 Open Access
Open Access
REVIEW
Molecular Mechanisms of Gemcitabine Resistance in Cholangiocarcinoma
1 Biomedical Sciences Program, Graduate School, Khon Kaen University, Khon Kaen, 40002, Thailand
2 Department of Biochemistry, Faculty of Medicine, Khon Kaen University, Khon Kaen, 40002, Thailand
3 Center for Translational Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, 40002, Thailand
4 Department of Forensic Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, 40002, Thailand
* Corresponding Author: Wunchana Seubwai. Email:
Oncology Research 2025, 33(12), 3679-3699. https://doi.org/10.32604/or.2025.069027
Received 12 June 2025; Accepted 28 September 2025; Issue published 27 November 2025
Abstract
Cholangiocarcinoma (CCA) is an aggressive cancer originating from bile duct epithelium. Surgical resection remains the primary curative treatment for CCA. However, most CCA patients are diagnosed at an advanced stage, which limits the applicability of surgical resection. Gemcitabine is widely used as a first-line chemotherapeutic agent for unresectable CCA. Its efficacy is often compromised by the development of drug resistance, which leads to poor clinical outcomes and low survival rates of CCA patients. At present, the mechanisms underlying gemcitabine resistance in CCA remain unclear. This review aimed to comprehensively summarize the current knowledge on the molecular mechanisms underlying gemcitabine resistance in CCA and highlight emerging therapeutic strategies that may overcome this resistance. Gemcitabine resistance arises through multiple mechanisms, including reduced drug uptake and increased efflux, impaired drug activation, enhanced DNA repair, apoptosis evasion, aberrations in cell-cycle progression, induction of epithelial–mesenchymal transition, metabolic reprogramming, alteration of tumor, and activation of oncogenic pathways contributes to gemcitabine resistance. A deeper understanding of gemcitabine resistance mechanisms highlights the need for combining gemcitabine with pathway-specific inhibitors, which hold promise for overcoming resistance and improving patient outcomes.Keywords
1 Cholangiocarcinoma: Classification, Epidemiology, and Treatment
Cholangiocarcinoma (CCA), also known as bile duct cancer, is a highly aggressive and fatal cancer. CCA originates from the epithelial cells lining the bile ducts. This cancer is the second most common primary liver cancer after hepatocellular carcinoma [1,2]. The location of the tumor has a significant influence on classification, clinical presentation, and treatment strategies. Tumors arising within the liver are classified as intrahepatic CCA, whereas those occurring outside the liver are classified as extrahepatic CCA [3,4]. The incidence of CCA has been increasing worldwide over recent decades, especially in Asia. The highest incidence of CCA has been reported in Northeast Thailand, where rates have reached up to 32 cases per 100,000 population [5,6]. In most Western countries, the age-standardized mortality rate for CCA is generally between 0.5 and 2.0 per 100,000 population [7].
Surgical resection remains the most potent curative treatment in the early stages of CCA, which offers the best chance for long-term survival [8,9]. Surgical resection is only feasible in a minority of patients with CCA; however, most are diagnosed at an advanced stage when the tumor has already spread beyond the biliary tree [10]. As such, chemotherapy is an alternative option to manage the CCA patients who are unable to meet the resection criteria. Many chemotherapeutic drugs, such as gemcitabine, capecitabine, and 5-fluorouracil, have been widely tested in clinical trials for CCA patients [10–12]. For nearly a decade, gemcitabine has been used for single and combination therapy with other chemotherapeutic drugs to treat CCA patients.
Gemcitabine is frequently used as a standard first-line chemotherapy agent for patients with advanced CCA [11,13,14]. Gemcitabine primarily exerts its anticancer effects by interfering with DNA synthesis, thereby inhibiting the growth of cancer cells. It is a nucleoside analog that acts as a prodrug. After entering cancer cells via nucleoside transporters, gemcitabine is phosphorylated by deoxycytidine kinase (dCK) into its active metabolites, including gemcitabine diphosphate (dFdCDP) and gemcitabine triphosphate (dFdCTP). dFdCDP inhibits ribonucleotide reductase, which is a key enzyme that converts ribonucleotides to deoxyribonucleotides. Inhibition of ribonucleotide reductase action by dFdCDP results in a reduction of the pool of deoxyribonucleotides, which are needed for DNA synthesis. In addition, dFdCTP is incorporated into DNA during replication, resulting in blocks to further DNA elongation and leading to apoptosis [15,16]. The clinical benefit of gemcitabine therapy in advanced CCA remains limited. The median overall survival (OS) for patients with advanced-stage CCA treated with gemcitabine-based chemotherapy remains less than two years (Table 1). The limited efficacy of gemcitabine is largely attributed to the development of chemoresistance, which can arise through various mechanisms, including reduced drug uptake, increased drug efflux, altered drug metabolism, enhanced DNA repair, and the activation of survival signaling pathways. Therefore, understanding the mechanisms underlying gemcitabine resistance is crucial for developing strategies to overcome this resistance and enhance the effectiveness of gemcitabine-based chemotherapy.
This review article provides a comprehensive overview of the molecular mechanisms underlying gemcitabine resistance in cholangiocarcinoma, including decreased drug uptake, increased drug efflux through ATP-binding cassette (ABC) transporters, impaired drug activation, enhanced DNA repair, evasion of apoptosis, dysregulation of cell-cycle progression, epithelial mesenchymal transition (EMT), metabolic reprogramming, the influence of the tumor microenvironment (TME), and the activation of survival signaling pathways. It also explores current and emerging therapeutic strategies aimed at overcoming gemcitabine resistance to improve treatment outcomes and survival rates for patients with advanced CCA.
2 Reduced Drug Uptake and Increased Drug Efflux Promote Gemcitabine Resistance
Gemcitabine resistance may result from a reduction in the intracellular concentration of the drug. This phenomenon may be caused by reduced drug uptake into cancer cells or increased drug efflux out of cancer cells [20]. The alteration in drug transport mechanisms can significantly limit the amount of gemcitabine that reaches its target within the cell, thereby reducing its effectiveness.
Gemcitabine requires nucleoside transporters, such as the human equilibrative nucleoside transporter 1 (hENT1), to enter cells. Several studies have indicated an association between high hENT1 expression and the efficacy of gemcitabine in various cancers. High hENT1 expression correlated with gemcitabine efficacy in patients with advanced leiomyosarcoma and angiosarcoma. A study in patients with advanced angiosarcoma and leiomyosarcoma found that patients with high hENT1 expression had significantly better progression-free survival (PFS) and OS than patients with low hENT1 expression. Leiomyosarcoma patients treated with gemcitabine exhibited PFS of 6.8 months vs. 3.2 months, and OS of 14.9 months vs. 8.5 months in patients with high hENT1 and low hENT1 expression, respectively. In addition, in angiosarcoma patients with gemcitabine, hENT1 overexpression was associated with a significant improvement in OS (20.6 vs. 10.8 months) and PFS (9.3 vs. 4.5 months) [21]. In CCA patients, the expression levels of hENT1 can act as a significant biomarker in predicting the survival outcomes of patients with advanced CCA undergoing gemcitabine-based chemotherapy. Clinical data from 40 patients with unresectable or recurrent CCA who received gemcitabine (1000 mg/m2) plus cisplatin (25 mg/m2) demonstrated that hENT1 expression was associated with treatment outcomes. CCA patients with high hENT1 expression had a median PFS of 24 weeks and a median OS of 52 weeks, whereas CCA patients with low hENT1 expression had a median PFS of 11 weeks and a median OS of 26 weeks [22]. Similar finds were reported from Hiroshima University Hospital, Japan [23] and the Cliniques universitaires Saint-Luc, Belgium [24].
In vitro studies have demonstrated the direct involvement of hENT1 in mediating gemcitabine resistance in CCA cell lines. First, gemcitabine-resistant CCA cell lines exhibit significantly lower levels of hENT1 expression compared to their parental cells or gemcitabine-sensitive CCA cell lines [25,26]. Second, knockdown of hENT1 expression by siRNA significantly increased the proliferation in gemcitabine-treated CCA cell lines compared with control siRNA [22].
ATP-Binding Cassette (ABC) transporters are a large family of membrane proteins involved in the efflux of a wide range of drugs and other molecules out of cells. These transporters play a significant role in the efflux of chemotherapeutic agents, resulting in a reduction of intracellular concentrations of chemotherapeutic drugs and chemotherapeutic resistance of cancer cells [27]. Overexpression of ABC transporters is strongly associated with tumor aggressiveness, progression, and poor patient prognosis in several cancers [28]. The overexpression of ABC transporters also plays a significant role in the multidrug resistance (MDR) and is associated with poor prognosis of CCA patients. In gemcitabine-resistant CCA cell lines, the overexpression of multidrug resistance-associated protein 1 (MRP1 or ABCC1) has been identified as a significant factor contributing to enhanced drug efflux and resistance [25]. A similar finding was found in CCA patients, MRP1 is significantly overexpressed in CCA tissues compared to non-tumor tissues. The overexpression of MRP1 was significantly correlated with shortened OS of CCA patients [29]. In addition, HuCCT1 cells, an intrahepatic CCA (ICC) cell line, expressed MRP1, MRP2, MRP4, MRP5, and MRP6, whereas KMBC cells, an extrahepatic CCA (ECC) cell line, expressed MRP1, MRP3, MRP4, and MRP5. MRP5 and MRP6 expressions were markedly elevated in HuCCT1 cells and MRP5 in KMBC cells after gemcitabine treatment. MRP5 and MRP6 knockdown significantly increased gemcitabine cytotoxicity in HuCCT1 and KMBC, respectively [30]. These findings indicate that the mechanisms underlying gemcitabine resistance differ between ICC and ECC.
3 Impaired Drug Activation Enhances Gemcitabine Resistance
Converting inactive gemcitabine into the active form requires multiple phosphorylation processes. The first and rate-limiting step is catalyzed by dCK. After phosphorylation processes, gemcitabine is converted into dFdCDP and dFdCTP. dFdCDP inhibits ribonucleotide reductase, whereas dFdCTP is incorporated into DNA, leading to chain termination and inhibition of DNA synthesis. Through these mechanisms, gemcitabine exerts its cytotoxic effects on cancer cells.
A reduction of dCK activity or expression can decrease gemcitabine activation and lead to gemcitabine resistance in pancreatic cancer [31,32]. There is a strong negative correlation between dCK levels and gemcitabine resistance in various murine tumors and human tumor xenografts [33]. A study on CCA cell lines demonstrated that forced increasing dCK expression through adenoviral transduction significantly enhances gemcitabine sensitivity [34]. In a phase II study of adjuvant chemotherapy using gemcitabine in patients with resected ICC and ECC, intratumoral expression of dCK was associated with longer recurrence-free survival (RFS). Median RFS was 34.95 months in dCK-positive CCA patients compared to 11.41 months in dCK-negative CCA patients [35]. This information indicated that dCK may serve as both a predictive biomarker and a therapeutic target in CCA.
4 Enhanced DNA Repair Mechanisms Increase Gemcitabine Resistance
Gemcitabine exerts its anti-tumor activity by incorporating into DNA, disrupting DNA synthesis, and interfering with the DNA replication process, inducing DNA damage, leading to induction of cancer cell death. Therefore, enhanced DNA repair capacity can counteract the DNA damage induced by gemcitabine and promote gemcitabine resistance in cancer cells [36].
Microarray analysis revealed that p53R2, ribonucleotide reductase regulatory TP53 inducible subunit M2B, is overexpressed in gemcitabine-resistant CCA cell lines [37]. Although the role of p53R2 on gemcitabine response in CCA remains unclear, the role of p53R2 in other cancers has been reported. Data from the TCGA revealed the amplification of p53R2 genes in several types of cancer. In addition, breast cancer patients with p53R2 gene amplification are associated with poor clinical outcomes [38].
5 Apoptosis Evasion Facilitates Gemcitabine Resistance
Induction of apoptosis is a common antitumor pathway of several chemotherapeutic drugs. Apoptosis is triggered by intracellular damage signals, especially DNA damage or nucleotide depletion. Cancer cells that acquire the ability to evade or delay apoptosis can survive chemotherapeutic drugs and develop drug resistance [39,40]. The downregulation of BAX and BAK (pro-apoptotic proteins) and caspase enzymes (caspase-3 and caspase-9) plays a significant role in chemotherapeutic resistance. In contrast, the overexpression of Bcl-2 (anti-apoptotic protein) is associated with resistance to chemotherapy in cancer cells [41,42].
The overexpression of anti-apoptotic proteins and the downregulation of pro-apoptotic proteins were commonly reported in CCA patients. Bcl-2 and XIAP expression are significantly higher in CCA tissues compared to non-cancerous tissues. High expression of these anti-apoptosis proteins in CCA is associated with lower OS and higher recurrence rates [43,44]. In contrast, BAX exhibits low expression in CCA when compared to adjacent non-tumor tissues [43]. Not only clinical observations, but also experimental evidence have demonstrated that apoptosis-related proteins play a critical role in gemcitabine resistance in CCA. In gemcitabine-resistant CCA organoids, inhibition of Bcl-xl, an anti-apoptotic protein, could overcome gemcitabine resistance and induce apoptosis [45].
p53 is a well-known tumor suppressor protein that plays a critical role in the cell cycle, DNA repair, senescence, and apoptosis. Under normal conditions, wild-type p53 is expressed at low levels. In contrast, p53 is activated in response to cellular stress induced by DNA damage, leading to cell cycle arrest or apoptosis. In gemcitabine-resistant CCA cell lines, alterations in apoptosis-related proteins and p53 significantly contribute to the development of drug resistance [25]. Elevation of p53 protein level was reported in 58.8%–77% of CCA patients [46–48]. p53 protein expression was significantly associated with poor differentiation and invasion of CCA [48]. Overexpression of the p53 protein often results from mutations, leading to a loss of its tumor suppressor function and/or the acquisition of oncogenic activities. Accumulation of the p53 mutant contributes to apoptosis evasion, chemoresistance. p53 gene mutations were detected in 61.1% CCA patients [48]. Mutant p53 was positively correlated with high hENT1 expression in ICC tissues. ICC patients with >4% mutant p53–positive tumor cells showed significantly higher hENT1 membrane positivity compared to those with ≤4%. In addition, ICC patients treated with adjuvant gemcitabine, those with >4% mutant p53–positive cells had longer DFS compared with ICC patients with ≤4% mutant p53 (18.5 vs. 6 months) [49]. This finding suggests that dysregulation of apoptosis-related proteins plays a critical role in CCA progression and resistance to chemotherapeutic drugs.
6 Aberration of Cell-Cycle Progression Contributes to Gemcitabine Resistance
Alterations of cell-cycle regulation are a hallmark of tumor progression and significantly contribute to therapeutic resistance [50]. Gemcitabine primarily targets rapidly dividing cells by incorporating into DNA during the Sphase, leading to chain termination and DNA damage. Therefore, the cytotoxic effect of gemcitabine depends on active cell proliferation and efficient engagement of the cell cycle.
Gemcitabine-resistant CCA cell lines tend to grow more slowly, with longer doubling times compared to their parental cells [25,51]. This slow proliferation rate may help cells evade gemcitabine cytotoxicity. Gemcitabine-resistant cell lines showed increased G1 phase arrest, which may reflect a slower proliferation rate. Cell cycle arrest in cancer cells may also be a protective mechanism against gemcitabine-induced cytotoxicity [52]. A transcriptomic analysis of gemcitabine-resistant CCA cells reveals significant alterations in genes involved in cell-cycle regulation, particularly at the G1/S transition phase, such as MCMs, histone-related genes, Rad51, PCNA, POLA2, DNA ligase I, and E2F1/2 [53].
7 EMT Promotes Gemcitabine Resistance
EMT is a dynamic process in which epithelial cells lose their polarity and cell-cell adhesion properties and transform into mesenchymal cells. This phenotypic shift is orchestrated by a network of signaling pathways, including TGF-β, Wnt/β-catenin, and PI3K/Akt, which activate transcription factors that regulate EMT-associated genes. EMT not only drives tumor progression and metastasis in CCA, but also significantly contributes to gemcitabine resistance [54,55].
In pancreatic cancer, EMT involves a shift from E-cadherin to N-cadherin expression. This cadherin switching leads to reduced expression and membrane localization of the equilibrative nucleoside transporter 1 (ENT1). ENT1 is crucial for gemcitabine uptake. Downregulation of ENT1 or hENT1 results in the induction of gemcitabine resistance in CCA [22–24] and pancreatic cancer [56].
8 Role of Cancer-Associated Fibroblasts (CAFs) in Gemcitabine Resistance
CAFs are key stromal components of the tumor microenvironment (TME) in CCA. CAFs play a central role in promoting tumor progression and chemoresistance. Derived from normal fibroblasts, CAFs acquire an activated phenotype characterized by enhanced secretion of growth factors (TGF-β, PDGF, FGF), cytokines, and extracellular matrix components. CAFs contribute to chemoresistance through multiple mechanisms, including promoting angiogenesis, remodeling the extracellular matrix, modulating immune responses, and facilitating cancer-cell survival and proliferation [57]. Among these mechanisms, the IL-6/STAT3 signaling pathway has emerged as a key contributor to gemcitabine resistance in CCA. CAF-derived IL-6 activates STAT3 in CCA cells, enhances survival, and reduces drug sensitivity. High serum levels of IL-6 were observed in CCA patients [58]. High IL-6R expression in patient CCA tissues correlates with poor OS and gemcitabine resistance [59].
Targeting CAFs presents a promising strategy to overcome gemcitabine resistance in CCA. Suppressing CAF activation by nintedanib inhibits the secretion of CAF-derived cytokines, such as IL-6 and IL-8, and enhances the antitumor activity of gemcitabine in ICC in vitro and in vivo [60]. Inhibiting CAF-cancer cell crosstalk using tocilizumab, a monoclonal antibody against the IL-6 receptor, can suppress STAT3 activation and restore gemcitabine sensitivity [59]. In addition, miR-206, a tumor-suppressive microRNA, has been shown to inhibit the transformation of normal fibroblasts into CAFs and enhance gemcitabine efficacy [61]. Together, these findings highlight the therapeutic potential of targeting the CAF-mediated microenvironment to improve treatment outcomes in CCA.
9 The Involvement of microRNAs in Regulating Gemcitabine Response
microRNAs (miRNAs) are small non-coding RNAs, approximately 18–25 nucleotides in length. miRNAs regulate gene expression post-transcriptionally by binding to the 3′ untranslated region of target mRNAs, leading to either mRNA degradation or translational repression. In cancer, miRNAs play critical roles as oncogenes or tumor suppressors. Aberrant expression of specific miRNAs has been identified in various tumor types and is often associated with tumor initiation, progression, and patient prognosis [62,63].
Several miRNAs have been identified as regulators of gemcitabine resistance in CCA. Oncogenic miRNAs promote gemcitabine resistance, whereas tumor suppressor miRNAs enhance gemcitabine sensitivity [64]. Upregulation of miR-130a-3p was found in gemcitabine-resistant CCA cells. miR-130a-3p promotes gemcitabine resistance by targeting peroxisome proliferator-activated receptor gamma (PPARγ), which has tumor-suppressive functions, including cell-cycle arrest and DNA repair. Inhibition of miR-130a-3p or activation of PPARγ by pioglitazone alleviates resistance and improves gemcitabine efficacy [65].
miR-122, miR-192, miR-29b and miR-155 were significantly increased in serum of CCA patients [66]. miR-29b, miR-205, and miR-221 have been identified as key miRNAs that influence the sensitivity of the HuH28 CCA cell line to gemcitabine. Based on a computational analysis, PIK3R1 and MMP-2 were identified as potential gene targets for these miRNAs. PIK3R1 and MMP-2 are involved in pathways that promote cell survival and proliferation, making them potential targets to enhance chemotherapy effectiveness [67].
10 Metabolic Reprogramming of Glucose Metabolism Enhances Gemcitabine Resistance
The relationship between glucose metabolism and gemcitabine resistance in CCA is a complex interplay of metabolic reprogramming and cellular adaptation mechanisms. CCA cells exhibit a high dependency on glucose metabolism, which is often linked to the development of resistance to gemcitabine. Gemcitabine resistance based on glucose metabolism is mediated through various pathways, including alterations in glycolysis, reactive oxygen species (ROS), and cancer stem cell (CSC) phenotypes [68,69].
CCA cells exhibit a preference for glycolysis over oxidative phosphorylation (OXPHOS) for energy production in the presence of oxygen [70,71]. This phenomenon is known as the Warburg effect. This metabolic reprogramming supports rapid cell proliferation and survival under stress conditions. Overexpression of glucose transporter 1 (GLUT1) [72,73], and several key glycolytic-related enzymes, such as pyruvate kinase M2(PKM2) [74], and lactate dehydrogenase A (LDHA) [75,76], have been reported in CCA. Overexpression of these glycolytic-related enzymes correlated with poor prognosis and gemcitabine resistance in CCA.
Overexpression of GLUT1 was significantly associated with non-papillary type, large tumor size, and short survival of CCA patients [72]. Expression of GLUT1 was increased in gemcitabine-treated CCA cell line. Silencing GLUT1 significantly increases the gemcitabine sensitivity of CCA cell in vitro and in vivo [73].
PKM2 contributes to gemcitabine resistance through both metabolic and non-metabolic mechanisms. Silencing PKM2 expression enhances pancreatic cancer cells to gemcitabine treatment. This effect of PKM2 is associated with the activation of the p38 MAPK pathway and subsequent phosphorylation of p53 at serine 46, culminating in caspase-3/7 activation and PARP cleavage [77]. PKM2 is not only involved in glycolysis but also in regulating oncogenic pathways. Nuclear PKM2 acts as a protein kinase and promotes tumor growth via the activation of oncogenic pathways such as STAT3 [78,79]. Inhibition of PKM2 using siRNA or an inhibitor enhances the gemcitabine sensitivity of ICC in vitro and in vivo [80].
Although the relationship between LDHA and gemcitabine resistance in CCA has not been directly reported, evidence from other cancers suggests that LDHA plays a critical role in gemcitabine resistance. Novel LDH-A inhibitors exhibit a synergistic effect on gemcitabine-treated pancreatic cancer cells under hypoxic conditions [81]. Altogether, the evidence mentioned above suggests that targeting glycolytic-associated molecules could be a potential strategy to overcome gemcitabine resistance in CCA cells.
ROS play a critical role in various cellular processes, including signal transduction and the regulation of apoptosis. Dysregulated ROS production has been implicated in the development of chemoresistance in cancer cells [82]. High-glucose conditions enhance ROS production in CCA, which plays a pivotal role in promoting the proliferation and migration of CCA cell lines [83].
Gemcitabine-resistant CCA cells shift their metabolism toward glycolysis, which reduces reliance on mitochondrial oxidative phosphorylation. This metabolic shift correlates with CSC-like features, which are associated with chemoresistance. The tumor tissues from CCA patients who do not respond to gemcitabine have low TCA cycle activity and high glucose levels, which is consistent with CSC metabolic signatures [84].
11 The Role of Hypoxia in Gemcitabine Resistance
Hypoxia is a common phenomenon in the TME. Rapidly growing tumors often exceed their blood supply, leading to regions of low oxygen tension. This hypoxic microenvironment can significantly influence cancer-cell behavior and treatment response to chemotherapeutic drugs. Hypoxic conditions can promote cancer-cell survival, proliferation, and metastasis, while also reducing the effectiveness of chemotherapeutic agents. Hypoxia is associated with treatment resistance and poor prognosis in CCA [85,86].
Hypoxia-inducible factor 1-alpha (HIF-1α) is a key transcription factor that regulates cellular adaptation to hypoxia and promotes tumor survival, angiogenesis, and metastatic progression. In CCA, overexpression of HIF-1α correlates with advanced tumor stage, increased tumor size, vascular invasion, and intrahepatic metastasis. Patients with HIF-1α-positive tumors exhibit significantly poorer survival outcomes compared to those with HIF-1α-negative tumors [86,87]. HIF-1α also plays a critical role in promoting gemcitabine resistance in CCA through several pathways. Elevated miR-210 sustains HIF-1α activity by suppressing HIF-3α, a negative regulator of HIF-1α. This feed-forward loop promotes cell-cycle arrest at G2/M phase and reduces gemcitabine sensitivity in CCA cells under hypoxia [88]. Overexpression of spindle and kinetochore-associated complex subunit 3 (SKA3) under hypoxia promotes gemcitabine resistance by enhancing fatty acid synthesis via the PARP1/HIF-1α axis. SKA3-overexpressing CCA cells show increased survival and reduced DNA damage after gemcitabine treatment under hypoxic conditions, both in vitro and in vivo [89].
12 Aberrant Activation of Signaling Pathways Mediates Gemcitabine Resistance
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that plays a role in cell proliferation, survival, and differentiation. EGFR is activated by binding to EGF, which triggers a cascade of intracellular signaling events that promote cell growth and survival. EGFR is overexpressed in several human tumors, including lung, breast, and liver cancers. Overexpression of EGFR is associated with aggressive tumor behavior and poor prognosis in several cancers and CCA [90,91]. In ECC, EGFR expression was significantly associated with lymph node metastasis, lymphatic vessels invasion, and perineural invasion. In addition, positive-EGFR expression was also correlated with shorter survival of ICC and ECC patients [91]. Dysregulation of EGFR signaling contributes to gemcitabine resistance. Gemcitabine-induced DNA damage leads to elevated ROS production, which contributes to the activation of stress-related kinases such as Src. This activation suppresses phosphatases that normally dephosphorylate EGFR, resulting in the promotion of phosphorylation of EGFR at tyrosine residues Y845 and Y1173. The activation of EGFR signaling pathways promotes cell survival and proliferation, which in turn reduces gemcitabine efficacy [92,93]. Increased phosphorylation of EGFR is observed in gemcitabine-resistant CCA cells. Erlotinib, a specific inhibitor of EGFR, significantly enhances the antitumor activity of gemcitabine with a synergistic effect in gemcitabine-resistant CCA cells. The synergistic effect of erlotinib on gemcitabine sensitivity suggests a significant role of EGFR signaling in gemcitabine resistance in CCA cells [52].
The PI3K/Akt/mTOR pathway is involved in cell growth, survival, metabolism, and chemotherapeutic resistance. This signaling pathway is activated through stimulation by various growth factors, cytokines, and hormones. Aberrant activation of PI3K/Akt/mTOR pathway has been frequently observed and associated with the progression and poor prognosis of CCA [94,95]. Inhibitions of PI3K/Akt/mTOR pathway by cannabidiol, NVP-BEZ235, and Tanshinone IIA have been shown to reduce proliferation and migration while increasing autophagy, apoptosis, and senescence in CCA cells [94,96,97].
Activation of the PI3K/Akt pathway has been linked to gemcitabine resistance in CCA. This activation supports cancer-cell survival and proliferation, thus diminishing the responsiveness of cancer cells to gemcitabine [98,99]. In contrast, inhibition of the PI3K/Akt pathway has been shown to restore gemcitabine sensitivity [100]. This evidence suggests that targeting the PI3K/Akt/mTOR pathway is a promising therapeutic target to overcome gemcitabine resistance in CCA.
The signal transducer and activator of transcription 3 (STAT3), a transcription factor which activated by various cytokines and growth factors. Once activated, STAT3 translocated to the nucleus, binds to DNA, and regulates the expression of genes involved in cancer progression [101]. Overexpression of STAT3 was associated with several poor clinical outcomes of CCA patients, including tumor size, vascular invasion, lymph node metastasis, shorter OS, and DFS [59,102]. IL-6 is a cytokine that activates the STAT3 signaling pathway. Increased IL-6/STAT3 activation can promote cancer-cell survival and reduce the sensitivity to gemcitabine [59]. Blockade of IL-6R can inhibit the CAF-CCA interaction and enhance gemcitabine sensitivity [59]. This approach represents a promising strategy for overcoming chemoresistance in CCA.
Wnt/β-catenin plays a crucial role in regulating various cellular processes in cancer cells, such as proliferation, differentiation, migration, apoptosis, and chemoresistance. Inhibition of Wnt/β-catenin signaling pathway using inhibitors (C59, XAV939, and PRI724) caused a reduction in the expression of its downstream target genes, namely AXIN2 and survivin. Furthermore, Supplementation of C59, XAV939, and PRI724 significantly enhanced the anti-tumor activity of gemcitabine in ICC and ECC cell lines [103].
13 Novel Therapeutic Strategies to Overcome Gemcitabine Resistance
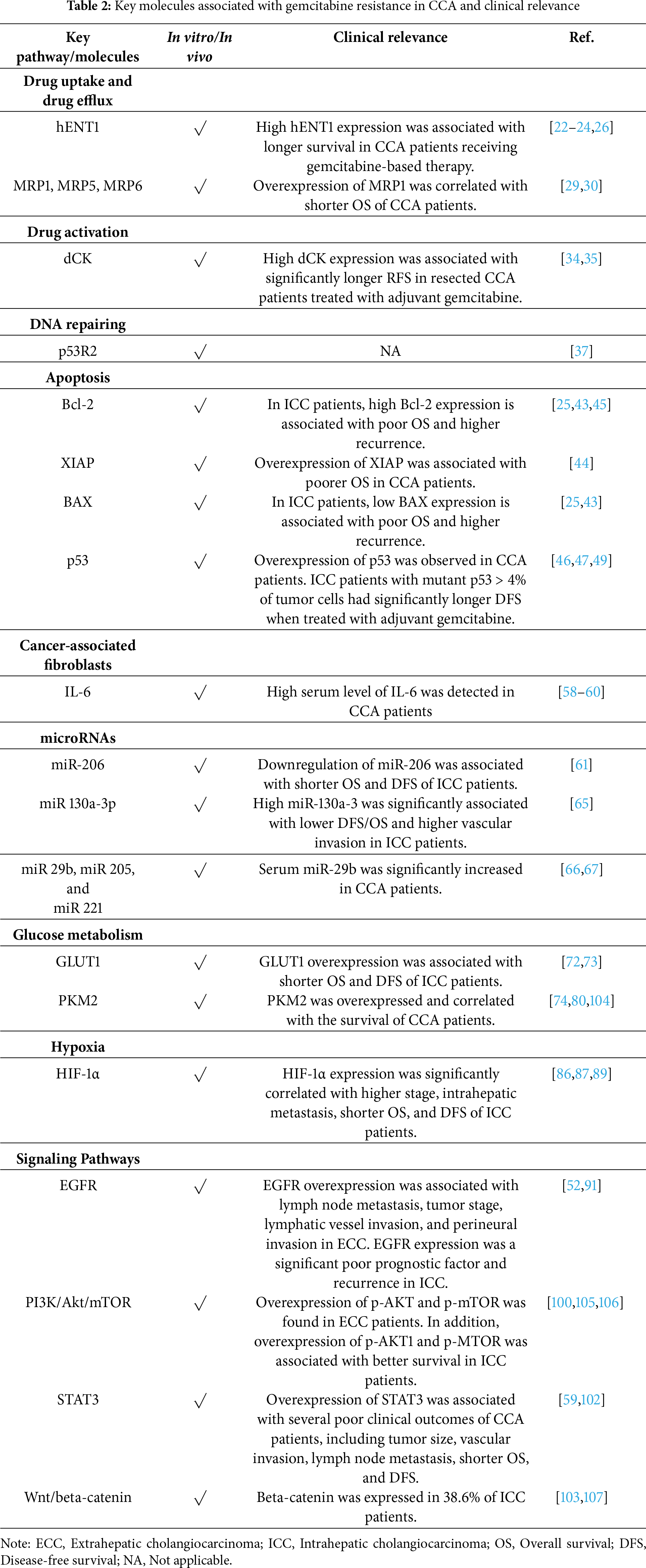
The mechanistic insights into gemcitabine resistance suggest potential therapeutic strategies for restoring drug sensitivity and improving clinical outcomes. The potential molecules related to gemcitabine resistance in CCA are summarized and demonstrated in Fig. 1 and Table 2.
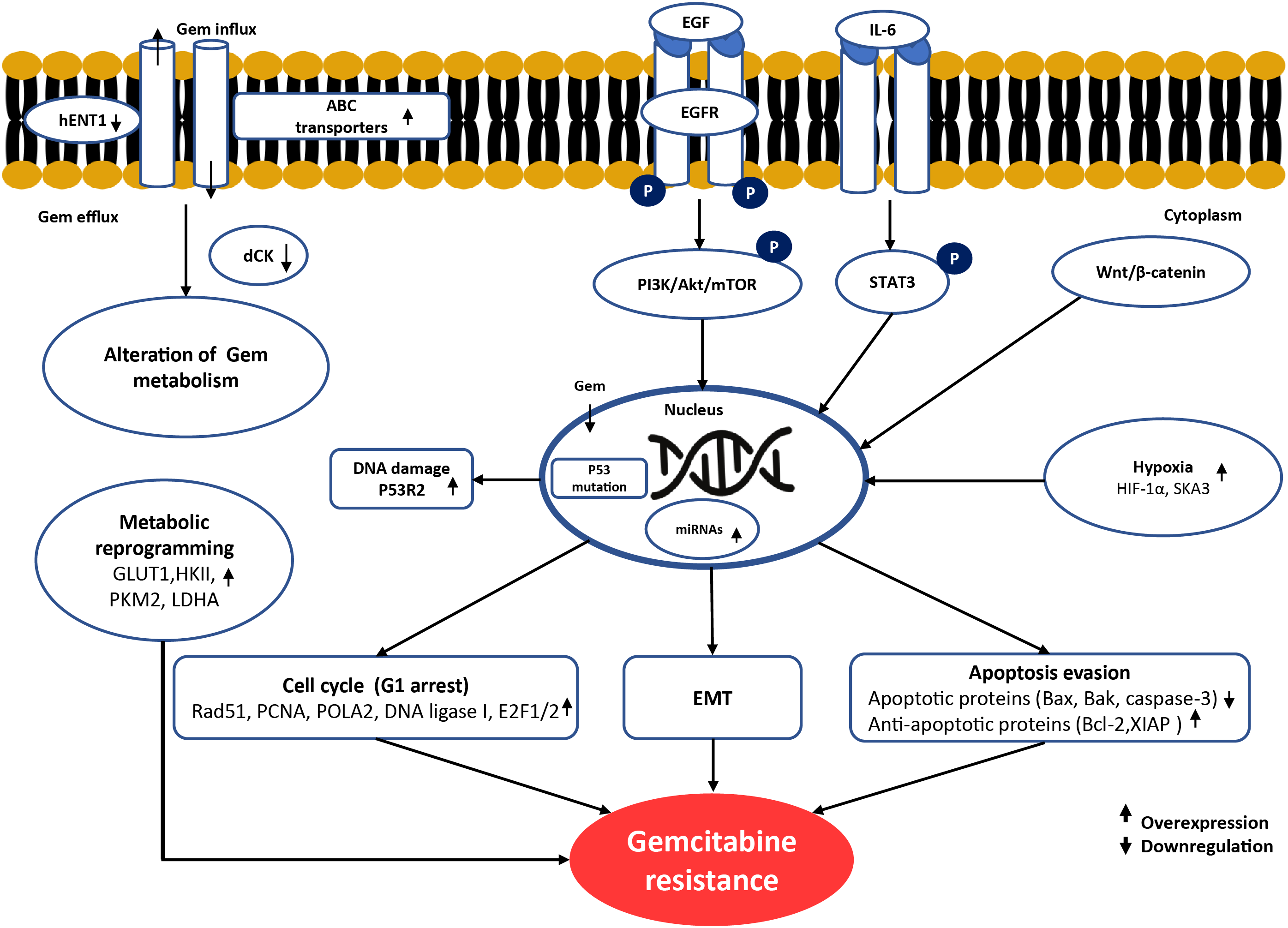
Figure 1: Schematic summary of the molecular mechanisms underlying gemcitabine resistance in cholangiocarcinoma. Gemcitabine resistance involves multiple alterations. At the cell membrane, reduced drug uptake through downregulation of hENT1 and increased drug efflux via ABC transporters limit intracellular gemcitabine accumulation. In cancer cells, impaired gemcitabine activation occurs due to reduced dCK expression. Dysregulation of apoptosis is observed through downregulation of BAX, BAK, and caspase-3, together with overexpression of anti-apoptotic proteins such as Bcl-2 and XIAP. Cell-cycle arrest at G1 phase driven by alterations in Rad51, PCNA, POLA2, DNA ligase I, and E2F1/2 reduces gemcitabine cytotoxicity by limiting incorporation during the S phase. Metabolic reprogramming, including overexpression of GLUT1, HKII, PKM2, and LDHA, enhances glycolysis and promotes gemcitabine resistance. EMT and aberrant activation of oncogenic signaling pathways, such as EGFR, PI3K/Akt/mTOR, STAT3, and Wnt/β-catenin, support survival and proliferation under stress conditions induced by gemcitabine. In the nucleus, enhanced DNA repair mediated by p53R2 (RRM2B) counteracts gemcitabine-induced DNA damage. In the tumor microenvironment, hypoxia-related factors (HIF-1α and SKA3) can induce gemcitabine resistance by promoting metabolic and signaling adaptations. Upward arrows (↑) indicate overexpression/activation, whereas downward arrows (↓) indicate downregulation/inactivation of the corresponding molecules. Created using Microsoft PowerPoint. Kidoikhammouan, S. (2025)
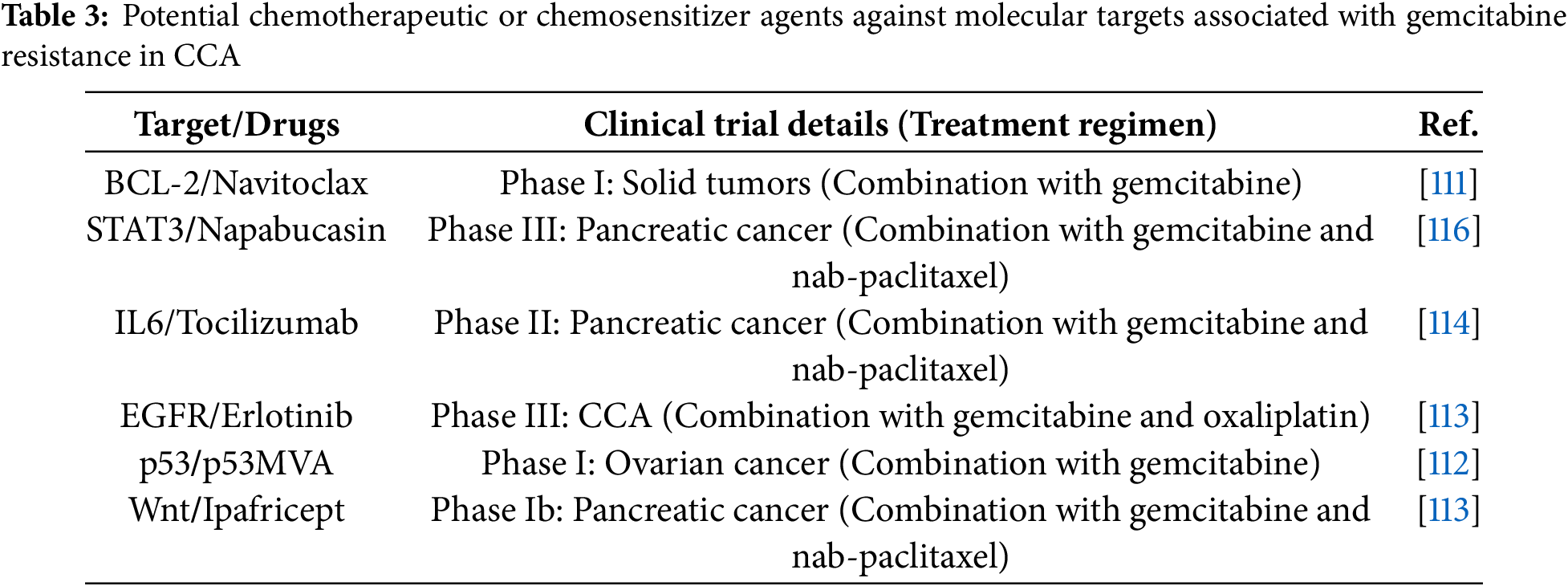
Several chemotherapeutic and chemosensitizing agents targeting gemcitabine resistance pathways have been evaluated in preclinical models and clinical trials of CCA and other cancers, as summarized in Table 3.
One of the promising strategies involves targeting drug transport mechanisms. For example, enhancing the expression or function of hENT1, which mediates gemcitabine uptake. On the other hand, inhibition of ABC transporters could prevent drug efflux and increase intracellular gemcitabine concentration in cancer cells. VX-710 (biricodar, INCEL), an inhibitor of P-glycoprotein (P-gp) and MRP1, has been investigated as a chemosensitizer in several cancers in preclinical and clinical studies. Although the combination of VX-710 with gemcitabine has not yet been investigated, studies with other chemotherapeutic agents have demonstrated promising results, such as recurrent small-cell lung cancer [108], ovarian cancer [109]. Similarly, dofequidar fumarate (MS-209), an orally bioavailable quinoline compound that inhibits P-gp and MDR-1, has also demonstrated potential in overcoming multidrug resistance in cancer therapy [110].
Another possible approach is the restoration of gemcitabine activation through the upregulation of dCK, the rate-limiting enzyme responsible for gemcitabine phosphorylation. Preclinical studies have demonstrated that increased dCK expression sensitizes CCA cells to gemcitabine, which suggests the potential for gene therapy or pharmacological induction.
Targeting enhanced DNA repair pathways also holds therapeutic potential. Upregulation of ribonucleotide reductase subunit p53R2 has been implicated in resistance by maintaining deoxyribonucleotide pools necessary for DNA repair. Inhibiting such compensatory repair mechanisms may sensitize tumor cells to gemcitabine-induced DNA damage.
Apoptosis evasion is a key contributor to gemcitabine in CCA. Therapies that restore pro-apoptotic signaling, such as upregulating BAX and caspases or inhibiting anti-apoptotic proteins like Bcl-2 and XIAP, have been proposed to resensitize cells to gemcitabine. Nowadays, there are several BCL-2 family inhibitors have been used in clinical trials, such as Venetoclax (a selective small-molecule inhibitor for targeting of Bcl-2) and Navitoclax. Navitoclax or ABT-263 is a pan-BCL-2 family inhibitor that inhibits Bcl-XL, Bcl-2, and Bcl-W. This pan-BCL-2 family inhibitor has been used in phase I and II clinical trials of several cancers. A phase I clinical trial on the combination of navitoclax with gemcitabine in patients with solid tumors found that the combination was generally well tolerated and exhibited a favorable safety profile in patients with advanced solid tumors [111].
Moreover, targeting mutant p53, which contributes to apoptosis resistance and chemoresistance, represents another promising avenue. In the phase 1 clinical trial, the combination of p53-expressing modified vaccinia Ankara virus (p53MVA) with gemcitabine was used to treat ovarian cancer patients. The result demonstrated that in 11 patients who received at least one treatment cycle, no complete responses were observed. Three patients achieved stable disease, and one patient had a partial response (64% tumor reduction) [112].
Alterations in cell-cycle progression contribute to a reduced cytotoxic response to gemcitabine. Agents that modulate cell-cycle regulators and promote S-phase entry may restore drug sensitivity by enhancing DNA incorporation of gemcitabine.
Emerging evidence also highlights the role of EMT and non-coding RNAs in chemoresistance. Inhibiting EMT-associated signaling pathways (such as TGF-β and Wnt/β-catenin) can reverse EMT-mediated drug resistance. The Phase Ib study of ipafricept (OMP-54F28), a Wnt pathway inhibitor, combined with gemcitabine and nab-paclitaxel in untreated stage IV pancreatic cancer patients showed that ipafricept can be administered with standard chemotherapy with reasonable tolerance. The study reported a clinical benefit rate of 81%, including 34.6% partial responses and 46.2% stable disease. Median progression-free survival was 5.9 months, and median overall survival was 9.7 months [113].
The modulation of miRNAs, such as suppressing oncogenic miR-130a-3p or restoring tumor-suppressive miR-424-5p, has demonstrated potential in sensitizing CCA cells to gemcitabine.
The TME, particularly CAFs, contributes to gemcitabine resistance via the secretion of cytokines such as IL-6, which activate the STAT3 signaling pathway in tumor cells. Targeting this interaction using monoclonal antibodies against IL-6R (tocilizumab) or reprogramming CAFs through miRNAs such as miR-206 may provide therapeutic benefit. The randomized Phase II study evaluated the combination of tocilizumab, an anti-IL-6 receptor antibody, with a first-line chemotherapy agent of gemcitabine and nab-paclitaxel in patients with advanced pancreatic cancer. Overall survival at 6 months showed no statistically significant difference between the combination (tocilizumab + gemcitabine/nab-paclitaxel) and chemotherapy alone (68.6% vs. 62.0%). However, overall survival at 18 months improved significantly with tocilizumab (27.1% vs. 7.0%) [114].
Metabolic reprogramming, including enhanced glycolysis and reduced oxidative phosphorylation, has also been linked to chemoresistance. Inhibitors of GLUT1 and key glycolytic enzymes, such as HK2, PKM2, and LDHA, have demonstrated efficacy in reversing resistance and restoring gemcitabine sensitivity.
Furthermore, hypoxia-induced pathways involving HIF-1α and its downstream effectors (miR-210 and SKA3) have been identified as mediators of drug resistance under low oxygen conditions. Therapeutic strategies targeting hypoxia signaling may be effective in overcoming resistance in hypoxic tumor regions.
Finally, aberrant activation of oncogenic signaling pathways, including EGFR, PI3K/Akt/mTOR, and STAT3, plays a central role in gemcitabine resistance. Pharmacological agents that inhibit these pathways, such as erlotinib (EGFR inhibitor), NVP-BEZ235 (PI3K/mTOR dual inhibitor), or cannabidiol (Akt/mTOR modulator), have shown synergistic effects with gemcitabine in preclinical models of CCA. A randomized phase 3 study investigated the efficacy of gemcitabine and oxaliplatin (GEMOX) with or without erlotinib in advanced CCA patients. The study found that more patients had an objective response in the chemotherapy plus erlotinib group than in the chemotherapy alone (40 patients vs. 21 patients) [115].
Collectively, these findings underscore the need for integrated therapeutic strategies that simultaneously target multiple resistance pathways. Further preclinical and clinical investigations are essential to validate these approaches and facilitate their translation into effective treatment regimens for patients with gemcitabine-resistant CCA.
In summary, overcoming gemcitabine resistance in CCA requires a multifaceted therapeutic approach that targets the diverse molecular alterations exploited by resistant cancer cells. These mechanisms include reduced drug uptake, enhanced drug efflux via ABC transporters, impaired drug activation, upregulated DNA repair pathways, evasion of apoptosis, dysregulation of cell-cycle progression, activation of EMT, metabolic reprogramming, influences from the TME, and aberrant activation of pro-survival signaling cascades. Targeting these pathways represents a promising strategy to restore gemcitabine sensitivity in CCA cells.
This intricate network of gemcitabine resistance pathways highlights the need for comprehensive therapeutic strategies that target multiple molecules within CCA cells. Rational combination therapies such as combination treatment of gemcitabine with agents that improve drug uptake, induce DNA damage, restore apoptosis, or inhibit key oncogenic pathways hold promise for overcoming chemoresistance.
Moreover, the emergence of computational drug discovery platforms and AI-based predictive models offers a powerful framework to identify compounds capable of reversing resistance-associated gene-expression profiles and predicting synergistic drug combinations. Tools such as the Connectivity Map (CMap), iLINCS, and L1000FWD facilitate transcriptomics-guided drug repurposing by matching disease-specific signatures with compounds that elicit opposing transcriptional effects. In parallel, deep learning models including MatchMaker and DeepSynergy enable synergy prediction based on molecular profiles and drug structure, thereby providing insight into rational combination strategies.
Through the strategic integration of mechanistic understanding, computational drug discovery, and precision-medicine approaches, the therapeutic landscape for gemcitabine-resistant CCA may be transformed from a clinical challenge to a tractable CCA with enhanced therapeutic opportunities and improved patient prognosis. Future research should prioritize validating promising targets in large-scale clinical trials, incorporating multi-omics data into predictive frameworks, and translating these findings into clinically applicable regimens. Such strategies may ultimately improve survival outcomes and quality of life for patients with CCA.
Acknowledgement: None.
Funding Statement: This work was supported by the Khon Kaen University Graduate School. Funding was provided to W. Seubwai and S. Kidoikhammouan by the program to admit high potential students for study and research at the Graduate School [631JH219].
Author Contributions: Sonexai Kidoikhammouan: Writing—original draft. Charupong Saengboonmee and Sopit Wongkham: Supervision. Wunchana Seubwai: Supervision, editing, and reviewing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Use of Artificial Intelligence: This manuscript used artificial intelligence tools, including ChatGPT (OpenAI, version GPT-5) and QuillBot for language enhancement and grammar correction. These tools were applied exclusively to improve the quality of the English writing and did not influence the scientific content. All final decisions regarding the text were made by the authors.
References
1. Sarcognato S, Sacchi D, Fassan M, Fabris L, Cadamuro M, Zanus G, et al. Cholangiocarcinoma. Pathologica. 2021;113(3):158–69. [Google Scholar] [PubMed]
2. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7(1):65. [Google Scholar] [PubMed]
3. Byrling J, Andersson B, Andersson R, Marko-Varga G. Cholangiocarcinoma–current classification and challenges towards personalised medicine. Scand J Gastroenterol. 2016;51(6):641–3. doi:10.3109/00365521.2015.1127409. [Google Scholar] [PubMed] [CrossRef]
4. Gopal P, Robert ME, Zhang X. Cholangiocarcinoma: pathologic and molecular classification in the era of precision medicine. Arch Pathol Lab Med. 2024;148(3):359–70. doi:10.5858/arpa.2022-0537-ra. [Google Scholar] [PubMed] [CrossRef]
5. Anchalee N, Thinkhamrop K, Suwannatrai AT, Titapun A, Loilome W, Kelly M. Spatio-temporal analysis of cholangiocarcinoma in a high prevalence area of Northeastern Thailand: a 10-year large scale screening program. Asian Pac J Cancer Prev. 2024;25(2):537–46. doi:10.31557/apjcp.2024.25.2.537. [Google Scholar] [PubMed] [CrossRef]
6. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–88. doi:10.1038/s41575-020-0310-z. [Google Scholar] [PubMed] [CrossRef]
7. Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol. 2022;77(6):1690–8. [Google Scholar] [PubMed]
8. Kodali S, Shetty A, Shekhar S, Victor DW, Ghobrial RM. Management of intrahepatic cholangiocarcinoma. J Clin Med. 2021;10(11):2368. doi:10.3390/jcm10112368. [Google Scholar] [PubMed] [CrossRef]
9. Melandro F, Nasto RA, Ginesini M, Balzano E, Bindi ML, Ghinolfi D, et al. A narrative review of intrahepatic cholangiocarcinoma: a surgical curative option. Chin Clin Oncol. 2023;12(2):13. doi:10.21037/cco-22-85. [Google Scholar] [PubMed] [CrossRef]
10. Luvira V, Nilprapha K, Bhudhisawasdi V, Pugkhem A, Chamadol N, Kamsa-ard S. Cholangiocarcinoma patient outcome in Northeastern Thailand: single-center prospective study. Asian Pac J Cancer Prev. 2016;17(1):401–6. doi:10.7314/apjcp.2016.17.1.401. [Google Scholar] [PubMed] [CrossRef]
11. Larsen FO, Mellergaard AH, Hoegdall DT, Jensen LH. Gemcitabine, capecitabine and oxaliplatin in advanced biliary tract carcinoma. Acta Oncol. 2014;53(10):1448–50. doi:10.3109/0284186x.2014.926026. [Google Scholar] [PubMed] [CrossRef]
12. Luvira V, Satitkarnmanee E, Pugkhem A, Kietpeerakool C, Lumbiganon P, Pattanittum P. Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma. Cochrane Database Syst Rev. 2021;9(9):CD012814. doi:10.1002/14651858.cd012814. [Google Scholar] [CrossRef]
13. Mohring C, Feder J, Mohr RU, Sadeghlar F, Bartels A, Mahn R, et al. First line and second line chemotherapy in advanced cholangiocarcinoma and impact of dose reduction of chemotherapy: a retrospective analysis. Front Oncol. 2021;11:717397. doi:10.3389/fonc.2021.717397. [Google Scholar] [PubMed] [CrossRef]
14. Hyung J, Kim B, Yoo C, Kim KP, Jeong JH, Chang HM, et al. Clinical benefit of maintenance therapy for advanced biliary tract cancer patients showing no progression after first-line Gemcitabine Plus Cisplatin. Cancer Res Treat. 2019;51(3):901–9. doi:10.4143/crt.2018.326. [Google Scholar] [PubMed] [CrossRef]
15. Gesto DS, Cerqueira NM, Fernandes PA, Ramos MJ. Gemcitabine: a critical nucleoside for cancer therapy. Curr Med Chem. 2012;19(7):1076–87. doi:10.2174/092986712799320682. [Google Scholar] [PubMed] [CrossRef]
16. Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs. 1995;6 Suppl 6:7–13. doi:10.1097/00001813-199512006-00002. [Google Scholar] [PubMed] [CrossRef]
17. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. doi:10.1056/nejmoa0908721. [Google Scholar] [PubMed] [CrossRef]
18. Charoentum C, Thongprasert S, Chewaskulyong B, Munprakan S. Experience with gemcitabine and cisplatin in the therapy of inoperable and metastatic cholangiocarcinoma. World J Gastroenterol. 2007;13(20):2852–4. doi:10.3748/wjg.v13.i20.2852. [Google Scholar] [PubMed] [CrossRef]
19. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: a phase 2 clinical trial. JAMA Oncol. 2019;5(6):824–30. doi:10.1001/jamaoncol.2019.0270. [Google Scholar] [PubMed] [CrossRef]
20. Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers. 2017;9(11):157. doi:10.3390/cancers9110157. [Google Scholar] [PubMed] [CrossRef]
21. Vincenzi B, Stacchiotti S, Collini P, Pantano F, Rabitti C, Perrone G, et al. Human equilibrative nucleoside transporter 1 gene expression is associated with gemcitabine efficacy in advanced leiomyosarcoma and angiosarcoma. Br J Cancer. 2017;117(3):340–6. doi:10.1038/bjc.2017.187. [Google Scholar] [PubMed] [CrossRef]
22. Kim J, Kim H, Lee JC, Kim JW, Paik WH, Lee SH, et al. Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS One. 2018;13(12):e0209104. doi:10.1371/journal.pone.0209104. [Google Scholar] [PubMed] [CrossRef]
23. Kobayashi H, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, et al. Human equilibrative nucleoside transporter 1 expression predicts survival of advanced cholangiocarcinoma patients treated with gemcitabine-based adjuvant chemotherapy after surgical resection. Ann Surg. 2012;256(2):288–96. doi:10.1097/sla.0b013e3182536a42. [Google Scholar] [PubMed] [CrossRef]
24. Borbath I, Verbrugghe L, Lai R, Gigot JF, Humblet Y, Piessevaux H, et al. Human equilibrative nucleoside transporter 1 (hENT1) expression is a potential predictive tool for response to gemcitabine in patients with advanced cholangiocarcinoma. Eur J Cancer. 2012;48(7):990–6. doi:10.1016/j.ejca.2011.11.006. [Google Scholar] [PubMed] [CrossRef]
25. Wattanawongdon W, Hahnvajanawong C, Namwat N, Kanchanawat S, Boonmars T, Jearanaikoon P, et al. Establishment and characterization of gemcitabine-resistant human cholangiocarcinoma cell lines with multidrug resistance and enhanced invasiveness. Int J Oncol. 2015;47(1):398–410. doi:10.3892/ijo.2015.3019. [Google Scholar] [PubMed] [CrossRef]
26. Mori R, Ishikawa T, Ichikawa Y, Taniguchi K, Matsuyama R, Ueda M, et al. Human equilibrative nucleoside transporter 1 is associated with the chemosensitivity of gemcitabine in human pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncol Rep. 2007;17(5):1201–5. doi:10.3892/or.17.5.1201. [Google Scholar] [CrossRef]
27. Pote MS, Gacche RN. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov Today. 2023;28(5):103537. doi:10.1016/j.drudis.2023.103537. [Google Scholar] [PubMed] [CrossRef]
28. Yang G, Wang XJ, Huang LJ, Zhou YA, Tian F, Zhao JB, et al. High ABCG4 expression is associated with poor prognosis in non-small-cell lung cancer patients treated with cisplatin-based chemotherapy. PLoS One. 2015;10(8):e0135576. doi:10.1371/journal.pone.0135576. [Google Scholar] [PubMed] [CrossRef]
29. Srimunta U, Sawanyawisuth K, Kraiklang R, Pairojkul C, Puapairoj A, Titipungul T, et al. High expression of ABCC1 indicates poor prognosis in intrahepatic cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13 Suppl:125–30. [Google Scholar] [PubMed]
30. Yang J, Sontag D, Gong Y, Minuk GY. Enhanced gemcitabine cytotoxicity with knockdown of multidrug resistance protein genes in human cholangiocarcinoma cell lines. J Gastroenterol Hepatol. 2021;36(4):1103–9. doi:10.1111/jgh.15289. [Google Scholar] [PubMed] [CrossRef]
31. Ohhashi S, Ohuchida K, Mizumoto K, Fujita H, Egami T, Yu J, et al. Down-regulation of deoxycytidine kinase enhances acquired resistance to gemcitabine in pancreatic cancer. Anticancer Res. 2008;28(4B):2205–12. [Google Scholar] [PubMed]
32. Dash S, Ueda T, Komuro A, Honda M, Sugisawa R, Okada H. Deoxycytidine kinase inactivation enhances gemcitabine resistance and sensitizes mitochondrial metabolism interference in pancreatic cancer. Cell Death Dis. 2024;15(2):131. doi:10.1038/s41419-024-06628-3. [Google Scholar] [PubMed] [CrossRef]
33. Kroep JR, Loves WJ, van der Wilt CL, Alvarez E, Talianidis I, Boven E, et al. Pretreatment deoxycytidine kinase levels predict in vivo gemcitabine sensitivity. Mol Cancer Ther. 2002;1(6):371–6. doi:10.1007/0-306-46843-3_56. [Google Scholar] [CrossRef]
34. Kamada M, Akiyoshi K, Akiyama N, Funamizu N, Watanabe M, Fujioka K, et al. Cholangiocarcinoma cell line TK may be useful for the pharmacokinetic study of the chemotherapeutic agent gemcitabine. Oncol Rep. 2014;32(2):829–34. doi:10.3892/or.2014.3227. [Google Scholar] [PubMed] [CrossRef]
35. Woo SM, Yoon KA, Hong EK, Park WS, Han SS, Park SJ, et al. DCK expression, a potential predictive biomarker in the adjuvant gemcitabine chemotherapy for biliary tract cancer after surgical resection: results from a phase II study. Oncotarget. 2017;8(46):81394–404. doi:10.18632/oncotarget.19037. [Google Scholar] [PubMed] [CrossRef]
36. Ma X, Fu T, Ke ZY, Du SL, Wang XC, Zhou N, et al. MiR-17-5p/RRM2 regulated gemcitabine resistance in lung cancer A549 cells. Cell Cycle. 2023;22(11):1367–79. doi:10.1080/15384101.2023.2207247. [Google Scholar] [PubMed] [CrossRef]
37. Sato J, Kimura T, Saito T, Anazawa T, Kenjo A, Sato Y, et al. Gene expression analysis for predicting gemcitabine resistance in human cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2011;18(5):700–11. doi:10.1007/s00534-011-0376-7. [Google Scholar] [PubMed] [CrossRef]
38. Iqbal W, Demidova EV, Serrao S, ValizadehAslani T, Rosen G, Arora S. RRM2B is frequently amplified across multiple tumor types: implications for DNA repair, cellular survival, and cancer therapy. Front Genet. 2021;12:628758. doi:10.3389/fgene.2021.628758. [Google Scholar] [PubMed] [CrossRef]
39. Hickman JA. Apoptosis and chemotherapy resistance. Eur J Cancer. 1996;32A(6):921–6. [Google Scholar] [PubMed]
40. Dogan E, Kara HG, Kosova B, Cetintas VB. Targeting apoptosis to overcome chemotherapy resistance. In: Sergi CM, editor. Metastasis. Brisbane, QLD, Austrilia: Exon Publications; 2022. [Google Scholar]
41. Neophytou CM, Trougakos IP, Erin N, Papageorgis P. Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers. 2021;13(17):4363. doi:10.3390/cancers13174363. [Google Scholar] [PubMed] [CrossRef]
42. Lopez A, Reyna DE, Gitego N, Kopp F, Zhou H, Miranda-Roman MA, et al. Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nat Commun. 2022;13(1):1199. doi:10.1038/s41467-022-28741-7. [Google Scholar] [PubMed] [CrossRef]
43. Kang Q, Zou H, Yang X, Cai JB, Liu LX, Xie N, et al. Characterization and prognostic significance of mortalin, Bcl-2 and Bax in intrahepatic cholangiocarcinoma. Oncol Lett. 2018;15(2):2161–8. [Google Scholar] [PubMed]
44. Zhou F, Xu J, Ding G, Cao L. Overexpressions of CK2beta and XIAP are associated with poor prognosis of patients with cholangiocarcinoma. Pathol Oncol Res. 2014;20(1):73–9. doi:10.1007/s12253-013-9660-y. [Google Scholar] [PubMed] [CrossRef]
45. Mi W, van Tienderen GS, Shi S, Broeders A, Monfils K, Roest HP, et al. Apoptosis regulators of the Bcl-2 family play a key role in chemoresistance of cholangiocarcinoma organoids. Int J Cancer. 2025;157(8):1694–708. doi:10.1002/ijc.35483. [Google Scholar] [PubMed] [CrossRef]
46. Puetkasichonpasutha J, Namwat N, Sa-Ngiamwibool P, Titapun A, Suthiphongchai T. Evaluation of p53 and its target gene expression as potential biomarkers of cholangiocarcinoma in thai patients. Asian Pac J Cancer Prev. 2020;21(3):791–8. doi:10.31557/apjcp.2020.21.3.791. [Google Scholar] [PubMed] [CrossRef]
47. Furubo S, Harada K, Shimonishi T, Katayanagi K, Tsui W, Nakanuma Y. Protein expression and genetic alterations of p53 and ras in intrahepatic cholangiocarcinoma. Histopathology. 1999;35(3):230–40. doi:10.1046/j.1365-2559.1999.00705.x. [Google Scholar] [PubMed] [CrossRef]
48. Liu XF, Zhang H, Zhu SG, Zhou XT, Su HL, Xu Z, et al. Correlation of p53 gene mutation and expression of P53 protein in cholangiocarcinoma. World J Gastroenterol. 2006;12(29):4706–9. [Google Scholar] [PubMed]
49. Deserti M, Relli V, Palloni A, Vasuri F, Malvi D, Degiovanni A, et al. Mutant p53 associates with human equilibrative nucleoside 1 upregulation and better response to adjuvant gemcitabine in intrahepatic cholangiocarcinoma patients. Int J Mol Sci. 2025;26(11):5259. doi:10.3390/ijms26115259. [Google Scholar] [PubMed] [CrossRef]
50. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi:10.1158/2159-8290.cd-21-1059. [Google Scholar] [PubMed] [CrossRef]
51. Li J, Hu Y, Zhang J, Zhang W, Yu J, Lu B. Establishment and characterization of three gemcitabine-resistant human intrahepatic cholangiocarcinoma cell lines. Sci Rep. 2025;15(1):4813. doi:10.21203/rs.3.rs-4900217/v1. [Google Scholar] [CrossRef]
52. Kidoikhammouan S, Lert-Itthiporn W, Deenonpoe R, Saengboonmee C, Obchoei S, Wongkham S, et al. Targeting EGFR activation to overcome gemcitabine resistance in cholangiocarcinoma. Anticancer Res. 2024;44(12):5393–404. doi:10.21873/anticanres.17366. [Google Scholar] [PubMed] [CrossRef]
53. Varamo C, Peraldo-Neia C, Ostano P, Basirico M, Raggi C, Bernabei P, et al. Establishment and characterization of a new intrahepatic cholangiocarcinoma cell line resistant to gemcitabine. Cancers. 2019;11(4):519. doi:10.3390/cancers11040519. [Google Scholar] [PubMed] [CrossRef]
54. Vaquero J, Guedj N, Claperon A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: from clinical evidence to regulatory networks. J Hepatol. 2017;66(2):424–41. doi:10.1016/j.jhep.2016.09.010. [Google Scholar] [PubMed] [CrossRef]
55. Brivio S, Cadamuro M, Fabris L, Strazzabosco M. Epithelial-to-mesenchymal transition and cancer invasiveness: what can we learn from cholangiocarcinoma? J Clin Med. 2015;4(12):2028–41. doi:10.3390/jcm4121958. [Google Scholar] [PubMed] [CrossRef]
56. Weadick B, Nayak D, Persaud AK, Hung SW, Raj R, Campbell MJ, et al. EMT-Induced gemcitabine resistance in pancreatic cancer involves the functional loss of equilibrative nucleoside transporter 1. Mol Cancer Ther. 2021;20(2):410–22. doi:10.1158/1535-7163.mct-20-0316. [Google Scholar] [PubMed] [CrossRef]
57. Ziani L, Chouaib S, Thiery J. Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol. 2018;9:414. doi:10.3389/fimmu.2018.00414. [Google Scholar] [PubMed] [CrossRef]
58. Goydos JS, Brumfield AM, Frezza E, Booth A, Lotze MT, Carty SE. Marked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical marker. Ann Surg. 1998;227(3):398–404. doi:10.1097/00000658-199803000-00012. [Google Scholar] [PubMed] [CrossRef]
59. Kittirat Y, Suksawat M, Thongchot S, Padthaisong S, Phetcharaburanin J, Wangwiwatsin A, et al. Interleukin-6-derived cancer-associated fibroblasts activate STAT3 pathway contributing to gemcitabine resistance in cholangiocarcinoma. Front Pharmacol. 2022;13:897368. doi:10.3389/fphar.2022.897368. [Google Scholar] [PubMed] [CrossRef]
60. Yamanaka T, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Hagiwara K, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer. 2020;122(7):986–94. doi:10.1038/s41416-020-0744-7. [Google Scholar] [PubMed] [CrossRef]
61. Yang R, Wang D, Han S, Gu Y, Li Z, Deng L, et al. MiR-206 suppresses the deterioration of intrahepatic cholangiocarcinoma and promotes sensitivity to chemotherapy by inhibiting interactions with stromal CAFs. Int J Biol Sci. 2022;18(1):43–64. doi:10.7150/ijbs.62602. [Google Scholar] [PubMed] [CrossRef]
62. Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 2016;17(10):1712. doi:10.3390/ijms17101712. [Google Scholar] [PubMed] [CrossRef]
63. Watanabe Y, Kanai A. Systems biology reveals MicroRNA-mediated gene regulation. Front Genet. 2011;2:29. [Google Scholar] [PubMed]
64. Wu Z, Jiang S, Chen Y. Non-coding RNA and drug resistance in cholangiocarcinoma. Noncoding RNA Res. 2024;9(1):194–202. doi:10.1016/j.ncrna.2023.11.003. [Google Scholar] [PubMed] [CrossRef]
65. Asukai K, Kawamoto K, Eguchi H, Konno M, Asai A, Iwagami Y, et al. Micro-RNA-130a-3p regulates gemcitabine resistance via PPARG in cholangiocarcinoma. Ann Surg Oncol. 2017;24(8):2344–52. doi:10.1245/s10434-017-5871-x. [Google Scholar] [PubMed] [CrossRef]
66. Loosen SH, Lurje G, Wiltberger G, Vucur M, Koch A, Kather JN, et al. Serum levels of miR-29, miR-122, miR-155 and miR-192 are elevated in patients with cholangiocarcinoma. PLoS One. 2019;14(1):e0210944. doi:10.1371/journal.pone.0210944. [Google Scholar] [PubMed] [CrossRef]
67. Okamoto K, Miyoshi K, Murawaki Y. miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PLoS One. 2013;8(10):e77623. doi:10.1371/journal.pone.0077623. [Google Scholar] [PubMed] [CrossRef]
68. Zhao H, Duan Q, Zhang Z, Li H, Wu H, Shen Q, et al. Up-regulation of glycolysis promotes the stemness and EMT phenotypes in gemcitabine-resistant pancreatic cancer cells. J Cell Mol Med. 2017;21(9):2055–67. doi:10.1111/jcmm.13126. [Google Scholar] [PubMed] [CrossRef]
69. Deng J, Guo Y, Hu X, Du J, Gu J, Kong L, et al. High glucose promotes pancreatic ductal adenocarcinoma gemcitabine resistance and invasion through modulating ROS/MMP-3 signaling pathway. Oxid Med Cell Longev. 2022;2022:3243647. doi:10.1155/2022/3243647. [Google Scholar] [PubMed] [CrossRef]
70. Hao L, Li S, Peng Q, Zhang J, Deng J, Hu X. Targeting glycolytic reprogramming in cholangiocarcinoma: a novel approach for metabolic therapy. J Inflamm Res. 2024;17:9665–81. doi:10.2147/jir.s497551. [Google Scholar] [PubMed] [CrossRef]
71. Pant K, Richard S, Peixoto E, Gradilone SA. Role of glucose metabolism reprogramming in the pathogenesis of cholangiocarcinoma. Front Med. 2020;7:113. doi:10.3389/fmed.2020.00113. [Google Scholar] [PubMed] [CrossRef]
72. Thamrongwaranggoon U, Sangkhamanon S, Seubwai W, Saranaruk P, Cha’on U, Wongkham S. Aberrant GLUT1 expression is associated with carcinogenesis and progression of liver fluke-associated cholangiocarcinoma. In Vivo. 2021;35(1):267–74. doi:10.21873/invivo.12255. [Google Scholar] [PubMed] [CrossRef]
73. Pei T, Peng X, Lang Q, Wang Y, Xue J, He Z, et al. Dysregulation of the miR-148a-GLUT1 axis promotes the progression and chemoresistance of human intrahepatic cholangiocarcinoma. Oncogenesis. 2020;9(2):19. doi:10.1038/s41389-020-0207-2. [Google Scholar] [PubMed] [CrossRef]
74. Thonsri U, Seubwai W, Waraasawapati S, Wongkham S, Boonmars T, Cha’on U, et al. Antitumor effect of shikonin, a PKM2 inhibitor, in cholangiocarcinoma cell lines. Anticancer Res. 2020;40(9):5115–24. doi:10.21873/anticanres.14515. [Google Scholar] [PubMed] [CrossRef]
75. Thonsri U, Seubwai W, Waraasawapati S, Sawanyawisuth K, Vaeteewoottacharn K, Boonmars T, et al. Overexpression of lactate dehydrogenase A in cholangiocarcinoma is correlated with poor prognosis. Histol Histopathol. 2017;32(5):503–10. [Google Scholar] [PubMed]
76. Yu Y, Liao M, Liu R, Chen J, Feng H, Fu Z. Overexpression of lactate dehydrogenase-A in human intrahepatic cholangiocarcinoma: its implication for treatment. World J Surg Oncol. 2014;12:78. doi:10.1186/1477-7819-12-78. [Google Scholar] [PubMed] [CrossRef]
77. Kim DJ, Park YS, Kang MG, You YM, Jung Y, Koo H, et al. Pyruvate kinase isoenzyme M2 is a therapeutic target of gemcitabine-resistant pancreatic cancer cells. Exp Cell Res. 2015;336(1):119–29. doi:10.1016/j.yexcr.2015.05.017. [Google Scholar] [PubMed] [CrossRef]
78. Chen X, Chen S, Yu D. Protein kinase function of pyruvate kinase M2 and cancer. Cancer Cell Int. 2020;20(1):523. doi:10.1186/s12935-020-01612-1. [Google Scholar] [PubMed] [CrossRef]
79. Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45(5):598–609. doi:10.1016/j.molcel.2012.01.001. [Google Scholar] [PubMed] [CrossRef]
80. Yu W, Zeng F, Xiao Y, Chen L, Qu H, Hong J, et al. Targeting PKM2 improves the gemcitabine sensitivity of intrahepatic cholangiocarcinoma cells via inhibiting beta-catenin signaling pathway. Chem Biol Interact. 2024;387:110816. doi:10.1016/j.cbi.2023.110816. [Google Scholar] [PubMed] [CrossRef]
81. Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, et al. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110(1):172–82. doi:10.1038/bjc.2013.681. [Google Scholar] [PubMed] [CrossRef]
82. Huang R, Chen H, Liang J, Li Y, Yang J, Luo C, et al. Dual role of reactive oxygen species and their application in cancer therapy. J Cancer. 2021;12(18):5543–61. doi:10.7150/jca.54699. [Google Scholar] [PubMed] [CrossRef]
83. Thonsri U, Wongkham S, Wongkham C, Hino S, Nakao M, Roytrakul S, et al. High glucose-ROS conditions enhance the progression in cholangiocarcinoma via upregulation of MAN2A2 and CHD8. Cancer Sci. 2021;112(1):254–64. doi:10.1111/cas.14719. [Google Scholar] [PubMed] [CrossRef]
84. Suksawat M, Phetcharaburanin J, Klanrit P, Namwat N, Khuntikeo N, Titapun A, et al. Metabolic phenotyping predicts gemcitabine and cisplatin chemosensitivity in patients with cholangiocarcinoma. Front Public Health. 2022;10:766023. doi:10.3389/fpubh.2022.766023. [Google Scholar] [PubMed] [CrossRef]
85. Seubwai W, Kraiklang R, Wongkham C, Wongkham S. Hypoxia enhances aggressiveness of cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2012;13 Suppl:53–8. [Google Scholar] [PubMed]
86. Thongchot S, Yongvanit P, Loilome W, Seubwai W, Phunicom K, Tassaneeyakul W, et al. High expression of HIF-1alpha, BNIP3 and PI3KC3: hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac J Cancer Prev. 2014;15(14):5873–8. doi:10.7314/apjcp.2014.15.14.5873. [Google Scholar] [PubMed] [CrossRef]
87. Morine Y, Shimada M, Utsunomiya T, Imura S, Ikemoto T, Mori H, et al. Hypoxia inducible factor expression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2011;58(110–111):1439–44. [Google Scholar] [PubMed]
88. Silakit R, Kitirat Y, Thongchot S, Loilome W, Techasen A, Ungarreevittaya P, et al. Potential role of HIF-1-responsive microRNA210/HIF3 axis on gemcitabine resistance in cholangiocarcinoma cells. PLoS One. 2018;13(6):e0199827. doi:10.1371/journal.pone.0199827. [Google Scholar] [PubMed] [CrossRef]
89. Chen Y, Xu X, Wang Y, Zhang Y, Zhou T, Jiang W, et al. Hypoxia-induced SKA3 promoted cholangiocarcinoma progression and chemoresistance by enhancing fatty acid synthesis via the regulation of PAR-dependent HIF-1a deubiquitylation. J Exp Clin Cancer Res. 2023;42(1):265. doi:10.1186/s13046-023-02842-7. [Google Scholar] [PubMed] [CrossRef]
90. Noordhuis MG, Eijsink JJ, Ten Hoor KA, Roossink F, Hollema H, Arts HJ, et al. Expression of epidermal growth factor receptor (EGFR) and activated EGFR predict poor response to (chemo)radiation and survival in cervical cancer. Clin Cancer Res. 2009;15(23):7389–97. doi:10.1158/1078-0432.ccr-09-1149. [Google Scholar] [PubMed] [CrossRef]
91. Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98(2):418–25. doi:10.1038/sj.bjc.6604129. [Google Scholar] [PubMed] [CrossRef]
92. Bartholomeusz C, Yamasaki F, Saso H, Kurisu K, Hortobagyi GN, Ueno NT. Gemcitabine overcomes erlotinib resistance in EGFR-overexpressing cancer cells through downregulation of Akt. J Cancer. 2011;2:435–42. doi:10.7150/jca.2.435. [Google Scholar] [PubMed] [CrossRef]
93. Song JW, Tan YX, Li SB, Zhang SK, Wan LM, Ji SP, et al. Gemcitabine-induced heparanase promotes aggressiveness of pancreatic cancer cells via activating EGFR signaling. Oncotarget. 2017;8(35):58417–29. doi:10.18632/oncotarget.16911. [Google Scholar] [PubMed] [CrossRef]
94. Yothaisong S, Dokduang H, Techasen A, Namwat N, Yongvanit P, Bhudhisawasdi V, et al. Increased activation of PI3K/AKT signaling pathway is associated with cholangiocarcinoma metastasis and PI3K/mTOR inhibition presents a possible therapeutic strategy. Tumour Biol. 2013;34(6):3637–48. doi:10.1007/s13277-013-0945-2. [Google Scholar] [PubMed] [CrossRef]
95. Javle MM, Yu J, Khoury T, Chadha KS, Iyer RV, Foster J, et al. Akt expression may predict favorable prognosis in cholangiocarcinoma. J Gastroenterol Hepatol. 2006;21(11):1744–51. doi:10.1111/j.1440-1746.2006.04373.x. [Google Scholar] [PubMed] [CrossRef]
96. Pongking T, Intuyod K, Thongpon P, Thanan R, Sitthirach C, Chaidee A, et al. Cannabidiol suppresses proliferation and induces cell death, autophagy and senescence in human cholangiocarcinoma cells via the PI3K/AKT/mTOR pathway. J Tradit Complement Med. 2024;14(6):622–34. doi:10.1016/j.jtcme.2024.04.007. [Google Scholar] [PubMed] [CrossRef]
97. Liu H, Liu C, Wang M, Sun D, Zhu P, Zhang P, et al. Tanshinone IIA affects the malignant growth of Cholangiocarcinoma cells by inhibiting the PI3K-Akt-mTOR pathway. Sci Rep. 2021;11(1):19268. doi:10.21203/rs.3.rs-538320/v1. [Google Scholar] [CrossRef]
98. Kittirat Y, Techasen A, Thongchot S, Loilome W, Thanan R, Yongvanit P, et al. Suppression of 14-3-3zeta in cholangiocarcinoma cells inhibits proliferation through attenuated Akt activity, enhancing chemosensitivity to gemcitabine. Oncol Lett. 2018;15(1):347–53. [Google Scholar] [PubMed]
99. Pan YR, Wu CE, Jung SM, Huang SC, Lin SH, Chou WC, et al. Mucin 4 confers gemcitabine resistance and an unfavorable prognosis in patients with cholangiocarcinoma via AKT activation. Int J Biol Sci. 2023;19(9):2772–86. doi:10.7150/ijbs.79126. [Google Scholar] [PubMed] [CrossRef]
100. Yoon H, Min JK, Lee JW, Kim DG, Hong HJ. Acquisition of chemoresistance in intrahepatic cholangiocarcinoma cells by activation of AKT and extracellular signal-regulated kinase (ERK)1/2. Biochem Biophys Res Commun. 2011;405(3):333–7. doi:10.1016/j.bbrc.2010.11.130. [Google Scholar] [PubMed] [CrossRef]
101. Wang HQ, Man QW, Huo FY, Gao X, Lin H, Li SR, et al. STAT3 pathway in cancers: past, present, and future. MedComm. 2022;3(2):e124. doi:10.1002/mco2.124. [Google Scholar] [PubMed] [CrossRef]
102. Gu D, Zhao X, Song J, Xiao J, Zhang L, Deng G, et al. Expression and clinical significance of interleukin-6 pathway in cholangiocarcinoma. Front Immunol. 2024;15:1374967. doi:10.3389/fimmu.2024.1374967. [Google Scholar] [PubMed] [CrossRef]
103. Delgado-Calvo K, Boulter L, Briz O, Rozyczko A, Olaizola P, Marin JJG, et al. Modulating Wnt/beta-catenin pathway activity to enhance chemosensitivity in cholangiocarcinoma. Biomed Pharmacother. 2025;188:118225. doi:10.1016/j.biopha.2025.118225. [Google Scholar] [PubMed] [CrossRef]
104. Qian Z, Hu W, Lv Z, Liu H, Chen D, Wang Y, et al. PKM2 upregulation promotes malignancy and indicates poor prognosis for intrahepatic cholangiocarcinoma. Clin Res Hepatol Gastroenterol. 2020;44(2):162–73. doi:10.1016/j.clinre.2019.06.008. [Google Scholar] [PubMed] [CrossRef]
105. Chung JY, Hong SM, Choi BY, Cho H, Yu E, Hewitt SM. The expression of phospho-AKT, phospho-mTOR, and PTEN in extrahepatic cholangiocarcinoma. Clin Cancer Res. 2009;15(2):660–7. doi:10.1158/1078-0432.ccr-08-1084. [Google Scholar] [PubMed] [CrossRef]
106. Lee D, Do IG, Choi K, Sung CO, Jang KT, Choi D, et al. The expression of phospho-AKT1 and phospho-MTOR is associated with a favorable prognosis independent of PTEN expression in intrahepatic cholangiocarcinomas. Mod Pathol. 2012;25(1):131–9. doi:10.1038/modpathol.2011.133. [Google Scholar] [PubMed] [CrossRef]
107. Gu MJ, Choi JH. Clinicopathological significance of E-cadherin, beta-catenin and epidermal growth factor receptor expression in intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2012;59(116):1241–4. [Google Scholar] [PubMed]
108. Gandhi L, Harding MW, Neubauer M, Langer CJ, Moore M, Ross HJ, et al. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer. 2007;109(5):924–32. doi:10.1002/cncr.22492. [Google Scholar] [PubMed] [CrossRef]
109. Seiden MV, Swenerton KD, Matulonis U, Campos S, Rose P, Batist G, et al. A phase II study of the MDR inhibitor biricodar (INCEL, VX-710) and paclitaxel in women with advanced ovarian cancer refractory to paclitaxel therapy. Gynecol Oncol. 2002;86(3):302–10. doi:10.1006/gyno.2002.6762. [Google Scholar] [PubMed] [CrossRef]
110. Dieras V, Bonneterre J, Laurence V, Degardin M, Pierga JY, Bonneterre ME, et al. Phase I combining a P-glycoprotein inhibitor, MS209, in combination with docetaxel in patients with advanced malignancies. Clin Cancer Res. 2005;11(17):6256–60. doi:10.1158/1078-0432.ccr-04-2316. [Google Scholar] [PubMed] [CrossRef]
111. Cleary JM, Lima CM, Hurwitz HI, Montero AJ, Franklin C, Yang J, et al. A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Invest New Drugs. 2014;32(5):937–45. doi:10.1007/s10637-014-0110-9. [Google Scholar] [PubMed] [CrossRef]
112. Hardwick NR, Frankel P, Ruel C, Kilpatrick J, Tsai W, Kos F, et al. p53-reactive T cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy. Clin Cancer Res. 2018;24(6):1315–25. doi:10.1158/1078-0432.ccr-17-2709. [Google Scholar] [PubMed] [CrossRef]
113. Dotan E, Cardin DB, Lenz HJ, Messersmith W, O’Neil B, Cohen SJ, et al. Phase Ib study of wnt inhibitor ipafricept with gemcitabine and nab-paclitaxel in patients with previously untreated stage IV pancreatic cancer. Clin Cancer Res. 2020;26(20):5348–57. doi:10.1158/1078-0432.ccr-20-0489. [Google Scholar] [PubMed] [CrossRef]
114. Chen IM, Johansen JS, Theile S, Silverman LM, Pelz KR, Madsen K, et al. Randomized phase II study of nab-paclitaxel and gemcitabine with or without tocilizumab as first-line treatment in advanced pancreatic cancer: survival and cachexia. J Clin Oncol. 2025;43(18):2107–18. doi:10.1200/jco.23.01965. [Google Scholar] [PubMed] [CrossRef]
115. Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13(2):181–8. doi:10.1016/s1470-2045(11)70301-1. [Google Scholar] [PubMed] [CrossRef]
116. Sonbol MB, Ahn DH, Goldstein D, Okusaka T, Tabernero J, Macarulla T, et al. CanStem111P trial: a Phase III study of napabucasin plus nab-paclitaxel with gemcitabine. Future Oncol. 2019;15(12):1295–302. doi:10.2217/fon-2018-0903. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF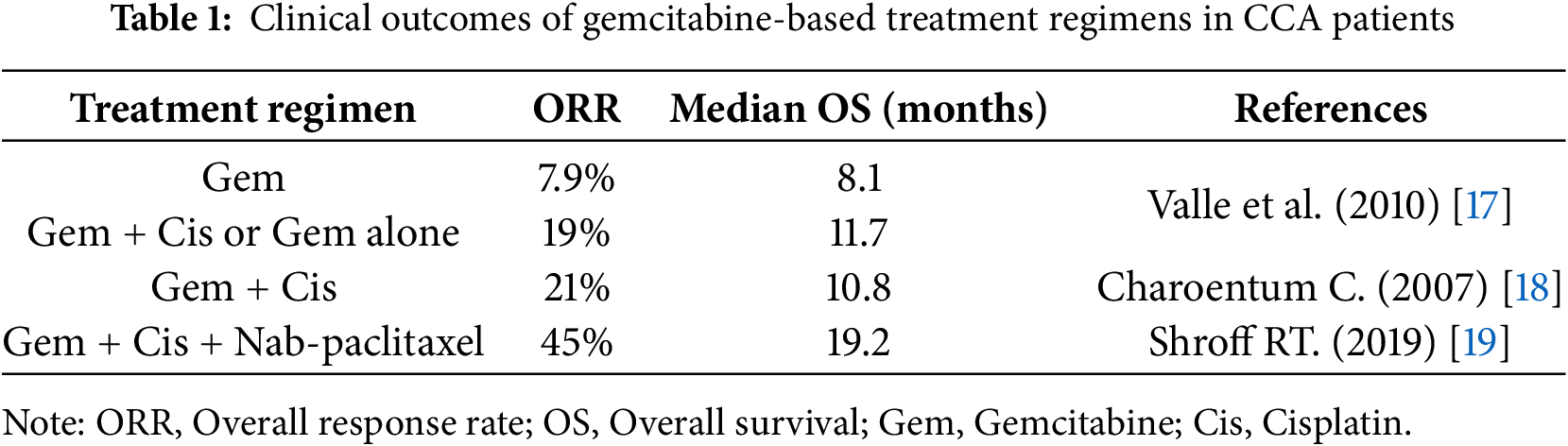
 Downloads
Downloads
 Citation Tools
Citation Tools