 Open Access
Open Access
ARTICLE
Diverse PD-1, CD163, and FOXP3 Profiles in Primary and Metastatic Microenvironments of Prostate Cancer
1 UroScience, State University of Campinas, Unicamp, Campinas, 13083-875, Brazil
2 Medicine School of Ribeirão Preto, University of São Paulo, Ribeirao Preto, 14049-900, Brazil
3 ImmunOncology, Pontifical Catholic University of Campinas, PUC-Campinas, Campinas, 13034-685, Brazil
4 INCT UroGen, National Institute of Science, Technology and Innovation in Genitourinary Cancer, Campinas, 13083-970, Brazil
5 Pathology Department, Pontifical Catholic University of Campinas, PUC-Campinas, Campinas, 13034-685, Brazil
6 Pathology Department, State University of Campinas, Unicamp, Campinas, 13083-888, Brazil
* Corresponding Author: Leonardo O. Reis. Email:
Oncology Research 2025, 33(11), 3417-3428. https://doi.org/10.32604/or.2025.068023
Received 19 May 2025; Accepted 27 August 2025; Issue published 22 October 2025
Abstract
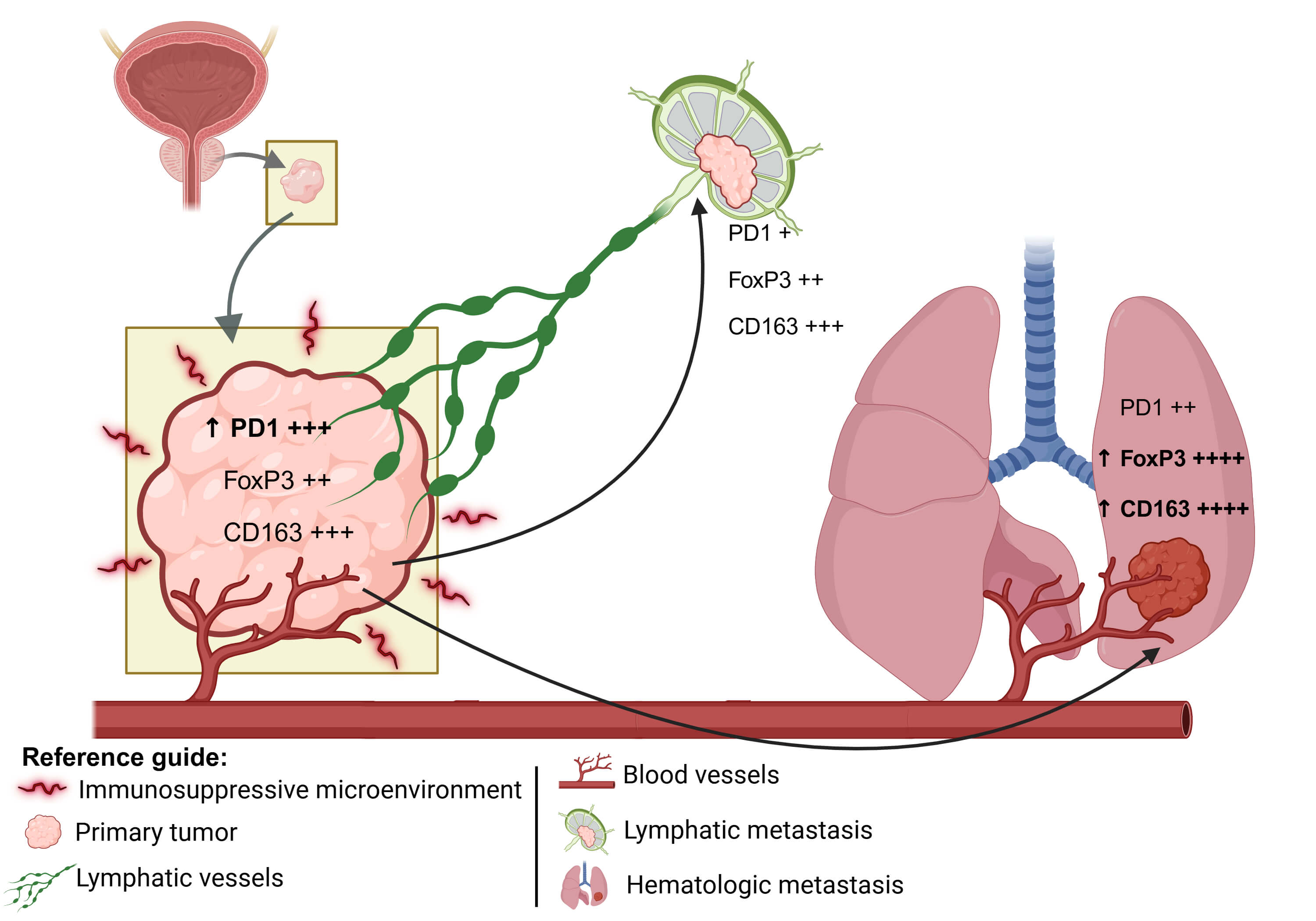
Objective: The tumor microenvironment plays a pivotal role in prostate cancer progression and may differ across metastatic sites. This study aimed to evaluate and compare the primary and metastatic prostate adenocarcinoma tumor microenvironment. Methods: A total of 27 formalin-fixed paraffin-embedded tissue samples derived from 17 patients diagnosed with prostate adenocarcinoma, including the primary tumors, and the corresponding metastatic lymphatic and hematogenous lesions from various anatomical sites. Immunohistochemical labeling was performed using antibodies against Cluster of Differentiation 3 epsilon chain (CD3e), CD8 alpha chain (CD8a), Cluster of Differentiation 68 (CD68), Cluster of Differentiation 163 (CD163), Forkhead box P3 (FOXP3), Cytotoxic T-Lymphocyte–Associated protein 4 (CTLA-4), B7 homolog 3 (B7-H3), Programmed cell death protein 1 (PD-1), and Marker of proliferation Ki-67 (Ki-67). Comparisons were made between primary and metastatic tumors to assess differences in immune cell infiltration, checkpoint expression, and proliferative indices. Results: Samples were classified into three groups: Primary Tumor n = 12, Lymphatic Metastasis n = 7, and Hematogenous Metastasis n = 10. FOXP3 (p = 0.0017) and CD163 (p = 0.0316) expression levels were significantly higher in the Hematogenous Metastasis compared to both the Primary Tumor and Lymphatic Metastasis. PD-1 showed a clear trend (p = 0.0577) toward higher levels in the Primary Tumor compared to both the Hematogenous Metastasis and Lymphatic Metastasis groups, suggesting distinct immunological landscapes depending on tumor location and progression. Conclusion: Diverse PD-1, CD163, and FOXP3 profiles were observed in primary and metastatic microenvironments of prostate cancer. These findings may contribute to the development of personalized therapeutic strategies and novel prognostic tools beyond conventional histological and TNM staging.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileProstate cancer (PC) is the second most common type of cancer diagnosed in men, following lung cancer [1]. Although only 6% of men with PC present with metastatic disease at diagnosis, approximately 90% of those who die from the disease exhibit metastases at the time of death [2,3]. Beyond complications such as pathological fractures and spinal cord compression, often requiring radiation therapy or orthopedic surgery, metastasis signifies disease progression to an incurable and lethal stage. This not only worsens the prognosis but also reinforces metastasis as one of the greatest concerns in the context of treatment strategies [4].
The current staging protocol (e.g., TNM) is based only on the parameters of the tumor cell (cell-autonomous process) and does not consider that the evolution of cancer reflects complex cellular and molecular interactions between the tumor and the patient’s immune system [5]. The prostate is considered an immunocompetent organ, filled with a small number of scattered leukocytes, mainly stromal and intraepithelial T and B lymphocytes, macrophages, and mast cells. In abnormal conditions, such as benign prostatic hyperplasia, prostatitis, proliferative inflammatory atrophy, and adenocarcinomas, the density and composition of immune infiltrates are frequently altered in comparison to normal tissue. Notably, specific immune cell profiles have been associated with cancer progression and may constitute key components of the anti-tumor immune response [6].
In this context, while primary tumors often develop within an increasingly immunosuppressive tumor microenvironment (TME), metastatic dissemination forces tumor cells to abandon the immunosuppressive “bunker” of the primary TME and colonize distant tissues where immune evasion mechanisms and stromal support are not yet established. In these nascent sites, disseminated cells are transiently vulnerable to immune surveillance, creating a potential window for therapeutic intervention [7].
Therefore, identifying precise prognostic markers that predict metastatic potential is critical to refine treatment choices, particularly for focal therapies, and enhance patient outcomes. Mapping the immune landscape across according to the International Society of Urological Pathology (ISUP) grades and between primary and metastatic lesions could provide prognostic insights that complement conventional histological grading and TNM staging [8].
2.1 Patient Samples and Ethical Approval
We used archival tissue samples (n = 28) obtained from 17 patients diagnosed with prostate adenocarcinoma after informed consent, and ethics committee approval of Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto of the Universidade de São Paulo (HCFMRP/USP), CAAE: 85464824.7.0000.5440. Primary tumors were defined as the original neoplastic tissue arising in the prostate gland, including infiltrative tumor extensions into adjacent organs such as the seminal vesicle, rectum, and bladder. Samples from these sites were considered part of the primary tumor group only if no evidence of metastatic dissemination was present. Metastatic lesions were classified separately as lymphatic or hematogenous metastases.
2.2 Definition and Classification of Tumor Samples
Patients who had previously undergone radiotherapy were excluded from the study. All samples were collected during surgical procedures, and none of the patients had received chemotherapy at the time of collection. However, detailed data on other treatments were limited, and paired pre- and post-treatment samples were not available. These factors were considered during data interpretation. Importantly, all patients were at advanced stages of prostate cancer and receiving androgen deprivation therapy (ADT) at the time of sampling, consistent with the standard of care. Thus, the immune profiles observed likely reflect the effects of castration-level hormonal suppression.
2.3 Histopathological Evaluation
Hematoxylin and eosin-stained slides of primary tumors and metastases were reviewed and graded according to the International Society of Urological Pathology (ISUP) consensus system, which refines Gleason scoring into five prognostic grade groups (ISUP grades 1–5) to enhance prognostic stratification [9].
2.4 Tissue Processing and Immunohistochemistry
Paraffin-embedded tissue blocks were sectioned on a Biocut 1130 rotary microtome (Reichert-Jung, Munich, Germany). After deparaffinization, slides underwent our overnight immunohistochemistry protocol. Endogenous peroxidase was quenched with 10-volume hydrogen peroxide for 15 minutes, and antigen retrieval was carried out in either 0.01 mol/L citrate buffer (pH 6) or Tris-EDTA (pH 8.9), per antibody requirements (Supplementary Table S1).
To block nonspecific binding, sections were incubated in Molico® skim milk, dried, and outlined with a hydrophobic barrier. Primary antibodies targeting proliferation (Ki-67), T cells (CD3e, CD8a, FOXP3), macrophage polarization (CD68, CD163), and immune checkpoints (PD-1, CTLA-4, B7-H3) were diluted in 1% BSA and applied overnight at 4°C, then incubated for 1 hour at 37°C. The catalog number, dilution ratio, and manufacturer’s information for all antibodies are provided in Supplementary Table S1.
After washing, slides were incubated with the appropriate anti-mouse or anti-rabbit IgG secondary antibody (1:200 in 1% BSA) for 90 minutes at 37°C, as detailed in Supplementary Table S1. Finally, slides were counterstained with hematoxylin, mounted using Entelan® resin (Merck, Darmstadt, Germany), and coverslipped.
2.5 Image Acquisition and Scoring
Imaging was performed on a Carl Zeiss Axio-Imager® A1 microscope (Carl Zeiss, Oberkochen, Germany) using AxioVision® software (version 4.9.1). For each slide, five randomly selected high-power fields (40× magnification) were captured under consistent illumination settings. Staining intensity was scored semi-quantitatively on a four-point scale: 1 = negative staining, 2 = weakly positive, 3 = positive, and 4 = strongly positive. For each case, the modal value (i.e., the most frequently observed score across the five fields) was recorded as the representative score for that sample.
2.6 Data Aggregation and Quantitative Analysis
To evaluate group-level trends, median values of individual sample scores were calculated per immunomarker and stratified by tissue origin: Primary Tumor, Lymphatic Metastasis, or Hematogenous Metastasis. Although inspired by the traditional H-score method, this approach does not apply proportional weighting and should therefore be considered a modified semi-quantitative H-score.
Statistical analyses were performed using the SAS System for Windows, version 9.4 (SAS Institute Inc., Cary, NC, USA), and R software (R Foundation for Statistical Computing, Vienna, Austria; version 4.3.1, https://www.R-project.org/). Descriptive measures were calculated to compare groups, including mean, standard deviation, minimum, maximum, and median values. Statistical comparisons were conducted using the Kruskal–Wallis test to assess differences in immunomarker expression across the three anatomical groups, followed by Dunn’s test for multiple comparisons when necessary. The significance level adopted for the study was 5% (p < 0.05) for all statistical analyses.
We obtained a total of 27 formalin-fixed paraffin-embedded tissue samples derived from 17 patients diagnosed with prostate adenocarcinoma. These samples included the primary tumors and the corresponding metastatic lymphatic and hematogenous lesions from various anatomical sites. Fig. 1 shows representative slides of H&E staining photographed at 40× and 100× magnification, highlighting areas of tumor and adjacent tissue. Sample origin and classification details are provided in Supplementary Tables S2 and S3.
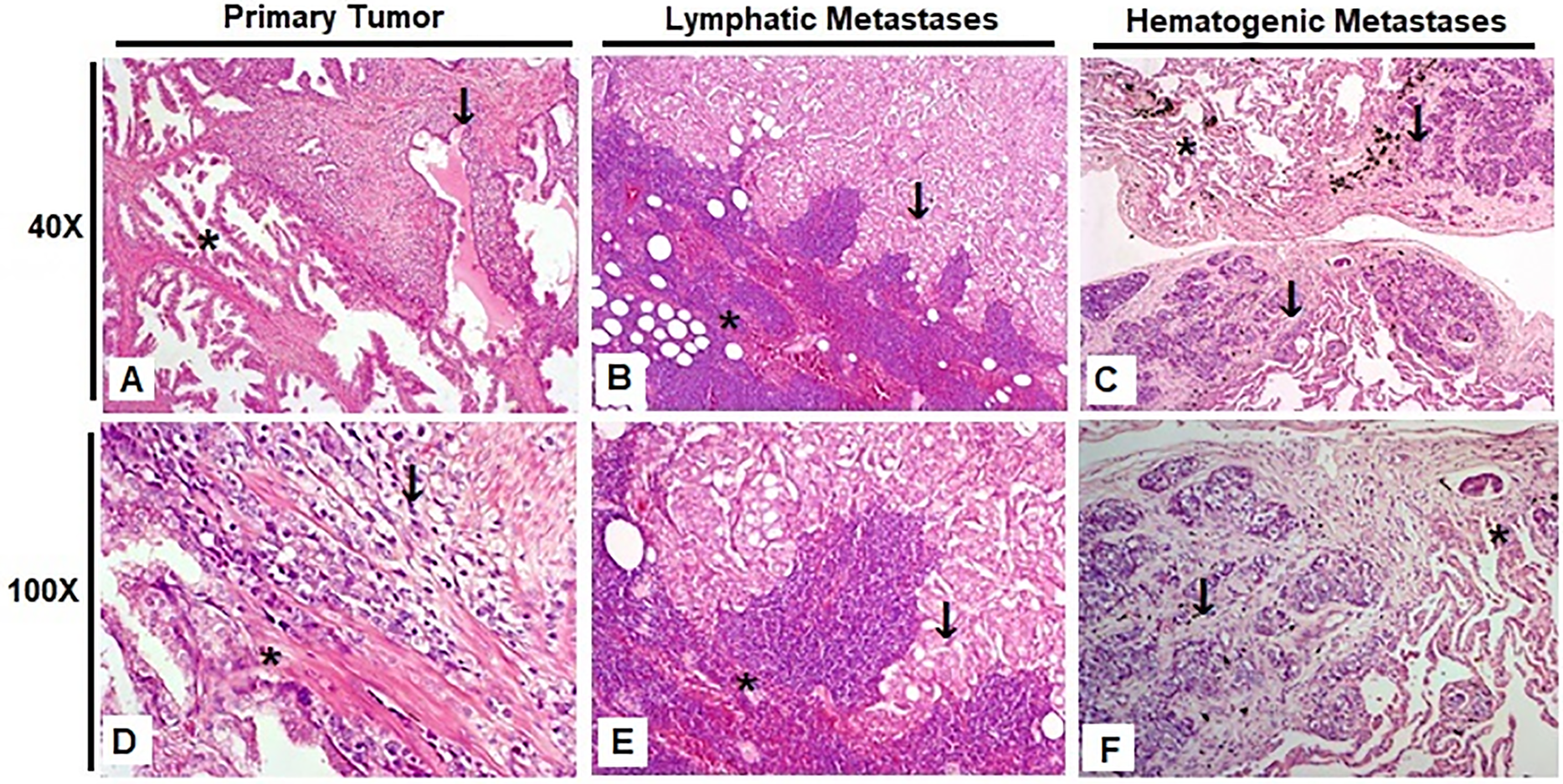
Figure 1: Representative hematoxylin and eosin (H&E) stained slides from the three experimental groups, photographed at 40× and 100× magnification. Scale bars: 200 µm (40×) and 50 µm (100×). (A,D) Prostate; (B,E) Obturated iliac lymph node; (C,F) Lung. Tumor regions are indicated by arrows, and adjacent non-neoplastic tissue is marked with asterisks
The samples were categorized into three distinct groups based on their origin and metastatic status. The first group, Primary Tumor, consisted of 12 samples and included tissues other than the prostate itself, such as the seminal vesicle, rectum, and bladder, which were infiltrated by the primary tumor. The second group, Lymphatic Metastasis, comprised 7 samples representing prostate cancer that had spread to lymph nodes. Lastly, the third group, Hematogenous Metastasis, included 10 samples of prostate cancer metastases found in distant sites such as bone, lung, and bone marrow, indicating dissemination through the bloodstream.
The distribution of marker expression across groups is summarized in Supplementary Table S4. FOXP3 (p = 0.0017) and CD163 (p = 0.0316) expression levels were significantly higher in the Hematogenous Metastasis compared to both the Primary Tumor and Lymphatic Metastasis. PD-1 showed a clear trend (p = 0.0577) toward higher levels in the Primary Tumor compared to both the Hematogenous Metastasis and Lymphatic Metastasis groups, Fig. 2.
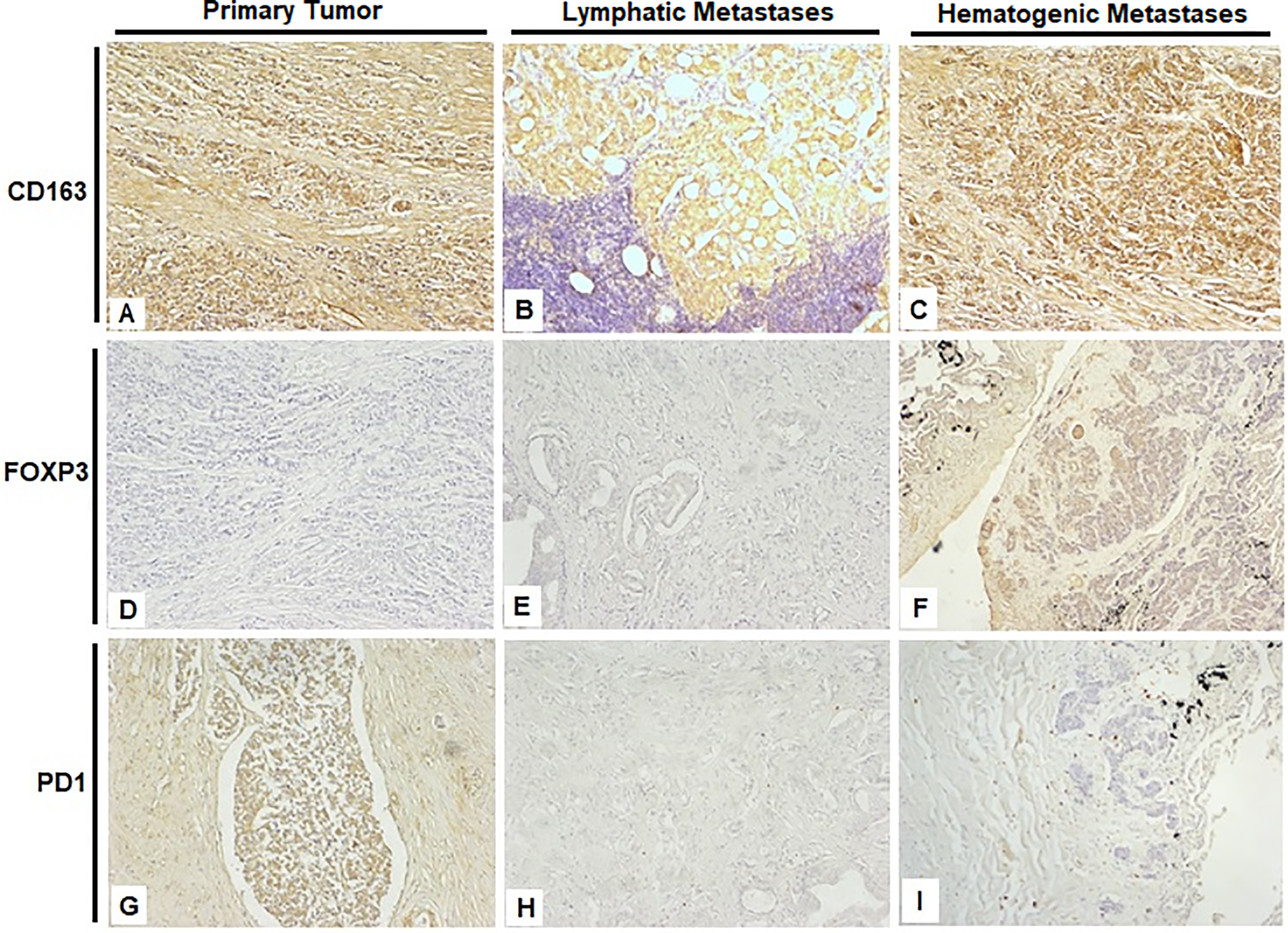
Figure 2: Representative immunohistochemical staining images from the three experimental groups, photographed at 40× magnification. (A,D,G) Prostate; (B,E,H) Obturated iliac lymph node; (C,F,I) Lung. Scale bar: 200 µm
Supplementary Figure S1 demonstrates the distribution and localization of CD3e, CD8a, CD68, CTLA-4, B7-H3, and Ki-67 across different tissues in the three experimental groups. Complete descriptive statistics and p-values are presented in Supplementary Table S5.
The distribution of positive immune cells was generally sparse, with no predominant spatial pattern observed in relation to either the tumor or peritumoral compartments. There was no consistent clustering or exclusion of immune cell subsets such as PD-1+ cells, FOXP3+ Tregs, or CD163+ macrophages.
For the subset of primary tumor samples, we also investigated whether immunomarker expression varied according to the histological ISUP grading system; no statistically significant differences were found across ISUP grades 1 to 5 (data not shown).
Our study demonstrates distinct immune profiles across primary tumors, lymphatic metastases, and hematogenous metastases in prostate cancer. Of the nine markers evaluated, FoxP3 and CD163 exhibited a significant increase in hematogenic metastasis compared to primary tumor and lymphatic metastasis, while PD-1 showed a clear trend to increase in the primary tumor compared to lymphatic and hematogenic metastasis.
FOXP3, a hallmark of regulatory T cells (Tregs), was elevated in both primary tumors and, most prominently, in hematogenous metastases, reflecting enhanced local immunosuppression and potentially worse prognosis. Tregs in the tumor microenvironment can generate an anti-tumor immune suppression response, favoring cancer progression [10].
CD163, indicative of M2-polarized macrophages, was also upregulated and correlates with tumor progression and metastasis; in bladder cancer, high CD163 expression predicts worse survival [11]. Notably, M2 macrophage infiltration has been linked to angiogenesis, suggesting a direct role in facilitating metastasis [12].
PD-1 is an immune checkpoint receptor expressed on activated T cells that plays a key role in downregulating anti-tumor immune responses. Its increased expression in lymphatic metastases suggests a more inflamed yet functionally exhausted microenvironment, potentially contributing to resistance to immunotherapy [13,14].
The higher expression of FOXP3 and CD163, specifically in the hematologic metastasis, suggests a more immunosuppressive tumor microenvironment at this stage of the disease, possibly reflecting greater infiltration of Tregs and M2-type macrophages, respectively. This observation is consistent with previous studies indicating the role of these cells in immune system escape and tumor progression in prostate cancer [15,16].
PD-1 expression, although with p slightly above the significance threshold, showed a pattern of variation between all groups, which may reflect the progressive involvement of T-cell exhaustion mechanisms during tumor evolution [17]. These observations are summarized in a conceptual model (Fig. 3), which illustrates the evolution of the tumor immune microenvironment from primary tumors to lymphatic and hematogenous metastases.
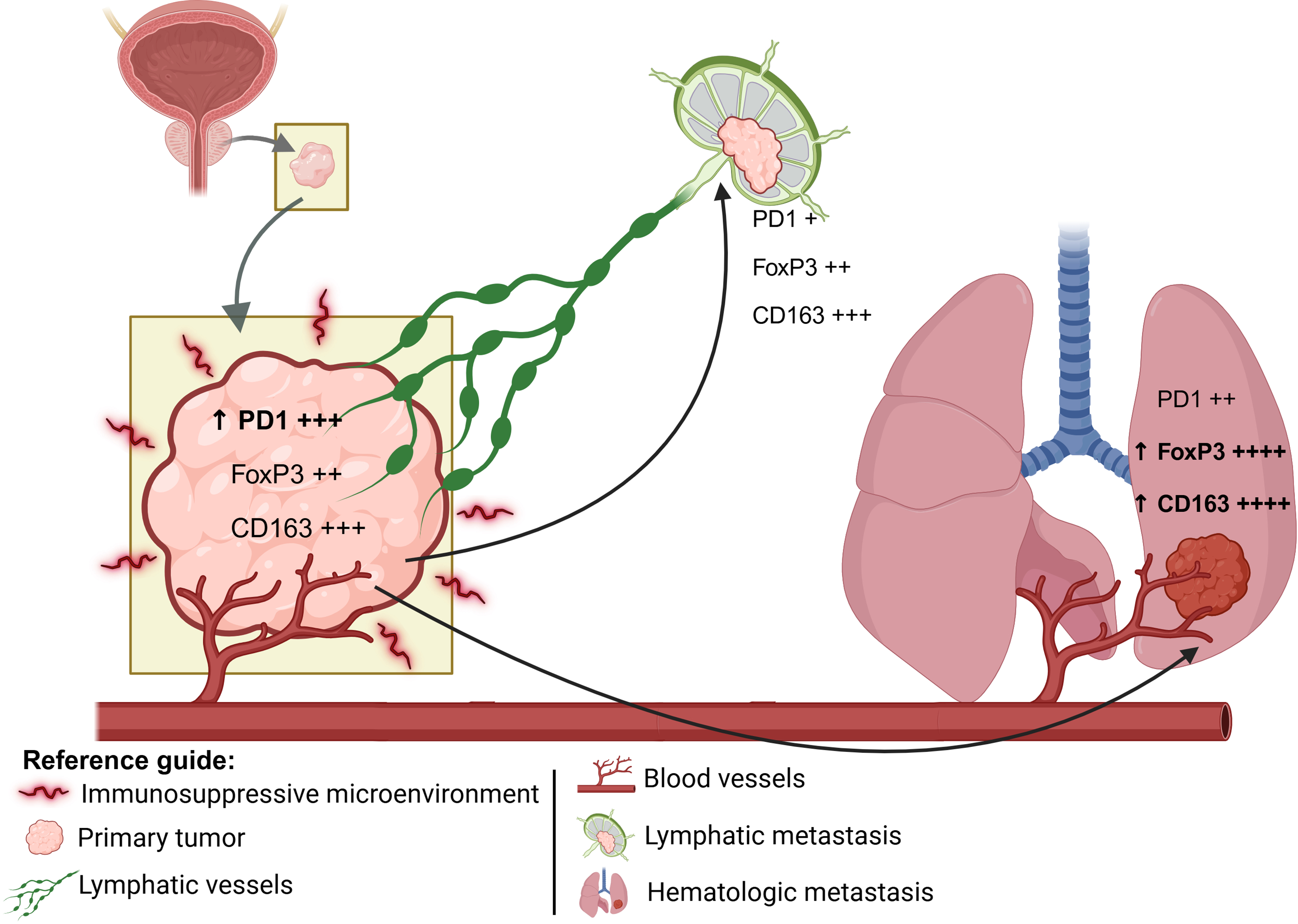
Figure 3: Schematic representation of the spatial progression of metastasis: the primary tumor represents an immunosuppressive environment; the lymphatic metastasis occurs closer to the primary site; and the hematogenous metastasis typically reaches more distant organs. The expression levels of PD1, FoxP3, and CD163 are represented semi-quantitatively, where “+” indicates low expression, “++” moderate expression, “+++” high expression, and “++++” very high expression. The upward arrow (↑) denotes a relative increase in expression compared to the previous metastatic site. Illustration created with BioRender.com
Interestingly, our findings also align with broader evidence suggesting that metastatic tumors, in general, exhibit fewer tumor-infiltrating lymphocytes (TIL) than primary tumors, creating a more immunologically inert milieu and potentially reducing the efficacy of checkpoint inhibitors in advanced stages [18,19]. Although some reports find comparable checkpoint expressions between primary and metastatic sites [18], others highlight significant heterogeneity, such as variable TIM-3 and PD-1 levels depending on tumor type and metastatic location [20]. In prostate cancer, primary tumors typically exhibit higher immune-checkpoint scores than early metastases, reflecting a more established immunosuppressive niche [21].
Supplementary Table S6 describes the potential roles and mechanisms of the selected immunomarkers in prostate cancer. The lack of significant differences in Ki-67, B7-H3, CD8a, CD68, CD3e, and CTLA-4 implies a more uniform presence of proliferative and cytotoxic populations, or perhaps their roles are less tightly linked to metastatic progression in our cohort. For example, Ki-67 has been described as a predictor of metastasis and mortality in treated prostate cancer [22], while B7-H3 expression correlates with disease progression [23]. The infiltration of CD8+ lymphocytes also serves as an independent prognostic factor [24], and immune cell infiltration in androgen-deprived prostate cancer has been characterized in detail [25]. Moreover, recent advances in T-cell engager therapy have opened new frontiers in prostate cancer immunotherapy [26]. These findings reinforce the prognostic potential of FOXP3, CD163, and PD-1 in characterizing the tumor immune microenvironment in metastatic prostate cancer.
Importantly, early metastatic niches appear more vulnerable than established primary tumors. Evidence indicates that the initial microenvironment is substantially distinct from the primary tumor, particularly in its lack of immediate immunosuppressive features—an aspect that may critically affect both tumor dynamics and the effectiveness of treatments across the progression of the disease [27,28].
In line with our findings, the primary tumor microenvironment develops progressively, fostering conditions that support neoplastic growth. In contrast, the initial metastatic sites present a less permissive environment, characterized by a distinct pattern of immunosuppressive cell recruitment [29]. Cancer-associated fibroblasts (CAFs) play a crucial role in the tumor microenvironment by promoting immunosuppression through extracellular matrix remodeling, cytokine secretion, and crosstalk with immune cells. In prostate cancer, CAFs support tumor progression and modulate the microenvironment partly via androgen receptor signaling, highlighting their importance as key stromal regulators contributing to tumor growth and therapy resistance [30].
Evidence in prostate cancer shows increased Treg infiltration in bone marrow metastases, promoting immune evasion [31], whereas primary tumors display elevated PD-1 on T cells, indicative of immune exhaustion [32]. Together, these differences underscore the metastatic microenvironment as both a therapeutic challenge and an opportunity—its nascent immunological state can be exploited by targeted strategies [33]. Moreover, as tumor cells disseminate, they exit the protective “bunker” of the primary niche and face heightened immune surveillance and stress, creating a transient window of vulnerability ripe for early intervention [7].
Heterogeneity in immune profiles between primary tumors and metastatic sites can profoundly affect treatment efficacy and drive resistance in advanced disease [34]. Although the extent of this heterogeneity remains debated [35], its impact on therapy selection is clear. Distinct anatomical sites can engage different immune-escape mechanisms, supporting the need for lesion-specific treatment strategies. Furthermore, primary tumors often exhibit greater immunogenicity than their metastatic counterparts, as reflected by higher scores on immunotherapy-predictive signatures [19].
Although our dataset does not include information on metastatic burden or progression-free survival, previous studies have reported associations between immune marker expression and advanced disease features in prostate cancer. High densities of FOXP3+ regulatory T cells have been linked to increased metastatic potential and poorer clinical outcomes [36,37]. Similarly, elevated infiltration by CD163+ M2 macrophages has been correlated with increased tumor aggressiveness and reduced progression-free survival, particularly in advanced and castration-resistant cases [38]. PD-1 expression on tumor-infiltrating lymphocytes has also been associated with higher Gleason scores and biochemical recurrence, suggesting its role in immune escape and disease progression [21].
In our cohort, we additionally examined whether immunomarker expression varied among primary tumors stratified by ISUP grade (1 to 5), and no statistically significant differences were observed. This absence of variation despite histopathological differences in the context of immunological diversity observed between primary tumor, lymphatic metastasis, and hematogenous metastasis supports the compartment hypothesis, in which the variability in the targeted immune markers appears to be driven more by differences in the tumor compartment than by variations of the primary tumor per se. These findings support the notion of a dynamic remodeling of the immune microenvironment along the metastatic cascade.
Moreover, evidence in advanced prostate cancer suggests that androgen receptor signaling contributes to the recruitment and polarization of immunosuppressive myeloid cells, including CD163+ macrophages, and enhances PD−1/PD-L1 immune checkpoint pathways, thereby reinforcing an immunosuppressive microenvironment that promotes therapeutic resistance in castration-resistant disease [39]. All patients in our cohort were receiving androgen deprivation therapy (ADT) with castration-level hormonal suppression at the time of sample collection, as per standard clinical management. This therapeutic context may have influenced the immune marker expression profiles observed, particularly those related to myeloid cell infiltration and checkpoint molecule upregulation. Notably, emerging evidence has expanded the scope of androgen receptor (AR)-mediated immune modulation beyond prostate cancer to other malignancies such as melanoma. In melanoma, AR activation has been linked to enhanced tumor invasiveness and impairment of critical immune cell functions, ultimately diminishing the efficacy of immune checkpoint therapies [40]. These observations underscore the potential for integrating AR-directed therapy with immune-targeted strategies in future cohort studies analyzing marker expression alongside clinical outcomes such as progression-free survival and metastatic burden.
The current study enhances our understanding of the tumor microenvironment across primary tumors, lymphatic metastases, and hematogenous metastases, suggesting that treatment strategies should be tailored to specific tumor sites due to distinct mechanisms and immune pathways. Although based on a small cohort (n = 17), the inclusion of 27 tumor samples from multiple metastatic sites provided consistent patterns, particularly through paired evaluations of primary and metastatic lesions, and highlights the potential for stage-specific therapeutic approaches.
In this context, it is important to recognize that organ-specific microenvironments can further influence immune cell infiltration at metastatic sites. Each tissue presents a unique set of biochemical cues, stromal architecture, and resident immune cells that modulate the recruitment and function of immune populations such as macrophages, regulatory T cells, and exhausted T lymphocytes [41]. For example, lung tissue often harbors a higher proportion of PD-1-expressing exhausted CD8+ T cells, contributing to localized immunosuppression, while other organs may promote alternative immunosuppressive mechanisms, such as Treg accumulation or M2 macrophage polarization [42].
These tissue-specific differences underscore the need for spatially informed interpretations of immune marker expression. However, due to the limited sample size in our study, we were not able to stratify the immune profiles by individual metastatic site. We acknowledge this limitation and highlight the importance of future prospective studies with larger, site-stratified cohorts to explore these mechanisms more deeply.
Study limitations: An important point to consider is the staging of the patients at the time of collection; many of the patients in our study already had metastatic disease in various tissues at the time the samples were taken, which may reflect different stages of tumor evolution compared to studies using only localized tumors or isolated metastases. Different treatments employed before collection or lack of treatment can also alter the expression of immune markers, such as PD-1, which is known to be induced by chronic tumor inflammation and chemotherapy [43]. The diversity of the metastatic sites analyzed (lymph nodes, bone marrow, lung, bladder) also imposes an additional degree of variability, as the local immune response can affect the infiltration and action of immune cells in different ways [44].
Another important limitation is the relatively small sample size, which restricted our ability to perform statistically robust subgroup analyses or establish reliable associations between immune marker expression and clinical or pathological features. Thus, the findings should be interpreted as descriptive and exploratory. It is important to highlight that, while the relatively small sample size represents a limitation, the present study provides exceptionally rare and valuable data by including matched samples of the primary tumor, lymphatic metastases, and hematogenous metastases—biological material that is seldom accessible, as these sites are not routinely biopsied or surgically removed in standard prostate cancer care. Our study’s rare dataset offers a unique opportunity to advance the biological understanding of metastatic progression while holding significant translational potential to inform future diagnostic strategies and guide the development of personalized therapeutic approaches in prostate cancer [45].
Recent studies have demonstrated that thermal ablation therapies, such as cryoablation, can modulate tumor-infiltrating immune cells by increasing CD8+ T cell infiltration and altering the CD4+/CD8+ ratio, potentially enhancing antitumor immunity. These immune changes reveal promising new targets for immunotherapy, suggesting that strategies aimed at boosting CD8+ T cell responses or modulating the CD4+/CD8+ balance could improve therapeutic outcomes [46]. Integrating such approaches with existing treatments may pave the way for innovative combinatorial therapies in cancer management.
Future studies should expand this analysis in larger, multicenter prospective cohorts to allow the comprehensive clinical annotation to further validate and expand upon our observations, as well as integrate spatial transcriptomics, multiplex imaging, and functional assays to validate the phenotypes and interactions suggested by the immunohistochemical data. Regarding the lack of a predominant spatial pattern observed in relation to either the tumor or peritumoral compartments, subsequent research employing double or triple staining methods, including multiplex immunofluorescence, would be valuable to uncover potential cell–cell interactions and their role in shaping immunosuppressive niches. Emerging spatially resolved technologies such as spatial transcriptomics, multiplex immunofluorescence, and spatial proteomics offer unprecedented resolution to map cellular phenotypes and interactions within their native tissue context. These approaches can elucidate the spatial organization of immune cell subsets, identify immunosuppressive niches, and reveal cell–cell communication networks that drive tumor progression and therapy resistance. Incorporating such advanced spatial analyses in future studies will be crucial to deepen our understanding of the immune microenvironment and to develop more precise, site-specific immunotherapeutic strategies in prostate cancer.
Additionally, characterizing the temporal evolution of the immune landscape—from localized to metastatic stages—may reveal critical windows of therapeutic opportunity, especially for immunotherapeutic interventions. Given the complexity and heterogeneity of metastatic progression, future studies with larger, site-stratified cohorts are essential to comprehensively understand the distinct immunosuppressive mechanisms and therapeutic vulnerabilities within different metastatic organ microenvironments. This approach will be pivotal for developing precise, site-specific immunotherapeutic strategies.
The findings of this study emphasize FOXP3, CD163, and PD-1 as key indicators of immune modulation across primary and metastatic prostate cancer. The dynamic shifts in immune landscapes, particularly the heightened immunosuppression in hematogenous metastases, underscore the need to tailor immunotherapeutic strategies by site and stage.
While lymphatic and hematogenous lesions share some features with primary tumors, their distinct immune contexts suggest different mechanisms of immune escape and therapeutic vulnerability. These findings reinforce the compartment hypothesis, in which variability in targeted marker expression is driven primarily by differences in the tumor microenvironment rather than by tissue type per se. Although limited by sample size and heterogeneity, our findings advocate larger, more diverse prospective cohorts to validate these markers and guide the development of precisely targeted interventions for metastatic prostate cancer.
Acknowledgement: The authors thank the institutional support.
Funding Statement: National Council for Scientific and Technological Development, CNPq, Research Productivity, grant numbers: 304747/2018-1; 310135/2022-2, INCT-UROGEN #408576/2024-3 (Leonardo O. Reis) and CAPES 88887.508226/2020-00-code 001, and 88887.974845/2024-00.
Author Contributions: Writing—Original Draft Preparation and Data Curation: Ana Clara Ciglioni Salustiano, Gabriela Barbosa; Visualization and Supervision: Leonardo O. Reis, Rodolfo Borges dos Reis; Writing—Review, Editing, and Funding Acquisition: Leonardo O. Reis; Co-Supervision and Pathological Report: Amílcar Castro de Mattos, Athanase Billis. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting this study’s findings are available within the article or its Supplementary Materials.
Ethics Approval: Approval was obtained from the Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da USP (HCFMRP/USP), institutional review board, CAAE: 85464824.7.0000.5440.
Informed Consent: Informed consent was obtained from the patients. Performed following the institutional ethical guidelines and standards in the 1964 Declaration of Helsinki and its later amendments.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://www.techscience.com/doi/10.32604/or.2025.068023/s1.
References
1. Feng DC, Li DX, Wu RC, Wang J, Xiao YH, Yoo KH, et al. Global burden and cross-country inequalities in urinary tumors from 1990 to 2021 and predicted incidence changes to 2046. Mil Med Res. 2025 Mar 17;12(1):12. doi:10.1186/s40779-025-00599-y. [Google Scholar] [PubMed] [CrossRef]
2. Desai MM, Cacciamani GE, Gill K, Zhang J, Liu L, Abreu A, et al. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw Open. 2022 Mar 1;5(3):e222246. doi:10.1001/jamanetworkopen.2022.2246. [Google Scholar] [PubMed] [CrossRef]
3. Jalalizadeh M, Roesch HRM, Korkes F, Dien-Trinh Q, Reis LO. Prostate cancer temporal and regional trends in Brazil. Oncol Res. 2024 Sep 18;32(10):1565–73. doi:10.32604/or.2024.052179. [Google Scholar] [PubMed] [CrossRef]
4. Reis LO. Old issues and new perspectives on prostate cancer hormonal therapy: the molecular substratum. Med Oncol. 2012 Sep;29(3):1948–55. doi:10.1007/s12032-011-9991-z. [Google Scholar] [PubMed] [CrossRef]
5. Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012 Feb 14;10:1–10. [Google Scholar]
6. Bindea G, Mlecnik B, Fridman WH, Pages F, Galon J. Natural immunity to cancer in humans. Curr Opin Immunol. 2010 Apr;22(2):215–22. doi:10.1016/j.coi.2010.02.006. [Google Scholar] [PubMed] [CrossRef]
7. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells—mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14:155–67. doi:10.1038/nrclinonc.2016.144. [Google Scholar] [PubMed] [CrossRef]
8. Ingels A, Mulders P, Serneels J, Oosterwijk E, Oyen WJG, Mulders PF. T-helper 1 immunoreaction influences survival in muscle-invasive bladder cancer: proof of concept. Ecancermedicalscience. 2014 Oct 14;8:486. doi:10.3332/ecancer.2014.486. [Google Scholar] [PubMed] [CrossRef]
9. Iczkowski KA, Van der Kwast TH, Egevad L, Epstein JI, Humphrey PA, Montironi R, et al. The 2019 International Society of Urological Pathology (ISUP) consensus conference on grading of prostatic carcinoma. Am J Surg Pathol. 2020 Aug;44(8):e87–99. doi:10.1097/pas.0000000000001678. [Google Scholar] [PubMed] [CrossRef]
10. Schreiber TH. The use of FOXP3 as a biomarker and prognostic factor for malignant human tumors. Cancer Epidemiol Biomarkers Prev. 2007 Oct;16(10):1931–4. doi:10.1158/1055-9965.epi-07-0396. [Google Scholar] [PubMed] [CrossRef]
11. Maniecki MB, Hasle H, Friis-Hansen L, Nielsen O, Iversen M, Bendix K, et al. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer. 2012 Nov 15;131(10):2320–31. doi:10.1002/ijc.27506. [Google Scholar] [PubMed] [CrossRef]
12. Lu Y, Zhou Y, Zhu K, Xu W, Zhang L, Zhang J, et al. M2 macrophage-secreted exosomes promote metastasis and increase vascular permeability in hepatocellular carcinoma. Cell Commun Signal. 2023 Oct 2;21(1):299. doi:10.1186/s12964-022-00872-w. [Google Scholar] [PubMed] [CrossRef]
13. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012 Apr 12;12(4):252–64. doi:10.1038/nrc3239. [Google Scholar] [PubMed] [CrossRef]
14. Schneider S, Riethdorf S, Huland H, Krech T, Heinzer H, Pantel K, et al. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis-impact on clinical outcome. Histopathology. 2018 Oct;73(4):573–84. doi:10.1111/his.13646. [Google Scholar] [PubMed] [CrossRef]
15. Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, et al. Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell. 2009 Oct 6;16(4):336–46. doi:10.1016/j.ccr.2009.08.016. [Google Scholar] [PubMed] [CrossRef]
16. Molina OE, Fioravantti P, Telesca D, Monti CR, Reis LO. High infiltration of CD209+ dendritic cells and CD163+ macrophages in the peritumor area of prostate cancer is predictive of late adverse outcomes. Front Immunol. 2023;14:1205266. doi:10.3389/fimmu.2023.1205266. [Google Scholar] [PubMed] [CrossRef]
17. Sharma M, Yang Z, Miyamoto H. Immunohistochemistry of immune checkpoint markers PD-1 and PD-L1 in prostate cancer. Medicine. 2019 Sep;98(38):e17257. doi:10.1097/md.0000000000017257. [Google Scholar] [PubMed] [CrossRef]
18. Mumba C, Muhimbe Z, Mapulanga V, Kawimbe M, Mutale K, Hamasuku A, et al. The effects of HIV and oncogenic human papillomavirus on the tumor immune microenvironment of penile squamous cell carcinoma. PLoS One. 2024;19:e0300729. doi:10.1371/journal.pone.0300729. [Google Scholar] [PubMed] [CrossRef]
19. Schlam I, Church SE, Hether TD, Chaldekas K, Hudson BM, White AM, et al. The tumor immune microenvironment of primary and metastatic HER2−positive breast cancers utilizing gene expression and spatial proteomic profiling. J Transl Med. 2021 Mar 10;19:113. doi:10.21203/rs.3.rs-625718/v1. [Google Scholar] [CrossRef]
20. Zhang X, Yin X, Zhang H, Sun G, Yang Y, Chen J, et al. Differential expression of TIM-3 between primary and metastatic sites in renal cell carcinoma. BMC Cancer. 2019 Jan 10;19(1):49. doi:10.1186/s12885-019-5273-5. [Google Scholar] [PubMed] [CrossRef]
21. Fiorentino V, Pepe L, Pizzimenti C, Zuccalà V, Pepe P, Cianci V, et al. PD-L1 expression in prostate cancer and gleason grade group: is there any relationship? Findings from a multi-institutional cohort. Pathol Res Pract. 2025 May;269:155916. doi:10.1016/j.prp.2025.155916. [Google Scholar] [PubMed] [CrossRef]
22. Pollack A, DeSilvio M, Khor LY, Li R, Al-Saleem T, Hammond ME, et al. Ki-67 staining is a strong predictor of distant metastasis and mortality for men with prostate cancer treated with radiotherapy and androgen deprivation. Cancer. 2004 Apr 1;100(7):1556–63. doi:10.1200/jco.2004.09.150. [Google Scholar] [PubMed] [CrossRef]
23. Bonk S, Tasdelen P, Kluth M, Hube-Magg C, Makrypidi-Fraune G, Möller K, et al. High B7-H3 expression is linked to increased risk of prostate cancer progression. Pathol Int. 2020 Oct;70(10):733–42. doi:10.1111/pin.12999. [Google Scholar] [PubMed] [CrossRef]
24. Ness N, Andersen S, Valkov A, Nordbakken CV, Al-Saad S, Donnem T, et al. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate. 2014 Oct;74(14):1452–61. doi:10.1002/pros.22862. [Google Scholar] [PubMed] [CrossRef]
25. Gannon PO, Poisson AO, Delvoye N, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. J Immunol Methods. 2009 Nov;348(1-2):9–17. doi:10.1016/j.jim.2009.06.004. [Google Scholar] [PubMed] [CrossRef]
26. Kwon WA, Joung JY. T-Cell engager therapy in prostate cancer: molecular insights into a new frontier in immunotherapy. Cancers. 2025 May 29;17(11):1820. doi:10.3390/cancers17111820. [Google Scholar] [PubMed] [CrossRef]
27. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013 Nov;19(11):1423–37. doi:10.1038/nm.3394. [Google Scholar] [PubMed] [CrossRef]
28. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74. doi:10.1016/j.cell.2011.02.013. [Google Scholar] [PubMed] [CrossRef]
29. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017 Feb 9;168(4):670–91. [Google Scholar]
30. Cioni B, Nevedomskaya E, Melis MHM, van Burgsteden J, Stelloo S, Hodel E, et al. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol Oncol. 2018;12(8):1308–23. doi:10.1002/1878-0261.12327. [Google Scholar] [PubMed] [CrossRef]
31. Zhao E, Wang L, Dai J, Kryczek I, Wei S, Vatan L, et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology. 2012 Feb 1;1(2):152–61. doi:10.4161/onci.1.2.18480. [Google Scholar] [PubMed] [CrossRef]
32. Calagua C, Russo J, Parikh M, Figler R, Anastos H, Kahnoski R, et al. Expression of PD-L1 in hormone-naïve and treated prostate cancer patients receiving neoadjuvant abiraterone acetate plus prednisone and leuprolide. Clin Cancer Res. 2017 Nov 15;23(22):6812–22. doi:10.1158/1078-0432.ccr-17-0807. [Google Scholar] [PubMed] [CrossRef]
33. Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006 Nov 17;127(4):679–95. doi:10.1016/j.cell.2006.11.001. [Google Scholar] [PubMed] [CrossRef]
34. Demuytere J, Ernst S, Van Ovost J, Cosyns S, Ceelen W. The tumor immune microenvironment in peritoneal carcinomatosis. Int Rev Cell Mol Biol. 2022;371:31–64. doi:10.1016/bs.ircmb.2022.04.015. [Google Scholar] [PubMed] [CrossRef]
35. Chen Y, Dai X, Wang J, Tao C, Wang Y, Zhu Q, et al. Heterogenous profiles between primary lung cancers and paired brain metastases reveal tumor evolution. Front Oncol. 2023;13:1026099. doi:10.3389/fonc.2023.1026099. [Google Scholar] [PubMed] [CrossRef]
36. Davidsson S, Ohlson AL, Andersson SO, Fall K, Meisner A, Fiorentino M, et al. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3+ regulatory T cells with respect to lethal prostate cancer. Mod Pathol. 2013 Mar;26(3):448–55. doi:10.1038/modpathol.2012.164. [Google Scholar] [PubMed] [CrossRef]
37. Flammiger A, Weisbach L, Huland H, Tennstedt P, Simon R, Minner S, et al. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur J Cancer. 2013 Apr;49(6):1273–9. doi:10.1016/j.ejca.2012.11.035. [Google Scholar] [PubMed] [CrossRef]
38. Han C, Deng Y, Xu W, Liu Z, Wang T, Wang S, et al. The roles of tumor-associated macrophages in prostate cancer. J Oncol. 2022 Sep 7;2022:8580043. doi:10.1155/2022/8580043. [Google Scholar] [PubMed] [CrossRef]
39. Cheng B, Huang H. Expanding horizons in overcoming therapeutic resistance in castration-resistant prostate cancer: targeting the androgen receptor-regulated tumor immune microenvironment. Cancer Biol Med. 2023 Aug;20(8):568–74. [Google Scholar]
40. Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017 Dec;14(12):717–34. doi:10.1038/nrclinonc.2017.101. [Google Scholar] [PubMed] [CrossRef]
41. Di Donato M, Cristiani CM, Capone M, Garofalo C, Madonna G, Passacatini LC, et al. Role of the androgen receptor in melanoma aggressiveness. Cell Death Dis. 2025;16:34. doi:10.1038/s41419-025-07350-4. [Google Scholar] [PubMed] [CrossRef]
42. Lavin Y, Kobayashi S, Leader A, Amir E-AD, Elefant N, Bigenwald C, et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell. 2017 May 4;169(4):750–65.e17. doi:10.1016/j.cell.2017.04.014. [Google Scholar] [PubMed] [CrossRef]
43. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015 Apr 13;27(4):450–61. doi:10.1016/j.ccell.2015.03.001. [Google Scholar] [PubMed] [CrossRef]
44. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018 May;24(5):541–50. doi:10.1038/s41591-018-0014-x. [Google Scholar] [PubMed] [CrossRef]
45. Coelho KBCA, Wosniaki DK, Pereira JL, Luz M, Albrecht L, Nardin JM, et al. Comparative analysis of cytokine expression profiles in prostate cancer patients. Biology. 2025 May 6;14(5):505. doi:10.3390/biology14050505. [Google Scholar] [PubMed] [CrossRef]
46. Cerqueira MA, Ferrari KL, de Mattos AC, Monti CR, Reis LO. T cells CD4+/CD8+ local immune modulation by prostate cancer hemi-cryoablation. World J Urol. 2020 Mar;38(3):673–80. doi:10.1007/s00345-019-02861-0. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools