 Open Access
Open Access
REVIEW
Current Perspectives on Umbilical Cord Abnormalities Including Blood Flow Parameters Based on Ultrasound Observations
1 School of Energy and Power Engineering, Shandong University, Jinan, 250061, China
2 Department of Ultrasound, Jinan Central Hospital, Jinan, 250000, China
* Corresponding Author: Jingying Wang. Email:
Molecular & Cellular Biomechanics 2022, 19(4), 209-219. https://doi.org/10.32604/mcb.2022.026082
Received 15 August 2022; Accepted 10 October 2022; Issue published 27 December 2022
Abstract
The umbilical cord is a vital structure between the fetus and placenta for the growth and well-being of the fetus. Although the umbilical cord may be the only organ that dies at the beginning of life, it is one of the most important parts of the feta-placental unit and plays a role in determining how life begins. In general, the prenatal examination of the umbilical cord is limited to the observation of the number of vessels and the evaluation of umbilical artery blood flow parameters. Pathologists have done more research on the morphological characteristics of the umbilical cord and linked them to perinatal outcomes. The introduction of advanced imaging technology makes it possible to study the characteristics of fetal umbilical cord from early to late gestation. Many studies have shown that the changes of umbilical cord structure may be related to pathological conditions, such as preeclampsia, fetal growth restriction. Prenatal morphometric umbilical cord characteristics and arterial blood flow parameters in normal and pathologic conditions are discussed in this review.Keywords
The umbilical cord and the placenta are important organs that anatomically bridge between the mother and the fetus. The umbilical cord delivers oxygen and nutrients to the fetus and removes carbon dioxide and waste elements to the placenta for exchanging. Account of allowing for two-way blood flow between the fetus and the placenta, the umbilical cord is called a lifeline. The fetus will die if the umbilical flow is interrupted for more than seven minutes [1]. The latest data show that about 20% of stillbirths are caused by umbilical cord abnormalities [2]. This is partly because current imaging diagnostic tools cannot achieve sufficient clarity during the umbilical cord examination [3]. Cases of fetal demise are inevitable, especially in the first and second trimesters when fetal heart rate monitoring is difficult to identify. Given the critical function of the umbilical cord in fetal development, it is of great significance to the theoretical and practical research of abnormal umbilical cord. If we can accurately grasp the influence of abnormal umbilical cord and blood flow, the clinical diagnosis and treatment will be more accurate, and ultimately protect the health of mother and child, vitally to the continuation of human race.
The shielding of the mother increases the difficulty of displaying the fetal structure. General physical examination methods are not able to involve the specific fetal structure and most instruments and equipment cannot be placed in the uterus. Diagnostic imaging provides crucial information about normal and diseased fetal structures [4]. Computed tomography (CT), X-ray, positron emission tomography (PET), ultrasound (US), single-positron emission computed tomography (SPECT), and magnetic resonance imaging (MRI) are common [5]. Advanced technologies have been widely used to enhance the imaging of the fetus structure. Although three-dimensional reconstruction of magnetic resonance imaging (MRI) is not routinely used in fetal examination, it can examine more detailed fetal structures or structures that cannot be detected by ultrasound due to fetal body occlusion. Fetal examination with high-energy ionizing radiation techniques such as X-rays, CT, and PET is harmful, and the safety of MRI during pregnancy is controversial [5,6].
During fetal development, oxygenated and nutritious blood is transferred from the placenta to the fetus through the umbilical vein. About half of the blood passes through a ductus venosus, which extends from the umbilical vein, into the inferior vena cava (IVC). The rest enters the portal vein to provide nutrition and oxygen to the liver. Most of blood entering the right atrium (RA) from the IVC enters the left atrium (LA) through the foramen ovale (FO). Blood from the superior vena cava (SVC) enters the RA, then the right ventricle (RV), and into the trunk of the pulmonary artery (PA). Most of the blood in the PA enters the aorta through the ductus arteriosus (DA). The fetal blood returns to the placenta through two umbilical arteries from the internal iliac artery.
When pulmonary respiration begins to function at birth, pulmonary blood pressure drops, blood flowing from the PA trunk to enter the left and right PA, and then back to the LA through the pulmonary veins. The FO and DA close and the pulmonary and systemic circulations are separated. When the umbilical cord is cut, the umbilical arteries, the umbilical vein and DV occlude.
3 Morphology and Function of Umbilical Cord
The human umbilical cord contains two arteries and a vein, which are surrounded and encompassed by Wharton’s jelly, the connective tissue of the umbilical cord. Two arteries spiral around the umbilical vein, and three vessels rotate in a helical way due to fetal motion, the different vessel arrangement from that in other parts of the human body. The umbilical venous blood is nutrient-high, which flows to the fetus, while the umbilical arterial blood is metabolic waste-high, which flows away from the fetus and to the placenta for exchange. Thus, the umbilical blood flow can indirectly reflect the state of the placenta and the fetus according to different flow directions. The umbilical cord enables the fetus to develop in an aquatic environment that facilitates pulmonary and joint development.
The umbilical cord is an important channel for gas exchange, nutrient supply and excretion of metabolites between the mother and the fetus. The fetal blood flow can be blocked at the time of umbilical cord compression, which even endangers fetal life. Therefore, abnormal umbilical cord, including abnormal shape/structure/position, changing umbilical blood flow waveform or flow velocity or resistance, etc. may affect fetal survival status, which is a meaningful and valuable study.
4 Anatomical Abnormalities of Umbilical Cord
It was observed that the umbilical cord grows non-uniformly as the gestational week progresses [7,8]. Approximate length of an average umbilical cord is 55–60 cm. Deviation of the length in either way both increases the risk of adverse perinatal outcomes, especially when above 100 cm and below 30 cm [9].
Compared with other fetal organs, the umbilical cord is difficult to be fully visualized by ultrasound. In general, the difficulty is encountered in tracking a long free-floating structure. In addition, it is difficult to sonographically monitor the umbilical cord because the fetus obscures some segments of it. The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) states only that the umbilical cord should be imaged and the number of blood vessels assessed, if available [10].
4.2 Number of Umbilical Vessels
Single umbilical artery (SUA), the most common structural anomaly, is congenital, in which one umbilical artery is missing. The incidence of SUA is 0.55% in general and 5.9% in high-risk fetuses; 11% of SUA cases are associated with congenital anomalies or chromosomal abnormalities [11]. Simple single umbilical artery (SSUA) is defined as SUA alone, without combined developmental or chromosomal abnormalities [12]. Fetuses with SSUA have a slightly higher rate of intrauterine growth restriction, lower neonatal birth weight, and an increased risk of maternal preterm delivery than normal fetuses [12], but increasing the frequency of the fetal sonographic testing is not generally considered to be required [13]. When the single umbilical artery and nuchal cord coexist, prolonged fetal heart rate monitoring is necessary. It can be detected easily during early pregnancy by ultrasound showing only one umbilical artery beside the bladder in transverse pelvic view. Two umbilical vessels in an “8” shape are seen in the transverse section of the umbilical cord in amniotic fluid (Fig. 1).
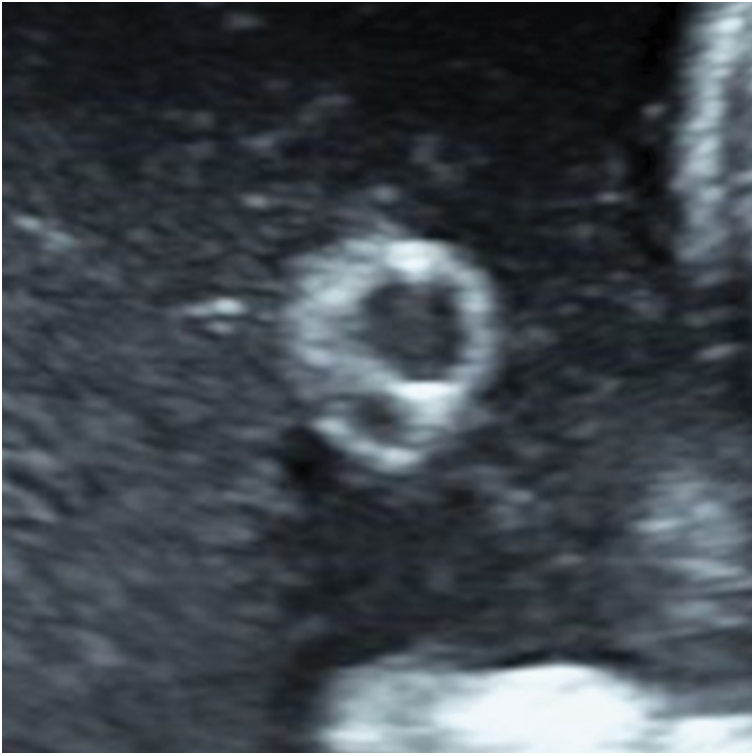
Figure 1: Single umbilical artery observed by ultrasound
4.2.2 Umbilical Artery Thrombosis
Another disease that causes changes in the number of umbilical vessels is umbilical artery thrombosis, which has a low incidence of 0.045% in high-risk pregnancies [14]. It occurs in all gestational weeks, which is closely related to adverse perinatal outcomes, such as intrauterine asphyxia, fetal growth restriction, and stillbirth. The main ultra-sonographic findings of umbilical artery embolism were as follows: an umbilical artery was filled with enhanced echo and no blood flow passed through or reviewing the previous ultrasound examination of three unobstructed umbilical vessels, only two unobstructed vessels were seen. Because it is difficult to get the direct sign that an umbilical artery is filled with thrombus by ultrasonography, it is often misdiagnosed as SUA [15,16]. Abnormal length of the umbilical cord, abnormal insertion of the umbilical cord, inflammation, torsion, true knot, and local stenosis of the umbilical cord can be accompanied by umbilical artery thrombosis [14,17]. When umbilical artery embolism is found or suspected in the third trimester, and once the fetal monitoring is abnormal, emergency cesarean section is the first choice to ensure fetal safety [17].
The umbilical cord is in the shape of a helix, called coiling of umbilical cord, which can be observed in the fetal period and after birth. Collins proposed from the perspective of pathology that the umbilical cord has its helix and twist. Twists are caused by fetal movement and can be untwisted after birth; helixes exist in the umbilical cord itself and cannot be untwisted after birth [18]. In the process of embryonic development, umbilical vessels grow faster than the surrounding connective tissue, resulting in self-helix, which exists in the early stage of pregnancy, and some gradually develops [19].
The mechanism of umbilical cord coiling formation is still inconclusive, and it is generally believed that it is the result of the umbilical inherent helix and fetal movement. Under the influence of fetal movement and other factors, the density of the umbilical cord coiling is constantly changing, the coiling is unevenly distributed on the whole umbilical cord, and the near fetal side is usually denser than the placental side and the middle segment [20]. The direction of the umbilical cord coiling includes left twist, right twist, mixed twist, and no twist; the left twist is more than the right and the proportion of mixed and non-twist is less [21].
The coiling of the umbilical cord can be divided into four patterns: undulating, rope, segmented, and linked patterns (Fig. 2) [8]. This classification is based on the density of the helix in the longitudinal direction of the umbilical cord. In the future, the physical characteristics of blood flow in segmental type and linked type will be focused on, which can reveal the potential defects of those patterns of umbilical cord abnormality. Whether the risk of adverse fetal outcomes with these four patterns can be identified in the fetal period remains to be studied [8].
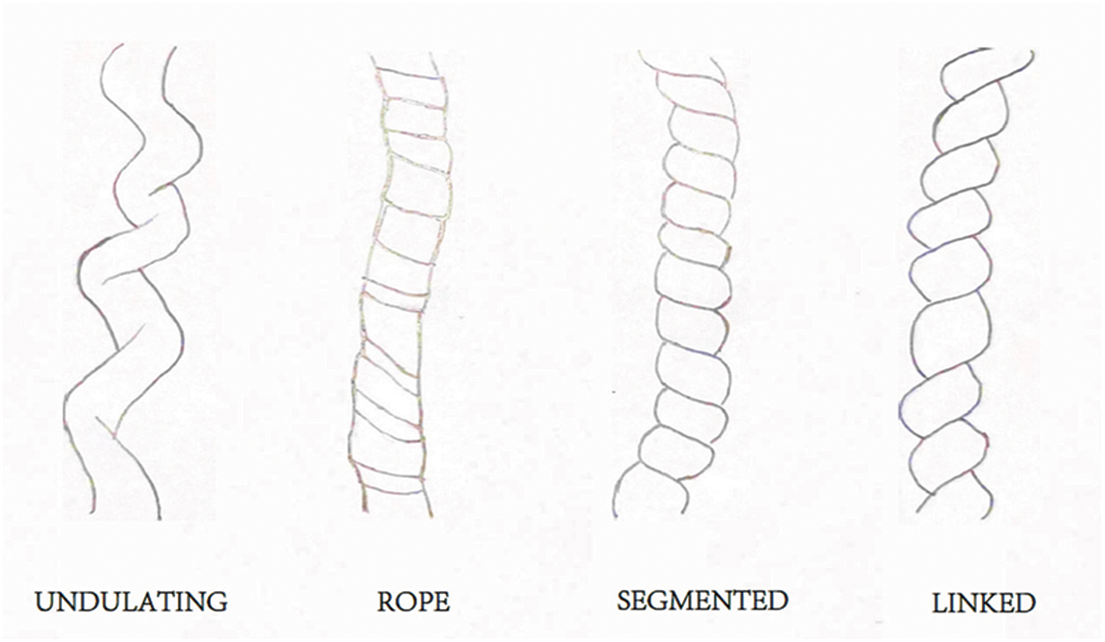
Figure 2: [8]: Schematic representation of four gross umbilical cord coiling patterns
Many studies have compared the umbilical cord coiling evaluated by ultrasound with that at birth and believed that the ultrasonic evaluation of the umbilical cord coiling is related to the birth coiling, but the consistency is not high. It is due to the inconsistency of the coiling in different segments of the umbilical cord, and the dynamic change during the whole gestation [22].
Hypercoiling of the umbilical cord can be shown as the shape of hemp rope and rat eye [23,24] by ultrasound, but these signs are greatly subjective. At present, the quantitative evaluation indexes of umbilical cord coiling by ultrasound include umbilical cord torsion pitch (Pitch) and umbilical coiling index (UCI), which are not used clinically, but important during autopsies and placental study and should be reported. Many studies have analyzed the relationship between umbilical cord hypercoiling and adverse pregnancy outcomes and found that the incidence of fetal distress, asphyxia, stillbirth, and growth restriction in the umbilical hyper-coiling group was significantly higher than that in the normal group. The effect of the umbilical cord coiling on blood flow is not clear, and the indexes of umbilical cord helical structure observed by ultrasound need to be further studied, including how to explain UCI clinically. Ultrasonic diagnosis of abnormal umbilical cord coiling for the prediction of adverse pregnancy outcomes needs evidence-based medical evidence to support [25].
4.4 Large and Lean Umbilical Cord
The diameter of umbilical cord is determined by the thickness of umbilical vessels and Wharton’s jelly. The increase of umbilical cord diameter is directly proportional to the gestational age until the third trimester [26]. Too large or too small umbilical cord diameter indicates that the fetus may be in some abnormal state.
Large umbilical cords are associated with fetal hydrops and maternal diabetes [27]. In the first trimester, fetuses with large umbilical cord have an increased risk of chromosomal abnormalities. Ultrasound assessment of umbilical cord diameter can not only improve the detection rate of macrosomia [28], but also improve the sensitivity of chromosome abnormality screening [29].
The overall thickness of the umbilical cord is uniform, and the umbilical cord diameter is determined by the thickness of the umbilical vessels and the amount of Wharton’s jelly. It is considered to be lean umbilical cord if the umbilical cord’s cross-sectional area is below the 10th centile of gestational age. Umbilical cord diameter measured in patients with miscarriage and preeclampsia was two and three SD below the mean, respectively [30]. Fetuses with lean umbilical cords are at increased risk for small gestational age (SGA) and delivery distress [31]. Some studies have pointed out that the umbilical cord of SGA is thinner than that of normal fetuses. Through electron microscopic observation, Wharton’s jelly and vascular composition are different from normal umbilical cords [32].
The umbilical vein varix (UVV) is relatively rare, with an overall incidence of 0.4%–1.1% [33–36]. Dilatation of the umbilical vein in the abdominal cavity of the fetus, known as intra-abdominal umbilical vein varix (Fig. 3), is common. A small number of UVV occur in the outer abdominal cavity, also known as intra-amniotic umbilical vein varix. UVV is generally limited to one segment and has a different length. Compared with the intra-amniotic UVV, the intra-abdominal UVV have a higher probability of chromosomal abnormalities [34,35]. Excluding chromosomal abnormalities and fetal structural abnormalities, the prognosis of intra-abdominal UVV is favorable, and the probability of serious complications is not high [34,35]. The extra-abdominal UVV is associated with fetal thrombosis, hematoma, obstruction of umbilical artery compression, and perinatal death [34,35]. Therefore, it is recommended that fetuses with UVV be followed weekly to 28 weeks of gestation and twice weekly thereafter to delivery at 36–37 weeks [36].
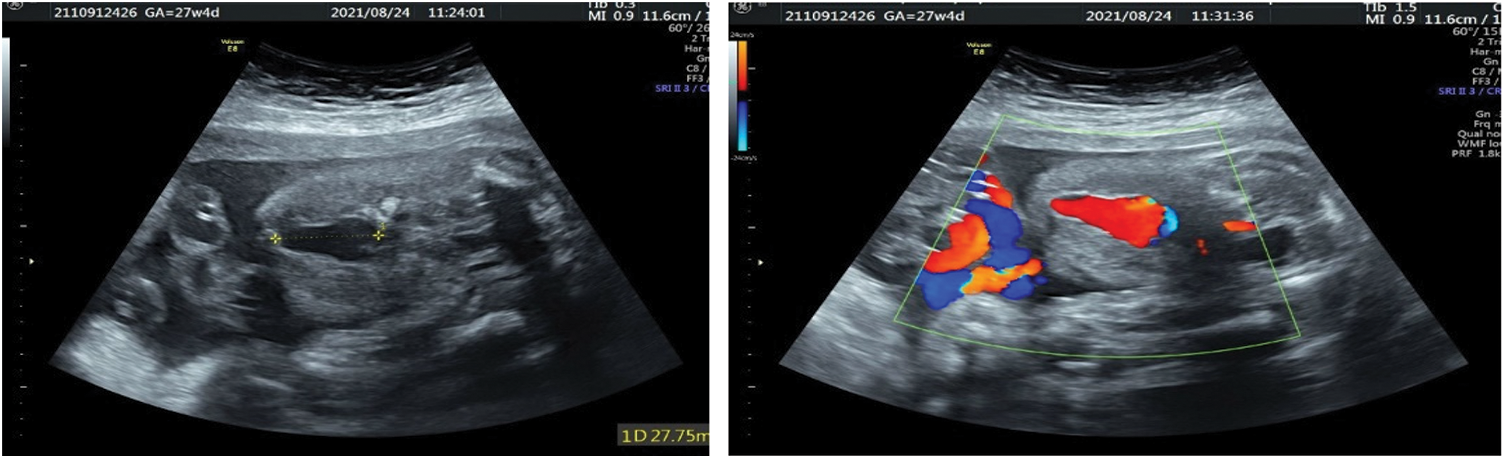
Figure 3: Fetal intra-abdominal varix at 27 weeks: Two-dimensional ultrasound showing the intra-abdominal UVV (left). Color Doppler imaging showing the blood flow pattern at the lesion (right)
Pathological findings of umbilical cord strictures often show a lack of Wharton’s jelly. The stenosis is usually near the fetal end of the umbilical cord, and thrombosis can occur. Most cases involve some single segment. Some cases of multiple segmental strictures of the umbilical cord are also reported [37]. Many hypotheses explain this disease, some think it is related to the local excessive torsion of the umbilical cord, and some think it is related to the loss of Wharton’s jelly [38]. Some suggest that it is caused by excessive local traction during the umbilical cord development and formation [39]; it can exist alone or coexist with umbilical cord torsion, velamentous insertion, etc. Umbilical cord stenosis is often associated with stillbirth.
Central and eccentric insertion accounted for more than 90% of the umbilical insertion position site. However, marginal insertion and velamentous insertion may also occur [40]. The former is considered as the position within 1–2 cm of the placental margin in the third trimester of pregnancy. In the latter situation, the umbilical vessel inserts into the fetal membranes between the amnion and chorion before insertion in the placenta, and is not protected by the Wharton’s jelly or the placenta chorionic plate [40]. Marginal cord insertion and velamentous cord insertion are noted in approximately 6.3% and 1.5% of singleton term deliveries [41]. Compared with marginal type, velamentous type was associated with more pregnancy complications [42]. Complications of velamentous insertion of the umbilical cord include compression during labor resulting in non-reassuring fetal status, potential rupture of membranous vessels, arterial or venous thrombi, or the presence of vasa previa [43]. Vasa previa is a rarely reported condition in which fetal blood vessels, unsupported by either the umbilical cord or placental tissue, traverse the fetal membranes within the lower segment of the uterus caudal to the presenting part. If the velamentous vessels are in the lower part of the uterus, these vessels are prone to rupture during delivery, resulting in rapid fetal blood loss.
In a multicenter study of pregnancy with vasa previa [44], the infant survival rate was 97% in cases where vasa previa was diagnosed by prenatal ultrasound, and only 44% in cases undiagnosed by prenatal ultrasound. Therefore, to get the favorable outcomes with this condition, it is necessary to obtain a relevant prenatal diagnosis and perform a cesarean section before the rupture of fetal membranes.
Another rare abnormal insertion is furcate cord insertion. The umbilical vessels dissociate from the trunk of the umbilical cord before they are inserted into the placenta and lack the protection of Wharton’s jelly. The separated vessel has an increased risk of aneurysm, thrombosis, or injury. In most cases, the furcate cord insertion has a good outcome; however, intrauterine fetal death occurs in about 1% of cases [45]. It is necessary to observe carefully and record the insertion of umbilical cord in detail in ultrasound scan [46].
5 Nuchal Cord and Presentation of Umbilical Cord
It is reported that the incidence of umbilical cord entangling the fetal neck is about 20%. It is not clear whether umbilical cord entanglement is associated with a significant increase in perinatal adverse outcomes. According to the experience of ultrasound doctors, the relationship between these two factors is unclear and less significant. However, some scholars believe that the relationship between nuchal cords and intrauterine fetal demise, though remains confirmation, is likely to be seriously underestimated. Nuchal cords are associated with the fetal blood supply, resulting in perinatal fetal hypoxia, asphyxia, and even stillbirth [47]. An analysis of the causes of fetal asphyxia shows that about 22% of neonatal asphyxia and 25% of severe neonatal asphyxia are reported in association with umbilical cord factors, most of which are related to an umbilical cord around the neck [48]. However many guidelines do not give a detailed description of how to check for umbilical cord entanglement.
When the fetal membrane is ruptured, the umbilical cord is protruded out of the cervix before the fetus, which is prolapse of umbilical cord. Presentation of umbilical cord means that when the fetal membrane is not ruptured, the umbilical cord is located in front of the fetal presentation or on one side, also known as invisible umbilical cord prolapse. Ultrasonography has high sensitivity and specificity for umbilical cord presentation. It has been reported that compared with fetuses with umbilical cord prolapse after rupturing of membranes, newborns with umbilical cord presentation detected by ultrasound before rupture of fetal membranes have a better prognosis [49]. Ultrasound screening for high-risk conditions before rupture of membranes can change the adverse pregnancy outcome associated with umbilical cord prolapse in newborns.
6 Clinical Application of Umbilical Artery Doppler Ultrasound
The umbilical artery is a special blood vessel that exists in the fetus, which sends fetal hypoxic blood to the placenta. The resistance of the placenta and umbilical blood flow gradually decreases as the pregnancy progressed, while the end-diastolic blood flow velocity increases. Doppler ultrasound showed that the end-diastolic blood flow signal (D) after the systolic peak (S), gradually increased, the S/D value decreased, and the resistance index (RI) and pulsatility index (PI) decreased.
In some abnormal conditions, the placental blood flow resistance increased, and the umbilical artery blood flow resistance increased accordingly. Ultrasound showed this situation that the end-diastolic blood flow disappeared or reversed (Fig. 4), and PI, RI, and S/D increased. The disappearance or reversal of end-diastolic blood flow is a characteristic change of severe deficiency of fetal-placental circulation, suggesting that the fetus is severely hypoxic or close to the decompensated stage of hypoxia. It is mainly found in intrauterine growth restriction and placental dysfunction. Combined with the maternal and fetal conditions, the appropriate delivery time can be chosen for the fetus according to the result of Doppler examination.
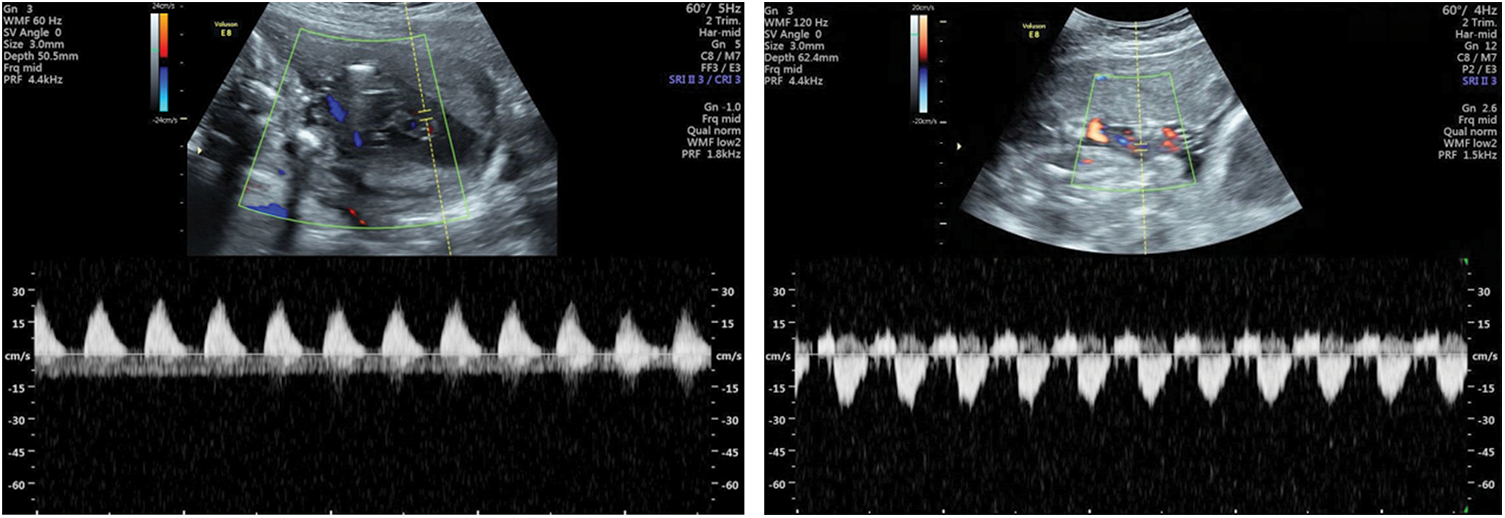
Figure 4: Umbilical arteries visualized by transabdominal color flow mapping and Doppler velocity waveforms: Loss of diastolic blood flow at 22 weeks (left); reversal of diastolic blood flow at 25 weeks (right)
The umbilical cord go through the process of embryonic development and intrauterine growth, and there is a two-way flow of blood in the umbilical cord. The umbilical cord abnormalities exist including anatomical abnormality (length, number of blood vessels, degree of helix, diameter, local dilatation, and stricture), abnormal positional relationship with the fetus (including umbilical cord entanglement, umbilical cord presentation), and an abnormal connection with the placenta. The fetus needs self-regulation to adapt to the umbilical changes. Some disease are reflected in umbilical cord blood flow. Abnormal flow also can directly cause changes in the geometry of the umbilical cord (for example, varix in the extra-abdominal segment is prone to thrombosis).
In the past, medicine mainly focused on the development of a single discipline, while multi-disciplinary integration to promote medical research has become a hot trend of scientific and technological development at present, especially the transformation of basic research results into clinical practices. The cross-integration of medicine and other disciplines has a long history, and the combination of biology and mechanics has given rise to the cross-discipline of biomechanics. Blood circulation mechanics, muscle mechanics, Young’s modulus, peripheral resistance of blood flow, and intercellular material transmembrane transport are all important derived from the development of this discipline concept.
The process of blood circulation includes blood flow, the deformation of blood cells and blood vessels, and the interaction between blood and blood vessels, which contains rich mechanical laws. The study of hemodynamics in arteries has been a hot topic in biomechanics and biomedical engineering. Hemodynamic factors, such as wall shear stress (WSS), wall shear stress gradient (WSSG), flow separation, secondary flow, and so on, have important effects on arterial endothelial cell injury, intimal thickening, intimal smooth muscle cell proliferation, intimal connective tissue junction, and aggregation of monocytes, platelets, and macrophages. If similar mechanical laws can be applied to the study of fetal blood circulation, it will help us to have a more thorough understanding of the fetus, a unique stage of human life.
Authorship: The authors confirm contribution to the paper as follows: study conception and design: X. Song, J. Wang; data collection: X. Song; analysis and interpretation of results: X. Song, J. Wang, M. Li; draft manuscript preparation: X. Song, M. Li, X. Yang, J. Wang. All authors reviewed the results and approved the final version of the manuscript.
Funding Statement: This research was funded by “Double First-Class” Foundation for the Talents of Shandong University, Grant No. 31380089963090.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Xing, X., Beihua, K. (2018). Obstetrics and gynecology. 9th edition Beijing, China: People’s Medical Publishing House. [Google Scholar]
2. Hammad, I. A., Blue, N. R., Allshouse, A. A., Silver, R. M., Gibbins, K. J. et al. (2020). Umbilical cord abnormalities and stillbirth. Obstetrics and Gynecology, 135(3), 644–652. DOI 10.1097/AOG.0000000000003676. [Google Scholar] [CrossRef]
3. Sepulveda, W., Wong, A. E., Gomez, L., Alcalde, J. L. (2009). Improving sonographic evaluation of the umbilical cord at the second-trimester anatomy scan. Journal of Ultrasound in Medicine, 28(6), 831–835. DOI 10.7863/jum.2009.28.6.831. [Google Scholar] [CrossRef]
4. Kim, E., Boyd, B. (2022). Diagnostic imaging of pregnant women and fetuses: Literature review. Bioengineering, 9(6), 236. DOI 10.3390/bioengineering9060236. [Google Scholar] [CrossRef]
5. Difonzo, M., Fliedel, L., Mignet, N., Andrieux, K., Alhareth, K. (2022). How could nanomedicine improve the safety of contrast agents for MRI during pregnancy? Sci, 4(1), 11. DOI 10.3390/sci4010011. [Google Scholar] [CrossRef]
6. Gatta, G., di Grezia, G., Cuccurullo, V., Sardu, C., Iovino, F. et al. (2021). MRI in pregnancy and precision medicine: A review from literature. Journal of Personalized Medicine, 12(1), 9. DOI 10.3390/jpm12010009. [Google Scholar] [CrossRef]
7. Georgiadis, L., Keski-Nisula, L., Harju, M., Räisänen, S., Georgiadis, S. et al. (2014). Umbilical cord length in singleton gestations: A Finnish population-based retrospective register study. Placenta, 35(4), 275–280. DOI 10.1016/j.placenta.2014.02.001. [Google Scholar] [CrossRef]
8. Ernst, L. M., Minturn, L., Huang, M. H., Curry, E., Su, E. J. (2013). Gross patterns of umbilical cord coiling: Correlations with placental histology and stillbirth. Placenta, 34(7), 583–588. DOI 10.1016/j.placenta.2013.04.002. [Google Scholar] [CrossRef]
9. Krzyżanowski, A., Kwiatek, M., Gęca, T., Stupak, A., Kwaśniewska, A. (2019). Modern ultrasonography of the umbilical cord: Prenatal diagnosis of umbilical cord abnormalities and assessement of fetal wellbeing. Medical Science Monitor, 25, 3170–3180. DOI 10.12659/MSM.913762. [Google Scholar] [CrossRef]
10. Salomon, L. J., Alfirevic, Z., Berghella, V., Bilardo, C., Hernandez-Andrade, E. et al. (2011). Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound in Obstetrics & Gynecology, 37(1), 116–126. DOI 10.1002/uog.8831. [Google Scholar] [CrossRef]
11. Ebbing, C., Kessler, J., Moster, D., Rasmussen, S. (2020). Single umbilical artery and risk of congenital malformation: Population-based study in Norway. Ultrasound in Obstetrics & Gynecology, 55(4), 510–515. DOI 10.1002/uog.20359. [Google Scholar] [CrossRef]
12. Yu, H., Cao, L., Ye, J. F., Ren, Y. Y., Zhang, Q. Y. et al. (2022). Isolated single umbilical artery brings adverse impacts on pregnant outcomes. Shanghai Med, 43(6), 369–374. [Google Scholar]
13. Voskamp, B. J., Fleurke-Rozema, H., Oude-Rengerink, K., Snijders, R. J. M., Bilardo, C. M. et al. (2013). Relationship of isolated single umbilical artery to fetal growth, aneuploidy and perinatal mortality: Systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology, 42(6), 622–628. DOI 10.1002/uog.12541. [Google Scholar] [CrossRef]
14. Sato, Y., Benirschke, K. (2006). Umbilical arterial thrombosis with vascular wall necrosis: Clinicopathologic findings of 11 cases. Placenta, 27(6–7), 715–718. DOI 10.1016/j.placenta.2005.05.008. [Google Scholar] [CrossRef]
15. Yang, H., Gao, Y. (2016). A case report of umbilical cord blood embolism. Journal of Practical Obstetrics and Gynecology, 32(6), 476. [Google Scholar]
16. Chen, J., Wei, X., Gao, C. (2017). Clinical analysis of umbilical cord blood embolism in two cases. Chinese Journal of Obstetrics and Gynecology, 52(5), 338–339. [Google Scholar]
17. Wei, J., Li, Q., Zhai, H. (2021). Umbilical artery thrombosis diagnosed at different gestational ages and fetal outcomes: A case series. BMC Pregnancy and Childbirth, 21(1), 1–5. DOI 10.1186/s12884-021-04264-9. [Google Scholar] [CrossRef]
18. Collins, J. C., Muller, R. J., Collins, C. L. (1993). Prenatal observation of umbilical cord abnormalities: A triple knot and torsion of the umbilical cord. American Journal of Obstetrics & Gynecology, 169(1), 102–104. DOI 10.1016/0002-9378(93)90139-A. [Google Scholar] [CrossRef]
19. StrongJr, T. H., Finberg, H. J., Mattox, J. H. (1994). Antepartum diagnosis of noncoiled umbilical cords. American Journal of Obstetrics and Gynecology, 170(5), 1729–1733. DOI 10.1016/S0002-9378(94)70348-5. [Google Scholar] [CrossRef]
20. de Laat, M. W., van Alderen, E. D., Franx, A., Visser, G. H., Bots, M. L. et al. (2007). The umbilical coiling index in complicated pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology, 130(1), 66–72. DOI 10.1016/j.ejogrb.2006.01.018. [Google Scholar] [CrossRef]
21. Malpas, P., Symonds, E. M. (1966). The direction of the helix of the human umbilical cord. Annals of Human Genetics, 29(4), 409–410. DOI 10.1111/j.1469-1809.1966.tb00539.x. [Google Scholar] [CrossRef]
22. Predanic, M., Perni, S. C., Chasen, S. T., Baergen, R. N., Chervenak, F. A. (2005). Assessment of umbilical cord coiling during the routine fetal sonographic anatomic survey in the second trimester. Journal of Ultrasound in Medicine, 24(2), 185–191. DOI 10.7863/jum.2005.24.2.185. [Google Scholar] [CrossRef]
23. Tang, L., Lin, Y., Zhu, Y. (2005). Two-dimensional and color doppler ultrasound analysis on diagnosis of fetal umbilical cord torsion. Chinese Journal of Ultrasound in Medicine, 21(8), 615–618. [Google Scholar]
24. Liu, Q., Fu, Q., Fu, S. (1999). Color doppler ultrasound diagnosis of umbilical cord torsion. Chinese Journal of Obsteics and Gynecology, 34(7), 431. [Google Scholar]
25. Li, S., Liao, Y., Luo, G. (2019). Evaluation of umbilical spiral structure in prenatal ultrasound based on evidence-based medicine. Chinese Journal of Obsteics and Gynecology, 54(2), 126–130. [Google Scholar]
26. Raio, L., Ghezzi, F., di Naro, E., Gomez, R., Franchi, M. et al. (1999). Sonographic measurement of the umbilical cord and fetal anthropometric parameters. European Journal of Obstetrics & Gynecology and Reproductive Biology, 83(2), 131–135. DOI 10.1016/S0301-2115(98)00314-5. [Google Scholar] [CrossRef]
27. Khong, T. Y., Mooney, E. E., Ariel, I., Balmus, N. C., Boyd, T. K. et al. (2016). Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Archives of Pathology & Laboratory Medicine, 140(7), 698–713. DOI 10.5858/arpa.2015-0225-CC. [Google Scholar] [CrossRef]
28. Cromi, A., Ghezzi, F., di Naro, E., Siesto, G., Bergamini, V. et al. (2007). Large cross-sectional area of the umbilical cord as a predictor of fetal macrosomia. Ultrasound in Obstetrics and Gynecology, 30(6), 861–866. DOI 10.1002/(ISSN)1469-0705. [Google Scholar] [CrossRef]
29. Ghezzi, F., Raio, L., di Naro, E., Franchi, M., Buttarelli, M. et al. (2002). First-trimester umbilical cord diameter: A novel marker of fetal aneuploidy. Ultrasound in Obstetrics and Gynecology, 19(3), 235–239. DOI 10.1046/j.1469-0705.2002.00650.x. [Google Scholar] [CrossRef]
30. Ghezzi, F., Raio, L., di Naro, E., Franchi, M., Brühwiler, H. et al. (2001). First-trimester sonographic umbilical cord diameter and the growth of the human embryo. Ultrasound in Obstetrics and Gynecology, 18(4), 348–351. DOI 10.1046/j.0960-7692.2001.00507.x. [Google Scholar] [CrossRef]
31. Raio, L., Ghezzi, F., di Naro, E., Franchi, M., Maymon, E. et al. (1999). Prenatal diagnosis of a lean umbilical cord: A simple marker for the fetus at risk of being small for gestational age at birth. Ultrasound in Obstetrics and Gynecology, 13(3), 176–180. DOI 10.1046/j.1469-0705.1999.13030176.x. [Google Scholar] [CrossRef]
32. Jakó, M., Surányi, A., Kaizer, L., Németh, G., Bártfai, G. (2019). Maternal hematological parameters and placental and umbilical cord histopathology in intrauterine growth restriction. Medical Principles and Practice, 28(2), 101–108. DOI 10.1159/000497240. [Google Scholar] [CrossRef]
33. Byers, B. D., Goharkhay, N., Mateus, J., Ward, K. K., Munn, M. B. et al. (2009). Pregnancy outcome after ultrasound diagnosis of fetal intra-abdominal umbilical vein varix. Ultrasound in Obstetrics and Gynecology, 33(3), 282–286. DOI 10.1002/uog.6233. [Google Scholar] [CrossRef]
34. Io, S., Kondoh, E., Iemura, Y., Minamiguchi, S., Chigusa, Y. et al. (2021). Severe fetal anemia as a consequence of extra-abdominal umbilical vein varix: A case report and review of the literature. Congenital Anomalies, 61(1), 4–8. DOI 10.1111/cga.12397. [Google Scholar] [CrossRef]
35. Gowda, S., Chakkalakkoombil, S. V., Bharathi, S., Barathi, D. (2019). Large fetal intra-abdominal umbilical vein varix: Antenatal sonographic diagnosis and follow-up. Journal of Obstetrics and Gynaecology Research, 45(9), 1936–1940. DOI 10.1111/jog.14045. [Google Scholar] [CrossRef]
36. Weissmann-Brenner, A., Simchen, M. J., Moran, O., Kassif, E., Achiron, R. et al. (2009). Isolated fetal umbilical vein varix—Prenatal sonographic diagnosis and suggested management. Prenatal Diagnosis, 29(3), 229–233. DOI 10.1002/pd.2219. [Google Scholar] [CrossRef]
37. Chew, M., Teoh, P. Y., Wong, Y. P., Tan, G. C. (2019). Multiple umbilical cord strictures in a case of intrauterine foetal demise. The Malaysian Journal of Pathology, 41(3), 365–368. [Google Scholar]
38. French, A. E., Gregg, V. H., Newberry, Y., Parsons, T. (2005). Umbilical cord stricture: A cause of recurrent fetal death. Obstetrics & Gynecology, 105(5), 1235–1239. DOI 10.1097/01.AOG.0000159041.55845.f7. [Google Scholar] [CrossRef]
39. Ling, S. Y., Hwang, J. L., Huang, L. W. (2006). Umbilical cord stricture causing intrauterine fetal death in a 22-week fetus. Taiwanese Journal of Obstetrics and Gynecology, 45(1), 73–75. DOI 10.1016/S1028-4559(09)60197-2. [Google Scholar] [CrossRef]
40. Ismail, K. I., Hannigan, A., O’Donoghue, K., Cotter, A. (2017). Abnormal placental cord insertion and adverse pregnancy outcomes: A systematic review and meta-analysis. Systematic Reviews, 6(1), 1–11. DOI 10.1186/s13643-017-0641-1. [Google Scholar] [CrossRef]
41. Ebbing, C., Kiserud, T., Johnsen, S. L., Albrechtsen, S., Rasmussen, S. (2013). Prevalence, risk factors and outcomes of velamentous and marginal cord insertions: A population-based study of 634,741 pregnancies. PLoS One, 8(7), e70380. DOI 10.1371/journal.pone.0070380. [Google Scholar] [CrossRef]
42. Ebbing, C., Johnsen, S. L., Albrechtsen, S., Sunde, I. D., Vekseth, C. et al. (2017). Velamentous or marginal cord insertion and the risk of spontaneous preterm birth, prelabor rupture of the membranes, and anomalous cord length, a population-based study. Acta Obstetricia et Gynecologica Scandinavica, 96(1), 78–85. DOI 10.1111/aogs.13035. [Google Scholar] [CrossRef]
43. Benirschke, K., Burton, G. J., Baergen, R. N. (2012). Anatomy and pathology of the umbilical cord. In: Pathology of the human placenta, pp. 309–375, Berlin, Heidelberg: Springer. [Google Scholar]
44. Oyelese, Y., Catanzarite, V., Prefumo, F., Lashley, S., Schachter, M. et al. (2004). Vasa previa: The impact of prenatal diagnosis on outcomes. Obstetrics & Gynecology, 103(5), 937–942. DOI 10.1097/01.AOG.0000123245.48645.98. [Google Scholar] [CrossRef]
45. Kosian, P., Henrich, W., Entezami, M., Weichert, A. (2020). Furcate insertion of the umbilical cord: Pathological and clinical characteristics in 132 cases. Journal of Perinatal Medicine, 48(8), 819–824. DOI 10.1515/jpm-2019-0459. [Google Scholar] [CrossRef]
46. Collins, J. H. (2021). The case for umbilical cord screening via ultrasound at 18–20 weeks. Medical Research Archives, 9(10). DOI 10.18103/mra.v9i10.2565. [Google Scholar] [CrossRef]
47. Chen, F., Wang, L. (2017). Correlation of impression depth of umbilical cord around neck with blood flow indexes of umbilical artery and fetus middle cerebral artery. Chinese Journal of Medical Imaging Technology, 33(9), 1371–1375. [Google Scholar]
48. Bian, X., Xu, Y., Yang, J. (1990). Clinical analysis of neonatal asphyxia. Chinese Journal of Obstetrics and Gynecology, 25(4), 235–236. [Google Scholar]
49. Hasegawa, J., Sekizawa, A., Ikeda, T., Koresawa, M., Ishiwata, I. et al. (2016). Clinical risk factors for poor neonatal outcomes in umbilical cord prolapse. The Journal of Maternal-Fetal & Neonatal Medicine, 29(10), 1652–1656. DOI 10.3109/14767058.2015.1058772. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2022 The Author(s). Published by Tech Science Press.
Copyright © 2022 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools