 Open Access
Open Access
ARTICLE
Serum Extracellular Vesicle-Associated GULP1 Is a Key Indicator of Hepatocellular Carcinoma
1 Department of Biochemistry, Kosin University College of Medicine, Seo-gu, Busan, 49267, Republic of Korea
2 Department of Molecular Diagnostics, Ugenecell, Songpa-gu, Seoul, 05557, Republic of Korea
3 Department of Gastroenterology, Ajou University School of Medicine, 164 Worldcup-ro, Yeongtong-gu, Suwon, 16499, Republic of Korea
4 Department of Biomedical Sciences, Ajou University Graduate School of Medicine, 164 Worldcup-ro, Yeongtong-gu, Suwon, 16499, Republic of Korea
5 Department of Molecular Medicine, Kyungpook National University School of Medicine, 680 Gukchaebosang-ro, Daegu, 41944, Republic of Korea
* Corresponding Authors: Jae Youn Cheong. Email: ; Jung Woo Eun. Email:
# These authors contributed equally to this work
Oncology Research 2026, 34(2), 13 https://doi.org/10.32604/or.2025.070392
Received 15 July 2025; Accepted 12 November 2025; Issue published 19 January 2026
Abstract
Objectives: Early detection of hepatocellular carcinoma (HCC) is a significant challenge due to the limited sensitivity of alpha-fetoprotein (AFP). This study aimed to assess serum-derived extracellular vesicle-encapsulated GULP PTB domain-containing engulfment adaptor 1 (EV-GULP1) as a novel, noninvasive biomarker for HCC detection and prognosis, leveraging the potential of tumor-specific molecules carried by small extracellular vesicles (EVs). Methods: The study utilized both internal and external cohorts of HCC patients and controls. Small EVs were isolated from serum samples, then characterized and validated to confirm their identity. The expression levels of EV-GULP1 were quantified using quantitative reverse transcription polymerase chain reaction (qRT-PCR). Results: EV-GULP1 expression was found to be significantly higher in HCC patients, including those with early-stage disease, when compared to control groups. It demonstrated superior diagnostic accuracy over AFP, achieving an area under the curve (AUC) of 0.919, and was particularly effective in detecting AFP-negative cases. Furthermore, high EV-GULP1 expression correlated with worse overall and disease-free survival outcomes. Conclusion: These findings highlight EV-GULP1 as a highly promising noninvasive biomarker for hepatocellular carcinoma. It offers improved diagnostic accuracy for early detection and better risk stratification for prognosis compared to the current standard, AFP.Keywords
Supplementary Material
Supplementary Material FileHepatocellular carcinoma (HCC) is the predominant form of primary liver cancer, accounting for approximately 90% of all liver cancer cases. This poses a significant health burden globally. It is the sixth most common cancer worldwide, and the third leading cause of cancer-related deaths globally [1–3]. Moreover, its annual incidence is expected to surpass 1 million new cases by 2025, with a 5-year survival rate of only 18% [4,5]. Major risk factors for HCC include chronic infection with the hepatitis B virus (HBV) or hepatitis C virus (HCV), excessive alcohol consumption, nonalcoholic fatty liver disease, aflatoxin B1 exposure, and smoking [6,7].
Despite significant advances in the understanding of the oncogenic processes of HCC, current surveillance methods primarily rely on abdominal ultrasonography and serum alpha-fetoprotein (AFP) measurements in high-risk populations, such as those with HBV, HCV, or liver cirrhosis (LC) [8,9]. However, abdominal ultrasonography has low sensitivity for early HCC detection, and liver biopsies pose medical challenges owing to the risk of bleeding. Thus, more effective and non-invasive HCC diagnostic methods are urgently required [10,11]. To overcome these limitations, liquid biopsy has emerged as a promising tool, offering the potential for early detection through the analysis of circulating biomarkers [12–14].
GULP PTB domain-containing engulfment adaptor 1 (GULP1), which is the human equivalent of ced-6 in Caenorhabditis elegans, plays a conserved role in phagocytosis [15]. It contributes to cellular processes, such as trogocytosis and engulfment of apoptotic cells, thereby maintaining tissue homeostasis [16]. Given its fundamental role in these processes, GULP1 may influence the development of diseases, such as cancer. GULP1 expression is often diminished in several cancer types, supporting its designation as a tumor suppressor gene [16,17]. However, emerging evidence reveals a contrasting trend in HCC, where GULP1 expression may be elevated and potentially oncogenic. In our earlier work, we not only observed that GULP1 was overexpressed in HCC cells, but also noted that the increased protein level of GULP1 correlated strongly with advanced disease stage and higher recurrence rates [18].
In the present study, we aimed to expand on our previous findings that GULP1 is not only overexpressed intracellularly and at the transcript level in HCC cells but also secreted into the extracellular environment via small extracellular vesicles (EVs). Based on this observation, we hypothesized that EV-associated GULP1 (EV-GULP1) in patient serum may serve as a novel biomarker for hepatocellular carcinoma (HCC), with enhanced diagnostic and prognostic value compared to the conventional marker, alpha-fetoprotein (AFP). Specifically, we investigated whether EV-GULP1 levels could improve HCC detection, particularly in early-stage and AFP-negative patients, and provide clinically relevant prognostic information.
2.1 Analysis of Publicly Available EV Datasets
To identify differentially expressed genes in EVs derived from patients with HCC, we analyzed publicly available RNA-seq data from the GSE199509 dataset (Gene Expression Omnibus, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE199509, accessed on 01 January 2025) [19]. This dataset includes the transcriptomic profiles of EVs isolated from patients with LC, early-stage HCC (eHCC), and advanced HCC (aHCC). Hierarchical clustering and differential expression analyses were performed to identify key upregulated genes in HCC-derived EVs.
2.2 Patient Recruitment and Clinical Data Collection
To ensure objectivity and reduce institutional bias, initial discovery and diagnostic evaluation were conducted using a discovery cohort composed exclusively of samples from three independent external biobanks: Seoul National University Hospital Biobank (Seoul, Republic of Korea), the Biobank of Keimyung University Dongsan Hospital (Daegu, Republic of Korea), and the Bank of KIRAMS Radiation (Seoul, Republic of Korea). This external discovery cohort included 29 healthy controls (HCs), 29 patients with chronic hepatitis (CH), 25 patients with LC, and 59 patients with HCC (Table 1).
All experiments were performed in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-BMR-KSP-16-365, AJIRB-BMR-SMP-17-189, AJOUIRB-KSP-2019-417, AJOUIRB-EX-2022-389 and AJOUIRB-EX-2024-332). Anonymous serum samples and clinical data were provided by the Ajou Human Bio-Resource Bank; the requirement for informed consent was waived.
For subsequent in-depth validation, an internal cohort was established using samples and clinical data from the Biobank of Ajou University Hospital (Suwon, Republic of Korea). The cohort comprised 29 HCs, 29 patients with CH, 31 patients with LC, and 65 patients with HCC. In contrast to the external cohort, the Ajou cohort was enriched with detailed clinical parameters, enabling extended analysis of the Barcelona Clinic Liver Cancer (BCLC) stage, overall survival (OS), and disease-free survival (DFS), particularly in patients with HCC (Table 2) [20].
The following clinical data were collected from all subjects: age, sex, platelet count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum AFP, protein induced by vitamin K absence II (PIVKA-II), etiology of liver disease, serum albumin, total bilirubin, and international normalized ratio (INR). Tumor stage and vascular invasion were assessed using the modified Union for International Cancer Control (mUICC) classification system [21]. Patients without AFP data were excluded from the receiver operating characteristic (ROC) and precision-recall (PR) curve analyses in both the discovery and validation cohorts.
The clinicopathological characteristics of the external and internal cohorts are summarized in Tables 1 and 2, respectively.
2.3 Serum Sample Collection and Processing
For serum collection, 5 mL whole blood was drawn from each participant and placed in serum separator tubes (without anticoagulants). Blood samples were left at room temperature for clotting and subsequently centrifuged to remove red blood cells. The serum fraction was transferred into RNA-free Eppendorf tubes and centrifuged again at 2000× g for 5 min at 4°C to eliminate residual cellular debris. All processed sera samples were stored at −80°C until further analysis.
2.4 Isolation of Serum-Derived Small EVs
Small EVs were isolated from human serum using ExoQuick (Catalog number EXOQ20A-1, System Biosciences, Mountain View, CA, USA) following the manufacturer’s protocol with slight procedural adjustments [22]. In brief, 300 μL of serum was mixed with 72 μL of ExoQuick and incubated at 4°C overnight to facilitate EV precipitation. The samples were subsequently centrifuged at 1500× g for 30 min at room temperature. Following the removal of the supernatant, we resuspended it in 100 μL of phosphate-buffered saline (PBS, pH 7.4) and preserved it at −80°C for downstream RNA and protein extraction.
2.5 Transmission Electron Microscopy (TEM)
The morphology of the isolated small EVs was visualized by TEM. EVs were fixed with 2% glutaraldehyde and 4% paraformaldehyde, and labeled with a 10-nm gold-conjugated anti-CD63 antibody (Catalog number sc-5275; 1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) before visualization. Images were acquired using a Sigma 500 electron microscope (Carl Zeiss, Oberkochen, Germany).
2.6 Nanoparticle Tracking Analysis (NTA)
The size distribution and concentration of serum-derived small EVs were determined by NTA using the NanoSight NS300 instrument (Malvern Panalytical, UK) equipped with a 405-nm laser. Three 60-s videos were recorded per sample, and the data were analyzed using NTA software (version 3.0).
For Western blotting, serum-derived small EVs and Hep3B total cell lysates were lysed in RIPA buffer (100 μL; Thermo Scientific, Waltham, MA, USA) and incubated on ice for 10 min. The Hep3B cell line was obtained from the Korean Cell Line Bank (KCLB, Seoul, Republic of Korea) and authenticated by short tandem repeat (STR) profiling. Mycoplasma contamination was routinely tested using PCR-based methods and found to be negative.
Total protein concentrations were measured using the bicinchoninic acid (BCA) assay (Catalog number 23225; Thermo Scientific). Equal amounts of protein (10 μg) were separated on 4%–20% Mini-PROTEAN TGX™ gels (Bio-Rad Laboratories, Hercules, CA, USA) and transferred onto polyvinylidene difluoride (PVDF) membranes (Amersham; GE Healthcare, Munich, Germany).
Membranes were blocked with 5% nonfat milk in TBS-T and incubated with the following primary antibodies: rabbit anti-CD9 (Catalog number ab92726; Abcam, Cambridge, UK; 1:2000), rabbit anti-CD63 (Catalog number ab134045; Abcam; 1:1000), and mouse anti-BiP/GRP78 (Catalog number 610979; BD Biosciences, San Jose, CA, USA; 1:1000). After washing, membranes were probed with HRP-conjugated secondary antibodies: anti-rabbit (Catalog number BR170-6515; Bio-Rad Laboratories Inc.; 1:3000) or anti-mouse (Catalog number BR170-6516; Bio-Rad Laboratories Inc.; 1:5000).
Full, uncropped, and unedited Western blot images are provided in Supplementary Fig. S1.
2.8 RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA was isolated from serum and EV samples using TRIzol-LS reagent (Catalog number 10296028; Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Reverse transcription was performed with the PrimeScript™ RT Master Mix (Catalog number RR036A; TaKaRa Bio, Otsu, Japan) for tissue- and cell-derived RNA, and with the miScript II RT Kit (Catalog number 218161; QIAGEN, Hilden, Germany) or the Biotechrabbit cDNA Synthesis Kit (Catalog number BR0400403; Biotechrabbit, Hennigsdorf, Germany) for serum EV-derived RNA. qRT-PCR was carried out using the AmfiSure qGreen Q-PCR Master Mix (Catalog number Q5602; GenDEPOT, Barker, TX, USA) on an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) or a Bio-Rad CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories).
The following primers were used: GULP1 (forward: 5′-AACGGGACCTGTTTGGAGCA-3′, reverse: 5′-ACCCCTCCTGCATCTCATCT-3′) and HMBS (reference gene: forward: 5′-GGAGGGCAGAAGGAAGAAAACAG-3′, reverse: 5′-CACTGTCCGTCTGTATGCGAG-3′).
To ensure consistency across different batches of EV analysis, we applied systematic quality control measures. HMBS was selected as the internal reference gene based on its validated expression stability in our previous studies [22–24]. All qRT-PCR reactions were performed in technical triplicate, and pooled healthy control samples were included in each run to monitor inter-batch variability. For qRT-PCR analysis of MIHA cells, the cells were provided by Dr. Roy-Chowdhury (Albert Einstein College of Medicine, Bronx, NY, USA), authenticated by STR profiling, and confirmed to be free of mycoplasma contamination.
Data are presented as mean values ± standard deviation of three experiments. The statistical significance of the differences between the two groups was assessed using the paired Student’s t-test or unpaired Welch’s t-test using GraphPad Prism (version 10.0; GraphPad Software, San Diego, CA, USA). One-way analysis of variance with Tukey’s post-hoc test was conducted for multi-group comparison. Violin plots were generated using GraphPad Prism to visualize the distribution of EV-GULP1 expression levels. All experiments were performed at least three times. Statistical significance was set at *p < 0.05. For the diagnostic evaluation of EV-GULP1, we performed ROC curve analysis on the ddCt (log2) values to determine the optimal cutoff. We used Youden’s Index, a widely accepted statistical method that identifies the optimal threshold by maximizing the sum of sensitivity and specificity. The analysis was conducted using MedCalc statistical software (MedCalc Software, version 22.018; Ostend, Belgium), and the optimal cutoff value for EV-GULP1 was determined to be 1.35. For the conventional biomarker AFP, we used the widely accepted clinical cutoff value of 20 ng/mL.
3.1 Serum-Derived Small EV-GULP1 Expression in HCC
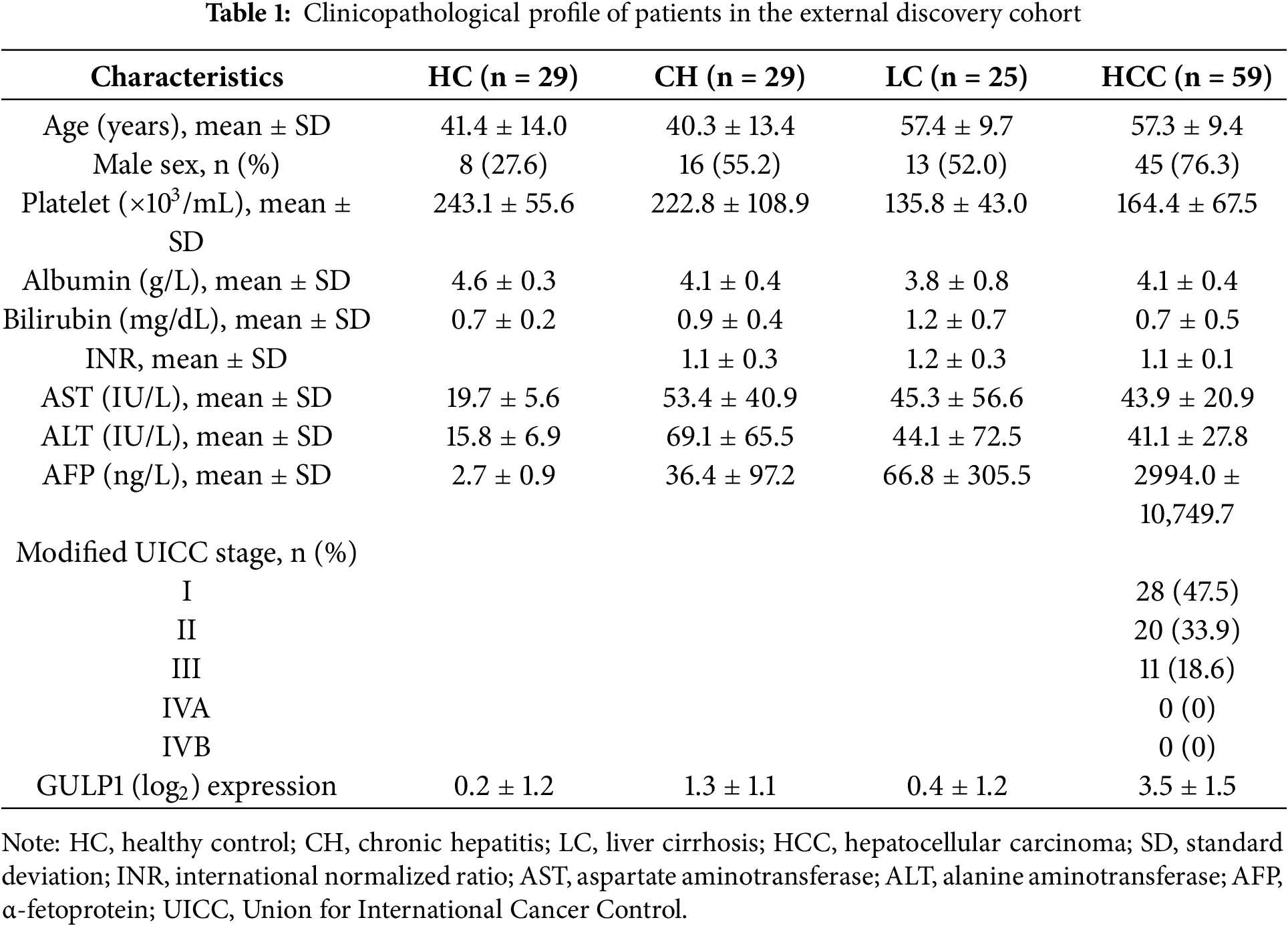
To investigate alterations in EV-encapsulated gene expression in HCC, we analyzed RNA-seq data from the GSE199509 dataset, comparing LC, eHCC, and aHCC cases, and validated our findings across discovery (n = 142) and validation (n = 154) cohorts. This comprehensive approach included serum-derived small EV isolation, qRT-PCR-based gene expression evaluation, and subsequent diagnostic and prognostic assessments. A summary of the workflow is presented in Fig. 1A.
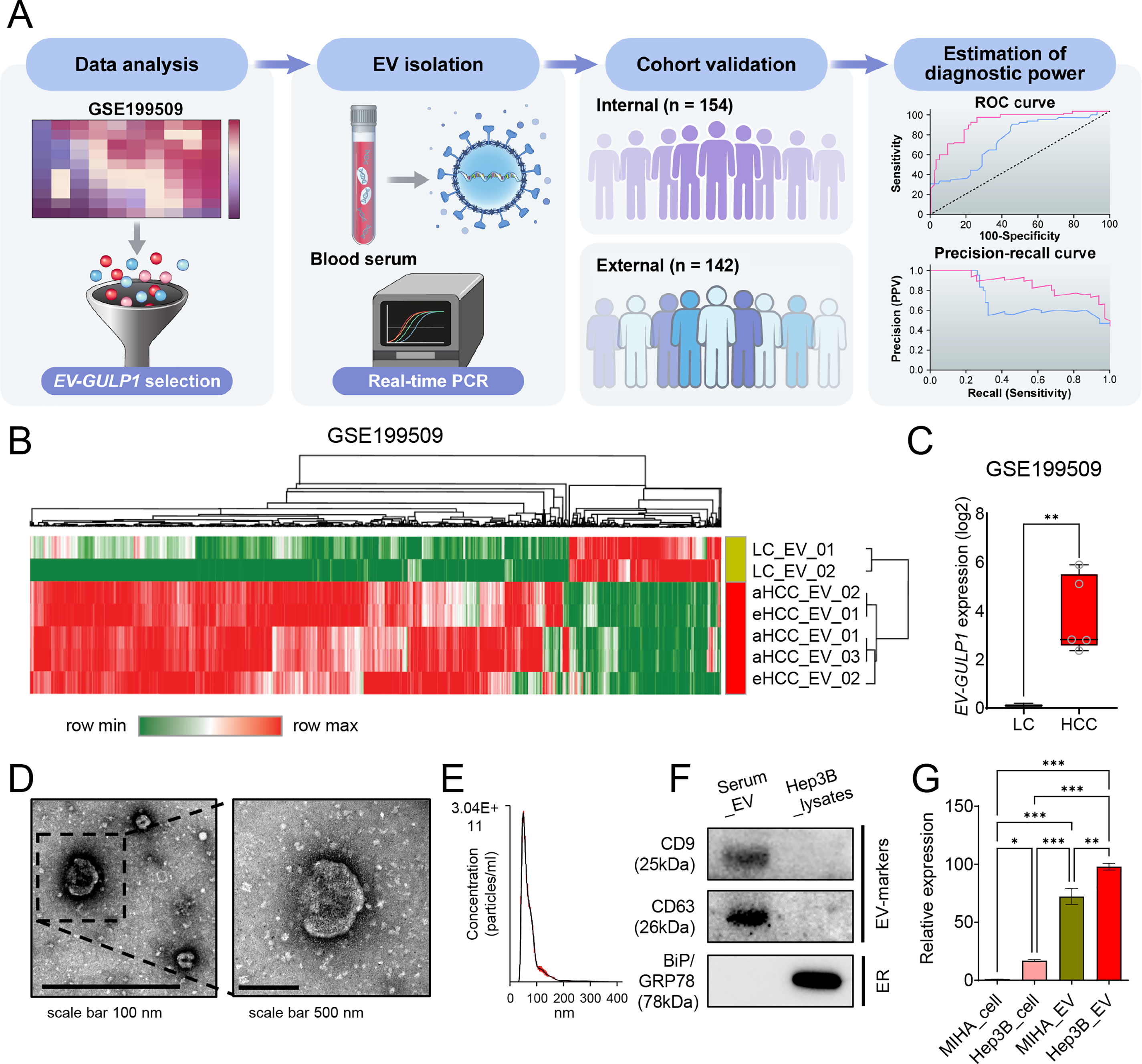
Figure 1: Serum-derived EV-GULP1 expression in patients with HCC. (A) Flow summary of the research process for small extracellular vesicle-encapsulated GULP1 (EV-GULP1) analysis in HCC. (B) Heatmap and hierarchical clustering analysis of RNA-seq data from the GSE199509 dataset comparing EVs derived from patients with liver cirrhosis (LC), early-stage HCC (eHCC), and advanced HCC (aHCC). GULP1 was identified as one of the most upregulated transcripts in HCC-derived EVs. (C) Box plot showing significantly elevated expression of EV-GULP1 in patients with HCC compared to patients with LC (p < 0.01). (D) Characterization of small EVs by transmission electron microscopy (TEM) confirmed the presence of vesicles with typical morphology and size. (E) Nanoparticle tracking analysis (NTA) revealed particle size distribution consistent with small EVs. (F) Western blot analysis of serum-derived small EVs and Hep3B cell lysates showed the presence of EV markers CD9 and CD63, whereas the negative and endoplasmic reticulum (ER) marker BiP/GRP78 was detected only in the cell lysate, confirming the purity of the isolated EVs. (G) Quantitative reverse transcription polymerase chain reaction analysis comparing GULP1 expression in MIHA cells, Hep3B cells, MIHA-derived EVs, and Hep3B-derived EVs, showing significantly increased expression of GULP1 in Hep3B EVs compared to MIHA EVs. *p < 0.05, **p < 0.01, and ***p < 0.001
Initially, we analyzed RNA-seq data from the GSE199509 dataset to identify differentially expressed genes in EVs from patients with HCC, including both eHCC and aHCC, compared with LC controls. Among the 39,276 detected genes, we initially selected genes with log2 (transcripts per million [TPM] + 1) values ≥ 0.1 in HCC group, based on their mean expression in LC, to define genes that are actively expressed in HCC-derived EVs. We then applied Welch’s t-test to compare expression between LC and HCC groups, selecting 554 genes with **p < 0.01 and fold change ≥ |3| as differentially expressed candidate genes.
Hierarchical clustering and heatmap analysis of the GSE199509 dataset showed that 554 genes were aberrantly regulated in HCC-derived EVs, which displayed a distinctive gene expression pattern that clearly distinguished the LC from HCC group (Fig. 1B). These results suggest that numerous EV-loaded genes may drive the development of HCC or may be altered in response to hepatocarcinogenesis. Building on our previous finding that serum GULP1 protein levels exhibit diagnostic potential in patients with HCC, we further determined whether EV-GULP1 retained similar clinical utility [18].
EV-GULP1 expression was significantly higher in both eHCC and aHCC than in LC, suggesting its potential role as a biomarker of HCC progression (Fig. 1C). To validate the presence and morphology of serum-derived EVs, we employed TEM. Microscopic images revealed vesicles with typical lipid bilayer membranes, and a higher-magnification view further confirmed their spherical morphology, consistent with that of small EVs (Fig. 1D). NTA was conducted to assess the size distribution and concentration of the isolated EVs, which showed that most vesicles were within the expected diameter range, confirming successful isolation (Fig. 1E). Western blot analysis verified the purity of the isolated EVs and the expression levels of GULP1 in serum-derived EVs and Hep3B_lysates. As shown in the serum_EV lane in Fig. 1F, the EV markers CD9 and CD63 were readily detected, whereas the non-EV marker BiP/GRP78 was absent, confirming the successful isolation and purity of the EV fraction. In contrast, Hep3B_lysates exhibited minimal expression of EV markers, but high BiP/GRP78 levels, indicating their intracellular origin and distinguishing them from EVs (Fig. 1F; Hep3B lysate lane).
To further characterize GULP1 expression in normal and HCC tissues, we measured its levels in MIHA (normal hepatocytes) and Hep3B (liver cancer) cells, as well as in their respective EVs (Fig. 1G). GULP1 expression was significantly higher in Hep3B cells than that in MIHA cells, suggesting its association with HCC. GULP1 levels were also elevated in Hep3B-derived EVs compared to those in MIHA-derived EVs, suggesting that EV-mediated transfer of GULP1 may contribute to HCC pathogenesis.
3.2 HCC Diagnostic Performance of EV-GULP1 Compared to AFP
The diagnostic performance of EV-GULP1 was further examined in an independent discovery cohort using sera samples obtained from the Seoul National University Hospital Biobank (Seoul, Republic of Korea), Biobank of Keimyung University Dongsan Hospital (Daegu, Republic of Korea), and the Bank of KIRAMS Radiation (Seoul, Republic of Korea). The discovery cohort included 29 HCs, 29 patients with CH, 25 patients with LC, and 59 patients with HCC, further stratified into mUICC stages I (n = 28), II (n = 20), and III (n = 11) (Table 1). To determine the relationship between EV-GULP1 expression and disease stage, we first examined its levels across different disease groups (Fig. 2A). EV-GULP1 expression was significantly higher in patients with mUICC I than in those with non-tumor conditions, and these elevated levels were maintained consistently in stages II and III rather than showing a gradual increase. This pattern suggests that EV-GULP1 undergoes a marked increase upon malignant transformation and remains elevated as the disease progresses, reinforcing its strong association with HCC rather than with incremental disease advancement. The ability of EV-GULP1 to differentiate HCC from non-tumor conditions was further analyzed using ROC curve analysis (Fig. 2B). The results demonstrated high diagnostic accuracy, with an AUC of 0.919 (***p < 0.001) for distinguishing HCC from non-tumor conditions, supporting its potential as a reliable biomarker.
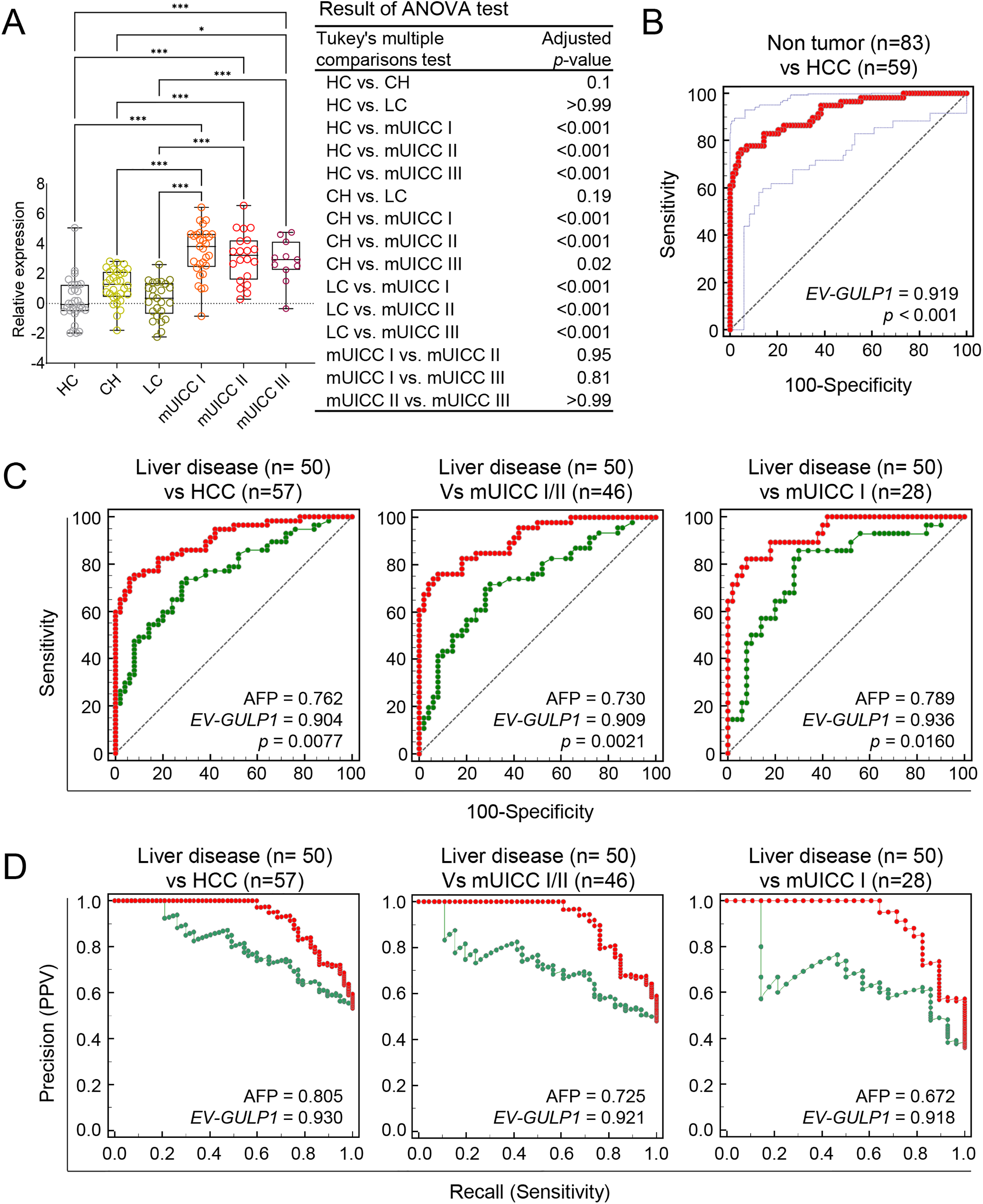
Figure 2: Diagnostic performance of EV-GULP1 compared to AFP for HCC detection. (A) Serum-derived EV-GULP1 expression levels in an external discovery cohort comprising healthy controls (HC), patients with chronic hepatitis (CH), patients with liver cirrhosis (LC), and those with HCC stratified by mUICC stages I, II, and III. EV-GULP1 levels were significantly increased in patients with HCC compared to healthy controls. (B) ROC curve analysis assessing the ability of EV-GULP1 to distinguish HCC from non-HCC conditions in the external discovery cohort, demonstrating high diagnostic accuracy. (C) ROC curve analysis comparing the performance of EV-GULP1 and AFP in differentiating HCC from patients with liver disease (CH and LC) in the external cohort. Patients with missing AFP values were excluded from the analysis. (D) PR curve analysis confirming that EV-GULP1 maintains superior classification performance compared to AFP in distinguishing HCC from liver disease in the external discovery cohort. Individuals lacking AFP data were not included in the analysis. Data are presented as means ± standard errors of the mean. *p < 0.05 and ***p < 0.001. ANOVA, analysis of variance; mUICC, modified Union for International Cancer Control; PPV, positive predictive value
A key challenge in HCC diagnosis is distinguishing malignant from non-malignant liver conditions, as traditional biomarkers, such as AFP, often yield positive results in patients with chronic liver disease, despite their intended use for cancer detection. Although AFP levels commonly remain negative in HCs, they frequently increase in patients with CH and LC, leading to false-positive results in non-malignant conditions [25,26]. Considering this limitation, assessing the ability of EV-GULP1 to differentiate HCC from the underlying liver disease is crucial for biomarker validation. Therefore, we performed an additional set of ROC and PR curve analyses within the external discovery cohort to evaluate EV-GULP1’s performance in distinguishing patients with HCC from those with liver disease.
Consequently, we conducted an independent analysis using an internal cohort to assess the diagnostic performance of EV-GULP1 in distinguishing HCC from non-malignant liver diseases including CH and LC. ROC analysis confirmed that EV-GULP1 maintained a strong ability to differentiate non-tumor conditions from HCC, achieving an AUC of 0.904 (p = 0.0077), whereas AFP showed a lower AUC of 0.762 (Fig. 2C, left panel). Additional analyses further reinforced its high diagnostic accuracy, with AUCs of 0.909 and 0.936 in different comparisons (p = 0.0021 and 0.016, respectively), which were superior to those of AFP (AUCs of 0.730 and 0.789, respectively) (Fig. 2C, middle and right panels, respectively). PR curve analysis, which accounted for class imbalance and provided a more clinically relevant evaluation, also highlighted the superior classification performance of EV-GULP1, with AUCs of 0.930, 0.921, and 0.918, whereas AFP exhibited lower AUCs of 0.805, 0.725, and 0.672, respectively (*p < 0.05) (Fig. 2D).
Furthermore, ROC curve analysis using the external cohort demonstrated that EV-GULP1 maintained high diagnostic accuracy in distinguishing HCC from non-tumor conditions, including HC and patients with CH or LC. EV-GULP1 achieved AUCs of 0.920, 0.925, and 0.948 across different comparisons—non-tumor vs. HCC, non-tumor vs. mUICC I/II, and non-tumor vs. mUICC I, respectively (p = 0.0183, 0.0054, and 0.0448, respectively) (Supplementary Fig. S2A). These values were consistently higher than those of AFP (AUCs of 0.815, 0.788, and 0.843, respectively), supporting the superior discriminative power of EV-GULP1 even in early-stage HCC. PR curve analysis using the external cohort also revealed that EV-GULP1 significantly outperformed AFP in terms of precision and recall, with AUCs of 0.917, 0.907, and 0.907, respectively, compared with 0.790, 0.707, and 0.664 for AFP, respectively (Supplementary Fig. S2B).
These findings underscore the ability of EV-GULP1 to accurately distinguish patients with HCC from healthy individuals or patients with chronic liver disease, addressing the key limitation of conventional biomarkers that often yield false positives in non-malignant conditions. Its capacity to maintain high specificity while preserving superior sensitivity for HCC detection highlights its strong clinical potential as a reliable and noninvasive biomarker, particularly in high-risk populations with chronic liver disease.
3.3 Additional Validation of EV-GULP1 as a Diagnostic Biomarker for HCC
To further evaluate the clinical relevance of EV-GULP1 expression across different HCC stages, we analyzed its levels using the BCLC and mUICC classification systems in our internal validation cohort (Table 2). The BCLC classification showed a stepwise increase in EV-GULP1 expression from BCLC stage 0 (very early stage) to stage C (advanced HCC), with a statistically significant difference between early and advanced disease stages (Fig. 3A). Similarly, the mUICC classification confirmed a progressive increase in EV-GULP1 levels as the severity of HCC increased (Fig. 3B). Thus, EV-GULP1 may be strongly associated with HCC progression and may serve as a valuable noninvasive biomarker for both early detection and disease stratification. Notably, to further evaluate its clinical applicability, we assessed the influence of demographic and etiological variables. Our analysis revealed that EV-GULP1 expression levels were not significantly affected by patient age, sex, or the etiology of their liver cancer (HBV, HCV, or alcohol), highlighting its potential as a universally robust biomarker (Supplementary Fig. S3A–C).
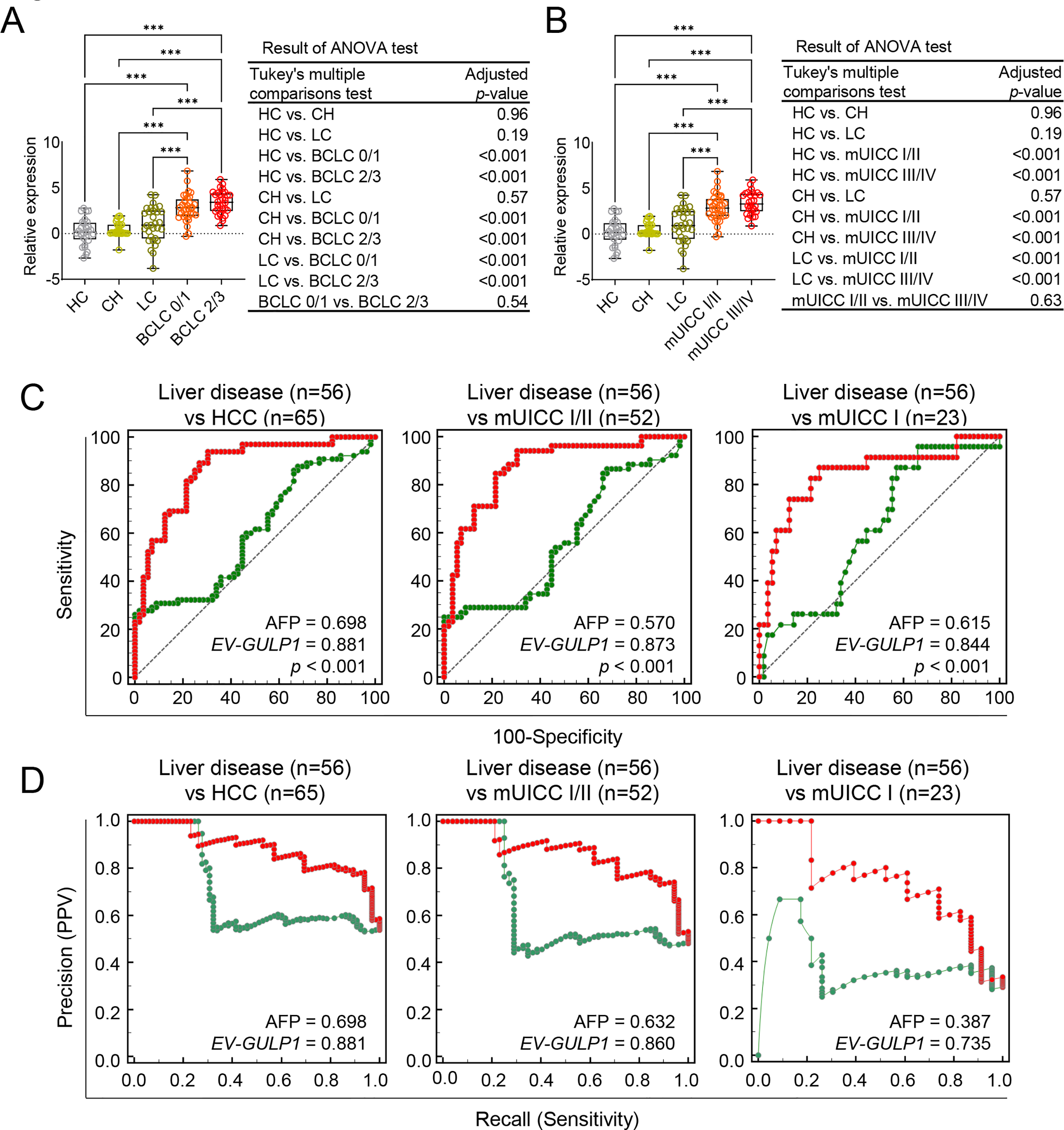
Figure 3: Internal validation of EV-GULP1 diagnostic performance in an independent cohort. (A) Serum-derived EV-GULP1 expression levels according to Barcelona Clinic Liver Cancer (BCLC) stages, showing significantly higher expression levels in patients with advanced-stage HCC. (B) Serum-derived EV-GULP1 expression levels stratified by modified Union for International Cancer Control (mUICC) stages, demonstrating a significant increase in patients with HCC compared to healthy controls. Data are presented as means ± standard errors of the mean. (C) ROC curve analysis evaluating the performance of EV-GULP1 and AFP in distinguishing HCC from patients with liver disease (chronic hepatitis and liver cirrhosis). Patients with missing AFP values were excluded from the analysis. (D) PR curve analysis confirming the superior precision and recall of EV-GULP1 over AFP in differentiating HCC from liver disease groups. Cases without available AFP values were excluded from the study. ***p < 0.001. ANOVA, analysis of variance; mUICC, modified Union for International Cancer Control; PPV, positive predictive value
Additionally, we assessed the ability of EV-GULP1 to differentiate HCC from other liver diseases (CH and LC) using ROC and PR analyses. EV-GULP1 demonstrated superior accuracy, with AUC values of 0.881, 0.873, and 0.844 for diagnosing all stages of HCC, mUICC stage I or II, and mUICC stage I, respectively, compared to AFP AUCs of 0.698, 0.570, and 0.615, respectively (Fig. 3C). Similarly, PR analysis confirmed the superior diagnostic performance of EV-GULP1, with AUCs of 0.881, 0.860, and 0.735, compared to AFP, which showed markedly lower AUCs of 0.698, 0.632, and 0.387, respectively (Fig. 3D).
To evaluate the diagnostic efficacy of serum EV-GULP1 compared to AFP in distinguishing HCC from non-tumor conditions, we performed ROC and PR curve analyses. ROC curve analysis demonstrated that EV-GULP1 exhibited significantly higher diagnostic accuracy than AFP across various disease stages. Specifically, for distinguishing non-tumor conditions (HC, CH, and LC) from HCC, EV-GULP1 achieved an AUC of 0.891, significantly outperforming AFP (AUC = 0.718, ***p < 0.001) (Supplementary Fig. S4A, left panel). This superior performance was also observed for early-stage HCC, as indicated by the mUICC stage I and II subgroup analysis, where EV-GULP1 exhibited an AUC of 0.893 compared to 0.691 for AFP (***p = 0.001) (Supplementary Fig. S4A, middle panel). In mUICC stage I, EV-GULP1 maintained a high AUC of 0.866, whereas AFP showed poor performance with an AUC of 0.540 (***p < 0.001) (Supplementary Fig. S4A, right panel). Thus, EV-GULP1 demonstrated greater sensitivity and specificity than AFP for detecting early-stage HCC.
To validate these findings further, we conducted PR curve analysis. The results of the PR analysis consistently demonstrated that EV-GULP1 maintained superior precision and recall compared to AFP. Across all HCC stages, EV-GULP1 had an AUC of 0.862, outperforming AFP (AUC = 0.690) (Supplementary Fig. S4B, left panel). In mUICC stages I and II, the advantage of EV-GULP1 was evident, with an AUC of 0.842, compared to 0.623 for AFP (Supplementary Fig. S4B, middle panel). Similarly, when distinguishing non-tumor conditions from mUICC stage I HCC, EV-GULP1 retained an AUC of 0.713, whereas AFP exhibited a significantly lower AUC of 0.250, further highlighting its limited sensitivity in early-stage detection (Supplementary Fig. S4B, right panel). Taken together, these results strongly suggest that EV-GULP1 is a highly promising biomarker with excellent potential for early detection of HCC, especially for distinguishing early-stage cancer from non-malignant liver conditions.
3.4 EV-GULP1 Positivity in Different Disease Groups and Its Comparison with AFP
To compare the detection rates of EV-GULP1 and AFP across different disease conditions, we analyzed their positivity rates in HCs, patients with liver disease, and patients at various stages of HCC (Fig. 4A). For this analysis, EV-GULP1 positivity was defined as a ddCt (log2) value of 1.35 or greater, while AFP positivity was defined as a value exceeding 20 ng/mL. Among the HCs, AFP showed no positivity (0%), whereas EV-GULP1 was detected in 17% of the cases, suggesting a slightly higher baseline presence in non-HCC conditions (Fig. 4A, first panel). In patients with liver disease, including CH and LC, AFP had a positivity rate of 39%, whereas EV-GULP1 was detected in 29% of the cases (Fig. 4A, second panel). The lower positivity rate of EV-GULP1 in liver disease compared to that of AFP suggests higher specificity, potentially reducing false positives from non-malignant liver conditions.
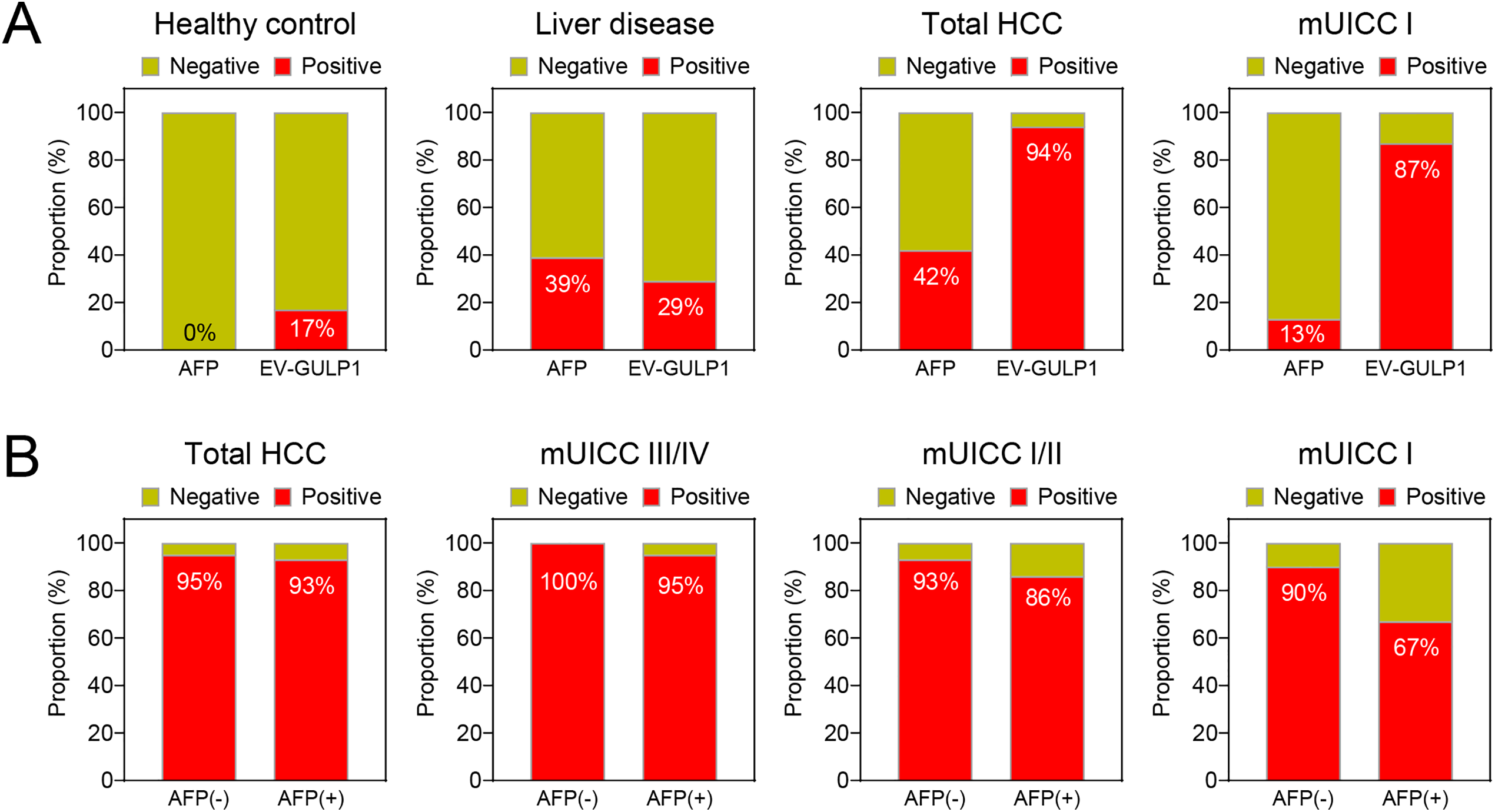
Figure 4: EV-GULP1 positivity in different disease groups and comparison with AFP. (A) Comparison of EV-GULP1 and AFP positivity rates across healthy controls, liver disease groups (chronic hepatitis and liver cirrhosis), and different HCC stages. EV-GULP1 exhibited significantly higher positivity rates than AFP in HCC, particularly in early-stage disease. (B) EV-GULP1 positivity rates in AFP-negative (−) and AFP-positive (+) patients with HCC, showing consistently high detection rates in both subgroups, reinforcing its diagnostic utility regardless of AFP status. mUICC, modified Union for International Cancer Control
AFP positivity increased to 42% in patients with HCC across all stages; however, EV-GULP1 demonstrated a significantly higher positivity rate of 94%, indicating superior sensitivity for HCC detection (Fig. 4A, third panel). In patients with mUICC stage I, AFP was positive in only 13% of cases, whereas EV-GULP1 exhibited a considerably higher positivity rate of 87%, further supporting its strong potential for detecting early-stage HCC (Fig. 4A, fourth panel). Although AFP retains its diagnostic utility, EV-GULP1 provides superior sensitivity, particularly in early-stage HCC, making it a reliable biomarker for liver cancer detection.
We further analyzed the positivity rates of EV-GULP1 in AFP-negative (AFP (−)) and AFP-positive (AFP (+)) patients with HCC across different stages. EV-GULP1 consistently exhibited high positivity rates in both AFP (−) and AFP (+) patients, with minimal variation between the two groups. In AFP (−) HCC cases, where AFP typically fails as a diagnostic marker, EV-GULP1 remained highly detectable, exceeding 90% positivity across all stages, confirming its high sensitivity, independent of AFP status (Fig. 4B). Specifically, EV-GULP1 was detected in 93% of AFP (−) cases in mUICC stage I/II, whereas its detection rate was 100% in advanced HCC cases of mUICC stage III/IV (Fig. 4B, second to fourth panels). Even in AFP (+) cases, EV-GULP1 positivity remained consistently high, with 86% detection in mUICC stage I/II and 95% detection in advanced HCC cases of mUICC stage III/IV, emphasizing its robustness as a diagnostic tool (Fig. 4B). These findings clearly demonstrate that EV-GULP1 provides reliable diagnostic performance regardless of AFP status.
3.5 Prognostic Significance of EV-GULP1 in HCC Survival Outcomes
To assess the prognostic significance of EV-GULP1 in HCC, we performed Kaplan–Meier survival analyses for OS and DFS based on EV-GULP1 expression levels. Patients with high EV-GULP1 expression levels exhibited significantly worse OS than those with low EV-GULP1 expression levels (hazard ratio [HR] = 2.42, 95% confidence interval [CI]: 1.09–5.42, p = 0.0297), indicating that elevated EV-GULP1 levels were associated with more aggressive disease and reduced survival rates (Fig. 5A). Similarly, patients with high EV-GULP1 expression levels had a significantly shorter DFS than those with low EV-GULP1 expression levels (HR = 2.57, 95% CI: 1.10–6.01, p = 0.023), suggesting that higher EV-GULP1 levels are correlated with a greater likelihood of early disease recurrence or progression following treatment (Fig. 5B). These findings highlight EV-GULP1 as a strong prognostic biomarker for HCC that can predict both reduced OS and shorter DFS. The significant association between high EV-GULP1 expression levels and poor survival outcomes supports its potential role in HCC progression and underscores its clinical relevance for patient risk stratification. EV-GULP1 may primarily indicate tumor aggressiveness and post-treatment recurrence, further emphasizing its utility as a prognostic biomarker for HCC management.

Figure 5: Prognostic significance of EV-GULP1 in HCC survival outcomes. (A) Kaplan–Meier survival curves for overall survival (OS) stratified by high and low EV-GULP1 expression groups. Patients with high EV-GULP1 expression levels exhibited significantly worse OS than those with low EV-GULP1 expression levels (p = 0.0297). (B) Kaplan–Meier survival curves for disease-free survival (DFS), showing a significant association between high EV-GULP1 expression and shorter DFS (p = 0.023). HR, hazard ratio; CI, confidence interval. *p < 0.05
Various guidelines have been established for primary liver cancer; however, recurrence and progression remain formidable challenges despite diverse therapeutic strategies, including surgical resection, ablation, and liver transplantation [27–29]. Although AFP has historically served as a conventional biomarker, its limited sensitivity for early-stage disease underscores the urgent need for novel indicators [30,31].
Recent developments in liquid biopsy, particularly EV analysis, have revealed promising avenues for overcoming this limitation [32–34]. Small EVs are abundantly released by tumor cells and encapsulate proteins, DNA, and various RNA species that mirror the molecular characteristics of their parent cells [35–37]. In the context of HCC, these findings offer a potent complement to standard surveillance methods as they allow minimally invasive, repeatable sampling that may detect subtle molecular signals preceding overt tumor recurrence.
Our previous study has reported that GULP1, initially recognized as a tumor suppressor in other malignancies, functions paradoxically as an oncogene in HCC [18]. Detailed mechanistic analyses revealed that GULP1 augments Wnt/β-catenin signaling—partly mediated via ARF6 activation—and drives epithelial–mesenchymal transition, thereby fostering metastatic dissemination [18]. Despite these mechanistic insights, the prior protein-based or ELISA-based measurement of GULP1 alone faced challenges, including relatively modest area under the curve values in some cohorts. Although serum GULP1 showed promise by outperforming traditional biomarkers such as AFP—particularly in early-stage HCC—its diagnostic utility was still not on par with more established markers, a limitation that other investigators have also pointed out [38].
In contrast, our recent findings on EV-GULP1 suggest a more tumor-specific and robust approach that may overcome many of these obstacles. Importantly, HCC cells secrete GULP1 through small EVs, implying that serum-derived EV-GULP1 levels could serve as a powerful surrogate marker of tumor activity. This aligns with recent research results indicating that EV cargo often reflects the aggressiveness of an underlying disease [39,40]. Our data further reinforce the clinical significance of GULP1 in that it outperforms AFP in detecting early-stage and AFP-negative tumors, offering higher specificity for HCC than for CH or LC.
However, this study had some limitations. First, our choice of a polymer-based precipitation method (e.g., ExoQuick) for EV isolation warrants discussion. While this approach offers key advantages in scalability, speed, and suitability for the small sample volumes often encountered in clinical biobanks, its main drawback is the potential co-isolation of non-vesicular proteins and macromolecules, which could affect sample purity. Alternative methods, such as ultracentrifugation—often considered the gold standard for high purity—or size-exclusion chromatography, were impractical for this study due to their requirements for larger sample volumes and more intensive labor. Given these constraints, the polymer-based method was the most feasible option, and its efficacy was supported by our preliminary validation experiments. Additionally, while the precise mechanism for GULP1 enrichment in EVs is unknown, we hypothesize it is driven by GULP1’s interaction with the ARF6-syntenin-ALIX exosome biogenesis pathway, a process likely enhanced by tumor-related stress like hypoxia, based on the previous studies [41,42]. Nonetheless, GULP1 expression may also fluctuate in response to LC, underlying inflammation, and coexisting treatments. Therefore, larger-scale validation studies are warranted to refine the cutoff values and strengthen the predictive accuracy of EV-GULP1. Combining GULP1 measurements with other extracellular biomarkers or novel liquid biopsy assays may enhance the prognosis and guide personalized therapeutics. Specifically, integrating GULP1 with advanced EV RNA panels could bolster the early detection of microscopic diseases, while simultaneous monitoring of circulating tumor DNA might offer insights into clonal evolution or emerging resistance. Overall, our findings position GULP1 at the intersection of oncogenic signaling and clinically actionable biomarker development. By demonstrating its pivotal role in HCC progression and its detectability in serum-derived EVs, this study proposes EV-GULP1 as both a practical clinical tool and a potential therapeutic target.
In summary, our findings support EV-GULP1 as a promising non-invasive biomarker for HCC. Nonetheless, the retrospective nature of our study involving only Korean cohorts necessitates broader geographic and ethnic validation. Although the sample size was sufficient to meet the primary endpoints, it was limited in-depth subgroup and longitudinal analyses. EVs were isolated via polymer-based precipitation, an established approach that may still contain trace non-vesicular components, underscoring the need to integrate orthogonal purification methods. In future research, harmonizing qRT-PCR cut-off values and confirming these results in prospective, multi-center trials will be essential to fully establish the clinical utility of EV-GULP1.
Acknowledgement: The biospecimens and data used in this study were provided by the Biobank of the Ajou University Hospital, a member of the Korea Biobank Network. We would like to thank Editage (www.editage.com) for the English language editing. ChatGPT (version 5 plus) was used for error checking and language refinement during the preparation of the manuscript.
Funding Statement: This work was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea (HR21C1003), and the Bio and Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science and ICT (RS-2022-NR070489, RS-2023-00210847, RS-2024-00422549, RS-2024-00463331, RS-2025-00521818, and RS-2025-00562556).
Author Contributions: Hyung Seok Kim performed bioinformatics analysis and wrote the manuscript and obtained financial support. Ju A Son performed internal cohort experiments. Minji Kang performed external cohort experiments. Soon Sun Kim interpreted clinical data. Geum Ok Baek managed the overall experiment. Moon Gyeong Yoon and Se Ha Jang assembled data. Dokyung Jung performed experiments. Ji Eun Han managed clinical data. Jae Youn Cheong wrote the manuscript, obtained financial support, and managed the project. Jung Woo Eun performed bioinformatics analysis, wrote and edited the manuscript, supervised the study, and obtained financial support. All the authors have read and agreed to the published version of the manuscript.
Availability of Data and Materials: The datasets used during the current study are available from the corresponding authors upon reasonable request.
Ethics Approval: All experiments were performed in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-BMR-KSP-16-365, AJIRB-BMR-SMP-17-189, AJOUIRB-KSP-2019-417, AJOUIRB-EX-2022-389, and AJOUIRB-EX-2024-332), each corresponding to distinct sample and data collection periods. Anonymous serum samples and clinical data were provided by the Ajou Human Source Bank, and the requirement for informed consent was waived by the IRB due to the retrospective design and use of fully anonymized biospecimens and non-identifiable information.
Conflicts of Interest: The authors declare no conflict of interest.
Supplementary Materials: The supplementary material is available online at https://www.techscience.com/doi/10.32604/or.2025.070392/s1.
Abbreviations
| AFP | Alpha-fetoprotein |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AUC | Area under the curve |
| BCLC | Barcelona Clinic Liver Cancer |
| CH | Chronic hepatitis |
| CI | Confidence interval |
| DFS | Disease-free survival |
| DNA | Deoxyribonucleic acid |
| eHCC | Early-stage hepatocellular carcinoma |
| ELISA | Enzyme-linked immunosorbent assay |
| ER | Endoplasmic reticulum |
| EV | Extracellular vesicle |
| EV-GULP1 | Extracellular vesicle-encapsulated GULP1 |
| GULP1 | GULP PTB domain-containing engulfment adaptor 1 |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HC | Healthy control |
| HR | Hazard ratio |
| INR | International normalized ratio |
| LC | Liver cirrhosis |
| mUICC | Modified Union for International Cancer Control |
| NTA | Nanoparticle tracking analysis |
| OS | Overall survival |
| PIVKA-II | Protein induced by vitamin K absence II |
| PR | Precision-recall |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| RNA | Ribonucleic acid |
| RNA-seq | RNA sequencing |
| ROC | Receiver operating characteristic |
| TEM | Transmission electron microscopy |
| TPM | Transcripts per million |
References
1. Safri F, Nguyen R, Zerehpooshnesfchi S, George J, Qiao L. Heterogeneity of hepatocellular carcinoma: from mechanisms to clinical implications. Cancer Gene Ther. 2024;31(8):1105–12. doi:10.1038/s41417-024-00764-w. [Google Scholar] [PubMed] [CrossRef]
2. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62. doi:10.1016/s0140-6736(22)01200-4. [Google Scholar] [PubMed] [CrossRef]
3. The L. Reversing the rise of liver cancer. Lancet. 2025;406(10504):665. doi:10.1016/s0140-6736(25)01530-2. [Google Scholar] [PubMed] [CrossRef]
4. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. doi:10.3322/caac.21834. [Google Scholar] [PubMed] [CrossRef]
5. Albarrak J, Al-Shamsi H. Current status of management of hepatocellular carcinoma in the gulf region: challenges and recommendations. Cancers. 2023;15(7):2001. doi:10.3390/cancers15072001. [Google Scholar] [PubMed] [CrossRef]
6. Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2(1):16018. doi:10.1038/nrdp.2016.18. [Google Scholar] [PubMed] [CrossRef]
7. Tran S, Zou B, Kam L, Lee K, Huang DQ, Henry L, et al. Updates in characteristics and survival rates of hepatocellular carcinoma in a nationwide cohort of real-world US patients, 2003–2021. J Hepatocell Carcinoma. 2023;10:2147–58. doi:10.2147/jhc.s420603. [Google Scholar] [PubMed] [CrossRef]
8. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–72. doi:10.1038/s41571-021-00573-2. [Google Scholar] [PubMed] [CrossRef]
9. Chen L, Wu T, Fan R, Qian YS, Liu JF, Bai J, et al. Cell-free DNA testing for early hepatocellular carcinoma surveillance. eBioMedicine. 2024;100:104962. [Google Scholar] [PubMed]
10. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–61. doi:10.1016/j.jhep.2019.08.025. [Google Scholar] [PubMed] [CrossRef]
11. Finn RS, Zhu AX. Evolution of systemic therapy for hepatocellular carcinoma. Hepatology. 2021;73( Suppl 1):150–7. doi:10.1002/hep.31306. [Google Scholar] [PubMed] [CrossRef]
12. Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–38. doi:10.1038/nrc.2017.7. [Google Scholar] [PubMed] [CrossRef]
13. Manea I, Iacob R, Iacob S, Cerban R, Dima S, Oniscu G, et al. Liquid biopsy for early detection of hepatocellular carcinoma. Front Med. 2023;10:1218705. doi:10.3389/fmed.2023.1218705. [Google Scholar] [PubMed] [CrossRef]
14. Liu M, Zhang Z, Zhang W, Liu SM. Advances in biomarker discovery using circulating cell-free DNA for early detection of hepatocellular carcinoma. WIREs Mech Dis. 2023;15(3):e1598. doi:10.1002/wsbm.1598. [Google Scholar] [PubMed] [CrossRef]
15. Liu QA, Hengartner MO. Human CED-6 encodes a functional homologue of the Caenorhabditis elegans engulfment protein CED-6. Curr Biol. 1999;9(22):1347–50. doi:10.1016/s0960-9822(00)80061-5. [Google Scholar] [PubMed] [CrossRef]
16. Gong J, Gaitanos TN, Luu O, Huang Y, Gaitanos L, Lindner J, et al. Gulp1 controls Eph/ephrin trogocytosis and is important for cell rearrangements during development. J Cell Biol. 2019;218(10):3455–71. doi:10.1083/jcb.201901032. [Google Scholar] [PubMed] [CrossRef]
17. Wang W, Li Y, Li S, Lin F, Guo J, Liu F, et al. The value of GULP1 in cancer prognosis and immunotherapy, validated from pan-cancer analysis to pancreatic cancer. Sci Rep. 2025;15(1):19021. doi:10.1038/s41598-025-99909-6. [Google Scholar] [PubMed] [CrossRef]
18. Kim HS, Yoon JH, Choi JY, Yoon MG, Baek GO, Kang M, et al. GULP1 as a novel diagnostic and predictive biomarker in hepatocellular carcinoma. Clin Mol Hepatol. 2025;31(3):914–34. doi:10.3350/cmh.2024.1038. [Google Scholar] [PubMed] [CrossRef]
19. Chen Y, Pan G, Yang Y, Wu H, Weng M, Wu Q, et al. Tumor exosomal RNPEP promotes lung metastasis of liver cancer via inducing cancer-associated fibroblast activation. Cancer Sci. 2025;116(3):792–807. doi:10.1111/cas.16417. [Google Scholar] [PubMed] [CrossRef]
20. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. doi:10.1016/s0140-6736(18)30010-2. [Google Scholar] [PubMed] [CrossRef]
21. Korean Liver Cancer Association, National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023;23(1):1–120. doi:10.17998/jlc.2022.11.07. [Google Scholar] [PubMed] [CrossRef]
22. Kim SS, Baek GO, Ahn HR, Sung S, Seo CW, Cho HJ, et al. Serum small extracellular vesicle-derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol Oncol. 2020;14(10):2646–59. doi:10.1002/1878-0261.12745. [Google Scholar] [PubMed] [CrossRef]
23. Ahn HR, Baek GO, Yoon MG, Son JA, You D, Yoon JH, et al. HMBS is the most suitable reference gene for RT-qPCR in human HCC tissues and blood samples. Oncol Lett. 2021;22(5):791. [Google Scholar] [PubMed]
24. Son JA, Weon JH, Baek GO, Ahn HR, Choi JY, Yoon MG, et al. Circulating small extracellular vesicle-derived splicing factor 3b subunit 4 as a non-invasive diagnostic biomarker of early hepatocellular carcinoma. J Exp Clin Cancer Res. 2023;42(1):288. doi:10.1186/s13046-023-02867-y. [Google Scholar] [PubMed] [CrossRef]
25. Manuc D, Preda CM, Sandra I, Baicus C, Cerban R, Constantinescu I, et al. Signification of serum alpha-fetoprotein levels in cases of compensated cirrhosis and hepatitis c virus without hepatocellular carcinoma. J Med Life. 2020;13(1):68–74. [Google Scholar] [PubMed]
26. Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, et al. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol. 2022;28(2):216–29. doi:10.3748/wjg.v28.i2.216. [Google Scholar] [PubMed] [CrossRef]
27. Li JK, Liu XH, Cui H, Xie XH. Radiofrequency ablation vs. surgical resection for resectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Clin Oncol. 2020;12(1):15–22. [Google Scholar] [PubMed]
28. Saraswat VA, Pandey G, Shetty S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S80–9. doi:10.1016/j.jceh.2014.05.004. [Google Scholar] [PubMed] [CrossRef]
29. Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer. 2018;7(3):235–60. [Google Scholar] [PubMed]
30. Qiao SS, Cui ZQ, Gong L, Han H, Chen PC, Guo LM, et al. Simultaneous measurements of serum AFP, GPC-3 and HCCR for diagnosing hepatocellular carcinoma. Hepatogastroenterology. 2011;58(110–111):1718–24. [Google Scholar] [PubMed]
31. Attia AM, Rezaee-Zavareh MS, Hwang SY, Kim N, Adetyan H, Yalda T, et al. Novel biomarkers for early detection of hepatocellular carcinoma. Diagnostics. 2024;14(20):2278. doi:10.3390/diagnostics14202278. [Google Scholar] [PubMed] [CrossRef]
32. Wang D, Shen Y, Qian H, Jiang J, Xu W. Emerging advanced approaches for liquid biopsy: in situ nucleic acid assays of extracellular vesicles. Theranostics. 2024;14(19):7309–32. doi:10.7150/thno.102437. [Google Scholar] [PubMed] [CrossRef]
33. Yen YT, Zhao C, Gao T, Yang JZ, Kang N, Pu J, et al. Tumour extracellular vesicle surface Protein-mRNA integration assay for early detection of epithelial ovarian cancer. eBioMedicine. 2025;119:105884. [Google Scholar] [PubMed]
34. Lehrich BM, Zhang J, Monga SP, Dhanasekaran R. Battle of the biopsies: role of tissue and liquid biopsy in hepatocellular carcinoma. J Hepatol. 2024;80(3):515–30. doi:10.1016/j.jhep.2023.11.030. [Google Scholar] [PubMed] [CrossRef]
35. Lopez K, Lai SWT, Lopez Gonzalez EJ, Davila RG, Shuck SC. Extracellular vesicles: a dive into their role in the tumor microenvironment and cancer progression. Front Cell Dev Biol. 2023;11:1154576. doi:10.3389/fcell.2023.1154576. [Google Scholar] [PubMed] [CrossRef]
36. Sohal IS, Kasinski AL. Emerging diversity in extracellular vesicles and their roles in cancer. Front Oncol. 2023;13:1167717. doi:10.3389/fonc.2023.1167717. [Google Scholar] [PubMed] [CrossRef]
37. Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A. 2015;112(12):E1433–42. doi:10.1073/pnas.1418401112. [Google Scholar] [PubMed] [CrossRef]
38. Yang J, Li X, Zheng S. Letter GULP1 as a novel diagnostic and predictive biomarker in hepatocellular carcinoma. Clin Mol Hepatol. 2025. doi:10.3350/cmh.2025.0216. [Google Scholar] [PubMed] [CrossRef]
39. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [Google Scholar] [PubMed]
40. Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-mediated metastasis: communication from a distance. Dev Cell. 2019;49(3):347–60. [Google Scholar] [PubMed]
41. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–85. doi:10.1038/ncb2502. [Google Scholar] [PubMed] [CrossRef]
42. He G, Peng X, Wei S, Yang S, Li X, Huang M, et al. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. 2022;21(1):19. doi:10.1186/s12943-021-01440-5. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools