into AgNPs between 200 and 800 nm. The AgNPs were characterized using TEM (JEOL, JEM2011) at a voltage of 120 kV. A suspension of 5 μL AgNPs was put on carbon-coated copper grids and dried in a desiccator with silica for 16 h. Furthermore, the hydrodynamic diameter, zeta potential, and polydispersity of the AgNPs were evaluated using dynamic light scattering (DLS) on a nanoparticle analyzer (NanoPartica SZ-100V2, Horiba, Japan), with a dispersion angle of 173° and a temperature of 25°C.
2.4 Preparation of Cellulose, Chitosan and AgNPs Composite Material
The composite material was prepared as follows: the cellulose fibers were dispersed in water to a consistency of 10%, and the chitosan was dissolved in 1% m/v acetic acid. Different concentrations (2% and 4%) and size (small and large) from silver nanoparticles and chitosan (4% and 8 %) were added, respectively, as seen in Table 2.

The paper-type composite material was made using the TAPPI T218 sp-02 Buchner method by suction and water elimination (Fig. 1) [34]. The composite material was allowed to dry air and subsequently weighed. The thickness of each material obtained was measured, and the final material was characterized by FTIR and SEM microscopy.

Figure 1: Buchner TAPPI T218 sp-02 method representation
2.5 Antibacterial Activity Evaluation from Composite Materials of Cellulose Fiber, Chitosan and AgNPs
An antibacterial test was adapted for the composite materials using the immersion method [12,35]. The technique consists of preparing a bacterial inoculum with a chosen common bacterium, in this case, Escherichia coli (ATCC 25922). An isolated bacterium colony was inoculated into 10 mL of nutrient broth suitable for the bacteria and incubated at 37°C for 24 h. Growth culture dilution with 20 mL of nutrient broth and 400 μL from inoculum were prepared. The optical density was measured with a spectrophotometer and diluted to achieve an absorbance of approximately 0.13 at 600 nm wavelength, which corresponds to 1 × 108 UFC/mL. The inoculum was further diluted to achieve a concentration of ≈ 5 × 105 UFC/mL; for this purpose, 100 μL was placed in 19.9 mL of nutrient broth and incubated again at 37°C for 24 h. Two serial dilutions 1/10 in saline solution (0.9% NaCl) were prepared from the inoculum. Then, 100 μL of each dilution was inoculated by surface extension on nutrient agar plates. This was repeated three times for each sample and the control. Finally, the plates were incubated at 37°C for 24 h. After the incubation period, the CFU in each plate was determined. The antimicrobial activity value was obtained according to Eq. (1).
where R is the antimicrobial activity coefficient, I correspond to the amount of colony forming units (CFU) found in the control, and T is the CFU found in the sample. An R-value ≥ 2 denotes antimicrobial property (or bacterial inhibition at least 99%). The antibacterial assay was repeated with each formulation prepared according to Table 1.
2.6 Mechanical Properties Evaluation from Composite Materials
From each composite material formulation, five pieces of 7.5 × 1.5 cm were cut for tensile analysis using an Universal Machine in stress-strain mode according to the ASTM D 3822-01 standard of the STPSF (Standard for Tensile Properties of Single Fibers) [36]. The values obtained were recorded and averaged for each condition.
The statistical significance was calculated to compare the means and identify differences between the experimental data from different materials and the control. Each test was carried out in triplicate. The Student’s t-test (p-value < 0.05) was utilized for this analysis, using Origin statistical software, version 9.0. The mean and standard deviation were used for descriptive analysis from the cellulose, chitosan, and silver nanoparticles characterization.
3.1 Characterization of Cellulose, Chitosan and AgNPs
The quality of the cellulose fiber obtained from agave bagasse was evaluated according to morphology analysis, and parameters commonly used in cellulose extraction via chemical processes, like kappa number and degree of polymerization, were assessed. The kappa number measures the lignin content in the pulp and the residual lignin remaining after the pulping process. The cellulose obtained from agave bagasse after pulping had a kappa number of 25.64 mL/g, which aligns with alkaline pulps obtained from bamboo (26.02 mL/g) [37]. This value is relatively low compared to alkaline pulps obtained from pine wood (64 mL/g) [38]. These differences are explained by the fact that the kappa number depends on the characteristics and properties of the raw material and the extraction conditions [38].
The kappa number represents an indirect measure of the amount of residual lignin in the fibers after pulping; a lower kappa number suggests the ease of bleaching that must be applied to remove the residual lignin [37]. The bleaching process removed lignin adequately, which spectroscopy infrared analysis (FTIR) corroborated. Fig. 2 shows the FTIR spectra for untreated agave bagasse, bagasse pulp, and bleached cellulose, which compare their chemical compositions. The FTIR spectrum compares agave bagasse, bagasse pulp, and bleached cellulose derived from bagasse. The bagasse spectrum displays peaks at 3335 cm−1 (O-H stretching vibrations), 2850 cm−1 (C-H aliphatic stretching), and significant features around 1600 and 1030 cm−1 (C = O stretching and C-O stretching, respectively). The bagasse pulp has similar peaks but more defined features, indicating partial purification. The bleached cellulose fiber exhibits pronounced peaks at 3335 and 1030 cm−1, with reduced intensity at 2850 and 1600 cm−1, suggesting higher cellulose purity and reduced hemicellulose and lignin content.

Figure 2: FTIR spectra of initial agave bagasse, bagasse pulp obtained from digester and bleached cellulose fiber
The polymerization degree is another crucial variable and refers to the length of repeated cellobiose chains that compose the cellulose [39]. Therefore, in this research, a DP of 650 was obtained for agave bagasse cellulose fibers obtained after bleaching and corresponds to what was reported in previous studies for alkaline cellulose from agave bagasse (625 DP) [3]. These results suggest that the pulping and bleaching processes reduces the lignin content and increase the cellulose purity. Also, the kappa number of the agave bagasse cellulose compared to other sources and the degree of polymerization evidences the potential of this raw material for cellulose production.
The SEM micrographs of Fig. 3 show the morphological differences of agave bagasse fibers during the extraction processes. The initial agave bagasse fiber is observed (Fig. 3A), which is a set of agglomerated cellulose fibers with a diameter of around 300 ± 25 µm and embedded in other polymers such as lignin and hemicelluloses, which after the alkaline pulping and bleaching process are individualized into cellulose fibers as seen in Fig. 3B. The Fiber Quality Analysis (FQA) of the cellulose fibers shows an average length of the fibers of 776 ± 25 µm and average diameter of 27.76 ± 8.1 µm, with a Curl Index of 0.091 ± 0.005 mm and a Kink Index of 24.1°. These morphological characteristics suggest that the agave cellulose fibers are suitable as support material for chitosan and AgNps and the development of composite materials.

Figure 3: SEM micrographs: (a) Agave bagasse fibers and (b) Cellulose fiber bleached
The chitosan used in this study had a molecular weight of 420 kDa and a deacetylation degree of 73%. Fig. 4 shows the FTIR spectrum of chitosan, confirming the presence of typical chitosan functional groups, such as amino and hydroxyl groups and glucose units that form its skeleton [14]. The peak at 3354 cm−1 corresponds to the O-H and N-H stretching vibration, indicating the presence of hydroxyl groups (-OH) and amino groups (-NH2) in the chitosan structure. The peak at 2873 cm−1 represents the aliphatic C-H stretching vibrations from the chitosan structure’s carbon chains. The peak at 1656 cm−1 is associated with the C = O (carbonyl) stretching, indicating the presence of amide I, which is relevant for the partial deacetylation of chitosan, derived from chitin. The 1571 cm−1 peak corresponds to N-H bending vibrations, which are linked to amide II, another indication of the polysaccharide structure of chitosan. The 1377 cm−1 peak is related to C-H angle deformation vibrations in the chitosan molecule skeleton, and the 1154 and 1060 cm−1 peaks are associated with C-O-C and C-O stretching vibrations, typical of glycosidic bonds present in polysaccharides such as chitosan [12,40].

Figure 4: FTIR chitosan spectra
The synthesized silver nanoparticles (AgNps) have a spherical shape, and they are within the nanometer scale (Fig. 5). The two syntheses carried out produced nanoparticles with a characteristic maximum absorbance peak at 420 nm that corresponds to the surface plasmon resonance that indicates the AgNP formation [41]. The TEM and DLS analyses showed that the small AgNPs (≤20 nm) had an average size of 15.4 ± 4.6 nm and a z potential of −18.9 ± 6.63 mV. On the other hand, the large AgNPs (≥20 nm) present an average size of 25.67 ± 10.5 nm with a z potential of −12.1 ± 11.2 mV. The zeta potential of silver nanoparticles is a crucial parameter that influences their suspension stability; for the larger nanoparticles, a zeta potential of −12.1 mV indicates that the nanoparticles have a relatively low surface charge, which can lead to moderate stability and a tendency to aggregate over time due to insufficient electrostatic repulsion between particles, this trend can be observed in Fig. 5a. In contrast, a zeta potential of −18 mV for the small AgNPs suggests a higher surface charge, providing better stability in suspension due to stronger electrostatic repulsion, which helps prevent aggregation as observed in Fig. 5b. However, both values fall within a range that may require additional stabilization methods, such as the use of surfactants or polymers, to ensure long-term stability and maintain the desired properties of the nanoparticles for various applications.
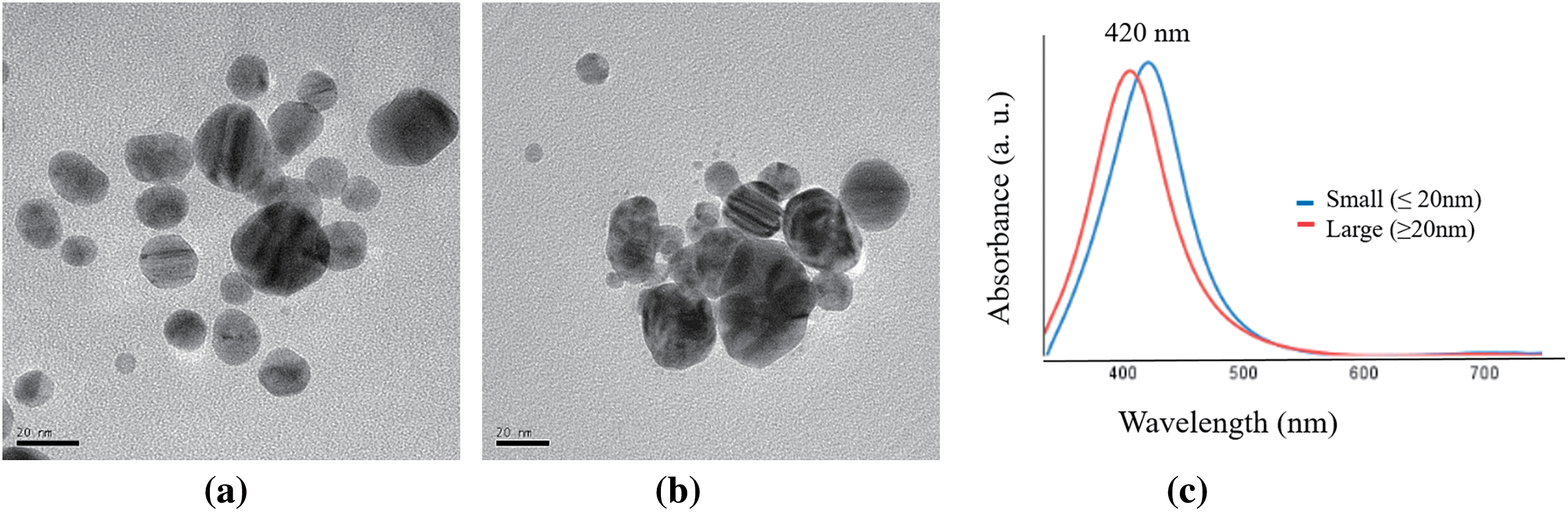
Figure 5: TEM micrographs and UV-Vis analysis: (a) Small (≤20 nm) AgNPs, (b) Large (≥20 nm) AgNPs and (c) UV–Vis absorption spectra of AgNPs
3.2 Paper-Type Composite Material of Agave Cellulose, Chitosan, and AgNPs
Fig. 6 shows the FTIR spectra of various composite materials based on cellulose agave fibers and their modifications with chitosan and silver nanoparticles (AgNPs). The spectrum of cellulose fibers shows peaks at 3335 cm−1 (O-H) and 1030 cm−1 (C-O), with a significant reduction at 1732 cm−1, indicating higher cellulose purity [42]. Cellulose modified only with chitosan exhibits broadening at 3335 cm−1 (O-H and N-H), with peaks at 2850 cm−1 (C-H) and 1030 cm−1 (C-O and C-N), reflecting the integration of chitosan [40]. Cellulose with AgNPs maintains broadening at 3335 cm−1 and peaks at 2850 and 1030 cm−1, suggesting nanoparticle interactions.

Figure 6: FTIR spectrum of composite materials of cellulose, chitosan, and AgNPs
The chitosan and nanoparticle incorporation significantly improve the tensile strength of composite materials. Fig. 7 shows that pure cellulose (Cel) presented the lowest value (13.67 ± 8.96 MPa), while the composite with chitosan (Cel + Ch) significantly increased the strength (42.33 ± 9.1 MPa) of the composite material. The inclusion of silver nanoparticles, both large (CelAgNPLarge) and small (CelAgNPSmall), improved the strength compared to pure cellulose, reaching values of 39.25 ± 7.55 MPa and 42.40 ± 3.21 MPa, respectively. Still, the values are comparable to the composite material only with chitosan added. The combination of large nanoparticles (CelAgNPLargeCh) and small nanoparticles (CelAgNPSmallCh) with chitosan increased the strength, reaching values of 69.60 ± 7.61 MPa and 73.90 ± 7.57 MPa, respectively. The highest tensile strength was reached by adding small nanoparticles and 8% chitosan (CelAgNPSmallCh+), reaching the maximum value of 119.85 ± 12.47 MPa. These results indicate the synergistic effect of adding small silver nanoparticles and higher chitosan concentration, giving the best mechanical properties and standing out as the most resistant composite material. This effect can be explained by the fact that there may be an increase in hydrogen bonding interactions between cellulose and chitosan.

Figure 7: Tensile strength of composites material of cellulose, chitosan, and AgNPs
Additionally, the hydroxyl groups of cellulose are negatively charged on its surface, which allows it to retain Ag+ nanoparticles [12]. The smaller nanoparticles have a zeta potential of −18.9 mV, which favors the dispersion of the nanoparticles and reduces their aggregation. It improves electrostatic interactions with the hydroxyl groups of cellulose; their homogeneous distribution allows filling voids together with chitosan in the fiber network, creating a “cementation effect” that reinforces the structure of the material by strengthening the network of bonds between the fibers, increasing the strength and mechanical stability of the composite [43]. This effect fills the voids within the composite material’s fiber network, significantly improving the mechanical properties.
3.3 Antibacterial Tests for Composite Material of Cellulose, Chitosan, and AgNPs
The antimicrobial activity of composite materials was evaluated against Escherichia coli. The activity was compared for pure cellulose, cellulose with chitosan, composites with chitosan, and silver nanoparticles of different sizes and concentrations, as shown in Fig. 8.

Figure 8: Antibacterial tests of composite materials: (a) Cellulose (Cel), (b) cellulose with chitosan (Cel + Ch), (c) Cellulose and silver nanoparticles large (CelAgNPlarge), (d) Cellulose and silver nanoparticles small (CelAgNPSmall), (e) Cellulose and silver nanoparticles large and chitosan (CelAgNPlargeCh) and (f) Cellulose and silver nanoparticles small and chitosan (CelAgNPSmallCh)
In the images of the antibacterial tests in Petri dishes (Fig. 8), it is observed that cellulose (Fig. 8a) has no antibacterial properties, evidenced by significant bacterial growth. When chitosan is incorporated (Fig. 8b), a reduction in bacterial growth is observed, attributed to its antibacterial properties. The chitosan mechanism involves electrostatic interactions between the NH3+ groups (positively charged) of chitosan and microbial cell membranes (negatively charged), which increases membrane permeability and causes the release of intracellular contents [44]. On the other hand, adding large (Fig. 8c) and small (Fig. 8d) silver nanoparticles improves the inhibition of bacterial growth considerably compared to chitosan alone. Larger nanoparticles are more effective because larger particles generally have a higher concentration of silver ions (Ag+) than small nanoparticles [45]. The interaction between AgNPs and microbial cell walls occurs due to the electrostatic attraction between the positively charged nanoparticles and the negatively charged microbial membrane [25]. This adhesion disrupts the structural integrity of the cell membrane, leading to depolarization and impaired cellular respiration, which ultimately causes membrane rupture and initiates cell death [25,46]. The combinations of silver nanoparticles and chitosan (Fig. 8e and f) do not show growth in Petri dishes, meaning there is antibacterial activity. However, it cannot be assured which components, whether chitosan or AgNP, perform the inhibition.
The bacterial growth images of the Petri dishes are corroborated by the measurement of the antimicrobial activity coefficient (R) and bacterial inhibition (%) in Table 3. The value of antimicrobial activity shows that pure cellulose (Cel) does not present antimicrobial activity, as indicated by an uncountable bacterial. In contrast, the cellulose composite with chitosan (Cel + Ch) shows a significant bacterial inhibition, with a value of 95.04% and an R-value of 6.44 ± 0.08, evidencing the antimicrobial effect for chitosan. The composite materials that include the large silver nanoparticles (CelAgNPlarge and CelAgNPlargeCh) achieve total bacterial inhibition (100%), suggesting that silver nanoparticles effectively eliminate bacteria, regardless of the chitosan presence. Similar inhibition results were observed for cellulose-AgNPs hydrogels [47]. Materials with small silver nanoparticles (CelAgNPSmall and CelAgNPSmallCh) also show almost complete inhibition (99.45% and 99.31%, respectively), although with slightly higher R values (2.26 ± 0.03 and 2.16 ± 0.04), indicating a very high antimicrobial efficacy, but somewhat lower compared to large nanoparticles. Overall, materials containing silver nanoparticles (AgNP), whether large or small, are more effective than the cellulose-chitosan composite. In contrast, combining chitosan and silver nanoparticles does not substantially improve the antibacterial activity compared to AgNPs alone. These findings state the role of the silver nanoparticles as antimicrobial agents in the composite materials.

In this study, eco-friendly composite materials were successfully obtained from agave bagasse, combined with chitosan and silver nanoparticles (AgNPs) of both small (≤20 nm) and large (≥20 nm) sizes, and thoroughly characterized. The cellulose exhibited high purity, as confirmed by its kappa number and degree of polymerization (DP), demonstrating its suitability as a base material for composite formation. Morphological and spectroscopic analyses verified the efficient removal of lignin and hemicellulose during bleaching, ensuring high cellulose quality. The green synthesis of silver nanoparticles through carbohydrate reduction methods proved to be a sustainable and effective alternative to traditional chemical synthesis, offering the advantage of producing nanoparticles with controlled size and properties. This environmentally friendly approach reduced the ecological footprint while yielding nanoparticles with enhanced stability and biological activity.
When incorporated into the cellulose matrix, both chitosan and AgNPs significantly enhanced the mechanical properties of the composites. The system containing small nanoparticles combined with higher concentrations of chitosan exhibited the highest tensile strength. Regarding antimicrobial efficacy, composites containing AgNPs, regardless of size, demonstrated near-complete bacterial inhibition against Escherichia coli, with inhibition rates approaching 100%. While chitosan contributed to the antimicrobial activity, its effect was less pronounced than silver nanoparticles’ effective action. These findings underscore the potential of cellulose-chitosan-AgNP composites for applications requiring both mechanical strength and robust antimicrobial properties, making them promising candidates for use in biomedicine, food packaging, and other industries where such characteristics are critical. Additionally, this study highlights the added value of utilizing agricultural residues such as agave bagasse for cellulose extraction and shrimp shells to produce chitosan, contributing to a more sustainable and efficient use of these byproducts.
Acknowledgement: The authors would like to thank the Department of Wood, Cellulose and Paper of CUCEI- University of Guadalajara (UDG), the Bioprocess Laboratory of CUTonala-UDG and POLIUNA National University of Costa Rica for providing the facilities and equipment to carry out this work.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Belkis Sulbarán-Rangel and Jorge Armando Caldera Siller; methodology, Jorge Armando Caldera Siller, Jenny Arratia-Quijada, and Marianelly Esquivel Alfaro; formal analysis, Belkis Sulbarán-Rangel and José Anzaldo-Hernandez; writing—original draft preparation, Belkis Sulbarán-Rangel; writing—review and editing, Salvador García Enríquez, José Anzaldo-Hernandez, Jenny Arratia-Quijada, and Marianelly Esquivel Alfaro. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest regarding the present study.
References
1. Yıldız ÜY, Keçili R, Hussain CM. Chapter 1—green and sustainable chemistry. In: Hussain CM, Keçili R editors. Green imprinted materials. Elsevier; 2024. p. 3–25. [Google Scholar]
2. Nowak PM. What does it mean that “something is green”? The fundamentals of a unified greenness theory. Green Chem. 2023;25(12):4625–40. doi:10.1039/D3GC00800B. [Google Scholar] [CrossRef]
3. Hernández J, Romero VH, Escalante A, Toriz G, Rojas O, Sulbarán-Rangel B. Agave tequilana bagasse as source of cellulose nanocrystals via organosolv treatment. BioResources. 2018;13(2):3603–14. doi:10.15376/biores.13.2.3603-3614. [Google Scholar] [CrossRef]
4. Palacios H, Hernandez J, Esquivel M, Toriz G, Rojas OJ, Sulbaran-Rangel B. Isolation and characterization of nanofibrillar cellulose from agave tequilana weber bagasse. Adv Mater Sci Eng. 2019;2019(1):7. doi:10.1155/2019/1342547. [Google Scholar] [CrossRef]
5. Khalid MY, Al Rashid A, Arif ZU, Ahmed W, Arshad H. Recent advances in nanocellulose-based different biomaterials: types, properties, and emerging applications. J Mater Res Technol. 2021;14(3):2601–23. doi:10.1016/j.jmrt.2021.07.128. [Google Scholar] [CrossRef]
6. Padzil FNM, Lee CH, Lee SH, Asa’ari AZM, Chin KL, Yasim-Anuar TAT, et al. Nanocellulose composites in the pulp and paper industry. In: Sapuan SM, Norrrahim MNF, Ilyas RA, Soutis C, editors. Industrial applications of nanocellulose and its nanocomposites. Sawston, UK: Woodhead Publishing; 2022. p. 375–95. [Google Scholar]
7. Salman MS, Sheikh MC, Hasan MM, Hasan MN, Kubra KT, Rehan AI, et al. Chitosan-coated cotton fiber composite for efficient toxic dye encapsulation from aqueous media. Appl Surf Sci. 2023;622(2):157008. doi:10.1016/j.apsusc.2023.157008. [Google Scholar] [CrossRef]
8. Du H, Parit M, Liu K, Zhang M, Jiang Z, Huang T-S, et al. Engineering cellulose nanopaper with water resistant, antibacterial, and improved barrier properties by impregnation of chitosan and the followed halogenation. Carbohydr Polym. 2021;270(3):118372. doi:10.1016/j.carbpol.2021.118372. [Google Scholar] [PubMed] [CrossRef]
9. Liu K, Wang Y, Liu W, Zheng C, Xu T, Du H, et al. Bacterial cellulose/chitosan composite materials for biomedical applications. Chem Eng J. 2024;494:153014. doi:10.1016/j.cej.2024.153014. [Google Scholar] [CrossRef]
10. Wahid F, Hu X-H, Chu L-Q, Jia S-R, Xie Y-Y, Zhong C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int J Biol Macromol. 2019;122(5):380–7. doi:10.1016/j.ijbiomac.2018.10.105. [Google Scholar] [PubMed] [CrossRef]
11. Lara-Topete GO, Castanier-Rivas JD, Gradilla-Hernández MS, González-López ME. Life cycle assessment of agave bagasse management strategies: PLA biocomposites versus conventional waste disposal practices. Sustain Chem Pharm. 2024;37:101435. doi:10.1016/j.scp.2024.101435. [Google Scholar] [CrossRef]
12. Montes de Oca-Vásquez G, Esquivel-Alfaro M, Vega-Baudrit JR, Jiménez-Villalta G, Romero-Arellano VH, Sulbarán-Rangel B. Development of nanocomposite chitosan films with antimicrobial activity from agave bagasse and shrimp shells. J Agric Food Res. 2023;14:100759. doi:10.1016/j.jafr.2023.100759. [Google Scholar] [CrossRef]
13. Bai L, Liu L, Esquivel M, Tardy BL, Huan S, Niu X, et al. Nanochitin: chemistry, structure, assembly, and applications. Chem Rev. 2022;122(13):11604–74. doi:10.1021/acs.chemrev.2c00125. [Google Scholar] [PubMed] [CrossRef]
14. Harugade A, Sherje AP, Pethe A. Chitosan: a review on properties, biological activities and recent progress in biomedical applications. React Funct Polym. 2023;191:105634. doi:10.1016/j.reactfunctpolym.2023.105634. [Google Scholar] [CrossRef]
15. Ambaye TG, Vaccari M, Prasad S, van Hullebusch ED, Rtimi S. Preparation and applications of chitosan and cellulose composite materials. J Environ Manag. 2022;301(4):113850. doi:10.1016/j.jenvman.2021.113850. [Google Scholar] [PubMed] [CrossRef]
16. Barik M, BhagyaRaj GVS, Dash KK, Shams R. A thorough evaluation of chitosan-based packaging film and coating for food product shelf-life extension. J Agric Food Res. 2024;16:101164. doi:10.1016/j.jafr.2024.101164. [Google Scholar] [CrossRef]
17. Doyo AN, Kumar R, Barakat MA. Recent advances in cellulose, chitosan, and alginate based biopolymeric composites for adsorption of heavy metals from wastewater. J Taiwan Inst Chem Eng. 2023;151(10):105095. doi:10.1016/j.jtice.2023.105095. [Google Scholar] [CrossRef]
18. Xu X, Yu J, Liu C, Yang G, Shi L, Zhuang X. Xanthated chitosan/cellulose sponges for the efficient removal of anionic and cationic dyes. React Funct Polym. 2021;160(31):104840. doi:10.1016/j.reactfunctpolym.2021.104840. [Google Scholar] [CrossRef]
19. Zhang D, Wang L, Zeng H, Yan P, Nie J, Sharma VK, et al. A three-dimensional macroporous network structured chitosan/cellulose biocomposite sponge for rapid and selective removal of mercury(II) ions from aqueous solution. Chem Eng J. 2019;363(6404):192–202. doi:10.1016/j.cej.2019.01.127. [Google Scholar] [CrossRef]
20. Peyravian N, Milan PB, Kebria MM, Mashayekhan S, Ghasemian M, Amiri S, et al. Designing and synthesis of injectable hydrogel based on carboxymethyl cellulose/carboxymethyl chitosan containing QK peptide for femoral head osteonecrosis healing. Int J Biol Macromol. 2024;270(1):132127. doi:10.1016/j.ijbiomac.2024.132127. [Google Scholar] [PubMed] [CrossRef]
21. Ravichandran S, Kandaswamy K, Muthu K. Evaluation of lupeol-chitosan nanoparticles infused cellulose acetate membranes for enhanced in-vitro anticancer and antidiabetic activities. Chemosphere. 2024;351(2):141149. doi:10.1016/j.chemosphere.2024.141149. [Google Scholar] [PubMed] [CrossRef]
22. Sultana T, Hossain M, Rahaman S, Kim YS, Gwon J-G, Lee B-T. Multi-functional nanocellulose-chitosan dressing loaded with antibacterial lawsone for rapid hemostasis and cutaneous wound healing. Carbohydr Polym. 2021;272(8):118482. doi:10.1016/j.carbpol.2021.118482. [Google Scholar] [PubMed] [CrossRef]
23. Zhao H, Zhang L, Zheng S, Chai S, Wei J, Zhong L, et al. Bacteriostatic activity and cytotoxicity of bacterial cellulose-chitosan film loaded with in-situ synthesized silver nanoparticles. Carbohydr Polym. 2022;281(4):119017. doi:10.1016/j.carbpol.2021.119017. [Google Scholar] [PubMed] [CrossRef]
24. Naganthran A, Verasoundarapandian G, Khalid FE, Masarudin MJ, Zulkharnain A, Nawawi NM, et al. Synthesis, characterization and biomedical application of silver nanoparticles. Materials. 2022;15(2):427. doi:10.3390/ma15020427. [Google Scholar] [PubMed] [CrossRef]
25. Fahim M, Shahzaib A, Nishat N, Jahan A, Bhat TA, Inam A. Green synthesis of silver nanoparticles: a comprehensive review of methods, influencing factors, and applications. JCIS Open. 2024;16:100125. doi:10.1016/j.jciso.2024.100125. [Google Scholar] [CrossRef]
26. Islam NF, Gogoi B, Saikia R, Yousaf B, Narayan M, Sarma H. Encouraging circular economy and sustainable environmental practices by addressing waste management and biomass energy production. Reg Sustain. 2024;5(4):100174. doi:10.1016/j.regsus.2024.100174. [Google Scholar] [CrossRef]
27. Aguilar-Méndez MA, San Martín-Martínez E, Ortega-Arroyo L, Cobián-Portillo G, Sánchez-Espíndola E. Synthesis and characterization of silver nanoparticles: effect on phytopathogen Colletotrichum gloesporioides. J Nanopart Res. 2010;13(6):2525–32. doi:10.1007/s11051-010-0145-6. [Google Scholar] [CrossRef]
28. TAPPI. T236 om-22 kappa number of pulp. STANDARD by technical association of the pulp and paper industry. Atlanta, GA, USA: Tappi Press; 2022. [Google Scholar]
29. TAPPI. T203 cm-22 alpha-, beta- and gamma-cellulose in pulp. STANDARD by Technical Association of the Pulp and Paper Industry. Atlanta, GA, USA: Tappi Press; 2022. [Google Scholar]
30. TAPPI. T271 om-07 fiber length of pulp and paper by automated optical analyzer using polarized light. STANDARD by technical association of the pulp and paper industry. Atlanta, GA, USA, 2007. [Google Scholar]
31. ASTM. D1795-13 standard test method for intrinsic viscosity of cellulose. West Conshohocken, PA, USA: ASTM International; 2013. [Google Scholar]
32. Kasaai MR, Arul J, Charlet G. Intrinsic viscosity-molecular weight relationship for chitosan. J Polym Sci B: Polym Phys. 2000;38(19):2591–8. doi:10.1002/(ISSN)1099-0488. [Google Scholar] [CrossRef]
33. Czechowska-Biskup R, Jarosińska D, Rokita B, Ulański P, Rosiak JM. Determination of degree of deacetylation of chitosan-comparision of methods. Prog Chem Appl Chitin Deriv. 2012;17:5–20. [Google Scholar]
34. TAPPI. T 218 sp-06 forming handsheets for reflectance testing of pulp (Büchner funnel procedure). STANDARD by technical association of the pulp and paper industry. Atlanta, GA, USA: Tappi Press; 2006. [Google Scholar]
35. Costa SM, Ferreira DP, Teixeira P, Ballesteros LF, Teixeira JA, Fangueiro R. Active natural-based films for food packaging applications: the combined effect of chitosan and nanocellulose. Int J Biol Macromol. 2021;177:241–51. doi:10.1016/j.ijbiomac.2021.02.105. [Google Scholar] [PubMed] [CrossRef]
36. ASTM. D3822-01 standard test method for tensile properties of single textile fibers. West Conshohocken, PA, USA: ASTM International; 2001. [Google Scholar]
37. Xin L-P, Yu B, Zhou Y. A dissolved lignin monitoring-based model for end-point control during displacement kraft pulping. BioResources. 2018;13(4):8529–38. doi:10.15376/biores.13.4.8529-8538. [Google Scholar] [CrossRef]
38. Małachowska E, Dubowik M, Lipkiewicz A, Przybysz K, Przybysz P. Analysis of cellulose pulp characteristics and processing parameters for efficient paper production. Sustainability. 2020;12(17):12–7. doi:10.3390/su12177219. [Google Scholar] [CrossRef]
39. Santos Ventura EM, Escalante Álvarez MA, Gutiérrez Becerra A, Sulbarán-Rangel B. Unbleached cellulose from waste corncob for isolation of cellulose nanocrystals. Emerg Mater Res. 2020;9(4):1258–65. doi:10.1680/jemmr.20.00073. [Google Scholar] [CrossRef]
40. Yasmeen S, Kanti Kabiraz M, Saha B, Rakibul Qadir M, Abdul Gafur M, Masum SM. Chromium (VI) ions removal from tannery effluent using chitosan-microcrystalline cellulose composite as adsorbent. Int Res J Pure Appl Chem. 2015;10(4):1–14. doi:10.9734/IRJPAC/2016/23315. [Google Scholar] [CrossRef]
41. Laime-Oviedo LA, Soncco-Ccahui AA, Peralta-Alarcon G, Arenas-Chávez CA, Pineda-Tapia JL, Díaz-Rosado JC, et al. Optimization of synthesis of silver nanoparticles conjugated with Lepechinia meyenii (Salvia) using plackett-burman design and response surface methodology—preliminary antibacterial activity. Processes. 2022;10(9):1727. doi:10.3390/pr10091727. [Google Scholar] [CrossRef]
42. Xu F-X, Zhang X, Zhang F, Jiang L-Q, Zhao Z-L, Li H-B. TG-FTIR for kinetic evaluation and evolved gas analysis of cellulose with different structures. Fuel. 2020;268(24):117365. doi:10.1016/j.fuel.2020.117365. [Google Scholar] [CrossRef]
43. Bhardwaj S, Bhardwaj NK, Negi YS. Effect of degree of deacetylation of chitosan on its performance as surface application chemical for paper-based packaging. Cellulose. 2020;27(9):5337–52. doi:10.1007/s10570-020-03134-5. [Google Scholar] [CrossRef]
44. Guarnieri A, Triunfo M, Scieuzo C, Ianniciello D, Tafi E, Hahn T, et al. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci Rep. 2022;12(1):8084. doi:10.1038/s41598-022-12150-3. [Google Scholar] [PubMed] [CrossRef]
45. Kaabipour S, Hemmati S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J Nanotechnol. 2021;12:102–36. doi:10.3762/bjnano.12.9. [Google Scholar] [PubMed] [CrossRef]
46. Majeed M, Hakeem KR, Rehman RU. Synergistic effect of plant extract coupled silver nanoparticles in various therapeutic applications- present insights and bottlenecks. Chemosphere. 2022;288(1):132527. doi:10.1016/j.chemosphere.2021.132527. [Google Scholar] [PubMed] [CrossRef]
47. Fadakar Sarkandi A, Montazer M, Harifi T, Mahmoudi Rad M. Innovative preparation of bacterial cellulose/silver nanocomposite hydrogels: in situ green synthesis, characterization, and antibacterial properties. J Appl Polym Sci. 2021;138(6):49824. doi:10.1002/app.49824. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools