 Open Access
Open Access
ARTICLE
Dual ligand-targeted Pluronic P123 polymeric micelles enhance the therapeutic effect of breast cancer with bone metastases
1 Jiaxing Key Laboratory for Photonanomedicine and Experimental Therapeutics, Department of Pharmaceutics, College of Medicine, Jiaxing University, Jiaxing, 314001, China
2 Department of Pharmacy, The General Hospital of Xinjiang Military Region, Urumqi, 830000, China
3 Department of Pathology and Medical Biology, University Medical Center Groningen, University of Groningen, Groningen, 9713 GZ, The Netherlands
4 Department of Pathology, Laboratory of Experimental Oncology, Erasmus MC, Rotterdam, 3015 GD, The Netherlands
5 Department of Pharmacy, Jiaxing Maternal and Child Health Care Hospital, Affiliated Hospital of Jiaxing University, Jiaxing, 314001, China
6 Qinghai Enlu Biotechnology Co., Ltd., Haidong, 810700, China
* Corresponding Authors: BAOYUE DING. Email: ; DONGFENG YIN. Email:
(This article belongs to the Special Issue: Cancer Metastasis)
Oncology Research 2024, 32(4), 769-784. https://doi.org/10.32604/or.2023.044276
Received 26 July 2023; Accepted 13 October 2023; Issue published 20 March 2024
Abstract
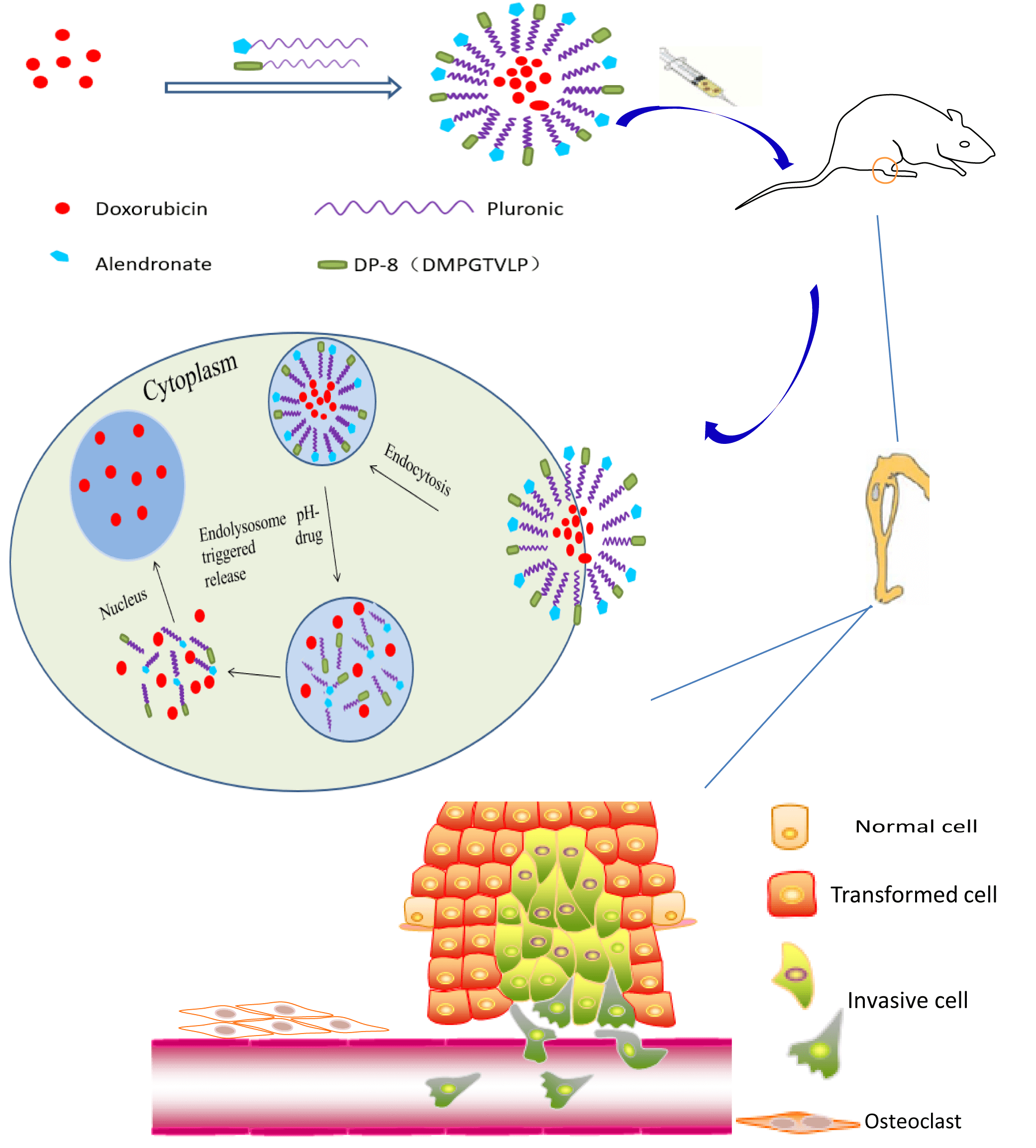
Bone metastasis secondary to breast cancer negatively impacts patient quality of life and survival. The treatment of bone metastases is challenging since many anticancer drugs are not effectively delivered to the bone to exert a therapeutic effect. To improve the treatment efficacy, we developed Pluronic P123 (P123)-based polymeric micelles dually decorated with alendronate (ALN) and cancer-specific phage protein DMPGTVLP (DP-8) for targeted drug delivery to breast cancer bone metastases. Doxorubicin (DOX) was selected as the anticancer drug and was encapsulated into the hydrophobic core of the micelles with a high drug loading capacity (3.44%). The DOX-loaded polymeric micelles were spherical, 123 nm in diameter on average, and exhibited a narrow size distribution. The in vitro experiments demonstrated that a pH decrease from 7.4 to 5.0 markedly accelerated DOX release. The micelles were well internalized by cultured breast cancer cells and the cell death rate of micelle-treated breast cancer cells was increased compared to that of free DOX-treated cells. Rapid binding of the micelles to hydroxyapatite (HA) microparticles indicated their high affinity for bone. P123-ALN/DP-8@DOX inhibited tumor growth and reduced bone resorption in a 3D cancer bone metastasis model. In vivo experiments using a breast cancer bone metastasis nude model demonstrated increased accumulation of the micelles in the tumor region and considerable antitumor activity with no organ-specific histological damage and minimal systemic toxicity. In conclusion, our study provided strong evidence that these pH-sensitive dual ligand-targeted polymeric micelles may be a successful treatment strategy for breast cancer bone metastasis.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools