 Open Access
Open Access
REVIEW
Unraveling Immunotherapy Resistance in Solid Tumors: Decoding Mechanisms and Charting Future Therapeutic Landscapes
1 State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China
2 State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, 611137, China
* Corresponding Authors: Cheng Peng. Email: ; Hailin Tang. Email:
# Huan Wang and Jindong Xie contributed equally to this work
Oncology Research 2025, 33(12), 3789-3800. https://doi.org/10.32604/or.2025.067592
Received 07 May 2025; Accepted 19 August 2025; Issue published 27 November 2025
Abstract
Solid tumors comprise the majority of the global cancer burden, with their incidence and associated mortality posing considerable challenges to public health systems. With population growth and aging, the burden of these tumors is anticipated to increase further in the coming decades. The progression of solid tumors depends on dynamic interactions between malignantly transformed cells and the tumor microenvironment (TME). Immune checkpoint inhibitor therapy improves T cell-mediated antitumor activity by suppressing regulatory pathways, such as programmed cell death protein 1/programmed death-ligand 1. Nonetheless, its widespread application is constrained by drug resistance. In this comprehensive review, we elucidate the latest advances in understanding the mechanisms underlying drug resistance, explore pioneering approaches, such as combination therapeutic regimens and nanoscale drug delivery platforms, and propose future avenues for research. These include investigating the intricacies of drug resistance pathways, refining combination therapy strategies, and modulating the TME, along with other key areas.Keywords
Solid tumors represent a large component of the global cancer burden [1,2]. For example, liver cancer accounted for approximately 905,700 new cases and 830,200 deaths worldwide in 2020, ranking among the leading causes of cancer-related mortality [3]. Similarly, in 2018, esophageal cancer accounted for over 572,000 new cases globally, with esophageal adenocarcinoma and esophageal squamous cell carcinoma being the predominant subtypes [4]. Furthermore, colorectal cancer ranks as the third most common malignancy globally, with a marked increase in incidence reported among the working-age population in 2021 [5]. The age-standardized incidence rate (ASIR) and age-standardized mortality rate (ASMR) for certain solid tumors have declined; however, the absolute number of cases continues to rise. For instance, by 2040, the number of liver cancer cases is projected to increase by 55% [3].
Solid tumors, defined as tissue masses originating from abnormal cellular proliferation, are frequently detected in organs, such as the lungs, breasts, and prostate glands [1]. These tumors demonstrate substantial clinical heterogeneity, and their pathogenesis involves complex mechanisms, including the accumulation of genetic mutations, epigenetic dysregulation, and the dynamic modulation of tumor microenvironment (TME). Surgical excision, radiotherapy, and chemotherapy form the foundation of existing therapeutic strategies. Nonetheless, challenges, such as drug resistance and postoperative recurrence, persist, with only marginal improvements observed in 5-year survival rates for patients with advanced-stage disease [6]. Despite the partial success of conventional therapies, including surgery, radiotherapy, and chemotherapy, drug resistance and tumor relapse continue to pose major challenges.
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, markedly enhancing treatment outcomes across several malignancies. By inhibiting immunosuppressive signaling pathways, ICIs restore T cell-mediated cytotoxicity against tumors, offering an innovative therapeutic option for solid tumors [7]. Notably, where conventional chemotherapy and targeted therapies fail, ICIs have exhibited exceptional clinical efficacy [8]. Nonetheless, the intricate immunosuppressive network within the TME contributes to overall response rates of less than 30%. Both primary resistance (absence of an initial therapeutic response) and acquired resistance (loss of efficacy after initial success) are intricately associated with tumor heterogeneity, antigen loss, and myeloid cell-mediated immunosuppression [9]. To this end, ongoing investigations focus on innovative combination strategies, such as combining ICIs with anti-angiogenic agents or enhancing tumor-specific recognition through engineered T cell receptors [10]. These cutting-edge approaches are undergoing rigorous clinical evaluation and show promise for overcoming existing therapeutic limitations. This article synthesizes current breakthroughs in elucidating drug resistance mechanisms and highlights promising avenues for future investigation.
2 Mechanisms of Immune Checkpoint Resistance
As a landmark advancement in cancer therapeutics, ICIs restore T-cell antitumor activity by obstructing signaling pathways, notably programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [7]. Two underlying mechanisms are involved: (i) binding of PD-1 receptors to PD-L1 ligands on T cells, which are overexpressed on tumor cells, leading to T-cell exhaustion; (ii) CTLA-4 competitively binds to cluster of differentiation (CD)80/CD86 co-stimulatory molecules, thereby inhibiting early-stage T-cell activation [11]. Despite clinical improvements in some patients receiving ICIs, most of them demonstrate limited therapeutic efficacy [12]. Notably, objective response rates (ORRs) range between 40% and 70% for malignancies, including microsatellite instability-high tumors, Hodgkin lymphoma, and melanoma, whereas most other cancer types demonstrate ORRs as low as 10% to 25% [13]. Importantly, even patients who initially respond to treatment may develop acquired secondary resistance, leading to disease progression [14].
2.1 Tumor-Intrinsic Mechanisms
A fundamental mechanism underlying drug resistance is the capacity of tumor cells to facilitate immune evasion through dynamic genetic alterations [15]. This strategy is characterized by two key processes: (i) downregulation of antigen presentation-related gene expression via mutations or epigenetic modifications (Fig. 1A) and (ii) upregulation of immunosuppressive molecule expression. For example, in ovarian cancer models, epigenetic silencing controlled by enhancer of zeste homolog 2-induced histone H3 lysine 27 trimethylation and DNA methyltransferase 1-mediated DNA methylation suppresses the expression of T helper 1-type chemokines C-X-C motif chemokine ligand 10 and C-X-C motif chemokine ligand 9. This suppression decreases tumor-infiltrating T cell density, thereby reducing the effectiveness of PD-L1 inhibitors.
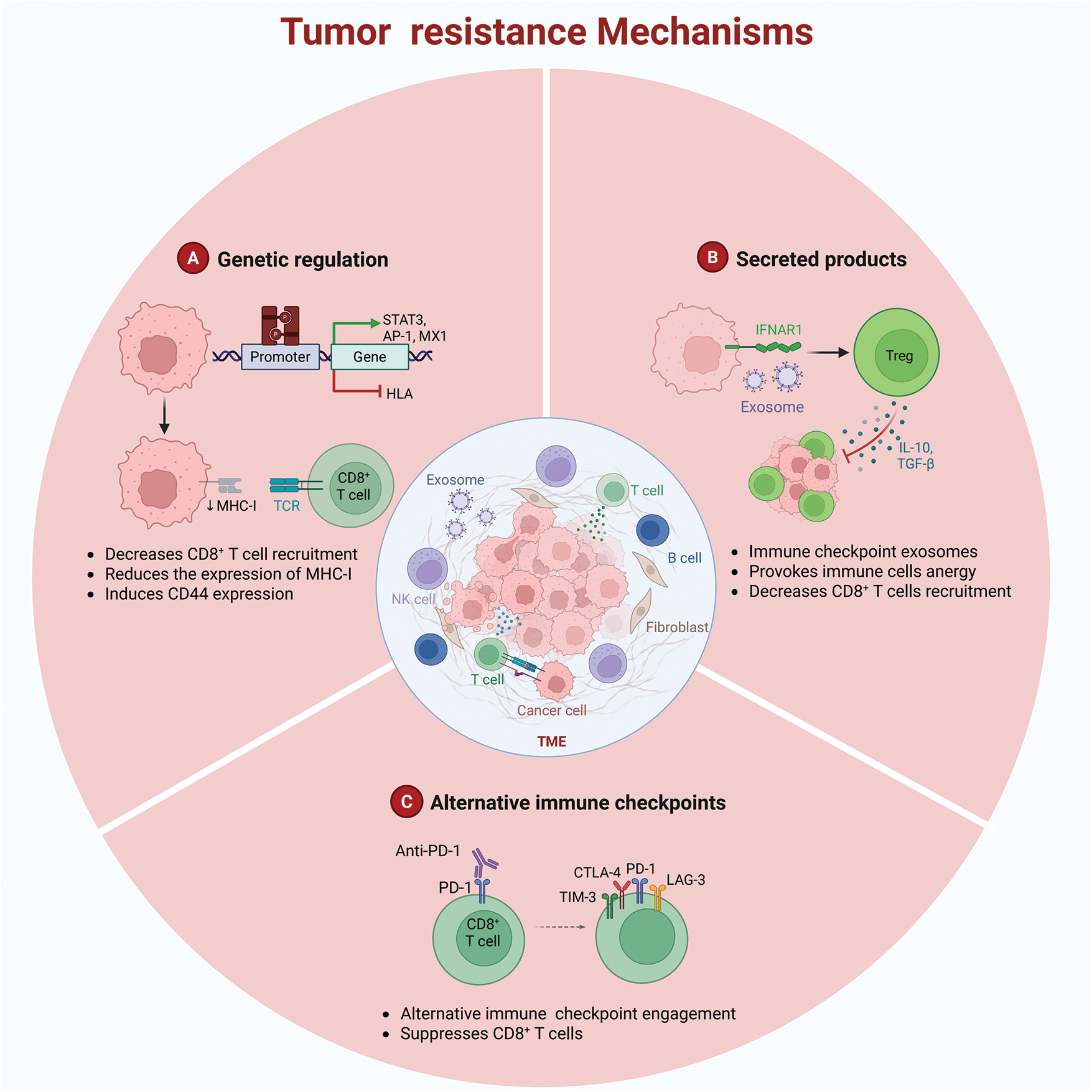
Figure 1: Tumor resistance mechanisms (Created using Biorender)
Moreover, primary resistance can originate from inadequate tumor antigenicity or defective antigen presentation. For example, the downregulation of major histocompatibility complex class I molecules is caused by mutations in phosphatidylinositol 3-kinase/protein kinase B and mitogen-activated protein kinase pathways [16]. Through epigenetic silencing (e.g., promoter methylation) or genetic alterations (e.g., β2-microglobulin mutations), tumor cells substantially reduce their antigen-presenting capacity, preventing recognition of tumor-associated antigens by T cells [17].
2.2 Immunosuppressive Remodeling of the TME
Immune cells residing in the TME demonstrate considerable heterogeneity [18]. Immunosuppressive cell populations, including myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and M2-type tumor-associated macrophages, release inhibitory cytokines, such as interleukin-10 and transforming growth factor-beta, which impair effector T-cell activity and contribute to drug resistance (Fig. 1B) [19]. MDSCs further exacerbate immunosuppression by suppressing the activation and expansion of natural killer cells and effector T cells, inducing Treg differentiation, and impairing the antigen-presenting capacity of antigen-presenting cells (APCs) [20]. Subsequently, they facilitate immune evasion [21].
Another substantial resistance mechanism is the compensatory activation of immune checkpoints. Beyond the PD-1/PD-L1 pathway, co-inhibitory molecules, such as CTLA-4, lymphocyte activation gene 3 (LAG-3), and T cell immunoglobulin and mucin-domain containing-3 (TIM-3), attenuate T-cell function, thereby limiting the effectiveness of immune checkpoint therapies (Fig. 1C) [22]. Both TIM-3 and PD-1 modulate immune responses by inhibiting T cell functionality. TIM-3 is an activation-induced inhibitory receptor that promotes T cell exhaustion and immune tolerance by interacting with ligands, such as galectin-9 and carcinoembryonic antigen-related cell adhesion molecule 1 [23]. Conversely, PD-1 attenuates T cell activation and proliferation by binding to PD-L1 or programmed death-ligand 2, thereby preserving immune homeostasis [24]. Importantly, TIM-3 and PD-1 can be co-expressed on exhausted T cells under specific conditions. This co-expression amplifies inhibitory signaling and synergistically suppresses T cell activity via distinct molecular mechanisms [25]. Within the TME, this co-expression facilitates tumor immune evasion. For example, in hepatocellular carcinoma and acute myeloid leukemia, the co-expression of TIM-3 and PD-1 is strongly related to T cell exhaustion and tumor progression [26].
Compensatory upregulation of TIM-3, LAG-3, or T cell immunoreceptor with Ig and ITIM domains occurs after PD-1 blockade. For example, in murine lung adenocarcinoma models, acquired resistance and tumor progression after an initial anti-PD-1 response were strongly related to increased TIM-3 expression. Sequential targeting of TIM-3 with blocking antibodies significantly enhanced survival rates. Moreover, the overexpression of CTLA-4 and LAG-3 on T cells has been observed in tumors showing resistance [27].
The TME encompasses the extracellular matrix (ECM), a noncellular component that provides essential biochemical signals and structural support to tumor cells [28]. Beyond functioning as a structural scaffold, the ECM participates in immune modulation and drug resistance. Its biomechanical properties and biochemical signaling can influence the immune microenvironment. For example, decellularized ECM can induce M2 macrophage polarization by optimizing its micromechanical properties, promoting anti-inflammatory responses and tissue rapid [29]. Additionally, the ECM can hinder drug diffusion and reduce treatment efficacy by increasing mechanical stiffness and altering its composition. Structural abnormalities in tumor ECM create physical barriers to drug delivery, fostering drug resistance [30]. Cancer-associated fibroblasts (CAFs) promote therapeutic resistance by remodeling the ECM to support tumor cell proliferation and survival while restricting immune cell infiltration and drug delivery. In pancreatic cancer, CAF-mediated excessive ECM deposition creates a dense stromal barrier that limits drug accessibility and induces chemoresistance [31].
The mechanisms by which tumors develop drug resistance may involve the following three aspects: A. Downregulation of antigen presentation-related gene expression via mutations or epigenetic modifications; B. Immunosuppressive cell populations releasing interleukin-10 and transforming growth factor-β, among others, leading to immune cell anergy; C. Compensatory activation of immune checkpoints resulting in reduced efficacy of immune checkpoint therapies.
3.1 Strategies of Combination Therapy
ICIs have achieved notable success in the treatment of solid tumors; nevertheless, the emergence of drug resistance remains a considerable challenge in clinical practice. To this end, combination therapies have emerged as a crucial approach, integrating diverse treatment modalities to synergistically enhance antitumor immune responses and mitigate drug resistance. The integration of chemotherapy with ICIs offers distinct advantages: chemotherapeutic agents directly destroy tumor cells while improving immune response by releasing tumor antigens and increasing tumor immunogenicity. This dual effect addresses the limitations of monotherapy by augmenting antigen presentation and enhancing tumor immunogenicity [32].
Likewise, the integration of targeted therapy with ICIs emphasizes precision-based therapeutic interventions. Targeted therapies inhibit tumor proliferation by blocking specific signaling pathways, whereas ICIs enhance systemic immune responses. Novel antibody-drug conjugates (ADCs), exemplifying a class of targeted agents, have demonstrated considerable potential in preclinical and clinical trials when combined with ICIs. They leverage antibody-mediated precision delivery mechanisms [33]. Radiotherapy, a localized treatment approach, works synergistically with ICIs by inducing radiation-induced immunogenic cell death (ICD). This mechanism not only controls local tumor lesions but also initiates systemic immune responses. In solid tumors, such as melanoma, combination therapy has enhanced immune cell infiltration and antigen release [34].
Immunomodulators and dual immune checkpoint blockade strategies broaden the scope of combination therapy. Tumor vaccines combined with ICIs specifically improve T-cell activation. The simultaneous inhibition of PD-1 and CTLA-4 has shown superior performance in tumors with high immunogenicity [35]. Interventions targeting the TME are equally vital. Anti-angiogenic agents ameliorate abnormal tumor vasculature and inhibit immunosuppressive cells, creating a favorable milieu for immunotherapy. Epigenetic modulators, such as histone deacetylase inhibitors, augment tumor immunogenicity by altering gene expression profiles, and their combination with ICIs has demonstrated synergistic effects in treatment-resistant tumors [32].
Recent advances in combination therapies have introduced innovative therapeutic strategies. A novel electroimmunotherapy combined with chimeric antigen receptor T cell (CAR-T) therapy has shown efficacy in solid tumors by inducing ICD and the remodeling of the TME. This, in turn, enhances CAR-T cell antitumor activity. Furthermore, substantial progress has been made in integrating ICIs with oncolytic viruses (OVs). The synergistic application of OVs and ICIs reshapes the immunosuppressive TME and enhances systemic immune responses [36]. Notably, preclinical studies have suggested that OVs engineered to express interleukin-12 and granulocyte-macrophage colony-stimulating factor, when combined with PD-1 blockade, exhibit robust antitumor activity even in poorly immunogenic solid tumors [37].
The combination of ICIs with other therapeutic modalities has markedly improved therapeutic outcomes. Nonetheless, it has increased the complexity and severity of adverse effects. Combination immunotherapy can precipitate a broad range of immune-related adverse events (irAEs) affecting multiple organ systems. In the gastrointestinal tract, irAEs may induce diarrhea and colitis [38]. Dermatologic manifestations include skin rashes and pruritus [39], with severe cases potentially escalating to Stevens-Johnson syndrome. Hematologic alterations, such as leukopenia and anemia, are frequently observed [40]. The incidence of all-grade adverse events escalates substantially upon combining chemotherapy with ICIs. In contrast, dual immunotherapy regimens (e.g., CTLA-4 + PD-1 inhibitors) are associated with an even higher risk of grade 3 or higher toxicities. To mitigate these adverse effects, a graded management approach, early intervention with corticosteroids, and personalized adjustments to treatment regimens, such as optimizing chemotherapy dosing, are warranted.
To summarize, combined therapeutic strategies enhance overall outcomes in solid tumors through multidimensional mechanisms. With ongoing clinical research and the advent of innovative therapies, this integrated strategy holds promise for overcoming drug resistance and improving long-term survival.
3.2 Potential of Nanomedicine Delivery Systems
Nanomedicine delivery systems, as emerging technological platforms, offer innovative strategies to address drug resistance. They substantially enhance the pharmacokinetic profiles of therapeutic agents via nanoparticle carriers, extending the half-life of drug circulation, improving bioavailability, and precisely modulating drug distribution patterns in vivo. Consequently, they enable the precise targeting of therapeutic sites. This capability allows immunotherapeutic drugs to accumulate in tumor tissues or specific immune cell regions, thereby enhancing antitumor efficacy while minimizing systemic toxicity [41].
To address resistance mechanisms in immune checkpoint therapy, nanocarrier systems offer distinct advantages through multidimensional strategies. They operate through three primary mechanisms:
1. Targeted ligand modifications facilitate drug-specific accumulation within the TME or immune cells, thereby minimizing nonspecific drug diffusion and enhancing drug concentration at lesion sites [42].
2. The sustained and controlled release capabilities of nanocarriers maintain effective drug concentrations in therapeutic areas, reducing the likelihood of resistance [43].
3. Combination strategies that co-deliver chemotherapeutic agents and immunomodulators address the limitations of monotherapy, generating synergistic antitumor immune responses [44].
Importantly, these systems extend beyond conventional drug delivery by exerting multifaceted immunomodulatory effects, such as inducing ICD to facilitate antigen release [45], enhancing the efficiency of APCs, and remodeling the TME through immunoregulatory molecule delivery.
Nanocarriers encompass a diverse array of types, each possessing unique physicochemical characteristics (Table 1). These include niosome and lipid-based formulations, polymeric, inorganic, hybrid, protein-based, and carbon-based nanoparticles. Lipid-based nanoparticles, such as liposomes, solid lipid nanoparticles, and nanostructured lipid carriers, are extensively used because of their inherent biocompatibility and biodegradability [46]. These properties improve drug solubility and bioavailability, reduce drug toxicity and adverse effects, and facilitate sustained and targeted drug delivery [47]. Nonetheless, limitations, such as stability issues during preparation and potential immune recognition and clearance in vivo may limit efficacy [47].
Polymeric nanoparticles, including poly(lactic-co-glycolic acid) and polyethylene glycol [48], are widely used for sustained and targeted drug delivery. These carriers possess high biocompatibility and controlled drug release profiles, enabling prolonged drug release [49]. However, complex preparation processes and low drug encapsulation efficiency present potential drawbacks [47]. Inorganic nanoparticles, such as gold nanoparticles, carbon nanotubes, and hydroxyapatite nanoparticles, exhibit high stability, favorable mechanical properties, and multifunctionality, which makes them suitable for both drug delivery and imaging [50]. Nevertheless, certain inorganic carriers may pose biotoxicity risks and limited biodegradability [50]. Hybrid nanoparticles, such as lipid-polymer hybrids or inorganic-organic hybrids, facilitate multifunctionality, including concurrent targeted drug delivery and imaging, thereby enhancing therapeutic efficacy [51]. Despite these advantages, their synthesis involves intricate processes, and compatibility challenges may emerge between constituent materials [52].
Protein-based nanoparticles, including recombinant casein micelles, have garnered considerable research interest because of their natural origin and biocompatibility. These carriers exhibit remarkable biocompatibility and biodegradability, which facilitate effective drug encapsulation and targeted delivery [53]. Nonetheless, they are susceptible to enzymatic degradation in vivo, and their stability may be affected by environmental stressors [52]. In contrast, carbon-based nanoparticles, such as graphene oxide and fullerenes, are used in drug delivery applications because of their distinctive physicochemical properties, such as high specific surface areas and exceptional drug-loading capacities [54]. However, certain carbon-based carriers may present biotoxicity risks and environmental hazards [55].
The selection of nanodrug carriers requires a thoughtful consideration of the specific clinical application along with the properties of both the carrier and the drugs. Lipid-based and polymeric carriers are extensively utilized because of their biocompatibility and controllability. In contrast, inorganic and hybrid carriers are utilized in specialized applications because of their multifunctionality and high stability. With the ongoing progress in nanotechnology, the development and clinical application of innovative nanocarriers are expected to increase.
Despite their noteworthy clinical potential, nanocarrier systems face substantial challenges in translational medicine. A more comprehensive monitoring framework is warranted for the long-term biosafety assessment of nanomaterials. Currently, there are no unified regulatory guidelines regarding the critical quality attributes of nanodrugs, such as the particle size, surface charge, and stability [56]. Moreover, insufficient data on long-term biocompatibility and immunogenicity have resulted in the discontinuation of some clinical trials because of toxicity concerns [56]. Furthermore, nanocarrier systems encounter a low conversion rate from preclinical research to clinical application. Despite extensive preclinical studies, less than 5% of nanodrugs receive regulatory approval [56]. Nonetheless, with ongoing progress in nanotechnology and translational research, these cutting-edge delivery systems hold promise to enhance existing immunotherapies and introduce novel pathways to address therapeutic challenges in solid tumors.
Immunotherapy targets immune checkpoints and has attained remarkable progress in the management of solid tumors; however, its clinical application continues to encounter substantial challenges, including drug resistance and interindividual variability in therapeutic efficacy. At the foundational research level, researchers should comprehensively elucidate the molecular mechanisms underlying ICIs, particularly emphasizing the complex network of downstream signaling pathways. Clarifying the regulatory principles governing these pathways will facilitate identifying the molecular basis for resistance mechanisms, thereby enabling the development of precise intervention strategies. Simultaneously, to overcome the limitations of monotherapy, researchers are actively investigating combination therapeutic approaches. These include integrating ICIs with CAR-T cell therapy or targeted agents [57] to enhance synergistic effects through optimized dosing ratios and administration schedules.
Personalized medicine provides novel opportunities to overcome immunotherapy resistance in solid tumors through precise diagnostic and therapeutic approaches. This field relies on the identification of biomarkers that can predict both treatment response and resistance. For example, TMEM92 is a pivotal biomarker of immune resistance in pancreatic cancer, and its elevated expression is associated with poor prognosis and suboptimal immunotherapy outcomes [58]. The identification of such biomarkers facilitates the selection of patients who are more likely to benefit from immunotherapy while enabling the development of alternative treatment regimens for patients exhibiting resistance.
Advanced single-cell multi-omics technologies, such as genomics, transcriptomics, and proteomics, provide insights into tumor heterogeneity and resistance mechanisms [59]. By examining cellular diversity within the TME, researchers can identify molecular pathways that drive resistance, thereby informing the development of targeted therapeutic strategies. These technologies facilitate personalized medicine to address tumor heterogeneity and treatment resistance [60]. Concurrently, multi-omics technologies can be synergistically integrated with artificial intelligence (AI), which shows considerable promise in predicting responses and mechanisms underlying resistance. AI can analyze multi-omics data, enabling the prediction of treatment responses and the optimization of personalized treatment plans, thereby improving both the precision and efficacy of therapeutic interventions [61].
The integration of emerging technologies has become crucial for facilitating scientific breakthroughs. Single-cell sequencing facilitates the dynamic analysis of tumor heterogeneity, whereas AI systems can predict individualized treatment responses [62]. The synergistic application of these technologies’ advances not only the discovery of biomarkers but also the development of personalized treatment regimens.
In the realm of technological innovation, researchers are exploring three avenues: (i) the ongoing identification of novel immune checkpoint molecules to broaden therapeutic targets [34]; (ii) the refinement of drug delivery systems through nanocarriers and ADCs; and (iii) the augmentation of anti-tumor immunity via metabolic reprogramming strategies. The advancement of these innovative methodologies necessitates a seamless integration with clinical translation. Upcoming clinical trials will focus on biomarker-guided patient stratification and utilize translational medicine platforms to expedite the application of laboratory discoveries into clinical practice.
Acknowledgement: Not applicable.
Funding Statement: This work was supported by the Open Research Fund of Chengdu University of Traditional Chinese Medicine Key Laboratory of Systematic Research of Distinctive Chinese Medicine Resources in Southwest China and Guangdong Basic and Applied Basic Research Foundation (2022B1515120087, Hailin Tang).
Author Contributions: Hailin Tang and Cheng Peng conceived the ideas and designed the study. Huan Wang and Jindong Xie wrote the original draft. Na Li, Qianwen Liu, Wenqi Song, and Wenkuan Chen validated the results. Hailin Tang and Cheng Peng critically revised the manuscript, reviewed, and edited the paper. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| TME | tumor microenvironment |
| ICI | immune checkpoint inhibitor |
| ORR | objective response rates |
| MDSCs | myeloid-derived suppressor cells |
| Tregs | regulatory T cells |
| APCs | antigen-presenting cells |
| ADCs | antibody-drug conjugates |
| ICD | immunogenic cell death |
References
1. Zhang X, Yu S, Li X, Wen X, Liu S, Zu R, et al. Research progress on the interaction between oxidative stress and platelets: another avenue for cancer? Pharmacol Res. 2023;191(9):106777. doi:10.1016/j.phrs.2023.106777. [Google Scholar] [PubMed] [CrossRef]
2. Li S, Dong R, Kang Z, Li H, Wu X, Li T. Exosomes: another intercellular lipometabolic communication mediators in digestive system neoplasms? Cytokine Growth Factor Rev. 2023;73(32):93–100. doi:10.1016/j.cytogfr.2023.06.005. [Google Scholar] [PubMed] [CrossRef]
3. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–606. doi:10.1016/j.jhep.2022.08.021. [Google Scholar] [PubMed] [CrossRef]
4. Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18(6):432–43. doi:10.1038/s41575-021-00419-3. [Google Scholar] [PubMed] [CrossRef]
5. Song Y, Wang X, Shen Y, Chen L, Yang L, Wang R, et al. Trends and cross-country inequality in the incidence of GI cancers among the working-age population from 1990 to 2021: a global burden of disease 2021 analysis. Gut. 2025;74(6):948–59. doi:10.1136/gutjnl-2024-333932. [Google Scholar] [PubMed] [CrossRef]
6. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi:10.3322/caac.21660. [Google Scholar] [PubMed] [CrossRef]
7. Xie J, Liu M, Deng X, Tang Y, Zheng S, Ou X, et al. Gut microbiota reshapes cancer immunotherapy efficacy: mechanisms and therapeutic strategies. iMeta. 2024;3(1):e156. doi:10.1002/imt2.156. [Google Scholar] [CrossRef]
8. Ma W, Xue R, Zhu Z, Farrukh H, Song W, Li T, et al. Increasing cure rates of solid tumors by immune checkpoint inhibitors. Exp Hematol Oncol. 2023;12(1):10. doi:10.1186/s40164-023-00372-8. [Google Scholar] [PubMed] [CrossRef]
9. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–23. doi:10.1016/j.cell.2017.01.017. [Google Scholar] [PubMed] [CrossRef]
10. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5. doi:10.1126/science.aar4060. [Google Scholar] [PubMed] [CrossRef]
11. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi:10.1038/nrc3239. [Google Scholar] [PubMed] [CrossRef]
12. Li S, Na R, Li X, Zhang Y, Zheng T. Targeting interleukin-17 enhances tumor response to immune checkpoint inhibitors in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2022;1877(4):188758. doi:10.1016/j.bbcan.2022.188758. [Google Scholar] [CrossRef]
13. Xu-Monette ZY, Zhang M, Li J, Young KH. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol. 2017;8:1597. doi:10.3389/fimmu.2017.01597. [Google Scholar] [PubMed] [CrossRef]
14. Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi:10.1056/nejmoa1200694. [Google Scholar] [PubMed] [CrossRef]
15. Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance mechanisms of anti-PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020;8:672. doi:10.3389/fcell.2020.00672. [Google Scholar] [PubMed] [CrossRef]
16. Zheng X, Chen J, Deng M, Ning K, Peng Y, Liu Z, et al. G3BP1 and SLU7 jointly promote immune evasion by downregulating MHC-I via PI3K/Akt activation in bladder cancer. Adv Sci. 2024;11(7):e2305922. doi:10.1002/advs.202305922. [Google Scholar] [PubMed] [CrossRef]
17. Haddad AF, Young JS, Gill S, Aghi MK. Resistance to immune checkpoint blockade: mechanisms, counter-acting approaches, and future directions. Semin Cancer Biol. 2022;86(Pt 3):532–41. doi:10.1016/j.semcancer.2022.02.019. [Google Scholar] [PubMed] [CrossRef]
18. Cao Z, Wang Z, Yang L, Li T, Tao X, Niu X. Reshaping the immune microenvironment and reversing immunosenescence by natural products: prospects for immunotherapy in gastric cancer. Semin Cancer Biol. 2025;110(1):1–16. doi:10.1016/j.semcancer.2025.02.002. [Google Scholar] [PubMed] [CrossRef]
19. Liu C, Xie J, Lin B, Tian W, Wu Y, Xin S, et al. Pan-cancer single-cell and spatial-resolved profiling reveals the immunosuppressive role of APOE+ macrophages in immune checkpoint inhibitor therapy. Adv Sci. 2024;11(23):e2401061. doi:10.1002/advs.202401061. [Google Scholar] [PubMed] [CrossRef]
20. Xie J, Lin X, Deng X, Tang H, Zou Y, Chen W, et al. Cancer-associated fibroblast-derived extracellular vesicles: regulators and therapeutic targets in the tumor microenvironment. Cancer Drug Resist. 2025;8:2. doi:10.20517/cdr.2024.152. [Google Scholar] [PubMed] [CrossRef]
21. Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362. doi:10.1038/s41392-021-00670-9. [Google Scholar] [PubMed] [CrossRef]
22. Alsaafeen BH, Ali BR, Elkord E. Resistance mechanisms to immune checkpoint inhibitors: updated insights. Mol Cancer. 2025;24(1):20. doi:10.1186/s12943-024-02212-7. [Google Scholar] [PubMed] [CrossRef]
23. Sim J, Park J, Kim S, Hwang S, Sung K, Lee JE, et al. Association of Tim-3/Gal-9 axis with NLRC4 inflammasome in glioma malignancy: Tim-3/Gal-9 induce the NLRC4 inflammasome. Int J Mol Sci. 2022;23(4):2028. doi:10.3390/ijms23042028. [Google Scholar] [PubMed] [CrossRef]
24. Kaufman B, Abu-Ahmad M, Radinsky O, Gharra E, Manko T, Bhattacharya B, et al. N-glycosylation of PD-L1 modulates the efficacy of immune checkpoint blockades targeting PD-L1 and PD-1. Mol Cancer. 2025;24(1):140. doi:10.1186/s12943-025-02330-w. [Google Scholar] [PubMed] [CrossRef]
25. Kimura K, Subramanian A, Yin Z, Khalilnezhad A, Wu Y, He D, et al. Immune checkpoint TIM-3 regulates microglia and Alzheimer’s disease. Nature. 2025;641(8063):718–31. [Google Scholar] [PubMed]
26. Liu F, Liu Y, Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J Hematol Oncol. 2018;11(1):126. [Google Scholar] [PubMed]
27. Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7(1):10501. doi:10.1038/ncomms10501. [Google Scholar] [PubMed] [CrossRef]
28. Aghlara-Fotovat S, Nash A, Kim B, Krencik R, Veiseh O. Targeting the extracellular matrix for immunomodulation: applications in drug delivery and cell therapies. Drug Deliv Transl Res. 2021;11(6):2394–413. doi:10.1007/s13346-021-01018-0. [Google Scholar] [PubMed] [CrossRef]
29. Luo P, Huang R, Wu Y, Liu X, Shan Z, Gong L, et al. Tailoring the multiscale mechanics of tunable decellularized extracellular matrix (dECM) for wound healing through immunomodulation. Bioact Mater. 2023;28:95–111. doi:10.1016/j.bioactmat.2023.05.011. [Google Scholar] [PubMed] [CrossRef]
30. Ding X, Liang Y, Zhou S, Wu Y, Salata P, Mikolajczk-Martinez A, et al. Targeting tumor extracellular matrix with nanoparticles to circumvent therapeutic resistance. J Control Release. 2025;383:113786. doi:10.1016/j.jconrel.2025.113786. [Google Scholar] [PubMed] [CrossRef]
31. Feng Y, Jiang Y, Yang L, Lu D, Li N, Zhang Q, et al. Targeting CAFs and extracellular matrix (ECM) in lung cancer: potential of adjuvants and nanoparticles. Bioorg Chem. 2025;162(3):108586. doi:10.1016/j.bioorg.2025.108586. [Google Scholar] [PubMed] [CrossRef]
32. Alsaafeen BH, Ali BR, Elkord E. Combinational therapeutic strategies to overcome resistance to immune checkpoint inhibitors. Front Immunol. 2025;16:1546717. doi:10.3389/fimmu.2025.1546717. [Google Scholar] [PubMed] [CrossRef]
33. Wei Q, Li P, Yang T, Zhu J, Sun L, Zhang Z, et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J Hematol Oncol. 2024;17(1):1. [Google Scholar] [PubMed]
34. Sun Q, Hong Z, Zhang C, Wang L, Han Z, Ma D. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct Target Ther. 2023;8(1):320. doi:10.1038/s41392-023-01522-4. [Google Scholar] [PubMed] [CrossRef]
35. Lao Y, Shen D, Zhang W, He R, Jiang M. Immune checkpoint inhibitors in cancer therapy—how to overcome drug resistance? Cancers. 2022;14(15):3575. doi:10.3390/cancers14153575. [Google Scholar] [PubMed] [CrossRef]
36. Liu X, Zhang J, Feng K, Wang S, Chen L, Niu S, et al. Efficacy and safety of oncolytic virus combined with chemotherapy or immune checkpoint inhibitors in solid tumor patients: a meta-analysis. Front Pharmacol. 2022;13:1023533. doi:10.3389/fphar.2022.1023533. [Google Scholar] [PubMed] [CrossRef]
37. Ahn HM, Jung BK, Hong J, Hong D, Yoon AR, Yun CO. Enhanced potency of immune checkpoint inhibitors against poorly immunological solid tumors by immune stimulatory oncolytic adenoviruses-mediated remodeling of the tumor microenvironment. Mol Med. 2025;31(1):175. doi:10.1186/s10020-025-01223-4. [Google Scholar] [PubMed] [CrossRef]
38. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8. doi:10.1001/jamaoncol.2018.3923. [Google Scholar] [PubMed] [CrossRef]
39. Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–8. doi:10.1016/j.currproblcancer.2016.12.001. [Google Scholar] [PubMed] [CrossRef]
40. Rached L, Laparra A, Sakkal M, Danlos FX, Barlesi F, Carbonnel F, et al. Toxicity of immunotherapy combinations with chemotherapy across tumor indications: current knowledge and practical recommendations. Cancer Treat Rev. 2024;127(1):102751. doi:10.1016/j.ctrv.2024.102751. [Google Scholar] [PubMed] [CrossRef]
41. Shukla S, Steinmetz NF. Emerging nanotechnologies for cancer immunotherapy. Exp Biol Med. 2016;241(10):1116–26. doi:10.1177/1535370216647123. [Google Scholar] [PubMed] [CrossRef]
42. Zang X, Zhao X, Hu H, Qiao M, Deng Y, Chen D. Nanoparticles for tumor immunotherapy. Eur J Pharm Biopharm. 2017;115:243–56. [Google Scholar] [PubMed]
43. Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy—strategies and perspectives. J Control Release. 2016;240(Suppl. 5):489–503. doi:10.1016/j.jconrel.2016.06.012. [Google Scholar] [PubMed] [CrossRef]
44. Ramasamy T, Ruttala HB, Munusamy S, Chakraborty N, Kim JO. Corrigendum to “Nano drug delivery systems for antisense oligonucleotides (ASO) therapeutics” [Journal of Controlled Release 352, (2022) 861–878]. J Control Release. 2023;354:34. doi:10.1016/j.jconrel.2022.12.047. [Google Scholar] [PubMed] [CrossRef]
45. Xiong J, Wu M, Chen J, Liu Y, Chen Y, Fan G, et al. Correction to “Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer”. ACS Nano. 2024;18(50):34420. doi:10.1021/acsnano.4c16033. [Google Scholar] [PubMed] [CrossRef]
46. Kumar A, Lunawat AK, Kumar A, Sharma T, Islam MM, Kahlon MS, et al. Recent trends in nanocarrier-based drug delivery system for prostate cancer. AAPS PharmSciTech. 2024;25(3):55. doi:10.1208/s12249-024-02765-2. [Google Scholar] [PubMed] [CrossRef]
47. Sohail M, Guo W, Li Z, Xu H, Zhao F, Chen D, et al. Nanocarrier-based drug delivery system for cancer therapeutics: a review of the last decade. Curr Med Chem. 2021;28(19):3753–72. doi:10.2174/0929867327666201005111722. [Google Scholar] [PubMed] [CrossRef]
48. Filho D, Guerrero M, Pariguana M, Marican A, Duran-Lara EF. Hydrogel-based microneedle as a drug delivery system. Pharmaceutics. 2023;15(10):2444. doi:10.3390/pharmaceutics15102444. [Google Scholar] [PubMed] [CrossRef]
49. Kanojiya P, Wadetwar R, Karemore M, Prasad S. The know-how of polymeric nanocarrier based vaginal drug delivery system: pitfalls, challenges and trends. Pharm Nanotechnol. 2024;13(3):432–447. doi:10.2174/0122117385283801231212114538. [Google Scholar] [PubMed] [CrossRef]
50. Pena Q, Wang A, Zaremba O, Shi Y, Scheeren HW, Metselaar JM, et al. Metallodrugs in cancer nanomedicine. Chem Soc Rev. 2022;51(7):2544–82. doi:10.1039/d1cs00468a. [Google Scholar] [PubMed] [CrossRef]
51. Pan JA, Cho H, Coropceanu I, Wu H, Talapin DV. Stimuli-responsive surface ligands for direct lithography of functional inorganic nanomaterials. Acc Chem Res. 2023;56(17):2286–97. doi:10.1021/acs.accounts.3c00226. [Google Scholar] [PubMed] [CrossRef]
52. Roszkowski S, Durczynska Z. Advantages and limitations of nanostructures for biomedical applications. Adv Clin Exp Med. 2025;34(3):447–56. [Google Scholar] [PubMed]
53. Bilal M, Qamar SA, Carballares D, Berenguer-Murcia A, Fernandez-Lafuente R. Proteases immobilized on nanomaterials for biocatalytic, environmental and biomedical applications: advantages and drawbacks. Biotechnol Adv. 2024;70(2):108304. doi:10.1016/j.biotechadv.2023.108304. [Google Scholar] [PubMed] [CrossRef]
54. Zheng S, Tian Y, Ouyang J, Shen Y, Wang X, Luan J. Carbon nanomaterials for drug delivery and tissue engineering. Front Chem. 2022;10:990362. doi:10.3389/fchem.2022.990362. [Google Scholar] [PubMed] [CrossRef]
55. Zare-Zardini H, Hatamizadeh N, Haddadzadegan N, Soltaninejad H, Karimi-Zarchi M. Advantages and disadvantages of using carbon nanostructures in reproductive medicine: two sides of the same coin. JBRA Assist Reprod. 2022;26(1):142–4. doi:10.5935/1518-0557.20210070. [Google Scholar] [PubMed] [CrossRef]
56. Namiot ED, Sokolov AV, Chubarev VN, Tarasov VV, Schioth HB. Nanoparticles in clinical trials: analysis of clinical trials, FDA approvals and use for COVID-19 vaccines. Int J Mol Sci. 2023;24(1):787. doi:10.3390/ijms24010787. [Google Scholar] [PubMed] [CrossRef]
57. Garg P, Pareek S, Kulkarni P, Horne D, Salgia R, Singhal SS. Next-generation immunotherapy: advancing clinical applications in cancer treatment. J Clin Med. 2024;13(21):6537. doi:10.3390/jcm13216537. [Google Scholar] [PubMed] [CrossRef]
58. Zhang S, Wan X, Lv M, Li C, Chu Q, Wang G. TMEM92 acts as an immune-resistance and prognostic marker in pancreatic cancer from the perspective of predictive, preventive, and personalized medicine. EPMA J. 2022;13(3):519–34. doi:10.1007/s13167-022-00287-0. [Google Scholar] [PubMed] [CrossRef]
59. An J, Lu Y, Chen Y, Chen Y, Zhou Z, Chen J, et al. Spatial transcriptomics in breast cancer: providing insight into tumor heterogeneity and promoting individualized therapy. Front Immunol. 2024;15:1499301. doi:10.3389/fimmu.2024.1499301. [Google Scholar] [PubMed] [CrossRef]
60. Xu Y, Wang X, Li Y, Mao Y, Su Y, Mao Y, et al. Multimodal single cell-resolved spatial proteomics reveal pancreatic tumor heterogeneity. Nat Commun. 2024;15(1):10100. doi:10.1038/s41467-024-54438-0. [Google Scholar] [PubMed] [CrossRef]
61. He X, Liu X, Zuo F, Shi H, Jing J. Artificial intelligence-based multi-omics analysis fuels cancer precision medicine. Semin Cancer Biol. 2023;88(9):187–200. doi:10.1016/j.semcancer.2022.12.009. [Google Scholar] [PubMed] [CrossRef]
62. Mao Y, Shangguan D, Huang Q, Xiao L, Cao D, Zhou H, et al. Emerging artificial intelligence-driven precision therapies in tumor drug resistance: recent advances, opportunities, and challenges. Mol Cancer. 2025;24(1):123. doi:10.1186/s12943-025-02321-x. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools