 Open Access
Open Access
REVIEW
The Role of Exosomes as a Key Factor of Cytostatic Resistance in Cancer: Mechanisms of Action, Potential Biomarkers, and Possible Exosome-Based Therapies
1 Department of Histology and Embryology, University of Medical Sciences in Poznan, Święcickiego 6 St. , Poznań, 60-781, Poland
2 Institute of Health Sciences, Collegium Medicum, University of Zielona Góra, Zyty 28 St., Zielona Góra, 65-046, Poland
* Corresponding Author: Sandra Kałużna. Email:
(This article belongs to the Special Issue: Identification of potential targets and biomarkers for cancers and the exploration of novel molecular mechanisms of tumorigenesis and metastasis)
Oncology Research 2026, 34(1), 2 https://doi.org/10.32604/or.2025.070356
Received 14 July 2025; Accepted 27 October 2025; Issue published 30 December 2025
Abstract
The last research focuses on the role of exosomes in cancer treatment. Exosomes are extracellular vesicles. They can be secreted by cancer cells, and they can modulate chemotherapy sensitivity. Determining exosomal content opens the possibility for guiding treatment strategies for cancer diseases. Exosomal microRNA are considered one of the prime candidates for exosomal biomarkers. Exosomal circular RNAs represent excellent biomarkers for liquid biopsy because of their stability in many types of cancer. Exosomal proteins remain reliable biomarkers also. Exosomes have emerged as promising therapeutic candidates. Their biological properties render them ideal vectors for drug delivery. Genetic modification of exosomes is an effective way to deliver material capable of modulating cellular pathways involved in drug resistance. Furthermore, exosomes have been explored as carriers for metal-chelating agents. Integrating exosome-based therapies with traditional anticancer agents aims to exploit the natural targeting abilities of exosomes to enhance drug delivery. Despite the dynamic development of this field, many mechanisms of exosome action remain incompletely understood. Therefore, it is necessary to conduct further studies that will allow for a better understanding of their role in the process of resistance and will enable the development of effective therapeutic strategies.Keywords
The incidence of cancer worldwide is increasing annually. Cancer is one of the most deadly diseases, causing about 20% of all deaths in Europe [1]. Cancer therapy is tailored to the individual patient based on the type and stage of the cancer. Treatment may include surgery, radiotherapy, chemotherapy, immunotherapy, or targeted therapy [2]. Chemotherapy involving cytotoxic drugs is the most commonly chosen treatment method. Cytostatics are toxic to cancer cells because they cause damage to the DNA of these cells or disrupt the processes necessary for their division. The result is inhibition of tumor development or its reduction [3].
A significant obstacle to chemotherapy’s success is drug resistance development. This resistance can be innate, present before treatment, or acquired, developing during treatment. The complex interaction between these factors contributes to developing resistance to various anticancer therapies. Intrinsic factors include pre-existing genetic mutations, tumor heterogeneity, and activated intracellular defence pathways. This leads to, among other things, the activation of intracellular defence pathways, which enable drug evasion by modulating oncogenic pathways, altering therapeutic targets, enhancing DNA repair, and activating survival mechanisms. In turn, extrinsic factors, primarily components of the tumor microenvironment (TME), actively contribute to developing cancer cells’ ability to evade the cytotoxic effects of anticancer drugs [4]. It is well established that multiple factors modulate the activity of recipient cells. Exosomes carry a variety of molecules derived mainly from cancer cells, epithelial and mesenchymal cells, and immune cells such as B lymphocytes and dendritic cells. Through this cargo, exosomes influence recipient cells, affecting their proliferation and functional activity in innate and adaptive immune systems. For instance, CD4+ and CD8+ T cells, including cytotoxic T lymphocytes (CTLs), can be directly or indirectly stimulated or suppressed in their proliferation and functions by exosome-mediated signaling [5].
Drug resistance is the primary and natural ability of cells to protect themselves from the toxic effects of drugs. It can also appear during the treatment of oncology patients as secondary drug resistance. Thus, cytostatic resistance is a severe challenge in the treatment of cancer. Cancer cells respond less to treatment or become completely resistant when it occurs. Acquired resistance significantly reduces the effectiveness of chemotherapy administered by physicians, and in many cases, contributes to the recurrence of cancer [6]. Up to 90% of cancer-related deaths are attributed to ineffective pharmacological treatment due to drug resistance [6,7]. Cancer cell plasticity may be key in this process. It has been shown that cancer cells can adapt to changing conditions to maintain their resistance, which results in a dynamically decreasing effectiveness of therapy [8].
Exosomes act as resistance vectors in many cancers, transferring resistant traits to treatment-sensitive cells. Understanding the mechanisms by which exosomes influence resistance is significant, as it may lead to the development of effective anticancer therapies. In breast cancer (BC), the second leading cause of cancer deaths among women, exosomal miR-9 can induce properties characteristic of cancer-associated fibroblasts (CAFs) [9]. In colorectal cancer, exosomal miR-1246 promotes mutations in tumor cells, induces M2 macrophage polarization, and remodels the tumor microenvironment by upregulating IL-10, transforming growth factor beta (TGFβ), and matrix metalloproteinase (MMP) expression [10]. In hepatocellular carcinoma (HCC), exosomal microRNAs (miRNAs) have also been shown to modulate tumor behavior and remodel the tumor microenvironment (TME) by regulating processes such as cell proliferation, metastasis, and immune response. [11]. In lung cancer, exosomes have been shown to modulate the tumor microenvironment by inducing phenotypic changes that hinder drug response. An example is TGF-β transported via exosomes, allowing cancer cells to transmit dormancy-inducing signals to neighboring cells, promoting the acquisition of a dormant phenotype. Consequently, dormant cells often exhibit reduced sensitivity to chemotherapy and targeted therapies, contributing to drug resistance [12].
The development of drug resistance in cancer cells is a highly complex process. Changes can occur at the molecular, cellular, and systemic levels. Contributing factors include: alterations in drug transport, changes in their metabolism or the target sites of action, dysregulation of apoptosis, modifications in DNA repair processes or the cell cycle. In addition, the phenotypic variability of the tumor and its microenvironment can have an impact.
Numerous studies have been conducted to better understand the resistance mechanisms to cytostatic drugs. It has been established that there are two main variants: congenital and acquired. They differ because the congenital variant exists before treatment, whereas the acquired variant develops in response to therapy, making it a greater challenge for physicians and researchers [6]. Signaling pathways that regulate fundamental biological processes, such as cell growth, proliferation, motility, angiogenesis, and survival, play a key role in drug resistance. Excessive activation of the phosphoinositide 3-kinase (PI3K) pathway is one of the most common cancer-related events in humans and contributes to tumor progression [13]. Conversely, the Gas6-Axl axis promotes cancer cell invasion by inducing multiple actin-dependent processes affecting cell dynamics, adhesion, and metabolism [14]. Disruptions in apoptotic pathways pose a significant challenge in cancer treatment. The primary role here is played by genes involved in regulating apoptosis, including p53 and members of the B-cell leukemia/lymphoma-2 (Bcl-2) family, which exhibit pro-survival activity in various cancers. It has been proven that a mutation in the TP53 gene encoding the transcription factor p53 inhibits the induction of apoptosis. As a result, cancers with TP53 mutations respond poorly to cytostatic therapy [15]. However, overexpression of Bcl-2 in cancer cells inhibits cell death pathways, resulting in lower effectiveness of anticancer drugs [16,17].
One of the most significant challenges in cancer therapy is multidrug resistance (MDR), characterised by cross-resistance to multiple drugs [6]. The most essential proteins contributing to MDR are drug transporters from the MDR protein family, including P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), which play a pivotal role [18–20]. The proteins involved in this process have been studied for many years. There is extensive literature on this subject, and our article does not focus strictly on this particular issue.
The role of the TME in shaping resistance to cytostatic drugs should not be underestimated, as its composition differs significantly from physiological conditions. Among the key components of the TME are the extracellular matrix (ECM), cytokines, chemokines, growth factors, and metabolic elements. ECM proteins play a crucial role in tumor progression and the development of treatment resistance. During neoplastic transformation, these proteins undergo functional changes that promote tumor cell survival and support their aggressive phenotype. The most prominent components of the ECM include collagens, elastin, laminins, fibronectin, lysyl oxidase (LOX), and transforming growth factor-beta-induced protein (TGFBI). Collagens contribute to tumor progression, metastasis formation, and modulation of the therapeutic response. Elastin, through elastosis, can facilitate changes that favor tumor progression. Laminins increase cancer cell resistance to apoptosis and stimulate tumor development through interactions with other proteins and cytokines. Fibronectin promotes cancer cell proliferation. LOX overexpression in tumor tissue and excessive collagen production enhance cell adhesion, migration, and invasiveness. A deeper understanding of ECM proteins in modulating the TME could serve as a foundation for developing novel therapeutic strategies to overcome drug resistance in cancer [21,22].
The information above highlights the significance of drug resistance and the ongoing extensive research on its development mechanisms. The history of cancer treatment goes back many years. Many different treatment methods have been modified and improved. New ones have emerged, such as the advent of targeted therapies, but surgery remains the same. Despite this, oncological treatment still has its limitations [2]. That’s why exosomes have attracted scientists’ attention. A relatively recent yet rapidly growing area of research focuses on exosomes, which play a key role in maintaining cellular homeostasis, clearing cellular debris, and facilitating intercellular communication [23]. Moreover, they regulate various processes, including immune responses, cancer development, tissue regeneration and progression of neurodegenerative diseases [5,24,25].
Exosomes have emerged as a significant area of research in cancer drug resistance because, unlike classical mechanisms, they show that cancer cells can “talk” to each other and their environment. They facilitate the transfer of miRNAs and proteins that confer a resistant phenotype to otherwise sensitive cells [26]. Exosomes are a subclass of bilayer membrane vesicles within the broader category of extracellular vesicles (EVs) [27]. They typically range in size from 30 to 150 nm [28,29]. They originate through invaginations within the endosomal compartment, subsequently forming multivesicular bodies (MVBs), which are released into the extracellular space [30,31]. The process of exosome formation is presented in Fig. 1.
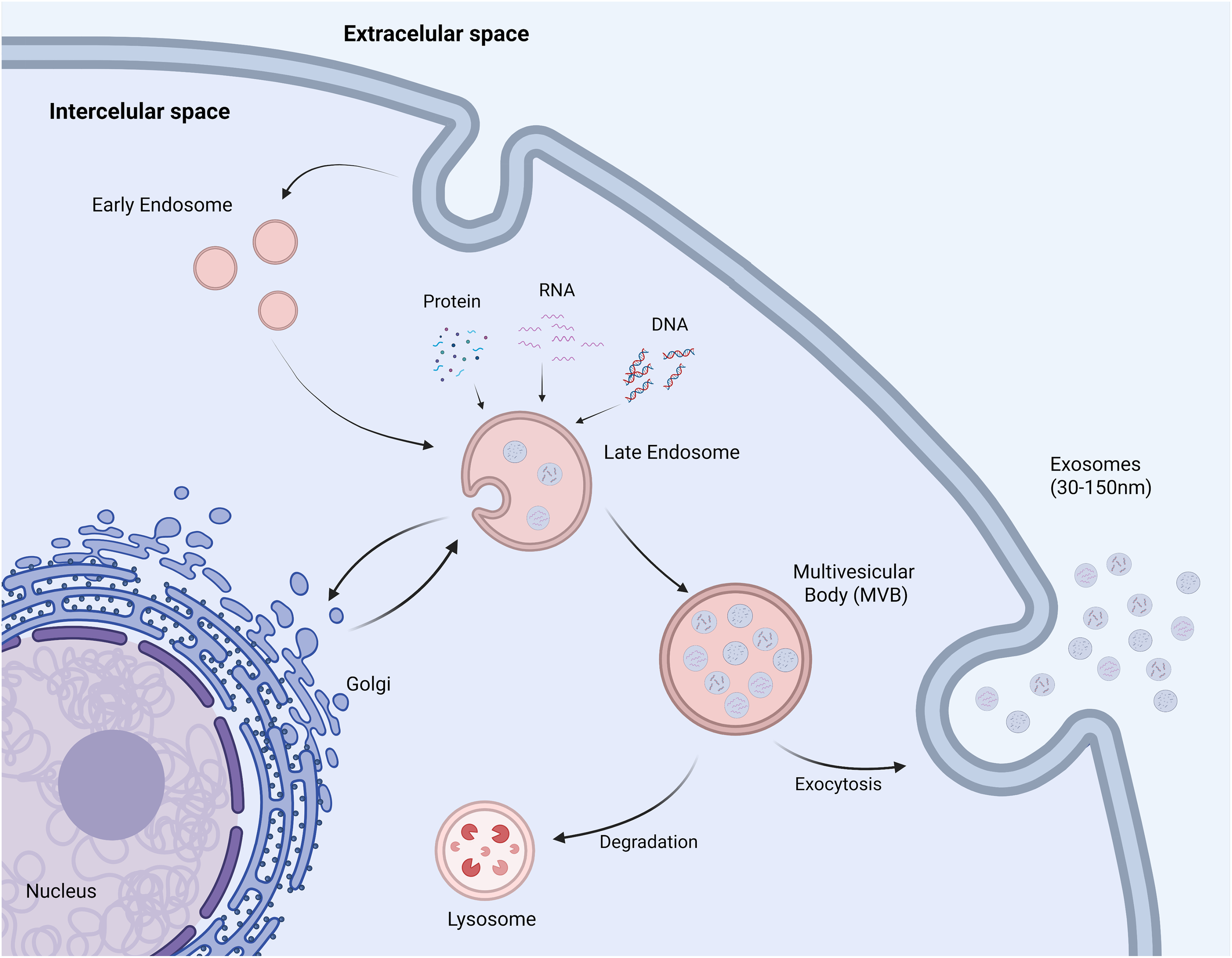
Figure 1: Biogenesis of exosomes. As a result of endocytosis, during which the plasma membrane becomes invaginated into the cell interior, vesicles called early endosomes are formed. As they mature, early endosomes transform into late endosomes, in which the membrane begins to fold and invaginate into their interior locally. This way, intraluminal vesicles (ILVs) are formed, into which selectively selected molecules such as proteins, lipids, RNA, or DNA are placed. Structures containing numerous ILVs are called multivesicular bodies (MVBs) and play a key role in further sorting of cellular cargo. MVBs can then fuse with the lysosome, leading to degradation of their contents, or fuse with the plasma membrane, releasing ILVs into the extracellular space as exosomes (Created in BioRender, accessed on 10 October 2024)
This review aims to explore the role of exosomes in cytotoxic resistance mechanisms and their potential as both diagnostic and therapeutic tools in cancer treatment.
2 Exosomes and Their Composition in the Context of Cytostatic Resistance
Exosomes are extracellular vesicles that mediate intercellular communication. Their composition is highly diverse, as they can contain proteins, lipids, DNA, and various forms of RNA, including mRNA and ncRNA. Exosomes secreted by cancer cells are referred to as tumor-derived exosomes (TEX) [25]. Studies have demonstrated that TEX modulate chemotherapy sensitivity by transferring a resistance phenotype to target cells [23].
One example concerns the P-gp protein. P-gp is a key factor in developing MDR in cancer cells [18]. Lopes-Rodrigues et al. were the first to demonstrate that vesicles secreted by cancer cells contain P-gp [32]. As a transport protein, it facilitates the efflux of chemotherapeutic agents such as paclitaxel (PTX), doxorubicin (DOX), and mitoxantrone from cancer cells. Consequently, their intracellular concentration decreases, leading to drug resistance [33,34]. Ovarian cancer (OC) data showed that exosomes from platinum-resistant A2780 cells contain higher P-gp than those from sensitive A2780, consistent with an exosomal role in MDR [35]. In turn, another study showed that human breast cancer cells are resistant to adriamycin (MCF7/ADM) secreted exosomes carrying P-gp proteins and ubiquitin carboxyl terminal hydrolase-L1 (UCH-L1) to the extracellular microenvironment. Exosomes interacted with adriamycin-sensitive human breast cancer cells (MCF7/WT), facilitating the transfer of a chemotherapy-resistant phenotype [36]. The researchers reached a similar conclusion regarding osteosarcoma and the DOX drug [37].
BCRP is another key factor contributing to the development of drug resistance. BCRP belongs to the ATP-binding cassette (ABC) family and is a defense mechanism against toxins and xenobiotics. It facilitates the excretion and restricts the absorption of potentially toxic substrates, including various chemotherapeutic agents used in cancer treatment [38]. Many sources indicate its essential role in shaping drug resistance [39–41]. Notably, Kong et al. were the first to suggest that BCRP is associated with and secreted via exosomes [42]. However, the preliminary observations by Kong et al., suggesting that BCRP is associated with and secreted via exosomes, require cautious interpretation, as the findings still need further validation and independent confirmation in different experimental models. It should be emphasized that this study was exploratory in nature, and the applied methodologies had limited capacity to demonstrate the interaction between BCRP and exosomes unequivocally. A significant interaction between BCRP exosomes and resistance development can be found in the studies of Kim et al. This study based on cell lines derived from triple-negative breast cancer (TNBC), examined BCRP and MDR1 and MRP1. MDR1, MRP1, and BCRP expression in extracellular vesicles (EVs) derived from breast cancer cells increased after chemotherapy in vitro, particularly in drug-resistant cell lines [43].
Another exosomal protein in drug resistance is programmed death-ligand 1 (PD-L1). PD-L1 is a membrane-bound ligand expressed on the surface of various cancer cells and exosomes, which is referred to as exosomal PD-L1 [44]. In esophageal squamous cell carcinoma (ESCC), elevated PD-L1 expression has been correlated with advanced clinical stage, poor prognosis, and resistance to chemotherapy [45,46]. These findings are further supported by in vitro studies showing that the paclitaxel-resistant EC-9706R cell line exhibits higher levels of exosomal PD-L1 than its paclitaxel-sensitive parental counterpart. Moreover, exosomes derived from EC-9706R cells enhanced invasion, migration, and resistance to paclitaxel in EC-9706 cells, potentially through regulation of the signal transducer and activator of transcription 3 (STAT3)/miR-21/phosphatase and tensin homolog (PTEN)/protein kinase B (AKT) axis [47]. Exosomal PD-L1 has also been detected in various cancers, including melanoma [48], breast cancer [49], glioma [50], stomach cancer [51], and lung cancer [52]. Moreover, exosomal PD-L1 plays a crucial role in enabling cancer cells to evade immune surveillance. PD-L1 binds to the programmed cell death protein 1 (PD-1) receptor on T cells, inhibiting their activity, ultimately suppressing the immune response [53].
Among the immunosuppressive molecules transported by exosomes, prostaglandin E2 (PGE2) and transforming growth factor beta (TGF-β) play a crucial role in conferring resistance to cytostatics by reducing cytotoxicity and promoting an immunosuppressive TME [25]. PGE2 activates signalling pathways such as PI3K/Akt and mitogen-activated protein kinase (MAPK) essential for cancer cell survival, proliferation, and chemotherapy resistance [54,55]. In the late stages of cancer, TGF-β promotes tumor progression by upregulating vascular endothelial growth factor (VEGF) expression [56]. Xiang et al. provided evidence supporting the immunosuppressive properties of exosomal PGE2 and TGF-β in cancer. They demonstrated that T cell-derived exosomes can induce myeloid-derived suppressor cells (MDSCs) accumulation in tumors. Moreover, tumor-derived exosomal PGE2 and TGF-β contribute to MDSC accumulation [57]. Additionally, studies have shown that exosomes derived from specific cancer cells can induce fibroblast differentiation into myofibroblasts. It was established that exosomally transported TGF-β is responsible for this differentiation. TGF-β, through activation of the SMAD family member 3 (SMAD3) signaling pathway, leads to the differentiation of fibroblasts into myofibroblasts [58].
A crucial mechanism underlying drug resistance in cancer cells is the transfer of non-coding RNAs (ncRNAs), including miRNAs and circular RNAs (circRNAs), via TEX [28]. The development of cancer therapies based on miRNAs, short hairpin RNAs (shRNAs), and small interfering RNAs (siRNAs) began in the early 21st century [2]. miRNAs are small, non-coding RNA molecules of 17–24 nucleotides in length, which maintain a high degree of conservatism. They act as gene regulators by binding to the 3′ untranslated region (3′ UTR) sequences of mRNA, inhibiting their expression [59]. They act as regulators of many key cellular processes, controlling several essential functions, including cell signaling, inflammatory processes related to cancer development, T cell and stem cell differentiation, and maintaining metabolic homeostasis [60]. TEX contribute to oncogenesis by influencing neighboring immune cells by transporting miRNAs, among other biomolecules. miRNAs that promote tumor growth are called oncomiRs [61]. Table 1 presents examples of miRNAs and their influence on drug resistance.
Jin et al. demonstrated the presence of exosomal miRNAs in the serum of patients with advanced colorectal cancer. The key exosomal miRNAs associated with chemoresistance included miR-21-5p, miR-1246, miR-1229-5p, and miR-96-5p [62]. For instance, miR-1246 can be transported via exosomes derived from OC cells. Oncogenic miR-1246 contributes to chemoresistance by suppressing caveolin-1 (Cav1) expression, a protein essential for forming caveolae-specialized membrane structures involved in cellular transport and signaling. In addition, exosomal miR-1246 promotes tumor development in the TME by acting on M2 macrophages, which promote oncogenic progression [60]. miR-1246 was also detected in exosomes from breast cancer [63], squamous cell carcinoma of the oral cavity [64] and glioma [65]. In the study by Mikamori et al., the authors demonstrated that exosomes play a key role in regulating miRNA levels and intercellular communication in PDAC. The study showed that the miR-155 loop confers chemoresistance in these cells. The mechanism was as follows: (1) prolonged exposure to gemcitabine (GEM) increases miR-155 expression in PDAC cells; (2) elevated miR-155 promotes both exosome secretion and anti-apoptotic activity, contributing to chemoresistance; (3) exosomes deliver miR-155 to other PDAC cells, propagating the resistance effect [66].
Many findings suggest exosomes can also release miRNA from tumor-associated macrophages (TAMs) [67]. Exosomes from TAMs carry, among others: mi365, miR-21, miR-223, miR-1246, miR-196 [68]. Conversely, exosomal miR-135b has been implicated in chemoresistance in colorectal cancer (CRC), gastric cancer (GC), and pancreatic cancer (PC) [69–71].
It has been proven that exosomal circRNAs also impact the development of chemoresistance. CircRNAs are a class of circular RNAs characterized by covalently closed-loop structures. They arise due to alternative splicing of exons or introns of genes [72]. The influence on chemoresistance is very often connected to the miRNA-RNA axis. Table 1 also presents examples of circRNA and their impact on drug resistance.
The use of small RNA-based therapies is associated with several challenges, the most critical being the inability to elicit clinically significant anticancer activity in clinical trials [2].
3 Exosomes and Intercellular Interactions
3.1 Effect of Exosomes on Neighboring Cells
Exosomes are present in various body fluids and are crucial in transferring biological materials from donor to recipient cells. Consequently, they are essential for intercellular communication, influencing the regulation of both physiological and pathological microenvironments [62]. Recently, extensive research has focused on the tumor TME, a critical factor in the development of chemoresistance. TEX influence neighboring cells, including fibroblasts, endothelial cells, and immune cells. Intercellular communication in the TME is also ensured by exosomes delivered by various microenvironment cells, including fibroblasts, macrophages, endothelial cells, adipocytes and pericytes [90].
Cancer-associated fibroblasts (CAFs) are key components of TME surrounding cancer cells. CAFs exhibit sustained activity, characterized by continuous stimulation of cellular metabolism, including collagen and elastin production and enzyme secretion. Sustained activation of fibroblasts in cancer tissue supports intensive proliferation and development of cancer cells. However, CAFs are abundant yet remain an insufficiently understood cell type in the TME, capable of promoting and inhibiting tumor growth, depending on the conditions and organ type [91]. The promotion of tumor progression by CAF has been confirmed, among others, in the case of liver cancer [91] or breast cancer [92]. CAFs promote the development of drug resistance in many processes, e.g., CAFs contribute to drug resistance through various mechanisms, such as inducing fibrous hyperplasia, which can directly lead to decreased drug perfusion, thereby reducing the effect of chemotherapy [93]. In another study, pancreatic stellate cells (PSCs) were shown to protect pancreatic ductal adenoma (PDAC) from the effects of gemcitabine. This suggests that CAFs within tumor tissues reshape the TME, fostering chemotherapy resistance [94]. The role of CAFs in tumor invasion and metastasis is becoming increasingly better known and described [95,96]. However, the involvement of exosomes in the development of cytoresistance based on the TME is a relatively new research trend. CAF-derived exosomes in PDAC, although not intrinsically resistant to gemcitabine, have been shown to promote chemoresistance by maintaining signaling with cancer cells during treatment. CAFs deliver exosomes containing miR-3173–5p affecting acyl-CoA synthetase long-chain family member 4 (ACSL4) secretion and inhibiting ferroptosis [93]. In research on chemoresistance in GC, CAFs have been shown to secrete exosomal miR-522, suppressing ferroptosis in GC tumor cells by targeting ALOX15 and preventing lipid-ROS accumulation, contributing to chemotherapy resistance. Another study demonstrates the interaction between CAFs and urothelial bladder cancer (UBC) cells. CAF-derived miR-146a-5p facilitates the formation and maintenance of the cancer stem cell (CSC) niche, promoting CSC-associated survival and invasiveness. CAFs interact with cancer cells through direct contact, such as cell adhesion mediated by SVEP1. Intercellular communication also occurs through regulating ARID1A and PRKAA2 gene expression in UBC cells, activating the signal transducer and activator of transcription 3 (STAT3) and mechanistic target of rapamycin (mTOR) signaling pathways. STAT3 activation enhances the expression of CSC markers, including SRY-box transcription factor 2 (SOX2) cluster of differentiation 44 (CD44), and integrin β1 [82].
Numerous studies suggest that the vascular endothelial cells, as a key component of the TME, play a crucial role in metastasis formation [97]. Angiogenesis, the process of forming new blood vessels, is closely associated with tumor development. Various tumor cell types secrete exosomes enriched with proangiogenic proteins, thereby promoting angiogenesis. Proangiogenic factors detected in exosomes include vascular endothelial growth factor (VEGF) transforming growth factor-beta (TGF-β) interleukin-6 (IL-6), interleukin-8 (IL-8), tissue inhibitor of metalloproteinases-1 (TIMP-1), and metalloproteinases-2 (TIMP-2) in glioblastoma multiforme; VEGF, basic fibroblast growth factor (bFGF), MMP9, and hepatocyte growth factor (HGF) in multiple myeloma; IL-6, VEGF, and MMP2 in melanoma; endothelin-1, IL-8, Thrombospondin 2 (TSP-2), urokinase-type plasminogen activator (uPA), and VEGF in lung adenocarcinoma; EGF-like repeats and discoidin I-like domain-3 (EDIL-3) in bladder cancer; and annexin II in breast cancer [98,99]. Additionally, tumor endothelial cells (TECs) not only stimulate the secretion and expression of proangiogenic molecules but also facilitate tumor metastasis. TECs show some variability depending on their origin and differ from normal endothelial cells (NECs) in their response to proangiogenic factors such as EGF, adrenomodulin, and VEGF. Autocrine VEGF secreted by TECs enhances their survival and promotes migration. Another key characteristic of TECs is their drug resistance [100].
As previously mentioned, exosomes derived from cancer cells and different cells associated with cancer can carry a variety of bioactive molecules, including proteins, lipids, and different types of RNA, such as miRNAs. These miRNAs can modulate endothelial cell function, influencing their migration, proliferation, and angiogenic potential, thereby promoting tumor progression. Exosomes derived from lung cancer tissue have been shown to selectively deliver miRNA-210 to endothelial cells, thereby promoting tumor angiogenesis [101]. Another study demonstrated higher expression of miR-92a in exosomes derived from chronic myeloid leukemia (CML) cells compared to endothelial cells. This resulted in enhanced cell migration and capillary formation [102]. In turn, Keklikoglou et al. demonstrated that taxanes and anthracyclines, two classes of cytotoxic drugs, induce increased exosome secretion from breast cancer cells, contributing to metastasis. Exosomes produced during chemotherapy are enriched in annexin-A6 (ANXA6), a Ca2+-dependent protein that promotes nuclear factor κB (NF-κB)-dependent activation of endothelial cells, induction of Chemokine (C-C motif) Ligand (CCL2), and expansion of Ly6C+CCR2+ monocytes, facilitating the development of lung metastases [103]. Disruption of tight junctions between endothelial cells increases vascular permeability, facilitating tumor cell extravasation and metastasis. Vascular permeability is also enhanced by the delivery of miRNAs through exosomes derived from cancer cells. In the study by Liu et al., exosomal miR-29a was shown to originate from epithelial-mesenchymal transition (EMT) in CRC cells. Exosomal miR-29a was transported to endothelial cells within the TME, increasing vascular permeability by downregulating the expression of intercellular junction proteins, including Zonula occludens-1 (ZO-1), Claudin-5, and Occludin. As a result, increased vascular permeability promotes the development and metastasis of CRC [104]. In turn, metalloproteinase 17 (ADAM17), present in circulating exosomes from CRC patients, may promote metastasis. ADAM17 affects vascular endothelial cells, increasing vascular permeability by modifying cadherins in the cell membrane [105].
Additionally, exosomes can influence the TME by modulating the immune response through their impact on immune cells, including macrophages and lymphocytes. This can suppress the immune response against tumor cells, thereby promoting tumor progression and therapy resistance. Immune cells produce various cytokines that negatively regulate immune function and promote tumor growth. Among these, TAMs are the most abundant [106]. TAMs originate from monocytes and represent the dominant immune cell type in the TME, comprising 15%–20% of the tumor mass. Macrophages differentiate into two main phenotypes. M1 macrophages exhibit a pro-inflammatory function, supporting the immune response and restricting tumor growth. Stimulated by T helper 1 (Th 1) cytokines, such as interferon gamma (IFN-γ) and lipopolysaccharide (LPS), which activate Toll-like receptors (TLRs) to promote inflammation. In contrast, M2 macrophages exhibit anti-inflammatory properties, promoting tumor progression, angiogenesis, and metastasis through stimulation by Th2 cytokines, including interleukin-4 (IL-4), IL-10, and TGF-β [107,108]. M2 macrophages contribute to cancer metastasis by secreting various enzymes in a paracrine manner. These enzymes degrade collagen components in the ECM, leading to structural breakdown and facilitating cancer cell migration [108]. Extensive research has documented the role of macrophages, particularly M2, in metastasis, angiogenesis, and tumor survival [109–111].
While interactions between cancer cells and M2 macrophages have been extensively studied, the mechanisms underlying M2 macrophage activation by cancer cells remain a topic of interest for researchers and clinicians. Exosomes, as key mediators of intercellular communication, may play a crucial role in this process. Exosomes targeting macrophages contribute to the formation of a metastatic niche, thereby complicating patient treatment. Zhao et al. demonstrated elevated expression of miR-934 in CRC, including colorectal cancer liver metastases (CRLM). They demonstrated that exosomal miR-934 induces M2 macrophage polarization by downregulating phosphatase and tensin homolog (PTEN) expression and activating the PI3K/AKT signaling pathway. These findings highlight the role of TEX in mediating interactions between tumors and TAMs within the metastatic microenvironment, playing a crucial role in CRLM [112]. Similarly, exosomal FGD5-AS1 from PC cells can drive macrophage polarization toward the M2 phenotype by activating the STAT3/NF-κB pathway. This mechanism involves the interaction of FGD5-AS1 with the p300 protein, which leads to STAT3 acetylation and, consequently, its translocation to the nucleus and an increase in the transcriptional activity of the STAT3/NF-κB pathway [113]. However, increased levels of exosomal miR-519a-3p were detected in serum in GC patients with liver metastases (LM). Exosomal miR-519a-3p initiates the MAPK/extracellular signal-regulated kinase (ERK) pathway activation by regulating DUSP2, which leads to macrophage polarization toward the M2 phenotype. Such polarized macrophages support the development of gastric cancer liver metastases (GC-LM) by stimulating angiogenesis and creating an intrahepatic premetastatic niche [114]. Exosomes derived from cholangiocarcinoma (CCA) containing LINC01812 play a key role in macrophage polarization toward the M2 phenotype. This process promotes perineural invasion (PNI) and tumor progression. LINC01812 interacts with the TUBB4B protein to activate the Notch signaling pathway in macrophages, leading to increased secretion of the chemokine CCL2. This LINC01812-Notch-CCL2 signaling axis promotes cancer cell migration and invasion. Additionally, LINC01812-containing exosomes can influence neuronal cells by enhancing their secretion of neurotrophic factors, further promoting PNI [115].
TAMs promote tumor immune evasion by recruiting immunosuppressive regulatory T cells (Tregs), thereby attenuating the cytotoxic activity of effector lymphocytes and weakening the host antitumor immune response [116]. Exosomes released from TAMs play a pivotal role in this process. Zhou et al. reported that microarray profiling of TAM-derived exosomes in epithelial ovarian cancer (EOC) identified enrichment of specific miRNAs, including miR-29a-3p and miR-21-5p. Transfection of these miRNAs into CD4+ T cells directly inhibited STAT3 signaling and disrupted the Treg/Th17 balance, synergistically affecting STAT3 suppression. These findings highlight that TAM-derived exosomes mediate crosstalk with T cells to establish an immunosuppressive tumor microenvironment, ultimately fostering tumor progression and metastasis [117]. Additionally, CD4+ T helper (Th) cells, which secrete IL-4, IL-13 and IL-10-producing Tregs, play a key role in macrophage polarization [118]. Moreover, Li et al. demonstrated that exosomal LINC01232 facilitates tumor immune evasion. TAMs secrete exosomal LINC01232, which promotes immune evasion by tumor cells. This mechanism involves direct binding of LINC01232 to E2F2, facilitating nuclear transport and jointly stimulating NBR1 gene transcription. Enhanced interaction between NBR1 and ubiquitinated major histocompatibility complex class I (MHC-I) via the ubiquitin domain promotes MHC-I degradation in autophagolysosomes. Consequently, this reduces MHC-I levels on the tumor cell surface, enabling immune evasion from CD8+ cytotoxic T lymphocytes (CTLs) [119]. Additionally, within the TME, cancer cells express PD-L1 on their surface, binding to PD-1 on T lymphocytes, inhibiting their ability to destroy tumor cells [110].
3.2 Exosomes and Inflammatory Processes
Exosomes may play a crucial role in inducing and modulating TME inflammation, thereby influencing cytostatic resistance development. Inflammation, both extratumoral (triggered by external factors such as infections or obesity) and intratumoral (driven by cancer mutations), plays a pivotal role in tumor progression. Inflammation and tumorigenesis share common signaling pathways and key regulatory molecules involved in apoptosis, proliferation, and angiogenesis. Chronic inflammation may promote tumor initiation, progression, and invasiveness by releasing bioactive proinflammatory factors that shape the TME [120,121]. Key proinflammatory factors associated with cancer include interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNF-α), along with the transcription factors NF-κB and STAT3 [121]. Numerous studies have confirmed the link between inflammation and cancer [122–124]. Thus, the role of exosomes in this process remains an active area of research.
Exosomes regulate inflammation, in part, by activating TLRs on the surface of monocytes. Bretz et al. isolated exosomes from malignant ascitic fluid in patients with OC. TEX were internalised by THP-1 monocytes, stimulating the secretion of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α. This mechanism involved exosomal interaction with TLR2 and TLR4 receptors, activating signaling pathways that induced NF-κB and STAT3 [125]. Haderk et al. demonstrated that TEX carrying noncoding RNAs, such as hY4, promote an inflammatory microenvironment and facilitate immune evasion by inducing PD-L1 expression. Exosomal transfer from chronic lymphocytic leukemia (CLL) cells to monocytes enhanced the secretion of cytokines, including CCL2, CCL4, and IL-6, while upregulating PD-L1 expression. This mechanism was TLR7-dependent, and its inhibition abrogated these effects [126].
The interaction between TEX and macrophages plays a crucial role in immune responses and inflammatory processes. Macrophages, essential for antigen presentation, phagocytosis, and immunomodulation, exhibit remarkable functional plasticity. The microenvironment shapes their phenotype, enabling them to perform diverse functions in inflammation [121]. GC-derived exosomes have been shown to activate NF-κB, stimulating the production of inflammatory cytokines, including G-CSF, IL-6, IL-8, IL-1β, CCL2, and TNF-α, thereby inducing a pro-inflammatory M1 macrophage response [127,128]. Furthermore, the interaction between breast cancer-derived exosomes and macrophages largely depends on TLR2 expression [127]. Exosomal proteins can also drive inflammation, with Annexin A2 (AnxA2) being a key example. TEX containing AnxA2 activate M1 macrophages, triggering NF-κB, STAT3, and p38 MAPK signaling pathways, thereby enhancing the secretion of proinflammatory cytokines, including IL-6 and TNF-α [129].
In the context of tumor exosome-mediated inflammation, it is essential to consider their impact on dendritic cells. Although the mechanisms by which TME and exosomes influence dendritic cells to promote tumorigenesis remain insufficiently understood. Dendritic cells are specialized antigen-presenting cells (APCs) responsible for recognizing, processing, and presenting antigens to T lymphocytes. This process is mediated by major histocompatibility complex (MHC) molecules, co-stimulatory proteins, and cytokines, ultimately leading to immune activation. Exosomes can be potent suppressors of immunity by inhibiting dendritic cell differentiation [121]. Additionally, TEX can activate dendritic cells, leading to increased production of inflammatory mediators, including IL-6 and prostaglandin E1 (PGE1), which subsequently enhances cancer cell invasion and metastasis [130].
MDSCs constitute a heterogeneous population of immature myeloid cells that play a crucial role in immune suppression, particularly in cancer. Under physiological conditions, MDSCs differentiate into monocytes, neutrophils, and dendritic cells. However, in the TME, exposure to proinflammatory cytokines and growth factors disrupts their maturation, resulting in the acquisition of an immunosuppressive phenotype [131]. However, in the TME, exposure to proinflammatory cytokines and growth factors disrupts their maturation, resulting in the acquisition of an immunosuppressive phenotype [132]. Conversely, glioma-derived exosomes carrying miR-10a or miR-21 are internalised by MDSCs, enhancing immunosuppression by increasing arginase activity, generating reactive oxygen species (ROS) and nitric oxide (NO), and secreting immunosuppressive cytokines [133].
The data mentioned above highlight the fundamental role of exosomes as mediators of intercellular communication, which is pivotal in developing resistance to cancer treatment. In summary, cancer cell-derived and tumour microenvironmental cell exosomes contribute to chemoresistance by transferring genetic material, modulating the immune response, and amplifying survival-related signalling pathways.
As previously mentioned, exosomes carry many types of molecules, such as proteins, lipids or RNAs [134]. Understanding what exosomes transport and under which conditions is crucial, as intercellular communication via exosomes is highly selective and not random. Therefore, determining exosomal content opens the possibility of identifying biomarkers that are valuable for diagnostics and guiding treatment strategies for various diseases, including cancer [134–136].
In recent years, numerous clinical trials have been conducted using exosomes, with approximately 50% of cancer therapy trials focusing on exosome biomarkers. Because exosomes are easily available in many biofluids, they offer a convenient source for biomarker testing [134,137,138]. Currently, the liquid biopsy is the most commonly used method, as it is minimally invasive [139]. Nonetheless, further validation is required, and exosome-derived biomarkers must be compared with established cancer biomarkers [140].
Exosomal miRNAs are considered one of the most abundant and stable molecules within exosomes, making them prime candidates as exosomal biomarkers [141]. Compared to normosomes, exosomal miRNAs originating from cancerous sources show a 30-fold increase in concentration, underscoring their diagnostic potential. This biomarker group is highly diverse, varying by cancer type and exosome source [139].
Among numerous miRNAs, potentially universal exosomal biomarkers include: iR-29a, miR-141-3p, miR-375, miR-106b-3p, miR-221/222, miR-200b-3p, miR-18a, miR-20b, miR-1307-5p, miR-519a-3p, miR-379-5p, and miR-410-3p [139]. In invasive submucosal colorectal cancer, biomarkers such as miR-181, miR-193 b, miR-195, and miR-411 have been identified and validated alongside previously established circulating cell-free serum (cf-miRNA) markers, demonstrating significant diagnostic performance in detecting lymph node metastasis [141]. Specifically, in colorectal cancer, miR-29a, miR-92a-3p, miR-128-3p promote metastasis, with miR-128-3p notably overexpressed at advanced tumor stage (III–IV) [139]. Additionally, exosomal miR-126, miR-1290, miR-23a, and miR-940 have emerged as promising diagnostic biomarkers in colorectal cancer, aiding in metastasis stage classification. Another study showed the downregulation of exosomal miRNA, such as miR-99b-5p and miR-150-5p, in CRC patients compared to healthy controls. Next, in esophageal cancer, exosomal miR-93-5p is believed to promote the proliferation of esophageal squamous carcinoma cells by suppressing the expression of p21 and cyclin D1 [138].
In breast cancer, exosomal miRNAs are used for cancer subtyping and setting a stemness and metastatic properties [140]. Scientists showed higher exosomal miR-101 and miR-372 levels in the serum of breast cancer patients compared to controls, highlighting miR-373’s potential as a breast cancer biomarker [135]. Several ovarian exosomal biomarkers have also been proposed for cancer diagnosis and monitoring disease progression. For example, the Lutgendorf group showed 22 exosomal RNA biomarkers strongly correlated with the progression class of OC [142]. In tissue sarcoma, miR-25-3p, miR-92a-3p, miR-34a, MT1-MMP, and MMP-14 have been implicated in disease progression [140]. Very valuable research was conducted by Gotanda et al., demonstrating that exosomal miR-328 could serve as a biomarker for assessing BCRP activity in the human intestines, because miR-328 negatively regulates BCRP expression [143]. Furthermore, exosomal miR-1246 in the body fluid of glioma patients has shown potential for monitoring glioma recurrence [65] and may also serve as a diagnostic biomarker for gastrointestinal cancers [144].
Exosomal circRNAs influence cancer proliferation, invasion, and drug resistance [145]. They represent excellent diagnostic and prognostic biomarkers for liquid biopsy because of their stability, long half-life, resistance to degradation, and broad distribution in many types of cancer [146]. In colorectal cancer, circRNAs such as circ-133 and circPABPC1 promote metastasis. Furthermore, analysis showed that these circRNAs can react with various miRNAs, enhancing metastatic foci formation [139]. For instance, hypoxic tumor-derived exosomal circ-133 promotes development of EMT in CRC through the miR-133a/GEF-H1/RhoA axis [146]. Esophageal squamous cell carcinoma is characterized by elevated levels of circRNAs hsa-circ-0001946 and hsa-circ-0043603, making them potential biomarkers of this cancer also [138]. Additionally, serum exosomes circMYC overexpression in nasopharyngeal carcinoma corelates with tumor size, lymph node metastasis, and survival rate [146].
Long non-coding RNAs (lncRNAs) have also emerged as promising biomarkers for cancer detection [139], as they may mediate cancer cell migration and invasion. Comparative analysis of exosomal RNA from CRC patients and healthy serum revealed 569 upregulated and 475 downregulated lncRNAs. For example, lncRNA UCA1 was overexpressed in the advanced stage of cancer, similar to lncRNA SPRY4-IT1. While the levels of lncRNA PVT1/VEGFA and LINC00161 were distinctly inverted [139]. In CRC patients, serum exosomal lncRNA FOXD2-AS1 has also demonstrated promising diagnostic potential [147]. In oral squamous-cell carcinoma (OSCC), it was shown that FOXD2-AS1 negatively regulated miR–185–5p, rather than the whole PLOD1/Akt/mTOR pathway activity, promoting cancer growth, invasion and migration [148]. Arima et al. reported that exosomal lncRNA brain cytoplasmic RNA 1 (BCYRN1) expression was significantly higher in bladder cancer patients than healthy individuals, contributing to enhanced proliferation, migration and invasion of cancer cells [149]. The lncRNAs have impressive potential to be biomarkers in gastric cancer (GC) because they are easy to detect in the fluids of the body. The studies showed some lncRNAs, such as LINC00152 or CASC15, to be crucial for tumour invasion, and low survival of GC patients [150].
It is important to note that exosomes also transport proteins that may serve as potential biomarkers. Despite their heterogeneity, exosomal proteins from diverse origins remain reliable prognostic, diagnostic, and predictive biomarkers in cancer treatment. For example, in colorectal cancer, the ADAM17 protein, which cleaves the E-cadherin junction, has enhanced vascular permeability and promotes metastasis [105]. Other potential protein biomarkers, such as HSPC111, Cyr61, and FMNL2, activate metabolic pathways, including XCL5-CXCR2 axis, αV β5/FAK/HIF-1α/STAT3/MMP2 pathway, and EGFL6/CKAP4/ERK axis, facilitating metastasis. In the case of hepatocellular cancer, exosomal protein biomarkers include CTLA-4, which activates the PTEN/CD44 signal pathway, and LOXL4, activating the FAK/Src pathway [139]. Barhoum group showed that exosomal protein MMP14 concentration is significantly higher in patients with adenoma vs. control patients [151]. Another protein, SUMO-specific protease 1 (SENP1), may serve as a prognostic biomarker for melanoma, influencing overall survival [152]. The other research group analysed lymphocyte migration regulation-related proteins (WASL, STK10 and WNK1) in the urine exosomes of lung cancer because of their non-exosomal analogous significant function in cancer pathogenesis. Finally, they demonstrated the tested proteins as potential lung cancer biomarkers [153]. In nasopharyngeal carcinoma, exosomal phosphatase and tensin homolog (PTEN) has been detected, which may assist in diagnosis and treatment planning for that type of cancer, as it plays a crucial role in radiotherapy and immunotherapy efficacy, tumour growth, and the immune microenvironment [154].
Importantly, some studies showed that exosomes can also inhibit cancer progression. Exosomes can convey biomarkers of cancer metastasis inhibition, such as miR-140-3p (which upregulate the expression of BCL9 and BCL2) and circFNDC3B (which inhibit miR-937-5p and upregulate TIMP3) in colorectal cancer [155,156]. In PC, miR-485-3p reduces PAK1 expression, while miRNA-339-5p decreases TGFBR3 level [139]. However, such reports are in the minority, and most indicate a positive correlation with the potential for cancer development. Nevertheless, there is no doubt that the identification of new exosomal biomarkers is crucial in modern medicine, but it remains challenging because of the complicated and yet non-standardised protocol of exosome isolation. Exosomes are secreted by all cell types, both healthy and cancerous. They mix in body fluids, making it difficult to assign a specific biomarker to a specific disease. The content of exosomes depends, among other things, on the physiological state of the organism and environmental conditions, which are variable. All this makes the analysis of exosomes very difficult. Therefore, further studies are necessary to assess exosomal cargos as early diagnostic biomarkers [151,157].
5 Therapeutic Strategies Involving Exosome Engineering
Exosomes have emerged as promising candidates for therapeutic applications in oncology due to their intrinsic ability to mediate intercellular communication by transferring proteins, lipids, and nucleic acids. Their favorable biological properties—including biocompatibility, low immunogenicity, and inherent targeting capabilities—render them ideal vectors for drug delivery. These attributes are particularly valuable in the development of innovative strategies aimed at overcoming chemoresistance in cancer therapy [158].
5.1 Engineering Exosomes as Drug Carriers
Genetic modification of exosomes is an effective way to confer new functions. This is achieved by fusing targeting ligands or peptides to exosomal transmembrane proteins, which are expressed in donor cells via plasmid transfection. The resulting exosomes display these ligands on their surface [159]. To date, several studies have investigated the use of exosomes as drug delivery vehicles. Tians’s comparative study showed that engineered exosomes displaying internalizing RGD peptide (iRGD) can effectively deliver DOX to αv integrin–positive breast cancer cells, resulting in significant tumor inhibition with minimal systemic toxicity [160]. Additional studies on breast cancer developed Tyr-Ile-Gly-Ser-Arg peptide (YIGSR) functionalized hybrid exosomes by combining exosomes from macrophages with lipid-polymeric nanoparticles to target laminin receptors overexpressed in breast cancer cells. These exosomes effectively delivered dasatinib, significantly enhancing drug uptake, inducing oxidative stress, and increasing apoptosis in tumor cells compared to non-targeted forms. In vivo, the system showed a 20-fold increase in drug bioavailability and a nearly 7-fold reduction in tumor size, demonstrating strong potential for precision breast cancer therapy [161]. The study by Wan presented a novel drug delivery system using extracellular vesicles (EVs) conjugated with aptamers to specifically target cancer cells. The study demonstrated that these targeted EVs effectively deliver anticancer drugs to breast cancer cells, enhancing therapeutic efficacy while minimising side effects [162]. Another researcher focused on PC and highlights engineered exosomes as highly promising drug carriers for this aggressive and hard-to-treat malignancy. Exosomes have been modified in several ways to enhance their drug delivery potential: by adding chondroitin sulfate for targeting CD44-positive tumor cells, using metabolic glycoengineering for precise delivery via click chemistry, and reassembling them with photosensitizers like chlorin e6 for imaging-guided photodynamic therapy. These strategies improve targeting, drug accumulation, and therapeutic precision in hard-to-treat pancreatic tumors [163]. Recently, investigators have examined advanced nanoparticle-based drug delivery systems (NDDS) for targeted CRC treatment. Researchers developed and characterised three distinct formulations: Bevacizumab-loaded chitosan nanoparticles (BEV-CHI-NP), polymeric micelles (BEV-PM), and BEV-conjugated exosomes enriched with AS1411 nucleolin-targeting DNA aptamer (AS1411) and N1-methyladenosine (AP-BEV + M1A-EXO). Among these, the AP-BEV + M1A-EXO formulation demonstrated superior efficacy, achieving a 65.4% reduction in tumor volume and significant decreases in tumor biomarkers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9), highlighting its potential as a precise and effective therapeutic strategy for CRC [164].
Alharbi et al. explored the use of nanotechnology to enhance the therapeutic effects of mangiferin and curcumin against OC. Researchers developed exosomal and liposomal nano-carriers to deliver these phytochemicals, aiming to improve their bioavailability and target the overactive PI3K/Akt/mTOR signaling pathway, which is central to OC progression. Computational analyses, including molecular docking and 100-nanosecond molecular dynamics simulations, demonstrated strong binding affinities of mangiferin and curcumin to key proteins in the pathway, suggesting that this nanotechnological approach could be a promising strategy in precision therapy for OC [165]. Interesting findings were made by Luo et al., who investigated the therapeutic potential of exosomes derived from expanded natural killer (eNK) cells in treating OC. These eNK-derived exosomes (eNK-EXO) were found to express typical NK cell markers and cytotoxic substances, demonstrating inherent anti-tumour activity against OC cells. Furthermore, when loaded with CIS, eNK-EXO enhanced the drug’s cytotoxic effects on resistant OC cells and reactivated suppressed NK cells within the TME, suggesting a dual mechanism of direct tumor inhibition and immune system modulation [166]. Pourmasoumi et al. explored the development of a targeted drug delivery system using exosomes conjugated with folic acid to deliver temozolomide (TMZ) and quercetin (QT) to glioblastoma (GBM) cells. Folic acid was utilized to enhance the targeting of exosomes to GBM cells that overexpress folate receptors. The co-delivery of TMZ and QT via these engineered exosomes resulted in increased cytotoxicity and apoptosis in GBM cells compared to treatments with either drug alone, indicating a synergistic effect and potential for improved therapeutic outcomes in GBM treatment [167]. Another study on GBM presents the development of hybrid nanocarriers combining exosomes and liposomes for targeted GBM treatment. These nanocarriers are labelled with a lipophilic NIR-II cyanine dye (NIR-C12) and functionalized with cyclic RGD peptides to enhance tumour targeting. The resulting NIR-C12-EL exhibits excellent colloidal stability and a high photothermal conversion efficiency of 62.28%, enabling effective NIR-II fluorescence imaging and photothermal therapy, which collectively contribute to prolonged survival in glioblastoma-bearing mice [168]. In a separate study, other researchers employed a similar exosome-based delivery strategy for Withaferin A. Withaferin A, a natural compound with anti-angiogenic properties, has been delivered using exosomes to enhance its therapeutic efficacy. Both intraperitoneal and oral administration of exosome-loaded Withaferin A showed a much stronger anti-tumour effect compared to free drugs in a human lung cancer xenograft mouse model [169].
5.2 Efflux Pump Inhibitors and Delivery of Chelating Agents
One of the major mechanisms behind chemoresistance is the upregulation of drug efflux transporters such as P-glycoprotein (P-gp/ABCB1) and multidrug resistance-associated proteins (MRPs). These ABC transporters actively remove cytotoxic drugs from the intracellular space, reducing their effectiveness. Engineered exosomes have shown promise in overcoming this mechanism by delivering inhibitors of these pumps directly to tumor cells, thereby enhancing intracellular drug retention and restoring chemosensitivity. In the literature, “pump inhibitors” delivered in exosomes are usually RNA molecules (miRNAs or siRNAs) targeting key regulatory genes associated with ABC-transport function [26,159,170].
Furthermore, exosomes have been explored as carriers for metal-chelating agents. Metal ions such as iron and copper are dysregulated, which has been implicated in both tumorigenesis and neurodegenerative disorders. Targeted delivery of chelators via exosomes may limit systemic toxicity and increase drug accumulation in specific tissues. This approach may increase drug accumulation in specific tissues and limit systemic toxicity [171–173].
Bellingham et al. discussed the potential of extracellular vesicles, particularly exosomes, as innovative carriers for targeted delivery of metal-chelating drugs to address disrupted metal ion homeostasis in neurodegenerative disorders. The authors emphasised that utilising exosomes for chelator delivery could enhance therapeutic specificity and efficiency while minimizing systemic toxicity. Furthermore, this strategy offers promising implications for precision medicine in treating conditions characterized by pathological metal accumulation, such as Alzheimer’s and Parkinson’s diseases [171]. Tao and Gao reviewed the potential of exosomes for diagnosing and treating neurodegenerative diseases. They emphasised exosomes’ ability to cross the blood-brain barrier, enabling targeted delivery of therapeutic agents, including metal-chelating drugs. Their analysis suggested that this approach could significantly improve the specificity and effectiveness of treatments for neurodegenerative disorders [172]. Nouri et al. further supported findings by Tao and Gao 2024 and Bellingham et al., highlighting exosomes as effective vehicles capable of crossing the blood-brain barrier to deliver targeted therapies, including metal-chelating drugs, for neurodegenerative diseases [173].
In the context of cancer, exosomes have been explored as drug delivery systems capable of transporting chemotherapeutics like paclitaxel and DOX directly to tumor cells, reducing systemic toxicity. Although their use for delivering metal-chelating agents in oncology is still limited, their potential in targeting tumors with metal ion dysregulation is significant [174].
5.3 Genetic Cargo Delivery: mRNA and miRNA
Exosomes can also be modified in order to deliver genetic material capable of modulating cellular pathways involved in drug resistance. To re-sensitise tumor cells to chemotherapeutic agents, miRNAs that downregulate resistance-associated genes or mRNAs encoding pro-apoptotic proteins can be packaged into exosomes [158,175].
Su et al. demonstrated that increasing the expression of miR-135b in drug-resistant non-small cell lung cancer (NSCLC) cells enhanced their sensitivity to CIS treatment by directly downregulating Frizzled-1 (FZD1) [176]. Zeng et al. investigated the role of exosomal miR-151a in glioblastoma multiforme (GBM). They found that miR-151a was downregulated in temozolomide (TMZ)-resistant GBM cells. Restoring miR-151a expression sensitised these cells to TMZ by inhibiting XRCC4-mediated DNA repair. Furthermore, exosomes from TMZ-resistant cells lacking miR-151a could transfer this resistance to sensitive cells. However, supplementing exosomal miR-151a reversed this effect, suggesting that exosomal miR-151a could be a therapeutic target to overcome chemoresistance in GBM [177]. Lou et al. explored the use of exosomes derived from adipose tissue-derived mesenchymal stem cells (AMSCs) modified to overexpress miR-122. They demonstrated that these exosomes could effectively deliver miR-122 to hepatocellular carcinoma (HCC) cells, leading to increased sensitivity to chemotherapeutic agents like sorafenib. In vivo experiments further showed that intra-tumour injection of these exosomes enhanced the antitumor efficacy of sorafenib, indicating that AMSC-derived exosomes carrying miR-122 could be a novel strategy to improve HCC chemosensitivity [178].
Exosomal delivery of therapeutic mRNAs represents a promising strategy to counteract resistance mechanisms in cancer.
5.4 Combining Exosome-Based Therapies with Conventional Drugs
Integrating exosome-based therapies with traditional anticancer agents aims to exploit the natural targeting abilities of exosomes to enhance drug delivery and efficacy. This combination can potentially:
• Enhance Drug Delivery: Exosomes can be engineered to carry chemotherapeutic drugs directly to tumor cells, improving drug accumulation at the tumor site and reducing systemic toxicity [179].
• Reverse Drug Resistance: By delivering specific molecules that inhibit resistance pathways, exosomes can sensitize tumor cells to conventional therapies [180].
• Modulate Immune Responses: Exosome-based treatments combined with immunotherapies can enhance anti-tumor immunity, potentially overcoming resistance mechanisms [181].
5.5 Examples of Combination Therapy Applications
Numerous studies have demonstrated the advantages of combining therapeutic agents, particularly in the management of complex or multifactorial diseases. In preclinical studies, bone marrow-derived mesenchymal stem cell (MSC) exosomes loaded with PAC successfully targeted breast cancer cells, increasing drug efficacy while reducing systemic toxicity [169]. Building on this concept, further research has demonstrated that exosomes can serve as efficient nanocarriers for PTX, significantly inhibiting tumor growth and metastasis. In particular, PTX-loaded exosomes have been shown to enhance drug accumulation at tumour sites while minimizing off-target effects [182,183]. In addition to PTX delivery, exosomes have also been employed as carriers for DOX, particularly in the context of drug-resistant cancers. Researchers have explored loading DOX into exosomes to target drug-resistant cancer cells. The exosome-mediated delivery system enhanced the cytotoxicity of DOX against resistant breast cancer cells, suggesting a potential strategy to overcome drug resistance [184]. Expanding on this strategy, another investigation focused on the co-delivery of DOX and miRNA-159 using targeted exosomes in triple-negative breast cancer (TNBC) models. This combined delivery system was shown to silence the T-cell factor 7 (TCF-7) gene effectively, resulting in enhanced anticancer activity without inducing significant toxicity. The study demonstrated a synergistic interaction between DOX and miR-159, offering a promising approach to overcoming therapeutic resistance in TNBC [185]. Further innovations have included engineering exosomes with dual-targeting ligands to optimize delivery efficiency. In a 2023 study, researchers developed exosomes functionalized with both iRGD and tLyp1 peptides, designed to improve specificity and uptake by breast cancer cells. These modified exosomes significantly enhanced DOX accumulation in both MCF-7 and MDA-MB-231 cell lines, leading to increased cytotoxic effects. This dual-targeting strategy offers a promising avenue for addressing drug resistance through more precise tumor targeting [186]. Another study showed that boron clusters (B12Br122−) enhanced DOX loading into milk-derived exosomes, creating exosome–dodecaborate (EDB) particles that overcame DOX resistance in breast cancer. EDB improved intracellular DOX delivery, inhibited P-glycoprotein-mediated efflux, and restored drug sensitivity, effectively suppressing tumor growth in resistant models [187].
Research conducted by Zhang et al. on OC demonstrated that CIS-loaded exosomes from umbilical cord blood M1 macrophages significantly boost cytotoxicity in OC cells, offering a promising strategy to overcome drug resistance [188]. Other studies by [189] have shown that exosomes from omental adipocytes influence epithelial ovarian cancer (EOC) progression and resistance to paclitaxel chemotherapy. The researchers found that these exosomes induce epithelial-to-mesenchymal transition (EMT) in EOC cells, enhancing their invasive capabilities. Additionally, the exosomes reduced the efficacy of PTX by promoting cancer cell proliferation and survival. MiRNA sequencing identified several miRNAs—miR-21, let-7b, miR-16, and miR-92a—as abundant in these exosomes, contributing to the observed effects [189]. Following this, X. Pan et al. examined the role of exosomal miR-4516 in promoting CIS resistance in OC. Researchers found that ovarian cancer stem cells (OCSCs) secrete exosomes enriched with miR-4516, which are taken up by CIS-resistant ovarian cancer cells (SKOV3/DDP). This uptake leads to the downregulation of the tumor suppressor gene GAS7, thereby enhancing the cells’ resistance to CIS. Overexpression of GAS7 was shown to reverse this resistance, both in vitro and in vivo, suggesting that targeting the miR-4516/GAS7 axis could be a potential therapeutic strategy to overcome CIS resistance in OC [190]. In a separate study, [191] explored that exosomal lncRNA PLADE from ascites enhances CIS sensitivity in high-grade serous OC by promoting R-loop formation. PLADE downregulates HNRNPD via VHL-mediated degradation, leading to DNA damage and increased apoptosis, and is notably reduced in CIS-resistant patients. Another experiment that explored the role of exosomes in reducing drug resistance was conducted by Zhao et al. The study showed that exosomes loaded with tetramethylpyrazine (EXO-TMP) reversed PTX resistance in A2780T OC cells by downregulating P-gp, ABCC1, and glutathione S-transferase Pi 1 (GSTP1) GSTP1, leading to increased apoptosis and improved drug sensitivity [192].
Another emerging area of interest involves the role of exosomes in overcoming resistance mechanisms in CML. The study by Du et al. showed that modified dendritic cell-derived exosomes (Dex) overcame drug resistance in CML, including T315I mutation, by activating NK and T cells via the NKG2D/NKG2D-L pathway, leading to effective leukemia cell killing and tumor suppression in mice [193]. Bellavia et al. engineered exosomes to express the interleukin-3 (IL-3) receptor ligand, allowing them to specifically target CML cells. These exosomes were loaded with either imatinib or BCR-ABL siRNA. The study demonstrated that these targeted exosomes effectively inhibited the growth of CML cells both in vitro and in vivo, suggesting a potential strategy to overcome drug resistance [194]. Javidi-Sharifi et al. found that fibroblast growth factor 2 (FGF2) from bone marrow stromal cells is secreted in exosomes, which are then taken up by leukemia cells, protecting them from tyrosine kinase inhibitors (TKIs). Inhibiting the fibroblast growth factor 2 and fibroblast growth factor receptor 1 (FGF2-FGFR1) signaling pathway reduced exosome secretion and sensitized leukemia cells to TKIs, indicating a potential therapeutic approach to counteract drug resistance [195].
Pancreatic cancer (PC) is an additional example of a malignancy characterized by poor prognosis and significant resistance to therapy. The study by Zhou et al. showed that bone marrow mesenchymal stem cell-derived exosomes loaded with PTX and gemcitabine monophosphate effectively penetrated pancreatic tumors, enhanced drug delivery, and inhibited tumor growth, offering a targeted strategy with reduced toxicity [196]. In pancreatic ductal adenocarcinoma (PDAC), exosomal miR-155 and circBIRC6 have been shown to promote gemcitabine and oxaliplatin resistance, respectively, by enhancing anti-apoptotic signaling and DNA repair pathways [66].
Liver cancer, particularly hepatocellular carcinoma, also poses significant therapeutic challenges. According to findings by Hwang et al., they showed that exosomal miR-6126 restores sorafenib sensitivity in resistant hepatocellular carcinoma by targeting CD44 and HK2, making it a promising therapeutic target to overcome drug resistance [197]. Another study demonstrated that engineered exosomes delivering a ferroptosis-inducing agent enhanced the efficacy of sorafenib in hepatocellular carcinoma. By combining exosome-based targeted delivery with a conventional drug, the approach helped overcome sorafenib resistance and boosted cancer cell death via ferroptosis [198]. The study by Li et al. showed that exosomes from bone marrow mesenchymal stem cells (BM-MSCs) modified with siRNA against GRP78 effectively delivered the siRNA to hepatocellular carcinoma cells, downregulated GRP78, and restored sorafenib sensitivity. Combined treatment significantly inhibited tumor growth and metastasis in resistant HCC models [199].
In the context of central nervous system malignancies, glioblastoma multiforme (GBM) stands out due to its poor prognosis and limited therapeutic success. The study by Han et al. introduced an innovative drug delivery system designed to traverse the blood-brain barrier (BBB) and enhance the efficacy of chemotherapeutic agents. This system utilizes engineered exosome membranes to camouflage thermoresponsive nanoparticles, enabling targeted delivery and controlled release of drugs within the brain. Although the study does not directly address drug resistance, the exosome-camouflaged, thermoresponsive delivery system enhances drug accumulation in brain tumors and may help overcome resistance by improving targeted penetration and controlled release across the blood-brain barrier [200]. Another study by Pourmasoumi et al. presents a novel approach to glioblastoma treatment by co-delivering temozolomide (TMZ) and quercetin (Qct) using folic acid (FA)-conjugated exosomes [167]. This strategy aims to overcome TMZ resistance and enhance drug delivery across the BBB. In the study by [201], glioblastoma cells were found to secrete exosomes containing the long noncoding RNA lnc-TALC. These exosomes are taken up by microglia, leading to their polarization into the M2 phenotype and subsequent production of complement component C5. The C5/C5a signaling pathway enhances DNA repair mechanisms in glioblastoma cells, contributing to resistance against TMZ chemotherapy. To counteract this resistance, the researchers employed a C5a receptor (C5aR1) antagonist, which effectively disrupted the exosome-mediated communication between glioblastoma cells and microglia. This intervention restored the sensitivity of glioblastoma cells to temozolomide, highlighting a potential therapeutic strategy to overcome chemoresistance in GBM [201]. Other researchers have contributed to this topic by examining the role of pacritinib, a STAT3 inhibitor, in overcoming TMZ resistance in glioblastoma. They found that M2-polarized glioblastoma-associated macrophages (GAMs) secrete exosomes enriched with miR-21, which contribute to TMZ resistance in glioblastoma cells. Treatment with pacritinib downregulated STAT3 activity in these macrophages, leading to a reduction in miR-21-enriched exosome secretion. This, in turn, sensitized glioblastoma cells to TMZ, suggesting that targeting the STAT3 pathway in GAMs can mitigate exosome-mediated chemoresistance [202]. In the study by Munoz et al., the authors identified the Sonic Hedgehog (SHH) signaling pathway as a key contributor to TMZ resistance in GBM cells. They discovered that miR-9 suppresses PTCH1, a negative regulator of SHH signaling, leading to ligand-independent activation of the SHH pathway and upregulation of the multidrug resistance gene MDR1. To counteract this, the researchers utilized bone marrow-derived mesenchymal stem cells (MSCs) engineered to deliver anti-miR-9 via exosomes and gap junctions. This approach successfully restored PTCH1 expression, inhibited SHH signaling, and sensitized GBM cells to TMZ, suggesting a potential therapeutic strategy to overcome chemoresistance [203].
In addition to the cancers discussed above, melanoma also presents distinct clinical and molecular challenges. Combining exosome-targeted strategies with conventional chemotherapy may improve treatment outcomes in drug-resistant cancers, as supported by recent studies. Kulkarni et al. demonstrated that growth hormone (GH) promotes drug resistance and migration in melanoma by enhancing the release of exosomes enriched with ABCC1, ABCB1, and other resistance-related proteins. Importantly, inhibition GH signaling with the receptor antagonist pegvisomant reduced the exosomal transfer of these factors and restored drug sensitivity. This work highlights how interfering with exosome-mediated communication can enhance the efficacy of classical anticancer therapies [204]. In the study by Feng et al., researchers developed an innovative cancer treatment strategy by combining exosome-based delivery with conventional photodynamic therapy to overcome drug resistance in melanoma. They engineered coordination-assembled Iridium(III) photosensitizers camouflaged with exosome membranes, enhancing tumor targeting and biocompatibility. Upon light activation, these nanoparticles induced multiple forms of cell death—apoptosis, autophagy, and ferroptosis—effectively eradicating melanoma tumors and inhibiting metastasis in mouse models. This approach exemplifies how integrating exosome-based delivery systems with traditional therapies can improve outcomes in drug-resistant cancers [205].
Nittayaboon et al. conducted a proteomic analysis of exosomes derived from butyrate-resistant CRC cells and identified proteins potentially involved in resistance to multiple anticancer drugs. These exosomal proteins may facilitate intercellular transfer of resistance traits, contributing to reduced treatment efficacy. Targeting such exosome-mediated mechanisms could help restore drug sensitivity and enhance the effectiveness of standard therapies in resistant CRC [206].
Lung cancer, one of the leading causes of cancer-related mortality worldwide, also demonstrates considerable treatment resistance. In the study by Saeed et al., researchers explored the use of camel milk-derived exosomes as nanocarriers for delivering curcumin (CUR) to lung cancer cells. They successfully isolated exosomes from camel milk and loaded them with CUR, achieving a 20% loading efficiency. In vitro experiments demonstrated that the exosome-encapsulated curcumin (ExoCUR) exhibited significantly enhanced cytotoxic effects against both drug-sensitive (A549) and taxol-resistant (A549TR) lung cancer cell lines compared to free curcumin. Molecular docking and dynamics simulations indicated that CUR has a strong binding affinity for the epidermal growth factor receptor (EGFR), comparable to the established drug gefitinib. Furthermore, CUR effectively downregulated EGFR and STAT3 expression in lung cancer cells, suggesting its potential to disrupt key signaling pathways involved in tumor progression [207]. These findings highlight the potential of camel milk-derived exosomes as effective and biocompatible delivery systems for curcumin, offering a promising strategy to overcome drug resistance in lung cancer therapy.
5.6 Exosome-Based Therapy—How to Study It?
Currently, the most optimal method for studying exosomes and testing exosome-based therapy seems to be xenograft in vivo models. Xenotransplants are widely used for the analysis of cancer cells’ tumorigenicity, tumor histology, and for testing of tumor response to novel therapies [208]. Many studies have already been undertaken using this model, due easy to label exosomes. For instance, the application of C-X-C chemokine receptor 4 (CXCR4)/TNF-related apoptosis-inducing ligand (TRAIL)-enriched exosomes in improving the efficacy of brain disease chemotherapy was tested [209]. NK cell-derived exosomes were studied in the primary liver cancer, hepatocellular carcinoma, using the orthotopic and subcutaneous tumor model also. Both are xenograft models but obtained by different techniques [210]. The other scientist analysed hypoxic exosomes on a mouse glioblastoma multiforme xenograft model [211].
Plasma of acute myeloid leukaemia (AML) patients is significantly enriched in exosomes. Chang-Sook Hong et al. showed that fully engrafted AML PDX (patient-derived xenograft) mice produced exosomes with properties similar to those of exosomes isolated from AML patients’ plasma [212,213]. This is particularly important because we know that most research models are not perfect and do not fully reflect the conditions prevailing in the patient’s body. Therefore, we believe that research using exosome-based therapy may yield the most and fastest positive results in AML research, rather than in other cancers.
However, when using exosome-based therapy, we must always remember the existing limitations. In the article, we mentioned several times the high heterogeneity of exosomes, which makes standardisation and schematic analysis difficult. The heterogeneity is often present within EVs isolated from a single cell type [214]. The other problem lies in achieving stable, large-scale production of exosomes. It is important to take into account established dosage plans for exosome treatment because of exosomes’ short half-life, which leads to short circulation time in vivo [215]. Key challenges include identifying the administration route. The costs of such therapy are still very high, making it difficult to disseminate on a large scale [158].
Nevertheless, exosomes, due to their low immunogenicity and capacity to pass through small pores, hold great promise in cancer therapy [181].
Exosomes are key in developing cancer resistance to cytostatics, acting as active mediators of intercellular communication within the tumor microenvironment. Through the transfer of transport proteins (such as P-gp, BCRP), immunosuppressive ligands (e.g., PD-L1, TGF-β, PGE2) and non-coding RNA molecules (miRNA, circRNA), exosomes promote the transfer of the resistant phenotype between cancer cells and between cancer cells and the surrounding microenvironment. These observations confirm their fundamental role in the modification of signaling pathways, regulation of apoptosis, angiogenesis and in shaping the immunosuppressive TME, which results in reduced efficacy of anticancer therapy.
Due to their presence in various body fluids and the ability to selectively transfer bioactive molecules, exosomes have great potential as non-invasive biomarkers used in diagnostics, monitoring treatment efficacy and prognosis of the course of cancer. At the same time, their participation in shaping therapeutic resistance makes them an attractive target for therapeutic interventions—both through blocking their secretion or inhibiting the transport of resistance, and through engineering exosomes as carriers of anticancer drugs. However, the clinical application of these strategies requires further deepening of knowledge about the biology of exosomes and their interactions within the tumor microenvironment (Fig. 2).
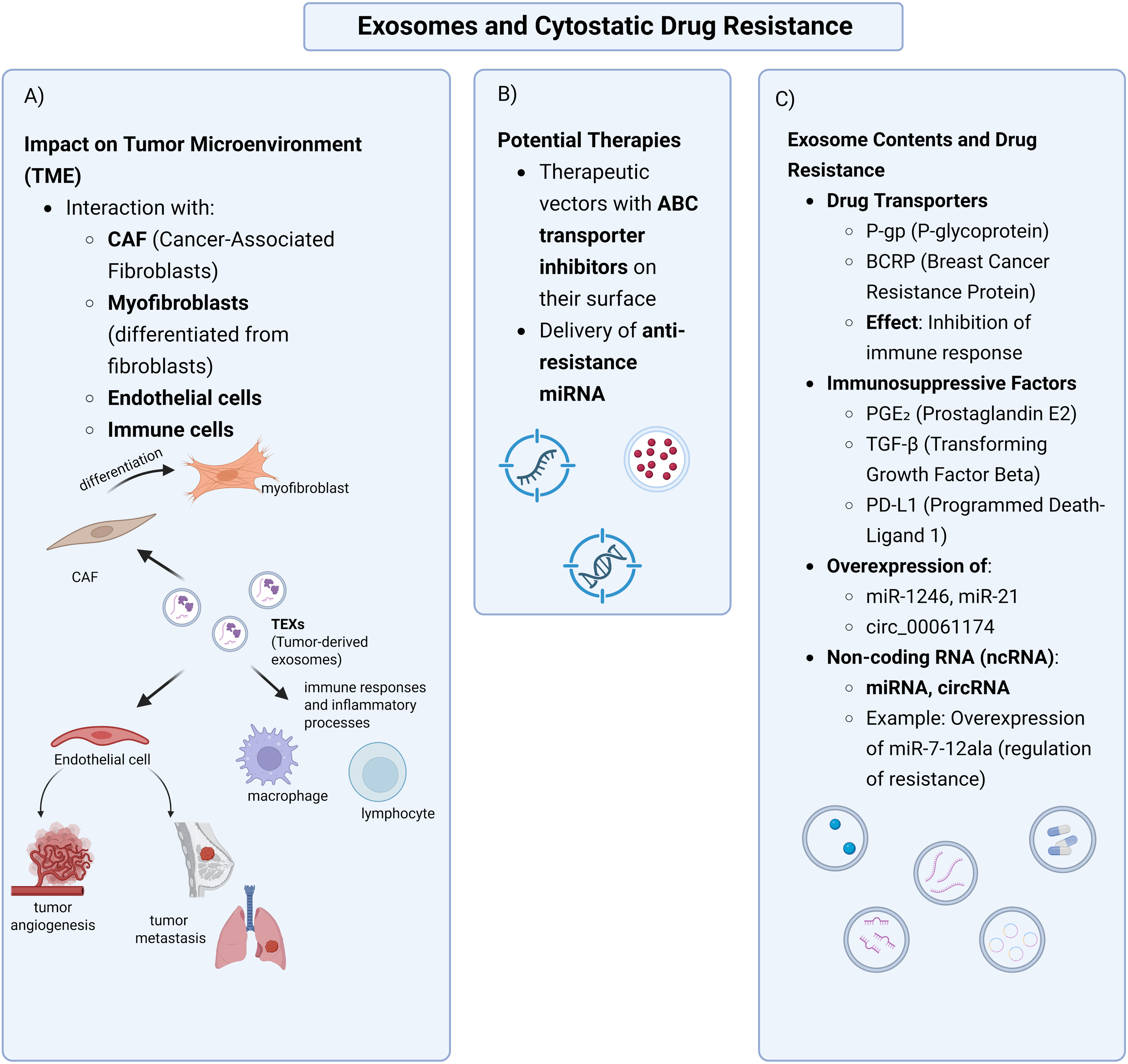
Figure 2: Exosomes and cytostatic drug resistance. (A) Tumour-derived exosomes (TEXs) interact with components of the tumour microenvironment (TME), including cancer-associated fibroblasts (CAFs), myofibroblasts, endothelial, and immune cells, contributing to TME remodelling, angiogenesis, metastasis, and immunomodulation. (B) Potential therapies involve exosome-based vectors delivering ABC transporter inhibitors or anti-resistance miRNAs. (C) Exosomes carry drug transporters (e.g., P-gp, BCRP), immunosuppressive factors (PGE2, TGF-β, PD-L1), and non-coding RNAs (miRNAs, circRNAs), which mediate drug resistance and immune escape. Overexpression of miR-1246, miR-21, and circ_0006174 is particularly implicated in multidrug resistance (Created in BioRender, accessed on 10 October 2024)
Despite the dynamic development of this field, many mechanisms of exosome action remain incompletely understood. Furthermore, we have to remember many limitations associated with exosome research itself. Currently, there’s no single, standardised protocol for exosome isolation due to their biodiversity [216]. Despite extensive efforts, in vitro analysis of isolated vesicles may still not fully reflect the conditions found in living organisms. Therefore, it is necessary to conduct further, targeted experimental and translational studies that will allow for a better understanding of their role in the process of resistance to treatment and will enable the development of effective therapeutic strategies based on their modification or modulation. Integrating knowledge from molecular biology, tumor immunology and nanomedicine may contribute to a future breakthrough in the treatment of chemotherapy-resistant tumors. At the same time, we must remember about nutritional aspects and preventive oncology, which can complement each other. And therapeutic interventions should be individualised [217].
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualisation, Sandra Kałużna, Monika Świerczewska, Karolina Wojtowicz; writing—original draft preparation, review and editing, Sandra Kałużna, Monika Świerczewska, Sylwia Ciesiółka, Małgorzata Partyka, Michał Nowicki, Karolina Wojtowicz; visualisation, Sandra Kałużna, Monika Świerczewska; supervision, Karolina Wojtowicz. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Gouveia TIA, Silva AMT, Freire MG, Sousa ACA, Alves A, Santos MSF. Multi-target analysis of cytostatics in hospital effluents over a 9-month period. J Hazard Mater. 2023;448(13):130883. doi:10.1016/J.JHAZMAT.2023.130883. [Google Scholar] [PubMed] [CrossRef]
2. Sonkin D, Thomas A, Teicher BA. Cancer treatments: past, present, and future. Cancer Genet. 2024;286–287:18–24. doi:10.1016/j.cancergen.2024.06.002. [Google Scholar] [PubMed] [CrossRef]
3. Pałaszewska-Tkacz A, Czerczak S, Konieczko K, Kupczewska-Dobecka M. Cytostatics as hazardous chemicals in healthcare workers’ environment. Int J Occup Med Environ Health. 2019;32(2):141–59. doi:10.13075/IJOMEH.1896.01248. [Google Scholar] [PubMed] [CrossRef]
4. Khan SU, Fatima K, Aisha S, Malik F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun Signal. 2024;22(1):1–26. doi:10.1186/S12964-023-01302-1. [Google Scholar] [PubMed] [CrossRef]
5. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020; 367:eaau6977. doi:10.1126/science.aau6977. [Google Scholar] [CrossRef]
6. Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. doi:10.3390/IJMS21093233. [Google Scholar] [PubMed] [CrossRef]
7. Tian Y, Wang X, Wu C, Qiao J, Jin H, Li H. A protracted war against cancer drug resistance. Cancer Cell Int. 2024;24(1):1–14. doi:10.1186/S12935-024-03510-2. [Google Scholar] [PubMed] [CrossRef]
8. Chatterjee N, Pulipaka B, Subbalakshmi AR, Jolly MK, Nair R. Unraveling the dangerous duet between cancer cell plasticity and drug resistance. Comput Syst Oncol. 2023;3(3):e1051. doi:10.1002/CSO2.1051. [Google Scholar] [CrossRef]
9. Guo QR, Wang H, Yan YD, Liu Y, Su CY, Chen HB et al.The role of exosomal microRNA in cancer drug resistance. Front Oncol. 2020;10:2165. doi:10.3389/FONC.2020.00472. [Google Scholar] [PubMed] [CrossRef]
10. Tan S, Xia L, Yi P, Han Y, Tang L, Pan Q, et al. Exosomal miRNAs in tumor microenvironment. J Exp Clin Cancer Res. 2020;39(1):1486. doi:10.1186/S13046-020-01570-6. [Google Scholar] [PubMed] [CrossRef]
11. Zelli V, Compagnoni C, Capelli R, Corrente A, Di Vito Nolfi M, Zazzeroni F, et al. Role of exosomal microRNAs in cancer therapy and drug resistance mechanisms: focus on hepatocellular carcinoma. Front Oncol. 2022;12:940056. doi:10.3389/FONC.2022.940056. [Google Scholar] [PubMed] [CrossRef]
12. Patra SK, Sahoo RK, Biswal S, Panda SS, Biswal BK. Enigmatic exosomal connection in lung cancer drug resistance. Mol Ther Nucleic Acids. 2024;35(2):102177. doi:10.1016/j.omtn.2024.102177. [Google Scholar] [PubMed] [CrossRef]
13. Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, et al. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010;295(1):110–23. doi:10.1016/j.canlet.2010.02.019. [Google Scholar] [PubMed] [CrossRef]
14. Zdzalik-Bielecka D, Poswiata A, Kozik K, Jastrzebski K, Schink KO, Brewinska-Olchowik M, et al. The GAS6-AXL signaling pathway triggers actin remodeling that drives membrane ruffling, macropinocytosis, and cancer-cell invasion. Proc Natl Acad Sci U S A. 2021;118(28):4296. doi:10.1073/pnas.2024596118. [Google Scholar] [PubMed] [CrossRef]
15. Daver NG, Maiti A, Kadia TM, Vyas P, Majeti R, Wei AH, et al. TP53-mutated myelodysplastic syndrome and acute myeloid leukemia: biology, current therapy, and future directions. Cancer Discov. 2022;12(11):2516–29. doi:10.1158/2159-8290.CD-22-0332. [Google Scholar] [PubMed] [CrossRef]
16. Kater L, Kater B, Jakupec MA, Keppler BK, Prokop A. KP772 overcomes multiple drug resistance in malignant lymphoma and leukemia cells in vitro by inducing Bcl-2-independent apoptosis and upregulation of Harakiri. J Biol Inorg Chem. 2021;26(8):897–907. doi:10.1007/S00775-021-01900-9. [Google Scholar] [PubMed] [CrossRef]
17. Kim J, Shim MK, Yang S, Moon Y, Song S, Choi J, et al. Combination of cancer-specific prodrug nanoparticle with Bcl-2 inhibitor to overcome acquired drug resistance. J Control Release. 2021;330:920–32. doi:10.1016/J.JCONREL.2020.10.065. [Google Scholar] [PubMed] [CrossRef]
18. Fletcher JI, Williams RT, Henderson MJ, Norris MD, Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist Updat. 2016;26:1–9. doi:10.1016/J.DRUP.2016.03.001. [Google Scholar] [PubMed] [CrossRef]
19. Biscetti F, Porreca CF, Bertucci F, Straface G, Santoliquido A, Tondi P, et al. TNFRSF11B gene polymorphisms increased risk of peripheral arterial occlusive disease and critical limb ischemia in patients with type 2 diabetes. Acta Diabetol. 2014;51(6):1025–32. doi:10.1007/s00592-014-0664-1. [Google Scholar] [PubMed] [CrossRef]
20. Karvar S, Biol TJ. The role of ABC transporters in anticancer drug transport. Turkish J Biol. 2014;38(6):800–5. doi:10.3906/biy-1407-3. [Google Scholar] [CrossRef]
21. Yu T-Y, Zhang G, Chai X-X, Ren L, Yin D-C, Zhang C-Y. Recent progress on the effect of extracellular matrix on occurrence and progression of breast cancer. Life Sci. 2023;332(4):122084. doi:10.1016/j.lfs.2023.122084. [Google Scholar] [PubMed] [CrossRef]
22. Zhang M, Zhang B. Extracellular matrix stiffness: mechanisms in tumor progression and therapeutic potential in cancer. Exp Hematol Oncol. 2025;14(1):1–34. doi:10.1186/S40164-025-00647-2. [Google Scholar] [PubMed] [CrossRef]
23. Krylova SV, Feng D. The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci. 2023;24(2):1337. doi:10.3390/IJMS24021337. [Google Scholar] [PubMed] [CrossRef]
24. Chen Y, Xie Y, Xu L, Zhan S, Xiao Y, Gao Y, et al. Protein content and functional characteristics of serum-purified exosomes from patients with colorectal cancer revealed by quantitative proteomics. Int J Cancer. 2017;140(4):900–13. doi:10.1002/IJC.30496. [Google Scholar] [PubMed] [CrossRef]
25. Taghikhani A, Farzaneh F, Sharifzad F, Mardpour S, Ebrahimi M, Hassan ZM. Engineered tumor-derived extracellular vesicles: potentials in cancer immunotherapy. Front Immunol. 2020;11:286. doi:10.3389/FIMMU.2020.00221. [Google Scholar] [PubMed] [CrossRef]
26. Li J, Gao N, Gao Z, Liu W, Pang B, Dong X, et al. The emerging role of exosomes in cancer chemoresistance. Front Cell Dev Biol. 2021;9:737962. doi:10.3389/fcell.2021.737962. [Google Scholar] [PubMed] [CrossRef]
27. Vergani E, Daveri E, Vallacchi V, Bergamaschi L, Lalli L, Castelli C, et al. Extracellular vesicles in anti-tumor immunity. Semin Cancer Biol. 2022;86(Pt 1):64–79. doi:10.1016/J.SEMCANCER.2021.09.004. [Google Scholar] [PubMed] [CrossRef]
28. Zhang C, Ji Q, Yang Y, Li Q, Wang Z. Exosome: function and role in cancer metastasis and drug resistance. Technol Cancer Res Treat. 2018;17(4):1159. doi:10.1177/1533033818763450. [Google Scholar] [PubMed] [CrossRef]
29. Ren B, Li X, Zhang Z, Tai S, Yu S. Exosomes: a significant medium for regulating drug resistance through cargo delivery. Front Mol Biosci. 2024;11:537. doi:10.3389/FMOLB.2024.1379822. [Google Scholar] [PubMed] [CrossRef]
30. Zhou E, Li Y, Wu F, Guo M, Xu J, Wang S, et al. Circulating extracellular vesicles are effective biomarkers for predicting response to cancer therapy. eBioMedicine. 2021;67(1):103365. doi:10.1016/J.EBIOM.2021.103365. [Google Scholar] [PubMed] [CrossRef]
31. Nikdoust F, Pazoki M, Mohammadtaghizadeh M, Aghaali MK, Amrovani M. Exosomes: potential player in endothelial dysfunction in cardiovascular disease. Cardiovasc Toxicol. 2022;22(3):225–35. doi:10.1007/S12012-021-09700-Y. [Google Scholar] [PubMed] [CrossRef]
32. Lopes-Rodrigues V, Di Luca A, Sousa D, Seca H, Meleady P, Henry M, et al. Multidrug resistant tumour cells shed more microvesicle-like EVs and less exosomes than their drug-sensitive counterpart cells. Biochim Biophys Acta. 2016;1860(3):618–27. doi:10.1016/J.BBAGEN.2015.12.011. [Google Scholar] [PubMed] [CrossRef]
33. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi:10.1038/NRC706. [Google Scholar] [PubMed] [CrossRef]
34. Li R, Dong C, Jiang K, Sun R, Zhou Y, Yin Z, et al. Rab27B enhances drug resistance in hepatocellular carcinoma by promoting exosome-mediated drug efflux. Carcinogenesis. 2020;41(11):1583–91. doi:10.1093/CARCIN/BGAA029. [Google Scholar] [PubMed] [CrossRef]
35. Shen J, Zhu X, Fei J, Shi P, Yu S, Zhou J. Advances of exosome in the development of ovarian cancer and its diagnostic and therapeutic prospect. Onco Targets Ther. 2018;11:2831–41. doi:10.2147/OTT.S159829. [Google Scholar] [PubMed] [CrossRef]
36. Ning K, Wang T, Sun X, Zhang P, Chen Y, Jin J, et al. UCH-L1-containing exosomes mediate chemotherapeutic resistance transfer in breast cancer. J Surg Oncol. 2017;115(8):932–40. doi:10.1002/JSO.24614. [Google Scholar] [PubMed] [CrossRef]
37. Torreggiani E, Roncuzzi L, Perut F, Zini N, Baldini N. Multimodal transfer of MDR by exosomes in human osteosarcoma. Int J Oncol. 2016;49(1):189–96. doi:10.3892/IJO.2016.3509. [Google Scholar] [PubMed] [CrossRef]
38. Natarajan K, Xie Y, Baer MR, Ross DD. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem Pharmacol. 2012;83(8):1084–103. doi:10.1016/J.BCP.2012.01.002. [Google Scholar] [PubMed] [CrossRef]
39. Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—an update. AAPS J. 2015;17(1):65–82. doi:10.1208/S12248-014-9668-6. [Google Scholar] [PubMed] [CrossRef]
40. Shigeta J, Katayama K, Mitsuhashi J, Noguchi K, Sugimoto Y. BCRP/ABCG2 confers anticancer drug resistance without covalent dimerization. Cancer Sci. 2010;101(8):1813–21. doi:10.1111/J.1349-7006.2010.01605.X. [Google Scholar] [PubMed] [CrossRef]
41. Guo J, Wang Q, Zhang Y, Sun W, Zhang S, Li Y, et al. Functional daidzein enhances the anticancer effect of topotecan and reverses BCRP-mediated drug resistance in breast cancer. Pharmacol Res. 2019;147:104387. doi:10.1016/J.PHRS.2019.104387. [Google Scholar] [PubMed] [CrossRef]
42. Kong JN, He Q, Wang G, Dasgupta S, Dinkins MB, Zhu G, et al. Guggulsterone and bexarotene induce secretion of exosome-associated breast cancer resistance protein and reduce doxorubicin resistance in MDA-MB-231 cells. Int J Cancer. 2015;137(7):1610–20. doi:10.1002/IJC.29542. [Google Scholar] [PubMed] [CrossRef]
43. Kim MW, Lee H, Lee S, Moon S, Kim Y, Kim JY, et al. Drug-resistant profiles of extracellular vesicles predict therapeutic response in TNBC patients receiving neoadjuvant chemotherapy. BMC Cancer. 2024;24(1):1–12. doi:10.1186/S12885-024-11822-9. [Google Scholar] [PubMed] [CrossRef]
44. Tang Y, Zhang P, Wang Y, Wang J, Su M, Wang Y, et al. The biogenesis, biology, and clinical significance of exosomal PD-L1 in cancer. Front Immunol. 2020;11:450. doi:10.3389/FIMMU.2020.00604. [Google Scholar] [PubMed] [CrossRef]
45. Xu W, Chen Y, Zhang Z, Jiang Y, Wang Z. Exosomal PIK3CB promotes PD-L1 expression and malignant transformation in esophageal squamous cell carcinoma. Med Oncol. 2023;40(8):394. doi:10.1007/S12032-023-02093-8. [Google Scholar] [PubMed] [CrossRef]
46. Qiu R, Wang W, Li J, Wang Y. Roles of PTEN inactivation and PD-1/PD-L1 activation in esophageal squamous cell carcinoma. Mol Biol Rep. 2022;49(7):6633–45. doi:10.1007/S11033-022-07246-Y. [Google Scholar] [PubMed] [CrossRef]
47. Wang H, Qi Y, Lan Z, Liu Q, Xu J, Zhu M, et al. Exosomal PD-L1 confers chemoresistance and promotes tumorigenic properties in esophageal cancer cells via upregulating STAT3/miR-21. Gene Ther. 2023;30(1–2):88–100. doi:10.1038/S41434-022-00331-8. [Google Scholar] [PubMed] [CrossRef]
48. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–6. doi:10.1038/S41586-018-0392-8. [Google Scholar] [PubMed] [CrossRef]
49. Yang Y, Li CW, Chan LC, Wei Y, Hsu JM, Xia W, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–4. doi:10.1038/S41422-018-0060-4. [Google Scholar] [PubMed] [CrossRef]
50. Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. 2018;4(3):1010. doi:10.1126/SCIADV.AAR2766. [Google Scholar] [PubMed] [CrossRef]
51. Fan Y, Che X, Qu J, Hou K, Wen T, Li Z, et al. Exosomal PD-L1 retains immunosuppressive activity and is associated with gastric cancer prognosis. Ann Surg Oncol. 2019;26(11):3745–55. doi:10.1245/S10434-019-07431-7. [Google Scholar] [PubMed] [CrossRef]
52. Kim DH, Kim HR, Choi YJ, Kim SY, Lee JE, Sung KJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. 2019;51(8):1–13. doi:10.1038/S12276-019-0295-2. [Google Scholar] [PubMed] [CrossRef]
53. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–27.e13. doi:10.1016/J.CELL.2019.02.016. [Google Scholar] [PubMed] [CrossRef]
54. Maharati A, Moghbeli M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun Signal. 2023;21(1):209. doi:10.1186/S12964-023-01225-X. [Google Scholar] [PubMed] [CrossRef]
55. Zhang H, Chi J, Hu J, Ji T, Luo Z, Zhou C, et al. Intracellular AGR2 transduces PGE2 stimuli to promote epithelial-mesenchymal transition and metastasis of colorectal cancer. Cancer Lett. 2021;518(2):180–95. doi:10.1016/J.CANLET.2021.06.025. [Google Scholar] [PubMed] [CrossRef]
56. Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G, et al. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol. 2007;72(1):152–61. doi:10.1124/MOL.106.029025. [Google Scholar] [PubMed] [CrossRef]
57. Xiang X, Poliakov A, Liu C, Liu Y, Bin DZ, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–33. doi:10.1002/IJC.24249. [Google Scholar] [PubMed] [CrossRef]
58. Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–30. doi:10.1158/0008-5472.CAN-10-1722. [Google Scholar] [PubMed] [CrossRef]
59. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–31. doi:10.1038/NRG1379. [Google Scholar] [PubMed] [CrossRef]
60. Kanlikilicer P, Bayraktar R, Denizli M, Rashed MH, Ivan C, Aslan B, et al.Corrigendum to ‘Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer [EBioMedicine 38 (2018) 100–112]. eBioMedicine. 2020;52. doi:10.1016/J.EBIOM.2020.102630. [Google Scholar] [PubMed] [CrossRef]
61. Otmani K, Rouas R, Lewalle P. OncomiRs as noncoding RNAs having functions in cancer: their role in immune suppression and clinical implications. Front Immunol. 2022;13:73. doi:10.3389/FIMMU.2022.913951. [Google Scholar] [PubMed] [CrossRef]
62. Jin G, Liu Y, Zhang J, Bian Z, Yao S, Fei B, et al. A panel of serum exosomal microRNAs as predictive markers for chemoresistance in advanced colorectal cancer. Cancer Chemother Pharmacol. 2019;84(2):315–25. doi:10.1007/S00280-019-03867-6. [Google Scholar] [PubMed] [CrossRef]
63. Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18(1):881. doi:10.1186/S13058-016-0753-X. [Google Scholar] [PubMed] [CrossRef]
64. Takeshita N, Hoshino I, Mori M, Akutsu Y, Hanari N, Yoneyama Y, et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer. 2013;108(3):644–52. doi:10.1038/BJC.2013.8. [Google Scholar] [PubMed] [CrossRef]
65. Qiu W, Guo X, Li B, Wang J, Qi Y, Chen Z, et al. Exosomal miR-1246 from glioma patient body fluids drives the differentiation and activation of myeloid-derived suppressor cells. Mol Ther. 2021;29(12):3449–64. doi:10.1016/J.YMTHE.2021.06.023. [Google Scholar] [PubMed] [CrossRef]
66. Mikamori M, Yamada D, Eguchi H, Hasegawa S, Kishimoto T, Tomimaru Y, et al. MicroRNA-155 controls exosome synthesis and promotes gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci Rep. 2017;7(1):1–14. doi:10.1038/srep42339. [Google Scholar] [PubMed] [CrossRef]
67. Yan Q, Liu J, Liu Y, Wen Z, Jin D, Wang F, et al. Tumor-associated macrophage-derived exosomal miR21-5p promotes tumor angiogenesis by regulating YAP1/HIF-1α axis in head and neck squamous cell carcinoma. Cell Mol Life Sci. 2024;81(1):445. doi:10.1007/S00018-024-05210-6. [Google Scholar] [PubMed] [CrossRef]
68. Li B, Cao Y, Sun M, Feng H. Expression, regulation, and function of exosome-derived miRNAs in cancer progression and therapy. FASEB J. 2021;35(10):2484. doi:10.1096/FJ.202100294RR. [Google Scholar] [PubMed] [CrossRef]
69. Qin Y, Li L, Wang F, Zhou X, Liu Y, Yin Y, et al. Knockdown of Mir-135b sensitizes colorectal cancer cells to oxaliplatin-induced apoptosis through increase of FOXO1. Cell Physiol Biochem. 2018;48(4):1627–37. doi:10.1159/000492284. [Google Scholar] [PubMed] [CrossRef]
70. Shao L, Chen Z, Soutto M, Zhu S, Lu H, Romero-Gallo J, et al. Helicobacter pylori-induced miR-135b-5p promotes cisplatin resistance in gastric cancer. FASEB J. 2019;33(1):264–74. doi:10.1096/FJ.201701456RR. [Google Scholar] [PubMed] [CrossRef]
71. Jiang W, Zhao S, Shen J, Guo L, Sun Y, Zhu Y, et al. The MiR-135b-BMAL1-YY1 loop disturbs pancreatic clockwork to promote tumourigenesis and chemoresistance. Cell Death Dis. 2018;9(2):7. doi:10.1038/S41419-017-0233-Y. [Google Scholar] [PubMed] [CrossRef]
72. Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428–42. doi:10.1016/J.MOLCEL.2018.06.034. [Google Scholar] [PubMed] [CrossRef]
73. Hasegawa S, Eguchi H, Nagano H, Konno M, Tomimaru Y, Wada H, et al. MicroRNA-1246 expression associated with CCNG2-mediated chemoresistance and stemness in pancreatic cancer. Br J Cancer. 2014;111(8):1572–80. doi:10.1038/BJC.2014.454. [Google Scholar] [PubMed] [CrossRef]
74. Wang H, Wang X, Zhang H, Deng T, Liu R, Liu Y, et al. The HSF1/miR-135b-5p axis induces protective autophagy to promote oxaliplatin resistance through the MUL1/ULK1 pathway in colorectal cancer. Oncogene. 2021;40(28):4695–708. doi:10.1038/S41388-021-01898-Z. [Google Scholar] [PubMed] [CrossRef]
75. Peng C, Li X, Yao Y, Nie Y, Fan L, Zhu C. MiR-135b-5p promotes cetuximab resistance in colorectal cancer by regulating FOXN3. Cancer Biol Ther. 2024;25(1). doi:10.1080/15384047.2024.2373497. [Google Scholar] [PubMed] [CrossRef]
76. Nishibeppu K, Komatsu S, Imamura T, Kiuchi J, Kishimoto T, Arita T et al.Plasma microRNA profiles: identification of miR-1229-3p as a novel chemoresistant and prognostic biomarker in gastric cancer. Sci Rep. 2020;10(1):1–11. doi:10.1038/s41598-020-59939-8. [Google Scholar] [PubMed] [CrossRef]
77. Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T, et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. JNCI J Natl Cancer Inst. 2015;107(7):289. doi:10.1093/JNCI/DJV135. [Google Scholar] [PubMed] [CrossRef]
78. Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38(1):1739. doi:10.1186/S13046-019-1095-1. [Google Scholar] [PubMed] [CrossRef]
79. Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben DavidG, et al. Transfer of miRNA in macrophage-derived exosomes induces drug resistance in pancreatic adenocarcinoma. Cancer Res. 2018;78(18):5287–99. doi:10.1158/0008-5472.CAN-18-0124. [Google Scholar] [PubMed] [CrossRef]
80. Qin X, Guo H, Wang X, Zhu X, Yan M, Wang X, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20(1):7. doi:10.1186/S13059-018-1604-0. [Google Scholar] [PubMed] [CrossRef]
81. Makinoya M, Miyatani K, Matsumi Y, Sakano Y, Shimizu S, Shishido Y, et al. Exosomal miR-493 suppresses MAD2L1 and induces chemoresistance to intraperitoneal paclitaxel therapy in gastric cancer patients with peritoneal metastasis. Sci Rep. 2024;14(1):209. doi:10.1038/S41598-024-60967-X. [Google Scholar] [PubMed] [CrossRef]
82. Zhuang J, Shen L, Li M, Sun J, Hao J, Li J, et al. Cancer-associated fibroblast-derived miR-146a-5p generates a niche that promotes bladder cancer stemness and chemoresistance. Cancer Res. 2023;83(10):1611–27. doi:10.1158/0008-5472.CAN-22-2213. [Google Scholar] [PubMed] [CrossRef]
83. Weiner-Gorzel K, Dempsey E, Milewska M, Mcgoldrick A, Toh V, Walsh A, et al. Overexpression of the microRNA miR-433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Med. 2015;4(5):745–58. doi:10.1002/CAM4.409. [Google Scholar] [PubMed] [CrossRef]
84. Shi L, Zhu W, Huang Y, Zhuo L, Wang S, Chen S, et al. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin Transl Med. 2022;12(7):167. doi:10.1002/CTM2.989. [Google Scholar] [PubMed] [CrossRef]
85. Zhang Y, Tan X, Lu Y. Exosomal transfer of circ_0006174 contributes to the chemoresistance of doxorubicin in colorectal cancer by depending on the miR-1205/CCND2 axis. J Physiol Biochem. 2022;78(1):39–50. doi:10.1007/S13105-021-00831-Y. [Google Scholar] [PubMed] [CrossRef]
86. Deng J, Pan T, Lv C, Cao L, Li L, Zhou X, et al. Exosomal transfer leads to chemoresistance through oxidative phosphorylation-mediated stemness phenotype in colorectal cancer. Theranostics. 2023;13(14):5057–74. doi:10.7150/THNO.84937. [Google Scholar] [PubMed] [CrossRef]
87. Zhao K, Cheng X, Ye Z, Li Y, Peng W, Wu Y, et al. Exosome-mediated transfer of circ_0000338 enhances 5-fluorouracil resistance in colorectal cancer through regulating MicroRNA 217 (miR-217) and miR-485-3p. Mol Cell Biol. 2021;41(5):475. doi:10.1128/MCB.00517-20. [Google Scholar] [PubMed] [CrossRef]
88. Zheng S, Tian Q, Yuan Y, Sun S, Li T, Xia R, et al. Extracellular vesicle-packaged circBIRC6 from cancer-associated fibroblasts induce platinum resistance via SUMOylation modulation in pancreatic cancer. J Exp Clin Cancer Res. 2023;42(1):7. doi:10.1186/S13046-023-02854-3. [Google Scholar] [PubMed] [CrossRef]
89. Xia W, Chen W, Ni C, Meng X, Wu J, Yang Q, et al. Chemotherapy-induced exosomal circBACH1 promotes breast cancer resistance and stemness via miR-217/G3BP2 signaling pathway. Breast Cancer Res. 2023;25(1):783. doi:10.1186/S13058-023-01672-X. [Google Scholar] [PubMed] [CrossRef]
90. Nail HM, Chiu CC, Leung CH, Ahmed MMM, Wang HMD. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J Biomed Sci. 2023;30(1):eaau6977. doi:10.1186/S12929-023-00964-W. [Google Scholar] [PubMed] [CrossRef]
91. Affo S, Nair A, Brundu F, Ravichandra A, Bhattacharjee S, Matsuda M, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39(6):883. doi:10.1016/j.ccell.2021.05.010. [Google Scholar] [PubMed] [CrossRef]
92. Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M, et al. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 MAPK signalling. Cancer Lett. 2019;442:320–32. doi:10.1016/J.CANLET.2018.10.015. [Google Scholar] [PubMed] [CrossRef]
93. Qi R, Bai Y, Li K, Liu N, Xu Y, Dal E, et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug Resist Updat. 2023;68:100960. doi:10.1016/J.DRUP.2023.100960. [Google Scholar] [PubMed] [CrossRef]
94. Dalin S, Sullivan MR, Lau AN, Grauman-Boss B, Mueller HS, Kreidl E, et al. Deoxycytidine release from pancreatic stellate cells promotes gemcitabine resistance. Cancer Res. 2019;79(22):5723–33. doi:10.1158/0008-5472.CAN-19-0960. [Google Scholar] [PubMed] [CrossRef]
95. Asif PJ, Longobardi C, Hahne M, Medema JP. The role of cancer-associated fibroblasts in cancer invasion and metastasis. Cancers. 2021;13(18):4720. doi:10.3390/CANCERS13184720. [Google Scholar] [PubMed] [CrossRef]
96. Ham IH, Lee D, Hur H. Cancer-associated fibroblast-induced resistance to chemotherapy and radiotherapy in gastrointestinal cancers. Cancers. 2021;13(5):1–17. doi:10.3390/CANCERS13051172. [Google Scholar] [PubMed] [CrossRef]
97. Pietilä M, Ivaska J, Mani SA. Whom to blame for metastasis, the epithelial-mesenchymal transition or the tumor microenvironment? Cancer Lett. 2016;380(1):359–68. doi:10.1016/J.CANLET.2015.12.033. [Google Scholar] [PubMed] [CrossRef]
98. Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116(13):2385–94. doi:10.1182/BLOOD-2009-08-239228. [Google Scholar] [PubMed] [CrossRef]
99. Ludwig N, Whiteside TL. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin Ther Targets. 2018;22(5):409–17. doi:10.1080/14728222.2018.1464141. [Google Scholar] [PubMed] [CrossRef]
100. Maishi N, Hida K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017;108(10):1921–6. doi:10.1111/CAS.13336. [Google Scholar] [PubMed] [CrossRef]
101. Cui H, Seubert B, Stahl E, Dietz H, Reuning U, Moreno-Leon L, et al. Tissue inhibitor of metalloproteinases-1 induces a pro-tumourigenic increase of miR-210 in lung adenocarcinoma cells and their exosomes. Oncogene. 2015;34(28):3640–50. doi:10.1038/ONC.2014.300. [Google Scholar] [PubMed] [CrossRef]
102. Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11(9):2783–97. doi:10.1016/J.APSB.2021.01.001. [Google Scholar] [PubMed] [CrossRef]
103. Keklikoglou I, Cianciaruso C, Güç E, Squadrito ML, Spring LM, Tazzyman S, et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol. 2019;21(2):190–202. doi:10.1038/S41556-018-0256-3. [Google Scholar] [PubMed] [CrossRef]
104. Liu K, Dou R, Yang C, Di Z, Shi D, Zhang C, et al. Exosome-transmitted miR-29a induces colorectal cancer metastasis by destroying the vascular endothelial barrier. Carcinogenesis. 2023;44(4):356–67. doi:10.1093/CARCIN/BGAD013. [Google Scholar] [PubMed] [CrossRef]
105. Li K, Xue W, Lu Z, Wang S, Zheng J, Lu K, et al. Tumor-derived exosomal ADAM17 promotes pre-metastatic niche formation by enhancing vascular permeability in colorectal cancer. J Exp Clin Cancer Res. 2024;43(1):713. doi:10.1186/S13046-024-02991-3. [Google Scholar] [PubMed] [CrossRef]
106. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi:10.1016/J.IMMUNI.2010.05.007. [Google Scholar] [PubMed] [CrossRef]
107. Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188434. doi:10.1016/J.BBCAN.2020.188434. [Google Scholar] [PubMed] [CrossRef]
108. Qiu Y, Lu G, Li N, Hu Y, Tan H, Jiang C. Exosome-mediated communication between gastric cancer cells and macrophages: implications for tumor microenvironment. Front Immunol. 2024;15:104601. doi:10.3389/FIMMU.2024.1327281. [Google Scholar] [PubMed] [CrossRef]
109. Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):87. doi:10.1186/S13046-017-0528-Y. [Google Scholar] [PubMed] [CrossRef]
110. Khalaf K, Hana D, Chou JTT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol. 2021;12:43. doi:10.3389/FIMMU.2021.656364. [Google Scholar] [PubMed] [CrossRef]
111. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–73. doi:10.1189/JLB.0609385. [Google Scholar] [PubMed] [CrossRef]
112. Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13(1):250. doi:10.1186/S13045-020-00991-2. [Google Scholar] [PubMed] [CrossRef]
113. He Z, Wang J, Zhu C, Xu J, Chen P, Jiang X, et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022;548:215751. doi:10.1016/J.CANLET.2022.215751. [Google Scholar] [PubMed] [CrossRef]
114. Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. 2022;41(1):402. doi:10.1186/S13046-022-02499-8. [Google Scholar] [PubMed] [CrossRef]
115. Wang Q, Sun Z, Guo J, Li H, Zhang J, Zhang B, et al. Tumor-derived exosomal LINC01812 induces M2 macrophage polarization to promote perineural invasion in cholangiocarcinoma. Cancer Lett. 2025;617:217596. doi:10.1016/J.CANLET.2025.217596. [Google Scholar] [PubMed] [CrossRef]
116. Jung M, Mertens C, Bauer R, Rehwald C, Brüne B. Lipocalin-2 and iron trafficking in the tumor microenvironment. Pharmacol Res. 2017;120(7):146–56. doi:10.1016/J.PHRS.2017.03.018. [Google Scholar] [PubMed] [CrossRef]
117. Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, et al. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6(12):1578–92. doi:10.1158/2326-6066.CIR-17-0479. [Google Scholar] [PubMed] [CrossRef]
118. DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29(2):309–16. doi:10.1007/S10555-010-9223-6. [Google Scholar] [PubMed] [CrossRef]
119. Li J, Wang K, Yang C, Zhu K, Jiang C, Wang M, et al. Tumor-associated macrophage-derived exosomal LINC01232 induces the immune escape in glioma by decreasing surface MHC-I expression. Adv Sci. 2023;10(17). doi:10.1002/ADVS.202207067. [Google Scholar] [PubMed] [CrossRef]
120. Singh N, Baby D, Rajguru J, Patil P, Thakkannavar S, Pujari V. Inflammation and cancer. Ann Afr Med. 2019;18(3):121–6. doi:10.4103/AAM.AAM_56_18. [Google Scholar] [PubMed] [CrossRef]
121. Othman N, Jamal R, Abu N. Cancer-derived exosomes as effectors of key inflammation-related players. Front Immunol. 2019;10:527. doi:10.3389/FIMMU.2019.02103. [Google Scholar] [PubMed] [CrossRef]
122. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. doi:10.1093/CARCIN/BGP127. [Google Scholar] [PubMed] [CrossRef]
123. Fernandes JV, Cobucci RNO, Jatobá CAN, De Medeiros Fernandes TAA, De Azevedo JWV, De Araújo JMG. The role of the mediators of inflammation in cancer development. Pathol Oncol Res. 2015;21(3):527–34. doi:10.1007/S12253-015-9913-Z. [Google Scholar] [PubMed] [CrossRef]
124. Landskron G, De La Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014(2):1–19. doi:10.1155/2014/149185. [Google Scholar] [PubMed] [CrossRef]
125. Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288(51):36691–702. doi:10.1074/JBC.M113.512806. [Google Scholar] [PubMed] [CrossRef]
126. Haderk F, Schulz R, Iskar M, Cid LL, Worst T, Willmund KV, et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol. 2017;2(13). doi:10.1126/SCIIMMUNOL.AAH5509. [Google Scholar] [PubMed] [CrossRef]
127. Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-κB. Sci Rep. 2014;4(1):24. doi:10.1038/SREP05750. [Google Scholar] [PubMed] [CrossRef]
128. Wu L, Zhang X, Zhang B, Shi H, Yuan X, Sun Y, et al. Exosomes derived from gastric cancer cells activate NF-κB pathway in macrophages to promote cancer progression. Tumour Biol. 2016;37(9):12169–80. doi:10.1007/S13277-016-5071-5. [Google Scholar] [PubMed] [CrossRef]
129. Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, et al. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res. 2017;15(1):93–105. doi:10.1158/1541-7786.MCR-16-0163. [Google Scholar] [PubMed] [CrossRef]
130. Shen Y, Guo D, Weng L, Wang S, Ma Z, Yang Y, et al. Tumor-derived exosomes educate dendritic cells to promote tumor metastasis via HSP72/HSP105-TLR2/TLR4 pathway. Oncoimmunology. 2017;6(12):e1362527. doi:10.1080/2162402X.2017.1362527. [Google Scholar] [PubMed] [CrossRef]
131. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–64. doi:10.1172/JCI80005. [Google Scholar] [PubMed] [CrossRef]
132. Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–9. doi:10.2353/AJPATH.2010.090777. [Google Scholar] [PubMed] [CrossRef]
133. Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, et al. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene. 2018;37(31):4239–59. doi:10.1038/S41388-018-0261-9. [Google Scholar] [PubMed] [CrossRef]
134. Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022;20(1):145. doi:10.1186/S12964-022-00959-4. [Google Scholar] [PubMed] [CrossRef]
135. Joyce DP, Kerin MJ, Dwyer RM. Exosome-encapsulated microRNAs as circulating biomarkers for breast cancer. Int J Cancer. 2016;139(7):1443–8. doi:10.1002/IJC.30179. [Google Scholar] [PubMed] [CrossRef]
136. Nasu M, Khadka VS, Jijiwa M, Kobayashi K, Deng Y. Exploring optimal biomarker sources: a comparative analysis of exosomes and whole plasma in fasting and non-fasting conditions for liquid biopsy applications. Int J Mol Sci. 2024;25(1):371. doi:10.3390/IJMS25010371. [Google Scholar] [PubMed] [CrossRef]
137. Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):209. doi:10.1186/S12943-022-01509-9. [Google Scholar] [PubMed] [CrossRef]
138. Zhang Y, Yue NN, Chen LY, Tian CM, Yao J, Wang LS, et al. Exosomal biomarkers: a novel frontier in the diagnosis of gastrointestinal cancers. World J Gastrointest Oncol. 2025;17(4):1–26. doi:10.4251/WJGO.V17.I4.103591. [Google Scholar] [PubMed] [CrossRef]
139. Zhong D, Wang Z, Ye Z, Wang Y, Cai X. Cancer-derived exosomes as novel biomarkers in metastatic gastrointestinal cancer. Mol Cancer. 2024;23(1):7. doi:10.1186/S12943-024-01948-6. [Google Scholar] [PubMed] [CrossRef]
140. Kok VC, Yu CC. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int J Nanomedicine. 2020;15:8019–36. doi:10.2147/IJN.S272378. [Google Scholar] [PubMed] [CrossRef]
141. Miyazaki K, Wada Y, Okuno K, Murano T, Morine Y, Ikemoto T, et al. An exosome-based liquid biopsy signature for pre-operative identification of lymph node metastasis in patients with pathological high-risk T1 colorectal cancer. Mol Cancer. 2023;22(1):551. doi:10.1186/S12943-022-01685-8. [Google Scholar] [PubMed] [CrossRef]
142. Lutgendorf SK, Thaker PH, Goodheart MJ, Arevalo JMG, Chowdhury MA, Noble AE, et al. Biobehavioral factors predict an exosome biomarker of ovarian carcinoma disease progression. Cancer. 2022;128(23):4157–65. doi:10.1002/CNCR.34496. [Google Scholar] [PubMed] [CrossRef]
143. Gotanda K, Hirota T, Saito J, Fukae M, Egashira Y, Izumi N, et al. Circulating intestine-derived exosomal miR-328 in plasma, a possible biomarker for estimating BCRP function in the human intestines. Sci Rep. 2016;6(1):1–8. doi:10.1038/srep32299. [Google Scholar] [PubMed] [CrossRef]
144. Wei C, Li Y, Huang K, Li G, He M. Exosomal miR-1246 in body fluids is a potential biomarker for gastrointestinal cancer. Biomark Med. 2018;12(10):1185–95. doi:10.2217/BMM-2017-0440. [Google Scholar] [PubMed] [CrossRef]
145. Mirzaei-nasab F, Majd A, Seyedena Y, Hosseinkhan N, Farahani N, Hashemi M. Integrative analysis of exosomal ncRNAs and their regulatory networks in liver cancer progression. Pract Lab Med. 2025;45(5):e00464. doi:10.1016/J.PLABM.2025.E00464. [Google Scholar] [PubMed] [CrossRef]
146. Zhang F, Jiang J, Qian H, Yan Y, Xu W. Exosomal circRNA: emerging insights into cancer progression and clinical application potential. J Hematol Oncol. 2023;16(1):19. doi:10.1186/S13045-023-01452-2. [Google Scholar] [PubMed] [CrossRef]
147. Ghani MU, Du L, Moqbel AQ, Zhao E, Cui H, Yang L, et al. Exosomal ncRNAs in liquid biopsy: a new paradigm for early cancer diagnosis and monitoring. Front Oncol. 2025;15:1615433. doi:10.3389/FONC.2025.1615433. [Google Scholar] [PubMed] [CrossRef]
148. Liu J, Zhang Y, Wu J, Liu X, Li L, Zhang J. LncRNA FOXD2-AS1 promotes the growth, invasion and migration of OSCC cells by regulating the MiR-185-5p/PLOD1/Akt/mTOR pathway. Cancer Genet. 2024;284–285(3):48–57. doi:10.1016/j.cancergen.2024.05.001. [Google Scholar] [PubMed] [CrossRef]
149. Arima J, Yoshino H, Fukumoto W, Kawahara I, Saito S, Li G, et al. LncRNA BCYRN1 as a potential therapeutic target and diagnostic marker in serum exosomes in bladder cancer. Int J Mol Sci. 2024;25(11):5955. doi:10.3390/IJMS25115955. [Google Scholar] [PubMed] [CrossRef]
150. Ghorbani A, Hosseinie F, Khorshid Sokhangouy S, Islampanah M, khojasteh-Leylakoohi F, Maftooh M, et al. The prognostic, diagnostic, and therapeutic impact of Long noncoding RNAs in gastric cancer. Cancer Genet. 2024;282–283(1):14–26. doi:10.1016/j.cancergen.2023.12.006. [Google Scholar] [PubMed] [CrossRef]
151. Barhoum M, Brassart-Pasco S, Dupont-Deshorgue A, Thierry A, Kanagaratnam L, Brassart B, et al. Circulating exosomal proteins as new diagnostic biomarkers for colorectal cancer (EXOSCOL01a pilot case-controlled study focusing on MMP14 potential. J Clin Lab Anal. 2025;39(9):281. doi:10.1002/JCLA.70016. [Google Scholar] [PubMed] [CrossRef]
152. Hsu CY, Chandramoorthy HC, Mohammed JS, Al-Hasnaawei S, Yaqob M, Kundlas M, et al. Exosomes as key mediators in immune and cancer cell interactions: insights in melanoma progression and therapy. Arch Dermatol Res. 2025;317(1):17. doi:10.1007/S00403-025-04237-4. [Google Scholar] [PubMed] [CrossRef]
153. Jin S, Liu T, Wang W, Li T, Liu Z, Zhang M. Lymphocyte migration regulation related proteins in urine exosomes may serve as a potential biomarker for lung cancer diagnosis. BMC Cancer. 2023;23(1):535. doi:10.1186/S12885-023-11567-X. [Google Scholar] [PubMed] [CrossRef]
154. Guo J, Zhang M, Li X, Wang J. PTEN as a prognostic factor for radiotherapy plus immunotherapy response in nasopharyngeal carcinoma. J Nanobiotechnol. 2025;23(1). doi:10.1186/S12951-025-03315-Z. [Google Scholar] [PubMed] [CrossRef]
155. Liu D, Chen C, Cui M, Zhang H. miR-140-3p inhibits colorectal cancer progression and its liver metastasis by targeting BCL9 and BCL2. Cancer Med. 2021;10(10):3358–72. doi:10.1002/CAM4.3840. [Google Scholar] [PubMed] [CrossRef]
156. Zeng W, Liu Y, Li WT, Li Y, Zhu JF. CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol Oncol. 2020;14(11):2960–84. doi:10.1002/1878-0261.12796. [Google Scholar] [PubMed] [CrossRef]
157. Yang H, Wu P, Li B, Huang X, Shi Q, Qiao L, et al. Diagnosis and biomarker screening of endometrial cancer enabled by a versatile exosome metabolic fingerprint platform. Anal Chem. 2024;96(44):17679–88. doi:10.1021/ACS.ANALCHEM.4C03726. [Google Scholar] [PubMed] [CrossRef]
158. Li J, Wang J, Chen Z. Emerging role of exosomes in cancer therapy: progress and challenges. Mol Cancer. 2025;24(1):13. doi:10.1186/s12943-024-02215-4. [Google Scholar] [PubMed] [CrossRef]
159. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–95. doi:10.7150/THNO.52570. [Google Scholar] [PubMed] [CrossRef]
160. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–90. doi:10.1016/j.biomaterials.2013.11.083. [Google Scholar] [PubMed] [CrossRef]
161. Tiwari P, Shukla RP, Yadav K, Sharma M, Bakshi AK, Panwar D, et al. YIGSR functionalized hybrid exosomes spatially target dasatinib to laminin receptors for precision therapy in breast cancer. Adv Healthc Mater. 2025;14(9):5. doi:10.1002/adhm.202402673. [Google Scholar] [PubMed] [CrossRef]
162. Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, et al. Aptamer-conjugated extracellular nanovesicles for targeted drug delivery. Cancer Res. 2018;78(3):798–808. doi:10.1158/0008-5472.CAN-17-2880. [Google Scholar] [PubMed] [CrossRef]
163. Shakerian N, Tafazoli A, Razavinia A, Sadrzadeh Aghajani Z, Bana N, Mard-Soltani M, et al. Current understanding of therapeutic and diagnostic applications of exosomes in pancreatic cancer. Pancreas. 2025;54(3):e255–67. doi:10.1097/MPA.0000000000002414. [Google Scholar] [PubMed] [CrossRef]
164. Uner B, Akyildiz EO, Kolci K, Reis R. Nanoparticle formulations for intracellular delivery in colorectal cancer therapy. AAPS PharmSciTech. 2025;26(3):81. doi:10.1208/s12249-025-03069-9. [Google Scholar] [PubMed] [CrossRef]
165. Alharbi HM, Alqahtani T, Alamri AH, Kumarasamy V, Subramaniyan V, Babu KS. Nanotechnological synergy of mangiferin and curcumin in modulating PI3K/Akt/mTOR pathway: a novel front in ovarian cancer precision therapeutics. Front Pharmacol. 2024;14:807. doi:10.3389/fphar.2023.1276209. [Google Scholar] [PubMed] [CrossRef]
166. Luo H, Zhou Y, Zhang J, Zhang Y, Long S, Lin X, et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions. Front Immunol. 2023;13:28. doi:10.3389/fimmu.2022.1087689. [Google Scholar] [PubMed] [CrossRef]
167. Pourmasoumi P, Abdouss M, Farhadi M, Jameie SB, Khonakdar HA. Co-delivery of temozolomide and quercetin with folic acid-conjugated exosomes in glioblastoma treatment. Nanomed. 2024;19(27):2271–87. doi:10.1080/17435889.2024.2395234. [Google Scholar] [PubMed] [CrossRef]
168. Liu Y, Li M, Gu J, Huang H, Xie H, Yu C, et al. Engineering of exosome-liposome hybrid-based theranostic nanomedicines for NIR-II fluorescence imaging-guided and targeted NIR-II photothermal therapy of subcutaneous glioblastoma. Colloids Surfaces B Biointerfaces. 2025;245:114258. doi:10.1016/j.colsurfb.2024.114258. [Google Scholar] [PubMed] [CrossRef]
169. Wang J, Zheng Y, Zhao M. Exosome-based cancer therapy: implication for targeting cancer stem cells. Front Pharmacol. 2017;7:4360. doi:10.3389/fphar.2016.00533. [Google Scholar] [PubMed] [CrossRef]
170. Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9(1):9412. doi:10.1038/S41392-024-01735-1. [Google Scholar] [PubMed] [CrossRef]
171. Bellingham SA, Guo B, Hill AF. The secret life of extracellular vesicles in metal homeostasis and neurodegeneration. Biol Cell. 2015;107(11):389–418. doi:10.1111/boc.201500030. [Google Scholar] [PubMed] [CrossRef]
172. Tao H, Gao B. Exosomes for neurodegenerative diseases: diagnosis and targeted therapy. J Neurol. 2024;271(6):3050–62. doi:10.1007/s00415-024-12329-w. [Google Scholar] [PubMed] [CrossRef]
173. Nouri Z, Barfar A, Perseh S, Motasadizadeh H, Maghsoudian S, Fatahi Y, et al. Exosomes as therapeutic and drug delivery vehicle for neurodegenerative diseases. J Nanobiotechnology. 2024;22(1):463. doi:10.1186/s12951-024-02681-4. [Google Scholar] [PubMed] [CrossRef]
174. Kibria G, Ramos EK, Wan Y, Gius DR, Liu H. Exosomes as a drug delivery system in cancer therapy: potential and challenges. Mol Pharm. 2018;15(9):3625–33. doi:10.1021/acs.molpharmaceut.8b00277. [Google Scholar] [PubMed] [CrossRef]
175. Salehi M, Kamali MJ, Arab D, Safaeian N, Ashuori Z, Maddahi M, et al. Exosomal microRNAs in regulation of tumor cells resistance to apoptosis. Biochem Biophys Rep. 2024;37(2):101644. doi:10.1016/j.bbrep.2024.101644. [Google Scholar] [PubMed] [CrossRef]
176. Su W, Mo Y, Wu F, Guo K, Li J, Luo Y, et al. miR-135b reverses chemoresistance of non-small cell lung cancer cells by downregulation of FZD1. Biomed Pharmacother. 2016;84:123–9. doi:10.1016/j.biopha.2016.09.027. [Google Scholar] [PubMed] [CrossRef]
177. Zeng A, Wei Z, Yan W, Yin J, Huang X, Zhou X, et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018;436:10–21. doi:10.1016/j.canlet.2018.08.004. [Google Scholar] [PubMed] [CrossRef]
178. Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8(1):122. doi:10.1186/s13045-015-0220-7. [Google Scholar] [PubMed] [CrossRef]
179. Zhang M, Hu S, Liu L, Dang P, Liu Y, Sun Z, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8(1):124. doi:10.1038/s41392-023-01382-y. [Google Scholar] [PubMed] [CrossRef]
180. Lv Y, Du X, Tang W, Yang Q, Gao F. Exosomes: the role in tumor tolerance and the potential strategy for tumor therapy. Pharmaceutics. 2023;15(2):462. doi:10.3390/PHARMACEUTICS15020462. [Google Scholar] [PubMed] [CrossRef]
181. Abedi A, Moosazadeh Moghaddam M, Kachuei R, Imani Fooladi AA. Exosomes as a therapeutic strategy in cancer: potential roles as drug carriers and immune modulators. Biochim Biophys Acta—Rev Cancer. 2025;1880(1):189238. doi:10.1016/J.BBCAN.2024.189238. [Google Scholar] [PubMed] [CrossRef]
182. Huang H, Shao L, Chen Y, Tang L, Liu T, Li J, et al. Synergistic strategy with hyperthermia therapy based immunotherapy and engineered exosomes−liposomes targeted chemotherapy prevents tumor recurrence and metastasis in advanced breast cancer. Bioeng Transl Med. 2022;7(2):357485. doi:10.1002/btm2.10284. [Google Scholar] [PubMed] [CrossRef]
183. Nguyen Cao TG, Kang JH, Kim W, Lim J, Kang SJ, You JY, et al. Engineered extracellular vesicle-based sonotheranostics for dual stimuli-sensitive drug release and photoacoustic imaging-guided chemo-sonodynamic cancer therapy. Theranostics. 2022;12(3):1247–66. doi:10.7150/thno.65516. [Google Scholar] [PubMed] [CrossRef]
184. Xu W, Wang K, Wang K, Zhao Y, Yang Z, Li X. Key magnetized exosomes for effective targeted delivery of doxorubicin against breast cancer cell types in mice model. Int J Nanomed. 2024;19:10711–24. doi:10.2147/IJN.S479306. [Google Scholar] [PubMed] [CrossRef]
185. Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding X, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnology. 2019;17(1):93. doi:10.1186/s12951-019-0526-7. [Google Scholar] [PubMed] [CrossRef]
186. Tran NH, Nguyen DD, Nguyen NM, Tran C, Nguyen Thi NT, Ho DT, et al. Dual-targeting exosomes for improved drug delivery in breast cancer. Nanomed. 2023;18(7):599–611. doi:10.2217/nnm-2022-0328. [Google Scholar] [PubMed] [CrossRef]
187. Bao Y, Chen Y, Deng X, Wang Y, Zhang Y, Xu L, et al. Boron clusters escort doxorubicin squashing into exosomes and overcome drug resistance. Adv Sci. 2025;12(7). doi:10.1002/advs.202412501. [Google Scholar] [PubMed] [CrossRef]
188. Zhang X, Liu L, Tang M, Li H, Guo X, Yang X. The effects of umbilical cord-derived macrophage exosomes loaded with cisplatin on the growth and drug resistance of ovarian cancer cells. Drug Dev Ind Pharm. 2020;46(7):1150–62. doi:10.1080/03639045.2020.1776320. [Google Scholar] [PubMed] [CrossRef]
189. Williams ME, Howard D, Donnelly C, Izadi F, Parra JG, Pugh M, et al. Adipocyte derived exosomes promote cell invasion and challenge paclitaxel efficacy in ovarian cancer. Cell Commun Signal. 2024;22(1):443. doi:10.1186/s12964-024-01806-4. [Google Scholar] [PubMed] [CrossRef]
190. Pan X, Shi X, Zhang H, Chen Y, Zhou J, Shen F, et al. Exosomal miR-4516 derived from ovarian cancer stem cells enhanced cisplatin tolerance in ovarian cancer by inhibiting GAS7. Gene. 2024;927:148738. doi:10.1016/j.gene.2024.148738. [Google Scholar] [PubMed] [CrossRef]
191. Liu H, Deng S, Yao X, Liu Y, Qian L, Wang Y, et al. Ascites exosomal lncRNA PLADE enhances platinum sensitivity by inducing R-loops in ovarian cancer. Oncogene. 2024;43(10):714–28. doi:10.1038/s41388-024-02940-6. [Google Scholar] [PubMed] [CrossRef]
192. Zhao C, Qiu L, Wu D, Zhang M, Xia W, Lv H, et al. Targeted reversal of multidrug resistance in ovarian cancer cells using exosome-encapsulated tetramethylpyrazine. Mol Med Rep. 2023;29(2):25. doi:10.3892/mmr.2023.13148. [Google Scholar] [PubMed] [CrossRef]
193. Du Z, Huang Z, Chen X, Jiang G, Peng Y, Feng W, et al. Modified dendritic cell-derived exosomes activate both NK cells and T cells through the NKG2D/NKG2D-L pathway to kill CML cells with or without T315I mutation. Exp Hematol Oncol. 2022;11(1):36. doi:10.1186/s40164-022-00289-8. [Google Scholar] [PubMed] [CrossRef]
194. Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics. 2017;7(5):1333–45. doi:10.7150/thno.17092. [Google Scholar] [PubMed] [CrossRef]
195. Javidi-Sharifi N, Martinez J, English I, Joshi SK, Scopim-Ribeiro R, Viola SK, et al. FGF2-FGFR1 signaling regulates release of Leukemia-Protective exosomes from bone marrow stromal cells. eLife. 2019;8:2269. doi:10.7554/eLife.40033. [Google Scholar] [PubMed] [CrossRef]
196. Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10(8):1563–75. doi:10.1016/j.apsb.2019.11.013. [Google Scholar] [PubMed] [CrossRef]
197. Hwang H, Kim J, Kim T-H, Han Y, Choi D, Cho S, et al. Exosomal miR-6126 as a novel therapeutic target for overcoming resistance of anti-cancer effect in hepatocellular carcinoma. BMC Cancer. 2024;24(1):1557. doi:10.1186/s12885-024-13342-y. [Google Scholar] [PubMed] [CrossRef]
198. Li X, Yu Q, Zhao R, Guo X, Liu C, Zhang K, et al. Designer exosomes for targeted delivery of a novel therapeutic cargo to enhance sorafenib-mediated ferroptosis in hepatocellular carcinoma. Front Oncol. 2022;12:7. doi:10.3389/fonc.2022.898156. [Google Scholar] [PubMed] [CrossRef]
199. Li H, Yang C, Shi Y, Zhao L. Exosomes derived from siRNA against GRP78 modified bone-marrow-derived mesenchymal stem cells suppress Sorafenib resistance in hepatocellular carcinoma. J Nanobiotechnology. 2018;16(1):103. doi:10.1186/s12951-018-0429-z. [Google Scholar] [PubMed] [CrossRef]
200. Han Z, Huang H, Li B, Zhao R, Wang Q, Liu H, et al. Engineering exosome membrane disguised thermal responsive system for targeted drug delivery and controlled release across the blood-brain barrier. Mater Today Bio. 2025;32(4):101656. doi:10.1016/j.mtbio.2025.101656. [Google Scholar] [PubMed] [CrossRef]
201. Li Z, Meng X, Wu P, Zha C, Han B, Li L, et al. Glioblastoma cell-derived lncRNA-containing exosomes induce microglia to produce complement C5, promoting chemotherapy resistance. Cancer Immunol Res. 2021;9(12):1383–99. doi:10.1158/2326-6066.CIR-21-0258. [Google Scholar] [PubMed] [CrossRef]
202. Chuang H-Y, Su Y, Liu H-W, Chen C-H, Chiu S-C, Cho D-Y, et al. Preclinical evidence of STAT3 inhibitor pacritinib overcoming temozolomide resistance via downregulating miR-21-enriched exosomes from M2 glioblastoma-associated macrophages. J Clin Med. 2019;8(7):959. doi:10.3390/jcm8070959. [Google Scholar] [PubMed] [CrossRef]
203. Munoz JL, Rodriguez-Cruz V, Walker ND, Greco SJ, Rameshwar P. Temozolomide resistance and tumor recurrence: halting the Hedgehog. Cancer Cell Microenviron. 2015;2(2). doi:10.14800/ccm.747. [Google Scholar] [PubMed] [CrossRef]
204. Kulkarni P, Basu R, Bonn T, Low B, Mazurek N, Kopchick JJ. Growth hormone upregulates melanoma drug resistance and migration via melanoma-derived exosomes. Cancers. 2024;16(15):2636. doi:10.3390/cancers16152636. [Google Scholar] [PubMed] [CrossRef]
205. Feng T, Tang Z, Karges J, Shen J, Jin C, Chen Y, et al. Exosome camouflaged coordination-assembled Iridium(III) photosensitizers for apoptosis-autophagy-ferroptosis induced combination therapy against melanoma. Biomaterials. 2023;301(24):122212. doi:10.1016/j.biomaterials.2023.122212. [Google Scholar] [PubMed] [CrossRef]
206. Nittayaboon K, Leetanaporn K, Sangkhathat S, Roytrakul S, Navakanitworakul R. Proteomic analysis of butyrate-resistant colorectal cancer-derived exosomes reveals potential resistance to anti-cancer drugs. Discov Med. 2024;36(185):1306. doi:10.24976/Discov.Med.202436185.121. [Google Scholar] [PubMed] [CrossRef]
207. Saeed M, Alshaghdali K, Moholkar D, Kandimalla R, Adnan Kausar M, Aqil F. Camel milk-derived exosomes as novel nanocarriers for curcumin delivery in lung cancer. Biomol Biomed. 2024;26(1):132–43. doi:10.17305/bb.2024.11267. [Google Scholar] [PubMed] [CrossRef]
208. Tudrej P, Kujawa KA, Cortez AJ, Lisowska KM. Characteristics of in vivo model systems for ovarian cancer studies. Diagnostics. 2019;9(3):120. doi:10.3390/DIAGNOSTICS9030120. [Google Scholar] [PubMed] [CrossRef]
209. Liu M, Hu Y, Chen G. The antitumor effect of gene-engineered exosomes in the treatment of brain metastasis of breast cancer. Front Oncol. 2020;10:1453. doi:10.3389/FONC.2020.01453. [Google Scholar] [PubMed] [CrossRef]
210. Kim HY, Min HK, Song HW, Yoo A, Lee S, Kim KP, et al. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: an in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 2022;29(1):2897–11. doi:10.1080/10717544.2022.2118898. [Google Scholar] [PubMed] [CrossRef]
211. Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7. doi:10.1073/PNAS.1220998110. [Google Scholar] [PubMed] [CrossRef]
212. Hong CS, Danet-Desnoyers G, Shan X, Sharma P, Whiteside TL, Boyiadzis M. Human acute myeloid leukemia blast-derived exosomes in patient-derived xenograft mice mediate immune suppression. Exp Hematol. 2019;76(4):60–6.e2. doi:10.1016/j.exphem.2019.07.005. [Google Scholar] [PubMed] [CrossRef]
213. Ma L, Guo H, Zhao Y, Liu Z, Wang C, Bu J, et al. Liquid biopsy in cancer current: status, challenges and future prospects. Signal Transduct Target Ther. 2024;9(1):1–36. doi:10.1038/S41392-024-02021-W. [Google Scholar] [PubMed] [CrossRef]
214. Mizenko RR, Feaver M, Bozkurt BT, Lowe N, Nguyen B, Huang KW, et al. A critical systematic review of extracellular vesicle clinical trials. J Extracell Vesicles. 2024;13(10):e12510. doi:10.1002/JEV2.12510. [Google Scholar] [PubMed] [CrossRef]
215. Al-Ani SA, Lee QY, Maheswaran D, Sin YM, Loh JS, Foo JB, et al. Potential of exosomes as multifunctional nanocarriers for targeted drug delivery. Mol Biotechnol. 2024;67(9):3391–414. doi:10.1007/S12033-024-01268-6. [Google Scholar] [PubMed] [CrossRef]
216. Solovicová V, Ďatková A, Bertók T, Kasák P, Vikartovská A, Lorencová L, et al. Advances in magnetic affinity-based isolation/detection of exosomes for robust diagnostics. Microchim Acta. 2025;192(4):102121. doi:10.1007/S00604-025-07048-6. [Google Scholar] [PubMed] [CrossRef]
217. Joshi RM, Telang B, Soni G, Khalife A. Overview of perspectives on cancer, newer therapies, and future directions. Endosc Ultrasound. 2024;10(3):105–9. doi:10.1097/ot9.0000000000000039. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF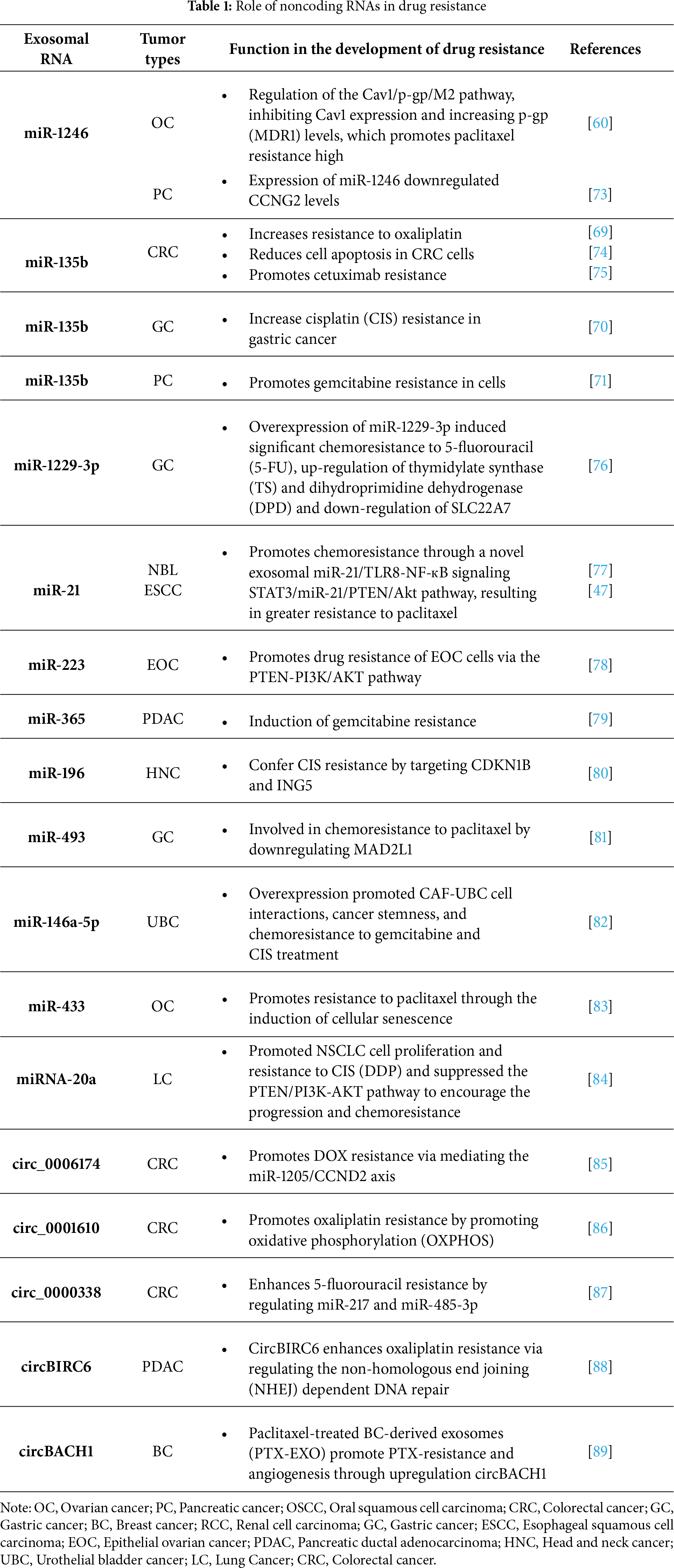
 Downloads
Downloads
 Citation Tools
Citation Tools