 Open Access
Open Access
ARTICLE
Investigation on the Anti-Cancer Effects of HER2-Targeted CAR-T Cells Engineered Using the PiggyBac Transposon System
1 Central Laboratory, Yan’an Hospital Affiliated to Kunming Medical University, Kunming, 650051, China
2 Key Laboratory of Tumor Immunological Prevention and Treatment of Yunnan Province, Yan’an Hospital Affiliated to Kunming Medical University, Kunming, 650051, China
3 Graduate School, Kunming Medical University, Kunming, 650050, China
4 Yunnan Cell Biology and Clinical Translation Research Center, Yan’an Hospital Affiliated to Kunming Medical University, Kunming, 650051, China
5 School of Life Science and Technology, ShanghaiTech University, Shanghai, 201210, China
* Corresponding Authors: Li-Wei Liao. Email: ; Lin Li. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Novel Biomarkers and Treatment Strategies in Solid Tumor Diagnosis, Progression, and Prognosis)
Oncology Research 2025, 33(11), 3447-3467. https://doi.org/10.32604/or.2025.065394
Received 11 March 2025; Accepted 11 August 2025; Issue published 22 October 2025
Abstract
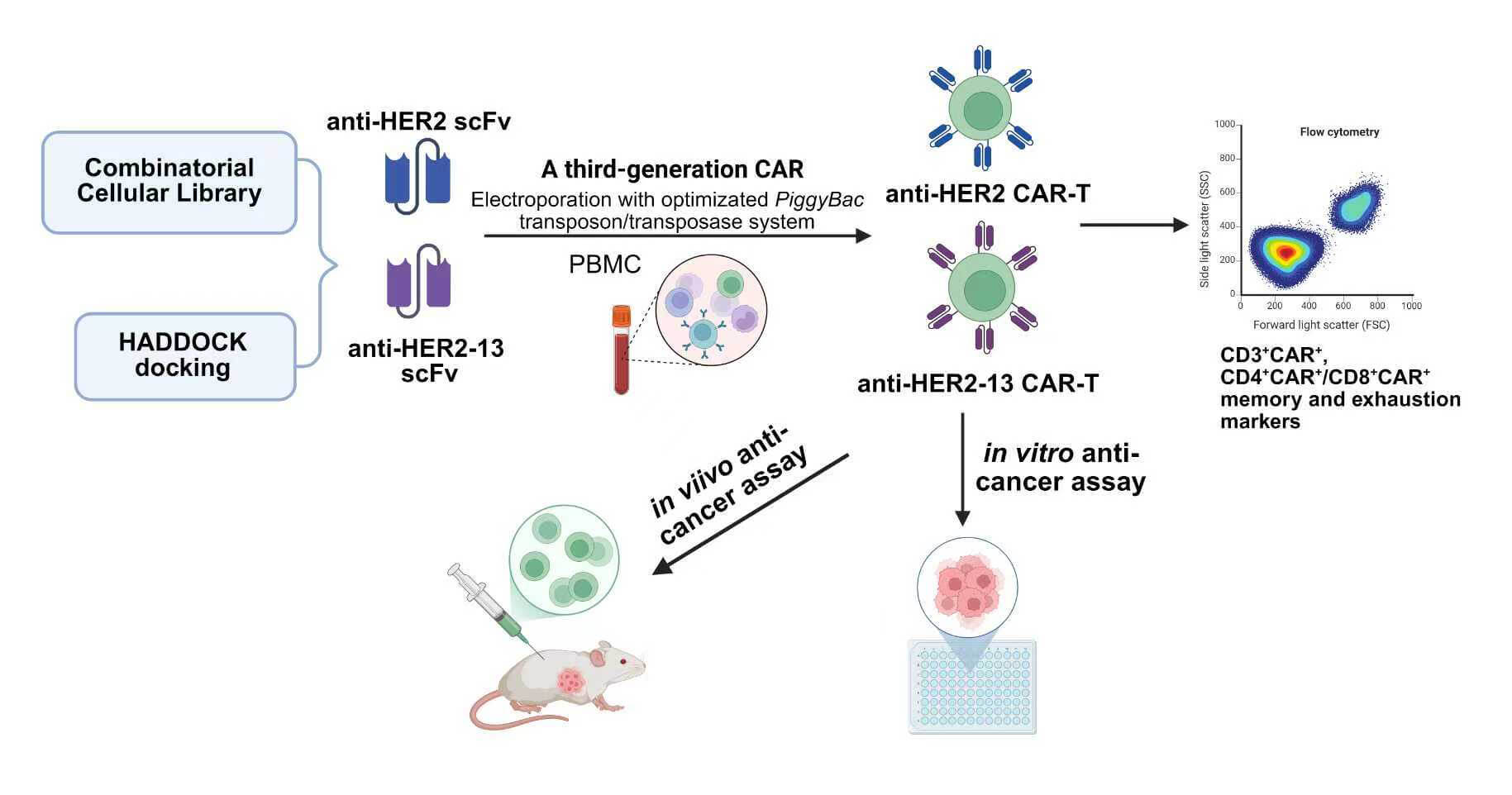
Background: Chimeric antigen receptor T (CAR-T) cell therapies have demonstrated significant clinical efficacy in hematological malignancies. However, their application to solid tumors remains substantially limited by multiple challenges, including the risk of off-target effects. Hence, optimizing CAR-T cells for stronger antigen binding is essential. Methods: In this study, we employed a classical anti-human endothelial growth factor receptor 2 (HER2) single-chain variable fragment (scFv) derived from trastuzumab, alongside an anti-HER2-13 scFv identified from a combinatorial cellular CAR library, for the construction of a third-generation CAR-T cell. Meanwhile, the phenotypes and both in vitro and in vivo functions of CAR-T cells transduced with the two scFvs via PiggyBac transposon-mediated gene transfer were compared. Results: The optimal ratio between the PiggyBac HER2-CAR-puro transposon and the Super PiggyBac transposase plasmid differed during the construction of the two HER2-targeted CAR-T cell types. The expansion abilities, CD3+CAR+ population, CD4+CAR+/CD8+CAR+ proportions, and memory and exhaustion markers between the two CAR-T groups were similar after using the optimized proportion of plasmid. Both CAR-T cell types exhibited significant antitumor activity, with the anti-HER2-13 CAR-T cells demonstrating superior target specificity. Therapeutic effects were observed with both CAR-T cells and trastuzumab in the MDA-MB-231HER2+ breast tumor xenograft model, with anti-HER2-13 CAR-T cells demonstrating slightly enhanced efficacy and no evident off-target toxicity. Conclusion: These results highlight the potential of anti-HER2-13 CAR-T cells to serve as a safer and more efficacious alternative in HER2-targeted therapy.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools