 Open Access
Open Access
REVIEW
Deashing of Agricultural Residues and Its Impact on Isolated Lignin Properties: A Mini Review
1 Department of Forest Products, Faculty of Forestry and Environment, IPB University, Bogor, 16680, Indonesia
2 Research Center for Biomass and Bioproducts, National Research and Innovation Agency (BRIN), Jl Raya Bogor KM 46, Cibinong, 16911, Indonesia
3 Faculty of Chemical and Energy Engineering, Universiti Teknologi Malaysia, Skudai, 81310, Malaysia
4 Institute of Bioproduct Development (IBD), Universiti Teknologi Malaysia, Skudai, 81310, Malaysia
5 Research Collaboration Center of Biomass-Based Nano Cosmetic, National Research and Innovation Agency (BRIN), Samarinda, 75118, Indonesia
* Corresponding Authors: Wasrin Syafii. Email: ; Widya Fatriasari. Email:
Journal of Renewable Materials 2025, 13(5), 865-884. https://doi.org/10.32604/jrm.2025.058804
Received 21 September 2024; Accepted 13 January 2025; Issue published 20 May 2025
Abstract
The significant amount of ash content in agricultural biomass presents an enormous challenge for efficient conversion processes. In addressing this issue, various deashing treatments have been tested and established, including simple leaching techniques, which can either be performed with or without the addition of chemical agents. These techniques hold promise for improving the deashing efficiency while potentially altering the structural and chemical composition of biomass, specifically lignin content, which is the key focus of this review. This review starts by exploring the presence of ash in agricultural residues and its impact on biomass properties. Next, this review examines deashing strategies aimed at reducing ash levels in biomass followed by analysis of the resulting changes in lignin physical and chemical properties as well as its thermal characteristics. The final part of this review is concluded by the discussion on the limitations of current approaches and the possible future directions to address the challenges covering the environmental impacts of the deashing treatments. A green process approach is emphasized as a sustainable solution with the aim to minimize negative environmental impacts associated with chemical usage during deashing. Finally, this review highlights the potential for ash recovery as a byproduct of the deashing processes, paving the way for an integrative, closed-loop approach within the biorefinery concept.Graphic Abstract
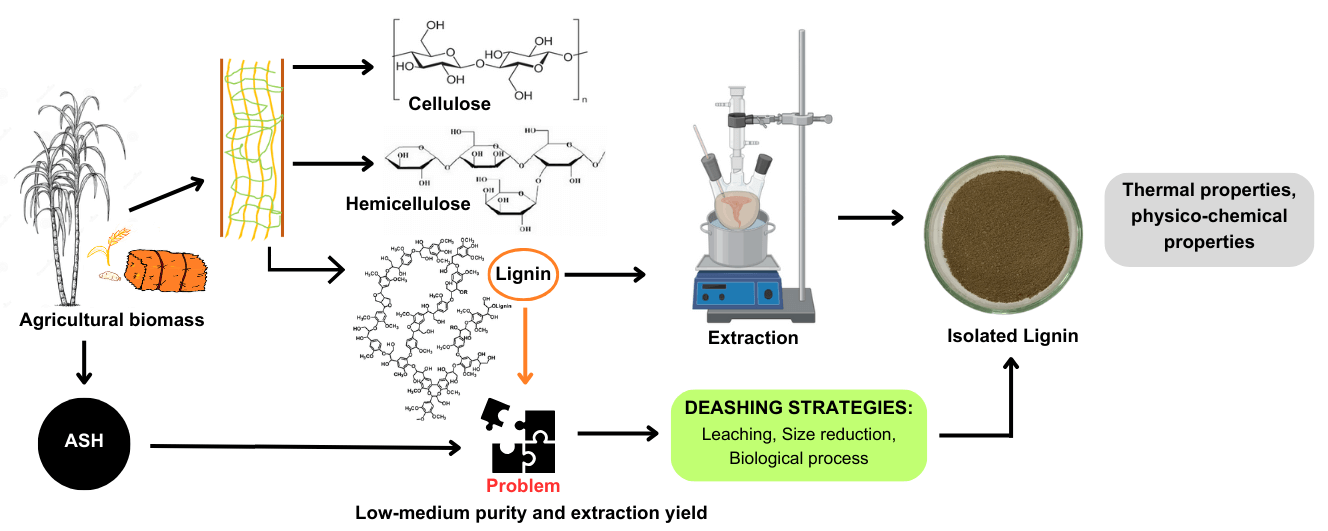
Keywords
Lignocellulose, which encompasses agricultural and forestry residues and energy crops, is widely employed as a raw material for biofuels and for biochemical production via various conversion procedures [1–3]. Several studies emphasize the conversion of biomass into products with additional value on the basis of the primary chemical component. However, in some cases, the process and product properties are affected by the minor elements contained in the biomass, such as ash. The ash fraction found in many sources of biomass differs in both its content and chemical makeup. These variations arise from factors such as geographic location, local ecosystems, cultivation conditions, and the surrounding environment [4]. Compared with forest waste, agricultural waste often has a significantly higher ash concentration [4]. The minerals found in the ash can be attached to the organic matrix of the biomass by replacing acidic hydrogen atoms in the phenolic groups and carboxylic acid and then forming salts [5]. There are two types of ash, namely, primary elements, such as Ti, P, Al, K, Fe, Mg, Ca, and Si, and secondary mineral elements, such as Ni, Mo, Hg, V, Cu, cadmium (Cd), chromium (Cr), and Ba. On the basis of the level of ash content, there are three groups of biomass, including those with a high ash content of >10 wt%, a medium ash content of 5–10 wt%, and a low ash content of <5 wt% [6]. A high ash content can influence the effectiveness of processes such as the thermomechanical conversion of biomass [7]. Their chemical composition and ability to undergo reactions influence the method by which biomass breaks down when exposed to heat. An elevated ash concentration can result in several issues with industrial machinery, including fouling, slag deposition, corrosion, sintering, and bed agglomeration [4,8].
Agricultural residues, such as rice, wheat, corn, sugarcane bagasse, and sugarcane residue, are rich in minerals and inorganic compounds, collectively referred to as ash. This ash content plays a significant role in influencing biomass conversion processes and the quality of the resulting products. Efficient feedstock management and resource utilization heavily depend on reducing ash levels. Among various agricultural biomasses, corn straw has been identified as having the highest ash content (~7.7 wt%), which is predominantly composed of elements such as SiO2, K2O, and CaO, with SiO2 being the most abundant component [4]. Numerous studies have focused on developing and implementing effective strategies to reduce the ash content in biomass, highlighting its importance in optimizing biomass utilization [7,9,10].
Among the abundantly available agricultural residues is sugarcane residue. Sugarcane residue is an agricultural byproduct generated in sugarcane fields after the stems are harvested. This biomass is often inefficiently managed, as it is predominantly burned or left in the field. While some of these materials are repurposed as cattle feed, more advanced applications—such as their use as fuel for sugar mills, electricity generation, compost production, coal substitutes, and mushroom cultivation—are only employed on a limited scale. Additionally, innovative products such as bioethanol, xylooligosaccharides, xylitol, reducing sugars, single-cell proteins [11], and biosurfactants [12] have been developed from sugarcane residue. Like other lignocellulosic biomasses, sugarcane residue contains key chemical constituents, including cellulose (21%–40.9%), hemicellulose (26%–35.5%), and lignin (17.4%–22.5%) [13–16]. However, its ash content, which can range from 2%–12% [17], poses a significant challenge, particularly in the extraction and utilization of lignin. Lignin is an aromatic biopolymer composed of phenylpropanoid units, including p-coumaryl (H), coniferyl (G), and sinapyl monolignols (S) [18], which are linked together by carbon‒carbon, ether, and ester bonds [19]. Lignin has prospective uses in biomaterials [20], cosmetics [21,22], medicines and biomedicine [23], and agriculture [24]. Lignin has many active groups, including phenolic hydroxyl groups, aliphatic groups, carboxyl groups, and methoxy groups [25], so it has antioxidant, antibacterial, and anti-ultraviolet (UV) properties [22], low cytotoxicity, and biocompatibility [22,26]. Lignin also contains functional groups that can bind well to diverse polymer matrices, increasing the mechanical strength of treated biomaterials [27]. The physicochemical characteristics of lignin might vary depending on the biomass source and extraction procedure. High ash levels often result in low lignin purity, adversely impacting the performance of lignin-based products. Few studies have explored lignin extraction from sugarcane residue, with one reporting the direct extraction of lignin through mineral acid precipitation of acid‒alkali liquor pretreatment. This process yielded lignin with purity levels ranging from 73.33%–82.67% and yields ranging from 27.80%–35.01% [28]. The properties of the extracted lignin are influenced by several factors, including extraction methods, pretreatment approaches, and the chemical agents used. Among these factors, the ash concentration plays a critical role in determining lignin purity. Therefore, pretreatment processes aimed at reducing the ash content before lignin extraction are essential for increasing the purity and improving the overall quality of lignin-derived products.
A comprehensive literature review by Puri et al. [4] highlighted the significant impact of ash on biomass pyrolysis yields. Their study revealed that ash catalyzes cracking reactions in primary pyrolysis vapor, leading to increased production of noncondensable gases and cracked vapors, which, in turn, degrade the quality of the resulting bio-oil. Additionally, ash contributes to catalyst poisoning, further hindering the pyrolysis process. To mitigate these effects, various methods have been explored to reduce ash content, including biological treatments, leaching, and the use of additives to increase the melting temperature of ash [29,30]. Deashing processes have also been shown to remove functional groups from the biomass surface, resulting in increased emission peaks of the volatile fractions in pretreated biomass [31]. Despite these advancements, the effects of ash content, composition, and deashing treatments on the chemical properties of biomass components—such as extracted lignin—remain largely unexamined. This review, therefore, aims to address this knowledge gap by investigating the influence of ash content on biomass, evaluating potential deashing methods for agricultural residues, and exploring extraction processes for lignin. Furthermore, the properties of lignin derived from deashed biomass should be analyzed, and strategies to increase its quality should be proposed. By discussing future challenges, this review underscores the importance of reducing the ash content to improve biomass utilization and lignin-based product performance.
The screening of literature was conducted on scientific databases such as Scopus, Web of Science, and Google Scholar from 2013 through 2024. The keywords included in the abstract or title were used in screening the literature, including deashing treatment, agricultural biomass, ash content, demineralization, lignin properties, and extraction methods. The types of literature used are journals, books, and chapter books. The selection of references was carried out via Publish and Perish software, and then the reference in the RIS (reference information system) was used as material for creating networks, overlays, and density visualizations via VOS Viewer software (Fig. 1a–c). According to Fig. 1, there are two clusters with a total link strength of 746. In Fig. 1a, cluster 1 is biomass as a center that correlates with ash, pyrolysis, property, demineralization, pretreatment, effect ash content, and lignin. In cluster 2 (Fig. 1b), deashing is the cluster core and is correlated with demineralization, ash content, effects, hemicellulose, cellulose, lignin, biomass, ash, pyrolysis, properties, and pretreatment. Fig. 1c shows the density visualization generated, in which biomass and demineralization resulted in a larger area than the other methods did. The demineralization of biomass has become a common pretreatment that is used for the removal of ash in biomass. Many publications have discussed these issues. The effect of lignin properties after this treatment has also been underscored but still opens opportunities to explore this area on the basis of its lower density, such as the impact of this treatment on cellulose and hemicellulose. An in-depth study of the effects of the deashing treatment on the cellulose and hemicellulose remaining in biomass could become a research subject in the future.

Figure 1: Network visualization of cluster 1 (a), cluster 2 (b), and density visualization (c) via VOS viewer software with reference to those selected via Publish and Perish software. Keywords include deashing treatment, agricultural biomass, ash content, demineralization, lignin properties, and extraction methods
2 Ash Content Variation of Agricultural Biomass
Various processes can convert agricultural residue biomass into thermal energy, liquids, solid or gaseous fuels, and other chemicals [32], but its high ash content makes it a challenging feedstock for chemical products and materials. The organic structural differences and strong nutritional needs during the growing period may result in greater ash contents in this biomass than in woody biomass [33,34]. Zajac et al. [35] conducted a study on the main components of ash from several biomass species, including woody biomass and agricultural biomass, which can be seen in Table 1. Almost all the woody biomass studied had a low ash content of less than 1%. The wood residue chips (municipal) contained 1.79% ash, which was most likely caused by excessive mineral compound contamination. A greater ash content was obtained in the agricultural biomass, with an average value of 8.33%.

Several ash contents of agricultural biomass have also been reported, such as coconut shell (0.31%), walnut shell (0.30%), banana peel (3.80%), cassava peel (5.23%) [36], and corn stover (9.65%) [37]. Harun et al. [32] reported that the ash content of kenaf was 2%, whereas that of rice husk was 11%. On the basis of previously reported ash content categories, the tested agricultural biomass presented varying levels of ash content, with an average ash content in the medium category. This variability is possible because each plant has different genes, physiologies, and morphologies [38]. In addition, different anatomical fractions of the same plant have different ash contents and compositions [39]. According to Antunes et al. [40], sugarcane bagasse has 5%–20% ash content, whereas sugarcane trash has 5%–7% ash content. Sugarcane trash is separated into two parts: green sugarcane tops and dry sugarcane leaves. The density of dry sugarcane leaves is three times greater than that of green sugarcane tops [41]. As a byproduct of agricultural activities, agricultural residue biomass also has an ash content that varies substantially depending on cultivation and harvesting practices [37]. Maraver et al. [42] conducted a comprehensive investigation on the ash content of several olive tree residual biomasses and discovered that olive leaves had the greatest ash content at nearly 9%, followed by bark (4.79%) and branches (3.78%). The high ash content of olive leaves could be due to soil contamination and chemicals during the collection process in this biomass harvesting practice. As reported, contamination by soil, stones, plastics, and metals can increase the ash content [43]. Another category of biomass that is widely distributed but regarded as detrimental is that of invasive plant species. It is therefore evident that the management of invasive plants to produce value-added products represents a crucial step in the control of this biomass. One such method of control is the conversion of biomass into an energy source [44,45]. The ash content of various invasive plant species has been investigated in multiple studies. For example, Reza et al. [44] reported an ash content of 3.91% in Acacia holosericea, Nunes et al. [45] reported an ash content of 0.52% in Acacia dealbata, and Robinia pseudoacacia was found to have an ash value of 5.14%. The ash content of certain invasive plant species may present challenges during the conversion process to certified wood pellets [45].
3 Deashing Strategy of Biomass and Its Effects on Biomass Quality
The deashing strategies of biomass (Fig. 2) can be adopted in coal deashing, as proposed by Dhawan et al. [31]. In addition to chemical deashing treatment, biological treatment and the addition of additives are among the effective approaches in the deashing technique. The variation effect on the deashing result relies on the composition and productivity of the biomass ash, the nature of the biomass, the surrounding conditions, and other pertinent considerations [4]. The alkali index, alkali ratio, and fouling index are considered factors in selecting the deashing technique [8].

Figure 2: Deashing strategies using different chemicals ranging from acidic to alkaline and the addition of oxidizing agents for reducing the ash content in biomass. Reconstructed from Dhawan et al. [31]
Leaching is a simple approach to reduce the ash content during biomass processing [4]. The leaching process directly connects biomass to a liquid solvent. Water, acids, and bases can be utilized as leaching solvents [29]. The biomass and leaching solvent interact, allowing unwanted solutes or minerals to diffuse from the solid biomass into the solvent. As a result, the components originally present in the biomass become separated [46]. Fig. 3 shows the leaching process of biomass using several solvent agents and a combination method for reducing the ash content.

Figure 3: Leaching process for reducing the ash content of biomass
Table 2 presents a method to remove ash from several biomasses, such as water leaching, acid leaching, bio-oil leaching, base leaching, ultrasound radiation, mechanical reduction, air classification, and bioleaching. Water leaching is considered a simple pretreatment method. The success of this process depends on various treatment conditions (the biomass-to-water ratio, duration, and temperature) of leaching, the distribution of particle sizes, and the characteristics of the material [47]. According to Yu et al. [48], leaching has been utilized as a pretreatment method for feedstocks to enhance the properties of diverse agricultural, energy crop biomass, and forestry. Several biomasses, including wheat straw, Jose tall wheatgrass, rice straw, switchgrass, corn stover, Miscanthus, and Douglas fir wood, were subjected to water leaching at a biomass/water ratio of 1:20 (kg/L) at room temperature. Some biomasses were leached for 2 h with stirring at 650–700 rpm, while rice straw and wheat straw were leached for 6 h with manual stirring every 30 min. In each case, the leaching process significantly reduced the ash content, with ash concentrations in rice straw, wheat straw, switchgrass, and Miscanthus ranging from 15% to 39%. Moreover, Wu et al. [49] removed ash from the wheat straw via tap water at room temperature for 30 min with stirring, and the biomass-to-water ratio was 1:12. The findings revealed a 26.09% reduction in the ash content of the wheat straw. The water leaching time and temperature can affect the ash content of the treated biomass. A study by Pattiya et al. [50] reported a decrease in the ash content of cassava residue in line with the duration of leaching. Increasing the leaching temperature to 80°C can reduce the ash content of cassava residue to between 37.9% and 45.49% during a 24-h leaching period.
Water leaching is capable of removing water-soluble components in biomass, particularly all chlorides and the majority of alkali metals [30]. Herbaceous biomass has a higher water-soluble content than woody biomass because of the interaction of alkali components and variations in cell wall structure [51,52]. As much as 92% sodium, 62% potassium, and up to 100% chloride can be removed from herbaceous biomass during the water-leaching process [53]. Liu et al. [54] applied water leaching to remove ash from pine bark and switchgrass. Water leaching has been shown to remove up to 40% of the inorganic contents. Owing to its low concentration of water-soluble inorganic compounds, simple water leaching is ineffective at diminishing the content of inorganic constituents in pine bark.
Water used as a leaching agent can be discharged directly into the field or recovered by distillation or reverse osmosis techniques subsequent to leaching [54]. Conversely, leaching with water results in an undesirably high moisture content of the treated biomass, particularly in the context of thermal applications [48]. Furthermore, some minerals in lignocellulosic biomass cannot be easily eliminated by water; therefore, dilute acid leaching has been developed to remove these elements from the biomass. Chin et al. [46] conducted experiments to evaluate the success of leaching in removing ash-forming elements via the use of water and dilute acetic acid (1 M) as extraction agents. The results indicated that the removal of each element with water as the agent of leaching was correlated with its water-soluble content. Leaching with acetic acid solutions was effective in eliminating most ash-forming elements, including those that are both water insoluble and soluble, from the lignocellulosic biomass. Higher ash removal efficiencies (>90%) can be achieved via the use of inorganic acid solutions such as hydrochloric acid and nitric acid [29,55,56]. Basic reagents such as NaOH have also been investigated as solvents in the leaching process; however, treatment with NaOH has been determined to be more effective for desulfurization than for mineral removal [29]. Furthermore, the delignification of biomass samples has been used as a reason for the adverse effects of NaOH treatment [56,57].
Leaching processes using bases and acids, particularly inorganic acids, are major environmental concerns. Compared with water-leaching processes, the use of alkaline solvents, such as NaOH, has been demonstrated to result in higher ash reduction rates. However, the application of NaOH has also been found to have an adverse effect on the chemical and physical structure of biomass because of its ability to dissolve lignocellulosic components [58]. As reported by Tabish et al. [56], the application of NaOH facilitates the dissolution of hemicellulose and lignin by breaking the ester bond between xylan and lignin. Moreover, the use of NaOH leaching as a pretreatment in the thermochemical conversion of biomass can result in a reduction in the C/H ratio. This is due to the dissolution of hydrocarbons during demineralization. Consequently, the heating value of the biomass is diminished [59]. The use of inorganic acid solutions, such as hydrochloric acid or sulfuric acid, in the context of biomass conversion can lead to a number of issues. First, the presence of hazardous elements, such as chlorine or sulfur, can result in the corrosion of metal components. Second, the generation of harmful gases is a further potential consequence of the use of acids [60]. The necessity for sample washing after the mixing or screening process also gives rise to an additional source of hazardous waste, particularly when higher acid concentrations are involved [55]. As a result, pretreatment leachate should not be used or discharged directly into the environment [54]. Alternatively, organic acids such as acetic acid are often used as leaching agents, which are considered to be more environmentally friendly. Javed [61] used hydrochloric acid, sulfuric acid, and acetic acid to reduce the ash fraction in wheat straw. Hydrochloric acid and sulfuric acid removed 41% and 20%, respectively, of the ash from the wheat straw, whereas the leaching efficiency of acetic acid was between the two, indicating that acetic acid is an effective leaching medium. This finding is congruent with the findings of Liu et al. [54], who utilized acetic acid at various concentrations as a leaching agent on switchgrass and pine bark, with ash removal efficiencies ranging from 35%–48%. On the other hand, in the study of Chin et al. [46], where acetic acid leaching was used, the amount of ash removed was 60%. Bioleaching is also referred to as an environmentally friendly leaching method and has been extensively investigated within the mining industry [62]. During bioleaching, microorganisms such as Aspergillus niger facilitate the conversion of solid compounds into soluble chemicals, thereby enabling the extraction and recovery of valuable elements [4]. Three microbial species were employed for the dissolution of inorganic elements from four lignocellulosic feedstocks: corncobs, wheat straw, switchgrass, and sorghum. These included two fungi (Fusarium oxysporum and Aspergillus niger) and one bacterium (Burkholderia fungorum) [63]. A. niger demonstrated the highest efficiency in the removal of up to 80% of the elements within 48 h, whereas sorghum proved to be a more responsive substrate to the bioleaching process. Zhang et al. [64] investigated Aspergillus niger fungus in a bioreactor for biomass leaching of sorghum straw with a 6.0% ash concentration. Compared with water leaching, sorghum feedstock considerably reduced the average residual ash concentration (3.63 ± 0.19%) after bioleaching (4.72 ± 0.13%).
Bio-oil is another alternative that shows great potential for the leaching of alkali and alkaline earth metals (AAEMs), such as Ca, Na, K, and Mg, present in biomass. Chen et al. [60] utilized six predominant compounds in bio-oil after the pyrolysis of biomass and four conventional solutions—HCl, H2O, simulated bio-oil (SBO), and aqueous phase bio-oil (APBO)—to extract alkali and alkaline earth metals (AAEMs) from various lignocellulosic biomasses, including fir sawdust, rice husk, and cotton stalk, through leaching pretreatment. The liquid/solid mass ratio was maintained at 20 in each experiment, and the experiment was conducted at 50°C for 2 h. The results indicated that the leaching of acetic acid could eliminate the preponderance of K in the biomass. Nonacid organic compounds (guaiacol, phenol, hydroxyacetone, ethylene glycol, and furfural) can increase the removal rates of Na, K, and Mg in biomass, but increasing the rate of Ca removal is difficult because Ca usually exists in the form of CaCO3 or Ca silicate, which are insoluble in water or other nonacidic solutions but can be removed by the leaching of HCl. These findings suggest that nonacidic compounds may play a key role in AAEM leaching.
Conventional leaching can be combined with physicochemical technologies such as direct/indirect ultrasound to provide better results [52]. Santos et al. [65] applied ultrasound radiation to remove metals and nonmetal elements from sugarcane straw. Experiments with HNO3, HCl, H2SO4, H2O2, and H2O solutions were performed in ultrasonic baths at various frequencies (25–130 kHz). The extraction temperature ranged from 30°C to 70°C, the sonication period ranged from 5 to 45 min, and the ultrasonic amplitude ranged from 10% to 70%. Compared with mechanical stirring (500 rpm), the use of ultrasonic energy increased the demineralization efficiency by up to 16%. As a result, the suggested ultrasound-assisted process may be regarded as a viable option for high-efficiency demineralization of sugarcane straw.
Mechanical size reduction is another method for removing biomass ash. Pattiya et al. [50] used a size reduction process to grind biomass to fewer than 2.0 mm particles, which were then sieved to sizes ranging from <0.250–2.000 mm. The cassava residue had the highest ash content among the particles smaller than 0.250 mm, whereas the larger particles had a much lower ash content. Similar results were observed for corn cob and soybean stalk residues separated into two grain sizes: 630–1000 mm and 1000–1400 mm. Samples with grain sizes of 1.00 to 1.40 mm had lower ash contents after analysis [55]. Particle size is also reported to have the most significant impact on leaching performance, with better removal efficiencies of inorganic constituents for larger particle sizes [54].
Another type of dry separation technology that may be used to remove ash from biomass is air classification. This approach, like particle size fractionation, separates particles on the basis of their size and shape while also considering particle density. The air classifier passes through the dispersed biomass particles. Smaller or lighter particles are caught in the airflow and carried to the outlet because they have higher aerodynamic resistance and a greater surface area-to-weight ratio [39]. As with sieving, air classification serves to sort biomass particles on the basis of their physical characteristics, potentially reducing the ash content of the biomass. Lacey et al. [66] investigated the effects of the air classification technique on pine forest residue biomass and discovered that it can reduce the inorganic content of pine forest residue.
4 Effect on Lignin Properties after Deashing Treatment
Deashing treatments have been reported to influence the characteristics of biomass, including its chemical components such as lignin. Table 3 summarizes the effects of the deashing treatment on the lignin characteristics of agricultural residue and its application. The following section discusses in more detail the effects of this treatment on the physical, chemical, and thermal properties of the lignin contained in the biomass.
4.1 Physical Properties of Lignin
As previously mentioned, leaching pretreatment has been proposed as a valuable technique for removing ash from biomass. However, this technique might result in unwanted moisture content in pretreated raw materials, which must subsequently be dried [48]. Furthermore, the choice of a leaching agent must be examined since it might impact the processed biomass and its byproducts. The reaction between acidic and basic solvents with a biomass amorphous structure facilitates the hydrolysis of cellulose and hemicellulose and biomass delignification [54–56]. On the other hand, water leaching of biomass can increase the concentration of lignin. This may be due to the water-insoluble nature of lignin, so once the water-soluble components are removed during the water-leaching process, the recoverable lignin in the biomass increases [51]. Smit et al. [9] reported that the preextraction technique resulted in a reduction in water-soluble or solvent-soluble extractives, which might have an impact on the quality of the lignin extracted.
4.2 Chemical Properties of Lignin
In general, the ash content in biomass affects the chemical properties of the biomass, such as the pH value. A higher ash content reduces the efficacy of lignin for greater applications, such as the development of superabsorptive biochar, due to cavity formation, which hampers its catalytic efficiency. The alkalinity or acidity of biomass is influenced by its ash concentration, particularly during pyrolysis (a high-temperature treatment above 500°C in the absence of oxygen), to generate biochar. In contrast to deashed biomass, ash-containing biomass is alkaline and is influenced primarily by the presence of metal oxides, including potassium (K), phosphorus (P), calcium (Ca), and magnesium (Mg) (e.g., Na2O, CaO, and K2O), inside the biomass. The decomposition of biomass removes alkaline mineral compounds, resulting in acidic biomass. The decomposition of biomass also leads to increased lignin content resulting from the formation of condensed aromatic structures [68]. A study by Manna et al. [68] investigated and compared the physical‒chemical properties of deashed wheat and rice straw biochars with respect to their potential as pyrazosulfuron-ethyl sorbents. The results revealed that deashed biomass increased the total carbon content and increased the pore volume and surface area of the biomass. Deashed lignocellulosic biomass may prevent ash minerals from binding to the organic matrix in the biomass. The binding of ash minerals may subsequently form salts via the substitution of acidic H atoms in phenolic groups and carboxylic acids. The formation of salts may come from major mineral elements (e.g., Ca, Mg, aluminum (Al), titanium (Ti), P, potassium, silicon (Si), and iron (Fe)) or minor mineral elements (e.g., copper (Cu), molybdenum (Mo), zinc (Zn), chromium (Cr), nickel (Ni), mercury (Hg), vanadium (V), cadmium (Cd), and barium (Ba)) contributed from the ash.
The effect of deashing on the chemical properties of lignin greatly depends on the percent composition of ash available in the biomass. High ash-containing biomass (>10 wt%) has a more significant effect on the reaction with lignin biomolecules than medium and low ash-containing biomasses do [4]. Compared with forestry waste, agricultural waste contains more ash, which results in significant chemical changes to lignin after the deashing process. A comparison study by Dai et al. [69] revealed that deashed lignin biochar has better pore structure characteristics than nonashed lignin biochar because of less severe ash deposition and blockage. The synthesis of lignin-based biochar for application as a carbon dioxide capture technology drastically increased its performance after deashing treatment as a result of the increase in micropore volume and specific surface area [70]. The existence of these micropores and surface areas allows the adsorption of CO2 molecules and lignin-based biochar through van der Waals interactions. Raw sugarcane bagasse has been treated with organosolv using ethanol as the solvent at 150°C–175°C, and an increased lignin trend was successfully obtained after the deashing effect [71]. Chotirotsukon et al. [71] reported that after treatment, modified lignin presented the greatest number of phenolic-OH functional groups, which resulted in high UV absorption and high antioxidant (free radical scavenging activity) properties. Washing wheat straw with acetic acid drastically removes alkali and alkaline earth elements from the biomass, which improves the production of bio-oil [72]. The presence of acetyl groups on lignin side chains is among the main sources of acetic acid present during pyrolysis.
The chemical properties of lignin and its purity are closely influenced by the type of treatment applied to the biomass. Different chemicals affect the lignin structure differently depending on the severity of the treatment applied; the presence of impurities such as ash and carbohydrates; the functional groups available, such as carboxyl, hydroxyl, and methoxyl groups; the structure of the phenylpropanoid moieties present; and the molar mass distribution inside the biomass. For example, lignins containing the highest content of total hydroxyl groups were produced when wheat straw biomass was treated with 30 mM H2SO4 and 50% w/w aqueous ethanol from an organosolv treatment [73]. The variation in terms of
4.3 Thermal Properties of Lignin
The presence of heavy metals, alkaline earth metals, and nonmetal elements in ash-containing biomass affects the thermal degradation of major polymeric biomolecules such as lignin [80]. Sodium (Na) and potassium (K) in ash have catalytic effects on the thermal degradation of lignin according to a study carried out by Thy et al. [81]. Moreover, the alkaline earth metals Ca and Mg have minor catalytic effects on the thermal degradation of lignin [82]. Heavy metals such as lead (Pb) may also have a catalytic influence on thermal degradation, leading to the demineralization of isolated lignin [81]. The ash content remaining in the biomass could be deposited on the biomass surfaces and decrease the surface area of the biomass particles at extended reaction times. This scenario limits heat and mass transfer within biomass particles, thus reducing the biomass conversion rate, product properties, and yield [83]. Fig. 4 below illustrates the effects of deashing on lignin biomass properties, which demonstrate improved properties in terms of the cavity, pore volume, surface area, and mineralization. Table 3 summarizes the effects of the deashing treatment on the lignin characteristics of agricultural residue and its application.
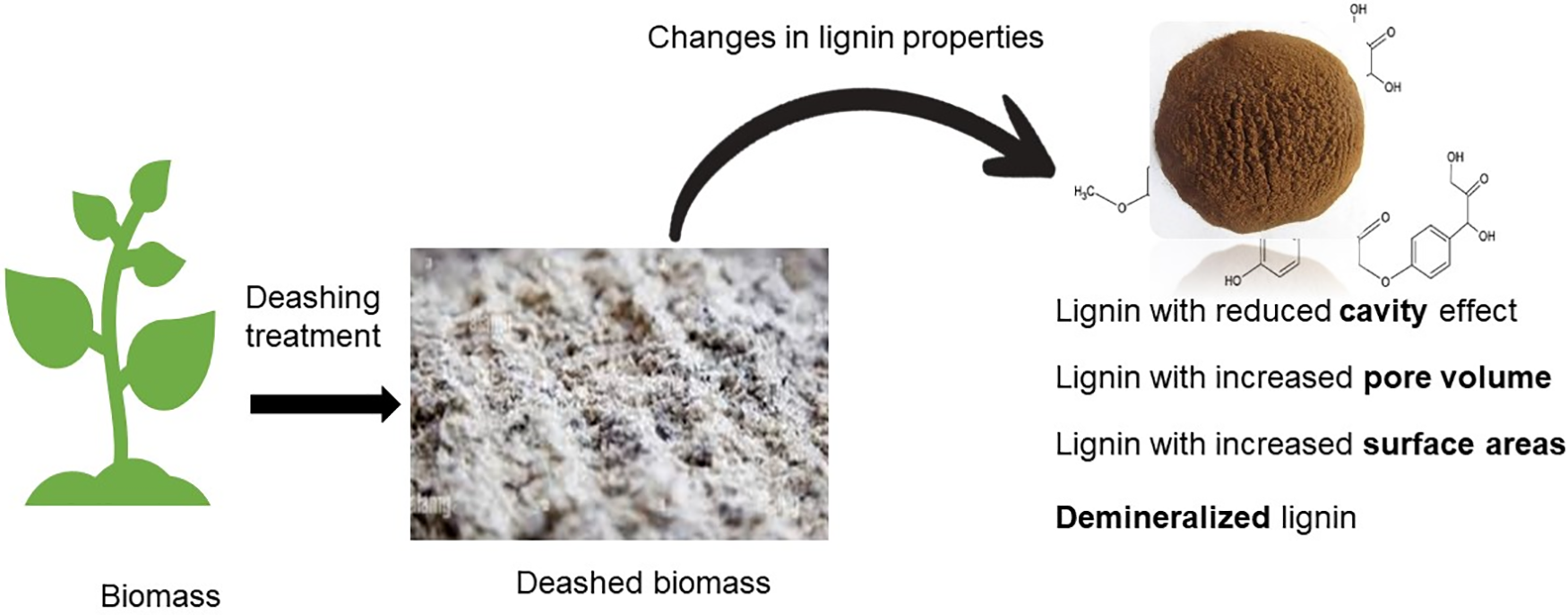
Figure 4: Effects of deashing on changes in lignin biomass properties
5 Future Challenges and Outlook
The utilization of lignin has become prominent in various applications. Many valorization methods have focused on obtaining the most feasible biorefinery platforms that are cost-effective. The heterogeneous and recalcitrant structure of lignin is the main hurdle to obtaining purified lignin. Optimizing a single deashing method that can fit all types of agricultural biomass might seem impossible, as different biomasses contain different chemical compositions. Thus, each type of biomass would require its own investigation and optimization, which can remove the maximum ash or mineral constituents with less lignin decomposition. Several studies have reported the drawbacks of the ash minerals contained in the biomass if not removed, such as the deposit of the minerals on the lignin, which reduces the lignin functionalities. A more sustainable method involving the use of biodegradable chemicals, which can result in higher lignin yields with minor side effects on the environment, is needed. The selection of the best deashing method is also crucial for avoiding undesired secondary reactions [72]. An important consideration is the determination of the specific type of ash before and after each demineralization process to enable an accurate understanding of the effect of each element on lignin properties [84].
From this point of view, the deashing treatment of agricultural biomass or other types of biomasses with high ash contents has the potential to obtain better lignin characteristics. This approach will be beneficial for further conversion of lignin into a value-added product, which minimizes the problem that occurs for further processing. It can also enhance the quality of lignin-based products. Extracted ash resulting from the deashing of biomass also has the potential for further use, such as Si as a fire-retardant agent, to improve the fire properties of composite products [85,86]. The utilization of extracted ash can complete the closed-loop strategy in biomass conversion in addition to the main target utilizing the chemical component of the deashed biomass. Therefore, deashing treatment can become an effective conversion approach, especially for high ash-content biomass; however, the impact not only on the chemical properties of biomass but also on environmental assessment and cost-effectiveness in all process streams still needs to be observed. For example, ash is detrimental to biomass conversion to sugars; however, demineralization can be utilized, allowing the generated ash to serve alternative functions, such as soil amendments and catalyst production for biorefineries [43,52]. Fig. 5 summarizes the biorefinery platform of deashed biomass to achieve sustainable processes with zero waste.
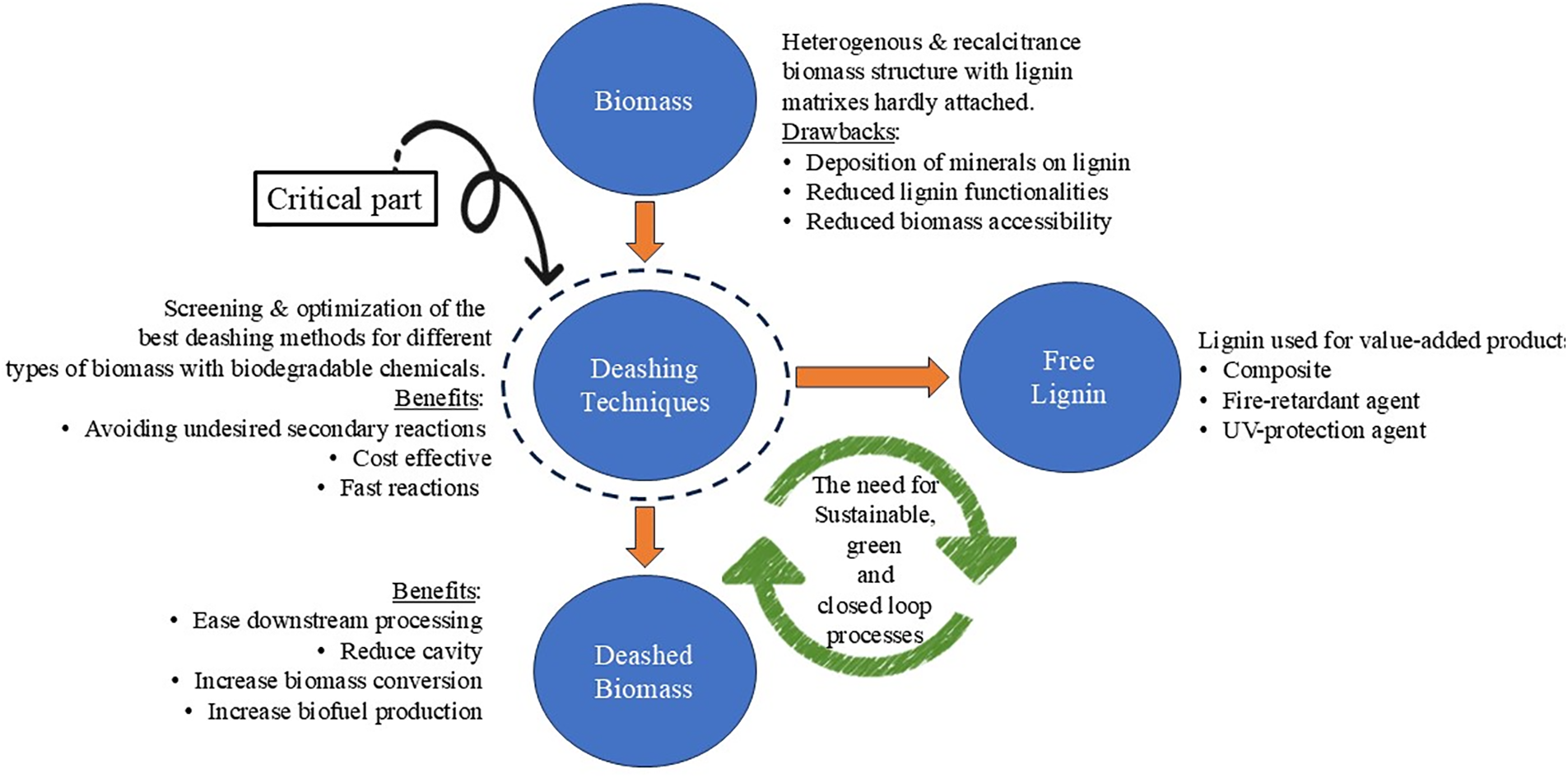
Figure 5: Biorefinery platform of deashed biomass
In conclusion, the ash content in agricultural biomass significantly affects biomass properties, in turn affecting lignin properties and various downstream applications. Deashing processes, when combined with optimized lignin extraction methods, can increase the efficiency and quality of biomass conversion, biofuel production, and environmental applications. Several strategies for deashing biomass have been introduced, with leaching treatment being one of the simple methods that can be chosen. After treatment, possible changes in the physical, chemical, and thermal properties of lignin, such as a reduced cavity effect of lignin, increases in the pore volume and surface area of lignin, and increased purity of lignin, were observed. Addressing these effects requires a full understanding of the interplay between lignin and ash contents and the development of tailored pretreatment and extraction strategies. Even though deashing pretreatment can improve the characteristics of biomass, side effects might be generated in the environment. Thus, green conversion processes can be considered a better approach not only for improving the quality of products but also for minimizing negative environmental effects. The utilization of green extraction solvents as replacements for acidic and alkaline extraction solvents would be a better solution, which would decrease the number of posttreatments needed after the process and may shorten the duration of the whole process. Another interesting topic that still requires further in-depth studies is the utilization of biological processes for biomass deashing. Even though the biological process might require a longer time, this process is seen to be more sustainable, mild in terms of reaction, and green.
Acknowledgement: The authors also gratefully recognize a research grant from the Japan-ASEAN and Technology Innovation Platform (JASTIP) for FY 2020–2021 with the research title “Utilization of biomass wastes for functional materials and chemicals”. This study is part of the E-ASIA project “Integrated biorefinery of sugarcane trash” in Work Package 4. This study is also the implementation area of a Letter of Intent (LOI) between the Institute of Bioproduct Development (IBD), Universiti Teknologi Malaysia (UTM), and the Research Center for Biomass and Bioproducts BRIN and the University of Mulawarman. The authors are grateful for the technical and scientific services provided by the Advanced Characterization Laboratories Cibinong-Integrated Laboratory of Bioproducts, National Research and Innovation Agency (BRIN) via the ELSA platform.
Funding Statement: This study was funded by the joint research collaboration of the Research Organization of Nanotechnology and Material, National Research and Innovation Agency (BRIN) (Grant number: 8/HK/II/2024) with the title Organosolv Lignin-Based Hydrogels from Sugarcane Leaves and Their Potential as Wound Dressings with Widya Fatriasari as the Principal Investigator. The authors are grateful for the research assistant program and for the Degree by Research (DBR) program of BRIN with scheme number: 20/III.10/HK/2024 and scholarship number 4637/II.5.4/SI.06.01/7/2024 for Eko Budi Santoso.
Author Contributions: Widya Fatriasari: conceptualization, writing—original draft, and writing—review, and editing; Eko Budi Santoso, Widya Fatriasari, Nur Izyan Wan Azelee, Deded Sarip Nawawi, and Wasrin Syafii: methodology and validation; Widya Fatriasari, and Wasrin Syafii: resources and project administration; Widya Fatriasari: investigation; Widya Fatriasari, Wasrin Syafii, and Deded Sarip Nawawi: visualization; Widya Fatriasari, and Nur Izyan Wan Azelee. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflict of interest to report regarding the present study.
References
1. Guo M, Song W, Buhain J. Bioenergy and biofuels: history, status, and perspective. Renew Sustain Energ Rev. 2015;42:712–25. doi:10.1016/j.rser.2014.10.013. [Google Scholar] [CrossRef]
2. Manikandan S, Vickram S, Sirohi R, Subbaiya R, Krishnan RY, Karmegam N, et al. Critical review of biochemical pathways to transformation of waste and biomass into bioenergy. Bioresour Technol. 2023;372:128679. doi:10.1016/j.biortech.2023.128679. [Google Scholar] [PubMed] [CrossRef]
3. Braghiroli FL, Passarini L. Valorization of biomass residues from forest operations and wood manufacturing presents a wide range of sustainable and innovative possibilities. Curr For Rep. 2020;6:172–83. doi:10.1007/s40725-020-00112-9. [Google Scholar] [CrossRef]
4. Puri L, Hu Y, Naterer G. Critical review of the role of ash content and composition in biomass pyrolysis. Front Fuels. 2024;2:1378361. doi:10.3389/ffuel.2024.1378361. [Google Scholar] [CrossRef]
5. Nik-Azar M, Hajaligol M, Sohrabi M, Dabir B. Mineral matter effects in rapid pyrolysis of beech wood. Fuel Process Technol. 1997;51(1–2):7–17. doi:10.1016/S0378-3820(96)01074-0. [Google Scholar] [CrossRef]
6. Stella Mary G, Sugumaran P, Niveditha S, Ramalakshmi B, Ravichandran P, Seshadri S. Production, characterization and evaluation of biochar from pod (Pisum sativumleaf (Brassica oleracea) and peel (Citrus sinensis) wastes. Int J Recycl Org Waste Agric. 2016;5:43–53. doi:10.1007/s40093-016-0116-8. [Google Scholar] [CrossRef]
7. Zhang S, Ban TP, Wen YX, Zhu JI, Wang YM, Hu H, et al. Removal of ash in biochar from carbonization by CO2-enhanced water leaching and its mechanism. J Fuel Chem Technol. 2023;51:544–51. doi:10.1016/S1872-5813(22)60059-8. [Google Scholar] [CrossRef]
8. Abioye KJ, Harun NY, Sufian S, Yusuf M, Jagaba AH, Ekeoma BC, et al. A review of biomass ash related problems: mechanism, solution, and outlook. J Energy Inst. 2024;112:101490. doi:10.1016/j.joei.2023.101490. [Google Scholar] [CrossRef]
9. Smit AT, Zomeren AV, Dussan K, Riddell LA, Huijgen WJJ, Dijkstra JW, et al. Biomass pre-extraction as a versatile strategy to improve biorefinery feedstock flexibility, sugar yields, and lignin purity. ACS Sustain Chem Eng. 2022;10(18):6012–22. doi:10.1021/acssuschemeng.2c00838. [Google Scholar] [PubMed] [CrossRef]
10. Gao Q, Ni L, He Y, Hou Y, Hu W, Liu Z. Effect of hydrothermal pretreatment on deashing and pyrolysis characteristics of bamboo shoot shells. Energy. 2022;247:123510. doi:10.1016/j.energy.2022.123510. [Google Scholar] [CrossRef]
11. Sari FP, Solihat NN, Azelee NIW, Fatriasari W. Biorefinery from plant biomass: a case study on sugarcane straw. In: Thomas S, Jose S, Sabu Mathew S, Samant L, editors. Plant biomass derived materials: sources, extractions, and applications. Boschstr, Weinheim, Germany: Wiley-VCH GmbH; 2024. [Google Scholar]
12. Karimah A, Hani IK, Laksana RPB, Ismayati M, Solihat NN, Sari FP, et al. Extraction of lignin from sugarcane trash and its potency as biosurfactant. Biores Technol Rep. 2023;24:101630. doi:10.1016/j.biteb.2023.101630. [Google Scholar] [CrossRef]
13. Hermiati E, Laksana RPB, Fatriasari W, Kholida LN, Thontowi A, Yopi et al. Microwave-assisted acid pretreatment for enhancing enzymatic saccharification of sugarcane trash. Biomass Convers Bioref. 2022;12(8):3037–54. doi:10.1007/s13399-020-00971-z. [Google Scholar] [CrossRef]
14. Menandro LMS, Cantarella H, Franco HCJ, Kölln OT, Pimenta MTB, Sanches GM, et al. Comprehensive assessment of sugarcane straw: implications for biomass and bioenergy production. Biofuels Bioprod Biorefin. 2017;11(3):488–504. doi:10.1002/bbb.1760. [Google Scholar] [CrossRef]
15. Balakrishnan M, Batra VS. Valorization of solid waste in sugar factories with possible applications in India: a review. J Environ Manag. 2011;92(11):2886–91. doi:10.1016/j.jenvman.2011.06.039. [Google Scholar] [PubMed] [CrossRef]
16. Jutakanoke R, Tolieng V, Tanasupawat S, Akaracharanya A. Ethanol production from sugarcane leaves by Kluyveromyces marxianus S1. 17, a genome-shuffling mediated transformant. BioResources. 2017;12(1):1636–46. doi:10.15376/biores.12.1.1636-1646. [Google Scholar] [CrossRef]
17. Franco H, Pimenta M, Carvalho J, Graziano Magalhães P, Rossell C, Braunbeck Ó, et al. Assessment of sugarcane trash for agronomic and energy purposes in Brazil. Scientia Agricola. 2013;70:305–12. doi:10.1590/S0103-90162013000500004. [Google Scholar] [CrossRef]
18. Meng Y, Lu J, Cheng Y, Li Q, Wang H. Lignin-based hydrogels: a review of preparation, properties, and application. Int J Biol Macromol. 2019;135:1006–19. doi:10.1016/j.ijbiomac.2019.05.198. [Google Scholar] [PubMed] [CrossRef]
19. de Sousa Nascimento L, da Mata Vieira FID, Horácio V, Marques FP, Rosa MF, Souza SA, et al. Tailored organosolv banana peels lignins: improved thermal, antioxidant and antimicrobial performances by controlling process parameters. Int J Biol Macromol. 2021;181:241–52. doi:10.1016/j.ijbiomac.2021.03.156. [Google Scholar] [PubMed] [CrossRef]
20. Sugiarto S, Leow Y, Tan CL, Wang G, Kai D. How far is Lignin from being a biomedical material? Bioact Mater. 2022;8:71–94. doi:10.1016/j.bioactmat.2021.06.023. [Google Scholar] [PubMed] [CrossRef]
21. Antunes F, Mota IF, Fangueiro JF, Lopes G, Pintado M, Costa PS. From sugarcane to skin: lignin as a multifunctional ingredient for cosmetic application. Int J Biol Macromol. 2023;234:123592. doi:10.1016/j.ijbiomac.2023.123592. [Google Scholar] [PubMed] [CrossRef]
22. Ariyanta HA, Santoso EB, Suryanegara L, Arung ET, Kusuma IW, Azman Mohammad Taib MN, et al. Recent progress on the development of lignin as future ingredient biobased cosmetics. Sustain Chem Pharm. 2023;32:100966. Elsevier B.V. doi:10.1016/j.scp.2022.100966. [Google Scholar] [CrossRef]
23. Domínguez-Robles J, Cárcamo-Martínez Á, Stewart SA, Donnelly RF, Larrañeta E, Borrega M. Lignin for pharmaceutical and biomedical applications—Could this become a reality? Sustain Chem Pharm. 2020;18:100320. doi:10.1016/j.scp.2020.100320. [Google Scholar] [CrossRef]
24. Kaur R, Sharma R, Chahal GK. Synthesis of lignin-based hydrogels and their applications in agriculture: a review. Chem Pap. 2021;75(9):4465–78. doi:10.1007/s11696-021-01712-w. [Google Scholar] [CrossRef]
25. Yun J, Wei L, Li W, Gong D, Qin H, Feng X, et al. Isolating high antimicrobial ability lignin from bamboo kraft lignin by organosolv fractionation. Front Bioeng Biotechnol. 2021;9:683796. doi:10.3389/fbioe.2021.683796. [Google Scholar] [PubMed] [CrossRef]
26. Menima-Medzogo JA, Walz K, Lauer JC, Sivasankarapillai G, Gleuwitz FR, Rolauffs B, et al. Characterization and in vitro cytotoxicity safety screening of fractionated organosolv lignin on diverse primary human cell types commonly used in tissue engineering. Biology. 2022;11:696. doi:10.3390/biology11050696. [Google Scholar] [PubMed] [CrossRef]
27. Solihat NN, Sari FP, Falah F, Ismayati M, Lubis MAR, Fatriasari W, et al. Lignin as an active biomaterial: a review. J Symb Logic. 2021;9(1):1–22. doi:10.23960/jsl191-22. [Google Scholar] [CrossRef]
28. Jiang L, Hu S, Sun LS, Su S, Xu K, He LM, et al. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour Technol. 2013;146:254–60. doi:10.1016/j.biortech.2013.07.063. [Google Scholar] [PubMed] [CrossRef]
29. Zafar MH, Kazmi M, Tabish AN, Ali CH, Gohar F, Rafique et al. An investigation on the impact of demineralization of lignocellulosic corncob biomass using leaching agents for its utilization in industrial boilers. Biomass Convers Biorefinery. 2020;10:1035–41. doi:10.1007/s13399-019-00528-9. [Google Scholar] [CrossRef]
30. Niu Y, Tan H, Hui SE. Ash-related issues during biomass combustion: alkali-induced slagging, silicate melt-induced slagging (ash fusionagglomeration, corrosion, ash utilization, and related countermeasures. Prog Energy Combust Sci. 2016;52:1–61. doi:10.1016/j.pecs.2015.09.003. [Google Scholar] [CrossRef]
31. Dhawan H, Sharma DK. Advances in the chemical leaching (inorgano-leachingbioleaching and desulphurization of coals. Int J Coal Sci Technol. 2019;6(2):169–83. doi:10.1007/s40789-019-0253-6. [Google Scholar] [CrossRef]
32. Yub Harun N, Jin Han T, Vijayakumar T, Saeed A, Afzal MT. Ash deposition characteristics of industrial biomass waste and agricultural residues. Mat Today: Proc. 2019;19:1712–21. doi:10.1016/j.matpr.2019.11.201. [Google Scholar] [CrossRef]
33. Vassilev SV, Baxter D, Andersen LK, Vassileva CG. An overview of the composition and application of biomass ash. Part 1. Phase-mineral and chemical composition and classification. Fuel. 2013;105:40–76. doi:10.1016/j.fuel.2012.09.041. [Google Scholar] [CrossRef]
34. Vassilev SV, Vassileva CG, Song YC, Li WY, Feng J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel. 2017;208(3):377–409. doi:10.1016/j.fuel.2017.07.036. [Google Scholar] [CrossRef]
35. Zając G, Szyszlak-Bargłowicz J, Gołębiowski W, Szczepanik M. Chemical characteristics of biomass ashes. Energies. 2018;11(11):2885. doi:10.3390/en11112885. [Google Scholar] [CrossRef]
36. Hills CD, Tripathi N, Singh RS, Carey PJ, Lowry F. Valorization of agricultural biomass-ash with CO2. Sci Rep. 2020;10(1):13801. doi:10.1038/s41598-020-70504-1. [Google Scholar] [PubMed] [CrossRef]
37. Polin JP, Carr HD, Whitmer LE, Smith RG, Brown RC. Conventional and autothermal pyrolysis of corn stover: overcoming the processing challenges of high-ash agricultural residues. J Anal Appl Pyrolysis. 2019;143:104679. doi:10.1016/j.jaap.2019.104679. [Google Scholar] [CrossRef]
38. Vassilev SV, Baxter D, Andersen LK, Vassileva CG, Morgan TJ. An overview of the organic and inorganic phase composition of biomass. Fuel. 2012;94(3):1–33. doi:10.1016/j.fuel.2011.09.030. [Google Scholar] [CrossRef]
39. Hu H, Westover TL, Cherry R, Aston JE, Lacey JA, Thompson DN. Process simulation and cost analysis for removing inorganics from wood chips using combined mechanical and chemical preprocessing. Bioenergy Res. 2017;10(1):237–47. doi:10.1007/s12155-016-9794-3. [Google Scholar] [CrossRef]
40. Antunes F, Mota IF, da Silva Burgal J, Pintado M, Santos Costa P. A review on the valorization of lignin from sugarcane by-products: from extraction to application. Biomass Bioenergy. 2022;166:106603. doi:10.1016/j.biombioe.2022.106603. [Google Scholar] [CrossRef]
41. Powar RV, Mehetre SA, Powar TR, Patil SB. End-use applications of sugarcane trash: a comprehensive review. Sugar Tech. 2022;24(3):699–714. doi:10.1007/s12355-022-01107-5. [Google Scholar] [CrossRef]
42. García-Maraver A, Terron LC, Ramos-Ridao A, Zamorano M. Effects of mineral contamination on the ash content of olive tree residual biomass. Biosyst Eng. 2014;118(1):167–73. doi:10.1016/j.biosystemseng.2013.12.009. [Google Scholar] [CrossRef]
43. Fitria, Kumar A, Dewa M, Liu J, Ha S, Yang B. Development of sulfonated carbon-based solid-acid catalysts derived from biorefinery residues and biomass ash for xylan hydrolysis. Biorese Technol Rep. 2023;24:101607. doi:10.1016/j.biteb.2023.101607. [Google Scholar] [CrossRef]
44. Reza MS, Ahmed A, Caesarendra W, Abu Bakar MS, Shams S, Saidur R, et al. Acacia holosericea: an invasive species for bio-char, bio-oil, and biogas production. Bioengineering. 2019;6(2):33. doi:10.3390/bioengineering6020033. [Google Scholar] [PubMed] [CrossRef]
45. Nunes LJR, Rodrigues AM, Loureiro LMEF, Sá LCR, Matias JCO. Energy recovery from invasive species: creation of value chains to promote control and eradication. Recycling. 2021;6(1):21. doi:10.3390/recycling6010021. [Google Scholar] [CrossRef]
46. Chin KL, H’ng PS, Paridah MT, Szymona K, Maminski M, Lee SH, et al. Reducing ash related operation problems of fast growing timber species and oil palm biomass for combustion applications using leaching techniques. Energy. 2015;90:622–30. doi:10.1016/j.energy.2015.07.094. [Google Scholar] [CrossRef]
47. Lam PY, Lim CJ, Sokhansanj S, Lam PS, Stephen JD, Pribowo A, et al. Leaching characteristics of inorganic constituents from oil palm residues by water. Indus Eng Chem Res. 2014;53(29):11822–7. doi:10.1021/ie500769s. [Google Scholar] [CrossRef]
48. Yu C, Thy P, Wang L, Anderson SN, Vandergheynst JS, Upadhyaya SK, et al. Influence of leaching pretreatment on fuel properties of biomass. Fuel Process Technol. 2014;128:43–53. doi:10.1016/j.fuproc.2014.06.030. [Google Scholar] [CrossRef]
49. Wu S, Chen J, Peng D, Wu Z, Li Q, Huang T. Effects of water leaching on the ash sintering problems of wheat straw. Energies. 2019;12(3):387. doi:10.3390/en12030387. [Google Scholar] [CrossRef]
50. Pattiya A, Chaow-U-Thai A, Rittidech S. The influence of pretreatment techniques on ash content of cassava residues. Int J Green Energy. 2013;10(5):544–52. doi:10.1080/15435075.2012.703629. [Google Scholar] [CrossRef]
51. Carrillo MA, Staggenborg SA, Pineda JA. Washing sorghum biomass with water to improve its quality for combustion. Fuel. 2014;116:427–31. doi:10.1016/j.fuel.2013.08.028. [Google Scholar] [CrossRef]
52. Fitria, Liu J, Yang B. Roles of mineral matter in biomass processing to biofuels. Biofuels Bioprod Bioref. 2023;17(3):696–717. doi:10.1002/bbb.2468. [Google Scholar] [CrossRef]
53. Saddawi A, Jones JM, Williams A, Le Coeur C. Commodity fuels from biomass through pretreatment and torrefaction: effects of mineral content on torrefied fuel characteristics and quality. Energy Fuels. 2012;26(11):6466–74. doi:10.1021/ef2016649. [Google Scholar] [CrossRef]
54. Liu X, Bi XT. Removal of inorganic constituents from pine barks and switchgrass. Fuel Process Technol. 2011;92(7):1273–9. doi:10.1016/j.fuproc.2011.01.016. [Google Scholar] [CrossRef]
55. Kukuruzović J, Matin A, Kontek M, Krička T, Matin B, Brandić I, et al. The effects of demineralization on reducing ash content in corn and soy biomass with the goal of increasing biofuel quality. Energies. 2023;16(2):967. doi:10.3390/en16020967. [Google Scholar] [CrossRef]
56. Tabish AN, Kazmi M, Hussain MA, Farhat I, Irfan M, Zeb H, et al. Biomass waste valorization by acidic and basic leaching process for thermochemical applications. Waste Biomass Valoriz. 2021;12(11):6219–29. doi:10.1007/s12649-021-01420-2. [Google Scholar] [CrossRef]
57. Shah TA, Ali S, Afzal A, Tabassum R. Effect of alkalis pretreatment on lignocellulosic waste biomass for biogas production. Int J Renew Energy Res. 2018;8(3):1318–26. doi:10.20508/ijrer.v8i3.7725.g7431. [Google Scholar] [CrossRef]
58. Lateef HU, Kazmi M, Tabish AN, Cheema II, Rashid MI. Effect of demineralization on physiochemical and thermal characteristics of wheat straw. Ener Sour Part A Recov Util Environ Eff. 2024;46(1):11254–63. doi:10.1080/15567036.2020.1791288. [Google Scholar] [CrossRef]
59. Siddiqi MH, Liu XM, Hussain MA, Qureshi T, Tabish AN, Lateef HU, et al. Evaluation of physiochemical, thermal and kinetic properties of wheat straw by demineralising with leaching reagents for energy applications. Energy. 2022;238(1):122013. doi:10.1016/j.energy.2021.122013. [Google Scholar] [CrossRef]
60. Chen D, Cen K, Chen F, Ma Z, Zhou J, Li M. Are the typical organic components in biomass pyrolyzed bio-oil available for leaching of alkali and alkaline earth metallic species (AAEMs) from biomass? Fuel. 2020;260:116347. doi:10.1016/j.fuel.2019.116347. [Google Scholar] [CrossRef]
61. Javed MA. Acid treatment effecting the physiochemical structure and thermal degradation of biomass. Renew Energy. 2020;159:444–50. doi:10.1016/j.renene.2020.06.011. [Google Scholar] [CrossRef]
62. Santhiya D, Ting YP. Bioleaching of spent refinery processing catalyst using Aspergillus niger with high-yield oxalic acid. J Biotechnol. 2005;116(2):171–84. doi:10.1016/j.jbiotec.2004.10.011. [Google Scholar] [PubMed] [CrossRef]
63. Zhang N, Wang L, Zhang K, Walker T, Thy P, Jenkins B, et al. Pretreatment of lignocellulosic biomass using bioleaching to reduce inorganic elements. Fuel. 2019;246:386–93. doi:10.1016/j.fuel.2019.02.138. [Google Scholar] [CrossRef]
64. Zhang N, Walker T, Jenkins B, Anderson S, Zheng Y. Bioleaching of Sorghum straw in bioreactors for biomass cleaning. Fermentation. 2021;7(4):270. doi:10.3390/fermentation7040270. [Google Scholar] [CrossRef]
65. Santos D, Giacobe K, Silva CM, Saldanha LF, Martins AF, Flores EMM, et al. Ultrasound-assisted demineralization process of sugarcane straw and its influence on the further biomass conversion. Sustainability. 2022;14(1):557. doi:10.3390/su14010557. [Google Scholar] [CrossRef]
66. Lacey JA, Aston JE, Westover TL, Cherry RS, Thompson DN. Removal of introduced inorganic content from chipped forest residues via air classification. Fuel. 2015;160:265–73. doi:10.1016/j.fuel.2015.07.100. [Google Scholar] [CrossRef]
67. Zhuang X, Gan Z, Chen D, Cen K, Ba Y, Jia D. An approach for upgrading bio-oil by using heavy bio-oil copyrolyzed with bamboo leached with light bio-oil. Fuel. 2023;330:125931. doi:10.1016/j.fuel.2022.125931. [Google Scholar] [CrossRef]
68. Manna S, Singh N, Purakayastha TJ, Berns AE. Effect of deashing on physico-chemical properties of wheat and rice straw biochars and potential sorption of pyrazosulfuron-ethyl. Arab J Chem. 2020;13(1):1247–58. doi:10.1016/j.arabjc.2017.10.005. [Google Scholar] [CrossRef]
69. Dai Q, Liu Q, Yılmaz M, Zhang X. Copyrolysis of sewage sludge and sodium lignosulfonate: kinetic study and methylene blue adsorption properties of the biochar. J Anal Appl Pyrolysis. 2022;165:105586. doi:10.1016/j.jaap.2022.105586. [Google Scholar] [CrossRef]
70. Cao W, Xu H, Zhang X, Xiang W, Qi G, Wan L, et al. Novel posttreatment of ultrasound assisting with acid washing enhance lignin-based biochar for CO2 capture: adsorption performance and mechanism. Chem Eng J. 2023;471:144523. doi:10.1016/j.cej.2023.144523. [Google Scholar] [CrossRef]
71. Chotirotsukon C, Jirachavala K, Raita M, Pongchaiphol S, Hararak B, Laosiripojana N, et al. Effects of thermal and physical modification on functional properties of organosolv lignin from sugarcane bagasse and its application in cosmeceutical products. Front Chem Eng. 2023;5:1099010. doi:10.3389/fceng.2023.1099010. [Google Scholar] [CrossRef]
72. Pagano M, Hernando H, Cueto J, Cruz P, Dufour J, Moreno I, et al. Insights on the acetic acid pretreatment of wheat straw: changes induced in the biomass properties and benefits for the bio-oil production by pyrolysis. Chem Eng J. 2022;454:140206. doi:10.1016/j.cej.2022.140206. [Google Scholar] [CrossRef]
73. Huijgen WJJ, Telysheva G, Arshanitsa A, Gosselink RJA, de Wild PJ. Characteristics of wheat straw lignins from ethanol-based organosolv treatment. Ind Crops Prod. 2014;59:85–95. doi:10.1016/j.indcrop.2014.05.003. [Google Scholar] [CrossRef]
74. Zouari M, Marrot L, DeVallance DB. Effect of demineralization and ball milling treatments on the properties of Arundo donax and olive stone-derived biochar. Int J Environ Sci Technol. 2024;21(1):101–14. doi:10.1007/s13762-023-04968-9. [Google Scholar] [CrossRef]
75. Basta AH, Fierro V, Saied H, Celzard A. Effect of deashing rice straws on their derived activated carbons produced by phosphoric acid activation. Biom Bioene. 2011;35(5):1954–9. doi:10.1016/j.biombioe.2011.01.043. [Google Scholar] [CrossRef]
76. Asadieraghi M, Wan Daud WMA. Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: effects of demineralization by diverse acid solutions. Energy Convers Manag. 2014;82:71–82. doi:10.1016/j.enconman.2014.03.007. [Google Scholar] [CrossRef]
77. Fierro V, Torné-Fernández V, Celzard A, Montané D. Influence of the demineralization on the chemical activation of Kraft lignin with orthophosphoric acid. J Hazard Mater. 2007;149(1):126–33. doi:10.1016/j.jhazmat.2007.03.056. [Google Scholar] [PubMed] [CrossRef]
78. Yin H, Huang X, Mu L, Xi X, Dong M, Huo Z. Insight into the structural feature, ash transformation behavior, and alkali metal heat release characteristic of industrial lignin via experimental and equilibrium evaluation. Fuel. 2023;343:127944. doi:10.1016/j.fuel.2023.127944. [Google Scholar] [CrossRef]
79. Castro-Díaz M, Vega MF, Díaz-Faes E, Barriocanal C, Musa U, Snape C. Evaluation of demineralized lignin and lignin-phenolic resin blends to produce biocoke suitable for blast furnace operation. Fuel. 2019;258:116125. doi:10.1016/j.fuel.2019.116125. [Google Scholar] [CrossRef]
80. Apaydın Varol E, Mutlu Ü. TGA-FTIR analysis of biomass samples based on the thermal decomposition behavior of hemicellulose, cellulose, and lignin. Energies. 2023;16(9):3674. doi:10.3390/en16093674. [Google Scholar] [CrossRef]
81. Thy P, Yu C, Jenkins BM, Lesher CE. Inorganic composition and environmental impact of biomass feedstock. Ener Fuels. 2013;27(7):3969–87. doi:10.1021/ef400660u. [Google Scholar] [CrossRef]
82. Thivolle-Cazat A, Net E, Labalette F, Marsac S. A short historical review of fast pyrolysis of biomass. OGST. 2013;68:621–783. [Google Scholar]
83. Naterer GF. Advanced heat transfer. 3rd ed. Boca Raton: CRC Press; 2021. doi:10.1201/9781003206125 [Google Scholar] [CrossRef]
84. Yang J, Feng Z, Gao Q, Ni L, Hou Y, He Y, et al. Ash thermochemical behaviors of bamboo lignin from kraft pulping: influence of washing process. Renew Energy. 2021;174:178–87. doi:10.1016/j.renene.2021.04.036. [Google Scholar] [CrossRef]
85. Manalindo AY, Arani AR, Quijano W. Fabrication and analysis of thermal insulation ceiling panel from corn husk fiber. Key Eng Mater. 2023;953(8):113–20. doi:10.4028/p-wZsX92. [Google Scholar] [CrossRef]
86. Iswanto AH, Manurung H, Sohail A, Hua LS, Antov P, Nawawi DS, et al. Physico-chemical, morphological, and fire characteristics of eco-friendly particleboard manufactured with phosphorylated lignin addition. J Renew Mater. 2024;12(7):1311–41. doi:10.32604/jrm.2024.052172. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF

 Downloads
Downloads
 Citation Tools
Citation Tools